Lumen-apposing metal stents (LAMSs) can be used for gastrojejunostomy (GJ) under natural orifice transluminal endoscopic surgery (NOTES) or EUS guidance. EUS-GJ requires both LAMS flanges to be properly placed. Proximal flange misplacement during LAMS deployment into the small bowel or the gallbladder has occasionally been salvaged by a bridging tubular SEMS. However, the only 2 reported instances of distal LAMS flange misplacement during EUS-GJ resulted in procedural failure. We report a successful NOTES approach to salvage distal LAMS flange misplacement during EUS-GJ.
An 80-year-old woman with metastatic pancreatic cancer and a biliary SEMS experienced symptoms of gastric outlet obstruction 4.5 months after diagnosis. Guidewire insertion across a stricture in the third part of the duodenum failed. She was not a surgical candidate, and direct EUS-GJ was chosen.
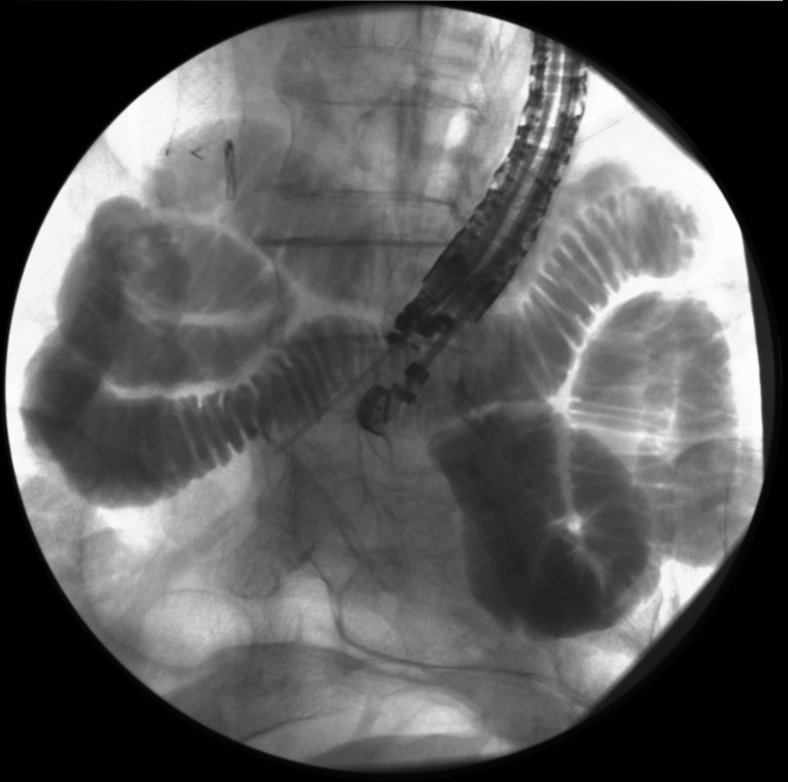
Loops of small bowel were initially distended with saline solution injection through a 22-gauge needle before puncture with a 19-gauge needle for enterography (Fig. 1). Small-bowel loops close to the duodenum were accessed freehand with a cautery-tipped LAMS delivery catheter. During catheter advancement, the bowel loop was inadvertently tented away from the stomach because the US window was momentarily lost. After deployment of a 15-mm by 10-mm LAMS (Fig. 2), the peritoneal cavity was identified endoscopically from the stomach through the LAMS. The EUS endoscope was exchanged for a therapeutic gastroscope (Video 1, available online at www.VideoGIE.org).
Figure 1.
EUS-guided enteroclysis before insertion of lumen-apposing metal stent.
Figure 2.
Distal flange of lumen-apposing metal stent after inadvertent intraperitoneal deployment.
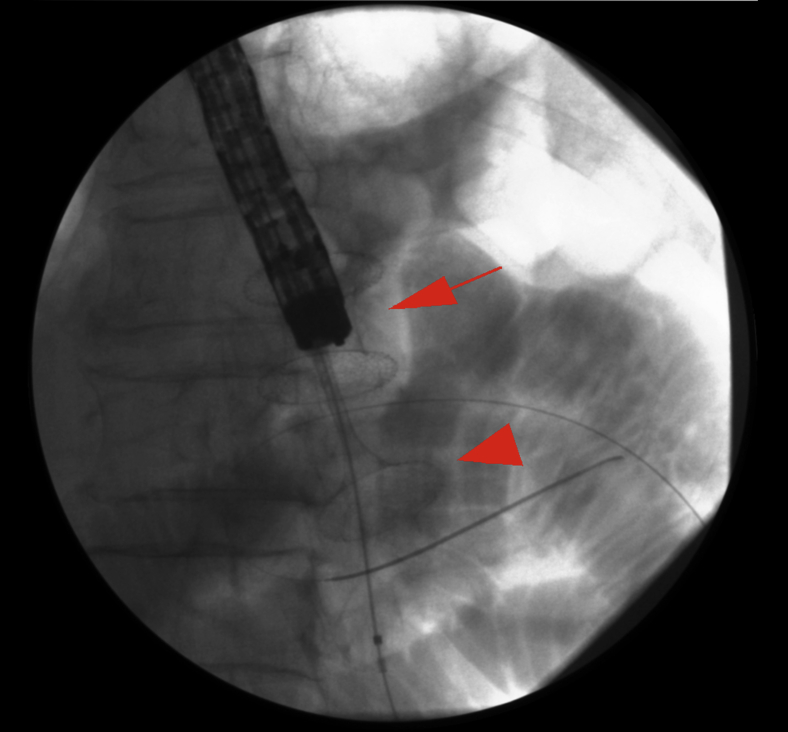
After LAMS balloon expansion up to 15 mm, the gastroscope was passed through the transgastric LAMS into the peritoneal cavity. A small-bowel loop was targeted under NOTES peritoneoscopy and fluoroscopy, and after gently being suctioned into the distal LAMS flange, its wall was cut with a needle-knife by the use of high coagulation settings (Fig. 3). A guidewire was passed through the needle-knife into the loop under combined fluoroscopy. Another 15-mm by 10-mm LAMS delivery catheter was advanced over the wire into the jejunal loop.
Figure 3.
Small-bowel suction into transgastric lumen-apposing metal stent before needle-knife incision.
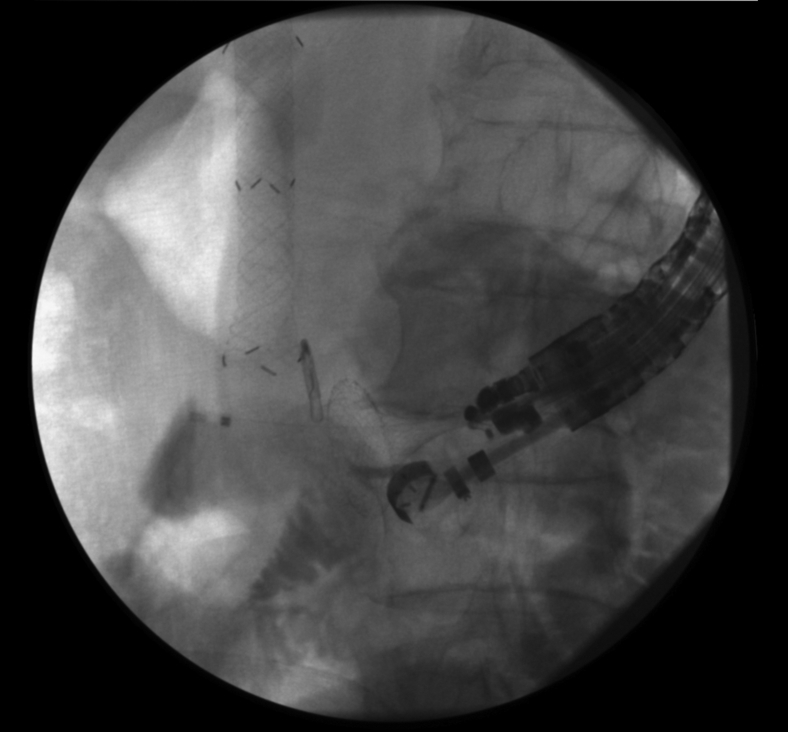
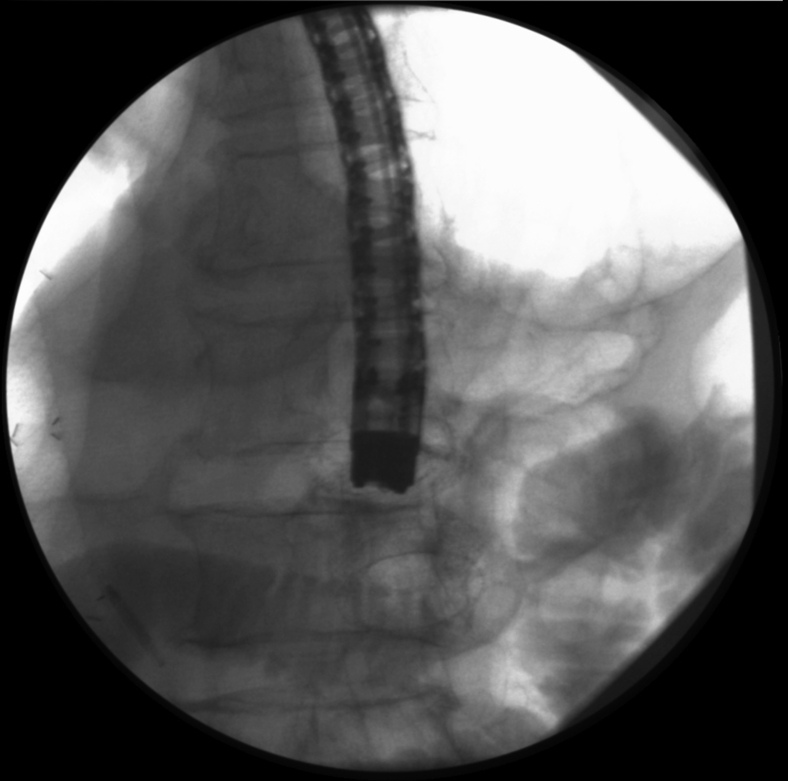
After deployment of the distal flange, the gastroscope-stent delivery assembly was withdrawn toward the stomach (Fig. 4), and the proximal flange of the second LAMS was deployed inside the originally misplaced transgastric LAMS by careful coupling of the distal flange of the second LAMS with the distal flange of the first LAMS. The second LAMS was thus deployed fully inside the initially misplaced LAMS (Fig. 5).
Figure 4.
Traction of second LAMS delivery catheter with distal flange deployed inside small bowel (arrowhead) into transgastric LAMS (arrow) to perform LAMS in LAMS. LAMS, lumen-apposing metal stent.
Figure 5.
Fluoroscopic view of second LAMS fully deployed within initially misplaced LAMS. Contrast material flushed through the gastroscope remains within the small bowel with no leakage. LAMS, lumen-apposing metal stent.
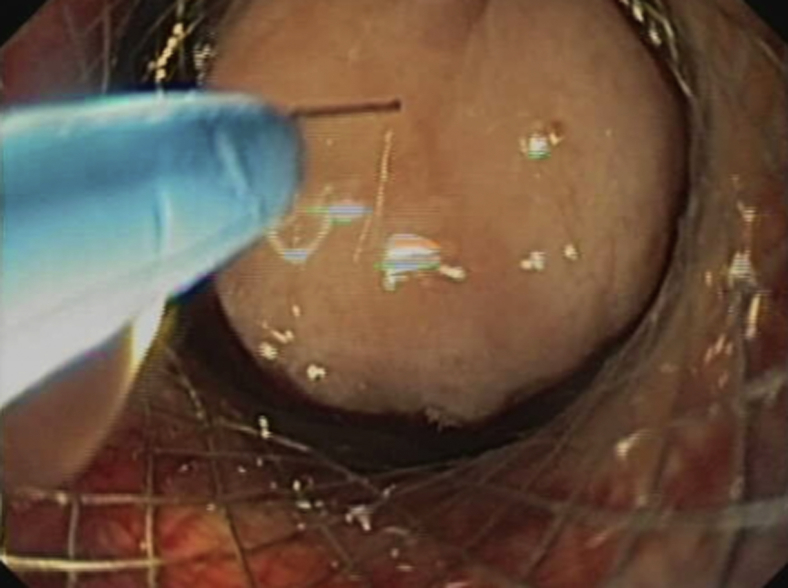
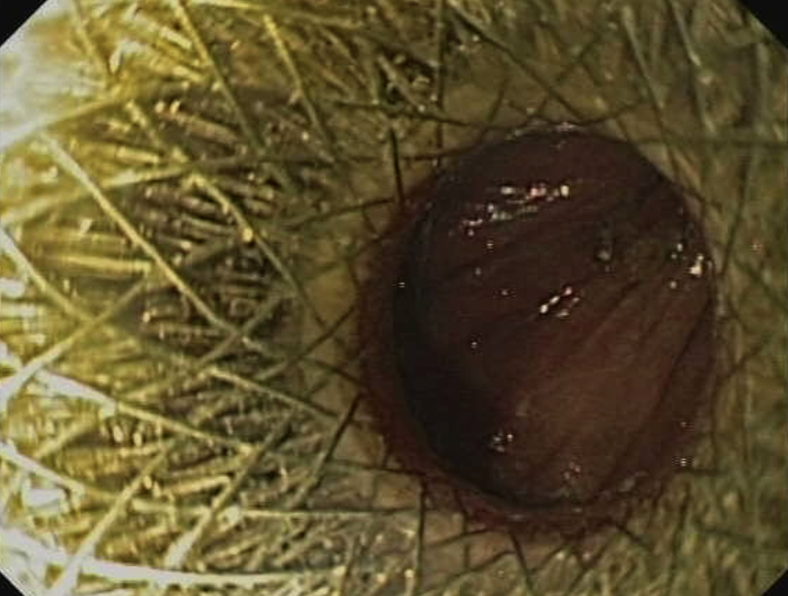
Endoscopy confirmed proper placement without leakage of contrast material (Figs. 5 and 6). The entire procedure was performed in the endoscopy unit with the patient under antibiotic prophylaxis, CO2 insufflation, and endoscopist-directed propofol sedation. Abdominal wall puncture for pneumperitoneum control was not required. No adverse events ensued. The patient started oral feeding within 24 hours and remained well throughout a 10-month follow-up, without any recurrent biliary or gastric outlet obstructive symptoms.
Figure 6.
Endoscopic view after deployment of second lumen-apposing metal stent, showing jejunal lumen and 2 metal stent meshes, 1 inside the other.
EUS-guided access into the small bowel is challenging because of the inherent mobility of the small bowel. In our case, this resulted in misplacement of the distal LAMS flange. The delivery catheter pushed away the small bowel without entering it, and this was missed on EUS. A transgastric misplaced LAMS was then used as an internal trocar for NOTES peritoneoscopy and successful LAMS-in-LAMS salvage of distal flange misplacement. The misplaced LAMS served as a trocar to facilitate passage of a standard therapeutic gastroscope. A double-channel gastroscope has been used by others to counter the mobility of the small bowel.
Despite the use of a standard single-channel therapeutic gastroscope in our case, guidewire stabilization, minimal CO2 insufflation, careful maneuvering, and fluoroscopic monitoring helped to successfully advance the LAMS delivery catheter into the small bowel during NOTES. By deployment of the second LAMS fully inside the previous LAMS, secure apposition was obtained, as evidenced by the good long-term outcome in our patient.
The available literature is consistent with a minimal risk of delayed migration when a LAMS is used to create a GJ, as opposed to when LAMSs are used for drainage of pancreatic fluid collections. During LAMS use for GJ, additional anchoring measures such as clipping or double-pigtail stents are probably unnecessary. However, initial access and LAMS deployment are highly challenging.
Disclosure
M. Perez-Miranda is a consultant for Boston Scientific, Gore and M.I. Tech. All other authors disclosed no financial relationships relevant to this publication.
Footnotes
Written transcript of the video audio is available online at www.VideoGIE.org.
Supplementary data
Performance of the “stent in stent” technique to salvage distal flange misplacement during EUS-guided gastrojejunostomy in a patient with a malignant gastric outlet obstruction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Performance of the “stent in stent” technique to salvage distal flange misplacement during EUS-guided gastrojejunostomy in a patient with a malignant gastric outlet obstruction.