Significance
Millions of individuals obtain insufficient sleep on a daily basis, which leads to impaired performance. Whether these decrements are caused by short sleep duration or extended wakefulness is unknown. In this study, healthy volunteers were randomized into either a chronically sleep-restricted or control protocol while living on a 20-h “day,” thus enabling short sleep without extended wakefulness. We demonstrate that chronic insufficient sleep, even without extended wakefulness, leads to neurobehavioral performance decrements at all times of the day, even when the circadian system is promoting arousal. These findings have implications for the understanding of basic physiology, the substantial population who chronically obtains insufficient sleep, and all of us who depend on sleep-restricted individuals working in safety-sensitive occupations.
Keywords: insufficient sleep, forced desynchrony, circadian, alertness, sleepiness
Abstract
Millions of individuals routinely remain awake for more than 18 h daily, which causes performance decrements. It is unknown if these functional impairments are the result of that extended wakefulness or from the associated shortened sleep durations. We therefore examined changes in objective reaction time performance and subjective alertness in a 32-d inpatient protocol in which participants were scheduled to wakefulness durations below 16 h while on a 20-h “day,” with randomization into standard sleep:wake ratio (1:2) or chronic sleep restriction (CSR) ratio (1:3.3) conditions. This protocol allowed determination of the contribution of sleep deficiency independent of extended wakefulness, since individual episodes of wakefulness in the CSR condition were only 15.33 h in duration (less than the usual 16 h of wakefulness in a 24-h day) and sleep episodes were 4.67 h in duration each cycle. We found that chronic short sleep duration, even without extended wakefulness, doubled neurobehavioral reaction time performance and increased lapses of attention fivefold, yet did not uniformly decrease self-reported alertness. Further, these impairments in neurobehavioral performance were worsened during the circadian night and were not recovered during the circadian day, indicating that the deleterious effect from the homeostatic buildup of CSR is expressed even during the circadian promotion of daytime arousal. These findings reveal a fundamental aspect of human biology: Chronic insufficient sleep duration equivalent to 5.6 h of sleep opportunity per 24 h impairs neurobehavioral performance and self-assessment of alertness, even without extended wakefulness.
Sleep is a vital process necessary for multiple physiological processes, including restoration of brain energy metabolism, neuronal reorganization, and repair following waking activity (1). Millions of individuals, however, routinely sleep less than 6 h per night on workdays (2), an amount shown to be insufficient for maintaining healthy physiological functioning (3, 4). Sleep loss can be the result of acute sleep deprivation (i.e., one extended wake episode with no sleep), acute sleep restriction (i.e., one extended wake episode with shortened sleep duration), or a cumulative buildup of insufficient sleep with wake durations greater than 16 h over consecutive days. These forms of sleep loss all predispose an individual to increased lapses of attention, errors, and accidents (5–8), with pronounced decrements of performance occurring during the circadian night (4, 7–9), when circadian rhythms promote sleep (9). It is unknown, however, if the impairments in neurobehavioral performance are the result of the homeostatic buildup of sleep pressure during extended wakefulness or from the inadequate amount of sleep. In prior studies, the impact of sleep deficiency has been confounded by the concurrent increase in wake duration, leading some to conclude that it is not sleep deficiency per se that impairs performance but rather a cumulative cost of additional wakefulness (6, 7). Thus, identifying the impact of short sleep in the absence of extended durations of wakefulness is essential to evaluating this fundamental aspect of human biology and to designing appropriate interventions for clinical, public health, and safety purposes.
We designed an intensive 32-d inpatient protocol to measure the effects of chronic sleep restriction (CSR) without extended wakefulness, which we define in the current study as wakefulness longer than 16 h, by randomizing healthy participants into either a control 1:2 sleep:wake schedule (Fig. 1A; n = 8) or a sleep-restricted 1:3.3 sleep:wake schedule (Fig. 1B; n = 9) on a 20-h “day.” The protocol utilized a forced desynchrony (FD) design, in which the intrinsic circadian pacemaker cycles at its endogenous ∼24.15-h period (10) during these 20-h days, resulting in sleep and wakefulness activities being evenly distributed relative to circadian timing over the FD protocol (11). Importantly, the protocol allowed for altering the finite amounts of sleep and wakefulness for the control (6.67 h sleep opportunity, 13.33 h wakefulness) and CSR (4.67 h sleep, 15.33 h wakefulness) conditions while maintaining the ratios equivalent to 8 or 5.6 h of sleep opportunity, respectively, on a 24-h day. The protocol also enabled observation of neurobehavioral and physiological changes at differing combinations of length of time awake and circadian phase, allowing for the uncoupling (i.e., “forced desynchrony”) of the two processes (12, 13).
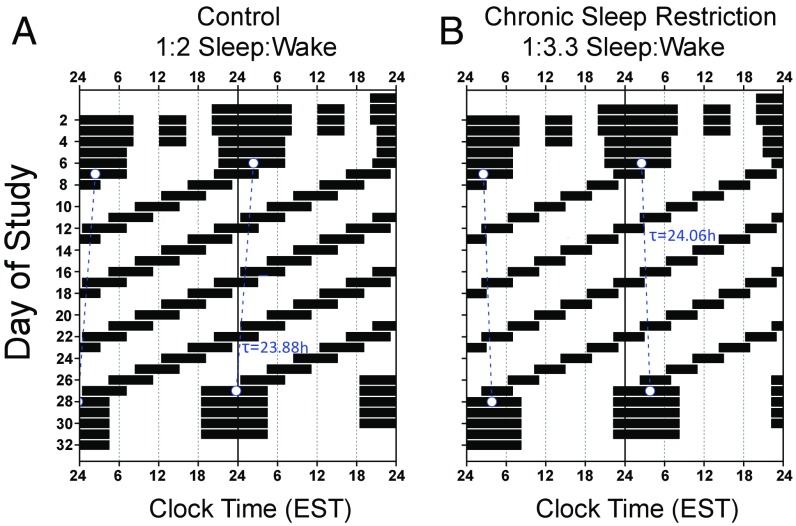
Fig. 1.
Raster plot of a (A) control (n = 8, 1:2 sleep:wake ratio) and (B) chronic sleep restriction (n = 9, 1:3.3 sleep:wake ratio) participant’s forced desynchrony protocol. Time is plotted on the horizontal axis and day of study is on the vertical axis. Days are double plotted such that each consecutive study day is plotted next to and below the previous day. Black bars represent sleep opportunities, and the blue dashed line represents each participant’s circadian period as derived from melatonin.
Results
During wakefulness, participants completed a series of cognitive neurobehavioral tests, including the psychomotor vigilance task (PVT) to test for sustained attention, visual analog scales (VAS) for self-reported alertness (14, 15), and Karolinska Sleepiness Scale (KSS) for self-reported sleepiness (8, 16). Mixed-effects statistical models were used to examine the effect of length of time awake, condition or sleep duration, circadian phase, beat cycle (i.e., time to complete a cycle of circadian and sleep:wake schedule combinations, which was ∼6 protocol days in this FD design), and their interaction. PVT reaction time (RT) metrics were the median RT and the number of lapses of attention (defined as RT responses >500 ms).
Influence of CSR Without Extended Wakefulness on Neurobehavioral Performance.
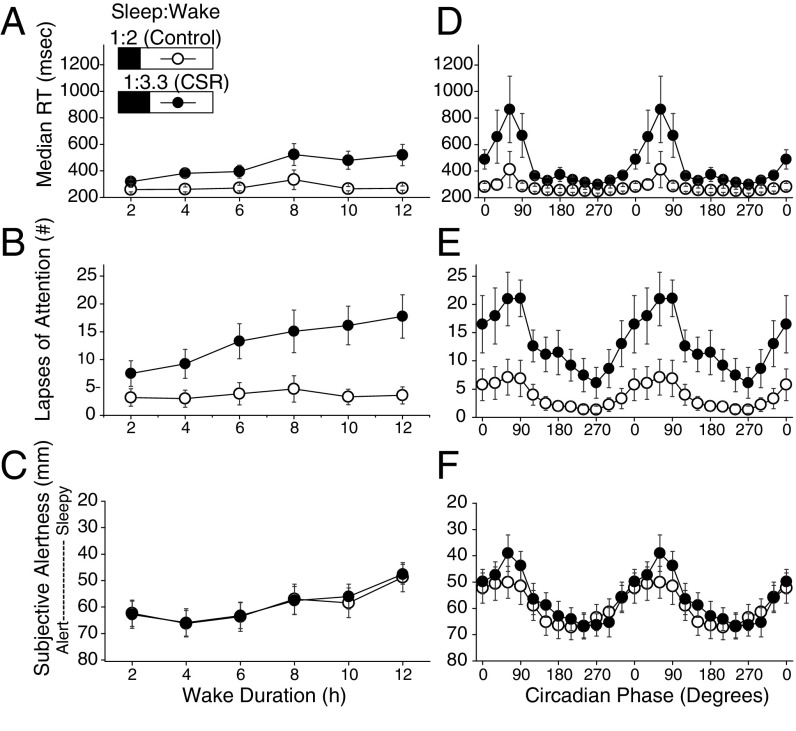
PVT median RT and lapses of attention remained relatively stable across wakefulness (i.e., increasing time awake) for the control condition, but became increasingly impaired in the CSR condition across wakefulness up to double that of the control condition for median RT (Fig. 2A, time awake x condition, P < 0.001) and up to a fivefold increase in lapses of attention (Fig. 2B, time awake x condition, P < 0.0001). Subjective alertness (VAS) significantly decreased (Fig. 2C, time awake, P < 0.0001) and subjective sleepiness (KSS) significantly increased (Fig. S1A, time awake, P < 0.0001) for both conditions across wakefulness. For both the control and CSR conditions, PVT performance peaked (i.e., shortest RT) at 270 circadian degrees (late circadian day) and reached its nadir at circadian 60° (mid circadian night) (Fig. 2 D and E), with significantly slower median RT and increased lapses of attention in the CSR condition across circadian phases (Fig. 2D, circadian phase x condition, P < 0.0001; Fig. 2E, circadian phase x condition, P < 0.0001). Subjective alertness peaked at 240 circadian degrees and reached its nadir at 60 circadian degrees (Fig. 2F) and subjective sleepiness, as expected, followed the opposite pattern, with a nadir at 240 circadian degrees and a peak at 60 circadian degrees (Fig. S1B). There were no control vs. CSR condition differences in subjective alertness (Fig. 2C, condition, P = 0.94; Fig. 2F, condition, P = 0.98) or sleepiness (Fig. S1A, condition, P = 0.90; Fig. S1B, condition, P = 0.62), suggesting a disassociation between objective performance and subjective alertness/sleepiness, as has been previously described during CSR (6, 17). When within–testing-session PVT and subjective alertness results were compared, there were large interindividual differences in the relationships (Fig. S2), with larger variation in the CSR group.
Fig. 2.
Psychomotor vigilance task median reaction time, PVT lapses of attention, and subjective alertness from the visual analog scales by duration of wakefulness and circadian phase. The control (n = 8, 1:2 sleep:wake ratio) condition is denoted by open circles and the CSR (n = 9, 1:3.3 sleep:wake ratio) condition is denoted by closed circles. Higher median RT and number of lapses and lower alertness scores (note inverted axis scales in C and F) indicate worse PVT performance and subjective alertness. (A–C) Median RT (A), lapses of attention (B), and subjective alertness (C) across wake duration. (D–F) PVT median RT performance (D), lapses of attention (E), and subjective alertness (F) across circadian phase (double plotted). Error bars represent SEM.
Impact of Acute and Chronic Exposure to Sleep Restriction During the Circadian Day and Night on Neurobehavioral Performance.
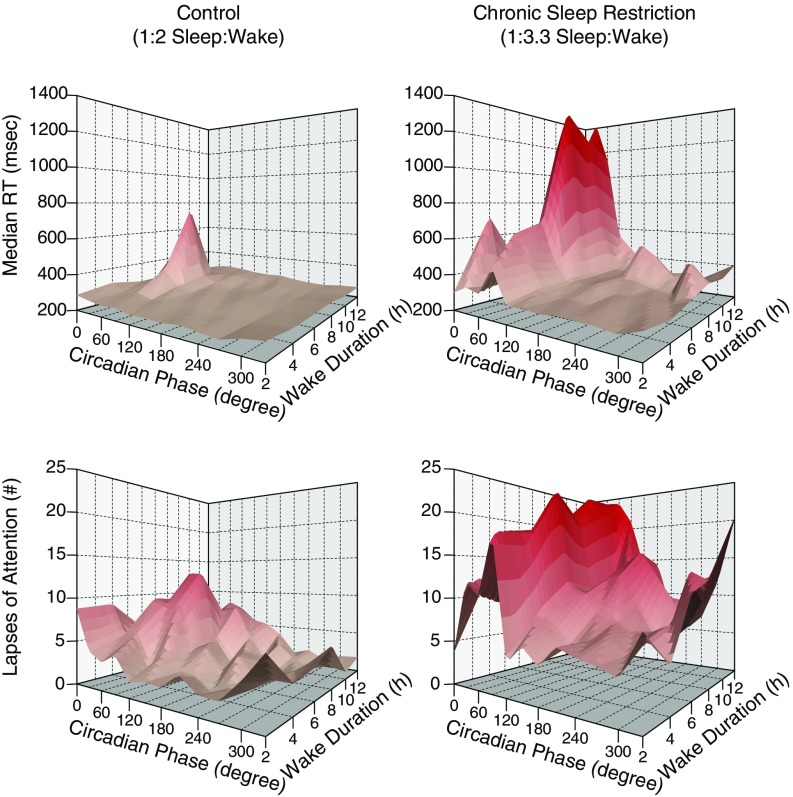
There was a significant interaction for impaired performance between time awake and circadian phase for increased median RT and number of lapses of attention in the CSR condition, such that the performance decrements observed when time awake increased were dependent on circadian phase (Fig. 3; circadian phase x time awake, P < 0.05; circadian phase x time awake, P < 0.0001), with much worse performance at testing associated with both more than ∼7 h awake and during the circadian night.
Fig. 3.
Interaction of circadian phase, hours of wakefulness, and PVT performance. Higher median RT and number of lapses indicate worse PVT performance. PVT median RT (Top), lapses of attention (Bottom), control condition (n = 8, Left), and CSR condition (n = 9, Right).
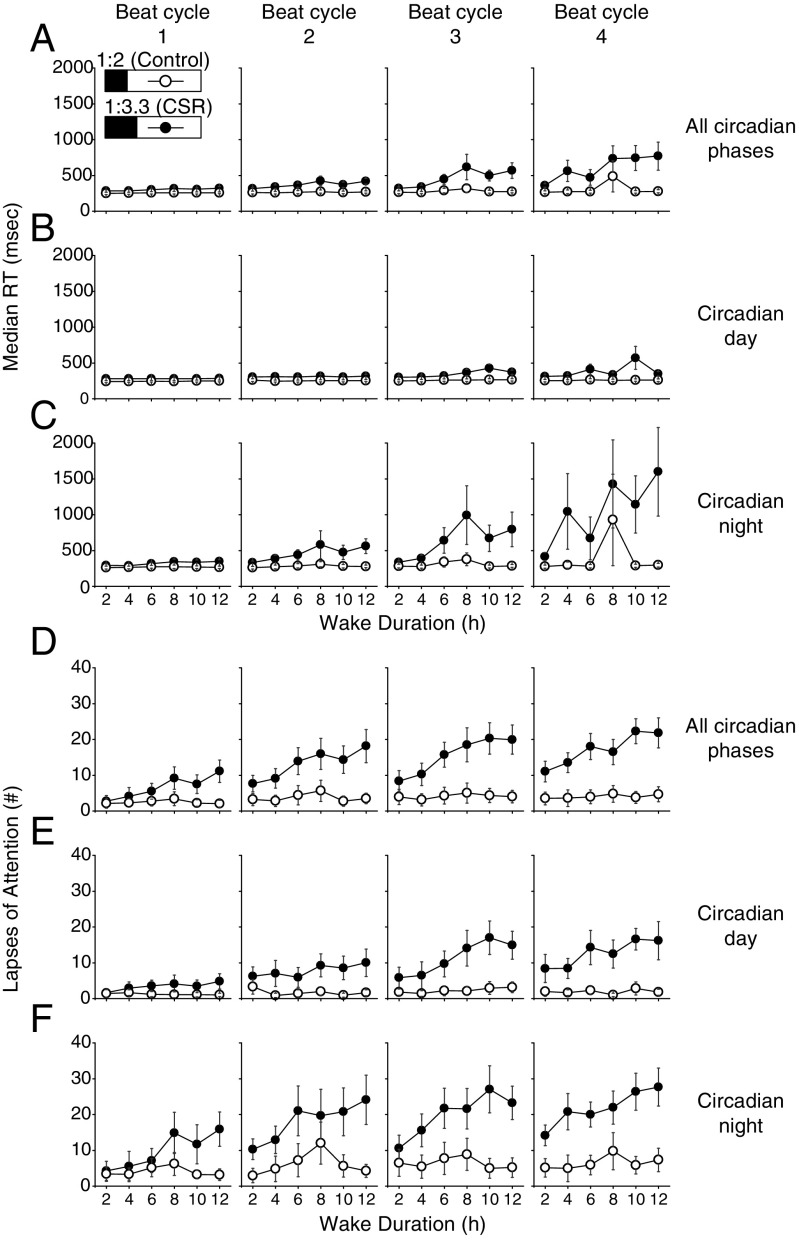
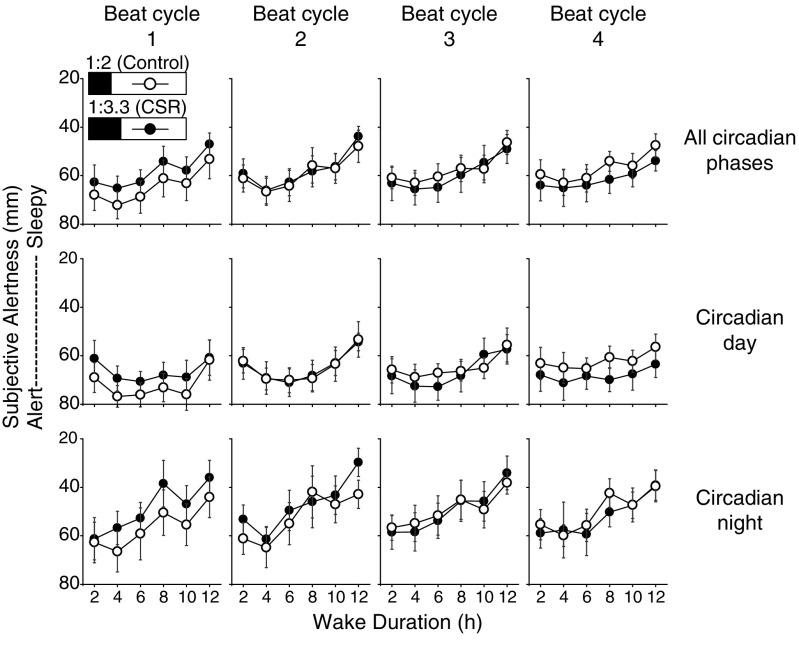
To quantify the chronic effects of sleep restriction and its interactions with time awake and circadian timing (i.e., circadian day or night), performance was analyzed as the FD protocol continued by beat cycle and by all circadian phases, circadian day, and circadian night. We found no difference in median RT in the control group across beat cycles but a significant increase in median RT for the CSR group across adjacent beat cycles 1 through 4 (3 adjusted beat cycle, all P < 0.05). There was a significant increase in the number of PVT lapses between adjacent beat cycles 1 through 4 (3 adjusted beat cycles, all P < 0.05) for both the CSR and control groups. When we compared the two conditions, we found a significant two-way interaction between condition x beat cycle for median RT in the CSR condition across all circadian phases (Fig. 4A, condition x beat cycles, P < 0.0001) and for tests occurring during the circadian day (Fig. 4B, condition x beat cycle, P < 0.0001), and an over fivefold slower median RT for tests occurring only during the circadian night (Fig. 4C, condition x beat cycle, P < 0.0001). There was a significant three-way interaction for condition x beat cycle x time awake for increased PVT lapses in the CSR condition across all circadian phases (Fig. 4D, condition x beat cycle x time awake, P < 0.0001), for tests only occurring during the circadian day (Fig. 4E, condition x beat cycle x time awake, P < 0.0001), and for tests occurring during the circadian night (Fig. 4F, condition x beat cycle x time awake, P < 0.0001). Given that we have previously reported that the circadian day can recover performance decrements observed during CSR with the same wake:sleep ratio but with extended wakefulness up to 32.85 h combined with an extended (10-h) sleep episode (5), this finding was remarkable. Differences between conditions in PVT median RT began at the second beat cycle (Fig. 4 A–C, all conditions, P < 0.05) and in lapses began at the first beat cycle (Fig. 4 D–F, all conditions, P < 0.05). We did not find any control vs. CSR condition differences in subjective alertness (Fig. 5, all conditions x beat cycle x time awake, P > 0.05) or subjective sleepiness scores (Fig. S3, all conditions x beat cycle x time awake, P > 0.05) across beat cycles of the protocol.
Fig. 4.
Acute and chronic impact of sleep restriction on PVT performance during the circadian day and night. The control (n = 8, 1:2 sleep:wake ratio) condition is denoted by open circles and the CSR (n = 9, 1:3.3 sleep:wake ratio) condition is denoted by closed circles. Higher median RT and number of lapses indicate worse PVT performance. Beat cycles (i.e., time to complete a cycle of circadian and sleep:wake schedule combinations, which was ∼6 protocol days in this forced desynchrony design) of the protocol are shown from Left to Right. Data are presented across wake duration for tests performed at all circadian phases (A and D) and during the circadian day (B and E) and circadian night (C and F). Error bars represent SEM.
Fig. 5.
Acute and chronic impact of sleep restriction on subjective alertness during the circadian day and night. The control (n = 8, 1:2 sleep:wake ratio) condition is denoted by open circles and the CSR (n = 9, 1:3.3 sleep:wake ratio) condition is denoted by closed circles. Higher alertness scores indicate higher subjective alertness; note inverted axis scales in all panels. Beat cycles (i.e., time to complete a cycle of circadian and sleep:wake schedule combinations, which was ∼6 protocol days in this forced desynchrony design) of the protocol are shown from Left to Right. Data are presented across wakefulness for tests performed at all circadian phases (Top) and during the circadian day (Middle) and circadian night (Bottom). Error bars represent SEM.
Sleep Measures for Control and CSR Conditions.
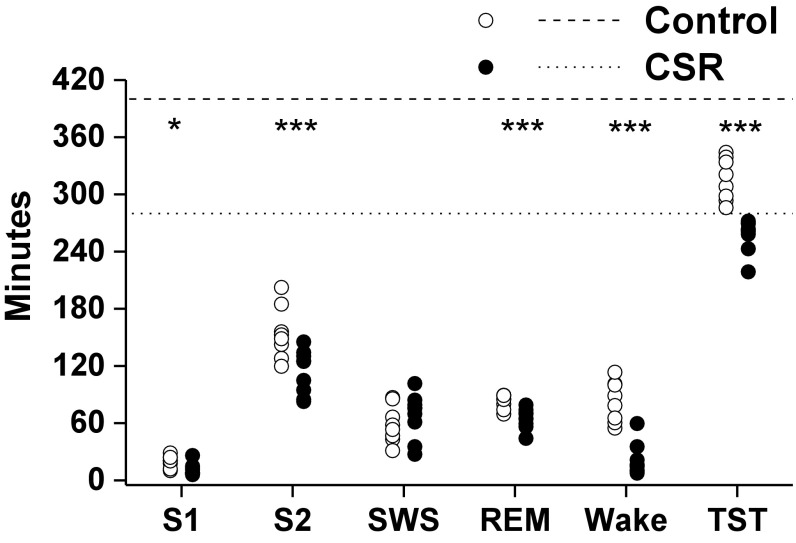
Compared with the control condition, during the FD protocol participants in the CSR condition had significantly lower total sleep time [TST; 4.3 ± 0.06 h vs. 5.2 ± 0.23 h; (mean ± SEM)], specifically in non-rapid eye movement (NREM) stage 1 sleep (P < 0.05, 11.3 ± 1.1 min vs. 19.8 ± 1.6 min), NREM stage 2 sleep (P < 0.001, 113.3 ± 3.9 min vs. 152.7 ± 8.4 min), and REM sleep (P < 0.001, 64.7 ± 3.7 min vs. 82.3 ± 6.3 min) but not in slow-wave sleep (SWS; P = 0.41, 67.6 ± 3.0 min vs. 59.0 ± 3.3 min) (Fig. 6). Participants in the CSR condition had significantly less wakefulness during the sleep opportunities (P < 0.001, 21.8 ± 3.4 min vs. 84.4 ± 13.9 min) corresponding to a significant condition effect for sleep efficiency between the CSR and control conditions, such that the CSR condition had higher sleep efficiency compared with the control (P < 0.0001, 92.0 ± 1.2% vs. 78.5 ± 3.5%).
Fig. 6.
Minutes of sleep opportunity spent in each sleep stage or wakefulness. The control (1:2 sleep:wake ratio) condition is denoted by open circles and the CSR (1:3.3 sleep:wake ratio) condition is denoted by closed circles. The dashed and dotted horizontal lines denote minutes of sleep opportunity provided for the control (6.67 h) and CSR (4.67 h) conditions, respectively. The asterisks represent significant differences between conditions; *P < 0.05 and ***P < 0.001. S1, non-rapid eye movement sleep stage 1; S2, NREM sleep stage 2; SWS, slow-wave sleep (NREM sleep stage 3+4); TST, total sleep time.
We also tested the effects of using a sleep metric (from each sleep episode before PVT, VAS, or KSS testing) instead of condition (control vs. CSR) in the analyses; wake duration, circadian phase, and length of time within the protocol were also included as variables. When NREM sleep stage 1, NREM sleep stage 2, SWS, or TST were included individually, all sleep metrics were significant covariates of the PVT median RT, PVT lapses, VAS, and KSS. When all sleep metrics were included in the model in a multivariable analysis, only TST was a significant covariate of the two PVT metrics and KSS, while NREM sleep stage 1 and TST were significant covariates of VAS.
Discussion
These findings reveal fundamental relationships among sleep duration, sleep timing, objective vigilant performance, and subjective alertness and sleepiness. Specifically, these data demonstrate that chronic short sleep duration, in the absence of extended wakefulness, impairs neurobehavioral performance, and that these impairments are worse during the circadian night. These impairments are also not recovered during the circadian day [which we observed in another chronic sleep restriction protocol with a longer wake and sleep episode (5)], indicating that the deleterious effect from the homeostatic buildup of CSR cannot be overcome by the strong circadian drive for daytime arousal (5). The current results differ from our study with the same 1:3.3 ratio but 10 h of sleep opportunity (5), in which performance during the circadian day was not different between CSR and control conditions; thus, these results emphasize the importance of sleep duration even in CSR conditions: 4.67 h of sleep opportunity is not sufficient for even short-term recovery (i.e., for the first 6 h after awakening) of neurobehavioral function as was found during CSR with a 10-h sleep opportunity (5).
The interactions among hours awake, control or CSR condition, circadian timing, and beat cycle (i.e., time into the protocol) demonstrate that short sleep durations may act both acutely and chronically via an incurred sleep debt. We have previously demonstrated that sleep restriction in humans affects at least two dissociable processes that act on different timescales (5): one associated with each wake episode, and one across multiple wake episodes. In a prior publication of a study using the same FD design and sleep:wake ratios but much longer durations of sleep and wakefulness (i.e., 42.85-h day with 10 h sleep, 32.85 h wakefulness) for 3 calendar weeks, acute sleep loss from the extended wake episodes caused neurobehavioral performance to deteriorate with each passing hour. Of especial interest was that this acute homeostatic process was rapidly reset by each 10-h sleep opportunity, restoring performance to normal levels for the first ∼6 h after waking (before the deterioration began). However, despite this rapid recovery of acute sleep loss from extended sleep opportunities (transiently returning performance to rested levels), the effects of CSR continued to build throughout that experiment; after the “normal” levels observed for the first few hours after awakening, vigilant performance deteriorated for each additional hour spent awake, with the rate of decay increasing as the CSR continued (5). In other FD designs with differing day lengths, including extended wakefulness (18) or shorter wakefulness and sleep episodes than the current protocol (19), there are similar reports of impaired performance, particularly during the circadian night. However, those reports differed from our study in three critical features: Much shorter exposure to insufficient sleep than the current protocol (i.e., only lasted one beat cycle), only studied males, and provided limited data on how much sleep individuals actually obtained before starting the study, so it is not documented whether individuals entered the FD portion (both control and sleep-restricted) with any self-imposed CSR (18, 19). The current findings parallel and extend these previous results, and indicate that extended wakefulness is not necessary for vigilant performance decrements during sleep restriction. Recent work has suggested that these performance decrements during acute sleep restriction may be due to regional changes in brain responsiveness across time awake and differing circadian phases (20). The increased sensitivity to the behavioral effects of each additional hour spent awake after chronic exposure to sleep restriction may be due to an increased density in adenosine receptors, as proposed in animal models (21, 22) and mathematical modeling (22); however, this is yet to be explored in humans. Nevertheless, whether the effects of sleep restriction are a result of this homeostatic imbalance between sleep and wakefulness amounts leading to a shift in the homeostatic setpoint, or to other hypothesized mechanisms, is a subject of open debate in the field (22, 23), though the results in the present manuscript contribute an important set of data to this debate. Furthermore, our findings of no significant differences in SWS, a marker of the acute sleep homeostatic process (24), suggest that an incomplete dissipation of sleep “pressure” may not cause the impaired performance in the CSR condition. It is important to highlight that this protocol did not include sleep deprivation (i.e., the maximum waking lengths were <17 h) and did include a chronic circadian desynchrony, though chronic circadian desynchrony has not been found to induce a cumulative impairment in this control group or in other extended forced desynchrony control conditions (5).
Our findings of performance decrements during both the circadian day and night are notable, as prior reports of a dose–response to chronic insufficient sleep have been conducted on a standard 24-h day+night cycle and/or included extended wakefulness. On a standard 24-h day+night cycle, scheduled decreases in sleep duration (e.g., 3, 4, 5, 6, or 7 h of sleep per night) are necessarily associated with extension in wake duration (e.g., 21, 20, 19, 18, or 17 h of wakefulness per day, respectively). In fact, Van Dongen et al. (6) concluded that the extension of wakefulness was primarily responsible for the observed deterioration of performance. One of the innovative features of our study was the decision to use a 20-h day+night cycle. This enabled us to study sustained sleep restriction (using short 4.67-h sleep episodes) without exposing participants to extended episodes of wakefulness (since all wake episodes were 15.33 h long), while simultaneously accounting for circadian phase, which is a confounding effect of CSR. In our study, which utilized a 20-h day+night cycle, the sleep–wake cycle was desynchronized from the underlying circadian rhythms, allowing us to distinguish the effects of circadian rhythms on performance from those induced by length of time awake. Therefore, we could assess the effects of performance both during the circadian day (i.e., habitual wake times) and circadian “night” (i.e., habitual sleep times). Previous dose–response studies (6, 7) only studied individuals’ performance during the circadian day. We show that the performance decrements are much larger during the circadian night compared with the circadian day. Failure to assess the impact of CSR at this most vulnerable circadian phase may undermine the generalizability of previous work.
Our observations may also highlight the importance of longer sleep episodes, rather than a “split sleep” schedule (in which an individual has two sleep episodes within a 24-h day), independent of length of time awake. Split sleep schedules have been suggested as a strategy to combat performance decrements associated with night shift work or inability to obtain adequate length of consolidated sleep, with reports of limited impairments in performance during such split sleep schedules (25, 26). However, chronic exposure to split sleep durations, with finite amounts of sleep similar to that tested in the current protocol, has not previously been evaluated. In a study of split sleep schedules under sleep restriction, Mollicone et al. (27) found that performance decrements occurred as a function of total amount of sleep per 24 h, with less sleep per 24 h worsening performance. However, the maximum wakefulness durations in that report consisted of 9.7 h, performance was not measured during the circadian night, and the schedule was only maintained for 10 d (27). In the present study, maximum wakefulness durations were a few hours longer and included measurements at all circadian phases. Moreover, differences in median RT performance between the control and CSR conditions only emerged during the second beat cycle (after about 2 calendar weeks of exposure); therefore, long-term short split sleep schedules may impair performance, whereas short-term split sleep schedules may sustain performance when a single longer sleep duration is not feasible (28, 29).
It is also important to note that there were no differences in subjective alertness or sleepiness between control and CSR conditions in this experiment; this disassociation between subjective alertness or sleepiness and objective performance may be detrimental to safety during work or daily activities (i.e., driving) when an individual may feel alert/not sleepy—and therefore decide to continue working or activities without more sleep or countermeasures—and yet exhibit performance impairment. This may be of particular importance to specific individuals, as this disassociation may be consistent within individuals (30) and can be exacerbated if a task requires physical labor (31). Thus, an individual’s failure to recognize the impairment is a public safety concern.
These data uncover fundamental interactions among sleep, the circadian system, neurobehavioral performance, and subjective alertness and sleepiness, and force a reconsideration of causes of impaired performance associated with sleep deficiency. The current study was not designed to mimic the exact circumstances under which chronic sleep restriction occurs in everyday life; rather, it was designed to elucidate physiological phenomena that can be the basis for future studies and interventions. Nonetheless, our findings do have implications for the millions of individuals who chronically obtain insufficient sleep, the substantial population who works during the biological night, and all of us who depend on individuals working in safety-sensitive occupations or activities who have not obtained sufficient sleep.
Materials and Methods
Participants.
Seventeen healthy volunteers [10 females; age 26.1 ± 4.4 y, 20.0 to 34.0 y; body mass index (BMI), 24.0 ± 3.6 kg/m2, 18.2 to 28.4 kg/m2; weight, 66.4 ± 12.1 kg, 47.2 to 88.9 kg (mean ± SD, range)] participated in the 32-d-long inpatient protocol. Participants were deemed medically and psychologically healthy based on self-reported health, psychological screening questionnaires, physical examination by a physician, laboratory testing of hematological or metabolic measures, and psychological evaluation from a clinical interview with a psychologist. Participants were also free of any sleep disorders, as determined by questionnaires and an overnight clinical sleep screening. Exclusion criteria consisted of a self-reported habitual sleep duration <7 or >9 h averaged across the entire week, history of night-shift work or transmeridian travel <3 mo before the study, BMI <18 or >29.9, age <18 or >35 y, pregnancy, and use of any prescription medication. For at least 3 wk before the inpatient protocol, participants maintained an approximate 10-h-per-night sleep schedule at their self-reported habitual timing that was verified by wrist actigraphy (Actiwatch-L Mini Mitter; Respironics), sleep logs, and call-ins to a time-stamped voicemail recording system immediately before going to bed and upon waking to minimize sleep loss before entering the Intensive Physiological Monitoring (IPM) Unit of the Brigham and Women’s Hospital Center for Clinical Investigation research facilities. During the 3 wk of at-home monitoring and throughout the protocol, participants abstained from any drug or over-the-counter medication use, caffeine, alcohol, nicotine, or other foreign substances, as verified via urine toxicology before and at admission to the IPM. All participants provided written informed consent, and all study procedures were approved by the Partners Healthcare Institutional Review Board. The study was registered as a clinical trial (NCT01581125).
Inpatient Protocol.
Participants arrived at the IPM ∼3 h after their habitual wake time and were admitted for 32 d to a sound-attenuated, temperature-controlled suite that was free from time cues. All events were scheduled related to the participant’s habitual timing, as determined from the 3 wk of home monitoring. To diminish any potential residual sleep loss upon entering the protocol, the first 3 d had 12-h overnight sleep opportunities and 4-h daytime nap opportunities. These “sleep satiation” days were followed by 2 baseline days of a 10-h overnight sleep opportunity at habitual timing. Participants were then scheduled to 24 cycles of a 20-h FD protocol over 20 calendar days and randomized to one of two sleep:wake FD conditions: control (1:2, 6.67 h sleep opportunity, 13.33 h wake; eight participants) or CSR (1:3.3, 4.67 h sleep, 15.33 h wake; nine participants). The participants were blinded to the specifics of the FD protocol (i.e., day length, time of day, date) and to which condition they were randomized. Following the FD protocol, participants were provided 5 recovery days of 10-h sleep opportunities at the same circadian phase as the baseline sleep opportunities. During scheduled wakefulness, participants were allowed to engage in sedentary activities (e.g., read, watch movies, talk or play board games with a researcher), and wakefulness was verified by continuous monitoring by research staff and continuous polysomnographic recordings (PSGs). Out of 396 PSG-scored wake periods, on average ≤1.9 min of sleep occurred per wake period (median 0 min, range 0 to 76 min). Lighting was maintained at dim (<4 lx) levels during scheduled wakefulness and 0 lx during scheduled sleep to minimize any shifts of circadian phase from light (32).
Sleep was recorded every sleep opportunity using PSG (Vitaport digital sleep recorder; TEMEC Instruments) from C3-A2, C4-A1, O1-A2, right and left electrooculogram, chin electromyogram, and electrocardiogram. Sleep was visually scored in 30-s epochs according to standard guidelines from brain region C3-A2 (33); SWS was defined as NREM sleep stage 3+4. If the C3-A2 trace contained an artifact, the C4-A1 trace was used to determine the sleep stage. Sleep efficiency was defined as (total sleep time/time in bed) × 100.
Performance Testing.
Participants completed a 35-min neurobehavioral test battery every 2 h during wakefulness, with the first set beginning at 2 h after scheduled wake time. This timing allowed for the dissipation of sleep inertia (i.e., the grogginess felt upon awakening) (34). The PVT was used to assess sustained vigilance via the participant’s reaction time to randomly presented millisecond counterstimuli across 10 min of testing (14). The interstimulus interval of the PVT varies between 2 and 10 s. Participants were instructed to only use their dominant thumb to respond to the stimulus and to attempt to be as accurate and fast as possible. Visual feedback was presented immediately after each stimulus.
Subjective alertness was assessed using VAS within the same performance battery as the PVT. The VAS prompted the participant to identify on a 100-mm horizontal line how they felt at that moment, with each end of the line labeled with the extremes of the subjective continuum (e.g., “alert” and “sleepy”) (15). Subjective sleepiness was determined via the KSS during the same performance battery, before each PVT test. The KSS is a questionnaire that assesses subjective sleepiness by asking the subject to rate on a scale of 1 to 9 how alert or sleepy they feel at the moment of the test, with 1 being most alert and 9 being most sleepy (16). Participants were not given any feedback on the “values” that they identified using the VAS.
Statistical Analysis.
Nonorthogonal spectral analysis of hourly serum melatonin was used to estimate intrinsic circadian period and circadian phase for each individual (5); all events were then binned for each participant in 60° circadian phase bins and referenced to the center of each bin. A circadian phase of 0° indicated the fit maximum of the circadian melatonin rhythm; therefore, circadian day was defined as 150 to 270 circadian degrees and circadian night was defined as 330 to 90 circadian degrees (5). Median reaction time, number of lapses of attention (RTs >500 ms), VAS, and KSS were analyzed using mixed-effects models with condition, length of time awake, and beat cycle as fixed effects and participant as a random effect to account for interparticipant differences. Hours awake 2, 4, 6, 8, 10, and 12 were used for analysis. We did not use the data from the hours awake 14-h bin for the CSR group, as the control group did not have data for that time point. All analyses were also performed with NREM sleep stage 1, NREM sleep stage 2, SWS, REM sleep, and/or TST as a covariate in place of condition (control vs. CSR) to test for any potential impact of any sleep-stage duration differences. Analyses for performance and vigilance measures were done using SAS 9.4 PROC MIXED and PROC GLIMMIX (unstructured) and for minutes of sleep comparisons using PROC TTEST.
Supplementary Material
Acknowledgments
We thank the participants and Center for Clinical Investigation staff for their support in conducting these studies. Funding: NIH (Grants KL2TR002370, F32DK107146, T32HL007901, K24HL105664, R01HL114088, R01GM105018, R01HL128538, P01AG009975, R21HD086392) and NSBRI (Grants HFP02802, HFP04201, HDP0006).
Footnotes
Conflict of interest statement: C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for Bose Corporation; Boston Celtics; Boston Red Sox; Columbia River Bar Pilots; Institute of Digital Media and Child Development; Klarman Family Foundation; Koninklijke Philips Electronics, N.V.; Samsung Electronics; Sleep Multimedia, Inc.; and Vanda Pharmaceuticals. He has also received education/research support from Cephalon, Inc., Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Optum, Philips Respironics, Inc., ResMed Foundation, San Francisco Bar Pilots, Schneider, Inc., and Sysco. He has received lecture fees from the Annual Congress of the German Sleep Society (DGSM), CurtCo Media Labs LLC, Global Council on Brain Health/AARP, Harvard School of Public Health, Integritas Communications Group, Maryland Sleep Society, National Sleep Foundation, University of Michigan, and Zurich Insurance Company, Ltd. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine (which C.A.C. directs) has received educational grant funding from Cephalon, Inc., Jazz Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., and Wake Up Narcolepsy. He is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc., and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms, including those involving the following commercial entities: Bombardier, Inc.; Continental Airlines; FedEx; Greyhound; and United Parcel Service. He owns or owned an equity interest in Lifetrac, Inc., Somnus Therapeutics, Inc., and Vanda Pharmaceuticals. He received royalties from McGraw Hill, Houghton Mifflin Harcourt, and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. His interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. E.B.K. has received travel reimbursement from the Sleep Technology Council, Wire In-Brain Conference, Free Health LLC, Employer Health Benefit Congress, The Society for Reproductive Investigation, and The Associated Professional Sleep Society, and has served as a consultant in cases involving transportation safety and sleep deprivation.
This article is a PNAS Direct Submission.
Data deposition: Data within the published figures have been deposited in Figshare (https://figshare.com/articles/Chronic_sleep_curtailment_even_without_extended_16-h_wakefulness_degrades_human_vigilance_performance/6205595).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706694115/-/DCSupplemental.
References
- 1.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Sleep Foundation . Executive Summary of the 2005 “Sleep in America” Poll. Natl Sleep Found; Washington DC: 2005. [Google Scholar]
- 3.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 5.Cohen DA, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 7.Belenky G, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 9.Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JF, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 12.Kleitman N. Sleep and Wakefulness as Alternating Phases in the Cycle of Existence. Univ Chicago Press; Chicago: 1939. [Google Scholar]
- 13.Czeisler CA, Weitzman Ed, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instrum. 1985;17:652–655. [Google Scholar]
- 15.Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- 16.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 17.Bermudez EB, et al. Prediction of vigilant attention and cognitive performance using self-reported alertness, circadian phase, hours since awakening, and accumulated sleep loss. PLoS One. 2016;11:e0151770. doi: 10.1371/journal.pone.0151770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, et al. Mismatch between subjective alertness and objective performance under sleep restriction is greatest during the biological night. J Sleep Res. 2012;21:40–49. doi: 10.1111/j.1365-2869.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 19.Kosmadopoulos A, et al. The efficacy of objective and subjective predictors of driving performance during sleep restriction and circadian misalignment. Accid Anal Prev. 2017;99:445–451. doi: 10.1016/j.aap.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Muto V, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353:687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- 21.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 22.McCauley P, et al. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips AJK, Klerman EB, Butler JP. Modeling the adenosine system as a modulator of cognitive performance and sleep patterns during sleep restriction and recovery. PLoS Comput Biol. 2017;13:e1005759. doi: 10.1371/journal.pcbi.1005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borbély AA. Processes underlying sleep regulation. Horm Res. 1998;49:114–117. doi: 10.1159/000023156. [DOI] [PubMed] [Google Scholar]
- 25.Kosmadopoulos A, et al. The effects of a split sleep-wake schedule on neurobehavioural performance and predictions of performance under conditions of forced desynchrony. Chronobiol Int. 2014;31:1209–1217. doi: 10.3109/07420528.2014.957763. [DOI] [PubMed] [Google Scholar]
- 26.Jackson ML, Banks S, Belenky G. Investigation of the effectiveness of a split sleep schedule in sustaining sleep and maintaining performance. Chronobiol Int. 2014;31:1218–1230. doi: 10.3109/07420528.2014.957305. [DOI] [PubMed] [Google Scholar]
- 27.Mollicone DJ, Van Dongen HP, Rogers NL, Dinges DF. Response surface mapping of neurobehavioral performance: Testing the feasibility of split sleep schedules for space operations. Acta Astronaut. 2008;63:833–840. doi: 10.1016/j.actaastro.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roach GD, et al. Are two halves better than one whole? A comparison of the amount and quality of sleep obtained by healthy adult males living on split and consolidated sleep-wake schedules. Accid Anal Prev. 2017;99:428–433. doi: 10.1016/j.aap.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Short MA, et al. The effect of split sleep schedules (6h-on/6h-off) on neurobehavioural performance, sleep and sleepiness. Appl Ergon. 2016;54:72–82. doi: 10.1016/j.apergo.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Leproult R, et al. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–R290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Mishima K, Satoh K, Shimizu T, Hishikawa Y. Physical activity increases the dissociation between subjective sleepiness and objective performance levels during extended wakefulness in human. Neurosci Lett. 2002;326:133–136. doi: 10.1016/s0304-3940(02)00335-x. [DOI] [PubMed] [Google Scholar]
- 32.Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Neurobiology of Sleep and Circadian Rhythms. Marcel Dekker; New York: 1999. pp. 149–180. [Google Scholar]
- 33.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Government Printing Office; Washington, DC: 1968. [Google Scholar]
- 34.Jewett ME, et al. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.