Significance
Tuft cells in the intestine are known to act as sentinels for infectious agents [e.g., helminths (worms) and bacterial microbiota] and express taste-signaling elements. In this work, the G protein-coupled receptor Sucnr1 was shown to be expressed specifically in tuft cells but not in other intestinal epithelial cells. Dietary succinate and perturbations in the microbiota activate tuft cells, and subsequently type 2 immunity, via tuft cell-expressed Sucnr1. Modulating this pathway using dietary succinate or specific Sucnr1 agonists may be a strategy for fighting bacterial and parasitic infections or other type 2 immune-related metabolic disorders such as obesity.
Keywords: Sucnr1, type 2 immunity, tuft cells, Trpm5, gustducin
Abstract
The hallmark features of type 2 mucosal immunity include intestinal tuft and goblet cell expansion initiated by tuft cell activation. How infectious agents that induce type 2 mucosal immunity are detected by tuft cells is unknown. Published microarray analysis suggested that succinate receptor 1 (Sucnr1) is specifically expressed in tuft cells. Thus, we hypothesized that the succinate–Sucnr1 axis may be utilized by tuft cells to detect certain infectious agents. Here we confirmed that Sucnr1 is specifically expressed in intestinal tuft cells but not in other types of intestinal epithelial cells, and demonstrated that dietary succinate induces tuft and goblet cell hyperplasia via Sucnr1 and the tuft cell-expressed chemosensory signaling elements gustducin and Trpm5. Conventional mice with a genetic Sucnr1 deficiency (Sucnr1−/−) showed diminished immune responses to treatment with polyethylene glycol and streptomycin, which are known to enhance microbiota-derived succinate, but responded normally to inoculation with the parasitic worm Nippostrongylus brasiliensis that also produces succinate. Thus, Sucnr1 is required for microbiota-induced but not for a generalized worm-induced type 2 immunity.
The intestinal epithelium comprises mature cells of two major lineages: absorptive enterocytes and secretory cells (1). Most mature differentiated cells in the intestinal epithelium are enterocytes; secretory cells constitute only a minor fraction. Secretory cells belong to four different classes: enteroendocrine cells, goblet cells, Paneth cells, and tuft cells (also known as brush cells) (1). Enteroendocrine cells function as hormone-secreting cells in response to macronutrients, goblet cells produce a protective mucus layer, and Paneth cells secrete niche factors to maintain intestinal stem cell homeostasis and also produce antimicrobial peptides (1). Despite being discovered decades ago (2–5), the function of tuft cells in the small intestine was only recently uncovered: They mediate host defense against parasitic infection or other pathogens by regulating an intestinal type 2 innate lymphoid cell (ILC2)–epithelial response circuit, and hence are involved in immune function (6–8).
Tuft cells express a number of taste-signaling elements, including transient receptor potential cation channel subfamily M member 5 (Trpm5), gustducin, and phospholipase β2 (Plcβ2) (7, 9–13). These signaling elements play critical roles in mediating the responses of tuft cells to parasitic infections (7). A hallmark feature of such infection is tuft and goblet cell hyperplasia (6–8, 14, 15). In wild-type mice, infections by helminth parasites or protozoans can induce tuft and goblet cell hyperplasia (6–8). In mice lacking gustducin or Trpm5, these responses are not observed (7). The transcription factor Pou2f3 is required for development of type II taste cells as well as tuft cells in the gut (8, 16). Mice deficient in Pou2f3, and therefore lacking tuft cells, cannot mount a tuft cell-mediated type 2 immune response toward infecting pathogens, similar to Trpm5−/− and gustducin−/− mice (8). Expulsion of the intestinal worm parasite Nippostrongylus brasiliensis from the gut is significantly delayed in Pou2f3−/− mice compared with wild-type mice (8). The current model suggests tuft cells detect parasitic infection via taste-signaling elements and secrete the proinflammatory cytokine IL-25, which subsequently triggers mucosal type 2 responses via IL-13–producing ILC2s and IL-13 receptor alpha-expressing intestinal epithelial progenitor cells (6–8, 17, 18). Given the importance of gustducin and Trpm5 for parasite type 2 immunity, which are downstream transduction elements for G protein-coupled taste receptors (GPCRs) (19, 20), we reasoned that activation of tuft cell-expressed GPCRs may cascade on the gustducin–Trpm5 pathway and trigger type 2 immunity, resembling the response to parasitic helminth or protozoan infections.
Previous microarray analysis of tuft cells suggested that succinate receptor 1 (Sucnr1), a GPCR with succinate as its cognate agonist (21), is expressed in tuft cells (11). As a metabolite of the Krebs cycle and microbial propionate synthesis, succinate is known to be secreted by certain parasites, bacteria, and inflamed tissues in pathological conditions (22–29). Thus, we hypothesized that the succinate–Sucnr1 axis is utilized by tuft cells to detect infectious agents.
Results
The Succinate Receptor Sucnr1 Is Specifically Expressed in Intestinal Tuft Cells.
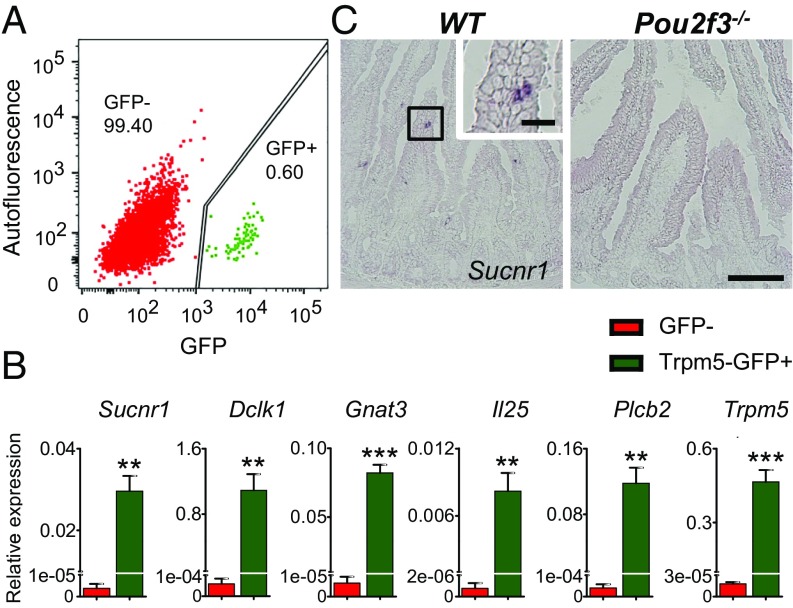
We began to search for the putative tuft cell-expressed receptor(s) responsible for detecting infectious agents by mining the microarray data of intestinal tuft cells (11). The succinate receptor Sucnr1 (also referred to as Gpr91) is expressed in tuft cells as a GPCR. To validate the expression of Sucnr1 in intestinal tuft cells, Trpm5-GFP–positive cells [the expression of green fluorescent protein (GFP) is driven by the Trpm5 promoter, and GFP is used as a surrogate marker for tuft cells] were isolated by fluorescence-activated cell sorting (7, 11) (Fig. 1A), and quantitative real-time PCR (qPCR) was performed using primer sets for established tuft cell markers as well as a Sucnr1 primer set (Fig. 1B). Nontuft intestinal epithelial cells (GFP-negative cells) were used as control. Sucnr1 and other established tuft cell markers [e.g., Dclk1, Gnat3 (encoding gustducin), Il25, Plcb2, Trpm5] were robustly expressed in tuft cells compared with nontuft intestinal epithelial cells. To directly visualize the expression of Sucnr1 in tuft cells and determine if Sucnr1 is found in other intestinal epithelial cells, we performed in situ hybridization of the jejunum tissue of wild-type mice using a Sucnr1 RNA probe (Fig. 1C). A few cells in the jejunum tissue of wild-type mice were stained positively with the Sucnr1 probe, but none were detected in mice deficient for Pou2f3, which results in the specific absence of tuft cells in the intestinal epithelium without affecting other types of intestinal epithelial cells (Fig. 1C) (8). Together, these data show that Sucnr1 is expressed selectively in tuft cells and not in other types of intestinal epithelial cells; this is also consistent with the recent finding that Sucnr1 is a tuft cell signature gene, demonstrated by profiling 53,193 individual epithelial cells from the small intestine and organoids of mice (30).
Fig. 1.
Specific expression of the Sucnr1 receptor in intestinal tuft cells. (A) Fluorescence-activated cell-sorting–based isolation of tuft cells (Trpm5-GFP–positive cells; green) and nontuft epithelial cells (GFP-negative cells; red). GFP: excitation, 488 nm; emission, 513 nm. Autofluorescence: excitation, 561 nm; emission, 585 nm. Numbers indicate percentages of cells in that population. (B) Expression of Sucnr1 and other tuft cell marker genes (Dclk1, Gnat3, Il25, Plcb2, Trpm5) in Trpm5-GFP–positive tuft cells and GFP-negative nontuft cells (control) was determined by real-time quantitative PCR. Data (mean ± SEM) are biological replicates (n = 3). **P < 0.01, ***P < 0.001 (Student’s t test). (C) In situ hybridization shows the presence of Sucnr1-expressing cells in the small intestine (jejunum) in wild-type (WT) but not in Pou2f3−/− mice. [Scale bars, 100 µm (low-magnification images) and 20 µm (Inset).]
Dietary Succinate Is Sufficient to Induce Tuft and Goblet Cell Hyperplasia.
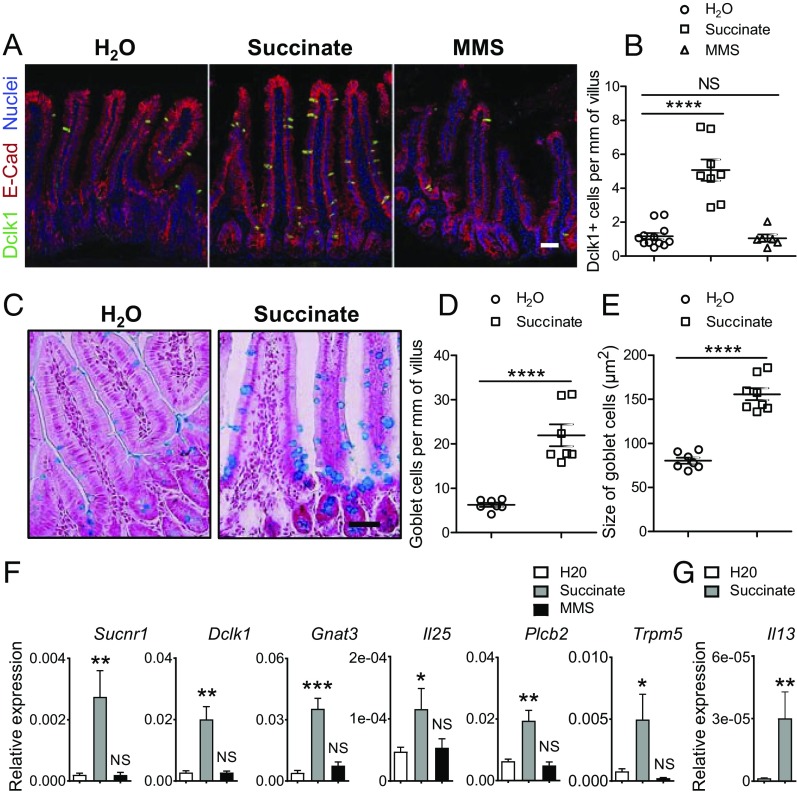
If the tuft cell-expressed Sucnr1 receptor is responsible for detecting succinate produced by pathogens or other sources, then ingestion of exogenous succinate would be expected to activate mucosal type 2 immunity, resulting in tuft and goblet cell expansion, goblet cell enlargement (production of mucus), and an increase in IL-13–expressing ILC2s. Dietary succinate (100 mM) was added to the drinking water of wild-type mice for 7 d, a time consistent with the turnover rate of gut epithelial cells (∼5 d) (31) and the peak of tuft cell hyperplasia in response to parasitic infection (day ∼7 of infection) (6). Mice drank normally (SI Appendix, Fig. S1) and had no apparent changes in body weight or behavior after consuming succinate. Immunostaining was performed with the antibody against the tuft cell marker Dclk1 to determine if feeding succinate induced tuft cell hyperplasia (32). There was a significant increase in the number of tuft cells in the small intestine of mice drinking water supplemented with succinate compared with plain water (Fig. 2 A and B). Parasitic helminth infections lead to tuft cell as well as goblet cell hyperplasia (6–8). To determine if succinate treatment, like parasitic helminth infection, also impacts goblet cell homeostasis, Alcian blue staining of the intestine of succinate-treated mice was used to detect goblet cells. As expected, the number and enlargement of goblet cells, features of a type 2 response, significantly increased after succinate treatment (Fig. 2 C–E and SI Appendix, Fig. S2A). To further validate our immunostaining results, we performed qPCR analyses of the jejunum of mice treated with succinate using primers for intestinal epithelial cell markers. qPCR data revealed a significant increase in the expression of genes encoding tuft cell markers (e.g., Sucnr1, Dclk1, Gnat3, Il25, Plcb2, Trpm5) (6–8) (Fig. 2F). The expression of Muc2 (a goblet cell marker) appeared slightly up-regulated, but this did not reach statistical significance, presumably due to the small sample size (n = 5 for water control and n = 6 for succinate treatment; SI Appendix, Fig. S2B). No changes in the expression of genes encoding Gcg (an enteroendocrine L-cell marker), Lyz1 (a Paneth cell marker), or Cdx2 (a general intestinal epithelial marker) were detected (SI Appendix, Fig. S2B). Since IL-13, predominantly produced by lamina propria ILC2s (33), mediates tuft cell hyperplasia, qPCR was performed to determine if the expression of Il13 is elevated in succinate-treated mice. As expected, Il13 was significantly increased after succinate feeding (Fig. 2G), consistent with the model that tuft cells, ILC2s, and epithelial progenitor cells comprise a response circuit that mediates epithelial remodeling associated with type 2 immunity (6).
Fig. 2.
Dietary succinate induces type 2 immune responses. Jejunum tissues were collected from wild-type mice after consumption of water (H2O) or water containing 100 mM succinate or 100 mM monomethyl succinate. (A) Tissues were immunostained with anti-Dclk1 (green) and anti–E-cadherin (red) antibody and counterstained with DAPI (blue). (Scale bar, 50 μm.) (B) Dclk1-positive cells per mm villus (n = 12 for H2O, n = 8 for succinate, and n = 6 for MMS treatment). (C) Goblet cell staining with Alcian blue/nuclear red. (Scale bar, 50 μm.) (D) Goblet cells per mm villus (n = 7 for H2O and succinate). (E) Goblet cell size (n = 7 for H2O and n = 8 for succinate). (F) qPCR results show increased expression of tuft cell marker genes (Sucnr1, Dclk1, Gnat3, Il25, Plcβ2, Trpm5) (n = 5 for H2O, n = 6 for succinate, and n = 3 for MMS treatment). (G) qPCR result shows increased expression of Il13 after succinate treatment (n = 14 for H2O and n = 10 for succinate). All data are biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; NS, not significant (Student’s t test). Data are mean ± SEM.
To confirm the specificity of the succinate activity, wild-type mice were given monomethyl succinate (MMS; 100 mM), an analog of succinate that does not activate Sucnr1, in the drinking water (21). Mice readily drank MMS-containing water (SI Appendix, Fig. S1). MMS did not induce a prototypical mucosal type 2 response, since no tuft cell hyperplasia was observed (Fig. 2 A, B, and F).
Sucnr1, Gustducin, and Trpm5 Are Required for a Succinate-Induced Type 2 Response.
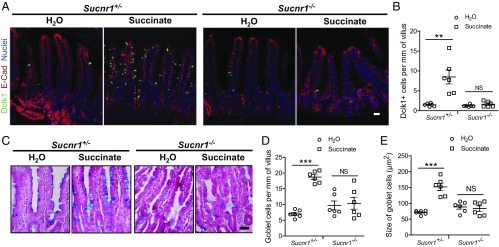
Given the effect of succinate on mucosal type 2 responses and the expression of the succinate receptor Sucnr1 in intestinal tuft cells, we hypothesized that succinate would most likely activate the Sucnr1 receptor to trigger type 2 responses. To confirm if the effect of succinate on mucosal immunity is mediated by the Sucnr1 receptor, we examined type 2 responses of Sucnr1−/− mice (28) in response to dietary succinate. Heterozygous Sucnr1+/− mice were used as controls. Heterozygous Sucnr1+/− mice that drank succinate-containing water for 7 d, like wild-type mice, showed a typical type 2 response, including tuft cell expansion (Fig. 3 A and B), increased goblet cell numbers (Fig. 3 C and D), and goblet cell enlargement (Fig. 3 C and E). In similarly treated Sucnr1−/− mice, neither tuft cells (Fig. 3 A and B) nor goblet cells increased in number (Fig. 3 C and D), and no goblet cell enlargement was noted (Fig. 3 C and E). No such difference was noted between Sucnr1+/− and Sucnr1−/− mice not treated with succinate (Fig. 3 B, D, and E). Collectively, these data suggest that the Sucnr1 receptor mediates the activity of succinate.
Fig. 3.
Sucnr1 is required for succinate-induced type 2 immune responses. Jejunum tissues were collected from Sucnr1+/− and Sucnr1−/− mice after consumption of water or water containing 100 mM succinate. (A) The tissues were immunostained with anti-Dclk1 (green) and anti–E-cadherin (red) antibodies and counterstained with DAPI (blue). (Scale bar, 50 μm.) (B) Dclk1-positive cells per mm of villus (n = 6 for each group). (C) Goblet cell staining with Alcian blue/nuclear red. (Scale bar, 50 μm.) (D) Goblet cell number per mm villus (n = 6 for each group). (E) Goblet cell size (n = 6 for each group). All data are biological replicates. **P < 0.01, ***P < 0.001; NS, not significant (Student’s t test). Data are mean ± SEM.
Recently, it has been shown that gustducin and Trpm5, two key taste-signaling components, orchestrate mucosal type 2 immunity in response to parasitic helminth or protozoan infections (7). Disruption of the gustducin–Trpm5 pathway affected type 2 immune responses (e.g., tuft cell expansion) in response to protozoans. To determine if gustducin and Trpm5 mediate the mucosal immunity-inducing activity of succinate, the type 2 responses of gustducin (Gnat3)- and Trpm5-deficient mice to succinate treatment were examined. Neither Gnat3−/− (34) nor Trpm5−/− (35) mice showed type 2 mucosal responses to succinate compared with water. There was no increase in the number of tuft cells (SI Appendix, Fig. S3 A and B) or of goblet cells (SI Appendix, Fig. S3 C–E) in Gnat3−/− or Trpm5−/− mice after succinate treatment. These data support a model in which gustducin and Trpm5 act downstream of the Sucnr1 receptor after activation with succinate.
Mice Deficient for Sucnr1 Show Normal Type 2 Responses to Infection with N. brasiliensis.
The feedback loop of tuft cells, ILC2s, and intestinal epithelial progenitor cells is initiated by the release of IL-25 from tuft cells in response to parasitic infections (6–8). We postulated that the failure to induce type 2 responses in Sucnr1−/− mice by succinate is due to the inability of tuft cells to sense succinate, thus not releasing IL-25 to initiate the effector system (e.g., intestinal remodeling by IL-13–producing ILC2s). Because adult N. brasiliensis can produce succinate (29), it was postulated that the Sucnr1 receptor would be a trigger for a robust type 2 response. However, both Sucnr1+/− and Sucnr1−/− mice inoculated with N. brasiliensis expressed a strong type 2 response (SI Appendix, Fig. S4 A, B, and D–F), consistent with what has been reported in wild-type mice (6–8). Worm burden at day 7 after N. brasiliensis infection was comparable between Sucnr1+/− and Sucnr1−/− mice (SI Appendix, Fig. S4C). Thus, Sucnr1 is not required for N. brasiliensis-induced type 2 immunity. Nevertheless, the fact that Sucnr1−/− mice mounted a strong type 2 immunity in response to N. brasiliensis infection but not to succinate suggests that Sucnr1−/− mice have an intact circuit for type 2 immunity and that the inability of succinate to induce type 2 responses in Sucnr1−/− mice is due to the deficiency of Sucnr1 in tuft cells.
Sucnr1 Is Required for Microbiota-Triggered Type 2 Immunity.
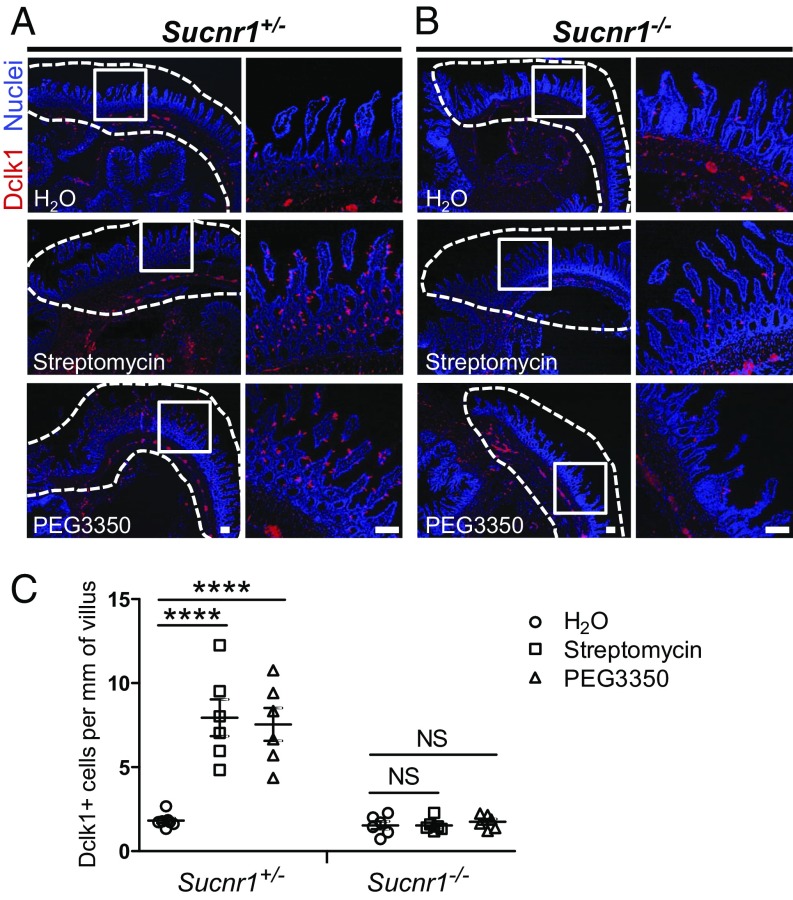
Recently, Ferreyra et al. (23) showed that conventional mice have less succinate in the cecum than do antibiotic- (e.g., streptomycin) or polyethylene glycol 3350 (PEG3350)-treated mice that also showed disrupted intestinal motility (23). To determine if this elevated level of succinate, produced by an altered microbiota, can lead to increased numbers and enlargement of tuft and goblet cells in the small intestine, littermate Sucnr1+/− and Sucnr1−/− mice were treated with streptomycin for 5 consecutive days and assayed 2 d later. Both the cecum and the distal ileum were collected for immune histochemical analysis. In Sucnr1+/− mice, increased numbers of tuft cells were noted in the distal part of the ileum but not in the cecum (Fig. 4 A and C). The increase in the number of tuft cells in the ileum was regional: Only the distal part showed an increase (Fig. 4 A and C). Similar results were noted for wild-type mice (SI Appendix, Fig. S5). In contrast, there was no increase in tuft cell number in the distal part of the ileum in any of the Sucnr1−/− mice treated with streptomycin (Fig. 4 B and C). Similarly, a robust increase in the number of tuft cells was observed in Sucnr1+/− but not in Sucnr1−/− mice after PEG3350 feeding for 10 d (Fig. 4). Goblet cell expansion and enlargement were also noted in Sucnr1+/− mice (SI Appendix, Fig. S6) but not in Sucnr1−/− mice (SI Appendix, Fig. S6) after treatment with either streptomycin or PEG3350. To confirm that streptomycin and PEG3350 alter the microbiome, 16S rRNA gene sequencing was performed using DNA extracted from cecal contents collected from treated and control mice. Hierarchical cluster analysis of the sequence reads (Dataset S1) showed that the cecal samples were clustered in a treatment-dependent but genotype-independent way (SI Appendix, Fig. S7), demonstrating that streptomycin and PEG3350 altered microbiota composition. These data suggest that Sucnr1 is required for microbiota-triggered type 2 immunity, presumably via an elevated level of succinate.
Fig. 4.
Altered microbiome triggers tuft cell expansion in the distal ileum. Littermate or age-matched Sucnr1+/− and Sucnr1−/− mice were either treated with streptomycin (gavage) for 5 consecutive days and then the distal ileum and cecum were collected 2 d later, or provided with a drinking bottle containing 10% PEG3350 for 10 d and the distal ileum and cecum were collected at day 10. (A and B) Representative images of the distal ileum (dashed lines) and cecum of Sucnr1+/− (A) and Sucnr1−/− (B) mice immunostained with anti-Dclk1 antibody (red) and counterstained with DAPI (blue), showing tuft cell expansion in the distal ileum in Sucnr1+/− mice but not in Sucnr1−/− mice after treatment with either streptomycin (Middle) or PEG3350 (Bottom). Tissues from mice drinking only water were used as controls (Top). (A and B, Left) Low magnification. (A and B, Right) High magnification of boxed areas. (Scale bars, 100 μm.) (C) Immunohistochemical quantification of tuft cells (Dclk1+) cells in the distal ileum (n = 7 for Sucnr1+/− with H2O treatment and n = 6 for other groups). ****P < 0.0001; NS, not significant (Student’s t test). All data are biological replicates. Data are mean ± SEM.
Discussion
Succinate, Inflammation, and Immunity.
Although tuft cells were first described about 50 y ago, their function has only recently been recognized (2, 5). It has been assumed that intestinal tuft cells serve as chemosensory cells for certain environmental or dietary factors due to the presence of chemosensory signaling elements (13). Recent work demonstrates that intestinal tuft cells detect parasitic infection and trigger type 2 immunity by secreting proinflammatory cytokine IL-25 (6–8). However, triggers for this response to infection remain speculative. In the current study, the succinate receptor Sucnr1 was found to be specifically expressed in intestinal tuft cells, and dietary and microbiota-produced succinate activated tuft cells and type 2 immunity via Sucnr1. The activity of Sucnr1 is mediated by the downstream taste-signaling elements gustducin and Trpm5. We demonstrated that the succinate–Sucnr1 axis in tuft cells is utilized by certain infectious agents to trigger type 2 immunity. Sucnr1 is not required for N. brasiliensis-induced type 2 immunity, suggesting that tuft cells may use multiple receptor mechanisms for detecting infectious agents.
In addition to its role as an intermediary of the citric acid cycle, succinate is known to act as an alarmin, initiating and propagating danger signals from tissue injury or inflammatory stimuli (28, 36). Thus, Sucnr1 has been implicated in the mechanisms governing diabetic retinopathy (37), cardiac hypertrophy (38), hepatic fibrosis (39), and necrosis (28), broadening its role in immune regulation. Succinate also acts as an endogenous danger signal to stabilize hypoxia-inducible factor 1α (HIF-1α), which subsequently regulates gene expression of IL-1β and other HIF-1α–dependent genes that leads to protein succinylation (40). Consistent with its role in immune regulation, we observed that tuft cell-expressed Sucnr1 also serves as a surveillance signal for detection of certain organisms in the intestine that produce succinate and trigger host response. Succinate activates Sucnr1 at millimolar or submillimolar ranges (21), and the type 2 immune responses were strongly evident when 100 mM succinate was ingested but barely detectable when 10 or 1 mM succinate was ingested (SI Appendix, Fig. S8). Recent work from De Vadder et al. (41) showed that dietary succinate is absorbed rapidly by intestinal epithelial cells and converted to glucose. The levels of succinate and other short-chain fatty acids were not altered in the cecum after succinate feeding, suggesting rapid metabolism of succinate in the small intestine. Thus, even when succinate was provided at a relatively high dose, due to its rapid metabolism by the gut, intestinal tuft cells were most likely stimulated by succinate at only a much lower dose. Additionally, succinate could potentially be metabolized by succinate-consuming microbes (42). These data suggest that the development of potent and stable Sucnr1 agonists could be used as a therapeutic tool to trigger type 2 immunity for protection or expulsion of infectious agents from the gut. There is also a possibility that succinate may alter the microbiota, and this may subsequently lead to an indirect effect on type 2 immunity. This is unlikely given that succinate cannot trigger type 2 immunity in mice deficient for Sucnr1, Trpm5, or gustducin.
Intestinal tuft cells are specialized epithelial cells that express taste-signaling machinery and act as an innate sensor for host defense. Similar cells are found in many other tissues, such as the upper respiratory tract, where they are generally referred to as solitary chemosensory cells (SCCs) (43–46). SCCs are located in critical positions for surveillance of bacterial infection by using bitter taste mechanisms. The similarity between SCCs in the upper respiratory tract and other bodily locations and intestinal tuft cells raises the possibility that Sucnr1 may also be utilized by SCCs to detect bacterial infection. This warrants further investigation.
Diet plays a significant role in shaping the microbiome. For instance, high-fat diet has been shown to reduce the formation of butyrate but increase succinate, inflammation, liver fat, and cholesterol in rats (47). The ability of succinate to trigger type 2 immunity in the small intestine may contribute to diet-induced inflammation. On the other hand, IL-25 treatment in obese mice fed a high-fat diet results in body weight loss, loss of adipose tissue mass, and improved glucose metabolism (48), mainly due to increased infiltration of ILC2s, type I and type II natural killer cells, eosinophils, and alternatively activated macrophages into the visceral adipose tissue (48). Given that succinate is able to trigger IL-25–mediated type 2 immunity, it is tempting to speculate that dietary succinate may have similar metabolic effects for improved glucose metabolism and reduced adipose tissue. In concordance with this notion, dietary succinate has been shown to improve glucose homeostasis. De Vadder et al. (41) attribute the effect of dietary succinate to intestinal gluconeogenesis, as succinate is efficiently converted into glucose in the intestinal mucosa. Whether the effect of succinate on IL-25 biology also contributes to the beneficial effects of succinate on glucose homeostasis remains to be determined. However, given the link between succinate, IL-25, and glucose homeostasis, it is likely that tuft cell-expressed Sucnr1 may also contribute to the effect of succinate on glucose homeostasis.
Streptomycin and PEG3350 treatments lead to succinate spikes in the cecum (23). Tsukahara and Ushida (24) showed that in pig large intestine, succinate accumulates during antibiotic-associated diarrhea and that there are changes in the constitution of succinate-producing flora. We did not observe any increase in the number of tuft cells in the cecum but noted significant tuft cell expansion in the distal ileum adjacent to the cecum. One possibility is that the succinate spike, produced by the microbiota, especially under anaerobic conditions, may spread into the distal ileum; therefore, this effect would be local and occur only in the ileal region next to the cecum. Alternatively, it is plausible that the microbiota in the distal ileum may also be altered by antibiotic or PEG3350 treatment. Previously, it has been shown that treatment with PEG3350 resulted in a significant increase in the relative abundance of members of the families Bacteroidaceae and Peptostreptococcaceae and a significant decrease in the relative abundance of members of the families Peptococcaceae, Eubacteriaceae, and Anaeroplasmataceae compared with samples before treatment (49). Our 16S gene-sequencing analysis of cecal microbiota showed that both streptomycin and PEG3350 altered microbiota in a treatment-specific way and that there were no apparent differences in microbiota between Sucnr1+/− and Sucnr1−/− mice. Consistent with data reported by Kashyap et al. (49), PEG3350 also led to an increase in the abundance of Bacteroidaceae. Nevertheless, because of profound changes in the composition of the microbiota, the exact microbiota species producing succinate after PEG3350 or streptomycin treatment is unknown, and this warrants further investigation. That there was no increase in tuft cell number in the cecum after streptomycin or PEG3350 treatment or infection with N. brasiliensis (6) suggests that tuft cells in the cecum and presumably in the colon may function differently.
An Additional Tuft Cell-Expressed Receptor(s) Mediates N. brasiliensis-Induced Type 2 Immunity.
Type 2 immunity has been studied extensively in the context of helminth infection and allergies, but bacterial and viral infections can also induce type 2 immunity (50). Such diversity in stimuli suggests multiple pathways may orchestrate the type 2 response (50). N. brasiliensis is an intestinal parasite that elicits robust type 2 immunity. About a 10-fold increase in the number of tuft cells was detected 7 d after inoculation with N. brasiliensis larvae in either Sucnr1+/− or Sucnr1−/− mice compared with naïve mice (SI Appendix, Fig. S4B), similar to what has been described previously for wild-type mice (6, 8). In comparison, dietary succinate appears to result in only about a 5-fold increase in tuft cells in the jejunum (Figs. 2B and 3B). The observation that N. brasiliensis induced an equally potent type 2 response in mice deficient in or heterozygous for Sucnr1 suggested that other tuft cell receptors are primarily activated by infection with N. brasiliensis, and those mechanisms remain to be determined.
Heligmosomoides polygyrus bakeri, Trichinella spiralis, and Tritrichomonas muris are all capable of inducing type 2 immunity (6–8), but their effects appear to be distinct from N. brasiliensis, which induces type 2 immunity in the entire small intestine (6). Like N. brasiliensis adults, H. polygyrus bakeri inhabits the duodenum, but the type 2 immune response appears to be weaker than that for N. brasiliensis (6–8). In contrast, the protozoan T. muris is present primarily in the distal intestine and appears to induce type 2 immunity in the distal intestine, albeit weaker than the effect produced by N. brasiliensis (7). All these data raise the possibility that tuft cells may have distinct receptor mechanisms for different pathogens to elicit type 2 immunity. Nevertheless, there may be shared downstream pathways (e.g., gustducin, Trpm5, and IL-25) that trigger mucosal responses.
Aside from helminths and protozoans, the microbiota may also evoke innate immunity via the tuft cell–ILC2 circuit. For example, microbiota-regulated IL-25 increases eosinophil number to provide protection during Clostridium difficile infection (51). Our data show that Sucnr1 is required for type 2 immunity caused by perturbation of the microbiota. Thus, Sucnr1 contributes to the immune surveillance function of tuft cells to detect certain pathogenic species in the gut. Therefore, activating tuft cell-expressed Sucnr1 may provide beneficial host effects for protection against bacterial infection and worm expulsion (presumably those eliciting weak type 2 responses on their own) or other metabolic syndromes.
Materials and Methods
Mouse Strains.
The Sucnr1-deficient mice were a kind gift from Novartis under a material transfer agreement (28) and were maintained at the Monell Center. C57BL/6 mice were obtained from the Jackson Laboratory. Pou2f3-deficient mice were generated in the I.M. laboratory (8), and Trpm5-GFP, Trpm5-deficient, and gustducin-deficient mice were generated in the R.F.M. laboratory (7). All mutant mice were maintained on the C57BL/6 genetic background. Both adult male and female mice (8 to 28 wk) were used. Littermate mice were housed in single-sex groups unless otherwise specified. All experiments were performed under National Institutes of Health guidelines for the care and use of animals in research and approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Treatment with Succinate, Streptomycin, and PEG3350.
Littermates or age-matched adult mice were provided with a drinking bottle containing 100 mM succinate (pH 7.1) or 100 mM monomethyl succinate (pH 7.1) in water for 7 d (individually housed during the treatment) (SI Appendix, Fig. S1). The intestinal tissue (the same area as described in SI Appendix, Materials and Methods) was harvested for immune histochemical analysis or qPCR analysis. Other mice were provided with a drinking bottle containing 10% PEG3350 for 10 consecutive days (housed in single-sex groups), and the distal ileum and cecum were collected at day 10. Adult mice were given an oral gavage of 20 mg streptomycin in 200 µL distilled H2O on a daily basis for 5 d (23) (housed in single-sex groups), and the distal ileum and cecum were harvested 2 d later. Cecal contents were collected from mice treated with streptomycin or PEG3350 and from control mice.
Details about immunostaining, histochemistry, infection with N. brasiliensis, cell sorting, RNA isolation and RT-qPCR for in vivo and in vitro tuft cells, 16S metagenomics sequencing, in situ hybridization, and statistical analysis are described in SI Appendix, Materials and Methods. The primers used for qPCR are listed in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank Drs. Amanda Littlewood-Evans and Jose M. Carballido for providing us with Sucnr1-deficient mice; members of the P.J. laboratory for discussion; and Drs. Gary Beauchamp, Danielle Reed, and Michael Tordoff for critical reading and editing of the manuscript. The work was supported by Monell institutional funds (to P.J.) and in part by NIH Core Grant P30 DC011735 (to R.F.M.). Research infrastructure was provided in part by NIH Grants G20OD020296 and S10OD018125.
Footnotes
Conflict of interest statement: W.L. and P.J. have filed a patent application related to the effect of succinate on type 2 immunity.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the National Center for Biotechnology Information (accession no. SRP134009).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720758115/-/DCSupplemental.
References
- 1.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 2.Isomäki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand [A] 1973;240(Suppl):1–35. [PubMed] [Google Scholar]
- 3.Nabeyama A, Leblond CP. “Caveolated cells” characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974;140:147–165. doi: 10.1002/aja.1001400203. [DOI] [PubMed] [Google Scholar]
- 4.Höfer D, Drenckhahn D. Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry. 1992;98:237–242. doi: 10.1007/BF00271037. [DOI] [PubMed] [Google Scholar]
- 5.Hammond JB, Ladeur L. Fibrillovesicular cells in the fundic glands of the canine stomach: Evidence for a new cell type. Anat Rec. 1968;161:393–411. doi: 10.1002/ar.1091610401. [DOI] [PubMed] [Google Scholar]
- 6.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howitt MR, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbe F, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Höfer D, Jöns T, Kraemer J, Drenckhahn D. From cytoskeleton to polarity and chemoreception in the gut epithelium. Ann N Y Acad Sci. 1998;859:75–84. doi: 10.1111/j.1749-6632.1998.tb11112.x. [DOI] [PubMed] [Google Scholar]
- 10.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 11.Bezençon C, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 12.Kokrashvili Z, et al. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology. 2009;137:598–606.e2. doi: 10.1053/j.gastro.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. Nippostrongylus brasiliensis: Histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp Parasitol. 1988;65:84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 15.Miller HR, Nawa Y. Immune regulation of intestinal goblet cell differentiation. Specific induction of nonspecific protection against helminths? Nouv Rev Fr Hematol. 1979;21:31–45. [PubMed] [Google Scholar]
- 16.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 19.Pérez CA, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 21.He W, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 22.Gilroy FV, Edwards MR, Norton RS, O’Sullivan WJ. Metabolic studies of the protozoan parasite, Crithidia luciliae, using proton nuclear magnetic resonance spectroscopy. Mol Biochem Parasitol. 1988;31:107–115. doi: 10.1016/0166-6851(88)90161-2. [DOI] [PubMed] [Google Scholar]
- 23.Ferreyra JA, et al. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014;16:770–777. doi: 10.1016/j.chom.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukahara T, Ushida K. Succinate accumulation in pig large intestine during antibiotic-associated diarrhea and the constitution of succinate-producing flora. J Gen Appl Microbiol. 2002;48:143–154. doi: 10.2323/jgam.48.143. [DOI] [PubMed] [Google Scholar]
- 25.Correa PR, et al. Succinate is a paracrine signal for liver damage. J Hepatol. 2007;47:262–269. doi: 10.1016/j.jhep.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toma I, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118:2526–2534. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouchani ET, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubic T, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 29.Saz DK, Bonner TP, Karlin M, Saz HJ. Biochemical observations on adult Nippostrongylus brasiliensis. J Parasitol. 1971;57:1159–1162. [PubMed] [Google Scholar]
- 30.Haber AL, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 32.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137:2179–2180; author reply 2180–2181. doi: 10.1053/j.gastro.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 33.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 35.Damak S, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 36.Rubić-Schneider T, et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 2017;72:444–452. doi: 10.1111/all.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapieha P, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 38.Aguiar CJ, et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun Signal. 2014;12:78. doi: 10.1186/s12964-014-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YH, Woo SH, Choi DH, Cho EH. Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells. Biochem Biophys Res Commun. 2015;463:853–858. doi: 10.1016/j.bbrc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vadder F, et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Fischbach MA, Sonnenburg JL. Eating for two: How metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10:336–347. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956;44:345–412. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- 45.Tizzano M, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee RJ, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476. doi: 10.1371/journal.pone.0080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kashyap PC, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–977. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buonomo EL, et al. Microbiota-regulated IL-25 increases eosinophil number to provide protection during Clostridium difficile infection. Cell Rep. 2016;16:432–443. doi: 10.1016/j.celrep.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.