Abstract
Emotional processing appears to be interlocked with perception, cognition, motivation, and action. These interactions are supported by the brain’s large-scale non-modular anatomical and functional architectures. An important component of this organization involves characterizing the brain in terms of networks. Two aspects of brain networks are discussed: brain networks should be considered as inherently overlapping (not disjoint) and dynamic (not static). Recent work on multivariate pattern analysis shows that affective dimensions can be detected in the activity of distributed neural systems that span cortical and subcortical regions. More broadly, the paper considers how we should think of causation in complex systems like the brain, so as to inform the relationship between emotion and other mental aspects, such as cognition.
Introduction
Why does emotion matter for cognition? Research in the past two decades has described how emotion interacts and is integrated with cognition [1]. Supporting these interactions are the brain’s non-modular anatomical and functional architectures [2–4]. Signal distribution and integration are the norm, allowing the confluence of information related to perception, cognition, emotion, motivation, and action. Thus, emotion is interlocked with all these mental domains via internetwork communication.
To better understand how brain networks inform the understanding of the interactions between emotion and cognition, we need to refine how they are conceptualized [5–7]. Here, two aspects of brain networks will be discussed: brain networks should be considered as 1) inherently overlapping, as well as 2) highly dynamic and context-sensitive. This discussion leads to the question of how emotions are represented in the brain. Even more broadly, the paper considers how we should think of causation in complex systems like the brain, so as to inform the relationship between emotion and other mental aspects, such as cognition.
From regions to networks: What’s right and what’s wrong with networks
Neuroscience has been always interested in circuits. Yet, the last 15 years have witnessed vigorous progress in neuroscience and network science analysis methods alike, with networks described at multiple levels, from micro (neuronal) to meso (pathways) to macro (whole-brain) levels [8]. In most instances, understanding structure-function mappings at the level of brain regions may be less productive because regions are not a meaningful computational unit in this regard [6]. Networks of brain regions collectively support complex behaviors. Thus, the network itself is the unit, not the brain region. Processes that support behavior are implemented by the interaction of multiple areas, which are dynamically recruited into multi-region coalitions.
Networks are overlapping, not disjoint
One of the goals of network analysis of brain data is to partition brain regions into clusters or “communities” that consist of regions that communicate more strongly (or that behave more alike) within the community than across it [9]. Most analyses describe networks in terms of disjoint sets, such that each brain region belongs to a single cluster of regions. But this assumes that brain areas compute a fairly well defined and specific function [6]. An alternative is to conceptualize networks as containing overlapping regions [10–13], such that specific areas belong to several intersecting networks [14]. In this manner, the processes carried out by an area will depend on its network affiliation (that is, the regions it clusters with) at a given time. What determines a region’s affiliation? An hypothesis is that the functional/behavioral context plays a pivotal role [15]. For example, region A will be part of network N1 during a certain context C1 but will be part of network N2 during another context C2.
These ideas resonate with the “flexible hub theory” [16], where some regions are suggested to flexibly shift their functional connectivity (that is, the degree to which signals from two regions co-vary in time) patterns as a function of task demands. To further understand potential network overlap, in a recent investigation of functional MRI (fMRI) data, we determined during rest and task conditions the distribution of “membership values,” that is, the extent to which each region participated across multiple networks ([17], see also [18]). Regions of the task-negative (or “default”) network, for example, participated in multiple networks simultaneously. Distributed participation was even more evident in a community of frontal and parietal regions important for attention and executive control, consistent with their multifunctional roles [19]. Overall, it is suggested that overlapping networks implement context-dependent computations that bring about behavioral flexibility.
Functional MRI is currently limited in its ability to inform the organization of brain networks at finer spatial resolutions. Such level is informed by recent neurotechniques that have precise control of cellular- and pathway-level activation and silencing, including optogenetics. A current debate is whether, within a structure, neuronal populations linked to appetitive and/or aversive processing are anatomically intermingled or segregated (for intermingled examples, see [20,21]; for segregated examples, see [22,23]). This literature is particularly relevant to inform the question of circuit/network overlap.
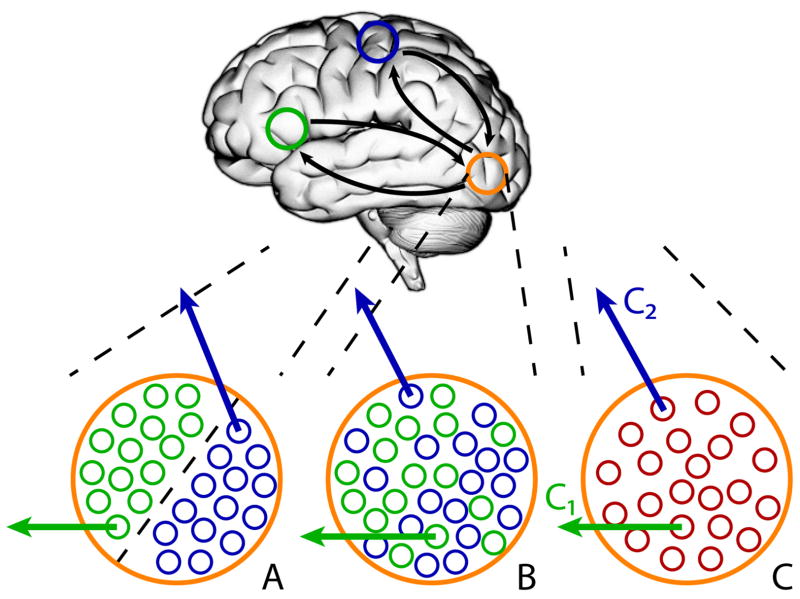
At least three scenarios are relevant. First, segregated populations within a region may be involved in interactions with separate regions (Figure 1A). In this case, we can refer to sub-regions of a larger anatomical region, which contains distinct populations of cells. In fact, in terms of networks, this situation could be described as involving disjoint and not overlapping networks. However, in some contexts, it is possible that the sub-regions involved, when combined, form noteworthy functional units, so the description in terms of overlapping networks may still be informative. Second, intermingled neuronal populations may exist, each of which is connected with different regions (Figure 1B). In this case, the neuronal sub-populations would be expected to exhibit different functional properties. Third, the same neurons may affiliate with different regions as a function of context (Figure 1C). For instance, neurons in a nucleus of the amygdala may affiliate with accumbens neurons in one context but with BNST neurons in a different one. In cellular-level studies, this possibility has not received as much attention yet. However, examples of how neurons or neuronal populations multiplex signals are well documented [24,25]. In such situations, multiple signals may be combined and transmitted even through a single “communication channel,” and even at the same time.
Figure 1.
Overlapping networks and neuronal populations. Blue and green circles represent brain regions of (relatively) separate networks. The orange circle indicates a region that is functionally linked to both networks. The small circles in A–C indicate neurons. (A) Segregated populations within a region are involved in interactions with separate brain regions/networks. (B) Intermixed neuronal populations within a region are involved in interactions with separate brain regions/networks. (C) The same population of neurons within a region is functionally connected with different brain regions/networks.
Networks are dynamic, not static
Brain networks are not static but evolve temporally. Functional connections vary as a function of context, and are altered by cognitive, emotional, and motivational variables (see [1]). Therefore, network organization must be understood dynamically [26–29]. Indeed, the growth of methods to describe time-varying functional connectivity has begun to yield novel characterizations of how network organization evolves [30–32].
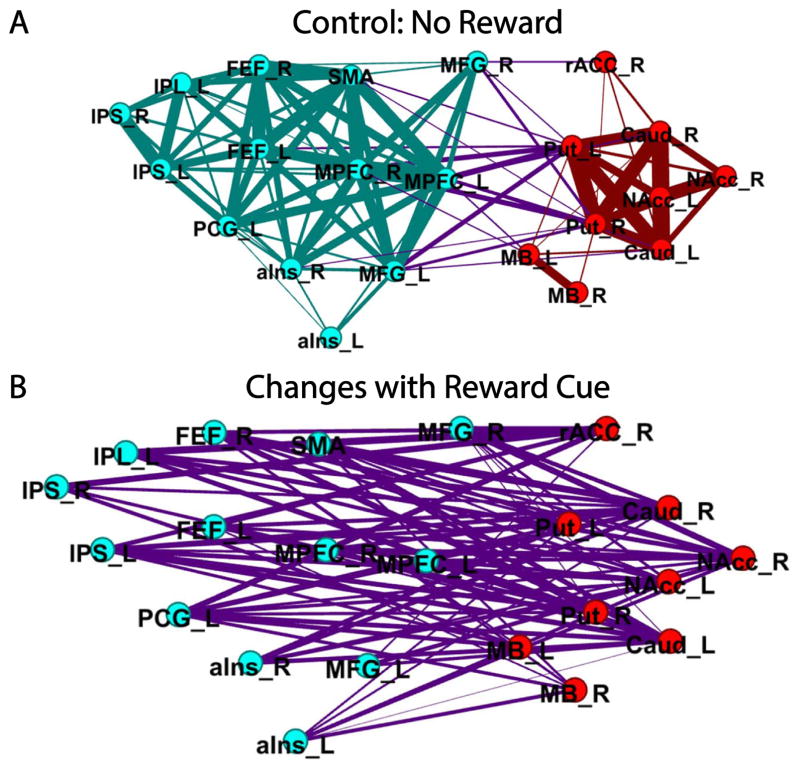
An example of changes to network organization is illustrated by a study in which participants performed a cognitive task during reward vs. no-reward conditions [33]. When a cue at the beginning of the trial signaled the possibility of reward, the functional connections between cortical regions important for cognitive processing and subcortical regions involved in reward processing increased systematically (Figure 2). In particular, the functional connections of the nucleus accumbens increased with all but one of the regions engaged by the task.
Figure 2.
Widespread changes in functional connectivity during cognitive-motivational interaction. (A) Representation of two communities during the control condition (no reward). Nodes indicate community organization (red: subcortical community; teal: cortical community) and edges indicate functional connections (purple: between-community edges). (B) Changes in reward vs. control connectivity between the two communities are shown. Reproduced with permission from Kinnison, Padmala, Choi, and Pessoa (2012).
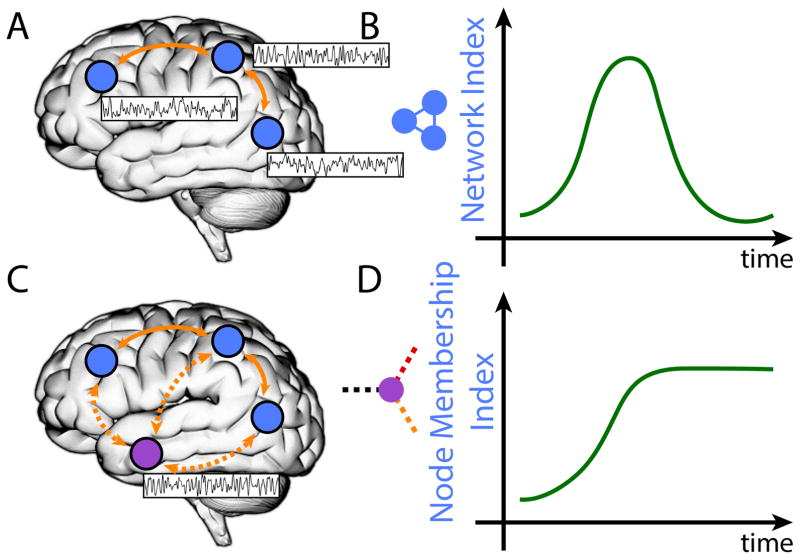
More generally, there are two important ways in which brain networks are dynamic. First, we can consider how specific networks evolve across time (Figure 3A–B). At the spatiotemporal resolution of fMRI, we and others have started to characterize how emotion influences the temporal unfolding of large-scale network organization [34–37]. In a recent study [36], periods of “anxious anticipation” were associated with transient and sustained changes to the salience, executive, and task-negative networks in the human brain. Notably, how the bed nucleus of the stria terminalis and the amygdala participated in network communication (as quantified by the measure of centrality) was altered during anxious states.
Figure 3.
Brain networks are dynamic. (A–B) Specific network properties (“network index”) evolve across time. (C–D) A region’s grouping with multiple networks evolves across time as indicated by the “membership index” (inset: white region and its functional connections to multiple networks). The region indicated in white increases its coupling with one of the networks and stays coupled with it for the remainder of the time.
Second, networks do not comprise fixed collections of regions. Networks are suggested to be dynamic coalitions of brain regions that form and dissolve to meet specific computational needs [6]. Accordingly, network descriptions need to specify how groupings of regions evolve temporally (Figure 3C–D). This poses several challenges, as the very notion of a network as a coherent unit is challenged. For instance, at what point does a coalition of regions become something other than, say, the salience network? Conceptualizing networks as inherently overlapping, as described previously, helps to mitigate this problem. For example, each node can be considered to be a member of multiple networks with a specific probability-like “membership value” [17], which fluctuates across time.
How are emotions represented in the brain?
Findings from pattern analyses of neuroimaging data show that affective dimensions and emotion categories can be detected in the activity of distributed neural systems that span cortical and subcortical regions. Indeed, attempts to classify brain states from distributed patterns of fMRI activation to predict these attributes have yielded high levels of specificity [38]. Some results indicate that emotion categories are not contained within any one region or system, but are represented as configurations across multiple brain networks [38]; for debate about emotion categories, see [39].
Interestingly, in one study [40], predictive patterns spanned multiple cortical and subcortical systems, with no single system being necessary or sufficient for predicting affective experience. Furthermore, predictive patterns were not reducible to activity in traditional “emotion-related” regions (e.g., amygdala) or resting-state networks (e.g., task-negative network).
When investigating the representation of emotions in the brain with fMRI, the spatial resolution of the technique must be considered, and the results interpreted with some caution. Although multi-voxel (distributed) representations have been proposed to be more sensitive spatially, consideration of Figure 1 highlights the fact that the issue is not simple, particularly when heterogeneous subcortical structures are involved. For instance, if the voxel size is larger than the tissue in Figure 1A, the subpopulations will be averaged or unsystematically linked to voxel placement; the situation is more problematic in Figure 1B, of course. Critically, Figure 1C highlights a scenario where the problem would exist regardless of the spatial resolution, and even cellular-level resolution would not solve it. This is because a region’s function will depend on the pattern of signal co-variation across regions.
This last case illustrates how the study of the brain basis of emotion benefits from studying functional relationships between regions. This could be done, for example, by employing machine learning to investigate patterns of functional connectivity ([41], see also [42]). In one study, we proposed combining functional connectivity with “functional fingerprint” analysis ([43], see also [44]). We used a meta-analytic approach to analyze the functional profiles of brain regions that exhibited co-activation with insular subdivisions (dorsal anterior, ventral anterior, and posterior insula). Functional profiles were determined based on interrogating fMRI study databases in terms of multiple task “domains” such as emotion, memory, attention, and action. The results suggested that all insular subdivisions are functionally diverse and that characterizations of the insula should move beyond cognition-emotion-interoception partitions.
I have proposed that the representation of emotion in the brain can be understood in terms of functionally integrated systems that involve large-scale cortical-subcortical networks that are sensitive to bodily signals [45]. The high degree of signal distribution and integration in the brain provides a nexus for the intermixing of information related to perception, cognition, emotion, motivation, and action. Importantly, as described above, the functional architecture consists of multiple overlapping networks that are dynamic and context-sensitive. Thus, how a given brain region affiliates with a specific network shifts as a function of task demands and brain state. Whereas the proposal of functionally integrated systems is consistent with multivariate and machine learning analyses of fMRI data indicating that emotional states are highly distributed, the model also predicts that brain “signatures” of affective dimensions are highly context-dependent, and may not generalize well across tasks and conditions.
How to think of causation in complex systems like the brain?
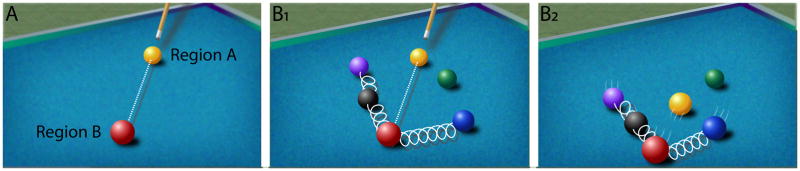
When we consider how emotion is represented in the brain and interacts with cognition, as in other areas of neuroscience, we tend to reason according to a billiard ball causal model (at least implicitly). In this model, force applied to a ball (external stimulus activating a region) leads to its movement until it hits the target ball (activation of an anatomically connected region) (Figure 4A). But this mode of thinking, which has been very productive in the history of science, is too impoverished when complex systems are considered [see 46]. When systems are not isolable, understanding the interrelatedness between “sub-systems” means that we should consider interactions between systems and integration of signals as the central elements to be unraveled.
Figure 4.
Causation in complex systems. (A) Simple billiard ball model of causation applied to the interactions between brain regions. (B) Some of the balls are connected by springs to suggest that they are coupled. When the yellow ball eventually hits the red one (B1), not only is the red ball affected but all of the ones coupled with it (B2). Thus, the overall goal of explanation is to understand the evolution of the coupled system as it evolves temporally (B2).
Whereas thinking of causation in complex systems is challenging, we can consider the situation illustrated in Figure 4B. When the initial force is applied to the yellow ball and it hits the target ball, the goal is to understand the evolution of the system of coupled elements as they interact with one another across time. In a related vein, an important goal for mind-brain scientists should be to understand the interactions between emotion and perception/cognition.
At the broadest level, the present discussion speaks to how we should study systems as complex as minds and brains. As advocated elsewhere [e.g. 47,48], the focus should not be on parts but on processes, which must be understood not solely in terms of their putative constituent elements but in terms of interactions and temporal evolution. In the brain, we will be often interested in describing the joint state of a set of regions, and how this joint state evolves through time. Consider the set of activity strengths for a set of brain regions: x1, x2, ···, xn. The vector x describes the current state of the system and x(t) describes its trajectory across time. At a particular time, the state of a set of regions can be represented as a point in multidimensional state space in which each axis represents a region’s activity. Therefore, the evolution of the system can be described as a spatio-temporal trajectory ([49], for an example involving cell recordings, see [50]).
In this view, we can advance our understanding of the emotional brain by studying and characterizing spatio-temporal trajectories (that is, how the joint activity across multiple brain regions evolves across time) of distributed systems believed to be central to emotional functions. And given the large-scale, overlapping, and dynamic nature of brain networks, emotion matters not only for closely associated motivational processes, but for perception, action, and cognition.
Highlights.
Interactions between emotion and cognition rely on large-scale distributed networks
Brain networks are composed of overlapping regions (not disjoint sets of regions)
Brain networks are dynamic, not static
Understanding interactions requires characterizing causation in complex systems
Acknowledgments
The author acknowledges funding from the National Institute of Mental Health (MH071589). I also thank Christian Meyer and Dan Levitas for help with figures and references.
Footnotes
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pessoa L. The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press; 2013. [Google Scholar]
- 2••.Markov NT, Ercsey-Ravasz M, Van Essen DC, Knoblauch K, Toroczkai Z, Kennedy H. Cortical high-density counterstream architectures. Science. 2013;342:1238406. doi: 10.1126/science.1238406. Based on monkey anatomical data, the authors propose that the cortical architecture is considerably more densely interconnected than previously thought. They propose a model that includes a core of highly interconnected regions and a periphery that is less interconnected. One of the consequences of the overall high connectivity is high global accessibility (the extent to which information in one part of a system can reach other parts via direct and/or indirect pathways) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bota M, Sporns O, Swanson LW. Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A. 2015;112:E2093–E2101. doi: 10.1073/pnas.1504394112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporns O. Networks of the Brain. MIT press; 2010. [Google Scholar]
- 6.Pessoa L. Understanding brain networks and brain organization. Physics of Life Reviews. 2014;11:400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornito A, Zalesky A, Bullmore E. Fundamentals of brain network analysis. Academic Press; 2016. [Google Scholar]
- 8.Swanson LW, Lichtman JW. From Cajal to Connectome and Beyond. Annu Rev Neurosci. 2016;39:197–216. doi: 10.1146/annurev-neuro-071714-033954. [DOI] [PubMed] [Google Scholar]
- 9.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 10.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 11.Palla G, Derenyi I, Farkas I, Vicsek T. Uncovering the overlapping community structure of complex networks in nature and society. Nature. 2005;435:814–818. doi: 10.1038/nature03607. [DOI] [PubMed] [Google Scholar]
- 12.Evans TS, Lambiotte R. Line graphs, link partitions, and overlapping communities. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80:016105. doi: 10.1103/PhysRevE.80.016105. [DOI] [PubMed] [Google Scholar]
- 13.Gopalan P, Blei D. Efficient discovery of overlapping communities in massive networks. Proceedings of the National Academy of Sciences. 2013;110:14534–14539. doi: 10.1073/pnas.1221839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh AR. Towards a network theory of cognition. Neural Networks. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 16••.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. The study found that the fronto-parietal network brain-wide functional connectivity pattern shifted more than those of other networks across a variety of task states and that these connectivity patterns could be used to identify the current task. The authors propose that this network is comprised of “flexible hubs” whose functional coupling shifts to meet task demands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najafi M, Mcmenamin BW, Pessoa L. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage. 2016;135:92–106. doi: 10.1016/j.neuroimage.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2014;88:212–227. doi: 10.1016/j.neuroimage.2013.10.046. The authors investigated network overlap with resting-state fMRI data. A Latent Dirichlet Allocation model revealed multiple interactions between brain networks, with many association regions belonging to at least two networks. The “default” and “dorsal attention” networks had the greatest proportion of regions participating in multiple networks. Overall, 44% of the vertices participated in multiple networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocchi L, Zalesky A, Fornito A, Mattingley JB. Dynamic cooperation and competition between brain systems during cognitive control. Trends in Cognitive Sciences. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 20•.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. The study identified overlapping populations of neurons in the basolateral amygdala projecting to the nucleus accumbens and to the centromedial amygdala, which undergo opposing synaptic changes following reward or fear conditioning, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. Independent hypothalamic circuits for social and predator fear. Nature Neuroscience. 2013;16:1731–1733. doi: 10.1038/nn.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93:1464–1479. e1465. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends in Neuroscience. 2010;33:111–120. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Akam T, Kullmann DM. Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nature Reviews Neuroscience. 2014;15:111–122. doi: 10.1038/nrn3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D-U. Complex networks: Structure and dynamics. Physics reports. 2006;424:175–308. [Google Scholar]
- 27.Palla G, Barabasi AL, Vicsek T. Quantifying social group evolution. Nature. 2007;446:664–667. doi: 10.1038/nature05670. [DOI] [PubMed] [Google Scholar]
- 28.Lambiotte R, Delvenne J-C, Barahona M. Laplacian dynamics and multiscale modular structure in networks. 2008 arXiv preprint arXiv:0812.1770. [Google Scholar]
- 29.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela JP. Community structure in time-dependent, multiscale, and multiplex networks. Science. 2010;328:876–878. doi: 10.1126/science.1184819. [DOI] [PubMed] [Google Scholar]
- 30.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helfrich RF, Knight RT. Oscillatory Dynamics of Prefrontal Cognitive Control. Trends Cogn Sci. 2016 doi: 10.1016/j.tics.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinnison J, Padmala S, Choi JM, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. Journal of Neuroscience. 2012;32:8361–8372. doi: 10.1523/JNEUROSCI.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 35.Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 36•.McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. Network analysis was employed to characterize how the organization of the salience, executive, and task-negative networks evolves during an extended period of “anxious anticipation.” For example, the salience network exhibited a transient increase in network efficiency followed by a period of sustained decreased efficiency. The amygdala became more central to network function (as assessed via betweenness centrality), and the extent to which the BNST became more central depended on self-reported anxiety. Overall, the study unraveled a progression of responses and network-level changes due to sustained threat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMenamin BW, Pessoa L. Localizing functional connectivity changes and discovering networks: The connectivity difference localizer method with application to anxiety states. 2017 [Google Scholar]
- 38.Kragel PA, LaBar KS. Decoding the Nature of Emotion in the Brain. Trends in Cognitive Sciences. 2016;20:444–455. doi: 10.1016/j.tics.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLoS Biology. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–1507. doi: 10.1093/brain/aws059. Machine learning was used to classify whole-brain resting-state functional connectivity patterns of depressed patients vs. controls. Classification accuracy exceeded 90%. Many of the most discriminating functional connections were located within or across the default mode network, what the authors called the “affective network” (including the amygdala and insula), and cerebellum. [DOI] [PubMed] [Google Scholar]
- 42•.Diano M, Tamietto M, Celeghin A, Weiskrantz L, Tatu MK, Bagnis A, Duca S, Geminiani G, Cauda F, Costa T. Dynamic Changes in Amygdala Psychophysiological Connectivity Reveal Distinct Neural Networks for Facial Expressions of Basic Emotions. Sci Rep. 2017;7:45260. doi: 10.1038/srep45260. Functional connectivity analysis revealed that amygdala interactions with other brain regions varied as a function of emotional expression (anger, fear, etc.). For each emotion, the amygdala interacted with a distinctive and spatially distributed set of structures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Uddin LQ, Kinnison J, Pessoa L, Anderson ML. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 2014;26:16–27. doi: 10.1162/jocn_a_00462. This study characterized the functional fingerprint of insular subdivisions, as well as the fingerprint of their co-activation partners (i.e., “target” regions). The neural partners of insular sub-regions were active at least some of the time in all of the task domains investigated, indicating that characterizations in terms of “cognitive” and “emotional” subdivisions neglect important aspects of insula function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauli WM, O’Reilly RC, Yarkoni T, Wager TD. Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci U S A. 2016;113:1907–1912. doi: 10.1073/pnas.1507610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pessoa L. A network model of the emotional brain. Trends in Cognitive Sciences. 2017 doi: 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Mannino M, Bressler SL. Foundational perspectives on causality in large-scale brain networks. Physics of Life Reviews. 2015;15:107–123. doi: 10.1016/j.plrev.2015.09.002. The authors attempt to clarify the concept of causality in large-scale brain networks both philosophically and scientifically. They argue that, given the topological complexity of its large-scale connectivity, the brain should be considered as a complex system and its causal influences treated as probabilistic in nature. [DOI] [PubMed] [Google Scholar]
- 47.Maturana HR, Varela FJ. The tree of knowledge: The biological roots of human understanding. New Science Library/Shambhala Publications; 1987. [Google Scholar]
- 48.Thompson E. Mind in life: Biology, Phenomenology, and the sciences of the mind. Cambridge, MA: Harvard University Press; 2007. [Google Scholar]
- 49.Buonomano DV, Maass W. State-dependent computations: spatiotemporal processing in cortical networks. Nat Rev Neurosci. 2009;10:113–125. doi: 10.1038/nrn2558. [DOI] [PubMed] [Google Scholar]
- 50.Mante V, Sussillo D, Shenoy KV, Newsome WT. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature. 2013;503:78–84. doi: 10.1038/nature12742. [DOI] [PMC free article] [PubMed] [Google Scholar]