Abstract
Homozygosity for the hemoglobin (Hb) S mutation (HbSS, sickle cell anemia) results in hemoglobin polymerization under hypoxic conditions leading to vaso-occlusion and hemolysis. Sickle cell anemia affects 1:500 African Americans and is a strong risk factor for kidney disease, although the mechanisms are not well understood. Heterozygous inheritance (HbAS; sickle cell trait) affects 1:10 African Americans and is associated with an increased risk for kidney disease in some reports. Using transgenic sickle mice, we investigated the histopathologic, ultrastructural, and gene expression differences with the HbS mutation. Consistent with progressive glomerular damage, we observed progressively greater urine protein concentrations (P=0.03), glomerular hypertrophy (P=0.002), and glomerular cellularity (P=0.01) in HbAA, HbAS and HbSS mice. Ultrastructural studies demonstrated progressive podocyte foot process effacement, glomerular basement membrane thickening with reduplication, and tubular villous atrophy with the HbS mutation. Gene expression studies highlighted the differential expression of several genes involved in prostaglandin metabolism (AKR1C18), heme and iron metabolism (HbA-A2, HMOX1, SCL25A37), electrolyte balance (SLC4A1, AQP6), immunity (RSAD2, C3, UBE2O), fatty acid metabolism (FASN), hypoxia hall-mark genes (GCK, SDC3, VEGFA, ETS1, CP, BCL2) as well as genes implicated in other forms of kidney disease (PODXL, ELMO1, FRMD3, MYH9, APOA1). Pathway analysis highlighted increased gene enrichment in focal adhesion, extracellular matrix-receptor interaction, and axon guidance pathways. In summary, using transgenic sickle mice, we observed that inheritance of the HbS mutation is associated with glomerular and tubular damage and identified several candidate genes and pathways for future investigation in sickle cell trait and sickle cell anemia-related kidney disease.
Keywords: Sickle cell anemia, sickle cell trait, kidney disease, transgenic mouse
Introduction
The sickle hemoglobin (Hb S) mutation β-globin gene (HBBE6V) has undergone selection by providing protection from P. falciparum malaria.1 This mutation is observed in 10% of African Americans and can reach a prevalence of up to 40% in certain regions of sub-Saharan African, the Middle East, and India.2
Inheritance of the Hb S mutation in the homozygous state (Hb SS, sickle cell anemia) is associated with hemoglobin polymerization under hypoxic conditions causing red blood cell deformity, extravascular and intravascular hemolysis, vaso-occlusion and many acute and chronic complications. The kidneys are among the most commonly affected organ systems. Chronic kidney disease (CKD) occurs in up to 60% of Hb SS adults,3,4 and is a consistent predictor for early mortality.5–7 Although renal biopsy tissues from Hb SS patients are infrequently available, small studies have demonstrated glomerular hypertrophy, membranoproliferative glomerulopathy, focal and segmental glomerulosclerosis, and hemosiderin deposition in the proximal tubules.8–12 The mechanisms for how the nephrons are damaged and how CKD develops in sickle cell anemia are poorly understood but may include hyperfiltration, ischemia-reperfusion injury, vasculopathy, and hypertension.13
Heterozygous inheritance of the Hb S mutation (Hb AS, sickle cell trait) is also associated with renal manifestations. These include defects in urine concentrating ability, an increased prevalence of hematuria, and a higher risk for renal medullary cancer than the general population.14 Large population studies have recently demonstrated that sickle cell trait is also associated with an increased risk for albuminuria, CKD and ESRD in African Americans.15,16 Consistent findings for the association of Hb AS with CKD were demonstrated in Hispanics and Latinos with a Caribbean background17 but not in a cohort of adults from the Democratic Republic of Congo.18 Renal tissue of Hb AS patients has been rarely analyzed but glomerular enlargement, mesangial proliferation, and iron deposition may also occur.10 The pathophysiologic mechanisms for how inheritance of Hb AS leads to CKD are also poorly understood.
Transgenic mice harboring the Hb S mutation have been developed and may improve our understanding of the pathophysiology occurring in the kidneys. Similar to what has been observed in humans, Hb SS mice have higher urine protein concentrations compared to Hb AA mice and frequently demonstrate glomerulosclerosis and membranoproliferative glomerulonephritis (MPGN)-like lesions.19–21 The Hb SS mice also demonstrate glomeruli that are hypertrophied with increased cellularity, thickened GBM, and mesangial expansion while the tubules have increased iron deposition, atrophy and increased basement membrane thickening.19,21–23 Hemizygous mice that express both mouse and human hemoglobin have been examined as a model for Hb AS.24,25 These studies have demonstrated enlarged glomeruli with increased cellularity, thickened GBM, and tubular degeneration in the hemizygous mice compared to control mice, although the hemizygous model is limited by the presence of mouse α chains which can interfere with Hb S polymerization as effectively as human γ chains do.26 To date, a comprehensive evaluation of urine, histopathology, electron microscopy, and gene expression changes occurring in the kidney of Hb AA, Hb AS, and Hb SS transgenic knockout mice exclusively expressing human α-, β-, and γ-globin has not been reported.
We conducted a study of transgenic sickle mice to determine whether progressive renal cortical damage is observed in Hb AA, Hb AS, and Hb SS mice. We also sought to identify genes that are differentially expressed in the renal cortex in order to highlight functional pathways by which the kidneys may be injured in sickle cell trait and sickle cell anemia.
Methods
Transgenic sickle mice
All animal procedures were conducted under protocols approved by the Illinois Institutional Animal Care and Use Committee at the University of Illinois at Chicago (UIC). Transgenic sickle cell mice (B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J) (Townes model, Jackson laboratory; Bar Harbor, U.S.A.) were bred and housed in the UIC Biologic Resources Laboratory. Studies were conducted in age- and gender-matched Hb AA, Hb AS, and Hb SS mice, all age 6 months or older, and hemoglobin genotype was confirmed by electrophoresis.
Blood and urine were collected on the day of animal sacrifice. Automated complete blood counts were obtained using the Advia 120 Hematology System (Siemens; Erlangen, Germany). Urine protein concentration was measured by the Bio-Rad dye method (Bio-Rad Laboratories Inc, Hercules, CA), and urine creatinine concentration by the Jaffé reaction, using the picric acid reagent supplied by Sigma Chemical Co., Saint Louis MO. Prior to harvesting the kidneys, the mice were perfused with PBS until clear fluid was returning to the heart. The kidneys were immediately weighed and the renal cortex was processed for histology, electron microscopy, and gene expression studies.
Histopathology
Masson trichrome, haemotoxylin and eosin, and Periodic acid-Schiff stained sections of the kidney were evaluated by renal pathologists blinded to the genotype of each specimen. Mesangial expansion was defined as increased matrix with increased mesangial cell number (>5 nuclei/mesangium). Mesangial expansion was graded based on the proportion of glomeruli per slide affected as follows: focal process if < 50% involvement or a diffuse process if ≥ 50% involvement. The perimeters of randomly selected glomeruli were measured using NanoZoomer Whole Slide Imaging (Hamamatsu Photon Imaging, Japan) and the nuclei per glomerulus were manually counted. Glomerular enlargement was defined as a glomerular perimeter > 300μM, which represented the upper quartile of measured glomeruli.
Electron microscopy
Renal cortical tissue was dissected into 1mm sections and samples were processed by the UIC, Electron Microscopy Service Core for transmission electron microscopy (TEM) imaging. Kidney tissues were fixed in buffered 2% p-formaldehyde + 2.5 % glutaraldehyde (pH, 7.2), rinsed with 0.1M sodium phosphate buffer, post-fixed with 1% osmium tetroxide, and dehydrated using an ascending series of ethanol solutions through 100%. They were then embedded in LX112 epoxy resin and polymerized at 60° C for 2–3 days. Toluidine blue stained semi-thin sections (0.5–1 μm) were taken to identify areas of interest, after which thin-sections (70–80 nm) were collected and stained with uranyl acetate and lead citrate, respectively. Specimens were examined using a JEOL JEM-1220 transmission electron microscope. Digital images were acquired using an Erlangshen ES1000W model 785 CCD camera and Digital Micrograph software 1.7.1. Electron micrographs of Hb AA, AS and SS mice were obtained and examined for glomerular and tubular differences between the three genotypes.
Gene expression
Renal cortical tissue was immediately frozen in liquid nitrogen after dissection. The RNA was extracted and processed by the UIC, Core Genomics Facility. A total of 15 samples (5 samples per genotype; all female and 9 months of age) were collected. RNA purification, labeling and hybridization were performed independently for each sample. An Affymetrix Mouse Gene Array 2.0 (Thermo Fisher Scientific, Waltham, MA) was employed for the study. This single array provides coverage of over 28,000 coding transcripts and >7,000 long intergenic non-coding transcripts. Total cellular RNA was isolated with the use of RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA quality control was performed with the use of automated electrophoresis system TapeStation (Agilent, Santa Clara CA). Labeling reactions and hybridizations were carried out according to the Whole Transcript (WT) Plus Target labeling protocol (Thermo Fisher Scientific, Waltham, MA). 100 ng of total RNA was used in each labeling reaction. The resulting labeled and fragmented ss-cDNA target was hybridized to the arrays. In order to minimize the batch-to-batch array variation, all hybridizations were performed with arrays from the same manufacturing lot. Hybridizations were followed by binding to a streptavidin-conjugated fluorescent marker. Detection of bound probe was achieved following laser excitation of the fluorescent marker and scanning of the resultant emission spectra using a scanning confocal laser microscope (Thermo Fisher Scientific, Waltham, MA). Signal acquisition was performed using the AGCC suite.
Probe level summarization of files was performed using Partek Genomics Suite (Partek, Saint Louis, MI). The Robust Multi-array Average (RMA) method was used to background correct and normalize hybridization signal intensities across all collected samples.27 Experimental groups of samples were compared using an additive model by linear regression to identify genes that are differentially expressed with inheritance of the Hb S mutation. Raw and FDR corrected P-values following the Benjamini-Hochberg procedure of differential expression were calculated. Differentially expressed transcripts were initially annotated according to the latest current release of Affymetrix NetAffx Analysis Center. This web-based resource facilitates for each individual transcript biological annotation and functional classification according to GO (http://www.geneontology.org), KEGG (http://www.genome.ad.jp/kegg) and Entrez Gene (http://www.ncbi.nlm.nih.gov) databases. We used the DAVID bioinformatics resources28 to develop gene enrichment pathway analysis using the 1029 genes that were differentially expressed. Hypoxia hallmark genes were obtained at: hhtp://www.broadinstitute.org/gsea/msigdb/cards/HALLMARK HYPOXIA and mapped to the orthologous genes in the mouse. Pathways with a Benjamini-adjusted P-value ≤ 0.01 were considered statistically significant.
Statistical Methods
Comparisons between each genotype were performed using the student’s t-test for linear variables and chi-square analysis for categorical variables. To determine whether inheritance of the Hb S gene (Hb AA to Hb AS to Hb SS) caused incremental damage, we used the test for linear trend for linear variables and Cochran’s test of linear trend for categorical variables. The renal pathologists were blinded to the genotype of each specimen and all mice were considered as a unique, independent variable. Analyses were performed using Systat 13 (Systat Software Corporation, USA) and mean values ± standard error of the mean are provided.
Results
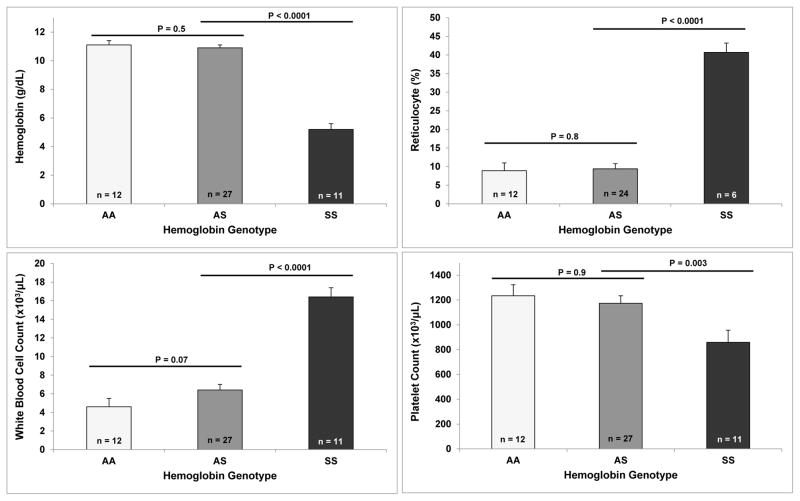
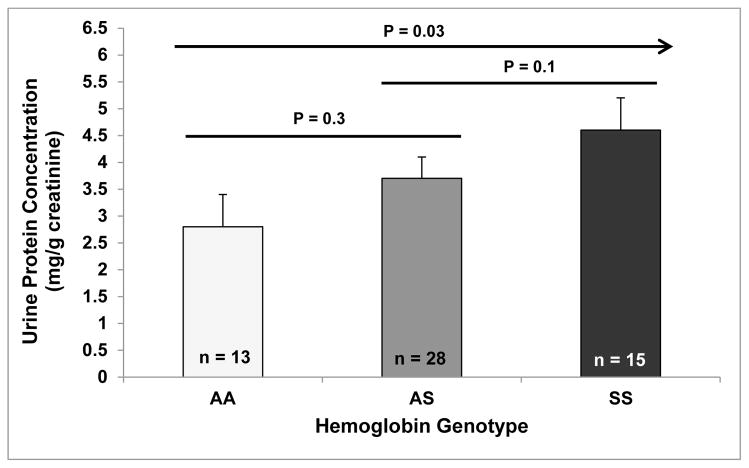
We conducted studies on 56 age- and gender-matched transgenic mice greater than 6 months of age who were in relatively healthy condition (Hb AA: n = 13, Hb AS: n = 28; Hb SS: n = 15). Baseline complete blood counts demonstrated that the Hb SS mice had more severe hemolysis, reflected by lower hemoglobin concentrations and higher reticulocyte percentages, a higher white blood cell count, and a lower platelet count compared to Hb AS and Hb AA mice (Figure 1). We observed progressively higher urine protein concentrations (P = 0.03 for trend) (Figure 2) in Hb AA, Hb AS and Hb SS mice. Expressed as the ratio to body weight, Hb SS mice had greater kidney masses (15.9 ± 0.6 mg/g body weight) compared to Hb AS and AA mice (12.6 ± 0.4 and 12.9 ± 0.6 mg/g body weight, respectively; P ≤ 0.002).
Figure 1.
Blood count parameters.
Figure 2.
Urine protein concentrations.
Histopathology
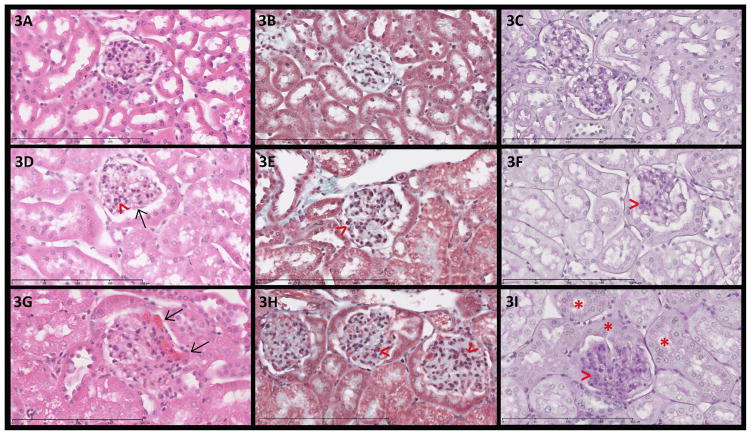
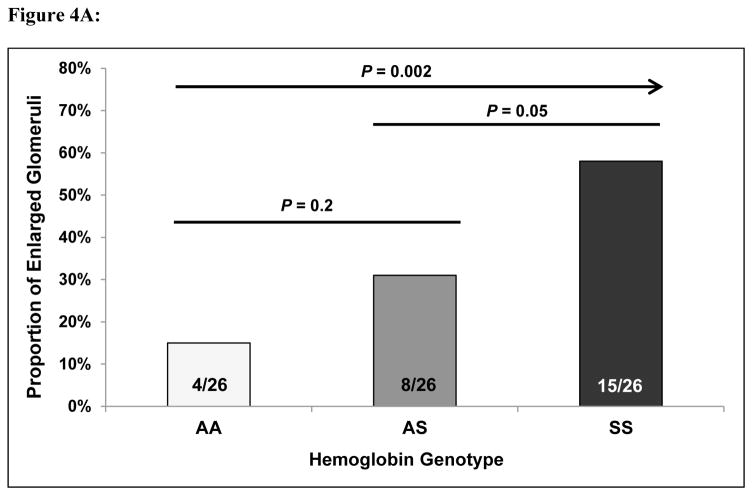
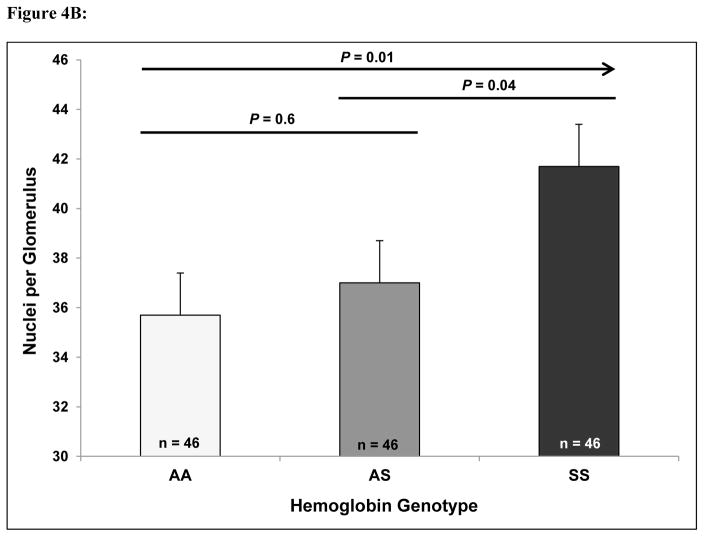
When grading the degree of mesangial expansion, a focal process (< 50% of glomeruli/slide affected) was observed in Hb AA and more so in Hb AS mice while a focal to a global process (≥ 50% of glomeruli/slide affected) was observed in Hb SS mice (Figure 3). Hemosiderin staining was moderately increased in the glomeruli and markedly increased in the tubules of the Hb SS mice versus the Hb AS and Hb AA mice. Progressively greater proportions of enlarged glomeruli were observed in Hb AA (15%), Hb AS (31%), and Hb SS (58%) transgenic mice (P = 0.002 for trend) (Figure 4A). Similarly, the number of nuclei per glomerulus progressively increased in Hb AA (35.7 ± 1.7), Hb AS (37.0 ± 1.7), and Hb SS (41.7 ± 1.7) mice (P = 0.01 for trend) (Figure 4B).
Figure 3. Histopathology of the kidney.
(original magnification 400x). 3A, 3D and 3G: H & E stained sections; 3B, 3E and 3H: Masson trichrome stained sections; 3C, 3F and 3I: Periodic acid-Schiff stained sections. 3A–C: Glomeruli from Hb AA mouse with normal cellularity and unremarkable tubules. 3D–F: Glomeruli from Hb AS mouse with mild focal segmental mesangial hypercellularity (>) and segmental congestion of glomerular capillary loops (→). 3G–I: Glomeruli from Hb SS mouse with global diffuse mesangial hypercellularity (>) and segmental congestion of glomerular capillary loops (→). 3I: Hb SS mouse with tubular and parietal epithelial deposition of cytoplasmic hemosiderin pigment (*).
Figure 4.
(A) Glomerular hypertrophy and (B) cellularity in Hb AA, Hb AS, and Hb SS mice.
Electron microscopy
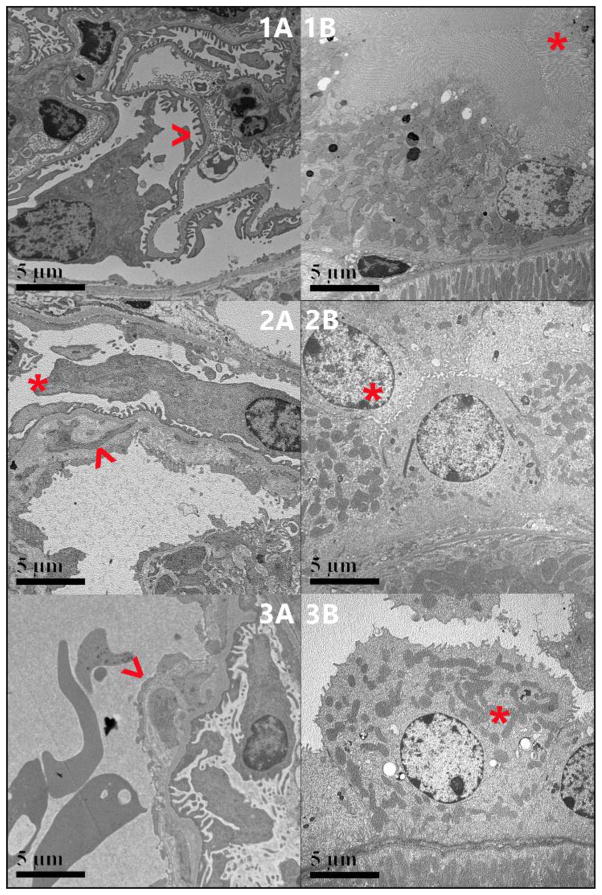
The Hb AA mice had predominantly intact foot processes and fairly uniform glomerular and tubular basement membranes (Figure 5A, 5B). Mesangial areas were also uniformly populated with cells and matrix. In comparison, the Hb AS mice had focal effacement of foot processes along with variably thickened glomerular basement membranes (GBM) and segmental subendothelial electron lucent widening. One of the two examined glomeruli demonstrated segmental areas of early basement membrane reduplication with mesangial interposition (Figure 5C). The Hb AS mice also had focal microvillous blunting and shortening of the tubular brush borders (Figure 5D). Similar, but more pronounced changes were observed in the Hb SS mice with both examined glomeruli showing easily identifiable areas of subendothelial electron lucent widening and more pronounced segmental areas of early basement membrane reduplication with mesangial interposition (Figure 5E). The Hb SS mice also had brush borders with focal marked attenuation of microvilli (Figure 5F).
Figure 5.
5A, 5C and 5E: Ultrastructure of glomeruli, 5B, 5D and 5F Ultrastructure of proximal convoluted tubules. 5A: Hb AA mouse: Glomerular basement membranes are overall fairly uniform in thickness with mainly intact foot processes ( < ). 5B. Hb AA mouse: Tubular basement membranes are overall fairly uniform in thickness with mainly intact brush borders (*). 5C AS: Glomerular basement membranes are variably thickened with focal effacement of foot processes (*). Both examined glomeruli have segmental subendothelial widening and one has early basement membrane reduplication with mesangial interposition (blue arrow). 5D. Hb AS: Tubular brush borders have focal microvillous blunting and shortening (*). 5E. Hb SS: Glomerular basement membranes are variably thickened with focal effacement of foot processes (*). Both examined glomeruli have segmental subendothelial widening and early basement membrane reduplication with mesangial interposition (blue arrow). 5F. SS: Tubular brush borders have focal marked attenuation with pronounced microvillous blunting (*).
Gene expression
Using an additive model with false discovery rate (FDR) < 0.01, 1029 genes were differentially expressed in the kidney cortex with inheritance of the Hb S mutation. The 10 genes with the strongest β-values are provided in Table 1. Other genes that were differentially expressed include those that are involved in maintaining the glomerular filtration barrier (PODXL, β 0.59, FDR 0.009) or have been implicated in diabetic nephropathy (ELMO1, β 0.28, FDR 0.003; FRMD3, β −0.11, FDR 0.009). Gene enrichment pathway analysis of the differentially expressed genes identified that focal adhesion (3.4-fold enrichment), extracellular matrix-receptor interaction (5.0-fold enrichment), and axon guidance (3.8-fold enrichment) were upregulated with inheritance of the Hb S mutation (Table 2). When focusing on hypoxia hallmark genes, we identified six additional genes differentially expressed with inheritance of the Hb S mutation (FDR < 0.01) (Table 3). The MYH9 gene, which co-segregates with APOL1 and has been implicated in kidney disease,29 was identified as a hypoxia gene differentially expressed with inheritance of the Hb S mutation (β 0.59, FDR 0.01). APOA1 encodes the major structural protein of HDL particles, including the APOL1-trypanasome lytic factor complex,30 and was also differentially expressed with the Hb S mutation (β −0.25, FDR 0.003). We did not observe significant changes in the expression of Hp, the main scavenger of cell-free hemoglobin in circulation, with the Hb S mutation (FDR 0.5).
Table 1.
Genes differentially expressed in the kidney cortex of Hb AS and Hb SS mice using an additive model.
| Gene | β value | FDR | Mechanistic Pathway |
|---|---|---|---|
| AKR1C18 | −1.12 | 0.007 | Oxidoreductase activity; catalyzes reduction of prostaglandins |
| HbA-A2 | 0.99 | 0.009 | Alpha chain of hemoglobin |
| HMOX1 | 0.93 | 0.0099 | Essential enzyme for heme degradation |
| SLC4A1 | 0.87 | 0.005 | Chloride/HCO3 exchange involved in kidney acid secretion |
| FASN | 0.87 | 0.007 | Regulates cholesterol biosynthesis |
| AQP6 | 0.80 | 0.006 | Kidney-specific water channel functioning in glomerular filtration |
| RSAD2 | 0.76 | 0.004 | IFNα/β signaling involved in CD4+cell activation and differentiation |
| C3 | 0.76 | 0.0099 | Central role in complement activation |
| SLC25A37 | 0.75 | 0.004 | Mitochondrial iron importer |
| UBE2O | 0.72 | 0.005 | Immune/antigen processing for ubiquination and proteosome degradation |
Table 2.
Pathways enriched in the kidney cortex of Hb AS and Hb SS mice.
| Pathways | Fold Enrichment | P value | Adjusted P value |
|---|---|---|---|
| Focal adhesion | 3.44 | 5.99 × 10−7 | 7.06 × 10−5 |
| Extracellular matrix-receptor interaction | 4.99 | 3.15 × 10−6 | 1.86 × 10−4 |
| Axon guidance | 3.84 | 6.64 × 10−6 | 2.61 × 10−4 |
Table 3.
Hypoxia hallmark genes differentially expressed in the kidney cortex of Hb AS and Hb SS mice using an additive model.
| Gene | β value | FDR | Mechanistic Pathway |
|---|---|---|---|
| GCK | −0.10 | 0.005 | Hexokinase involved in glucose metabolism |
| SDC3 | 0.35 | 0.008 | Interacts with actin cytoskeleton to organize cell shape |
| VEGFA | 0.43 | 0.008 | Induces proliferation and migration of endothelial cells for angiogenesis |
| ETS1 | 0.22 | 0.009 | Transcriptional factor involved in cell senescence and death |
| CP | 0.29 | 0.009 | Ferroxidase involved in iron metabolism |
| BCL2 | 0.31 | 0.0099 | Regulates apoptosis and attenuates NLRP1-inflammasome activation |
Discussion
Using a transgenic sickle mouse model, we observed progressive glomerular changes with inheritance of Hb AS and Hb SS. Urine protein concentration, glomerular hypertrophy, and glomerular cellularity progressively increased in Hb AS and Hb SS mice. Ultrastructural studies supported our findings of progressive glomerular damage with Hb AS and Hb SS, demonstrating incremental degrees of podocyte foot process effacement and glomerular basement membrane reduplication with mesangial interposition. In addition, progressive tubular damage, reflected by incremental brush border microvillous abnormalities by TEM, was observed. Gene expression profiling of the kidney cortex identified 1) several genes involved in plausible pathways for sickle cell trait and sickle cell anemia-related nephropathy, 2) genes implicated in focal segmental glomerulosclerosis or diabetic nephropathy, and 3) a gene enrichment pathway analysis emphasizing focal adhesion, extracellular matrix-receptor interactions, and axon guidance with inheritance of the Hb S mutation.
Initial reports demonstrated that Hb AS was twice as common in African Americans with ESRD,31 although a subsequent study did not find an association between Hb AS and ESRD after adjusting for the APOL1 G1/G2 risk variants.32 More recently, large cohort studies of African Americans have shown that Hb AS is associated with a 1.8-fold increased risk for incident CKD15 and a 2.0-fold increased risk for developing ESRD,16 independent of APOL1 risk variant status. The association of Hb AS and CKD has also been demonstrated in a cohort of Caribbean background17 but not in an adult cohort from the Democratic Republic of Congo.18 The evaluation of glomerular and tubular changes in Hb AS patients has been less frequently reported but cortical infarcts with sickled red blood cells plugging glomerular capillaries was observed in a case report of a Hb AS patient with nephrotic syndrome and moderate increases in glomerular size and tubular atrophy were observed in a case series of 10 Hb AS patients.10,33 To our knowledge, this is the first report describing progressive histopathologic and ultrastructural abnormalities with inheritance of the Hb S mutation. Also unique to our study is the histopathologic and ultrastructural evidence that glomerular and tubular damage is occurring in Hb AS kidneys, providing support to the epidemiologic findings that Hb AS is a risk factor for CKD.
Among our top 10 differentially expressed genes, we identified several candidate genes with plausible mechanisms for kidney disease in sickle cell trait and sickle cell anemia. AKR1C18, which is involved in prostaglandin metabolism, was down regulated in Hb AS and Hb SS mice. This is particularly interesting because prostaglandins have been implicated in the hyperfiltration of sickle cell anemia,34 progressively higher proportions of Congolese children have hyperfiltration with co-inheritance of the Hb S mutation (Hb AA 6%, Hb AS 16%, and Hb SS 30%),35 and we observed progressive glomerular hypertrophy in Hb AS and Hb SS mice. Genes implicated in hemoglobin, heme and iron metabolism (HbA-A2, HMOX1, and SLC25A37), were also upregulated with the Hb S mutation. These candidate genes have been implicated in hypertensive nephropathy (HbA-A2),36 sickle cell nephropathy (HMOX1),37,38 and play critical roles in cell-free heme and iron metabolism (HMOX1, SLC25A37).39,40 HbA-A2 is expressed in arterial endothelial cells and interacts with eNOS to regulate blood vessel tone.41 Upregulation of HbA-A2, as was observed in the kidney cortex of Hb AS and Hb SS mice, may lead to reduced NO diffusion across the vessel wall and to increased arterial reactivity in response to adrenergic agonists such as phenylephrine. Two genes involved in electrolyte and acid-base balance, SLC4A1 and AQP6, were also differentially expressed with the Hb S mutation. SLC4A1 functions as a chloride/bicarbonate exchange and mutations in SLC4A1 have been implicated in distal renal tubular acidosis42,43 and in hemolytic anemias.44 AQP6 encodes an intracellular transmembrane protein involved in anion transport, including nitrate, as well as urea and glycerol, and has proposed functions in glomerular filtration, tubular endocytosis, and acid-base regulation.45,46 Genes implicated in the innate immune system (RSAD2), complement system activation (C3), and immune antigen processing (UBE2O) were also upregulated in the kidneys of Hb AS and Hb SS mice. These genes are also particularly interesting because progressive MPGN-like lesions, which can be associated with immune-complex or C3 deposition, were observed in the Hb AS and Hb SS mice by TEM and C3 deposition has been previously described in the GBM and mesangium of Hb SS patients in some studies.9,10,47–50 FASN is involved in fatty acid metabolism and is upregulated under high glycemic conditions or in chronic renal failure.51,52 Increased expression of FASN is associated with extracellular matrix deposition52 and we observed mesangial expansion in the Hb AS and Hb SS mice. In addition to these candidate genes, other genes that have been implicated in focal segmental glomerulosclerosis (PODXL)53 and diabetic nephropathy (ELMO1 and FRMD3)54,55 were differentially expressed in Hb AS and Hb SS mice.
APOL1 is only present in some primates and humans and evolved to confer immunity against Trypanosoma brucei rhodesiense infection.56 MYH9 co-segregates with APOL1 and risk variants in both MYH9 and APOL1 have been implicated in sickle cell nephropathy.57 MYH9 may play a role in maintaining capillary wall integrity against hydraulic pressure and contribute to podocyte foot process retraction under pathologic conditions and we observed increased MYH9 expression in the kidney cortex of Hb AS and Hb SS mice.29,58 APOA1 promotes oxidant scavenging and is a major structural protein of the APOL1-HDL trypanosome lytic factor.59 We observed lower APOA1 expression in the kidney cortex of Hb AS and Hb SS mice.
Hypoxia hallmark genes that were differentially expressed with the Hb S mutation included genes involved in glucose metabolism (GCK), maintenance of the actin cytoskeleton (SDC3), cell senescence and apoptosis (ETS1, BCL2), endothelial cell migration and angiogenesis (VEGF), and iron metabolism (CP). VEGF stimulates endothelial cell proliferation and increases vascular permeability.60 Increased VEGF expression has been observed in the kidneys of human and animal diabetic models and has been implicated in glomerular and tubular hypertrophy in response to nephron loss.61 Ceruloplasmin is a ferroxidase that facilitates the transport of iron across cell membranes and has both antioxidant and pro-oxidant properties.62 Increased urine concentrations of ceruloplasmin have been identified as biomarkers of early glomerular disease in diabetes63 and correlate with kidney disease stage in Hb SS adults.64 Future studies investigating expression patterns and polymorphisms in these candidate genes may improve our understanding behind the mechanisms and susceptibilities for CKD in sickle cell trait and sickle cell anemia.
We also conducted a gene set enrichment analysis from the genes that were differentially expressed in Hb AS and Hb SS mice and identified three major pathways: focal adhesion, extracellular matrix-receptor interaction and axon guidance. Focal adhesion of sickled red blood cells can lead to ischemia-reperfusion injury with the generation of reactive oxygen species.65 Consistent with increased oxidative stress occurring in Hb AS and Hb SS kidneys, significantly higher advanced oxidation protein products have been observed in the kidneys of Hb AS and Hb SS mice compared to Hb AA mice.66 Extracellular matrix-receptor interactions are involved in anchoring cells to the extracellular matrix and basement membrane. Interestingly, we observed glomerular basement membrane reduplication with mesangial interposition in Hb AS and Hb SS mice. Axon guidance plays an integral role in cell migration and differentiation and this pathway is disrupted after ischemia-reperfusion injury in the kidneys.67 These pathways all represent biologically feasible mechanisms for kidney damage in sickle cell trait and sickle cell anemia and may represent targets for therapies to prevent CKD and improve kidney function.
In conclusion, we provide evidence of progressive glomerular and tubular damage occurring in the kidneys of transgenic mice with Hb AS and Hb SS supporting recent epidemiological data that Hb AS is associated with kidney disease. We have also identified several candidate genes and pathways that may be implicated in sickle cell trait and sickle cell anemia-related CKD. Our model is limited by using transgenic mice, although we observed similar pathologic lesions to what has been demonstrated in patients. Future studies confirming the histopathologic and ultrastructural lesions in people with sickle cell trait and evaluating the role of these candidate genes and pathways in sickle cell trait and sickle cell anemia-related nephropathy will provide valuable pathophysiological information and guide the development of future therapies.
Background
Sickle cell anemia (HbSS) and sickle cell trait (HbAS) are both associated with an increased risk for kidney disease, although the mechanisms are not well understood.
Translational Significance
We observed progressive glomerular and tubular damage, determined by increasing urine protein concentrations, histopathologic changes, and ultrastructural abnormalities, in transgenic HbAS and HbSS mice. Gene expression studies identified the differential expression of several candidate genes with inheritance of the HbS mutation and pathway analysis highlighted increased gene enrichment of several pathways for future investigation in sickle cell trait and sickle cell anemia-related kidney disease.
Acknowledgments
The authors are grateful to Figen Seiler and Zarema Arbieva for their assistance in processing samples at the University of Illinois at Chicago, Research Resources Center in the Electron Microscopy Services and Core Genomics Facilities, respectively.
The project described was supported by the National Institutes of Health through grants K23HL125984 (S.L.S), R01HL111656 and R01HL127342 (R.F.M.).
Footnotes
All authors have read the journal’s policy on conflicts of interest and there are no conflicts of interest to disclose.
Authorship:
S.L.S. designed and performed research, analyzed the data, and wrote the paper. J.R.S., A.S., S.S., K.P.G., A.K.S. performed research, analyzed the data, and wrote the paper. X.Z. designed the genetic analyses, analyzed the data and wrote the paper. J.A.A., R.F.M., and V.G.R. designed the research, analyzed the data, and wrote the paper. All authors read and agree to the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med. 2013;3(10):a011783. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 4.Saraf SL, Zhang X, Kanias T, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol. 2014;164(5):729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 6.Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115(8):614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 7.Darbari DS, Wang Z, Kwak M, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8(11):e79923. doi: 10.1371/journal.pone.0079923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejani A, Phadke K, Adamson O, Nicastri A, Chen CK, Sen D. Renal lesions in sickle cell nephropathy in children. Nephron. 1985;39(4):352–355. doi: 10.1159/000183404. [DOI] [PubMed] [Google Scholar]
- 9.Bhathena DB, Sondheimer JH. The glomerulopathy of homozygous sickle hemoglobin (SS) disease: morphology and pathogenesis. J Am Soc Nephrol. 1991;1(11):1241–1252. doi: 10.1681/ASN.V1111241. [DOI] [PubMed] [Google Scholar]
- 10.Elfenbein IB, Patchefsky A, Schwartz W, Weinstein AG. Pathology of the glomerulus in sickle cell anemia with and without nephrotic syndrome. Am J Pathol. 1974;77(3):357–374. [PMC free article] [PubMed] [Google Scholar]
- 11.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326(14):910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- 12.Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010;89(1):18–27. doi: 10.1097/MD.0b013e3181ca59b6. [DOI] [PubMed] [Google Scholar]
- 13.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11(3):161–171. doi: 10.1038/nrneph.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naik RP, Haywood C., Jr Sickle cell trait diagnosis: clinical and social implications. Hematology Am Soc Hematol Educ Program. 2015;2015:160–167. doi: 10.1182/asheducation-2015.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312(20):2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik RP, Irvin MR, Judd S, et al. Sickle Cell Trait and the Risk of ESRD in Blacks. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer HJ, Stilp AM, Laurie CC, et al. African Ancestry-Specific Alleles and Kidney Disease Risk in Hispanics/Latinos. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukendi K, Lepira FB, Makulo JR, Sumaili KE, Kayembe PK, Nseka MN. Sickle cell trait is not associated with chronic kidney disease in adult Congolese patients: a clinic-based, cross-sectional study. Cardiovasc J Afr. 2015;26(3):125–129. doi: 10.5830/CVJA-2014-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Paepe ME, Trudel M. The transgenic SAD mouse: a model of human sickle cell glomerulopathy. Kidney Int. 1994;46(5):1337–1345. doi: 10.1038/ki.1994.403. [DOI] [PubMed] [Google Scholar]
- 20.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107(4):1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278(5339):873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 22.Bank N, Aynedjian HS, Qiu JH, et al. Renal nitric oxide synthases in transgenic sickle cell mice. Kidney Int. 1996;50(1):184–189. doi: 10.1038/ki.1996.301. [DOI] [PubMed] [Google Scholar]
- 23.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi CT, Gladwin M, Diwan B, et al. Pathophysiology of a sickle cell trait mouse model: human alpha(beta)(S) transgenes with one mouse beta-globin allele. Blood Cells Mol Dis. 2001;27(6):971–977. doi: 10.1006/bcmd.2001.0469. [DOI] [PubMed] [Google Scholar]
- 25.Diwan BA, Gladwin MT, Noguchi CT, Ward JM, Fitzhugh AL, Buzard GS. Renal pathology in hemizygous sickle cell mice. Toxicol Pathol. 2002;30(2):254–262. doi: 10.1080/019262302753559597. [DOI] [PubMed] [Google Scholar]
- 26.Rhoda MD, Domenget C, Vidaud M, et al. Mouse alpha chains inhibit polymerization of hemoglobin induced by human beta S or beta S Antilles chains. Biochim Biophys Acta. 1988;952(2):208–212. doi: 10.1016/0167-4838(88)90117-3. [DOI] [PubMed] [Google Scholar]
- 27.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 28.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Colares VS, Titan SM, da Pereira AC, et al. MYH9 and APOL1 gene polymorphisms and the risk of CKD in patients with lupus nephritis from an admixture population. PLoS One. 2014;9(3):e87716. doi: 10.1371/journal.pone.0087716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 31.Derebail VK, Nachman PH, Key NS, Ansede H, Falk RJ, Kshirsagar AV. High prevalence of sickle cell trait in African Americans with ESRD. J Am Soc Nephrol. 2010;21(3):413–417. doi: 10.1681/ASN.2009070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks PJ, Langefeld CD, Lu L, et al. Sickle cell trait is not independently associated with susceptibility to end-stage renal disease in African Americans. Kidney Int. 2011;80(12):1339–1343. doi: 10.1038/ki.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Femi-Pearse D, Odunjo EO. Renal cortical infarcts in sickle-cell trait. Br Med J. 1968;3(5609):34. doi: 10.1136/bmj.3.5609.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jong PE, de Jong-Van Den Berg TW, Sewrajsingh GS, Schouten H, Donker AJ, Statius van Eps LW. The influence of indomethacin on renal haemodynamics in sickle cell anaemia. Clin Sci (Lond) 1980;59(4):245–250. doi: 10.1042/cs0590245. [DOI] [PubMed] [Google Scholar]
- 35.Aloni MN, Ngiyulu RM, Nsibu CN, et al. Congolese children with sickle cell trait may exhibit glomerular hyperfiltration: A case control study. J Clin Lab Anal. 2017 doi: 10.1002/jcla.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tain YL, Huang LT, Chan JY, Lee CT. Transcriptome analysis in rat kidneys: importance of genes involved in programmed hypertension. Int J Mol Sci. 2015;16(3):4744–4758. doi: 10.3390/ijms16034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraf SL, Zhang X, Shah B, et al. Genetic Variants and Cell-Free Hemoglobin Processing in Sickle Cell Nephropathy. Haematologica. 2015 doi: 10.3324/haematol.2015.124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geard A, Pule GD, Chetcha Chemegni B, et al. Clinical and genetic predictors of renal dysfunctions in sickle cell anaemia in Cameroon. Br J Haematol. 2017 doi: 10.1111/bjh.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Aguilar M, Baines CP. Physiological and pathological roles of mitochondrial SLC25 carriers. Biochem J. 2013;454(3):371–386. doi: 10.1042/BJ20121753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18(2):414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 41.Straub AC, Lohman AW, Billaud M, et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Liu KX, He JW, et al. Identification of two novel mutations in the SLC4A1 gene in two unrelated Chinese families with distal renal tubular acidosis. Arch Med Res. 2012;43(4):298–304. doi: 10.1016/j.arcmed.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Shao L, Xu Y, Dong Q, Lang Y, Yue S, Miao Z. A novel SLC4A1 variant in an autosomal dominant distal renal tubular acidosis family with a severe phenotype. Endocrine. 2010;37(3):473–478. doi: 10.1007/s12020-010-9340-6. [DOI] [PubMed] [Google Scholar]
- 44.Fawaz NA, Beshlawi IO, Al Zadjali S, et al. dRTA and hemolytic anemia: first detailed description of SLC4A1 A858D mutation in homozygous state. Eur J Haematol. 2012;88(4):350–355. doi: 10.1111/j.1600-0609.2011.01739.x. [DOI] [PubMed] [Google Scholar]
- 45.Michalek K. Aquaglyceroporins in the kidney: present state of knowledge and prospects. J Physiol Pharmacol. 2016;67(2):185–193. [PubMed] [Google Scholar]
- 46.Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci U S A. 1999;96(10):5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardo V, Strauss J, Kramer H, Ozawa T, McIntosh RM. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. II. Clinicopathologic study of seven patients. Am J Med. 1975;59(5):650–659. doi: 10.1016/0002-9343(75)90226-0. [DOI] [PubMed] [Google Scholar]
- 48.Bakir AA, Hathiwala SC, Ainis H, et al. Prognosis of the nephrotic syndrome in sickle glomerulopathy. A retrospective study. Am J Nephrol. 1987;7(2):110–115. doi: 10.1159/000167444. [DOI] [PubMed] [Google Scholar]
- 49.Iskandar SS, Morgann RG, Browning MC, Lorentz WB. Membranoproliferative glomerulonephritis associated with sickle cell disease in two siblings. Clin Nephrol. 1991;35(2):47–51. [PubMed] [Google Scholar]
- 50.Vogler C, Wood E, Lane P, Ellis E, Cole B, Thorpe C. Microangiopathic glomerulopathy in children with sickle cell anemia. Pediatr Pathol Lab Med. 1996;16(2):275–284. [PubMed] [Google Scholar]
- 51.Szolkiewicz M, Nieweglowski T, Korczynska J, et al. Upregulation of fatty acid synthase gene expression in experimental chronic renal failure. Metabolism. 2002;51(12):1605–1610. doi: 10.1053/meta.2002.36302. [DOI] [PubMed] [Google Scholar]
- 52.Hao J, Liu SX, Zhao S, Liu QJ, Liu W, Duan HJ. High-fat diet causes increased serum insulin and glucose which synergistically lead to renal tubular lipid deposition and extracellular matrix accumulation. Br J Nutr. 2012;107(1):74–85. doi: 10.1017/S0007114511002613. [DOI] [PubMed] [Google Scholar]
- 53.Barua M, Shieh E, Schlondorff J, Genovese G, Kaplan BS, Pollak MR. Exome sequencing and in vitro studies identified podocalyxin as a candidate gene for focal and segmental glomerulosclerosis. Kidney Int. 2014;85(1):124–133. doi: 10.1038/ki.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leak TS, Perlegas PS, Smith SG, et al. Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann Hum Genet. 2009;73(2):152–159. doi: 10.1111/j.1469-1809.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freedman BI, Langefeld CD, Lu L, et al. Differential effects of MYH9 and APOL1 risk variants on FRMD3 Association with Diabetic ESRD in African Americans. PLoS Genet. 2011;7(6):e1002150. doi: 10.1371/journal.pgen.1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashley-Koch AE, Okocha EC, Garrett ME, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol. 2011;155(3):386–394. doi: 10.1111/j.1365-2141.2011.08832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13(1):65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 59.Widener J, Nielsen MJ, Shiflett A, Moestrup SK, Hajduk S. Hemoglobin is a co-factor of human trypanosome lytic factor. PLoS Pathog. 2007;3(9):1250–1261. doi: 10.1371/journal.ppat.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106(4):148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 61.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 62.Giurgea N, Constantinescu MI, Stanciu R, Suciu S, Muresan A. Ceruloplasmin - acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease. Med Sci Monit. 2005;11(2):RA48–51. [PubMed] [Google Scholar]
- 63.Yamazaki M, Ito S, Usami A, et al. Urinary excretion rate of ceruloplasmin in non-insulin-dependent diabetic patients with different stages of nephropathy. Eur J Endocrinol. 1995;132(6):681–687. doi: 10.1530/eje.0.1320681. [DOI] [PubMed] [Google Scholar]
- 64.Jerebtsova M, Saraf SL, Lin X, et al. Identification of ceruloplasmin as a biomarker of chronic kidney disease in urine of sickle cell disease patients by proteomic analysis. Am J Hematol. 2018;93(2):E45–E47. doi: 10.1002/ajh.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ, Group CS. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am J Hematol. 2011;86(6):484–489. doi: 10.1002/ajh.22012. [DOI] [PubMed] [Google Scholar]
- 66.Charrin E, Ofori-Acquah SF, Nader E, et al. Inflammatory and oxidative stress phenotypes in transgenic sickle cell mice. Blood Cells Mol Dis. 2016;62:13–21. doi: 10.1016/j.bcmd.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294(4):F739–747. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]