Abstract
The number of persons with dementia (PWD) in the United States is expected to reach 16 million by 2050. Due to the behavioral and psychological symptoms of dementia, caregivers face challenging in-home care situations that lead to a range of negative health outcomes such as anxiety and depression for the caregivers and nursing home placement for PWD. Supporting Family Caregivers with Technology for Dementia Home Care (FamTechCare) is a multisite randomized controlled trial evaluating the effects of a telehealth intervention on caregiver well-being and PWD behavioral symptoms. The FamTechCare intervention provides individualized dementia-care strategies to in-home caregivers based on video recordings that the caregiver creates of challenging care situations. A team of dementia care experts review videos submitted by caregivers and provide interventions to improve care weekly for the experimental group. Caregivers in the control group receive feedback for improving care based on a weekly phone call with the interventionist and receive feedback on their videos at the end of the 3-month study. Using linear mixed modeling, we will compare experimental and control group outcomes (PWD behavioral symptoms and caregiver burden) after 1 and 3 months. An exploratory descriptive design will identify a typology of interventions for telehealth support for in-home dementia caregivers. Finally, the cost for FamTechCare will be determined and examined in relation to hypothesized effects on PWD behavioral symptoms, placement rates, and caregiver burden. This research will provide the foundation for future research for telehealth interventions with this population, especially for families in rural or remote locations.
Keywords: dementia, caregiver, telehealth intervention
The number of persons diagnosed with dementia is expected to triple by 2050, reaching 16 million Americans with projected annual costs of $1.1 billion for dementia care (Alzheimer’s Association, 2017). Ensuring that persons with dementia (PWD) are able to remain in the community as long as possible is essential to meet the healthcare needs of the expanding aging population. There are currently 15.9 million in-home dementia caregivers in the United States, saving the healthcare system an estimated $230 million annually by providing an estimated 18.2 billion hours of unpaid care (Alzheimer’s Association, 2017). Caregivers of a PWD face many challenges, causing caregiving to be considered a chronic stressor that may lead to depression, insomnia, psychotropic medication use, and increased morbidity and mortality (Monin & Schulz, 2009; Talley & Montgomery, 2013; Vitaliano, Zhang, & Scanlan, 2003).
Behavioral and psychological symptoms of dementia cause these challenging care situations for caregivers and are a leading cause of nursing home placement for PWD (Balestreri, Grossberg, & Grossberg, 2005; Kunik et al., 2010). To intervene in challenging care situations, healthcare providers are guided by the Need-driven Dementia-compromised Behavior Model to explain how fixed and modifiable factors contribute to behavioral symptoms such as physical aggression, wandering, withdrawal, and disruptive vocalizations (Algase et al., D. L. Algase et al., 1996, 2005). Recently this model has been expanded to include elements beyond PWD needs also considering other PWD factors, caregiver factors, and environmental factors (Kales, Gitlin, & Lyketsos, 2015). However, deciphering behavioral symptoms within this model requires dementia care expertise beyond the scope of lay caregivers who also report their own memory issues, sleep disruption, strain, and fatigue (Carlozzi et al., 2017). Traditionally, caregivers use retrospective recall to describe challenging care situations to healthcare providers, but caregivers may not have a clear recall or holistic perception of the situation, limiting the ability of the care provider to intervene and provide effective interventions. Without effective evidence-based interventions, a community-based PWD may receive higher rates of psychoactive medications and premature nursing home placement (Bradford et al., 2012; Fuchs-Lacelle, Hadjistavropoulos, & Lix, 2008).
Behavioral and psychological symptoms of dementia that occur in up to 90% of affected persons at some time require expertise to decipher both the behaviors and the precipitating and contextual factors (Kunik et al., 2010). Available in-home technology provides a tool to link caregivers to dementia experts without leaving home. Such technology supports video recording that provides the contextual information specific to care situations for observation by dementia care experts. Caregivers of older adults are motivated to use technology as a caregiving resource, particularly technologies that allow for personalized professional consultation and caregiving guidance (American Association of Retired Persons, 2016).
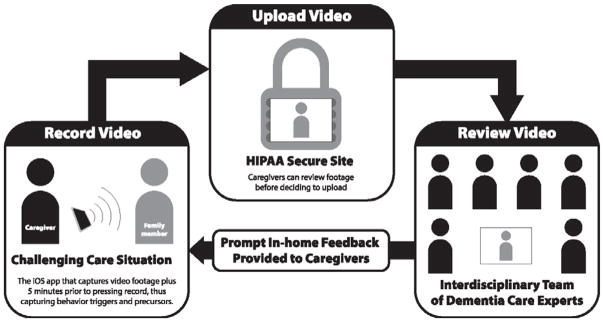
The Supporting Family Caregivers with Technology for Dementia Home Care (FamTechCare) clinical trial tests a telehealth intervention in which: (1) caregivers video record care situations they find challenging using an innovative video-recording application and upload the videos to a HIPAA-secure website; (2) an interdisciplinary team of dementia experts reviews the recordings; and (3) based on the recordings, the dementia experts provide individualized interventions and feedback to the caregiver at home (Figure 1). The overall hypothesis is that PWD behavioral and psychological symptoms and subsequent caregiver burden will be significantly reduced for those in the experimental group, who receive feedback based on videos submitted weekly over the 3-month trial, compared to the control group. The control caregivers receive feedback based on their verbal reports via telephone weekly over 3 months. At the end of their participation they receive feedback based on review of their submitted videos. The overall objective of this trial is to determine the effects of the FamTechCare intervention on PWD behavioral symptoms and caregiver related outcomes (e.g., burden, confidence, depression) over the 3-month study period. Additional goals are to identify precursors of behavioral symptoms, develop a typology of telehealth interventions for in-home caregivers, and evaluate ease of use, satisfaction, and cost effectiveness of the intervention. This paper reports the trial protocol for the multicomponent FamTechCare intervention.
Figure 1.
Supporting Family Caregivers with Technology for Dementia Homecare (FamTechCare) Study Diagram.
Graphic Design by Chris Lorenzen © 2016 for K.N. Williams.
Methods
Study Sites
The FamTechCare randomized clinical trial is funded by the National Institute of Nursing Research and is being implemented at the University of Kansas and the University of Iowa. Participants at both sites are primarily recruited from locations within a 2-hour driving distance. Each site has a goal of enrolling four PWD-caregiver dyads during each quarter of the study with a planned enrollment of 88 total dyads over the four-year study.
Recruitment
Participants at the Kansas site are primarily recruited through the University of Kansas Alzheimer Disease Center. The Kansas research team includes clinician investigators affiliated with the center and the local Alzheimer’s Association chapter. The Kansas site also uses a number of community outreach activities and a memory care clinic to recruit participants.
The University of Iowa recruitment primarily targets family caregivers in the surrounding communities through advertisements in local newspapers and local magazines that focus on older adults, through presentations to civic groups and organizations, and at regional conferences for caregivers. A mass email system at the University of Iowa has also been used for recruitment.
Eligibility
Eligibility is assessed by a research team member over the telephone, by email, or in person. To be eligible: (1) the caregiver must provide in-home care to a PWD at least weekly, (2) the PWD must be diagnosed with dementia (of any etiology, including unknown); and (3) both the caregiver and the PWD must provide consent. Exclusion criteria for a PWD include Huntington’s disease, alcohol-related dementia, schizophrenia, bipolar disorder, deafness, or developmental delay. As part of the study, caregivers agree to send videos to a HIPAA-secure website every week for review and feedback, commit to a weekly call from an interventionist over the 3-month trial period; and complete a series of survey measures at baseline, 1-month, and 3-month data collection points. Caregivers may have varied relationships to the PWD including spouse, child or spouse of child, friend or neighbor, or paid paraprofessional caregiver.
Human Subject Protections
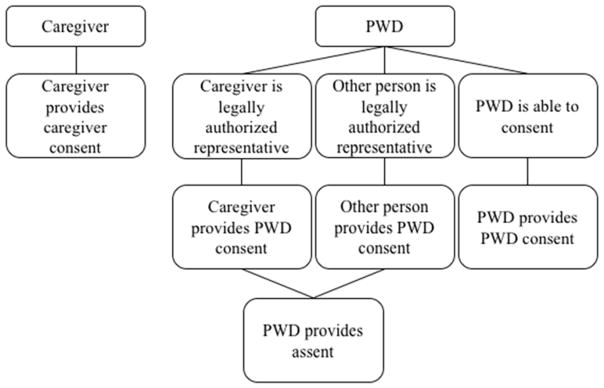
Consent is obtained from both the caregiver and the PWD. Persons with dementia identified as independent decision makers provided signed informed consent. The PWD who consent independently must demonstrate their competency to provide informed consent by correctly verbalizing facts about the study such as the study purpose and what they would do if they decided they no longer wanted to participate. For persons with dementia who are unable to independently consent due to cognitive impairment, consent is obtained from their legally authorized representative along with verbal or written assent from the PWD. Consent for use of study data for secondary analysis, for developing caregiver training materials, and for scholarly presentations is included. The consent process is illustrated in Figure 2.
Figure 2.
Consent Flowchart.
Human subject approval was obtained from each University Institutional Review Board. To protect the confidentiality of participants, participants can opt out of sharing videos beyond the immediate research team. Videos used for future training and presentation will have facial features blurred. A data safety committee, composed of experts external to the study, meets annually and as needed to review study conduct, progress, and unexpected events.
Randomization
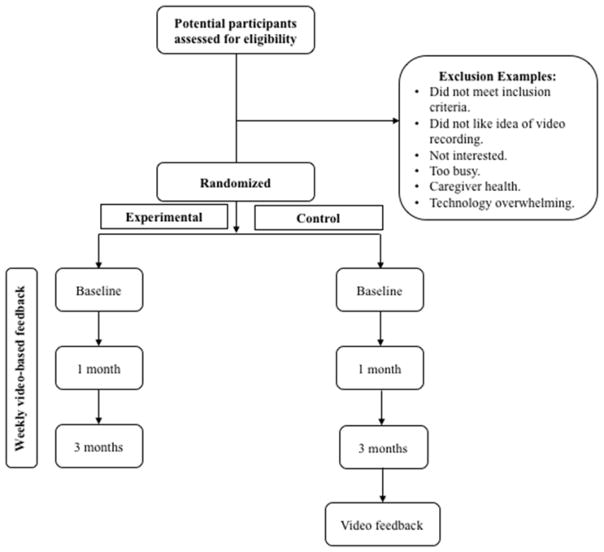
Following consent, each PWD-caregiver dyad is randomly assigned to the experimental (FamTechCare intervention) or control (weekly phone call) group (Figure 3). The randomization scheme uses a 1:1 allocation ratio developed by the study statistician. A blocking strategy was used to ensure equal numbers of experimental and control groups per each quarter. Following completion of the informed consent documents, the randomization list is referenced and both the research team and participants are made aware of their group assignment.
Figure 3.
Study Design.
Sample Size and Power Considerations
The proposed sample size of 88 caregiver-PWD dyads, with 44 dyads at each of the two study sites, will provide sufficient power to detect between-group differences in outcomes measures. Statistical power was estimated for between-group differences in caregiver-reported burden and other outcomes at 1 and 3 months based on published reports of large (average .81) effect sizes in studies testing psycho-educational interventions like FamTechCare (Gallagher-Thompson & Coon, 2007). Power is estimated at 92% for detecting differences in 70 dyads using an assigned Type I error of 0.05 and assuming average overall effect sizes of 0.81. The sample size was increased to 88 to allow for 20% attrition. To reach enrollment projections, the recruitment goal was four dyads each quarter (3-month period) per site. Enrollment was staggered to reduce equipment and personnel costs.
Feasibility Study
The protocol was developed based on experiences from a feasibility study (Williams, Arthur, Niedens, Moushey, & Hutfles, 2013). That study involved interviews with caregiver participants and focus groups with dementia care professionals that identified strategies to bolster study enrollment including limiting complexity of the technology, incorporating investigator recruitment time, using multiple sites, and emphasizing ease of use and caregiver control of recordings for privacy.
Procedures
Following enrollment, caregivers in both experimental and control groups are trained to record, review, and transmit recordings using the tablet-based app. The equipment includes an iPad Mini™, Bluetooth remote, and iPad stand. The iPad is connected to the participant’s password-protected home Internet. If the participant does not have Internet access or a secure network, a wireless hotspot is provided free of charge by the study team along with the standard study equipment. All materials are provided at no cost to the participants and are returned following completion of the study. The equipment is portable and can be transported easily.
Behavior Capture© is the application used to record and submit videos of the PWD. Behavior Capture© was developed by Behavior Imaging Solutions (https://behaviorimaging.com/) with National Institutes of Health support for the purpose of remote behavioral monitoring of children with autism. Behavior Capture© is innovative compared to other video recording tools because it has a buffering capacity that captures antecedents by saving both retrospective and prospective video of challenging care situations. When the Behavior Capture© application is open, it is always recording but not saving video data. Once “record” is triggered by the caregiver (either manually on the iPad or on the Bluetooth remote), the video will be saved. Because the buffer includes the time before and after the caregiver triggers the capture, it provides content for evaluating antecedent factors as well as consequences. As required by the Institutional Review Board, caregivers can review each video and decide whether to delete or upload the video to the HIPAA-secure Behavior Connect© website for review by the expert team.
Each caregiver is encouraged to submit videos specific to one of the priority problems they identified upon enrollment, but there is flexibility to change the focus if new priorities develop. It is important to note that caregivers submit videos of the care situation that they want feedback on each week, thus the intervention is caregiver-directed and caregivers maintain control over what content is reviewed. A research team member screens the submitted videos within 24 hours to identify and address any immediate safety concerns.
A team of dementia care experts meets weekly to review videos and identify specific interventions for each unique PWD-caregiver dyad. Each site has a separate video review team that integrates health care professionals with multiple years of experience caring for persons with dementia in fields including nursing, geriatric psychiatry, social work, psychology, or as a family caregiver. Other specialties such as speech pathology, dentistry, or occupational therapy are consulted as needed. Team members attend the video review meeting in person or remotely using Zoom© web conferencing. Zoom© provides a share-screen feature, so all group members can watch the videos simultaneously and discuss and develop dyad-specific interventions. Members of the expert team provide feedback and interventions based on their clinical expertise and evidence-based dementia care protocols. To assure consistency of approach, both sites evaluate videos within the framework of the Need-Based Dementia Compromised Behavior Model and use a protocol manual developed by a member of the Kansas research team (Algase et al., 1996; Niedens, 2010).
Caregivers in both the experimental and control groups receive a weekly call from the interventionist, who is a healthcare professional with dementia care experience. During this call, consent is reaffirmed and non-routine events (healthcare service utilization, prescribed medication changes, as-needed medication usage, and current problems) are assessed. All participants receive interventions. Experimental group caregivers receive weekly feedback from the expert team based on the recorded videos. Control group caregivers receive feedback based on their verbal reports to the interventionist. All interventionists attend the video review meetings, but the control interventionist is excluded during the review of the control group videos so that interventions provided to control caregivers are based only on the phone conversation. This process simulates a usual care control condition so that we evaluate the effects of caregiver support based on the video data versus the traditional retrospective verbal-report. The control interventionist has access to the same evidence-based dementia resources and understands the quality and type of interventions presented to the experimental group because they are active participants in the experimental video review meeting. The control interventionist does not view control group videos until the end of the 3-month study period and at that time provides control group caregivers with feedback from the experts based on the review of their submitted videos.
Outcome Measures
Caregiver and PWD outcomes include PWD behavioral symptoms, PWD and caregiver medication use, caregiver burden, competence, confidence, depression, sleep disturbances, desire to institutionalize the PWD, and satisfaction with home monitoring and use of the technology. Caregiver confidence is rated in relation to each challenging care situation identified by caregivers upon enrollment, providing an additional measure of the effectiveness of the interventions. Data is collected at three points throughout the study: baseline, 1 month, and 3 months.
A home visit is made to complete data collection surveys at the three time points. Alternatively, surveys are mailed to participants to complete with available assistance over the telephone. Measures take less than 1 hour total to complete and were selected to limit participant burden. Caregivers receive $75 at each data collection, totaling up to $225 for submitting videos and completing surveys as part of the study. During the baseline data collection, the Functional Assessment Staging of Dementia (FAST) is administered (Sclan & Reisberg, 1992) to establish the PWD’s stage of dementia, and the caregiver generates a list of three aspects of care on which they want to focus during the study. Lists of medications for the PWD and caregiver are obtained. Caregiver-reported outcome measures that are completed at baseline, 1 month, and 3 months are described in Table 1. At 3 months, the assessment of dementia stage is repeated (to assess for changes in behavioral symptoms that may occur due to disease progression), and a satisfaction with home monitoring survey is completed. Data from the surveys along with notes summarizing the expert team interventions and weekly caregiver contacts are stored in the Research Electronic Data Capture (REDCap).
Table 1.
Trial Measures for Caregiver-reported Outcomes
| Concept | Instrument | Description (Reliability based on previous trials) | Measure Focus | Testing Occasion |
|---|---|---|---|---|
| Demographics | Investigator-developed demographic form. | Sample characteristics. | Caregiver & PWD | T1 |
| Dementia severity | Functional Assessment Staging of Dementia (Sclan & Reisberg, 1992). | 16-item, dichotomous yes-no scale for seven major functional levels (normal adult to severe dementia). Intrarater reliability = .86; interrater reliability = .87. | PWD | T1, T3 |
| Caregiver burden*† | Zarit Burden Interview-Short (Bedard et al., 2001). | 12-item, 5-point Likert scale (never to nearly always) for two domains (personal strain and role strain). Alpha = .88. | Caregiver | T1, T2, T3 |
| Caregiver confidence* | Adapted Caregiver Target Problems Questionnaire (Teri, McCurry, Logsdon, & Gibbons, 2005). | Three caregiver-identified target problems, 5-point Likert scale for frequency of occurrence (none to daily or more often), severity of problem (trivial to severe), and level of confidence managing problem (unable to very confident). | Caregiver | T1, T2, T3 |
| Caregiver depression* | CES-D (Radloff, 1977). | 20-item, 4-point Likert scale (rarely or none of the time -less than 1 day in past week - most or all of the time - 5–7 days in past week) in 4 domains (depressed affect, positive affect, somatic activity, and interpersonal). Alpha = .85. | Caregiver | T1, T2, T3 |
| Caregiver sleep disturbance* | Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). | 19-item, 4-point Likert scale with question dependent anchor (not during the past month to 3 or more times a week; no problem at all to a very big problem; very good to very bad) for seven domains (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Alpha = .83. | Caregiver | T1, T2, T3 |
| Desire to Institutionalize*† | Desire to Institutionalize Scale Modified (McCaskill, Burgio, Decoster, & Roff, 2011). | 6-item, dichotomous yes-no scale for single domain (desire to institutionalize PWD). Alpha .69–.77. | Caregiver | T1, T2, T3 |
| Medication utilization*† | Investigator-developed medication tracking form. | Record of prescription and over-the-counter medication utilization. | Caregiver & PWD | T1, Weekly |
| Occurrence and bother of PWD behavioral symtpoms*† | Revised Memory and Problem Behavior Checklist (Roth et al., 2003). | 24-item, 5-point Likert scale (never to daily or more often) for behaviors that occurred at least weekly, plus how bothersome the behavior is to caregiver on 5-point Likert scale (not at all to extremely). Alpha (number of problems) = 0.78; alpha (reaction) = .87. | Caregiver | T1, T2, T3 |
| Perceived caregiving competence* | Short Sense of Competence Questionnaire (Vernooij-Dassen et al., 1999). | 7-item, 5-point Likert scale (strongly disagree to strongly agree) on three domains (satisfaction with PWD, satisfaction with caregiving performance, and consequences of caregiving). Alpha = .76. | Caregiver | T1, T2, T3 |
| Satisfaction with home monitoring† | Investigator developed satisfaction form. | 12-item, 5-point Likert scale (strongly disagree to strongly agree) assessing ease of use and perceived satisfaction with the home-monitoring unit and telehealth feedback. | Caregiver | T3 |
| FamTechCare Dissemination† | The Duke Diffusion of Innovation in Long-Term Care (McConnell et al., 2012). | 50-item, 6-point Likert scale (strongly disagree to strongly agree) on 11 domains (relative advantage, compatibility, complexity, observability, image, trialability, voluntariness, leadership, communication openness, accuracy, and timeliness). Alpha .5–.95. | Expert Team | Trial End |
Objective 1 = Determine effects of FamTechCare on outcome measures.
Objective 2 = Identify precursors to disruptive behaviors and develop a typology of interventions for caregiver in-home use.
Objective 3 = Evaluate ease of use, satisfaction, and cost effectiveness using traditional cost methods and Data Envelopment Cost-efficiency Analysis.
Note: T1 = Baseline, T2 = 1 month, T3 = 3 months.
Analyses of Caregiver-reported Outcomes
Experimental and control groups will be compared at baseline, using appropriate tests (i.e., Mann–Whitney U, t-tests, or chi-square) to confirm baseline group equivalency. Potential covariates, such as dyad relationship type (spouse, child), gender combination (male-male etc.), dementia type (Alzheimer’s disease versus vascular dementia), and caregiver age will be examined (Cohen-Mansfield, 2005; Forbes-Thompson, Gajewski, Scott-Cawiezell, & Dunton, 2006; Kovach et al., 2005; Scott, Vojir, Jones, & Moore, 2005). The outcome variables for the first objective are (1) PWD behavioral symptoms and psychoactive medication use and (2) caregiver burden, depression, sleep disturbance, confidence for managing identified problems, competence, and desire to institutionalize the PWD.
Linear mixed modeling will be used to test between group differences in the outcome variables after 1 and 3 months. Mixed modeling accounts for the correlation of outcome measures for the same subject across time points and missing data (Brown & Prescott, 2006). The fixed effects in the model will include group (experimental or control), site, data collection time, and all two-factor and three-factor interactions. A significant treatment by time interaction effect will indicate differences between the two groups. The analysis will be adjusted for important covariates such as age, race, relationship, and stage of dementia, identified with baseline comparisons. Anticipating at least 70 subjects, seven covariate factors can be included and will meet statistical requirements for 5 to 10 subjects per covariate factor (Tabachnick & Fidell, 2000).
Model fit will be evaluated with Akaike information criterion (AIC) and likelihood ratio tests. Parameter estimates from the fitted mixed models will be used to test the specific comparisons of interest (i.e., comparing experimental and control groups in terms of mean change in PWD behavioral symptoms and psychoactive medication use, caregiver burden, and other outcomes from baseline to 1 month and from baseline to month 3). Significant differences between the two groups, if they are in the expected direction, will support the hypothesis.
Typology of Caregiver Needs and Provider Interventions
Qualitative descriptive analysis will be used to explore the needs expressed by caregivers, reflected in submitted videos, and the interventions provided to caregivers based on video review. A typology of needs and interventions for video-based dementia telehealth interventions will be developed with a goal of informing future research and practice using telehealth technology (Elo & Kyngas, 2008). Qualitative descriptive analysis is ideal for categorizing this information without extensive interpretation (Sandelowski, 2010). The typology will organize behaviors, care needs, and interventions into categories that allow for comparison between experimental and control groups and for interventions provided based on video and phone call verbal reports. Comparison of caregiver reported needs and actual interventions provided will also provide information about potential benefits of dementia telehealth support and can inform health care practices of providers who support families living with dementia at home.
Ease of Use, Satisfaction, and Cost-effectiveness Analyses
Process-based costing will be used to determine costs of the FamTechCare intervention, including those costs for resources used by caregivers, the interventionist, and the expert team. Primary resource costs will include video-recording equipment and training, review of video data and care-planning, and providing specific feedback and caregiver instruction for managing behaviors. Mileage, materials, other study equipment, and video conferencing will also be included. We will tabulate all resources used and calculate the cost of the FamTechCare and control interventions.
Data envelopment analysis (DEA) will be used to analyze the net effects of the intervention on costs (Hollingsworth, 2008). This approach tests the hypothesis that the cost of FamTechCare will be offset by reductions in PWD behavioral symptoms and caregiver burden. We will calculate a simple cost-effectiveness ratio: the incremental cost of FamTechCare in relation to any reductions in PWD behavioral symptoms, caregiver burden, and caregiver desire for NH placement. We will examine whether added costs for the video versus phone only intervention, are associated with greater reductions in each outcome, and the cost for any increased effects.
Discussion
Recruitment and Enrollment
The FamTechCare trial is currently in year 3 of the 4-year study. The greatest challenge has been enrollment. The challenges of recruiting people to participate in clinical trials for Alzheimer’s disease and related dementias has been characterized as a “crisis” and second only to a lack of adequate funding as a major barrier to progress for improving dementia care (Fargo, Carrillo, Weiner, Potter, & Khachaturian, 2016). To facilitate recruitment, the study is registered in both ClinicalTrials.gov and the Alzheimer’s Association’s TrialMatch@alz.org. Biweekly web conferences with research teams at both sites have been instrumental in discussing challenges and sharing ideas for resolving issues.
To meet enrollment projections, both teams have enrolled more than the originally planned four caregiver-PWD dyads during each quarter to accelerate enrollment and have enrolled more than one caregiver per PWD if enrollment criteria are met. The Iowa site successfully advertised in local magazines, newspapers, and a hospital newsletter; met with healthcare providers and civic groups; sent mass email notifications to the University community; participated in an Alzheimer’s walk and lay caregiver conferences; and mailed letters to potential participants based on the hospital system electronic medical records system. We developed “meet the expert team” brochures that provide a picture and brief biography for each expert team member and have recently begun advertising on Facebook. Enrollment has expanded outside of our immediate area (using mailed equipment and phone contact). To date, dyads from six states have participated.
At the Iowa site, we estimate that about 40% of persons who contact the team about potential participation will enroll. During the first 3 years of the study, the Iowa team enrolled 33 of the 78 caregivers who contacted the team about the study (42%). Reasons that contacts failed to enroll included: lost to follow-up (n=14), objection to video recording (n=8), did not meet inclusion criteria (n=7), not interested (n=5), caregiver health issues (n=4), not the primary caregiver (n=3), lives too far away (n=2), refusal of care recipient (n=1), and anticipated NH placement (n=1).
The Kansas team, based at an NIA-designated Alzheimer’s Disease Center of Excellence, utilizes a different recruitment strategy. Enrollment at this site focuses primarily on recruitment from the affiliated Memory Care Clinic. The study team has secured a partial HIPAA waiver to perform limited pre-screening of individual medical records for those with upcoming appointments at the University of Kansas Health System Memory Care Clinic. Study staff identified potentially qualified individuals and alerted the clinicians with an easily identifiable referral prompt in the chart. Clinicians refer participants based on their assessment of caregiver need and appropriateness for the study. The team does not record the number of individuals approached by clinicians or specific clinical reasoning if individuals were not approached. Clinician judgement is always deferred to regarding referral, and no information from the medical record is kept unless written, informed consent of medical records release is obtained. The Kansas team also encouraged participation through community talks and print advertisement.
During the first 3 years of the study, the Kansas team assessed 135 caregiver contacts for eligibility and enrolled 26 caregiver-PWD dyads. Reasons for exclusion for 109 contacts included: not interested (n=44), lost to follow-up (n=17), too overwhelmed (n=7), or concerned about the technology (n=2). Factors for exclusion related to care recipients included living alone (n=16), too ill or facing imminent nursing home placement (n=11), or privacy concerns (n=8).
Privacy and Security of Video Data
Privacy and related security concerns are a key issue for potential participants, including the many older adult spousal caregivers in the study. Although older adults express a desire to use technology, many also convey concerns about sharing videos and other information related to sensitive care activities and behaviors over the internet (Boise et al., 2013). To address this concern, participants are taught how to review each video they record and to delete those they decide not to submit for review. Toileting and bathing are common challenges in caring for a PWD that many caregivers do not feel comfortable recording. In response, the team has suggested using audio only recording for these situations by setting the camera to face the wall or by talking directly to the camera immediately following the challenging care tasks. Although this fails to capture visual information about the care situation and the antecedents, this approach has been used to provide interventions for ADL care.
Study participants in both groups are asked to submit at least one video each week pertaining to the challenging care situations they identified. The first five participants submitted a range of 0–5 videos weekly over the twelve week study, with an average of one video weekly. Control group caregivers may be less motivated to submit weekly videos because feedback based on video review is delayed for this group. Team members work to establish trust with the caregivers during recruitment and weekly calls. In addition to suggestions to improve care, reinforcement of strengths in care such as communication strategies and management of challenging care situations are emphasized.
Technology
Anticipating the challenge for some caregivers, especially spouses, in learning the technology for the study, we conducted a feasibility study at the start of the trial to determine the optimal set-up and user interface for in-home caregivers (Williams, Pennathur, Bossen, & Gloeckner, 2016). Participants were recruited from a local senior center and trained in using the iPad to record and upload videos. Participants were video recorded providing a return demonstration. Computer-assisted behavioral coding was then used to identify critical challenges. The analysis was shared with the Behavior Imaging company and resulted in some changes to the software. This pilot also established that an iPad Mini, rather than an iPod Touch (cell phone size) device, was preferred by older adult users. Technology support was used to preset the iPads by deleting programs other than the capture and upload applications to limit complexity. Training caregivers has been successful using both in-person and phone-supported sessions.
Additional efforts have focused on familiarizing caregivers with the iPad and the Behavior Connect© program. During the initial visit, a research team member shows the family how to turn the iPad on and off, how to plug it in and place it in the stand, and how to record and upload a video. Following the demonstration, the caregiver is asked to practice uploading a video while the research team member is there to provide assistance as needed and ensure all steps are followed to complete the process. Participants are provided with a manual, which is used during the demonstration, with screenshots of the step-by-step process from turning on the iPad though uploading and deleting the video.
The challenges with technology for the caregivers in our study are consistent with the findings about caregivers in general and their perceptions about the use of technology to assist with their caregiving responsibilities. For example, a nationally representative survey of caregivers found that 71% reported being interested in using technology to help them with their caregiving tasks; however, only 59% reported that they are likely to use technology that is currently available to them. Further, only 7% are currently using or have used technology in the past to assist with these tasks (American Association of Retired Persons, 2016).
Many families have experienced significant technology challenges, with issues ranging from confusion about how to turn on the iPad to how to use the camera and how to review and upload videos. One unanticipated issue was the numerous updates that Apple© issues for their iPads. Some of the updates required the caregivers to log back into the iPad or to create a new passcode and make changes to the study specific settings. Another unforeseen issue was having the Behavior Capture© software passwords expire during the study period, requiring assignment of a new password. Both of these instances prevented families from recording and uploading videos until the technological complications were corrected. As the study progressed and these ongoing issues due to updates became apparent to the study team, participants were told that technology issues might be experienced and that they could contact the team at any time for help in resolving them. Research team members also checked with caregivers during weekly calls to see if there were any problems. If so, and they had not been resolved, a member of the team would call the participant to set up a time to help with the problem and have the caregiver send a test video to be sure the system was working.
Limitations
The FamTechCare clinical trial has a number of limitations related to the design and conduct of the study. Data submitted and therefore the problems addressed are caregiver-driven. This provides a more pragmatic approach than traditional clinical trials, that makes it more acceptable to participants, but also creates threats to validity (Ford & Norrie, 2016; Kalkman et al., 2017). The data submitted by caregivers may not reflect all the challenges that they experience and because they can review videos and not submit them, analysis and the ability to provide interventions may be limited. Review of the videos prior to submission, was required as a human subject protection and may have been an important factor in a caregiver’s decision to participate. However, reviewing videos prior to submission may influence caregiver behaviors, discussion with the interventionist, and responses to the expert feedback. Caregivers can also direct the team to provide feedback on different problems than originally identified. This complicates evaluation of intervention effectiveness but meets study goals of providing a caregiver-directed intervention. The purpose of this trial is not to test the effects of specific interventions on PWD, rather to test wither video technology supports persons providing care for PWD.
The control group also receives feedback on ways to improve care. This differs from usual care (most caregivers do not receive a weekly contact) making it difficult to evaluate effectiveness. Different interventionists have worked with participants during the trial. Although each was provided with a standardized orientation to the role, differences in their intervention and documentation skills and possible bias due to awareness of group assignment are also potential limitations. Because caregivers evaluate the effectiveness of the interventions indirectly, and only at 1 and 3 months, information about the effectiveness of specific weekly interventions is limited. Despite the potential value of this information, it must be balanced with limiting participation burden for caregivers who are often overwhelmed with caregiving.
Conclusions
The results of this study will provide new knowledge about the use of available telehealth technologies using video recordings to link caregivers with dementia care experts to increase skills in managing PWD behavioral symptoms and challenging care situations and to reduce the stress and burden for caregivers. If effective, reductions in healthcare system usage and caregiver intentions for nursing home placement may also result. The study will also add knowledge about caregiver and provider identified needs for telehealth interventions for the growing population of caregivers of PWD living in the community. The goal is to utilize technology to support caregivers and thereby enable care recipients with dementia to remain living at home.
Acknowledgments
This research is supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR014737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Kansas Alzheimer’s Disease Center (P30AG035982) provided essential infrastructure and recruitment support
Footnotes
The authors declare no conflicts of interest.
ClinicalTrials.gov registration NCT02483520
References
- Algase DL, Beck C, Kolanowski A, Berrent S, Richards K, Beattie E. Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. American Journal of Alzheimer’s Disease and Other Dementias. 1996;11(6):10–19. [Google Scholar]
- Algase DL, Beck C, Kolanowski A, Whall A, Berent S, Richards K, Beattie E. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. American Journal of Alzheimer’s Disease. 1996;11(6):10–19. [Google Scholar]
- Alzheimer’s Association. 2017 Alzheimer’s Disease Facts and Figures. 2017 Retrieved from https://www.alz.org/documents_custom/2017-facts-and-figures.pdf.
- American Association of Retired Persons. Caregivers & Technology: What they want and need. 2016 Retrieved from http://www.aarp.org/content/dam/aarp/home-and-family/personal-technology/2016/04/Caregivers-and-Technology-AARP.pdf.
- Balestreri L, Grossberg A, Grossberg GT. Behavioral and Psychological Symptoms of Dementia as a Risk Factor for Nursing Home Placement. International Psychogeriatrics. 2005;12(S1):59–62. doi: 10.1017/S1041610200006773. [DOI] [Google Scholar]
- Boise L, Wild K, Mattek N, Ruhl M, Dodge HH, Kaye J. Willingness of older adults to share data and privacy concerns after exposure to unobtrusive in-home monitoring. Gerontechnology : international journal on the fundamental aspects of technology to serve the ageing society. 2013;11(3):428–435. doi: 10.4017/gt.2013.11.3.001.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A, Shrestha S, Snow AL, Stanley MA, Wilson N, Hersch G, Kunik ME. Managing pain to prevent aggression in people with dementia: a nonpharmacologic intervention. American Journal of Alzheimer’s Disease and Other Dementias. 2012;27(1):41–47. doi: 10.1177/1533317512439795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. 2. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Carlozzi NE, Sherman CW, Angers K, Belanger MP, Austin AM, Ryan KA. Caring for an individual with mild cognitive impairment: a qualitative perspective of health-related quality of life from caregivers. Aging & Mental Health. 2017:1–9. doi: 10.1080/13607863.2017.1341468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J. Nonpharmacological interventions for persons with dementia. Alzheimer’s Care Quarterly. 2005;6(2):129–145. [Google Scholar]
- Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimer’s & Dementia. 2016;12(11):1113–1115. doi: 10.1016/j.jalz.2016.10.001. doi: https://doi.org/10.1016/j.jalz.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Forbes-Thompson S, Gajewski B, Scott-Cawiezell J, Dunton N. An exploration of nursing home organizational processes. Western Journal of Nursing Research. 2006;28(8):935–954. doi: 10.1177/0193945906287053. [DOI] [PubMed] [Google Scholar]
- Ford I, Norrie J. Pragmatic Trials. New England Journal of Medicine. 2016;375(5):454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- Fuchs-Lacelle S, Hadjistavropoulos T, Lix L. Pain assessment as intervention: a study of older adults with severe dementia. Clinical Journal of Pain. 2008;24(8):697–707. doi: 10.1097/AJP.0b013e318172625a. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychology and Aging. 2007;22(1):37–51. doi: 10.1037/0882-7974.22.1.37. [DOI] [PubMed] [Google Scholar]
- Hollingsworth B. The measurement of efficiency and productivity of health care delivery. Health Economics. 2008;17(10):1107–1128. doi: 10.1002/hec.1391. [DOI] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ : British Medical Journal. 2015:350. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman S, van Thiel G, van der Graaf R, Zuidgeest M, Goetz I, Grobbee D, van Delden J. The Social Value of Pragmatic Trials. Bioethics. 2017;31(2):136–143. doi: 10.1111/bioe.12315. [DOI] [PubMed] [Google Scholar]
- Kovach CR, Noonan PE, Schlidt AM, Wells T. A model of consequences of need-driven, dementia-compromised behavior. Journal of Nursing Scholarship. 2005;37(2):134–140. doi: 10.1111/j.1547-5069.2005.00025_1.x. discussion 140. [DOI] [PubMed] [Google Scholar]
- Kunik ME, Snow AL, Davila JA, McNeese T, Steele AB, Balasubramanyam V, … Morgan RO. Consequences of aggressive behavior in patients with dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(1):40–47. doi: 10.1176/appi.neuropsych.22.1.40. [DOI] [PubMed] [Google Scholar]
- Monin JK, Schulz R. Interpersonal effects of suffering in older adult caregiving relationships. Psychology and Aging. 2009;24(3):681–695. doi: 10.1037/a0016355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedens M. The Neuropsychiatric symptoms of dementia: A visual guide to response considerations. Leawood, Kansas: Alzheimer’s Association Heart of America Chapter; 2010. [Google Scholar]
- Sandelowski M. What’s in a name? Qualitative description revisited. Res Nurs Health. 2010;33(1):77–84. doi: 10.1002/nur.20362. [DOI] [PubMed] [Google Scholar]
- Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. International Psychogeriatrics. 1992;4(Suppl 1):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- Scott J, Vojir C, Jones K, Moore L. Assessing nursing homes’ capacity to create and sustain improvement. Journal of Nursing Care Quality. 2005;20(1):36–42. doi: 10.1097/00001786-200501000-00007. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4. Needham Heights, MA: Allyn & Bacon; 2000. [Google Scholar]
- Talley RC, Montgomery R. Caregiving across the lifespan. New York NY: Springer; 2013. [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin. 2003;129(6):946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Williams K, Pennathur P, Bossen A, Gloeckner A. Adapting Telemonitoring Technology Use for Older Adults. Research in Gerontological Nursing. 2016;9(1):17–23. doi: 10.3928/19404921-20150522-01. [DOI] [PMC free article] [PubMed] [Google Scholar]