Abstract
Predicting biotic resistance to highly invasive strains of “killer algae” (Caulerpa spp.) requires understanding the diversity and feeding preferences of native consumers, including sea slugs in family Oxynoidae. Past studies reported low algal host specificity for Oxynoe (6 spp.) and Lobiger (4 spp.), but these taxonomically challenging slugs may represent species complexes of unrecognized specialists that prefer different Caulerpa spp. Here, we assess global diversity of these genera by integrating gene sequences with morphological data from microscopic teeth and internal shells, the only hard parts in these soft-bodied invertebrates. Four delimitation methods applied to datasets comprising mtDNA and/or nuclear alleles yielded up to 16 species hypotheses for samples comprising five nominal taxa, including five highly divergent species in Lobiger and five in Oxynoe. Depending on the analysis, a further four to six species were recovered in the O. antillarum-viridis complex, a clade in which mitochondrial divergence was low and nuclear alleles were shared among lineages. Bayesian species delimitation using only morphological data supported most candidate species, however, and integrative analyses combining morphological and genetic data fully supported all complex members. Collectively, our findings double the recognized biodiversity in Oxynoidae, and illustrate the value of including data from traits that mediate fast-evolving ecological interactions during species delimitation. Preference for Caulerpa spp. and radular tooth characteristics covaried among newly delimited species, highlighting an unappreciated degree of host specialization and coevolution in these taxa that may help predict their role in containing outbreaks of invasive algae.
Keywords: Caulerpa, Cryptic species, Heterobranch, Oxynoe, Sacoglossa, Species delimitation
1. Introduction
Biodiversity affects the aggregate properties of a community, including biotic resistance to invasion; identifying the species native to a region and defining their niche can thus be critical to predict vulnerability to non-indigenous species (Stachowicz et al., 2007; Molnar et al., 2008; Palumbi et al., 2009). An emerging global threat to coastal ecosystems is invasion by Caulerpa spp., dubbed “killer algae” for their rapid spread and dramatic effects on native communities (Meinesz, 1999; Williams and Smith, 2007). A strain of the Indo-Pacific C. taxifolia had devastating ecological and economic impacts in the Mediterranean (Meinesz et al., 2001); transiently appeared in Japan (Komatsu et al., 2003); and was eradicated by a costly campaign in California, U.S.A. (Anderson, 2005). Invasive strains of C. taxifolia also altered habitats and community structure in southeastern Australia (Schaffelke et al., 2002; York et al., 2006; Byers et al., 2010), and the Mediterranean has been invaded by an Australian strain of C. cylindracea (Klein and Verlaque, 2008; Piazzi and Balata, 2008; Bulleri et al., 2010; Altamirano et al., 2014).
Invasive Caulerpa spp. produce toxic secondary metabolites that inhibit competitors and repel generalist grazers (Boudouresque et al., 1996; Gollan and Wright, 2006; Paul et al., 2007). The only known Caulerpa specialists are sea slugs in clade Sacoglossa (Gastropoda: Heterobranchia), including all species of Oxynoe and Lobiger, and some Elysia spp. (Baumgartner et al., 2009; Burfeind et al., 2009; see Supplemental File 1 for taxonomic details). These taxa constitute the guild within native communities with the greatest potential to undergo niche expansion and consume introduced Caulerpa spp., or serve as biological control, but it is unclear whether sacoglossans specialize on particular Caulerpa spp. Slugs may rapidly evolve preference for invasive algae (Trowbridge, 2002, 2004); however the Mediterranean natives O. olivacea and L. serradifalci were ineffective at controlling C. taxifolia due to low population growth rates, and feeding behavior that spread algal fragments (Thibaut and Meinesz, 2000). Regional vulnerability to invasion may thus depend in part on the biology of native sacoglossans. Intentional introduction of sacoglossans with non-dispersive larvae has also been proposed as biocontrol for Caulerpa (Coquillard et al., 2000). However, the ecology, larval biology, and diversity of tropical sacoglossans remain poorly understood, making it difficult to propose taxa for use in biocontrol (Krug et al., 2015, Krug et al., 2016). Feeding preferences and field surveys suggested three Australian species (L. viridis, O. viridis, E. tomentosa) were generalists, consuming or associating with at least half of assayed Caulerpa spp. (Raven et al., 2001; Baumgartner et al., 2009). However, an alternative hypothesis is that these nominal taxa each represent a complex of cryptic species that may specialize on subsets of available Caulerpa spp. but that were not distinguished in prior studies (Krug et al., 2013). Delimiting species is thus a prerequisite for biocontrol applications, and to establish local guild properties that predict invasion resistance.
Current systematics generally recognizes six Oxynoe spp. and four Lobiger spp. (see Supplemental File 1), but external variation among nominal conspecifics suggests some taxa comprise species complexes. Taxonomic progress on soft-bodied invertebrates is impeded by their lack of discrete characters and a dwindling pool of expertise (Costello et al., 2010; Tittensor et al., 2010). Sea slugs have deformable bodies; the hard parts of oxynoideans are a thin internal shell and the radula, a chitinous ribbon of microscopic teeth used to puncture the algal cell wall during feeding. As radular traits coevolve with host algae, tooth characters may aid in delimiting species partitioned among different Caulerpa spp. However, morphotypes of Oxynoe and Lobiger have rarely been split based on tooth shape (Jensen, 1980).
Molecular species delimitation has accelerated the discovery of biodiversity, but marine taxa vary widely in divergence rates at the cytochrome c oxidase I (COI) gene, a problem for barcoding: distances between sister species may be negligible for cnidarians (Huang et al., 2008), ~2% for fish (Hebert et al., 2003, 2010), yet > 8% for sacoglossans (Krug et al., 2013). Methods such as iBPP (Solís-Lemus et al., 2014) integrate coalescent modeling of multi-locus divergence with the evolution of morphological traits, but were developed for vertebrates in which continuous characters abound in organized skeletal elements absent in most marine invertebrates. Although morphological traits are routinely used to evaluate species hypotheses generated from molecular data (e.g., Puillandre et al., 2009), no prior analysis has delimited molluscan species by integrating continuous trait and sequence data in a hypothesis-testing framework. (e.g., Yeates et al., 2011). Such approaches may be especially useful in resolving recent radiations in which ecologically important traits under selection may diverge faster than neutral molecular markers. Here, we assess diversity in Oxynoe and Lobiger by applying four methods of species delimitation to molecular datasets, generating a range of candidate species (CS, sensu Vieites et al., 2009) hypotheses. We then integrate DNA sequences with morphological data from tooth and shell traits in one Bayesian analysis to resolve incongruity among hypotheses. Our results show the utility of incorporating fast-evolving characters into species delimitation analyses, and the extent to which biodiversity has been underestimated among Caulerpa consumers.
2. Materials and methods
2.1. Sample collection and host use
Separate collections (~1 kg) of each Caulerpa sp. were made by SCUBA or snorkeling at field sites, and slugs removed in the laboratory (Tables 1, S1). Host alga was assessed for each collection of specimens from field associations and direct feeding observations. Both Oxynoe and Lobiger spp. have a clear ovoid shell partly covered by tissue flaps (parapodia); an elongated tail; and colored ocelli (ringed spots) or stripes visible on mantle tissue through the shell. Lobiger spp. have two paired parapodial extensions lined with bright colors that unfurl in a defensive reaction. Slugs were relaxed in MgCl2 isotonic with seawater and photographed. Tissue clips were preserved in 100% ethanol (EtOH); bodies were fixed in either 100% EtOH, or in 5% formalin and subsequently transferred to 70% EtOH. Some preserved specimens were obtained from colleagues with photographs of the live animal, or from museums (Table S1).
Table 1.
Sampling localities and site codes for specimens of Lobiger and Oxynoe.
| Region | Collecting Site (code) | Latitude, Longitude |
|---|---|---|
| Atlantic | ||
| Bahamas | ||
| Sweetings Cay (SWE) | 26°37′38″N, 77°50′00″W | |
| Compass Cay (COMP) | 24°16′29″N, 76°30′35″W | |
| Cat Island (CAT) | 24°18′19″N, 75°30′50″W | |
| Northern Exumas (NEX) | 23°41′30″N, 76°00′60″W | |
| Plana Cays (PLA) | 22°36′40″N, 73°33′50″W | |
| Florida and FL Keys, U.S. | ||
| Lake Surprise, Key Largo (LKS) | 25°10′51″N, 80°23′00″W | |
| Key Largo (KLA) | 25°05′13″N, 80°27′12″W | |
| Anne’s Beach (GEI) | 24°50′58″N, 80°44′30″W | |
| Geiger Beach, Key West (GEI) | 24°33′59″N, 81°40′15″W | |
| Tarpon Springs, Tampa (TAM) | 28°09′10″N, 82°46′15″W | |
| U.S. Virgin Islands, St. Thomas (USVI) | 18°20′00″N, 64°55′45″W | |
| Martinique, Sainte-Anne (MAR) | 14°26′34″N, 60°53′30″W | |
| Curaçao (CUR) | ||
| Piscadera Bay | 12°07′24″N, 68°58′12″W | |
| Playa Kanoa | 12°10′30″N, 68°51′52″W | |
| Panama, Bocas del Toro (PAN) | 09°20′10″N, 82°14′45″W | |
| Pacific | ||
| Mexico | ||
| Puerto Escondido (MEX) | 15°51′31″N, 97°03′45″W | |
| El Anclote, Nayarit (EAN) | 20°46′15″N, 105°31′00″W | |
| Isla San Jose, Baja (ISJ) | 24°56′08″N, 110°33′00″W | |
| Panama, Isla de Canal de Afuera, Veraguas (CDA) | 07°41′54″N, 81°37′15″W | |
| Japan | ||
| Chinen, Okinawa (JPN) | 26°10′24″N, 127°54′45″E | |
| Zanpa, Okinawa (JPN) | 26°26′07″N, 127°42′50″E | |
| Guam, Asan Bay (GUA) | 13°28′11″N, 144°42′10″E | |
| Mo’orea, Cook’s Bay (MOR) | 17°29′00″S, 149°49′15″W | |
| Australia | ||
| Hastings Point, New South Wales (AUS) | 28°21′30″S, 153°34′45″E | |
| Point Cartwright, Mooloolaba, Queensland (AUS) | 26°40′45″S, 153°07′59″E | |
| Lord Howe Island (HOW) | 31°31′00″S, 159°04′00″E | |
| South Africa, Shaka’s Rock | 29°31′15″S, 31°13′55″E | |
| Durban, KwaZulu, Natal (SAF) |
2.2. Molecular data acquisition: DNA amplification and sequencing, microsatellite genotyping
DNA was extracted using Qiagen DNeasy blood and tissue kits from ~1 mm3 of tail tissue from specimens of Oxynoe (N = 125) and Lobiger (N = 12). Polymerase chain reactions (PCR) amplified portions of three loci: COI (primers from Folmer et al., 1994, modified as LCO, CAACAAAYCATAARGATATTGG, and HCO, ACTTCWGGGTGHCCAAARAAYCA); the mitochondrial large ribosomal subunit rRNA (16S) gene (primers ARL and BRH from Palumbi, 1996); and the nuclear histone 3 (H3) gene (primers H3F and H3R from Colgan et al., 2000). M13 viral tails were added to improve amplification and sequencing quality (Regier and Shi, 2005). Reactions included Promega GoTaq Green master mix and BSA. Thermal cycler profiles used 5 min of denaturation at 95 °C, followed by 40 cycles of 94 °C for 30 s, 40–45 °C (COI), 52 °C (16S, H3) or 56 °C (28S) for 30 s, and 72 °C for 90 s, with a final extension at 72 °C for 10 min. Purified PCR products were cycle-sequenced (Retrogen, San Diego CA) using primers or M13 sequences. Forward and reverse sequencing chromatograms were edited in Geneious Pro 6.1.6 (Biomatters Inc., Auckland NZ), and aligned using ClustalX (Chenna et al., 2003) under default settings. For 16S, sequences were aligned using a model of secondary structure (Krug et al., 2015); ambiguous regions masked by the least stringent criteria in Gblocks v.0.91b (Castresana, 2000) were omitted from the final alignment. Sequences were obtained for 94% (COI), 98% (16S), and 99% (H3) of samples; two Oxynoe specimens yielded only H3 sequences, and two only microsatellite data. NCBI accession numbers are given in Supplemental Table S1.
Allelic phase of most H3 genotypes was resolved using PHASE 2.1.1 (Stephens and Donnelly, 2003) with default parameters, generating input files from alignments of unresolved genotypes in SEQPHASE (Flot, 2010). Phase of genotypes with < 90% probability (or with more than three heterozygous positions) was resolved using a TOPO-TA bacterial cloning kit (LifeTechnologies, Carlsbad CA). Unpurified H3 amplicons (~1 μL) were ligated into the PCR 4.0 plasmid following manufacturer’s recommended protocols, then used to transform TOP-10 chemically competent E. coli cells. Transformed cells were spread onto LB plates containing 50 μg/μL ampicillin and incubated at 37 °C for > 24 h. Individual colonies (n = 4–10 per sample) were used as template DNA in PCR reactions with two primer pairs, M13-21/M13-REV and T3/T7, to amplify the inserted H3 gene. Samples displaying one amplified product of ~500 bp were sequenced. Alleles resolved by cloning were used in five runs of PHASE 2.1.1 to predict all remaining genotypes with > 90% confidence.
Microsatellite loci were developed from genomic DNA of O. antillarum and scored for six species in the O. antillarum-viridis complex (see Section 3). Next-generation sequencing via Illumina MiSeq (Illumina Inc., San Diego CA) was performed using a genomic library (Fernandez-Silva et al., 2013). Tissues were treated with CTAB lysis buffer followed by DNA isolation with 24:1 chloroform:isoamyl alcohol. Microsatellite loci were identified and primers designed with MSATCOMMANDER v1.0.8 (Faircloth, 2008). Primer pairs were tested with a touchdown thermal cycler profile: denaturation at 95 °C for 15 min; 19 cycles of 94 °C for 30 s, 65–55 °C for 30 s using decrements of 0.5 °C, and 72 °C for 90 s; 19 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 90 s; and 72 °C for 10 min. Only three polymorphic trinucleotide loci amplified for > 95% of complex members (N = 93 specimens), but no locus preferentially amplified in any one CS (Table S2). These three loci were genotyped at the GenoSeq facility of UCLA (Los Angeles CA), and traces were visualized in Geneious 6.1.6. Peaks of height ≥200 relative fluorescent units reflecting a product size of 200–300 bp were assigned as alleles, using a bin width of 1–1.5 bp, retaining alleles outside of designated bins as imperfect repeats (Chambers and MacAvoy, 2000).
2.3. Phylogenetic and population genetic analyses
To resolve evolutionary relationships, phylogenies were constructed for (1) concatenated mitochondrial DNA (mtDNA) using COI + 16S haplotypes, and (2) H3 alleles, for all specimens. Maximum Likelihood (ML) analyses were run in RAxML v7.2.7 (Stamatakis, 2006) via the CIPRES web portal (Miller et al., 2010) with 103 bootstrap pseudoreplicates (determined during the run); jModelTest (Posada, 2008) and AIC scores were used to select the GTR + Γ model for mtDNA, and the Jukes-Cantor model for H3 data. For Bayesian Inference (BI) analyses, three independent runs were performed with a parallelized version of BayesPhylogenies 2.0.4 (Pagel and Meade, 2004) and four cores of a 2.93 GHz W3540 Xeon processor. For each run, two MCMC chains were sampled for 1.5 × 107 generations, with trees sampled every 103 generations and the first 50% of trees discarded as burnin. Two mixture models (GTR +Γ, with four rate multipliers) were used to account for heterogeneity in rates and base frequencies across sites. Inspection of harmonic mean ln(L) scores and model parameters in Tracer v1.4 (Rambaut and Drummond, 2007) confirmed that all chains reached stationarity. The posterior samples from all chains were combined and a 50% majority-rule consensus tree constructed in BayesTrees (http://www.evolution.rdg.ac.uk/BayesTrees.html). Bayesian posterior probabilities (PP) ≥0.9, or ML bootstrap support (BS) ≥70%, were taken as significant (Douady et al., 2003; Huelsenbeck and Rannala, 2004).
Delimitation using the multispecies coalescent model may recover subpopulations as species (Sukumaran and Knowles, 2017). To determine if O. antillarum and O. viridis were genetically subdivided, analysis of molecular variance (AMOVA) was run in Arlequin v.3.5.1.3 (Excoffier and Lischer, 2010). For O. viridis, COI data were edited to 647 bp due to a few short sequences. For O. antillarum, Florida sites (KLA, LKS, GEI, TAM) were undifferentiated and pooled for final analyses. Overall and pairwise FST values were calculated from haplotype frequencies; pairwise COI distances were also analyzed by ΦST to partition covariances within (ΦSC) and among (ΦST) populations after Tamura-Nei (TrN) correction. For both species, genetic diversity indices were also calculated using COI data for each sampling site, and Tajima’s D (Tajima, 1989) and Fu’s FS (Fu, 1997) were calculated to assess deviations from neutrality. For (a) O. antillarum, and (b) O. viridis + O. sp. 8, COI haplotype networks were also constructed using a 95% parsimony criterion in TCS v.1.2.1 (Clement et al., 2000).
2.4. Species delimitation I: ABGD analysis of pairwise COI distances
Pairwise TrN distances for aligned COI haplotypes were calculated in MEGA 6.06 (Tamura et al., 2013) and analyzed by Automated Barcode Gap Discovery (ABGD; Puillandre et al., 2012) for each genus separately, using a range of priors (pmin = 0.004; pmax = 0.08), with 10 recursive steps and 20 bins. The pmax value was based on prior studies in sacoglossans in which divergent mtDNA clades were supported as CS if the minimum TrN-corrected COI distance to the sister group was 6–8% (Krug et al., 2013, 2015, 2016). A value of X = 1.5 was used as the sensitivity for gap detection, with X the minimum relative gap width required to delimit a CS; groups of sequences that differed by > 1.5 times the maximum intraspecific divergence were separated into CS. Initial ABGD analyses recovered five morphologically distinct Lobiger CS and five divergent Oxynoe CS in the Indo-West Pacific (IWP); remaining samples belonged to the O. antillarum-viridis complex, including putative O. antillarum (n = 44), O. azuropunctata-like Caribbean specimens (n = 8), IWP O. viridis (n = 30), and eastern Pacific samples (n = 10) (see Section 3). Subsequent delimitation focused on generating alternative hypotheses for complex members, as ABGD grouped IWP “O. viridis” with eastern Pacific samples but split O. azuropunctata-like samples into three CS.
2.5. Species delimitation II: GMYC analysis of COI haplotypes
We next analyzed mtDNA using the tree-based General Mixed Yule-Coalescent model (GMYC; Pons et al., 2006, Fujisawa and Barraclough, 2013) implemented in the R package SPLITS (Ezard et al., 2013). GMYC employs a ML search to find the best-fitting model of speciation, delimiting provisional species using the Yule model (Nee et al., 1994) and neutral coalescent model dynamics. Aligned COI haplotypes for all O. antillarum-viridis complex members were analyzed in RAxML, and the best-scoring ML tree was ultrametricized via nonparametric rate smoothing, employing the chronos function in the “ape” package (v3.1-1; Paradis et al., 2004, Paradis, 2013) for R. The tree was evaluated using a single threshold model to estimate the transition from among-to within-species branching, with the number of candidate species estimated within a 95% confidence interval. The ML score for the regime of delimited species entities was compared to that of a null model (no distinct species) by likelihood ratio test.
2.6. Species delimitation III: Structure analysis of multilocus nuclear data
East Pacific samples were not delimited as a species by ABGD or GMYC, but comprised a geographically isolated mtDNA lineage, and were traditionally considered a distinct species (Supplemental File 1). A combined analysis of microsatellite and H3 allele frequencies was performed to test whether O. antillarum-viridis complex members could be differentiated by nuclear loci in STRUCTURE v2.3.4 (Pritchard et al., 2000), testing whether CS (based on prior delimitation or the “East Pacific species” hypothesis) were assigned to different gene pools. Specimens with data for at least three of four nuclear loci (N = 93) were analyzed using a presence-absence format under a null model of sequence evolution, assuming no directional bias towards increasing allele size over time. The default admixture model and the option to analyze allele frequencies of each individual independently from simulated populations were enabled. Three replicate runs were conducted for each value of K hypothetical populations from 2 to 10, for 5 × 105 generations, discarding the first 10% as burnin. The best-fit K value was determined using the web interface for STRUCTURE HARVESTER (Evanno et al., 2005; Earl and vonHoldt, 2012). STRUCTURE results from replicate runs were combined for each value of K using the clustering algorithm in CLUMPP (Jakobsson and Rosenberg, 2007).
2.7. Species delimitation IV: BPP analysis of mtDNA and nuclear H3 alleles
Using Bayesian Phylogenetics and Phylogeography v3.3 (BPP; Yang and Rannala, 2014), mtDNA and nuclear H3 sequence data were analyzed to assess support for each CS previously supported by ABGD, GMYC, or STRUCTURE; subpopulations of O. antillarum were not treated as CS (e.g., Carstens et al., 2013; Sukumaran and Knowles, 2017). Input data were concatenated COI + 16S haplotypes (1063 bp) plus phased H3 alleles for 116 specimens representing 11 CS of Oxynoe. ‘A11’ analyses (Yang, 2015) were implemented to delimit species and infer the species tree, given a large number of potential populations. Runs employed rjMCMC algorithm 1, with α = 2, m = 1, gamma-distributed priors on θ and τ of (2, 2 × 103), and a heredity multiplier estimated using a gamma prior (4, 4) given the smaller expected Ne of mtDNA. Changing β values for priors did not alter conclusions. Fine-tune parameters were optimized in pilot runs using the autotune option, to obtain acceptance proportions close to 30% (Yang, 2015); runs were then performed with the following values: GBtj, 102; GBspr, 10−4, update θs, 10−3, update τs, 5 × 10−4; mix, 0.07; and locus/heredity rate, 0.5. Two analyses were run for 2.5 × 106 generations after a 105 burnin, sampling every 250 generations to minimize autocorrelation. Support for species hypotheses, defined by a priori labeling of individuals in the imap file, was taken as PP ≥0.90.
2.8. Species delimitation V: Integrating morphological and molecular data with iBPP
Final support for all CS was assessed using Integrative Bayesian Phylogenetics and Phylogeography (iBPP; Solís-Lemus et al., 2014). A guide tree was generated for one exemplar per CS of Oxynoe (N = 11) plus two Lobiger spp. Sequence data for a fourth locus, the nuclear large rRNA (28S) gene, were added using described amplification procedures, and a published secondary structure model to guide alignments (Krug et al., 2015). The concatenated four-gene alignment (2850 bp) was analyzed using RAxML, with one GTR + Γ model of sequence evolution per gene region, and 103 bootstrap pseudoreplicates. After rooting, outgroups and O. sp. 9 (for which trait data were unavailable) were pruned from the guide tree.
Morphological data were obtained for 20 Oxynoe specimens (1–4 exemplars per CS) by dissecting out the shell and radula, a bent row of teeth with ascending and descending limbs (Jensen, 1980). The pharynx was dissected from exemplars, dissolved in 10% NaOH for > 72 hr, and the exposed radula rinsed and mounted. Prepared stubs were sputter-coated to a thickness of 200 Å with an Emitech K550x coater, and visualized with a Hitachi S-3000 N variable pressure scanning electron microscope (SEM) at an accelerating voltage of 10–15 kV. The shell was dissected from specimens and similarly imaged. Measurements were taken from SEM images using the FIJI interface for ImageJ (Schindelin et al., 2012); data for an O. azuropuncata specimen were added from Jensen (1980), yielding data for 21 total specimens (Table S3A). Seven traits were scored (where applicable, for the active tooth): (1) tip angle (Fig. S1A); (2) angle between the lateral ridge and the tooth base (Fig. S1B); (3) tooth length:depth-at-base ratio (Fig. S1C); (4) length of the 20th hair-like denticle (Fig. S1D); number of teeth in (5) ascending, and (6) descending, limbs (Fig. S1E); and (7) tooth depth 10 μm from the tip (Fig. S1F). Body length was measured from head to tip of tail, and shell length from anterior to posterior margin. Four traits (tip angle, ridge-base angle, length:depth ratio, denticle length) were uncorrelated with body size; all others were corrected for allometric growth effects (Lleonart et al., 2000) (Fig. S2, Table S3B). Final trait values were uncorrelated with each other after Bonferroni correction.
Initial iBPP inputs were (a) the guide tree with 10 terminals, (b) concatenated COI + 16S haplotypes (1063 bp) and phased H3 allele sequences for 114 specimens; and (c) morphological data for eight traits from 21 exemplars. Runs used the same parameters as BPP analyses, with fine-tuning parameters set to yield appropriate step lengths (GBtj, 0.5; GBspr, 10−6; update θs, 10−3; update τs, 4 × 10−4; mixing step, 0.09; change locus/heredity rates, 0.4). Four runs were performed for 2.5 × 106 generations each, with a 2 × 105 burnin sampling every 250 generations. To ensure results were not solely due to the guide tree and genetic data, three runs with different random seeds were performed using only morphology data, setting ‘useseqdata = 0’. Each run lasted 106 generations after a 1.25 × 105 burnin; fine-tuning parameters were GBtj, 1.0; GBspr, 4 × 10−5; update θs, 7 × 10−4; update τs, 10−4; mixing step, 0.04; and traitHsq, 0.56. Significant support for descendant species was taken as PP ≥0.90 for a node, pooled across runs. Gamma-distributed priors on θ were (2, 2 × 103) and on τ (2, 2 × 104); runs using smaller β values increased nodal support but also variance among runs, and did not alter conclusions. Morphology-only analyses were repeated after randomizing species labels for trait data in the O. antillarum-viridis complex, expected to reduce nodal support if trait data were informative; if the guide tree were driving delimitation results, we expected little drop in support values. Three independent runs were performed as described, and posterior probabilities pooled across runs. In addition to the continuous data included in iBPP analyses, we collected data on host use (multi-state trait), larval type (binary trait), and geographical range; all data were used to assess whether delimited taxa met the criteria for species under the general metapopulation lineage concept (de Queiroz, 2005). Input files for molecular phylogenetic and species delimitation analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ch6b3.
3. Results
3.1. Delimitation of divergent candidate species in Lobiger and Oxynoe
Phylogenetic analyses recovered three highly divergent mtDNA clades in Lobiger (one Caribbean, two IWP), each > 19% distant at COI (Fig. 1). Some Pacific specimens lacked mantle lines, and had inner parapodial surfaces rimmed with orange and dotted with black spots, loosely matching L. viridis. The divergent Lobiger sp. 1 had blue lines on the mantle, and parapodia rimmed with white and orange-red bands. The Caribbean L. souverbii complex was split into three CS by ABGD across all pmax values. All three CS were > 8% divergent, whereas intraspecific distances were < 1% for specimens of L. sp. 3. The largest specimen, with thin dark lines on the mantle, was tentatively identified as L. souverbii. The other CS had fewer, thicker blue lines. In Lobiger, only four positions were variable at the H3 locus. Two alleles were sampled in L. sp. 1, a third was fixed in specimens of L. sp. 3, and a fourth allele was fixed in all specimens of L. viridis, L. souverbii and L. sp. 4 (Fig. S3). Together, the ABGD results, distribution of H3 alleles and morphological features collectively distinguished five species in Lobiger from regions where only two species are generally recognized (Supplemental File 1). The remainder of this study focused on Oxynoe.
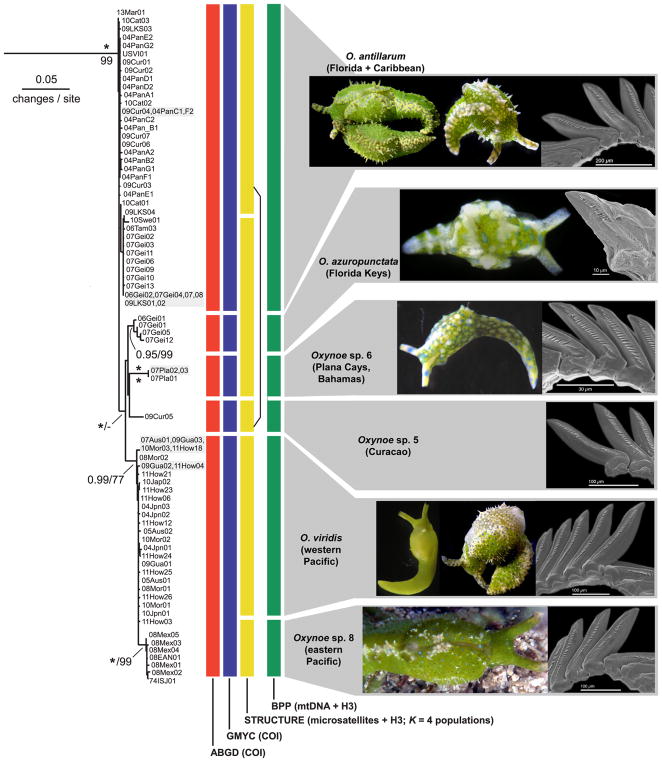
Fig. 1.
Phylogenetic relationships among mtDNA lineages in Oxynoe and Lobiger. Topology and branch lengths are from ML analysis, with significant support values given as bootstrap percentage (below branch) or posterior probability from BI analyses (above branch); asterisk = 100% or 1.0. Horizontal shaded bars indicate candidate species delimited by ABGD and ≥8% divergent at COI from their closest relative; external morphology of exemplars is shown, with SEM views of radular teeth for Oxynoe if available. Location codes given in Table 1, isolate codes in Table S1.
ABGD analysis recognized six Oxynoe CS down to low pmax values: (a) five deeply divergent Pacific lineages, and (b) the O. antillarum-viridis complex, comprising all Caribbean (N = 52), eastern Pacific (N = 10), and remaining western Pacific (N = 30) samples. Five Pacific CS were each > 16% divergent from their closest relative, and morphologically distinct. Sister to the rest of Oxynoe (but lacking support on the mtDNA tree), O. sp. 1 had a distinctive cream-colored body with black reticulations (Fig. 1). Three specimens were ≤0.3% divergent at COI, but their closest relative was > 22% divergent. On the H3 gene tree, O. sp. 1 was also sister to the rest of Oxynoe, and was ≥8% genetically divergent from all other species at the H3 locus (Fig. S3).
A second lineage (BS = 91, PP = 1.0) contained two fully supported subclades > 21% divergent at COI (Fig. 1). Within each CS, maximum COI divergence was < 0.7%. Comprising two specimens from Lord Howe Island, Australia, O. sp. 7 had a yellow body covered in blue ocelli darkening towards the center. The sister lineage (O. sp. 9) comprised two specimens from South Africa with green bodies, sparse blue spots and blue-tipped rhinophores. Position of the clade (O. sp. 7 + O. sp. 9) was congruent on the H3 gene tree; H3 divergence between these taxa was ≥2.1%, while distance to other species was ≥9% (Fig. S3).
Remaining mtDNA haplotypes formed a supported clade (BS = 93, PP = 1.0) in which three fully supported subclades formed a polytomy (Fig. 1). One subclade (O. sp. 2) comprised specimens with mint-green papillae, orange patches, and dark spots along the parapodia; COI distances within O. sp. 2 were ≤2.1%. A second subclade (O. sp. 3) comprised specimens < 1% divergent at COI, possessing blue ocelli with a central black spot and white parapodial margins peppered with black dots, characters absent in western Pacific ‘O. viridis’. Minimum COI distance between O. sp. 2 and O. sp. 3 was 19%. Although the topology of the H3 gene tree was largely unresolved, specimens of O. sp. 3 (N = 13) were fixed for one H3 allele that was ≥2.1% divergent from any other allele. Allelic diversity was high in O. sp. 2 (eight alleles sampled from 13 individuals), and alleles formed a divergent (> 1.8% distant) and supported clade (BS = 92).
3.2. mtDNA-based species delimitation and population structure in the O. antillarum-viridis complex
The remaining mtDNA clade (O. antillarum-viridis complex) was > 17% divergent from O. sp. 2 and O. sp. 3, but included lineages among which COI distance was ≤7.3% (Fig. 2). Nominal O. antillarum formed an early-branching grade paraphyletic with respect to the remaining complex members; COI haplotypes of O. antillarum were ≤1.1% divergent, but ≥3.0% divergent from their nearest relative. Nested within O. antillarum was a lineage supported in BI analysis comprising four subclades: three Caribbean lineages, and all remaining Pacific haplotypes. Specimens identified as O. azuropunctata formed a supported clade in which COI haplotypes were ≤1.3% divergent, but > 2.7% divergent from O. antillarum. One uniformly pale green specimen from Curaçao (O. sp. 5) was > 3.7% divergent from O. antillarum, while three specimens from the Bahamas (O. sp. 6) were externally similar to O. azuropunctata but > 4% divergent from their closest relative. In the 4th clade (BS = 77; PP = 0.99), O. viridis from the western and central Pacific was paraphyletic with respect to a subclade (BS = 99; PP = 1.0) comprising all East Pacific samples. COI haplotypes of O. viridis were maximally 1.2% divergent. The East Pacific lineage was termed O. sp. 8 given the a priori hypothesis of a distinct species in this region, although no valid name has been proposed for this taxon; within O. sp. 8, COI haplotypes were ≤0.8% divergent, while distance from O. viridis was 1.2–2.7%.
Fig. 2.
Phylogenetic relationships among mtDNA haplotypes in the O. antillarum-viridis complex, and hypotheses generated from four species delimitation methods. Topology, branch lengths and support values as given in Fig. 1 (BS below, PP above branch); asterisk = full support. Vertical bars indicate specimens grouped as species entities by ABGD (red) or GMYC (blue) analyses of COI data; STRUCTURE analysis of nuclear allele frequencies (yellow); or BPP analysis of mtDNA + H3 allele sequences (green). Segments joined by the black bar formed a single cluster at K = 4 gene pools in STRUCTURE. Grey horizontal bars denote candidate species supported by at least one delimitation method, showing external and radular morphology. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At low pmax values, ABGD split the complex into four species, lumping O. antillarum with O. azuropunctata, and O. viridis with O. sp. 8. Less restrictive ABGD analyses recovered 10 Oxynoe spp., separating O. antillarum and O. azuropunctata, but still grouping O. viridis and O. sp. 8 (Fig. 2, red bars). The GMYC model similarly separated four Caribbean lineages as CS, but lumped O. viridis with O. sp. 8 (Fig. 2, blue bars).
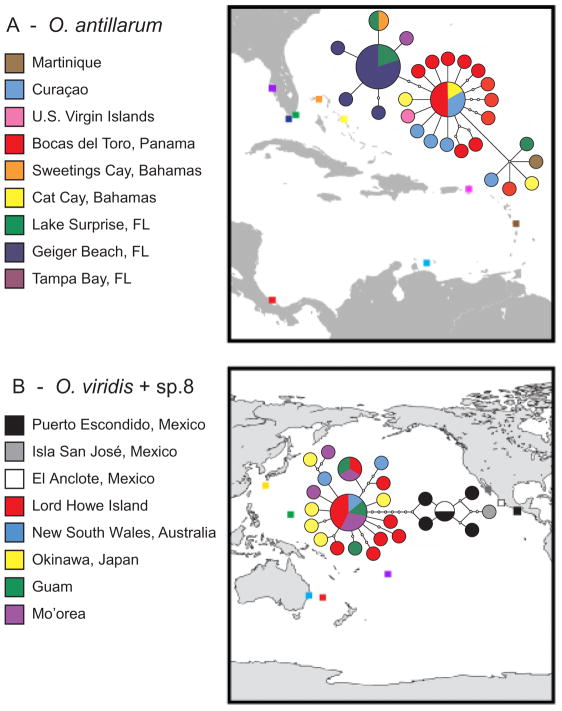
Our sampling permitted tests of intraspecific phylogeography and population genetics based on COI data for the well-sampled taxa O. antillarum and O. viridis. The 27 haplotypes sampled from 42 specimens of O. antillarum formed one network to which O. azuropunctata, O. sp. 5, and O. sp. 6 did not connect under a 95% parsimony criterion (Fig. 3A). The inferred ancestral haplotype was shared by six Caribbean specimens from Panama, Curaçao and the Bahamas, and differed from other haplotypes by five or fewer substitutions. The most common haplotype was sampled in 10 Floridian specimens. Starburst patterns in Caribbean and Floridian sub-networks, combined with significant, negative Fu’s FS and Tajima’s D tests for Florida and Panama, were consistent with recent expansions (Table 3A). In population genetic analyses of O. antillarum, overall FST was highly significant (Table 4A; P < 0.00001), as was ΦST (Table 4B; P < 0.00001). Florida was differentiated from Curaçao (P = 0.003) and Panama (P = 0.00001) in pairwise FST comparisons, and from all other sites in ΦST comparisons (P < 0.005) (Table 4C).
Fig. 3.
Statistical parsimony network of COI haplotypes from (A) Oxynoe antillarum, and (B) O. viridis and O. sp. 8. Frequency of haplotypes is indicated by size of the corresponding circle, with the smallest colored circles representing singletons, and open dots representing unsampled haplotypes; each line segment represents one substitution. Color indicates sampling sites marked by squares.
Table 3.
Genetic diversity and selective neutrality tests at the COI locus for populations represented by multiple samples in (A) Oxynoe antillarum, and (B) O. viridis.
| CAT | FLA | CUR | PAN | ||
|---|---|---|---|---|---|
| A | |||||
| n | 3 | 16 | 6 | 14 | |
| # of haplotypes | 3 | 7* | 5* | 12* | |
| Expected # of haplotypes | 2.17 | 4.01 | 2.99 | 5.74 | |
| Mean # of pairwise differences | 2.00 ± 1.51 | 1.38** ± 0.89 | 1.67 ± 1.13 | 3.11** ± 1.72 | |
| Haplotype diversity (h) | 1.00 ± 0.27 | 0.63 ± 0.14 | 0.93 ± 0.12 | 0.97 ± 0.04 | |
| % of private haplotypes | 66.7 | 93.8 | 80.0 | 91.7 | |
| # of variable sites | 3 | 11 | 5 | 21 | |
| Nucleotide diversity (π) | 0.0030 ± 0.0029 | 0.0020 ± 0.0015 | 0.0025 ± 0.0020 | 0.0047 ± 0.0030 | |
|
| |||||
| JAP | GUA | MOR | AUS | HOW | |
|
| |||||
| B | |||||
| n | 5 | 3 | 5 | 3 | 10 |
| # of haplotypes | 5 | 3 | 4 | 3 | 8* |
| Expected # of haplotypes | 3.45 | 2.30 | 3.21 | 2.39 | 4.64 |
| Mean # of pairwise differences | 3.60 ± 2.19 | 2.67 ± 1.92 | 2.80 ± 1.77 | 3.33 ± 2.32 | 2.76** ± 1.59 |
| Haplotype diversity (h) | 1.00 ± 0.13 | 1.00 ± 0.27 | 0.90 ± 0.16 | 1.00 ± 0.27 | 0.93 ± 0.077 |
| % of private haplotypes | 100 | 33 | 50 | 66 | 75 |
| # of variable sites | 9 | 4 | 7 | 5 | 13 |
| Nucleotide diversity (π) | 0.0056 ± 0.0040 | 0.0040 ± 0.0036 | 0.0043 ± 0.0031 | 0.0051 ± 0.0044 | 0.0042 ± 0.0027 |
Mean pairwise differences, h, and π given with 95% confidence intervals.
Fu’s FS significantly negative (P < 0.0005).
Tajima’s D significantly negative (P < 0.02).
Table 4.
Population genetic structure in Oxynoe antillarum based on AMOVA analysis of COI haplotypes;
| Source of variation | Degrees of freedom | Sum of squares | Variance components | % of variation |
|---|---|---|---|---|
| A | ||||
| Among populations | 3 | 3.44 | 0.08 | 17.08*** |
| Within populations | 35 | 14.31 | 0.41 | 82.92 |
| Total | 38 | 17.74 | 0.49 | |
|
| ||||
| Source of variation | Degrees of freedom | Sum of squares | Variance components | % of variation |
|
| ||||
| B | ||||
| Among populations | 3 | 27.05 | 0.91 | 46.28*** |
| Within populations | 35 | 36.95 | 1.06 | 53.72 |
| Total | 38 | 63.99 | 1.97 | |
|
| ||||
| CAT | CUR | PAN | FLA | |
|
| ||||
| C | ||||
| CAT | – | 0.04 | 0.02 | 0.26 |
| CUR | −0.05 | – | 0.05 | 0.25 |
| PAN | −0.07 | −0.04 | – | 0.21 |
| FLA | 0.65 | 0.64 | 0.53 | – |
P < 0.00001.
(A) Results from conventional FST analysis using haplotype frequencies. (B) Results from ΦST analysis using Tamura-Nei (TrN)-corrected genetic distances. (C) Pairwise differences among populations, based on FST analysis of haplotype frequencies (above diagonal) or ΦST analysis of TrN genetic distances (below diagonal). Bolded values are significant; P < 0.005.
The Pacific COI network included 18 haplotypes from O. viridis (N = 26) and six haplotypes from O. sp. 8 (N = 7) (Table 3B, Fig. 3B). Haplotypes from O. viridis formed a starburst network consistent with a recent expansion or selective sweep; the most common haplotype was sampled at four sites, and differed from other haplotypes by 1–4 substitutions (Fig. 3B). The subnetwork of haplotypes from O. sp. 8 was separated from the ancestral O. viridis haplotype by ≥8 substitutions. Within O. viridis, one haplotype was sampled from seven specimens and one from three specimens; 16 unique haplotypes were sampled, including six from Lord Howe Island, the only site for which Fu’s FS and Tajima’s D were significantly negative (FS = −3.85, P = 0.005; D = −1.82, P = 0.017). In O. viridis, overall FST was significant (Table 5), but neither ΦST nor any pairwise comparisons were significant.
Table 5.
Results from AMOVA on COI haplotypes from Oxynoe viridis, based on FST analysis of haplotype frequencies.
| Source of variation | Degrees of freedom | Sum of squares | Variance components | Percentage of variation |
|---|---|---|---|---|
| Among populations | 4 | 2.35 | 0.02 | 4.53* |
| Within populations | 21 | 10.00 | 0.48 | 95.47 |
| Total | 25 | 12.35 | 0.50 |
P = 0.02
3.3. Multilocus tests of species hypotheses in the O. antillarum-viridis complex
The O. sp.8 hypothesis was not supported by mtDNA, but H3 allele distributions suggested differences among CS within the complex (Fig. S3). A range of low-frequency, private alleles were recovered from both O. antillarum and O. viridis, but among complex members, only O. viridis had a common private allele (#16). Allele #14 was sampled at high frequencies in O. antillarum (0.41), O. sp. 8 (0.75), and O. sp. 5 (1.0), while #15 was sampled at high frequencies in O. antillarum (0.42), O. azuropunctata (1.0) and O. sp. 6 (1.0), but both were rare in O. viridis (0.05). We therefore tested whether clustering analyses would support different gene pools (species hypotheses) based on nuclear loci, especially with regard to O. sp. 8.
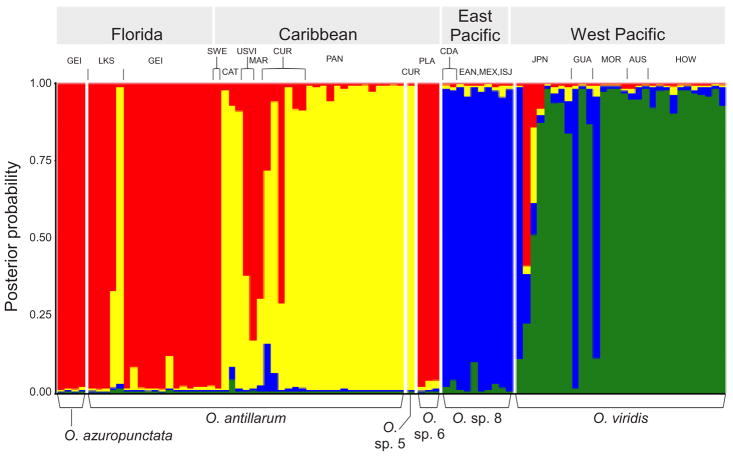
Analyses by STRUCTURE supported four gene pools with high probabilities of population assignment in the O. antillarum-viridis complex, whereas K ≥5 lowered assignment probabilities without revealing meaningful structure (Fig. 4). At K = 2, individuals were partitioned into Atlantic and Pacific populations with strong support (P < 0.01) (Fig. S4A). At K = 3, Pacific Oxynoe were distinct from Atlantic populations that reflected differences between Florida and Caribbean samples (Fig. S4B). At the favored number of K = 4 genetic entities, Pacific samples split into two populations principally comprising O. viridis (green) and O. sp. 8 (blue), with one specimen each from Japan, Guam and Mo’orea assigned to the East Pacific gene pool (Fig. 4).
Fig. 4.
Plot from two pooled STRUCTURE analyses of nuclear alleles in the O. antillarum-viridis complex, with individual assignment probabilities to K = 4 gene pools indicated by height of colored bars. Sampling sites and geographic regions from which specimens were sampled given above plot; species hypotheses given below plot.
In the western Atlantic, specimens were divided into two populations but assignments were more reflective of sampling site (Florida versus Caribbean) than species identifications. Specimens of O. azuropunctata and O. antillarum from Florida grouped with one O. antillarum from SWE (northern Bahamas), one O. antillarum from USVI, and O. sp. 6 from the Bahamas (Fig. 4, red). As the specimen from SWE assigned to the Florida population also had a COI haplotype shared with a Florida slug, this specimen may have dispersed from Florida to the Bahamas. The remaining Caribbean O. antillarum formed a population with O. sp. 5 from Curaçao (Fig. 4, yellow), although assignment probabilities were lower for some Bahamas and USVI samples. Only two of 18 Floridian specimens of O. antillarum (from Lake Surprise) were assigned to the Caribbean population; one also had a COI haplotype from the Caribbean subnetwork (Fig. 3A), and likely represents a recent immigrant to Florida from the Caribbean.
Collectively, population genetic analyses indicated that Florida and Caribbean O. antillarum comprised subpopulations that were minimally divergent in their mtDNA, and shared migrants among neighboring sites; these populations were thus deemed unsuitable as CS for delimitation under the multispecies coalescent (Sukumaran and Knowles, 2017). In contrast, the East Pacific lineage designated O. sp. 8 was highly differentiated from west Pacific O. viridis, and was reasonably considered a CS and not a subpopulation, given that O. viridis lacked appreciable genetic structure across the sampled Indo-West Pacific.
3.4. BPP and iBPP: Integrating tooth characters in species delimitation
Using BPP, simultaneous species delimitation and species-tree inference supported 11 species with nearly complete support (run 1, PP = 0.9972; run 2, PP = 0.9964). Simultaneous inference of the species tree did not strongly support any one tree, but the topscoring trees all recovered sister relationships for (O. sp. 7 + O. sp. 9), (O. sp. 2 + O. sp. 3), and a monophyletic O. antillarum-viridis complex. Both ML and BI analyses of the concatenated four-gene alignment supported a topology similar to that of the mtDNA phylogram (Fig. 5). Oxynoe sp. 1 was fully supported as sister to the rest of Oxynoe, within which a clade of O. sp. 7 and O. sp. 9 was fully supported, and sister to the remaining species. Oxynoe sp. 2 and O. sp. 3 formed a clade with marginal support (69% BS), sister to the fully supported O. antillarum-viridis complex. Within the complex, relationships were unresolved except that O. viridis and O. sp. 8 were supported as sister taxa in both analyses (98% BS, 0.96 PP).
Fig. 5.
Integrative species delimitation and phylogenetic relationships in Oxynoe. Topology of the uncollapsed cladogram based on ML analysis of four loci served as the guide tree for species delimitation using iBPP (after pruning O. sp. 9). Support values above a branch are the bootstrap % (left) and posterior probability (right) of that node. Values below a branch are posterior probabilities of the two descendent lineages representing distinct species based on iBPP analyses of three datasets: (a) before slash, both genetic and morphological data; (b) between slashes, morphological data only; (c) after slash, morphological data only, randomized for members of the O. antillarum-viridis complex. Asterisk = 100% or 1.0.
All 10 tested candidate species received 100% support in independent iBPP analyses integrating molecular and morphological data (Fig. 5). When iBPP was run using only morphological data, all species received significant support except two pairs that were collapsed: O. viridis + O. sp. 8 (PP = 0.43), and O. sp. 2 + O. sp. 3 (PP = 0.65) (Fig. 5). However, when species labels were randomized with respect to trait data for members of the O. antillarum-viridis complex, support was non-significant (PP < 0.4) for all species in the complex, as well as O. sp.2 and O. sp.3. (Fig. 5). Thus, morphological data alone delimited eight Oxynoe spp., indicating radular characters have substantial value for species delimitation.
Covariance of radular morphology and host suggested functional differences in tooth shape. Oxynoe sp. 1 had deeply concave teeth with wedge-shaped tips, and fed on finely branching Caulerpa spp. (Table 2). Slugs that ate ‘sea grapes’ (C. racemosa complex, possibly including C. cylindracea; Belton et al., 2014) had concave, pointed teeth with a length:depth ratio of > 4.2 (O. antillarum, some O. viridis, O. sp. 2, O. sp. 7). Slugs eating feather-like algae in the C. sertularioides complex (O. sp. 5, O. sp. 8, some O. viridis) had slightly concave teeth with a length:depth ratio < 4.0. Taxa that fed on C. cupressoides or C. paspaloides had deep, convex teeth and short denticles (O. azuropunctata, O. sp. 6) or deep, flat, pointed teeth (Oxynoe sp. 3), and generally had the lowest tooth length:depth ratios. Australian specimens of O. viridis did not feed on C. cupressoides during 24 hr of observation, but fed immediately when offered C. racemosa, indicating at least local host preference. In contrast, L. viridis and L. sp. 1 fed on C. racemosa and C. cupressoides at multiple sites, suggesting less specialization in Lobiger.
Table 2.
Algal host use in Oxynoe spp. based on field associations and laboratory feeding observations.
| species | Collection site | Host Caulerpa species |
|---|---|---|
| O. antillarum | Curaçao | C. racemosa, C. sertularioides |
| Florida Keys | C. cupressoides, C. paspaloides | |
| O. azuropunctata | Florida Keys | C. cupressoides |
| C. paspaloides, C. sertularioidesa | ||
| O. viridis | Guam | C. scalpelliformis |
| Okinawa, Japan | C. racemosa, C. serrulata, C. cupressoides | |
| Lord Howe Island | C. racemosab | |
| O. sp. 1 | Guam | C. verticillata, C. filicoides |
| Mabini, Philippines | C. verticillata | |
| O. sp. 2 | Lord Howe Island | C. racemosab |
| O. sp. 3 | Lord Howe Island | C. cupressoides |
| O. sp. 5 | Curaçao | C. sertularioides |
| O. sp. 6 | Bahamas | C. cupressoides or C. paspaloides |
| O. sp. 7 | Lord Howe Island | C. racemosab |
| O. sp. 8 | Mexico | C. scalpelliformis |
Possibly C. cylindracea, not taxonomically clarified at time of collection (Belton et al., 2014).
4. Discussion
4.1. Integrative species delimitation for soft-bodied marine animals
Molecular approaches have been increasingly used to delimit species in soft-bodied marine taxa, highlighting where morphological work is needed to describe historically overlooked taxa (Ward, 2009; Bucklin et al., 2011). However, notable challenges remain; the size of the bar-coding gap and pace of lineage sorting varies among taxa due to differences in mutation rates, Ne, or time since speciation (Beltrán et al., 2002; Hickerson et al., 2006; Meier et al., 2006, 2008), and introgression may spread alleles between species (Zink and Barrowclough, 2008; Kindler et al., 2012). It thus remains difficult to predict a priori which loci, and how many, should be used to delimit species, or what to do if some sister species are highly divergent yet others do not show reciprocal monophyly (Dupuis et al., 2012). Accommodating discordance among methods and integrating phenotypic characters remain problematic (Carstens et al., 2013). Despite common claims of “integrative” taxonomy in the literature (Yeates et al., 2011), studies of marine invertebrates have rarely if ever integrated morphological and molecular data into one combined delimitation analysis, a particular challenge for taxa with deformable bodies.
Here, we identified a clade of sea slugs in which levels of divergence were highly variable among pairs of sister species. Our initial screen recovered five species per genus that were ≥16% divergent from their nearest relative, and morphologically distinctive. At present, there are six generally recognized species of Oxynoe (including three not sampled here), and four of Lobiger (including two not sampled here). The 11 pseudocryptic species (i.e., previously unrecognized but morphologically distinguishable) uncovered here thus more than doubles species diversity in these genera; current taxonomy underestimates the species richness of these Caulerpa feeders by at least twofold (Table 6).
Table 6.
Biodiversity in family Oxynoidae, as reflected by current taxonomy (see Supplemental File 1) versus new candidate species identified herein.
| Genus | Described species
|
CS found in present study | Total | |
|---|---|---|---|---|
| Sampled in present study | Unsampled in present study | |||
| Lobiger | 2 | 2 | 3 | 7 |
| Oxynoe | 3 | 3 | 8 | 14 |
| Roburnella | 0 | 1 | – | 1 |
| Total | 5 | 6 | 11 | 22 |
The O. antillarum-viridis complex was problematic as rates of divergence among its six COI lineages (1.3–7.0%) were well below interspecific distances among other Oxynoe spp., and other sacoglossans (generally > 8%; Krug et al., 2013, 2015, 2016). Strikingly, Pacific and Caribbean lineages were much less divergent (4.3% TrN-distance) than the presumed geminate sacoglossans Elysia velutinus (Caribbean) and E. sp. 6 (East Pacific), which are 11.6% divergent (Krug et al., 2015, 2016). This suggests either a slow-down in the molecular clock for the O. antillarum-viridis complex relative to the rest of Oxynoe, or else most Oxynoe spp. are comparatively old, and E. velutinus-E. sp. 6 diverged long before closure of the Panamanian Isthmus (Marko, 2002).
Analyses of different molecular datasets also yielded discordant results for the number of species in the O. antillarum-viridis complex: O. sp. 8 was lumped with O. viridis based on mtDNA, but split by clustering analyses of nuclear alleles. Conversely, Caribbean samples were split into four CS by mtDNA, but only two gene pools by nuclear allele frequencies, which corresponded better to geography than provisional species identities. However, all 11 species were fully supported by BPP, and differences in morphology, host use and range indicated each was likely an independently evolving metapopulation lineage (de Queiroz, 2005). We thus tested whether delimitation analyses integrating ecologically relevant tooth characters would improve support for CS that were lumped in one or more prior analyses that used only genetic data.
To our knowledge, no prior study integrated morphological and molecular data from a soft-bodied animal in one species delimitation analysis. iBPP using a guide tree strongly supported all 10 tested species, including six in the O. antillarum-viridis complex. Using only shell length and radular tooth measurements, iBPP delimited five complex members, lumping O. viridis and O. sp. 8, whereas randomizing species labels collapsed all complex members. The East Pacific O. sp. 8 was supported as distinct when morphology was combined with genetic data, supporting the longstanding hypothesis of a distinct species in this biogeographical region. A misleading title on the species description led to the name ‘O. panamensis’ being misapplied to East Pacific specimens, but O. sp. 8 remains formally undescribed (Supplemental File 1). Although we lacked radular data for O. sp. 9, its sampling site (South Africa) suggests O. natalensis should be formally resurrected for this taxon, pending full morphological analysis.
No prior work had suggested multiple rare species of Oxynoe were present in the Caribbean. Oxynoe azuropunctata is distinguished from O. antillarum by blue spots and rounded papillae, and non-feeding larvae (Jensen, 1980). Both ABGD and GMYC split O. antillarum from O. azuropunctata, but also identified rare lineages (O. sp. 5 and O. sp. 6), each more distant from O. azuropunctata than the latter was from O. antillarum. Differences in tooth shape confirmed that O. sp. 5 was distinct from named species. Tooth shape for O. sp. 6 was similar to O. azuropunctata, which also occurred on the same host alga, but O. sp. 6 had a longer shell relative to its body length, short denticles and more teeth in its ascending radular limb.
Our approach has advantages over traditional taxonomy which suffered from a lack of formalization regarding characters that reliably show species-level differences, and lack of access to quantitative methodology; the resulting subjectivity commonly led to cycles of lumping and splitting. In contrast, integrative analyses incorporating trait data can objectively resolve species complexes, which are ubiquitous in marine heterobranchs (Wilson et al., 2013; Cooke et al., 2014; Churchill et al., 2014; Krug et al., 2013, 2016). Data on morphology, ecology, larval type and distribution can now be used to formally describe each CS (Krug et al., in press).
4.2. Intraspecific phylogeography and population structure
Recognizing subpopulations as CS runs the risk of false positives in species delimitation using the multispecies coalescent (Sukumaran and Knowles, 2017). Despite the wide geographic range of O. viridis, no sampling sites were genetically differentiated. High gene flow may impede divergence and explain the lack of closely related species in the IWP; the closest relative (O. sp. 8) was separated by the Eastern Pacific Barrier, ~5000 km of uninterrupted deep water that impedes migration in many benthic taxa (Baums et al., 2012; Cowman and Bellwood, 2013). Our data support recognizing O. sp. 8 as a species and not a subpopulation of O. viridis.
In contrast, there was a marked genetic discontinuity between O. antillarum lineages sampled from Florida versus the greater Caribbean. In each regional population, the COI network formed a starburst pattern consistent with recent expansion, which may have followed Pleistocene sea-level fluctuations that eliminated suitable habitat (Hearty and Neumann, 2001). Genetic isolation of Florida is congruent with studies of other sacoglossans (Krug et al., 2011; Rico, 2012) and some fishes (Taylor and Hellberg, 2006; Jackson et al., 2014). The fast-moving Florida Current, which feeds into the Gulf Stream, may represent a biophysical boundary that molluscan larvae rarely cross, instead being advected into the North Atlantic. The individual in Sweetings Cay, Bahamas that grouped with Florida at both mitochondrial and nuclear loci may have dispersed from Florida as a larva and been carried by the Gulf Stream to the Bahamas; conversely, the specimen from Key Largo with both Caribbean mtDNA and nuclear genotypes likely represented a recent immigrant, indicating migration across the Gulf Stream does occur, albeit infrequently. A similarly deep phylogeographic divide, with rare migrant individuals sampled in Florida and the Bahamas, was recently reported for another sacoglossan (Ellingson and Krug, 2016). Biophysical barriers and cycles of transient allopatry during the Pleistocene may have produced both genetically subdivided populations within species, and also a rapid radiation of Atlantic taxa in the O. antillarum-viridis complex, consistent with high Caribbean endemicity across marine heterobranchs (Ornelas-Gatdula and Valdés, 2012; Espinoza et al., 2014; Malaquias, 2014; Krug et al., 2016; Valdés et al., 2016).
A striking split was also seen between Florida and the Caribbean in the nuclear genome of O. antillarum. At K = 3, the divide between Floridian and Caribbean populations was stronger than the split between western and eastern Pacific Oxynoe spp., despite the difference in geographic isolation (< 500 km between Florida and isolated Caribbean sites, versus > 6000 km for the closest eastern and western Pacific sites). At K = 4, the predominant signal in the Caribbean remained the divergence between Florida and Caribbean populations of O. antillarum. Notably, no H3 alleles were unique to O. azuropunctata, O. sp. 5, or O. sp. 6, each being fixed for one of the common alleles sampled in O. antillarum; some microsatellite alleles were also shared across species. In prior studies of > 200 sacoglossan species, including surveys of > 100 individuals for some Caribbean taxa, no H3 alleles were shared between species when there was intraspecific polymorphism (Krug et al., 2013, 2015, 2016). Each rare species (O. azuropunctata, O. sp. 5 and O. sp. 6) contained nuclear alleles typical of co-occurring O. antillarum, but not alleles that were common in other populations of O. antillarum, a pattern more consistent with introgression than incomplete lineage sorting. Further study is needed to determine whether selection maintains these species in the face of gene flow, or if a slow-down in the rate of lineage sorting impeded divergence in nuclear and mitochondrial genomes alike. However, mtDNA divergence and morphology distinguished O. sp. 5 and O. sp. 6, whereas the FL and Caribbean subpopulations of O. antillarum were not considered distinct CS given negligible divergence in mtDNA and similar host use, external appearance and radular morphology.
4.3. Implications for assessing marine biodiversity and biocontrol
Our results double the biodiversity of family Oxynoidae, adding 11 new CS to the 11 recognized species (see Supplemental File 1): three CS in Lobiger, five highly divergent CS in Oxynoe, and three CS in the O. antillarum-viridis complex (Table 6). Variable divergence among Oxynoe spp. illustrates the risk of assessing biodiversity with uniform barcoding cutoffs: a 2% threshold would not differentiate O. sp. 8 from O. viridis, but would greatly overestimate diversity in most sacoglossan genera (Hebert et al., 2003), yet the 10% threshold proposed for other heterobranch groups would detect only six Oxynoe spp. (Malaquias and Reid, 2008). Our approach compliments recent work in other sea slugs showing that nominal species may comprise five to 10 pseudocryptic taxa (Krug et al., 2013; Jensen et al., 2014). True species richness in sacoglossans has been systematically under-estimated by a conservative taxonomy overly reliant on characters of limited value (e.g., shell features) while overlooking key traits (e.g., colored mantle striations in Lobiger; radular tooth shape, mantle spotting in Oxynoe).
Our findings indicate Caulerpa consumers are more specialized than reflected in the ecological literature. Moreover, tooth morphology covaried among Oxynoe spp. with algal preference, suggesting host use imposes selection on radular characters (Jensen, 1983). Notably, teeth had high length:width ratios (> 4) and were generally concave for species feeding on the C. racemosa complex (O. antillarum; O. viridis from Australia and Japan; O. sp. 2, O. sp. 7). In contrast, taxa feeding on feathery fronds of the C. sertularioides complex (O. viridis from Guam, O. sp. 5, O. sp. 8) had teeth with length:depth ratios < 4, high ridge-base angles, and longer denticles. Taxa feeding on the upright, thick-bladed C. cupressoides had either short, convexly curved teeth with narrow points (O. azuropuncta, O. sp. 6) or long and deep teeth (O. sp. 3). The three rare Caribbean species were not sampled on C. racemosa, the most common host of O. antillarum, and sister taxa O. sp. 2 and O. sp. 3 fed on different algae; disruptive selection on algal host may thus reduce competition or hybridization and contribute to speciation in this complex.
An ideal biocontrol agent for invasive algae would produce non-dispersive larvae, associated with short generation times and rapid population growth (Coquillard et al., 2000). Such larvae are only known from O. azuropunctata and O. sp. 1, neither of which feeds on invasive species, and are therefore sub-optimal for biocontrol. Intentional introduction of any Oxynoe species would likely be of limited utility, compared to species of Elysia that are both non-dispersive and generalist Caulerpa consumers (e.g., E. subornata). However, given the extent of previously unrecognized diversity in Oxynoidae, there may yet be unsampled species with better combinations of host preference and dispersal ability for biocontrol applications.
Our work indicates that both species diversity and dietary specialization is greater in Oxynoe than previously thought, and may explain the range of host preferences reported for nominal “O. viridis.” Notably, experiments using Australian specimens could have included at least four species (O. viridis, O. sp. 2, O. sp. 3, and O. sp. 7) (Baumgartner et al., 2009). Differences in feeding preferences among species may determine whether a local guild can limit the success of invasive Caulerpa spp. Renewed study is warranted to assess the true degree of specialization and capacity for host shifting over ecological time scales, within and among the diversity of shelled Caulerpa consumers here shown to exist in the Pacific and western Atlantic.
Supplementary Material
Acknowledgments
For donating specimens or assistance with sampling, we thank C. Blackburn, Y. Buske, K. and T. Eve, J. Sigwart, K. Kocot, D. Marshall, C. Meyer and the Moorea Biocode Project, M. Phuong, P. Schupp, G. Rouse and H. Wägele. We thank A. Meade for a parallelized version of BayesPhylogenies, and for comments that improved the paper, C. Barrett, K. Fisher, and three reviewers. This study was supported by awards from the U.S. National Science Foundation [DEB-0817084, DEB-1355190, and OCE 11-30072 to PJK; DEB-1355177 to AAV], the U.S. National Institutes of Health [grant number GM-61331 supporting DR], and the LaKretz Endowment for Environmental Biology at Cal State L.A. The Australian Museum provided support for NGW and fieldwork on Lord Howe Island. Special thanks to Joseph R. Pawlik for the invitation to participate in research cruises to the Bahamas, supported by awards from the U.S. National Science Foundation (OCE 0095724 and PEHS 0550468 to JRP).
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ympev.2018.02.027.
References
- Altamirano M, Andreakis N, Souza-Egipsy V, Zanolla M, de la Rosa J. First record of Caulerpa cylindracea (Caulerpaceae, Chlorophyta) in Andalusia (Southern Spain) Anales del Jardín Botánico de Madrid. 2014;71:1–9. [Google Scholar]
- Anderson L. California’s reaction to Caulerpa taxifolia: a model for invasive species rapid response. Bio Inv. 2005;7:1003–1016. [Google Scholar]
- Baumgartner F, Motti C, deNys R, Paul N. Feeding preferences of specialist marine herbivores align with quantitative variation in seaweed secondary metabolites. Mar Ecol Prog Ser. 2009;396:1–12. [Google Scholar]
- Baums IB, Boulay J, Polato NR, Hellberg ME. No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol Ecol. 2012;21:5418–5433. doi: 10.1111/j.1365-294X.2012.05733.x. [DOI] [PubMed] [Google Scholar]
- Belton GS, Reine WF, Huisman JM, Draisma S, Gurgel D, Frederico C. Resolving phenotypic plasticity and species designation in the morphologically challenging Caulerpa racemosa–peltata complex (Chlorophyta, Caulerpaceae) J Phycol. 2014;50:32–54. doi: 10.1111/jpy.12132. [DOI] [PubMed] [Google Scholar]
- Beltrán M, Jiggins C, Bull V, Linares M, Mallet J, McMillan W, Bermingham E. Phylogenetic discordance at the species boundary: comparative gene genealogies among rapidly radiating Heliconius butterflies. Mol Biol Evol. 2002;19:2176–2190. doi: 10.1093/oxfordjournals.molbev.a004042. [DOI] [PubMed] [Google Scholar]
- Boudouresque C, Lemée R, Mari X, Meinesz A. The invasive alga Caulerpa taxifolia is not a suitable diet for the sea urchin Paracentrotus lividus. Aquat Bot. 1996;53:245–250. [Google Scholar]
- Bucklin A, Steinke D, Blanco-Bercial L. DNA barcoding of marine metazoa. Annu Rev Mar Sci. 2011;3:471–508. doi: 10.1146/annurev-marine-120308-080950. [DOI] [PubMed] [Google Scholar]
- Bulleri F, Balata D, Bertocci I, Tamburello L, Benedetti-Cecchi L. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: from passenger to driver of ecological change. Ecology. 2010;91:2205–2212. doi: 10.1890/09-1857.1. [DOI] [PubMed] [Google Scholar]
- Burfeind D, Tibbetts I, Udy J. Grazing rates of Elysia tomentosa on native and introduced Caulerpa taxifolia. Hydrobiologia. 2009;632:355–358. [Google Scholar]
- Byers JE, Wright J, Gribben P. Variable direct and indirect effects of a habitat-modifying invasive species on mortality of native fauna. Ecology. 2010;91:1787–1798. doi: 10.1890/09-0712.1. [DOI] [PubMed] [Google Scholar]
- Carstens B, Pelletier T, Reid N, Satler J. How to fail at species delimitation. Mol Ecol. 2013;22:4369–4383. doi: 10.1111/mec.12413. [DOI] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Chambers G, MacAvoy E. Microsatellites: consensus and controversy. Comp Biochem Physiol B. 2000;126:455–476. doi: 10.1016/s0305-0491(00)00233-9. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson T, Higgins D, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nuc Acid Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill CK, Valdés Á, Foighil DÓ. Molecular and morphological systematics of neustonic nudibranchs (Mollusca: Gastropoda: Glaucidae: Glaucus), with descriptions of three new cryptic species. Invert Syst. 2014;28:174–195. [Google Scholar]
- Clement M, Posada D, Crandall K. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Colgan D, Ponder W, Eggler P. Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences. Zool Scripta. 2000;29:29–63. [Google Scholar]
- Cooke S, Hanson D, Hirano Y, Ornelas-Gatdula E, Gosliner TM, Chernyshev AV, Valdés Á. Cryptic diversity of Melanochlamys sea slugs (Gastropoda, Aglajidae) in the North Pacific. Zool Scripta. 2014;43:351–369. [Google Scholar]
- Coquillard P, Thibaut T, Hill D, Gueugnot J, Mazel C, Coquillard Y. Simulation of the mollusc Ascoglossa Elysia subornata population dynamics: application to the potential biocontrol of Caulerpa taxifolia growth in the Mediterranean Sea. Ecol Model. 2000;135:1–16. [Google Scholar]
- Costello M, Coll M, Danovaro R, Halpin P, Ojaveer H, Miloslavich P. A census of marine biodiversity knowledge, resources, and future challenges. PloS One. 2010;5:e12110. doi: 10.1371/journal.pone.0012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman P, Bellwood D. Vicariance across major marine biogeographic barriers: temporal concordance and the relative intensity of hard versus soft barriers. Proc Roy Soc B. 2013;280:20131541. doi: 10.1098/rspb.2013.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Queiroz K. Ernst Mayr and the modern concept of species. Proc Natl Acad Sci USA. 2005;102:6600–6607. doi: 10.1073/pnas.0502030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douady C, Delsuc F, Boucher Y, Doolittle W, Douzery E. Comparison of Bayesian and maximum likelihood bootstrap measures of phylogenetic reliability. Mol Biol Evol. 2003;20:248–254. doi: 10.1093/molbev/msg042. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Roe A, Sperling F. Multi-locus species delimitation in closely related animals and fungi: one marker is not enough. Mol Ecol. 2012;21:4422–4436. doi: 10.1111/j.1365-294X.2012.05642.x. [DOI] [PubMed] [Google Scholar]
- Earl DA, vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res. 2012;4:361. [Google Scholar]
- Ellingson RA, Krug PJ. Reduced genetic diversity and increased reproductive isolation follow population-level loss of larval dispersal in a marine gastropod. Evolution. 2016;70:18–37. doi: 10.1111/evo.12830. [DOI] [PubMed] [Google Scholar]
- Espinoza E, DuPont A, Valdés A. Molecular data reveal an undescribed cryptic species of Costasiella Pruvot-Fol, 1951 (Euthyneura: Sacoglossa) in the Bahamas. Amer Malacol Bull. 2014;32:173–182. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer H. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Ezard T, Fujisawa T, Barraclough T. R Package Version 1.0-18/r45. 2013. SPLITS: SPecies’ LImits by Threshold Statistics. [Google Scholar]
- Faircloth BC. msatcommander: detection of microsatellite repeat arrays and automated, locus-specific primer design. Mol Ecol Res. 2008;8:92–94. doi: 10.1111/j.1471-8286.2007.01884.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Silva I, Whitney J, Wainwright B, Andrews KR, Ylitalo-Ward H, Bowen BW, Toonen RJ, Goetze E, Karl SA. Microsatellites for next-generation ecologists: a post-sequencing bioinformatics pipeline. PLoS one. 2013;8:e55990. doi: 10.1371/journal.pone.0055990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flot JF. SeqPHASE: a web tool for interconverting PHASE input/output files and FASTA sequence alignments. Mol Ecol Res. 2010;10:162–166. doi: 10.1111/j.1755-0998.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech. 1994;3:294–299. [PubMed] [Google Scholar]
- Fu YX. Statistical test of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Barraclough T. Delimiting species using single-locus data and the generalized mixed yule coalescent (GMYC) approach: a revised method and evaluation on simulated datasets. Syst Biol. 2013;62:707–724. doi: 10.1093/sysbio/syt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JR, Wright JT. Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Freshwat Res. 2006;57:685–694. [Google Scholar]
- Hearty P, Neumann A. Rapid sea level and climate change at the close of the last interglaciation (MIS 5e): evidence from the Bahama Islands. Quart Sci Rev. 2001;20:1881–1895. [Google Scholar]
- Hebert P, Ratnasingham S, deWaard J. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc Roy Soc B. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P, deWaard J, Landry J. DNA barcodes for 1/1000th of the animal kingdom. Biol Lett. 2010;6:359–362. doi: 10.1098/rsbl.2009.0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickerson M, Meyer C, Moritz C. DNA barcoding will often fail to discover new animal species over broad parameter space. Syst Biol. 2006;55:729–739. doi: 10.1080/10635150600969898. [DOI] [PubMed] [Google Scholar]
- Huang D, Meier R, Todd P, Chou L. Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. J Mol Evol. 2008;66:167–174. doi: 10.1007/s00239-008-9069-5. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J, Rannala B. Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst Biol. 2004;53:904–913. doi: 10.1080/10635150490522629. [DOI] [PubMed] [Google Scholar]
- Jackson A, Semmens B, de Mitcheson Y, Nemeth R, Heppell S, Bush P, Aguilar-Perera A, et al. Population structure and phylogeography in Nassau grouper (Epinephelus striatus), a mass-aggregating marine fish. PloS one. 2014;9:e97508. doi: 10.1371/journal.pone.0097508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Jensen KR. Oxynoe azuropunctata, n.sp., a new sacoglossan from the Florida Keys (Mollusca: Opisthobranchia) J Moll Stud. 1980;46:282–292. [Google Scholar]
- Jensen KR. Factors affecting feeding selectivity in herbivorous ascoglossa (Mollusca: Opisthobranchia) J Exp Mar Biol Ecol. 1983;66:135–148. [Google Scholar]
- Jensen KR, Krug PJ, DuPont A, Nishina M. A review of taxonomy and phylogenetic relationships within Costasiella (Mollusca, Heterobranchia, Sacoglossa), with description of a new species. J Moll Stud. 2014;80:562–574. [Google Scholar]
- Kindler E, Arlettaz R, Heckel G. Deep phylogeographic divergence and cytonuclear discordance in the grasshopper Oedaleus decorus. Mol Phylogenet Evol. 2012;65:695–704. doi: 10.1016/j.ympev.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Klein J, Verlaque M. The Caulerpa racemosa invasion: a critical review. Mar Poll Bull. 2008;56:205–225. doi: 10.1016/j.marpolbul.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Ishikawa T, Yamaguchi N, Hori Y, Ohba H. But next time?: Unsuccessful establishment of the Mediterranean strain of the green seaweed Caulerpa taxifolia in the Sea of Japan. Biol Inv. 2003;5:275–278. [Google Scholar]
- Krug PJ, Berriman JS, Valdés AA. Phylogenetic systematics of the shelled sea slug genus Oxynoe Rafinesque, 1814 (Heterobranchia: Sacoglossa), with integrative descriptions of seven new species. Invert Syst. in press. [Google Scholar]
- Krug PJ, Händeler K, Vendetti J. Genes, morphology, development and photosynthetic ability support the resurrection of Elysia cornigera (Heterobranchia: Plakobranchoidea) as distinct from the ‘solar–powered’ sea slug E timida. Invert Syst. 2011;25:477–489. [Google Scholar]
- Krug PJ, Vendetti J, Rodriguez A, Retana J, Hirano Y, Trowbridge C. Integrative species delimitation in photosynthetic sea slugs reveals twenty candidate species in three nominal taxa studied for drug discovery, plastid symbiosis or biological control. Mol Phylogenet Evol. 2013;69:1101–1119. doi: 10.1016/j.ympev.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug PJ, Vendetti J, Valdés A. Molecular and morphological systematics of Elysia Risso, 1818 (Heterobranchia: Sacoglossa) from the Caribbean region. Zootaxa. 2016;4148:1–137. doi: 10.11646/zootaxa.4148.1.1. [DOI] [PubMed] [Google Scholar]
- Krug PJ, Vendetti J, Ellingson R, Trowbridge C, Hirano Y, Trathen D, Rodriguez A, Swennen C, Wilson N, Valdés A. Species selection favors dispersive life histories in sea slugs, but higher per-offspring investment drives shifts to short-lived larvae. Syst Biol. 2015;64:983–999. doi: 10.1093/sysbio/syv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleonart J, Salat J, Torres G. Removing allometric effects of body size in morphological analysis. J Theoret Biol. 2000;205:85–93. doi: 10.1006/jtbi.2000.2043. [DOI] [PubMed] [Google Scholar]
- Malaquias M. New data on the heterobranch gastropods (‘opisthobranchs’) for the Bahamas (tropical western Atlantic Ocean) Mar Biodiver Rec. 2014;7:e27. [Google Scholar]
- Malaquias M, Reid DG. Systematic revision of the living species of Bullidae (Mollusca: Gastropoda: Cephalaspidea), with a molecular phylogenetic analysis. Zool J Linn Soc. 2008;153:453–543. [Google Scholar]
- Marko P. Fossil calibration of molecular clocks and the divergence times of geminate species pairs separated by the Isthmus of Panama. Mol Biol Evol. 2002;19:2005–2021. doi: 10.1093/oxfordjournals.molbev.a004024. [DOI] [PubMed] [Google Scholar]
- Meier R, Shiyang K, Vaidya G, Ng P. DNA barcoding and taxonomy in diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55:715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- Meier R, Zhang G, Ali F. The use of mean instead of smallest interspecific distances exaggerates the size of the ‘barcoding gap’ and leads to misidentification. Syst Biol. 2008;57:809–813. doi: 10.1080/10635150802406343. [DOI] [PubMed] [Google Scholar]
- Meinesz A. Killer Algae. University of Chicago Press; Chicago: 1999. [Google Scholar]
- Meinesz A, Belsher T, Thibaut T, Antolic B, Mustapha K, Boudouresque C, Chiaverini D, et al. The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean. Bio Inv. 2001;3:201–210. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE); 14 Nov. 2010; New Orleans, LA. 2010. pp. 1–8. [Google Scholar]
- Molnar J, Gamboa R, Revenga C, Spalding M. Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ. 2008;6:485–492. [Google Scholar]
- Nee S, May R, Harvey P. The reconstructed evolutionary process. Philosoph Trans Roy Soc Lond Ser B. 1994;344:305–311. doi: 10.1098/rstb.1994.0068. [DOI] [PubMed] [Google Scholar]
- Ornelas-Gatdula E, Valdés A. Two cryptic and sympatric species of Philinopsis (Mollusca, Opisthobranchia, Cephalaspidea) in the Bahamas distinguished using molecular and anatomical data. J Moll Stud. 2012;78:313–320. [Google Scholar]
- Pagel M, Meade A. A phylogenetic mixture model for detecting pattern–heterogeneity in gene sequence or character-state data. Syst Biol. 2004;53:571–581. doi: 10.1080/10635150490468675. [DOI] [PubMed] [Google Scholar]
- Palumbi S. Nucleic acids II: the polymerase chain reaction. In: Hillis D, Moritz C, Mable B, editors. Molecular Systematics. Sinauer Associates; Sunderland: 1996. pp. 205–247. [Google Scholar]
- Palumbi S, Sandifer P, Allan J, Beck M, Fautin D, Fogarty M, Halpern B, Incze L, Leong JA, Norse E, Stachowicz J, Wall D. Managing for ocean biodiversity to sustain marine ecosystem services. Front Ecol Environ. 2009;7:204–211. [Google Scholar]
- Paradis E. Molecular dating of phylogenies by likelihood methods: a comparison of models and a new information criterion. Mol Phylogenet Evol. 2013;67:436–444. doi: 10.1016/j.ympev.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Paul V, Arthur K, Ritson-Williams R, Ross C, Sharp K. Chemical defenses: from compounds to communities. Biol Bull. 2007;213:226–251. doi: 10.2307/25066642. [DOI] [PubMed] [Google Scholar]
- Piazzi L, Balata D. The spread of Caulerpa racemosa var. cylindracea in the Mediterranean Sea: an example of how biological invasions can influence beta diversity. Mar Environ Res. 2008;65:50–61. doi: 10.1016/j.marenvres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Pons J, Barraclough T, Gomez-Zurita J, Cardoso A, Duran D, Hazell S, Kamoun S, Sumlin W, Vogler A. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol. 2006;55:595–609. doi: 10.1080/10635150600852011. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012;21:1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Bayla M, Boisselier MC, Cruaud C, Samadi S. An integrative approach to species delimitation in Benthomangelia (Mollusca: Conoidea) Biol J Linn Soc. 2009;96:696–708. [Google Scholar]
- Rambaut A, Drummond A. Tracer v1. 4: MCMC Trace Analyses Tool. 2007 Available from: < http://beast.bio.ed.ac.uk/Tracer >.
- Raven J, Walker D, Jensen K, Handley L, Scrimgeour C, McInroy S. What fraction of the organic carbon in sacoglossans is obtained from photosynthesis by kleptoplastids? An investigation using the natural abundance of stable carbon isotopes. Mar Biol. 2001;138:537–545. [Google Scholar]
- Regier JC, Shi D. Increased yield of PCR product from degenerate primers with nondegenerate, nonhomologous 5′ tails. Biotechniques. 2005;38:34–38. doi: 10.2144/05381BM02. [DOI] [PubMed] [Google Scholar]
- Rico DM. Masters Thesis. California State Univ; Los Angeles: 2012. How much does larval duration matter when it comes to dispersal? A genetic test with Caribbean molluscs. [Google Scholar]
- Schaffelke B, Murphy N, Uthicke S. Using genetic techniques to investigate the sources of the invasive alga Caulerpa taxifolia in three new locations in Australia. Mar Poll Bull. 2002;44:204–210. doi: 10.1016/s0025-326x(01)00202-8. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís-Lemus C, Knowles LL, Ané C. Bayesian species delimitation combining multiple genes and traits in a unified framework. Evolution. 2014;69:492–507. doi: 10.1111/evo.12582. [DOI] [PubMed] [Google Scholar]
- Stachowicz J, Bruno J, Duffy J. Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst. 2007;38:739–766. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Amer J Human Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran J, Knowles LL. Multispecies coalescent delimits structure, not species. Proc Nat Acad Sci USA. 2017;114:1607–1612. doi: 10.1073/pnas.1607921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MS, Hellberg ME. Comparative phylogeography in a genus of coral reef fishes: biogeographic and genetic concordance in the Caribbean. Mol Ecol. 2006;15:695–707. doi: 10.1111/j.1365-294X.2006.02820.x. [DOI] [PubMed] [Google Scholar]
- Thibaut T, Meinesz A. Are the Mediterranean ascoglossan molluscs Oxynoe olivacea and Lobiger serradifalci suitable agents for a biological control against the invading tropical alga Caulerpa taxifolia? Comptes Rendus de l’Académie des Sciences-Series III-Sciences de la Vie. 2000;323:477–488. doi: 10.1016/s0764-4469(00)00151-7. [DOI] [PubMed] [Google Scholar]
- Tittensor D, Mora C, Jetz W, Lotze H, Ricard D, Berghe E, Worm B. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- Trowbridge CD. Local elimination of Codium fragile ssp tomentosoides: indirect evidence of sacoglossan herbivory? J Mar Biol Assoc UK. 2002;82:1029–1030. [Google Scholar]
- Trowbridge CD. Emerging associations on marine rocky shores: specialist herbivores on introduced macroalgae. J Anim Ecol. 2004;73:294–308. [Google Scholar]
- Valdés A, Medrano SM, Bhave VJ. A new species of Cuthona (Heterobranchia: Nudibranchia: Tergipedidae) from the Caribbean Sea. Nautilus. 2016;130:72–78. [Google Scholar]
- Vieites DV, Wollenberg K, Andreone F, Kohler J, Glaw F, Vences M. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc Nat Acad Sci USA. 2009;106:8267–8272. doi: 10.1073/pnas.0810821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD. DNA barcode divergence among species and genera of birds and fishes. Mol Ecol Res. 2009;9:1077–1085. doi: 10.1111/j.1755-0998.2009.02541.x. [DOI] [PubMed] [Google Scholar]
- Williams SL, Smith JE. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evol Syst. 2007;38:327–359. [Google Scholar]
- Wilson NG, Maschek JA, Baker BJ. A species flock driven by predation? Secondary etabolites support diversification of slugs in Antarctica. PloS one. 2013;8:e80277. doi: 10.1371/journal.pone.0080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Rannala B. Unguided species delimitation using DNA sequence data from multiple loci. Mol Biol Evol. 2014;31:3125–3135. doi: 10.1093/molbev/msu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. The BPP program for species tree estimation and species delimitation. Curr Zool. 2015;61:854–865. [Google Scholar]
- Yeates DK, Seago A, Nelson L, Cameron SL, Joseph LE, Trueman JW. Integrative taxonomy, or iterative taxonomy? Syst Entomol. 2011;36:209–217. [Google Scholar]
- York P, Booth D, Glasby T, Pease B. Fish assemblages in habitats dominated by Caulerpa taxifolia and native seagrasses in south-eastern Australia. Mar Ecol Prog Ser. 2006;312:223–234. [Google Scholar]
- Zink R, Barrowclough G. Mitochondrial DNA under siege in avian phylogeography. Mol Ecol. 2008;17:2107–2121. doi: 10.1111/j.1365-294X.2008.03737.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.