Abstract
Animal and human studies have shown that both early-life traumatic events and ongoing stress episodes affect neurodevelopment, however, it remains unclear whether and how they modulate normative adolescent neuro-maturational trajectories. We characterized effects of early-life (age 0–5) and ongoing stressors (age 14–17) on longitudinal changes (age 14 to17) in grey matter volume (GMV) of healthy adolescents (n = 37). Timing and stressor type were related to differential GMV changes. More personal early-life stressful events were associated with larger developmental reductions in GMV over anterior prefrontal cortex, amygdala and other subcortical regions; whereas ongoing stress from the adolescents’ social environment was related to smaller reductions over the orbitofrontal and anterior cingulate cortex. These findings suggest that early-life stress accelerates pubertal development, whereas an adverse adolescent social environment disturbs brain maturation with potential mental health implications: delayed anterior cingulate maturation was associated with more antisocial traits – a juvenile precursor of psychopathy.
Introduction
Adolescence is a critical developmental stage during which a cascade of biological changes leads to profound structural modifications in the brain. The protracted maturation of human adolescents1,2 makes brain development particularly sensitive to ongoing environmental stressors3. Studies in clinical populations and animal models have also shown that neurodevelopmental trajectories are influenced by incubated effects of early-life stressors4,5. In this study we examined the effects of early-life stress, experienced from birth until 5 years of age, on brain developmental trajectories of healthy adolescents (14–17 years old), while also considering cerebral effects of ongoing stressors.
Cross-sectional studies have provided converging evidence for the effects of early-life and pubertal stress on developmental susceptibility of grey matter volume (GMV)6,7. However, inferences on developmental trajectories require longitudinal designs. Previous neurodevelopmental studies on the effects of early-life adversity have used longitudinal designs in (sub-) clinical cohorts8–10, but those studies cannot distinguish general developmental effects from cohort-specific effects. By discriminating between the effects of different types of stress, exerted at different developmental times, we characterize the stress susceptibility of grey matter maturation in a normative sample during the final window of pubertal plasticity11–13.
Stress activates the production of glucocorticoids that influence receptors distributed throughout the brain, with particularly high concentrations in the prefrontal cortex, hippocampus, and amygdala14,15. These stress-sensitive brain regions are particularly susceptible during adolescence, when hormonal stress sensitivity is enhanced16,17, leading to stress-related reductions in GMV18–21. One interpretation of volumetric reductions, particularly in animals and adults, has been linked to the toxic effects of glucocorticoids causing dendritic spine loss or even cell death3,22. However, recent studies have interpreted structural changes, particularly during adolescence, as accelerated maturation of neural circuits associated with emotional processing due to an evolutionary prioritization of adult-like functioning23. Brain volumetric changes may be the net result of differential effects of current stress and stress experienced early in life, different maturational profiles of prefrontal and limbic structures, and the interaction between stress occurrence and developmental state1,24–26. Beside timing of stress occurrence, the nature of the stressors might also diversely impact neurodevelopmental trajectories27. Here we consider two distinct stressor categories, that is personal negative life events (such as illness, parental divorce, etc.) and adverse social environments. For the latter, we account for the fact that children of different ages are predominantly sensitive to different social environments – the relationship with parents has a profound impact during early childhood28,29, whereas peer relationships become increasingly important during adolescence with poor relations forming a potent stress-factor in that time-window30,31.
This study disambiguates cerebral effects of early childhood events from current pubertal stress, evoked by personal and by social circumstances, on neurodevelopmental trajectories. Those trajectories are estimated from a structural index of brain development (GMV) measured between mid and late adolescence in 37 adolescents tested at 14 and 17 years of age (Fig. 1). We apply whole-brain statistical inferences. We expect that regions with a high distribution of glucocorticoid receptors will show stress-effects on brain development32, namely the prefrontal cortex, amygdala, and hippocampus. Finally, we consider the behavioral relevance of the longitudinal GMV changes observed in adolescents and focus on traits known to provide risk factors for the occurrence of psychopathology later in life, that is callous unemotional traits and internalizing symptoms4,33,34.
Figure 1.
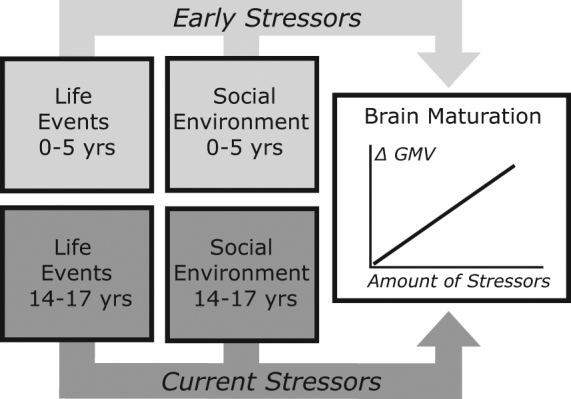
Model of early childhood and current adolescent factors influencing pubertal neural development. The amount of stressors, i.e. negative personal life events and social environment, affect the magnitude of change (positive or negative) in grey matter volume (GMV).
Results
Effects of early childhood stress
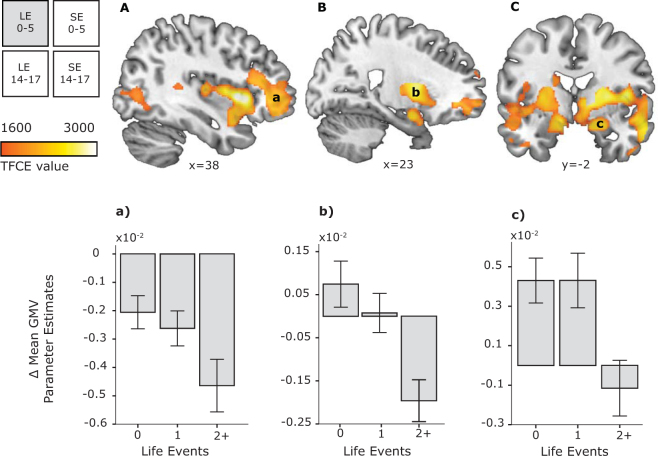
Changes in brain structure between ages 14 and 17 were significantly modulated by early-life events. Namely, adolescents who experienced more negative personal early-life events (before age 5) showed larger GMV decreases. These decreases occurred in subcortical structures such as the putamen, insula, caudate, and thalamus, as well as cortical areas spanning the prefrontal, frontal, posterior cingulate and temporal cortex (Fig. 2, Table 1). A similar direction, but different type of GMV changes occurred in the amygdala – more negative personal early-life events were associated with a lack of growth in this region between ages 14 and 17. Having experienced no and one negative personal life event was even associated with an increase in amygdala volume. These effects were not related to baseline differences at age 14 in respect to early-life events (see S2 in Supplementary Information). Variations in early social environment were not associated with significant GMV changes. (See S3 in Supplementary Information for model controlling scanner-type effects.) General developmental changes are addressed in Supplementary Information, Tables S3 and S4.
Figure 2.
Personal early-life events modulate grey matter volume (GMV) changes in the (A) prefrontal cortex, (B) insula, and (C) amygdala (statistical maps thresholded at TFCE PFWE < 0.05 overlaid on representative structural images). For visualization purposes, the number of adverse life events (LE) was split into three categories: 0, 1, 2+ (two or more) negative events. Graphs show parameter estimates of GMV change between ages 14 and 17. SE, social environment; TFCE, threshold-free cluster-enhancement; FWE, family-wise error; x and y indicate medio-lateral and antero-posterior location of the structural section in stereotactic space, respectively. Error bars represent +/− 1 SE.
Table 1.
Effects of Personal Early-Life Events on grey matter volume changes between age 14 and 17.
| Anatomical Region | Side | BA | K | x | y | z | PFWE | TFCE | Mean parameter estimates per category | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | +2 | |||||||||
| Middle frontal gyrus | L | 47/46 | 5490 | −26 | 46 | 9 | 0.022 | 2044 | −0.0021 | −0.0027 | −0.0049 |
| Frontal pole | L | 11 | −20 | 62 | −9 | 0.029 | 1852 | −0.0014 | −0.0021 | −0.0060 | |
| Superior frontal gyrus | L | 11 | −18 | 51 | 3 | 0.029 | 1837 | −0.0006 | −0.0012 | −0.0017 | |
| Posterior cingulate cortex | R | 23 | 1273 | 3 | −36 | 28 | 0.034 | 1746 | −0.0033 | −0.0042 | −0.0071 |
| L | 23 | −8 | −30 | 30 | 0.034 | 1722 | −0.0013 | −0.0015 | −0.0023 | ||
| Anterior insula | R | 48 | 29243 | 38 | 14 | 0 | 0.005 | 3006 | 0.0017 | −0.0005 | −0.0030 |
| Putamen | R | 26 | 2 | 8 | 0.008 | 2751 | 0.0008 | 0.0003 | −0.0021 | ||
| Insula | R | 48 | 44 | 0 | 8 | 0.008 | 2739 | −0.0006 | −0.0018 | −0.0036 | |
| Orbitofrontal cortex | R | 47 | 44 | 45 | −15 | 0.012 | 2459 | −0.0007 | −0.0017 | −0.0055 | |
| Amygdalae | R | 34 | 22 | −2 | −18 | 0.012 | 2429 | 0.0051 | 0.0047 | −0.0009 | |
| L | 34/25 | −12 | 3 | −14 | 0.012 | 2429 | 0.0024 | −0.0011 | −0.0031 | ||
| Medial parietal cortex | L | 7 | 1260 | −8 | −64 | 44 | 0.040 | 1648 | −0.0016 | −0.0032 | −0.0049 |
| Postcentral sulcus | L | 2 | −24 | −39 | 40 | 0.041 | 1632 | −0.0003 | −0.0004 | −0.0007 | |
| Medial parietal cortex | R | 7 | 3 | −68 | 39 | 0.042 | 1625 | −0.0029 | −0.0041 | −0.0058 | |
| Supramarginal gyrus | R | 48 | 42 | 50 | −39 | 32 | 0.049 | 1560 | −0.0019 | −0.0023 | −0.0039 |
| Superior temporal gyrus | R | 42 | 5 | 57 | −33 | 20 | 0.050 | 1551 | −0.0011 | −0.0003 | −0.0026 |
| Middle temporal gyrus | L | 37 | 260 | −45 | −58 | 9 | 0.044 | 1607 | −0.0018 | −0.0030 | −0.0047 |
| Middle occipital gyrus | L | 39 | −33 | −70 | 15 | 0.048 | 1570 | −0.0011 | −0.0009 | −0.0019 | |
| Inferior temporal gyrus | R | 20 | 14 | 56 | −14 | −34 | 0.047 | 1564 | −0.0036 | −0.0016 | −0.0053 |
| Middle occipital gyrus | R | 19 | 2111 | 39 | −72 | 0 | 0.032 | 1797 | −0.0004 | −0.0014 | −0.0025 |
| Inferior temporal gyrus | R | 37 | 52 | −66 | −6 | 0.037 | 1703 | −0.0032 | −0.0036 | −0.0062 | |
| Middle temporal gyrus | R | 21 | 50 | −46 | 12 | 0.037 | 1699 | −0.0037 | −0.0043 | −0.0056 | |
| Inferior occipital gyrus | L | 19 | 317 | −34 | −74 | −8 | 0.033 | 1749 | −0.0005 | −0.0016 | −0.0023 |
BA, Brodmann Area; K, number of voxels in a cluster; PFWE, combined peak-cluster level value; TFCE, threshold free cluster enhancement statistic; R, right; L, left. Note:Mean parameter estimates are split into categories of 0, 1, 2 or more early-life events for interpretational purposes. Table presents MNI coordinates of anatomically relevant markers of the cluster. Clusters have more than one local maxima, for a complete list see Supplementary Information Table S1.
Effects of current adolescent stress
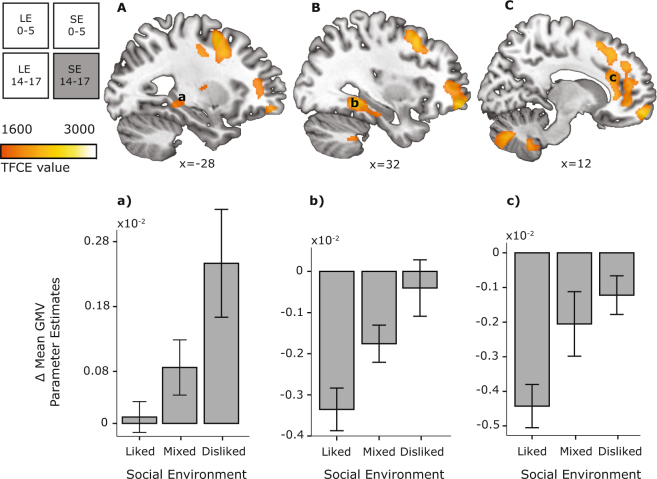
Negative personal life events during adolescence were not associated with significant modulations of grey matter maturation. However, variations in the peer environment were associated with GMV changes in the anterior cingulate, parahippocampus, and prefrontal cortex (Fig. 3, Table 2). Namely, adolescents disliked by their peers showed smaller GMV decreases in those cortical regions, and even an increased GMV in the hippocampus. (See S3 in Supplementary Information for model controlling scanner-type effects.) General developmental changes are addressed in Supplementary Information, Tables S3 and S4.
Figure 3.
Adolescent peer social environment modulates GMV changes in the (A) left hippocampus, (B) right parahippocampal gyrus, and (C) anterior cingulate cortex (TFCE PFWE < 0.05). For visualization purposes, adolescent peer environment was grouped into three categories: liked (>0.5 on social preference scale), mixed (0 to 0.5 on social preference scale), and disliked (<0 on social preference scale). Graphs show parameter estimates of GMV change between ages 14 and 17. Other conventions as in Fig. 2. Error bars represent +/− 1 SE.
Table 2.
Effects of Peer Environment on grey matter volume changes between age 14 and 17.
| Anatomical Region | Side | BA | K | x | y | z | PFWE | TFCE | Mean parameter estimates per category | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liked | Mixed | Disliked | |||||||||
| Orbitofrontal cortex | R | 11 | 14197 | 12 | 60 | −14 | 0.014 | 2350 | −0.0052 | −0.0022 | −0.00003 |
| Middle frontal gyrus | R | 9 | 39 | 10 | 50 | 0.016 | 2290 | −0.0041 | −0.0035 | −0.0004 | |
| Frontal pole | R | 11 | 28 | 56 | −12 | 0.016 | 2283 | −0.0057 | −0.0018 | 0.0004 | |
| Anterior cingulate cortex | R | 32 | 9 | 32 | 21 | 0.022 | 2024 | −0.0036 | −0.0023 | −0.0003 | |
| Middle frontal gyrus | L | 47 | 427 | −24 | 39 | 6 | 0.030 | 1845 | −0.0002 | −0.0001 | −0.00007 |
| L | 46 | −20 | 40 | 15 | 0.035 | 1760 | −0.001 | −0.0007 | −0.0004 | ||
| Inferior frontal gyrus/Middle frontal gyrus | R | 46 | 61 | 40 | 34 | 26 | 0.045 | 1648 | −0.0053 | −0.0034 | −0.0019 |
| Fusiform gyrus | R | 37 | 631 | 32 | −42 | −9 | 0.013 | 2446 | −0.0056 | −0.0027 | −0.0007 |
| Parahippocampal gyrus | R | 20 | 32 | −22 | −21 | 0.039 | 1727 | −0.0009 | 0.001 | 0.0032 | |
| Putamen | L | 26 | −28 | −10 | 8 | 0.048 | 1612 | −0.0008 | 0.00005 | −0.0001 | |
| Parahippocampal gyrus | L | 27 | 450 | −14 | −33 | −9 | 0.024 | 1976 | −0.0011 | 0.0004 | 0.0020 |
| Hippocampus/Parahippocampal gyrus | L | 37 | −26 | −36 | −8 | 0.035 | 1772 | −0.0001 | 0.0006 | 0.0026 | |
| Middle temporal gyrus | L | 37 | 237 | −58 | −66 | 8 | 0.028 | 1900 | −0.0046 | −0.002 | −0.001 |
| Middle temporal gyrus | L | 21 | 305 | −52 | −44 | 0 | 0.031 | 1840 | −0.0063 | −0.0038 | −0.002 |
| Medulla | L | 1093 | −2 | −48 | −63 | 0.029 | 1882 | 0.00003 | 0.001 | 0.0019 | |
| Cerebellum | R | 10 | −52 | −46 | 0.029 | 1877 | −0.0012 | 0.0005 | 0.0023 | ||
| Vermis | L/R | 1603 | 0 | −63 | −34 | 0.017 | 2236 | −0.0012 | 0.0012 | 0.0025 | |
| Cerebellum | R | 9 | −74 | −36 | 0.021 | 2087 | −0.0007 | 0.00003 | 0.0026 | ||
| Cerebellum | L | 631 | −14 | −84 | −34 | 0.032 | 1817 | −0.0002 | 0.0008 | 0.0029 | |
| Cerebellum | L | 12 | −9 | −50 | −46 | 0.049 | 1597 | −0.0008 | 0.0002 | 0.0013 | |
| Cerebellum | R | 8 | 20 | −22 | −30 | 0.049 | 1598 | 0.00008 | 0.0019 | 0.0026 | |
BA, Brodmann Area; K, number of voxels in a cluster; PFWE, combined peak-cluster level value; TFCE, threshold free cluster enhancement statistic; R, right; L, left. Note:Mean parameter estimates are split into liked (>0.5 on social preference scale), mixed (0 to 0.5 on social preference scale), and disliked (<0 on social preference scale) for interpretational purposes. Table presents MNI coordinates of anatomically relevant markers of the cluster. Clusters have more than one local maxima, for a complete list see Supplementary Information Table S2.
Interaction effects of early and current stressors
Two additional models tested for the interaction of early and current stressors as well as the effect of socioeconomic status (SES) on GMV changes described above. In the first model, the interaction of negative personal early-life events and the adolescent peer environment did not significantly modulate GMV, showing that early and current stress are independently related to neurodevelopmental maturation. In the second model, SES also did not significantly modulate GMV changes. All effects of early and adolescent stressors described previously remained the same in both models.
Behavioral relevance of longitudinal GMV changes in adolescents
We also assessed the behavioral relevance of longitudinal GMV changes observed in our adolescent sample. We explored whether volumetric changes in regions affected by early-life stress were related to the presence of internalizing symptoms and adolescent social stress to callous-unemotional traits. Correlational analysis between grey matter volume changes (controlled for early and current life events, early social environment, and gender) and callous-unemotional traits showed a positive relationship localized to the right anterior cingulate cortex (r = 0.39, p = 0.018, Bayes Factor = 6.05). Namely, a smaller developmental GMV decrease in the right anterior cingulate cortex was associated with the presence of more callous-unemotional traits (Fig. 4). The correlation between right anterior cingulate cortex GMV changes and callous-unemotional traits remained significant when controlling for adolescent social environment (r = 0.33, p = 0.048). This relationship was not present for the bilateral orbitofrontal cortex (r = 0.28, p = 0.091, Bayes Factor = 1.53). There were no significant associations between internalizing symptoms and developmental GMV changes (p > 0.09).
Figure 4.
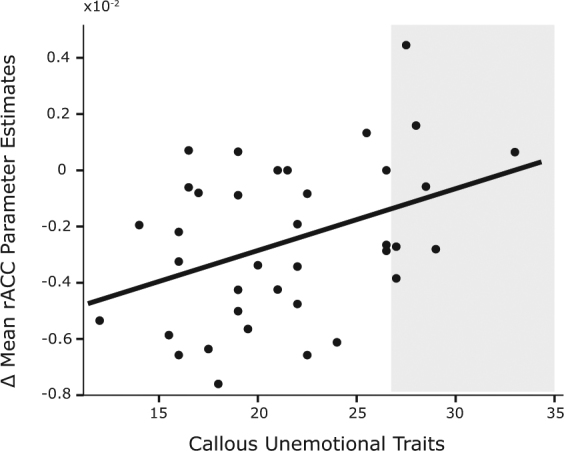
Scatterplot of the association between parameter estimates of GMV change in right anterior cingulate cortex and callous unemotional traits (r = 0.39). Each dot represents a participant (n = 37). Shaded area represents scores above the cutoff point for individuals at risk88.
Discussion
In this study, we tested the contribution of early childhood and current stress factors on brain maturation of adolescents between mid and late puberty, while differentiating between personal and social stress. Cerebral developmental trajectories were accelerated by early childhood personal stress, and delayed or disrupted by current social stress. Namely, the maturational decrease in GMV over the anterior prefrontal cortex, amygdala, putamen and insula was stronger in those adolescents that experienced negative personal life events during early childhood. In contrast, the maturational decrease in GMV over the anterior cingulate cortex, the parahippocampal gyrus, and the prefrontal cortex was smaller in those adolescents that experience ongoing social stress. Early and current stress were independently related to GMV changes and did not have a cumulative effect on neurodevelopmental maturational profiles. Furthermore, stress-related modulations of the developmental trajectory of the anterior cingulate cortex were already behaviorally relevant, accounting for a significant portion of variance in the adolescents’ antisocial traits.
These observations provide the first empirical evidence that, in typically developing children, even moderate early-life stress can incubate long-lasting effects, leading to increased neural pruning during puberty. The effects of early-life stress are functionally and spatially distinct from current social stress, which modulates neurodevelopmental trajectories in the opposite direction, and over different neural structures. This study also suggests that the neurodevelopmental effects of ongoing social stress may be related to adolescents’ behavioral traits. These findings qualify how pubertal neural plasticity depends on a combination of type and timing of the stressors experienced by a child.
This study shows that negative personal early-life events are associated with larger reductions in subcortical and prefrontal GMV, in line with findings from sub-clinical cohorts of adolescents dealing with severe traumatic events19,21,35,36. Elaborating on previous evidence indicating how childhood maltreatment flattens the growth of the amygdala between early to mid-adolescence10, here we show that the trajectory of amygdala development continues to be impacted by early-life stress during the second half of puberty. Namely, even moderate negative personal events occurring early in life, such as illness, bias pubertal neurodevelopmental patterns. This bias consists of an increased reduction of grey matter volume, within a prefrontal-amygdala circuit known to control emotional reactivity6,37. The direction and location of these findings fit with the notion that early-life stress leads to faster pubertal brain maturation23,38, possibly as a consequence of accelerated synaptic pruning1,39. Rodent models have shown that early-life stressors alter the regulation of glucocorticoids and hypothalamic corticotropin-releasing factor (CRF), leading to long-term hypothalamic-pituitary-adrenal axis disturbances40. Early-life stress may also prematurely activate structures of the emotion regulation circuit, fixating the brain into an adult-like configuration41 with precocious myelination of amygdala axons42 and earlier emergence of adult-like long term potentiation (LTP)43.
Early maturation may be the outcome of an adaptive mechanism at a time of heightened stress23. However, it might also prevent the brain from adjusting to the current environment by means of the developmental plasticity usually afforded by adolescence, leading to later costs for mental and physical health44. Dendritic spine density in the prefrontal cortex is strongly influenced by stress-related modulations of the noradrenergic system, and of the GABAergic system within the basolateral amygdala and the hippocampus45,46. In mice, deviations in dendritic spine density induced during puberty are detrimental for optimal cognition in adulthood47,48. The present findings fit with those neurobiological observations and open the way to test whether the GMV changes reported here are driven by structural neuronal changes49,50.
In adults, acute stress decreases GMV25,51,52. In adolescents, the effects of recent stress on GMV are less clear. While animal models report GMV decreases in the frontal cortex and hippocampus16, the handful of existing human studies point to GMV increases in these same regions in children and adolescents suffering from PTSD53,54. GMV increases have also been reported in the anterior cingulate cortex, parahippocampal gyrus, and temporal cortex following recent or perceived stress in adolescents and young adults55,56; as well as inferior temporal gyrus increases related to social rejection sensitivity in the latter group57. Here we add to those findings by showing that, in adolescents, negative peer environment leads to both increased hippocampal GMV as well as a lack of GMV reduction in anterior cingulate cortex and prefrontal cortex. These observations suggest that stress during adolescence delays or disrupts the physiological reduction of GMV previously reported in cortical structures1,58.
This study suggests that the effects of current social stress on cingulate development might already influence the emergence of adolescents’ antisocial traits. This observation confirms, on a longitudinal scale, previous cross-sectional studies in children and adolescents reporting an increase in prefrontal GMV in relation to conduct problems and callous unemotional traits59,60. A meta-analysis in adults similarly identified increased cingulate gyrus volume as a neural correlate of antisocial behavior61. The anterior cingulate gyrus is involved in the control of cognitive and emotional behavior and through its links to the amygdala, involved in affective processing and empathy62,63. The delayed or disrupted structural maturation of this region may partially explain the deficiencies in social behavior, especially empathy, observed in those participants with callous unemotional traits. This in turn may be related to the risk of developing psychopathy later in life33,64.
This study benefits from the strengths of a longitudinal design allowing for reliable and accurate tests of developmental changes. Our findings indicate that experiencing mildly stressful events early in life can already change neural maturation in puberty. In turn, these findings may help to characterize the developmental processes evoked by traumatic experiences and related emotional problems. However, there are some limitations that should be considered. It might be argued that the reliability of those inferences is limited by the moderate sample size of this study (n = 37). However, that limitation on sensitivity should be weighed against the specificity afforded by the accurate characterization of the developmental profile of each participant from birth until 17 years, including neuro-developmental trajectories during puberty.
The findings of this study relate increased GMV reductions to the exposure of early-life stress. This is in contrast to a few adolescent cross-sectional or between-group studies reporting amygdalar or hippocampal GMV increases or null effects9,54,65,66. Inconsistencies in the field may be related to stressor-type or measurement period27. For example, it has been suggested that early enlargement of the amygdala may occur in response to adversity, later followed by premature volume reduction26. We also did not find associations between early-life induced GMV changes and internalizing symptoms while other studies have related structural changes in the prefrontal cortex, ACC and amygdala to internalizing problems4,67,68. This may be related to the fact that in this study, we assess developmental trajectories in contrast to generally reported end-point group difference measures. Finally, since increased hippocampal volume related to negative peer environment was particularly affected by measurement on different scanners in our sample, it needs to be replicated in future longitudinal studies and treated tentatively. The longitudinal design of this study, coupled with its focus on healthy children, distinguishes genuine developmental effects from incidental cohort differences. Future follow-up studies might be able to address why some adolescents develop stress susceptibility while others become stress resilient69.
Conclusions
These findings suggest that brain maturation between mid and late adolescence is particularly sensitive to adverse personal events early in life and to adverse social events during adolescence. Increased grey matter reduction in the prefrontal cortex and several subcortical regions was associated with negative personal early-life events. This observation is consistent with the idea that early-life stress accelerates pubertal development. In contrast, brain maturation was disrupted by the effect of concurrent adolescent social stress. This suggests that both early as well as later stressors can bias neurodevelopmental trajectories, which in turn may affect mental health outcomes. Having defined the relative contribution of time-delineated stressors on the maturation of neural circuits during adolescence, this study opens the way to understanding stress susceptibility and resilience later in adulthood.
Materials and Methods
Participants
All actively participating children from the Nijmegen Longitudinal Study on Child and Infant Development (n = 116) were approached to take part in this imaging study. Anatomical scans were obtained from participants at 14 (M = 14.6, SD = 0.17) and 17 years of age (M = 17.09, SD = 0.15). Forty-nine at the first imaging time-point and ninety-six at the second imaging time-point agreed to participate. Participants who could not undergo magnetic resonance imaging (MRI) or who had missing data at one of the two time-points were excluded from these analyses. The final sample consisted of 37 adolescents (15 boys). Participants did not have a history of psychiatric disorders or neurological illness (as indicated by parent/guardian report). Table 3 presents the characteristics of the sample. Written informed consent was obtained from parents and participants during each measurement wave. The study was approved by the local ethics committee (CMO region Arnhem – Nijmegen) and was conducted in compliance with these guidelines.
Table 3.
Sample characteristics.
| Age (years) | Mean (SD) | Min/Max | |
|---|---|---|---|
| Pubertal Development | |||
| Testosterone levels [pg/ml] | 14 | Boys: 42.67 (34.23); Girls: 11.08 (6.52) |
4.4/149.4; 1.7/26.54 |
| 17 | Boys: 146.43 (73.99); Girls: 23.93 (12.27) |
54.06/296.69; 9.10/51.11 |
|
| PDS | 14 | Boys: 2.38 (0.33) Girls: 2.89 (0.38)^ |
2/3.2; 2/3.4 |
| 17 | Boys: 3.57 (0.38); Girls: 3.40 (0.41) |
2.75/4; 2.5/4 |
|
| Cognitive Functioning | |||
| Bayley cognitive development | 1.25 | 108.73 (14.498) | 71/137 |
| Peabody verbal ability | 5 | 111.43 (16.227) | 82/136 |
| Academic performance TR | 16 | 4.82 (1.36)^ | 1/7 |
| Learning progress TR | 16 | 4.37 (1.19)^^ | 2/7 |
| Adequate school behavior TR | 16 | 4.84 (1.22)^^^ | 2/6 |
| SES During Childhood | |||
| Education mother | 1.25 | 5.24 (1.66) | 2/7 |
| Education father | 1.25 | 5.27 (1.68) | 2/7 |
| Work mother | 1.25 | 3.24 (2.06) | 0/6 |
| Work father | 1.25 | 3.81 (1.49) | 0/6 |
| Measures of Interest | |||
| Personal early-life events | 0–5 | 1.43 (1.19) | 0/4 |
| Parent-child interaction scores | 0–5 | −0.06 (3.05) | −7.86/4.61 |
| Personal current life events | 14–17 | 1 (1.05) | 0/4 |
| Peer ratings | 16 | 0.15 (0.76) | −2.18/1.33 |
| Internalizing symptoms [CBCL] | 17 | raw scores: 5.51 (5.71); T scores: 50 (9.94) |
0/28; 33/75 |
| Callous unemotional traits [ICU] | 17 | 21.46 (4.94) | 12/33 |
PDS, Pubertal Development Scale; SES, social economic status; TR, teacher report, based on a 7-point scale (S1 in Supplementary Information provides details on Cognitive Functioning measures); CBCL, Child Behaviour Checklist; ICU, Inventory of Callous Unemotional Traits. ^n = 34; ^^n = 30; ^^^n = 32. Note: There were no significant correlations (p < 0.05) of SES with any early-life or current stressors, pubertal development (testosterone values at age 14 and 17), nor symptomatology (internalizing symptoms, ICU). The restricted range of SES scores in this sample is fairly representative of the Dutch population of families with children in the same age range (for more information on the wider NLS sample and SES see75). Concerning associations between cognitive functioning and stress, childhood IQ scores (Peabody) at age 5 were not significantly correlated with early-life stressors assessed up until this age (amount of negative personal events [r = −0.01, p = 0.953]; parent-child interaction quality [r = 0.165, p = 0.33]). The three indices of cognitive functioning during adolescence were also not correlated with either early or later stressors (p > 0.05). The amount of early-life events was moderately correlated with current events (r = 0.332, p = 0.045).
Life events
The experience of early-life events (before age 5) and current life events (between age 14 and 17) were assessed via parent report. All life event reports were collected within one to two years after the event had taken place. This meant that for early-life events, reports were taken at 15 months, 28 months, and 5 years. For current adolescent life events, the report was taken at age 17 for reports until age 14. The life events questionnaire consisted of items selected from Sarason, Johnson, and Siegel’s Life Experiences Survey70 and Coddington’s Life Events Scale for Children71 based on the likelihood they would have an aversive influence on the child’s development72. Both measures have been widely used in international research73,74. The life events questionnaire remained the same at all measurement times and has previously been used in this longitudinal study75,76. Items require a ‘yes’ or ‘no’ response. The score represents the total number of negative personal life events in the given assessment period and was calculated for events that took place until early childhood (until age 5) and during late adolescence (i.e., between ages 14 and 17).
Social environment
We used age-relevant measures of the individual’s social environment (SE). The quality of parent-child interactions was used as an index of early SE. Poor parent-child interaction quality has previously been shown to be related to elevated childhood cortisol levels in the NLS cohort29. Parent-child interactions were assessed at 15 months, 28 months, and 5 years of age during a home visit29,75. Video recordings of these interactions were rated by four trained observers on five 7-point scales: Supportive Presence, Respect for Child’s Autonomy, Structure and Limit Setting, Quality Instruction, and Hostility. An average score for SE before age 5 was taken across all scales (with reversed coding for hostility scores) and time-points for each child77. In case of a missing assessment (n = 2 cases) the average was computed based on the two remaining time points.
Peer environment (social preference) between age 14 and 17 was assessed in the classroom with a well-established sociometric measure previously used in this cohort78,79. Children were asked to nominate classmates who they liked (“Who do you like the most?”) and disliked (“Who do you like the least?”). Students were asked to nominate at least one classmate, excluding self-nominations. There was no maximum number of nominations. For each question, the number of nominations that a child received was counted and standardized within the classroom, to control for differences in classroom size. A score for social preference was calculated by subtracting the liked least from the liked most score. This difference score was again standardized within classrooms.
Socioeconomic Status
SES scores were computed based on education (7-point scale) and occupation (6-point scale) levels for both parents in line with previous reports on this cohort75. The levels of education and occupation for the two parents were first standardized and then summed to create a single score per parent. The final SES score was derived by taking the average score of the mother and father.
Behavioral measures of psychopathology
Internalizing symptoms at age 17 were measured using the Child Behaviour Checklist (CBCL)80. The CBCL is a parent-report questionnaire used to assess the frequency of emotional and behavioral problems exhibited by the adolescent in the past six months. The parent rated each behavior or symptom on a three-point Likert scale (not true, somewhat or sometimes true, very true or often true). Items from the scales anxious/depressive, withdrawn/depressive, and somatic complaints were summed to provide a score for internalizing symptoms.
Specific aspects of socialization during adolescence was measured with the Inventory of Callous Unemotional Traits 81. Self-report and parent-report versions of the questionnaire were used to assess the occurrence and intensity of affective features of callousness such as lack of empathy, disregard for others, and shallow affect. It consisted of 24 items scored on a four-point Likert scale (not at all true, somewhat true, very true, definitely true). The self-report and parent-report versions were significantly correlated with each other (r = 0.42, p = 0.013). A mean score was created from both versions to increase consistency of the measure. For two participants with missing data (1 self-report, 1 parent-report) the available score was used for further analysis.
The statistical threshold for correlations of GMV with psychopathology measures were set to p < 0.025 (multiple correction for number of regions tested for each measure).
Imaging parameters
Structural T1 images were acquired at 3 Tesla using Siemens MAGNETOM Trio or PRISMA systems (acquired at the same site; 18 participants at age 17) with a 32-channel coil. Images were acquired using the same MPRAGE sequence (TR = 2300 ms; TE = 3.03 ms; 192 sagittal slices; 1.0 × 1.0 × 1.0 mm voxels; FOV = 256 mm). To ensure that there were no differences in the quality of T1 images acquired on the TRIO and PRISMA scanners at age 17, these normalized and smoothed GM images were checked using the “Check sample homogeneity” function in CAT12 (Computation Anatomy Toolbox). One participant was identified as a potential outlier for manual inspection. After manually checking the data for artefacts, it was included in the analyses.
Voxel Based Morphometry
Magnetic resonance images were processed using the Matlab toolbox SPM12 [Statistical Parametric Mapping (www.fil.ion.ucl.uk/spm)]. Each MR image was checked for artifacts or anatomical abnormalities and alignment to the anterior commissure. Using a pairwise longitudinal registration approach82 a Jacobian difference map was generated as well as a “halfway space” image, which was subsequently segmented into white matter, grey matter (GM), and cerebrospinal fluid (CSF). Diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) was used for inter-subject registration of the GM “halfway” images to a group average template image83. The GM “halfway” image was multiplied by the Jacobian difference map for each participant. This subsequent GM difference map was transformed and resampled at an isotropic voxel size of 1.5 mm, resulting in spatially normalized, Jacobian scaled, and smoothed (8 mm FWHM Gausian kernel) images in Montreal Neurological Institute (MNI) space. Data quality of normalized and smoothed difference images was checked with CAT12 using the “Check sample homogeneity” function. This function did not indicate any potential outliers - based on a mean correlation of the sample below 2 standard deviations. The GM images were entered into a multiple regression analysis with standardized scores of life events and social environment as early (0–5 years) and current (14–17 years) stressors entered as covariates. Gender and average (age 14 and 17) grey and white matter total brain volume (TBV) were entered as covariates of no interest. To minimize boundary effects, a binary mask of the group template was used to exclude voxels outside of the brain. Statistical significance was assessed using non-parametric permutation tests using the Threshold Free Cluster Enhancement (TFCE) Toolbox in SPM12 (Version 90; http://dbm.neuro.uni-jena.de/tfce/) with 5000 permutations. After TFCE, the statistical threshold was set to p < 0.05 adjusted for family wise error at a whole brain level. TFCE suppresses random noise that may have a similar intensity as the real signal, but lacks spatial continuity (smoothness). The TFCE values at each voxel represent a combination of spatially distributed cluster size and height information. In other words, the TFCE statistic summarizes the cluster-wise evidence at each voxel. There is no initial threshold for voxel level inference. Statistical inference is based on the distribution of TFCE values - derived from the non-parametric permutations. This type of approach is particularly beneficial for VBM data84,85. Anatomical inference was drawn by superimposing images on a standard SPM single-subject T1 template, the group-specific average template (created in DARTEL), and subject-specific T1 scans standardized in MNI space.
Behavioral relevance of longitudinal GMV changes
To relate the longitudinal GMV changes observed in adolescents to psychopathology, that is callous unemotional traits and internalizing symptoms, GMV changes were extracted from the relevant significant clusters. To achieve anatomical specificity, an overlap was taken between the significant cluster and the brain area, based on the Automated Anatomical Labeling (AAL) Atlas86. As such, the grey matter estimates reflected only the significant changes in an anatomically defined area. To test the association between adolescent-social-stress-related volumetric changes and callous unemotional traits, we identified regions significantly modulated by adolescent social stress that have previously been also identified as part of the callous unemotional neuro-profile in adolescent samples59,60,87, namely the anterior cingulate cortex (only the right hemisphere in our study) and bilateral orbital frontal cortex. Parameter estimates of grey matter volume changes of these two regions were entered into SPSS and JASP for correlational analysis.
To test the association between early-life stress-induced volumetric changes and internalizing symptomology, we identified regions modulated by negative personal early-life events that have previously been suggested as developmental targets for internalizing disorders, namely the amygdala-prefrontal circuit and anterior cingulate cortex4,67,68. GMV changes were extracted from these relevant clusters and analyzed for associations with internalizing symptoms, following the same procedure described for callous unemotional traits.
Finally, two post-hoc analyses were conducted to rule out the effects of additional stressors. The first model included standardized SES scores as an additional regressor in the previously described multiple regression analysis. The second model explored the interaction between early and current stressors. The standardized interaction scores of personal early-life events and adolescent peer environment (the two significant predictors of GMV changes) were entered into the original multiple regression analysis. For both analyses, all other parameters were kept the same as in the original model.
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
Code Availability
The code used to analyze the data is available from the corresponding author upon request.
Electronic supplementary material
Acknowledgements
This work was supported by a European Research Council starting grant (ERC_StG2012_313749 awarded to KR) and a FP7-HEALTH-2013-INNOVATION grant (602805-2). The Netherlands Organisation for Scientific Research supported IT (Vici grant 453-08-002), HCMN (Research Talent Grant 406-13-022), and JLP (Research Talent Grant 406-12-110). IV was supported by a Marie Curie Individual Fellowship (MSCA-IF-2014 EF 660397) within the European Union’s Horizon 2020 Framework Programme. The authors would like to thank all the families participating in the Nijmegen Longitudinal Study who made this research possible.
Author Contributions
A.T., I.V., H.C.M.N., J.L.P., S.S., A.H.N.C., I.T., K.R. were involved in the design of the study. Data was collected by A.T., H.C.M.N., S.S., J.L.P. Data analysis was performed by A.T. Observational and sociometric data was prepared by S.S. and J.L.P. (respectively). The manuscript was written by A.T., I.V., K.R., I.T. with comments and edits from the other authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Ivan Toni and Karin Roelofs jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27439-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giedd JN, et al. Puberty-related influences on brain development. Mol. Cell. Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Tamnes CK, et al. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 4.Fareri DS, Tottenham N. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb, B., Harker, A., Mychasiuk, R., de Melo, S. R. & Gibb, R. Stress and prefrontal cortical plasticity in the developing brain. Cogn. Dev. 1–12, 10.1016/j.cogdev.2017.01.001 (2017).
- 6.Cohen MM, Tottenham N, Casey BJ. Translational developmental studies of stress on brain and behavior: Implications for adolescent mental health and illness? Neuroscience. 2013;249:53–62. doi: 10.1016/j.neuroscience.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrion VG, Wong SS. Can traumatic stress alter the brain? Understanding the implications of early trauma on brain development and learning. J. Adolesc. Heal. 2012;51:S23–S28. doi: 10.1016/j.jadohealth.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 9.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry. 2001;50:305–309. doi: 10.1016/S0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 10.Whittle S, et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:940–953. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Herting MM, et al. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Hum. Brain Mapp. 2014;35:5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TV, et al. Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex. 2013;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koolschijn PCMP, Peper JS, Crone EA. The influence of sex steroids on structural brain maturation in adolescence. PLoS One. 2014;9:e83929. doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziedzic N, Ho A, Adabi B, Foilb AR, Romeo RD. Shifts in hormonal stress reactivity during adolescence are not associated with changes in glucocorticoid receptor levels in the brain and pituitary of male rats. Dev. Neurosci. 2014;36:261–268. doi: 10.1159/000362873. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 16.Romeo RD. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res. 2017;1654(Pt B):185–191. doi: 10.1016/j.brainres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 17.McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.De Brito SA, et al. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J. Child Psychol. Psychiatry Allied Discip. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- 19.Hodel AS, et al. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks SJ, et al. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metab. Brain Dis. 2014;29:311–321. doi: 10.1007/s11011-014-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson JL, et al. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol. Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 23.Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol. Psychiatry. 2013;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn, M. et al. Mismatch or allostatic load? Timing of life-adversity differentially shapes gray matter volume and anxious-temperament. Soc. Cogn. Affect. Neurosci. 537–547, 10.1093/scan/nsv137 (2015). [DOI] [PMC free article] [PubMed]
- 26.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 28.Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeekens S, Riksen-Walraven JM, van Bakel HJA. Cortisol reactions in five-year-olds to parent-child interaction: The moderating role of ego-resiliency. J. Child Psychol. Psychiatry Allied Discip. 2007;48:649–656. doi: 10.1111/j.1469-7610.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg L, Morris AS. Adolescent development. Annu. Rev. Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc. Natl. Acad. Sci. 2013;110:117180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frick PJ, White SF. Research Review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 34.Forbes MK, Tackett JL, Markon KE, Krueger RF. Beyond comorbidity: Toward a dimensional and hierarchical approach to understanding psychopathology across the life span. Dev. Psychopathol. 2016;28:971–986. doi: 10.1017/S0954579416000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. USA. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edmiston EEE, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med. 2011;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyborowska A, Volman I, Smeekens S, Toni I, Roelofs K. Testosterone during puberty shifts emotional control from pulvinar to anterior prefrontal cortex. J. Neurosci. 2016;36:6156–6164. doi: 10.1523/JNEUROSCI.3874-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.2307/1131166. [DOI] [PubMed] [Google Scholar]
- 39.Tamnes CK, et al. Development of the cerebral cortex across adolescence: A multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. J. Neurosci. 2017;37:3302–16. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rincon-Cortes M, Sullivan RM. Early life trauma and attachment: Immediate and enduring effects on neurobehavioral and stress axis development. Front. Endocrinol. (Lausanne). 2014;5:1–15. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee DG, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. USA. 2013;110:15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono M, et al. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156:1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 43.Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res. Mol. Brain Res. 2003;115:55–62. doi: 10.1016/S0169-328X(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 44.Belsky, J. & Shalev, I. Contextual adversity, telomere erosion, pubertal development, and health: Two models of accelerated aging, or one? Dev. Psychopathol. 1–17 (2016). [DOI] [PubMed]
- 45.Albrecht A, et al. Neurobiological consequences of juvenile stress: A GABAergic perspective on risk and resilience. Neurosci. Biobehav. Rev. 2017;74:21–43. doi: 10.1016/j.neubiorev.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afroz, S., Shen, H. & Smith, S. S. α4βδ GABAA receptors reduce dendritic spine density In CA1 hippocampus and impair relearning ability Of adolescent female mice: Effects of a GABA agonist and a stress steroid. Neuroscience (2017). [DOI] [PMC free article] [PubMed]
- 48.Afroz S, Parato J, Shen H, Smith SS. Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines. Elife. 2016;5:1–23. doi: 10.7554/eLife.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddock, R. J. & Buonocore, Michael, H. MR spectroscopic studies of the brain in psychiatric disorders. Curr. Top. Behav. Neurosci. 199–251, 10.1007/7854_2011_197 (2012). [DOI] [PubMed]
- 51.Papagni SA, et al. Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress. 2011;14:227–32. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, et al. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrion VG, et al. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: An MRI study. Psychiatry Res. - Neuroimaging. 2009;172:226–234. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Walsh ND, et al. General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. NeuroImage Clin. 2014;4:308–318. doi: 10.1016/j.nicl.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, et al. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage. 2014;92:1–7. doi: 10.1016/j.neuroimage.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, et al. Regional gray matter volume is associated with rejection sensitivity: A voxel-based morphometry study. Cogn. Affect. Behav. Neurosci. 2014;14:1077–1085. doi: 10.3758/s13415-014-0249-z. [DOI] [PubMed] [Google Scholar]
- 58.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/S0012162201001591. [DOI] [PubMed] [Google Scholar]
- 59.De Brito SA, et al. Size matters: Increased grey matter in boys with conduct problems and callousunemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 60.Fairchild G, et al. Brain structure abnormalities in adolescent girls with conduct disorder. J. Child Psychol. Psychiatry Allied Discip. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoki Y, Inokuchi R, Nakao T, Yamasue H. Neural bases of antisocial behavior: A voxel-based meta-analysis. Soc. Cogn. Affect. Neurosci. 2014;9:1223–1231. doi: 10.1093/scan/nst104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 63.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blair RJR, Peschardt KS, Budhani S, Mitchell DGV, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:262–275. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 65.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian adoptees study pilot. J. Child Psychol. Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 66.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herringa RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. USA. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hostinar CE, Gunnar MR. The developmental effects of early life stress: An overview of current theoretical frameworks. Curr. Dir. Psychol. Sci. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J. Consult. Clin. Psychol. 1978;46:932–946. doi: 10.1037/0022-006X.46.5.932. [DOI] [PubMed] [Google Scholar]
- 71.Coddington R. The significance of life events as etiological factors in the diseases of children. J. Psychosom. Res. 1972;16:7–18. doi: 10.1016/0022-3999(72)90018-9. [DOI] [PubMed] [Google Scholar]
- 72.Gersten JC, Langner TS, Eisenberg JG, Simcha-Fagan O. An evaluation of the etiologic role of stressful life-change events in psychological disorders. J. Health Soc. Behav. 1977;18:228–244. doi: 10.2307/2136351. [DOI] [PubMed] [Google Scholar]
- 73.Abela JR. The hopelessness theory of depression: A test of the diathesis-stress and causal mediation components in third and seventh grade children. J. Abnorm. Child Psychol. 2001;29:241–54. doi: 10.1023/A:1010333815728. [DOI] [PubMed] [Google Scholar]
- 74.Johnston C. Parent characteristics and parent-child interactions in families of nonproblem children and ADHD children with higher and lower levels of oppositional-defiant behavior. J. Abnorm. Child Psychol. 1996;24:85–104. doi: 10.1007/BF01448375. [DOI] [PubMed] [Google Scholar]
- 75.Smeekens S, Riksen-Walraven JM, Van Bakel HJA. Multiple determinants of externalizing behavior in 5-year-olds: A longitudinal model. J. Abnorm. Child Psychol. 2007;35:347–361. doi: 10.1007/s10802-006-9095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niermann HCM, et al. Infant attachment predicts bodily freezing in adolescence: Evidence from a prospective longitudinal study. Front. Behav. Neurosci. 2015;9:1–10. doi: 10.3389/fnbeh.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erickson M, Sroufe LA, Egeland B. The relationship between quality of attachment and behavior problems in preschool in a high-risk sample. Monogr. Soc. Res. Child Dev. 1985;50:147–166. doi: 10.2307/3333831. [DOI] [PubMed] [Google Scholar]
- 78.Pouwels, J. L. et al. Predicting adolescents’ bullying participation from developmental trajectories of social status and behavior. Child Dev. 1–20, 10.1111/cdev.12794 (2017). [DOI] [PubMed]
- 79.Pouwels JL, Lansu TAM, Cillessen AHN. Participant roles of bullying in adolescence: Status characteristics, social behavior, and assignment criteria. Aggress. Behav. 2016;42:239–253. doi: 10.1002/ab.21614. [DOI] [PubMed] [Google Scholar]
- 80.Achenbach, T. M. Manual for the child behavior checklist/4-18 and 1991 profile. University of Vermont, Department of Psychiatry (1991).
- 81.Frick, P. J. The Inventory of Callous-Unemotional Traits. Unpublished rating scale. The University of New Orleans (2003).
- 82.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 2013;6:1–9. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Nickerson LD, Nichols TE, Gao JH. Comparison of a non-stationary voxelation-corrected cluster-size test with TFCE for group-level MRI inference. Hum. Brain Mapp. 2017;38:1269–1280. doi: 10.1002/hbm.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 86.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 87.Sebastian CL, et al. Grey matter volumes in children with conduct problems and varying levels of callous-unemotional traits. J. Abnorm. Child Psychol. 2016;44:639–649. doi: 10.1007/s10802-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Docherty M, Boxer P, Huesmann LR, O’Brien M, Bushman B. Assessing callous-unemotional traits in adolescents: Determining cutoff scores for the Inventory of Callous and Unemotional Traits. J. Clin. Psychol. 2017;73:257–278. doi: 10.1002/jclp.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.