Abstract
Objectives
Recently, the density score of coronary artery calcium (CAC) has been shown to be associated with a lower risk of cardiovascular disease (CVD) events at any level of CAC volume. Whether risk factors for CAC volume and CAC density are similar or distinct is unknown. We sought to evaluate the associations of CVD risk factors with CAC volume and CAC density scores.
Methods
Baseline measurements from 6,814 participants free of clinical CVD were collected for the Multi-Ethnic Study of Atherosclerosis. Participants with detectable CAC (n=3,398) were evaluated for this study. Multivariable linear regression models were used to evaluate independent associations of CVD risk factors with CAC volume and CAC density scores.
Results
Whereas most CVD risk factors were associated with higher CAC volume scores, many risk factors were associated with lower CAC density scores. For example, diabetes was associated with a higher natural logarithm (ln) transformed CAC volume score [standardized β= 0.44 (95% confidence interval 0.31, 0.58) ln-units] but a lower CAC density score [β= −0.07 (−0.12, −0.02) density units]. Chinese, African-American, and Hispanic race/ethnicity were each associated with lower ln CAC volume scores [β= −0.62 (−0.83, −0.41), −0.52 (−0.64, −0.39), and −0.40 (−0.55, −0.26) ln-units, respectively], and higher CAC density scores [β= 0.41 (0.34, 0.47), 0.18 (0.12, 0.23), and 0.21 (0.15, 0.26) density units, respectively] relative to Non-Hispanic White.
Conclusions
In a cohort free of clinical CVD, CVD risk factors are differentially associated with CAC volume and density scores, with many CVD risk factors inversely associated with the CAC density score after controlling for the CAC volume score. These findings suggest complex associations between CVD risk factors and these components of CAC.
Introduction
As a marker of underlying coronary artery atherosclerosis, CAC has been observed to be a strongly associated with atherosclerotic CVD risk.1 The predominant metric used to quantify CAC is the Agatston score, which is comprised of the two-dimensional area of CAC and a four-point multiplicative factor based on the maximum density within each plaque.2 Thus, both a greater area of CAC and a higher density of CAC will increase the Agatston score.
However, in a prior study from the Multi-Ethnic Study of Atherosclerosis, a higher average CAC density score was found to be associated with a lower risk of CVD events when adjusted for the CAC volume score.3 This finding is consistent with studies in the literature that have found sparsely calcified atherosclerotic plaques to more frequently result in coronary events compared to heavily calcified plaques.4–6 Taken together, these findings suggest that a higher density of CAC may be associated with lower, rather than higher, CVD risk.3
These findings also highlight the need to evaluate the associations of CVD risk factors with CAC separated into its components of volume and density. Risk factors for CVD have previously been linked to CAC Agatston scores,7, 8 yet the associations of CVD risk factors with CAC volume and density scores are unknown. Given the apparently opposite associations of CAC volume and density with CVD events, investigating determinants of these CAC components may yield further insight into the relationship between CAC and CVD. Therefore, we aimed to elucidate the associations of CVD risk factors with CAC volume and density scores to further investigate the inverse association of CAC density with CVD risk.
Methods
MESA Study Design
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study started in July 2000 designed to evaluate subclinical CVD in a multi-ethnic cohort. The detailed study design has previously been reported.9 Briefly, community-dwelling individuals aged 45–84 years were recruited from six study sites: Baltimore, MD; Chicago, IL; Winston-Salem, NC; Los Angeles, CA; New York, NY; and St. Paul, MN. The study population had an approximate ethnic composition of 38% Non-Hispanic White (NHW), 28% African-American, 23% Hispanic, and 11% Asian (predominantly Chinese). Participants with any history of clinically apparent CVD or major obstacles to follow-up were excluded. Measurements at the first examination between July 2000 and July 2002 included a participant questionnaire, collection of fasting blood samples, and cardiac CT imaging. The institutional review boards of the six study centers have each approved the study protocol. Written informed consent was obtained from all participants.
Computed Tomography
Cardiac CT scans were performed using either a cardiac-gated electron-beam CT (EBCT) scanner at the Chicago, Los Angeles, and New York sites (Imatron C-150; Imatron, South San Francisco, CA) and an electrocardiogram-triggered multidetector CT (MDCT) system at the Baltimore, Winston-Salem, and St. Paul sites (Lightspeed, General Electric Medical Systems, Waukesha, WI or Volume Zoom, Siemens, Erlangen, Germany). All scans included a phantom of known calcium concentration for calibration. A minimum of 35 contiguous tomographic slices were obtained. Duplicate scans were performed consecutively (within 15 minutes) for each participant and read centrally at the MESA CT Reading Center using custom software developed to measure calcified plaque for this study.10
Coronary Artery Calcium Scoring
The CAC scoring protocol has been described in detail previously.10 A calcified plaque was defined as any area greater than 5.5 mm3 (EBCT) or 4.6 mm3 (MDCT) of attenuation >130 Hounsfield units (Hu). A volume score was obtained by multiplying the total area of calcified plaque by the slice thickness (3mm for EBCT or 2.5 mm for MDCT). For Agatston scores, individual calcified plaque areas were multiplied by a density factor of 1, 2, 3 or 4 corresponding to the maximum Hu attenuation within each plaque (130–199 Hu=1, 200–299 Hu=2, 300–399 Hu=3, 400+ Hu=4).2 These plaque-specific scores were then summed to produce the Agatston score. Results from the duplicate scans of each participant were averaged for final volume and Agatston scores.
The average CAC density score of all defined plaques for each participant was obtained by dividing the Agatston score (Agatston = Area*Density Factor) by the total CAC area. CAC area was derived by dividing the CAC volume by the CT scan slice thickness.3 As the CAC density score can only be determined in participants with identified CAC volume, participants with CAC volume equal to zero were excluded.
Risk Factor Assessment
Participants completed self-report questionnaires on pertinent health history including tobacco use, alcohol consumption, medical diagnoses, family history of CVD, medication use, typical walking pace, annual income, and level of educations. Tobacco use and alcohol consumption were classified as: never, former, or current. Body mass index (BMI) was computed as weight (kg)/squared height (m2). Hip and waist circumference were also measured and used to determine the waist-hip ratio (WHR). Resting systolic and diastolic blood pressure (SBP, DBP) measurements were taken in triplicate after 5 minutes of rest from the right arm of participants in the seated position with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL). The average of the latter two measurements was used in analyses.
Venous blood samples were collected from participants after a 12-hour overnight fast and measured for high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, total cholesterol, and glucose levels. Diabetes was defined as either fasting glucose levels >125 mg/dL or use of hypoglycemic medications or insulin. Gender, age, total cholesterol, HDL, SBP, antihypertensive use, and tobacco usage were used to calculate the Global Framingham Risk Score (GFRS).11 Serum C-reactive protein and fibrinogen concentrations were measured using the BN II nephelometer (Dade Behring Inc., Deerfield, IL). Serum interleukin-6 (IL-6) concentration was measured using ultrasensitive ELISA (Quantikine HS Human interleukin-6 Immunoassay, R& D Systems, Minneapolis, MN).
Statistical Analyses
The exposure variables chosen for this analysis were many traditional and non-traditional cardiovascular risk factors captured in the MESA, and included age, gender, race/ethnicity, total cholesterol, HDL-C, SBP, DBP, antihypertensive medication use, smoking status and amount, diabetes, statin use, BMI, waist circumference, hip circumference, WHR, GFRS, family history of myocardial infarction (MI), typical walking pace, alcohol consumption status and amount, IL-6, fibrinogen, and CRP concentrations. The outcome variables were the CAC volume score and the CAC density score.
The participants were divided into quartiles of CAC volume and density scores. Quartile means and percentages were computed for continuous and categorical exposure variables, respectively. Analyses of covariance (ANCOVA) were performed to determine associations of exposure variables across quartiles of CAC volume and density scores adjusted for age, gender, and the CAC density score (for models evaluating the CAC volume score) or the CAC volume score (for models evaluating the CAC density score). These covariates were standardized to their mean values of 66.4 years, 57% male, and 257.9 mm3 of CAC volume and 2.69 units of CAC density.
Multivariable linear regressions analyses were performed to evaluate the associations of the exposure variables with CAC volume and density scores. For regression analyses, continuous variables were all scaled to standard deviation units. Because CAC volume has a highly skewed distribution, CAC volume scores were log transformed (i.e. natural logarithm [ln] volume score) to reduce skewness. First, associations between individual exposure variables and CAC volume and density scores were adjusted for age, gender, and CAC volume or density scores, henceforth referred to as “minimally adjusted” regression models. Next, we created an intermediate multivariable model incorporating all exposure variables. Then, exposure variables found in the 2013 ACC/AHA ASCVD Pooled Cohort Equations,12 statin use, and any exposure variables with p<0.10 in the intermediate models were forced into the final “fully-adjusted” multivariable models. The exposure variables included in both CAC volume and density final models were age, gender, race/ethnicity, annual income, total cholesterol, HDL, SBP, smoking status, diabetes status, antihypertensive use, statin use, BMI, walking pace, and IL-6. Level of education, family history of MI, alcohol consumption amount, and CRP were also included in the final CAC volume model. Variables excluded from both final models were smoking amount, hip circumference, waist circumference, WHR, alcohol consumption status, and fibrinogen.
Beta coefficients for continuous variables are per standard deviation change in the exposure variable. All statistical analyses were performed in SPSS version 22 (IBM Corporation, Armonk, NY). Statistical significance was defined as a two-tailed p-value of less than 0.05.
Results
Of the 6,814 MESA participants, 3,398 had CAC volume scores >0 and were retained in the analysis. Table 1 displays the cohort characteristics stratified by quartiles of ascending CAC volume scores. Variables that showed a significant monotonic increase with increasing quartiles included SBP, former and current smoking status, diabetes, antihypertensive medication use, statin use, BMI, waist circumference, hip circumference, WHR, GFRS, family history of MI, and IL-6. The proportions of Chinese and Hispanics relative to NHW had a significant monotonic decrease across ascending quartiles.
Table 1.
Cohort characteristics by quartiles of CAC volume scores in the Multi-Ethnic Study of Atherosclerosis
| Total Cohort |
Volume Quartile 1 |
Volume Quartile 2 |
Volume Quartile 3 |
Volume Quartile 4 |
p value* |
|
|---|---|---|---|---|---|---|
|
|
||||||
| CAC volume score range (mm^3) | 2–4992 | 2.3–24.5 | 24.6–85.2 | 85.2–273.5 | 273.5–4991.9 | |
| N | 3398 | 852 | 847 | 850 | 849 | |
| CAC density score (density unit) | 2.69 | 2.07 | 2.70 | 2.91 | 3.09 | <0.01 |
| Age (years) | 66.35 | 62.95 | 65.17 | 67.25 | 70.05 | <0.01 |
| Male | 58% | 46% | 53% | 61% | 72% | <0.01 |
| Ethnicity | ||||||
| Caucasian | 44% | 32% | 38% | 49% | 58% | <0.01 |
| Chinese | 12% | 19% | 15% | 10% | 4% | <0.01 |
| African American | 24% | 26% | 26% | 22% | 23% | 0.17 |
| Hispanic | 20% | 23% | 22% | 19% | 16% | 0.01 |
| Education | ||||||
| <High school | 19% | 21% | 20% | 18% | 16% | 0.07 |
| High school+ | 35% | 31% | 34% | 37% | 39% | 0.02 |
| College+ | 46% | 48% | 46% | 45% | 45% | 0.61 |
| Annual Income | ||||||
| <$50,000 | 63% | 64% | 66% | 63% | 61% | 0.33 |
| $50,000–$99,999 | 24% | 22% | 23% | 26% | 26% | 0.20 |
| $100,000+ | 13% | 14% | 12% | 12% | 13% | 0.52 |
| Total cholesterol (mg/dL) | 194.62 | 193.59 | 192.43 | 195.78 | 196.68 | 0.10 |
| HDL (mg/dL) | 49.41 | 50.43 | 49.49 | 48.94 | 48.79 | 0.18 |
| Systolic BP (mmHg) | 130.81 | 127.53 | 131 | 131.48 | 133.23 | <0.01 |
| Diastolic BP (mmHg) | 72.57 | 71.42 | 72.96 | 72.53 | 73.36 | <0.01 |
| Smoking status | ||||||
| Never | 45% | 52% | 46% | 42% | 38% | <0.01 |
| Former | 42% | 37% | 42% | 44% | 46% | 0.01 |
| Current | 13% | 11% | 12% | 13% | 16% | 0.04 |
| Smoking amount (Pack-years) | 14.70 | 12.11 | 12.29 | 14.88 | 19.52 | <0.01 |
| Diabetes | 18% | 12% | 15% | 19% | 25% | <0.01 |
| Hypertension meds | 46% | 37% | 43% | 48% | 55% | <0.01 |
| Statins | 20% | 15% | 19% | 21% | 25% | <0.01 |
| BMI (kg/m^2) | 28.36 | 27.07 | 28.07 | 28.79 | 29.52 | <0.01 |
| Waist circumference (cm) | 99.69 | 99.33 | 99.03 | 100.89 | 102.5 | <0.01 |
| Hip circumference (cm) | 105.32 | 102.6 | 105.09 | 106.21 | 107.42 | <0.01 |
| WHR | 0.95 | 0.94 | 0.94 | 0.95 | 0.95 | <0.01 |
| GFRS | 18.34 | 16.63 | 18.06 | 18.71 | 19.97 | <0.01 |
| Family history of MI | 45% | 38% | 45% | 50% | 60% | <0.01 |
| Typical walking pace | ||||||
| No walking | 5% | 2% | 7% | 5% | 7% | <0.01 |
| Stroll | 26% | 22% | 27% | 26% | 27% | 0.09 |
| Normal | 49% | 56% | 45% | 48% | 48% | <0.01 |
| Brisk | 18% | 18% | 18% | 19% | 17% | 0.75 |
| Stride | 2% | 2% | 2% | 1% | 1% | 0.10 |
| Alcohol consumption status | ||||||
| Never | 19% | 24% | 22% | 17% | 15% | <0.01 |
| Former | 25% | 22% | 29% | 25% | 25% | 0.03 |
| Current | 55% | 54% | 49% | 57% | 60% | <0.01 |
| Alcohol consumption (drinks/week) | 5.62 | 5.25 | 5.17 | 5.38 | 6.62 | 0.02 |
| IL-6 (mg/dL) | 1.67 | 1.58 | 1.59 | 1.72 | 1.81 | 0.01 |
| Fibrinogen (mg/dL) | 353.80 | 348.43 | 353.07 | 354.00 | 359.73 | 0.09 |
| CRP (mg/dL) | 3.79 | 3.48 | 3.44 | 4.10 | 4.15 | 0.06 |
Participants stratified by quartiles of ascending CAC volume score. Analysis includes only participants with CAC volume score >0. Means and frequencies were adjusted for age, gender, and CAC density score by ANCOVA. The covariates of age, gender (proportion of males), and CAC density score were normalized to their mean values of 66.35 years, 57% male, and 2.69 density units, respectively.
Abbreviations: BMI=Body Mass Index, WHR=waist-hip ratio, GFRS=Global Framingham Risk Score, MI=myocardial infarction, IL-6=interleukin-6, CRP=C-reactive protein,
for trend across quartiles
Table 2 displays the cohort characteristics stratified by quartiles of ascending CAC density scores. Across ascending quartiles, the proportion of Chinese increased monotonically, whereas NHW decreased monotonically. Family history of MI, BMI, waist circumference, the GFRS, CRP, and IL-6 decreased monotonically across increasing quartiles.
Table 2.
Cohort characteristics by quartiles of CAC density score in the Multi-Ethnic Study of Atherosclerosis
| Total Cohort |
Density Quartile 1 |
Density Quartile 2 |
Density Quartile 3 |
Density Quartile 4 |
p value* |
|
|---|---|---|---|---|---|---|
|
|
||||||
| CAC density score range (density units) | 0.83–4.00 | 0.83–2.23 | 2.23–2.79 | 2.70–3.17 | 3.18–4.00 | |
| N | 3398 | 850 | 849 | 850 | 849 | |
| CAC volume (mm^3) | 2.69 | 70.49 | 178.38 | 411.68 | 370.93 | <0.01 |
| Age (years) | 66.35 | 65.79 | 66.68 | 66.23 | 66.71 | <0.01 |
| Male | 58% | 61% | 58% | 56% | 56% | <0.01 |
| Ethnicity | ||||||
| Caucasian | 44% | 52% | 48% | 48% | 29% | <0.01 |
| Chinese | 12% | 6% | 7% | 11% | 25% | <0.01 |
| African American | 24% | 22% | 27% | 25% | 23% | 0.03 |
| Hispanic | 20% | 21% | 19% | 17% | 23% | 0.01 |
| Education | ||||||
| < High school | 19% | 20% | 17% | 17% | 21% | 0.04 |
| High school+ | 35% | 39% | 36% | 37% | 29% | <0.01 |
| College+ | 46% | 42% | 47% | 46% | 50% | 0.05 |
| Annual Income | ||||||
| <$50,000 | 63% | 64% | 62% | 65% | 64% | 0.53 |
| $50,000–$99,999 | 24% | 26% | 27% | 24% | 21% | 0.04 |
| $100,000+ | 13% | 11% | 12% | 12% | 16% | 0.03 |
| Total cholesterol (mg/dL) | 194.62 | 193.74 | 195.96 | 194.61 | 194.15 | 0.62 |
| HDL (mg/dL) | 49.41 | 48.35 | 49.15 | 49.09 | 51.06 | <0.01 |
| Systolic BP (mmHg) | 130.81 | 131.37 | 131.12 | 131.46 | 129.29 | 0.12 |
| Diastolic BP (mmHg) | 72.57 | 72.73 | 72.82 | 72.34 | 72.37 | 0.73 |
| Smoking status | ||||||
| Never | 45% | 43% | 44% | 43% | 48% | 0.14 |
| Former | 42% | 43% | 44% | 43% | 41% | 0.62 |
| Current | 13% | 14% | 13% | 14% | 11% | 0.42 |
| Smoking amount (Pack-years) | 14.70 | 16.35 | 14.95 | 15.38 | 12.13 | 0.01 |
| Diabetes | 18% | 22% | 19% | 15% | 16% | 0.01 |
| Hypertension meds | 46% | 50% | 46% | 45% | 42% | 0.08 |
| Statins | 20% | 24% | 17% | 20% | 19% | 0.01 |
| BMI (kg/m^2) | 28.36 | 29.25 | 28.96 | 28.14 | 27.09 | <0.01 |
| Waist circumference (cm) | 99.69 | 102.10 | 101.21 | 98.64 | 96.79 | <0.01 |
| Hip circumference (cm) | 105.32 | 106.86 | 106.90 | 105.13 | 102.40 | <0.01 |
| WHR | 0.95 | 0.95 | 0.95 | 0.94 | 0.94 | <0.01 |
| GFRS | 18.34 | 18.99 | 18.51 | 18.42 | 17.44 | <0.01 |
| Family history of MI | 45% | 52% | 50% | 49% | 43% | <0.01 |
| Typical walking pace | ||||||
| No walking | 5% | 6% | 8% | 6% | 2% | <0.01 |
| Stroll | 26% | 27% | 26% | 25% | 25% | 0.83 |
| Normal | 49% | 49% | 48% | 50% | 51% | 0.72 |
| Brisk | 18% | 18% | 16% | 18% | 20% | 0.24 |
| Stride | 2% | 1% | 1% | 2% | 2% | 0.20 |
| Alcohol consumption status | ||||||
| Never | 19% | 15% | 17% | 21% | 24% | <0.01 |
| Former | 25% | 26% | 27% | 27% | 21% | 0.01 |
| Current | 55% | 58% | 55% | 51% | 55% | 0.06 |
| Alcohol consumption (drinks/week) | 5.62 | 6.46 | 5.17 | 5.38 | 5.47 | 0.11 |
| IL-6 (mg/dL) | 1.67 | 1.77 | 1.76 | 1.64 | 1.53 | <0.01 |
| Fibrinogen (mg/dL) | 353.80 | 359.20 | 354.72 | 349.19 | 352.07 | 0.14 |
| CRP (mg/dL) | 3.79 | 4.07 | 4.20 | 3.45 | 3.45 | 0.04 |
Participants stratified by quartiles of ascending CAC density. Analysis includes only participants with CAC volume >0. Means and frequencies were adjusted for age, gender, and CAC volume by ANCOVA. The covariates of age, gender (proportion of males), and CAC volume were normalized to their mean values of 66.35 years, 57% male, and 257.86 mm3, respectively.
Abbreviations: BMI=Body Mass Index, WHR=waist-hip ratio, GFRS=Global Framingham Risk Score, MI=myocardial infarction, IL-6= interleukin-6, CRP=C-reactive protein,
for trend across quartiles
Table 3 displays the associations of CVD risk factors with ln CAC volume score in minimally-adjusted and fully-adjusted multivariable regression models, which included adjustment for the CAC density score. The adjusted R2 for the fully-adjusted model was 0.49. The CAC density score was positively associated with the ln CAC volume score (β= 0.94 ln-units, fully-adjusted model). Compared to NHW, the other three races/ethnicities were each significantly associated with a lower ln CAC volume score and had the strongest inverse associations (β= −0.62, −0.52, and −0.40 ln-units for Chinese, African-American, and Hispanic, respectively, fully-adjusted model). Age, male gender, college or greater level of education, total cholesterol, diabetes, antihypertensive medication use, BMI, family history of MI, and amount of alcohol consumption were all positively associated with the ln CAC volume score. Annual income greater than $100,000, average and brisk walking paces, and CRP were inversely associated with the ln CAC volume score.
Table 3.
Associations of cardiovascular disease risk factors with ln CAC volume scores in the Multi-Ethnic Study of Atherosclerosis
| Minimally-adjusted | Fully-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| β | 95% CI | p value | β | 95% CI | p value | |||
| CAC density score (/0.695 density unit) | 0.88 | 0.84 | 0.93 | <0.01 | 0.94 | 0.89 | 0.99 | <0.01 |
| Age (/9.53 yrs) | 0.34 | 0.30 | 0.38 | <0.01 | 0.31 | 0.25 | 0.37 | <0.01 |
| Male | 0.50 | 0.41 | 0.58 | <0.01 | 0.53 | 0.41 | 0.65 | <0.01 |
| Ethnicity | ||||||||
| Caucasian (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Chinese | −0.77 | −0.91 | −0.63 | <0.01 | −0.62 | −0.83 | −0.41 | <0.01 |
| African-American | −0.29 | −0.40 | −0.19 | <0.01 | −0.52 | −0.64 | −0.39 | <0.01 |
| Hispanic | −0.34 | −0.45 | −0.23 | <0.01 | −0.40 | −0.55 | −0.26 | <0.01 |
| Level of education | ||||||||
| < High school (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| High school+ | 0.18 | 0.06 | 0.30 | <0.01 | 0.11 | −0.05 | 0.27 | 0.16 |
| College+ | 0.09 | −0.03 | 0.21 | 0.13 | 0.17 | 0.00 | 0.33 | 0.04 |
| Annual income | ||||||||
| <$50,000 (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| $50,000–$99,999 | 0.09 | −0.02 | 0.20 | 0.09 | −0.04 | −0.17 | 0.08 | 0.49 |
| $100,000+ | −0.05 | −0.18 | 0.09 | 0.50 | −0.22 | −0.38 | −0.06 | 0.01 |
| Total cholesterol (/36.46 mg/dL) | 0.03 | −0.02 | 0.07 | 0.25 | 0.06 | 0.01 | 0.11 | 0.03 |
| HDL (/14.49 mg/dL) | −0.06 | −0.11 | −0.02 | 0.01 | −0.04 | −0.10 | 0.01 | 0.13 |
| Systolic BP(/21.65 mmHg) | 0.09 | 0.05 | 0.14 | <0.01 | 0.05 | 0.00 | 0.10 | 0.07 |
| Diastolic BP (/10.22 mmHg) | 0.06 | 0.02 | 0.11 | 0.01 | ||||
| Smoking status | ||||||||
| Never (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Former | 0.19 | 0.10 | 0.28 | <0.01 | 0.05 | −0.05 | 0.16 | 0.33 |
| Current | 0.27 | 0.13 | 0.40 | <0.01 | 0.15 | −0.01 | 0.31 | 0.06 |
| Smoking amount (/24 pack-years) | 0.13 | 0.09 | 0.17 | <0.01 | ||||
| Diabetes | 0.36 | 0.26 | 0.47 | <0.01 | 0.44 | 0.31 | 0.58 | <0.01 |
| Hypertension meds | 0.30 | 0.21 | 0.38 | <0.01 | 0.21 | 0.11 | 0.32 | <0.01 |
| Statins | 0.25 | 0.14 | 0.35 | <0.01 | 0.10 | −0.03 | 0.22 | 0.13 |
| BMI (/5.30 kg/m^2) | 0.19 | 0.15 | 0.23 | <0.01 | 0.09 | 0.03 | 0.15 | <0.01 |
| Waist circumference (/13.87 cm) | 0.18 | 0.14 | 0.23 | <0.01 | ||||
| Hip circumference (/11.21 cm) | 0.18 | 0.13 | 0.22 | <0.01 | ||||
| WHR (/0.077) | 0.11 | 0.06 | 0.15 | <0.01 | ||||
| Family history of MI | 0.34 | 0.25 | 0.42 | <0.01 | 0.20 | 0.10 | 0.29 | <0.01 |
| Typical walking pace | ||||||||
| No walking (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Stroll | −0.09 | −0.29 | 0.11 | 0.36 | −0.16 | −0.40 | 0.08 | 0.20 |
| Average pace | −0.25 | −0.43 | −0.06 | 0.01 | −0.27 | −0.51 | −0.04 | 0.02 |
| Brisk | −0.24 | −0.44 | −0.03 | 0.02 | −0.29 | −0.55 | −0.04 | 0.02 |
| Stride | −0.52 | −0.91 | −0.13 | 0.01 | −0.45 | −0.91 | 0.00 | 0.05 |
| Alcohol consumption status | ||||||||
| Never (reference) | -- | -- | -- | -- | ||||
| Former | −0.07 | 0.08 | 0.34 | 0.32 | ||||
| Current | 0.17 | 0.11 | 0.33 | 0.04 | ||||
| Alcohol consumption (/9.1 drinks/week) | 0.01 | 0.00 | 0.01 | <0.01 | 0.05 | 0.00 | 0.10 | 0.03 |
| IL-6 (/1.27 mg/dL) | 0.09 | 0.05 | 0.13 | <0.01 | 0.05 | 0.00 | 0.11 | 0.06 |
| Fibrinogen (/75.93 mg/dL) | 0.06 | 0.01 | 0.10 | 0.01 | ||||
| CRP (/6.29 mg/dL) | 0.04 | 0.00 | 0.08 | 0.06 | −0.07 | −0.13 | −0.01 | 0.04 |
Beta coefficients for continuous variables are per one standard deviation change in exposure variable. Analysis includes only participants with CAC volume scores >0. The left column shows minimally-adjusted analyses (adjustment for age, gender, and CAC density). All listed exposure variables were then included in an intermediate multivariable model (not shown). Only those variables previously shown to be strongly tied to CVD risk (i.e. components of the pooled cohort equations and statin use) and any others that yielded p<0.10 were included in the final fully-adjusted multivariable model, shown on the right.
Abbreviations: ln=natural log, BMI=Body Mass Index, WHR=waist-hip ratio, MI=myocardial infarction, IL-6=interleukin-6, CRP=C-reactive protein
Table 4 shows associations of risk factors with CAC density in minimally-adjusted and fully-adjusted multivariable models. The adjusted R2 for the fully-adjusted model was 0.41. The ln CAC volume score was positively associated with the CAC density score (β=0.44 density units). With NHW as the reference, race/ethnicity variables were among the strongest positive correlates of the CAC density score (β= 0.41, 0.18, 0.21 density units for Chinese, African American, and Hispanic, respectively, fully-adjusted model). Age, annual income greater than $100,000, HDL-C, and brisk walking pace were positively associated with the CAC density score, while male gender, diabetes, and BMI were inversely associated with the CAC density score. Compared to no walking, stroll, average pace, and stride had borderline positive associations with the CAC density score in the fully-adjusted model that were not statistically significant. Total cholesterol and systolic BP had borderline inverse associations with the CAC density score in the fully-adjusted model that were not statistically significant.
Table 4.
Associations of cardiovascular disease risk factors with CAC density scores in the Multi-Ethnic Study of Atherosclerosis
| Minimally-adjusted | Fully-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| β | 95% CI | p value | β | 95% CI | p value | |||
| Ln CAC volume score (/1.62 ln-units) | 0.41 | 0.39 | 0.43 | <0.01 | 0.44 | 0.42 | 0.46 | <0.01 |
| Age (/9.53 yrs) | 0.03 | 0.01 | 0.05 | 0.02 | 0.02 | 0.00 | 0.05 | 0.05 |
| Male | −0.04 | −0.08 | 0.00 | 0.07 | −0.07 | −0.11 | −0.02 | <0.01 |
| Ethnicity | ||||||||
| Caucasian (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Chinese | 0.40 | 0.34 | 0.46 | <0.01 | 0.41 | 0.34 | 0.47 | <0.01 |
| African-American | 0.09 | 0.05 | 0.14 | <0.01 | 0.18 | 0.12 | 0.23 | <0.01 |
| Hispanic | 0.13 | 0.09 | 0.18 | <0.01 | 0.21 | 0.15 | 0.26 | <0.01 |
| Level of education | ||||||||
| < High school (reference) | -- | -- | -- | -- | ||||
| High school+ | −0.07 | −0.13 | −0.02 | 0.01 | ||||
| College+ | −0.01 | −0.07 | 0.04 | 0.60 | ||||
| Annual income | ||||||||
| <$50,000 (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| $50,000–$99,999 | −0.05 | −0.09 | 0.00 | 0.06 | 0.00 | −0.05 | 0.05 | 0.99 |
| $100,000+ | 0.06 | −0.01 | 0.12 | 0.07 | 0.12 | 0.06 | 0.19 | <0.01 |
| Total cholesterol (/36.46 mg/dL) | −0.01 | −0.03 | 0.01 | 0.52 | −0.02 | −0.04 | 0.00 | 0.07 |
| HDL (/14.49 mg/dL) | 0.04 | 0.02 | 0.06 | <0.01 | 0.03 | 0.00 | 0.05 | 0.03 |
| Systolic BP (/21.65 mmHg) | −0.02 | −0.04 | 0.00 | 0.04 | −0.02 | −0.04 | 0.00 | 0.06 |
| Diastolic BP (/10.22 mmHg) | −0.01 | −0.03 | 0.01 | 0.29 | ||||
| Smoking status | ||||||||
| Never (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Former | −0.04 | −0.08 | 0.01 | 0.09 | 0.01 | −0.03 | 0.05 | 0.58 |
| Current | −0.06 | −0.12 | 0.00 | 0.05 | −0.02 | −0.09 | 0.04 | 0.49 |
| Smoking amount (/24 pack-years) | −0.03 | −0.05 | −0.01 | 0.01 | ||||
| Diabetes | −0.08 | −0.13 | −0.03 | <0.01 | −0.07 | −0.12 | −0.02 | <0.01 |
| Hypertension meds | −0.06 | −0.10 | −0.02 | <0.01 | −0.03 | −0.07 | 0.01 | 0.15 |
| Statins | −0.06 | −0.10 | −0.01 | 0.02 | −0.04 | −0.08 | 0.02 | 0.17 |
| BMI (/5.30 kg/m^2) | −0.08 | −0.10 | −0.06 | <0.01 | −0.04 | −0.06 | −0.02 | <0.01 |
| Waist circumference (/13.87 cm) | −0.07 | −0.09 | −0.05 | <0.01 | ||||
| Hip circumference (/11.21 cm) | −0.08 | −0.10 | −0.06 | <0.01 | ||||
| WHR (/0.077) | −0.02 | −0.04 | 0.00 | 0.02 | ||||
| Family history of MI | −0.08 | −0.11 | −0.04 | <0.01 | ||||
| Typical walking pace | ||||||||
| No walking (reference) | -- | -- | -- | -- | -- | -- | -- | -- |
| Stroll | 0.11 | 0.02 | 0.20 | 0.02 | 0.08 | −0.01 | 0.18 | 0.09 |
| Average pace | 0.14 | 0.05 | 0.22 | <0.01 | 0.09 | −0.01 | 0.18 | 0.06 |
| Brisk | 0.14 | 0.05 | 0.23 | <0.01 | 0.12 | 0.02 | 0.22 | 0.02 |
| Stride | 0.27 | 0.09 | 0.44 | <0.01 | 0.17 | −0.01 | 0.35 | 0.06 |
| Alcohol consumption status | ||||||||
| Never (reference) | -- | -- | -- | -- | ||||
| Former | −0.11 | −0.17 | −0.05 | <0.01 | ||||
| Current | −0.11 | −0.16 | −0.06 | <0.01 | ||||
| Alcohol consumption (9.1 drinks/week) | −0.01 | −0.04 | 0.01 | 0.20 | ||||
| IL-6 (/1.27 mg/dL) | −0.04 | −0.06 | −0.02 | <0.01 | −0.01 | −0.03 | 0.01 | 0.36 |
| Fibrinogen (/75.93 mg/dL) | −0.02 | −0.04 | 0.00 | 0.05 | ||||
| CRP (/6.29 mg/dL) | −0.02 | −0.04 | 0.00 | 0.02 | ||||
Beta coefficients for continuous variables are per one standard deviation change in exposure variable. Analysis includes only participants with CAC volume scores >0. The left column shows minimally-adjusted analyses (adjustment for age, gender, and ln CAC volume). All listed exposure variables were then included in an intermediate multivariable model (not shown). Only those variables previously shown to be strongly tied to CVD risk (i.e. components of the pooled cohort equations and statin use) and any others that yielded p<0.10 were included in the final fully-adjusted multivariable model, shown on the right.
Abbreviations: ln=natural log, BMI=Body Mass Index, WHR=waist-hip ratio, MI=myocardial infarction, IL-6=interleukin-6, CRP=C-reactive protein
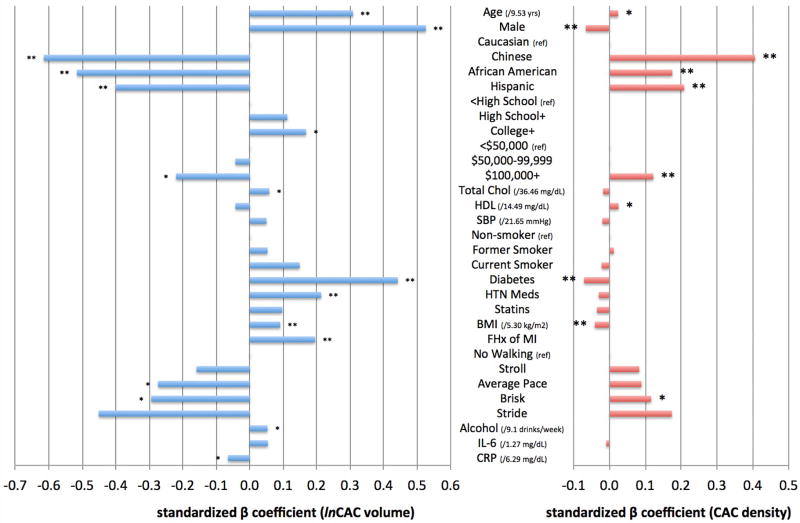
Figure 1 graphically summarizes the magnitude and direction of the associations between CVD risk factors and CAC volume and CAC density scores. β-coefficients from the fully-adjusted multivariable regression models are plotted. For risk factors retained in the multivariable models, the associations for volume and density scores tended to be in opposite directions. The associations for race/ethnicity were as strong or stronger than most of the traditional risk factor associations.
Figure 1. Independent associations of CVD risk factors with CAC volume and density scores.
Standardized β coefficients from multivariable regression models. Variables on the vertical axis are the exposure variables. The outcome variable is either (a) ln CAC volume score (mm3) or (b) CAC density score (density unit). Single asterisk indicates p<0.05 and double asterisk indicates p<0.01. Coefficients for ln CAC volume and CAC density scores cannot be quantitatively compared due to inherent unit differences between measurements of CAC volume and CAC density scores.
Discussion
In a large, multi-ethnic cohort of community-living individuals with detectable CAC who were free of clinical CVD, we found that many CVD risk factors were generally associated with CAC volume and CAC density scores in opposite directions. For instance, diabetes and higher BMI were associated with higher CAC volume but lower CAC density scores. Moreover, HDL-C, a risk factor known to be inversely associated with coronary heart disease risk13 and faster walking paces were associated with a higher CAC density score. These observations align with previous findings from this cohort demonstrating that a higher CAC density score is associated with a lower risk of CVD events at any level of CAC volume.3 Taken together, CAC density appears to be a quantifiable aspect of CAC that is inversely associated with CVD risk factors and CVD events, despite the strong overall association of CAC with subclinical coronary artery disease. While CVD risk factors appeared to have relatively stronger associations with CAC volume scores than CAC density scores among individuals with calcified atherosclerosis, a higher CAC density score may nonetheless be a marker of a more favorable CVD risk factor profile.
We also found striking associations between race/ethnicity and CAC volume and density scores. Specifically, NHW participants showed higher CAC volume and lower CAC density scores compared to the other race/ethnicity groups after multivariable adjustment. The distribution of CAC as quantified by the Agatston score has previously been observed to vary significantly by race/ethnicity in this cohort.14 Race/ethnicity may influence the development of CAC through its volume and density components, and this may merit further investigation.
Our findings also support the observation that the Agatston scoring method of weighting CAC scores to account for higher CAC density may be suboptimal. Several studies have demonstrated the associations of CVD risk factors with higher CAC Agatston scores,7, 8, 15 but to our knowledge no previous study has differentiated CVD risk factor associations with CAC volume and CAC density independently. As many CVD risk factors were associated with a lower CAC density score, it appears that the Agatston score may obscure important associations between CVD risk factors and CAC composition.
In this observational study, the associations of statin use with CAC volume and density scores were not statistically significant. Clinical trials have suggested that statins do not attenuate (and may actually increase) the progression of CAC.16–18 In a recent meta-analysis of eight randomized clinical trials, statins appeared to increase calcified plaque while decreasing non-calcified plaque in coronary arteries assessed with intravascular ultrasound.19 Our findings may be distorted due to confounding by indication, as participants prescribed statins tend to have a greater burden of CVD risk factors. Furthermore, annual income was found to have a positive association with CAC density and an inverse association with CAC volume. These observations suggest that there may be additional factors not captured in the models that influence CAC volume and density.
Among the strengths of this study are its large sample size, broad range of CVD risk factors, and the multi-ethnic nature of the study population. The study also has important limitations. First, the density score does not measure the density of individual plaques, but rather an average of all calcified plaques identified in the coronary tree. The density score is arbitrarily capped at a maximum of four for all plaque densities greater than 400 Hu, potentially attenuating the inverse association of CVD risk factors with CAC density. Adults with clinical CVD at baseline were excluded from participating in the MESA, and participants without detectable CAC (and thus no density score) were excluded from this analysis by necessity; as such, the study sample was middle aged and had detectable CAC but no clinically apparent CVD. Whether results will generalize to other populations is presently uncertain. Finally, in multiple linear regression models reporting the associations of several exposure variables with the outcomes of CAC volume and density scores, some of the significant associations observed may have been due to chance as a consequence of multiple testing.
In conclusion, we demonstrated the differential associations CVD risk factors with CAC volume and density scores. CVD risk factors were generally associated with higher CAC volume scores, but some CVD risk factors were also associated with lower CAC density scores. NHW race was also strongly associated with higher CAC volume but lower CAC density scores. These findings suggest a complex association between CVD risk factors and CAC volume and density components, and highlight a limitation of the Agatston method of CAC scoring. Future studies should address whether modification of CVD risk factors might have a dual effect of reducing CAC volume and increasing CAC density.
Key Questions.
What is already known about this subject?
Coronary artery calcium (CAC) is a robust marker of subclinical cardiovascular disease (CVD). Recently, the density score of CAC has been shown to be inversely associated with CVD events after adjusting for the CAC volume score. Determinants of the CAC density score are unknown.
What does this study add?
This study demonstrates that many demographic and CVD risk factors tend to have opposite associations with CAC volume and density scores, with many CVD risk factors being inversely associated with the CAC density score.
How might this impact on clinical practice?
As favorable risk factor profiles among individuals with detectable CAC appear to be associated with a higher CAC density score, assessment of the CAC density score may provide additional insight into an individual’s overall risk of CVD.
Acknowledgments
Funding and Acknowledgements: The Multi-Ethnic Study of Atherosclerosis was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. This project was partially supported by the National Institutes of Health, Grant 5T35HL007491. The authors would like to thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: Matthew A. Allison: Merck Advisory Board. The remaining authors have nothing to disclose.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
References
- 1.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. New England Journal of Medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. Jama. 2014;311:271–8. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 5.Shemesh J, Apter S, Itzchak Y, Motro M. Coronary calcification compared in patients with acute versus in those with chronic coronary events by using dual-sector spiral CT. Radiology. 2003;226:483–8. doi: 10.1148/radiol.2262011903. [DOI] [PubMed] [Google Scholar]
- 6.Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, Becker C, Reiser M, Steinbeck G, Boekstegers P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. The American journal of cardiology. 2003;91:714–8. doi: 10.1016/s0002-9149(02)03411-2. [DOI] [PubMed] [Google Scholar]
- 7.Oei HH, Vliegenthart R, Hofman A, Oudkerk M, Witteman JC. Risk factors for coronary calcification in older subjects. The Rotterdam Coronary Calcification Study. European heart journal. 2004;25:48–55. doi: 10.1016/j.ehj.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 12.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 13.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 14.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–7. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 15.Lambrechtsen J, Gerke O, Egstrup K, Sand NP, Norgaard BL, Petersen H, Mickley H, Diederichsen AC. The relation between coronary artery calcification in asymptomatic subjects and both traditional risk factors and living in the city centre: a DanRisk substudy. Journal of internal medicine. 2012;271:444–50. doi: 10.1111/j.1365-2796.2011.02486.x. [DOI] [PubMed] [Google Scholar]
- 16.Houslay ES, Cowell SJ, Prescott RJ, Reid J, Burton J, Northridge DB, Boon NA, Newby DE. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart (British Cardiac Society) 2006;92:1207–12. doi: 10.1136/hrt.2005.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V, Lahiri A, Leischik R, Moshage W, Schartl M, Siffert W, Steinhagen-Thiessen E, Sinitsyn V, Vogt A, Wiedeking B, Erbel R. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. 2006;113:427–37. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- 18.Saremi A, Bahn G, Reaven PD. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT) Diabetes care. 2012;35:2390–2. doi: 10.2337/dc12-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. Journal of the American College of Cardiology. 2015;65:1273–82. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]