Abstract
Protein interacting with C-kinase 1 (PICK1) is a peripheral membrane protein that controls insulin granule formation, trafficking, and maturation in INS-1E cells. However, global Pick1-knockout mice showed only a subtle diabetes-like phenotype. This raises the possibility that compensatory effects from tissues other than pancreatic beta cells may obscure the effects of insulin deficiency. To explore the role of PICK1 in pancreatic islets, we generated mice harboring a conditional Pick1 allele in a C57BL/6J background. The conditional Pick1-knockout mice exhibited impaired glucose tolerance, profound insulin deficiency, and hyperglycemia. In vitro experiments showed that the ablation of Pick1 in pancreatic beta cells selectively decreased the initial rapid release of insulin and the total insulin levels in the islets. Importantly, the specific ablation of Pick1 induced elevated proinsulin levels in the circulation and in the islets, accompanied by a reduction in the proinsulin processing enzymes prohormone convertase 1/3 (PC1/3). The deletion of Pick1 triggered the specific elimination of chromogranin B in pancreatic beta cells, which is believed to control granule formation and release. Collectively, these data demonstrate the critical role of PICK1 in secretory granule biogenesis, proinsulin processing, and beta cell function. We conclude that the beta cell–specific deletion of Pick1 in mice led to hyperglycemia and eventually to diabetes.
INTRODUCTION
Pancreatic beta cell dysfunction plays an important role in the pathogenesis of both type 1 and type 2 diabetes (Herold et al., 2015; Yabe and Seino, 2016). The secretion of insulin by pancreatic beta cells in response to glucose is critical for the regulation of glucose homeostasis. The dysfunction of this process is the hallmark of early-stage diabetes (Alberti and Zimmet, 1998). In healthy pancreatic beta cells, insulin maturation can generally be divided into three stages: first, during the biosynthesis of preproinsulin, the signal peptide of preproinsulin is cleaved concurrent with its translocation to the endoplasmic reticulum (ER), and in the ER, proper disulfide bonds are formed and folded in proinsulin (Papa, 2012); second, proinsulin is transferred to the Golgi apparatus, in which hexamerization is believed to occur; and finally, proinsulin is packaged into immature secretory granules (ISGs) that are gradually processed into the final mature insulin granules (Halban, 1994). This maturation process occurs through the joint action of three types of proteases, prohormone convertase 1/3 (PC1/3), prohormone convertase 2 (PC2), and carboxypeptidase E (CPE), which cleave proinsulin into mature insulin and c-peptide (Smeekens et al., 1992).
Previous studies have reported that prediabetes, type 2 diabetes (T2D), and maturity-onset diabetes of the young (MODY) all share pathological features, including elevated proinsulin levels in the plasma, a dilated ER, and impaired glucose tolerance (American Diabetes Association, 2010). Indeed, because proinsulin exerts only 5% of the biological activity of mature insulin, an increase in circulating proinsulin is believed to limit the action of mature insulin and, consequently, to contribute to worsening glucose tolerance and ultimately hyperglycemia.
Recent genomewide association studies have identified nine single nucleotide polymorphisms (SNPs) associated with either altered proinsulin levels or proinsulin-to-insulin conversion (Strawbridge et al., 2011). Clinical observations suggest that an elevated proinsulin-to-insulin level is a risk factor that facilitates the onset of T2D in apparently healthy individuals (Pfutzner et al., 2004). Nevertheless, the regulatory molecules that control proinsulin processing and insulin granule biogenesis have not yet been fully elucidated.
Protein interacting with C-kinase 1 (PICK1) is a peripheral membrane protein that contains a PDZ domain and a BAR domain (Xu and Xia, 2006). The PDZ domain enables PICK1 to interact with and regulate the trafficking of membrane proteins, such as the AMPA receptor subunit GluR2 (Xia et al., 1999). The BAR domain is capable of sensing membrane curvature and facilitating vesicle formation (Madsen et al., 2008). We previously reported that PICK1 forms heteromeric complexes with islet cell autoantigen 69 kD (ICA69) in immature SG of pancreatic beta cells (Cao et al., 2013). While traditional Pick1-knockout mice display mildly impaired glucose tolerance, they do not exhibit the full features of diabetes in vivo (Holst et al., 2013). This finding prompted us to further evaluate the role of PICK1 in disease onset in more detail. In this study, we explore the role of PICK1 in proinsulin processing and insulin secretion using beta cell–specific Pick1-conditional knockout mice. We demonstrate a critical new role of PICK1 in hyperglycemia and that the modulation of Pick1 could be used therapeutically to improve beta cell function in diabetes.
RESULTS
Generation of pancreatic beta cell–specific conditional Pick1-knockout mice
To determine the role of PICK1 in pancreatic beta cells, we intercrossed mice harboring the rat insulin promoter 2 (RIP2)-driven Cre transgene (Gannon et al., 2000; Cui et al., 2011) with mice harboring a floxed Pick1 allele (Gardner et al., 2005). PCR genotyping was performed 4 wk after birth using DNA from the ears of the mice. In this study, the control mice included wild-type (WT) and Pick1loxp–/– plus Cre+ mice (Supplemental Figure S1a). These control mice are referred to as WT mice below. The RIP2-Cre+: Pick1loxp+/+ knockout mice (referred to as Pick1 cKO hereafter) contain an ablation of Pick1 that is predominantly limited to pancreatic beta cells (Kulkarni et al., 1999, Postic et al., 1999). To confirm the specific knockout of Pick1 in the islets, a Western blot analysis using a PICK1 antibody was performed with protein extracts from the brain, pancreas, testis, and isolated islets from both WT mice and Pick1 cKO mice at the age of 4 mo. As RIP-Cre is expressed at a low level in the hypothalamus (Gannon et al., 2000), PICK1 expression in this tissue was also tested. Compared with the WT mice, the PICK1 protein was mostly depleted in the islets that were isolated from the cKO mice (Supplemental Figure S1b). PICK1 protein level was slightly decreased in the pancreas (Supplemental Figure S1, c and g) but remained unchanged in the whole brain, testis, and hypothalamus of the Pick1 cKO mice (Supplemental Figure S1, d–g). These results confirmed that the Pick1 knockout was pancreatic beta cell–specific.
Pick1 deficiency resulted in insufficient insulin content and triggered hyperglycemia
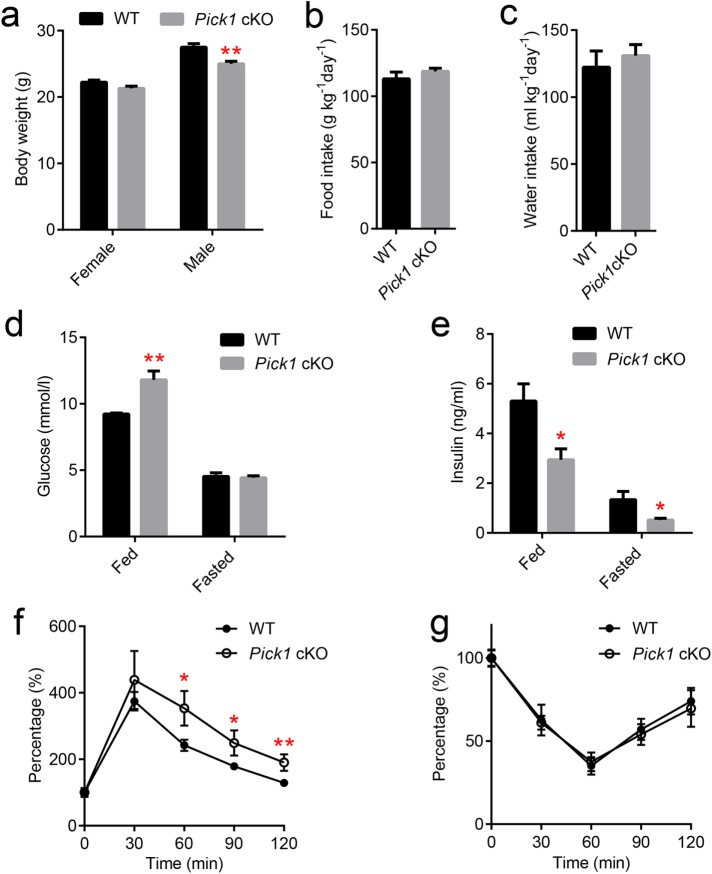
We then examined the metabolic phenotype of the Pick1 cKO mice. The body weights of 4-mo-old male Pick1 cKO mice were significantly lower than those of their WT littermates (Figure 1a). No changes in food or water intake were observed between the Pick1 cKO and the WT mice (Figure 1, b and c). To investigate the role of PICK1 in the maintenance of glucose homeostasis in adult mice, blood glucose levels were subsequently monitored. When the mice were fed, the glucose level was significantly higher in the Pick1 cKO mice than in their WT littermates. However, after a longer period of starvation (16 h), their glucose levels were similar (Figure 1d). We also observed that the plasma insulin levels in the fed and fasted Pick1 cKO mice were significantly decreased relative to those of the age-matched WT mice (Figure 1e). Similarly, the oral glucose tolerance test revealed impaired glucose tolerance in the Pick1 cKO mice (Figure 1f) without any changes in the insulin sensitivity in peripheral tissues (Figure 1g), implying that a lack of insulin production and/or secretion may be a cause of hyperglycemia.
FIGURE 1:
Pick1 cKO mice are glucose-intolerant. (a–c) Adult WT male mice aged 4–5 mo and their Pick1 cKO littermates were examined to determine body weight and water and food intake, n = 5. (d, e) Glucose and insulin were measured under fed and fasted conditions, n = 8. (f) OGTT was performed on male WT and Pick1 cKO mice after a 16-h fast, n = 5. (g) ITT was performed on male WT and Pick1 cKO mice after a 2-h fast, n = 5. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; two-sided Student’s t test.
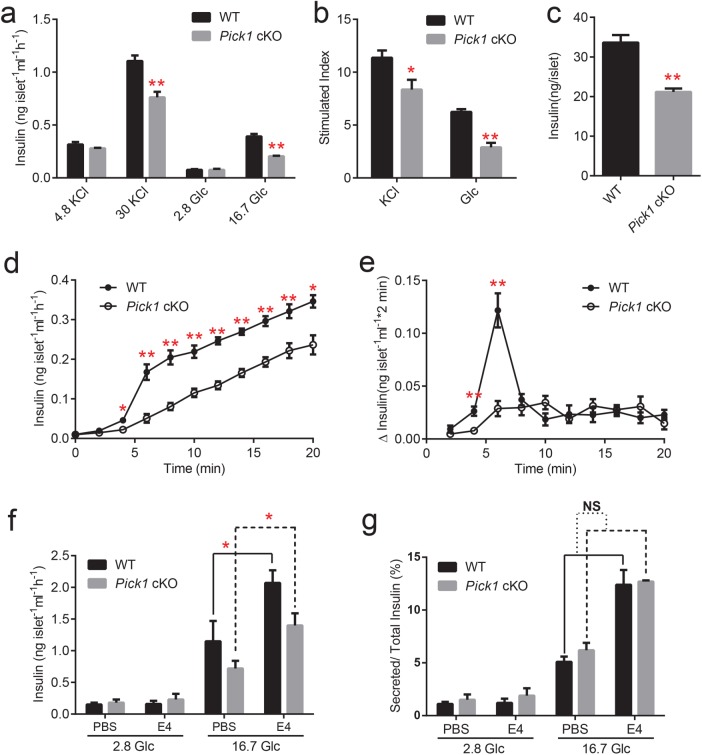
Next, we carried out in vitro tests on isolated islets. As shown in Figure 2, a and b, glucose-stimulated insulin secretion was significantly reduced in the Pick1 cKO mice compared with that in the WT mice. Additionally, the cKO islets exhibited a 25% decrease in insulin secretion stimulated by high potassium (Figure 2b). Indeed, the reduced insulin secretion by the Pick1 cKO islets was partially due to the insufficient insulin content (Figure 2c), suggesting that a defect in insulin production is largely responsible for the hyperglycemia caused by the conditional knockout of Pick1. Furthermore, insulin content was evaluated in INS-1E cells with reduced PICK1 protein expression. Transient knockdown of Pick1 by shRNA transfection into INS-1E cells led to a decrease in the insulin content after 72 h compared with that of the controls. Furthermore, the overexpression of Pick1 increased insulin levels in the INS-1E cells (Supplemental Figure S1g). Next, we measured the time course of insulin secretion from isolated islets. Similar to the glucose-stimulated insulin secretion (GSIS) observations, the WT islets responded to high glucose with a biphasic time course that was characterized by a transient rapid phase of release, followed by a slower sustained phase, whereas the Pick1 cKO islets maintained a steady speed of insulin secretion (Figure 2d). The peak insulin secretion rate, which was reached 4–6 min after the glucose challenge, was three times higher in the WT islets than in the Pick1 cKO islets (Figure 2e). The decrease of secreted insulin is mainly due to the total insulin reduction, because there was no difference between wild-type and PICK1 KO islets in the ratio of secreted to total islet insulin amount (Figure 2g).
FIGURE 2:
Impaired insulin secretion from isolated Pick1 cKO islets. (a) GSIS was performed on isolated islets stimulated with 4.8 or 30 mM KCl or 2.8 or 16.7 mM glucose (Glc). (b) The stimulated index was also calculated following KCl or Glc treatment. (c) Insulin content in the isolated islets was measured by ELISA. (d) Isolated WT and Pick1 cKO islets were incubated in the presence of 16.7 mM Glc at 2-min intervals for 20 min. (e) Same data as shown in d, but plotted as release per 2 min. (f, g) Secreted insulin was tested from isolated WT and Pick1 cKO islets incubated with or without E4. Data are represented as mean ± SEM, n = 3, three to four groups per type of mouse, 10–0 islets per group. *p < 0.05, **p < 0.01; two-sided Student’s t test.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced in enteroendocrine L cells of the gut, which can directly activate pancreatic β cells and augment its glucose-induced insulin secretion in a cAMP-dependent pathway (Orskov, 1992; Flint et al., 1998). Exendin-4 (E4) is a 39-amino acid peptide incretin mimetic that exhibits glucoregulatory activities similar to those observed with the mammalian incretin hormone GLP-1(Thorens et al., 1993; Campbell and Drucker, 2013).To investigate whether PICK1’s effect on insulin secretion is related to the GLP-1-dependent pathway, we treated the isolated islets with E4 in KRBH buffer containing 2.8 or 16.7 mM glucose. Compared with PBS control medium, E4 indeed significantly enhanced insulin secretion in high-glucose medium in both WT and Pick1 knockout islets, but there is no difference between the two genotypes. In addition, when normalized to total insulin, there is no difference in the ratio of insulin secreted (Figure 2, f, g). Therefore, PICK1 is not likely to be implicated in the GLP-1-dependent pathway in insulin granule trafficking.
Pick1 cKO islets showed an abnormal insulin expression pattern
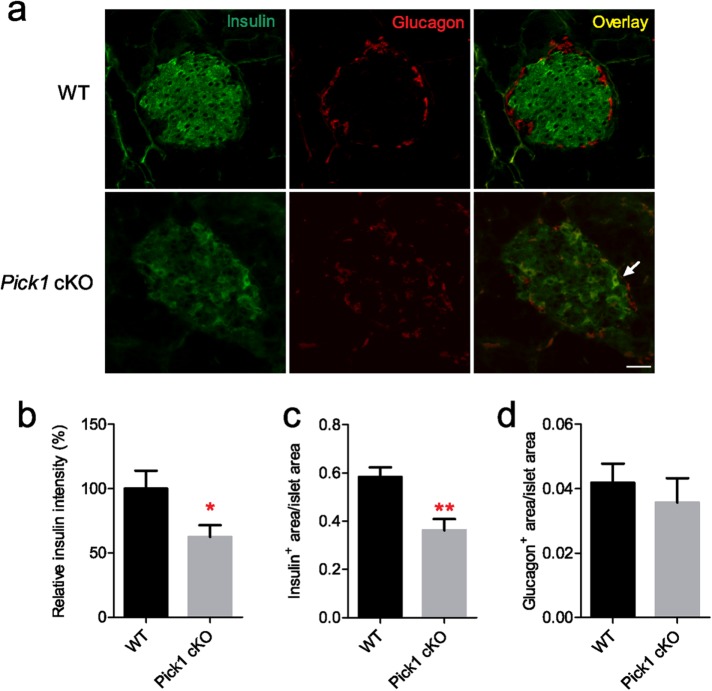
To determine the effect of deleting PICK1 on β-cell mass in pancreatic islets, immunohistochemical staining with insulin and glucagon was applied to pancreatic cryosections on 18-wk-old Pick1 cKO mice and their WT littermates. We observed a universal ablation of the insulin signal from Pick1cKO islets (Figure 3a). Quantitative analysis revealed that the relative insulin intensity was significantly reduced by 37% after knockout of PICK1 (Figure 3b). The ratio of insulin-positive area to total pancreatic area significantly decreased as well, while the ratio of glucagon-positive area to total pancreatic area did not change (Figure 3d). In some occurrences, we also observed dual-positive cells in the Pick1 knockout islet section, which comprised both insulin and glucagon signals, a sign usually indicating the onset of diabetes (Cui et al., 2011). Collectively, these results corroborate the insufficient insulin content and hyperglycemia that were observed in the Pick1 cKO mice.
FIGURE 3:
Disrupted pancreatic beta cells in Pick1 cKO islets. (a) Representative insulin and glucagon immunostaining images of pancreas from WT and Pick1 cKO mice. Scale bar, 50 μm. Arrow indicates insulin and glucagon dual-positive cells. (b) Quantification of relative insulin intensity. (c, d) Quantification of insulin-positive area/islet area and glucagon-positive area/islet area from WT and Pick1 cKO mice, n = 6; 50–60 islets were calculated from each genotype. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; two-sided Student’s t test.
Pick1 deficiency triggered proinsulin accumulation
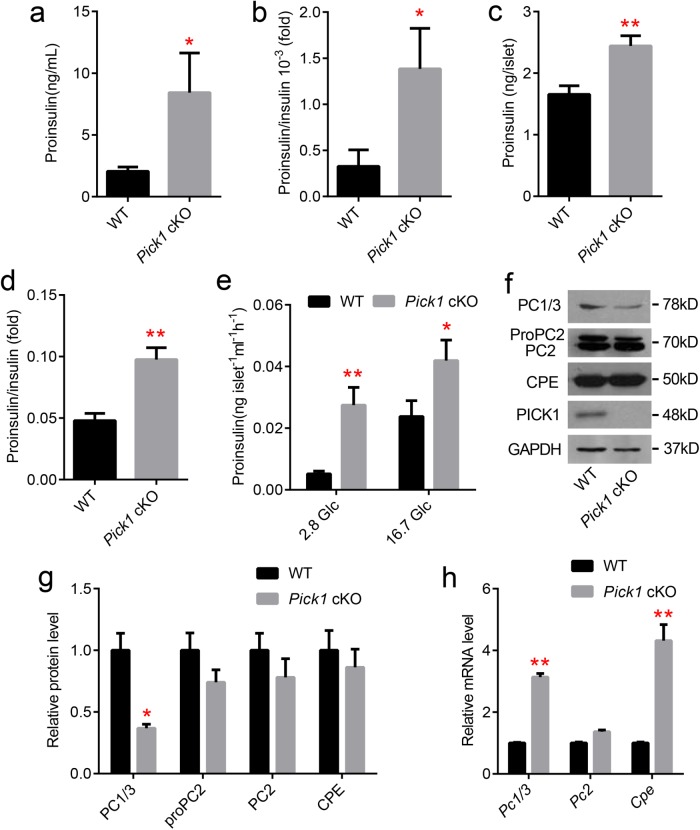
Because PICK1 was previously identified in Golgi apparatus and secretory granules, we examined its role in insulin maturation. First, we examined the proinsulin level. The circulating proinsulin level, which is associated with an elevated proinsulin/insulin ratio, was increased approximately fourfold in the Pick1 cKO animals (Figure 4, a and b). Consistent with these results, the proinsulin content and proinsulin/insulin ratio were also significantly higher in the Pick1 cKO islets than tin the WT islets (Figure 4, c and d). Furthermore, the Pick1 cKO islets also secreted more proinsulin during the glucose stimulation process. Surprisingly, the proinsulin level secreted under basal glucose stimulation (2.8 mmol glucose) was extraordinarily high (approximately fivefold higher than that from the WT islets; Figure 4e). Taken together, the lack of Pick1 in pancreatic beta cells promotes proinsulin production and accumulation.
FIGURE 4:
Elevated proinsulin in islets and serum from Pick1 cKO mice. (a–d) Proinsulin and proinsulin/insulin levels from WT and Pick1 cKO serum (a and b, n = 8) and isolated islets (c and d, n = 3, three groups per type of mouse, 10–20 islets per group). (e) Secreted proinsulin level stimulated with 2.8 or 16.7 mM Glc, n = 3. (f–h) mRNA and protein levels of PC1/3, PC2, and CPE from isolated islets of WT and Pick1 cKO mice. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; two-sided Student’s t test.
To investigate the underlying cause of the poor proinsulin processing in the Pick1 cKO mice, we evaluated the expression pattern of the proinsulin-processing enzymes PC1/3, PC2, and CPE. We observed an obvious decrease in the expression of PC1/3 and no change in the expression of CPE and PC2 in the Pick1 cKO islets (Figure 4, f and g). These results show that Pick1 deficiency likely disrupts secretory granule (SG) biosynthesis. Consequently, to compensate for poor proinsulin processing, the Pc1/3 and Cpe transcripts showed robust up-regulation rather than a decrease (Figure 4h). Thus, our data confirm the direct role of Pick1 in proinsulin processing.
Specific elimination of chromogranin B in beta cells from Pick1 cKO islets
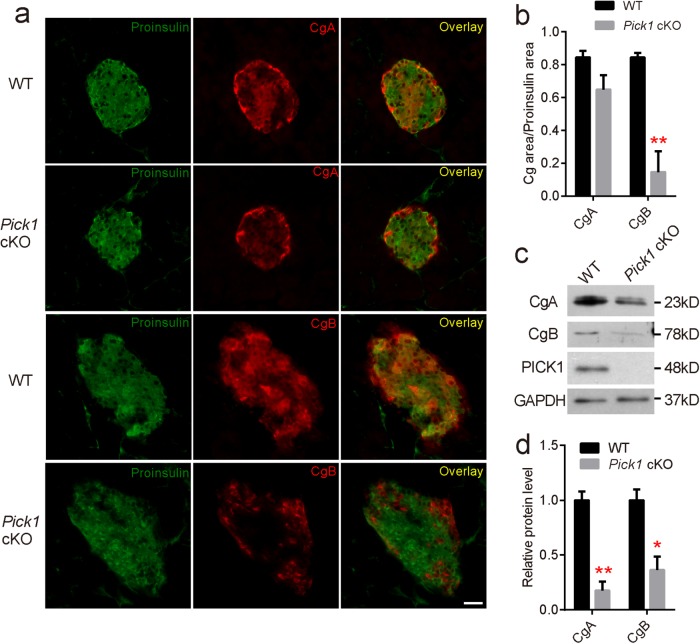
To evaluate the possibility that PICK1 is also involved in granule biogenesis and protein sorting, we assessed the most abundant and ubiquitously expressed granins, chromogranin A (CgA) and chromogranin B (CgB), which are implicated as the major constituents of dense-core secretory granules in endocrine cells. Proinsulin and chromogranin costaining revealed that CgA was moderately expressed in pancreatic beta cells but was highly expressed at the edges of the islets, which likely comprise the α cells, in both the WT and Pick1 cKO islets (Figure 5a). In sharp contrast, CgB expression was restricted to proinsulin-negative cells in the Pick1 cKO islets, while its expression was universal in the WT islets (Figure 5a). This observation indicates a sharp decrease in CgB in the pancreatic beta cells from the Pick1 cKO islets, which is consistent with the quantification analysis (Figure 5b). As shown in Supplemental Figure S2, b and c, the costaining of insulin and CgB also revealed the same expression pattern. Moreover, a dramatic decrease in the protein expression of both CgA (CgA product, beta-granin) and CgB was observed in the Pick1 cKO islets (Figure 5c).
FIGURE 5:
Dramatic decrease in CgB in pancreatic beta cells from Pick1 cKO mice. (a) Pancreatic sections from 5-mo-old mice of the indicated genotypes were stained with antibodies against proinsulin (green) and CgA (red) or proinsulin (green) and CgB (red). Scale bar represents 50 μm. (b) Quantification analysis of the Cg/proinsulin area. n = 3. 25 islets were calculated per genotype. (c) Western blot analysis of CgA and CgB in isolated islets from WT and Pick1 cKO mice. (d) Quantification of CgA and CgB in isolated islets from WT and Pick1 cKO mice. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01; two-sided Student’s t test.
Proinsulin accumulation induced ER stress and compensatory insulin transcription in Pick1 cKO islets
Abnormal accumulation of proinsulin resulted from lower PC1/3 and PC2 expression levels in Pick1 cKO islets and could lead to ER stress; thus, several downstream genes that are involved in the unfolded protein response (UPR) were examined using quantitative reverse transcription-PCR (RT-PCR). As expected, many UPR target genes, including Chop, Xbp1, Atf4, and the downstream Bip, were significantly increased in the Pick1 cKO islets (Figure 6a). We concluded that proinsulin accumulation that was triggered by Pick1 deficiency in islets led to ER stress. To further evaluate the function of the Pick1 cKO islets, we measured the mRNA levels of several genes that are involved in the regulation of insulin transcription. As shown in Figure 6b, the mRNA levels of Pdx1, Ins1, and Ins2 were significantly up-regulated in the Pick1 cKO islets, indicating that the compensatory increase in the proinsulin protein level was largely due to the increased mRNA levels of Pdx1, Ins1, and Ins2.
FIGURE 6:
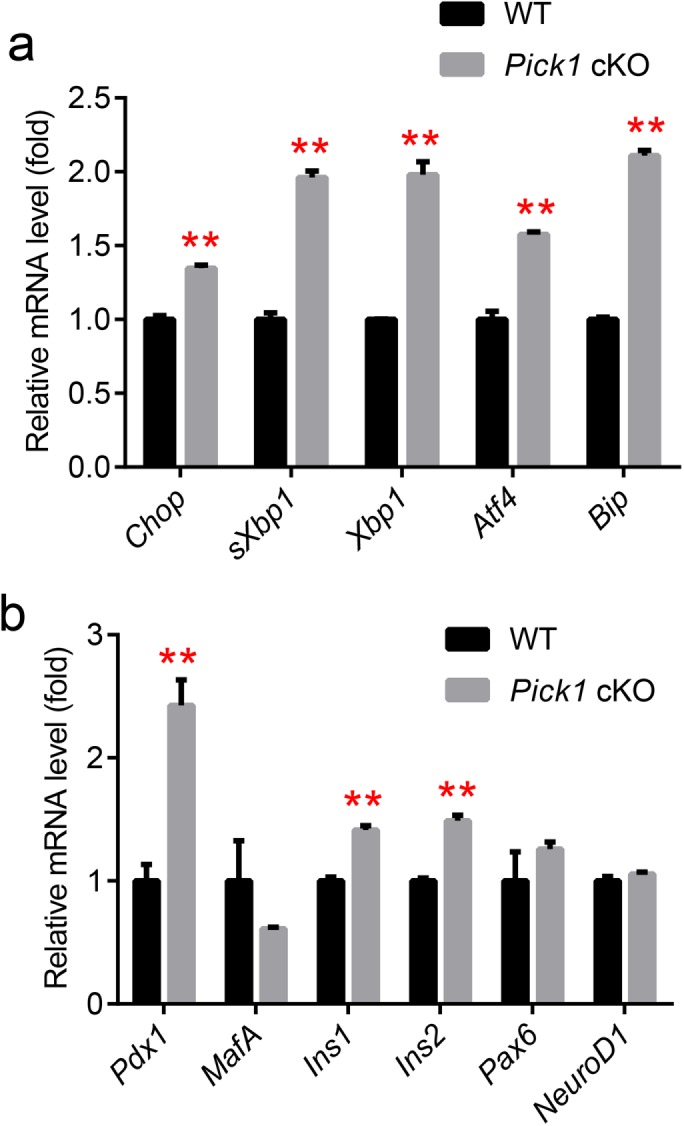
Compensatory activation of insulin synthesis. (a, b) Quantitative analysis of the mRNA expression levels in islets from WT and Pick1 cKO mice, n = 3. Genes related to UPR (a) and insulin transcription (b). Data are represented as mean ± SEM. **p < 0.01; two-sided Student’s t test.
DISCUSSION
In this in vivo study, we provide evidence that Pick1 is critical for both beta cell function and glucose homeostasis. Pick1 cKO mice displayed significant glucose intolerance and hyperglycemia as early as 5 mo of age, which was not due to insulin resistance but was instead related to an insulin production/maturation defect. This defect was coupled with the reduced expression of PC1/3 in the islets, which was accompanied by a specific loss of CgB in pancreatic beta cells.
Results from the current study suggest that insufficient insulin storage accounts for the phenotype, a conclusion supported by multiple lines of evidence. First, in INS1-E cells, transient transfection of Pick1 shRNA led to impaired insulin content, while the overexpression of Pick1 decreased the level of insulin, indicating that the rapid regulation of Pick1 can alter insulin content. Second, Pick1 cKO islets exhibited a marked reduction in the beta cell area that was paralleled by decreased insulin content. Specifically, Pick1 cKO islets are also characterized by the loss of the initial rapid phase of insulin secretion. The release of readily releasable pool (RRP) granules accounts for the first phase of GSIS, and mobilization of a subsequent supply of new granules for release by mobilization accounts for the second phase (Prentki et al., 2013). The loss of PICK1 may reduce the size of mature insulin in RRP, while new synthesized granules may be slightly affected or some compensation mechanism may work later.
In line with previous observations in global Pick1-knockout mice, our study also reveals that poor proinsulin processing/insulin maturation in islets is the key defect that accounts for insulin deficiency induced by Pick1 deletion. Importantly, significantly elevated circling proinsulin was first identified, along with proinsulin accumulation in islets in the Pick1 cKO mice. The increased ratio of circulating proinsulin to insulin found in Pick1 cKO animals suggests a high risk of diabetes. Moreover, the proinsulin secretion that was evoked by 16.7 mM glucose increased by 50%, while 2.8 mM glucose increased the proinsulin secretion fivefold in vitro, indicating that elevated proinsulin leakage can be triggered even under basal conditions. Thus, PICK1 appears to be particularly important for the maintenance of proinsulin granule trafficking and insulin granule maturation, likely by regulating granule-specific sorting channels.
Altogether, these data strongly suggest that the beta cell–specific deletion of Pick1 leads to hyperglycemia in mice. Global Pick1-knockout mice show only a subtle diabetes-like phenotype (Cao et al., 2013), likely because of a secondary phenotype that includes defects in other tissues or organs, possibly from the brain, digestive system, and pancreas. Because these organs are all hormone-rich and PICK1 plays a key role in secreted granule trafficking, it is possible that some of the hormones countering the action of insulin could also be reduced in global Pick1-knockout mice. This could lead to a mild net effect on glucose homeostasis. In fact, it was reported that a severe reduction in growth hormone (GH) storage in the pituitary and impaired secretion of both insulin and GH in response to physiological stimuli were found in global Pick1-knockout mice (Holst et al., 2013). GH treatment is known to be diabetogenic and could negatively affect glucose tolerance (Yakar et al., 2004); the loss of GH and impaired GH secretion might compensate for the absence of PICK1 in pancreatic beta cells in global knockout mice.
Furthermore, our work on proinsulin processing regulated by Pick1 confirms the roles of PC1/3 in insulin granule maturation. We found that the reduced expression of PC1/3 is accompanied by poor insulin maturation. PC1/3 is believed to be more important than PC2, since the PC3-null pancreas extracts contained over 85% proinsulin-like material, while PC2-null mice only exhibited a significant elevation of proinsulin to ∼35% (Zhu et al., 2002; Furuta et al., 1998). As a consequence, compensatory activation of the transcription of Pc1/3 and Cpe and a mild increase in ER stress trigger branches of the UPR, such as Xbp1, Chop and Atf4, were observed in Pick1 cKO islets. Although apoptosis has been implicated in beta cells, we failed to detect increased TUNEL/caspase 3-positive cells in the islets, which should accompany ER stress.
In further support of a causative role of PICK1 in insulin granule biogenesis, CgB was found to be selectively ablated in pancreatic beta cells from Pick1 cKO islets. CgA and CgB have previously been hypothesized to play key roles in granule biogenesis and protein sorting. CgA was reported to play an on/off switch role in secretory granule biogenesis (Kim et al., 2001), while CgB is more effective than CgA at inducing secretory granule formation in nonneuroendocrine NIH3T3 and COS-7 cells (Huh et al., 2003), thus enhancing vesicular storage and the release of catecholamines from PC12 cells (Zhang et al., 2014). CgB-deficient mice exhibit somewhat impaired glucose clearance and reduced insulin release but normal insulin sensitivity (Obermuller et al., 2010). CgB-knockout islets lack the initial rapid phase of stimulated secretion, and proinsulin is stored and released twice as much in CgB-knockout islets as in WT islets (Obermuller et al., 2010), which is consistent with our Pick1 cKO islet data. It is hypothesized that PICK1 regulates insulin granule formation via CgB, but the underlying molecular mechanisms remain to be investigated.
In conclusion, our findings raise the possibility that the ablation of Pick1 in pancreatic beta cells is associated with beta cell failure, hyperglycemia, and the development of diabetes. In light of the critical role of PICK1 in proinsulin processing and insulin granule maturation, we hypothesize that it could serve as a therapeutic target for the development of new drugs for the prevention and treatment of diseases related to proinsulin accumulation and/or CgB-elevated pancreatic islet cell tumors (Taupenot et al., 2003).
MATERIALS AND METHODS
Antibodies
The mouse anti-PICK1 polyclonal antibody was generated as previously described (Cao et al., 2007). The mouse anti-PC1/3 (1:1000 for Western blotting) antibody was purchased from Abnova, the mouse anti-PC2 (1:1000 for Western blotting) was purchased from EMD Millipore Corporation, and the mouse anti-CPE (1:2000 for Western blotting) was purchased from BD-Biosciences. The guinea pig anti-insulin (1:500 for immunostaining) and rabbit anti-CgA and CgB (1:800 for Western blotting, 1:200 for immunostaining) antibodies were purchased from Abcam. The mouse anti-proinsulin antibody (1:50 for immunostaining) was obtained from R&D Systems. The mouse anti-glucagon antibody (1:50 for immunostaining) was purchased from Sigma. The rabbit anti-SgII antibody (1:100 for immunostaining) was purchased from Novocastra Laboratories. The Alexa Fluor 488– and Alexa Fluor 647–conjugated secondary antibodies were obtained from Molecular Probes. The horseradish peroxidase (HRP)–labeled secondary antibody was purchased from Amersham BioSciences (GE Healthcare).
Cell culture and transfection
INS-1E cells were cultured in a humidified atmosphere containing 5% CO2 in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol, 10 mM HEPES, 100 U/ml penicillin, and 100 µg/ml streptomycin. The culture was passaged every 3 d. Transient transfection was performed using Lipofectamine 2000.
Animal care
Male and female C57BL/6J mice, 4–6 wk of age, were purchased from the Animal and Plant Care Facility of Hong Kong University of Science and Technology. All mice were maintained in an environmentally controlled facility under standard light (12 h light/dark cycle) and temperature conditions and were given free access to water and a standard rodent chow. Three to four male/female mice from the same parents were maintained in an open cage with corncob bedding. The mice were assessed to ensure that they were fit for the experiments, and the procedures were selected to minimize pain, stress, and other discomfort to the mice. The microbiological status of the mice was monitored by the facility using sentinels. All animal procedures were approved by the Animal Ethics Committee of the Hong Kong University of Science and Technology.
Generation of conditional Pick1 knockout mice
The conditional Pick1-KO mice were generated by crossing a floxed pick1 mouse line with the RIP2-Cre transgenic line. The RIP2-Cre transgenic mice (Cui et al., 2011) were first crossed with Pick1-targeted mice, which were generated by homologous recombination as previously described (Gardner et al., 2005), referred to as Pick1 loxp/loxp in this paper. Then the RIP2-Cre: Pick1loxp+/− mice were bred with each other to generate the final conditional knockout mice (RIP2-Cre: Pick1lopx/loxp). WT and RIP2-Cre littermates were used as controls. All experiments were performed with 18- to 20-wk-old male mice.
Genotyping PCR and quantitative RT-PCR
Pick1 floxed mice were genotyped by PCR using DNA harvested from their ears with the following primers: forward, 5′-ATCCCTGAACTTGAGAGGTGGAG-3′, and reverse, 5′-TCACTTGCCAGAGGAGAAAACTG-3′. The PCR product of the WT allele is a 400–base pair band, and that from the floxed allele is 200 base pairs. Islets from the WT and conditional Pick1 KO mice were used for quantitative RT-PCR analysis. Total RNA was extracted with TRIzol reagent (Invitrogen) as described in the manufacturer’s instructions, and cDNA was prepared by reverse transcription using a First Strand cDNA synthesis kit (Fermentas). Quantitative RT-PCR was performed with SYBR Green on an ABI7900 fast real-time PCR system (Applied Biosystems). GAPDH was used as the control gene. The delta delta Ct method was used to compare the gene expression differences between the genotypes. The primer information is provided in Supplemental Table S1.
Mouse islet isolation
Mouse islet isolation was performed as previously described (Cao et al., 2007). Briefly, in their home cages, the mice were starved overnight before the test and then transferred to the laboratory. Each mouse was given an intraperitoneal administration of a mixed anesthetic, which included a combination of 75/1/0.2 mg/kg of propofol/medetomidine/fentanyl. After 10 min, when the loss of the pedal withdrawal reflex was observed, a blood sample was collected by cardiac puncture. After 30 min, the blood samples were centrifuged at 100 × g for 10 min, and the serum was collected for enzyme-linked immunosorbent assay (ELISA).
For the islet isolation, collagenase V (0.9 mg/ml) (Sigma) was dissolved in Hank’s balanced salt solution (HBSS) and injected into the pancreas through the common bile duct. The distended pancreas was isolated and incubated in collagenase V solution (0.9 mg/ml) at 37°C for 10–14 min. The islets were separated on a Histopaque 1.077 g/ml (Sigma) density gradient and hand-picked under a dissecting microscope. The islets were cultured in a humidified atmosphere containing 5% CO2 in an RPMI 1640 medium supplemented with 10% FBS, 1 mM sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin, and 100 µg/ml streptomycin. After overnight recovery, the islets were used for the subsequent functional analyses.
OGTT and ITT
An oral glucose tolerance test (OGTT) and an insulin tolerance test (ITT) were performed as previously described (Li et al., 2010). For the OGTT, the mice were starved overnight, and glucose was delivered by oral gavage at 2.5 g/kg body weight after initial measurement of fasting blood glucose. For the ITT, mice were fasted for 2 h and then received an injection of regular human insulin in saline (0.5 U/kg; Roche). Blood glucose was determined 0, 30, 60, and 120 min after the glucose or insulin load with a One Touch Ultra 2 glucometer (Lifescan, Milpitas, CA).
Immunocytochemistry
The pancreas was fixed, processed, stained, and quantified as previously described (Cao et al., 2013). The pancreas was isolated and fixed with 4% paraformaldehyde and 4% sucrose in PBS for at least 72 h at 4°C and then cryoprotected by incubation in gradient sucrose–phosphate-buffered saline (PBS) solutions at 4°C (10% sucrose for 1 h, 20% sucrose for 1 h, and 30% sucrose overnight). Cryosections (15 µm thick) were used for immunohistochemical analysis. Sections were rinsed with PBS, permeabilized, and blocked with 10% normal goat serum plus 0.2% Triton X-100 in PBS for 1 h at room temperature and then incubated overnight with primary antibodies at 4°C in a humidified atmosphere. After gentle washing with PBS and incubation with fluorescent secondary antibodies for 1 h at room temperature, the sections were dehydrated and mounted with the VectaMount Solution (Vector Labs).
Western blotting and protein analysis
Tissue lysates (5–20 μg protein) were analyzed by SDS–PAGE and Western blotting (Cao et al., 2013).
Glucose-stimulated insulin secretion
For the glucose stimulation assay, the islets were preincubated in Krebs Ringer bicarbonate HEPES buffer (KRBH, 120 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 10 mM NaHCO3, and 30 mM HEPES, pH 7.4) containing 2.8 mM glucose for 1 h at 37°C. The islets were then transferred to 1 ml KRBH buffer containing 2.8 or 16.7 mM glucose for another 1-h incubation at 37°C. The medium was collected and centrifuged at 500 × g for 5 min. The supernatant was used for insulin/proinsulin measurements. Total insulin was extracted with acid ethanol. For the KCl stimulation assay, the islets were preincubated in KRBH buffer containing 4.8 mM KCl for 1 h at 37°C and transferred to 1 ml KRBH buffer containing 4.8 mM or 30 mM KCl for a 30-min incubation at 37°C. For the time course experiment, the islets were preincubated in KRBH buffer containing 2.8 mM glucose for 1 h. The islets were then incubated in 1 ml KRBH buffer containing 16.7 mM glucose, and the supernatant from each time point was collected to measure the insulin concentration and to generate time course curves.
ELISA
The serum insulin and proinsulin and the insulin from the medium and islet supernatants collected from the GSIS were tested using the Ultra-sensitive mouse insulin ELISA Kit (Crystal Chem) or the Proinsulin rat/mouse ELISA Kit (Mercodia Insulin/Proinsulin).
Statistics
The level of significance of the difference between the data sets was assessed using a two-sided Student’s unpaired t test. The results are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Tung Wailin (Hong Kong University of Science and Technology) for technical assistance. This work was supported in part by the Research Grants Council of the Hong Kong SAR, China (16146516, 16102914, 663613, 768113M, 17127015, N_HKUST625/15, HKUST10/CRF/12R, C4011-14R, T13-607/12R, AoE/M-05/12, and AoE/M-604/16), the Hong Kong Scholars Program, and the National Natural Science Foundation of China (31401152).
Abbreviations used:
- CgA
chromogranin A
- CgB
chromogranin B
- CPE
carboxypeptidase E
- ER
endoplasmic reticulum
- GH
growth hormone
- GLP-1
Glucagon-like peptide-1
- GSIS
glucose-stimulated insulin secretion
- ICA69
autoantigen 69 kD
- ISG
immature secretory granules
- MODY
maturity-onset diabetes of the young
- PC1/3
prohormone convertase 1/3
- PC2
prohormone convertase 2
- PICK1
protein interacting with C-kinase 1
- RRP
readily releasable pool
- RT-PCR
reverse transcription-PCR
- SG
secretory granule
- T2D
type 2 diabetes
- UPR
unfolded protein response.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-03-0204) on January 3, 2018.
REFERENCES
- Alberti KG, Zimmet PZ. (1998). (2010). Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med (7), 539-553. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care (Suppl 1), S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. (2013). Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab , 819-837. [DOI] [PubMed] [Google Scholar]
- Cao M, Mao Z, Kam C, Xiao N, Cao X, Shen C, Cheng KK, Xu A, Lee KM, Jiang L, Xia J. (2013). PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance. PLoS Biol , e1001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Xu J, Shen C, Kam C, Huganir RL, Xia J. (2007). PICK1-ICA69 heteromeric BAR domain complex regulates synaptic targeting and surface expression of AMPA receptors. J Neurosci , 12945-12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wang Z, Cheng Q, Lin R, Zhang XM, Leung PS, Copeland NG, Jenkins NA, Yao KM, Huang JD. (2011). Targeted inactivation of kinesin-1 in pancreatic beta-cells in vivo leads to insulin secretory deficiency. Diabetes , 320-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. (1998). Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest , 515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. (1998). Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem , 3431-3437. [DOI] [PubMed] [Google Scholar]
- Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. (2000). Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis , 139-142. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. (2005). Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron , 903-915. [DOI] [PubMed] [Google Scholar]
- Halban PA. (1994). Proinsulin processing in the regulated and the constitutive secretory pathway. Diabetologia , S65-S72. [DOI] [PubMed] [Google Scholar]
- Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, Ledizet M, Sosenko JM, Krischer JP, Palmer JP, Type 1 Diabetes TrialNet Study Group (2015). β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest , 1163-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Madsen KL, Jansen AM, Jin C, Rickhag M, Lund VK, Jensen M, Bhatia V, Sorensen G, Madsen AN, et al. (2013). PICK1 deficiency impairs secretory vesicle biogenesis and leads to growth retardation and decreased glucose tolerance. PLoS Biol , e1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh YH, Jeon SH, Yoo SH. (2003). Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem , 40581-40589. [DOI] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng JH, Eiden LE, Loh YP. (2001). Chromogranin A, an on/off" switch controlling dense-core secretory granule biogenesis. Cell , 499-509. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. (1999). Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell , 329-339. [DOI] [PubMed] [Google Scholar]
- Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, et al. (2010). Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab , 154-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Eriksen J, Milan-Lobo L, Han DS, Niv MY, Ammendrup-Johnsen I, Henriksen U, Bhatia VK, Stamou D, Sitte HH, et al. (2008). Membrane localization is critical for activation of the PICK1 BAR domain. Traffic , 1327-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermuller S, Calegari F, King A, Lindqvist A, Lundquist I, Salehi A, Francolini M, Rosa P, Rorsman P, Huttner WB, Barg S. (2010). Defective secretion of islet hormones in chromogranin-B deficient mice. PLoS One , e8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C. (1992). Glucagon-like peptide-1, a new hormone of the entero-insular axis. Diabetologia , 701-711. [PubMed] [Google Scholar]
- Papa FR. (2012). Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med , a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfutzner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lubben G, Forst T. (2004). Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care , 682-687. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. (1999). Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol C , 305-315. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM, Madiraju SR. (2013). Metabolic signaling in fuel-induced insulin secretion. Cell Metab , 162-185. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Montag AG, Thomas G, Albiges-Rizo C, Carroll R, Benig M, Phillips LA, Martin S, Ohagi S, Gardner P, et al. (1992). Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci USA , 8822-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, Petrie JR, Travers ME, Bouatia-Naji N, Dimas AS, et al. (2011). Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes , 2624-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT. (2003). The chromogranin–secretogranin family. N Engl J Med , 1134-1149. [DOI] [PubMed] [Google Scholar]
- Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. (1993). Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes , 1678-1682. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. (1999). Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron , 179-187. [DOI] [PubMed] [Google Scholar]
- Xu J, Xia J. (2006). Structure and function of PICK1. Neurosignals , 190-201. [DOI] [PubMed] [Google Scholar]
- Yabe D, Seino Y. (2016). Type 2 diabetes via beta-cell dysfunction in East Asian people. Lancet Diabetes Endocrinol , 2-3. [DOI] [PubMed] [Google Scholar]
- Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. (2004). Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest , 96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Biswas N, Gayen JR, Miramontes-Gonzalez JP, Hightower CM, Mustapic M, Mahata M, Huang CT, Hook VY, Mahata SK, et al. (2014). Chromogranin B: intra- and extra-cellular mechanisms to regulate catecholamine storage and release, in catecholaminergic cells and organisms. J Neurochem , 48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. (2002). Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA , 10293-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.