Abstract
Treatment guidelines for chronic pain recommend non-pharmacologic modalities as part of a comprehensive management plan. Chronic pain is common among people living with HIV/AIDS, but there is little data to guide the choice of non-pharmacologic therapies in this complex population. We performed a mixed-methods feasibility study of Mindfulness Based Stress Reduction (MBSR) versus health education control with 32 inner city, HIV-infected participants. Outcome measures included: the Brief Pain Inventory, Perceived Stress Scale, HIV Symptoms Index, autonomic function testing, and audiotaped focus groups. Post-intervention, participants reported modest improvements in pain measures and perceived stress, but no effect of group assignment was observed. At 3-month follow-up, 79% of MBSR participants were still practicing, and pain intensity was improved, whereas in the control group pain intensity had worsened. Qualitative analysis revealed a strong sense of community in both groups, but only MBSR was perceived as useful for relaxation and pain relief.
Keywords: HIV, Mindfulness Based Stress Reduction (MBSR), chronic pain, mixed methods
Introduction
Pain is very common among people living with HIV/AIDS (PLWHA). A recent meta-analysis reported a point prevalence of pain in HIV of 54%, with pain prevalence increasing to 83% when participants were asked to recall over the past 3 months.1 Estimates of the prevalence of chronic pain among PLWHA vary based on the population described and the method of ascertainment, ranging from 39–85%.2–4 Relieving chronic pain is a priority for patients and their health care providers. However doing so is difficult. Pharmacologic treatment options are often limited by medical co-morbidity and polypharmacy. In particular, the use of prescription opioids for chronic pain is constrained by the risk of problematic use,5 and limited evidence of benefit.6 Non-pharmacologic interventions are considered an important part of the management of chronic pain in general. Proposed mechanisms of action include improved pain coping, and amelioration of deconditioning that often results from, and in turn exacerbates, chronic pain. However patients with limited resources have difficulty obtaining such treatments. Thus there is a tremendous need in the HIV community for low-cost, non-pharmacologic interventions for chronic pain. However there has been little study of non-pharmacologic interventions for chronic pain in PLWHA. The data that do exist are mainly from small pilots describing acupuncture,7 hypnosis,8,9 and physical therapy,10 and a larger but non-randomized study of cognitive behavioral therapy.11 Potentially complicating the design and implementation of such interventions is the known difficulty of keeping some HIV-populations engaged. Overall HIV-populations exhibit 25–35% “no-show” rates, and this rate is significantly higher among certain sub-populations, particularly minorities with lower income and education levels.12
Mindfulness Based Stress Reduction (MBSR) is an 8-week course developed to reduce stress and symptoms, including pain, in medically ill people.13,14 It includes a variety of mindfulness practices including breathing techniques, body awareness meditations, and yoga. There have been numerous studies of MBSR for the treatment of chronic pain outside the realm of HIV including general chronic pain populations,15–17 and more specific conditions such as tension headache,18 fibromyalgia,19 low back pain,20,21 rheumatoid arthritis,22 and pediatric chronic pain.23 A systematic review of this literature is currently underway. MBSR is attractive for use in the HIV clinic because it is conducted in a group setting, allowing the treatment of several patients at once, requires little or no specialized equipment, and can accommodate patients with varying levels of fitness and health. Several studies have explored the use of MBSR in PLWHA, examining a variety of outcomes including markers of immune function (number and function of natural killer cells, CD4+ cell counts), psychological symptoms (e.g. depression, perceived stress), physical symptoms, and medication adherence.24–29 However all of these studies are limited by the lack of a randomized design and/or a rigorous control group. For most, the control consisted of care as usual, a wait list, or brief educational sessions. None included weekly classes. These controls are problematic because they do not mimic the structure, continuity, and social environment provided by the MBSR course. A supportive social environment has been shown to effect a variety of health-related outcomes in HIV including: depression,30,31 quality of life,32,33 medication adherence,34,35 retention in care,34 cognitive symptoms,36 stress,37 and wellbeing.38 There is also some evidence to support a link between social support and immune function,39,40 with some41–43 but not all44 studies finding an association. The importance of controlling for social environment has been recognized in studies of MBSR outside the realm of HIV, in which a control condition, designed to mimic the social but not the mindful aspects of MBSR (referred to as the health enhancement program45), has been increasingly employed.
We describe herein a mixed methods, randomized, controlled pilot study of MBSR for the treatment of chronic pain in HIV. We became interested in studying MBSR for chronic pain in HIV because of its successful use in other complex populations.46 Also MBSR (like other inwardly-focused meditation techniques) has effects on the autonomic nervous system,47 which is commonly dysregulated in HIV,48 and can be a source of altered body awareness (referred to as interoception).49 In addition, MBSR is well-known, well-defined, designed for use in a medical setting, and contains a variety of different mindfulness techniques. This last attribute was particularly attractive to us in the context of this partially qualitative pilot study, because we would be able to explore whether one or more of these techniques might warrant greater focus in future studies using a more tailored intervention. Finally MBSR has a group-based, in-person format which has the benefit of being cost-effective and also providing a potentially therapeutic social environment. We designed the study with the following goals in mind. First we sought to determine the feasibility of MBSR “as-is” in an inner city, predominantly minority population of PLWHA, including retention in the program, acceptance of the techniques, and qualitative data on what (if any) aspects of the course were helpful for chronic pain. We chose not to adapt the MBSR intervention beforehand because there is no prior published experience with MBSR and chronic pain in HIV, and so we thought it best to expose participants to the intervention “as-is” first so that the knowledge gained could be used to tailor a future intervention. We also sought to produce preliminary evidence for effect of MBSR (as compared to an intervention that controlled for supportive social environment) on the following quantitative measures: pain intensity, pain interference, symptom burden and perceived stress. Finally we sought to gather preliminary data as to whether the MBSR course had effects on autonomic function as measured by a standardized battery of autonomic function tests.50
Methods
Participants
Participants were recruited from prior research studies or referred by primary care physicians at our institution. Included participants were English speaking, HIV-infected adults who had been experiencing neuropathic and/or musculoskeletal pain for at least 3 months. In recognition of the medical and psychiatric complexity of patients with co-morbid HIV and chronic pain we sought to be as inclusive as possible, thus our only other inclusion criterion was that (in the opinion of the PI and referring physician) patients should be medically and psychiatrically stable enough to come regularly to the intervention and participate in a non-disruptive manner. All procedures were performed according to a protocol approved by the Institutional Review Board at our institution. All participants provided written informed consent.
Study Procedures
At baseline participants underwent a standardized assessment of autonomic function consistent with clinical guidelines,51,52 which includes: quantitative sudomotor axon reflex testing, heart rate variability in response to paced deep breathing, and heart rate and blood pressure responses to Valsalva maneuver and tilt table testing.53 Participants were advised to refrain from smoking and caffeine consumption on the day of testing, and to delay taking any medications that might interfere with the testing. Symptom questionnaires were also completed at this visit including: the HIV Symptom Index,54 the Brief Pain Inventory (short form),55 and the Perceived Stress Scale.56
Following the baseline visit, ten randomly selected participants attended an audiotaped focus group designed to elicit their experiences of chronic pain in the context of HIV, and their perceptions of mind-body treatments for pain. All focus groups were facilitated by the same investigator, a PhD candidate in health psychology (MCG), who did not participate in any of the intervention sessions. Following the baseline focus group, participants were randomized 1:1 to either MBSR or control. The MBSR course has been described elsewhere.13,14 We conducted the course “as-is” without alteration in content. Briefly, the course consists of 8 weekly classes conducted in-person, in a group setting, and comprised of guided meditations, gentle movement exercises, and group discussion. The MBSR teacher was a social worker specializing in HIV and geriatrics, who had previous experience leading MBSR groups in a New York City HIV clinic.
The control group also consisted of 8 weekly classes. We wanted the content of these classes to be interesting enough to engage participants and so we consulted with our clinic’s community advisory board (CAB) whose members are all PLWHA, several of whom also have chronic pain. The CAB recommended an interactive rather than didactic format, and provided the following topics as they relate to HIV-associated chronic pain: family dynamics, exercise, communicating with healthcare providers, medication adherence, community resources, complementary and alternative treatments, addiction, and nutrition. We then sought out facilitators appropriate for each topic. The facilitators included physicians (neurology and psychiatry), a psychologist, social workers, and non-medical experts in fitness, nutrition, and community resources for promoting health.
All participants (MBSR and control groups) received reminder phone calls prior to the classes, and were called again if they missed class in order to address barriers to attendance (e.g. transportation). All sessions for both MBSR and control were conducted at the medical center where the participants were receiving their primary HIV care. Sessions were observed by an investigator (JRP) and field notes were made immediately after the sessions.
Individual post-intervention visits were conducted during which the questionnaires and autonomic assessment were repeated. Two additional focus groups were also conducted, one for the MBSR group and one for the control group. The purpose of these focus groups was to elicit participants’ experience of the interventions, including any effect on symptoms, and suggestions for improvement. All focus groups and interviews were conducted with the goal of achieving theme saturation, and were concluded when the moderator perceived that no new information was being produced. The final study visit occurred three months later. At this visit participants again completed the questionnaires and MBSR participants underwent an interview regarding whether they had continued any of the practices.
Measures
Pain intensity and pain interference were measured using the Brief Pain Inventory.55 Pain intensity was defined as the average of four items (scored 0–10): worst, least and average pain over the last 24 hours, and pain “right now.” Pain interference was defined as the average of seven items (scored 0–10) which rate the degree to which pain interferes with: general activity, mood, walking, work, relations with others, sleep and enjoyment of life. Symptom burden was defined using the HIV symptom index which is a 20-item questionnaire which queries a variety of symptoms (constitutional, gastrointestinal, pulmonary, psychiatric and neurologic) relevant to HIV.54 Perceived stress was quantified using the Perceived Stress Scale, which is a 10-item questionnaire which rates frequency of experiences such as feeling “nervous and stressed.”56 Autonomic function was quantified using the Modified Composite Autonomic Severity Score (mCASS), which is calculated from the data obtained in the autonomic function testing as previously described.57 However since the mCASS is designed to measure autonomic dysfunction, and may therefore not be sensitive to changes that remain within normal limits, we also examined other exploratory markers of autonomic function including: evoked sweat output, heart rate response to deep breathing, Valsalva ratio, resting heart rate, resting blood pressure, respiratory rate, and baroreflex sensitivity (adrenergic and vagal).58
Analyses
The focus of the study was on the qualitative aspects, and also on feasibility, acceptability, and effect sizes. The number of patients included in the study was based on the number of patients that could be comfortably accommodated in the two groups (MBSR and control), and also based on the needs of the qualitative analysis, rather than a power calculation for the quantitative analysis. Descriptive statistics were performed for quantitative data including attendance and retention in the intervention. Correlations between baseline variables were explored using Pearson’s correlation coefficients. Repeated measures analysis of variance (ANOVA) was performed to determine the effect of group assignment on pain intensity and other symptoms over the study period. All analyses were two-tailed and performed at the α=0.05 level using SPSS version 22. Due to the pilot nature of this study, small numbers, and low power to detect quantitative change no correction for multiple comparisons was made. Qualitative data were analyzed using a six-step thematic analysis method as described by Braun and Clarke.59 While similar to grounded theory, thematic analysis does not require a pre-existing theoretical framework and need not be directed toward theory development, constraints which were not directly useful in our study. We chose inductive and essentialist/realist approaches, the former meaning that we coded data without trying to fit it to a pre-existing framework, and the latter meaning that we sought to report the experiences of our participants without interpreting them in the context of a societal discourse.59 We began with transcribed audio-recordings from the focus groups and interviews, and also with reference to notes written by investigators during the study procedures. The data analysis began with detailed reading of the transcripts by three of the co-investigators independently (JRP, MK, MCG) in order to become immersed in the data. During these readings the investigators initially attempted to identify interesting features of the data that might ultimately form patterns and the basis for themes. With subsequent readings, codes were identified, recorded and organized in an iterative process of moving repeatedly through the data. Commonality in codes was then sought and codes were grouped or combined into themes as appropriate. Themes were then reviewed and refined, and the data were assessed for theme saturation. The coding and identification of themes and subthemes were discussed and developed collaboratively. Quotes supporting the themes and sub-themes were identified by MK and JRP.
Results
Participants and baseline quantitative results
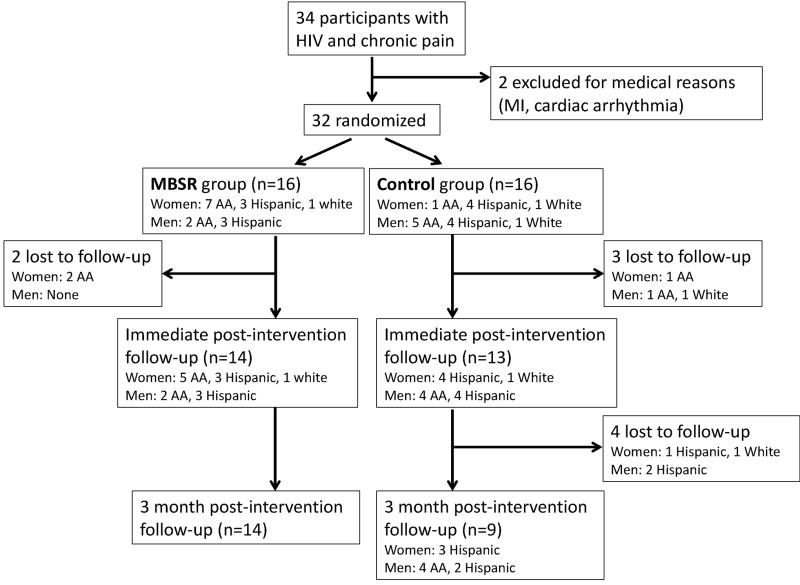
Thirty-four HIV-infected adults enrolled in the study, two were subsequently excluded, one due to a myocardial infarction which occurred shortly after the baseline visit, and the other on account of a cardiac arrhythmia detected during the autonomic testing. As shown in the table, the sample (N=32) was predominantly minority, with a fairly equal distribution of men and women, which is representative of the chronic pain patients in our clinic. All participants had public forms of insurance (27 with Medicaid, 3 with Medicare based on disability status, and 2 with Medicare based on age). Seventeen (53%) participants lived alone, of whom three had been widowed. Of the 15 participants who did not live alone, eight resided with a spouse or life partner, and seven resided with other family members, typically children and/or grandchildren. No participants were currently employed in a traditional full time job, although three identified with a specific profession (two in the arts and one in technology). Several reported informal part-time work (e.g. dog-walking, babysitting), and many, particularly the women, were caregivers to family members, such as grandchildren. Four participants spoke about having been incarcerated in the past, although this was not specifically queried.
Table.
Participant characteristics at baseline
| Overall (N=32) | MBSR (n=16) | Control (n=16) | |
|---|---|---|---|
|
| |||
| Age, years | 52 (8.0) | 53 (6.3) | 51 (9.8) |
|
| |||
| Gender | |||
| Female | 53% | 69% | 38% |
| Male | 47% | 31% | 62% |
|
| |||
| Ethnicity | |||
| African-American | 47% | 56% | 38% |
| Hispanic | 44% | 38% | 50% |
| Non-Hispanic, white | 9% | 6% | 13% |
|
| |||
| CD4+ cells/mm3 | 675 (322) | 674 (323) | 677 (333) |
|
| |||
| Source of pain | |||
| Musculoskeletal | 53% | 50% | 56% |
| Neuropathic | 22% | 19% | 25% |
| Both | 25% | 31% | 19% |
All participants were treated with combination antiretroviral therapy (CART), and the mean CD4+ count was above 600 cells/mm3. The majority (69%) had undetectable viral loads. An additional 25% had viral loads under 500 copies/ml. Only one participant had poorly controlled HIV with a viral load above 45,000 copies/ml, which was thought to be due to poor medication adherence. The majority of participants (88%) had at least one past and/or current psychiatric diagnosis including 22 (69%) with depressive disorders, 11 (34%) with anxiety disorders, and 1–2 patients with each of the following: post-traumatic stress disorder, bipolar disorders, adjustment disorders and somatization/hypochondriasis.
The majority of patients (53%) experienced musculoskeletal pain including fibromyalgia, chronic low back pain, osteoarthritis, myofascial pain, and osteonecrosis. Others experienced neuropathic pain (22%) including HIV-associated distal symmetric polyneuropathy, HIV-associated vacuolar myelopathy, and post-herpetic neuralgia. The remaining participants (25%) had both musculoskeletal and neuropathic pain.
On a scale of 0–10, mean baseline pain intensity was 5.8 (1.9) and mean baseline pain interference was 6.5 (2.4). There were multiple correlations between baseline symptom measures including: pain interference and pain intensity (r=0.70, p>.001), perceived stress and symptom burden (r=0.64, p>.001), perceived stress and pain interference (r=0.51, p=.003), and pain interference and symptom burden (r=0.46, p=.008). Autonomic function testing at baseline, summarized by the mCASS, was mildly abnormal in 52% of participants, with 42% of participants exhibiting normal or equivocal results, and the remainder (6%) exhibiting moderate autonomic dysfunction.
Baseline focus group
There were five men and five women in the in the baseline focus group (6 African-American, three Hispanic, and one white). The baseline focus group was performed prior to randomization, but the participants went on to be randomized evenly between the control and MBSR groups (five in each). Much of the discussion focused on topics that are known to be important generally in the experience of chronic pain, such as pain interference and the interaction between pain and mood. In addition, two themes more specific to the experience of HIV-associated chronic pain also emerged.
The first theme was balancing the need for privacy with the desire for social support. Due to their HIV status and prior experiences with disclosure, many participants considered sharing their health issues to be high stakes. This reticence extended to chronic pain, which they were hesitant to discuss with others. For example, a Hispanic man shared that he did not speak freely about his neuropathic pain: “I can definitely relate… on the disclosure issue. For me it’s the trust. I mean a small circle of friends, I do talk to them. But when I mean a small circle of friends, I can count them on one hand.” Another participant, an older Hispanic woman, recalled that when she first developed neuropathy she was hesitant to tell anyone, but related it to her experience of sharing her HIV-status, which was difficult but worthwhile: “My feet, now they’re starting also to bother me. But like I said, I’m glad I could talk to my sister… She’s always there for me… When I told her about my HIV … in the beginning, she took it so bad, so seriously… But I’m so glad that I told her.”
The second theme was the interrelatedness of the chronic pain and HIV experiences. For example, one African-American man said: “Especially the pain, and then when I put HIV in there as well, (it) has taken away my youth.” An African-American woman said: “You don’t want to take nothing (for pain) because you’ve gotta deal with the HIV meds.” A Hispanic man said: “I go for pain management but… I’m not sure if this is part of the HIV.”
We also specifically inquired about the perception of mind-body treatments, and found that participants were generally open to them, as illustrated by the following quotes from three African-American male participants, to which the group expressed general agreement: 1) “The mind does control the body. If you can conquer that, you can conquer this;” 2) “Change your thinking, change your life. I don't remember quite where I heard it. But those words resonated with me.” 3) “You sort of like trigger that into a more mellow thinking… It helps. It’s a tool to take you away from stressful situations.”
Feasibility of the MBSR intervention
Overall the MBSR intervention was found to be feasible, in so far as we were able to maintain a core group of participants sufficient to establish a sense of continuity and progress. MBSR was also feasible in terms of participant acceptance of the practices. Participants actively engaged with the MBSR instructor and were willing to try what she asked them to do even when it seemed unfamiliar. For example, one Hispanic man who had a history of bilateral hip replacements and was physically inactive, was able to perform some simple modified yoga poses on the floor, despite initial trepidation, and was visibly pleased afterward. The MBSR group was also successful in establishing a calm and supportive atmosphere in which participants were verbally gentle with one another.
The tone in the control group was palpably different. Conversations were lively and active, and participants spent a fair amount of time critiquing the information being presented, which they appeared to enjoy doing. They were respectful with one another in their interactions, but there were active exchanges of viewpoints and a tendency toward offering advice from personal experience.
The main challenge to feasibility was maintaining consistent attendance, which was an issue for both the MBSR and control groups equally, and was quite labor intensive (see also figure 1). Many of the participants (n=20) had pre-existing relationships with members of the research team either through the clinic or prior research studies. We actively capitalized on this pre-existing rapport, and sought to develop similar rapport with participants who were new to us. We took time to talk with participants before and after the sessions. We called every participant prior to each session to encourage them to attend, and to convey that they were welcome and valued as part of the group. We also called participants after missed sessions to remind them they were still welcome, and to address potential barriers to attendance. The main barriers cited by participants were scheduling conflicts with other responsibilities or appointments, transportation, and the weather (the sessions were conducted in New York City in early 2014 during which time there were multiple snow storms). The mean number of classes attended by participants was 4.8 for both the MBSR and control groups. Attendance was not predicted by participant demographic factors (age, gender, ethnicity, whether or not they lived alone) or by whether or not participants had a pre-existing relationship with the research team. However higher baseline pain interference was associated with less frequent attendance (r=−.357, p=0.045).
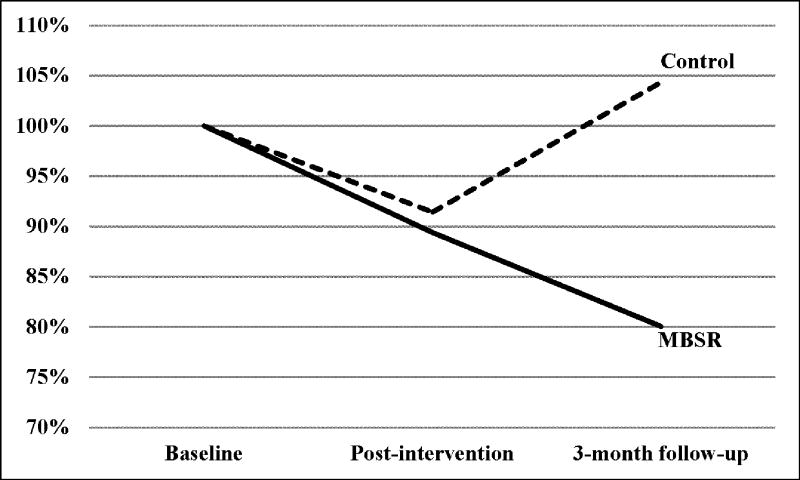
Figure 1.
Percent change in pain intensity over the course of the study
Immediate post-intervention quantitative results
All of the quantitative symptom measures improved modestly from baseline to post intervention, with small effect sizes (Cohen’s d ranging from 0.18 to 0.33). The change reached statistical significance for perceived stress (p=0.033) and pain interference (p=0.015), and trend level for pain intensity (p=0.08). However there was no difference between the MBSR and control groups (p > 0.2 for all). There were no significant differences from baseline to post-intervention in any of the measures of autonomic function.
Immediate post-intervention qualitative results: the common theme of community
Qualitative analysis of the post-intervention focus groups revealed one theme common to both the MBSR and control groups: “community.” Participants from both groups valued the opportunity to come together and connect with people with similar experiences. Participants from the MBSR group expressed the following. A Hispanic woman said: “We got to learn about each other’s health issues and the stress that we go through… And that makes you feel, like you know I'm not the only one.” An African-American woman said: “We were in this room to connect and to tell each other what was going on because we are all basically in the same place.” Another African-American woman added: “What was most helpful for me was having the place to talk to people like myself. And when I spoke about having HIV and medication, and me being tired of being sick, other people could identify with me. And I sat here and I cried, and I just let go of everything that I had been holding on to for years.” A Hispanic woman said: “But you feel like the connection because you know that the person is going through a similar thing like you. And with the class and everything, I mean it seems like it helped.” Participants from the control group expressed similar sentiments. Three African-American men expressed the following: “It was great to come together with people who have similar medical issues;” and “For me, like I said, this class was wonderful… I have this chronic back pain and fatigue due to my virus… I need a goal, you know, to get me out. And this group motivated me… I’m like: okay, I’m gonna get myself together and I’m gonna calm down and I’m gonna be around these people who share the same issues that I have. And I leave the group feeling a little enlightened;” and “Other people who are going through something similar… you can talk to them about… how they alleviate some of the stuff that… might help you deal with some of the stuff you going through.” A Hispanic man said: “I loved hearing different people talk about what they’re going through and how they deal with it. And it gives me an idea of how I can change myself… when it comes to my pain.”
Immediate post-intervention qualitative results: additional themes from the MBSR group and contrasts with the control group
In addition to the theme of “community,” which was common to both groups, the MBSR group had two additional themes that had no correlate in the control group: 1) MBSR techniques are useful for relaxation and relieving pain; and 2) practice of MBSR techniques has benefit that extends beyond the practice time.
Examples in support of the first theme included the following. African-American women contributed these two quotes: “I worry a lot… When I find myself getting into that place, I tell myself now to breathe, breathe in, breathe out, and I can feel the difference… My main issue was dealing with chronic pain, and I was kind of skeptical that meditation could actually help with that to ease it, but it does;” and “I had more energy, more calmer, and best of all the pain wasn’t bothering me so much.” A white woman said: “My body didn’t tense up as it usually does…Usually I tense up and then I react. And I just realized my mind has more control than my body.” A Hispanic man said: “I learned how to count to 20 before I react, not ten, 20. It doesn’t take the pain away, but it does ease it.”
The second theme was that the practice of MBSR techniques has benefit that extends beyond the practice time. A Hispanic man said: “The sessions here made me feel very, very peaceful. It was like I was in a very serene place. I opened the doors on to 5th Avenue to take the bus and that serenity was still with me.” An African-American woman agreed: “I went home and I was just like: I'm gonna have peace this weekend… Because I was like so – like he said, in sync and it was serenity.” A Hispanic woman added: “I used to rush and rush… so now I'm slowing down and taking my time.”
In contrast, the control group felt that their experience did not provide anything they could take with them. A Hispanic man said: “You know what I don’t like about these type of – I’ll say seminars… is that you all get a little bit of information and you all drop everything, which is sad… Everything just comes to a halt.” When asked whether the control group led to any change, the group agreed with a Hispanic woman who answered: “Nothing changes at all.”
Immediate post-intervention qualitative results: suggestions for improvement of the MBSR intervention
Participants from the MBSR group were also specifically asked how the course could be improved to better suit their needs. Overall there was a diversity of opinion, but consensus emerged on two points. The first was that there should be more time for conversation, as demonstrated by the following exchange between four participants (two Hispanic men, a Hispanic woman, and an African-American woman): “She kept saying: okay, be quiet.” “It was the only thing that I didn’t like.” “Me, too.” “Being told to be quiet.” “We had a chance to not just hear what everybody has to say, but to talk to each other. And we were being curtailed from doing that.” The second theme was that there should be less sitting still, which tended to exacerbate pain, for example (from an African-American woman and a Hispanic woman respectively): “What I didn’t like is the sitting for a long period of time. It helped when we did the yoga exercises for me because it loosened up my bones or whatever. But the last part was the sitting… I can't sit in one spot like that.” “Me too, because my right leg always numbs when I'm sitting too long, all the way down to my feet it gets numb.”
Three-month post-intervention follow-up results
All participants were contacted three months after the intervention. Of the 16 participants in each group, 14 MBSR participants returned for follow-up, whereas only 9 control participants returned (as depicted in figure 1). Participants who did not return for follow-up were re-contacted and encouraged to do so until the study team deemed that further contact would be futile and/or harassing. As illustrated in figure 2, at 3-month follow-up the average pain intensity of the participants in the MBSR group had continued to improve representing a 20% reduction from baseline, a relatively large effect size (Cohen’s d = 0.76), and a change in the median pain intensity from 5.6 to 3.9. Meanwhile for participants in the control group, pain intensity had reverted, and was 4% greater than baseline.
Figure 2.
Study participant flow chart
Analysis of the interviews with the MBSR participants at 3-month follow-up revealed that 11/14 (79%) were still practicing one or more of the techniques they had learned including seated meditation, walking meditation, mindful breathing, and yoga. Six were practicing every day or almost every day, four were practicing 2–3 days per week, and one was practicing about once a week.
Discussion
Chronic pain is very common among PLWHA and effective, low-cost, non-pharmacologic treatments are needed. Mindfulness Based Stress Reduction (MBSR) is attractive as a possible treatment because it has been used for pain and symptom management outside the context of HIV, and there is some preliminary data that it could be beneficial in HIV for immunologic function, psychological and physical symptoms, and medication adherence.24–29 However prior studies of MBSR in HIV have not included a rigorous control, which is crucial in studies with self-reported outcomes such as pain. We undertook the present pilot study to assess the feasibility of MBSR in an inner city, predominantly minority, HIV-infected population; to gather preliminary evidence as to whether MBSR was effective as a treatment for pain; and to determine whether MBSR had effects on autonomic function as measured by a standardized battery of clinical diagnostic testing.
At baseline we found that participants’ pain experience was closely related to their stress level and overall symptom burden. This is consistent with the recent findings of other authors studying chronic pain in HIV who recently reported that mood and pain are closely related in HIV.60 We also found a high prevalence of autonomic dysfunction (58%) most of which was mild, consistent with our prior findings.48 During the intervention phase, we found that the MBSR course was feasible, in that participants were accepting of and engaged in the practices, and we were able to establish a sense of continuity and progress throughout the sessions. However we exerted considerable effort in trying to support attendance, and even with this effort, on average participants attended only slightly more than half the sessions. We suspect that this is largely a feature of the population rather than the intervention, since it was an issue in the MBSR and control groups equally. However this experience suggests that such interventions might work best if they are implemented in the context of existing infrastructure, such as care coordination programs.
Following the MBSR and control interventions, there was modest quantitative improvement overall in the pain measures and perceived stress, but this was no different in the MBSR versus control groups. Neither MBSR nor the control intervention had any discernable effect on autonomic function. The qualitative data revealed similarities and differences in the ways participants viewed MBSR vs. control. The main similarity was also the dominant theme in both groups, “community.” This finding suggests that our control intervention was successful in mimicking the social aspects of MBSR, but more importantly demonstrates the strong degree to which participants in both groups bonded with each other over the shared experience of chronic pain and HIV. At the end of the study, one Hispanic woman who had been in the control group commented that although she had attended groups for PLWHA before she had never enjoyed them, and that this group seemed to really understand her. Although it is possible that this strong bonding will not be reproducible, we believe that this finding constitutes a rationale to more explicitly emphasize a social component in future interventions for HIV and chronic pain, for example by incorporating peer-mentorship models.
Other than the feeling of community the MBSR and control groups were perceived quite differently. MBSR participants reported that the intervention was helpful for their pain, and helped them relax and feel more peaceful. When asked how the MBSR course could be improved, participants voiced that they would prefer less sitting (which exacerbated their pain) and more social interaction. In contrast, members of the control group felt that nothing had changed as a result of the course, although they had enjoyed participating.
At 3-month follow-up we had tremendous difficulty getting members of the control group to return for assessment, and those that did return (n=9) had lost whatever small improvements in symptoms they had experienced. In contrast, all of the MBSR participants who had completed the intervention (n=14) returned at three months. The MBSR group at 3-month follow-up demonstrated continued improvement in symptoms, and 79% were still regularly practicing one or more of the techniques they had learned, with some having developed a consistent daily practice. We believe that the MBSR participants were more likely to return because their ongoing practice made them feel a continued connection to the study, whereas for the control participants there was no enduring effect once the camaraderie of the group was gone.
Overall the results of this pilot study provide important guidance for future work seeking to examine the role of MBSR and other mind-body therapies for symptom management in HIV. First, we found that a mind-body intervention such as MBSR was feasible, well-accepted, and considered beneficial by our sample of inner city, predominantly minority, HIV-infected participants. Second, we found that both MBSR and a control intervention designed to mimic the social aspects of MBSR were similarly effective in leading to short-term improvements in pain and perceived stress. This finding highlights the importance of a proper control group in this type of study, and also demonstrates the potential benefit of structured social interaction among patients with co-morbid HIV and chronic pain, suggesting that a social component should be considered in the design of non-pharmacologic interventions for this population. However longer-term benefit was seen only in the MBSR group, suggesting that MBSR had an additional effect that accrued after the social support of the group was removed. The follow-up interviews suggest that this effect could be attributable to continued practice of the mindfulness techniques. As one participant, a Hispanic man, said, “Before, I would just explode and want to fight when I felt pain, I would wanna go get high… I learned that… if I just sit down… with a little bit of time and effort it will subside, it will pass.”
The mechanism by which MBSR might lead to reductions in pain remains uncertain, particularly since MBSR is an amalgam of multiple mindfulness techniques from different traditions which may have different effects. We were unable to demonstrate any autonomic physiologic change, although in retrospect the measurement technique we chose, although the gold standard for the clinical measurement of autonomic dysfunction, was likely insufficiently sensitive. Prior studies attempting to measure autonomic changes due to mindfulness techniques such as MBSR, have typically used healthy volunteers and made the measurements while the subject was actively practicing.61–63 We chose to measure autonomic function in a resting “non-mindful” state with the rationale that it would be more indicative of durable change, but in hindsight it would have been preferable to maximize the likelihood of detecting change by measuring autonomic function during mindfulness practice, especially in light of the small sample size and the high prevalence of autonomic dysfunction in HIV.
This study has limitations. Since the study was intended to be an exploratory pilot, the sample size is small and so the characteristics of individual participants have the potential to affect the outcome greatly. This is especially true because we were studying a group intervention, where group dynamics play an important role. By chance, more women were randomized to MBSR, since we did not stratify the randomization by gender. While we are not aware of any literature reporting gender differences in response to MBSR, there are well documented gender differences in the pain experience including higher prevalence of many chronic pain conditions in women, and gender differences in experimental pain perception.64 It has been proposed that there may be central mechanisms which predispose women to pain and make them more difficult to treat including less active descending inhibitory mechanisms.64 Thus having more women in the MBSR group may have decreased the likelihood of demonstrating an effect. However having more women in the MBSR group and more men in the control group may also have contributed to differences the groups’ atmosphere, with the MBSR group being calmer and the control group being more active. In the future it would be preferable to stratify randomization by gender. Despite extensive efforts, we did not achieve full attendance in either group, with the mean number of classes attended being 4.8 (60%) for both groups. Thus we cannot know if a post-intervention difference would have been observed if participants had attended more classes. The time point at which a between group difference was observed, three-month follow-up was confounded by a loss to follow-up that was significantly greater in the control group. We hypothesize that this is because most participants in the MBSR group were still practicing the techniques they had learned and thus still felt more connected with the study. However we cannot exclude other causes. Due to this uneven loss to follow-up, the difference in pain intensity observed at 3-months must be interpreted with caution and cannot be considered definitive.
Conclusions
In summary, this study provides preliminary evidence that MBSR may be a helpful tool in reducing chronic pain associated with HIV, and that some of this effect is likely related to the supportive social environment of the course. In future studies, a tailored mindfulness intervention which contains more opportunity for social interaction, and less time sitting still, might be more successful. Strategies to maximize attendance also must be considered, such as deploying the intervention in the context of a clinic-based care coordination program.
Acknowledgments
This work was supported by a grant (K23 NS066789) from the National Institute of Neurological Disorders and Stroke to Dr. Robinson-Papp, and the CTSA grant (UL1 TR000067) awarded to the Mount Sinai School of Medicine. The authors thank Dr. Monica Rivera Mindt and Vanessa Guzman for their contributions which include critical review of the statistical analyses and manuscript. Figure production was aided by Daniel’s XL Toolbox addin for Excel, version 6.53, by Daniel Kraus, Würzburg, Germany.
Contributor Information
Mary Catherine George, Icahn School of Medicine at Mount Sinai Department of Neurology, Research Program Manager, fax: 212-987-3301, mary-catherine.george@mssm.edu.
Arada Wongmek, Icahn School of Medicine at Mount Sinai Department of Neurology, Visiting Research Scholar, fax: 212-987-3301, arada.wongmek@mssm.edu.
Michelle Kaku, Icahn School of Medicine at Mount Sinai Department of Neurology, Neurology Resident, fax: 212-987-3301, michelle.kaku@mssm.edu.
Alexandra Nmashie, Icahn School of Medicine at Mount Sinai Department of Neurology, Research Coordinator, fax: 212-987-3301, alexandra.nmashie@mssm.edu.
Jessica Robinson-Papp, Icahn School of Medicine at Mount Sinai Department of Neurology, Assistant Professor, fax: 212-987-3301, jessica.robinson-papp@mssm.edu.
References
- 1.Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: A systematic review. J Int AIDS Soc. 2014;17:18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12(9):1004–1016. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KA, Gay C, Portillo CJ, et al. Symptom experience in HIV-infected adults: A function of demographic and clinical characteristics. J Pain Symptom Manage. 2009;38(6):882–893. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlin JS, Cen L, Praestgaard A, et al. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. J Pain Symptom Manage. 2012;43(3):638–645. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson-Papp J, Elliott K, Simpson DM, Morgello S for the Manhattan HIV Brain Bank. Problematic prescription opioid use in an HIV-infected cohort: The importance of universal toxicology testing. J Acquir Immune Defic Syndr. 2012;61(2):187–193. doi: 10.1097/QAI.0b013e3182683c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 7.Shlay JC, Chaloner K, Max MB, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. JAMA. 1998;280(18):1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman D, George MC, Schnur J, et al. Hypnosis for the treatment of HIV neuropathic pain: a preliminary report. Pain Med. 2013;14(7):1048–1056. doi: 10.1111/pme.12074. [DOI] [PubMed] [Google Scholar]
- 9.Langenfeld MC, Cipani E, Borckardt JJ. Hypnosis for the control of HIV/AIDS-related pain. Int J Clin Exp Hypn. 2002;50(2):170–188. doi: 10.1080/00207140208410097. [DOI] [PubMed] [Google Scholar]
- 10.Mgbemena O, Westfall AO, Ritchie CS, et al. Preliminary outcomes of a pilot physical therapy program for HIV-infected patients with chronic pain. AIDS Care. 2015;27(2):244–247. doi: 10.1080/09540121.2014.940272. [DOI] [PubMed] [Google Scholar]
- 11.Trafton JA, Sorrell JT, Holodniy M, et al. Outcomes associated with a cognitive-behavioral chronic pain management program implemented in three public HIV primary care clinics. J Behav Health Serv Res. 2012;39(2):158–173. doi: 10.1007/s11414-011-9254-y. [DOI] [PubMed] [Google Scholar]
- 12.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clin Infect Dis. 2010;50(5):752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 13.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 14.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 15.Wong SY, Chan FW, Wong RL, et al. Comparing the effectiveness of mindfulness-based stress reduction and multidisciplinary intervention programs for chronic pain: A randomized comparative trial. Clin J Pain. 2011;27(8):724–734. doi: 10.1097/AJP.0b013e3182183c6e. [DOI] [PubMed] [Google Scholar]
- 16.Marchand WR. Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and zen meditation for depression, anxiety, pain, and psychological distress. J Psychiatr Pract. 2012;18(4):233–252. doi: 10.1097/01.pra.0000416014.53215.86. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Omidi A, Zargar F. Effect of mindfulness-based stress reduction on pain severity and mindful awareness in patients with tension headache: A randomized controlled clinical trial. Nurs Midwifery Stud. 2014;3(3):e21136. doi: 10.17795/nmsjournal21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75(6):500–510. doi: 10.1016/j.jpsychores.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain. A systematic review. BMC Complement Altern Med. 2012;12 doi: 10.1186/1472-6882-12-162. 162-6882-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmer G, Blum J, Rulf J, Pier J. Mindfulness-based stress reduction for failed back surgery syndrome: A randomized controlled trial. J Am Osteopath Assoc. 2010;110(11):646–652. [PubMed] [Google Scholar]
- 22.Fogarty FA, Booth RJ, Gamble GD, Dalbeth N, Consedine NS. The effect of mindfulness-based stress reduction on disease activity in people with rheumatoid arthritis: A randomised controlled trial. Ann Rheum Dis. 2015;74(2):472–474. doi: 10.1136/annrheumdis-2014-205946. [DOI] [PubMed] [Google Scholar]
- 23.Jastrowski Mano KE, Salamon KS, Hainsworth KR, et al. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med. 2013;19(6):8–14. [PubMed] [Google Scholar]
- 24.Seyed Alinaghi S, Jam S, Foroughi M, et al. Randomized controlled trial of mindfulness-based stress reduction delivered to human immunodeficiency virus-positive patients in Iran: Effects on CD4(+) T lymphocyte count and medical and psychological symptoms. Psychosom Med. 2012;74(6):620–627. doi: 10.1097/PSY.0b013e31825abfaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: A randomized, wait-list controlled trial. J Pain Symptom Manage. 2012;43(2):161–171. doi: 10.1016/j.jpainsymman.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gayner B, Esplen MJ, DeRoche P, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med. 2012;35(3):272–285. doi: 10.1007/s10865-011-9350-8. [DOI] [PubMed] [Google Scholar]
- 27.Jam S, Imani AH, Foroughi M, SeyedAlinaghi S, Koochak HE, Mohraz M. The effects of mindfulness-based stress reduction (MBSR) program in Iranian HIV/AIDS patients: A pilot study. Acta Med Iran. 2010;48(2):101–106. [PubMed] [Google Scholar]
- 28.Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: A small randomized controlled trial. Brain Behav Immun. 2009;23(2):184–188. doi: 10.1016/j.bbi.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson FP, Mathews HL, Witek-Janusek L. Psycho-endocrine-immune response to mindfulness-based stress reduction in individuals infected with the human immunodeficiency virus: A quasiexperimental study. J Altern Complement Med. 2003;9(5):683–694. doi: 10.1089/107555303322524535. [DOI] [PubMed] [Google Scholar]
- 30.Vyavaharkar M, Moneyham L, Corwin S, Saunders R, Annang L, Tavakoli A. Relationships between stigma, social support, and depression in HIV-infected African American women living in the rural southeastern United States. J Assoc Nurses AIDS Care. 2010;21(2):144–152. doi: 10.1016/j.jana.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breet E, Kagee A, Seedat S. HIV-related stigma and symptoms of post-traumatic stress disorder and depression in HIV-infected individuals: Does social support play a mediating or moderating role? AIDS Care. 2014;26(8):947–951. doi: 10.1080/09540121.2014.901486. [DOI] [PubMed] [Google Scholar]
- 32.Oetzel J, Wilcox B, Archiopoli A, et al. Social support and social undermining as explanatory factors for health-related quality of life in people living with HIV/AIDS. J Health Commun. 2014;19(6):660–675. doi: 10.1080/10810730.2013.837555. [DOI] [PubMed] [Google Scholar]
- 33.Bekele T, Rourke SB, Tucker R, et al. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care. 2013;25(3):337–346. doi: 10.1080/09540121.2012.701716. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: Results from the steps study. J Assoc Nurses AIDS Care. 2014;25(5):405–413. doi: 10.1016/j.jana.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124–133. [PubMed] [Google Scholar]
- 36.Atkins JH, Rubenstein SL, Sota TL, et al. Impact of social support on cognitive symptom burden in HIV/AIDS. AIDS Care. 2010;22(7):793–802. doi: 10.1080/09540120903482994. [DOI] [PubMed] [Google Scholar]
- 37.Lopez CR, Antoni MH, Fekete EM, Penedo FJ. Ethnic identity and perceived stress in HIV+ minority women: The role of coping self-efficacy and social support. Int J Behav Med. 2012;19(1):23–28. doi: 10.1007/s12529-010-9121-x. [DOI] [PubMed] [Google Scholar]
- 38.Chesney MA, Chambers DB, Taylor JM, Johnson LM. Social support, distress, and well-being in older men living with HIV infection. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S185–93. doi: 10.1097/00126334-200306012-00016. [DOI] [PubMed] [Google Scholar]
- 39.Ironson G, Hayward H. Do positive psychosocial factors predict disease progression in HIV-1? A review of the evidence. Psychosom Med. 2008;70(5):546–554. doi: 10.1097/PSY.0b013e318177216c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 41.Persson L, Gullberg B, Hanson BS, Moestrup T, Ostergren PO. HIV infection: Social network, social support, and CD4 lymphocyte values in infected homosexual men in malmo, sweden. J Epidemiol Community Health. 1994;48(6):580–585. doi: 10.1136/jech.48.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young J, De Geest S, Spirig R, et al. Stable partnership and progression to AIDS or death in HIV infected patients receiving highly active antiretroviral therapy: Swiss HIV cohort study. BMJ. 2004;328(7430):15. doi: 10.1136/bmj.328.7430.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theorell T, Blomkvist V, Jonsson H, Schulman S, Berntorp E, Stigendal L. Social support and the development of immune function in human immunodeficiency virus infection. Psychosom Med. 1995;57(1):32–36. doi: 10.1097/00006842-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ironson G, O'Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacCoon DG, Imel ZE, Rosenkranz MA, et al. The validation of an active control intervention for mindfulness based stress reduction (MBSR) Behav Res Ther. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakhan SE, Schofield KL. Mindfulness-based therapies in the treatment of somatization disorders: A systematic review and meta-analysis. PLoS One. 2013;8(8):e71834. doi: 10.1371/journal.pone.0071834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubia K. The neurobiology of meditation and its clinical effectiveness in psychiatric disorders. Biol Psychol. 2009;82(1):1–11. doi: 10.1016/j.biopsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Robinson-Papp J, Sharma S, Simpson DM, Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J Neurovirol. 2013;19(2):172–180. doi: 10.1007/s13365-013-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craig AD. How do you feel? interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 50.Low PA, Benarroch EE. Clinical autonomic disorders. 3. Wolters Kluwer, Lippincott Williams & Williams; 2008. Laboratory evaluation of autonomic failure; p. 130. [Google Scholar]
- 51.England JD, Gronseth GS, Franklin G, et al. Practice parameter: Evaluation of distal symmetric polyneuropathy: Role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):177–184. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 52.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 53.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 54.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 55.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 57.Robinson-Papp J, Sharma S, Dhadwal N, Simpson DM, Morgello S. Optimizing measures of HIV-associated neuropathy. Muscle Nerve. 2015;51(1):56–64. doi: 10.1002/mus.24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J, Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007;64(3):381–386. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- 59.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3 77-77-101. [Google Scholar]
- 60.Merlin JS, Walcott M, Ritchie C, et al. 'Two pains together': Patient perspectives on psychological aspects of chronic pain while living with HIV. PLoS One. 2014;9(11):e111765. doi: 10.1371/journal.pone.0111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann Behav Med. 2006;32(3):227–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- 62.Nyklicek I, Mommersteeg PM, Van Beugen S, Ramakers C, Van Boxtel GJ. Mindfulness-based stress reduction and physiological activity during acute stress: A randomized controlled trial. Health Psychol. 2013;32(10):1110–1113. doi: 10.1037/a0032200. [DOI] [PubMed] [Google Scholar]
- 63.Nijjar PS, Puppala VK, Dickinson O, et al. Modulation of the autonomic nervous system assessed through heart rate variability by a mindfulness based stress reduction program. Int J Cardiol. 2014 doi: 10.1016/j.ijcard.2014.08.116. [DOI] [PubMed] [Google Scholar]
- 64.Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: A systematic review. Pain. 2010;150(2):309–318. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]