Abstract
Neuroengineering is faced with unique challenges in repairing or replacing complex neural systems that are composed of many interacting parts. These interactions form intricate patterns over large spatiotemporal scales and produce emergent behaviors that are difficult to predict from individual elements. Network science provides a particularly appropriate framework in which to study and intervene in such systems by treating neural elements (cells, volumes) as nodes in a graph and neural interactions (synapses, white matter tracts) as edges in that graph. Here, we review the emerging discipline of network neuroscience, which uses and develops tools from graph theory to better understand and manipulate neural systems from micro- to macroscales. We present examples of how human brain imaging data are being modeled with network analysis and underscore potential pitfalls. We then highlight current computational and theoretical frontiers and emphasize their utility in informing diagnosis and monitoring, brain–machine interfaces, and brain stimulation. A flexible and rapidly evolving enterprise, network neuroscience provides a set of powerful approaches and fundamental insights that are critical for the neuroengineer’s tool kit.
Keywords: neuroengineering, graph theory, network neuroscience, neuroimaging, control theory
1. INTRODUCTION
Could we graft new connections into the brain to give someone back the abilities they had preinjury (1)? Could we decode the thoughts of someone who is caged inside their own body (2, 3)? Could we develop adaptive brain–computer interfaces that evolve and adapt to remain effective for a child whose brain is continuously developing (4, 5)? Answering these and many other seemingly overambitious questions is the fundamental aim of neuroengineering (6), a relatively new domain of biomedical engineering that develops and uses computational and empirical techniques to understand and modulate the properties of neural systems. Particularly exciting frontiers of neuroengineering include neuroimaging; neural interfaces; neural prosthetics and robotics; and more general techniques for regeneration, enhancement, and refinement of neural systems (7).
In the era of big data, neural systems are no exception to the rule of ever-increasing petabytes streaming into servers around the world (8). However, in many other arenas, the amount of data being gathered has not posed an insurmountable obstacle. What is the fundamental difference that causes neuroscientific data to be so challenging? Is it a lack of a mechanistic understanding of how the brain works (9, 10)? Or is it an inability to physically construct the hardware required to liaise with neural systems for effective interventions (5)? We argue that fundamental to both of these problems is the challenge of dealing with complex relational data (11). In developing a data science to meet these rising demands (12), we must acknowledge that these data are far from independent: Instead, data from neural systems are inherently relational data (13).
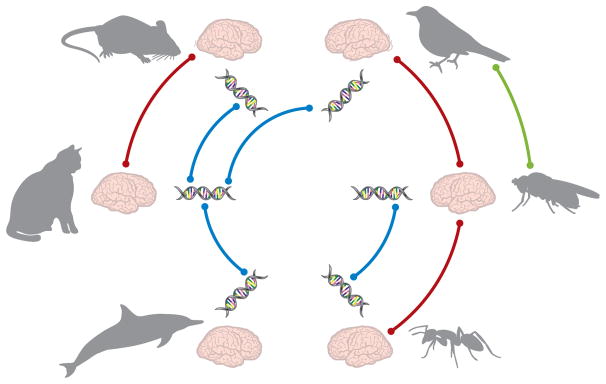
Relational data can be defined as any data that codify relationships between elements (14). The nervous system is composed of units across many spatial scales (genes, neurons, cortical columns, areas) that are related to one another in many different ways (anatomical connections, functional relationships, material similarities) (Figure 1) (15, 16). These relationships form intricate patterns—of synaptic connections, gene coexpression, connectome fingerprints—that may differ across species (17, 18) or across cohorts within a single species (e.g., in health versus disease) (19, 20). From these patterns stem the very complicated phenomena of development, behavior, and cognition (21, 22).
Figure 1.
Relational data in biological systems. Repeating genotypic and phenotypic patterns emerge frequently in the study of biological systems. These biological patterns are expressed across multiple scales of granularity. Illustrated here are three different scales of biological elements (behavioral, structural, and genetic) in different animal species, with lines representing conceptual relationships between elements. At the macroscale, we observe behavioral similarities across different species, such as the ability to fly in birds and fruit flies. However, a closer lens on the neurological substrate of this behavior may tell a different story: that mesoscale structural brain architecture differs significantly between birds and fruit flies, and is more similar between insects (e.g., fruit flies and ants) and between mammals (e.g., mice and cats). Despite differences in structural brain architecture, we might find that animals of different species share commonalities in genetic code that manifest similarly in physical attributes. Although differences in each element reveal unique qualities of each individual animal species, examining relational data can provide a more comprehensive view of the functional role of each element ecologically.
Biological patterns like these are particularly difficult to study for several reasons. First, the governing principles of pattern formation are often challenging to infer (23–25), so mechanistic insights are difficult to come by. Second, it is not always straightforward to simplify patterns using coarse graining or other dimensionality-reduction approaches while maintaining the richness of the neurophysiologically relevant information (10). Although retaining necessary information while simplifying the patterns is difficult, so is studying each element in the pattern: With thousands and sometimes millions of elements, the set of interactions between them—particularly if they evolve over time—quickly becomes enormous and complicated. Indeed, as researchers in many fields have now come to realize, the intricacies of relational data call for a new conceptual and mathematical framework (26, 27).
2. THE PECULIAR APPROPRIATENESS OF NETWORK SCIENCE
Network science is an emerging interdisciplinary field that combines theories from statistical mechanics, computational techniques from computer science, statistics, applied mathematics, engineering, and visualization approaches to probe, perturb, and predict the behavior of complex systems in technology, biology, and sociology (28). Although historically developed to understand social interactions or friendship webs similar to those supported (or elicited) by Facebook or Twitter, network science is a peculiarly appropriate framework in which to tackle the challenges of neural data sciences to better engineer artificial and natural neural systems. In particular, rather than reducing complex relational data to a list of independent parts, network science provides tools to explicitly characterize the pattern of interactions between neural elements (29). In addition to these descriptive tools, it provides benchmark graphical models for statistical comparison and inference (30–32), mathematical models to quantify and predict the flow of information (33) or communication (34) through neural circuits, and predictive tools to forecast how networks might change in response to injury (35) or therapeutic interventions (36).
How does one go about translating neural systems into the language of network science (19, 37–43)? The first critical step is to determine which constituent elements are the fundamental units of interest that are measurable in the particular experiment under consideration (44). These elements—which might be single neurons, neuronal ensembles, genes, or large-scale brain areas—are treated as nodes or vertices in the network (45). Then, one must define the connections, interactions, or relationships of interest between network nodes. These links—which might be white matter tracts between large-scale brain areas, chemical or electrical synapses between neurons, or coexpression patterns among genes—are treated as network edges. Once nodes and edges have been defined, the network itself—the pattern of edges linking the nodes—can be studied from the point of view of a graph in mathematics (46, 47).
3. DEFINING A NETWORK SCIENCE OF NEURAL SYSTEMS
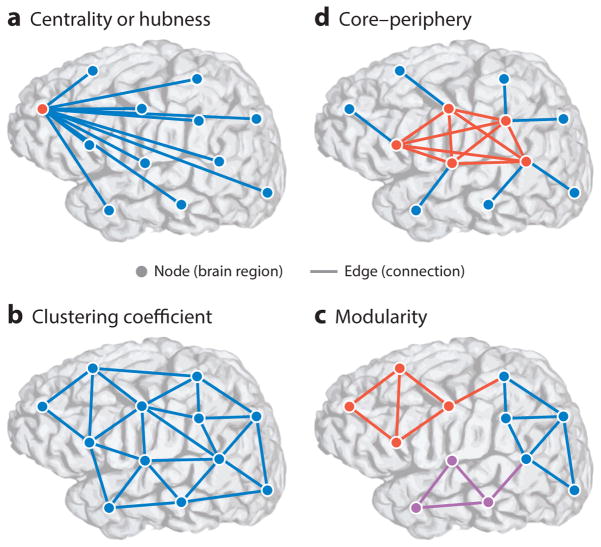
In the field of mathematics, a graph 𝒢 = (𝒱, ℰ) is composed of a node set 𝒱 and an edge set ℰ (46, 47). This information is stored in an adjacency matrix A, whose elements indicate the strength of edges between nodes. The representation of data in a graph enables the investigator to characterize the patterns of connectivity locally, surrounding a single node, or globally, taking into account all edges. In addition to local and global structure, tools are available to probe so-called mesoscale structure in the graph, which can be defined as structure that is present at intermediate length scales in the system (Figure 2).
Figure 2.
Multiscale topology in brain networks. Brain networks express fundamental organizing principles across multiple spatial scales. Brain networks are modeled as a collection of nodes (representing regions of interest with presumably coherent functional responsibilities) and edges (structural connections or functional interactions between brain regions). (a) Node centrality describes the importance of individual nodes in terms of their connectivity relative to other nodes in the network. Nodes with more connections or stronger edges tend to be hubs (red ), whereas nodes with fewer connections tend to be isolated (blue). (b) Clustering coefficient, a measure of connectivity between the neighbors of a node, is another local measure of network topology. Unlike network topologies with strong hubness qualities, as in panel a, networks with strong clustering coefficient demonstrate a high density of triangles that is believed to facilitate local information processing. (c) Modularity is a mesoscale topological property that captures communities of nodes that are tightly connected to one another and weakly connected to nodes in other communities. Modular organization underlies a rich functional specialization within individual communities. Here, nodes of different communities are colored red, blue, or purple. (d ) Networks with core–periphery structure exhibit a set of tightly connected nodes (core, red ) sparsely connected to a set of isolated nodes (periphery, blue). This organization is in stark contrast to the modular organization in panel c. The core–periphery architecture is characteristic of networks that integrate information from isolated regions in a central area.
To give the reader some simple intuitions, we briefly describe examples of local, mesoscale, and global statistics that can be computed from graphs of neural systems. [Note that we leave questions of topology (48) and network optimization (49) to later, more forward-looking sections of this review.] First, a common local statistic that has proven particularly effective in characterizing neural systems is the clustering coefficient of a node, which can be defined as the fraction of a node’s neighbors that are also connected to one another (50). In essence, this statistic is sensitive to the density of triangles in the graph, and is thought to play a nontrivial role in local information processing in neural systems (51; for caveats, see References 52 and 53). A common global statistic that has proven useful in characterizing neural systems is the characteristic path length, which is defined as the average shortest path between all possible node pairs (28). This statistic is sensitive to long-distance connections that provide shortcuts from one side of the network to another, and is thought to play a role in the swift transmission of information across the system (43). Interestingly, early research demonstrated that humans displaying brain-wiring patterns with shorter characteristic path lengths also had a higher intelligence quotient (IQ) than those with longer characteristic path lengths (54), suggesting that network statistics are sensitive to architectures that support healthy human cognitive function. However, note that short characteristic path lengths do not appear to be the full story (42, 55), and measures of segregated information processing also play an important role in brain function (56).
In addition to local and global structure, mesoscale organization provides a window into the properties of groups of nodes. Two common mesoscale structures are modularity and core–periphery structure. A network with modular structure is one that contains groups of nodes known as modules; the nodes in a module are more densely connected to other nodes in the same module than to nodes in other modules (57, 58). This modular architecture is thought to support specialization of function, each module performing a different role in support of neurophysiological processes from synchronization to cognition (56). In contrast, a network with core–periphery structure contains both a set of core nodes that are densely interconnected with all of the other nodes in the network and a set of periphery nodes that are sparsely interconnected with all other nodes in the network (59, 60). This organization is thought to support the integration of information across neuronal assemblies, neural circuits, or large-scale functional modules (61), in each of which the top-down web is often referred to as a rich club (62).
The multiscale nature of these network tools is particularly useful for neural systems, which are thought to perform inherently different computations at different levels of the network hierarchy (63). For example, information is thought to be processed in local cortical areas before being passed across modules along rich-club edges [in a so-called small world (42, 55)], allowing integrative computations and coherent behavioral responses (64, 65). Understanding this multiscale architecture and its functional role in neural system dynamics is critical for developing effective interventions that capitalize on existing structure and dynamic properties, rather than fighting against them.
4. HOW DO WE BUILD BRAIN NETWORKS?
Using the mathematical tools of network science to understand neural data requires one to explicitly build network models. How does one go about doing so? This topic warrants a full review of its own, describing methods to build brain networks from spiking data (66), calcium transients and microelectrode arrays (67), mesoscale tract tracing (55), gene expression (68), and large-scale neuroimaging (43) across species from cat (69) and macaque (70) to Caenorhabditis elegans (64), mouse (71), rat (72), Drosophila (18, 73), and human (74) (to mention only a few examples). Because we cannot do justice to the full richness of this question here, we focus our presentation on human brain imaging, which has historically provided the largest source of data for testing the utility of network science to characterize complex neural systems. Thousands of healthy subjects and patient populations have been scanned, primarily by magnetic resonance imaging (MRI), to identify both structural and functional properties of the nervous system. Given the dominant role of imaging, here we familiarize the reader with the strategies used to transform brain scans into data structures that are amenable to network analysis. However, we emphasize that the network tools we describe are fully translatable to other neural systems, and also commonly applied in electroencephalography (EEG) (75–77), magnetoencephalography (MEG) (78), electrocorticography (ECOG) (79, 80), and functional near-IR spectroscopy (fNIRS) (81, 82) (for a more thorough review of these application areas, see Reference 13).
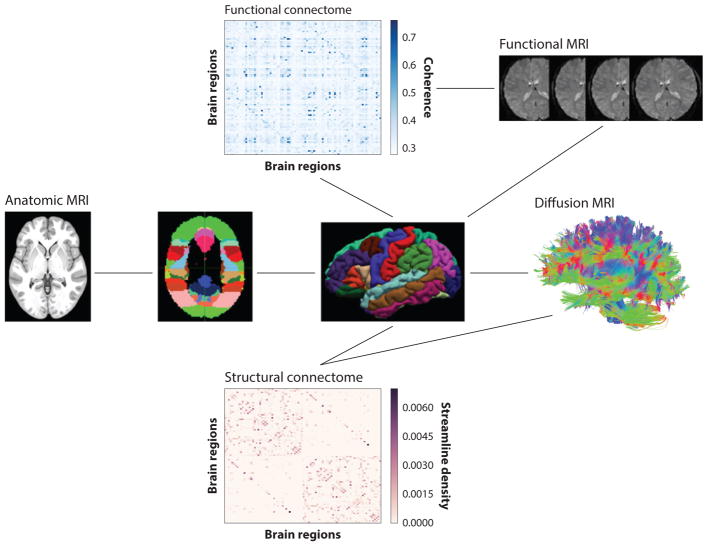
The most common measurements of human brain connectivity, whether they are functional or structural networks, rely on scans obtained by MRI. There are three basic types of brain scans that are typically used in network construction. The first is an anatomic scan. This is a T1-weighted, high-resolution (<1-mm isotropic) sequence that can distinguish gray matter from underlying white matter. Many software tools are available for segmenting these two types of tissues and for partitioning the gray matter into a set of local regions that form the network nodes (Figure 3). There are many atlases available for partitioning the gray matter, varying from ~50 to 1,000 separate regions. The second type of scan is a functional MRI (fMRI). This is a series of T2*-weighted scans acquired at sampling rates as fast as 2.21 Hz (although more typically at 0.5 Hz) and at a lower spatial resolution (~3 mm nonisotropic). This tissue contrast is sensitive to changes in blood oxygen level–dependent (BOLD) signals, which vary as a function of cortical activity (whether neuronal or synaptic). These scans can be acquired with the subject at rest, the so-called resting-state fMRI, or with the subject performing a particular task, the so-called task-based fMRI (83, 84). The time series of brain activity, averaged across all voxels in each local region, can then be extracted. To create an adjacency matrix reflecting functional connectivity, pairs of time series are related by correlation, partial correlation, wavelet-filtered correlation, or coherence within a particular frequency band (85).
Figure 3.
Constructing connectomes from magnetic resonance imaging (MRI) data. To generate human connectomes with MRI, an anatomic scan delineating gray matter is partitioned into a set of nodes. This scan is combined with either diffusion scans of white matter structural connections or time series of brain activity measured by functional MRI, resulting in a weighted connectivity matrix.
The third method is based on diffusion imaging. It involves the acquisition of a set of scans, each of which is sensitive to the magnitude of water diffusion in a particular direction in three dimensions. The set of oriented diffusion scans is then combined to estimate voxel-wise distributions of water diffusion (86). The brain is then seeded uniformly at subvoxel resolution, and a probable path of diffusion through the full volume is calculated, resulting in a virtual tract of diffusion, referred to as a streamline. Streamlines are virtual estimates of possible water diffusion that can correspond to true white matter fascicles or tracts. The white matter fascicles and tracts are thought to be the primary means for information sharing between distinct gray matter regions, analogous to wiring that connects distinct computer modules (65, 74). To create an adjacency matrix reflecting this structural connectivity, the strength of connectivity between pairs of regions can be estimated by the number of streamlines, the density of streamlines, or the number of streamlines normalized by the length they traverse (87, 88). The set of all real connections in the brain is referred to as the human connectome. Although it is not yet possible to characterize this full set of connections, it can be approximated by the streamlines reconstructed with diffusion imaging. This lower-dimensional connectome can then be characterized with the tools of network science. Given that it is an approximation, it is valuable to test the robustness of any particular network property across a range of atlases or spatial resolutions.
5. WHAT DO BRAIN NETWORKS OFFER NEUROENGINEERING?
After building networks from imaging data, one can then use these networks to address pressing questions in neuroengineering. Although we cannot exhaustively cover all possible uses of these tools currently in the literature, we highlight their utility in neural mapping and connectivity estimation, diagnosis and monitoring, and rehabilitation and treatment.
5.1. Diagnosis and Monitoring
Accurately diagnosing disorders of the human connectome and monitoring their progression are particularly critical applications of network-based tools for neural systems. Diseases thought to be accompanied by connectome abnormalities or alterations include schizophrenia (78, 89, 90), autism (91, 92), epilepsy (80, 93), and Alzheimer disease (94–96), among others (19, 97). The pattern of alterations in a given condition can be described in the form of a graph, as can the patterns that are similar or different between a pair of conditions. In some cases, these network changes occur early in a disease, offering potential as diagnostic biomarkers (98). In support of this possibility, several studies have demonstrated that by incorporating network statistics as features in machine learning algorithms, it is possible to classify groups of individuals with and without a particular condition, from aging (99) to major depression (100). Whereas diagnosis and classification are binary decisions, one can also continuously monitor brain networks within a single individual (101) either during drastic changes, such as those accompanying disease progression, or during minor changes in mental state, such as those induced by driving fatigue (102).
5.2. Rehabilitation and Treatment
The sensitivity of network measures to brain state offers the generalizable potential for graph statistics to be used as indicators of the efficacy of rehabilitation and treatment. Initial studies support this potential efficacy by demonstrating appreciable changes in network organization induced by memory rehabilitation treatment [a broad intervention useful across multiple clinical conditions (103)], seizure therapy [an intervention for severe depression (75)], and motor imagery [a frequent intervention for stroke (104)]. Common techniques to effect these interventions include neurofeedback, in which humans learn to control the activity or connectivity in certain parts of their brain to enhance mental function (105, 106). Notably, graph statistics of functional network architecture have proven sensitive to cognitive workload during these interventions, offering task-independent markers for monitoring and matching participants ability and task difficulty during neurofeedback training (107). Neurofeedback approaches often utilize exquisitely calibrated brain–computer interfaces (108), systems that can also function as neural prosthetics. When these techniques are applied to clinical populations, several pressing questions arise due to limited resources: Who will benefit the most? Can we choose the intervention that best fits a given individual? Interestingly, emerging data suggest that organizational characteristics of a person’s functional network architecture as measured by EEG can be used to predict who will be receptive to motor imagery treatment (77, 109). These initial studies underscore the potential of network representations of neural data to provide sensitive and specific markers of the receptiveness of neural circuits to induced network structure change (110).
5.3. Neural Mapping and Neural Connectivity Estimation
Although neuroengineering is often thought of as a field of clinical translation, basic science plays a fundamental role in giving the investigator the knowledge and understanding necessary to intervene in a way that benefits the system. A particularly exciting current frontier in neuroengineering involves mapping neural systems by using a variety of imaging techniques (111) and estimating the connectivity between neural elements by using sophisticated statistical algorithms (112). In these contexts, network science offers explicit tools to characterize the maps and to use empirical estimates of connectivity to inform the design of new networks. Indeed, the concept of network design is a relatively new one in biological systems. When applied to the neural domain, network design includes the building of computational models of neural dynamics, as well as physical models (113), via micropatterning, microfabricated multielectrode arrays, and low-density neuronal culture techniques (114). Together, these algorithmic and empirical approaches provide exciting avenues to map the neural connectome across scales and species (13), and to better understand the dynamics that produce cognition and behavior (21).
6. CONSTRUCTING AND USING BRAIN NETWORKS FOR NEUROENGINEERING
This brief survey of the literature demonstrates that brain networks offer exciting capabilities in addressing pressing questions in neuroengineering. In this section, we describe important considerations in constructing and using brain networks in the context of human imaging. Although we focus on human neuroimaging, these (or similar) considerations are likely to be important in the collection and analysis of other types of data (multiunit recording, optical imaging) as they become available for network analysis.
6.1. Image Acquisition
The rapid growth of imaging-based network science has been accompanied by a parallel recognition that functional and structural MRI data can be corrupted by a broad array of technical, physiologic, and anatomic factors that, if not handled properly, can lead to major errors in network modeling. The good news is that MRI is a mature technology, and it is rare for data to be corrupted by artifacts secondary to unreliable hardware or poor pulse sequence designs. The bad news is that brain imaging is commonly corrupted by more subtle physical–anatomic properties that can be difficult to surmount with conventional hardware and pulse sequences (115). Both diffusion-weighted imaging (used for structural connectomics) and T2*-weighted imaging (used to detect changes of BOLD signals in functional connectomics) are highly sensitive to susceptibility artifacts. The most troublesome cause is an air–tissue interface that leads to very localized nonlinear image distortion and signal irregularity. For example, the medial and inferior temporal cortex and orbital frontal cortex of the brain are adjacent to air-filled petrous and ethmoid sinuses. The resulting artifacts lead to missing streamlines projecting into these areas or unreliable estimates of functional activity within the distorted gray matter regions. These distortions are difficult to correct post hoc. The degree of signal dropout and missing data varies enormously between individual subjects. Thus, network analyses that are aggregated over a population need to carefully evaluate the influence of missing data on the underlying connectivity matrices.
A second challenge in brain MRI, particularly for diffusion imaging, is the effect of eddy currents. Eddy currents are loops of electrical current induced within brain tissue by the changing magnetic field required to generate images. This causes spatial distortion within each image slice and can be particularly impactful in diffusion imaging. A third challenge, also leading to geometric distortion, arises from magnetic field inhomogeneity and phase-encoding errors. There are numerous software tools available for correcting both types of distortion post hoc.
6.2. Pitfalls Unique to Functional Imaging Networks
Ideally, all functional connections, whether for a resting-state (116–118) or a task-based network, would be determined by patterned brain activity reflecting inherent cognitive processes. However, numerous other sources of noise can contribute to spurious increases in functional connectivity (119–121). One of the most important influences on functional connectivity is variation in the amplitude or rate of the cardiac and respiration cycles. Respiration rate (~0.2 Hz) and depth of breathing can clearly influence the local BOLD signal (122). Cardiac rate (~1 Hz) also influences the BOLD signal (123). These effects are regionally complex, with respiration effects more apparent near the ventricles and cardiac effects more apparent near the largest arteries. Higher-order effects of the cardiac and respiratory cycles may also be present in the tissue beyond a simple linear projection of the pulse and bellows signals. For example, chest wall expansion will influence global magnetic field inhomogeneity, and CSF pulsatility (via the cardiac pulse) may be periodic with the chest expansion. Thus, the influence of both on functional connectivity analyses will depend on an individual subject’s physiologic state and unique anatomy.
There are many retrospective strategies for removing the effects of cardiac and respiratory cycle variation on the BOLD time series from each voxel. If heart rate and respiration have been independently measured, then software tools such as RETROICOR can be employed (124). RETROICOR uses a Fourier expansion of nonbrain physiologic signals with 8–20 regressors. Although this method works well for both linear and higher-order artifacts, there is a trade-off in that increasing the number of regressors during RETROICOR correction will remove a greater amount of relevant brain signal (125).
For many experimental situations, independent measurements of heart rate and respiration are not available, and methods other than RETROICOR are needed. Here, we discuss techniques based on independent component analysis (ICA) of resting-state MRI data. ICA decomposes the functional time series for all voxels into patterns of activity consisting of a set of spatial maps, each of which has a corresponding time series that, when added linearly, sums to the original voxel-wise time series. A set of ICA components represents both brain activity and noise components. Ideally, these sources of brain and nonbrain activity are independent. If so, then these latter noise components can be removed and a new noise-free times series can be reconstructed. The challenge, then, is to find an unbiased, efficient way to identify those components reflecting noise. Manual classification of ICA components is very difficult and requires expert knowledge. One semiautomated ICA-based X-noiseifier, called FIX (126), uses a machine learning approach to aid this process. For each ICA component, a large number of distinct spatial and temporal features are generated, each describing the proportion of temporal fluctuations observed at high frequencies. These features are fed into a multilevel classifier. After training by hand classification across a sufficient number of data sets, the classifier can then be used with new data sets.
An alternative approach is to estimate pulse and respiratory variability for a subject directly from an independent set of fMRI data, utilizing temporal ICA (127). This method assumes that nonbrain physiologic noise is spatially stationary. For example, noise associated with the carotid arteries will be in the same location across different resting-state MRI scans from the same subject. Once the underlying and independently derived spatial weighting matrix is identified by ICA in one data set, it can be applied to a separate resting-state MRI time series from the same subject to produce the temporal pattern of noise. The resulting cardiac and respiratory estimators can then be used with RETROICOR or similar correction methods. Although this method works well, it requires an independent sample of functional data.
It has been assumed that global BOLD activity, measured over the whole brain, will remain constant across a time series. Any fluctuation would be due to instrumentation issues or nonbrain physiologic effects. However, recent studies have examined the effect of removing the global mean signal from the time course on subsequent connectivity analyses. Interestingly, multiple studies show that a significant portion of the global mean signal is in fact related to the average signal within particular resting-state brain networks (128, 129) and that removing the global signal can result in spurious negative correlations (130) and reduce the reproducibility of many network metrics (131). Despite these disadvantages, global signal regression can be helpful in developmental and clinical cohorts to correct for motion-related artifacts (132, 133).
Indeed, it is almost impossible for a person to remain motionless in an MRI scanner. Breathing, swallowing, and volitional movements can create motion that propagates to the head. A brain placed in the MRI field will become magnetized over ~6 s. If the brain moves, then it will no longer be magnetized in the same direction, and there can be a massive increase in the signal until the brain has remagnetized in the new magnetic orientation. To account for the effects of this motion-induced noise, a variety of retrospective methods have been proposed. Most assume that the change in signal intensity will be global, occurring within a single time sample of the resting-state MRI time series. One of the most common methods is to use linear transformations to fit each time sample to one time point. The resulting transformation weights (translations and rotations) can be used to adjust global signal intensity or be included in a regression model as a covariate of noninterest (134, 135). However, the “filtering” of time-series data with motion parameters is problematic because they do not model continuous motion directly. Rather, they capture net displacement at the temporal resolution of the sampling frequency (~0.5 Hz). If the head is displaced rapidly, and returns to the same position within a single sampling period, then there is no net displacement, but a large signal spike in the data. This will profoundly alter the strength of connectivity between areas with a common motion-induced signal change. This type of signal change has been described as the “predominant effect of motion” in a sample of patients ranging from 8 to 23 years old (132). Software has been developed to address these potential artifacts from rapid motion (or radio-frequency spikes). Rather than using the realignment information, these methods search for global spikes in signal intensity.
There is one final challenge with head motion artifacts. Within each volumetric acquisition, a stack of slices are acquired sequentially, typically by sampling first the odd slices and then the even slices. It is not uncommon for a brief head movement to demagnetize a subset of slices, causing artifacts in every other slice. These artifacts are not detected in the transformation matrix and may not alter global signal intensity. New tools are emerging to detect unexpected signal spikes within single slices (136). For a useful study benchmarking confound regression strategies for the control of motion artifacts in studies of functional connectivity, see Reference 133.
6.3. Pitfalls Unique to Structural Imaging Networks
There are many sampling schemes for acquiring a set of oriented diffusion scans. One such scheme is diffusion tensor imaging (DTI), which samples an object at a uniformly spaced set of angles and at a constant magnetic gradient strength. When the gradient strength or number of directions is increased, the angular resolution improves [Q-ball and high–angular resolution diffusion imaging (HARDI)], but with the trade-off of a reduced signal-to-noise ratio in the scans. Multiple shells of gradient strengths (multishell diffusion imaging) or a uniform distribution across gradient strength and direction [diffusion spectrum imaging (DSI)] can be applied (137). Critically, each of these methods requires a different mathematical technique for converting diffusion images to probabilistic estimates of local water diffusion, resulting in variable success at modeling the connectivity in different brain areas where there can be water diffusion in multiple directions (the crossing-fiber problem). Methods using lower angular resolution, such as DTI, consistently underestimate the number of possible streamlines by an order of magnitude compared with multishell and DSI methods. Missing connections can also arise because an insufficient number of seeds are introduced to generate the underlying streamline set. Whatever the cause, allowing for missing data can significantly alter graph metrics (83). By contrast, commonly used algorithms for generating streamlines can create noisy, anatomically implausible connections that must be removed by length and/or angle thresholds. Most current algorithms for generating streamline connections suffer from a length bias (138, 139). The shorter a connection is, the easier it will be to reconstruct. Thus, a structural network will be more likely to represent short connections than long connections. Similarly, the odds are greater that a streamline will be reconstructed if it is in a thick white matter fascicle with many fibers oriented in a common direction than if it is in a thin fascicle. To address the length bias, some authors normalize the streamline count between two gray matter regions by the physical size of those same regions. Clearly, standardization in these acquisition, reconstruction, and counting procedures is essential for reproducibility and generalizability.
7. FRONTIERS IN COMPUTATIONAL SCIENCE AND SYSTEMS ENGINEERING
With these empirical considerations in mind, it is clear that brain imaging has provided a fertile test bed for developing and testing novel tools from network science. Yet, it is also clear that this is only the first wave of innovation. Indeed, network neuroscience offers the field of neuro-engineering two distinct sets of frontiers: one in the development of computational and systems engineering approaches and the other in translating current and future advances directly to clinical populations. In this section, we briefly review new directions in algorithmic development, computational architectures, signal processing techniques, and statistics that support the extraction, representation, and characterization of meaningful relational patterns in neural data. We also discuss the nascent application of control theory to these networks, and highlight its potential utility in guiding clinical interventions.
7.1. Dynamic and Multilayer Networks
A common challenge in applying network analyses to neural data is that the processes we often wish to understand are inherently dynamic processes (140–142). Rehabilitation, response to treatment, monitoring disease progression, and tracking brain–computer interface learning are all evolving processes that occur over a range of timescales. Yet, networks in their simplest forms are static: The members of a fixed set of network nodes are connected by a single estimate of connectivity. In extending these static descriptions to incorporate time, the applied mathematics community has defined so-called multilayer networks (143). Colloquially, a multilayer network is a network that contains different layers, and in which the edges in a given layer represent a different type of relationship than the edges in another layer. Perhaps the simplest type of multilayer network is a temporal network, in which each layer is a time window and the edges within that layer represent relationships that are true in that time window (144). When each layer is tied to the next by identity links (edges between node i in layer l and node i in layer l ±1), the static graph representation as an adjacency matrix is expanded to a dynamic graph representation as an adjacency tensor (145, 146), providing important mathematical advantages to help surmount common statistical challenges present in these data.
The tools of temporal networks are particularly useful in modeling plasticity and learning with the aim of predicting recovery (147). In initial efforts, temporal networks have been used to reveal common patterns of network reconfiguration that occur as healthy adult individuals learn a new motor–visual skill over the course of days to weeks (61, 148–151). Interestingly, individuals who displayed greater network flexibility, particularly in areas of the brain that are critical for cognitive control (151), learned more quickly than individuals with less-flexible brains (148). Although these studies initially applied temporal network techniques to motor skill learning with the aim of informing rehabilitation after stroke (152), many open questions remain about how sensitive these techniques might be to neural or cognitive plasticity underlying other types of learning (153, 154), or to other dynamic processes that are important to neuroengineering in other clinical contexts.
Beyond temporal networks, one can extend the multilayer network construct to represent relationships across different imaging modalities (155), such as calcium transients and local field potentials, or structural MRI and EEG, or diffusion imaging and fMRI (156). Alternatively, one could consider letting each layer represent a different frequency band (157, 158) or a different patient in a clinical cohort. Indeed, the potential applications of these multilayer representations across neuroimaging contexts are surprisingly broad, and future efforts will likely include a careful assessment of their utility in uncovering conserved and variable properties of networked neural systems.
7.2. Statistical Tools, Frameworks, and Null Models
An important burgeoning area of research involves building, testing, and validating appropriate statistical methods and models for network inference. Because networks are not simple mathematical objects, the tools required to capture and compare them extend beyond what traditional statistics offers (159). Many efforts have focused on developing sophisticated permutation-based methods for network comparison (160, 161), and some have extended these methods to assess differences in network functions (rather than univariate statistics) (162–164), for example, by building on tools developed in the field of functional data analysis (165–167). In addition to comparing networks, one often wishes to understand whether the network structure or dynamics that one observes in empirical data is expected or unexpected. Answering these questions depends on the development of appropriate static (164, 168, 169) and dynamic (146) network null models. Statistical considerations also extend to estimating the connectivity itself (32, 170), assessing its significance (162), and measuring its relationship to behavior or symptomatology (171). Finally, a nascent area of inquiry lies in building statistical models of networks (31, 172, 173) in order to understand their generative principles (23–25, 30).
7.3. Algebraic Topology
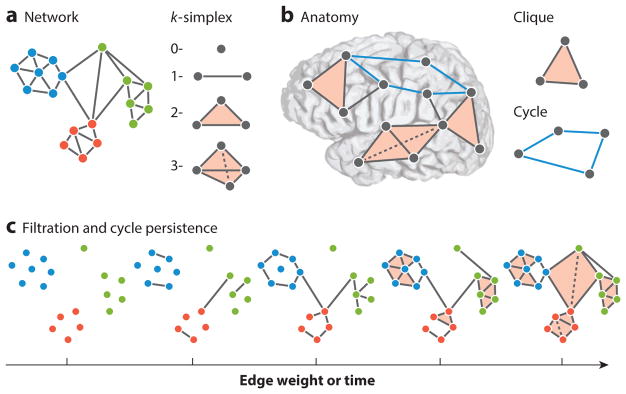
Although extremely powerful, network science is largely built on the tools of graph theory, which inherently treat the dyad (a single connection between two nodes) as the fundamental unit of interest. Recent evidence, however, indicates that sensor networks, technological networks, and even neural networks display higher-order interactions that simply cannot be reduced to pairwise relationships (174, 175). To address this growing realization, we can turn to recent advances in applied algebraic topology (48), which reframes the problem of relational data in terms of simplices or collections—rather than pairs—of vertices (Figure 4) (176). This added sensitivity enables algebro-topological tools to offer mechanisms for neural coding (177, 178), distinguish disparate classes of graph models (179), and separate healthy from clinical populations (180). The framework also offers useful tools to consider the evolution of simplices over time, drawing on the notion of a filtration, and tools to identify and track hollow cavities in networks—structures that are otherwise invisible to common graph metrics (176). We anticipate that the next few years will see an increasing interest in better understanding the role of these higher-order interactions in healthy cognition versus disease, as well as their sensitivity as biomarkers for tracking effects of training and rehabilitation.
Figure 4.
Tools for higher-order interactions from algebraic topology. (a) The human connectome is a complex network architecture that contains both dyadic and higher-order interactions. Graph representations of the human connectome encode only dyadic relationships, leaving higher-order interactions unaccounted for. A natural way in which to encode higher-order interactions is in the language of algebraic topology, which defines building blocks called simplices (176): A 0-simplex is a node, a 1-simplex is an edge between two nodes, a 2-simplex is a filled triangle, and so on. (b) These building blocks enable the description of two distinct structural motifs that are thought to play very different roles in neural computations (178): Cliques, which are all-to-all connected subgraphs, are thought to facilitate integrated computations, and cycles or cavities, which are collections of n-simplices arranged to have an empty geometric boundary, are thought to facilitate segregated codes and computations. (c) Additional tools available to the investigator include filtrations and persistent homology. Filtrations represent weighted simplicial complexes as a series of unweighted simplicial complexes, and can be used to study networks that change over time or display hierarchical structure across edge weights. Filtrations allow one to follow cycles from one complex to another and quantify how long they live (via the number of complexes in which they are consecutively present). Because this is a study of the persistence of a cycle, it is referred to as the persistent homology of the weighted simplicial complex.
7.4. Network Control Theory
The final exciting frontier that we discuss in this review—which bridges computational science and systems engineering—is the development and application of network control theory (181, 182) to neural systems (Figure 5). Indeed, neural control engineering (183) is slowly evolving into neuro–network control engineering, as the control problems become tuned to the underlying graph architecture of the dynamical processes (184). In general, these applications take one of two forms: those that seek to understand how neural systems control themselves and those that investigate how one can exogenously control a neural system, steering it away from pathological dynamics and toward healthy dynamics.
Figure 5.
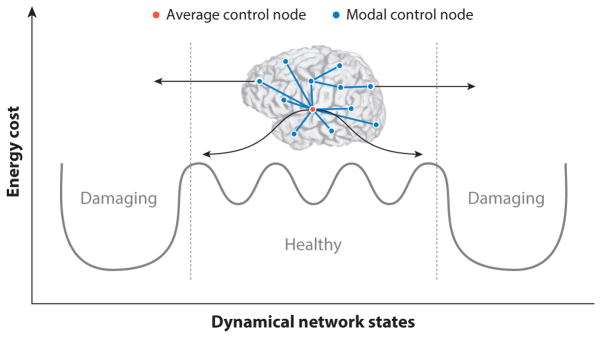
Brain network regulation and control can help navigate dynamical states. To accomplish behavioral and cognitive goals, brain networks internally navigate a complex space of dynamical states. Putative brain states may be situated in various peaks and troughs of an energy landscape, requiring the brain to expend metabolic energy to move from the current state to the next state. Within the space of possible dynamical states, there are easily accessible states and harder-to-reach states; in some cases, the accessible states are healthy, whereas in other cases, they may contribute to dysfunction, and similarly for the harder-to-reach states. Two commonly observed control strategies that are quantifiable in brain networks are average control and modal control. In average control, highly central nodes navigate the brain towards easy-to-reach states. In contrast, modal control nodes tend to be isolated brain regions that navigate the brain toward hard-to-reach states that may require additional energy expenditure (185). As a self-regulation mechanism for preventing transitions towards damaging states, the brain may employ cooperative and antagonistic push–pull control strategies (80). In such a framework, the propensity for the brain to transition toward a damaging state might be competitively limited by opposing modal and average controllers whose goal would be to pull the brain toward less damaging states.
In the first case, we seek to understand how neural systems control themselves. To address this question, we can construct a model of brain dynamics in which the current brain state depends on (a) the previous brain state, (b) the wiring pattern that structurally connects network nodes (brain regions), and (c) the control input. Assuming that this is a linear, time-invariant, noise-free, and discrete-time model, we can infer which brain regions are predisposed to affect the system, and in what ways. Early efforts along these lines revealed that regions in the brain’s executive system are well poised to push the brain into difficult-to-reach states, far away on an energy landscape (185). Moreover, the brain’s densely interconnected rich club is poised to form the ground state of the system, being the least energetically costly target state (186). Interestingly, these control principles of the brain, built on the organization of white matter tracts, are significantly altered in individuals who have experienced traumatic brain injury (187), suggesting their utility in clinical applications. However, it is also important to be cautious; these predictions are based on linear network control, whereas the brain is a nonlinear dynamical system; therefore, interpretations should be validated in additional studies (188). For example, demonstrating that individual differences in cognitive control function are correlated with individual differences in network control statistics will be an important first step (189), as will demonstrating that these statistics change over developmental timescales in which cognitive control emerges in children (190). Moreover, exploring the applicability of nonlinear control strategies, including linearization of nonlinear systems, will be an important avenue of inquiry for future research (188).
The second context in which network control theory offers a powerful tool set for neuroengineers concerns how to exogenously control a neural system and accurately predict the outcome on neurophysiological dynamics—and, by extension, cognition and behavior. Indeed, how to target, tune, and optimize stimulation interventions is one of the most pressing challenges in the treatment of Parkinson disease and epilepsy, for example (7). More broadly, this question directly affects the targeting of optogenetic stimulation in animals (191) and the use of invasive and non-invasive stimulation in humans (e.g., deep brain, grid, transcranial magnetic, transcranial direct current, and transcranial alternating current stimulation) (192). As a case study, consider medically refractory epilepsy, in which network techniques can be used to identify seizure onset zones (79, 93, 193) and network control theory can be used to detect drug-resistant seizures (194), inform the development of a distributed control algorithm to quiet seizures using grid stimulation (195), and identify areas of the network to target during resective surgery (80).
8. CONCLUSIONS AND FUTURE DIRECTIONS
8.1. Toward Clinical Translation
Together, these exciting computational frontiers have the potential to directly inform clinical practice. Indeed, several of the main translational challenges of neuroengineering are ripe for the incorporation of network data. These opportunities begin at the earliest stages of clinical diagnosis and monitoring, when variation between individuals—and, indeed, even variation within a single individual—stymies progress in tuning medication, stimulation, brain–machine interfaces, neuroprosthetics, and physical or cognitive–behavioral therapy to offer individuals a better quality of life (Figure 6). Concerted efforts in mapping relational architectures in neural and behavioral data in the form of graphs and networks will be critical to obtaining a more holistic understanding of mental health, as well as greater insights into optimizing interventions. For example, the changes in brain network architecture following stroke (196) could be explicitly linked to the changes in motor performance of that patient (197) to identify the class of rehabilitation strategies predicted to maximize that person’s recovery. Such mappings could directly occur in the traditional sense, using empirical measurements performed in research or hospital settings. But even beyond these traditional contexts, the most tantalizing possibilities include the use of digital data from social network games and platforms on smartphones to accurately phenotype individuals and the health of their mental state, with the goal of better guiding intervention strategies for the clinically unwell (198, 199).
Figure 6.
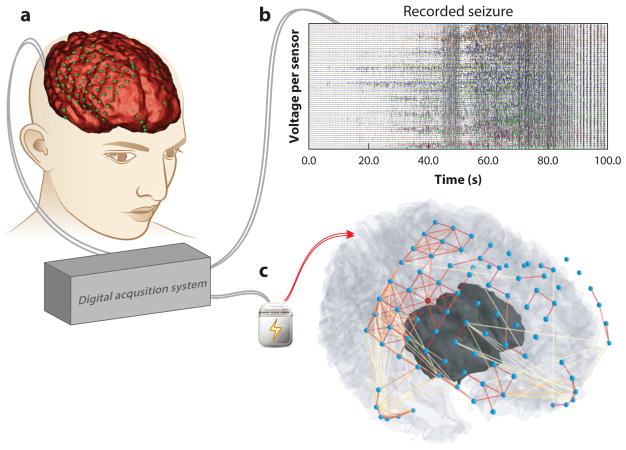
Clinical translation of network neuroscience tools. Network neuroscience offers a natural framework for improving tools to diagnose and treat brain network disorders. (a) For drug-resistant epilepsy patients, invasive monitoring of brain activity to localize brain tissue where seizures originate and to plan resective surgery is challenging, because the neural processes generating seizures are poorly understood. Epileptic brain signals, electrical fields produced by the firing of neuron populations, are sensed by electrodes that rest on the surface of the brain, beneath the dura, and are recorded by a digital acquisition system. Shown is a three-dimensional reconstruction of a patient’s brain (red ) with electrodes colocalized ( green) to anatomical features. (b) Recorded brain signals are studied by clinical practitioners to characterize spatial and temporal behavior of the patient’s seizure activity. Each line represents a time-varying voltage fluctuation from each electrode sensor. (c) Inferred functional connections from a single time slice during the patient’s recorded seizure demonstrates rich relationships in neural dynamics between brain regions and are not visually evident in panel b (nodes, blue; strong connections, red links; weak connections, yellow links). Functional connectivity patterns demonstrate strong interactions around the brain regions in which seizures begin and weak projections to the brain regions in which seizures spread. Objective tools in network neuroscience can usher in an era of personalized algorithms capable of mapping epileptic network architecture from neural signals and pinpointing implantable neurostimulation devices to specific brain regions for intervention (79, 80, 192).
8.2. Extensions Beyond Neuroengineering
Before concluding, we note that the mathematical methods and conceptual frameworks discussed in this review are more generally applicable beyond the specific realm of brain connectivity. From genes (200) to the musculoskeletal system (201), from the central to the peripheral nervous system (1), and from injured neural tissue in brains causing cognitive deficits to neural tissue in muscles causing pain (16), network science offers an approach that spurns reductionism in favor of holistic maps and models of complex interconnected systems. Indeed, future research may benefit from considering the nervous system as embedded or embodied in a broader context, as only one part of an interconnected web of networks supporting human life (202, 203).
8.3. Conclusion and Future Outlook
In this review, we have sought to introduce an exciting and emerging frontier in neuroengineering: a network science of brain connectivity. In addition to outlining the mathematical underpinnings of the field, we have briefly described some marked initial successes in which the tools of network neuroscience have been brought to bear on neural mapping and connectivity estimation, diagnosis and monitoring, and rehabilitation and treatment. However, we have also been careful to describe common pitfalls and associated limitations, in an effort to offer a balanced guide to incorporating these techniques into the reader’s own research practices. In the last few sections, we have taken the liberty of speculating about some important frontiers that we believe will become increasingly critical to the questions posed by neuroengineering in the near future, both from a computational point of view and from a view toward clinical impact. In closing, we underscore yet again that the strength and novelty of network neuroscience lie in its grasp of the full complexities of relational data, facilitating transformative approaches to understanding, fixing, and building brains.
Acknowledgments
We thank Chad Giusti, Jason Kim, and Matthew Hemphill for helpful comments on an earlier version of this manuscript. D.S.B. and A.N.K. acknowledge support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Army Research Laboratory and the Army Research Office (contract numbers W911NF-10-2-0022 and W911NF-14-1-0679), the National Institute of Mental Health (2-R01-DC-009209-11), the National Institute of Child Health and Human Development (1R01HD086888-01), the Office of Naval Research, and the National Science Foundation (BCS-1441502, BCS-1430087, BCS-1631550, and CAREER PHY-1554488). S.T.G. was supported by the Institute for Collaborative Biotechnologies through grant W911NF-09-0001 from the US Army Research Office. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Chen HI, Jgamadze D, Serruya MD, Cullen DK, Wolf JA, Smith DH. Neural substrate expansion for the restoration of brain function. Front Syst Neurosci. 2016;10:1. doi: 10.3389/fnsys.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006;7:523–34. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 3.Christophel TB, Hebart MN, Haynes JD. Decoding the contents of visual short-term memory from human visual and parietal cortex. J Neurosci. 2012;32:12983–89. doi: 10.1523/JNEUROSCI.0184-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putze F, Schultz T. Adaptive cognitive technical systems. J Neurosci Methods. 2014;234:108–15. doi: 10.1016/j.jneumeth.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Krusienski DJ, Grosse-Wentrup M, Galan F, Coyle D, Miller KJ, et al. Critical issues in state-of-the-art brain–computer interface signal processing. J Neural Eng. 2011;8:025002. doi: 10.1088/1741-2560/8/2/025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiLorenzo DJ, Bronzino JD. Neuroengineering. Boca Raton, FL: CRC; 2007. [Google Scholar]
- 7.Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, et al. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60:610–24. doi: 10.1109/TBME.2013.2244890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser JI, Kording KP. The development and analysis of integrated neuroscience data. Front Comput Neurosci. 2016;10:11. doi: 10.3389/fncom.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valiant LG. What must a global theory of cortex explain? Curr Opin Neurobiol. 2014;25:15–19. doi: 10.1016/j.conb.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Craver CF. Beyond reduction: mechanisms, multifield integration and the unity of neuroscience. Stud Hist Philos Biol Biomed Sci. 2005;36:373–95. doi: 10.1016/j.shpsc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Bullmore E, Barnes A, Bassett DS, Fornito A, Kitzbichler M, et al. Generic aspects of complexity in brain imaging data and other biological systems. NeuroImage. 2009;47:1125–34. doi: 10.1016/j.neuroimage.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson IH, Kording KP. How advances in neural recording affect data analysis. Nat Neurosci. 2011;14:139–42. doi: 10.1038/nn.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betzel RF, Bassett DS. Multi-scale brain networks. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.11.006. In press. https://doi.org/10.1016/j.neuroimage.2016.11.006. [DOI] [PMC free article] [PubMed]
- 14.Long B, Zhang Z, Yu PS. Relational Data Clustering: Models, Algorithms, and Applications. Boca Raton, FL: CRC; 2010. [Google Scholar]
- 15.Conaco C, Bassett DS, Zhou H, Arcila ML, Degnan SM, et al. Functionalization of a protosynaptic gene expression network. PNAS. 2012;109:10612–18. doi: 10.1073/pnas.1201890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Bassett DS, Winkelstein BA. Stretch-induced network reconfiguration of collagen fibres in the human facet capsular ligament. J R Soc Interface. 2016;13:20150883. doi: 10.1098/rsif.2015.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Heuvel MP, Bullmore ET, Sporns O. Comparative connectomics. Trends Cogn Sci. 2016;20:345–61. doi: 10.1016/j.tics.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Shih CT, Sporns O, Yuan SL, Su TS, Lin YJ, et al. Connectomics-based analysis of information flow in the Drosophila brain. Curr Biol. 2015;25:1249–58. doi: 10.1016/j.cub.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–47. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fornito A, Bullmore ET. Connectomics: a new paradigm for understanding brain disease. Eur Neuropsychopharmacol. 2015;25:733–48. doi: 10.1016/j.euroneuro.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Medaglia JD, Lynall ME, Bassett DS. Cognitive network neuroscience. J Cogn Neurosci. 2015;27:1471–91. doi: 10.1162/jocn_a_00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misic B, Sporns O. From regions to connections and networks: new bridges between brain and behavior. Curr Opin Neurobiol. 2016;40:1–7. doi: 10.1016/j.conb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vertes PE, Alexander-Bloch AF, Gogtay N, Giedd JN, Rapoport JL, Bullmore ET. Simple models of human brain functional networks. PNAS. 2012;109:5868–73. doi: 10.1073/pnas.1111738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vertes PE, Alexander-Bloch AF, Bullmore ET. Generative models of rich clubs in Hebbian neuronal networks and large-scale human brain networks. Philos Trans R Soc Lond B. 2014;369:20130531. doi: 10.1098/rstb.2013.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betzel RF, Avena-Koenigsberger A, Goñi J, He Y, de Reus MA, et al. Generative models of the human connectome. NeuroImage. 2016;124:1054–64. doi: 10.1016/j.neuroimage.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilosof S, Porter MA, Pascual M, Kefi S. The multilayer nature of ecological networks. 2016 doi: 10.1038/s41559-017-0101. arXiv:1511.04453 [q-bio] [DOI] [PubMed] [Google Scholar]
- 27.Proulx SR, Promislow DE, Phillips PC. Network thinking in ecology and evolution. Trends Ecol Evol. 2005;20:345–53. doi: 10.1016/j.tree.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Newman MEJ. Networks: An Introduction. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 29.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Pavlovic DM, Vértes PE, Bullmore ET, Schafer WR, Nichols TE. Stochastic blockmodeling of the modules and core of the Caenorhabditis elegans connectome. PLOS ONE. 2014;9:e97584. doi: 10.1371/journal.pone.0097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson SL, Hayasaka S, Laurienti PJ. Exponential random graph modeling for complex brain networks. PLOS ONE. 2011;6:e20039. doi: 10.1371/journal.pone.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindquist MA, Xu Y, Nebel MB, Caffo BS. Evaluating dynamic bivariate correlations in resting-state fMRI: a comparison study and a new approach. NeuroImage. 2014;101:531–46. doi: 10.1016/j.neuroimage.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misic B, Betzel RF, Nematzadeh A, Goni J, Griffa A, et al. Cooperative and competitive spreading dynamics on the human connectome. Neuron. 2015;86:1518–29. doi: 10.1016/j.neuron.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 34.Misic B, Sporns O, McIntosh AR. Communication efficiency and congestion of signal traffic in large-scale brain networks. PLOS Comput Biol. 2014;10:e1003427. doi: 10.1371/journal.pcbi.1003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel TP, Ventre SC, Geddes-Klein D, Singh PK, Meaney DF. Single-neuron NMDA receptor phenotype influences neuronal rewiring and reintegration following traumatic injury. J Neurosci. 2014;34:4200–13. doi: 10.1523/JNEUROSCI.4172-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gratton C, Lee TG, Nomura EM, D’Esposito M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:124. doi: 10.3389/fnsys.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser M. A tutorial in connectome analysis: topological and spatial features of brain networks. NeuroImage. 2011;57:892–907. doi: 10.1016/j.neuroimage.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. NeuroImage. 2013;80:426–44. doi: 10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- 39.van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol. 2015;126:1468–81. doi: 10.1016/j.clinph.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. The application of graph theoretical analysis to complex networks in the brain. Clin Neurophysiol. 2007;118:2317–31. doi: 10.1016/j.clinph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 42.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 43.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 44.Butts CT. Revisiting the foundations of network analysis. Science. 2009;325:414–16. doi: 10.1126/science.1171022. [DOI] [PubMed] [Google Scholar]
- 45.Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, et al. Whole-brain anatomical networks: Does the choice of nodes matter? NeuroImage. 2010;50:970–83. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Bollobas B. Random Graphs. New York: Academic; 1985. [Google Scholar]
- 47.Bollobas B. Graph Theory: An Introductory Course. Berlin: Springer; 1979. [Google Scholar]
- 48.Ghrist R. Amazon.com: Createspace. v1.0. 2014. Elementary Applied Topology. [Google Scholar]
- 49.Avena-Koenigsberger A, Goñi J, Betzel RF, van den Heuvel MP, Griffa A, et al. Using Pareto optimality to explore the topology and dynamics of the human connectome. Philos Trans R Soc Lond B. 2014;369:20130530. doi: 10.1098/rstb.2013.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–42. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 51.Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci. 2011;31:8259–570. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinov M, Bassett DS. Emerging evidence of connectomic abnormalities in schizophrenia. J Neurosci. 2011;31:6263–65. doi: 10.1523/JNEUROSCI.0382-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassett DS, Lynall ME. Network methods to characterize brain structure and function. In: Gazzaniga M, Ivry RB, Mangun GR, editors. Cognitive Neuroscience: The Biology of the Mind. 5 Cambridge, MA: MIT Press; 2015. [Google Scholar]
- 54.Li Y, Liu Y, Li J, Qin W, Li K, et al. Brain anatomical network and intelligence. PLOS Comput Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bassett DS, Bullmore ET. Small world brain networks revisited. Neuroscientist. 2016 doi: 10.1177/1073858416667720. In press. https:doi.org/10.1177/1073858416667720. [DOI] [PMC free article] [PubMed]
- 56.Sporns O, Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–40. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porter MA, Onnela JP, Mucha PJ. Communities in networks. Not AMS. 2009;56:1082–97. [Google Scholar]
- 58.Fortunato S. Community detection in graphs. Phys Rep. 2010;486:75–174. [Google Scholar]
- 59.Everett MG, Borgatti SP. Peripheries of cohesive subsets. Soc Netw. 1999;21:397–407. [Google Scholar]
- 60.Borgatti SP, Everett MG. Models of core/periphery structures. Soc Netw. 1999;21:375–95. [Google Scholar]
- 61.Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST. Task-based core–periphery organization of human brain dynamics. PLOS Comput Biol. 2013;9:e1003171. doi: 10.1371/journal.pcbi.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bassett DS, Siebenhühner F. Multiscale network organization in the human brain. In: Pesenson MZ, editor. Multiscale Analysis and Nonlinear Dynamics: From Genes to the Brain. New York: Wiley; 2013. [Google Scholar]
- 64.Bassett DS, Greenfield DL, Meyer-Lindenberg A, Weinberger DR, Moore SW, Bullmore ET. Efficient physical embedding of topologically complex information processing networks in brains and computer circuits. PLOS Comput Biol. 2010;6:e1000748. doi: 10.1371/journal.pcbi.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. Conserved and variable architecture of human white matter connectivity. NeuroImage. 2011;54:1262–79. doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Muldoon SF, Soltesz I, Cossart R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. PNAS. 2013;110:3567–72. doi: 10.1073/pnas.1216958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bettencourt LM, Stephens GJ, Ham MI, Gross GW. Functional structure of cortical neuronal networks grown in vitro. Phys Rev E. 2007;75:021915. doi: 10.1103/PhysRevE.75.021915. [DOI] [PubMed] [Google Scholar]
- 68.Fulcher BD, Fornito A. A transcriptional signature of hub connectivity in the mouse connectome. PNAS. 2016;113:1435–40. doi: 10.1073/pnas.1513302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hilgetag CC, Kaiser M. Clustered organization of cortical connectivity. Neuroinformatics. 2004;2:353–60. doi: 10.1385/NI:2:3:353. [DOI] [PubMed] [Google Scholar]
- 70.Markov NT, Ercsey-Ravasz M, Van Essen DC, Knoblauch K, Toroczkai Z, Kennedy H. Cortical high-density counterstream architectures. Science. 2013;342:1238406. doi: 10.1126/science.1238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubinov M, Ypma R, Watson C, Bullmore E. Wiring cost and topological participation of the mouse brain connectome. PNAS. 2015;112:10032–37. doi: 10.1073/pnas.1420315112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Heuvel MP, Scholtens LH, de Reus MA. Topological organization of connectivity strength in the rat connectome. Brain Struct Funct. 2016;221:1719–36. doi: 10.1007/s00429-015-0999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaiser M. Neuroanatomy: Connectome connects fly and mammalian brain networks. Curr Biol. 2015;25:R416–18. doi: 10.1016/j.cub.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, et al. Mapping the structural core of human cerebral cortex. PLOS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng ZD, McClinctock SM, Lisanby SH. Brain network properties in depressed patients receiving seizure therapy: a graph theoretical analysis of peri-treatment resting EEG. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2203–6. doi: 10.1109/EMBC.2015.7318828. [DOI] [PubMed] [Google Scholar]
- 76.Toppi J, Ciaramidaro A, Vogel P, Mattia D, Babiloni F, et al. Graph theory in brain-to-brain connectivity: a simulation study and an application to an EEG hyperscanning experiment. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2211–14. doi: 10.1109/EMBC.2015.7318830. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Xu P, Guo D, Yao D. Prediction of SSVEP-based BCI performance by the resting-state EEG network. J Neural Eng. 2013;10:066017. doi: 10.1088/1741-2560/10/6/066017. [DOI] [PubMed] [Google Scholar]
- 78.Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. PNAS. 2009;106:11747–52. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khambhati AN, Davis KA, Oommen BS, Chen SH, Lucas TH, et al. Dynamic network drivers of seizure generation, propagation and termination in human neocortical epilepsy. PLOS Comput Biol. 2015;11:e1004608. doi: 10.1371/journal.pcbi.1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khambhati A, Davis K, Lucas T, Litt B, Bassett DS. Virtual cortical resection reveals push–pull network control preceding seizure evolution. Neuron. 2016;91:1170–82. doi: 10.1016/j.neuron.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niu H, Wang J, Zhao T, Shu N, He Y. Revealing topological organization of human brain functional networks with resting-state functional near infrared spectroscopy. PLOS ONE. 2012;7:e45771. doi: 10.1371/journal.pone.0045771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Lin X, Fu G, Sai L, Chen H, et al. Mapping the small-world properties of brain networks in deception with functional near-infrared spectroscopy. Sci Rep. 2016;6:25297. doi: 10.1038/srep25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bassett DS, Brown J, Deshpande V, Carlson J, Grafton ST. Conserved and variable architecture of human white matter connectivity. NeuroImage. 2011;54:1262–79. doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, et al. Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage. 2010;50:970–83. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, Telesford QK, Giusti C, Lim KO, Bassett DS. Choosing wavelet methods, filters, and lengths for functional brain network construction. PLOS ONE. 2016;11:e0157243. doi: 10.1371/journal.pone.0157243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–52. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Hermundstad AM, Brown KS, Bassett DS, Aminoff EM, Frithsen A, et al. Structurally-constrained relationships between cognitive states in the human brain. PLOS Comput Biol. 2014;10:e1003591. doi: 10.1371/journal.pcbi.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, et al. Structural foundations of resting-state and task-based neural activity in the human brain. PNAS. 2013;110:6169–74. doi: 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–48. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage Clin. 2015;7:732–41. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Burns SP, Santaniello S, Yaffe RB, Jouny CC, Crone NE, et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. PNAS. 2014;111:E5321–30. doi: 10.1073/pnas.1401752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He Y, Chen Z, Gong G, Evans A. Neuronal networks in Alzheimer’s disease. Neuroscientist. 2009;15:333–50. doi: 10.1177/1073858409334423. [DOI] [PubMed] [Google Scholar]
- 95.Poza J, Garcia M, Gomez C, Bachiller A, Carreres A, Hornero R. Characterization of the spontaneous electroencephalographic activity in Alzheimer’s disease using disequilibria and graph theory. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5990–93. doi: 10.1109/EMBC.2013.6610917. [DOI] [PubMed] [Google Scholar]
- 96.Tijms BM, Wink AM, de Haan W, van der Flier WM, Stam CJ, et al. Alzheimer’s disease: connecting findings from graph theoretical studies of brain networks. Neurobiol Aging. 2013;34:2023–36. doi: 10.1016/j.neurobiolaging.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 97.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15:683–95. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 98.Zhu H, Zhou P, Alcauter S, Chen Y, Cao H, et al. Changes of intranetwork and internetwork functional connectivity in Alzheimer’s disease and mild cognitive impairment. J Neural Eng. 2016;13:046008. doi: 10.1088/1741-2560/13/4/046008. [DOI] [PubMed] [Google Scholar]
- 99.Petti M, Toppi J, Pichiorri F, Cincotti F, Salinari S, et al. Aged-related changes in brain activity classification with respect to age by means of graph indexes [sic] Conf Proc IEEE Eng Med Biol Soc. 2013;2013:4350–53. doi: 10.1109/EMBC.2013.6610509. [DOI] [PubMed] [Google Scholar]
- 100.Sacchet MD, Prasad G, Foland-Ross LC, Thompson PM, Gotlib IH. Elucidating brain connectivity networks in major depressive disorder using classification-based scoring. Proc IEEE Int Symp Biomed Imaging. 2014;2014:246–49. doi: 10.1109/ISBI.2014.6867855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toppi J, Anzolin A, Petti M, Cincotti F, Mattia D, et al. Investigating statistical differences in connectivity patterns properties at single subject level: a new resampling approach. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6357–60. doi: 10.1109/EMBC.2014.6945082. [DOI] [PubMed] [Google Scholar]
- 102.Zhao C, Zhao M, Yang Y, Gao J, Rao N, Lin P. The reorganization of human brain networks modulated by driving mental fatigue. IEEE J Biomed Health Inform. 2016 doi: 10.1109/JBHI.2016.2544061. In press. [DOI] [PubMed] [Google Scholar]
- 103.Toppi J, Mattia D, Anzolin A, Risetti M, Petti M, et al. Time varying effective connectivity for describing brain network changes induced by a memory rehabilitation treatment. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6786–89. doi: 10.1109/EMBC.2014.6945186. [DOI] [PubMed] [Google Scholar]
- 104.Ge R, Zhang H, Yao L, Long Z. Motor imagery learning induced changes in functional connectivity of the default mode network. IEEE Trans Neural Syst Rehabil Eng. 2015;23:138–48. doi: 10.1109/TNSRE.2014.2332353. [DOI] [PubMed] [Google Scholar]
- 105.Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, et al. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage Clin. 2014;5:245–55. doi: 10.1016/j.nicl.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Banca P, Sousa T, Duarte IC, Castelo-Branco M. Visual motion imagery neurofeedback based on the hMT+/V5 complex: evidence for a feedback-specific neural circuit involving neocortical and cerebellar regions. J Neural Eng. 2015;12:066003. doi: 10.1088/1741-2560/12/6/066003. [DOI] [PubMed] [Google Scholar]
- 107.Fels M, Bauer R, Gharabaghi A. Predicting workload profiles of brain-robot interface and electromygraphic neurofeedback with cortical resting-state networks: personal trait or task-specific challenge? J Neural Eng. 2015;12:046029. doi: 10.1088/1741-2560/12/4/046029. [DOI] [PubMed] [Google Scholar]
- 108.Andersen RA, Kellis S, Klaes C, Aflalo T. Toward more versatile and intuitive cortical brain–machine interfaces. Curr Biol. 2014;24:R885–97. doi: 10.1016/j.cub.2014.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang R, Yao D, Valdes-Sosa PA, Li F, Li P, et al. Efficient resting-state EEG network facilitates motor imagery performance. J Neural Eng. 2015;12:066024. doi: 10.1088/1741-2560/12/6/066024. [DOI] [PubMed] [Google Scholar]
- 110.Sporns O. The human connectome: origins and challenges. NeuroImage. 2013;80:53–61. doi: 10.1016/j.neuroimage.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 111.Christopoulos VN, Boeff DV, Evans CD, Crowe DA, Amirikian B, et al. A network analysis of developing brain cultures. J Neural Eng. 2012;9:046008. doi: 10.1088/1741-2560/9/4/046008. [DOI] [PubMed] [Google Scholar]
- 112.Kafashan M, Ching S. Optimal stimulus scheduling for active estimation of evoked brain networks. J Neural Eng. 2015;12:066011. doi: 10.1088/1741-2560/12/6/066011. [DOI] [PubMed] [Google Scholar]
- 113.Kanagasabapathi TT, Massobrio P, Barone RA, Tedesco M, Martinoia S, et al. Functional connectivity and dynamics of cortical-thalamic networks co-cultured in a dual compartment device. J Neural Eng. 2012;9:036010. doi: 10.1088/1741-2560/9/3/036010. [DOI] [PubMed] [Google Scholar]
- 114.Chang JC, Brewer GJ, Wheeler BC. Neuronal network structuring induces greater neuronal activity through enhanced astroglial development. J Neural Eng. 2006;3:217–26. doi: 10.1088/1741-2560/3/3/004. [DOI] [PubMed] [Google Scholar]
- 115.Viallon M, Cuvinciuc V, Delattre B, Merlini L, Barnaure-Nachbar I, et al. State-of-the-art MRI techniques in neuroradiology: principles, pitfalls, and clinical applications. Neuroradiology. 2015;57:441–67. doi: 10.1007/s00234-015-1500-1. [DOI] [PubMed] [Google Scholar]
- 116.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–67. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 118.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aurich NK, Filho JA. Evaluating the reliability of different preprocessing steps to estimate graph theoretical measures in resting state fMRI data. Front Neurosci. 2015;9:48. doi: 10.3389/fnins.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brooks JCW, Faull OK, Pattinson KTS, Jenkinson M. Physiological noise in brainstem fmri. Front Hum Neurosci. 2013;7:623. doi: 10.3389/fnhum.2013.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marchitelli R, Minati L, Marizzoni M, Bosch B, Bartrés-Faz D, et al. Test–retest reliability of the default mode network in a multi-centric fMRI study of healthy elderly: effects of data-driven physiological noise correction techniques. Hum Brain Mapp. 2016;37:2114–32. doi: 10.1002/hbm.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 123.Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38:306–20. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–67. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 125.Beall EB, Lowe MJ. The non-separability of physiologic noise in functional connectivity MRI with spatial ICA at 3T. J Neurosci Methods. 2010;191:263–76. doi: 10.1016/j.jneumeth.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 126.Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–68. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. NeuroImage. 2007;37:1286–300. doi: 10.1016/j.neuroimage.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andellini M, Cannat V, Gazzellini S, Bernardi B, Napolitano A. Test–retest reliability of graph metrics of resting state MRI functional brain networks: a review. J Neurosci Methods. 2015;253:183–92. doi: 10.1016/j.jneumeth.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 132.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciric R, Wolf DH, Power JD, Roalf DR, Baum G, et al. Benchmarking confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2017.03.020. In press. https://doi.org/10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed]
- 134.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time series. Magn Reson Med. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 135.Lemieux L, Salek-Haddadi A, Lund TE, Laufs H, Carmichael D. Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging. 2007;25:894–901. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 136.Tierney TM, Weiss-Croft LJ, Centeno M, Shamshiri EA, Perani S, et al. FIACH: a biophysical model for automatic retrospective noise control in fMRI. NeuroImage. 2016;124:1009–20. doi: 10.1016/j.neuroimage.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 137.Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion–weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–23. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 138.Pestilli F, Yeatman JD, Rokem A, Kay KN, Wandell BA. Evaluation and statistical inference for human connectomes. Nat Methods. 2014;11:1058–63. doi: 10.1038/nmeth.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yeh CH, Smith RE, Liang X, Calamante F, Connelly A. Correction for diffusion MRI fibre tracking biases: the consequences for structural connectomic metrics. NeuroImage. 2016;142:150–62. doi: 10.1016/j.neuroimage.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 140.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–78. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calhoun VD, Miller R, Pearlson G, Adal T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–74. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the connectome: the dynome. Neuron. 2014;83:1319–28. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kivel M, Arenas A, Barthelemy M, Gleeson JP, Moreno Y, Porter MA. Multilayer networks. J Complex Netw. 2014;2:203–71. [Google Scholar]
- 144.Holme P, Saramaki J. Temporal networks. Phys Rep. 2012;519:97–125. [Google Scholar]
- 145.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela JP. Community structure in time-dependent, multiscale, and multiplex networks. Science. 2010;328:876–78. doi: 10.1126/science.1184819. [DOI] [PubMed] [Google Scholar]
- 146.Bassett DS, Porter MA, Wymbs NF, Grafton ST, Carlson JM, Mucha PJ. Robust detection of dynamic community structure in networks. Chaos. 2013;23:013142. doi: 10.1063/1.4790830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reinkensmeyer DJ, Burdet E, Casadio M, Krakauer JW, Kwakkel G, et al. Computational neurorehabilitation: modeling plasticity and learning to predict recovery. J Neuroeng Rehabil. 2016;13:42. doi: 10.1186/s12984-016-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. PNAS. 2011;108:7641–46. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mantzaris AV, Bassett DS, Wymbs NF, Estrada E, Porter MA, et al. Dynamic network centrality summarizes learning in the human brain. J Complex Netw. 2013;1:83–92. [Google Scholar]
- 150.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Grafton ST. Cross-linked structure of network evolution. Chaos. 2014;24:013112. doi: 10.1063/1.4858457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18:744–51. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heitger MH, Ronsse R, Dhollander T, Dupont P, Caeyenberghs K, Swinnen SP. Motor learning–induced changes in functional brain connectivity as revealed by means of graph-theoretical network analysis. NeuroImage. 2012;61:633–50. doi: 10.1016/j.neuroimage.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 153.Mattar MG, Thompson-Schill SL, Bassett DS. The network architecture of value learning. 2016 doi: 10.1162/netn_a_00021. arXiv:1607.04169 [q-bio] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Karuza EA, Thompson-Schill SL, Bassett DS. Local patterns to global architectures: influences of network topology on human learning. Trends Cogn Sci. 2016;20:629–40. doi: 10.1016/j.tics.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muldoon SF, Bassett DS. Network and multilayer network approaches to understanding human brain dynamics. Philos Sci. 2016;83:710–20. [Google Scholar]
- 156.Nicosia V, Skardal PS, Latora V, Arenas A. Spontaneous synchronization driven by energy transport in interconnected networks. 2014 arXiv:1405.5855v2 [nlin] [Google Scholar]
- 157.Brookes MJ, Tewarie PK, Hunt BA, Robson SE, Gascoyne LE, et al. A multi-layer network approach to MEG connectivity analysis. NeuroImage. 2016;132:425–38. doi: 10.1016/j.neuroimage.2016.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Domenico M, Sasai S, Arenas A. Mapping multiplex hubs in human functional brain network. Front Neurosci. 2016;10:326. doi: 10.3389/fnins.2016.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kolaczyk ED. Statistical Analysis of Network Data: Methods and Models. Berlin: Springer; 2009. [Google Scholar]
- 160.Simpson SL, Lyday RG, Hayasaka S, Marsh AP, Laurienti PJ. A permutation testing framework to compare groups of brain networks. Front Comput Neurosci. 2013;7:171. doi: 10.3389/fncom.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. NeuroImage. 2015;123:253–68. doi: 10.1016/j.neuroimage.2015.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ginestet CE, Simmons A. Statistical parametric network analysis of functional connectivity dynamics during a working memory task. NeuroImage. 2011;55:688–704. doi: 10.1016/j.neuroimage.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 163.Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. NeuroImage. 2012;59:2196–207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Betzel RF, Medaglia JD, Papadopoulos L, Baum G, Gur RC, et al. The modular organization of human anatomical brain networks: accounting for the cost of wiring. Netw Neurosci. 2016 doi: 10.1162/NETN_a_00002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramsay JO, Silverman BW. Functional Data Analysis. Berlin: Springer; 2005. [Google Scholar]
- 166.Ramsay JO, Silverman BW. Applied Functional Data Analysis: Methods and Case Studies. Berlin: Springer; 2002. [Google Scholar]
- 167.Ramsay JO. Functional Data Analysis. New York: Wiley; 2006. [Google Scholar]
- 168.Papadopoulos L, Puckett J, Daniels KE, Bassett DS. Evolution of network architecture in a granular material under compression. Phys Rev E. 2016;94:032908. doi: 10.1103/PhysRevE.94.032908. [DOI] [PubMed] [Google Scholar]
- 169.Bassett DS, Owens ET, Porter MA, Manning ML, Daniels KE. Extraction of force-chain network architecture in granular materials using community detection. Soft Matter. 2015;11:2731–44. doi: 10.1039/c4sm01821d. [DOI] [PubMed] [Google Scholar]
- 170.Lepage KQ, Ching S, Kramer MA. Inferring evoked brain connectivity through adaptive perturbation. J Comput Neurosci. 2013;34:303–18. doi: 10.1007/s10827-012-0422-8. [DOI] [PubMed] [Google Scholar]
- 171.Shehzad Z, Kelly C, Reiss PT, Craddock RC, Emerson JW, et al. A multivariate distance-based analytic framework for connectome-wide association studies. NeuroImage. 2014;93:74–94. doi: 10.1016/j.neuroimage.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Simpson SL, Moussa MN, Laurienti PJ. An exponential random graph modeling approach to creating group-based representative whole-brain connectivity networks. NeuroImage. 2012;60:1117–26. doi: 10.1016/j.neuroimage.2012.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Klimm F, Bassett DS, Carlson JM, Mucha PJ. Resolving structural variability in network models and the brain. PLOS Comput Biol. 2014;10:e1003491. doi: 10.1371/journal.pcbi.1003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ganmor E, Segev R, Schneidman E. The architecture of functional interaction networks in the retina. J Neurosci. 2011;31:3044–54. doi: 10.1523/JNEUROSCI.3682-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ganmor E, Segev R, Schneidman E. Sparse low-order interaction network underlies a highly correlated and learnable neural population code. PNAS. 2011;108:9679–84. doi: 10.1073/pnas.1019641108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giusti C, Ghrist R, Bassett DS. Two’s company, three (or more) is a simplex: algebraic-topological tools for understanding higher-order structure in neural data. J Comput Neurosci. 2016;41:1–14. doi: 10.1007/s10827-016-0608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Giusti C, Pastalkova E, Curto C, Itskov V. Clique topology reveals intrinsic geometric structure in neural correlations. PNAS. 2015;112:13455–60. doi: 10.1073/pnas.1506407112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Curto C. What can topology tell us about the neural code? 2016 arXiv:1605.01905 [q-bio] [Google Scholar]
- 179.Sizemore A, Giusti C, Bassett DS. Classification of weighted networks through mesoscale homological features. J Complex Netw. 2016;2016:cnw013. [Google Scholar]
- 180.Kim E, Kang H, Lee H, Lee HJ, Suh MW, et al. Morphological brain network assessed using graph theory and network filtration in deaf adults. Hear Res. 2014;315:88–98. doi: 10.1016/j.heares.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 181.Liu YY, Slotine JJ, Barabási AL. Controllability of complex networks. Nature. 2011;473:167–73. doi: 10.1038/nature10011. [DOI] [PubMed] [Google Scholar]
- 182.Pasqualetti F, Zampieri S, Bullo F. Controllability metrics, limitations and algorithms for complex networks. IEEE Trans Control Netw Syst. 2014;1:40–52. [Google Scholar]
- 183.Schiff SJ. Neural Control Engineering: The Emerging Intersection Between Control Theory and Neuroscience. Cambridge, MA: MIT Press; 2011. [Google Scholar]
- 184.Motter AE. Networkcontrology. Chaos. 2015;25:097621. doi: 10.1063/1.4931570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, et al. Controllability of structural brain networks. Nat Commun. 2015;6:8414. doi: 10.1038/ncomms9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Betzel RF, Gu S, Medaglia JD, Pasqualetti F, Bassett DS. Optimally controlling the human connectome: the role of network topology. Sci Rep. 2016;29:30770. doi: 10.1038/srep30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gu S, Betzel RF, Cieslak M, Delio PR, Grafton ST, et al. Optimal trajectories of brain state transitions. NeuroImage. 2017;148:305–17. doi: 10.1016/j.neuroimage.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cornelius SP, Kath WL, Motter AE. Realistic control of network dynamics. Nat Commun. 2013;4:1942. doi: 10.1038/ncomms2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Medaglia JD, Gu S, Pasqualetti F, Ashare RL, Lerman C, et al. Cognitive control in the controllable connectome. 2016 arXiv:1606.09185 [q-bio] [Google Scholar]
- 190.Tang E, Giusti C, Baum G, Gu S, Kahn AE, et al. Structural drivers of diverse neural dynamics and their evolution across development. 2016 arXiv:1607.01010 [q-bio.NC] [Google Scholar]
- 191.Ching S, Ritt JT. Control strategies for underactuated neural ensembles driven by optogenetic stimulation. Front Neural Circuits. 2013;7:54. doi: 10.3389/fncir.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Muldoon SF, Pasqualetti F, Gu S, Cieslak M, Grafton ST, et al. Stimulation-based control of dynamic brain networks. PLOS Comput Biol. 2016;12:e1005076. doi: 10.1371/journal.pcbi.1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hao S, Subramanian S, Jordan A, Santaniello S, Yaffe R, et al. Computing network-based features from intracranial EEG time series data: application to seizure focus localization. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5812–15. doi: 10.1109/EMBC.2014.6944949. [DOI] [PubMed] [Google Scholar]
- 194.Santaniello S, Burns SP, Golby AJ, Singer JM, Anderson WS, Sarma SV. Quickest detection of drug-resistant seizures: an optimal control approach. Epilepsy Behav. 2011;22:S49–60. doi: 10.1016/j.yebeh.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ching S, Brown EN, Kramer MA. Distributed control in a mean-field cortical network model: implications for seizure suppression. Phys Rev E. 2012;86:021920. doi: 10.1103/PhysRevE.86.021920. [DOI] [PubMed] [Google Scholar]
- 196.Crofts JJ, Higham DJ, Bosnell R, Jbabdi S, Matthews PM, et al. Network analysis detects changes in the contralesional hemisphere following stroke. NeuroImage. 2011;54:161–69. doi: 10.1016/j.neuroimage.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Motor chunking is correlated with activation of the human sensorimotor putamen. Neuron. 2012;74:936–46. doi: 10.1016/j.neuron.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Onnela JP, Rauch SL. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology. 2016;41:1691–96. doi: 10.1038/npp.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Torous J, Kiang MV, Lorme J, Onnela JP. New tools for new research in psychiatry: a scalable and customizable platform to empower data driven smartphone research. JMIR Ment Health. 2016;3:e16. doi: 10.2196/mental.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Beagan JA, Gilgenast TG, Kim J, Plona Z, Norton HK, et al. Local genome topology can exhibit an incompletely rewired 3D folding state during somatic cell reprogramming. Cell Stem Cell. 2016;18:611–24. doi: 10.1016/j.stem.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Murphy AC, Muldoon SF, Baker D, Lastowka A, Bennett B, et al. Structure, function, and control of the musculoskeletal network. arXiv. 2016;2016 doi: 10.1371/journal.pbio.2002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Baldassano SN, Bassett DS. Topological distortion and reorganized modular structure of gut microbial co-occurrence networks in inflammatory bowel disease. Sci Rep. 2016;6:26087. doi: 10.1038/srep26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Steinway SN, Biggs MB, Loughran TPJ, Papin JA, Albert R. Inference of network dynamics and metabolic interactions in the gut microbiome. PLOS Comput Biol. 2015;11:e1004338. doi: 10.1371/journal.pcbi.1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]