Abstract
Background
Prior trials comparing a strategy of optimal medical therapy with or without revascularization have not shown that revascularization reduces cardiovascular events in patients with stable ischemic heart disease (SIHD). However, those trials only included participants in whom coronary anatomy was known prior to randomization and did not include sufficient numbers of participants with significant ischemia. It remains unknown whether a routine invasive approach offers incremental value over a conservative approach with catheterization reserved for failure of medical therapy in patients with moderate or severe ischemia.
Methods
The ISCHEMIA Trial is a National Heart, Lung, and Blood Institute supported trial, designed to compare an initial invasive or conservative treatment strategy for managing SIHD patients with moderate or severe ischemia on stress testing. Five thousand one-hundred seventy-nine participants have been randomized. Key exclusion criteria included estimated glomerular filtration rate (eGFR) <30 ml/min, recent myocardial infarction (MI), left ventricular ejection fraction <35%, left main stenosis >50%, or unacceptable angina at baseline. Most enrolled participants with normal renal function first underwent blinded coronary computed tomography angiography (CCTA) to exclude those with left main coronary artery disease (CAD) and without obstructive CAD. All randomized participants receive secondary prevention that includes lifestyle advice and pharmacologic interventions referred to as optimal medical therapy (OMT). Participants randomized to the invasive strategy underwent routine cardiac catheterization followed by revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, when feasible, as selected by the local Heart Team to achieve optimal revascularization. Participants randomized to the conservative strategy undergo cardiac catheterization only for failure of OMT. The primary endpoint is a composite of cardiovascular (CV) death, nonfatal myocardial infarction (MI), hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest. Assuming the primary endpoint will occur in 16% of the conservative group within 4 years, estimated power exceeds 80% to detect an 18.5% reduction in the primary endpoint. Major secondary endpoints include the composite of CV death and nonfatal MI, net clinical benefit (primary and secondary endpoints combined with stroke), angina-related symptoms and disease-specific quality of life, as well as a cost-effectiveness assessment in North American participants. Ancillary studies of patients with advanced chronic kidney disease and those with documented ischemia and non-obstructive coronary artery disease are being conducted concurrently.
Conclusions
ISCHEMIA will provide new scientific evidence regarding whether an invasive management strategy improves clinical outcomes when added to optimal medical therapy in patients with SIHD and moderate or severe ischemia.
Background
Evidence from clinical trials of stable ischemic heart disease (SIHD) supports the use of intensive lifestyle and pharmacologic interventions to inhibit the progression of atherosclerosis and reduce the likelihood of major adverse cardiac events, including myocardial infarction (MI) and cardiovascular (CV) death.1 In addition to this secondary prevention, many patients also undergo routine cardiac catheterization and revascularization as part of their management. Clinical trial evidence to date, however, has failed to demonstrate that an initial invasive strategy (routine cardiac catheterization and planned revascularization plus optimal medical therapy [OMT)] reduces death or MI as compared with an initial conservative strategy (OMT alone) in SIHD patients.2-4 The substantial difference between how physicians often treat SIHD patients and what published evidence shows suggests that there remains considerable doubt about whether previous trials have fully and properly addressed this issue.
Rationale for the ISCHEMIA Trial
The scientific evidence underlying the management of SIHD is limited in two major ways. First, in most of the pivotal randomized comparisons between medicine and coronary artery bypass graft surgery (CABG), “medical therapy” did not include any of the pharmacologic therapies that have been shown to favorably affect prognosis and that we now consider foundational: aspirin, statins, and renin-angiotensin-aldosterone system inhibitors. Thus, previous trials essentially compared CABG surgery with medical therapy for symptom control alone. Second, more recent trials using multifaceted, contemporary medical therapy did not include large numbers of higher-risk subjects, such as those with moderate or severe ischemia, to test whether an invasive approach with OMT reduces risk in these patients with more advanced SIHD as compared with OMT alone.
Most studies support the concept that ischemia identifies patients at increased risk for death and MI, and that revascularization of such patients improves prognosis.5,6,7 In an observational study of 13,969 patients with mean 8.7 year follow-up ≥10% inducible ischemia by SPECT was associated with improved late survival in those clinically selected to undergo early revascularization.8 In the COURAGE serial nuclear substudy, an apparently strong relationship was found between the extent of core laboratory assessed residual moderate-to-severe ischemia 6-18 months and subsequent death or MI in 314 patients, but this was not significant after multivariable adjustment.9 In a separate, larger COURAGE report based on site interpretation of the extent of ischemia in 1,381 patients with baseline SPECT imaging, no significant relationship was present between the rate of death and MI and ischemia severity (quantified as ≥3 vs. <3 ischemic segments).10 Furthermore, in a follow-up COURAGE core laboratory study in 621 patients, the extent of baseline ischemia did not correlate with the rate of death, MI, or acute coronary syndrome after mean follow-up of 4.7 years.11 Thus, there is contradictory evidence regarding the risk imposed by moderate-to-severe ischemia, and the putative benefit from revascularization.
Previous strategy trials of SIHD management were designed to select patients after diagnostic catheterization rather than at the time when initial invasive evaluation was being considered. We believe there was a tendency for physicians not to enroll patients with higher risk coronary anatomy in those strategy trials. Hence, knowledge of the coronary anatomy likely introduced a selection bias that may have weakened the potential to demonstrate the superiority of revascularization. Thus, the motivating premise for the ISCHEMIA Trial was that we still do not know whether routine cardiac catheterization and revascularization, when feasible, improves prognosis in such higher-risk patients with more severe ischemia who may derive the greatest clinical benefit from an invasive approach. Given the cost and potential complications of invasive management of SIHD patients—many of whom routinely undergo PCI with insufficient evidence that this is the best approach to improve patient outcomes—it is essential from a public health perspective that a trial adequately powered for important outcomes be performed to support rational patient-centric care decisions and to assure health policy makers and payers that the care of these patients will provide the highest value possible for the resources consumed. In addition to the primary aim of assessing the occurrence of clinical events, angina-related quality of life is of major interest. There is recent trial evidence to support a placebo effect of PCI in selected patients with angina, single-vessel CAD, and good exercise tolerance.12 While the ISCHEMIA trial was not designed to include a sham procedure, it will carefully evaluate prospective quality of life measures over the long-term, where the placebo effect may have less impact.
Methods
Funding from NIH grants U01HL105907, U01HL105462, U01HL105561, and U01HL105565 were used to support the research and creation of this paper. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Trial Overview and Aims
Figure 1 shows the study design. Trial endpoints are listed in Table 1. The primary aim of the ISCHEMIA trial is to determine whether an initial invasive strategy of cardiac catheterization and optimal revascularization, if feasible, in addition to OMT, will reduce major adverse cardiovascular events in participants with SIHD and moderate or severe ischemia compared with an initial conservative strategy of OMT alone, with catheterization reserved for failure of OMT. The original grant application submitted to the National Heart, Lung, and Blood Institute (NHLBI) in 2010 proposed and was later funded for a 5-component primary endpoint13: cardiovascular death, MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest. We sought and obtained approval from the Data and Safety Monitoring Board (DSMB) and NHLBI to make the 2-component endpoint of cardiovascular death or MI the primary with a plan specified in the original protocol that included a change back to the 5-component endpoint to retain power if needed. Indeed, this 2-component endpoint occurred less frequently than projected and, as pre-specified in the ISCHEMIA Trial protocol, an independent panel was convened to review the impact of the lower event rate on power. An independent panel was needed because the DSMB had been unblinded. The panel advised NHLBI and study leadership to change the primary outcome measure from the 2-component endpoint to the 5-component composite endpoint that was originally proposed in the grant application to increase power (see statistics section) and to extend follow-up. Table 2 provides a timeline of this and other important changes during the trial. The projected average follow-up will be approximately 3.5 years with a range of 1.5 to 7 years.
Figure 1. Study Design.
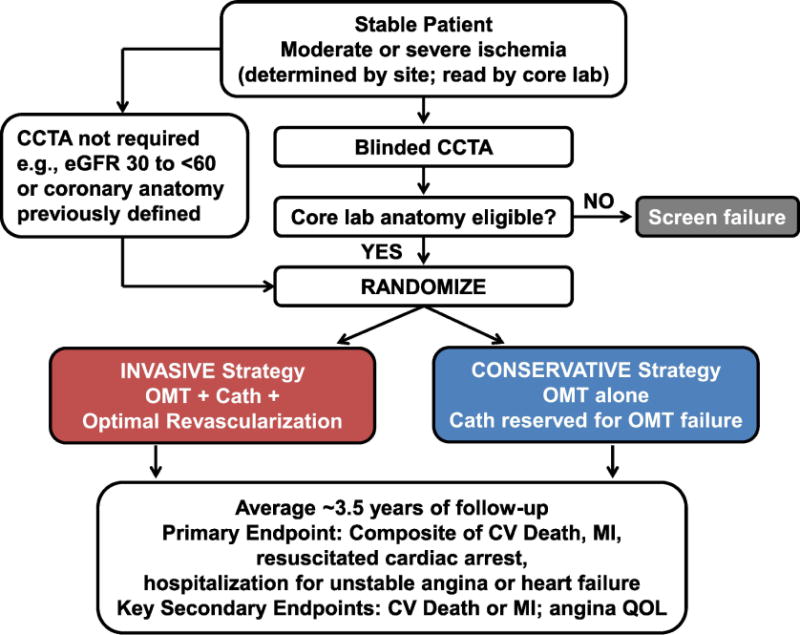
Patients who underwent stress testing for clinical indications at enrolling sites were screened for eligibility if the site determined that moderate or severe ischemia was present on a stress imaging test, or severe ischemia was present on a non-imaging exercise tolerance test. Consenting participants were enrolled and most underwent blinded CCTA. (CCTA was usually performed in participants with normal renal function and not performed in participants with eGFR <60 mL/min). Participants with left main stenosis ≥50% or no obstructive disease were excluded. If prior CCTA or cardiac catheterization demonstrated the absence of significant left main stenosis and the presence of significant obstructive disease in other coronary arteries, a study CCTA was not required. Eligible participants were randomized to invasive or conservative management strategies (see text for details). The primary endpoint is a composite of cardiovascular death, nonfatal myocardial infarction, resuscitated cardiac arrest, hospitalization for unstable angina, and hospitalization for heart failure. The composite of cardiovascular death or nonfatal myocardial infarction is a key secondary endpoint. Patients with advanced chronic kidney disease and moderate or severe ischemia on stress testing were considered for the ISCHEMIA-CKD ancillary trial. Participants with no obstructive disease who qualified for enrollment with stress echocardiography were considered for the CIAO-ISCHEMIA ancillary study.
Lab, laboratory; CCTA, coronary computed tomography angiography; cath, cardiac catheterization; CV, cardiovascular; MI, myocardial infarction; QOL, quality of life.
Table 1.
Primary and Secondary Endpoints
| Primary Endpoint | 1. Composite of cardiovascular death, nonfatal myocardial infarction, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest. |
| Major Secondary Endpoints | 1. Composite of cardiovascular death or nonfatal myocardial infarction 2. Angina per SAQ Angina Frequency Scale |
| Other Secondary Endpoints | 1. Composite of cardiovascular death, nonfatal myocardial infarction, or stroke 2. Composite of cardiovascular death, nonfatal myocardial infarction, hospitalization for unstable angina, hospitalization for heart failure, resuscitated cardiac arrest, or stroke 3. Individual components of the primary endpoint 4. All-cause death 5. Stroke 6. Composite endpoints incorporating all cause death 7. Composite endpoints incorporating other definitions of MI1 8. Disease-specific quality of life per SAQ Quality of Life Scale 9. Health resource utilization, costs, and cost-effectiveness |
See Table S5 for definitions of clinical endpoints.
SAQ = Seattle Angina Questionnaire
Table 2.
Timeline of Design Modifications
| Date | Design Modification |
|---|---|
| Jul 2011 | NHLBI awards grants based on 5-component primary endpoint. |
| Jul - Oct 2011 | Discussion of elevation of 2-component composite of CV death or MI to primary endpoint. |
| Oct 2011 | DSMB approved the 2-component endpoint to become primary, with contingency plan. |
| Jan 2012 | Protocol Version 1.0 finalized. Initial design was to randomize 8,000 participants with primary endpoint of CV death or MI. Protocol specified potential change from 2- to 5-component primary endpoint based on projected aggregate event rate. All 5 event types adjudicated from onset. |
| Jan 2014 | DSMB approves non-imaging exercise test for entry into the trial. |
| Aug 2014 | Recruitment was slower than expected, and leadership reviewed precision and power under reduced recruitment assumptions. |
| Oct 2014 | Increase in the number of sites permitted to randomize participants prior to core laboratory review of ischemia severity on stress test. |
| Apr 2015 | DSMB endorsed the plan to reduce sample size from 8,000 to 5,000, extend recruitment by 6 months, and extend follow-up by 6 months to December 2018. |
| Jul 2015 | Pre-specified first analysis for monitoring and projecting the final aggregate number of primary endpoint events. |
| Aug 2016 | NHLBI approved plan to reduce sample size from 8,000 to 5,000 |
| Sep 2016 | Protocol addendum issued describing changes to sample size and average duration of follow-up. |
| May 2017 | Independent Advisory Panel convened by NHLBI. |
| Jun 2017 | Without knowledge of trial results by treatment group, NHLBI approved Independent Advisory Panel recommendation to make the 5-component composite the primary endpoint. 2-component CV death or MI became a key secondary endpoint. Extension of follow-up to June 2019, pending final approval. |
CV = cardiovascular; MI = myocardial infarction; DSMB = Data and Safety Monitoring Board; NHLBI = National Heart, Lung, and Blood Institute.
Secondary aims are to determine whether an initial invasive strategy compared to a conservative strategy will improve: 1) the composite of CV death or MI; 2) angina symptoms and quality of life, as assessed by the Seattle Angina Questionnaire (SAQ) Angina Frequency and Quality of Life (QOL) scales;14 3) all-cause mortality; 4) net clinical benefit assessed by adding stroke to the primary composite endpoint; and 5) individual components of the composite endpoints. The protocol also pre-specified that additional endpoints may be constructed for sensitivity analyses to aid interpretation of the primary and secondary analyses using the clinical event adjudication committee (CEC) primary and secondary definitions of MI. Similarly, while health status outcomes (e.g., angina as measured by the SAQ) will be analyzed as continuous variables, categorization of these outcomes may be performed to facilitate clinical interpretability of the results. Analyses of disease-specific quality of life, as assessed by the SAQ QOL scale and other QOL measures, and health resource utilization, costs, and cost-effectiveness were funded under a separate award.
Additional Studies Embedded in the ISCHEMIA Trial
Long-term follow-up
We plan to seek funding to assess long-term all-cause mortality. This plan was included in the original protocol which states “Dependent on additional funding, telephone or email follow-up every 6 months or ascertainment of database information on vital status may continue after all clinic visits have been completed, unless prohibited by local regulations.” The consent form template includes possible contact for up to 20 years.
ISCHEMIA-CKD and CIAO-ISCHEMIA
An ancillary trial of patients with SIHD, moderate or severe ischemia, and advanced chronic kidney disease with eGFR <30 mL/min/1.73m2 or on dialysis (NCT01985360) and an ancillary study of patients with moderate or severe ischemia and no obstructive CAD on CCTA (NCT02347215) are being conducted concurrently.
Site Selection
Clinical Sites, PCI Operators, Cardiac Surgeons, and Institutional Resources
Each participating clinical site required an established PCI and CABG program with operators that met trial performance criteria (see online supplement Table S2) willing to utilize a collaborative Heart Team approach to evaluating optimal revascularization strategies and care practices for all patients. Eligible sites were required to have access to ≥64-slice coronary computed tomography angiography (CCTA) with willingness to use a low dose radiation protocol, and have experience in clinical research. The online supplement lists participating clinical sites, NHLBI program staff, Data and Safety Monitoring Board members, and trial committee members.
Patient Population Eligibility Criteria
Patients were considered eligible for inclusion if they had SIHD and moderate or severe ischemia on stress imaging or severe ischemia on non-imaging exercise tolerance testing (hereafter referred to collectively as moderate or severe ischemia), were ≥21 years of age, and able to give informed consent. Eligible participants were clinically stable, and had either angina controlled medically or silent ischemia. Eligibility criteria are listed in Table 2. Ischemia severity was determined by sites and will be reported according to independent core laboratory review. Prior cardiac catheterization was not an exclusion criterion, but recent cardiac catheterization was discouraged with a plan to monitor and cap randomization of patients with a recent diagnostic angiogram if a high frequency was observed.
Screening, Enrollment, and Randomization
Monthly screening logs were submitted to the Clinical Coordinating Center during the initial phase of enrollment to facilitate central monitoring of recruitment methods. Consented patients who met all clinical eligibility criteria (see Table 3) were enrolled via an interactive web response system (IXRS). After informed consent and enrollment, anatomic eligibility was further assessed based on the absence of ≥50% left main stenosis, and presence of obstructive coronary disease in those who had blinded CCTA (see below). Core laboratories independently reviewed stress test images, non-imaging exercise tests, ECGs, CCTAs, and, post-randomization, reports from protocol-assigned cardiac catheterization laboratory and CABG procedures. Enrollment began in the U.S. in July 2012 with sequential initiation of sites in 37 countries worldwide over >4 years. A total of 5,179 participants were randomized at 320 sites. Enrollment and randomization ended in January 2018. See Figure 2.
Table 3.
Inclusion and Exclusion Criteria*
|
Inclusion Criteria (at enrollment) 1. At least moderate ischemia on a qualifying stress test 2. Participant is willing to give informed consent 3. Age ≥ 21 years |
|
Exclusion Criteria 1. LVEF <35% 2. History of unprotected left main stenosis ≥50% on prior CCTA or prior cardiac catheterization (if available) 3. Finding of “no obstructive coronary artery disease” (<50% stenosis in all major epicardial vessels) on prior CCTA or prior catheterization, performed within 12 months 4. Coronary anatomy unsuitable for either PCI or CABG 5. Unacceptable level of angina despite maximal medical therapy 6. Very dissatisfied with medical management of angina 7. History of noncompliance with medical therapy 8. Acute coronary syndrome within the previous 2 months 9. PCI within the previous 12 months 10. Stroke within the previous 6 months or spontaneous intracranial hemorrhage at any time 11. History of ventricular tachycardia requiring therapy for termination, or symptomatic sustained ventricular tachycardia not due to a transient reversible cause 12. NYHA class III-IV heart failure at entry or hospitalization for exacerbation of chronic heart failure within the previous 6 months 13. Non-ischemic dilated cardiomyopathy or hypertrophic cardiomyopathy 14. End stage renal disease on dialysis or estimated glomerular filtration rate <30 ml/min (not an exclusion criterion for CKD ancillary trial, see CKD ancillary trial) 15. Severe valvular disease or valvular disease likely to require surgery or percutaneous valve replacement during the trial 16. Allergy to radiographic contrast that cannot be adequately pre-medicated, or any prior anaphylaxis to radiographic contrast 17. Planned major surgery necessitating interruption of dual antiplatelet therapy (note that patients may be eligible after planned surgery) 18. Life expectancy less than the duration of the trial due to non-cardiovascular comorbidity 19. Pregnancy (known to be pregnant; to be confirmed pre-CCTA and/or randomization, if applicable) 20. Patient who, in the judgment of the patient’s physician, is likely to have significant unprotected left main stenosis (those who are able to undergo CCTA will have visual assessment of the left main coronary artery by the CCTA core laboratory) 21. Enrolled in a competing trial that involves a non-approved cardiac drug or device 22. Inability to comply with the protocol 23. Exceeds the weight or size limit for CCTA or cardiac catheterization at the site 24. Canadian Cardiovascular Society Class III angina of recent onset, or angina of any class with a rapidly progressive or accelerating pattern 25. Canadian Cardiovascular Society Class IV angina, including unprovoked rest angina 26. High risk of bleeding which would contraindicate the use of dual antiplatelet therapy 27. Cardiac transplant recipient 28. Prior CABG, unless CABG was performed more than 12 months ago and coronary anatomy has been demonstrated to be suitable for PCI or CABG to accomplish complete revascularization of ischemic areas (CCC approval required) |
ISCHEMIA Protocol Version 2.0
LVEF = left ventricular ejection fraction; CCTA = coronary computed tomography angiography; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft surgery; NYHA = New York Heart Association; CKD = chronic kidney disease; CCC = Clinical Coordinating Center.
Figure 2. Timeline of Active Sites, Enrollments, and Randomizations.
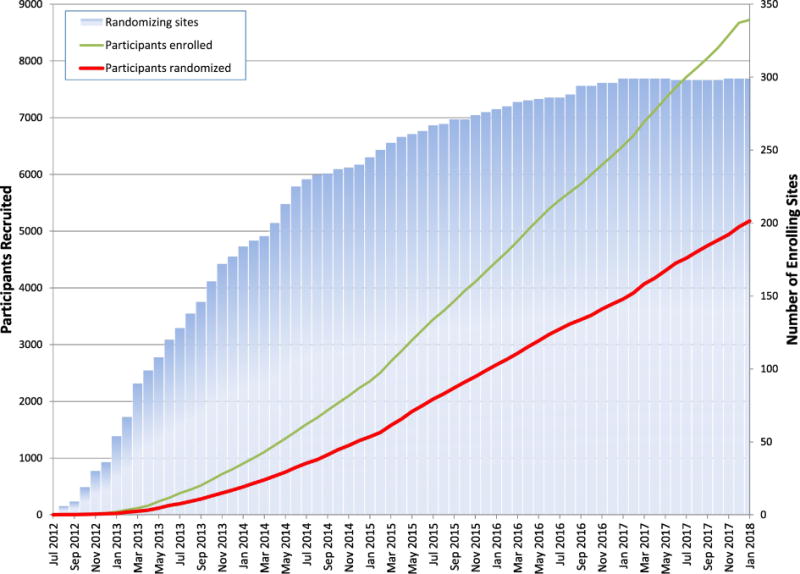
The figure shows the cumulative number of randomizing sites, participant enrollments, and participant randomizations between July 2012 and January 2018. Although 320 sites randomized at least one participant, some of those sites closed and transferred participants to another site, with a final number of 299 active sites at the end of the recruitment phase.
Stress Testing
Patients with moderate or severe ischemia (see Table 4) as judged by sites on a stress test performed for clinical indications, preferably within the prior 3 months, were identified and screened for eligibility. Stress test data (e.g., images and ECG recordings) were transferred electronically to the relevant core laboratory. The core laboratories independently interpreted baseline stress tests for all trial participants. At the start of the trial, stress imaging tests were the only modalities permitted to determine patient eligibility. Trial leadership sought DSMB approval to include non-imaging exercise testing for the following reasons: 1) non-imaging exercise testing is the most widely used modality worldwide; 2) this modality is recommended as preferred by the American College of Cardiology/American Heart Association guidelines for patients with an interpretable ECG who can exercise;1 and 3) inclusion of non-imaging exercise tests will improve the trial’s relevance to real world practice. To minimize the risk of including individuals with falsely positive stress tests and lesser amounts of ischemia, we solicited input from exercise testing experts and established strict eligibility criteria that were consensus driven based on published data. Exercise testing entry criteria required all of the following: 1) history of stable or exercise test-induced typical angina; 2) an interpretable resting ECG (e.g., no resting ST segment depression ≥1 mm, no left ventricular hypertrophy with repolarization abnormalities); 3) at least 2 leads showing new exercise-induced ST segment depression ≥1.5 mm or a single lead ≥2 mm as compared to the baseline tracing occurring at ≤7 METS or peak heart rate <75% of age-predicted maximum confirmed by the ECG core laboratory;15-18 and 4) anatomic confirmation of eligibility and more stringent CCTA anatomic eligibility to reduce the false positive rate and select individuals with stenoses subserving greater amounts of myocardium at higher risk of events (see Table 4 and “CCTA” below). In January 2014, the DSMB approved non-imaging exercise tolerance tests for entry into the trial.
Table 4.
Ischemia Eligibility Criteria by Stress Test Modality
| Stress Test Modality | Diagnostic criteria |
|---|---|
| Nuclear perfusion via SPECT or PET | ≥10% myocardium ischemic1 |
| Echocardiography | ≥3/16 segments with stress-induced severe hypokinesis or akinesis |
| Cardiac Magnetic Resonance | Perfusion: ≥12% myocardium ischemic, and/or Wall motion: ≥3/16 segments with stress-induced severe hypokinesis or akinesis |
| Exercise Test without Imaging2 (criteria 1-4 must all be met) | 1. Clinical history of typical angina or typical angina during the exercise test 2. Absence of resting ST-segment depression ≥1.0 mm or confounders that render exercise ECG non-interpretable (LBBB, LVH with repolarization, pacemaker, etc.) 3. As compared to the baseline tracing, additional exercise-induced horizontal or downsloping ST-segment depression ≥1.5 mm in 2 leads or ≥2.0 mm in any lead; ST-segment elevation ≥1mm in a non-infarct territory. 4. Either of the following: a. Workload at which ST-segment criteria are met is not to exceed completion of stage 2 of a standard Bruce protocol or 7 METs if a non-Bruce protocol is used or b. ST segment criteria are met at <75% of the maximum predicted HR Note: Anatomic eligibility must be confirmed |
To fulfill an expanded definition of moderate ischemia, participants needed to achieve all 4 of the following:
1. History of typical angina, or chest pain during exercise stress.
2. HR ≤75% predicted maximum.
3. Workload not greater than stage 2 of Bruce Protocol or 7 METs.
4. Nuclear imaging: ≥5% ischemic myocardium; Echocardiography: ≥2 segments with dobutamine- or exercise-induced severe hypokinesis or akinesis.
Non-imaging exercise test criteria were developed to approximate severe ischemia, taking into account the potentially higher false positive rate. Anatomic eligibility confirmation was required and CCTA eligibility criteria were more stringent for participants enrolled using non-imaging exercise stress tests, requiring ≥70% stenosis in the proximal or mid left anterior descending, proximal or mid right coronary artery, or proximal left circumflex (or circumflex equivalent).
SPECT = single photon emission computed tomography; PET = positron emission tomography; ECG = electrocardiogram; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; HR = heart rate; MET = metabolic equivalent of task; Ischemia criteria across imaging modalities were selected to achieve a projected rate of CV death or MI of 5% per year.41
Initially, most sites were asked to wait for core laboratory review and agreement with site interpretation regarding the presence of moderate or severe ischemia before randomization as an operational procedure. In October 2014, most sites were no longer asked to wait for pre-randomization core laboratory confirmation of moderate or severe ischemia. The intent of this operational change was to simplify the work flow and therefore increase recruitment, and to respond to site feedback that the process was not necessary based on high rates of concordance. Rates of concordance between each site and core lab interpretations were continuously reviewed and corrective actions employed for future enrollment at sites as needed.
Role of the Stress Test Core Laboratories
The goals of the Stress Test Core Laboratories were to: 1) provide independent interpretation of pre-enrollment ischemia severity as defined by the sites; 2) provide continuous oversight of site stress tests screened for the trial through monitoring, education, and training on stress test protocols, image acquisition, processing, interpretation, and reporting of the ISCHEMIA eligibility criteria; and 3) provide expert quality control of site performance and interpretation of stress testing through expert review of image quality and interpretation, and provide retraining if needed.
CCTA
A blinded CCTA was performed in most participants with eGFR ≥60 mL/min/1.73m2 to identify and exclude participants with either significant unprotected left main disease (defined as ≥50% stenosis) or those without obstructive CAD (<50% stenosis in all major epicardial coronary arteries). The goal was to perform CCTA within 15 days of enrollment. Anatomic eligibility confirmation was required and CCTA criteria were more stringent for participants enrolled after a non-imaging exercise test. For such patients, ≥70% stenosis in the proximal or mid left anterior descending, proximal or mid right, or proximal left circumflex coronary artery (or circumflex equivalent) on CCTA was required. Participants and their physicians were advised if the CCTA demonstrated anatomic eligibility for the trial, in which case no further details were provided, or if the participant was excluded due to significant left main CAD or absence of obstructive CAD, in which case the CCTA was unblinded at the site and could be used clinically. The CCTA was also unblinded to the sites if: 1) other incidental findings ethically mandated unblinding and protocol ineligibility, such as a lung mass; 2) the participant failed to meet entry criteria prior to randomization for any other reason; or 3) for planning of revascularization after randomization at the discretion of site investigators. In general, participants with eGFR <60 ml/min/1.73m2 did not undergo a CCTA due to the risk of developing contrast associated acute kidney injury. Such patients were excluded by the site if significant left main stenosis was suspected based on clinical and stress test data. In a small number of cases, CCTA was performed prior to enrollment for clinical indications. When this occurred, or when a prior cardiac catheterization demonstrated the absence of significant left main stenosis and the presence of significant obstructive disease in other coronary arteries, a study CCTA was not required and attempts were made to collect the report and/or images to document atherosclerotic burden at baseline.
Role of the CCTA Core Lab
The primary purpose of the CCTA Core Lab was to identify individuals with significant unprotected left main coronary artery stenosis, defined as CCTA-identified >50% luminal diameter narrowing. Such patients were excluded from randomization due to safety concerns. The secondary purpose was to identify individuals who did not have any coronary artery stenosis >50% in any major epicardial coronary artery or branch vessel (>70% if the qualifying stress test was a non-imaging exercise test; see Table 4). Such patients were excluded from randomization to enhance appropriate selection of intended participants.
Optimal Medical Therapy
To achieve the trial’s primary aim, it is critical that OMT be applied equally to both treatment groups. The OMT recommendations were based on guideline-directed medical therapy for secondary prevention.1,19,20 When new medications were approved, evidence became available, or guidelines for secondary prevention were updated and published during the trial, they were considered for incorporation into the study. The goals of medical therapy are provided in Table 5.
Table 5.
Goals of Medical Therapy
| RISK FACTOR | GOALS | |
|---|---|---|
| Behavioral | ||
| Smoking | Smoking cessation1 | |
| Physical activity | ≥30 minutes of moderate intensity ≥5 times/week | |
| Saturated fat | <7% calories | |
| Physiological | ||
| Blood pressure | Systolic blood pressure <130 mm/Hg1,2 | |
| LDL cholesterol | LDL-C <70 mg/dl (1.8 mmol/L)1 | |
| Body Mass Index (kg/m2) | Initial BMI 25-27.5 >27.5 | Weight Loss Goal BMI <25 10% relative weight loss |
| Diabetes | <8%.3 A more stringent HbA1c goal (such as <7%) may be appropriate for selected individuals.4 | |
| Pharmacological agents | Indications | |
| Aspirin | All participants, 75-162 mg daily1 | |
| Statin | All participants, maximum tolerated dose of high-intensity statin (atorvastatin 40-80 mg or rosuvastatin 20-40 mg)1 | |
| ACEi/ARB | Use for hypertension, diabetes, eGFR <60 or LVEF <40%1 | |
| Beta blocker | Use for history of MI or LVEF <40%1 | |
| P2Y12 receptor antagonist | Use for participants with contraindication to aspirin; In combination with aspirin for participants who receive PCI (duration depends on BMS vs. DES); post-MI/ACS for 1 year |
|
| Ezetimibe | Use for participants unable to reach LDL-C goal on maximally tolerated statin dose in countries without access to evolocumab provided to trial participants | |
| Evolocumab | Use for participants unable to reach LDL-C goal on maximally tolerated statin dose in countries with access to evolocumab provided to trial participants | |
This risk factor goal is included in the trial’s definition of optimal medical therapy
This risk factor goal changed from <140 mmHg to <130 mmHg in April 2018.
Appropriate for participants with a history of severe hypoglycemia, advanced microvascular or macrovascular complications, extensive comorbid conditions, and those with long-standing diabetes in whom a goal of <7% is difficult to attain.
May be appropriate for participants with a short duration of diabetes and a long life expectancy if this can be achieved without significant hypoglycemia or other adverse effects of treatment.
BMI = body mass index; HbA1c = hemoglobin A1c; MI = myocardial infarction; ACEi/ARB = angiotensin converting enzyme inhibitor/angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; BMS = bare metal stent; DES = drug-eluting stent; ACS = acute coronary syndrome; LDL-C = low-density lipoprotein cholesterol.
The site study team worked in collaboration with the participant’s personal physician to achieve OMT goals. Study coordinators were trained to provide lifestyle counseling focused on smoking cessation, nutrition, physical activity, and medication adherence. Pharmacologic secondary prevention therapy included antiplatelet therapy and medications to control low-density lipoprotein (LDL) cholesterol (principally high-intensity statin therapy), blood pressure, and angina. To improve medication adherence, the trial was able to provide certain medications at no cost to participants in some countries (see online supplement Table S4). Pedometers were donated for all participants to encourage regular exercise.
ISCHEMIA treatment algorithms were developed and recommended for management of LDL cholesterol, blood pressure, and angina (see Figure S1). The Clinical Coordinating Center monitors attainment of risk factor goals and provides monthly reports to sites regarding their performance. Other methods to optimize medical therapy included investigator meetings, webinars, monthly newsletters, emails, dedicated phone calls with site study teams, and in-person meetings with individual investigators when possible, to review participant level data and provide specific feedback regarding site performance in achieving risk factor control. Participants were provided cards and key tags that list the risk factor goals.
Optimal Revascularization
The goal of the invasive strategy in ISCHEMIA is revascularization of all ischemic territories (based on results of stress test findings and/or diagnostic catheterization), incorporating fractional flow reserve (FFR) for selection of target vessels, where appropriate. Trial guidelines for optimal revascularization apply to participants randomized to the invasive strategy and to participants in the conservative management strategy who require cardiac catheterization due to failure of OMT alone. The selection of PCI vs. CABG (or medical therapy only in cases of non-obstructed coronary arteries, diffuse small vessel disease, etc.) was left to the discretion of the heart team per local standards and expertise, but guided by several general principles as outlined below. Criteria for site selection based on revascularization performance are in the online supplement Table S2. FFR was recommended for all lesions with a site-assessed diameter stenosis of <80% unless noninvasive evidence of ischemia was already present in that myocardial territory. FFR was also encouraged if angiographic appearance suggested a non-significant stenosis but there was inducible ischemia on stress testing in that vascular bed.
PCI
PCI was performed with a goal of relieving all areas of significant ischemia detected by noninvasive imaging and/or anatomy with FFR testing for lesions of borderline significance (see FFR algorithm, Figure S2). Drug-eluting stents (DES) were used routinely unless extended dual antiplatelet therapy was precluded. FDA-approved everolimus-eluting stents or slow-release zotarolimus-eluting stents were strongly recommended for participants receiving DES. To optimize DES use, the trial secured donations of everolimus-eluting stents and slow-release zotarolimus-eluting stents to be provided at no cost to participants in all countries, for use in the protocol-assigned initial invasive procedure (see online supplement Table S4). The use of other DES, bioresorbable scaffolds, and bare metal stents was discouraged.
CABG
It was recommended that all coronary arteries >1.5 mm in diameter with ≥70% stenosis be revascularized unless these territories were known not to be ischemic on the basis of noninvasive or invasive testing. If FFR indicated hypoperfusion to the myocardium beyond a ≥50% stenosis, these vessels were also targeted for grafting. The goal was complete arterial revascularization when technically and anatomically feasible.
Criteria to Select PCI vs. CABG
Criteria to Select PCI vs. CABG. The overarching goal was to select the revascularization approach that provided the most complete relief of ischemia while balancing the risk of procedure-related death, MI, or stroke. The choice of PCI or CABG was determined by the local Heart Team (interventional cardiologist and cardiac surgeon). Guidelines from professional societies and appropriateness use criteria were incorporated into the decision process.21-23 It was recommended that the Heart Team review complex cases (such as those with multivessel CAD or whenever in doubt) to determine the optimal strategy of revascularization for the individual participant. The general principles of this decision process are outlined in Table S2.
Role of the Angiographic Core Lab
The angiographic core lab reviewed the protocol-assigned angiograms (including staged procedures) for participants randomized to INV and review all suspected endpoint event-related angiograms in both groups triggered by the CEC, blinded to the randomized treatment assignment. The goals of the Angiographic Core Laboratory were to: 1) independently confirm that enrollment procedures effectively excluded patients with obstructive unprotected left main CAD; 2) independently confirm a high prevalence of obstructive non-left main-related CAD in the enrolled population; 3) describe the baseline burden of CAD in patients randomized to the invasive strategy; 4) provide independent assessment of PCI quality and practices; and 5) enable independent assessment of the appropriateness of use of PCI vs. CABG vs. neither, and the completeness of ischemic and anatomic revascularization among participants assigned to the invasive strategy.
Adherence to Protocol-Assigned Management
In patients assigned to the conservative strategy, cardiac catheterization is reserved for failure of OMT. Failure of OMT was defined as unacceptable ischemia-related symptoms despite maximally tolerated medical therapy. Sites are instructed that at least two anti-anginal drugs from different drug classes should be added to beta-blocker therapy and titrated to maximally tolerated doses before medical therapy is considered to have failed. Participants who cannot tolerate beta-blockers should be taking maximally tolerated doses of medications from three different anti-anginal drug classes before medical therapy is considered to have failed due to an unacceptable level of angina. Elective revascularization for unacceptable symptoms despite maximal medical therapy and urgent revascularization for unstable angina or MI represents adherence to the protocol. Performance of cardiac catheterization with or without revascularization in the absence of these clinical indications is considered non-adherent to the assigned strategy.
Although complete revascularization was the goal in patients randomized to the invasive strategy, it was recognized that some participants in the invasive treatment arm may not have coronary anatomy suitable for revascularization. This occurred when cardiac catheterization revealed the absence of obstructive CAD (despite the findings of CCTA, when performed) or the presence of severe, complex anatomy that could not be safely and effectively revascularized by either PCI or CABG as determined by the site Heart Team. The absence of revascularization in these settings was considered adherent to the assigned strategy. A small proportion of participants had CABG directly after randomization based on prior known coronary anatomy. In other circumstances, if cardiac catheterization was not performed, this was considered evidence of non-adherence to the invasive strategy. The rate of non-adherence to each assigned strategy is closely monitored.
Measures were taken to maximize adherence to the assigned strategy in both groups. Patients were excluded from enrollment if they had an unacceptable level of angina despite maximal medical therapy (daily angina without ability to further titrate medical or anti-anginal therapy), were dissatisfied with medical management of angina (defined as a patient response “extremely bothersome” to the SAQ question about how bothersome it is to take pills for angina), had a history of nonadherence to medical therapy, Canadian Cardiovascular Society Class III or IV angina of recent onset, or angina of any class with a rapidly progressive or accelerating pattern. Sites were advised not to randomize individuals who expressed a clear preference for undergoing revascularization or not undergoing revascularization during the informed consent process. In keeping with current clinical practice guidelines, sites are discouraged from performing routine stress tests during follow-up after randomization in the absence of new symptoms. Sites are provided with monthly reports that include anti-anginal medication use in participants who were experiencing angina at least once a week at their last visit. If a site notifies the CCC of a possible elective catheterization, they are asked to submit corroborating clinical information about angina symptom severity, pharmacologic management of angina, heart rate, and blood pressure. Measures to maximize adherence to the invasive strategy included training site investigators to conduct thorough informed consent to prevent inclusion of participants unwilling to accept invasive procedures, including CABG, after assignment to the INV strategy. Performance of CCTA helped prevent inclusion of individuals with no obstructive CAD in whom revascularization would not be performed. The study strongly encouraged completion of catheterization and revascularization in the INV group within 30 days after randomization to avoid the occurrence of endpoints prior to revascularization. If the angiographic core laboratory noted that INV participants may not have received complete revascularization, they notified the CCC of this fact. In turn, the CCC notified participating sites regarding cases of incomplete revascularization. Figure 3 is a schema of invasive procedures and protocol adherence in the trial.
Figure 3. Invasive Procedures and Protocol Adherence.
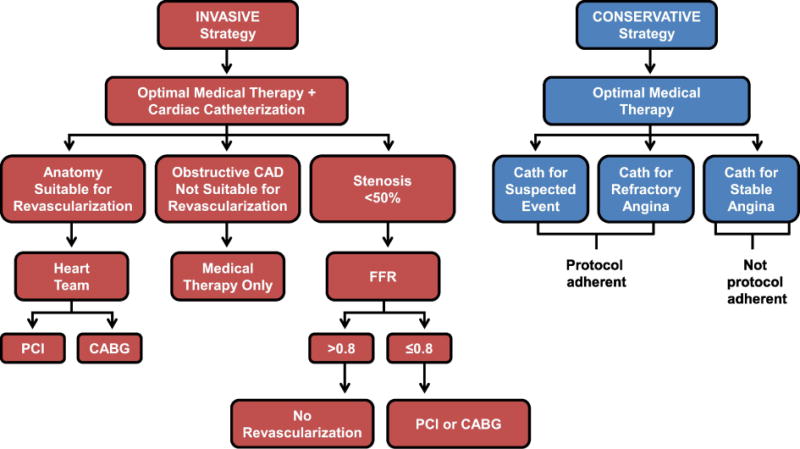
In addition to receiving optimal medical therapy, participants randomized to the invasive strategy were to undergo cardiac catheterization followed by complete revascularization of all ischemic territories when feasible. For complex anatomy, the local Heart Team recommended the optimal method of revascularization. FFR was recommended for stenosis <50% if PCI was considered and stress imaging showed ischemia in the corresponding territory. FFR was also recommended for stenosis <80% if PCI was considered and stress imaging did not show ischemia in the corresponding territory (see Figure S2a). Use of instantaneous wave-free ratio instead of FFR (where available) was permitted, using a cutoff of ≤0.89 for physiologic significance. Among participants randomized to the conservative strategy, urgent revascularization for diagnosed or suspected acute coronary syndrome or elective revascularization for unacceptable symptoms despite maximal medical therapy is adherent to the protocol. Cardiac catheterization with or without revascularization in the absence of these clinical indications is non-adherent to the protocol for the conservative strategy.
CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; FFR, fractional flow reserve; iFR®, instantaneous wave-free ratio.
Data Collection and Follow-up Assessments
The full study dataset is collected for participants who entered the randomized phase of the study. The primary data collection system for ISCHEMIA is a web-based electronic data capture system, a validated Electronic Record, Electronic Signatures (ERES) compliant platform (21 CFR Part 11). All participant level data collected at any point in the trial, except source documents requested by the CEC and economic and QOL supplemental information, are entered into this system. Electronic case report forms are the source for all baseline and follow-up data collected and entered by sites and entered by the central core laboratories (CCTA, ECG, angiography, and 4 types of stress testing).
Resource use is being collected on the case report form for the initial treatment phase and includes details about the diagnostic testing strategy used following randomization. In addition, we are collecting data on any invasive cardiac procedures used, including cardiac catheterization and coronary revascularization. Selected procedural resource use details include time in the catheterization laboratory, operating room, specific procedures performed, stents placed, ICU days, and total hospital days post procedure. Data on the patient’s initial pharmacological regimen were also collected. Follow-up data collection includes hospitalizations, selected outpatient care, major diagnostic tests, and medication use. Custodial and nursing home stay data are also collected.
Hospital bills for patients in the United States only are being collected by the Economic Study Coordinating Center at the Duke Clinical Research Institute. Medicare Cost Report Worksheets C and D-1 Part 2 are being obtained from each US hospital where an ISCHEMIA baseline or follow-up hospitalization is reported. Physician fees will be estimated using the Medicare Fee Schedule.
Collection of QOL data, including the follow-up QOL questionnaire validated scales, is performed at baseline and during follow-up at 3, 12, 24, and 36 months after randomization and at the final ISCHEMIA visit by trained telephone interviewer staff from the Economics and QOL Coordinating Center for participants enrolled in North America and by the site coordinator in sites outside North America that elected to participate in this aspect of the study. Disease-specific health status is captured by the SAQ24 and is supplemented with the Rose Dyspnea Questionnaire to capture dyspnea. These are supplemented with the Duke Activity Status Index, Rand General Health rating and EuroQOL-5D for generic assessments of function. Depressive symptoms are captured with the 8-item Patient Health Questionnaire,25,26 stress with the Perceived Stress Scale27,28 and optimism with the Life Orientation Test-Revised.29
More frequent assessments of angina and dyspnea are assessed with the 7-item SAQ,14 the Rose Dyspnea Questionnaires and the Visual Analogue Scale of the EQ-5D by site coordinators at every site at every study visit through 36 months and the final closeout visit, and entered directly into the study EDC system.
Capture of Events
When designing the trial we recognized there could be bias in the diagnosis and/or reporting of events in the different treatment arms. Although hospitalization for unstable angina or heart failure would be more susceptible to bias, we recognize that MI is also subject to bias; epidemiologic data demonstrate that “silent MIs” are not uncommon, which in part may represent failure to recognize symptoms or seek hospitalization.
We therefore implemented several methods from trial inception to mitigate bias in the ascertainment of events. These include carefully constructed data collection forms that focus sites on endpoint events, screening of angiographic and ECG core laboratory data, site investigator and coordinator education and reminders about the importance of complete event ascertainment and reporting, extensive cross variable checks, random and for-cause document reviews, and queries to ensure complete ascertainment and site reporting of any potential endpoint events. Programmed database algorithms identify possible missed events (e.g., marker elevation; hospitalization for other reasons such as chest pain, dyspnea, or pneumonia; and change in New York Heart Association and/or Canadian Cardiovascular Society class on study visits). These triggers result in requests for event forms to be completed and source documents provided by sites for CEC review. ECG’s are obtained at 2 years and reviewed by the core laboratory to assess for the occurrence of silent Q wave MI.
Monitors visit sites, with regular visits to all sites in certain countries and periodic visits to selected other sites. Individual participant medical records are reviewed for unreported or missed hospitalizations by monitors and/or by site coordinators. Site coordinators and/or monitors are requested to look for unreported hospitalizations in national, regional, or health insurance databases where available. In the US medical bills are cross checked against reported hospitalizations. In addition, variation in average vs. observed event rates at sites are reviewed. Sites with unexpectedly low event rates are subject to additional monitoring. All countries with available data will have death indexes reviewed for participants lost to follow-up.
Procedural complications including anaphylaxis, renal failure, bleeding, and major adverse cardiac events arising from CCTA, cardiac catheterization, PCI, and CABG are collected in the case report forms. Quality assurance monitoring included review of 85% of PCI reports when procedure-related hospitalization was longer than one night and 85% of all CABG reports for a 3.5 year period. Other adverse events that may possibly be related to procedures such as bleeding during follow-up are not systematically captured, but may be reported on hospitalization forms.
Clinical Event Adjudication Committee (CEC)
Data collected for suspected events are provided to an independent CEC comprised of physician reviewers masked to treatment assignment that adjudicate the following endpoints based on study definitions: cause of death, MI, hospitalization for unstable angina, hospitalization for heart failure, resuscitated cardiac arrest (events that occur out of hospital or in the emergency department), and stroke. MI was classified using two definitions. The primary definition used the local hospital biomarker reference limit to determine abnormal values. For types 4A and 5 MI, CK-MB was the preferred biomarker. The secondary definition employed the Third Universal Definition of Myocardial Infarction30 and the manufacturer’s suggested 99th percentile reference limit for the assay to determine abnormal values, and for types 4A and 5 MI troponin was the preferred biomarker. MI was classified using the two definitions due to continued evolution of MI criteria at the time the study was initiated. (See Table S5 for definitions of endpoint events.)
Statistical Considerations
Sample size estimation
ISCHEMIA originally planned to randomize 8,000 participants over 4 years with an average follow-up of 3.7 years. To achieve this sample it was estimated that more than 10,000 participants would be enrolled, accounting for screen failures. The sample size was estimated to provide 90% power to detect a 15% relative reduction in the primary composite endpoint assuming the primary endpoint occurs within 4 years in 20% of the conservative strategy group and 17% of the invasive strategy group. An annual rate of CV death or MI in patients with at least moderate ischemia was estimated to be ~5% using data from the COURAGE trial9 and several observational stress imaging registries.31-41 A modest relative reduction was used in power calculations in light of ISCHEMIA’s strategy trial design which includes patients without known coronary anatomy and the expectation that not all patients in the target population would be suitable for revascularization or benefit equally from an invasive strategy. The modest between-group difference was also intended to account for attenuation of the treatment effect by non-adherence to the randomized treatment strategy. For example, in writing our grant proposal, we assumed that the rate of discretionary catheterization in CON participants might be as high as 70% within 6 years. The between-group difference would be larger if a high adherence rate was realized. Participants in the conservative group who undergo catheterization for unacceptable angina despite maximal OMT are considered protocol-adherent, because the conservative strategy allows for catheterization after OMT failure, but such catheterizations may reduce power for the primary endpoint if they prevent the occurrence of primary endpoint events. Such potential attenuation was incorporated in sample size calculations by specifying a between-group difference that is smaller than it would be hypothetically if catheterization was never performed in the conservative strategy.
Slower than expected recruitment ultimately led study leadership to request approval from NHLBI to reduce the target sample size from 8,000 to 5,000 randomized participants with an extension of the recruitment period. This request was approved by NHLBI in August 2016. To mitigate the loss of statistical power follow-up was also extended.
Considerations for changing the primary endpoint
To ensure an adequate number of endpoint events for the primary analysis, the initial ISCHEMIA protocol (version 1.0 dated January 18, 2012) included a contingency plan to allow changing the primary endpoint from the 2-component to the 5-component endpoint after trial initiation by following a process with safeguards incorporated to protect against bias and inflation of the type-I error rate. At various points in the trial, the primary endpoint event rate and statistical power were re-estimated using updated assumptions derived from blinded pooled analyses (not by treatment group) of the accumulating trial data. In accordance with the protocol, triggered by a lower than projected number of primary endpoint events due to a lower event rate and smaller sample size, an independent panel was convened by NHLBI in May 2017 for the purpose of reviewing relevant blinded aggregate study data and advising the NHLBI director and study leadership. The panel reviewed data showing that the 2-component primary endpoint event rate was lower than originally projected and that power for this endpoint may fall below 60%. To increase statistical power, the panel recommended reverting to the 5-component primary endpoint, and making the 2-component endpoint a key secondary endpoint. The panel’s recommendation was approved by the Director of the Division of Cardiovascular Sciences, NHLBI in June 2017 and simultaneously adopted by the ISCHEMIA Trial leadership. The reduced sample size, expanded primary endpoint composite, and extended follow-up were estimated to provide at least 80% power to detect an 18.5% reduction in the 4-year incidence of the primary endpoint under assumptions consistent with the accumulating aggregate study data.
Analysis of the Primary Endpoint
The primary endpoint is time from randomization until the first occurrence of any event from the primary composite of CV death, MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest. Comparisons by treatment group will follow the intention-to-treat principle. The occurrence rate of the primary endpoint will be compared across treatment groups using the Cox proportional hazards model. Estimation of event rates with 95% confidence intervals not assuming proportional hazards will be performed as an important secondary analysis. Patients who are lost to follow-up before the planned end of data collection will be censored at the time of last known status. To account for heterogeneity among trial participants, the primary Cox model will be adjusted for a pre-specified set of prognostically important baseline covariates including age, sex, renal function, ejection fraction, and diabetes. Covariates were selected on the basis of their established prognostic importance in other SIHD cohorts, highly complete data capture, and a sufficient range of values for risk to vary among patients meeting trial eligibility criteria. Secondary analyses will be performed to examine whether the estimated treatment effect is similar for all participants or whether it varies according to specific participant characteristics. These analyses will be conducted using the Cox model by estimating interactions between treatment group and specific baseline characteristics. Multiple endpoint events in the same individual (e.g., recurrent MI) will not be considered in the primary results analysis but will be analyzed in planned secondary analyses. Subgroups of particular interest include those defined by baseline ischemia severity, diabetes, new onset or worsening angina, and optimization status of medical therapy at baseline. For those who undergo CCTA, baseline extent and severity of CAD are of particular interest. To supplement the conventional estimation approaches described above for the primary and secondary endpoints, we will perform additional supporting analyses using the Bayesian statistical framework in order to make probability statements about the likelihood of a clinically important difference in either direction.
Analysis of Economic Outcomes
The primary comparison of costs will be to test total US study costs out to the end of study follow-up. If the invasive strategy is shown to be superior in analysis of clinical outcomes, cost-effectiveness analyses that quantify the incremental cost required to add an extra life year with the invasive strategy relative to the conservative strategy will be conducted. In cost-effectiveness sensitivity analyses, utility weights to estimate the incremental cost per quality-adjusted life year gained between randomized strategies will be incorporated.
Analysis of Quality of Life Outcomes
Quality of life measures will be summarized using descriptive statistics, including mean, standard deviation, median and interquartile ranges (25th and 75th percentiles) at each time point. Statistics will be reported separately for each treatment group, both for the absolute score and for change from baseline. While the primary analyses will be conducted using continuous health status scores, categorization of the results into clinically interpretable categories (e.g. any angina vs. none) will be performed to facilitate interpretation. Because patients are expected to experience a nonlinear improvement in QOL following randomization, with rapid gains during the first 6 months followed by continued gradual improvement through the end of follow-up, we will use piecewise linear growth curves with knots at 3 and 6 months to quantify changes through 3 months, between 3 and 6 months, and between 6 months and the end of follow-up. QOL analyses will therefore fit these curves utilizing hierarchical linear (for continuous outcomes) or generalized linear (for dichotomous and ordinal outcomes) models and will include patient-specific random effects for the growth-curve parameters, corresponding fixed-effect parameters representing the trajectory for an “average” patient, fixed effects for treatment and treatment-by-trajectory interaction terms, and adjustment for baseline QOL as a covariate. The key outcome of interest to be compared between treatment groups is the average QOL over the duration of follow-up, calculated as area under the mean model-estimated growth curve for each treatment group. Estimated QOL at each follow-up time point, and the difference between treatment groups, will be reported, along with 95% confidence intervals. This framework will be used for all QOL measures obtained via the brief symptom/QOL assessment to include all patients enrolled in ISCHEMIA, with the full QOL assessment, obtained only at 3, 12, 24 and 36 months, being conducted among sites participating in this aspect of the study, but employing growth curves with only a single knot at 12 months. Collectively, these analyses will provide detailed insights into the health status (symptoms, function and quality of life) benefits of an invasive management strategy over a conservative one.
Interim Monitoring
An independent Data and Safety Monitoring Board (DSMB) was appointed by the NHLBI to monitor participant safety and provide recommendations regarding terminating, continuing, or modifying the study protocol if concerns arise. Reports to the DSMB are generated by an independent analytic center at Vanderbilt University by staff who are not involved in daily trial operations. Interim treatment group comparisons are planned for the primary endpoint at least 3 times during the study and will be monitored with the use of two-sided symmetric O’Brien-Fleming type boundaries.42,43 An α-spending function will be used to control the overall type-I error probability for the primary endpoint at 5%.
Ancillary Studies
The ISCHEMIA-CKD trial will be described in more detail in a separate trial design paper. The CIAO-ISCHEMIA ancillary study (Changes in Ischemia and Angina over One year among ISCHEMIA trial screen failures with non-obstructive coronary artery disease on CT angiography) examines the relationship between ischemia, symptoms, and atherosclerosis in patients without angiographically obstructive CAD. CIAO-ISCHEMIA enrolls participants who were excluded from the ISCHEMIA trial due to non-obstructive CAD on CCTA (defined as <50% stenosis in all epicardial vessels). Enrollment is restricted to participants who underwent stress echocardiography. CIAO-ISCHEMIA participants have angina assessed at baseline and at 6 and 12 months from the pre-enrollment stress test. Stress echocardiography is repeated approximately 12 months after the pre-enrollment stress test. The primary objective is to investigate the association between change in angina severity and change in ischemia severity over one year. Secondary analyses will focus on the correlation between ischemia severity, angina severity, and severity of non-obstructive atherosclerosis on CCTA at baseline.
Summary
The ISCHEMIA Trial was designed to address important gaps in our scientific knowledge about the best way to manage SIHD patients with moderate or severe ischemia. The results of ISCHEMIA will inform professional society guidelines, health policy, and clinical practice.
Supplementary Material
Acknowledgments
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565
Disclaimer:
This article refers to work supported by National Heart, Lung, and Blood Institute grant U01HL105907, in-kind donations from Abbott Vascular; Medtronic, Inc.; St. Jude Medical, Inc.; Volcano Corporation; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc.; and by financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
We are grateful to the ISCHEMIA Trial participants for their contribution to the knowledge base that leads to improved medical care. We wish to acknowledge that the ISCHEMIA Trial represents a large-scale collaboration among well over 1,000 healthcare professionals that comprise site study teams, committees, core laboratories, and coordinating centers. To all these dedicated individuals, we express our sincere gratitude for their collective efforts (see Tables S1 and S3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 3.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. The New England journal of medicine. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Hochman JS, Williams DO, et al. Medical Therapy With Versus Without Revascularization in Stable Patients With Moderate and Severe Ischemia: The Case for Community Equipoise. J Am Coll Cardiol. 2016;67:81–99. doi: 10.1016/j.jacc.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 7.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 8.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–24. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 10.Shaw LJ, Weintraub WS, Maron DJ, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164:243–50. doi: 10.1016/j.ahj.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Mancini GB, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 13.NIH RePORT-The ISCHEMIA trial. https://projectreporter.nih.gov/project_info_description.cfm?aid=9265117&icde=38661984&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball= Accessed March 23, 2018.
- 14.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640–7. doi: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Mark DB, Shaw L, Harrell FE, Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–53. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 17.Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol. 1984;3:772–9. doi: 10.1016/s0735-1097(84)80254-5. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s heart disease: a textbook of cardiovascular medicine. Tenth. Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- 19.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Task Force M, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 21.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–41. doi: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K., SR The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr Ann. 2002;32:509–21. [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 28.CSW G. Perceived stress in a probability sample of the United States. In: Oskamp SSS, editor. The social psychology of health: Caremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 29.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Shaw LJ, Hendel RC, Cerquiera M, et al. Ethnic differences in the prognostic value of stress technetium-99m tetrofosmin gated single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;45:1494–504. doi: 10.1016/j.jacc.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LJ, Hachamovitch R, Heller GV, et al. Noninvasive strategies for the estimation of cardiac risk in stable chest pain patients. The Economics of Noninvasive Diagnosis (END) Study Group. Am J Cardiol. 2000;86:1–7. doi: 10.1016/s0002-9149(00)00819-5. [DOI] [PubMed] [Google Scholar]
- 33.Lertsburapa K, Ahlberg AW, Bateman TM, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2008;15:745–53. doi: 10.1007/BF03007355. [DOI] [PubMed] [Google Scholar]
- 34.Yoshinaga K, Chow BJ, Williams K, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Bangalore S, Yao SS, Chaudhry FA. Usefulness of stress echocardiography for risk stratification and prognosis of patients with left ventricular hypertrophy. Am J Cardiol. 2007;100:536–43. doi: 10.1016/j.amjcard.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Yao SS, Qureshi E, Sherrid MV, Chaudhry FA. Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease. J Am Coll Cardiol. 2003;42:1084–90. doi: 10.1016/s0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 37.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doesch C, Seeger A, Doering J, et al. Risk stratification by adenosine stress cardiac magnetic resonance in patients with coronary artery stenoses of intermediate angiographic severity. JACC Cardiovasc Imaging. 2009;2:424–33. doi: 10.1016/j.jcmg.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50:1174–9. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 41.Shaw LJ, Berman DS, Picard MH, et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7:593–604. doi: 10.1016/j.jcmg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 43.Lan KDD. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


