Abstract
Background
Osteoporosis and related fractures, decreased physical activity, and metabolic dysfunction are serious health concerns for postmenopausal women. Soy protein might counter the negative effects of menopause on bone and metabolic health due to the additive or synergistic effects of its bioactive components.
Objective
To evaluate the effects of ovariectomy (OVX) and a soy-protein diet (SOY) on bone outcomes in female, low-capacity running (LCR) rats selectively bred for low aerobic fitness as a model of menopause.
Methods
At 27 weeks of age, LCR rats (N = 40) underwent OVX or sham (SHAM) surgery and were randomized to one of two isocaloric and isonitrogenous plant-protein-based dietary treatments: 1) soy-protein (SOY; soybean meal); or, 2) control (CON, corn-gluten meal), resulting in four treatment groups. During the 30-week dietary intervention, animals were provided ad libitum access to food and water; body weight and food intake were measured weekly. At completion of the 30-week intervention, body composition was measured using EchoMRI; animals were fasted overnight, euthanized, and blood and hindlimbs collected. Plasma markers of bone formation (osteocalcin, OC; N-terminal propeptide of type I procollagen, P1NP) and resorption (tartrate-resistant acid phosphatase, TRAP5b; C-terminal telopeptide of type I collagen, CTx) were measured using ELISA. Tibial trabecular microarchitecture and cortical geometry were evaluated using μCT; and torsional loading to failure was used to assess cortical biomechanical properties. Advanced glycation end-product (AGE) content of the femur was measured using a fluorimetric assay, and was expressed relative to collagen content measured by a colorimetric OH-proline assay. Two-factor ANOVA or ANOVCA was used to test for significant main and interactive effects of ovarian status (OV STAT: OVX vs. SHAM) and DIET (SOY vs. CON); final body weight was included as a covariate for body-weight-dependent cortical geometry and biomechanical properties.
Results
OVX had significantly greater CTx than SHAM; SOY did not affect bone turnover markers. OVX adversely affected trabecular microarchitecture as evidenced by reduced BV/TV, trabecular thickness (Tb.Th), trabecular number (Tb.N), and connectivity density (Conn.D), and by increased trabecular separation (Tb.Sp) and structural model index (SMI). SOY increased BV/TV only in ovary-intact animals. There was no effect of OVX or SOY on tibial cortical geometry. In SHAM and OVX rats, SOY significantly improved whole-bone strength and stiffness; SOY also increased tissue-level stiffness and tended to increase tissue-level strength (p = 0.067). There was no effect of OVX or SOY on AGE content.
Conclusion
Soy protein improved cortical bone biomechanical properties in female low-fit rats, regardless of ovarian hormone status.
Abbreviations: LCR, low-capacity runners; OVX, ovariectomy; SHM, Sham; SOY, Soy-protein-based diet; CON, control diet; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Tb.N, trabecular number; Conn.D, connectivity density; Tmax, maximal torque; Ks, torsional stiffness; G, shear modulus of elasticity; Su, ultimate tensile strength; OC, osteocalcin; P1NP, N-terminal propeptide of type I procollagen; TRAP5b, tartrate-resistant acid phosphatase; CTx, C-terminal telopeptide of type I collagen
Keywords: Osteoporosis, Menopause, Soy protein, Ovariectomy, Bone
Highlights
-
•
Ovariectomized low-capacity running (LCR) rats exhibit cancellous bone loss characteristic of human menopause.
-
•
Soy protein improved tibial whole-bone and tissue-level biomechanical properties in ovariectomized and ovary-intact rats.
-
•
Advanced glycation end-product (AGE) content of the femoral diaphysis was not affected by ovariectomy or soy protein.
-
•
Estrogen receptor-α-independent mechanisms might be responsible for the improvements in cortical strength observed with soy.
1. Introduction
1.1. Postmenopausal bone loss
The cessation of ovarian hormone production that defines menopause predisposes women to osteoporosis and the metabolic syndrome (Spritzer and Oppermann, 2013). Ovarian hormone loss has many consequences, and the loss of estrogen is especially detrimental to bone (Seeman, 2004; Frost, 1999). Postmenopausal osteoporosis affects 30% of women (Foundation, I.O, 2015), and a woman aged 50 years has a 40–50% chance of suffering an osteoporotic fracture in her remaining lifetime (Johnell and Kanis, 2005; van Staa et al., 2001). While total body bone mineral density (BMD) declines during menopause, the loss of endogenous estrogen appears to have a greater negative impact on cancellous bone than on cortical bone (Berning et al., 1996; Seeman, 2013). The hip, vertebrae and epiphysis and metaphysis of long bones are largely cancellous bone, which in part explains why fragility fractures most frequently occur at these sites (Reeve et al., 1999; Kanis, 1994).
Postmenopausal bone loss has been attributed to increased remodeling rate with a disproportionate increase in bone resorption relative to formation (Teitelbaum, 2000), resulting in net bone loss and increased resorptive bone area, which increase fracture risk (Bala and Seeman, 2015). Estrogen promotes bone formation by stimulating osteoblast differentiation and function and attenuates bone resorption by reducing osteoclastogenesis and osteoclast activity (Kameda et al., 1997; Eastell et al., 2016). The effects of estrogen loss on osteoblasts and osteoclasts are mediated by increased RANK and decreased Wnt signaling (Eastell et al., 2016; Fujiwara et al., 2016; Dong et al., 2016; Liang et al., 2014; Zhang et al., 2016).
1.2. Postmenopausal metabolic dysfunction
Menopause often results in weight gain, physiologically unfavorable changes in body composition, and increased risk of the metabolic syndrome (Stefanska et al., 2015). Compared with premenopausal women, postmenopausal women have an increased risk of metabolic syndrome characteristics (Kwasniewska et al., 2012), including preferential expansion of intra-abdominal adiposity and the consequent increased risk of insulin resistance and dyslipidemia (reviewed in (Stefanska et al., 2015)). Abdominal obesity (Cohen et al., 2013), insulin resistance (Arikan et al., 2012), hyperglycemia (Terzi et al., 2015) and dyslipidemia (Mandal, 2015) might contribute to the detrimental changes in bone's structural and material properties (Yamauchi et al., 2015; Ramos-Junior et al., 2017; Pelton et al., 2012; You et al., 2011) that result in increased fracture risk postmenopause (Tanaka et al., 2013; Felson et al., 1993; Zhao et al., 2007). In addition, following menopause, many women significantly reduce their physical activity level (Hunter et al., 2001; Duval et al., 2014), which further accelerates bone loss (Dallanezi et al., 2016) and the onset of metabolic dysfunction (Duval et al., 2014).
1.3. Soybean consumption and health benefits
According to a survey conducted by the United Soybean Board, current soy protein consumption in the US is at its highest level, with 78% of US adults reporting consumption of soy foods or beverages (Board, U.S, 2015) and 38% consuming soy at least once per week (Board, U.S, 2015). Soy protein has received considerable interest for its potential ability to improve both bone and metabolic health in postmenopausal women (Bawa, 2010; Messina, 2016). Epidemiological evidence suggests that dietary soy intake is positively associated with bone mineral content (BMC) and BMD (Greendale et al., 2002; Messina, 2010). Consumption of whole soy foods is associated with lower fracture risk (Zhang et al., 2005; Koh et al., 2009) and attenuated rates of bone loss in prospective, cohort-studies in Asian populations (Ikeda et al., 2006; Ho et al., 2003) with the greatest beneficial effect in postmenopausal women (Ikeda et al., 2006). Soy also improves metabolic health outcomes in postmenopausal women (Messina, 2016). In experimental animals, dietary soy protein intake is associated with reduced adiposity, blood glucose and insulin, and with improvements in lipid profile and insulin sensitivity (Chen et al., 2013; Torre-Villalvazo et al., 2008; Lavigne et al., 2000). Thus, in addition to its direct effects on bone, soy protein might also indirectly affect bone by improving metabolic health risk factors. In particular, because soy improves glycemic control, it might reduce accumulation of AGE in bone and, thus, enhance bone's material properties. While the effects of soy protein on bone and on metabolic health indicators have been examined independently (Messina, 2016), the indirect skeletal benefits of soy protein ingestion due to improved metabolic health have not been investigated.
1.4. Reductionist versus whole food approach to soy health research
To date, the trend in soy research has been reductionist, with the end goal of identifying the bioactive component with health-promoting effects (Reinwald et al., 2010; Reinwald and Weaver, 2010). However, soy is a complex food with a multitude of bioactive components that might have additive, synergistic, or even antagonistic effects (Reinwald et al., 2010; Reinwald and Weaver, 2010). Studies that investigate soy as it is regularly consumed as part of a well-balanced Western diet are needed (Klein et al., 2010). The purpose of the present study was not to identify the bioactive molecule responsible for the beneficial effects of soy, nor to isolate the effects of soy isoflavones. Rather, the purpose of the present study was to compare the effects of soybean meal to those of corn-gluten meal on bone outcomes in ovariectomized and ovary-intact LCR rats.
1.5. Study purpose and animal model
Here, we compared the effects of a soybean-meal (SOY) versus a control corn-gluten-meal diet (CON) on bone outcomes in female, ovariectomized (OVX) low-capacity-running (LCR) rats, as a rodent model of human menopause. Specifically, we examined the effects of SOY on tibial trabecular microarchitecture, cortical geometry and biomechanical properties, plasma markers of bone formation and resorption, and AGE content. We hypothesized that SOY would improve bone outcomes relative to CON and that the benefits of SOY would be greater in OVX animals due to their greater potential to respond.
2. Materials and methods
2.1. Experimental design and animal protocol
A two-by-two factorial experimental design (ovarian status: OVX vs. SHAM; and diet: SOY vs. CON) was used to test the main and interactive effects of ovarian status and diet on bone outcomes in skeletally mature LCR rats as a model of human menopause. Human postmenopausal bone loss occurs after skeletal maturity, so animal models of menopause should be in skeletally mature animals. To isolate effects of OVX or dietary treatments on skeletal outcomes, animals should be at a developmental stage during which skeletal mass is stable (Kalu, 1984), which occurs at an age of 6–24 months in female rats (Kalu et al., 1989). LCR rats selectively bred and tested for running capacity at the University of Michigan as previously described (Koch and Britton, 2007) were received at 25 weeks of age and immediately placed on a soy-maintenance diet (Harlan Inc., Madison, WI, USA) for two weeks prior to surgery and group randomization. This paper reports bone outcomes from a parent longitudinal study investigating the effects of OVX and SOY on inflammation and adipose tissue (Cross et al., 2017), in which female LCR rats underwent ovariectomy (OVX) or sham surgery (SHAM) at 27 weeks of age, prior to being fed either a soybean meal (SOY) or corn-gluten meal control (CON) diet for 28–30 weeks. Animals were pair-housed in a temperature-controlled environment (~22 °C) with a 12 h–12 h light/dark cycle maintained throughout the experimental period. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri and the National Institutes of Health animal care guidelines were strictly followed.
2.2. Animal surgeries
Rats were anesthetized using inhaled isofluorane (2%) and then bilateral OVX, or SHAM surgery was performed. For OVX, the whole ovary was removed. For SHAM, the ovary was externalized before being replaced back inside the body cavity. A single 2.5-cm midline incision along the dorsal surface was made followed by two bilateral incisions through the muscle layer to expose the ovaries. Upon completion of surgery, wound clips were used to close the incision and acetaminophen (500 mg/kg) was administered.
2.3. Animal diets
Following surgery, OVX and SHAM LCR rats were randomly assigned to either the SOY or CON diet (Table 1), resulting in four experimental groups (n = 10 per group): SHAM:CON, SHAM:SOY, OVX:CON, or OVX:SOY. All animals were allowed ad libitum access to the SOY or CON diet and to water. The SOY and CON diets were isocaloric and isonitrogenous with carbohydrate, protein and fat providing 63, 25 and 12% of energy, respectively (Table 1). The protein sources in the SOY and CON diets were soybean meal (48% protein) and corn-gluten meal (60% protein), respectively. Soybean meal is produced by grinding dehulled, oil-extracted whole soybeans (INFOcenter, S.M, n.d.). In addition to high-quality protein, soybean meal contains bioactive components (e.g., isoflavones, and soyasaponins). Corn-gluten meal is a by-product of corn wet milling process and corn starch production.
Table 1.
Diet ingredients for the soybean meal (SOY) and corn-gluten meal (CON) diets.
| Diet ingredients (g/kg diet)a | SOYb | CONc |
|---|---|---|
| Soybean meal (48% protein) | 260.0 | 0 |
| Corn gluten meal (60% protein) | 0 | 188.0 |
| Corn | 357.5 | 388.0 |
| Wheat, soft | 230.25 | 230.5 |
| Wheat, middlings | 46.0 | 73.0 |
| D,L-methionine | 1.0 | 1.0 |
| l-lysine HCl | 1.0 | 8.0 |
| Soybean oil | 20.0 | 16.0 |
| Cellulose | 50.60 | 58.93 |
| Mineral mix | 5.0 | 5.0 |
| Calcium phosphate dibasic | 8.0 | 10.0 |
| Calcium carbonate | 13.0 | 13.0 |
| Sodium chloride, iodized | 2.5 | 2.5 |
| Magnesium oxide | 0.5 | 0.5 |
| Vitamin mix | 4.0 | 4.0 |
| Chloride | 0.40 | 1.57 |
| Red food color | 0.25 | 0 |
Diets prepared by Harlan Laboratories (Madison, WI) using food-grade ingredients.
SOY diet provided ~590 mg genistein + daidzein (aglycone equivalents)/kg diet.
CON diet provided <15 ppm isoflavones.
2.4. Animal sacrifice and tissue collection
Rats were euthanized between 55 and 57 weeks of age. Rats were euthanized over a span of two weeks due to the time required to perform time-consuming assessments (e.g., EchoMRI) the day of sacrifice. Equivalent numbers of animals from each group were sacrificed each day so that there were no difference among groups in age at sacrifice. Prior to sacrifice, body composition was assessed by EchoMRI. After a 5-h fast, animals were euthanized via carbon dioxide and then exsanguinated via cardiac puncture. Blood was collected into a tube containing K3EDTA, mixed, placed on ice, and then centrifuged at 7000g for 10 min at 4 °C to obtain plasma. Plasma was aliquoted and stored at −80 °C for subsequent analysis of metabolic health outcomes (glucose and insulin) and markers of bone formation (N-terminal propeptide of type I procollagen, P1NP; and osteoclacin, OC), and bone resorption (tartrate resistant acid phosphatase isoform 5b, TRAP5b; and, C-terminal telopeptide of type 1 collagen, CTx). Left tibiae were collected, cleaned of soft tissue, wrapped in 1× phosphate-buffer saline (PBS)-soaked gauze, and frozen at −80 °C for subsequent biomechanical testing and determination of trabecular microarchitecture and cortical geometry. Left femora were similarly removed and frozen for determination of hydroxyl-proline (OH-proline) and AGE content.
2.5. Plasma glucose, insulin and bone formation and resorption markers
Fasting glucose and insulin concentrations were measured by a clinical diagnostic service at the University of Missouri (Clinical Pathology Services, LLC) with an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea CA). The bone formation markers OC and P1NP, and the resorption markers CTx and TRAP5b were measured in plasma using commercially available, rodent-specific ELISA kits (ImmunoDiagnostic Systems, Fountain Hills, AZ; kit #s: OC (AC-12F1), P1NP (AC-33F1), CTx (AC-06F1), TRAP5b (SB-TR102)). All assays were run on the same day to avoid inter-assay variation; all samples were run in duplicate. The intra-assay CVs were: OC = 3.14%, P1NP = 6.23%, CTx = 1.79%, and TRAP5b = 3.88%. The resorptive index was calculated as the ratio of CTx to TRAP5b and is an indicator of osteoclast activity per cell (Rissanen et al., 2008).
2.6. Tibia trabecular microarchitecture and cortical geometry
Micro-computed tomographic (μCT) imaging of the tibia was performed using a high-resolution (32-μm slice increment) imaging system (Siemens INVEON Micro SPECT/CT (Siemens Medical, Malvern, PA). The methods used were in accordance with guidelines for the use of μCT in rodents (Bouxsein et al., 2010). Scans were acquired using an isotropic voxel size of 31.6 μm and a peak X-ray tube potential of 80 KvP and 500 uA, 600 ms exposure at a medium-high magnification using a bin of 2. In a single rotation, 360 projections were collected at one-degree increments and calibration images were collected prior to data acquisition. Images were reconstructed in real-time using a Feldkamp cone beam filtered back projection algorithm (2D-FDP). Trabecular bone microarchitecture was evaluated in a region of interest that began 1 mm from the point at which the growth cartilage begins to transition into the proximal tibia metaphysis and extended 1 mm distally (Supplementary Fig. 1A). Cortical bone cross-sectional geometry was evaluated in the tibia mid-diaphysis between the crest of the tibia and the distal edge of the tibiofibular joint in a 0.5-mm region of interest 0.25 mm proximal and 0.25 mm distal to the mid-slice (Supplementary Fig. 1B). The optimize threshold function was used to delineate mineralized bone from soft tissue. Segmentation thresholds of 214 mg/cm3 and 570 mg/cm3 were used for evaluation of trabecular and cortical bone, respectively. Scans were analyzed using BoneJ software (Doube et al., 2010), a subset of ImageJ (ver. 1.50d) (NIH public domain). The following 3D outcomes for trabecular microarchitecture were measured: total volume (TV, volume of region of interest), bone volume (BV, volume of region segmented as bone), bone volume fraction (BV/TV), connectivity density (Conn.D, degree of trabeculae connectivity normalized to TV), structural model index (SMI, indicator of trabecular structure 0 for parallel plates and 3 for cylindrical rods (Hildebrand and Ruegsegger, 1997), trabecular number (Tb.N, average number of trabeculae per unit length calculated as 1/(Tb.Th + Tb.Sp) (Bruker, n.d.), trabecular thickness (Tb.Th, mean trabecular thickness), trabecular separation (Tb.Sp, distance between trabeculae), degree of anisotropy (DA, 1 = isotropic and >1 = anisotropic). Cortical morphometric outcomes included: tibia length, total cross-sectional area inside the periosteal envelope (Tt.Ar), marrow area (Ma.Ar), cortical bone area (Ct.Ar), cortical area fraction (Ct.Ar/Tt.Ar, percent), average cortical thickness (Ct.Th), and minimum, maximum and polar moments of inertia (Imin, Imax, K, respectively) were determined.
Supplementary Fig. 1.
Trabecular hone microarchitecture was evaluated in a region of interest that began 250 μm from the point at which the growth cartilage begins to transition into the proximal tibia metaphysis and extended 1 mm distally (A). Cortical bone cross-sectional geometry was evaluated in the tibia mid-diaphysis between the crest of the tibia and the distal edge of the tibiofibular joint in a 0.5-mm region of interest 0.25 mm proximal and 0.25 mm distal to the mid-slice (B).
2.7. Tibial biomechanical properties
Torsional loading to failure was used to assess whole-bone and tissue-level biomechanical properties of the tibia. The distal and proximal ends of the left tibia were embedded in a steel cylindrical holder that was then placed in a test fixture of the analyzer (TA-HDi, Stable Micro Systems, Surrey, UK). A cross-bar was used to prevent the proximal end of the holder/tibia from rotating about its long axis while the distal end was rotated about its long axis at a speed of 10 mm/s with a load cell of 5 kg. The machine's control software (Stable Micro Systems, Surrey, UK) measured cable force (F) in grams and applied torque (T). The load displacement curve from this analysis is analogous to the torque-twist curve, which is used along with geometrical properties determined from μCT (i.e. length of specimen and polar moment of inertia) to calculate: maximal torque at fracture (Tmax), torsional stiffness (Ks), the shear modulus of elasticity (G), and the ultimate tensile strength or maximal shear stress (Su), as previously described (Hinton et al., 2015).
2.8. Advanced glycation end-product and collagen content
Advanced glycation end-product content of the femur diaphysis was measured using a fluorimetric assay, and was expressed relative to hydroxy-proline (OH-proline) content measured by a colorimetric OH-proline assay. Femurs were flushed of marrow, acid-hydrolyzed for 3 h at 124 °C with 6 N HCL (Fisher Scientific), dried overnight and reconstituted with 0.001 N HCL. The hydrolysate was used for determination of OH-proline and AGE content. For OH-proline, an OH-proline (Sigma-Aldrich) standard curve was prepared. Chloramine T and Erlich's reagent (Sigma-Aldrich) were used for the colorimetric assay; standards and samples were read at 558 nm using disposable cuvettes. For AGE content, a quinine (Fisher) standard curve was prepared; and, fluorescence was measured at an excitation wavelength of 360 nm and an emission wavelength of 460 nm (Tang et al., 2007).
2.9. Statistical analysis
Two-way ANCOVA was used to test for significant main effects between ovarian status (OVX vs. SHM) and diet (CON vs. SOY), as well as for interactions between ovarian status and diet. In the case of a significant interaction, post-hoc comparisons were made using the Least Significant Difference test to locate the interaction. Because bone size, BMD, and strength tend to increase with body weight, analyses for cortical bone outcomes included final body weight as a covariate (Jepsen et al., 2015). Data are means ± SEM or body-weight-adjusted means ± SEM; and a P-value ≤0.05 was considered statistically significant. All analyses were performed by using SPSS software (SPSS/15.0, SPSS, Chicago, IL, USA).
3. Results
3.1. Animal characteristics
Final body mass, body fat, lean body mass and fasting insulin were significantly greater in OVX vs. SHM (these data will be published as a part of the parent study, but are also included in Table 2 to provide context for the bone outcomes reported here). Uterine mass relative to body mass was significantly reduced in OVX compared with SHM (Table 2). SOY significantly reduced body fat (%) and increased lean mass (%) compared with CON (Table 2). There was a significant main effect of ovarian status on body mass (OVX = 353 ± 10 g; SHM = 308 ± 9 g, p = 0.002, body fat (OVX = 24.8 ± 1.4%; SHM = 15.3 ± 1.3%, p < 0.0001, lean body mass (69.8 ± 1.3%; SHM = 78.3 ± 1.2%, p < 0.0001), uterine mass (OVX = 0.05 ± 0.05 g/kg BW; SHM = 0.36 ± 0.05 g/kg BW, p < 0.0001), and insulin (OVX = 2.43 ± 0.34 ng/mL; SHM = 1.31 ± 0.33 ng/mL, p = 0.025). There was a significant DIET main effect for body fat (SOY = 16.5 ± 1.3%; CON 23.6 ±1.4%, p < 0.0001), and lean body mass (SOY = 77.4 ± 1.2%; CON = 70.8 ±1.3%, p < 0.0001).
Table 2.
Characteristics of LCR rats fed a soybean (SOY) or corn gluten meal (CON) diet for 30 weeks following ovariectomy (OVX) or sham (SHAM) surgery.
| Outcome | SHAM |
OVX |
2-factor ANOVA p-values |
||||
|---|---|---|---|---|---|---|---|
| CON | SOY | CON | SOY | OV STAT | DIET | INT | |
| Food intake (g/d) | 16.2 ± 0.3 | 16.8 ± 0.3 | 16.1 ± 0.4 | 16.7 ± 0.3 | 0.846 | 0.810 | 0.942 |
| Body mass (g) | 325 ± 13 | 291 ± 13 | 359 ± 15 | 348 ± 13 | 0.002 | 0.101 | 0.399 |
| Body fat (%) | 19.6 ± 1.8 | 11.0 ± 1.8 | 27.6 ± 2.1 | 22.1 ± 1.8 | <0.0001 | 0.001 | 0.425 |
| LBM (%) | 74.6 ± 1.8 | 82.1 ± 1.8 | 67.0 ± 2.0 | 72.7 ± 1.8 | <0.0001 | 0.001 | 0.611 |
| Uterine mass (g/kgBW) | 0.29 ± 0.07 | 0.43 ± 0.07 | 0.05 ± 0.08 | 0.06 ± 0.07 | <0.0001 | 0.298 | 0.356 |
| Glucose (mg/dL) | 190 ± 18 | 175 ± 18 | 217 ± 21 | 182 ± 18 | 0.373 | 0.199 | 0.594 |
| Insulin (ng/mL) | 1.46 ± 0.48 | 1.17 ± 0.45 | 2.57 ± 0.51 | 2.28 ± 0.45 | 0.025 | 0.539 | 1.000 |
Data are means ± SE. SHAM, sham surgery. OVX, ovariectomy. CON, control corn gluten meal diet. SOY, soybean meal diet. OV STAT, ovarian status: OVX vs. SHAM. DIET, dietary treatment: SOY vs. CON. INT, ovarian status × diet interaction. There was a significant main effect for OV STAT for body mass [(g): OVX = 353 ± 10; SHAM = 308 ± 9, p = 0.002], body fat [(%): OVX = 24.8 ± 1.4; SHAM = 15.3 ± 1.3, p < 0.0001], lean body mass [(%): 69.8 ± 1.3; SHAM = 78.3 ± 1.2, p < 0.0001], uterine mass [(g/kg BW): OVX = 0.05 ± 0.05; SHAM = 0.36 ± 0.05, p < 0.0001], and insulin [(ng/mL): OVX = 2.43 ± 0.34; SHM = 1.31 ± 0.33, p = 0.025]. There was a significant DIET main effect for body fat [(%): SOY = 16.5 ± 1.3; CON 23.6 ± 1.4, p < 0.0001], and lean body mass [(%): SOY = 77.4 ± 1.2; CON = 70.8 ± 1.3, p < 0.0001].
3.2. Plasma markers of bone formation and resorption
As shown in Fig. 1, CTx was significantly greater in OVX rats compared with SHM (OVX = 7.5 ± 0.4 ng/mL; SHM = 5.4 ± 0.4 ng/mL, p < 0.0001) and TRAP5b trended lower in OVX (OVX = 3.2 ± 0.5 ng/mL vs. SHM = 4.4 ± 0.5 ng/mL, p = 0.09). Consequently, the resorptive index, which reflects osteoclast activity relative to osteoclast number was also increased by OVX (OVX = 2.88 ± 0.28 vs. SHM = 1.56 ± 0.28, p = 0.002). OC trended higher in OVX vs. SHM (OVX = 136.5 ± 11.0 ng/mL; SHM = 110.7 ± 10.4 ng/mL, p = 0.09); OVX had no effect on P1NP (Fig. 1). There was no effect of SOY on markers of plasma bone formation or resorption (Fig. 1).
Fig. 1.
Bone formation (Osteocalcin; P1NP) and resorption (TRAP5b; CTX) markers in SHAM-CON, SHAM-SOY, OVX-CON, and OVX-SOY LCR rats at sacrifice. Data are means ± SEM (n = 8–10 animals per group). Significant OV STAT main effects for OC [(ng/mL): OVX = 136.5 ± 11.0a; SHAM = 110.7 ± 10.4b, p = 0.09], CTx [(ng/mL): OVX = 7.5 ± 0.4a; SHAM = 5.4 ± 0.4b, p < 0.0001], and TRAP5b [(U/L): OVX = 3.2 ± 0.5b vs. SHAM = 4.4 ± 0.5a, p = 0.09].
3.3. Tibial trabecular microarchitecture
OVX adversely affected trabecular microarchitecture (Fig. 2). OVX significantly decreased BV/TV, Tb.Th, Tb.N and Conn.D, and significantly increased Tb.Sp and SMI relative to SHM. There was a significant main effect of OV STAT on BV/TV (OVX = 10.2 ± 2.8%; SHM = 44.0 ± 2.6%, p < 0.0001, Tb.Th (OVX = 0.125 ± 0.012 mm; SHM = 0.200 ± 0.011 mm, p < 0.0001), Tb.Sp (OVX = 0.622 ± 0.028 mm; SHM = 0.297 ± 0.025 mm, p < 0.0001), Tb.N (OVX = 0.812 ± 0.113 1/mm; SHM = 2.076 ± 0.100 1/mm, p < 0.0001), Conn.D (OVX = 13.33 ± 2.23 1/mm3; SHM = 35.93 ± 1.98 1/mm3, p < 0.0001), SMI (OVX = 2.43 ± 0.18; SHM = 1.24 ± 0.16, p < 0.0001). There was a significant interaction between DIET and OVSTAT for BV/TV (p = 0.020), such that SOY increased BV/TV only in SHM rats (Fig. 2).
Fig. 2.
Trabecular microarchitecture of the tibia: bone volume fraction (BV/TV); trabecular thickness (Tb.Th); trabecular separation (Tb.Sp); connectivity density (Conn.D); structural model index (SMI); degree of anisotropy (DA) in SHAM-CON, SHAM-SOY, OVX-CON, and OVX-SOY LCR rats at sacrifice weeks of age. Data are means ± SEM (n = 8–10 animals per group); means with different letter superscripts are significantly different. There was a significant main effect of OV STAT on BV/TV [(%): OVX = 10.2 ± 2.8b; SHM = 44.0 ± 2.6a, p < 0.0001], Tb.Th [(mm): OVX = 0.125 ± 0.012b; SHM = 0.200 ± 0.011a, p < 0.0001], Tb.Sp [(mm): OVX = 0.622 ± 0.028a; SHM = 0.297 ± 0.025b, p < 0.0001], Tb.N [(1/mm): OVX = 0.812 ± 0.113b; SHM = 2.076 ± 0.100a, p < 0.0001], Conn.D [(1/mm3): OVX = 13.33 ± 2.23b; SHM = 35.93 ± 1.98a, p < 0.0001], SMI [OVX = 2.43 ± 0.18; SHM = 1.24 ± 0.16, p < 0.0001].
3.4. Tibial cortical geometry
There was no effect of OVX or SOY on tibia length or cortical geometry (Table 3).
Table 3.
Tibial cortical geometry of LCR rats fed a soybean meal (SOY) or corn gluten meal (CON) diet for 30 weeks following ovariectomy (OVX) or sham (SHAM) surgery.
| Outcome | SHAM |
OVX |
2-factor ANOVA p-values |
||||
|---|---|---|---|---|---|---|---|
| CON | SOY | CON | SOY | OV STAT | DIET | INT | |
| Length (mm) | 39.2 ± 0.6 | 39.5 ± 0.7 | 38.1 ± 0.7 | 38.9 ± 0.6 | 0.264 | 0.412 | 0.656 |
| Tt.Ar (mm2) | 5.33 ± 0.14 | 5.59 ± 0.16 | 5.35 ± 0.17 | 5.29 ± 0.14 | 0.394 | 0.527 | 0.296 |
| Ma.Ar (mm2) | 1.16 ± 0.04 | 1.17 ± 0.05 | 1.16 ± 0.05 | 1.28 ± 0.04 | 0.254 | 0.141 | 0.172 |
| Ct.Ar (mm2) | 4.23 ± 0.04 | 4.23 ± 0.05 | 4.23 ± 0.05 | 4.11 ± 0.04 | 0.254 | 0.141 | 0.172 |
| Ct.Th (mm) | 0.77 ± 0.02 | 0.78 ± 0.02 | 0.79 ± 0.02 | 0.75 ± 0.02 | 0.689 | 0.476 | 0.181 |
| Tt.Ar/L (mm2/mm) | 7.41 ± 0.19 | 7.13 ± 0.22 | 7.16 ± 0.23 | 7.39 ± 0.20 | 0.985 | 0.911 | 0.212 |
| Imax (mm) | 2.54 ± 0.16 | 2.82 ± 0.18 | 2.83 ± 0.19 | 2.57 ± 0.17 | 0.951 | 0.951 | 0.122 |
| Imin (mm) | 1.95 ± 0.10 | 2.08 ± 0.11 | 1.82 ± 0.12 | 1.79 ± 0.10 | 0.085 | 0.618 | 0.442 |
| K (mm4) | 6.52 ± 0.48 | 6.48 ± 0.47 | 6.59 ± 0.48 | 6.39 ± 0.43 | 0.975 | 0.795 | 0.850 |
Data are adjusted means ± SE with final body mass (length, Tt.Ar, Tt.Ar/L, Imax, Imin, K) or with final body mass and Tt.Ar (Ma.Ar and Ct.Ar) as covariate(s) in the model. SHAM, sham surgery. OVX, ovariectomy. CON, control corn gluten meal diet. SOY, soybean meal diet. OV STAT, ovarian status: OVX vs. SHAM. DIET, dietary treatment: SOY vs. CON. INT, ovarian status × diet interaction.
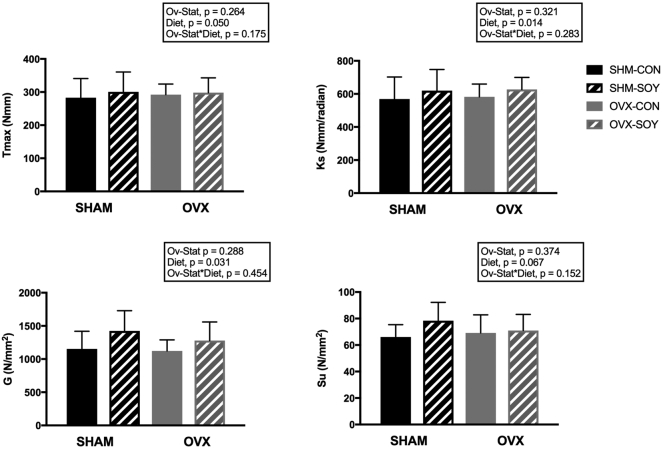
3.5. Tibial biomechanical properties
OVX had no effect on whole-bone or tissue-level biomechanical properties of cortical bone (Fig. 3). SOY significantly increased whole-bone strength (SOY = 310.0 ± 10.8; CON = 275.8 ± 11.9 Nmm, p = 0.050) and stiffness (SOY = 645.3 ± 22.9; CON = 552.1 ± 25.2 Nmm/rad, p = 0.014). Tissue-level strength (i.e., ultimate tensile strength, Su) and stiffness (i.e., shear modulus of elasticity, G) were also increased with SOY: G (SOY = 1363 ± 68; CON = 1124 ± 75 N/mm2, p = 0.031), and Su (SOY = 75.7 ± 3.1; CON = 66.4 ± 3.5 N/mm2, p = 0.067).
Fig. 3.
Biomechanical properties of the tibial diaphysis: maximum torque (Tmax); torsional stiffness (Ks); ultimate tensile strength (Su); and, shear modulus of elasticity (G) in SHAM-CON, SHAM-SOY, OVX-CON, and OVX-SOY LCR rats at sacrifice. Data are means ± SEM adjusted with final body weight as a covariate (n = 8–10 animals per group). There was a significant main effect for DIET on Tmax [(Nmm): SOY = 310.0 ± 10.8a; CON = 275.8 ± 11.9b, p = 0.050], Ks [(Nmm/rad): SOY = 645.3 ± 22.9a; CON = 552.1 ± 25.2b, p = 0.014], G [(N/mm2): SOY = 1363 ± 68a; CON = 1124 ± 75b, p = 0.031], and Su [(N/mm2): SOY = 75.7 ± 3.1a; CON = 66.4 ± 3.5b, p = 0.067].
3.6. AGE content
There were no effects of ovarian status or diet on AGE content relative to collagen in the femur diaphysis (SHM-CON: 2.18 ± 0.29; SHM-SOY: 1.96 ± 0.29; OVX-CON: 1.90 ± 0.27; OVX-SOY: 1.92 ± 0.27 ng/μg OH-proline).
4. Discussion
4.1. Study summary
We examined the effects of ovarian hormone loss and a soy-protein diet on bone outcomes in our newly established rodent model of human menopause, the low running capacity (LCR) rat (Vieira-Potter et al., 2015). Similar to aging, postmenopausal women, LCR rats have reduced physical activity and develop metabolic dysfunction with loss of ovarian hormones (Vieira-Potter et al., 2015), making them more translatable to human menopause than other animal models. Moreover, in the present study, because OVX and the soy dietary intervention were implemented during a period of stable skeletal mass, the observed effects of OVX and SOY were not confounded by skeletal changes associated with development (Kalu, 1984). In this study, we extended our previous findings to show that the loss of ovarian hormones via ovariectomy (OVX) adversely affects cancellous bone in the LCR rat model. Further, we found that the SOY diet improved tibial whole-bone and tissue-level biomechanical properties of cortical bone in both ovariectomized and ovary-intact rats.
4.2. Metabolic effects of ovariectomy and soy protein diet
As confirmation of our previous work (Vieira-Potter et al., 2015; Park et al., 2016), loss of ovarian hormones following OVX in LCR rats resulted in greater adiposity and fasting insulin compared to SHM controls. OVX decreased uterine weight as expected. Other studies have shown high expression of ER-α in reproductive tissues (Gallo et al., 2005). SOY had no effect on uterine mass, as characteristic of SERMs, including soy isoflavones, which bind ER-β (Gallo et al., 2005; Pie et al., 2006). SOY significantly reduced body fat and increased lean mass, regardless of ovarian hormone status. These findings are in agreement with others that have shown soy beans (Park et al., 2013) or soy protein reduces adipose tissue mass in estrogen-replete and -deficient female rats and male rats (Chen et al., 2013; Torre-Villalvazo et al., 2008; Lavigne et al., 2000). The beneficial effects of soy protein on body composition have been attributed to reduced adipocyte size and altered adipose and hepatic expression of genes involved in lipid metabolism (Torre-Villalvazo et al., 2008; Frigolet et al., 2011).
4.3. Effects of ovariectomy and soy protein on markers of bone resorption and formation
Increased bone turnover is a characteristic feature of postmenopausal bone loss (Garnero et al., 1996), particularly during the first five to ten years after cessation of menses (Eastell et al., 2016; Taguchi et al., 1998; Khosla et al., 2011). The increased remodeling rate is of clinical significance, as accelerated bone turnover is positively associated with fracture risk (Riggs and Melton 3rd, 2002). In the present study, OVX resulted in a marked increase in CTx, and OC also tended to be increased. Traditionally, OC has been viewed as a bone formation marker because it is synthesized by osteoblasts. However, because OC is released from the bone matrix during bone resorption, it is also an indicator of bone resorption. In the present study, OC and CTx were significantly and positively correlated (r = 0.431, p = 0.008), suggesting that some of the OC in circulation resulted from bone resorption. TRAP5b, which correlates with osteoclast number (Rissanen et al., 2008), tended to be reduced by OVX. The decrease in TRAP5b is likely due to the significant loss of cancellous bone where the majority of osteoclasts reside (Rissanen et al., 2008). Consequent to the changes in CTx and TRAP5b, the resorptive index was significantly increased by OVX, as previously reported (Rissanen et al., 2008). The bone formation marker P1NP was not affected by OVX, suggesting that bone resorption was increased relative to formation following OVX. Increased bone remodeling markers are correlated with elevated bone remodeling assessed via histomorphometry in ovariectomized rodents (Lin et al., 2015) and with decreased BMD (Dong et al., 2016; Park et al., 2013; Arjmandi et al., 1996; Zhang et al., 2007).
Contrary to our hypothesis, SOY had no impact on plasma markers of bone turnover. The few studies that examined the effects of soy on bone turnover markers in ovariectomized rats reported mixed results. Soybeans increased serum OC and decreased urinary DPD in rats (Park et al., 2013), while in another study of ovariectomized rats, black or yellow soybeans decreased OC and DPD (Byun et al., 2010). Similar to results of the present study, soy protein with or without high levels of isoflavones did not affect serum or urinary markers of bone formation or resorption in ovariectomized rats (Arjmandi et al., 1996; Arjmandi et al., 1998). These discrepant results might be due to the limitations of circulating bone turnover markers, which do not distinguish between skeletal sites or cortical versus cancellous bone (Marcus et al., 2013).
4.4. Effects of ovariectomy on tibial trabecular microarchitecture and cortical biomechanical properties
OVX negatively affected trabecular bone volume and microarchitecture of the proximal tibia, but did not impact cortical geometry or biomechanical properties of the tibial diaphysis. Specifically, in the present study, OVX reduced BV/TV, Tb.Th, Tb.N, and Conn.D, and increased Tb.Sp. OVX also resulted in more rod-like trabeculae (i.e., increased SMI). Others have reported similar deterioration of trabecular microarchitecture in the tibia, femur, or lumbar vertebrae following OVX (Goulet et al., 2011; Parfitt, 1992; Bourrin et al., 2002; Maimoun et al., 2012; Hu et al., 2015; Dai et al., 2008; Ahn et al., 2014; Li et al., 2011; Bagi et al., 1996; Cai et al., 2005; Blum et al., 2003; Jee and Yao, 2001), and BV/TV of the proximal tibia was significantly reduced in LCR rats 4 weeks after OVX relative to LCR rats that underwent sham surgery (Goulet et al., 2011). Tb.N reflects osteoclast activity (Parfitt, 1992; Bourrin et al., 2002); therefore, we conclude that the loss of trabeculae observed in the present study was likely mediated by increased osteoclast activity, which is consistent with the significant increase in CTx in OVX animals. The decrease in cancellous bone strength observed following ovariectomy has been attributed to the loss of trabecular bone mass and deterioration of trabecular microarchitecture (Maimoun et al., 2012; Hu et al., 2015; Dai et al., 2008; Ahn et al., 2014; Li et al., 2011; Bagi et al., 1996), rather than to changes in nanomechanical (material) properties (Hu et al., 2015). The deleterious changes in trabecular microarchitecture are clinically significant, as fragility fractures associated with early phase of menopausal bone loss usually occur in cancellous bone (Eastell et al., 2016).
In contrast to the marked effects of ovarian hormone loss observed in cancellous bone, OVX had no effect on cortical cross-sectional geometry at the tibial mid-diaphysis. Others have also reported that OVX does not affect cortical area of long bone diaphyses (Zhang et al., 2007; Cai et al., 2005; Blum et al., 2003). This might be because cortical bone loss following ovariectomy is slow to develop (Jee and Yao, 2001). The differential pattern of bone loss is consistent with that observed in human menopause, which is characterized by rapid initial phase of cancellous bone loss, followed by slower decline in cortical bone (Eastell et al., 2016). The discrepant expression of ER-α and ER-β in cancellous versus cortical bone (Bord et al., 2001) might explain this different pattern bone loss. Osteocytes, osteoclasts, and osteoblasts in cortical bone express ER-α, while both ER-α and ER-β are expressed in cancellous bone (Khosla et al., 2011; Bord et al., 2001). Because ER-α and ER-β heterodimers have lower affinity for estradiol, cancellous bone is less sensitive to estrogen and its actions, i.e., greater estrogen concentrations are required to elicit receptor binding. Consequently, cancellous bone is more readily affected by loss of ovarian hormone production at menopause. In addition, OVX is associated with decreased ER-α protein expression in bone (Li et al., 2011), further exacerbating the decrease in endogenous estrogen.
4.5. Effects of soy protein on trabecular microarchitecture
In the present study, ovary-intact animals fed the SOY diet had significantly greater trabecular bone volume compared to those fed the CON diet. Although data on the effects of soy protein in non-osteporosis models are sparse, others have also reported that a diet containing soy protein isolate favorably affects cancellous bone. Soy protein isolate increased BV/TV in male mice (Yan et al., 2015) and trabecular bone mineral density in young, ovary-intact female rats (Chen et al., 2008). In the OVX rats, the SOY diet did not preserve trabecular microarchitecture, similar to previous reports that a soy protein diet with or without added isoflavones did not protect against deleterious changes in BV/TV, Tb.Th, Tb.N, Tb.Sp, SMI or Conn.D following ovariectomy in rats (Cai et al., 2005; Devareddy et al., 2006). These results suggest that the skeletal effects of soy protein are dependent on endogenous estrogen status.
4.6. Effects of soy protein on cortical biomechanical properties
In the present study, cortical geometry was unaffected by SOY. Despite this, rats fed SOY had greater whole-bone strength (Tmax), stiffness (Ks), and tissue-level stiffness (G) than rats fed CON, regardless of ovarian hormone status. The soy isoflavone genistein increased cortical whole-bone strength and stiffness in osteoporotic OVX rats (Azboy et al., 2016; Bitto et al., 2008). Cortical bone strength is determined by bone quantity, cross-sectional geometry, and whole-cortex tissue composition (Donnelly, 2011). Therefore, because cortical geometry was not altered by SOY, the effects of SOY on whole-bone strength and stiffness were likely due to changes in these parameters at the tissue-level, which are determined by characteristics of the protein matrix and mineral.
Bone is a composite biomaterial, consisting of a mineralized protein matrix, which is 90% type I collagen and 10% non-collagenous proteins, such as osteocalcin and osteopontin (Bala and Seeman, 2015). Enzymatic intermolecular crosslinking of lysine residues by lysyl oxidase within and between collagen fibrils stabilizes collagen and improves its mechanical properties (Bala and Seeman, 2015). Both the total number of enzymatic crosslinks and the ratio of immature to mature enzymatic crosslinks affect mechanical properties. Non-enzymatic crosslinking occurs via a series of reactions, the initial reaction occurring between an aldehyde group of a sugar (e.g. glucose) and the ε-amino group of hydroxylysine or lysine. Ultimately, non-enzymatic crosslinks, which are known as advanced glycation end-products (AGEs), form within and across collagen fibers. AGEs accumulate in bone with age and disease, negatively affecting biomechanical properties (Karim et al., 2013). AGE accumulation in diabetic or aging bone increases brittleness, crack propagation, and fracture propensity (Osterhoff et al., 2016). We hypothesized that SOY would reduce AGE content of bone and thus improve tissue-level biomechanical properties. SOY had no effect on AGE content and therefore does not explain the effect of SOY on cortical tissue-level biomechanical properties. Likewise, contrary to our hypothesis, OVX had no effect on AGE content in the femoral diaphysis. This might be because OVX rats did not exhibit hyperglycemia compared to ovary intact animals. It is worth noting, however, that cancellous bone has a much higher AGE content than cortical bone (Karim et al., 2013; Michalsky et al., 1993), and we might have detected effects of ovarian status or diet in cancellous bone.
The chemical properties and structure of the bone mineral phase, which is poorly crystallized non-stoichiometric apatite, influence the tissue-level mechanical properties. Mineralization increases strength in compression and stiffness in tension, but dramatically reduces toughness. As crystal size increases, the surface area of interaction with the collagen increases limiting fibril deformation. Assessment of mineral composition and crystal structure require sophisticated techniques (e.g., Fourier transform infrared spectroscopy, backscatter electron microscopy, scanning small- and wide-angle X-ray scattering. Thus, although we know that soy protein increases bone Ca content (Gaffney-Stomberg et al., 2014; Figard et al., 2006) and whole bone BMC/BMD, the effects of soy protein on mineral composition or crystal structure have not been investigated to date. Cortical bone has a greater proportion of ER-α (Eastell et al., 2016) and soy isoflavones bind ER-β with greater affinity (Kuiper et al., 1998). Therefore, we hypothesize that the benefits of soy protein on tissue-level strength and stiffness are due to changes in the collagen matrix and its mineralization, independent of ER-mediated effects.
4.7. Mechanisms by which soy protein exerts beneficial effects
We hypothesized that SOY would increase plasma markers of bone formation, decrease plasma markers of bone resorption, and improve trabecular microarchitecture, cortical geometry and biomechanical properties of the tibia, particularly in the OVX rats due to their greater potential to respond to the soy phytoestrogens because of the loss of ovarian hormone production. However, soy protein had equivalent beneficial effects on cortical whole-bone and tissue-level biomechanical properties in both ovariectomized and ovary-intact LCR rats, and favorably impacted trabecular bone volume only in ovary-intact rats. Since improvements in cortical biomechanical properties occurred with SOY in both OVX and ovary-intact rats, soy isoflavones do not appear to be acting as “estrogen replacements” (i.e., acting via ER-α). Instead, ER-α-independent mechanisms are likely responsible for the improvements we observed with SOY.
We hypothesize that the beneficial effects of soy protein on cancellous bone observed in the present study might be mediated via soy isoflavone binding to ER-β, as this isoform of the ER is expressed in cancellous, but not cortical bone (Bord et al., 2001). There is both in vivo and in vitro evidence that soy isoflavones alter expression of genes encoding proteins involved in osteoclast and osteoblast differentiation, proliferation, and activity. In vivo, isoflavones increase osteoblast differentiation and proliferation by activating Smad(s) (Zhang et al., 2012), decrease RANKL levels (Yu et al., 2015), and increase osteoblast OPG expression (Yu et al., 2015). Soy isoflavones also increase expression of β-catenin and Wnts 3a and 7b in primary osteoblasts (Yu et al., 2015). Interestingly, osteoblasts have a membrane ER that binds daidzein and is structurally similar to ER-β, but not ER-α (de Wilde et al., 2006), which is expected as isoflavones preferentially bind ER-β. Physiologic concentrations of daidzein activate ERK1/2 and induce phosphorylation of transcription factors for early genes controlling osteoblast differentiation and proliferation (de Wilde et al., 2006). In vitro studies show that both soy protein matrix and soy isoflavones are required for optimal ER activity (Rando et al., 2009).
Other possible mechanisms include the relative high concentration of arginine in soy protein. Arginine is a GH secretagogue (Ghigo et al., 2001) and therefore increases GH and IGF-I in vivo (Isidori et al., 1981). Both GH and IGF-I are osteogenic with some of the effects of GH mediated via IGF-I (Yakar et al., 2002; Locatelli and Bianchi, 2014; Ohlsson et al., 1998). In addition, the relatively high levels of arginine and lysine compared to animal-based proteins, might increase calcium absorption (Bihuniak et al., 2014). Soy protein increases expression of intestinal calcium transporters, specifically TRPV6, in rats (Gaffney-Stomberg et al., 2014). Thus, increased intestinal calcium absorption following soy consumption might explain the beneficial effects of soy independent of ovarian hormone status. In recent years, there has been growing interest in the effects of β-conglycinin, the primary storage protein in soy beans, on bone health (Akao et al., 2015). Deamidated soybean β-conglycinin suppresses parathyroid hormone (PTH) secretion and reduces bone resorption in OVX rats (Akao et al., 2015). Lastly, oxidative stress, leading to increased inflammation and bone resorption, might play a causal role in age- and menopause-associated bone loss (Zhang et al., 2011). Soybean flour or soy isoflavones increase antioxidant defenses (Razzeto et al., 2015) and reduce oxidative stress (Sankar et al., 2015). Therefore, it is possible that the beneficial skeletal effects of soy protein are due to the anti-oxidant properties of isoflavones and peptides (Ma et al., 2016; Agyei, 2015).
4.8. Study strengths and limitations
A strength of the present study is the applicability of the OVX LCR rat model to human menopause, as OVX provides insight into some of the menopause-associated changes such as increased adiposity and insulin resistance (IR) (Rogers et al., 2009; Walton et al., 1993). In addition, the long-term feeding of SOY and CON (30 weeks) allowed us to examine the effects of our dietary intervention well beyond the initial phase of rapid bone loss and increased turnover that follows OVX (Kalu et al., 1989). The use of torsional loading to test cortical biomechanical properties of the tibia was also a strength of the present study because it allowed us to assess both whole-bone and tissue-level biomechanical properties, both of which are important determinants of overall bone strength and fracture risk. However, the present study is not without limitations. While OVX is often used to simulate human menopause, the abrupt decline in ovarian hormones is not representative of the more gradual decline that occurs with human menopause. This sudden decline in ovarian hormones following OVX may make clinical translation of the results more challenging, as extended studies in animals have demonstrated increased bone mineral content, bone area, and body weight, which is not compatible with the human condition (Chen et al., 1995; Bagi et al., 1993; Thompson et al., 1995). In addition, since a large number of osteoporosis-related fractures occur at the ends of long bones, vertebrae or hip, future studies should also test the biomechanical properties of trabecular bone (e.g., vertebrae via compression loading) following OVX and SOY.
4.9. Conclusions
In summary, the present study highlights that a soy-protein-based diet improves tibia cortical biomechanical properties in female low-fit rats, regardless of ovarian hormone status. These results support our hypothesis that a soy-protein-based diet might improve metabolic and bone outcomes in both menopausal and premenopausal women. Moreover, our results support the benefits of dietary soy protein, rather than the isolated, individual components.
The following are the supplementary data related to this article.
Conflicts of interest
The authors declare that no conflict of interest exists in this study. This study was joint-funded by the National Center for Complementary and Integrated Health (NCCIH), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI) (Grant number: P50AT006273). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health.
Transparency document
Transparency document.
Acknowledgments
V.V·P, T.M.Z, S.L.B, and L.G.K designed the research. M.W.R, L.C·O, E.L.S. and T.M.Z. conducted research. P.S.H., R.K.D., and L.C.O. analyzed data; P.S.H. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
The Transparency document associated this article can be found, in online version.
References
- Agyei D. Bioactive proteins and peptides from soybeans. Recent Pat. Food Nutr. Agric. 2015;7:100–107. doi: 10.2174/2212798407666150629134141. [DOI] [PubMed] [Google Scholar]
- Ahn H., Seo D.H., Kim H.S., Choue R. Calorie restriction aggravated cortical and trabecular bone architecture in ovariectomy-induced estrogen-deficient rats. Nutr. Res. 2014;34:707–713. doi: 10.1016/j.nutres.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Akao M., Abe R., Sato N., Hasegawa-Tanigome A., Kumagai H., Kumagai H. Prevention of osteoporosis by oral administration of phytate-removed and deamidated soybean beta-conglycinin. Int. J. Mol. Sci. 2015;16:2117–2129. doi: 10.3390/ijms16012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan S., Tuzcu A., Bahceci M., Ozmen S., Gokalp D. Insulin resistance in type 2 diabetes mellitus may be related to bone mineral density. J. Clin. Densitom. 2012;15:186–190. doi: 10.1016/j.jocd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Arjmandi B.H., Alekel L., Hollis B.W., Amin D., Stacewicz-Sapuntzakis M., Guo P. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J. Nutr. 1996;126:161–167. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- Arjmandi B.H., Getlinger M.J., Goyal N.V., Alekel L., Hasler C.M., Juma S. Role of soy protein with normal or reduced isoflavone content in reversing bone loss induced by ovarian hormone deficiency in rats. Am. J. Clin. Nutr. 1998;68:1358S–1363S. doi: 10.1093/ajcn/68.6.1358S. [DOI] [PubMed] [Google Scholar]
- Azboy I., Ozkaya M., Demir T., Demirtas A., Kagan Arslan A., Ozkul E. Biomechanical properties of osteoporotic rat femurs after different hormonal treatments: genistein, estradiol, and estradiol/progesterone. SICOT J. 2016;2:24. doi: 10.1051/sicotj/2016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi C.M., Mecham M., Weiss J., Miller S.C. Comparative morphometric changes in rat cortical bone following ovariectomy and/or immobilization. Bone. 1993;14:877–883. doi: 10.1016/8756-3282(93)90318-5. [DOI] [PubMed] [Google Scholar]
- Bagi C.M., Deleon E., Ammann P., Rizzoli R., Miller S.C. Histo-anatomy of the proximal femur in rats: impact of ovariectomy on bone mass, structure, and stiffness. Anat. Rec. 1996;245:633–644. doi: 10.1002/(SICI)1097-0185(199608)245:4<633::AID-AR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bala Y., Seeman E. Bone's material constituents and their contribution to bone strength in health, disease, and treatment. Calcif. Tissue Int. 2015;97:308–326. doi: 10.1007/s00223-015-9971-y. [DOI] [PubMed] [Google Scholar]
- Bawa S. The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J. Osteoporos. 2010;2010 doi: 10.4061/2010/891058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berning B., Kuijk C.V., Kuiper J.W., Bennink H.J., Kicovic P.M., Fauser B.C. Effects of two doses of tibolone on trabecular and cortical bone loss in early postmenopausal women: a two-year randomized, placebo-controlled study. Bone. 1996;19:395–399. doi: 10.1016/s8756-3282(96)00219-0. [DOI] [PubMed] [Google Scholar]
- Bihuniak J.D., Sullivan R.R., Simpson C.A., Caseria D.M., Huedo-Medina T.B., O'Brien K.O. Supplementing a low-protein diet with dibasic amino acids increases urinary calcium excretion in young women. J. Nutr. 2014;144:282–288. doi: 10.3945/jn.113.185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A., Burnett B.P., Polito F., Marini H., Levy R.M., Armbruster M.A. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and oestradiol. Br. J. Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S.C., Heaton S.N., Bowman B.M., Hegsted M., Miller S.C. Dietary soy protein maintains some indices of bone mineral density and bone formation in aged ovariectomized rats. J. Nutr. 2003;133:1244–1249. doi: 10.1093/jn/133.5.1244. [DOI] [PubMed] [Google Scholar]
- Board, U.S . Bite: The Data is Delicious. 2015. Consumer attitudes about nutrition, health and soyfoods.http://www.soyconnection.com/bite-2015/ [cited 2017 January 10, 2017]; 22:[Available from: [Google Scholar]
- Bord S., Horner A., Beavan S., Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J. Clin. Endocrinol. Metab. 2001;86:2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- Bourrin S., Ammann P., Bonjour J.P., Rizzoli R. Recovery of proximal tibia bone mineral density and strength, but not cancellous bone architecture, after long-term bisphosphonate or selective estrogen receptor modulator therapy in aged rats. Bone. 2002;30:195–200. doi: 10.1016/s8756-3282(01)00661-5. [DOI] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Bruker, Morphometric Parameters Measured by Skyscan™ CT-analyser Software: Kontich, Belgium.
- Byun J.S., Han Y.S., Lee S.S. The effects of yellow soybean, black soybean, and sword bean on lipid levels and oxidative stress in ovariectomized rats. Int. J. Vitam. Nutr. Res. 2010;80:97–106. doi: 10.1024/0300-9831/a000010. [DOI] [PubMed] [Google Scholar]
- Cai D.J., Zhao Y., Glasier J., Cullen D., Barnes S., Turner C.H. Comparative effect of soy protein, soy isoflavones, and 17beta-estradiol on bone metabolism in adult ovariectomized rats. J. Bone Miner. Res. 2005;20:828–839. doi: 10.1359/JBMR.041236. [DOI] [PubMed] [Google Scholar]
- Chen H.K., Ke H.Z., Jee W.S., Ma Y.F., Pirie C.M., Simmons H.A. Droloxifene prevents ovariectomy-induced bone loss in tibiae and femora of aged female rats: a dual-energy X-ray absorptiometric and histomorphometric study. J. Bone Miner. Res. 1995;10:1256–1262. doi: 10.1002/jbmr.5650100816. [DOI] [PubMed] [Google Scholar]
- Chen J.R., Singhal R., Lazarenko O.P., Liu X., Hogue W.R., Badger T.M. Short term effects on bone quality associated with consumption of soy protein isolate and other dietary protein sources in rapidly growing female rats. Exp. Biol. Med. (Maywood) 2008;233:1348–1358. doi: 10.3181/0802-RM-63. [DOI] [PubMed] [Google Scholar]
- Chen J.R., Zhang J., Lazarenko O.P., Cao J.J., Blackburn M.L., Badger T.M. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013;27:3514–3523. doi: 10.1096/fj.12-226464. [DOI] [PubMed] [Google Scholar]
- Cohen A., Dempster D.W., Recker R.R., Lappe J.M., Zhou H., Zwahlen A. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J. Clin. Endocrinol. Metab. 2013;98:2562–2572. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross T.L., Zidon T.M., Welly R.J., Park Y.M., Britton S.L., Koch L.G. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Sci. Rep. 2017;7:9261. doi: 10.1038/s41598-017-08965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R., Ma Y., Sheng Z., Jin Y., Zhang Y., Fang L. Effects of genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J. Bone Miner. Metab. 2008;26:342–349. doi: 10.1007/s00774-007-0830-4. [DOI] [PubMed] [Google Scholar]
- Dallanezi G., Freire B.F., Nahas E.A., Nahas-Neto J., Corrente J.E., Mazeto G.M. Physical activity level of post-menopausal women with low bone mineral density. Rev. Bras. Ginecol. Obstet. 2016;38:225–230. doi: 10.1055/s-0036-1583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A., Heberden C., Chaumaz G., Bordat C., Lieberherr M. Signaling networks from Gbeta1 subunit to transcription factors and actin remodeling via a membrane-located ERbeta-related protein in the rapid action of daidzein in osteoblasts. J. Cell. Physiol. 2006;209:786–801. doi: 10.1002/jcp.20767. [DOI] [PubMed] [Google Scholar]
- Devareddy L., Khalil D.A., Smith B.J., Lucas E.A., Soung do Y., Marlow D.D. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone. 2006;38:686–693. doi: 10.1016/j.bone.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Dong X.L., Li C.M., Cao S.S., Zhou L.P., Wong M.S. A high-saturated-fat, high-sucrose diet aggravates bone loss in ovariectomized female rats. J. Nutr. 2016;146:1172–1179. doi: 10.3945/jn.115.225474. [DOI] [PubMed] [Google Scholar]
- Donnelly E. Methods for assessing bone quality: a review. Clin. Orthop. Relat. Res. 2011;469:2128–2138. doi: 10.1007/s11999-010-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doube M., Klosowski M.M., Arganda-Carreras I., Cordelieres F.P., Dougherty R.P., Jackson J.S. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval K., Prud'Homme D., Rabasa-Lhoret R., Strychar I., Brochu M., Lavoie J.M. Effects of the menopausal transition on dietary intake and appetite: a MONET group study. Eur. J. Clin. Nutr. 2014;68:271–276. doi: 10.1038/ejcn.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R., O'Neill T.W., Hofbauer L.C., Langdahl B., Reid I.R., Gold D.T. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Zhang Y., Hannan M.T., Anderson J.J. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J. Bone Miner. Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- Figard H., Mougin F., Gaume V., Berthelot A. Combined intervention of dietary soybean proteins and swim training: effects on bone metabolism in ovariectomized rats. J. Bone Miner. Metab. 2006;24:206–212. doi: 10.1007/s00774-005-0673-9. [DOI] [PubMed] [Google Scholar]
- Foundation, I.O Osteoporosis epidemiology. 2015. https://www.iofbonehealth.org/epidemiology cited 2017; Available from:
- Frigolet M.E., Torres N., Uribe-Figueroa L., Rangel C., Jimenez-Sanchez G., Tovar A.R. White adipose tissue genome wide-expression profiling and adipocyte metabolic functions after soy protein consumption in rats. J. Nutr. Biochem. 2011;22:118–129. doi: 10.1016/j.jnutbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Frost H.M. On the estrogen-bone relationship and postmenopausal bone loss: a new model. J. Bone Miner. Res. 1999;14:1473–1477. doi: 10.1359/jbmr.1999.14.9.1473. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Piemontese M., Liu Y., Thostenson J.D., Xiong J., O'Brien C.A. RANKL (receptor activator of NFkappaB ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J. Biol. Chem. 2016;291:24838–24850. doi: 10.1074/jbc.M116.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney-Stomberg E., Cao J.J., Lin G.G., Wulff C.R., Murphy N.E., Young A.J. Dietary protein level and source differentially affect bone metabolism, strength, and intestinal calcium transporter expression during ad libitum and food-restricted conditions in male rats. J. Nutr. 2014;144:821–829. doi: 10.3945/jn.113.188532. [DOI] [PubMed] [Google Scholar]
- Gallo D., Zannoni G.F., Apollonio P., Martinelli E., Ferlini C., Passetti G. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: effects on bone, uterus, and lipid profile. Menopause. 2005;12:589–600. doi: 10.1097/01.GME.0000156348.61767.D5. [DOI] [PubMed] [Google Scholar]
- Garnero P., Sornay-Rendu E., Chapuy M.C., Delmas P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- Ghigo E., Aimaretti G., Arvat E., Camanni F. Growth hormone-releasing hormone combined with arginine or growth hormone secretagogues for the diagnosis of growth hormone deficiency in adults. Endocrine. 2001;15:29–38. doi: 10.1385/ENDO:15:1:029. [DOI] [PubMed] [Google Scholar]
- Goulet G.C., Halonen N.R., Koch L.G., Britton S.L., Zernicke R.F., Kozloff K.M. Osteoblast response to ovariectomy is enhanced in intrinsically high aerobic-capacity rats. Calcif. Tissue Int. 2011;88:325–335. doi: 10.1007/s00223-010-9457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale G.A., Fitzgerald G., Huang M.H., Sternfeld B., Gold E., Seeman T. Dietary soy isoflavones and bone mineral density: results from the study of women's health across the nation. Am. J. Epidemiol. 2002;155:746–754. doi: 10.1093/aje/155.8.746. [DOI] [PubMed] [Google Scholar]
- Hildebrand T., Ruegsegger P. Quantification of bone microarchitecture with the structure model index. Comput. Methods Biomech. Biomed. Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- Hinton P.S., Shankar K., Eaton L.M., Rector R.S. Obesity-related changes in bone structural and material properties in hyperphagic OLETF rats and protection by voluntary wheel running. Metabolism. 2015;64:905–916. doi: 10.1016/j.metabol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Ho S.C., Woo J., Lam S., Chen Y., Sham A., Lau J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos. Int. 2003;14:835–842. doi: 10.1007/s00198-003-1453-9. [DOI] [PubMed] [Google Scholar]
- Hu S., Li J., Liu L., Dai R., Sheng Z., Wu X. Micro/nanostructures and mechanical properties of trabecular bone in ovariectomized rats. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G.R., Weinsier R.L., Gower B.A., Wetzstein C. Age-related decrease in resting energy expenditure in sedentary white women: effects of regional differences in lean and fat mass. Am. J. Clin. Nutr. 2001;73:333–337. doi: 10.1093/ajcn/73.2.333. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Iki M., Morita A., Kajita E., Kagamimori S., Kagawa Y. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese population-based osteoporosis (JPOS) study. J. Nutr. 2006;136:1323–1328. doi: 10.1093/jn/136.5.1323. [DOI] [PubMed] [Google Scholar]
- INFOcenter, S.M Soybean Meal: Composition. http://www.soymeal.org/composition.html Available from:
- Isidori A., Lo Monaco A., Cappa M. A study of growth hormone release in man after oral administration of amino acids. Curr. Med. Res. Opin. 1981;7:475–481. doi: 10.1185/03007998109114287. [DOI] [PubMed] [Google Scholar]
- Jee W.S., Yao W. Animal models of bone diseases. Introduction. J. Musculoskelet. Neuronal Interact. 2001;1:183–184. [PubMed] [Google Scholar]
- Jepsen K.J., Silva M.J., Vashishth D., Guo X.E., van der Meulen M.C. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. 2015;30:951–966. doi: 10.1002/jbmr.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O., Kanis J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005;16(Suppl. 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- Kalu D.N. Evaluation of the pathogenesis of skeletal changes in ovariectomized rats. Endocrinology. 1984;115:507–512. doi: 10.1210/endo-115-2-507. [DOI] [PubMed] [Google Scholar]
- Kalu D.N., Liu C.C., Hardin R.R., Hollis B.W. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology. 1989;124:7–16. doi: 10.1210/endo-124-1-7. [DOI] [PubMed] [Google Scholar]
- Kameda T., Mano H., Yuasa T., Mori Y., Miyazawa K., Shiokawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J.A. Blackwell Science; Oxford; Cambridge, Mass.: 1994. Osteoporosis. (x, 254 p.) [Google Scholar]
- Karim L., Tang S.Y., Sroga G.E., Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos. Int. 2013;24:2441–2447. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Melton L.J., 3rd, Riggs B.L. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J. Bone Miner. Res. 2011;26:441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M.A., Nahin R.L., Messina M.J., Rader J.I., Thompson L.U., Badger T.M. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J. Nutr. 2010;140:1192S–1204S. doi: 10.3945/jn.110.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L.G., Britton S.L. Evolution, atmospheric oxygen, and complex disease. Physiol. Genomics. 2007;30:205–208. doi: 10.1152/physiolgenomics.00043.2007. [DOI] [PubMed] [Google Scholar]
- Koh W.P., Wu A.H., Wang R., Ang L.W., Heng D., Yuan J.M. Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese Health Study. Am. J. Epidemiol. 2009;170:901–909. doi: 10.1093/aje/kwp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kwasniewska M., Pikala M., Kaczmarczyk-Chalas K., Piwonnska A., Tykarski A., Kozakiewicz K. Smoking status, the menopausal transition, and metabolic syndrome in women. Menopause. 2012;19:194–201. doi: 10.1097/gme.0b013e3182273035. [DOI] [PubMed] [Google Scholar]
- Lavigne C., Marette A., Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- Li X., Song Q.S., Wang J.Y., Leng H.J., Chen Z.Q., Liu Z.J. Simvastatin induces estrogen receptor-alpha expression in bone, restores bone loss, and decreases ERalpha expression and uterine wet weight in ovariectomized rats. J. Bone Miner. Metab. 2011;29:396–403. doi: 10.1007/s00774-010-0231-y. [DOI] [PubMed] [Google Scholar]
- Liang H.D., Yu F., Lv P., Zhao Z.N., Tong Z.H. Role of Sost in Wnt signal pathway in osteoporosis rats and regulating effect of soybean isoflavones on Wnt signal pathway. Mol. Biol. Rep. 2014;41:4447–4454. doi: 10.1007/s11033-014-3315-2. [DOI] [PubMed] [Google Scholar]
- Lin S., Huang J., Fu Z., Liang Y., Wu H., Xu L. The effects of atorvastatin on the prevention of osteoporosis and dyslipidemia in the high-fat-fed ovariectomized rats. Calcif. Tissue Int. 2015;96:541–551. doi: 10.1007/s00223-015-9975-7. [DOI] [PubMed] [Google Scholar]
- Locatelli V., Bianchi V.E. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Liu R., Zhao Z., Zhang Z., Cao Y., Ma Y. A novel peptide from soybean protein isolate significantly enhances resistance of the organism under oxidative stress. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimoun L., Brennan-Speranza T.C., Rizzoli R., Ammann P. Effects of ovariectomy on the changes in microarchitecture and material level properties in response to hind leg disuse in female rats. Bone. 2012;51:586–591. doi: 10.1016/j.bone.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Mandal C.C. High cholesterol deteriorates bone health: new insights into molecular mechanisms. Front. Endocrinol. (Lausanne) 2015;6:165. doi: 10.3389/fendo.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Robert, Dempster David W., Luckey Marjorie, Cauley Jane A., editors. Osteoporosis. 4 ed. Elsevier; 2013. [Google Scholar]
- Messina M. Insights gained from 20 years of soy research. J. Nutr. 2010;140:2289S–2295S. doi: 10.3945/jn.110.124107. [DOI] [PubMed] [Google Scholar]
- Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8 doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalsky M., Norris-Suarez K., Bettica P., Pecile A., Moro L. Rat cortical and trabecular bone collagen glycosylation are differently influenced by ovariectomy. Biochem. Biophys. Res. Commun. 1993;192:1281–1288. doi: 10.1006/bbrc.1993.1555. [DOI] [PubMed] [Google Scholar]
- Ohlsson C., Bengtsson B.A., Isaksson O.G., Andreassen T.T., Slootweg M.C. Growth hormone and bone. Endocr. Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- Osterhoff G., Morgan E.F., Shefelbine S.J., Karim L., Mcnamara L.M., Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47(Suppl. 2):S11–S20. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A.M. Implications of architecture for the pathogenesis and prevention of vertebral fracture. Bone. 1992;13(Suppl. 2):S41–S47. doi: 10.1016/8756-3282(92)90196-4. [DOI] [PubMed] [Google Scholar]
- Park Y., Moon H.J., Paik D.J., Kim D.Y. Effect of dietary legumes on bone-specific gene expression in ovariectomized rats. Nutr. Res. Pract. 2013;7:185–191. doi: 10.4162/nrp.2013.7.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.M., Rector R.S., Thyfault J.P., Zidon T.M., Padilla J., Welly R.J. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2016;310(3):E190–E199. doi: 10.1152/ajpendo.00434.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton K., Krieder J., Joiner D., Freeman M.R., Goldstein S.A., Solomon K.R. Hypercholesterolemia promotes an osteoporotic phenotype. Am. J. Pathol. 2012;181:928–936. doi: 10.1016/j.ajpath.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pie J.E., Park J.H., Park Y.H., Ryu Y.M., Kim K.N., Suh S.W. Effect of genistein on the expression of bone metabolism genes in ovariectomized mice using a cDNA microarray. J. Nutr. Biochem. 2006;17:157–164. doi: 10.1016/j.jnutbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ramos-Junior E.S., Leite G.A., Carmo-Silva C.C., Taira T.M., Neves K.B., Colon D.F. Adipokine chemerin bridges metabolic dyslipidemia and alveolar bone loss in mice. J. Bone Miner. Res. 2017;32(5):974–984. doi: 10.1002/jbmr.3072. [DOI] [PubMed] [Google Scholar]
- Rando G., Ramachandran B., Rebecchi M., Ciana P., Maggi A. Differential effect of pure isoflavones and soymilk on estrogen receptor activity in mice. Toxicol. Appl. Pharmacol. 2009;237:288–297. doi: 10.1016/j.taap.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Razzeto G.S., Lopez V.R., Gimenez M.S., Escudero N.L. Soybean flour induces a greater increase of the antioxidant defenses in rats fed with a normocaloric diet compared with a hypercaloric diet. J. Sci. Food Agric. 2015;95:607–613. doi: 10.1002/jsfa.6795. [DOI] [PubMed] [Google Scholar]
- Reeve J., Walton J., Russell L.J., Lunt M., Wolman R., Abraham R. Determinants of the first decade of bone loss after menopause at spine, hip and radius. QJM. 1999;92:261–273. doi: 10.1093/qjmed/92.5.261. [DOI] [PubMed] [Google Scholar]
- Reinwald S., Weaver C.M. Soy components vs. whole soy: are we betting our bones on a long shot? J. Nutr. 2010;140:2312S–2317S. doi: 10.3945/jn.110.124008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinwald S., Akabas S.R., Weaver C.M. Whole versus the piecemeal approach to evaluating soy. J. Nutr. 2010;140:2335S–2343S. doi: 10.3945/jn.110.124925. [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Melton L.J., 3rd Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J. Bone Miner. Res. 2002;17:11–14. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- Rissanen J.P., Suominen M.I., Peng Z., Halleen J.M. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif. Tissue Int. 2008;82:108–115. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- Rogers N.H., Perfield J.W., 2nd, Strissel K.J., Obin M.S., Greenberg A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P., Zachariah B., Vickneshwaran V., Jacob S.E., Sridhar M.G. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: the molecular mechanisms. Exp. Gerontol. 2015;63:67–75. doi: 10.1016/j.exger.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr. Osteoporos. Rep. 2004;2:90–96. doi: 10.1007/s11914-004-0016-0. [DOI] [PubMed] [Google Scholar]
- Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1218–1225. doi: 10.1093/gerona/glt071. [DOI] [PubMed] [Google Scholar]
- Spritzer P.M., Oppermann K. Weight gain and abdominal obesity at menopause. Climacteric. 2013;16:292. doi: 10.3109/13697137.2012.753874. [DOI] [PubMed] [Google Scholar]
- Stefanska A., Bergmann K., Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv. Clin. Chem. 2015;72:1–75. doi: 10.1016/bs.acc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Taguchi Y., Gorai I., Zhang M.G., Chaki O., Nakayama M., Minaguchi H. Differences in bone resorption after menopause in Japanese women with normal or low bone mineral density: quantitation of urinary cross-linked N-telopeptides. Calcif. Tissue Int. 1998;62:395–399. doi: 10.1007/s002239900451. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kuroda T., Saito M., Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos. Int. 2013;24:69–76. doi: 10.1007/s00198-012-2209-1. [DOI] [PubMed] [Google Scholar]
- Tang S.Y., Zeenath U., Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Terzi R., Dindar S., Terzi H., Demirtas O. Relationships among the metabolic syndrome, bone mineral density, bone turnover markers, and hyperglycemia. Metab. Syndr. Relat. Disord. 2015;13:78–83. doi: 10.1089/met.2014.0074. [DOI] [PubMed] [Google Scholar]
- Thompson D.D., Simmons H.A., Pirie C.M., Ke H.Z. FDA guidelines and animal models for osteoporosis. Bone. 1995;17:125S–133S. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- Torre-Villalvazo I., Tovar A.R., Ramos-Barragan V.E., Cerbon-Cervantes M.A., Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J. Nutr. 2008;138:462–468. doi: 10.1093/jn/138.3.462. [DOI] [PubMed] [Google Scholar]
- van Staa T.P., Dennison E.M., Leufkens H.G., Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- Vieira-Potter V.J., Padilla J., Park Y.M., Welly R.J., Scroggins R.J., Britton S.L. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am. J. Phys. Regul. Integr. Comp. Phys. 2015;308:R530–R542. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C., Godsland I.F., Proudler A.J., Wynn V., Stevenson J.C. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur. J. Clin. Investig. 1993;23:466–473. doi: 10.1111/j.1365-2362.1993.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Yakar S., Rosen C.J., Beamer W.G., Ackert-Bicknell C.L., Wu Y., Liu J.L. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Yamaguchi T., Nawata K., Tanaka K., Takaoka S., Sugimoto T. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine. 2015;48:279–286. doi: 10.1007/s12020-014-0292-0. [DOI] [PubMed] [Google Scholar]
- Yan L., Graef G.L., Nielsen F.H., Johnson L.K., Cao J. Soy protein is beneficial but high-fat diet and voluntary running are detrimental to bone structure in mice. Nutr. Res. 2015;35:523–531. doi: 10.1016/j.nutres.2015.04.012. [DOI] [PubMed] [Google Scholar]
- You L., Sheng Z.Y., Tang C.L., Chen L., Pan L., Chen J.Y. High cholesterol diet increases osteoporosis risk via inhibiting bone formation in rats. Acta Pharmacol. Sin. 2011;32:1498–1504. doi: 10.1038/aps.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Liu Z., Tong Z., Zhao Z., Liang H. Soybean isoflavone treatment induces osteoblast differentiation and proliferation by regulating analysis of Wnt/beta-catenin pathway. Gene. 2015;573:273–277. doi: 10.1016/j.gene.2015.07.054. [DOI] [PubMed] [Google Scholar]
- Zhang X., Shu X.O., Li H., Yang G., Li Q., Gao Y.T. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch. Intern. Med. 2005;165:1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lai W.P., Leung P.C., Wu C.F., Wong M.S. Short- to mid-term effects of ovariectomy on bone turnover, bone mass and bone strength in rats. Biol. Pharm. Bull. 2007;30:898–903. doi: 10.1248/bpb.30.898. [DOI] [PubMed] [Google Scholar]
- Zhang Y.B., Zhong Z.M., Hou G., Jiang H., Chen J.T. Involvement of oxidative stress in age-related bone loss. J. Surg. Res. 2011;169:e37–e42. doi: 10.1016/j.jss.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Zhang J., Lazarenko O.P., Wu X., Tong Y., Blackburn M.L., Gomez-Acevedo H. Differential effects of short term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Hu M., Chu T., Lin L., Wang J., Li X. Sclerostin antibody prevented progressive bone loss in combined ovariectomized and concurrent functional disuse. Bone. 2016;87:161–168. doi: 10.1016/j.bone.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.J., Liu Y.J., Liu P.Y., Hamilton J., Recker R.R., Deng H.W. Relationship of obesity with osteoporosis. J. Clin. Endocrinol. Metab. 2007;92:1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.