Abstract
Restoration of insulin-independence and normoglycemia has been the overarching goal in diabetes research and therapy. While whole organ and islet transplantation have become gold standard procedures in achieving glucose control in diabetic patients, the profound lack of suitable donor tissues severely hampers the broad application of these therapies. Here, we describe current efforts aimed at generating a sustainable source of functional human stem cell-derived insulin-producing islet cells for cell transplantation and present state-of-the-art efforts to protect such cells via immune modulation and encapsulation strategies.
Graphical Abstract
Sneddon et al. describe current efforts aimed at generating a sustainable source of functional human stem cell-derived insulin-producing islet cells for cell transplantation for the treatment of diabetes and present state-of-the-art efforts to protect such cells via immune modulation and encapsulation strategies.
Over the last century, improvements in the synthesis and delivery of recombinant insulin have substantially decreased the morbidity and mortality associated with diabetes mellitus. Despite these advances, more than 400 million people across the world who are affected by diabetes mellitus continue to suffer from devastating secondary complications, including diabetic nephropathy, retinopathy, and neuropathy. Intensified metabolic control has reduced or prevented the development and progression of secondary complications in two landmark trials in patients with type I (Diabetes Control and Complications Trial Research Group et al., 1993) and type 2 (Holman et al., 2008; UK Prospective Diabetes Study (UKPDS) Group, 1998a; UK Prospective Diabetes Study (UKPDS) Group., 1998b) diabetes mellitus. Unfortunately, the tighter control associated with intensified regimens has been limited by the inherent risks of hypoglycemia. Excellent metabolic control without the need for exogenous insulin can be achieved with beta cell replacement, either through solid organ pancreas transplantation or pancreatic islet transplantation. Both strategies for beta cell replacement stabilize or minimize progression of the secondary complications associated with diabetes mellitus, providing stable long-term allograft function as demonstrated by insulin independence and normalization of glycated hemoglobin (HbA1C) levels.
Despite the increasing success of both strategies for beta cell replacement, broader application of islet and pancreas transplantation is severely limited by the number of available donor pancreases and the need for life-long immunosuppression; as a result, only a small fraction of people with diabetes mellitus can currently benefit from these therapies. Creating an unlimited source of insulin-producing cells from stem cells will permit widespread application of beta cell replacement to achieve insulin independence. As this source of beta cells moves closer to clinical translation, it is important to review the current state of the art in beta cell replacement with a focus on successful encapsulation and immune modulation strategies that can be applied to stem cell-derived cells.
Clinically viable transplantation strategies for treating diabetes
Whole pancreas organ transplantation
Advances in surgical technique and refinement of immunosuppression have dramatically improved the success of pancreas transplantation performed for diabetes mellitus. The traditional indication for solid organ pancreas transplant has been in recipients with type I diabetes (T1D) and end-stage renal disease, and the procedure is most commonly performed simultaneously with a kidney transplant (SPK). One-year allograft success, as defined by insulin independence, is approximately 90% at most centers performing this operation. Long-term results continue to improve with evolution of better immunosuppressive regimens, with five- and ten-year pancreas graft survival rates at 73% and 56%, respectively (A. C. Gruessner and R. W. G. Gruessner, 2016). Marked improvements in successful transplantation, as defined by long-term insulin independence, have increased the indications for pancreas transplantation to include pre-uremic T1D recipients with life threatening diabetes secondary to hypoglycemic unawareness. Type 2 diabetic (T2D) recipients now represent 9% of all SPK recipients, and their early allograft success is comparable to the T1D SPK recipients (Kandaswamy et al., 2018).
Pancreas transplantation requires a strong cardiovascular system to tolerate both the initial procedure as well as the potential complications associated with transplantation of a fragile organ containing digestive enzymes. Pancreas transplants involve the intraperitoneal placement of the pancreas in a heterotopic location. The reconstructed donor pancreas most often receives its arterial blood supply from recipient iliac vessels, portal (SMV) or systemic (iliac vein) venous drainage, and enteric exocrine drainage of the donor pancreas through an anastomosis between the donor duodenal segment and the recipient ileum. The technical success of pancreatic transplants is around 90–95%. Early loss of the pancreas allograft is related to thrombosis of the pancreas allograft or leaks of pancreatic enzymes resulting in infection, necessitating removal of the allograft. A technically successful allograft results in almost immediate insulin independence. Furthermore, pre-transplant insulin requirements and body mass index (BMI) do not impact the ability to achieve insulin independence, and the vast majority of pancreas transplant recipients with technically successful allografts achieve insulin independence (Kandaswamy et al., 2018). Stable function of the pancreas allograft prevents recurrence of diabetic nephropathy in the simultaneously transplanted kidney and can prevent progression of retinopathy and neuropathy (Fioretto et al., 1998). For that reason, solid organ pancreas transplantation has become the gold standard for beta cell replacement for patients healthy enough to sustain this taxing procedure. Of note, the widespread application of the technique is limited by the rigors of the procedure as well as the scarce availability of suitable donor pancreases.
Pancreatic islet transplantation
Transplantation of isolated islets provides a gentler alternative to whole organ transplantation. A brief history of the events leading to this success is important for understanding future strategies for beta cell replacement using stem cell-derived beta cells. Although pancreatic islet transplantation had been conducted in animal models of diabetes since the early 1980’s, successful clinical trials in T1D recipients were elusive until the Edmonton protocol was published in 2000 (Shapiro et al., 2000). To achieve insulin independence in seven consecutive patients, islets were isolated from deceased donor pancreases and infused into the portal vein of the liver. Two to three infusions of islets from different donors were required to obtain insulin independence, which was initially achieved in all seven patients. The success was attributed to a regimen that minimized exposure to immunosuppressive agents toxic to beta cells, such as steroids or lymphodepleting induction therapy. Instead, the immunosuppressive regimens consisted of low-dose calcineurin inhibitors (CNI) to minimize nephron- and beta-cell toxicity. Despite their initial insulin independence, all seven patients lost islet function within two years.
This initial experience pointed out some of the problems associated with intraportal infusion of islets, as well as the necessity for more potent immunosuppression. The requirement for 2–3 donor infusions to create an early state of insulin independence was likely related to poor engraftment of the donor islets. The instant blood-mediated inflammatory reaction (IBMIR) and ischemia contributed to islet loss shortly after infusion into the portal system (Eich et al., 2007). The marginal islet mass that survives and engrafts following intraportal infusion is consistent with the finding that insulin independence has been more common in recipients with low BMIs and lower insulin requirements (Barton et al., 2012; Shapiro et al., 2017).
The problems with marginal mass following islet transplantation are further exacerbated by the alloimmune and recurrent autoimmune responses against the islet transplant. In fact, significant improvements in long-term insulin independence have been achieved by using more potent immunosuppression. Recent international registry data have reported 3-year insulin independence rates following intraportal infusion of islets at nearly 50% (Shapiro et al., 2017). Improvements in long-term insulin independence required the same potent lymphodepleting induction regimens that were found to be successful in solid organ pancreas transplants (Barton et al., 2012). Novel immunosuppressive regimens involving maintenance based on blocking immune costimulation (CTLA4-Ig, Belatacept) or leukocyte adhesion (anti-LFA1, Raptiva) have further increased the 3-year insulin independence rates to 70% (Posselt et al., 2010). This type of immunosuppression is attractive in that these agents specifically target immune cells without toxic effects on beta cells or kidneys. Importantly, insulin independence was achieved in several cases with a single infusion of donor islets. A cost comparison between solid organ pancreas transplantation and pancreatic islet transplantation has demonstrated comparable costs to achieve insulin independence (Moassesfar et al., 2016). Unfortunately, the very limited number of pancreatic islets available for transplantation severely restricts the widespread use of this technology.
Stem cell-derived therapies for the treatment of diabetes
Recent advances in the differentiation of human stem cells towards pancreatic islet cells now suggest clear and tangible alternatives to the more conventional treatment options for T1D and T2D described above. Remarkable progress has been made with regard to the generation of functional beta cells from human stem cell populations over the last decade. The underlying strategy is to closely recapitulate the path that pluripotent stem cells take during embryogenesis, from the formation of definitive endoderm, to pancreatic endoderm, to endocrine progenitors, and finally pancreatic islet cells (Figure 1) (D’Amour et al., 2005; 2006; Guo et al., 2013; Kroon et al., 2008; Rezania et al., 2011; 2012). More recently, efforts have been placed on generating single hormone positive beta cells capable of glucose-stimulated insulin secretion (GSIS) (Millman et al., 2016; Pagliuca et al., 2014; Rezania et al., 2014; Russ et al., 2015; S. Zhu et al., 2016). Efforts in several laboratories are focused on generating fully functional beta and islet cells that could soon replace human islets as a source for insulin-producing cells. Indeed, clinical trials using stem cell-derived pancreas progenitors capable of developing into functional beta and islet cells are currently underway. Here, we review the current state of generating human embryonic stem cell (hESC)-derived pancreatic islet cell types.
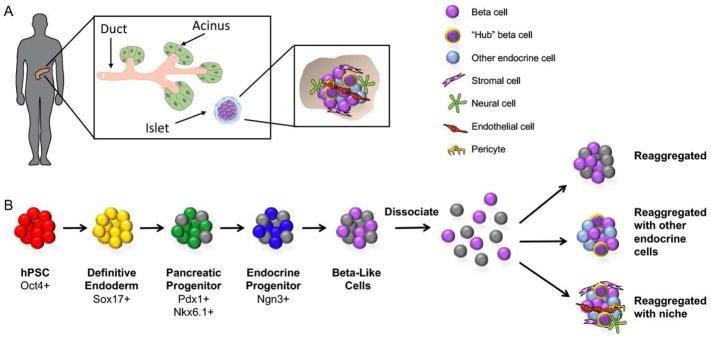
Figure 1.
Strategies for generating human pluripotent stem cell (hPSC)-derived pancreatic islets for transplantation. A) The pancreas is comprised of both an exocrine and an endocrine compartment. The latter is composed of small spherical mini-organs called the islets of Langerhans (inset). Each islet contains insulin-producing beta cells, along with other hormone-producing endocrine cells (alpha, delta, epsilon, and PP cells). A subset of beta cells are so-called “hub” cells, which orchestrate the coordinated release of insulin across the islet in response to glucose. The endocrine cells do not exist in isolation, but rather in the context of various types of niche cells, including stromal, neural, endothelial, and perivascular cells. B) The generation of beta-like cells from hPSCs involves the promotion of pluripotent cells through a series of intermediate progenitor stages. At the completion of directed differentiation, the clusters of beta-like cells can be dissociated and reaggregated into islet-like clusters of defined size and composition. The reaggregation with other endocrine cell types (themselves potentially derived from hPSCs) may lead to improved glucose homeostatic function, potentially through the specialization of some hPSC-derived beta-like cells into hub cells. Lastly, endocrine cells can be reaggregated along with niche cells to more closely recapitulate interactions with the local microenvironment that are critical for beta cell function.
Optimizing the stem cell niche to generate islet-like structures
Despite the rapid progress in generating insulin-producing cells from hESC populations, the formation of fully functional cells perfectly replicating all aspects of endogenous beta cells has remained elusive. Considering that beta cells in vivo do not exist in isolation, but rather in the context of intricate, three-dimensional structures of the islets of Langerhans, generation of a ‘full complement’ of islet cells should be considered beneficial (Figure 1). Indeed, the maintenance of beta cell function has been reported to be highly dependent on the complex islet cytoarchitecture, with isolated beta cells behaving differently from those within intact islets (Cabrera et al., 2006; Halban et al., 1982; Ravier et al., 2005; Wojtusciszyn et al., 2008). In single, isolated beta cells, for instance, the GSIS is compromised compared to intact islets, due to both elevated basal insulin secretion and reduced maximal insulin secretion in response to glucose (Benninger et al., 2011; Halban et al., 1982). Along with other mechanisms for coordinating cell-cell communication, gap junctions such as Connexin-36 play an important role in maintaining beta cell coupling and islet synchronization of insulin oscillations (Benninger et al., 2011; Ravier et al., 2005). As we consider the methods for generating the most optimally-functioning hESC-derived unit for diabetic rescue, it will also be important to consider methods for recapitulating endogenous islet structure, cell-cell contacts, and communication with the islet microenvironment.
In rodent models, pancreatic islets typically consist of a beta cell core, surrounded by a mantle of alpha, delta, and pancreatic polypeptide (PP) cells, which secrete other hormones important for proper glucose homeostasis (Bosco et al., 2010; Schaeffer et al., 2011). This architectural arrangement favors homologous cell-cell contacts and is important for normal islet function; rodent models of diabetes exhibit abnormal islet architecture, with alpha, delta, and PP cells intermingling with beta cells within the islet core (Wojtusciszyn et al., 2008). In contrast to rodent islets, human islets favor heterologous cell-cell contacts, with more evenly distributed endocrine types found across an islet and less compartmentalization into peripheral and central compartments (Bosco et al., 2010). The relative proportions of the endocrine subtypes can also differ between murine and human islets, with the former dominated by beta cells (~80%) and the latter more evenly comprised of alpha (40%) and beta (50%) cells (Pan and Wright, 2011), although different proportions have also been noted (Bonner-Weir et al., 2015; Kilimnik et al., 2012). Disparities in composition and structure between mouse and human islets have been linked to functional differences. In murine islets, for instance, beta-beta intercellular contacts are believed to coordinate homogeneous and synchronous release of insulin, whereas human islets, which lack as many homologous beta cell contacts, display asynchronous insulin release following glucose stimulation (Wojtusciszyn et al., 2008). Exciting work is emerging from single cell sorting and sequencing that supports the notion of distinct beta cell subtypes in human islets (Dorrell et al., 2016).
Another determinant of beta cell function is communication with cells in the islet niche, which is comprised of multiple non-endocrine cell types, including endothelial, perivascular, neuronal, and mesenchymal cells (Hayden et al., 2008; Lammert et al., 2003a; Reinert et al., 2014). In particular, pancreatic islets are highly vascularized; each beta cell is believed to make one or more contacts with an endothelial cell, and together they form specialized ultrastructural features at their interface (Bonner-Weir and Orci, 1982; Henderson and Moss, 1985). During pancreatic development, endothelial cells are initially recruited into the burgeoning islet by vascular endothelial growth factor A (VEGF-A), which is secreted by endocrine cells (Lammert et al., 2003b). It is the endothelial cells that then synthesize and secrete the components of the islet basement membrane, which is believed to serve as a critical modulator of beta cell growth, survival, and function (Lammert et al., 2003a; Nikolova et al., 2006; Oberg-Welsh, 2001; Stendahl et al., 2009; Wang and Rosenberg, 1999). Work by the Otonkoski group demonstrated the presence of two basement membranes around the endothelium and beta cells in human islets (Otonkoski et al., 2008; Virtanen et al., 2008). Given the specialized relationship between beta cells and supporting cells in their natural environment, it stands to reason that recapitulation of some elements of the endogenous beta cell niche may lead to improved survival and function of hESC-derived beta cells in vitro.
The vascular islet niche consists not only of endothelial cells, but also of mural cells called pericytes. Pericytes wrap around endothelial cell tubes to provide structural support and generate functional, mature blood vessels (Gutiérrez et al., 2009) and modulate endothelial cell proliferation, survival, and function (Jain, 2003). The role of pericytes in normal islet function is not completely understood, but one study using genetic ablation suggested their loss in the adult murine islet results in impaired insulin expression, total insulin content, and glucose-clearing (Sasson et al., 2016).
Given the importance of islet cytoarchitecture for endocrine function, culture systems can be designed to recapitulate elements of the intricate three-dimensional structure of the developing islet. Various methods have been used to generate engineered pancreatic islets, or so-called “pseudo-islets,” in vitro (Kojima, 2014). Multiple studies have reported improved function of reaggregated pseudo-islets, either in vitro or in vivo after transplantation. There is also evidence that beta cells reaggregated with endothelial progenitor cells demonstrate improved GSIS compared to pseudo-islets comprised only of beta cells (Penko et al., 2011).
In summary, more work is needed to determine the cellular composition and architectural arrangement of human islets that is optimal for function. Challenges will likely include decisions regarding how to source the desired human niche cells, particularly given that there is a lack of truly specific markers for some of these cell types, and that the supply may be limiting. One option is the generation of the niche cells themselves from hESCs or iPSCs. Another challenge is that current techniques for creating pseudo-islets rely on the property of self-organization among endocrine and non-endocrine cells; 3D printing of islet tissues may someday be possible and could instead allow enforcement of desired architecture. Future work aimed at incorporating flow in microfluidic devices, as has been done in various other systems with “organ-on-a-chip” approaches, may allow even more sophisticated optimization of function while reducing negative effects of waste product accumulation. Re-engineering additional components of a pancreatic islet with the optimal composition of endocrine and non-endocrine niche cells, including vascular niche cells and extracellular matrix (ECM) components, may lead to an optimally functional beta cell population for transplantation into patients.
Applying lessons learned from whole organ pancreas and islet transplantation to stem cell derived beta cells
There is growing appreciation for the fact that the transplant site itself influences the functionality and survival of islets. Unlike the insulin independence that always occurs following a technically successful pancreas transplant, the marginal mass of engrafted islets associated with islets infused through the portal vein often requires multiple infusions from different deceased donors (Hering et al., 2016). Furthermore, it is more challenging to create states of insulin independence in patients with higher BMIs and higher insulin requirements (Balamurugan et al., 2014). Portal infusion limits the amount of tissue that can be infused secondary to the development of portal hypertension. In addition, isolated islets engraft poorly secondary to IBMIR and ischemia injuries (Eich et al., 2007). Of equal significance, islets infused into the portal vein are not retrievable, and this remains a concern for the clinical translation of any trials using hESC-derived beta cells until it has been unequivocally proven that such cells are equivalent to fully mature human islet cells and lack any possibility of neoplastic transformation. However, while the risk of teratoma formation from undifferentiated stem cells cannot be completely ignored at present, further optimizing the generation of fully differentiated islet and beta cells from stem cells should eliminate this concern.
Considering the information gained from solid organ and islet transplantation, it is clear that a more optimal site for islet tissue is necessary. Multiple investigators are optimizing subcutaneous/intramuscular sites for islet transplantation to enhance engraftment and permit removal of the graft in the event of tissue dysfunction or transformation (Bertuzzi et al., 2018; Gamble et al., 2018). Similarly, exploring optimal sites that permit retrieval of hESC-derived beta cells will be important for clinical translation. Successful pancreas transplantation of T2D patients suggests that given an unlimited source of beta cells, beta cell replacement may provide a benefit for the vast majority of people suffering from T2D (Kandaswamy et al., 2018). Of note, transplantation of any allogeneic cell source requires mitigation of the recipient immune system.
Immune modulation in beta cell replacement therapy
The recent success in the use of beta cell replacement to treat T1D, combined with advances in the generation of hESC/iPS-derived beta cells, provides a roadmap for effective treatment of the disease. However, both the alloimmune and autoimmune systems remain major barriers to the widespread adoption of cell replacement therapies in T1D and, potentially, T2D. Current immunosuppressive drugs are effective but require life-long treatment, and in many cases lead to side effects and toxicities that make the adoption of this treatment strategy limited to only the most severe disease cases. Here, we focus on new efforts to understand and control immunity, with the goal of creating a combinatorial therapy that takes advantage of drugs, bioengineering, and gene editing to bring beta cell replacement to the larger diabetes community.
Control of alloimmune responses against beta cells
In T1D patients, transplanted allogeneic beta cells face alloimmune-mediated rejection that can eliminate the graft within days if not suppressed. T cells are the principal drivers of alloimmunity; their activation results from T cell receptor (TCR) recognition of antigenic peptides presented by the major histocompatibility complexes (MHCs). The highly polymorphic proteins of the MHC, referred to as human leukocyte antigens (HLAs) in humans, are the major targets of alloimmune attacks. Minor histocompatibility antigens, such as male antigens in female recipients or mitochondrial antigens, can also trigger rejection responses, although at a slower pace. MHC class I is expressed on all cell types, including beta cells, and beta cells overexpress MHC I in inflamed conditions (Hamilton-Williams et al., 2003). MHC class II is expressed on antigen presenting cells (APCs) such as B cells, dendritic cells, and macrophages and can be induced on non-APCs, including beta cells (Foulis and Farquharson, 1986) (Pujol-Borrell et al., 1987). After transplantation, MHCs released by donor cells are taken up by recipient APCs, broken down into antigenic fragments, and presented by recipient MHC on the surface of recipient APC. This normal process of T cell activation is referred to as the “indirect” pathway in the context of transplantation. This is in contrast to the “direct” pathway that is unique to transplantation immunology. In the direct pathway, TCRs on the recipient’s T cells bind to allogeneic MHC expressed on donor cells, leading to T cell activation and direct attack of the graft cells. Both direct and indirect T cells contribute to graft rejection and often act cooperatively to carry out the attacks (Brennan et al., 2009; Jiang et al., 2004; Makhlouf et al., 2003).
The current approach to control transplant rejection is to globally suppress T cell activation by inhibiting TCR signaling with calcineurin inhibitors, prevent T cell proliferation with mycophenolate, and dampen inflammation with steroids. In addition to this triple-drug regimen, transplant patients often receive ‘induction’ therapies, defined as short-term treatments in the peri-transplant period. Clinical islet transplant experiences have shown that stronger induction therapies that deplete T-cells, combined with blockade of inflammatory cytokines, TNFα and IL-1, lead to better long-term islet graft function (Barton et al., 2012).
Although immunosuppression protects grafts from rejection, it globally compromises the patients’ immune system, leaving them vulnerable to infections and malignancies. An ideal strategy would be to train the immune system to accept the transplanted graft as self and avoid chronic global immunosuppression. Immune tolerance to transplanted tissue has been achieved in many preclinical animal models, and potential tolerance-promoting therapies are currently under development for humans.
Most commonly used immunosuppressive drugs inhibit both effector cells and tolerance-promoting immune regulatory mechanisms (Table 1). However, different drugs have varying selectivity in inhibiting anti-graft effector responses versus tolerance-promoting regulatory T cells (Tregs) (Furukawa et al., 2016). For example, the use of calcineurin inhibitors decreases the percentages of Tregs, whereas rapamycin use is associated with an increase in percentages of Tregs. CTLA-4Ig (belatacept) inhibits effector T cell activation and clonal expansion but can also impair Treg homeostasis and function at high doses (Bour-Jordan et al., 2004; Salomon and Bluestone, 2001). Thus, by selecting immunosuppressive drugs and their dosing, it may be possible to preferentially inhibit effector cells and to leverage the immune system’s self-control mechanisms to reduce the burden of immunosuppression. Achieving immune tolerance requires the immune system to be aware of the antigen and to actively avoid activation toward that antigen. Tolerance to self-antigens is achieved through a combination of deletion, cell-intrinsic checkpoints, and suppression by regulatory mechanisms such as Tregs, IL-10, and transforming growth factor β (TGFβ) signaling. By blocking immune activation, currently available immunosuppressive drugs also inhibit these tolerance-inducing mechanisms. Thus, attaining tolerance is very inefficient using currently available immunosuppressive drugs.
Table 1.
Immune modulators for alloimmune and autoimmune responses
| Drug | Effect | Use in transplant | Use in T1D patients |
|---|---|---|---|
| ATG1 | delete T cells, NK cells | induction IS1, treatment of rejection | * does not prevent disease progression (NCT00515099) (Gitelman et al., 2013) |
| anti-CD25 | delete activated T cells | induction IS | * does not prevent disease progression (NCT00198146) |
| Calcineurin inhibitors | block TCR signaling | maintenance IS | * remission while on IS (Bougnères et al., 1990; Feutren et al., 1986; Stiller et al., 1984) |
| Mycophenolate | block T and B cell proliferation | maintenance IS | * does not prevent disease progression (NCT00100178) |
| Steroid | anti-inflammatory | induction IS, may be used as maintenance, treatment of rejection | toxic to beta cells |
| Rapamycin | block mTOR signaling, cell cycle and metabolism | maintenance IS | * enhances Treg function (NCT02803892, NCT00525889) |
| CTLA-4Ig | block T cell costimulation | maintenance IS | * transient halt of islet function decline |
| Anti-CD3 | partial agonist of TCR, depleting effector T cells | * induction IS, treatment of rejection | * transient halt of islet function decline (NCT00378508, NCT00627146, NCT00678886) |
| LFA3Ig | selectively target memory and effector T cells over Tregs | * no additional benefit when added to conventional IS | * transient halt of islet function decline (NCT00965458) |
| Anti-CD20 | deplete B cells | * failed to treat antibody-mediated rejection (Becker et al., 2004; Vo et al., 2008) | * transient halt of islet function decline (NCT00279305) |
| BMT1 | immunological reset | * can achieve tolerance using donor BMT in some patients | * transient halt of islet function decline (NCT00821899) (Esmatjes et al., 2010; Gu et al., 2014) |
| Treg cell therapy | Promote Treg-mediated suppression | * safe in early phase clinical trials | * safe in early phase clinical trial |
This table summarizes immune modulatory therapies used in controlling transplant rejection. Many of these drugs have also been explored to induce diabetes remission in patients with recent onset of type 1 diabetes.
abbreviations: ATG, anti-thymocyte globulin; IS, immunosuppressants; BMT, bone marrow transplant.
data from clinical trials with ClinicalTrials.gov Identifier number provided in parenthesis.
In the past two decades, there have been concerted research efforts to develop strategies for inducing transplantation tolerance. One such strategy is to construct donor-recipient hematopoietic chimeras. The presence of donor immune cells will educate the recipient immune cells to accept cells of donor origin as self, thus preventing graft rejection (Kawai and Sachs, 2013). One challenge with this approach is the instability of the mixed chimeric state, resulting in either the host or the donor immune system taking over. When donor cells dominate, the risk of graft-versus-host disease becomes high; when recipient cells prevail, tolerance towards the donor is often lost. An alternative strategy is to promote Tregs using adoptive cell therapy (Tang and Bluestone, 2013). A challenge with adoptive Treg therapy is the high frequency of donor-reactive effector T cells that can overwhelm Treg-mediated suppression. Reduction of donor-reactive T cells is needed for Tregs to control the rejection responses (Lee et al., 2014; Wells et al., 1999). Emerging preclinical evidence suggests that Treg cell therapy may stabilize hematopoietic chimeras; thus, the two therapies may synergize to achieve more reliable and durable tolerance.
Control of recurrent autoimmune attack of transplanted beta cells
In T1D patients, islet autoantigen-specific T cells can also attack and destroy transplanted beta cells and thus contribute to the rejection of transplanted beta cells (Burke et al., 2015; Stegall et al., 1996). MHC class I expression on islet cells is necessary for islet destruction by recurrent autoimmunity (Young et al., 2004), implicating a role of autoreactive CD8 T cells. Additionally, islet antigens expressed by the transplanted beta cells can be presented by recipient APCs and activate autoreactive CD4 T cells to destroy the graft (Kupfer et al., 2005). These autoreactive T cells can directly kill beta cells, make cytokines toxic to beta cells, and activate innate immune cells such as NK cells and macrophages to kill beta cells.
Strategies for the control of recurrent autoimmune rejection of transplanted beta cells can be adopted from those developed to control autoimmune responses in T1D patients. Extensive research efforts have been devoted to the restoration of immune self-tolerance in T1D. Interestingly, many of the commonly used immunosuppressive drugs for preventing transplant rejection are not effective at inducing diabetes remission (Table 1). Interestingly, the majority of the commonly used immunosuppressive drugs for preventing alloimmune-mediated transplant rejection can induce short-term diabetes remission with no long-term efficacy (Table 1). The ineffectiveness of conventional drugs at suppressing autoimmune responses is also evident from recurrent autoimmune attacks against islets in immunosuppressed recipients of pancreas and kidney transplants (Burke et al., 2015). T cell and B cell targeting therapies, such as anti-CD3, Abatacept (a CTLA-4Ig molecule similar to Belatacept described above), and anti-CD20, lead to a transient halt in the decline of beta cell function in some newly-diagnosed T1D patients (Hagopian et al., 2013; Herold et al., 2005; 2002; Keymeulen et al., 2005; 2010; Orban et al., 2011; Pescovitz et al., 2009; Sherry et al., 2011) (Pescovitz et al., 2009). Anti-thymocyte globulin, a drug commonly used to induce T cell depletion in organ transplantation, does not protect against beta cell loss when given at a high dose (Gitelman et al., 2013) (Gitelman et al., 2016) and had short-term benefit when given at a low dose in combination with G-CSF (Haller et al., 2015). In contrast, another T cell targeting agent, LFA-3Ig (Alefacept), stabilizes beta cell mass in patients with new-onset disease. A commonality among anti-CD3, Abatacept, and Alefacept is that they are relatively selective for effector T cells, while sparing and even boosting regulatory T cells (Bluestone et al., 2008; Long et al., 2017; Rigby et al., 2015; Tintle et al., 2011). Thus, their therapeutic efficacy may be due to their ability to simultaneously thwart effector T cells while promoting regulation. These hints of efficacy in controlling autoimmune attack of islets provide guidance in designing more effective combination therapies to induce long-term tolerance.
In addition to the broad immunomodulatory strategies described above, there are also ongoing efforts in developing islet antigen-specific therapies. Preclinical data show that it is possible to target effector cells specific for dominant islet antigens using tolerogenic peptide vaccine or nanoparticles. Efficacy of such strategies in patients remains to be demonstrated in clinical trials (Ludvigsson et al., 2012; Wherrett et al., 2011). Although many of these studies have shown some effects in small patient subsets, and new antigen therapy approaches are underway in several programs, it will be essential to determine the precise antigen, timing, and method of administration to maximize and effectively test efficacy. A challenge for antigen-specific immunotherapy is the number of islet antigens that are targeted by the immune system; disarming T cells specific for one or a subset of the islet antigens alone will not likely be sufficient to change the disease course. In addition, the repertoires of islet antigen specificities are likely to be distinct in different patients at different disease stages, making it difficult to know which specificities to target. Approaches that induce dominant antigen-specific regulation are likely to be more effective and easier to apply to diverse patient populations.
Treg cell therapy offers many attractive therapeutic attributes with regards to dominant antigen-specific regulation of effector T cell responses. For example, Tregs control unwanted immune responses via ‘linked suppression’ and ‘infectious tolerance’. In ‘linked suppression’, Tregs activated by one antigen can suppress responses to other antigens presented in the same tissue microenvironment. In ‘infectious tolerance’, Tregs create a tolerogenic tissue microenvironment so that other T cells activated in the vicinity adopt a regulatory instead of an effector cell fate. This allows tolerance to propagate over time beyond the persistence of the initiating Tregs. These properties ensure that Tregs of limited specificity can exert specific, localized, dominant, and durable control of other immune cells. Indeed, single infusion of islet antigen-specific Tregs can induce life-long remission of diabetes in autoimmune non-obese diabetes mice (Tang et al., 2004). Early phase trials of Treg cell therapy in type 1 diabetes patients are currently underway, demonstrating the feasibility and safety of this approach in patients (Bluestone et al., 2015; Marek-Trzonkowska et al., 2012).
Genetic engineering of stem cell derived beta cells to prevent immune detection/rejection
Stem cell derived beta and islet cells are now very close to their endogenous counterparts residing within islets with regard to functional properties, indicating that the generation of mature beta cells from stem cells is within grasp. However, issues concerning the prevention of immune destruction of such cells by the recipient immune system remains an unsolved problem. Most notably, the immune system of T1D patients that already targets native beta cells is likely primed to target transplanted stem cell-derived beta cells. Moreover, allogeneic responses to transplanted hESC-derived beta cells pose a significant obstacle for the implementation of this new technology for both T1D and T2D patients.
Current therapeutic strategies described above designed to promote immune tolerance to transplant antigens and islet self-antigens can be applied to beta cell replacement therapy in diabetic patients. In addition, stem cell technology offers further opportunities to engineer beta cells to avoid immune attacks. The advent of novel gene editing tools, most notably the CRISPR/Cas9 system (Lin et al., 2014), provides unique opportunities to disassemble MHC components in hESCs with the goal of preventing presentation of alloantigens by the stem cell-derived cells. Such a strategy would also prevent presentation of auto-antigens by the stem cell-derived beta cells and recognition by memory autoimmune cells in T1D patients. As described above, T cell recognition of polymorphic MHC molecules is the principal driver of alloimmunity. In T1D patients, recurrent autoimmune attacks of transplanted beta cells also depend on MHC class I expression on islet cells (Young et al., 2004). Activated T cells can directly kill beta cells, make cytokines toxic to beta cells, and activate innate immune cells such as NK cells and macrophages to kill beta cells. Thus, eliminating MHC class I and class II expression can potentially reduce alloimmune and autoimmune-mediated rejection of beta cells.
There are 6 expressed MHC class I genes in the human genome: HLA-A, B, C, E, G, and F. HLA-A, B and C are highly expressed by most nucleated cells and are most polymorphic (thus alloimmunogenic), whereas HLA-E, F, G are less polymorphic and have a more restricted expression pattern (Shiina et al., 2009). Beta 2 microglobulin (β2M) facilitates the proper folding and cell surface expression of class I MHC proteins, and deletion of β2M is thought to be an efficient way to abolish all HLA I expression. However, experimental data in mice show that the dependency on β2M for MHC I expression is not absolute and β2M deficient cells are promptly rejected by CD8 T cells recognizing MHC I (Bix and Raulet, 1992; Glas et al., 1992). Therefore, individual deletion of HLA I genes may be needed to reduce alloimmunogenicity of beta cells. Individual targeting of HLA I genes also offers the opportunity to preserve less polymorphic HLA I alleles, thus ensuring immune surveillance of the graft cells against infections and malignancy transformation. MHC class II expression is more restricted to professional APCs in steady state. Inflammation can lead to broader expression of HLA II. IFNγ and TNF together can induce HLA II expression on beta cells (Pujol-Borrell et al., 1987). Thus, abolishing HLA class II expression may be needed to silence alloimmune responses against beta cells. An efficient way to prevent expression of HLA II genes is to eliminate the master transactivator of HLA class II genes, CIITA (C. H. Chang et al., 1996).
Ablating HLA expression on beta cells not only decreases their alloimmunogenicity, but also reduces autoimmune recognition of the cells. However, islet autoantigens from the grafted cells can still be presented by host APCs, leading to activation of autoreactive T cells, and can injure beta cells by producing cytotoxic cytokines and activating innate immune cells. Moreover, beta cells react to inflammation by producing inflammatory cytokines and chemokines themselves, leading to the amplification of the immune response against the graft (Eizirik et al., 2009). Much of the beta cell toxicity induced by cytokines and pro-inflammatory response of the beta cells is mediated by the transcription factor NFκB and the signal transducer STAT1 (Eizirik et al., 2009). Controlling NFκB activation by over-expression of A20 and suppressing STAT1 activation by over expression of SOCS1 can be effective in quenching the cascade of inflammatory responses in beta cells (Chong et al., 2001; 2002; Grey et al., 1999; Solomon et al., 2011). Furthermore, employing other immune modulatory approaches, such as enhancing immune checkpoints via forced expression of PDL1, may also be applied to protect beta cells against autoreactive T cells.
Additional considerations should be given to NK cells that may be activated against HLA-ablated stem cell-derived beta cells. NK cell cytotoxicity is regulated by an array of activating and inhibitory receptors (Lanier, 2008). NK-mediated killing requires the engagement of an activating receptor and an absence of signaling by the inhibitory receptors. Islet beta cells are known to express ligands for NK activating receptors such as Rae1 in mice and MICA/B in humans (Hankey et al., 2002; Ogasawara et al., 2004). Class I HLA are major ligands for NK inhibitory receptors. Thus, complete ablation of class I HLA expression on stem-cell-derived beta cells may render them vulnerable to NK-mediated killing. HLA-E and G are less polymorphic than HLA-A, B and C and can be expressed in islet endocrine cells (Cirulli et al., 2006) and both molecules have strong inhibitory effect on NK cells. By keeping HLA-E and G alleles intact while deleting HLA-A, B, and C, it might be possible to achieve reduction of T cell responses while maintaining inhibition of NK cells.
Lastly, it is important to consider graft immune surveillance when designing immunoengineering strategies of stem cell therapies. Reducing immune recognition of stem-cell derived grafts will also impair the efficacy of the immune system to control neoplastic growth and infection of the graft cells. A potential solution for reducing immunogenicity while preserving some immune surveillance would be to retain a single HLA gene in the stem cells while eliminating others. Single HLA-expressing cells can be more easily matched with recipients to avoid alloimmune rejection and a bank of stem cells can be constructed that contain an array of single HLA stem cell lines to allow matching with most of the patient populations. For example, HLA-A2 is expressed on 50% of Caucasians and Asians and beta cells derived from stem cells that express only HLA-A2 could be used for 50% of patients from these ethnic backgrounds. However, the tradeoff of retaining one HLA is that autoreactive T cells can recognize and attack the beta cells. Therefore, immune modulation will still be needed to protect the graft in patients with an underlying autoimmune disease.
Current status and future direction of cell encapsulation strategies
Despite efforts to develop immune privileged beta cells through tolerogenic immunotherapies or genetic manipulation of stem cell-derived beta cells, there is likely to be continued assault on the target tissue by both the alloimmune and autoimmune responses. A supportive approach would entail the use of a semipermeable membrane/capsule to protect the transplanted beta cells from the patient’s immune system, while allowing for sufficient mass transfer to maintain cell viability and ensuring favorable insulin secretion kinetics (Figure 2). Moreover, encapsulation would allow patient protection from the risk of stem cell-derived beta cell oncogenic transformation. Although the notion of an encapsulation strategy that can promote both beta cell survival and protection from immunity has been an aspiration for decades, the optimal device remains yet to be developed, as semipermeable membranes that protect cells from the immune system typically have not been sufficiently permeable to the nutrients necessary to sustain their viability. However, the use of a suitable encapsulation membrane as a physical barrier between the recipient and hESC-derived beta cells could enable the safe use of these therapies while reducing or eliminating the need of immunosuppression (de Vos et al., 2010; Desai and Shea, 2017; Vaithilingam and Tuch, 2011); (Vegas et al., 2016). An ideal encapsulation device should 1) provide ample blood supply to sustain the survival and function of a beta cell mass sufficient for the maintenance of normoglycemia; 2) be biocompatible; 3) provide an immune protective environment to prevent sensitization and rejection; 4) allow secreted insulin to rapidly exit the device for transport via systemic circulation; and 5) contain any potentially tumorigenic cells. Achieving effective glucose homeostasis using beta cell replacement therapy will require finding ways of preventing both alloimmune and autoimmune responses. Polymeric microcapsules have yielded some promising results for allogeneic cell transplantation with immune suppression, but few approaches have been effective for non-immune suppressed encapsulation due to mechanical rupture of the membrane, biochemical instability, and broad membrane pore size distributions. Most importantly, few, if any, encapsulation strategies have been able to protect islets long-term from both an allogeneic and autoimmune response in a spontaneously autoimmune setting (Desai and Shea, 2017).
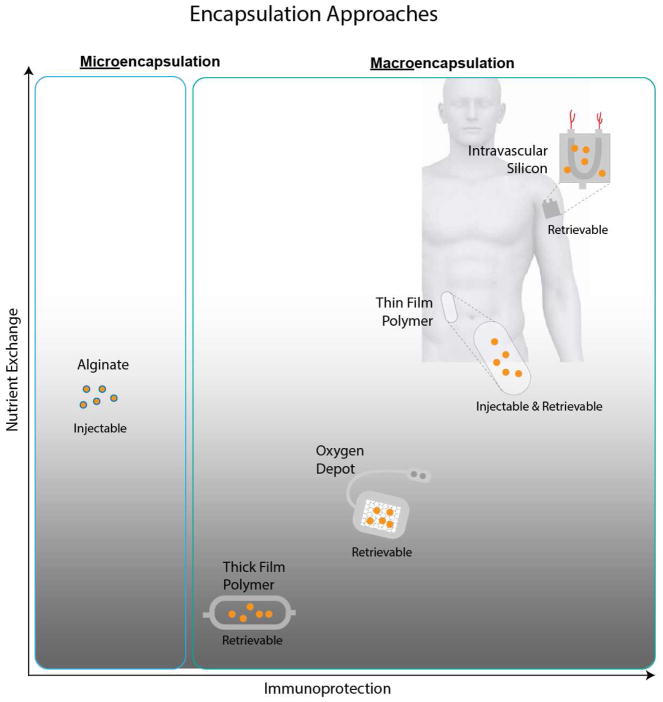
Figure 2.
Cell encapsulation strategies for islet transplantation. While encapsulation with expanded polytetrafluoroethylene (ePTFE) and alginate have been the conventional approaches, more recent advances include the use of microfabricated silicon and nanotemplated polymer thin films, such as polycaprolactone (PCL). A hybrid approach utilizes an oxygen depot to enhance cell viability. Pros and cons of the various strategies center around ease of implantation (injectable or not), ability for retrieval (if needed), getting oxygen to and insulin from the cells, and protection provided by the membrane to cells from attack by the patient’s immune system.
Encapsulation strategies in the clinic
Despite decades of work on encapsulation strategies, only a handful of technologies have made it to the clinic. Perhaps the approach most widely studied and furthest along is based on microencapsulation, in which microcapsules formed from a thin layer of alginate and with a diameter in the range of 300 to 400 μm have been used to encapsulate allogeneic islets. These capsules were delivered intraperitoneally and reduced exogenous insulin requirements (Basta et al., 2011). Clinical trials have also focused on confirming the safety of alginate microcapsules with xenogeneic islets. Specifically, these trials looked at the safety of xenotransplantation of 10,000 to 20,000 IEQ/kg body weight of alginate encapsulated porcine islets in the peritoneal cavity.
One of the limitations of many encapsulation strategies is the lack of sufficient oxygen to maintain islet function. To overcome this challenge, the company Beta-O2 developed the βAir™ device to provide exogenous oxygen to transplanted islets. The disc-shaped device consists of a compartment containing islets, encapsulated in an alginate hydrogel slab, adjacent to a gas chamber where cells receive oxygen by diffusion through a gas permeable membrane (Barkai et al., 2013). The subcutaneously implanted device contains ports that allow for daily filling with oxygen (Neufeld et al., 2013). A case report for this device in a single patient reported that islets retained function for the 10-month duration of the study, with modest reduction in exogenous insulin requirements (Ludwig et al., 2013).
Another strategy that can potentially enhance islet survival after transplantation is the enhancement of microvasculature (Gu et al., 2001). Two devices that have reached clinical trials, the Theracyte device and Sernova cell pouch (Pepper et al., 2015), aim to pre-vascularize a subcutaneous site prior to administration of the cells through a port. The Theracyte device is thought to block key immune molecules (Kumagai-Braesch et al., 2013; Sörenby et al., 2008), and is composed of a two-membrane pouch. The outer membrane has a 5 μm pore size to support cell infiltration and promote angiogenesis throughout the device. The inner membrane has a pore size diameter of 0.4 μm for immunoisolating the islets adjacent to the vasculature. This device was most recently adapted to house stem cells by Viacyte (Agulnick et al., 2015; Robert et al., 2018). This system, termed Encaptra, has a single membrane that is immunoisolating, while allowing oxygen and nutrients to pass, and it is in Phase I/II clinical trial to assess safety and efficacy (NCT02239354). In contrast to Encaptra, the Sernova cell pouch is not immunoisolating. The device is inserted under the skin for 30 days to enable vascular integration within the device. Subsequently, a series of rods are removed to expose channels that can be filled with transplanted islets. The three-year Phase I/II clinical study using this device recently terminated after recruiting 3 patients (NCT01652911) (Gala-Lopez et al., n.d.).
Microfabricated encapsulation devices
Two major areas for the successful design of next generation cell encapsulation devices are providing effective immunoprotection and presenting sufficient mass transfer between the outside environment and the encased islets. As discussed above, the semipermeable membranes must exhibit a precisely controlled pore size to separate soluble inflammatory mediators that are on a scale of nanometers in size while exhibiting exceptional uniformity in pore size distribution to provide sufficient immunoprotection. Microfabricated silicon membranes can be used to achieve such levels of high precision control over pore sizes, as illustrated by examples like nanoporous biocapsules (Desai et al., 1999; 2004; Leoni and Desai, 2004) and Nanogland (Sabek et al., 2013). The surfaces of silicon membranes can also be selectively grafted with biocompatible polymer thin films to ensure functional performance over extended time periods, making them suitable for biological applications (Li et al., 2011; Melvin et al., 2010; J. Zhu and Marchant, 2006).
The nanoporous biocapsule and Nanogland were designed with L-shaped pore paths with perpendicular microchannels and parallel nanochannels to the membrane surface. This L-shaped design effectively prevents diffusion of larger immune components, but allows hindered diffusion of small molecules due to the indirect, long diffusion distance. This effect was observed in the Nanogland device where nanochannels with 3.6 and 5.7 nm pore sizes showed a reduction in glucose diffusivity by 40% and 25% compared with the molecule in the bulk medium (Sabek et al., 2013). Besides the long diffusion distance, solutes also face reduced diffusion as their size approaches the molecular dimension of the pores. Dechadilok and Deen reviewed hindered transport theory for both diffusive and convective hindrance factors in which uncharged, spherical particles travel in the long cylindrical and slit pores of uniform cross-section (DechadilokWilliam M Deen, 2006). Depending on the mode of transport, it is crucial to design immunoprotective membranes with size exclusion properties for the larger immune components (e.g. cytokines, antibodies) while still permitting the passage of smaller molecules (e.g. glucose, insulin). Although nano-sized pores are ideal to restrict the passage of immune components, encased cell functions and viability as well as insulin secretion kinetics could be greatly impacted under the diffusive transport approach.
Recently, approaches to create controlled nanopores in materials other than silicon have also been explored. A nanoporous thin-film cell encapsulation device made from polycaprolactone (PCL) was developed using a nanotemplating technique (Nyitray et al., 2015), whereby well-defined pore sizes are created by growing nanorods and then casting a film onto this substrate. While still maintaining flexibility, the material is engineered to have precise nanoscale pores and showed cell viability in allogeneic mouse models for up to 90 days. The device was also able to support the function of stem cell-derived insulin-producing cells for up to 6 months (R. Chang et al., 2017) and block antigen recognition from antigen specific T cells. The lack of foreign body response, in combination with rapid neovascularization around the device, demonstrates its promise for cell encapsulation.
A faster mass transfer of oxygen and nutrients to the encapsulated beta cells could be achieved with an intravascular device. Moreover, convective transport with an applied pressure gradient results in more efficient mass transfer where solutes actively move along with solvent flux (Song et al., 2016), which, in turn, enhances insulin secretion kinetics. In one configuration, capsules are constructed from silicon semipermeable membranes with straight slit pores. In contrast to the previous L-shaped pores, these straight pores exhibited shorter distances for molecules to travel resulting in higher permeability. This pore geometry optimizes the trade-off between selectivity and permeability of the membranes. The permeability – selectivity analysis for ultrafiltration showed that membranes with monodispersed slit-shaped pores exhibited greater selectivity at a given value of permeability than membranes with cylindrical pores for pore size below 100 nm (Kanani et al., 2010). With the relatively thin silicon membranes, convection-dominated mass transport is advantageous, because it efficiently transports solutes such as glucose, oxygen, and insulin between the device and adjacent vascular system (Song et al., 2017).
Conclusions
Rapid advances in device technology, immune modulation, and generation of functional pancreatic islet cells from human stem cells may soon provide novel therapeutic avenues for the treatment of diabetic patients. While significant technical hurdles remain in all these areas, the advent of new encapsulation technologies, novel immune modulation approaches, and improved stem cell differentiation protocols paired with gene editing technologies carry the promise of revolutionizing modern cell therapy approaches for both T1D and T2D patients.
Acknowledgments
Funding
J.B.S., Q. T., J.A.B., P.S., and M.H. are part of the UCSF Diabetes Endocrinology Research Center (P30 DK063720). S.R. is supported by funds from the NIH (U01EB025136) and the JDRF Encapsulation Consortium. M.H. receives support from the NIH (DK105831, U01EB025136).
Footnotes
Declaration of Interests
J. S., Q.T., P. S. and M. H. serve on the scientific advisory board for Encellin Inc. Q. T. serves on the scientific advisory board for Encellin, Inc. T. D. is the Founder and serves on the scientific advisory board for Encellin Inc. S. R. is the Founder and has an ownership of Silicon Kidney. M. H. is on the scientific advisory board of Semma, Inc. and holds stock options in Viacyte, Inc.
R. S. holds the US Patent 9,802,158 - Ultrafiltration membrane device, bioartificial organ, and related methods.
M. H. in an inventor on the US Patent No. 9,850,465 - Generation of Thymic Epithelial Progenitor Cells In Vitro as well as provisional US Patent applications: Case 2015-203-3 PCT: PCT/US16/28963 (filed 4/22/1): Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro; Case 2016-019-4 PCT: PCT/US16/44096 (filed 7/26/16): Generation of human beta cell equivalents from pluripotent stem cells in vitro; Case 2018-064-1 PRV: (filed 12/12/2017): The use of parathyroid gland cells and their secreted factors to promote islet beta cell engraftments. App 62/597,825
T. D. is an inventor on the Patent Application PCT/US2016/023808 Entitled: Thin Film Cell Encapsulation Device, Ref. SF2015-176-2.
Q. T. and J. A. B. are inventors on the patents Regulatory T Cells Suppress Autoimmunity. US Patent # 7,722,862,B2 (Issued.5/25/2010) and Expansion of alloantigen-reactive regulatory T cells. US Patent # 14/382.537 (Issued 10/31/2017)
Q. T. and P. S. are inventors on the patent application Case 2018-064-1 PRV: (filed 12/12/2017): The use of parathyroid gland cells and their secreted factors to promote islet beta cell engraftments. App 62/597,825
J. A. B. is also inventor on the Patent CD127 expression inversely correlates with Foxp3 and suppresses function of CD4+ Tregs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4:1214–1222. doi: 10.5966/sctm.2015-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan AN, Naziruddin B, Lockridge A, Tiwari M, Loganathan G, Takita M, Matsumoto S, Papas K, Trieger M, Rainis H, Kin T, Kay TW, Wease S, Messinger S, Ricordi C, Alejandro R, Markmann J, Kerr-Conti J, Rickels MR, Liu C, Zhang X, Witkowski P, Posselt A, Maffi P, Secchi A, Berney T, O’Connell PJ, Hering BJ, Barton FB. Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am J Transplant. 2014;14:2595–2606. doi: 10.1111/ajt.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, Yavriyants K, Azarov D, Zimermann B, Maimon S, Shabtay N, Balyura M, Rozenshtein T, Vardi P, Bloch K, de Vos P, Rotem A. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22:1463–1476. doi: 10.3727/096368912X657341. [DOI] [PubMed] [Google Scholar]
- Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O’Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TWH, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AMJ. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta G, Montanucci P, Luca G, Boselli C, Noya G, Barbaro B, Qi M, Kinzer KP, Oberholzer J, Calafiore R. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care. 2011;34:2406–2409. doi: 10.2337/dc11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- Benninger RKP, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol (Lond) 2011;589:5453–5466. doi: 10.1113/jphysiol.2011.218909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi F, Colussi G, Lauterio A, De Carlis L. Intramuscular islet allotransplantation in type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2018;22:1731–1736. doi: 10.26355/eurrev_201803_14588. [DOI] [PubMed] [Google Scholar]
- Bix M, Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. Journal of Experimental Medicine. 1992;176:829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Sullivan BA, Weir GC. Human Islet Morphology Revisited: Human and Rodent Islets Are Not So Different After All. J Histochem Cytochem. 2015;63:604–612. doi: 10.1369/0022155415570969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnères PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–1272. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TV, Jaigirdar A, Hoang V, Hayden T, Liu FC, Zaid H, Chang CK, Bucy RP, Tang Q, Kang SM. Preferential priming of alloreactive T cells with indirect reactivity. Am J Transplant. 2009;9:709–718. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GW, Vendrame F, Virdi SK, Ciancio G, Chen L, Ruiz P, Messinger S, Reijonen HK, Pugliese A. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr Diab Rep. 2015;15:121. doi: 10.1007/s11892-015-0691-5. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- Chang R, Faleo G, russ HA, Parent AV, Elledge SK, Bernards DA, Allen JL, Villanueva K, Hebrok M, Tang Q, Desai TA. Nanoporous Immunoprotective Device for Stem-Cell-Derived β-Cell Replacement Therapy. ACS Nano. 2017;11:7747–7757. doi: 10.1021/acsnano.7b01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MM, Thomas HE, Kay TW. gamma-Interferon signaling in pancreatic beta-cells is persistent but can be terminated by overexpression of suppressor of cytokine signaling-1. Diabetes. 2001;50:2744–2751. doi: 10.2337/diabetes.50.12.2744. [DOI] [PubMed] [Google Scholar]
- Chong MMW, Thomas HE, Kay TWH. Suppressor of cytokine signaling-1 regulates the sensitivity of pancreatic beta cells to tumor necrosis factor. J Biol Chem. 2002;277:27945–27952. doi: 10.1074/jbc.M110214200. [DOI] [PubMed] [Google Scholar]
- Cirulli V, Zalatan J, McMaster M, Prinsen R, Salomon DR, Ricordi C, Torbett BE, Meda P, Crisa L. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes. 2006;55:1214–1222. doi: 10.2337/db05-0731. [DOI] [PubMed] [Google Scholar]
- D’Amour K, Agulnick A, Eliazer S, Kelly O, Kroon E, Baetge E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour K, Bang A, Eliazer S, Kelly O, Agulnick A, Smart N, Moorman M, Kroon E, Carpenter M, Baetge E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006 doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- de Vos P, Spasojevic M, Faas MM. Treatment of diabetes with encapsulated islets. Adv Exp Med Biol. 2010;670:38–53. doi: 10.1007/978-1-4419-5786-3_5. [DOI] [PubMed] [Google Scholar]
- Dechadilok P, Deen William M. Hindrance Factors for Diffusion and Convection in Pores. Industrial & Engineering Chemistry Research. 2006;45:6953–6959. doi: 10.1021/ie051387n. [DOI] [Google Scholar]
- Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2017;16:338–350. doi: 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TA, Chu WH, Rasi G, Sinibaldi-Vallebona P, Guarino E, Ferrari M. Microfabricated biocapsules provide short-term immunoisolation of insulinoma xenografts. Biomed Microdevices. 1999;1:131–138. doi: 10.1023/A:1009948524686. [DOI] [PubMed] [Google Scholar]
- Desai TA, West T, Cohen M, Boiarski T, Rampersaud A. Nanoporous microsystems for islet cell replacement. Adv Drug Deliv Rev. 2004;56:1661–1673. doi: 10.1016/j.addr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M. Human islets contain four distinct subtypes of β cells. Nat Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich T, Eriksson O, Lundgren T Nordic Network for Clinical Islet Transplantation. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356:2754–2755. doi: 10.1056/NEJMc070201. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- Esmatjes E, Montaña X, Real MI, Blanco J, Conget I, Casamitjana R, Rovira M, Gomis R, Marin P. Regeneration of insulin production by autologous bone marrow blood autotransplantation in patients with type 1 diabetes. Diabetologia. 2010;53:786–789. doi: 10.1007/s00125-010-1660-9. [DOI] [PubMed] [Google Scholar]
- Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- Foulis AK, Farquharson MA. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986;35:1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Wisel SA, Tang Q. Impact of Immune-Modulatory Drugs on Regulatory T Cell. Transplantation. 2016;100:2288–2300. doi: 10.1097/TP.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gala-Lopez BL, Pepper AR, Pepper Dinyari P, Malcolm AJ, Kin T, Pawlick LR, Senior PA, Shapiro AMJ. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch - preliminary experience. CellR. n.d;4:e2132. [Google Scholar]
- Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets. 2018;10:80–94. doi: 10.1080/19382014.2018.1428511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Harris KM, Kanaparthi S, Phippard D, Ding L, Bluestone JA, Ehlers MR ITN START Study Team. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59:1153–1161. doi: 10.1007/s00125-016-3917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, Ding L, Bluestone JA, Ehlers MR START Study Team. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306–316. doi: 10.1016/S2213-8587(13)70065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas R, Franksson L, Ohlén C, Höglund P, Koller B, Ljunggren HG, Kärre K. Major histocompatibility complex class I-specific and -restricted killing of beta 2-microglobulin-deficient cells by CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1992;89:11381–11385. doi: 10.1073/pnas.89.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C. A20 inhibits cytokine-induced apoptosis and nuclear factor kappaB-dependent gene activation in islets. Journal of Experimental Medicine. 1999;190:1135–1146. doi: 10.1084/jem.190.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruessner AC, Gruessner RWG. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377–385. doi: 10.1097/MOT.0000000000000331. [DOI] [PubMed] [Google Scholar]
- Gu Y, Gong C, Peng X, Wei L, Su C, Qin M, Wang X, Li F. Autologous hematopoietic stem cell transplantation and conventional insulin therapy in the treatment of children with newly diagnosed type 1 diabetes: long term follow-up. Chin Med J. 2014;127:2618–2622. [PubMed] [Google Scholar]
- Gu Y, Tabata Y, Kawakami Y, Balamurugan AN, Hori H, Nagata N, Satake A, Cui W, Qi M, Misawa Y, Toma M, Miyamoto M, Nozawa M, Inoue K. Development of a new method to induce angiogenesis at subcutaneous site of streptozotocin-induced diabetic rats for islet transplantation. Cell Transplant. 2001;10:453–457. [PubMed] [Google Scholar]
- Guo T, Landsman L, Li N, Hebrok M. Factors Expressed by Murine Embryonic Pancreatic Mesenchyme Enhance Generation of Insulin-Producing Cells From hESCs. Diabetes. 2013;62:1581–1592. doi: 10.2337/db12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Hagopian W, Ferry RJ, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P, Wollheim C, Blondel B, Meda P, Niesor E, Mintz D. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology. 1982;111:86–94. doi: 10.1210/endo-111-1-86. [DOI] [PubMed] [Google Scholar]
- Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448–455. doi: 10.1172/JCI78492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci USA. 2003;100:6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey KG, Drachenberg CB, Papadimitriou JC, Klassen DK, Philosophe B, Bartlett ST, Groh V, Spies T, Mann DL. MIC expression in renal and pancreatic allografts. Transplantation. 2002;73:304–306. doi: 10.1097/00007890-200201270-00029. [DOI] [PubMed] [Google Scholar]
- Hayden MR, Patel K, Habibi J, Gupta D, Tekwani SS, Whaley-Connell A, Sowers JR. Attenuation of endocrine-exocrine pancreatic communication in type 2 diabetes: pancreatic extracellular matrix ultrastructural abnormalities. J Cardiometab Syndr. 2008;3:234–243. doi: 10.1111/j.1559-4572.2008.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347–356. doi: 10.1113/expphysiol.1985.sp002920. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AMJ, Stock PG, Turgeon NA Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2016;39:1230–1240. doi: 10.2337/dc15-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16:550–557. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kanani DM, Fissell WH, Roy S, Dubnisheva A, Fleischman A, Zydney AL. Permeability - Selectivity Analysis for Ultrafiltration: Effect of Pore Geometry. J Memb Sci. 2010;349:405. doi: 10.1016/j.memsci.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry MA, Prentice MA, Fox A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Pancreas. Am J Transplant. 2018;18(Suppl 1):114–171. doi: 10.1111/ajt.14558. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Curr Opin Organ Transplant. 2013;18:402–407. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C, Candon S, Waldmann H, Ziegler AG, Chatenoud L, Pipeleers D. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M. Quantification of islet size and architecture. Islets. 2012;4:167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N. In vitro reconstitution of pancreatic islets. Organogenesis. 2014;10:225–230. doi: 10.4161/org.28351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson L, Kadoya K, Bang A, Kelly O, Eliazer S, Young H, Richardson M, Smart N, Cunningham J, Agulnick A, D’Amour K, Carpenter M, Baetge E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008 doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A. The TheraCyte™ device protects against islet allograft rejection in immunized hosts. Cell Transplant. 2013;22:1137–1146. doi: 10.3727/096368912X657486. [DOI] [PubMed] [Google Scholar]
- Kupfer TM, Crawford ML, Pham K, Gill RG. MHC-mismatched islet allografts are vulnerable to autoimmune recognition in vivo. J Immunol. 2005;175:2309–2316. doi: 10.4049/jimmunol.175.4.2309. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003a;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003b;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. doi: 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni L, Desai TA. Micromachined biocapsules for cell-based sensing and delivery. Adv Drug Deliv Rev. 2004;56:211–229. doi: 10.1016/j.addr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Li L, Marchant RE, Dubnisheva A, Roy S, Fissell WH. Anti-biofouling Sulfobetaine Polymer Thin Films on Silicon and Silicon Nanopore Membranes. J Biomater Sci Polym Ed. 2011;22:91–106. doi: 10.1163/092050609X12578498982998. [DOI] [PubMed] [Google Scholar]
- Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 2014;3:e04766. doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SA, Thorpe J, Herold KC, Ehlers M, Sanda S, Lim N, Linsley PS, Nepom GT, Harris KM. Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes. Cell Immunol. 2017;319:3–9. doi: 10.1016/j.cellimm.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J, Samuelsson U, Elding Larsson H, Åman J, Kärdell G, Neiderud Helsingborg J, Lundström G, Albinsson E, Carlsson A, Nordvall M, Fors H, Arvidsson CG, Edvardson S, Hanås R, Larsson K, Rathsman B, Forsgren H, Desaix H, Forsander G, Nilsson N-Ö, Åkesson CG, Keskinen P, Veijola R, Talvitie T, Raile K, Kapellen T, Burger W, Neu A, Engelsberger I, Heidtmann B, Bechtold S, Leslie D, Chiarelli F, Cicognani A, Chiumello G, Cerutti F, Zuccotti GV, Gomez Gila A, Rica I, Barrio R, Clemente M, López Garcia MJ, Rodriguez M, Gonzalez I, Lopez JP, Oyarzabal M, Reeser HM, Nuboer R, Stouthart P, Bratina N, Bratanic N, de Kerdanet M, Weill J, Ser N, Barat P, Bertrand AM, Carel JC, Reynaud R, Coutant R, Baron S. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110:19054–19058. doi: 10.1073/pnas.1317561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf L, Yamada A, Ito T, Abdi R, Ansari MJI, Khuong CQ, Winn HJ, Auchincloss H, Sayegh MH. Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice. J Am Soc Nephrol. 2003;14:2168–2175. doi: 10.1097/01.asn.0000079041.15707.a9. [DOI] [PubMed] [Google Scholar]
- Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin ME, Fissell WH, Roy S, Brown DL. Silicon induces minimal thromboinflammatory response during 28-day intravascular implant testing. ASAIO J. 2010;56:344–348. doi: 10.1097/MAT.0b013e3181d98cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moassesfar S, Masharani U, Frassetto LA, Szot GL, Tavakol M, Stock PG, Posselt AM. A Comparative Analysis of the Safety, Efficacy, and Cost of Islet Versus Pancreas Transplantation in Nonuremic Patients With Type 1 Diabetes. Am J Transplant. 2016;16:518–526. doi: 10.1111/ajt.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T, Ludwig B, Barkai U, Weir GC, Colton CK, Evron Y, Balyura M, Yavriyants K, Zimermann B, Azarov D, Maimon S, Shabtay N, Rozenshtein T, Lorber D, Steffen A, Willenz U, Bloch K, Vardi P, Taube R, de Vos P, Lewis EC, Bornstein SR, Rotem A. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE. 2013;8:e70150. doi: 10.1371/journal.pone.0070150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber H, Ferrara N, Melton D, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Nyitray CE, Chang R, Faleo G, Lance KD, Bernards DA, Tang Q, Desai TA. Polycaprolactone Thin-Film Micro- and Nanoporous Cell-Encapsulation Devices. ACS Nano. 2015;9:5675–5682. doi: 10.1021/acsnano.5b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg-Welsh C. Long-term culture in matrigel enhances the insulin secretion of fetal porcine islet-like cell clusters in vitro. Pancreas. 2001;22:157–163. doi: 10.1097/00006676-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(Suppl 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of Functional Human Pancreatic β Cells In Vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Penko D, Mohanasundaram D, Sen S, Drogemuller C, Mee C, Bonder CS, Coates PTH, Jessup CF. Incorporation of endothelial progenitor cells into mosaic pseudoislets. Islets. 2011;3:73–79. doi: 10.4161/isl.3.3.15392. [DOI] [PubMed] [Google Scholar]
- Pepper AR, Pawlick R, Gala-Lopez B, MacGillivary A, Mazzuca DM, White DJG, Toleikis PM, Shapiro AMJ. Diabetes Is Reversed in a Murine Model by Marginal Mass Syngeneic Islet Transplantation Using a Subcutaneous Cell Pouch Device. Transplantation. 2015;99:2294–2300. doi: 10.1097/TP.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, McElroy J, Ramos MD, Kerlan RK, Fong L, Vincenti F, Bluestone JA, Stock PG. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90:1595–1601. doi: 10.1097/TP.0b013e3181fe1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol-Borrell R, Todd I, Doshi M, Bottazzo GF, Sutton R, Gray D, Adolf GR, Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987;326:304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Güldenagel M, Charollais A, Gjinovci A, Caille D, Söhl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- Reinert RB, Cai Q, Hong JY, Plank JL, Aamodt K, Prasad N, Aramandla R, Dai C, Levy SE, Pozzi A, Labosky PA, Wright CVE, Brissova M, Powers AC. Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development. 2014;141:1480–1491. doi: 10.1242/dev.098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YHC, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014 doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania AA, Bruin JEJ, Riedel MJM, Mojibian MM, Asadi AA, Xu JJ, Gauvin RR, Narayan KK, Karanu FF, O’Neil JJJ, Ao ZZ, Warnock GLG, Kieffer TJT. Maturation of Human Embryonic Stem Cell-Derived Pancreatic Progenitors Into Functional Islets Capable of Treating Pre-existing Diabetes in Mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T, De Mesmaeker I, Stangé GM, Suenens KG, Ling Z, Kroon EJ, Pipeleers DG. Functional Beta Cell Mass from Device-Encapsulated hESC-Derived Pancreatic Endoderm Achieving Metabolic Control. Stem Cell Reports. 2018;10:739–750. doi: 10.1016/j.stemcr.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, Blelloch R, Szot GL, Arvan P, Hebrok M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015 doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabek OM, Ferrati S, Fraga DW, Sih J, Zabre EV, Fine DH, Ferrari M, Gaber AO, Grattoni A. Characterization of a nanogland for the autotransplantation of human pancreatic islets. Lab Chip. 2013;13:3675–3688. doi: 10.1039/c3lc50601k. [DOI] [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- Sasson A, Rachi E, Sakhneny L, Baer D, Lisnyansky M, Epshtein A, Landsman L. Islet pericytes are required for beta-cell maturity. Diabetes. 2016 doi: 10.2337/db16-0365. [DOI] [PubMed] [Google Scholar]
- Schaeffer M, Hodson DJ, Lafont C, Mollard P. Endocrine cells and blood vessels work in tandem to generate hormone pulses. J Mol Endocrinol. 2011;47:R59–66. doi: 10.1530/JME-11-0035. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, Kneteman N, Rajotte R. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen [see comments] N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13:268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- Solomon M, Flodström-Tullberg M, Sarvetnick N. Beta-cell specific expression of suppressor of cytokine signaling-1 (SOCS-1) delays islet allograft rejection by down-regulating Interferon Regulatory Factor-1 (IRF-1) signaling. Transpl Immunol. 2011;24:181–188. doi: 10.1016/j.trim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S. An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport. Lab Chip. 2017;17:1778–1792. doi: 10.1039/c7lc00096k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S. Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Sci Rep. 2016;6:23679. doi: 10.1038/srep23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model. Transplantation. 2008;86:364–366. doi: 10.1097/TP.0b013e31817efc78. [DOI] [PubMed] [Google Scholar]
- Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996;61:1272–1274. doi: 10.1097/00007890-199604270-00027. [DOI] [PubMed] [Google Scholar]
- Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller CR, Dupré J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, von Graffenried B, Wolfe BM. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science (New York, NY) 1984;223:1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang L, Chiricozzi A, Cardinale I, Duan S, Bowcock A, Krueger JG, Guttman-Yassky E. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. e1–4. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998a;352:837–853. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998b;352:854–865. [PubMed] [Google Scholar]
- Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud. 2011;8:51–67. doi: 10.1900/RDS.2011.8.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016 doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51:1181–1191. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nuñez G, Tang A, Sayegh M, Hancock WW, Strom TB, Turka LA. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Palmer JP, Raskin P, Rodriguez H, Schatz D, Wilson DM, Krischer JP, Skyler JS Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- Young HY, Zucker P, Flavell RA, Jevnikar AM, Singh B. Characterization of the role of major histocompatibility complex in type 1 diabetes recurrence after islet transplantation. Transplantation. 2004;78:509–515. doi: 10.1097/01.tp.0000128907.83111.c6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Marchant RE. Dendritic saccharide surfactant polymers as antifouling interface materials to reduce platelet adhesion. Biomacromolecules. 2006;7:1036–1041. doi: 10.1021/bm050611p. [DOI] [PubMed] [Google Scholar]
- Zhu S, russ HA, Wang X, Zhang M, Ma T, Xu T, Tang S, Hebrok M, Ding S. Human pancreatic beta-like cells converted from fibroblasts. Nat Commun. 2016;7:10080. doi: 10.1038/ncomms10080. [DOI] [PMC free article] [PubMed] [Google Scholar]