Abstract
Background
Nicotinamide riboside (NR) is a nicotinamide adenine dinucleotide (NAD+) precursor which is present in foods such as milk and beer. It was reported that NR can prevent obesity, increase longevity, and promote liver regeneration. However, whether NR can prevent ethanol-induced liver injuries is not known. This study aimed to explore the effect of NR on ethanol induced liver injuries and the underlying mechanisms.
Methods
We fed C57BL/6 J mice with Lieber-DeCarli ethanol liquid diet with or without 400 mg/kg·bw NR for 16 days. Liver injuries and SirT1-PGC-1α-mitochondrial function were analyzed. In in vitro experiments, HepG2 cells (CYP2E1 over-expressing cells) were incubated with ethanol ± 0.5 mmol/L NR. Lipid accumulation and mitochondrial function were compared. SirT1 knockdown in HepG2 cells were further applied to confirm the role of SirT1 in the protection of NR on lipid accumulation.
Results
We found that ethanol significantly decreased the expression and activity of hepatic SirT1 and induced abnormal expression of enzymes of lipid metabolism in mice. Both in vivo and in vitro experiments showed that NR activated SirT1 through increasing NAD+ levels, decreased oxidative stress, increased deacetylation of PGC-1α and mitochondrial function. In SirT1 knockdown HepG2 cells, NR lost its ability in enhancing mitochondrial function, and its protection against lipid accumulation induced by ethanol.
Conclusions
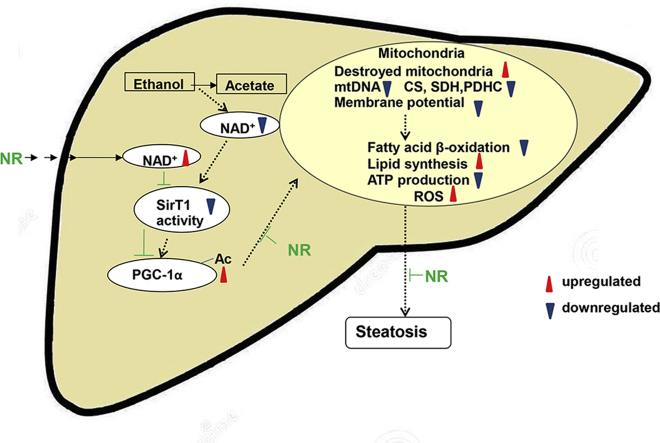
NR can protect against ethanol induced liver injuries via replenishing NAD+, reducing oxidative stress, and activating SirT1-PGC-1α-mitochondrial biosynthesis. Our data indicate that SirT1 plays an important role in the protection of NR against lipid accumulation and mitochondrial dysfunctions induced by ethanol.
Abbreviations: ADH, alcohol dehydrogenase; ALD, Alcoholic liver disease; ALDH, aldehyde dehydrogenase; CPT-1α, carnitine palmitoyl transterase-1α; CR, calorie restriction; CS, citrate synthase; CYP2E1, cytochrome P450 2E1; FASN, fatty acid synthase; NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMPT, nicotinamide phosphoribosyl transferase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide mononucleotide adenylyl transferase; NR, nicotinamide riboside; NRF-1, nuclear respiratory factor 1; NRF-2, nuclear respiratory factor 2; NRK, nicotinamide riboside kinase; PDHC, pyruvate dehydrogenase complex; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PPAR-α, peroxisome proliferator- activated receptor α; PPAR-γ, peroxisome proliferator-activated receptor γ; ROS, reactive oxygen species; SDH, succinate dehydrogenase; SirT1, sirtuin 1; SirT3, sirtuin 3; T2D, type 2 diabetes; TCA cycle, tricarboxylic acid cycle; TFAM, mitochondrial transcriptional factor; UCP2, uncoupling protein-2
Keywords: Alcoholic liver disease, NAD+, Nicotinamide Riboside, SirT1, Oxidative stress, Mitochondrial biosynthesis
Graphical abstract
Highlights
-
•
NR could reverse ethanol induced hepatic steatosis and oxidative stress.
-
•
Boosting NAD+, NR enhanced mitochondrial functions by regulating SirT1/ PGC-1α.
-
•
SirT1 played an important role in NR's protection against alcohol liver injuries.
1. Introduction
Alcoholic liver disease (ALD) is a common clinical complication of long-term alcohol abuse. Its morphological features include alcoholic fatty liver (steatosis), alcoholic hepatitis, and alcoholic cirrhosis. Fatty liver is a uniform and early response of the liver to ethanol consumption. The prevention of the formation of fatty liver is the key to prevent the damages to livers induced by alcohol.
The metabolism of alcohol is mainly catalyzed by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). During long term heavy drinking or binge drinking, cytochrome P450 2E1 (CYP2E1) is activated, playing a critical role in alcohol metabolism [1]. The metabolism of ethanol increases the conversion of nicotinamide adenine dinucleotide (NAD+) to NADH which decreases the ratio of NAD+/NADH [2]. NAD+ is an essential coenzyme of mitochondrial oxidative phosphorylation and energy metabolism and also the substrate of NAD+ consuming enzymes [3]. Steatosis induced by high-fat diet triggers the reduction in nicotinamide phosphoribosyl transferase (NAMPT)-mediated NAD+ biosynthesis and contribute to the pathogenesis of type 2 diabetes (T2D) [4]. Decline in nuclear NAD+ during aging, perhaps due to defects in nicotinamide mononucleotide adenylyl transferase (NMNAT) which regulates NAD+ synthesis from nicotinamide mononucleotide (NMN), causes impairment in mitochondrial homeostasis [5]. The function of NAD+ was further tested by inactivation of poly (ADP-ribose) polymerase 1, a major cellular NAD+ consumer. Increasing tissue NAD+ levels and activates mitochondrial metabolism in brown adipose tissue and muscle, culminates in a solid protection against metabolic disease [6]. Hence, an understanding of how alcohol affects NAD homeostasis and NAD+ consuming enzymes may provide an important insight into the mechanisms of ALD.
Sirtuin 1 (SirT1), is an NAD+-dependent class III protein deacetylase and/or ADP-ribosyltransferase, whose activation is regulated by the intracellular level of NAD+, inhibited by the reaction product nicotinamide (NAM) [7]. So far, the relationship between SirT1 and liver damage is not conclusive, even though most studies favor the protective effect of SirT1. Either protein or mRNA expression of SirT1 has been found to be suppressed with alcohol consumption in human livers with alcoholic hepatitis or animal alcoholic models 8, 9. Activation of SirT1 by resveratrol prevents alcoholic liver steatosis [10]. In contrast, increased SirT1 expression has been found in nuclei of hepatocytes in mice with significant liver tumor burden compared to control mice on a B6C3 background [11]. SirT1 acts as a metabolic and redox sensor of changes in nutrient and energy status, such as calorie restriction and fasting [12], during which processes SirT1 is induced and mitochondrial biogenesis increases in mice 13, 14. One target of SirT1 is peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which plays a central role in glucose/fatty acid metabolism in fasting 14, 15. Resveratrol increased SirT1 activity and then induced mitochondrial activity through modulating PGC-1α functions, renders the animals resistant to diet-induced obesity [16]. Taken together, it's supposed that regulating SirT1 activity and improving mitochondrial biogenesis might be important mechanisms for the protection against ALD.
Nicotinamide riboside (NR), a form of vitamin B3 and NAD+ precursor, is converted to bioavailable NAD+, via nicotinamide riboside kinase (NRK) and NMNAT, or by the action of nucleoside phosphorylase and NAM salvage 17, 18. The level of NAD+ in human blood rose 2.7-folds with a single oral dose of NR in a pilot study [19]. Numerous studies have shown that NR supplementation increases NAD+ level, enhances mitochondrial biogenesis and oxidative metabolism, protects against metabolic disease, neurodegenerative disorders and age-related physiological decline in mammals 20, 21, 22. In this study, we will explore whether NR can prevent alcohol induced liver injuries, and whether the protective mechanism is via regulating SirT1 and mitochondrial function in liver.
2. Materials and methods
2.1. Animal experiments
All animal experiments were approved by the Animal Care and Utilization Committee of Sun Yat-sen University. Mice were housed in colony cages with a 12-h light, 12-h dark cycle in a temperature-controlled environment. Eight-week-old male C57BL/6 J mice were randomly divided into 3 groups: control group (CTRL), ethanol group (EtOH, Chronic plus binge ethanol model), and NR supplement group (EtOH+NR). Mice in ethanol group and NR supplement group were fed with Lieber- DeCarli ethanol liquid diet (TROPHIC Animal Feed High-tech Co., Ltd., Nantong, China) for 10 days, while the controls mice were pair-fed as previously described [23]. NR (ChromaDex Corporate, Irvine, CA, USA) was added to the diet at a dose of 400 mg/kg according to literatures 20, 21, 24.
2.2. Statistical analysis
Results were expressed as mean±SEM from three independent experiments. Comparisons between groups were analyzed by one-way ANOVA followed by Student-Newman-Keuls test. P < 0.05 was considered to be significant. SPSS software 20.0 was used for the analyses.
Other materials and methods are described in details in the Supplementary information.
3. Results
3.1. NR alleviates hepatic steatosis and oxidative stress in mice fed with Chronic-plus-binge ethanol
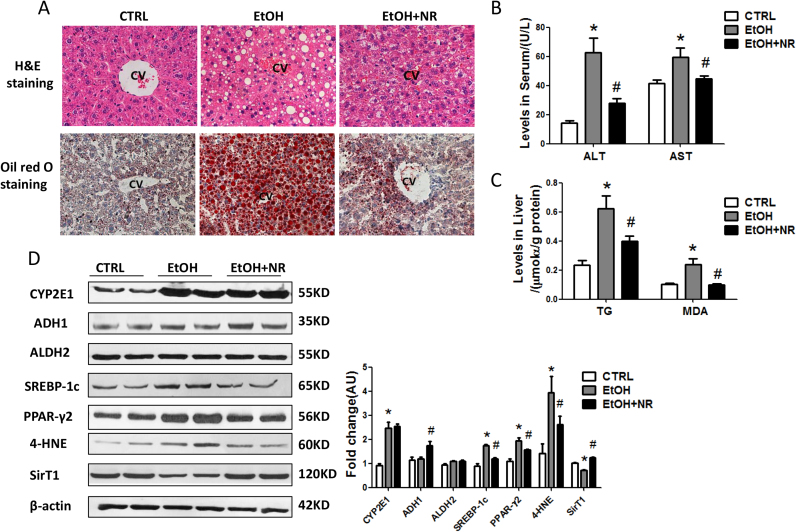
Mice were pair-fed and there were no differences in body weight among the three groups (Fig. S1A, B). Fat accumulated in ethanol group, but only a small number of tiny lipid droplets were observed in NR treated mice as shown in H&E staining and Oil red O staining (Fig. 1A). Ethanol exposure elevated serum ALT and AST and liver triglycerides while NR significantly decreased their levels (Fig. 1B, C). The ratio of liver-to-body weight increased by ethanol compared to controls, while it was slightly reduced by NR (Fig. S1C). The expressions of sterol regulatory element binding protein-lc (SREBP-1c) and peroxisome proliferator-activated receptorγ2 (PPAR-γ2), as well as PPAR-γ mRNA, were markedly elevated by ethanol, which were reduced by NR (Fig. 1D, S1D).
Fig. 1.
NR alleviates hepatic steatosis and oxidative stress in mice fed with Chronic-plus-binge ethanol. Mice were treated with chronic-plus-binge ethanol feeding and NR supplementation for 16 days. (A) Representative images of mouse liver, H&E staining and oil red O staining with 200 × magnification. (B) Serum ALT and AST levels. (C) Liver TG and MDA levels. (D) Western blot analysis of alcohol metabolic enzymes (CYP2E1, ADH1 and ALDH2), lipid metabolic regulators (SREBP-1c and PPAR-γ2), 4-HNE and SirT1 in mouse liver. Results were expressed as fold changes of control. Data are expressed as mean±SEM. n = 6–8/group, *P < 0.05 compared with the CTRL group; #P < 0.05 compared with the EtOH group. Abbreviations: NR, nicotinamide riboside; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TG, triglyceride; MDA, malondialdehyde CYP2E1, cytochrome P450 2E1; ADH1, alcohol dehydrogenase 1; ALDH2, aldehyde dehydrogenase 2; SREBP-1c, sterol regulatory element binding protein-lc; PPAR-γ2, peroxisome proliferator-activated receptor γ2; 4-HNE, 4-hydroxynonenal; SirT1, sirtuin 1; PGC-1α, peroxisome proliferator- activated receptor γ coactivator-1α.
Ethanol significantly induced hepatic CYP2E1 expression, which wasn’t decreased by NR. We didn’t find different expressions of ADH1 and ALDH2 among the three groups. Ethanol increased the levels of hepatic MDA and 4-hydroxynonenal, two major products of lipid peroxidation, and NR markedly decreased them (Fig. 1C, D). SirT1 was significantly decreased in liver of ethanol fed mice compared to controls, and NR restored SirT1 to normal (Fig. 1D). There was also a decrease in the hepatic inflammatory transcripts TNF-α and IL-6 in NR-treated mice compared to mice of ethanol group (Fig. S1D).
3.2. NR prevents ethanol induced metabolic disorders and energy imbalance in mice
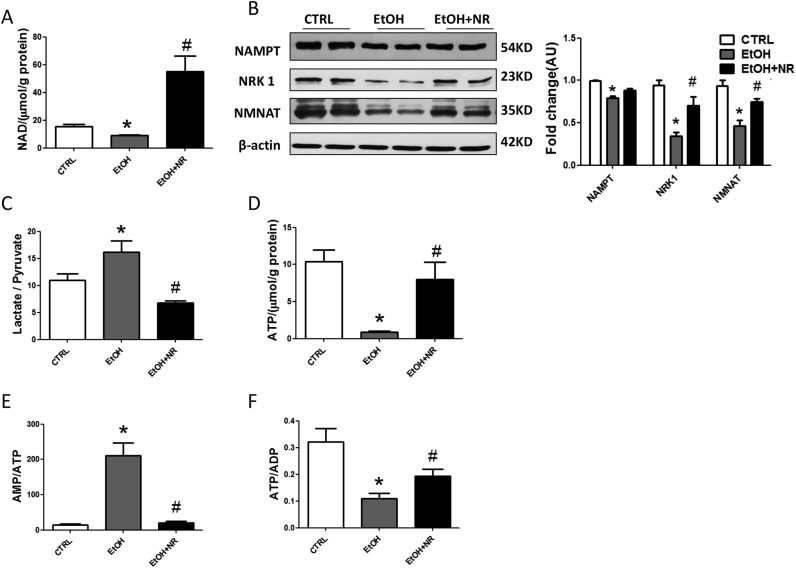
NAD+ is the central coenzyme for fuel oxidation. In this study, the level of hepatic NAD+ and NADP+ were decreased in ethanol group compared to controls, while NR prominently increased them (Fig. 2A, S2A). The levels of NMN and NAM, two metabolites of NR, were comparable in the three groups (Fig. S2B). Expressions of hepatic NRK1, NAMPT and NMNAT were down-regulated in ethanol group, contributing to the reduction of NAD+. NR enhanced the expressions of NRK1 and NMNAT, but had no significant effect on NAMPT (Fig. 2B). Increased liver lactate/pyruvate ratio induced by ethanol confirmed the reduction of cytosolic NAD+/NADH ratio, while NR increased the cytosolic NAD+/NADH ratio (Fig. 2C).
Fig. 2.
NR prevents ethanol induced metabolic disorders and energy imbalance in mice. (A) Liver NAD+ level. (B) Western blot analysis of NAMPT, NMNAT and NRK1 in mouse liver. Results were expressed as fold changes of control. (C) The ratio of lactate/pyruvate. (D) Liver ATP level. (E) The ratios of AMP/ATP and ATP/ADP. Data are expressed as mean±SEM. n = 6–8/group,*P < 0.05 compared with the CTRL group; #P < 0.05 compared with the EtOH group. Abbreviations: NR, nicotinamide riboside; NAD+, nicotinamide adenine dinucleotide; NAMPT, nicotinamide phosphoribosyl transferase; NMNAT, nicotinamide mononucleotide adenylyl transferase; NRK1, nicotinamide riboside kinase 1; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate.
NAD+ is a signaling molecule linking cellular energy status. Our results showed ethanol treatment significantly decreased hepatic ATP and ADP concentrations, accompanied by increased AMP/ATP and decreased ATP/ADP ratios (Fig. 2D–F). The AMP was not changed by ethanol (Fig. S2C). However, NR effectively improved energy homeostasis, indicated by increased ATP and ADP concentrations, decreased AMP/ATP ratio, and increased ATP/ADP ratio (Fig. 2D–F, S2C).
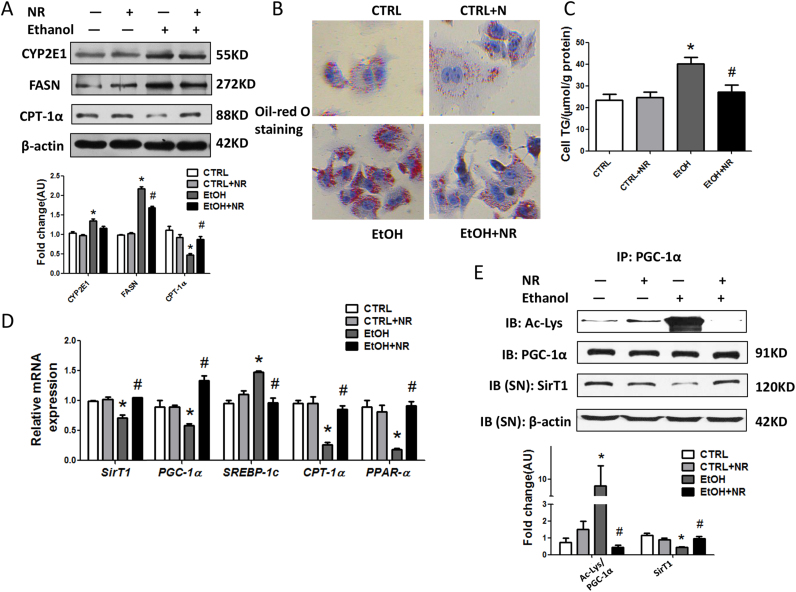
3.3. NR reverses ethanol induced mitochondrial dysfunctions in mice
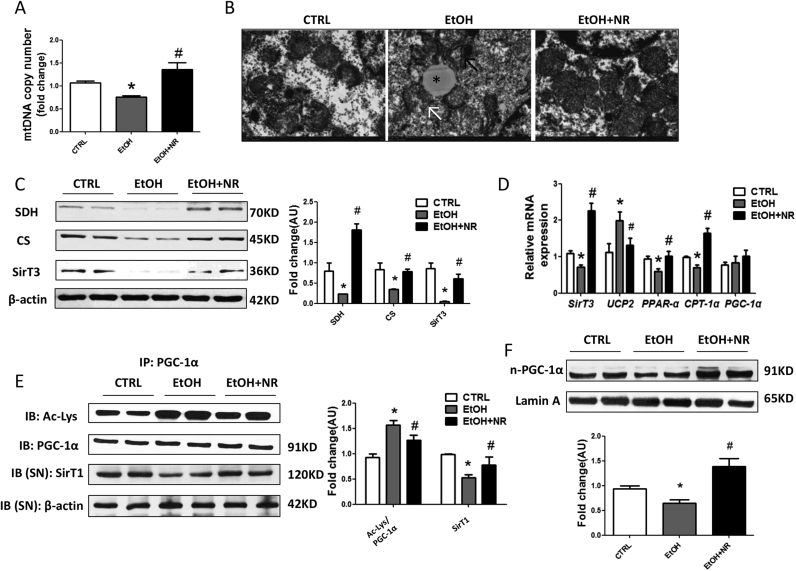
Next, we found the mitochondrial contents in livers of ethanol group were about 70% of those in controls (Fig. 3A). The transmission electronic microcopy study demonstrated the ultrastructural abnormalities of the mitochondria, characterized by apparent disruptions in the cristae and pale and granular matrix (Fig. 3B). Further, significant reductions of both expression and mRNA of SirT3, a deacetylase distributed in mitochondria, succinate dehydrogenase (SDH) and citrate synthase (CS) protein levels, two rate-limiting enzymes in tricarboxylic acid (TCA) cycle, PPAR-α and carnitine palmitoyl transterase-1α (CPT-1α) mRNAs, were all observed in ethanol treated mice (Fig. 3C, D). Consistent with the degradation of ATP levels, a significant higher uncoupling protein-2 (UCP2) mRNA level, an indicator of increased uncoupling of oxidation from ATP synthesis, was found in livers of ethanol fed mice (Fig. 3D). Importantly, NR rescued the abnormalities of mitochondrial function.
Fig. 3.
NR reverses alcohol induced mitochondrial dysfunctions in mice. (A) Mitochondrial abundance in mouse liver. (B) Representative electron micrographs from livers (magnification, 5 000 ×); swollen mitochondria with disrupted membranes (black arrows), pale matrix (white arrow) and lipid droplets (*). (C) Western blot analysis of SDH, CS and SirT3 in mouse liver. Results were expressed as fold changes of control. (D) Relative mRNA levels of SirT3, UCP2, PPAR-α, CPT-1α and PGC-1α by quantitative real-time PCR. (E) Acetylated lysine and total PGC-1α expression in mouse liver by immunoprecipitation and western blot analysis. Results were expressed as fold changes of control. (F) Western blot analysis of nuclear PGC- 1α in mouse liver. Results were expressed as fold changes of control. Data are expressed as mean±SEM. n = 6–8/group, *P < 0.05 compared with the CTRL group; #P < 0.05 compared with the EtOH group. Abbreviations: NR, nicotinamide riboside; SDH, succinate dehydrogenase; CS, citrate synthase; SirT3, sirtuin3; UCP2, uncoupling protein-2; PPAR-α, peroxisome proliferator-activated receptor α; CPT-1α, carnitine palmitoyl transterase-1α; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; SirT1, sirtuin 1; IP, immunoprecipitation.
PGC-1α is one target of SirT1. There were no changes in mRNA and total protein levels of PGC-1α after ethanol or NR treatment (Fig. 3D, E). Interestingly, we found nuclear localization of PGC-1α was down-regulated by ethanol and up-regulated by NR (Fig. 3F) which provided another evidence of the replenishment of mitochondrial fitness and metabolic flexibility by NR. The acetylation of PGC-1α was significantly increased by alcohol consumption, indicated lowered hepatic SirT1 deacetylase activity, which was up-regulated by NR (Fig. 3E).
3.4. NR alleviates ethanol induced oxidative stress and lipid accumulation in HepG2 cells
Ethanol induced severe oxidative stress via inducing CYP2E1 overexpression in HepG2 cells, shown by the reduction of GSH and increase of ROS in whole cell lysates and superoxide in mitochondria, while NR attenuated oxidative stress, without decreasing CYP2E1 (Fig. 4A, S3A–C). Ethanol induced lipid accumulation in HepG2 cells, as evidenced by elevated lipid droplets in Oil-red O staining, BODIPY staining, cellular TG levels, expression of fatty acid synthase (FASN) and mRNA of SREBP-1c, and reduced mRNAs of CPT-1α and PPAR-α. NR significantly alleviated lipid accumulation in HepG2 cells (Fig. 4A–D, S4A). We also found that SirT1 protein expression, mRNA levels and deacetylase activity were significantly decreased upon ethanol incubation, while NR revered these changes (Fig. 4D, E).
Fig. 4.
NR supplementation alleviates ethanol induced lipid accumulation in HepG2 cells. (A) Western blot analysis of CYP2E1, FASN and CPT-1α in cells. Results were expressed as fold changes of control. (B) Representative images of HepG2 cells, oil red O staining with 200 × magnification. (C) Cellular triglyceride levels. (D) Relative mRNA level of SirT1, PGC-1α, SREBP-1c, CPT-1α and PPAR-α by quantitative real-time PCR. (E) Acetylated lysine and total PGC-1α expression in cells by immunoprecipitation and western blot analysis. Results were expressed as fold changes of control. Data are expressed as mean±SEM from three independent experiments. *P < 0.05 compared with the CTRL group; #P < 0.05 compared with the EtOH group. Abbreviations: NR, nicotinamide riboside; CYP2E1, cytochrome P450 2E1; FASN, fatty acid synthase; CPT-1α, carnitine palmitoyl transterase-1α; SREBP- 1c, sterol regulatory element binding protein-lc; PPAR-α, peroxisome proliferator- activated receptor α; SirT1, sirtuin 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; IP, immunoprecipitation.
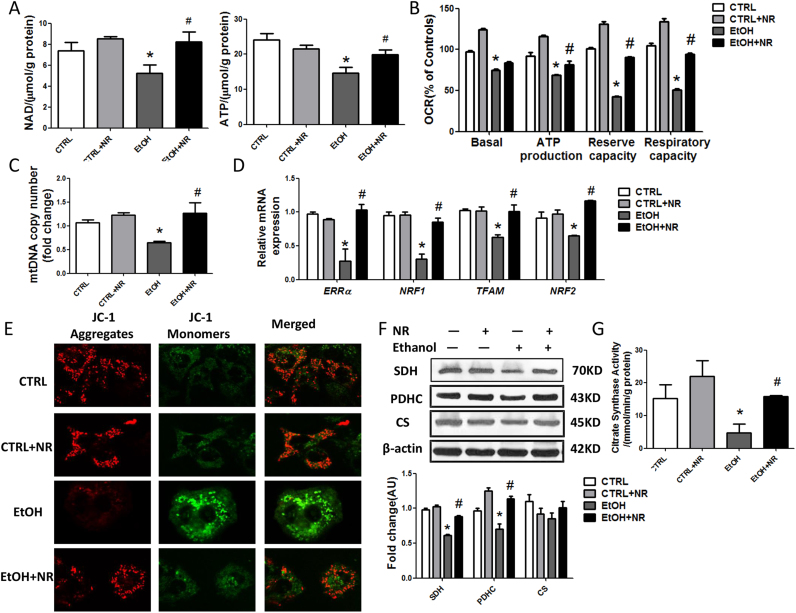
3.5. NR alleviates ethanol induced mitochondrial dysfunctions in HepG2 cells
Ethanol decreased the levels of NAD+ and ATP in HepG2 cells, which were upregulated by NR (Fig. 5A). The oxygen consumption rate (OCR) in HepG2 cells was abolished by ethanol. The basal OCR and reserve oxidative capacity, which is the FCCP-sensitive OCR, and respiratory capacity, which is the rotenone sensitive OCR, were all decreased by ethanol. These changes were reversed by NR, indicating that NR improved mitochondrial aerobic respiration (Fig. 5B, S5A). Next, we detected a reduction in mtDNA abundance by ethanol and NR increased the mtDNA copies (Fig. 5C). Consistently, the estrogen-related receptor α (ERRα), which mediates many of the downstream effects of PGC-1α on mitochondrial biosynthesis and energy metabolism, was markedly decreased by ethanol, as was the ERRα/PGC-1α target, nuclear respiratory factor-1 (NRF1) and its target, mitochondrial transcription factor A (TFAM), and nuclear respiratory factor-2 (NRF2). NR increased the expressions of ERRα, NRF1, NRF2 and TFAM, promoting mitochondrial biogenesis (Fig. 5D).
Fig. 5.
NR alleviates ethanol induced mitochondrial dysfunctions in HepG2 cells. (A) Cellular NAD+ and ATP level. (B) Oxygen consumption rates (OCRs). Results were expressed as fold changes of control. (C) Mitochondrial abundance in cells. (D) Relative mRNA levels of ERRα, NRF1, NRF2 and TFAM by quantitative real-time PCR. (E) Representative images of JC-1 staining with 1000 × magnification of HepG2 cells. Green fluorescence: JC-1 monomers; Red fluorescence: JC-1 aggregates. (F) Western blot analysis of SDH, PDHC and CS in cells. Results were expressed as fold changes of control. (G) Citrate synthase activity was assayed. Data are expressed as mean±SEM from three independent experiments. *P < 0.05 compared with the Scramble siRNA group. Abbreviations: NR, nicotinamide riboside; NAD+, nicotinamide adenine dinucleotide; ATP, adenosine triphosphate; ERRα: estrogen- related receptor α; NRF1: nuclear respiratory factor-1; TFAM: mitochondrial transcription factor A; NRF2: nuclear respiratory factor-2; SDH, succinate dehydrogenase; CS, citrate synthase; PDHC, pyruvate dehydrogenase complex.
Further, we investigated changes in mitochondrial membrane potential of HepG2 cells. Using the membrane-permeant JC-1 dye, we observed fluorescence shift from red to green as mitochondria got depolarized in ethanol-treated cells compared to controls. NR resulted in an increase in the red/green fluorescence intensity ratio (Fig. 5E). Furthermore, ethanol induced a significant increase in the release of cytochrome c levels into the cytoplasm, confirmed damages occurred in mitochondria (Fig. S5B). Besides, ethanol caused disruptions in TCA cycle, as the expressions of SDH and pyruvate dehydrogenase complex (PDHC), and CS activity was reduced, though no obvious difference was found in CS protein (Fig. 5F, G). NR supplementation decreased the release of cytochrome c and enhanced the function of TCA cycle.
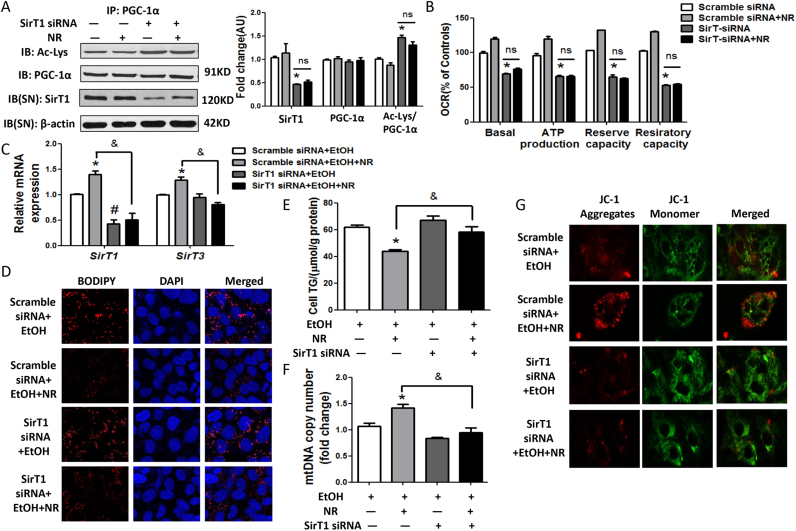
3.6. Improvement of mitochondrial functions by NR requires SirT1-PGC-1α axis
To determine the role of SirT1, in the protection of NR on liver functions, HepG2 cells were transfected with SirT1-siRNA. This strategy effectively reduced endogenous SirT1 level and its deacetylase activity, without affecting the expressions of SirT3, PGC-1α and PPAR-α, except that CPT-1α was blocked (Fig. 6A, S6A). Knocking-down of SirT1 caused the mitochondrial dysfunctions, as indicated by the decreased OCRs, mtDNA copies, expressions of mitochondrial biosynthesis regulators and membrane markers voltage-dependent anion-selective channel (VDAC), prohibitin 1 and depolarization of the mitochondria, which couldn’t be corrected by NR (Figs. S6B-F,6B). Superoxide dismutase 1 (SOD1) expression was stimulated by the depletion of SirT1 (Fig. S6F).
Fig. 6.
Improvement of mitochondrial functions by NR requires SirT1-PGC-1α axis. (A) Acetylated lysine and total PGC-1α expression in cells by immunoprecipitation and western blot analysis. Results were expressed as fold changes of control. (B) Oxygen consumption rates (OCRs). Results were expressed as fold changes of control. *P < 0.05 compared with the Scramble siRNA group. (C) Relative mRNA levels of SirT1 and SirT3 by quantitative real-time PCR. (D) Representative images of HepG2 cells stained with BODIPY (red fluorescence) with 1000 × magnification. Nuclear localization was stained with DAPI (blue fluorescence). (E) Cellular triglyceride levels. (F) Mitochondrial abundance in cells. (G) Representative images of JC-1 staining with 1000 × magnification of HepG2 cells. Green fluorescence: JC-1 monomers; Red fluorescence: JC-1 aggregates. Data are expressed as mean±SEM from three independent experiments. *P < 0.05 and #P < 0.05 compared with the Scramble siRNA+EtOH group; &P < 0.05 compared with the Scramble siRNA+EtOH+NR group. Abbreviations: NR, nicotinamide riboside; SirT1, sirtuin 1; SirT3, sirtuin 3; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; IP, immunoprecipitation.
Next, HepG2 cells transfected with SirT1 siRNA or scramble siRNA were further treated with ethanol, with or without NR. The levels of SirT1 mRNA were much lower in ethanol treated SirT1 siRNA-transfected cells. SirT3 couldn’t be activated by NR in the presence of SirT1 siRNA and ethanol (Fig. 6C). The effects of NR on reducing the lipid accumulation and oxidative stress induced by ethanol were greatly weakened in the presence of SirT1 siRNA (Fig. 6D, E, S7A-B). Knockdown of SirT1 completely blocked the ability of NR to rescue mtDNA copies (Fig. 6F). Further, we found that mitochondria got depolarized in SirT1 siRNA treated cells and NR lost the ability to return to normal (Fig. 6G). Together, these data indicate that SirT1 plays an important role in the effects of NR on regulating lipid accumulation,oxidative stress and mitochondrial dysfunctions induced by ethanol.
Finally, mice were fed with ethanol diet added with 200 mg/kg NAM to serve as a SirT1 inhibitor. We found that SirT1 was significantly decreased in NAM- fed mice (Fig. S8A). NAM indeed exacerbated lipid accumulation in the hepatocytes and also exaggerated levels of serum ALT, AST, and hepatic MDA induced by ethanol (Fig. S8B-D). Although NAM reversed the reduced NAD+ levels by ethanol, the level of ATP was much lower in the NAM group (Fig. S8E). This data confirmed in vivo that inhibition of SirT1 is one key step for the formation of ALD.
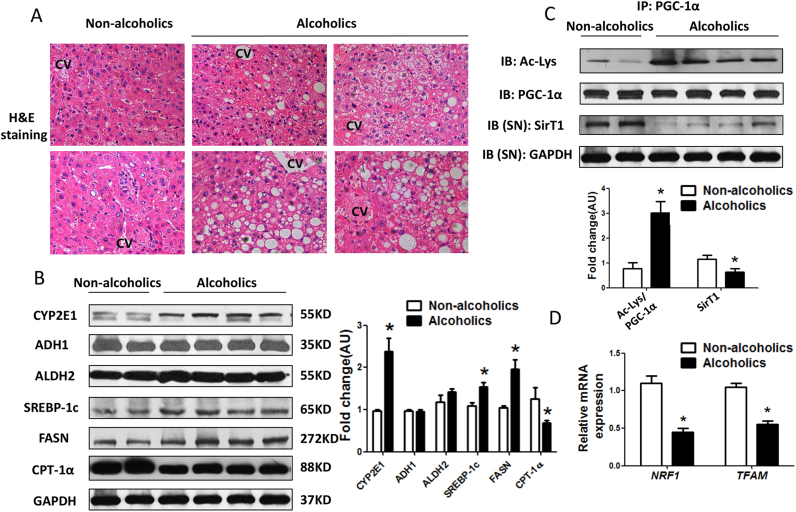
3.7. Decreased SirT1/PGC-1α may contribute to the development of human ALD
We investigated the clinical relevance of SirT1-PGC-1α axis regulated mitochondrial biogenesis with ALD in human liver samples. Firstly, lipid accumulated in alcoholics, while the histology of livers was normal in non-alcoholics (Fig. 7A). CYP2E1 was induced by chronic alcohol consumption, while ADH1 and ALDH2 were comparable in two groups (Fig. 7B). The expression of SREBP-1c, and FASN were elevated, while CPT-1α, as well as SirT1 protein and its deacetylase activity were severely decreased in the liver of alcoholics (Fig. 7B, C). As a result, hepatic NRF1 and TFAM mRNA levels were indeed reduced in alcoholics compared to non- alcoholics (Fig. 7D). Collectively, these data confirmed our data in mice model and HepG2 cells that the impairments of the SirT1/PGC-1α/mitochondrial biogenesis contributed to the development of human ALD.
Fig. 7.
Decreased SirT1/PGC-1α may contribute to the development of human ALD. Human livers were collected from patients with or without long term alcohol drinking. (A) Representative images of human liver, H&E staining with 200 × magnification. (B) Western blot analysis of alcohol metabolic enzymes (CYP2E1, ADH1 and ALDH2), lipid metabolic regulators (SREBP-1c, FASN and CPT-1α) in liver. Results were expressed as fold changes of control. (C) Acetylated lysine and total PGC-1αexpression in cells by immunoprecipitation and western blot analysis. Results were expressed as fold changes of control. (D) Relative mRNA levels of NRF-1 and TFAM by quantitative real-time PCR. Data are expressed as mean±SEM from three independent experiments. *P < 0.05 compared with the non-alcoholics group. Abbreviations: CYP2E1, cytochrome P450 2E1; ADH1, alcohol dehydrogenase 1; ALDH2, aldehyde dehydrogenase 2; SREBP-1c, sterol regulatory element binding protein-lc; FASN, fatty acid synthase; CPT-1α, carnitine palmitoyl transterase-1α; SirT1, sirtuin 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α.
4. Discussion
In this study, we reported that NR can alleviate ethanol induced liver injuries. We demonstrated the key role of PGC-1α deacetylation by SirT1 on the protection of NR against ALD. NR replenishes cellular NAD+ pool, then boosts the activities of SirT1 and PGC-1α, reduces oxidative stress, restores mitochondrial biogenesis and aerobic respiration, and finally reverses alcohol induced liver injuries.
Chronic-plus-binge ethanol feeding mimics the drinking pattern of alcoholic hepatitis patients who have had a history of heavy drinking for many years and with recent excessive alcohol consumption [23]. In the literature, nicotinic acid was reported to ameliorate chronic ALD in mice via the salvage pathway [25], but high-dose nicotinic acid produces a painful flushing response that limits its application. Nicotinamide counteracts alcohol-induced alteration of hepatic protein metabolism by ameliorating the redox state [26], however, nicotinamide is now considered as a SirT1 inhibitor. NMN also increased NAD+ levels, which could be a useful intervention related to metabolic disorders [27], but it isn’t found in dietary constituents. Recently, NR has been identified as an NAD+ precursor, existing in foods, without side effects of flushing [17]. Boosting NAD+ concentrations and NAD+ dependent enzymes can be therapeutic in certain metabolic disorders, such as obesity, NAFLD and T2D, even hepatocellular carcinoma [28]. In our study, NR increased the levels of hepatic NAD+, probably due to reversing the decreased expressions of NRK1 and NMNAT by ethanol, both of which are key enzymes in NAD+ biosynthesis through NR salvage pathway, then stimulated the activity of SirT1, alleviating alcoholic liver disease.
Upon continued exposure to ethanol, CYP2E1 was induced and oxidative stress occurred. Activated SirT1 has a regulatory effect on oxidative stress in many tissues. SirT1 overexpression can prevent oxidative stress-induced apoptosis through p53 deacetylation in mesangial cells [29]. Mn-SOD induced by resveratrol via nuclear SirT1 reduced oxidative stress and participated in cardiomyocyte protection [30]. Resveratrol activates the SirT1/AMPK and NRF2/antioxidant defense pathway in a rat periodontitis model [31]. Activation of SirT1 with NR could deacetylate and activate PGC-1α transcriptional activity, then lead to higher expression of target antioxidant genes, subsequently protect against oxidative stress in skeletal muscle and BAT in obesity model [20]. Similarly, we found that NR decreased hepatic MDA and 4-HNE in ethanol fed mice and decreased ROS levels in HepG2 cells. In addition, NR supplementation weakened ethanol induced oxidative stress in a SirT1-dependent way in HepG2 cells.
SirT1 plays a critical role in the regulation of hepatic lipogenesis, fatty acid oxidation via modification of the acetylation status of a wide range of transcriptional regulators such as p53, PPARα, and PGC-1α 32, 33, 34, 35. Growing evidence from human and rodent studies showed that ALD, NAFLD and obesity are closely associated with impairment of hepatic SirT1 signaling 9, 20, 21, as in our present findings. Overexpression of SirT1 in vivo and in vitro decreased TG levels and activation of hepatic SirT1 by resveratrol alleviates alcoholic liver steatosis 9, 10, 36. AMPK activation and reduced SREBP-1c activity were also involved in the function of SirT1 on lipid regulation 10, 37. In our study, we found that NR enhanced hepatic SirT1 expression and its deacetylase activity, increasing PGC-1α activity and decreasing SREBP-1c activity. Alleviating alcoholic liver disease by NR is SirT1 dependent.
PGC-1α is a key regulator of mitochondrial biogenesis. In alcoholic injury models, the function of PGC-1α is not conclusive. In some studies, alcohol significantly reduces hepatic PGC-1α mRNA and expression in chronic or acute ethanol treated rat models 8, 38. Whereas, in other models, ethanol induced the upregulation of hepatic PGC-1α and TFAM mediated mitochondrial biogenesis as an adaptive response to enhance alcohol metabolism in the liver 39, 40. SirT1 can deacetylate PGC-1α and promote mitochondrial biogenesis 14, 41. Our results indicated that NR could retain the deacetylation of PGC-1α and increase activation of PGC-1α, thus modulating mitochondrial biosynthesis and oxidative metabolism, preventing ethanol induced liver injuries.
In conclusion, NR prevents alcohol induced liver injuries. Activation of SirT1 plays an important role in the protective effect of NR on livers. NR serves as an NAD+ precursor, regulates intracellular NAD homeostasis, activates SirT1-PGC-1α axle, refurbishes mitochondrial function and regulates lipid metabolism, reversing liver injuries induced by alcohol. NR is a promising food supplement to prevent the adverse effects induced by alcohol consumption.
Declarations of interest
None.
Author contributions
S.W. and L.Y. contributed to the conception and design of the study and final approval of the version to be submitted. X.L. and Z.Z. participated in the drafting the article and revising it critically for the human subjects experiments. W.L. provided technical and material support. T.W and Q.Y. performed animal studies and implemented data. M.Y. and N.P. contributed to the acquisition of LC-MS data. L.P. and Y. H. performed cell studies and implemented data. M.Y. and R.J. contributed to the clinical study and data analysis. B.L. contributed to the revision the grammar.
Financial support
This work was supported by the National Natural Science Foundation of China (81370528 and 81573142, to L.Y. in study design and the decision to submit the article for publication; 81461168028 and 81672276, to Z.Z. in the analysis of data), Natural Science Foundation of Guangdong Province of China (2015A030313078, to L.Y. in the collection of data), Key Projects of Sun Yat-sen University of China (15kjc09d, to L.Y. in the interpretation of data), Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (2016TQ03R517 to L.Y. in the interpretation of data).
Patient consent
Written consent form was provided to all participants prior to participant.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.04.006.
Contributor Information
Xiaojun Lin, Email: linxj@sysucc.org.cn.
Zhenfeng Zhang, Email: zhangzhf@gzhmu.edu.cn.
Lili Yang, Email: yangll7@mail.sysu.edu.cn.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Caro A.A., Cederbaum A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 2.Comporti M., Signorini C., Leoncini S. Ethanol-induced oxidative stress: basic knowledge. Genes Nutr. 2010;5:101–109. doi: 10.1007/s12263-009-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto C., Menzies K.J., Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshino J., Mills K.F., Yoon M.J. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes A.P., Price N.L., Ling A.J. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai P., Canto C., Oudart H. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar-Arnal L., Katada S., Orozco-Solis R. NAD(+)-SIRT1 control of H3K4 trimethylation through circadian deacetylation of MLL1. Nat. Struct. Mol. Biol. 2015;22:312–318. doi: 10.1038/nsmb.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieber C.S., Leo M.A., Wang X. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem. Biophys. Res. Commun. 2008;370:44–48. doi: 10.1016/j.bbrc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Yin H., Hu M., Liang X. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 2014;146:801–811. doi: 10.1053/j.gastro.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajmo J.M., Liang X., Rogers C.Q. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson K.J., Humphries J.R., Niemeyer D.J. The effect of alcohol on Sirt1 expression and function in animal and human models of hepatocellular carcinoma (HCC) Adv. Exp. Med. Biol. 2015;815:361–373. doi: 10.1007/978-3-319-09614-8_21. [DOI] [PubMed] [Google Scholar]
- 12.Canto C., Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nisoli E., Tonello C., Cardile A. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers J.T., Lerin C., Haas W. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 15.Jager S., Handschin C., St-Pierre J. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagouge M., Argmann C., Gerhart-Hines Z. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforov A., Dolle C., Niere M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trammell S.A., Schmidt M.S., Weidemann B.J. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canto C., Houtkooper R.H., Pirinen E. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariani K., Menzies K.J., Ryu D. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Ryu D., Wu Y. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 23.Bertola A., Mathews S., Ki S.H. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conze D.B., Crespo-Barreto J., Kruger C.L. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum. Exp. Toxicol. 2016;35:1149–1160. doi: 10.1177/0960327115626254. [DOI] [PubMed] [Google Scholar]
- 25.Dou X., Shen C., Wang Z. Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice. J. Nutr. Biochem. 2013;24:1520–1528. doi: 10.1016/j.jnutbio.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpi E., Lucidi P., Cruciani G. Nicotinamide counteracts alcohol-induced impairment of hepatic protein metabolism in humans. J. Nutr. 1997;127:2199–2204. doi: 10.1093/jn/127.11.2199. [DOI] [PubMed] [Google Scholar]
- 27.Uddin G.M., Youngson N.A., Sinclair D.A. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front Pharmacol. 2016;7:258. doi: 10.3389/fphar.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tummala K.S., Gomes A.L., Yilmaz M. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Kume S., Haneda M., Kanasaki K. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic. Biol. Med. 2006;40:2175–2182. doi: 10.1016/j.freeradbiomed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Tanno M., Kuno A., Yano T. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaki N., Cristina O.R., Inagaki Y. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014;75:222–229. doi: 10.1016/j.freeradbiomed.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Ponugoti B., Kim D.H., Xiao Z. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers J.T., Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You M., Jogasuria A., Taylor C. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015;4:88–100. doi: 10.3978/j.issn.2304-3881.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derdak Z., Villegas K.A., Harb R. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J. Hepatol. 2013;58:785–791. doi: 10.1016/j.jhep.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez T., Li Y.M., Yin S. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017;66:601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Z., Liang X., Rogers C.Q. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaung W.W., Jacob A., Ji Y. Suppression of PGC-1alpha by ethanol: implications of Its Role in Alcohol Induced Liver Injury. Int. J. Clin. Exp. Med. 2008;1:161–170. [PMC free article] [PubMed] [Google Scholar]
- 39.Caro A.A., Bell M., Ejiofor S. N-acetylcysteine inhibits the up-regulation of mitochondrial biogenesis genes in livers from rats fed ethanol chronically. Alcohol Clin. Exp. Res. 2014;38:2896–2906. doi: 10.1111/acer.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han D., Ybanez M.D., Johnson H.S. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: biogenesis, remodeling, and functional alterations. J. Biol. Chem. 2012;287:42165–42179. doi: 10.1074/jbc.M112.377374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerhart-Hines Z., Rodgers J.T., Bare O. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material