Abstract
Background
Molecular characterization of nonmuscle invasive bladder cancer (NMIBC) may provide a biologic rationale for treatment response and novel therapeutic strategies.
Objective
To identify genetic alterations with potential clinical implications in NMIBC.
Design, setting, and participants
Pretreatment index tumors and matched germline DNA from 105 patients with NMIBC on a prospective Institutional Review Board-approved protocol underwent targeted exon sequencing analysis in a Clinical Laboratory Improvement Amendments-certified clinical laboratory.
Outcome measurements and statistical analysis
Comutation patterns and copy number alterations were compared across stage and grade. Associations between genomic alterations and recurrence after intravesical bacillus Calmette-Guérin (BCG) were estimated using Kaplan-Meier and Cox regression analyses.
Results and limitations
TERT promoter mutations (73%) and chromatin-modifying gene alterations (69%) were highly prevalent across grade and stage, suggesting these events occur early in tumorigenesis. ERBB2 or FGFR3 alterations were present in 57% of high-grade NMIBC tumors in a mutually exclusive pattern. DNA damage repair (DDR) gene alterations were seen in 30% (25/82) of high-grade NMIBC tumors, a rate similar to MIBC, and were associated with a higher mutational burden compared with tumors with intact DDR genes (p < 0.001). ARID1A mutations were associated with an increased risk of recurrence after BCG (hazard ratio = 3.14, 95% confidence interval: 1.51–6.51, p = 0.002).
Conclusions
Next-generation sequencing of treatment-naive index NMIBC tumors demonstrated that the majority of NMIBC tumors had at least one potentially actionable alteration that could serve as a target in rationally designed trials of intravesical or systemic therapy. DDR gene alterations were frequent in high-grade NMIBC and were associated with increased mutational load, which may have therapeutic implications for BCG immunotherapy and ongoing trials of systemic checkpoint inhibitors. ARID1A mutations were associated with an increased risk of recurrence after BCG therapy. Whether ARID1A mutations represent a predictive biomarker of BCG response or are prognostic in NMIBC patients warrants further investigation.
Patient summary
Analysis of frequently mutated genes in superficial bladder cancer suggests potential targets for personalized treatment and predictors of treatment response, and also may help develop noninvasive tumor detection tests.
Keywords: AT-Rich interaction domain 1A, Bacillus Calmette-Guérin, DNA damage repair, Genomics, Immunotherapy, Nonmuscle invasive bladder cancer, Targeted therapy
1. Introduction
Of the estimated 429 000 people to be diagnosed with bladder cancer in the industrialized world each year, 70–80% will have nonmuscle invasive bladder cancer (NMIBC) [1,2]. Half of all NMIBC patients will experience tumor recurrence within 5 yr, and 20–30% will progress to secondary MIBC [3]. Ultimately, as many as 10–15% of patients presenting with NMIBC will die of bladder cancer [4].
Previous investigations into NMIBC genetics have been limited by their inability to comprehensively profile tumors for multiple cancer-associated genes [5]. More recently, MIBC were comprehensively investigated by The Cancer Genome Atlas (TCGA) and other groups using next-generation sequencing (NGS), leading to the identification of potential biomarkers and targets for therapeutic intervention [6,7]. However, very few NMIBC tumors have been examined with NGS methods to date, and these investigations have been limited by a lack of clinical annotation, the absence of restaging transurethral resection (TUR) to ensure appropriate tumor staging, or a failure to differentiate between primary and recurrent tumors [8–11].
In this study, we examined primary treatment-naive index tumors from a cohort of patients with NMIBC using a massively parallel, targeted, exon capture-based NGS platform to define the prevalence of genetic alterations and their potential clinical implications.
2. Patients and methods
2.1 Patients and samples
Targeted NGS with a 341 or updated 410 cancer-associated gene panel was performed on formalin-fixed paraffin embedded sections of treatment-naive index tumors along with matched germline DNA for 105 patients with NMIBC as part of an Institutional Review Board-approved protocol (Supplementary Fig. 1, Supplementary Table 1, Supplementary data) [12]. A board-certified genitourinary pathologist reviewed representative hematoxylin and eosin slides to confirm grade, stage, and urothelial histology. All patients underwent evaluation and TUR by a urologic oncologist at Memorial Sloan Kettering Cancer Center (MSK). All tumors profiled were newly diagnosed and untreated. Patients who received perioperative mitomycin or other adjuvant perioperative therapies were excluded. All high-grade T1 (HGT1) tumors had restaging TUR and confirmation of uninvolved detrusor muscle. Treatment and management was at the discretion of the treating urologic oncologist. Patients managed with TUR were followed at MSK with cystoscopy and urine cytology every 3 mo for the 1st yr, then every 3–6 mo. Recurrence was defined as histological proven cancer on biopsy or TUR. See Supplementary data for additional details.
For comparison purposes, we also evaluated the frequency of genomic alterations seen in 40 pretreatment index tumors in patients with primary MIBC who were sequenced with MSK-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) on the same Institutional Review Board-approved protocol and 98 MIBC specimens from patients in the TCGA study who were reported to have no prior history of NMIBC (Supplementary data).
2.2 Statistical analysis
Alterations in oncogenes were deemed significant if they were recurrent or known functional missense mutations or amplifications (Supplementary data). Alterations in tumor suppressor and DNA damage repair genes were deemed significant if truncating mutations (nonsense, frameshift indels), recurrent missense mutations, or homozygous deletions were present (Supplementary data). Fisher’s exact tests were used to analyze categorical associations. Kruskal-Wallis and Wilcoxon tests were used for continuous variables. Cox regression modeling was used to determine the association between genomic alterations and recurrence after bacillus Calmette-Guérin (BCG). The Kaplan-Meier method and log-rank test were used for estimations of recurrence free survival. A p value of <0.05 was considered statistically significant. All analyses were conducted using R v.3.3.1. (https://cran.r-project.org/bin/windows/base/old/3.3.1/)
3. Results
3.1 Patient demographics and treatment
To characterize the genomic landscape of NMIBC, we analyzed 105 tumors across the disease spectrum comprising low-grade Ta (LGTa; n = 23), high-grade Tis (HGTis; n = 12), high-grade Ta (HGTa; n = 32), and HGT1 (n = 38) for alterations in 341 cancer-associated genes. Information on patient demographics and treatments are listed in Table 1, Supplementary Table 2, and Supplementary Table 3. The median follow-up for the NMIBC cohort managed by TUR with or without adjuvant intravesical therapy (n = 100) was 24.4 mo, with recurrences occurring in 46 patients. Treatment following resection for the 23 low-grade tumors included surveillance only (48%), intravesical mitomycin (39%), and intravesical BCG (13%). The 82 high-grade tumors were treated with intravesical BCG (81%), observation (12%), or immediate radical cystectomy in five patients (4 = pT1N0, 1 = pTaN0). Additional details on the NMIBC cohort and the MIBC samples used for comparison purposes are available in the Supplementary data, Supplementary Table 2, and Supplementary Table 3.
Table 1.
Clinicopathologic characteristics of NMIBC cohorts
| Characteristics | Low-grade NMIBC (%), n = 23 |
High-grade NMIBC (%), n = 82 |
High-grade NMIBC treated BCG without maintenance (%), n = 62 |
|
|---|---|---|---|---|
| Median age (range) | 67 (25, 80) | 70 (36, 87) | 69.8 (37.3, 87.3) | |
| Sex | ||||
| Male | 13 (56) | 67 (82) | 51 (82) | |
| Female | 10 (43) | 15 (18) | 11 (18) | |
| Smoking | ||||
| Current | 3 (13) | 13 (16) | 9 (15) | |
| Former | 13 (57) | 46 (56) | 35 (56) | |
| Never | 7 (30) | 23 (28) | 18 (29) | |
| Stage | ||||
| Ta | 23 (100) | 32 (39) | 26 (42) | |
| Tis | 0 | 12 (15) | 12 (19) | |
| T1 | 0 | 38 (46) | 24 (39) | |
| No. of tumors | ||||
| Single | 18 (78) | 45 (55) | 33 (53) | |
| Multiple | 5 (22) | 37 (45) | 29 (46) | |
| Tumor size (cm) | ||||
| <3 | 16 (70) | 46 (56) | 36 (58) | |
| ≥3 | 7 (30) | 36 (44) | 26 (42) | |
| Concomitant CIS | ||||
| No | 23 (100) | 43 (52) | 30 (48) | |
| Yes | 0 (0) | 39 (47) | 32 (52) | |
| Treatment | ||||
| BCG | 3 (13) | 66 (80) | 62 (100) | |
| MMC | 9 (39) | 1 (1) | 0 (0) | |
| Observation | 11 (48) | 10 (12) | 0 (0) | |
| Immediate cystectomy | 0 (0) | 5 (6) | 0 (0) |
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; MMC = mitomycin; MIBC = muscle invasive bladder cancer; NMIBC = nonmuscle invasive bladder cancer; TUR = transurethral resection.
3.2 Genomic landscape of NMIBC
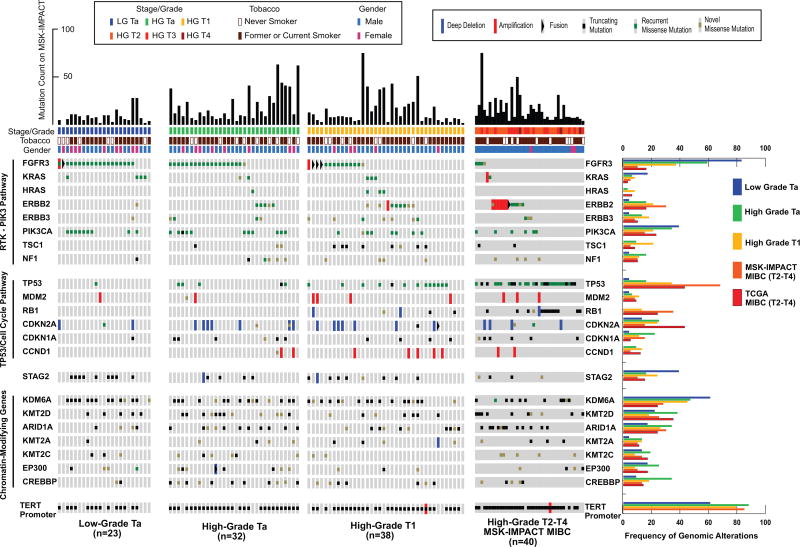
The most frequently altered genes in NMIBC were the TERT promoter (73%), FGFR3 (49%), KDM6A (38%), PIK3CA (26%), STAG2 (23%), ARID1A (21%), and TP53 (21%) (Fig. 1, Supplementary Figs. 2 and 3, Supplementary Table 4). The associations between genetic alterations with tumor stage and grade are presented in Supplementary Tables 5 and 6. TERT promoter mutations were identified in 61% (14/23) of LGTa, 88% (28/32) of HGTa, and 79% (30/38) of HGT1 tumors. The TERT promoter region was not sequenced by TCGA, as the whole-exome sequencing method employed interrogated only protein-coding regions. However, TERT promoter mutations were identified in 85% (34/40) of the MSK-MIBC patients. TERT promoter mutations were present at similar frequency across stage (p = 0.2) and grade (p = 0.15). Alterations in chromatin modifying genes were also highly prevalent in NMIBC, occurring in 69% (72/105) of the tumors (Fig. 1, Supplementary Fig. 4). The most commonly altered chromatin-modifying genes were KDM6A (38%) and ARID1A (21%). KDM6A was altered in 52% (12/23) of LGTa, 38% of HGTa (12/32), 32% (12/38) of HGT1, and 25% (10/40) of MSK-MIBC, and 24% of TCGA-MIBC tumors. ARID1A mutations were identified in 9% (2/23) of LGTa, 28% (9/32) of HGTa, 18% (7/38) of HGT1, 30% (12/40) of MSK-MIBC, and 24% of TCGA-MIBC tumors. While KDM6A alterations were identified more often in LGTa, there were no statistically significant associations with grade (p = 0.15) or stage (p = 0.44). Similarly, ARID1A mutation did not correlate with either grade (p = 0.23) or stage (p = 0.44).
Fig. 1.
An overview of the genomic landscape of NMIBC by grade and stage with comparison to MIBC.
HGTa = high-grade Ta; LGTa = low-grade Ta; MIBC = muscle invasive bladder cancer; MSK-IMPACT = Memorial Sloan Kettering Cancer Center-Integrated Mutation Profiling of Actionable Cancer Targets; NMIBC = nonmuscle invasive bladder cancer; TCGA = The Cancer Genome Atlas.
Another frequently altered gene was STAG2, present in 23% of the NMIBC cohort (LGTa = 39% [9/23]; HGTa = 16% [5/32]; HGT1 = 24% [9/380], 5% of MSK-MIBC [2/40], and 15% of TCGA-MIBC; Fig. 1). Studies are conflicted over whether STAG2 mutations are associated with aneuploidy and aggressive bladder tumors or associated with lower grade and l-stage disease [10,11,13,14]. We found truncating STAG2 mutations in our cohort to be associated with LGTa tumors (p = 0.046).
3.3 Genomic pathways of NMIBC
To better define the prevalence and co-occurrence patterns of potentially actionable genomic alterations in NMIBC, we analyzed known cancer-associated genes within the context of their canonical pathway and cellular function. Alterations in the receptor tyrosine kinase/phosphatidylinositol 3-kinase pathway were present in 79% (83/105) of NMIBC tumors (Fig. 1, Supplementary Fig. 5). Consistent with previous studies, FGFR3 mutations were associated with lower grade and stage (LGTa = 83% [19/23]; HGTa = 59% [19/32]; HGT1 = 34% [13/38]; MSK-MIBC = 8% [3/40]; TCGA-MIBC = 16%) [5]. Four FGFR3 fusions were identified in the NMIBC cohort, including a FGFR3-TNIP2 fusion predicted to be activating due to its in-frame FGFR3 kinase domain and a recent NGS report identifying another bladder tumor with a FGFR3-TNIP2 fusion [15]. We also identified a FGFR3-TACC3 fusion in a LGTa tumor. While FGFR3 fusions have predominantly been reported only in high-grade tumors, there are a few low-grade papillary bladder cell lines known to harbor FGFR3-TACC3 fusions [16,17].
Interestingly, mutations in ERBB2/HER2 were identified in a mutually exclusive pattern with alterations in FGFR3 (Fig. 1, Supplementary Table 7, Supplementary Fig. 6). With the exception of a single LGTa tumor that harbored an ERBB2 missense mutation of unknown significance (R103Q), ERBB2 alterations were only present in high-grade NMIBC tumors. Six of the 12 Tis specimens analyzed had ERBB2 alterations, including hotspot S310F missense mutations in three tumors, amplification in one, and mutations of unknown significance in two tumors (S728F and S305C, respectively). ERBB2 alterations were associated with higher grade (p = 0.01) and stage (p = 0.05) but not after adjusting for multiple genomic comparisons (p = 0.60 and p = 0.20, respectively). While FGFR3 was associated with lower-grade and lower-stage disease, alterations in FGFR3 were seen in 39% (32/82) of high-grade NMIBC. In total, 57% (47/82) of high-grade NMIBC tumors had alterations in either ERBB2 or FGFR3, suggesting that trials testing targeted inhibitors of these kinases should be further explored (Supplementary Fig. 7A).
An alteration in either the TP53 pathway or a gene with a central role in cell cycle regulation was identified in 47% (49/105) of NMIBC tumors, most commonly in high-grade tumors (Fig. 1, Supplementary Fig. 8). An expected stepwise increase in TP53/MDM2 alteration rates were seen with stage (p < 0.001) and grade (p < 0.001) from 9% in LGTa (2/23), 19% in HGTa (6/32), 45% in HGT1 (17/38), and 75% in MSK-MIBC (30/40). Furthermore, alterations in cell cycle regulation genes (RB1, Cyclin D1 [CCND1], CDKN1A [p21], or CDKN2A) were more common in higher stage (p = 0.028) and grade (p = 0.009) disease, increasing from 13% in LGTa (3/23), to 41% in HGTa (13/32), 42% in HGT1 (16/38), and 53% (21/40) in MSK-MIBC (Supplementary Fig. 8).
3.4 DNA damage repair and mutational burden
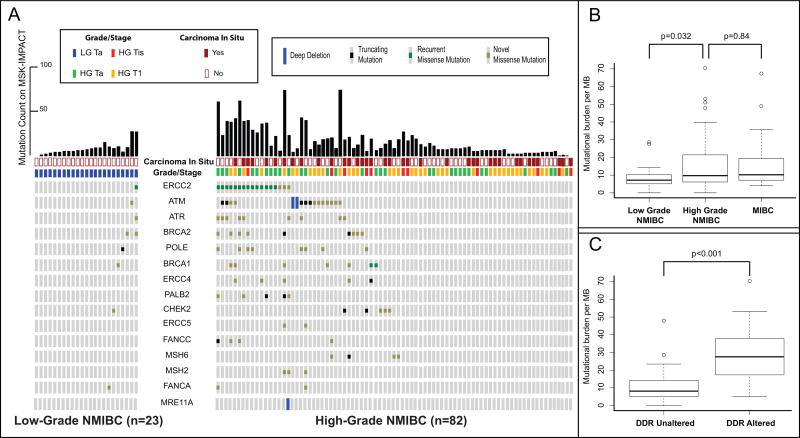
As recent reports suggest that DNA damage repair (DDR) gene alterations are associated with sensitivity to cisplatin, immunotherapy, and radiation therapy in MIBC [18–20], we assessed the prevalence of alterations in these genes in NMIBC. Deleterious DDR gene alterations were identified in 30% (25/82) of high-grade NMIBC tumors, but in only 4% (1/23) of low-grade tumors (p = 0.012; Fig. 2A). This rate of deleterious DDR gene alterations in high-grade NMIBC was similar to the MSK-MIBC cohort (33% [13/40]). ERCC2 missense mutations were the most common DDR gene alteration, occurring in 17% (14/82) of high-grade NMIBC tumors and 20% of MSK-MIBC (8/40). As has been shown in MIBC, the majority of missense mutations in NMIBC were found to cluster around conserved helicase domains (Supplementary Fig. 9). Less frequent DDR gene alterations were also observed in ATM, BRCA1, BRCA2, ERCC4, PALB2, CHECK2, FANCC, and MSH6 (Fig. 2A). One LGTa with five total mutations had a truncating POLE H1901Lfs*15 mutation that is likely not functionally relevant given its location near the 3’ end of the protein and the absence of a hypermutation phenotype. The remaining DDR gene alterations in NMIBC were missense variants of unknown significance.
Fig. 2.
(A) A comparison of DNA damage repair gene alterations found in high and low grade NMIBC. (B) Box and whisker plot comparing mutational burden per megabase between low-grade NMIBC (n = 23), high-grade NMIBC (n = 82), and MIBC (n = 40). (C) Box and whisker plot comparing mutational burden per megabase between high-grade NMIBC tumors with altered and unaltered DNA damage repair genes (n = 82).
DDR = DNA damage repair; MB = megabase; MIBC = muscle invasive bladder cancer; MSK-IMPACT = Memorial Sloan Kettering Cancer Center-Integrated Mutation Profiling of Actionable Cancer Targets; NMIBC = nonmuscle invasive bladder cancer.
Since MIBC has one of the largest mutational burdens of all tumor types studied by TCGA, we used mutational burden per megabase on targeted exon sequencing to infer associations between mutational burden and clinical variables [21]. High-grade NMIBC tumors had a larger median mutational burden per megabase than low-grade NMIBC (9, interquartile rage [IQR]: 6–21 vs 7, IQR: 5–10, p = 0.032) and had a mutational burden similar to MIBC (10, IQR: 7–19, p = 0.84; Fig. 2B). Furthermore, tumors with deleterious DDR gene alterations had a significantly higher mutational burden as compared with tumors with intact DDR genes (26, IQR: 15–36 vs 8, IQR: 5–12, p < 0.001; Fig. 2C, Supplementary Fig. 10). Similarly, ERCC2-mutated tumors had a significantly higher mutational burden than ERCC2 wild-type tumors (29, IQR: 23–37 vs 8, IQR: 6–14, p < 0.001; Supplementary Fig. 11).
3.5 Genomic correlates with recurrence after BCG therapy
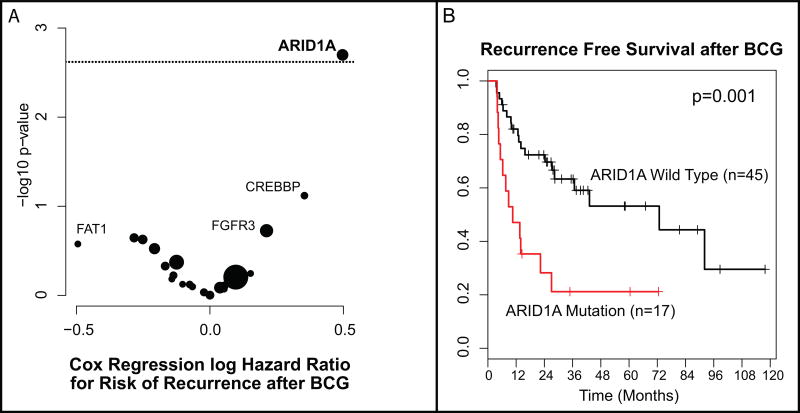
With the objective of identifying genomic alterations associated with recurrence after BCG, we examined 62 patients within the NMIBC cohort who had high-grade disease and were treated with a 6-wk induction course of BCG without maintenance therapy. Tumor recurrence occurred in 52% (32/62) of patients at a median follow-up of 24 mo. When evaluating all genes in the 341-gene panel that were altered in at least five tumors in the 62-patient cohort, only ARID1A mutations were significantly associated with an increased risk of recurrence after BCG treatment (hazard ratio [HR] = 3.14, 95% confidence interval [CI]: 1.51–6.51, p = 0.002; Table 2, Fig. 3). This held true even when correcting for multiple comparisons (p = 0.04) and when ARID1A missense mutations of unknown significance were included (HR = 3.08, 95% CI: 1.49–6.35, p = 0.002; Table 2, Supplementary Fig. 12). ARID1A mutations were also associated with tumor recurrence within the larger cohort of 100 patients managed by TUR with or without adjuvant intravesical therapy (HR = 2.07, 95% CI: 1.10–3.88, p = 0.024). No significant associations were identified between the examined clinical variables and recurrence free survival in either the 62 patient BCG cohort or the larger 100 patient cohort (Supplementary Table 8).
Table 2.
Cox regression between genomic alterations and recurrence after BCG therapy (n = 62, 32 events)
| Gene | HR | 95% CI | p value | False discovery rate |
|---|---|---|---|---|
| ARID1A | 3.14 | (1.51, 6.51) | 0.002 | 0.04 |
| FGFR3 | 1.63 | (0.79, 3.37) | 0.188 | 0.9 |
| KRAS | 0.79 | (0.18, 3.38) | 0.75 | 0.94 |
| ERBB2 | 0.56 | (0.21, 1.46) | 0.236 | 0.9 |
| PIK3 | 0.62 | (0.26, 1.52) | 0.299 | 0.9 |
| TSC1 | 0.84 | (0.29, 2.43) | 0.755 | 0.94 |
| TP53 | 1.09 | (0.51, 2.32) | 0.819 | 0.94 |
| CDKN2A | 0.68 | (0.24, 1.94) | 0.469 | 0.94 |
| CDKN1A | 0.95 | (0.33, 2.73) | 0.924 | 0.97 |
| CCND1 | 0.72 | (0.17, 3.03) | 0.656 | 0.94 |
| STAG2 | 1.13 | (0.46, 2.78) | 0.785 | 0.94 |
| KDM6A | 0.75 | (0.36, 1.53) | 0.424 | 0.94 |
| EP300 | 1.13 | (0.34, 3.71) | 0.847 | 0.94 |
| CREBBP | 2.26 | (0.92, 5.55) | 0.076 | 0.8 |
| TERT | 1.25 | (0.51, 3.11) | 0.624 | 0.94 |
| ERCC2 | 0.52 | (0.18, 1.49) | 0.226 | 0.9 |
| KMT2D | 1 | (0.38, 2.62) | 0.994 | 0.99 |
| RBM10 | 1.42 | (0.43, 4.68) | 0.568 | 0.94 |
| FAT1 | 0.32 | (0.04, 2.37) | 0.265 | 0.9 |
| FBXW7 | 0.73 | (0.22, 2.39) | 0.597 | 0.94 |
| MGA | 0.86 | (0.26, 2.84) | 0.799 | 0.94 |
BCG = bacillus Calmette-Guérin; CI = confidence interval; HR = hazard ratio.
Fig. 3.
(A) Volcano plot of effect size (log odds ratio) by significance (−log10 p value) for the association between specific genomic alterations and recurrence after bacillus Calmette-Guérin (BCG). Results are from Cox regression analysis (n = 62). The horizontal dotted line indicates an adjusted p-value <0.05. Bubble size is proportional to the total number of alterations. (B) Kaplan-Meier curve for recurrence after BCG Therapy for tumors with ARID1A truncating mutations compared to ARID1A wild-type (n = 62).
As reports associating TP53 and treatment outcomes in NMIBC are conflicting [22], we examined recurrence free survival in TP53-altered tumors alone and in combination with MDM2 and found no association with recurrence after BCG (p = 0.94 and p = 0.36, respectively; Supplementary Fig. 13). Furthermore, tumors with alterations in ERBB2 and FGFR3 had similar recurrence rates after BCG as wild type tumors (p = 0.3), further supporting adjuvant trials of targeted inhibitors of these kinases (Supplementary Fig. 7B).
Since mutational count is associated with response to systemic checkpoint inhibitor immunotherapy, we also examined the association between mutational count in NMIBC with recurrence after BCG immunotherapy [18]. We were unable to identify an association between mutational count on MSK-IMPACT and tumor recurrence after BCG (HR = 0.96, 95% CI: 0.83–1.1, p = 0.3).
4. Discussion
Most sequencing efforts in bladder cancer have focused on MIBC. Yet the majority of bladder cancer patients are diagnosed with NMIBC, the treatment of which imposes substantial burden to patients and the global health care system [3]. To our knowledge, our study is the largest NGS effort to focus on NMIBC to date. Our objective was to identify genetic alterations with potential clinical implications in addressing the many unmet needs of NMIBC patients.
One major unmet need is reliable screening and surveillance biomarkers to replace invasive cystoscopies. The high prevalence of TERT promoter mutations and chromatin-modifying gene alterations seen in every stage and grade in our study not only suggests that these events likely occur early in urothelial carcinogenesis, but it also supports the development of noninvasive methods to detect these alterations in urine as a potential screening and/or surveillance biomarker [23,24].
Another issue in NMIBC has been the lack of progress in treatment outcomes and therapeutic options. For over 40 yr, BCG has been the most effective intravesical therapy for high-risk disease, yet pretreatment biomarkers that reliably predict which patients will benefit from BCG treatment are still needed [3]. We found that patients whose tumors harbored an ARID1A mutation had significantly worse recurrence-free survival after an induction course of BCG. ARID1A mutations are associated with a poor prognosis in several cancers and have been shown to be associated with high-grade bladder cancer [25,26], but to our knowledge no prior study has examined the association between ARID1A mutations and BCG outcomes. Further work is needed to clarify whether ARID1A mutations are a predictive biomarker for BCG therapy or whether they identify a patient cohort with an overall worse prognosis. However, if such an association is confirmed in an independent cohort, not only could ARID1A mutations serve as a potential biomarker for BCG response, but drugs that can reverse the epigenetic consequences of ARID1A inactivation, such as inhibitors of the EZH2 methyltransferase, may have therapeutic benefit for such patients [27].
Additionally, our study confirmed the presence of multiple other potentially actionable targets in NMIBC. FGFR3 inhibitors are currently being investigated in patients with persistent or recurrent NMIBC following BCG treatment, but the utility of these targeted agents has been limited in part by the systemic toxicity of currently available agents. With the development of highly selective and less toxic kinase inhibitors, it would be reasonable to test such agents as alternatives or adjuncts to BCG in patients whose tumors harbor an FGFR3 or ERBB2 alteration. Advances in intravesical delivery systems may also allow targeted agents to be administered with limited systemic toxicity in the future.
Another important finding from our investigation was the high frequency of DDR gene alterations in high-grade NMIBC, with ERCC2 missense mutations being the most common. For several decades, a two-pathway model of low-grade papillary and high-grade invasive bladder cancer has been postulated, yet there is marked heterogenicity in molecular profiles and clinical outcomes [5]. We found DDR gene alterations and ERCC2 mutations in particular to be associated with a larger mutational burden that might underlie the so-called genomically unstable pathway in bladder cancer development [5]. Impaired DNA repair may allow the permissive accumulation of multiple molecular alterations resulting in growth advantage and invasive capabilities [28]. Additionally, the high mutational burden in NMIBC might have important implications for the use of systemic checkpoint inhibitor immunotherapies. These agents have recently demonstrated significant activity in MIBC and are now being tested in NMIBC patients [18]. Several studies have found an association between higher mutational burden and predicted neoantigen burden with antiprogrammed cell death-1/programmed death-ligand 1 response in patients with metastatic solid tumors, including bladder cancer [18]. The role that mutational burden plays in dictating BCG response also warrants further investigation. While we were unable to find a statistically significant association on MSK-IMPACT between mutational burden and BCG response, this relationship will need to be further explored in a larger cohort and with whole exome sequencing. The exact mechanism of action for BCG immunotherapy has remained elusive, so leveraging the knowledge recently gained from correlative studies of systemic checkpoint inhibitors may prove fruitful [29].
There are important limitations to this study. While this is the largest cohort of NMIBC tumors analyzed to date using NGS methods, when parsed out by grade and stage the absolute numbers in each subgroup were relatively small and longer follow-up will be needed. To facilitate future larger-scale analyses, we have made all genomic and clinical data from this study publically available through the cBioPortal for Cancer Genomics [30]. Furthermore, intertumor and intratumor heterogenicity may impact our results. We also found tissue-based sequencing of carcinoma in situ specimens to be particularly challenging, with nearly two-thirds of samples demonstrating inadequate tumor purity for analysis. To overcome these limitations, alternative approaches are currently being explored, including sequencing of cytology specimens, urinary cell-free DNA, and urinary exosomes. Future advances and refinements in single-cell sequencing may also overcome current limitations.
5. Conclusions
NGS of treatment-naive index tumors from patients with NIMBC identified that the majority of tumors had at least one potentially actionable alteration that could serve as a drug target in clinical trials of novel intravesical or systemic therapy. High rates of DDR gene alterations were identified in high-grade NMIBC tumors, which might have implications for BCG immunotherapy and systemic checkpoint inhibition in NMIBC patients. ARID1A mutations may be associated with earlier recurrence after BCG and may be a potential therapeutic target.
Supplementary Material
High-grade nonmuscle invasive bladder cancer has high rates of DNA damage repair gene alterations, mutational burden, and actionable alterations, making trials of novel targeted agents and immunotherapies warranted. ARID1A mutations are associated with recurrence after bacillus Calmette-Guérin therapy and may be a predictive biomarker and a potential therapeutic target.
Acknowledgments
Funding/Support and role of the sponsor: Supported by The Sidney Kimmel Center for Prostate and Urologic Cancers, the Michael and Zena Wiener for Therapeutics Program in Bladder Cancer, Pin Down Bladder Cancer, the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, and the National Cancer Institute Cancer Center Core Grant Number P30-CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Bernard H. Bochner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pietzak, Bagrodia, Cha, Solit, Bochne.
Acquisition of data: Pietzak, Bagrodia, Cha, Li, Baez, Rosenberg, Bajorin, Dalbagni, Al-Ahmadie, Bochner.
Analysis and interpretation of data: Pietzak, Cha, Iyer, Isharwal, Berger, Zehir, Schultz, Rosenberg, Bajorin, Dalbagni, Al-Ahmadie, Solit, Bochner.
Drafting of the manuscript: Pietzak, Iyer, Solit, Bochner.
Critical revision of the manuscript for important intellectual content: Pietzak, Bagrodia, Drill, Iyer, Isharwal, Berger, Zehir, Schultz, Dalbagni, Al-Ahmadie, Solit, Bochner.
Statistical analysis: Drill, Ostrovnaya.
Obtaining funding: Bochner, Solit.
Administrative, technical, or material support: Baez.
Supervision: Ostrovnaya, Solit, Bochner.
Other: None.
Financial disclosures: Bernard H. Bochner certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Schned AR, Andrew AS, Marsit CJ, Kelsey KT, Zens MS, Karagas MR, et al. Histological classification and stage of newly diagnosed bladder cancer in a population-based study from the Northeastern United States. Scand J Urol Nephrol. 2008;42:237–42. doi: 10.1080/00365590801948166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219–27. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60:493–500. doi: 10.1016/j.eururo.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim PH, Cha EK, Sfakianos JP, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur Urol. 2015;67:198–201. doi: 10.1016/j.eururo.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazier JB, Rao SR, McLean CM, et al. Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Nat Commun. 2014;5:3756. doi: 10.1038/ncomms4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordentoft I, Lamy P, Birkenkamp-Demtröder K, et al. Mutational context and diverse clonal development in early and late bladder cancer. Cell Rep. 2014;7:1649–63. doi: 10.1016/j.celrep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Balbas-Martinez C, Sagrera A, Carrillo-de-Santa-Pau E, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nat Genet. 2013;45:1464–9. doi: 10.1038/ng.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo G, Sun X, Chen C, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45:1459–63. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon DA, Kim T, Diaz-Martinez LA, et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science. 2011;333:1039–43. doi: 10.1126/science.1203619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor CF, Platt FM, Hurst CD, Thygesen HH, Knowles MA. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum Mol Genet. 2014;23:1964–74. doi: 10.1093/hmg/ddt589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22:259–67. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 16.Williams SV, Hurst DC, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Hum Mol Genet. 2013;22:795–803. doi: 10.1093/hmg/dds486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sfakianos JP, Cha EK, Iyer G, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68:970–7. doi: 10.1016/j.eururo.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai NB, Scott SN, Zabor EC, et al. Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer. 2016;122:3715–23. doi: 10.1002/cncr.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–53. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. 2016;34:2141–7. doi: 10.1200/JCO.2015.65.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Zhang G, Tian Y. p53 status correlates with the risk of recurrence in non-muscle invasive bladder cancers treated with bacillus Calmette-Guerin: a meta-analysis. PLoS One. 2015;10:e0119476. doi: 10.1371/journal.pone.0119476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–6. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 24.Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–9. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balbas-Martinez C, Rodríguez-Pinilla M, Casanova A, et al. ARID1A alterations are associated with FGFR3-wild type, poor-prognosis, urothelial bladder tumors. PLoS One. 2013;8:e62483. doi: 10.1371/journal.pone.0062483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitler BG, Aird KM, Garipov A, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–8. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartek J, Lukas J, Bartkova J. DNA damage response as an anti-cancer barrier: damage threshold and the concept of "conditional haploinsufficiency". Cell Cycle. 2007;6:2344–7. doi: 10.4161/cc.6.19.4754. [DOI] [PubMed] [Google Scholar]
- 29.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol. 2014;11:153–62. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 30.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.