Abstract
Animal models have been critical in building evidence that the prenatal experience and intrauterine environment are capable of exerting profound and permanent effects on metabolic health through developmental programming of obesity. However, despite physiological and evolutionary similarities, nonhuman primate models are relatively rare. The common marmoset monkey (Callithrix jacchus) is a New World monkey that has been used as a biomedical model for well more than 50 years and has recently been framed as an appropriate model for exploring early-life impacts on later health and disease. The spontaneous, multifactorial, and early-life development of obesity in the common marmoset make it a valuable research model for advancing our knowledge about the role of the prenatal and placental mechanisms involved in developmental programming of obesity. This paper provides a brief overview of obesity in the common marmoset, followed by a discussion of marmoset reproduction and placental characteristics. We then discuss the occurrence and utility of variable intrauterine environments in developmental programming in marmosets. Evidence of developmental programming of obesity will be given, and finally, we put forward future directions and innovations for including the placenta in developmental programming of obesity in the common marmoset.
INTRODUCTION
The emergence of adult metabolic disorders in children is a worldwide health concern (21). The pediatric obesity epidemic is incalculably problematic given that obese children are at risk for all of the major chronic health conditions that burden adults (21, 58). Undoubtedly, the pediatric obesity epidemic is partially influenced by our external environments of caloric excess and sedentary behaviors (41). However, there is increasing evidence that the prenatal experience and intrauterine environment are capable of exerting profound and permanent effects on metabolic health through developmental programming (5, 17, 27, 57).
Birth weight is an outcome of particular interest in developmental programming research. Low birth weight followed by rapid catchup growth during infancy is known as “centile crossing” and is a well-established phenotype in developmental programming of obesity (36, 37). Human studies have demonstrated that centile crossing is associated with childhood obesity and have also linked centile crossing to other metabolic disorders such as diabetes and hypertension (2, 9, 11, 33, 36, 59). Although low birth weight is typically the outcome of fetal “undernutrition” during the prenatal period, high birth weight is a common outcome of fetal “overnutrition,” particularly in pregnancies characterized by maternal obesity and excessive gestational weight gain. High birth weight is also an established risk factor for the development of obesity later in life, and the U-shaped association between birth weight and adulthood obesity is widely accepted (2, 8, 39).
Animal models have been critical to our understanding of the pathogenesis of obesity. The short lifespans of most animals when compared with humans permit relatively rapid assessment of short-term and long-term health consequences as well as intergenerational effects (50, 64). Animal models also allow for control over environmental and genetic factors, which are key components of developmental programming processes that cannot be controlled for in human population studies. Although there are limitations to every animal model, the knowledge gleaned from a variety of animal models allows us to continually build and strengthen the field of developmental programming.
The common marmoset monkey (Callithrix jacchus) is a small-bodied nonhuman primate that has been used as a biomedical model for more than 50 years (1). It has recently claimed space within the domain of developmental programming research programs (49). In this review, we introduce researchers to the extensive work done in the field of developmental programming in the marmoset by presenting evidence from our team’s decades of research demonstrating the current position of this nonhumanprimate. The purpose of this paper is to review the current evidence from two domains of the marmoset model: 1) marmoset placental phenotypes and 2) the marmoset as a model of pediatric obesity, with the goal of bringing together these two domains to yield an emerging marmoset model for developmental programming of obesity in which there are future directions for exploring prenatal, placental, and postnatal factors.
All research described herein was conducted under appropriate animal care and use committee guidelines and permits.
AN INTRODUCTION TO THE COMMON MARMOSET MONKEY
The common marmoset monkey is a New World monkey that is part of the Cebidae family and the subfamily Callitrichinae, which also includes tamarins, lion tamarins, and Goeldi’s monkeys (20, 49, 71). The callitrichines are native to South and Central America, with common marmosets living primarily in the coastal region of northeastern Brazil (20, 49).
A defining characteristic of the marmoset is its small body size. In the wild, average adult weights range from 320 to 340 g, and in captivity adult weights range from 283 to 500 g (1, 65). This is similar in size to the Sprague-Dawley rat that is commonly used in research (49), making it one of the least expensive nonhuman primates to maintain in research laboratories (1). Because of their small size, several marmoset breeding groups can be housed in a single 12 × 12 in. room, and at the Southwest National Primate Research Center, maintenance costs are ∼20% less than those for larger Old World species (baboons and macaques).
As a nonhuman primate, the marmoset monkey exhibits physiological similarities with humans, and in particular, marmosets in captive colonies spontaneously develop high body weight and relatively high fat concentrations (42, 65). The captive environment in which food availability is high and physical activity requirements are low is similar to modern human environments and suggests that increased adiposity occurs through similar underlying physiological processes (64). Much like humans and other animal models of obesity, overweight and obese marmoset monkeys develop a suite of obesity-associated sequelae, including alterations in glucose, lipid, and cholesterol levels (Table 1) (65, 70). Multiple organ pathologies have been reported in association with obsesity in marmosets, including hepatomegaly, hepatic glycogen deposition, and nonalcoholic steatohepatitis, tubular and glomerular alterations in the kidney, arteriosclerosis, microvascular cerebral hemorrhage, and pancreatic islet hypertrophy (Refs. 26 and 70 and Tardif SD, personal communication).
Table 1.
Metabolic markers for obese (>80th percentile relative fat) and nonobese (<80th percentile relative fat) adult marmosets
| Obese | Nonobese | |
|---|---|---|
| n | 14 | 50 |
| Age, yr | 3.3 ± 1.4 | 4.2 ± 1.5 |
| Lean weight, g | 291.7 ± 34.0 | 275.0 ± 34.4 |
| Fat weight, g | 77.9 ± 12.0 | 36.6 ± 18.4 |
| Glucose, mg/dl | 202.3 ± 67.9 | 161.2 ± 38.6 |
| Hb A1c, % | 5.23 ± 0.86 | 4.52 ± 0.80 |
| Cholesterol, mg/dl | 167.4 ± 70.2 | 151.2 ± 41.4 |
| HDL, mg/dl | 71.6 ± 21.3 | 67.2 ± 27.4 |
| LDL, mg/dl | 41.6 ± 25.4 | 59.8 ± 30.3 |
| VLDL, mg/dl | 58.08 | 24.61 |
| Triglycerides, mg/dl | 420.8 | 163.5 |
Values are means ± SD or median. Boldface values are significant (P < 0.01) and differ between sexes. Hb A1c, hemoglobin A1c; HDL, high-denisity lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein. Republished from Tardiff et al. (65) with permission from Elsevier.
Marmosets exhibit a fast life history that allows for efficient assessment of pregnancy as well as short- and long-term offspring health outcomes within the standard 5-yr grant period. Infants begin weaning at ∼1 mo of age (42, 67), the onset of puberty begins as early as 7–8 mo, and sexual maturity is typically reached between 11 and 12 mo of age (1, 67). Within an average of 43 days after pairing (Rutherford JN, unpublished data), first conception can occur at ∼14–15 mo. After an average gestation period of 143 days, first births can occur at ∼19–20 mo of age (1). Because postpartum ovulation typically occurs 10–20 days after delivery and usually results in conception and delivery, marmosets have a relatively short interbirth interval that averages 162 days compared with the 1- to 2-yr interbirth interval in macaques (13, 67). With this short interbirth interval, an established mating pair of marmosets can produce offspring twice a year throughout their lifespan, which averages 6 yr in captivity (1, 49). In addition to their ability to frequently reproduce, marmosets regularly carry litters. In captive colonies, twins, triplets, quadruplets, and, rarely, quintuplets are produced (20, 63, 67). In the wild, twins are the most common litter size, although triplet litters in wild callitrichines have been observed (3, 10, 54). How this variation in litter size presents a “natural experiment” for exploring intrauterine conditions that are shaped by different degrees of fetal demand and maternal supply is discussed in the next section.
LITTER SIZE AND NATURAL VARIABILITY IN INTRAUTERINE CONDITIONS: IMPLICATIONS FOR DEVELOPMENTAL PROGRAMMING OF OBESITY
Marmoset litters are multizygotic, resulting from fertilization of multiple ova, rather than monozygotic (63, 67). The evolutionary history of twinning within the Callitrichinae is complicated and as yet unresolved but likely involved combined changes in resource availability and predation risk that favored multiple births to support what even now is very high infant mortality (reviewed in Ref. 47). Recent work has demonstrated genetic underpinnings of the gestation of multiples, with GDF9, BMP15, BMP4, and WFIKKN likely playing a role in regulation of ovulation number (71).
Despite the evolutionary or genetic antecedents, litter size is highly variable within marmoset mothers, and the heritability is low (20, 49, 71). There is a positive association between maternal body weight (a reflection of maternal investment capacity) and the number of ovulations in a given cycle (P = 0.011) (63). Therefore, the litter sizes that a female carries throughout her reproductive lifespan vary depending on her weight at the time of ovulation. This suggests a system that is ecologically and energetically flexible, with ovarian function tied to metabolic condition, as seen in sheep (19).
Although mothers of triplets are heavier at the outset of pregnancy and gain more weight during pregnancy, they do not gain weight in a manner that is proportional to twin-bearing mothers. Thus, the maternal-to-fetal mass ratio is reduced by 24.9% in triplet pregnancies compared with twin pregnancies (46). This difference suggests that additional fetuses create intrauterine conditions of high fetal demand with limited maternal energetic resources, an intrauterine condition with major implications for developmental programming. Consequences of that deficit are reflected by the poorer perinatal outcomes of triplet infants; historically, triplets are born at lower birth weights, exhibit centile crossing, and experience much higher perinatal mortality than do their twin counterparts (7, 24, 25, 62). Here, we propose that, although not directly analogous to active maternal restriction, the low maternal energetic supply and high fetal demand of triplet litters represents a model of an “undernourished” or restricted intrauterine condition, whereas twin pregnancies represent a “control” intrauterine condition.
One of the tenets of developmental programming is that intrauterine conditions characterized by alterations in fetal access to nutrients and resources leads to alterations in fetal physiological development, which may predispose the individual to obesity or other chronic illnesses (4, 5, 64). Certainly, other key exposures include maternal chronic and acute psychosocial stress and environmental stressors; our focus here is on intrauterine nutritional allocation. Many animal models of developmental programming experimentally alter the intrauterine conditions (reviewed in Ref. 68), yet in this model the marmoset monkey’s unique feature of gestating multiples with a shared placental mass leads to natural differences in intrauterine conditions based on differences in litter size without experimental manipulation (49). The natural variation in litter size creates intrauterine conditions in which the developing fetuses compete differently, depending on litter size, for available nutrients and resources, with a single placenta regulating this availability for the entire litter. The different intrauterine conditions between twin and triplet pregnancies produce differences in birth and growth outcomes that are in alignment with the tenets of developmental programming.
Consistent with our model of a restricted intrauterine condition, triplets are born at significantly lower weights than twins (Table 2). Among male and female newborns, the average birth weight for twins was 30.24 g, and triplets demonstrated a lower average birth weight of 27.73 g (62). In a more recent study, the mean birth weight of twin females was significantly higher than the mean birth weight of triplet females, 31.17 and 28.72 g, respectively (P = 0.002) (50). These differences in birth weight suggest that developmental programming mechanisms may be at play during prenatal development, and indeed the weight differences between individual fetuses of triplet and twin litters are evident by approximately day 120 of the 143-day gestation period (Fig. 1) (7, 46). Knowing this developmental timeline allows for generating specific hypotheses about maternal nutritional status, placental function, and fetal development at critical developmental time points.
Table 2.
Placental and fetal characteristics in the marmoset monkey based on litter size
| Twin | Triplet | Ref. No(s). | |
|---|---|---|---|
| Average fetal weight, g | + | − | 50, 53, 61 |
| Average total litter weight, g | − | + | 53 |
| Placental weight, g | Difference not significant | Difference not significant | 53 |
| Fetal/placental weight ratio | − | + | 53 |
| Trabecular volume, ml | Difference not significant | Difference not significant | 52 |
| Trabecular surface area, cm2 | − | + | 52 |
| Trabecular surface area/fetus | + | − | 52 |
| IGF-II concentration, ng/ml | Difference not significant | Difference not significant | 51 |
| IGF-II concentration/fetus | + | − | 51 |
, Litter category that demonstrates larger value; −, litter category that demonstrates smaller value. Republished with permission (49).
Fig. 1.
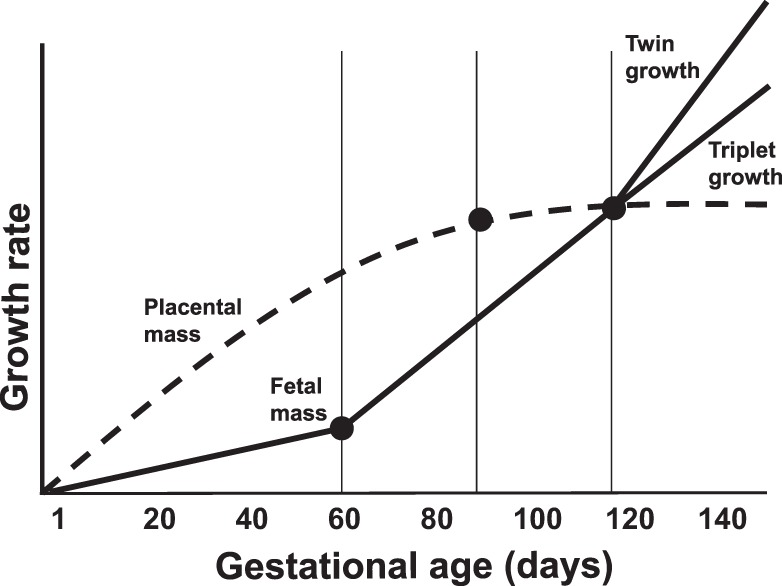
Schematic timeline of marmoset fetoplacental development across 143-day gestation, indicating critical time points (●) of the model. The most rapid period of placental growth occurs between day 0 and day 100, at which time placental growth is completed. Fetal growth is slow during early gestation until day 60, when it begins to rapidly increase through day 120. Day 90 is the midway point of this growth rate period. The most rapid increase in fetal growth rate occurs after day 120, and at this time point twin growth rate exceeds triplet growth rate.
PLACENTAL PHENOTYPES REFLECT INTRAUTERINE CONDITIONS
The marmoset placenta provides a range of opportunities and challenges in studies of the intrauterine environment and their applicability to humans. Marmoset placentas, like those of all the other anthropoid primates, are hemochorial, meaning that fetal and maternal blood are separated by a very thin membrane comprising the fetal capillary endothelium, a small amount of fetal mesodermal connective tissue, a layer of cytotrophoblast that becomes less prominent as gestation progresses, and a highly attenuated epithelial tissue called syncytiotrophoblast (34).
Marmoset placentas differ from the placentas of humans and more traditional nonhuman primate models such as rhesus macaques in the organization of their microscopic structural elements of the maternal-fetal interface as well as in the degree to which the placenta invades the uterine wall and remodels the maternal blood supply. Whereas the microscopic structural elements of human and macaque placentas present finger-like villi, the marmoset placenta is described as trabecular, wherein small bridges run through the tissue connecting villi to each other. Monkey, ape, and human placentas invade and burrow into the uterine wall, yet the human placenta engages in the most extensive cellular remodeling and colonizing of the maternal spiral arterioles that supply the endometrium. It is not yet clear what the functional significance of these differences are, but the differences likely limit direct translation from marmoset to human, a limitation shared by most nonhuman primate species currently used in research.
Rodents, too, have a hemochorial placenta, which strengthens their standing as an important model of intrauterine biology and outcomes. However, rodent placentas are neither villous nor trabecular but rather described as labyrinthine, wherein the maternal-fetal interface comprises many small connected chambers through which fetal capillaries course (44). From a functional perspective, the myriad endocrine processes of both the human and nonhuman primate placenta are localized in the syncytiotrophoblast of the maternal-fetal interface, whereas the site of maternofetal exchange in the rodent placenta, the labyrinth, has no similar endocrine function (32). Although a review of the placentas of all research models is beyond the purview of our paper, it is clear that nonhuman primate placentas share more similarities with human placentas than do rodent placentas.
Placental phenotypes (e.g., measures such as gross absolute and relative weight, volumes and surface areas of microscopic components, nutrient transporter location and density, and epigenomic profiles, among many others) are robust indicators of the intrauterine conditions and can reflect placental strategies taken to mediate maternal and fetal signals (23, 48). Indeed, placental phenotypes in marmosets reflect the very different intrauterine conditions of twin and triplet pregnancies. In a study of full-term twin and triplet pregnancies, fetal-to-placental weight ratios were significantly higher for triplet pregnancies [adjusted mean = 9.037, 95% CI (8.415, 9.660)] than for twin pregnancies [adjusted mean = 7.203, 95% CI (6.434, 7.972)] (53). This difference translates to 9 g of fetal mass produced per 1 g of triplet placenta compared with 7 g of fetal mass produced per 1 g of twin placenta. Although the triplet placenta is more efficient in production of fetal mass (i.e., increased fetal-to-placental weight ratio), the allocation of placental tissue per fetus is significantly reduced for triplets [adjusted mean = 3.183, 95% CI (3.056, 3.310)] compared with twins [adjusted mean = 4.724, 95% CI (4.562, 4.886)]. This reduction in the allocation of placental mass from which to access maternal resources may be a limiter of fetal growth in triplet pregnancies that contributes to lower birth weights and reinforces our model of a restricted intrauterine environment in larger litter sizes (53).
Microscopic morphometric analyses of the placental trabecular surface area, which is analogous to the villous surface area in the human placenta, have shown that although absolute surface area of the placental tissue in contact with maternal blood is increased in the triplet placenta, there is a significant difference between twins and triplets in the surface area per fetus, with triplets demonstrating a 25% reduction in surface area per fetus (4,300 vs. 5,700 cm2, P = 0.001) (52). This per-fetus reduction in trabecular surface area is consistent with the phenotype in human intrauterine growth restriction (IUGR) pregnancies in which IUGR placentas demonstrate a significantly reduced villous surface area compared with controls (M = 8.19 ± 2.88 vs. 10.02 ± 1.83 m2 , P < 0.01) (6). In other animal models and human pregnancy, low concentrations of insulin-like growth factor II (IGF-II) are related to IUGR (14, 16). Consistent with this, placental insulin-like growth factor (IGF-II) concentrations per fetus (i.e., total IGF-II/no. of fetuses) are significantly reduced by 42% in triplets compared with twins (t = 2.33, P = 0.03) (46, 49). Furthermore, triplet placentas produced more microscopic surface area relative to the volume of the underlying tissue than twin placentas (P = 0.008) (37). This expansion of surface area relative to available volume may be one way the triplet placenta attempts to compensate for the dramatic increase in fetal mass without a commensurate increase in placental mass. We are currently exploring relationships between microscopic surface area and interhemal membrane distance, along with the localization and density of various nutrient transporters, to delineate a more complex map of potential and actual nutrient transport across the placenta over a range of different litter sizes and birth conditions. Taken together, the summary of the marmoset triplet fetoplacental phenotype demonstrated in Table 2 is consistent with a nutrition- and growth-restricted intrauterine environment and thus provides a foundation for conceptualizing early-life growth and development as a frame for pediatric (and later in life, adult) obesity.
EARLY-LIFE EMERGENCE OF THE OBESE PHENOTYPE AND EVIDENCE OF DEVELOPMENT PROGRAMMING IN THE MARMOSET MONKEY
Obesity in marmosets has been characterized by both body mass percentile (i.e., body mass >90th percentile in adulthood at 17 mo) and body fat (i.e., total body fat or relative body fat >80th percentile, which corresponds to body fat ≥14% in late adolescence at 12 mo) (64, 65). Similar to classifications of obesity in other model systems, such as rodents (28) and sheep (56), the marmoset model of obesity reflects significant differences in metabolic markers that are similar to those seen in obese humans: higher fasting blood glucose, hemoglobin A1c, triglycerides, and very low-density lipoprotein (Table 1) (18, 65, 70). Like humans and other primates, sex differences are evident in marmosets, with females demonstrating a higher propensity to store fat (64). It should be noted that the marmoset criterion of ≥14% body fat is relatively low compared with the 20–35% body fat criterion that has been suggested for human adolescents (60). Yet, as shown here, ≥14% body fat in the marmoset is associated with alterations in metabolic markers, and the fat and lean growth patterns are distinctly different between marmosets destined to become obese. Relevant findings from studies using this marmoset obesity classification criteria are summarized in Table 3.
Table 3.
Summary of relevant findings from studies of marmoset obesity
| Subjects | Study Design | Relevant Findings | Ref. No. |
|---|---|---|---|
| 64 Adult marmosets | Obese (relative fat >80th percentile; n = 14); nonobese (relative fat <80th percentile; n = 50) | Obese marmosets had significantly higher fat weight, fasting glucose, Hb A1c, VLDL, and triglyceride concentrations than nonobese marmosets (P < 0.01; see Table 1); females had significantly higher body weights (411.0 ± 52.5 vs. 386.0 ± 47.6), fat weight (55.5 ± 21.7 vs. 39.3 ± 23.2), and relative proportion of fat than males (0.132 ± 0.04 vs. 0.097 ± 0.05, all P < 0.009). | 65 (Study 1) |
| 49 Marmosets observed from birth through 24 mo | Obese (relative fat >90th percentile at 24 mo; n = 13); nonobese (relative fat <90th percentile in early adulthood at 24 mo; n = 36) | Marmosets classified as obese at 17–24 mo were significantly heavier at birth and at 2, 4, and 6 mo of age than their nonobese counterparts (birth: F = 9.854, P < 0.004; 2 mo: F = 12.77, P < 0.001; 4 mo: F = 13.43, P < 0.001; 6 mo: F = 12.96, P < 0.001). | 65 (Study 2) |
| 31 Marmosets observed from birth through 12 mo; animals were fed either a control diet or HFD | Obese (>14% body fat at 6 and/or 12 mo; n = 15); nonobese (<14% body fat at 6 and/or 12 mo; n = 16) | Compared with nonobese individuals, marmosets classified as obese at 12 mo demonstrated
|
42 |
| 30 Marmosets observed from birth to 12 mo | Obese (≥14% body fat at 12 mo; n = 15); nonobese (<14% body fat at 12 mo; n = 15) | Compared with nonobese individuals, marmosets classified as obese at 12 mo
|
45 |
| 39 Marmosets observed from birth thru 12 mo | Obese (≥14% body fat at 6 and 12 mo; n = 18); nonobese (<14% body fat at 6 and 12 mo; n = 21) | Marmosets classified as obese at 6 mo demonstrated a trend for higher fasting glucose (123.2 ± 8.7 vs.101.1 ± 7.4 mg/dl, P = 0.063), significantly lower insulin sensitivity than nonobese individuals (mean QUICKI = 0.378 ± 0.029 vs. 0.525 ± 0.019, P = 0.003; n = 11), and no significant difference in leptin, and leptin was not associated with %body fat (r = 0.140, P = 0.453). Compared with nonobese marmosets, marmosets classified as obese at 12 mo demonstrated significantly higher fasting glucose (129.3 ± 9.1 vs. 106.1 ± 6.5 mg/dl, P = 0.042), significantly higher insulin (16.45 ± 3.05 vs. 1.01 ± 0.25 μU/ml, P < 0.001), significantly lower insulin sensitivity (0.317 ± 0.010 vs. 0.513 ± 0.018, P < 0.001), and significantly higher leptin (1.18 ± 0.15 vs. 0.73 ± 0.09 ng/ml, P = 0.014). Obese marmosets had a decreased ability to clear glucose, and oral glucose tolerance tests demonstrated significantly higher mean glucose values at 15 (159 ± 13 vs. 121 ± 11 mg/dl, P = 0.032), 30 (173 ± 15 vs. 136 ± 10 mg/dl, P = 0.043), and 120 min (154 ± 23 vs. 110 ± 14 mg/dl, P = 0.016). | 43 |
| 187 Adult marmosets | Obese (relative fat >80th percentile; n = 39); nonobese (relative fat <80th percentile; n = 148) | Compared with nonobese marmosets, obese marmosets demonstrated significantly higher total mass (444.85 ± 48.97 vs. 365.79 ± 35.98 g, P = 0.00001), lean mass (313.72 ± 35.15 vs. 286.29 ± 26.13 g, P = 0.00001), fat mass (77.21 ± 13.40 vs. 29.43 ± 12.16 g, P = 0.00001), blood glucose (242.50 ± 56.30 vs.176.63 ± 45.75 mg/dl, P = 0.00001), and serum triglyceride (430.06 ± 620.14 vs. 97.25 ± 57.57 mg/dl, P < 0.05). | 70 |
HFD, high-fat diet.
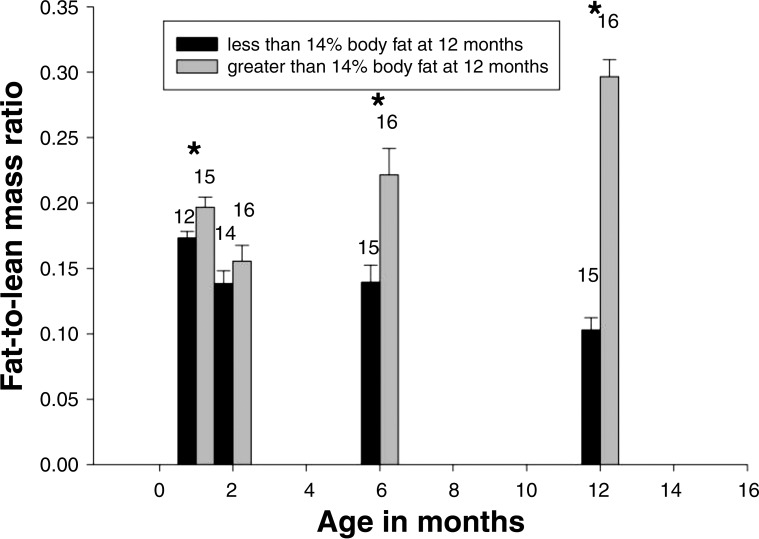
A primary advantage of the marmoset as a model for developmental programming of obesity is the early-life emergence of the obese phenotype. Previous longitudinal studies have shown that significant differences in body mass between individuals who became obese in adulthood and those who did not were present at birth and at 2, 4, and 6 mo of age (birth: F = 9.854, P < 0.004; 2 mo: F = 12.77, P < 0.001; 4 mo: F = 13.43, P < 0.001; 6 mo: F = 12.96, P < 0.001) (42, 64). Significant differences in fat-to-lean mass ratio between individuals destined to be obese and nonobese individuals were present by 1 mo of age. During this first month, individuals destined to become obese demonstrated higher fat depositions for every gram of lean body mass (42). After 1 mo of age, individuals destined to become obese showed a distinctly different pattern of fat deposition between 2 and 12 mo, with an increase in fat-to-lean mass ratios in those who became obese and a decrease in those who did not become obese (Fig. 2). Thus, infants that become obese and those that do not demonstrate very different patterns of growth throughout the first year of life, and these differences emerge as early as 1 mo of age. Although these patterns are not in perfect alignment with the fat-to-lean mass ratio gains in humans, the marmoset does allow for exploring the underlying mechanisms of greater proportions of fat gain that contribute to centile crossing in both species. Beyond the very different growth patterns, Power et al. (43) has shown that metabolic consequences of early-onset obesity are also apparent in the first year of life, with those destined to become obese showing impaired glucose homeostasis early on.
Fig. 2.
Fat-to-lean mass ratio for nonobese (<14% body fat at 12 mo; black bars) and obese (>14% body fat at 12 mo; gray bars) individuals at 1, 2, 6, and 12 mo of age. Standard error of the mean is shown, and the numbers above each bar are the no. of subjects measured. *Significant differences between groups (P<0.05). Figure adapted from Power et al. (42) with permission.
One of the most salient findings for developmental programming of pediatric obesity in marmosets comes from observations in juvenile growth rates. Tardif and Bales (62) identified a later juvenile growth period in which the average daily growth rate was 0.83 g/day compared with 1.15 g/day in the early growth period that preceded. During this later growth period, which on average began at 5.3 mo of age, there was a significant interactive effect of birth weight (F = 10.44, df = 1, 46; P = 0.002) and litter size (F = 48.93, df = 1, 3.02; P = 0.006) (62). In twins, there was a positive association such that low-birth-weight twins exhibited slower later growth rates, and these individuals went on to be lower-weight adults. Yet low-birth-weight triplets exhibited a centile crossing growth pattern with faster later growth rates and the highest rate of body mass gain during this period, leading to higher weights in adulthood. This demonstration that low-birth-weight triplets are more likely to centile cross and develop obesity later in life supports our model of the triplet-restricted intrauterine environment, wherein lower birth weights are followed by postnatal growth outcomes, consistent with the centile crossing observations in human populations.
As the developmental programming window opens into the postnatal period, feeding patterns may contribute to the postnatal growth patterns and obesity outcomes later in life. Indeed, marmosets are classified as obese at 12 mo, demonstrate very different feeding patterns, and start the weaning process at a significantly earlier time point than their nonobese counterparts (day 24 ± 1.0 vs. 28 ± 1.0, P = 0.021) (45). Additionally, efficiency of liquid diet consumption (i.e., grams of diet per lick) significantly predicted obesity status at 12 mo (r2 = 0.142, r = 0.376, P = 0.04).
Diet and maternal size are two other factors that have demonstrated independent effects on postnatal growth patterns that lead to obesity in marmosets (42). High-fat diet exposure had a significant effect at 6 mo, and 58% of subjects exposed to the high-fat diet were classified as obese (>14% body fat) compared with 25% of the control diet subjects that were classified as obese at 6 mo (χ2 = 3.860, df = 1, P = 0.049). At 12 mo of age, the relationship between high-fat diet and obesity was no longer significant. Twenty-six of the 31 subjects were categorized identically at 6 and 12 mo of age. A subset of four subjects (only one with high-fat diet exposure) crossed over from the normal-weight group at 6 mo to the obese group at 12 mo. Of the subjects that crossed over from the normal-weight group to the obese group, only one was exposed to the high-fat diet, but all were the offspring of relatively large mothers (i.e., 12.9–17% body fat). This suggests that early high-fat diet exposure is not necessary for marmosets to become obese, and the risk of obesity is potentially associated with being the offspring of a relatively large mother. However, when comparing high-fat diet and glucose-enriched diets in a subset of adult marmosets (n = 23; average age = 4.16 yr), Wachtman et al. (70) observed that obese phenotypes were more likely to emerge with a glucose-enriched rather than a high-fat diet, and these obese subjects demonstrated rapid and persistent elevations in hemoglobin A1c after only 16 wk of diet exposure. Thus, it is possible that other dietary challenges may contribute to obese phenotypes in young marmosets as well and that the dietary response may be related to intrauterine conditions and litter size. This has yet to be explored.
That maternal size may have an independent effect on offspring obesity is of particular interest given the discussed relationship between maternal weight and litter size and suggests that developmental programming mechanisms contribute to an individual’s response to diet and propensity toward obesity. Combined, the differences in placental characteristics, birth outcomes, and postnatal growth patterns, along with the early-life occurrence of obesity that can be altered by maternal condition and high-fat diet exposure, position the marmoset for further expansion as a model for developmental programming of pediatric obesity.
WHERE WE ARE NOW AND WHERE WE ARE HEADED: THE FUTURE OF THE MODEL
Here we have synthesized the current evidence demonstrating extensive differences in placental and fetal outcomes based on litter size along with links among litter size, postnatal growth patterns, and obesity outcomes. Together, the evidence positions the marmoset as a useful model for developmental programming of pediatric obesity. Furthermore, ours and others’ work shows that the placenta is a strong candidate as an agent of these developmental programming effects (48). The natural occurrence of variable litter size in marmosets as well as the development of obesity in infancy and adolescence presents the unique opportunity for exploring placental, prenatal, and postnatal mechanisms of programming obesity. An immediate question our group is addressing is how placental phenotypes are related to obesity later in life. This alone has the potential to reveal structural and functional strategies that the primate placenta is capable of exploiting while negotiating maternal supply and fetal demands in utero. Our group is currently exploring the intra- and intergenerational relationships among maternal early life characteristics, metabolic factors during pregnancy (e.g., weight, weight gain, metabolic hormones), placental structure and nutrient transport systems (with special emphasis on glucose and amino acid transporters), and offspring outcomes such as birth weight, perinatal mortality, weight gain during growth, and the development of obesity. For example, we have shown that a female marmoset’s birth weight is associated with the relative size of her offspring’s placenta (Rutherford JN, unpublished data), and the size of the placenta a female is born with predicts how many offspring she will produce in adulthood (49). The mechanisms linking these intergenerational reproductive and metabolic processes remain unclear and thus reveal a gap for further exploration.
Additionally, the recent mapping of the marmoset genome now provides the opportunity to explore the genetic and epigenetic underpinnings of placental and offspring phenotypes. With our increasing knowledge about the lasting impacts of placental function on health outcomes in offspring, the placenta will be a promising target for intervention and treatment strategies. Yet a great deal of work remains to establish the underlying placental mechanisms at play. Human and rodent studies have demonstrated alterations in placental epigenetic profiles of several gene loci, including LEP, ABCA1, Igf2r, and Dlk1, in relation to maternal energetic status (e.g., maternal obesity, gestational weight gain, diabetes, high-fat diet, etc.) (15, 22, 29, 55). It is likely that placental epigenetic profiles differ in marmosets, depending on maternal weight, and that placental epigenetics influence the obesity outcomes that are seen in relation to maternal weight and litter size. Further exploration of placental epigenetic profiles in the marmoset is a promising path toward elucidating gene pathways that can be targeted for intervention and treatment strategies. Indeed, ours is the first research group to explore how marmoset placental epigenetic profiles relate to maternal metabolic factors during pregnancy and offspring health outcomes (Riesche L, Wildman DE, Armstrong DL, Weckle A, Bell AF, Tardif SD, Ross CN, Patil CL, and Rutherford JN, unpublished data).
Beyond future placental directions, a recent exploration of genetic mechanisms involved in programming metabolic syndrome in marmosets demonstrated that antenatal glucocorticoid exposure caused persistent increases in 11β-HSD1 gene expression in adipose tissue of offspring (35). This suggests a novel mechanism for developmental programming in response to antenatal glucocorticoid exposure and demonstrates the feasibility of linking antenatal/prenatal exposures to tissue-specific molecular outcomes in offspring. Exciting opportunities exist for studies of similar design paired with complementary omic assays. Characterization of the marmoset metabolome compared with the human metabolome has revealed that although there are differences between the two species, utilizing targeted metabolomics in marmoset studies will provide insight into an evolutionary understanding of primate metabolic responses (18).
Because similarities in altered metabolic markers are seen in marmosets and humans when obesity is present, the marmoset model has the potential to be used for testing drugs and other obesity interventions such as diet and exercise. Indeed, techniques for engaging marmosets in aerobic exercise have been successfully developed (40). Utilizing these techniques in the context of our marmoset developmental programming model may elucidate differences in exercise response between nonobese and obese individuals who were exposed to different intrauterine conditions. Another important feature of this model is the ease with which infants can be manipulated, including cross-fostering of infants to different parents. In this way, marmosets can be used in a fashion similar to rodent models, allowing for the decoupling of pre- and postnatal factors that may drive or ameliorate obesity development.
Various animal models, including the sheep (38), the baboon (12, 30), and rodents (reviewed in Ref. 31), have contributed to our understanding of developmental programming, with each having its own strengths and weaknesses. Likewise, the marmoset model has both strengths and weaknesses. The aspects of marmoset biology that present such fascinating avenues for study, such as the genetics and physiology of multiple births, the interaction of litter size and birth weight, and the great variability of offspring outcomes are evolutionary derived traits that mean the model is valuable for some questions but not others. For example, the evolved physiology of multiple births in the marmoset differs from that in the human such that marmosets may be a poor model in which to test etiologies or therapeutics related to complications of multiple gestations in humans. Additionally, more research will have to be done to understand whether there are any effects of placental sex in the marmoset, as the shared placental mass is formed from fusion of all embryos in the litter, and mixed-sex litters occur with high frequency. On the other hand, the association of routine gestation of multiple fetuses with a nonhuman primate placentation offers the opportunity to study naturally occurring variation in the prenatal environment and to decouple pre- and postnatal environments through cross-fostering. The small size of the marmoset also brings both pros and cons. Their small size and associated faster life history means that they can be a more efficient model than Old World monkeys for the study of lifespan phenomena, such as the effects of the prenatal environment on adult phenotypes. However, their small size places limits on certain types of sampling (e.g., high volume blood sampling, instrumentation).
Perspectives and Significance
Different animal models each bring their own strengths and weaknesses to any area of study. The development of multiple model species for any given area of study creates the highest likelihood of having the best model for any given question and the best chance of assessing the robustness of any given finding across models. As a unique nonhuman primate model, the marmoset model is poised to contribute to the field of developmental programming by providing an integrated understanding of the prenatal, placental, and postnatal physiology involved in developmental programming and allows us to generate an evolutionary perspective about the physiological mechanisms that may be at play across primates. Weighing advantages and disadvantages, we contend that the early-life emergence of obesity combined with the fast life history of the marmoset provides an efficient and economical model for implementing intervention and treatment strategies that will allow for immediate, short-term, long-term, and intergenerational effects to be explored in a feasible time frame.
GRANTS
Funding was provided in part by the Robert Wood Johnson Future of Nursing Scholars program (Riesche) and by the National Institutes of Health (R01-DK-077639 to S.D.Tardif and R01-HD-076018 to J. N. Rutherford).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.R., S.D.T., C.N.R., V.A.d., and J.N.R. analyzed data; L.R., S.D.T., C.N.R., T.Z., and J.N.R. interpreted results of experiments; L.R. and J.N.R. prepared figures; L.R. and J.N.R. drafted manuscript; L.R., S.D.T., C.N.R., V.A.d., T.Z., and J.N.R. edited and revised manuscript; L.R., S.D.T., C.N.R., V.A.d., T.Z., and J.N.R. approved final version of manuscript; S.D.T., C.N.R., and J.N.R. conceived and designed research; S.D.T., C.N.R., V.A.d., and J.N.R. performed experiments.
ACKNOWLEDGMENTS
We thank Drs. Linda Scott, Aleeca Bell, Crystal Patil, and Derek Wildman for overall support and constructive comments on earlier versions of this article as well as the animal care staff at Southwest National Primate Research Center and the University of Texas Health Science Center at San Antonio Barshop Center for Longevity and Aging.
REFERENCES
- 1.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med 53: 339–350, 2003. [PubMed] [Google Scholar]
- 2.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res 36: 73–84, 2008. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 3.Bales K, O’Herron M, Baker AJ, Dietz JM. Sources of variability in numbers of live births in wild golden lion tamarins (Leontopithecus rosalia). Am J Primatol 54: 211–221, 2001. doi: 10.1002/ajp.1031. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The fetal and infant origins of adult disease. BMJ 301: 1111, 1990. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJP. The developmental origins of adult disease. J Am Coll Nutr 23, Suppl: 588S–595S, 2004. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, Ghosh SK, Chhabra S. Surface area of chorionic villi of placentas: an index of intrauterine growth restriction of fetuses. J Obstet Gynaecol Res 34: 487–493, 2008. doi: 10.1111/j.1447-0756.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 7.Chambers PL, Hearn JP. Embryonic, foetal and placental development in the common marmoset monkey (Callithrix jacchus). J Zool 207: 545–561, 1985. doi: 10.1111/j.1469-7998.1985.tb04951.x. [DOI] [Google Scholar]
- 8.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep 13: 27–33, 2013. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes 39: 633–641, 2015. doi: 10.1038/ijo.2015.13. [DOI] [PubMed] [Google Scholar]
- 10.Dixson AF, Anzenberger G, Monteiro Da Cruz MA, Patel I, Jeffreys AJ. DNA fingerprinting of freeranging groups of marmosets in Northeast Brazil. In: Paternity in Primates: Genetic Tests and Theories, edited by Martin RD, Dixson AF, and Wickings EJ. Basel, Switzerland: Karger, 1992, p. 192–202. [Google Scholar]
- 11.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes 30: 610–617, 2006. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 12.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ Jr, Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE. Feto-placental adaptations to maternal obesity in the baboon. Placenta 30: 752–760, 2009. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fooden J. Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). Chicago, IL: Field Museum of Natural History, 2000. doi: 10.5962/bhl.title.7192. [DOI] [Google Scholar]
- 14.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta 24: 803–812, 2003. doi: 10.1016/S0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 15.Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J, Boudadi E, Gross MS, Taurelle J, Vigé A, Breton C, Reusens B, Remacle C, Vieau D, Ekström TJ, Jais JP, Junien C. Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS One 5: e14398, 2010. doi: 10.1371/journal.pone.0014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res 65, Suppl 3: 28–33, 2006. doi: 10.1159/000091503. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 1: 6–18, 2010. doi: 10.1017/S2040174409990171. [DOI] [PubMed] [Google Scholar]
- 18.Go YM, Liang Y, Uppal K, Soltow QA, Promislow DE, Wachtman LM, Jones DP. Metabolic characterization of the common marmoset (Callithrix jacchus). PLoS One 10: e0142916, 2015. [Correction in PLoS One 11: e0147880, 2016.] doi: 10.1371/journal.pone.0142916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn RG, Doney JM. The interaction of nutrition and body condition at mating on ovulation rate and early embryo mortality in Scottish Blackface ewes. J Agric Sci 85: 465–470, 1975. doi: 10.1017/S0021859600062341. [DOI] [Google Scholar]
- 20.Harris RA, Tardif SD, Vinar T, Wildman DE, Rutherford JN, Rogers J, Worley KC, Aagaard KM. Evolutionary genetics and implications of small size and twinning in callitrichine primates. Proc Natl Acad Sci USA 111: 1467–1472, 2014. doi: 10.1073/pnas.1316037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 299: R711–R722, 2010. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houde AA, Guay SP, Desgagné V, Hivert MF, Baillargeon JP, St-Pierre J, Perron P, Gaudet D, Brisson D, Bouchard L. Adaptations of placental and cord blood ABCA1 DNA methylation profile to maternal metabolic status. Epigenetics 8: 1289–1302, 2013. doi: 10.4161/epi.26554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113: 1–13, 2007. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 24.Jaquish CE, Gage TB, Tardif SD. Reproductive factors affecting survivorship in captive Callitrichidae. Am J Phys Anthropol 84: 291–305, 1991. doi: 10.1002/ajpa.1330840306. [DOI] [PubMed] [Google Scholar]
- 25.Jaquish CE, Tardif SD, Toal RL, Carson RL. Patterns of prenatal survival in the common marmoset (Callithrix jacchus). J Med Primatol 25: 57–63, 1996. doi: 10.1111/j.1600-0684.1996.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JA, Grindley J, Crowell AM, Makaron L, Kohli R, Kirby M, Mansfield KG, Wachtman LM. The common marmoset as a model for the study of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Vet Pathol 52: 404–413, 2015. doi: 10.1177/0300985814537839. [DOI] [PubMed] [Google Scholar]
- 27.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J Anat 215: 36–51, 2009. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leopoldo AS, Lima-Leopoldo AP, Nascimento AF, Luvizotto RA, Sugizaki MM, Campos DH, da Silva DC, Padovani CR, Cicogna AC. Classification of different degrees of adiposity in sedentary rats. Braz J Med Biol Res 49: e5028, 2016. doi: 10.1590/1414-431X20155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesseur C, Armstrong DA, Murphy MA, Appleton AA, Koestler DC, Paquette AG, Lester BM, Marsit CJ. Sex-specific associations between placental leptin promoter DNA methylation and infant neurobehavior. Psychoneuroendocrinology 40: 1–9, 2014. doi: 10.1016/j.psyneuen.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DS, Bertrand HA, McMahan CA, McGill HC Jr, Carey KD, Masoro EJ. Preweaning food intake influences the adiposity of young adult baboons. J Clin Invest 78: 899–905, 1986. doi: 10.1172/JCI112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res 2011: 592408, 2011. doi: 10.1155/2011/592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update 9: 531–539, 2003. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro POA, Victora CG. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obes Rev 6: 143–154, 2005. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 34.Mossman HW. Vertebrate Fetal Membranes. New Brunswick, NJ: Rutgers University Press, 1987. doi: 10.1007/978-1-349-09065-5. [DOI] [Google Scholar]
- 35.Nyirenda MJ, Carter R, Tang JI, de Vries A, Schlumbohm C, Hillier SG, Streit F, Oellerich M, Armstrong VW, Fuchs E, Seckl JR. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11beta-hydroxysteroid dehydrogenase type 1. Diabetes 58: 2873–2879, 2009. doi: 10.2337/db09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320: 967–971, 2000. doi: 10.1136/bmj.320.7240.967. [Erratum in BMJ 320: 1244, 2000.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res 65, Suppl 3: 65–69, 2006. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan V, Veiga-Lopez A. Reproduction Symposium: developmental programming of reproductive and metabolic health. J Anim Sci 92: 3199–3210, 2014. doi: 10.2527/jas.2014-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettitt DJ, Jovanovic L. Birth weight as a predictor of type 2 diabetes mellitus: the U-shaped curve. Curr Diab Rep 1: 78–81, 2001. doi: 10.1007/s11892-001-0014-x. [DOI] [PubMed] [Google Scholar]
- 40.Phillips KA, Hambright MK, Hewes K, Schilder BM, Ross CN, Tardif SD. Take the monkey and run. J Neurosci Methods 248: 27–31, 2015. doi: 10.1016/j.jneumeth.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popkin B. Global dynamics in childhood obesity: reflections on a life of work in the field. In: Pediatric Obesity: Etiology, Pathogenesis, and Treatment edited by Freemark MS. London; Totowa, NJ: Humana, 2010, p. 3–11. doi: 10.1007/978-1-60327-874-4_1. [DOI] [Google Scholar]
- 42.Power ML, Ross CN, Schulkin J, Tardif SD. The development of obesity begins at an early age in captive common marmosets (Callithrix jacchus). Am J Primatol 74: 261–270, 2012. doi: 10.1002/ajp.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Power ML, Ross CN, Schulkin J, Ziegler TE, Tardif SD. Metabolic consequences of the early onset of obesity in common marmoset monkeys. Obesity (Silver Spring) 21: E592–E598, 2013. doi: 10.1002/oby.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinkenberger J, Werb Z. The labyrinthine placenta. Nat Genet 25: 248–250, 2000. doi: 10.1038/76985. [DOI] [PubMed] [Google Scholar]
- 45.Ross CN, Power ML, Artavia JM, Tardif SD. Relation of food intake behaviors and obesity development in young common marmoset monkeys. Obesity (Silver Spring) 21: 1891–1899, 2013. doi: 10.1002/oby.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutherford JN. Fetal signaling through placental structure and endocrine function: illustrations and implications from a nonhuman primate model. Am J Hum Biol 21: 745–753, 2009. doi: 10.1002/ajhb.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutherford JN. Litter Size Effects on Placental Structure and Function In Common Marmoset Monkeys (Callithrix jacchus): Implications For Intrauterine Resource Allocation Strategies. Ann Arbor, MI: ProQuest Dissertations, 2007. [Google Scholar]
- 48.Rutherford JN. The Primate Placenta as an Agent of Developmental and Health Trajectories Across the Life Course. New York: Springer, 2013, p. 27–53. doi: 10.1007/978-1-4614-4060-4_2. [DOI] [Google Scholar]
- 49.Rutherford JN. Toward a nonhuman primate model of fetal programming: phenotypic plasticity of the common marmoset fetoplacental complex. Placenta 33, Suppl 2: e35–e39, 2012. doi: 10.1016/j.placenta.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutherford JN, deMartelly VA, Donna GLC, Ross CN, Tardif SD. Developmental origins of pregnancy loss in the adult female common marmoset monkey (Callithrix jacchus). PLoS One 9: e96845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutherford JN, Eklund A, Tardif S. Placental insulin-like growth factor II (IGF-II) and its relation to litter size in the common marmoset monkey (Callithrix jacchus). Am J Primatol 71: 969–975, 2009. doi: 10.1002/ajp.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutherford JN, Tardif SD. Developmental plasticity of the microscopic placental architecture in relation to litter size variation in the common marmoset monkey (Callithrix jacchus). Placenta 30: 105–110, 2009. doi: 10.1016/j.placenta.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutherford JN, Tardif SD. Placental efficiency and intrauterine resource allocation strategies in the common marmoset pregnancy. Am J Phys Anthropol 137: 60–68, 2008. doi: 10.1002/ajpa.20846. [DOI] [PubMed] [Google Scholar]
- 54.Savage A, Soto L, Medina F, Emeris G, Soltis J. Litter size and infant survivorship in wild groups of cotton-top tamarins (Saguinus oedipus) in Colombia. Am J Primatol 71: 707–711, 2009. doi: 10.1002/ajp.20696. [DOI] [PubMed] [Google Scholar]
- 55.Sferruzzi-Perri AN, Vaughan OR, Haro M, Cooper WN, Musial B, Charalambous M, Pestana D, Ayyar S, Ferguson-Smith AC, Burton GJ, Constancia M, Fowden AL. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J 27: 3928–3937, 2013. doi: 10.1096/fj.13-234823. [DOI] [PubMed] [Google Scholar]
- 56.Shasa DR, Odhiambo JF, Long NM, Tuersunjiang N, Nathanielsz PW, Ford SP. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int J Obes 39: 695–701, 2015. doi: 10.1038/ijo.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons R. Developmental origins of adult metabolic disease. Endocrinol Metab Clin North Am 35: 193–204, 2006. doi: 10.1016/j.ecl.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Speakman JR. Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu Rev Nutr 33: 289–317, 2013. doi: 10.1146/annurev-nutr-071811-150711. [DOI] [PubMed] [Google Scholar]
- 59.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 109: 194–199, 2002. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 60.Sweeting HN. Measurement and definitions of obesity in childhood and adolescence: a field guide for the uninitiated. Nutr J 6: 32, 2007. doi: 10.1186/1475-2891-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tardif S, Power M, Layne D, Smucny D, Ziegler T. Energy restriction initiated at different gestational ages has varying effects on maternal weight gain and pregnancy outcome in common marmoset monkeys (Callithrix jacchus). Br J Nutr 92: 841–849, 2004. doi: 10.1079/BJN20041269. [DOI] [PubMed] [Google Scholar]
- 62.Tardif SD, Bales KL. Relations among birth condition, maternal condition, and postnatal growth in captive common marmoset monkeys (Callithrix jacchus). Am J Primatol 62: 83–94, 2004. doi: 10.1002/ajp.20009. [DOI] [PubMed] [Google Scholar]
- 63.Tardif SD, Jaquish CE. Number of ovulations in the marmoset monkey (Callithrix jacchus): relation to body weight, age and repeatability. Am J Primatol 42: 323–329, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 64.Tardif SD, Power ML, Ross CN, Rutherford JN. Body mass growth in common marmosets: toward a model of pediatric obesity. Am J Phys Anthropol 150: 21–28, 2013. doi: 10.1002/ajpa.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring) 17: 1499–1505, 2009. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comp Med 53: 364–368, 2003. [PubMed] [Google Scholar]
- 68.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 92: 287–298, 2007. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- 70.Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, Mansfield KG. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity (Silver Spring) 19: 1145–1156, 2011. doi: 10.1038/oby.2010.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Worley KC, Warren WC, Rogers J, Locke D, Muzny DM, Mardis ER, Weinstock GM, Tardif SD, Aagaard KM, Archidiacono N, Rayan NA, Batzer MA, Beal K, Brejova B, Capozzi O, Capuano SB, Casola C, Chandrabose MM, Cree A, Dao MD, de Jong PJ, del Rosario RC-H, Delehaunty KD, Dinh HH, Eichler EE, Fitzgerald S, Flicek P, Fontenot CC, Fowler RG, Fronick C, Fulton LA, Fulton RS, Gabisi RA, Gerlach D, Graves TA, Gunaratne PH, Hahn MW, Haig D, Han Y, Harris RA, Herrero J, Hillier LDW, Hubley R, Hughes JF, Hume J, Jhangiani SN, Jorde LB, Joshi V, Karakor E, Konkel MK, Kosiol C, Kovar CL, Kriventseva EV, Lee SL, Lewis LR, Liu Y, Lopez J, Lopez-Otin C, Lorente-Galdos B, Mansfield KG, Marques-Bonet T, Minx P, Misceo D, Moncrieff JS, Morgan MB, Nazareth LV, Newsham I, Nguyen NB, Okwuonu GO, Prabhakar S, Perales L, Pu L-L, Puente XS, Quesada V, Ranck MC, Raney BJ, Raveendran M, Deiros DR, Rocchi M, Rodriguez D, Ross C, Ruffier M, Ruiz SJ, Sajjadian S, Santibanez J, Schrider DR, Searle S, Skaletsky H, Soibam B, Smit AFA, Tennakoon JB, Tomaska L, Ullmer B, Vejnar CE, Ventura M, Vilella AJ, Vinar T, Vogel J-H, Walker JA, Wang Q, Warner CM, Wildman DE, Witherspoon DJ, Wright RA, Wu Y, Xiao W, Xing J, Zdobnov EM, Zhu B, Gibbs RA, Wilson RK; Marmoset Genome Sequencing and Analysis Consortium . The common marmoset genome provides insight into primate biology and evolution. Nat Genet 46: 850–857, 2014. doi: 10.1038/ng.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]