Abstract
Pulmonary hypertension describes a heterogeneous disease defined by increased pulmonary artery pressures, and progressive increase in pulmonary vascular resistance due to pathologic remodeling of the pulmonary vasculature involving pulmonary endothelial cells, pericytes, and smooth muscle cells. This process occurs under various conditions, and although these populations vary, the clinical manifestations are the same: progressive dyspnea, increases in right ventricular (RV) afterload and dysfunction, RV-pulmonary artery uncoupling, and right-sided heart failure with systemic circulatory collapse. The overall estimated 5-yr survival rate is 72% in highly functioning patients, and as low as 28% for those presenting with advanced symptoms. Metabolic theories have been suggested as underlying the pathogenesis of pulmonary hypertension with growing evidence of the role of mitochondrial dysfunction involving the major proteins of the electron transport chain, redox-related enzymes, regulators of the proton gradient and calcium homeostasis, regulators of apoptosis, and mitophagy. There remain more studies needed to characterize mitochondrial dysfunction leading to impaired vascular relaxation, increase proliferation, and failure of regulatory mechanisms. The effects on endothelial cells and resulting interactions with their microenvironment remain uncharted territory for future discovery. Additionally, on the basis of observations that the “plexigenic lesions” of pulmonary hypertension resemble the unregulated proliferation of tumor cells, similarities between cancer pathobiology and pulmonary hypertension have been drawn, suggesting interactions between mitochondria and angiogenesis. Recently, mitochondria targeting has become feasible, which may yield new therapeutic strategies. We present a state-of-the-art review of the role of mitochondria in both the pathobiology of pulmonary hypertension and potential therapeutic targets in pulmonary vascular processes.
Keywords: mitochondria, pulmonary hypertension
OUR CURRENT UNDERSTANDING OF PULMONARY HYPERTENSION
The first pathological description of pulmonary arterial hypertension (PAH) was described in a German publication in 1891. The article entitled On Sclerosis of the Pulmonary Artery: From the Medical Clinic of Leipzig, described the autopsy findings of patients who suffered from a constellation of progressive dyspnea, cyanosis, fatigue, and ultimately heart failure with “general hydrops” (generalized edema) (136). Pathologically, the patients all had enlarged right ventricles and pulmonary arteries with notable “congestion”. It was not until 50 yr later, however, that the hemodynamic implications of these pathological findings would truly be known, with the development of right heart catheterization (115). In 1951, the first article fully describing the clinical entity of pulmonary hypertension (PH), as we understand it today, was published (39). This allowed for a greater understanding of the pre-mortem features of PH. In 1973, the World Health Organization (WHO) recognized three specific categories or etiologies of PH based on additional pathological data (73), and 14 years later, the first series was published (51).
Currently, we recognize five categories of patients with PH, defined as “groups” by the WHO. These groups have been periodically updated, as our understanding of the underlying mechanisms expands. PH is now defined by both hemodynamic measures and pathological findings, unchanged from 70 yr ago when first described by Dresdale et al. (39). Hemodynamically, PH is a mean pulmonary artery pressure ≥25 mmHg at rest. This basic hemodynamic measure applies to all five WHO groups. PAH, which is a subgroup of PH and is WHO group 1 PH, is characterized in addition by a normal pulmonary artery wedge pressure (≤15 mmHg) and a pulmonary vascular resistance >3.0 Wood units (>240 dynes·s−1·cm−5).
Pathologically, there is greater variation among the different WHO groups. Classically, WHO group 1, which includes idiopathic PAH, is characterized by hypertrophic pulmonary artery remodeling with arteriolar muscularization, intimal fibrosis, and in situ thrombosis with neovascularization and occasionally the presence of “plexiform” lesions. In WHO group 1 PH, these hypertrophic and plexigenic lesions lead to increased vascular resistance within the pulmonary system and ultimately to maladaptive responses of the right ventricle to an increased pressure load. In this category, what remains unknown is exactly what mechanism initiates the maladaptive response of the vasculature.
HISTORIC MECHANISMS OF PULMONARY HYPERTENSION
PH was considered as a disease of vascular sclerosis and vasoconstriction (arteriolar muscularization), possibly due to “vascular hyperreactivity”, and frequently complicated by thrombotic disease, leading to increased vascular resistance throughout the pulmonary system. It was suggested that the protective mechanism of transient hypoxic pulmonary vasoconstriction was dysregulated or constitutively active, leading to remodeling, and, thus, the phenotype described above (51). As such, the first mechanisms underlying PH to be studied involved vasoactive pathways within the vasculature. These early studies led to the vast majority of available therapies, namely endothelin-1, nitric oxide (NO), and prostacyclin pathways.
The first and most potent vasodilator of the vascular smooth muscle to be discussed is NO. Originally termed “endothelium-derived relaxing factor” by Furchgott and Zawadzki (50), this vasoactive compound is synthesized by NO synthase in endothelial cells (eNOS), which is then secreted as a dissolved gas to be taken up by nearby vascular smooth muscle cells (SMCs). Within SMCs, NO increases production of cGMP via activation of soluble guanylate cyclase (sGC), which, in turn, decreases calcium influx, thus promoting relaxation of the SMC and vasodilation when in concert with other surrounding SMCs. This effect on cGMP also leads downstream to decreased DNA synthesis, thus inhibiting proliferation of SMCs. Patients with PAH have been found to be deficient in NO and its downstream products (83). Low NO is potentially due to inactivation of eNOS by aberrant phosphorylation in vascular endothelial cells (56). Several therapeutic options specifically address these deficiencies by delivering NO directly to the lungs via inhalation, or by increasing its downstream effector molecule cGMP either via decreased breakdown (phosphodiesterase inhibitors) (81), or via upregulation by stimulating soluble guanylate cyclase (55).
Similar to NO, another important pulmonary vasodilator is the prostaglandin PGI2 or prostacyclin. This product of the arachidonic acid pathway is synthesized in the endothelium in response to vascular injury or stress, and it is released in a paracrine fashion, exerting its action on nearby vascular SMCs, platelets, and other endothelial cells. Prostacyclin acts on the prostacyclin receptor through G protein-coupled receptors, resulting in increased adenylate cyclase activity, thereby increasing cAMP levels. This has various effects depending on the cell type affected, including decreased cytosolic calcium and increased breakdown of myosin light chains in SMCs leading to vasodilation and inhibition of platelet aggregation through multiple pathways, including via inhibition of thromboxane (126). Additionally, prostacyclin signaling may lead to downstream expression of endothelial NOS, leading to NO production via peroxisome proliferator-activated receptor (PPAR) activation, as well playing an important antiproliferative role via other analogs of PPAR (44). NOS levels are also reduced in platelets of patients with PAH, highlighting the role of NO as an important regulator of platelet function (13). Patients with PAH have been shown to have decreased levels of prostacyclin synthase (168), contributing to dysregulated endothelial and vascular SMCs in the pathobiology of this disease. Since the discovery of prostacyclin as an important regulator of pulmonary vasculature, several therapeutics have been developed. Initially, the focus was on improving delivery of synthetic prostacyclin (14, 126), but more recently, efforts to identify the importance of the prostacyclin receptor itself are under way with new nonprostanoid targeted therapies (148).
The third classic pathway in the pathophysiology of PH is the endothelin-1 (ET-1) pathway. ET-1 is a potent vasoconstrictor of vascular SMCs, as well as a promoter of their proliferation. Its activity is mediated through the receptors ET-A and ET-B, the antagonism of which has been a target of therapy in PH since the 1990s. Both ET-A and ET-B act upon G protein-coupled receptors, affecting concentrations of inositol triphosphate (IP3); ET-A increases IP3, thereby stimulating calcium release into the cytosol, leading to SMC constriction, whereas ET-B has the opposite effect on IP3, ultimately leading to vasodilation and clearance of ET-1. Patients with PAH have been found to have increased circulating and lung tissue levels of ET-1, as well as increased expression of ET-A receptors. Perhaps most significantly, levels of ET-1 have been found to correlate with disease severity (24). Additionally, ET-1 is known to activate RhoA/Rho kinase, a pathway that separately has been shown to significantly contribute to pulmonary vasoconstriction in murine models of both early and late-stage PH. Furthermore, inhibition of Rho kinase demonstrated hemodynamic improvement in both models, even when other vasodilator therapies were unsuccessful. Alternatively, when ET-1 is directly antagonized, there is partial reversal of pulmonary vasoconstriction (116, 172). These data suggest that the contribution of reversible pulmonary arterial vasoconstriction to PH pathophysiology persists even to late-stage disease. In 2001, the U.S. Food and Drug Administration approved the first endothelin receptor antagonist that nonspecifically binds to ET-A and ET-B. Newer drugs have been developed to specifically target the ET-A receptor (81); however, the clinical significance of the ET-1 receptor selectivity is not clear.
The final “pathway” that is of great importance to our understanding of PH, as well as the basis of therapeutic intervention, is calcium handling and regulation within vascular SMCs. As noted in several pathways, as described above, the flow of calcium into different compartments within the cell determines the contractile nature of the SMC (58). Early interventions in PH simply blocked the influx of calcium into the cytosol via calcium channel blockers. Clinically, however, very few patients with PH have initial or sustained responses to calcium channel blockade (81, 149). Given its importance in all known pathways, calcium handling remains an important mechanism by which PH may develop and is discussed further below.
As better understanding of vascular and endothelial biology has emerged over the past decade, additional pathways, particularly those involving the mitochondria, have been found to play significant roles in the development of PH. Comprehensive reviews by Archer et al. (11), Huetsch et al. (79), Paulin et al. (123), Pugliese et al. (128), and Schumacker et al. (146) have described the various pathways and mechanisms by which mitochondrial dysfunction may play a role in the development of pulmonary hypertension, and have offered insights into future therapeutic applications. However, despite this growing literature, therapeutic development has lagged behind the basic and translational sciences. As such, only the aforementioned pathways have been translated so far into actual therapies for PH. In addition to recapitulating previously described pathways, we aim to suggest a paradigm shift in the characterization, and thus, the diagnosis and treatment of pulmonary hypertension, expanding upon already known mechanisms and pointing toward new therapeutics.
GENETIC UNDERPINNINGS OF PULMONARY HYPERTENSION
On the basis of the well-known form of familial or hereditary PH, the genetic underpinning of PH has been well established. The most prevalent genetic mutations occur in the bone-morphogenetic protein receptor-2 (BMPR2), which is a member of the transforming growth factor-beta (TGF-β) superfamily. BMPR2 deficiency has been associated with apoptosis resistance, increased inflammatory responses, and increased proliferation, some of which are related to defects in multiple mitochondrial pathways (32, 35, 151). Additionally, several other receptor types within this superfamily have been identified in cohorts of patients with PH, including BMPR1, activin receptor-like kinase 1 (ACVRL1), and endoglin, as well as BMP-related SMADs (131, 152). Recently, several researchers have proposed a “two-hit” hypothesis of PAH given the incomplete penetrance and variable expressivity of phenotype with the above mutations, parallel to that seen with neoplastic lesions. Additional somatic mutations have been observed in the lungs of patients with PAH, although it is not exactly clear whether these result from or lead to these characteristic plexigenic lesions (7, 97). Federici et al. (47) then demonstrated that similar DNA damage and mutations were more easily induced in pulmonary vascular cells from the relatives of patients with idiopathic or heritable forms of PH, than from healthy controls. These findings further suggest that DNA sensitivity to damage may be a precursor to pulmonary vascular disease.
Epigenetic modification of genes has also been found to play a role in the development of PAH, particularly microRNA (miRNA) regulation of gene expression (see Table 1). Microarray profiles of patients with PAH were analyzed, identifying over 20 different miRNAs across several studies that regulate expression of various genes and signaling pathways germane to the development of PH (45, 76, 113, 156). Several miRNAs specifically target BMPR2-related pathways, whereas the majority are unrelated, and without a clear underlying connection. The table highlights a number of these small noncoding RNAs and their relationship with BMPR2, modified from Negi and Chan (113). This is not a comprehensive list of all epigenetic phenomena known to effect pulmonary hypertension, as this list is continually expanding. Rather, this is a brief review of some of the microRNAs relevant to our discussions below.
Table 1.
Targets and effects of relevant microRNAs
| miRNA | Target and Effect |
|---|---|
| miR-17/92 | Antagomir attenuates PH in animal models by directly targeting BMPR2. |
| miR-20a | Antagomir prevents development of remodeling in PH animal models by directly targeting BMPR2 (21). |
| miR-302 | Cyclic feedback relationship with BMPR2, inhibits PASMC proliferation and migration. |
| miR-21 | Reduces expression of BMPR2, though in vivo inhibitors attenuate hypoxic vasoconstriction and subsequent vascular remodeling; targets the HIF pathway. |
| miR-322 | Acts upon BMPR1a and SMADs and promotes proliferation of PASMCs. |
| miR-125a | Increases protein concentrations of BMPR2 in PAECs leading to inhibition of cell proliferation. Hypoxia leads to upregulation of miR-125a in mouse models. However, in human subjects with PH, miR-125a circulating levels are decreased when compared with normal controls (78). |
| miR-138 and miR-25 | Impair calcium signaling via downregulation of a component of the mitochondrial calcium uniporter. This increases cytosolic calcium within pulmonary arterial SMCs leading to vasoconstriction and a proproliferative environment (76). |
| miR-204 and BRD4 | Downregulation of miR-204 leads to upregulation of the “epigenetic reader” bromodomain-containing protein 4 (BRD4), which, in turn, leads to overexpression of the oncogenes NFAT, survivin, and Bcl-2. The upregulation of these genes has been implicated in abnormal cellular proliferation in cancer cells, as well as in patients with pulmonary hypertension (106). |
PH, pulmonary hypertension; BMPR2, bone-morphogenetic protein receptor-2; PASMCs, pulmonary artery smooth muscle cells; HIF, hypoxia-inducible factor; PAECs, pulmonary artery endothelial cells; SMCs, smooth muscle cells.
BRIEF OVERVIEW OF CELL TYPES AFFECTED IN PULMONARY HYPERTENSION
There is complex interplay between the various cells that make up the pulmonary vasculature, with dysregulated intercellular communication as the origin of PH. It was first hypothesized that SMCs and myofibroblasts were the critical cell types affected in PH; however, newer evidence points to complicated communications between all cells within the vasculature. In 2005, Sakao et al. (141) hypothesized that the cascade of events that lead to the vascular remodeling characteristic of pulmonary vascular disease begins with early apoptosis of the pulmonary endothelial cell, leading to hyperproliferation of “apoptosis-resistant” endothelial cells. In general, endothelial cells display a propensity to proliferate, a necessary feature to rapidly repair when injured. There are, however, different subpopulations that possess a greater propensity for proliferation than others. Alvarez et al. (8) demonstrated that pulmonary microvascular cells, in particular, grow nearly two times faster than other populations. Furthermore, they demonstrated that the pulmonary vasculature contains a significant proportion of progenitor cells with much higher vasculogenic capacity. Sakao et al. (141) further demonstrated that when naïve endothelial cells were placed in media that was conditioned with apoptotic cells, the plated endothelial cells would adopt an apoptosis-resistant phenotype. Helenius et al. (74) also demonstrated this phenotype, a result of vascular SMC migration and downregulation of CD39 on the surface of endothelial cells leading to extracellular accumulation of ATP, and the resultant increase in perivascular inflammatory cells. These disorganized and hyperproliferative endothelial cells are precursors to the plexiform lesions in small precapillary pulmonary arterioles, which are the histopathologic hallmarks of PAH (157).
Plexiform lesions form in response to specific stimuli or injury, which can include hypoxemia, shear stress, inflammation, drug, or toxin, likely in a genetically susceptible host. Injury alters endothelial cell proliferation, apoptosis, and homeostatic functions, such as coagulation pathways, and response to growth factors and vasoactive agents (80). Defects in growth-suppressive genes and increased levels of angiogenic factors, such as PDGF and VEGF have been found in plexiform lesions (140, 184), which are often characterized by clonal populations of endothelial cells, suggesting these sites play a role in endothelial proliferation (167). Additionally, the crosstalk between endothelial cells and pericytes is important to vascular remodeling. Ricard et al. (134) hypothesized that pulmonary endothelial cell dysfunction leads to abnormal microvascular pericyte distribution, causing pulmonary arterial medial thickening, via abnormal fibroblast growth factor-2 and IL-6 signaling.
SMCs and myofibroblasts also play an important role in the vascular remodeling of precapillary arterioles. SMCs migrate distally along the arteriole toward the respiratory acinus, adding SMCs to precapillary pulmonary arterioles that were previously nonmuscularized. A new layer of myofibroblasts and extracellular matrix forms between the endothelium and the internal elastic lamina, termed the neointima. This altered extracellular matrix has increased expression of collagens, matrix metalloproteinase 19, disintegrin, and metalloprotease 33 in both intimal and medial layers (75, 154). The cellular mechanisms of these processes are not well understood; however, hypoxia models suggest that fibroblasts in the adventitia may be the first to differentiate to myofibroblasts and lead to the cascade of migration and proliferation (154). Evidence suggests that the serotonylation of fibronectin by tissue transglutaminase likely plays a role in this tissue migration, as demonstrated in hypoxia-induced PH animal models (125, 176). Neovascularization occurs following the formation of the neointima, with blood vessels forming in the now thickened adventitia and media (80, 128). There is also evidence from animal models that SMCs play a role in balancing cytosolic and mitochondrial ROS in response to cyclic stretching, leading to downstream expression of growth factors for both endothelial cells and SMCs. In models already demonstrating a propensity for proliferation, this leads to a so-called “feed forward” mechanism of growth (174).
Macrophages and lymphocytes have also been found histologically near plexiform lesions in a subset of patients, suggesting an inflammatory component to this pathogenesis (167), although the specific contribution from the adaptive immune system is not well characterized. Maston et al. (102) found that genetic deletion of the recombination-activating gene 1 in mice (RAG1−/−), which lack mature B and T cells, results in diminished right ventricular systolic pressures and less vascular remodeling compared with wild-type mice that were exposed to hypoxia. In fact, they found that RAG1−/− mice that were given T helper 17 cells developed PH independent of hypoxia. IL-13, a T-helper type-2 cell effector cytokine, has also been implicated in the pathogenesis of PAH. In one study, IL-13 stimulated cellular proliferation in human pulmonary artery SMCs (27). Additionally, chronic inflammation or immune dysregulation may be the inciting injury that causes PAH to develop in patients with human immunodeficiency virus infection or in patients with connective tissue diseases. For example, some patients with systemic lupus erythematosus have had clinical benefit of their PH from immunosuppressive therapy, underscoring the role inflammation may have in subsets of patients (38, 107).
METABOLIC PATHWAYS AND MITOCHONDRIA IN PULMONARY HYPERTENSION—THE “METABOLIC THEORY”
The “metabolic theory” of disease suggests that alterations in the bioenergetics of an organism lead to dysfunctional processes downstream, with the subsequent development of disease. This theory has been most thoroughly described in cancer biology (28, 37, 147, 159, 170), but more recently, it has been expanded to the pathobiology of PH (10, 65, 109, 147).
Specifically, the metabolic shift within an organism from energy production predominantly via aerobic respiration to that of glycolysis and fermentation, leads to a number of adaptive and maladaptive downstream effects. Endothelial cells are very sensitive to this change, particularly as they are the first to sense an internal environment low in oxygen and their importance in signaling to surrounding cells. Notably, endothelial cells from different vascular beds are quite different in their responses to stress, circulating factors, and surrounding cells (54). Because of their unique environment, some of the cells within the pulmonary vasculature rely more heavily on glycolysis, such as the pulmonary microvascular endothelial cells, which use aerobic glycolysis as the predominant source of energy (120). Other cells, such as the pulmonary artery endothelial cells, however, depend more highly on cellular respiration for their energy requirements (121, 180). These differences between cell types allow the pulmonary vasculature to be highly sensitive to small changes in oxygen concentration, and a metabolic shift to increased glycolysis is important in inducing a signaling cascade that leads to rapid vasoconstriction of the pulmonary bed to preserve ventilation-perfusion matching (177). In patients with PH, this metabolic shift occurs at higher or even normal oxygen concentrations (128). This “glycolytic shift” in the face of normoxia has been termed the “Warburg effect” after the German physician who first described this phenomenon in the 1920s, and it is associated with a more highly proliferative phenotype (54, 146, 170, 177).
Aside from simply being less efficient in energy production, this process leads to sudden shifts in reactive oxygen species (ROS) production with impaired handling of oxidative stress (22, 48), alterations in oxygen-sensing potassium channels (Kv 1.5 channels), resultant shifts in cytosolic calcium, and constriction of the pulmonary vasculature (11).
Mitochondrial and cellular biology rely on the presence of ROS for signaling and internal regulation; however, the hallmark of metabolic or mitochondrial disease is an imbalance of oxidative stress (89, 94, 146) (see Fig. 1). Typically, the production and removal of ROS are tightly regulated, particularly mitochondrial ROS (mtROS). This allows for changes in ROS content within compartments to signal downstream targets, some of which include signal transducers and transcription factors that regulate apoptosis, cellular proliferation, angiogenesis, and even gene expression. In the vascular compartment, NADPH oxidases (NOXs) are a significant source of ROS and mtROS (62). Multiple endogenous and exogenous oxidants activate NADPH, and many have been used to induce cellular injury in animal and in vitro models. Examples include hyperoxia, hypoxia, inhaled particles, xanthine oxidase, cigarette smoke, and other ROS themselves, all of which contribute to mitochondrial dysfunction by overwhelming enzymes within the OXPHOS metabolic pathway (9, 18, 69, 158). For example, Ghouleh et al. (57) recently demonstrated increased expression of Nox-1 in the pulmonary endothelium of patients with PH. This correlated to increased overall ROS production and increased expression of an antagonist to bone morphogenetic protein (BMP) and the proangiogenic factor sonic hedgehog. Conversely, deficiency in Nox-1 expression within PASMCs, which leads to decreased mtROS production, was shown to be associated with SMC proliferation and vascular remodeling (82). Other studies of hypoxia-induced pulmonary hypertension have demonstrated overexpression of Nox-4 with associated increase in ROS levels and downregulation of thioredoxin 2, a mitochondrial redox regulator (1). This is to highlight the fact that different cellular compartments may experience different degrees and different types of ROS, a fact that has significant impact on downstream expression (173).
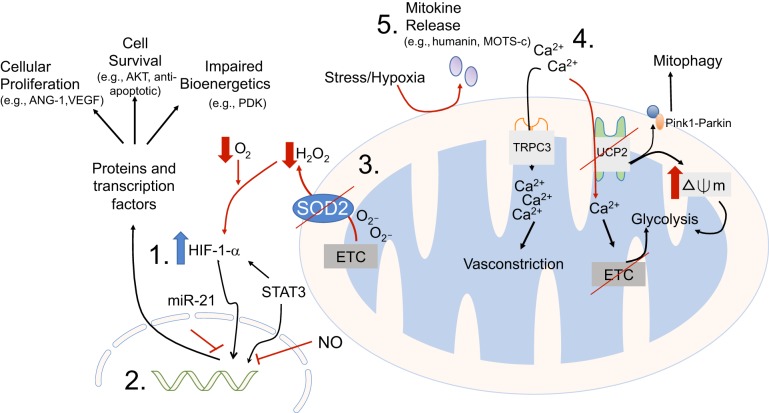
Fig. 1.
The potential mitochondrial derangements that may be present in pulmonary hypertension are many and varied. This cartoon illustrates some of the areas where molecular or targeted therapies are being explored. 1) HIF-1α pathway has been perhaps the most explored metabolic pathway in pulmonary hypertension, and has both upstream and downstream targets; 2) HIF-1α regulates expression of many genes. In this area, there have been multiple noncoding RNA molecules with potential to alter downstream gene expression; 3) upstream from HIF-1α, regulation of appropriate redox signaling through augmentation of redox enzyme SOD2 has proven effective in animal models; 4) calcium homeostasis is necessary for preventing hyperpolarization of membrane potential, and subsequent glycolytic transition; and 5) small signaling molecules derived from injured mitochondrial may prevent propagation of maladaptive phenotypes by attenuating proangiogenic and antiapoptotic responses.
Ultimately, unregulated oxidative stress leads to dysfunction and ultimately removal of impaired mitochondria through a process called mitophagy (4). In the pathobiology of disease, this contributes to reduced mitochondrial mass and impairs ATP production, further promoting a glycolytic state (89, 138). Specifically in PH, glycolysis promotes hyperpolarization of the inner mitochondrial membrane, preventing the release of proapoptotic chemicals and, in part, leading to a so-called “apoptosis-resistant” phenotype (170). Additionally, inhibitors of apoptosis are released from mitochondria when cells are under stress. These changes, in addition to other proangiogenic factors that are upregulated or altered in patients with PAH, are the basis of the metabolic theory of PH.
The resistance to apoptosis is a major factor in the pathobiology of pulmonary hypertension. First described in tumor cells, Dohi et al. (37) identified mitochondrial pools of the caspase inhibitor survivin were shown to be released into the cytosol when tumor cells had received proapoptotic signals. McMurtry et al. (104) then demonstrated that survivin is also upregulated in patients with PAH, as well as in monocrotaline rat models of PAH. Furthermore, they were able to show that levels of survivin expression in PASMCs correlated with the severity of disease, and when survivin was inhibited, measures of pulmonary hypertension were attenuated in this animal model. Michelakis et al. (108) provided further evidence of the role of apoptosis-resistant PASMCs in the development of pulmonary hypertension, when they used the metabolic modulator dichloroacetate (DCA). In addition to promoting oxidative phosphorylation via activation of pyruvate dehydrogenase, DCA also depolarizes the mitochondrial membrane via upregulation of Kv 1.5 channels, which then leads to caspase activation and increased apoptosis. Administration of this molecule to rats that developed PH after exposure to chronic hypoxia successfully reversed evidence of the disease in these animals. They then demonstrated that administration of DCA along with chronic hypoxia prevented the development of pulmonary hypertension. McMurtry et al. (105) recapitulated this effect in a model of monocrotaline-induced PH, again demonstrating an increase in apoptosis leading to reversal of the PH phenotype.
For some time, the transcription factor hypoxia inducible factor 1α (HIF-1α) has been at the center of this theory. HIF-1α expression, which controls energy metabolism, erythropoiesis, vasomotor tone, and angiogenesis, is typically upregulated by hypoxia (11). However, Fijalkowska et al. (49) demonstrated that pulmonary endothelial cells in idiopathic PAH patients have greater HIF-1α accumulation under normoxia and hypoxia, as compared with controls. The expression of HIF-1α and its transcriptional target carbonic anhydrase IX were also increased in the endothelial cells of blood vessels with plexiform lesions in vivo (49). HIF-1α pathway is thought to be regulated by KLF5, a transcription factor that when genetically silenced, attenuates hypoxia-induced pulmonary hypertension (93). Chettimada et al. (25, 26) also found that increased glucose-6-phosphate dehydrogenase activity increased HIF-1α, which directed cells to synthesize less contractile proteins, and more proliferative proteins in PASMCs.
Increased HIF-1α is also caused by decreased levels of NO and manganese superoxide dismutase (MnSOD or SOD2) activity. Sato et al. first published on SOD2 deficiency in fawn-hooded rats, leading to the spontaneous development of pulmonary hypertension (142), and the induction of other isoforms of superoxide dismutase has been associated with the development of pulmonary hypertension (132), although exact mechanisms had not been clear. Fijalkowska et al. (49) showed that decreases in SOD2 in normal endothelial cells was sufficient to increase HIF-1α expression under normoxia. Furthermore, HIF-1α knockout mice exhibit increased numbers of mitochondria, suggesting that increased expression of HIF-1α likely contributes to decreased mitochondria, often seen in PH endothelial cells. Additionally, Bonnet et al. (19) demonstrated this same mechanism in the fawn-hooded rats, further establishing this connection. SOD2 is the enzyme responsible for converting superoxide, which is produced early in the electron transport chain, into H2O2. The presence of this redox molecule is necessary for signaling to “oxygen-sensors” that the environment is normoxic by keeping Kv 1.5 channels open, thereby preventing stabilization of HIF-1α. If SOD2 is deficient, then H2O2 levels fall. When SOD2 activity is restored, HIF-1α expression is again suppressed (10).
As most mitochondrial proteins, SOD2 is not produced within the mitochondria, but rather is nuclear-derived, and assembled within the cytosol, then requires transport into the mitochondria. This process is regulated by the inducible heat shock protein iHSP70. Afolayan et al. (3) describe the process whereby impairment of this chaperone mechanism due to low ATP levels leads to increased cytosolic degradation of SOD2, and thus decreased mitochondrial levels of the enzyme, impairing conversion of superoxide. This further impairs redox signaling as described above. Similarly, SOD1 deficiency leads to impaired conversion of superoxide to hydrogen peroxide. However, the mechanism seems to be different in that SOD1 (−/−) mice also develop PH spontaneously, but the phenotype is not augmented by chronic hypoxia, and appears to be driven by activation of the transcription factor NFAT (132). Bonnet et al. demonstrated that NFAT activation led to changes in calcium handling (increased cytosolic Ca2+), and decreased density of Kv 1.5 channels leading to similar mechanistic outcome, as described above (20).
Interestingly, new evidence suggests that there may be alternative mechanisms responsible for chronic hypoxia-induced PH compared with acute hypoxia-induced pulmonary vasoconstriction (HPV). Sommer et al. (150) demonstrated that SMCs of mice with Cox4i2−/− (a subtype of cytochrome c, or complex IV of the electron transport chain (ETC) were resistant to acute HPV, although continued to develop characteristic PH when exposed to chronic hypoxia. In this model, HIF-1α stabilization was not affected, suggesting that mitochondrial ROS production alone is not sufficient for its stabilization.
Additionally, both pulmonary and total body NO is reduced in PAH patients (59, 83, 98). In normoxia, the presence of NO mimics the effects of hypoxia, and HIF-1α levels increase, whereas low levels of NO decrease HIF-1α levels. In hypoxia, however, increasing levels of NO reduce HIF-1α in the endothelial cells of normal hosts (95) by blocking cellular respiration, which enables a higher level of overall intracellular O2 that results in HIF-1α degradation (68). In the endothelial cells of patients with PH, high levels of NO resulted in increased HIF-1α expression under normoxia, whereas low levels of supplemented NO reduced HIF-1α. High levels of NO decreases the need for the endothelial cells to synthesize their own NO. This suggests that the loss of NO production, via decreased endothelial NO synthesis, may result in the activation of HIF-1α under normoxia in patients with PH (49).
NO regulates cellular respiration and mitochondrial biogenesis. In models of primary pulmonary hypertension, there is decreased eNOS activity, which is associated with mitochondrial impairment and decreased ATP levels and dysregulated endothelial angiogenesis (2, 85). When treated with inhaled nitric oxide or an NO donor, such as detaNONOate, mitochondrial biogenesis was restored along with ATP levels (2). Similarly, Xu et al. (180) showed that the endothelial cells of patients with PH had decreased mitochondrial dehydrogenase activity and lower numbers of mitochondria and mitochondrial DNA content per cell, all of which increased after exposure to NO. This restoration of mitochondrial mass and return to appropriate redox balance in response to increased NO appears to be mediated by PGC-1α with downstream effects on AMPKα, Sirt-1, and eNOS. In fetal lambs, this balance was negatively affected by increased oxygen levels (2). Xu et al. (180) also found that although ATP content under normoxia was similar in the endothelial cells of subjects with PH, as compared with normal controls, cellular ATP levels did not change significantly in PH cells under hypoxia. This suggests that the endothelial cells of normal subjects are more dependent on cellular respiration for energy under hypoxia than PH cells. Additionally, the endothelial cells of subjects with PH were found to have a threefold greater glycolytic rate, which provides evidence of altered metabolism in these cells.
More recently, a role for the transcription factor STAT3 has been described. Originally known for its role in acute phase reactions, such as activation by the cytokine IL-6 and interacting with JAK (182), STAT3 has also been identified as a promotor of VEGF and other angiogenic factors. Its role in vessel proliferation and prosurvival mechanisms has again been demonstrated in tumor cell lines (114), and evidence has accumulated for a similar role in PH. STAT3 activation is significantly increased in endothelial cells of subjects with PH and specifically localizes to areas of plexiform lesions. Furthermore, when STAT3 is inhibited, so is the hyperproliferative phenotype observed in PH (101). STAT3 leads to the activation of survivin, NFAT, and Bcl-2, all of which promote an apoptosis-resistant environment (123), and promotes HIF-1α expression and signaling, further supporting a proremodeling phenotype (101, 155). Independent of its transcriptional activity, STAT3 has also been shown to directly regulate mitochondrial function. Wegrzyn et al. (175) discovered that STAT3 localizes to complexes I and II of the ETC, and further work has identified its role in regulating a number of functions, including calcium homeostasis (182).
As alluded to earlier, intracellular calcium handling is a complex process and an important function of mitochondria that has only more recently been understood. Calcium homeostasis has a role in mitochondrial respiration, oxygen-sensing, and cell survival, among others (66). Increases in mitochondrial calcium leads to activation of mitochondrial and tricarboxylic acid (TCA) cycle enzymes, accelerating oxidative metabolism and, thereby, increasing ROS production (41). Oxygen-sensitive potassium channels (Kv 1.5) affect L-type voltage-gated calcium channels. As the surface expression of potassium channels declines in the presence of hypoxia (low H2O2), there is a downstream increased influx of calcium via these L-type calcium channels. In pulmonary SMCs, this leads to contraction (thus, vasoconstriction) acutely (10, 169).
In addition to the classic L-type calcium channels, a group of incompletely understood nonselective channels has been identified that regulates calcium signaling in vascular cells, called transient receptor potential channels (TRPCs) (163, 171, 183, 190). In patients and in animal models with PH, there are also greater numbers of calcium-sensitive receptors (CaSR), particularly on SMCs. These CaSRs enhance calcium transport through certain TRPCs, which play a significant role in the pathogenesis of PH (163). TRPC3 has been found to localize to the inner mitochondrial membrane of SMCs and augment mitochondrial influx of calcium (188). Wang et al. (171) demonstrated that the presence of TRPC3 led to increased vasoconstriction, and, thus, increased systemic hypertension in an animal model. Furthermore, inhibition of TRPC3 by telmesartan reduced ROS production and improved mitochondrial respiration. Additionally, Teshima et al. (165) identified that overexpression of the mitochondrial protein, uncoupling protein-2 (UCP2) in cardiomyocytes prevented excessive influx of calcium into mitochondria and reduced ROS production. Conversely, if mitochondrial calcium is reduced, mitochondrial function is impaired. Dromparis et al. (40) observed that when the mitochondrial protein UCP-2 was deficient in pulmonary SMCs, calcium transfer into mitochondria from nearby endoplasmic reticulum (ER) declined, resulting in a switch to glycolysis. Additionally, these UCP2-deficient cells demonstrated impairment in calcium-sensitive pyruvate dehydrogenase, an important enzyme in the TCA cycle, further perpetuating the glycolysis pathway. Similarly, overexpression of the protein Nogo-B, a protein that tethers the ER to the mitochondria, allowing for efficient calcium transfer, has been associated with the development of PH. During ER stress, ATF6 is activated, and Nogo-B is upregulated leading to mitochondrial hyperpolarization, suppression of Kv 1.5 channels, and stabilization of HIF-1α, thereby stimulating a prosurvival and apoptosis-resistant environment. Conversely, inhibition of Nogo-B promotes apoptosis in PASMCs and prevents development of the PH phenotype in animal models (160).
As illustrated by Sutendra et al. (159) and Dromparis et al. (40), the proximity of mitochondria to ER also plays an important regulatory role. There is evidence that ER wraps around mitochondria, marking several points of contact for the fission protein DLP1 to promote mitochondrial fission. This process is enhanced during periods of stress or hypoxia, when DLP1 is upregulated (88). Delmotte et al. (33, 34) expand upon this relationship with sarcoplasmic reticulum (SR) and describe a mechanism by which mitochondria move within cells based on cytosolic concentrations of calcium. They demonstrate that calcium concentrations and, thus, mitochondrial movement, is affected by certain inflammatory cytokines, leading to decreased proximity to SR and potential inability to meet metabolic demands of the cell, resulting in increased metabolic stress. Also, interestingly, during hypoxia, mitochondria have been shown to localize to the perinuclear region within a cell. This is associated with increased nuclear ROS accumulation and subsequent oxidative base damage to so-called hypoxic responsive elements of certain genes. Specifically, modification to VEGF promoter regions allows incorporation of HIF-1α, thereby upregulating VEGF expression (6, 122). Al-Mehdi et al. (6) demonstrated that when mitochondrial localization was inhibited, overall ROS production was not altered, although nuclear oxidative base damage was impaired, thereby preventing hypoxia-induced upregulation of VEGF. These observations suggest that alterations in ROS production alone is not sufficient to effect phenotypic changes, but rather location of mitochondria within the cell plays an important role in the development of PH. With ongoing advances in subcellular imaging, such as CEPIA and live imaging techniques (135, 162), the importance of the physical relationships of organelles and their molecular messengers (i.e., calcium and ROS) may be further elucidated in future studies.
Uncoupling proteins have other important effects on mitochondria. In addition to regulation of calcium handling, Teshima et al. (165) also demonstrated that increased UCP2 was associated with maintenance of mitochondrial membrane potential, suppression of cell-death markers, and ultimately with cardioprotection. The importance of well-regulated mitochondrial membrane potential has already been discussed, and hyperpolarization of this membrane has been clearly associated with the development of PH. Pak et al. (118) demonstrated that the mitochondrial membrane potentials of SMCs of subjects with PH were hyperpolarized when compared with SMCs of normal controls. This was recapitulated in monocrotaline-induced PH animal models, and again in UCP2-knockout mice. Characteristic of other PH models, the UCP2-knockout mice also demonstrated the proproliferative and antiapoptotic phenotype.
Following from the identification of UCP2 as an important mediator in the development of a PH-phenotype in SMCs, interest arose in exploring other cell types. Our group examined the role of UCP2 in endothelial cells. We used intermittent hypoxia as a model of oxidant-induced PH to identify the role of mitophagy via mitochondrial UCP2 in the development of PH (72). Mitophagy is the selective autophagy of mitochondria, and is an important quality control mechanism that eliminates damaged mitochondria, although defects in mitophagy have been implicated in certain cancers and a number of pulmonary diseases (4, 28, 87, 139). Specifically, the imbalance between mitochondrial biogenesis and mitochondrial turnover leads to functional impairment of the cell. This relationship has been demonstrated in various pathological processes, as well as in the process of aging (119). In terms of its role in the development of pulmonary hypertension, there is evidence of increased mitophagy and decreased mitochondrial biogenesis in human patients with PAH, as well as in experimental mouse models of the disease (72, 138).
Mitophagy is initiated by a change in mitochondrial membrane potential, which leads to the accumulation of PTEN-induced kinase 1 (Pink1) on the outer mitochondrial membrane, leading to the recruitment of cytoplasmic Parkin and subsequent ubiquitination of damaged mitochondria (61). Oxidative injury increases mitophagy (4, 187) and excessive mitophagy can lead to cell death (12). Our group found that the loss of endothelial UCP2 increased levels of mitophagy-associated proteins Pink1 and Parkin, which led to increased mitophagy. Additionally, Haslip et al. (72) demonstrated decreased levels of PGC-1α, a transcriptional cofactor involved in multiple pathways promoting mitochondrial biogenesis. Increased mitophagy and inadequate mitochondrial biosynthesis were shown to be associated with increased apoptosis in the endothelium. These changes were associated with physiological evidence of PH in mice, such as increased right ventricular systolic pressure and right ventricular hypertrophy. We also found that even at room air, the loss of endothelial UCP2 resulted in increased Pink1 and Parkin, and resulted in the development of spontaneous PH, emphasizing the role of endothelial mitophagy and the UCP2 pathway in the development of pulmonary vascular remodeling.
Aside from alterations in mitochondrial proteins and pathways, mitochondrial DNA (mtDNA) itself plays a significant role in the regulation of mitochondrial functioning. Although there is evidence of nuclear DNA damage contributing to pulmonary hypertension (133), the effects of mitochondrial DNA damage and mutations have been most thoroughly explored in cancer biology (23). Significant to our discussion, mtDNA is exquisitely more sensitive to oxidative damage than is nuclear DNA, especially when comparing exogenous (i.e., xanthine oxidase) vs. mitochondria-derived ROS (29, 36, 63), thereby increasing the risk of function-altering mutations in the genome (23). Interestingly, when mitochondrial oxidative repair enzymes are downregulated, there is increased cytotoxicity and subsequent apoptosis. Conversely, when these enzymes are overexpressed, there seems to be a protective effect on the cell (146). Ruchko et al. (137) demonstrated in pulmonary arterial endothelial cells that when mtDNA specifically was exposed to exogenous oxidant stress, there was increased mitochondrial dysfunction (as measured by changes in mitochondrial membrane potential as described above), and subsequent increased apoptosis. In this model, if mtDNA repair mechanisms were upregulated via the overexpression of Ogg1, mitochondrial membrane potential was spared, and these cells were protected against oxidant-induced apoptosis. These protective effects of Ogg1 were reproduced in other forms of oxidant injury, including ventilator-induced and hyperoxia-induced lung injury (71). Further studies also demonstrated a protective effect on barrier function of endothelial cells, which is not only important in models of lung injury and PH, but also inflammatory models and sepsis (29). Similarly, work that was done by our laboratory demonstrated the role of mitochondrial dysfunction and impaired turnover on worse outcomes in sepsis-induced lung injury. Using a model of MKK3-deficient mice, we identified increased turnover of defective mitochondria through a PGC-1 (peroxisome proliferator-activated receptor γ coactivator 1)-mediated mechanism, resulting in decreased ROS production, decreased apoptosis, as well as inflammation and improvements in survival (100).
Attendant to mitochondrial dysfunction and subsequent degradation of mitochondria via mitophagy, is the breakdown and recycling of mtDNA. It has been demonstrated in other models (i.e., sepsis, atherosclerosis, and cancer biology) that mtDNA is released from cells after apoptosis (and perhaps mitophagy alone), either in toto or as fragments (146, 178). These fragments are expressed as damage-associated molecular patterns (DAMPs) and have been demonstrated to play a role in innate immunity, and as such, inflammation-mediated end-points, such as plaque rupture and endothelial remodeling (178). DAMPs have been found to play a major role in activating Toll-like receptors (178), including TLR4 and TLR9. TLR4 can be found on tumor cells, and its activations leads to tumor progression (92). Additionally, TLR4 activation of platelets is associated with development of PH (16), and in models of sickle cell disease, DAMPs are associated with development of the vasculopathy that leads to PH (127). TLR9 activation, however, leads to further damage and fragmentation of mtDNA, suggesting a “feed-forward” mechanism for continued mitochondrial injury (86). DAMPS are also recognized by NOD-like receptors (NLRs), which trigger additional responses by immune cells. Notably, the NLRP3 inflammasome is activated by mitochondria-associated DAMPs and has been associated with the pathogenesis of PH (112). Additionally, when the receptor P2X7R, and upstream activator of NLR, was inhibited, the development of PH in an animal model was attenuated (185). This further suggests that mitochondrial damage is associated with perivascular inflammation and the development of PH.
Additional signaling mechanisms have been described, including “mitokines”, or mitochondrial-derived peptides. Mitokines are released in response to mitochondrial stress or dysfunction in one organ and can signal certain responses of mitochondria in other tissues (161). A number of candidate molecules have been identified, including most prominently humanin and MOTS-c, both of which have been shown to play protective roles in metabolic disease (84). Humanin was first identified in Alzheimer’s disease, but Widmer et al. (179) demonstrated that this peptide is also expressed in vascular endothelial cells, and its expression is upregulated in the presence of endothelial injury or dysfunction with higher levels being associated with improvement in blood flow, a surrogate for endothelial function. Humanin is thought to exert its protective effects through improved NO bioavailability and through both proapoptotic and antiapoptotic mechanisms (84, 166, 179). Zhang et al. (189) demonstrated that when mice were fed a diet supplemented with humanin, they expressed increased antiangiogenic proteins and inhibited angiopoietin-1, a protein that has been implicated in SMC hyperplasia in PH models (53, 96, 111), which led to attenuated vascular remodeling and fibrosis. Of note, exogenous humanin administration was also associated with increased expression of STAT3 in this model (189). Further supporting its role in mitochondrial protection, Thummasorn et al. (166) showed significant decreases in myocardial infarction size when exogenous humanin was infused before ischemic injury, and concomitant decrements in mitochondrial ROS production. Additionally, the expression of proapoptotic proteins, such as Bax and procaspase-3, was attenuated in the presence of exogenous humanin.
In addition to humanin, newer mitokines have been discovered that exert similarly “metaboloprotective” effects on cells and cellular systems. Mitochondrial open reading frame of the 12S rRNA-c (MOTS-c) and another related group called small humanin-like peptides 1–8 have been shown to act similarly to humanin in their ability to increase mitochondrial biogenesis and, thus, increase oxygen consumption and decrease ROS production. Although these molecules act on similar targets as humanin, such as the AMPK pathway, there is not yet sufficient evidence regarding their roles in disease outside of insulin-resistance and aging (84).
FUTURE DIRECTIONS AND THERAPEUTICS IN PH; WHY UNDERSTANDING MITOCHONDRIAL DYSFUNCTION IN PH IS RELEVANT TO POTENTIAL THERAPEUTICS
Current therapies do not cure the disease, and appear to have limited effects on the underlying pathobiology, which we now appreciate involves major changes in endothelial and SMC behavior, with the evolution of a glycolytic, apoptosis-resistant, and proliferative cellular phenotype, enhanced by a complex interplay of inflammation, metabolic derangements, and mitochondrial processes.
Prior to focusing on therapeutics, it may be prudent to reclassify PH based on molecular phenotype. Defining patients by clinical criteria alone is no longer sufficient to produce the advances needed in treating this disease (42). Gurtu and Michelakis (67) argue that a diagnostic, therapeutic, and research-oriented approach to PH should mimic the “precision medicine” paradigm of cancer research and therapeutics. In changing this paradigm, we may improve our approaches to treatment and continue to spark more novel therapeutics in the future (15).
One such approach would be to further characterize the metabolomics of PH. For example, Lewis GD makes a strong argument for profiling of the NO pathway, and more importantly, of “NO responsiveness” in patients with PH (90), to better understand how patients may benefit from therapy. In a separate report, Lewis et al. (91) demonstrate strong correlations with metabolic profiles and clinical phenotypes, and possibly with clinical outcomes.
Other potential areas of exploration regarding future improved characterization and diagnostics include evaluation of mitochondrial subunits themselves. There are currently well-established methods for comparing circulating mitochondrial DNA and nuclear DNA in other human diseases, namely, in cancer (145, 186). Given the numerous similarities to cancer pathobiology, it would not be surprising that we find similar correlations between circulating mitochondrial DNA numbers in PH and disease phenotype or outcomes. Furthermore, Farha et al. (46) demonstrated that independent of germline mutations, mitochondrial genetics may play a role in predisposing groups to the development of PH, making this a very interesting area for further exploration.
The treatment of PH can capitalize on already achieved milestones in precision medicine, by coopting treatments that already exist in other fields. Targeting transcription factors (e.g., STAT3, mTORC, Akt, PI3K, FoxO, NFAT, and NF-κB), in addition to dysregulated metabolic and mitochondrial signaling networks, may reverse established disease (31, 70, 117, 138). As noted by Tuder et al. (169), upregulation of HIF-1α/β alone leads to the activation of more than 100 genes individually involved in bioenergetics, apoptosis, angiogenesis, and so on, making this area ripe for therapeutic intervention. This makes the repurposing of targeted cancer or immunologic therapies a promising prospect for the treatment of PH (130). To this end, certain cancer treatments have been tested in animal models of PH and have largely provided promising results, particularly with EGFR and PDGFR inhibition demonstrating positive effects on hemodynamics, remodeling, and survival in experimental PH (17, 77, 99, 144). Unfortunately, many of these have not borne out in human studies (64, 110).
Directly targeting mitochondrial function has been studied in other disease processes, as well and may prove effective in PH. Agrawal and Mabalirajan (5) provide a useful model for considering therapeutics for mitochondria, named the 3Rs: repair, replacement, and reprogramming. In our discussion above, we have touched upon each of these areas of mitochondrial biology. In terms of repair, antioxidants and other ROS scavengers have long been considered in treatment of many disease processes, including PH, and most have proven either ineffective or not feasible. MitoQ and other mitochondrial-specific scavengers of ROS have been shown to improve mitochondrial functioning in PH (117a), as well as other metabolic disease states, such as diabetes and heart disease (164), a well as cigarette smoke-induced lung injury (9, 69). Additionally, direct repair of mitochondrial DNA has been demonstrated by the addition of exogenous mitochondria-targeted fusion proteins Ogg1 and Endo III (29). This was associated with normalization of ROS production and apoptotic mechanisms in pulmonary endothelial cells. Although this has not been directly associated with reversal of pulmonary hypertension, there are several corollary studies to suggest this strategy may provide some benefit (29, 35, 86).
Other more novel repair mechanisms have included mitochondrial regeneration or mitochondrial transplantation. Replacement of diseased or defective mitochondria via improved mechanisms of mitophagy is being explored. As noted earlier, there may be a role in inhibition of MKK3 in improving mitochondria turnover (100). Similarly, replacement or upregulation of PGC-1α may have similar beneficial effects on mitochondria turnover. Agrawal and Mabalirajan (5) describe the use of pyrroloquinoline quinone as a promoter of this pathway and may improve the balance of healthy mitochondria. Through a similar mechanism, there is evidence that salicylate, the main ingredient of aspirin, also promotes mitochondrial biogenesis through the expression of PGC-1α (181). Separately, aspirin has also been shown to attenuate hemodynamic changes and RV remodeling in monocrotaline rat models through inhibition of ERK 1/2 pathways (52).
More novel approaches to replace mitochondria have been described, including transfer of healthy mitochondria to injured cells (60, 103, 153). Zhu et al. (191) describe the successful transplantation of femoral artery-derived mitochondria into pulmonary artery endothelial and smooth muscle cells with subsequent reductions in hypoxia-induced vasoconstriction. Although they had greater success with direct intracellular transplantation, they were also able to demonstrate the feasibility and efficacy of an intravenous method of mitochondrial delivery. Additionally, in a case series of pediatric patients requiring extracorporeal membrane oxygenation after myocardial dysfunction resulting from ischemia-reperfusion injury, mitochondria were successfully autotransplanted from healthy skeletal muscles to diseased myocardium, demonstrating safety in human patients (43).
Other molecular approaches, such as targeting NOTCH3 signaling with DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) and mTORC1/2 with rapamycin have shown promise in the treatment of PH (130). SOD2 augmentation with DNA methylation inhibitors or SOD mimetic therapy (MnTBAP) (10, 79) and augmentation of mtDNA repair mechanisms such as hOgg1 (10, 79, 130) are novel approaches targeting specific metabolic pathways that are promising.
In terms of “reprogramming” mitochondria, we have already described the potential therapeutic role of epigenetic manipulation with the use of small noncoding RNA in the treatment or suppression of the PH phenotype in animal models. Further studies are actively pursuing applications in human models of disease and have shown potential (21, 30, 129).
Mitochondria play a number of important regulatory and homeostatic roles, particularly within the vasculature. As summarized, dysfunction of this complex system has been associated with many of the phenotypic changes expressed in PH. Through a better understanding of both the molecular pathways of this system, as well as the regulatory mechanisms of mitochondria themselves, we will have a more systematic and focused approach to the diagnosis and treatment of this heterogeneous and perplexing disease entity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Research Training Grant T32-HL-007778 and Research Project Grant R01-HL-138396; Veterans Administration, Office of Research and Development Grant 11858595; Department of Defense, Congressionally Directed Medical Research Program Grant PR150809; and Flight Attendant Medical Research Institute Grant 150074.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.M. prepared figures; J.D.M. and I.B. drafted manuscript; J.D.M., I.B., Y.Z., W.H.F., and P.J.L. edited and revised manuscript; P.J.L. approved final version of manuscript.
REFERENCES
- 1.Adesina SE, Wade BE, Bijli KM, Kang BY, Williams CR, Ma J, Go YM, Hart CM, Sutliff RL. Hypoxia inhibits expression and function of mitochondrial thioredoxin 2 to promote pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L599–L608, 2017. doi: 10.1152/ajplung.00258.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, Konduri GG. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L40–L49, 2016. doi: 10.1152/ajplung.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, Pritchard K, Konduri GG. Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Physiol Lung Cell Mol Physiol 306: L351–L360, 2014. doi: 10.1152/ajplung.00264.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal S, Mannam P, Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 311: L433–L452, 2016. doi: 10.1152/ajplung.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal A, Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am J Physiol Lung Cell Mol Physiol 310: L103–L113, 2016. doi: 10.1152/ajplung.00320.2015. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 5: ra47, 2012. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, Thistlethwaite PA, Tuder RM, Erzurum SC, Geraci MW, Coldren CD. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 1153–1160, 2010. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 9.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 306: L840–L854, 2014. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer SL. Acquired mitochondrial abnormalities, including epigenetic inhibition of superoxide dismutase 2, in pulmonary hypertension and cancer: therapeutic implications. Adv Exp Med Biol 903: 29–53, 2016. doi: 10.1007/978-1-4899-7678-9_3. [DOI] [PubMed] [Google Scholar]
- 11.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42, 2013. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, Dweik RA. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 302: L512–L520, 2012. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badesch DB, McLaughlin VV, Delcroix M, Vizza CD, Olschewski H, Sitbon O, Barst RJ. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl S: S56–S61, 2004. doi: 10.1016/j.jacc.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Barnes JW, Tonelli AR, Heresi GA, Newman JE, Mellor NE, Grove DE, Dweik RA. Novel methods in pulmonary hypertension phenotyping in the age of precision medicine (2015 Grover Conference series). Pulm Circ 6: 439–447, 2016. doi: 10.1086/688847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer EM, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM. Genetic deletion of Toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circ Res 114: 1596–1600, 2014. doi: 10.1161/CIRCRESAHA.114.303662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgart B, Guha M, Hennan J, Li J, Woicke J, Simic D, Graziano M, Wallis N, Sanderson T, Bunch RT. In vitro and in vivo evaluation of dasatinib and imatinib on physiological parameters of pulmonary arterial hypertension. Cancer Chemother Pharmacol 79: 711–723, 2017. doi: 10.1007/s00280-017-3264-2. [DOI] [PubMed] [Google Scholar]
- 18.Bongard RD, Myers CR, Lindemer BJ, Baumgardt S, Gonzalez FJ, Merker MP. Coenzyme Q1 as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung. Am J Physiol Lung Cell Mol Physiol 302: L949–L958, 2012. doi: 10.1152/ajplung.00251.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 104: 11418–11423, 2007. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 35: 3203–3211, 2014. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 22.Buehler PW, Baek JH, Lisk C, Connor I, Sullivan T, Kominsky D, Majka S, Stenmark KR, Nozik-Grayck E, Bonaventura J, Irwin DC. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am J Physiol Lung Cell Mol Physiol 303: L312–L326, 2012. doi: 10.1152/ajplung.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene 25: 4663–4674, 2006. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 24.Chaumais MC, Guignabert C, Savale L, Jaïs X, Boucly A, Montani D, Simonneau G, Humbert M, Sitbon O. Clinical pharmacology of endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension. Am J Cardiovasc Drugs 15: 13–26, 2015. doi: 10.1007/s40256-014-0095-y. [DOI] [PubMed] [Google Scholar]
- 25.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L287–L300, 2015. doi: 10.1152/ajplung.00229.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chettimada S, Joshi SR, Alzoubi A, Gebb SA, McMurtry IF, Gupte R, Gupte SA. Glucose-6-phosphate dehydrogenase plays a critical role in hypoxia-induced CD133+ progenitor cells self-renewal and stimulates their accumulation in the lungs of pulmonary hypertensive rats. Am J Physiol Lung Cell Mol Physiol 307: L545–L556, 2014. doi: 10.1152/ajplung.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho WK, Lee CM, Kang MJ, Huang Y, Giordano FJ, Lee PJ, Trow TK, Homer RJ, Sessa WC, Elias JA, Lee CG. IL-13 receptor α2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 304: L112–L124, 2013. doi: 10.1152/ajplung.00101.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chourasia AH, Boland ML, Macleod KF. Mitophagy and cancer. Cancer Metab 3: 4, 2015. doi: 10.1186/s40170-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol Lung Cell Mol Physiol 301: L892–L898, 2011. doi: 10.1152/ajplung.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension. Translating microRNA biology in pulmonary hypertension. It will take more than “miR” words. Am J Respir Crit Care Med 195: 167–178, 2017. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Church AC, Martin DH, Wadsworth R, Bryson G, Fisher AJ, Welsh DJ, Peacock AJ. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-α: a potential novel anti-inflammatory strategy in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 309: L333–L347, 2015. doi: 10.1152/ajplung.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies RJ, Holmes AM, Deighton J, Long L, Yang X, Barker L, Walker C, Budd DC, Upton PD, Morrell NW. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-β in pulmonary artery smooth muscle cells: role of proinflammatory cytokines. Am J Physiol Lung Cell Mol Physiol 302: L604–L615, 2012. doi: 10.1152/ajplung.00309.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca2+ in human airway smooth muscle cells. Am J Physiol Cell Physiol 303: C244–C256, 2012. doi: 10.1152/ajpcell.00414.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmotte P, Zavaletta VA, Thompson MA, Prakash YS, Sieck GC. TNFα decreases mitochondrial movement in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 313: L166–L176, 2017. doi: 10.1152/ajplung.00538.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608, 2015. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol 283: L205–L210, 2002. doi: 10.1152/ajplung.00443.2001. [DOI] [PubMed] [Google Scholar]
- 37.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 114: 1117–1127, 2004. doi: 10.1172/JCI200422222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 22: 358–363, 2003. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 39.Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension. I. Clinical and hemodynamic study. Am J Med 11: 686–705, 1951. doi: 10.1016/0002-9343(51)90020-4. [DOI] [PubMed] [Google Scholar]
- 40.Dromparis P, Paulin R, Sutendra G, Qi AC, Bonnet S, Michelakis ED. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ Res 113: 126–136, 2013. doi: 10.1161/CIRCRESAHA.112.300699. [DOI] [PubMed] [Google Scholar]
- 41.Dromparis P, Sutendra G, Michelakis ED. The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 88: 1003–1010, 2010. doi: 10.1007/s00109-010-0670-x. [DOI] [PubMed] [Google Scholar]
- 42.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, Michelakis E, Morrell NW, Stenmark K, Tuder RM, Newman J; ATS Committee on Pulmonary Hypertension Phenotypes . An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med 189: 345–355, 2014. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 154: 286–289, 2017. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Falcetti E, Hall SM, Phillips PG, Patel J, Morrell NW, Haworth SG, Clapp LH. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 1161–1170, 2010. doi: 10.1164/rccm.201001-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fares WH, Pandit KV, Kaminski N. Novel Mechanisms of Disease: Network Biology and MicroRNA Signaling in Pulmonary Hypertension. In: Pulmonary Hypertension: Basic Science to Clinical Medicine, edited by Maron BA, Zamanian RT, Waxman AB. Cham: Springer International Publishing, 2016, p. 123–133. doi: 10.1007/978-3-319-23594-3_7. [DOI] [Google Scholar]
- 46.Farha S, Hu B, Comhair S, Zein J, Dweik R, Erzurum SC, Aldred MA. Mitochondrial haplogroups and risk of pulmonary arterial hypertension. PLoS One 11: e0156042, 2016. doi: 10.1371/journal.pone.0156042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Federici C, Drake KM, Rigelsky CM, McNelly LN, Meade SL, Comhair SA, Erzurum SC, Aldred MA. Increased mutagen sensitivity and DNA damage in pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 219–228, 2015. doi: 10.1164/rccm.201411-2128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fessel JP, Flynn CR, Robinson LJ, Penner NL, Gladson S, Kang CJ, Wasserman DH, Hemnes AR, West JD. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. Am J Respir Cell Mol Biol 49: 778–787, 2013. doi: 10.1165/rcmb.2012-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 51.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 70: 580–587, 1984. doi: 10.1161/01.CIR.70.4.580. [DOI] [PubMed] [Google Scholar]
- 52.Gao H, Cheng Y, Zong L, Huang L, Qiao C, Li W, Gong B, Hu J, Liu H, Wang X, Zhao C. Aspirin attenuates monocrotaline-induced pulmonary arterial hypertension in rats by suppressing the ERK/MAPK pathway. Clin Exp Hypertens 39: 34–41, 2017. doi: 10.1080/10641963.2016.1210620. [DOI] [PubMed] [Google Scholar]
- 53.García-Lucio J, Argemi G, Tura-Ceide O, Diez M, Paul T, Bonjoch C, Coll-Bonfill N, Blanco I, Barberà JA, Musri MM, Peinado VI. Gene expression profile of angiogenic factors in pulmonary arteries in COPD: relationship with vascular remodeling. Am J Physiol Lung Cell Mol Physiol 310: L583–L592, 2016. doi: 10.1152/ajplung.00261.2015. [DOI] [PubMed] [Google Scholar]
- 54.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 68: 1–12, 2004. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369: 330–340, 2013. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh S, Gupta M, Xu W, Mavrakis DA, Janocha AJ, Comhair SA, Haque MM, Stuehr DJ, Yu J, Polgar P, Naga Prasad SV, Erzurum SC. Phosphorylation inactivation of endothelial nitric oxide synthesis in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 310: L1199–L1205, 2016. doi: 10.1152/ajplung.00092.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghouleh IA, Sahoo S, Meijles DN, Amaral JH, de Jesus DS, Sembrat J, Rojas M, Goncharov DA, Goncharova EA, Pagano PJ. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin Sci (Lond) 131: 2019–2035, 2017. doi: 10.1042/CS20160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilbert G, Ducret T, Savineau JP, Marthan R, Quignard JF. Caveolae are involved in mechanotransduction during pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L1078–L1087, 2016. doi: 10.1152/ajplung.00198.2015. [DOI] [PubMed] [Google Scholar]
- 59.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 172: 352–357, 2005. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 60.Gollihue JL, Rabchevsky AG. Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35: 70–79, 2017. doi: 10.1016/j.mito.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13: 378–385, 2012. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000. doi: 10.1161/01.ATV.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 63.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol 280: L1300–L1308, 2001. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 64.Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Manéglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 126: 3207–3218, 2016. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev 22: 543–551, 2013. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol Cell Physiol 267: C313–C339, 1994. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 67.Gurtu V, Michelakis ED. A paradigm shift is needed in the field of pulmonary arterial hypertension for its entrance into the precision medicine era. Circ Res 119: 1276–1279, 2016. doi: 10.1161/CIRCRESAHA.116.309689. [DOI] [PubMed] [Google Scholar]
- 68.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302: 1975–1978, 2003. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 69.Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol 305: L737–L746, 2013. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 70.Harvey LD, Chan SY. Emerging metabolic therapies in pulmonary arterial hypertension. J Clin Med 6: 43, 2017. doi: 10.3390/jcm6040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol 304: L287–L297, 2013. doi: 10.1152/ajplung.00071.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol 35: 1166–1178, 2015. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatano S, Strasser T (). Primary Pulmonary Hypertension. Report on a WHO Meeting. Geneva, Switzerland: World Health Organization, 1975, p. 7–45. [Google Scholar]
- 74.Helenius MH, Vattulainen S, Orcholski M, Aho J, Komulainen A, Taimen P, Wang L, de Jesus Perez VA, Koskenvuo JW, Alastalo TP. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 308: L1046–L1057, 2015. doi: 10.1152/ajplung.00340.2014. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, König P, Wilkens H, Haitchi HM, Hoefler G, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol 308: L1002–L1013, 2015. doi: 10.1152/ajplung.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong Z, Chen KH, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, Wu D, Mewburn J, Ormiston ML, Archer SL. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med 195: 515–529, 2017. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu J, Xu Q, McTiernan C, Lai YC, Osei-Hwedieh D, Gladwin M. Novel targets of drug treatment for pulmonary hypertension. Am J Cardiovasc Drugs 15: 225–234, 2015. doi: 10.1007/s40256-015-0125-4. [DOI] [PubMed] [Google Scholar]
- 78.Huber LC, Ulrich S, Leuenberger C, Gassmann M, Vogel J, von Blotzheim LG, Speich R, Kohler M, Brock M. microRNA-125a in pulmonary hypertension: regulator of a proliferative phenotype of endothelial cells. Exp Biol Med (Maywood) 240: 1580–1589, 2015. doi: 10.1177/1535370215579018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huetsch JC, Suresh K, Bernier M, Shimoda LA. Update on novel targets and potential treatment avenues in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L811–L831, 2016. doi: 10.1152/ajplung.00302.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl S: 13S–24S, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 81.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 82.Iwata K, Ikami K, Matsuno K, Yamashita T, Shiba D, Ibi M, Matsumoto M, Katsuyama M, Cui W, Zhang J, Zhu K, Takei N, Kokai Y, Ohneda O, Yokoyama T, Yabe-Nishimura C. Deficiency of NOX1/nicotinamide adenine dinucleotide phosphate, reduced form oxidase leads to pulmonary vascular remodeling. Arterioscler Thromb Vasc Biol 34: 110–119, 2014. doi: 10.1161/ATVBAHA.113.302107. [DOI] [PubMed] [Google Scholar]
- 83.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158: 917–923, 1998. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 84.Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595: 6613–6621, 2017. doi: 10.1113/JP274472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konduri GG, Afolayan AJ, Eis A, Pritchard KA Jr, Teng RJ. Interaction of endothelial nitric oxide synthase with mitochondria regulates oxidative stress and function in fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 309: L1009–L1017, 2015. doi: 10.1152/ajplung.00386.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuck JL, Obiako BO, Gorodnya OM, Pastukh VM, Kua J, Simmons JD, Gillespie MN. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 308: L1078–L1085, 2015. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16: 992–1003, 2014. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee H, Yoon Y. Mitochondrial fission: regulation and ER connection. Mol Cells 37: 89–94, 2014. doi: 10.14348/molcells.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leopold JA, Maron BA. Molecular mechanisms of pulmonary vascular remodeling in pulmonary arterial hypertension. Int J Mol Sci 17: 761, 2016. doi: 10.3390/ijms17050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis GD. The emerging role of metabolomics in the development of biomarkers for pulmonary hypertension and other cardiovascular diseases (2013 Grover Conference series). Pulm Circ 4: 417–423, 2014. doi: 10.1086/677369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis GD, Ngo D, Hemnes AR, Farrell L, Domos C, Pappagianopoulos PP, Dhakal BP, Souza A, Shi X, Pugh ME, Beloiartsev A, Sinha S, Clish CB, Gerszten RE. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol 67: 174–189, 2016. doi: 10.1016/j.jacc.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Yang F, Wei F, Ren X. The role of Toll-like receptor 4 in tumor microenvironment. Oncotarget 8: 66,656–66,667, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, He Y, Xu Y, Huang X, Liu J, Xie M, Liu X. KLF5 mediates vascular remodeling via HIF-1α in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L299–L310, 2016. doi: 10.1152/ajplung.00189.2015. [DOI] [PubMed] [Google Scholar]
- 94.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795, 2006. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J Biol Chem 273: 15257–15262, 1998. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 96.Maarman G, Lecour S, Butrous G, Thienemann F, Sliwa K. A comprehensive review: the evolution of animal models in pulmonary hypertension research: are we there yet? Pulm Circ 3: 739–756, 2013. doi: 10.1086/674770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, Chung WK, Benjamin N, Elliott CG, Eyries M, Fischer C, Gräf S, Hinderhofer K, Humbert M, Keiles SB, Loyd JE, Morrell NW, Newman JH, Soubrier F, Trembath RC, Viales RR, Grünig E. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat 36: 1113–1127, 2015. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machado RF, Londhe Nerkar MV, Dweik RA, Hammel J, Janocha A, Pyle J, Laskowski D, Jennings C, Arroliga AC, Erzurum SC. Nitric oxide and pulmonary arterial pressures in pulmonary hypertension. Free Radic Biol Med 37: 1010–1017, 2004. doi: 10.1016/j.freeradbiomed.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 99.Maihöfer NA, Suleiman S, Dreymüller D, Manley PW, Rossaint R, Uhlig S, Martin C, Rieg AD. Imatinib relaxes the pulmonary venous bed of guinea pigs. Respir Res 18: 32, 2017. doi: 10.1186/s12931-017-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, Merkel J, Kang MJ, Dela Cruz CS, Ahasic AM, Pisani MA, Trentalange M, West AP, Shadel GS, Elias JA, Lee PJ. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol 306: L604–L619, 2014. doi: 10.1152/ajplung.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 102.Maston LD, Jones DT, Giermakowska W, Howard TA, Cannon JL, Wang W, Wei Y, Xuan W, Resta TC, Gonzalez Bosc LV. Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L609–L624, 2017. doi: 10.1152/ajplung.00531.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion 34: 127–134, 2017. doi: 10.1016/j.mito.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 104.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95: 830–840, 2004. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 106.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, Chabot S, Ruffenach G, Henry S, Breuils-Bonnet S, Tremblay È, Nadeau V, Lambert C, Paradis R, Provencher S, Bonnet S. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res 117: 525–535, 2015. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 107.Mensah KA, Yadav R, Trow TK, Brunet CM, Fares WH. Lupus-associated pulmonary arterial hypertension: variable course and importance of prompt recognition. Case Rep Med 2015: 328435, 2015. doi: 10.1155/2015/328435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 109.Michelakis ED, Weir EK. The metabolic basis of vascular oxygen sensing: diversity, compartmentalization, and lessons from cancer. Am J Physiol Heart Circ Physiol 295: H928–H930, 2008. doi: 10.1152/ajpheart.00697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minami M, Arita T, Iwasaki H, Muta T, Aoki T, Aoki K, Yamasaki S, Matsushima T, Kato K, Takenaka K, Tanimoto K, Kamimura T, Ogawa R, Akashi K, Miyamoto T. Comparative analysis of pulmonary hypertension in patients treated with imatinib, nilotinib and dasatinib. Br J Haematol 177: 578–587, 2017. doi: 10.1111/bjh.14608. [DOI] [PubMed] [Google Scholar]
- 111.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54, Suppl: S20–S31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal 23: 1329–1350, 2015. doi: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Negi V, Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2: e91327, 2017. doi: 10.1172/jci.insight.91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21: 2000–2008, 2002. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 115.Nossaman BD, Scruggs BA, Nossaman VE, Murthy SN, Kadowitz PJ. History of right heart catheterization: 100 years of experimentation and methodology development. Cardiol Rev 18: 94–101, 2010. doi: 10.1097/CRD.0b013e3181ceff67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 117.Ortiz LA, Champion HC, Lasky JA, Gambelli F, Gozal E, Hoyle GW, Beasley MB, Hyman AL, Friedman M, Kadowitz PJ. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol 282: L1209–L1221, 2002. doi: 10.1152/ajplung.00144.2001. [DOI] [PubMed] [Google Scholar]
- 117a.Pak O, Scheibe S, Esfandiary A, Gierhardt M, Sydykov A, Logan A, Fysikopoulos A, Veit F, Hecker M, Kroschel F, Quanz K, Erb A, Schafer K, Fassbinder M, Alebrahimdehkordi N, Ghofrani HA, Schermuly RT, Brandes RP, Seeger W, Murphy MP, Weissmann N, Sommer N. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J. [Ahead-of-print 2018 Feb 1.] doi: 10.1183/13993003.01024-2017. [DOI] [PubMed] [Google Scholar]
- 118.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, Sydykov A, Haag D, Esfandiary A, Kojonazarov B, Veit F, Fuchs B, Weisel FC, Hecker M, Schermuly RT, Grimminger F, Ghofrani HA, Seeger W, Weissmann N. Mitochondrial hyperpolarization in pulmonary vascular remodeling. Mitochondrial uncoupling protein deficiency as disease model. Am J Respir Cell Mol Biol 49: 358–367, 2013. doi: 10.1165/rcmb.2012-0361OC. [DOI] [PubMed] [Google Scholar]
- 119.Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 56: 182–188, 2014. doi: 10.1016/j.exger.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 120.Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Stevens T. Essential role of lactate in controlling the rapid proliferation of pulmonary microvascular endothelial cells (Abstract) FASEB J 23, 2009. [Google Scholar]
- 121.Parra-Bonilla G, Alvarez DF, Cioffi DL, Stevens T. Glucose restriction inhibits glycolytic flux and limits proliferation in lung microvascular endothelial cells (Abstract). FASEB J 22, 2008. [Google Scholar]
- 122.Pastukh V, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, Borchert GM, Gillespie MN. An oxidative DNA “damage” and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am J Physiol Lung Cell Mol Physiol 309: L1367–L1375, 2015. doi: 10.1152/ajplung.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paulin R, Meloche J, Bonnet S. STAT3 signaling in pulmonary arterial hypertension. JAKSTAT 1: 223–233, 2012. doi: 10.4161/jkst.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 115: 148–164, 2014. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 125.Penumatsa KC, Fanburg BL. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L309–L315, 2014. doi: 10.1152/ajplung.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pluchart H, Khouri C, Blaise S, Roustit M, Cracowski JL. Targeting the prostacyclin pathway: beyond pulmonary arterial hypertension. Trends Pharmacol Sci 38: 512–523, 2017. doi: 10.1016/j.tips.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol 308: L314–L324, 2015. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 185: 409–419, 2012. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 130.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramiro-Diaz JM, Nitta CH, Maston LD, Codianni S, Giermakowska W, Resta TC, Gonzalez Bosc LV. NFAT is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol 304: L613–L625, 2013. doi: 10.1152/ajplung.00408.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci 17: 17, 2016. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmüller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 129: 1586–1597, 2014. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 135.Rivera-Ingraham GA, Rocchetta I, Bickmeyer U, Meyer S, Abele D. Spatial compartmentalization of free radical formation and mitochondrial heterogeneity in bivalve gills revealed by live-imaging techniques. Front Zool 13: 4, 2016. doi: 10.1186/s12983-016-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Romberg E. Ueber Sklerose der Lungenarterie: Ausdermedicinischen Klinik zu Leipzig. Dtsch Arch Klin Med 48: 197–206, 1891. [Google Scholar]
- 137.Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L530–L535, 2005. doi: 10.1152/ajplung.00255.2004. [DOI] [PubMed] [Google Scholar]
- 138.Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berl) 93: 229–242, 2015. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 131: 1691–1702, 2015. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Said SI. Mediators and modulators of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 291: L547–L558, 2006. doi: 10.1152/ajplung.00546.2005. [DOI] [PubMed] [Google Scholar]
- 141.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 142.Sato K, Webb S, Tucker A, Rabinovitch M, O’Brien RF, McMurtry IF, Stelzner TJ. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. Am Rev Respir Dis 145: 793–797, 1992. doi: 10.1164/ajrccm/145.4_Pt_1.793. [DOI] [PubMed] [Google Scholar]
- 144.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet 13: 878–890, 2012. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 7: 7, 2010. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV; GRIPHON Investigators . Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 373: 2522–2533, 2015. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 149.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 111: 3105–3111, 2005. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 150.Sommer N, Hüttemann M, Pak O, Scheibe S, Knoepp F, Sinkler C, Malczyk M, Gierhardt M, Esfandiary A, Kraut S, Jonas F, Veith C, Aras S, Sydykov A, Alebrahimdehkordi N, Giehl K, Hecker M, Brandes RP, Seeger W, Grimminger F, Ghofrani HA, Schermuly RT, Grossman LI, Weissmann N. Mitochondrial complex IV subunit 4 isoform 2 is essential for acute pulmonary oxygen sensing. Circ Res 121: 424–438, 2017. doi: 10.1161/CIRCRESAHA.116.310482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, Upton P, Morrell NW. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 859–872, 2015. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, Humbert M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 62, Suppl: D13–D21, 2013. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 153.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103: 1283–1288, 2006. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest 122, Suppl: 326S–334S, 2002. doi: 10.1378/chest.122.6_suppl.326S. [DOI] [PubMed] [Google Scholar]
- 155.Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119: 1164–1172, 2015. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stevens HC, Deng L, Grant JS, Pinel K, Thomas M, Morrell NW, MacLean MR, Baker AH, Denby L. Regulation and function of miR-214 in pulmonary arterial hypertension. Pulm Circ 6: 109–117, 2016. doi: 10.1086/685079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stevens T, Gillespie MN. The hyperproliferative endothelial cell phenotype in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol L546–L547, 2007. doi: 10.1152/ajplung.00246.2007. [DOI] [PubMed] [Google Scholar]
- 158.Sturrock A, Seedahmed E, Mir-Kasimov M, Boltax J, McManus ML, Paine R III. GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury. Am J Physiol Lung Cell Mol Physiol 302: L343–L351, 2012. doi: 10.1152/ajplung.00276.2011. [DOI] [PubMed] [Google Scholar]
- 159.Sutendra G, Dromparis P, Kinnaird A, Stenson TH, Haromy A, Parker JM, McMurtry MS, Michelakis ED. Mitochondrial activation by inhibition of PDKII suppresses HIF1α signaling and angiogenesis in cancer. Oncogene 32: 1638–1650, 2013. doi: 10.1038/onc.2012.198. [DOI] [PubMed] [Google Scholar]
- 160.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, Michelakis ED. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 3: 88ra55, 2011. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab 19: 558–573, 2014. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 162.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun 5: 4153, 2014. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang H, Yamamura A, Yamamura H, Song S, Fraidenburg DR, Chen J, Gu Y, Pohl NM, Zhou T, Jiménez-Pérez L, Ayon RJ, Desai AA, Goltzman D, Rischard F, Khalpey Z, Black SM, Garcia JG, Makino A, Yuan JX. Pathogenic role of calcium-sensing receptors in the development and progression of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L846–L859, 2016. doi: 10.1152/ajplung.00050.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tang X, Luo Y-X, Chen H-Z, Liu D-P. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 5: 175, 2014. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Teshima Y, Akao M, Jones SP, Marbán E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res 93: 192–200, 2003. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 166.Thummasorn S, Apaijai N, Kerdphoo S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc Ther 34: 404–414, 2016. doi: 10.1111/1755-5922.12210. [DOI] [PubMed] [Google Scholar]
- 167.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D4–D12, 2013. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 159: 1925–1932, 1999. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 169.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 185: 260–266, 2012. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang B, Xiong S, Lin S, Xia W, Li Q, Zhao Z, Wei X, Lu Z, Wei X, Gao P, Liu D, Zhu Z. Enhanced mitochondrial transient receptor potential channel, canonical type 3-mediated calcium handling in the vasculature from hypertensive rats. J Am Heart Assoc 6: 6, 2017. doi: 10.1161/JAHA.117.005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 9: 287–296, 2009. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ward JPT. Physiological redox signalling and regulation of ion channels: implications for pulmonary hypertension. Exp Physiol 102: 1078–1082, 2017. doi: 10.1113/EP086040. [DOI] [PubMed] [Google Scholar]
- 174.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L196–L203, 2015. doi: 10.1152/ajplung.00097.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial Stat3 in cellular respiration. Science 323: 793–797, 2009. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL. Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L1273–L1279, 2012. doi: 10.1152/ajplung.00082.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med 353: 2042–2055, 2005. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 17: 363–375, 2017. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304: H393–H397, 2013. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 104: 1342–1347, 2007. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yan Y, Yang X, Zhao T, Zou Y, Li R, Xu Y. Salicylates promote mitochondrial biogenesis by regulating the expression of PGC-1α in murine 3T3-L1 pre-adipocytes. Biochem Biophys Res Commun 491: 436–441, 2017. doi: 10.1016/j.bbrc.2017.07.074. [DOI] [PubMed] [Google Scholar]
- 182.Yang R, Rincon M. Mitochondrial Stat3, the need for design thinking. Int J Biol Sci 12: 532–544, 2016. doi: 10.7150/ijbs.15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. doi: 10.1152/ajplung.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88: E2–E11, 2001. doi: 10.1161/01.RES.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 185.Yin J, You S, Liu H, Chen L, Zhang C, Hu H, Xue M, Cheng W, Wang Y, Li X, Shi Y, Li N, Yan S, Li X. Role of P2X7R in the development and progression of pulmonary hypertension. Respir Res 18: 127, 2017. doi: 10.1186/s12931-017-0603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci 89: 65–71, 2011. doi: 10.1016/j.lfs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 187.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol 1: 19–23, 2013. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 189.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE-deficient mice. Life Sci 91: 199–206, 2012. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou C, Townsley MI, Alexeyev M, Voelkel NF, Stevens T. Endothelial hyperpermeability in severe pulmonary arterial hypertension: role of store-operated calcium entry. Am J Physiol Lung Cell Mol Physiol 311: L560–L569, 2016. doi: 10.1152/ajplung.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu L, Zhang J, Zhou J, Lu Y, Huang S, Xiao R, Yu X, Zeng X, Liu B, Liu F, Sun M, Dai M, Hao Q, Li J, Wang T, Li T, Hu Q. Mitochondrial transplantation attenuates hypoxic pulmonary hypertension. Oncotarget 7: 48925–48940, 2016. doi: 10.18632/oncotarget.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]