Abstract
With every deep inspiration (DI) or sigh, the airway wall stretches, as do the airway smooth muscle cells in the airway wall. In response, the airway smooth muscle cell undergoes rapid stretch-induced cytoskeletal fluidization. As a molecular mechanism underlying the cytoskeletal fluidization response, we demonstrate a key role for the actin-severing protein cofilin. Using primary human airway smooth muscle cells, we simulated a DI by imposing a transient stretch of physiological magnitude and duration. We used traction microscopy to measure the resulting changes in contractile forces. After a transient stretch, cofilin-knockdown cells exhibited a 29 ± 5% decrease in contractile force compared with prestretch conditions. By contrast, control cells exhibited a 67 ± 6% decrease (P < 0.05, knockdown vs. control). Consistent with these contractile force changes with transient stretch, actin filaments in cofilin-knockdown cells remained largely intact, whereas actin filaments in control cells were rapidly disrupted. Furthermore, in cofilin-knockdown cells, contractile force at baseline was higher and rate of remodeling poststretch was slower than in control cells. Additionally, the severing action of cofilin was restricted to the release phase of the transient stretch. We conclude that the actin-severing activity of cofilin is an important factor in stretch-induced cytoskeletal fluidization and may account for an appreciable part of the bronchodilatory effects of a DI.
Keywords: airway smooth muscle, cofilin, cytoskeleton, deep inspiration, fluidization, stretch
INTRODUCTION
Within hollow organs such as lung, heart, great vessels, gut, and bladder, resident cells are routinely subjected to transient stretches. These stretches, in turn, can have profound physiological effects (20, 32, 33, 35, 36, 67). In the healthy young adult, for example, a deep inspiration (DI) occurs spontaneously at a frequency of roughly six times per hour (3, 59, 61). Each DI stretches the airways and their constituent human airway smooth muscle (HASM) cells. In response to that stretch, contractile tone of HASM diminishes promptly but then recovers slowly, over the time scale of minutes (38, 59, 65), and with the next DI this process is repeated. This effect is strong, in the sense that it abolishes airway smooth muscle contractile force almost completely, and, during induced bronchospasm, it is the most effective bronchodilator known (3, 28, 34, 46, 53, 61, 63). Although important contrary evidence has been noted (43, 44, 54), the preponderance of experimental evidence obtained in vitro, ex vivo, and in vivo, in animal and human studies, confirms a strong bronchodilatory effect of a DI (13, 38, 41, 45, 46, 48, 59, 61, 63, 65, 68). In individuals with asthma during a spontaneous asthma attack, however, this salutary effect of a DI is dramatically impaired or even reversed (13, 45, 46, 61, 63), as first noted by Salter more than 150 years ago (61).
The beneficial effects of a DI have been attributed to the fact that a transient stretch causes the prompt transition of the HASM cytoskeleton from a solidlike contracted state to a fluidlike relaxed state in a process called cytoskeletal fluidization (12, 38, 59, 65). The mechanism of fluidization induced by transient stretch has been attributed to disruption of actin-myosin interactions and other weak bonds within the cytoskeletal lattice, thus leading to a prompt decrease in contractile force and stiffness (12, 23, 38, 48, 51, 55, 65). In parallel with disruption of weak bonds, it is possible that rapid disassembly of filamentous actin (F-actin) might also contribute to the fluidization response (12, 57).
To probe this possibility, we examined the role of the actin-severing protein cofilin, a well-characterized actin disassembly protein. Cofilin is known to play a central role in cell division, regulation of cell shape, and cell migration (1, 2, 4, 5). Additionally, cofilin has been suggested to play an important role during smooth muscle contraction and remodeling (29, 52, 64, 71), but its potential contribution to stretch-induced cytoskeletal fluidization has not been examined. The goal of this study was to test the hypothesis that the fluidization response of HASM to a DI is mediated in large part by the severing actions of cofilin.
To test this hypothesis, we used small interfering RNA (siRNA) to suppress the expression level of cofilin in primary HASM cells. After establishing the effective knockdown of cofilin in these cells, we employed optical magnetic twisting cytometry (10) and cell-mapping rheometry (11) to measure cytoskeletal remodeling rates and cell traction dynamics in response to transient stretch. In addition, structural and molecular probes were used to investigate F-actin reorganization. We found that, compared with control cells, in cofilin-knockdown cells, 1) basal contractile forces and F-actin content were enhanced, 2) the fluidization response was appreciably blunted, and 3) the actin filament network remained largely intact after a transient stretch mimicking a DI. These results establish that, in addition to perturbing actin-myosin bonds and other weak bonds (23, 48), a DI also fluidizes the HASM cytoskeleton through the specific actions of cofilin-mediated severing of actin.
MATERIALS AND METHODS
Primary HASM cell culture and pharmacological interventions.
HASM cells were obtained from R. Panettieri (University of Pennsylvania) and grown in Ham’s F-12 nutrient mixture supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 200 μg/ml amphotericin B, 12 mM NaOH, 1.6 mM CaCl2, 2 mM l-glutamine, and 25 mM HEPES. To develop a contractile phenotype, HASM cells were serum-starved for 48 h before all experiments, and only early-passage (passages 3–8) cells were used. All protocols and use of human cells were approved by the Harvard Institutional Review Board. The following pharmacological interventions were used to modulate the baseline traction of HASM cells: 30 min of incubation in ML-7 (30 μM), 30 min of incubation in 10% FBS, 15 min of incubation in histamine (10 μM), and 15 min of incubation in N-acylethanolamide phosphate (1 μM).
siRNA transfection.
HASM cells were plated overnight and then transfected with 0.2% (by volume) Lipofectamine 3000 with either noncoding siRNAs for scrambled control (catalog no. 6568, Cell Signaling Technology) or 25 nM cofilin-targeted siRNA (catalog no. 6267, Cell Signaling Technology). The targeting sequence of this siRNA is located on exon 5 (65626886 to 65622282) of cofilin-1 and shares little homology with cofilin-2 and actin depolymerization factor (ADF); thus this transfection is unlikely to affect the expression of a similar protein, such as ADF and cofilin-2. We did not examine cofilin-2 level, but we did confirm that ADF expression is indeed not affected by our siRNA transfection (data not shown). To test our knockdown efficiency, protein expression was determined by Western blotting at 72 h.
Traction force microscopy and cell stretch.
The methodology by which tractions are measured and stretches are imposed is described in detail by Krishnan et al. (38). Briefly, fluorescent beads are embedded near the surface of a collagen-coated gel, and the bead positions are measured. The cell is then plated on that gel. The cell attaches to, spreads upon, and contracts against the gel, thereby exerting contractile forces against the gel, which cause the gel to deform elastically. Displacements of the beads are measured as the gel deforms and used to compute the traction forces (11).
Here we report traction as contractile moment, as first described by Butler et al. (11). Briefly, the contractile moment of a cell is a weighted sum of all local traction forces that are exerted on the substrate, where the local traction force is weighted by its distance from the cell centroid. The value of the contractile moment is that any spatial distribution of tractions, no matter how complicated, can be reduced to an equivalent force dipole. The contractile moment is therefore analogous to the dipole moment in electrostatics. This force dipole comprises two point forces, F, that are equal in magnitude, opposite in direction, and separated by a distance d. The contractile moment is then Fd, which is a single number and has the units of energy. If the forces remain fixed but the distance between them increases, then the contractile moment increases, and conversely.
After baseline tractions are determined in this fashion, we impose on the gel a transient strain of 10% linear amplitude that is isotropic in the plane; such a strain imposed on the gel necessarily stretches the cell on which the gel is adherent (11). When this imposed strain is subsequently released, the tractions are measured once again.
Optical magnetic twisting cytometry and bead tracking.
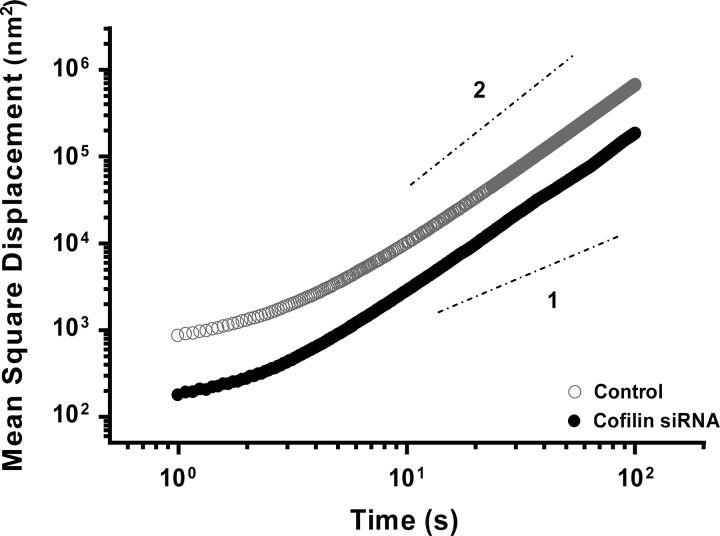
RGD peptide-coated 5-μm iron microbeads were added to serum-deprived HASM cells, which were incubated for 20 min to allow formation of focal adhesions on the beads. Spontaneous bead motions were recorded to measure cytoskeletal remodeling. As previously described, the overall rate of remodeling was quantified by the mean square displacement (MSD) over time (10, 19, 65).
Western blotting and immune labeling.
To evaluate knockdown efficiency, protein expression was determined by Western blotting at 72 h posttransfection. All experiments were performed at 72 h to ensure adequate protein knockdown. As previously described (42), HASM cells were washed twice with cold PBS, and cell lysates (10 μg) were collected on ice with phosphatase inhibitor cocktail (Roche). Equal amounts of protein lysates from each condition were separated using NuPAGE 10-well 4–12% Bis-Tris protein gel (Thermo Fisher, Waltham, MA) and then transferred onto a nitrocellulose membrane. The membrane was cut into two pieces, which were probed separately with cofilin antibody (catalog no. 5175, Cell Signaling Technology) and GAPDH antibody (catalog no. 627408, GeneTex) and then with secondary antibody (catalog nos. 205718 and 97040, Abcam). Because the antibody we used to probe cofilin expression is cofilin-1-specific (catalog no. 5175, Cell Signaling Technology), the targeting antigen of this antibody shares little homology with similar proteins, such as ADF and cofilin-2. We used GAPDH as our loading control, because the variation of GAPDH expression among different donors/transfections is less than onefold (data not shown).
For evaluation of phosphorylated cofilin, HASM cells were flash-frozen with liquid nitrogen, and cell lysates were collected on ice. To obtain phosphorylated and total cofilin levels, the same amount of protein (10 μg) from one sample was loaded onto two separate gels: one gel was used to determine the total cofilin level using cofilin antibody (catalog no. 5175, Cell Signaling Technology), and the other was used to determine the level of phosphorylated (Ser3) cofilin (catalog no. 3313, Cell Signaling Technology). The specificity of phosphorylated cofilin antibody (catalog no. 3313, Cell Signaling Technology) is confirmed by the manufacturer and previous studies (14, 37). The ratio of phosphorylated to total cofilin was computed and is presented in Fig. 6.
Fig. 6.
Cofilin knockdown reduces cytoskeletal remodeling in HASM cells. Mean square displacement (MSD) of microbeads adherent to cytoskeleton from control and cofilin-knockdown cells; cofilin knockdown leads to a slower rate of remodeling, as evidenced by a downward shift of the MSD curve. Dashed lines indicate diffusion exponents: diffusive (slope 1) and ballistic (slope 2).
For cofilin and actin fluorescence imaging, HASM cells were fixed using 4% paraformaldehyde and treated for 15 min with 1% Triton X-100. F-actin was then labeled with Alexa Fluor 488-phalloidin (Thermo Fisher). Cofilin was labeled with cofilin antibody (1:500 dilution; catalog no. 5175, Cell Signaling Technology) overnight and then with fluorescent secondary antibody (1:200 dilution; Thermo Fisher). For live actin imaging, HASM cells were incubated with a silicon-rhodamine (SiR)-actin imaging kit following the manufacturer’s protocol (Cytoskeleton, Denver, CO). All images were obtained at the same settings using a fluorescence microscope (model DMi8, Leica, Wetzlar, Germany) and processed using the same setting in ImageJ, as previously described (9).
Statistics.
All statistics were performed in Matlab (MathWorks, Natick, MA). A standard two-tailed t-test was used for the data set with two groups. A one-way ANOVA followed by post hoc multiple-comparison test with Tukey-Kramer correction was used to compare data sets of three or more groups. P < 0.05 was considered statistically significant.
RESULTS
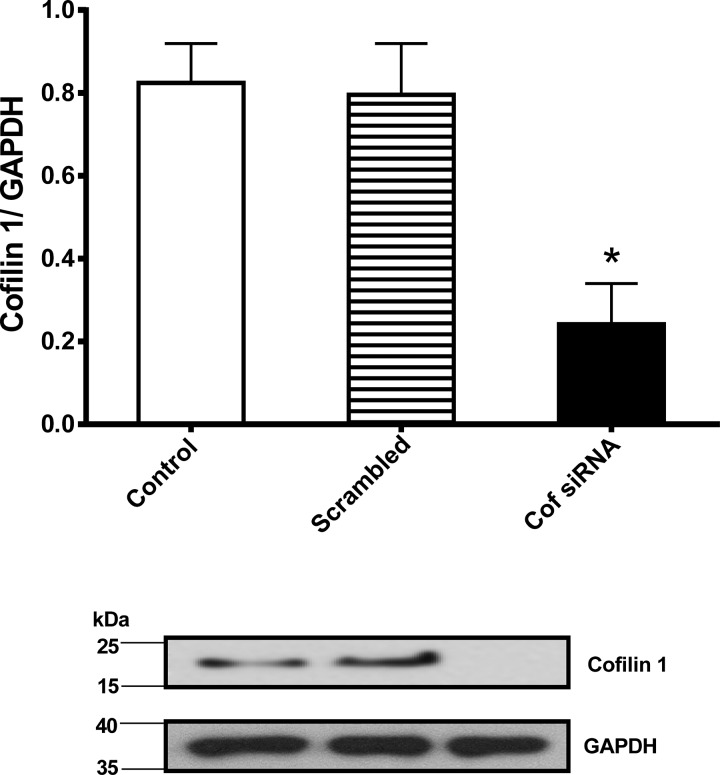
Transfection efficiency was confirmed in HASM cell lysates at 72 h after siRNA transfection. Cofilin level was then evaluated by Western blotting, with GAPDH used for loading control. Three separate transfections were performed on HASM cells from three different donors, and cofilin expression was normalized to GAPDH loading control. Cofilin expression was significantly lower in the knockdown group than in control HASM cells normalized to loading control (0.29 ± 0.11 vs. 0.84 ± 0.16, n = 5, P = 0.0026; Fig. 1). As expected, the scrambled control showed no difference in normalized cofilin expression (0.8 ± 0.21, n = 5, P = 0.78; Fig. 1).
Fig. 1.
Cofilin knockdown in human airway smooth muscle (HASM) cells. Quantification (top) of a representative Western blot (bottom) showing substantially reduced protein expression levels of cofilin (Cof) at 72 h after siRNA transfection. Values are means ± SD; n = 5. *P < 0.05 (by ANOVA).
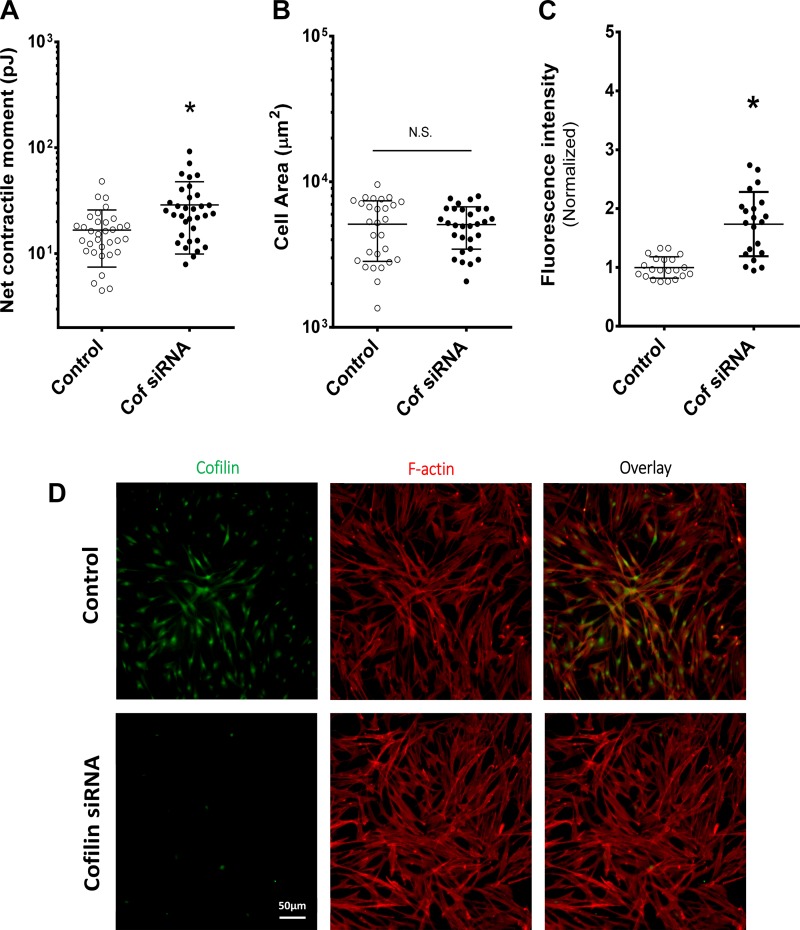
To further determine how cofilin influences cell structure and mechanical properties, we assayed cell traction forces and cytoskeletal structure (F-actin). First, using traction force microscopy, we measured the integrated contractile force generated by each cell. Before stretch, the baseline contractile moment was significantly higher in the cofilin-knockdown than control group (28.8 ± 3.3 vs. 16.6 ± 1.6 pJ, n = 33, P = 0.0015; Fig. 3A). The contractile moment depends on traction forces and their spatial distribution, but cofilin knockdown did not show a difference in spreading area compared with control (n = 28, P = 0.79; Fig. 2B). However, the cofilin knockdown in HASM cells did lead to structural changes in the cytoskeleton, especially the F-actin content (Fig. 2D). The normalized fluorescence intensity of F-actin was significantly higher for cofilin-knockdown than control HASM cells (n = 21, P = 0.00012; Fig. 2C).
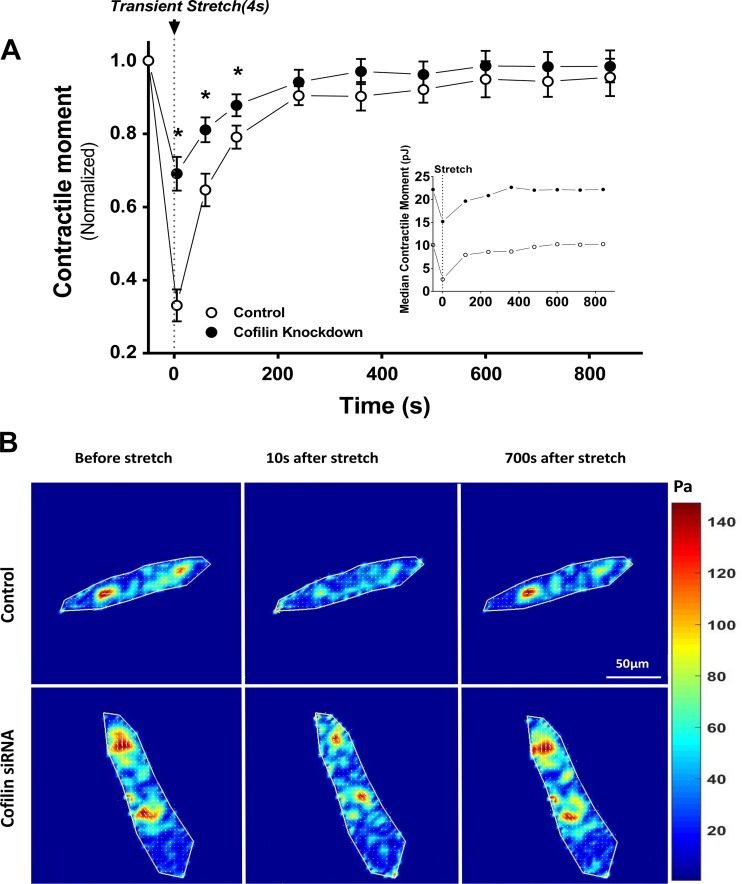
Fig. 3.
Cofilin regulates transient stretch-induced fluidization. A: normalized net contractile moment after a single transient isotropic stretch in control (n = 27) and cofilin-knockdown (n = 25) single isolated HASM cells. Inset: median of unnormalized contractile moment. Traction reduction is significantly less in cofilin-knockdown than control cells at 10, 70, and 130 s. Values are means ± SE.*P < 0.05 (by ANOVA). B: representative traction maps of control and cofilin-knockdown HASM cells at baseline and 10 and 700 s after stretch.
Fig. 2.
Cofilin knockdown enhances traction force and filamentous actin (F-actin) in HASM cells. A: corresponding traction force is significantly greater in cofilin-knockdown cells. Values are means ± SD; n = 33 per group. *P < 0.05 (by 2-tailed t-test). B: area of cell spreading is similar in control and cofilin-knockdown cells. Values are means ± SD; n = 28 per group. NS, not significant [P > 0.05 (by 2-tailed t-test)]. C: normalized fluorescence intensity of F-actin is significantly higher in cofilin-knockdown than control HASM cells. Values are means ± SD; n = 21 per group. *P < 0.05 (by 2-tailed t-test). D: fluorescent-labeled F-actin (red) and cofilin (green) images from control and cofilin-knockdown HASM cells.
HASM cells are subjected to stretches of 4% of their length during every breath and as much as 12–25% during a sigh or DI (3, 22, 31). In response to comparable stretches imposed in vitro, the HASM cell fluidizes (38, 59, 65). To explore the extent to which cofilin and filament disassembly account for this fluidization response, we applied 10% linear isotropic strain on control and cofilin-knockdown HASM cells and then traced the corresponding changes in traction assessed by traction microscopy (59) (Fig. 3). Consistent with our previous results (12, 38, 59, 65), transient stretches in the physiological range induced prompt fluidization and decreased contractile forces to 0.33 ± 0.04 of the prestretch values. However, the same stretch applied to cofilin-knockdown cells led to significantly less fluidization (n = 28 per group, P = 0.00013), with contractile force dropping to only 0.71 ± 0.05 of the prestretch value. Control and cofilin-knockdown cells recovered to their baseline traction within 15 min. As expected, cofilin knockdown led to greater baseline traction forces (Fig. 3A, inset). The magnitude of absolute traction decrements induced by a stretch was roughly the same in control and cofilin-knockdown cells, but the proportional changes in traction relative to baseline were much smaller in cofilin-knockdown than control cells (Fig. 3A).
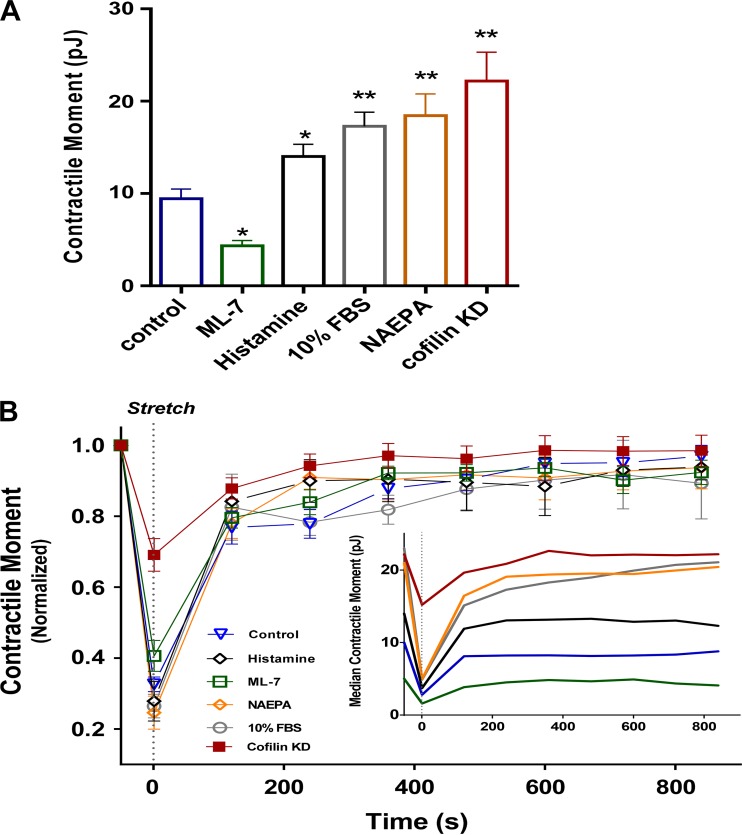
To explore the effect of different levels of baseline traction, we pretreated HASM cells with a variety of media that appreciably augment or attenuate baseline traction levels: ML-7 (30 μM), histamine (10 μM), 10% FBS, and N-acylethanolamide phosphate (1 μM). These pretreatments created baseline tractions that varied over a range of roughly fourfold (Fig. 4A). With the notable exception of cofilin knockdown, the greater the baseline traction (in absolute terms and in pJ), the greater was the absolute stretch-induced traction decrement (Fig. 4B, inset). This was not so with cofilin knockdown, however, where the absolute baseline traction was larger, but the absolute stretch-induced decrement, in most cases, was smaller. With the notable exception of cofilin knockdown, stretch caused traction to decrease to ∼30% of baseline in each instance (Fig. 4B), thus showing a potent fluidization response that depends only weakly, if at all, on the level of baseline traction. In the case of cofilin knockdown, however, stretch decreased traction to ∼70% of baseline (Fig. 4B), thereby establishing a severely blunted fluidization response.
Fig. 4.
Except in the case of cofilin knockdown, the fluidization response is roughly a constant fraction of baseline traction. A: at baseline, traction exerted by pharmacologically treated [ML-7, histamine, FBS, N-acylethanolamide phosphate (NAEPA), and cofilin knockdown (KD)] HASM cells is substantially different from that exerted by control cells. Values are means ± SE; n = 7 per group. *P < 0.05, **P < 0.001 (by ANOVA). B: despite large differences in prestretch (baseline) tractions in response to a 10% transient stretch, normalized traction changes are roughly a constant fraction of baseline traction. Values are means ± SE; n = 5 per group in all cases, except cofilin knockdown. Inset: median of unnormalized contractile moments.
Across interventions that produced a fourfold range of baseline tractions, the only case that stands out is that of cofilin knockdown. Together, these data indicate that the magnitude of the stretch-induced traction decrement was proportional to the baseline traction in the case of cofilin knockdown, where the decrement was systematically smaller.
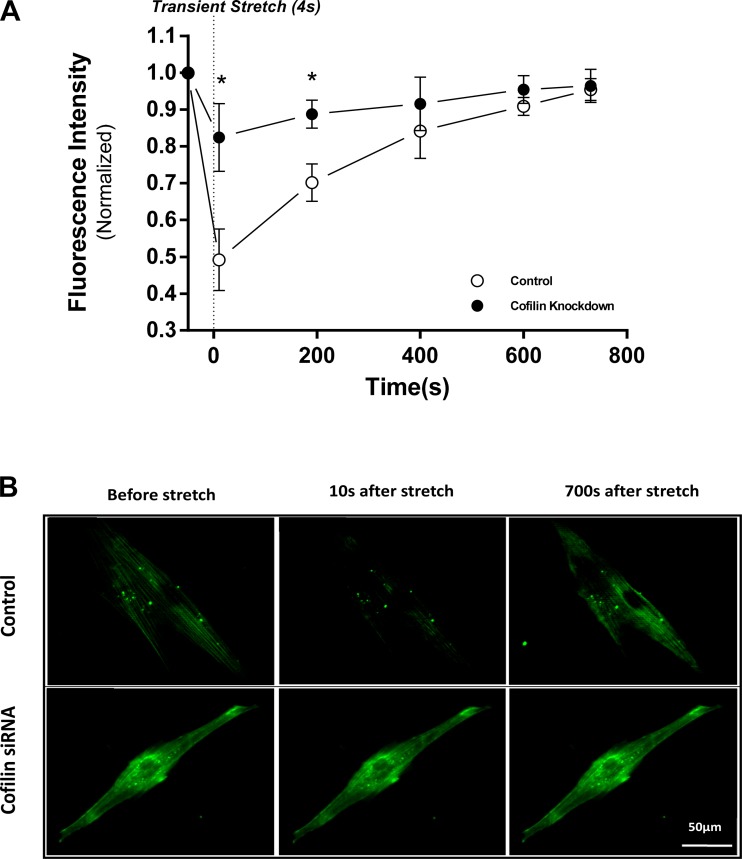
To evaluate F-actin dynamics during the fluidization response, we incubated HASM cells in control and cofilin-knockdown groups with SiR-actin (Fig. 5), which labels F-actin in live cells. In the control group, the normalized fluorescence intensity of F-actin dropped to 0.49 ± 0.04 of the prestretch value immediately after stretch, whereas in the cofilin-knockdown group, actin filaments remained largely intact and fluorescence intensity dropped to 0.825 ± 0.05, which is significantly less than in the control group (n = 9, P = 0.0009). Similar to traction dynamics, F-actin intensity remained significantly lower than control until ∼300 s after stretch. The F-actin intensity of control and cofilin-knockdown cells recovered to baseline within 15 min.
Fig. 5.
Cofilin regulates poststretch actin filament disassembly. A: normalized changes in actin fluorescence intensity after a single transient isotropic stretch in control and cofilin-knockdown HASM cells. Actin intensity is significantly higher in cofilin-knockdown than control cells at 10 and 200 s after a transient stretch. Values are means ± SD; n = 9. *P < 0.05 (by ANOVA). B: representative images of actin fluorescence at baseline and 10 and 700 s after stretch.
To probe the cytoskeletal remodeling rate of HASM cells, we used microbeads coated with integrin-binding peptide sequence RGD. Such an attached microbead can move spontaneously only if associated cytoskeletal structures to which it is attached remodel (10). Therefore, these spontaneous molecular-scale motions of an attached bead have been used as a reporter of the remodeling rate of cytoskeletal reorganization (59). MSD was measured over an average of 500 microbeads attached on ∼200 cells per group, and the evolution of MSD on the nanometer scale revealed the expected power law behavior (10, 65). However, there was a dramatic downward shift of the MSD curve in cofilin-knockdown compared with control cells (Fig. 6). These results indicate that cofilin knockdown leads to a slower cytoskeletal remodeling rate, suggesting a relatively more stable and solidlike cytoskeleton in cofilin-knockdown than control cells.
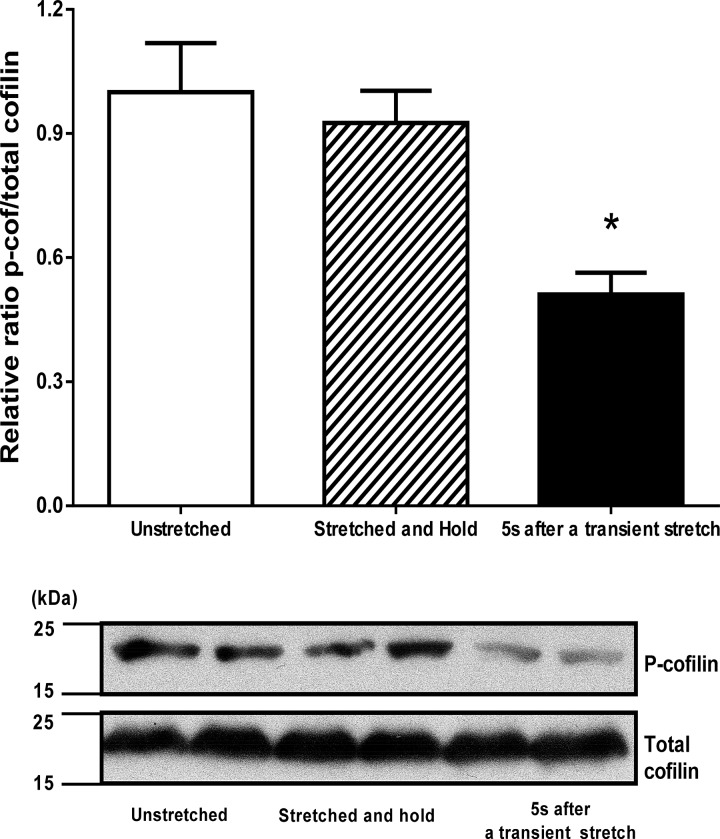
The actin-severing function of cofilin is inhibited by phosphorylation of cofilin at Ser3 through LIM kinase (56, 70). To explore when, during a transient stretch, cofilin is activated, HASM cells were flash-frozen in three different conditions: 1) at rest without stretch, 2) after a stretch-and-hold maneuver, and 3) after completion of a 4-s transient stretch. In each of these conditions, the levels of cofilin phosphorylation were evaluated. In unstretched HASM cells, the level of phosphorylated cofilin relative to total cofilin was 0.24 ± 0.05 (n = 4; Fig. 7). After the stretch-and-hold maneuver, the phosphorylation level was unchanged (0.22 ± 0.03, n = 4, P = 0.53). After transient stretch, however, the phosphorylation level significantly decreased (0.12 ± 0.02, n = 4, P = 0.0069). Together, these observations suggest that cofilin becomes dephosphorylated and activated only after release of the initial stretch, i.e., during the unloading phase of the transient stretch (Fig. 7). That activation of cofilin is restricted to the release phase is consistent with our previous finding that the fluidization response is also restricted to the release phase (12, 38, 65).
Fig. 7.
Cofilin dephosphorylates immediately after a transient stretch. Top: level of phosphorylated cofilin (p-Cof) decreases significantly only after a transient stretch. Values are means ± SD; n = 4. *P < 0.05 (by ANOVA). However, the stretch-and-hold maneuver does not alter phosphorylated cofilin (p-cofilin) levels compared with unstretched airway smooth muscle. Bottom: representative immunoblot.
DISCUSSION
The principal findings of this study are that the actin-severing protein cofilin plays a critical role in the stretch-induced fluidization response and that the mechanism by which cofilin mediates this fluidization response is rapid disassembly of the actin filaments. These findings reveal a basic molecular mechanism of cytoskeletal fluidization in response to a transient stretch.
Airway smooth muscle possesses a force-generating capacity sufficient to close virtually every airway in the lung (8). The bronchospastic effects of HASM contraction can be largely offset, however, and the airway narrowing can be mostly mitigated through the dynamic loading of the muscle that is induced by spontaneous DIs (21, 45, 46, 53, 69). A DI can prevent excessive airway narrowing through stretch-induced fluidization followed by slow resolidification (38, 59, 65). It has been established that the resolidification response is facilitated by the action of the cytoskeletal repair protein zyxin (59). Until now, however, the underlying molecular mechanism of fluidization has remained unclear. Here we used siRNA to suppress the expression of cofilin and, thus, explore the role of cofilin in the stretch-induced fluidization response.
Without stretch, cofilin knockdown in HASM cells demonstrated a variety changes consistent with the known action of cofilin as an actin-severing protein. For example, F-actin staining suggests an accumulation of stress fibers in the cofilin-knockdown cells (Fig. 2C), which is consistent with previous findings (62). In addition, we observed significantly higher baseline traction force in cofilin-knockdown than control cells (Fig. 2A). This increase in baseline traction is likely caused by the enhanced accumulation of F-actin due to the diminished severing action of cofilin. For microbeads attached to the cytoskeleton in cofilin-knockdown compared with control cells, there was also a dramatic decrease in spontaneous motions (Fig. 5), suggesting a much slower rate of cytoskeletal remodeling and a more solidlike and stable cytoskeleton. Consistent with our finding, when another actin-severing protein, gelsolin, is suppressed in HASM cells, Mikami et al. observed similar phenotypic changes, including an elevated ratio of F- to G-actin and a slower rate of cytoskeletal remodeling (49). Overall, suppression of cofilin expression in HASM cells led to a more solidlike contractile phenotype, which is consistent with the latch state that is suspected to prevail in the asthmatic lung during a spontaneous asthma attack (39).
More importantly, when HASM cells are subjected to 10% transient stretch, the control cells fluidized immediately after stretch (Fig. 3A), as previously described (12, 38, 59, 65). However, the same stretch applied to cofilin-knockdown cells led to substantially less fluidization (Figs. 3 and 4). Furthermore, in control cells, the actin filaments were rapidly disrupted by transient stretch (Fig. 5A), which is consistent with previous findings (12, 57). However, in cofilin-knockdown cells, actin filaments remained largely intact, even after a transient stretch (Fig. 5A). It has been shown that direct application of physical force can contribute to the stretch-induced fluidization response through perturbation of actin-myosin binding and disruption of other nonspecific weak bonds (22, 23, 26, 48). Here, our results further demonstrate that a specific molecular mechanism, cofilin-mediated severing of actin, is also a key contributor to this fluidization response.
While it remains unclear how mechanical stretch triggers the severing action of cofilin, recent evidence indicates that physical forces may be essential (15, 16, 18, 24, 30, 47). Previously, we used microbeads to show that the fluidization response is restricted to the release phase of a transient stretch, where the traction of a cell after transient stretch quickly drops. During a stretch-and-hold maneuver, by contrast, traction force and stiffness of a stretched cell become elevated and largely sustained (65). Here, we showed that the activation of cofilin, similarly, is restricted to the release phase (Fig. 7), whereas the stretch-and-hold maneuver did not alter the phosphorylation level of cofilin. In support of this interpretation, Hayakawa et al. demonstrated that relaxed actin filaments are preferentially severed by cofilin (30). Thus it is likely that a transient stretch triggers the severing action of cofilin by releasing tension from a stretched cytoskeleton (7, 40, 58, 60).
The rate at which the severing action of cofilin occurs can be enhanced through cooperative binding and other actin-binding proteins (6, 27, 50, 66). However, this does not fully explain why the rate of cytoskeletal depolymerization in the unstretched state is relatively slow (7, 40, 58, 60), whereas the fluidization response after a transient stretch is virtually instantaneous (38, 59, 65). It has been established that F-actin displays spontaneous random disorder in its helical twist angle (17), and similar twist angle can also be achieved by the binding of cofilin (25). It remains unknown, however, if twist distortions of the filament that are induced by a stretch can enhance the binding and severing ability of cofilin. Such a possibility is supported by our previous finding that a transient stretch can dramatically enhance the remodeling rate of the cytoskeleton (65).
In conclusion, at the level of single-cell mechanics and structure, we provide biophysical evidence that cofilin induces rapid cytoskeletal disassembly and, thereby, comprises a major molecular mechanism accounting for the HASM fluidization response to a transient stretch. This evidence suggests, further, that abnormal cofilin responses during transient stretch might impair the cytoskeletal fluidization response, which otherwise helps maintain a dynamically equilibrated open airway. Together, these findings provide a novel molecular mechanism to explain how transient stretch induced by a DI can fluidize the HASM, as described more than 150 years ago by Salter (61), and may elucidate a novel target for potential asthma therapy.
GRANTS
This work was funded by National Cancer Institute Grant 1U01-CA-202123, National Heart, Lung, and Blood Institute Grants R01-HL-107561-01, PO1-HL-120839, R21-HL-123522, and T32-HL-007118, and Canadian Institutes of Health Operating Grants MOP-13505 and MOP-97988. B. Lan is supported by Eye’s High Fellowship Award (University of Calgary).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.L., R.K., J.P.B., Q.L., W.C.C., and J.J.F. conceived and designed research; B.L., R.W., and R.P. performed experiments; B.L., R.K., C.Y.P., R.W., R.P., and J.J.F. analyzed data; B.L., R.K., C.Y.P., R.W., R.P., J.P.B., Q.L., W.C.C., and J.J.F. interpreted results of experiments; B.L. and J.J.F. prepared figures; B.L., R.K., J.P.B., and J.J.F. drafted manuscript; B.L., R.K., C.Y.P., J.P.B., Q.L., W.C.C., and J.J.F. edited and revised manuscript; B.L., R.K., C.Y.P., R.W., R.P., J.P.B., Q.L., W.C.C., and J.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Reynold Panettieri for providing primary HASM cells; Drs. Xiaofeng Jiang, Qiyu Wang, and Maoyu Sun for technical support; and Drs. Jennifer Mitchel, Yoav Green, and Stephen Decamp for critical reading of the manuscript.
REFERENCES
- 1.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell 24: 13–23, 2006. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15: 185–230, 1999. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 3.Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol 19: 195–198, 1964. doi: 10.1152/jappl.1964.19.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol 20: 187–195, 2010. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo-Cordero JJ, Magalhaes MAO, Eddy RJ, Hodgson L, Condeelis J. Functions of cofilin in cell locomotion and invasion. Nat Rev Mol Cell Biol 14: 405–415, 2013. doi: 10.1038/nrm3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J Cell Sci 124: 3305–3318, 2011. doi: 10.1242/jcs.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brieher W. Mechanisms of actin disassembly. Mol Biol Cell 24: 2299–2302, 2013. doi: 10.1091/mbc.E12-09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown RH, Mitzner W. The myth of maximal airway responsiveness in vivo. J Appl Physiol (1985) 85: 2012–2017, 1998. doi: 10.1152/jappl.1998.85.6.2012. [DOI] [PubMed] [Google Scholar]
- 9.Burgess A, Vigneron S, Brioudes E, Labbé J-C, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA 107: 12564–12569, 2010. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater 4: 557–561, 2005. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 11.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Krishnan R, Zhou E, Ramachandran A, Tambe D, Rajendran K, Adam RM, Deng L, Fredberg JJ. Fluidization and resolidification of the human bladder smooth muscle cell in response to transient stretch. PLoS One 5: e12035, 2010. doi: 10.1371/journal.pone.0012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin LYM, Bossé Y, Pascoe C, Hackett TL, Seow CY, Paré PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J 40: 45–54, 2012. doi: 10.1183/09031936.00065411. [DOI] [PubMed] [Google Scholar]
- 14.Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol 5: 1083–1089, 2003. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 15.De La Cruz EM, Martiel J-L, Blanchoin L. Mechanical heterogeneity favors fragmentation of strained actin filaments. Biophys J 108: 2270–2281, 2015. doi: 10.1016/j.bpj.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson RB. Forcing filament fragmentation with cofilin. Biophys J 108: 2094–2095, 2015. doi: 10.1016/j.bpj.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egelman EH, DeRosier DJ. Image analysis shows that variations in actin crossover spacings are random, not compensatory. Biophys J 63: 1299–1305, 1992. doi: 10.1016/S0006-3495(92)81716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elam WA, Kang H, De la Cruz EM. Biophysics of actin filament severing by cofilin. FEBS Lett 587: 1215–1219, 2013. doi: 10.1016/j.febslet.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 20.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol 281: L529–L533, 2001. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 21.Fredberg JJ. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol 106: 615–624, 2000. doi: 10.1067/mai.2000.109429. [DOI] [PubMed] [Google Scholar]
- 22.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997. doi: 10.1164/ajrccm.156.6.9611016. [DOI] [PubMed] [Google Scholar]
- 23.Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 159: 959–967, 1999. doi: 10.1164/ajrccm.159.3.9804060. [DOI] [PubMed] [Google Scholar]
- 24.Galkin VE, Orlova A, Egelman EH. Actin filaments as tension sensors. Curr Biol 22: R96–R101, 2012. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galkin VE, Orlova A, Kudryashov DS, Solodukhin A, Reisler E, Schröder GF, Egelman EH. Remodeling of actin filaments by ADF/cofilin proteins. Proc Natl Acad Sci USA 108: 20568–20572, 2011. doi: 10.1073/pnas.1110109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavara N, Roca-Cusachs P, Sunyer R, Farré R, Navajas D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys J 95: 464–471, 2008. doi: 10.1529/biophysj.107.124180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grintsevich EE, Yesilyurt HG, Rich SK, Hung R-J, Terman JR, Reisler E. F-actin dismantling through a redox-driven synergy between MICAL and cofilin. Nat Cell Biol 18: 876–885, 2016. doi: 10.1038/ncb3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gump A, Haughney L, Fredberg J. Relaxation of activated airway smooth muscle: relative potency of isoproterenol vs. tidal stretch. J Appl Physiol (1985) 90: 2306–2310, 2001. doi: 10.1152/jappl.2001.90.6.2306. [DOI] [PubMed] [Google Scholar]
- 29.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol 195: 721–727, 2011. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes JM, Hoppin FG Jr, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol 32: 25–35, 1972. doi: 10.1152/jappl.1972.32.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887, 2002. doi: 10.1161/01.RES.0000039537.73816.E5. [DOI] [PubMed] [Google Scholar]
- 33.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9: 1–34, 2007. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 34.Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol (1985. 91: 506–515, 2001. doi: 10.1152/jappl.2001.91.1.506. [DOI] [PubMed] [Google Scholar]
- 35.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17: 979–983, 1999. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol 11: 75–81, 2010. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 37.Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity 29: 404–413, 2008. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan R, Park CY, Lin Y-C, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS One 4: e5486, 2009. doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan R, Trepat X, Nguyen TTB, Lenormand G, Oliver M, Fredberg JJ. Airway smooth muscle and bronchospasm: fluctuating, fluidizing, freezing. Respir Physiol Neurobiol 163: 17–24, 2008. doi: 10.1016/j.resp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys J 88: 1387–1402, 2005. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan B, Deng L, Donovan GM, Chin LYM, Syyong HT, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC-Y, Swyngedouw NE, Banaem SM, Paré PD, Seow CY. Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L1–L10, 2015. doi: 10.1152/ajplung.00222.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan B, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC-Y, Solomon D, Paré PD, Deng L, Seow CY. Rho-kinase mediated cytoskeletal stiffness in skinned smooth muscle. J Appl Physiol (1985) 115: 1540–1552, 2013. doi: 10.1152/japplphysiol.00654.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laprad AS, Lutchen KR. The dissolution of intact airway responsiveness from breathing fluctuations: what went wrong? J Appl Physiol (1985) 110: 1506–1507, 2011. doi: 10.1152/japplphysiol.00356.2011. [DOI] [PubMed] [Google Scholar]
- 44.LaPrad AS, Szabo TL, Suki B, Lutchen KR. Tidal stretches do not modulate responsiveness of intact airways in vitro. J Appl Physiol (1985) 109: 295–304, 2010. doi: 10.1152/japplphysiol.00107.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med 186: 225–232, 2012. doi: 10.1164/rccm.201202-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim TK, Pride NB, Ingram RH Jr. Effects of volume history during spontaneous and acutely induced air-flow obstruction in asthma. Am Rev Respir Dis 135: 591–596, 1987. [DOI] [PubMed] [Google Scholar]
- 47.McCullough BR, Grintsevich EE, Chen CK, Kang H, Hutchison AL, Henn A, Cao W, Suarez C, Martiel J-L, Blanchoin L, Reisler E, De La Cruz EM. Cofilin-linked changes in actin filament flexibility promote severing. Biophys J 101: 151–159, 2011. doi: 10.1016/j.bpj.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mijailovich SM, Butler JP, Fredberg JJ. Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys J 79: 2667–2681, 2000. doi: 10.1016/S0006-3495(00)76505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikami M, Zhang Y, Danielsson J, Joell T, Yong HM, Townsend E, Khurana S, An SS, Emala CW. Impaired relaxation of airway smooth muscle in mice lacking the actin-binding protein gelsolin. Am J Respir Cell Mol Biol 56: 628–636, 2017. doi: 10.1165/rcmb.2016-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikati MA, Breitsprecher D, Jansen S, Reisler E, Goode BL. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. J Mol Biol 427: 3137–3147, 2015. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizrahi N, Zhou EH, Lenormand G, Krishnan R, Weihs D, Butler JP, Weitz DA, Fredberg JJ, Kimmel E. Low intensity ultrasound perturbs cytoskeleton dynamics. Soft Matter 8: 2438–2443, 2012. doi: 10.1039/c2sm07246g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno-Domínguez A, El-Yazbi AF, Zhu H-L, Colinas O, Zhong XZ, Walsh EJ, Cole DM, Kargacin GJ, Walsh MP, Cole WC. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J Biol Chem 289: 20939–20952, 2014. doi: 10.1074/jbc.M114.553743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadel JA, Tierney DF. Effect of a previous deep inspiration on airway resistance in man. J Appl Physiol 16: 717–719, 1961. doi: 10.1152/jappl.1961.16.4.717. [DOI] [PubMed] [Google Scholar]
- 54.Noble PB, Jones RL, Cairncross A, Elliot JG, Mitchell HW, James AL, McFawn PK. Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J Appl Physiol (1985) 114: 1460–1471, 2013. doi: 10.1152/japplphysiol.01489.2012. [DOI] [PubMed] [Google Scholar]
- 55.Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol 37: 264–272, 2007. doi: 10.1165/rcmb.2006-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E. Actin filament severing by cofilin. J Mol Biol 365: 1350–1358, 2007. doi: 10.1016/j.jmb.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pender N, McCulloch CA. Quantitation of actin polymerization in two human fibroblast sub-types responding to mechanical stretching. J Cell Sci 100: 187–193, 1991. [DOI] [PubMed] [Google Scholar]
- 58.Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol 103: 2747–2754, 1986. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosner SR, Pascoe CD, Blankman E, Jensen CC, Krishnan R, James AL, Elliot JG, Green FH, Liu JC, Seow CY, Park J-A, Beckerle MC, Paré PD, Fredberg JJ, Smith MA. The actin regulator zyxin reinforces airway smooth muscle and accumulates in airways of fatal asthmatics. PLoS One 12: e0171728, 2017. doi: 10.1371/journal.pone.0171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rottner K, Stradal TEB. Actin dynamics and turnover in cell motility. Curr Opin Cell Biol 23: 569–578, 2011. doi: 10.1016/j.ceb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Salter HH. On Asthma: Its Pathology and Treatment. London: Churchill, 1868. [Google Scholar]
- 62.Sidani M, Wessels D, Mouneimne G, Ghosh M, Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, Eddy R, Soll D, Condeelis J. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol 179: 777–791, 2007. doi: 10.1083/jcb.200707009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang DD. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir Res 16: 134, 2015. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umeki N, Hirose K, Uyeda TQP. Cofilin-induced cooperative conformational changes of actin subunits revealed using cofilin-actin fusion protein. Sci Rep 6: 20406, 2016. doi: 10.1038/srep20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7: 265–275, 2006. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Paré PD, Seow CY. Effects of length oscillation on the subsequent force development in swine tracheal smooth muscle. J Appl Physiol (1985) 88: 2246–2250, 2000. doi: 10.1152/jappl.2000.88.6.2246. [DOI] [PubMed] [Google Scholar]
- 69.Wong WD, Wang L, Paré PD, Seow CY. Bronchodilatory effect of deep inspiration in freshly isolated sheep lungs. Am J Physiol Lung Cell Mol Physiol 312: L178–L185, 2017. doi: 10.1152/ajplung.00321.2016. [DOI] [PubMed] [Google Scholar]
- 70.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393: 809–812, 1998. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 71.Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem 283: 36522–36531, 2008. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]