Abstract
Public health researchers may assume, based on the fetal origins literature, that “scarring” of birth cohorts describes the population response to modern-day stressors. We contend, based on extensive literature concerned with selection in utero, that this assumption remains questionable. At least a third and likely many more of human conceptions fail to yield a live birth. Those that survive to birth, moreover, do not represent their conception cohort. Increasing data availability has led to an improved understanding of selection in utero and its implications for population health. The literature describing selection in utero, however, receives relatively little attention from social scientists. We aim to draw attention to the rich theoretical and empirical literature on selection in utero by offering a typology that organizes this diverse work along dimensions we think important, if not familiar, to those studying population health. We further use the typology to identify important gaps in the literature. This work should interest social scientists for two reasons. First, phenomena of broad scholarly interest (i.e., social connectivity, bereavement) affect the extent and timing of selection in utero. Second, the life-course health of a cohort depends in part on the strength of such selection. We conclude by identifying new research directions and with a reconciliation of the apparent contradiction between the “fetal origins” literature and that describing selection in utero.
Keywords: Cohort selection, Pregnancy loss, Population stressors, Reproductive suppression, Evolutionary theory, Life course health
Highlights
-
•
The “fetal origins” narrative does not parsimoniously characterize the population health response to stressors in utero.
-
•
The strength of selection in utero varies substantially across populations and over time.
-
•
Cohort health among live births depends, in part, on the strength of such selection.
-
•
Increasing data availability on pregnancy losses indicates that males in the 2nd and 3rd trimester appear especially sensitive to selection in utero.
-
•
We conclude by reconciling the apparent contradiction between the “fetal origins” literature and that describing selection in utero.
1. Introduction
Scholars have long recognized that pregnancy serves as a critical period for maturation that shapes health over the life span. More recently, population health researchers from a diverse set of disciplines have examined pregnancy through the lens of evolutionary biology. This perspective treats pregnancy within the life history of the mother such that she chooses not only between current and future reproduction but also between reproductive effort and somatic (i.e., her non reproductive biology) maintenance (Vitzthum, 2008). An evolutionary perspective makes explicit the trade-offs inherent in reproductive decisions. In this paper, we introduce social scientists to research concerned with selection in utero—a mechanism anticipated by an evolutionary perspective on gestation, documented by epidemiology, and important to understanding population health.
Natural selection has had at least 150 billion gestations, or “experiments,” from which to conserve mutations that increase the likelihood of a woman yielding reproductively fit offspring. These conserved mutations sum to a strategy often labeled in the literature as “reproductive suppression (Wasser & Barash, 1983).” This strategy either blocks conception (e.g., amenorrhea) or ends gestation when mothers do not likely have sufficient resources to sustain the gestation or infancy of reproductively competent offspring. We refer to mechanisms that end gestations as “selection in utero.”
Population health scholars appear more familiar with the fetal origins literature than with that concerned with selection in utero. The “fetal origins” hypothesis asserts that exposure in utero to the maternal stress response increases the likelihood of life-limiting metabolic syndrome and chronic disease (Barker, 1998, Gluckman and Hanson, 2004, Gluckman et al., 2008, Perrone et al., 2016). Acceptance of this narrative has stimulated calls for interventions to reduce life years lost to disease “programmed” in utero (Wijesuriya et al., 2010, Koletzko et al., 2014). The theory underlying this narrative essentially posits that natural selection conserved mechanisms whereby a fetus adapts in utero to environmental circumstances stressing the prospective mother (Bateson, Barker, Clutton-Brock, Deb, & D’Udine, 2004). Whereas these adaptations may have improved the reproductive fitness of offspring through much of evolutionary time, many appear maladaptive in recent centuries.
Much research extends the fetal origins narrative to examine the lifelong “scarring” consequences of insults during pregnancy (e.g., Schulz, 2010; Dancause, Laplante, Fraser, Brunet, & Ciampi, 2012). These extensions typically do not invoke theory regarding the conservation of a stress mechanism during pregnancy. Rather, the work reports teratogenic effects of various exposures during critical periods in fetal development that afflict cohorts well after infancy. Although the quality of study designs and ability to rule out plausible rival explanations ranges widely in these studies, the field generally agrees that, among surviving birth cohorts, many teratogens during pregnancy cause morbidity later in life.
Public health researchers may assume, based on the fetal origins literature, that “scarring” best describes the population response to modern-day stressors. We contend, based on extensive literature concerned with selection in utero, that this assumption remains questionable. At least a third and likely many more of human conceptions fail to yield a live birth (Wilcox et al., 1988, Boklage, 1990). Those that survive to birth, moreover, do not represent their conception cohort. Increasing availability of data describing the fate of human conception cohorts has led to an improved understanding of selection in utero and its implications for population health.
The literature we describe should interest social scientists for two reasons. First, many phenomena they study (e.g., social connectivity, bereavement) may affect the extent and timing of selection in utero. Signals of such selection (e.g., low secondary sex ratios, low male twin ratios) appear strong, for example, in birth cohorts of mothers socially connected to disaster-affected areas (Catalano et al., 2013, Catalano et al., 2016). In addition, threats transmitted to pregnant women via the social environment likely affect the probability that a gestation survives to live birth (Catalano et al., 2017).
Second, social scientists interested in health over the life course routinely compare birth cohorts subjected to varying levels of an exposure of interest (e.g., poverty in childhood). These studies often assume that the health of cohorts at birth, over short time periods, remains relatively constant. This assumption, however, may not hold given empirical work in which heightened selective pressure on some cohorts but not others yields an uneven “playing field” at birth. Failure to account for this circumstance may lead to spurious results, which some have argued may lead to the failure (for example) of studies on prenatal famine and long-term mortality to reject the null (see review by Lumey, Stein, & Susser, 2011).
The literature describing selection in utero receives relatively little attention from population health scholars. To illustrate this point, a recent search for “selection in utero” from 1997–2017 in five population health and epidemiology journals (i.e., Social Science & Medicine, American Journal of Public Health, International Journal of Epidemiology, American Journal of Epidemiology, and Epidemiology) yielded only 13 articles. We presume that this lack of attention arises for several reasons. “Fetal origins” research may appear to contradict or diminish the relevance of selection in utero to health of infants once born. In addition, literature concerned with selection in utero comes from diverse fields, including anthropology, demography, epidemiology, evolutionary biology, human ecology, medicine, and sociology. Each of these fields uses their preferred theoretical motivation, terminology, study designs, and publication outlets. This circumstance poses a challenge for scholars trying to integrate the work into population health.
We aim to draw the attention of social scientists to the rich theoretical and empirical literature on selection in utero by offering a typology that organizes the work along dimensions we think important, if not familiar, to those studying population health. We further use the typology to identify important gaps in the literature. We conclude by reconciling the apparent contradiction between the “fetal origins” literature and that describing selection in utero.
2. Magnitude and timing of selection in utero
Most of the research into conception, implantation, and gestation has come from the clinical community. Estimates of the magnitude of pregnancy losses, from conception to birth, range from 30 to 70 percent (Wilcox et al., 1988, Boklage, 1990). Researchers derive these estimates from prospective cohort studies of healthy couples intending to conceive, as well as from integration of empirical observations across distinct periods of pregnancy. Wilcox and colleagues’ prospective examination of 221 healthy women remains the most highly cited study of the incidence of early pregnancy loss (Wilcox et al., 1988). The Authors detected pregnancies based on the presence of elevated urinary concentrations of human chorionic gonadotropin (hCG) at the expected time of implantation. They report that 22% of pregnancies ended before clinical detection and that 31% of pregnancies overall ended before a live birth.
Boklage (1990) integrates results from Wilcox and colleagues, as well as others, to focus on the challenging issue of identifying the fraction of fertilizations that evade clinical pregnancy detection. Based on a series of calculations and assumptions, Boklage concludes that at least 70 percent of natural conceptions will not survive beyond the sixth week of gestation. In addition, among pregnancies that survive to the sixth week, Boklage estimates that 10 percent will end in spontaneous loss. A separate meta-analysis estimates a cumulative incidence of 11 to 22 percent of non-elective pregnancy loss between the 5th and 20th week of gestation (Ammon Avalos, Galindo, & Li, 2012). This meta-analysis coheres with a large cohort study in Denmark in which 20.8 percent of clinically detected pregnancies that mothers intended to carry to term ended in a spontaneous loss (Buss et al., 2006). A recent population-based study of clinically detected pregnancies in Denmark reports a cumulative incidence of spontaneous loss of 11 percent (Bruckner, Mortensen, & Catalano, 2016). Researchers consider the quality of Denmark’s ascertainment of these losses as among the best in the world (Kamper-Jorgensen, 2011).
The literature does not converge on a precisely bounded estimate of the extent of selection in utero. Much of the divergence arises from different estimates of loss in the first month of pregnancy. By contrast, the literature converges on the estimate that, beyond the sixth week of gestation, spontaneous loss occurs among 10 to 22 percent of pregnancies that mothers intend to carry to term (Ammon Avalos et al. 2012).
3. Putative biological mechanisms
For our typology, we define the beginning of gestation, and pregnancy, identically—that is, after implantation of the embryo into the uterine lining. Genetic, immunologic, and endocrinology research implicates numerous mechanisms by which pregnancy loss occurs. The literature typically attributes pregnancy loss to factors unique to either the mother or to the conceptus. Among mothers, numerous anatomical, endocrine, immunological, blood-related, and genetic factors may contribute. However, none of these factors shows a high prevalence or confers a strong increased risk of pregnancy loss (Rai and Regan, 2006, Jauniaux et al., 2006).
Among couples who experience recurrent pregnancy loss after the sixth week of gestation, chromosomal anomalies in either parent occurs ten times more frequently than that of the general population (Branch, Gibson, & Silver, 2010). This evidence indicates that many chromosomal abnormalities appear incompatible with fetal development even among embryos that successfully implant. These anomalies, however, occur in less than six percent of all couples with recurrent pregnancy loss. In addition, several chromosomal anomalies among fetuses that lead to developmental defects can also survive to term (e.g., trisomy 21, also referred to as Down syndrome; see Morris, Wald, & Watt, 1999).
Human chorionic gonadotropin (hCG), a glycoprotein in maternal serum, appears as early as the second week after fertilization. Because hCG promotes maintenance of the corpus luteum and stimulates progesterone secretion from the placenta, the literature has assumed the hormone prevents miscarriage (Haig, 1993). Indeed, low levels of hCG predict spontaneous loss (Goetzl et al., 2004, Sasaki et al., 2008). But clinical trials using hCG to reduce spontaneous loss have not shown improvement in pregnancy outcome (Devaseelan, Fogarty, & Regan, 2010). Low levels of hCG, therefore, may signal, but not cause, subsequent pregnancy loss.
During the 10th to 12th week of gestation, after all major organs have formed, a local peak of spontaneous loss occurs (Goldhaber & Fireman, 1991). These losses may arise in part from a failure in the late first trimester to remodel the spiral arteries in the mother’s endometrium (Lyall et al., 2001, Burton and Fowden, 2015). This failure prevents adequate blood flow to the developing fetus. After the 12th week, placental dysfunction, as well as pathophysiology consistent with preterm parturition, are thought to lead to pregnancy loss (Silver, Branch, Goldenberg, Iams, & Klebanoff, 2011). The literature has characterized very few biological mechanisms that precede parturition, and instead suggests that multiple, redundant “biological clock” factors initiate parturition (Menon, Bonney, Condon, Mesiano, & Taylor, 2016).
4. Typology
We provide a typology of the literature that we deem germane to understanding the population health implications of selection in utero. We used our judgment to arrive at a typology which met the three following criteria. First, social scientists interested in health could replicate our assignment of articles to cells. Second, any empirical article concerned with selection in utero would “fit” into just one cell of the typology. Third, and most important, the dimensions defining the typology should have “heuristic” power in that social scientist would agree that empty or less populated cells imply important research agendas. We arrived at this typology based on the substantial time and energy we have devoted over the past ten years to understanding the disparate literature on this topic. Whereas other methods (e.g., latent Dirichlet allocation) use more objective algorithms to discover latent organizing features of a broad literature, we believe that our typology better provides social scientists with a clear understanding of the key themes of selection in utero that pertain to population health.
We attempted to minimize the subjectivity of literature included in the taxonomy in several ways. First, we created a taxonomy which allowed for a wide range of study designs, data types, and methodological approaches. This broad range may avoid preferential populating of the taxonomy by a discipline that favors a particular method or study design. Second, when identifying an exemplary article for a particular cell, we chose those with higher citation counts―an empirical (albeit imperfect) gauge of broad impact. Third, two doctoral students working in the field reviewed a draft of the manuscript with the aim of ensuring that the authors did not overlook important contributions. Fourth, we reviewed the journals in our reference list to ensure that we included a broad set of disciplines.
We organize peer-reviewed publications using the typology outlined in Table 1. Rather than attempting an exhaustive review of this interdisciplinary literature, we summarize papers that we judge to have influenced the field (based on citation volume) and/or serve as an as exemplar of a broader set of papers in that area. In addition, we introduce each section of the typology with the theoretical argument that would predict a set of empirical findings for that section.
Table 1.
Typology of research concerned with selection in utero, with key examples from the literature.
|
Inference to which gestations |
||||
|---|---|---|---|---|
| Population | Males | Other frail subgroup | ||
|
Endemic Selection |
Based on observed losses | 1 | 3 | 5 |
| Wilcox et al., 1988: In prospective study of 221 women, 31% of pregnancies end before a live birth. | Macdorman et al., 2007: Using US Vital Statistics, they report a 10% excess in male fetal deaths (vs. females). | Hardy et al., 2016: 60% of 8,319 tissue samples from spontaneous abortions show a chromosomal anomaly; percent elevated for mothers >35 years. | ||
| Avalos et al., 2012: Meta-analysis which estimates spontaneous pregnancy loss of 11-22% between 5th and 20th week of gestation. | Byrne & Warburton, 1987: In a clinical series of 683 cases, sex ratio (M:F) of chromosomally normal pregnancy losses is 1.30. | Nybo Anderson et al., 2000: In prospective study of 1.2 million pregnancies, risk of spontaneous loss increases rapidly with maternal age >35 years. | ||
| Cousens et al., 2011: Using official fetal death registration data, they report 2% fetal loss in 3rd trimester. | Orzack et al., 2015: Using 139,00 embryos from in vitro fertilization clinics, Authors estimate that, at conception, 50.2% of embryos are male. | Harlap & Shiono, 1980: Self-reported maternal alcohol use in 2nd trimester varies positively with pregnancy loss. | ||
| Flenady et al., 2011: Risk of loss >20th week elevated among mothers with lower education level. | ||||
|
Inferred from live births or fertility histories |
2 | 4 | 6 | |
| Casterline, 1989: Spontaneous pregnancy loss estimates from self-report in World Fertility Survey range from 3.7 to 14.9%. | Tyson, Parikh, Langer, Green, & Higgins, 2008: Males more common (54%) among 4,000 periviable live births delivered 22 - 25 weeks. | Leridon, 1977: Strong relation between advanced maternal age and pregnancy loss. | ||
| Zeitlin et al., 2002: In pooled analysis of 24 studies, males show a 12% increased odds of delivery <37 weeks (vs. females). | Gage, Fang, O’Neill, & DiRienzo, 2010: Based on US vital statistics, excess fetal loss among non-Hispanic blacks could account for the live birth health advantage of light, frail infants. | |||
| Platt et al., 2004: Health advantages at birth of some allegedly “high-risk” pregnancies may arise due to selection against frail fetuses late in gestation. | ||||
| Epidemic Selection | Based on observed losses | 7 | 9 | 11 |
| Haberg et al., 2013: Mother’s influenza during pregnancy varies positively with pregnancy loss >12th week. | Catalano et al., 2005: In California, the risk of male relative to female losses >20th week rises one month after rises in the unemployment rate. | |||
| Strand et al., 2012: Increased risk of fetal death late in pregnancy during high temperatures. | Bruckner et al., 2010: The terrorist attacks of September 11, 2001 correspond with a nationwide rise in male (relative to female) fetal loss >20th week. | |||
| Faiz et al., 2012: Modest increase in pregnancy loss >20 weeks following exposure to specific air pollutants. | ||||
| Hogue et al., 2013: Positive relation between self-reported significant life events and loss >20 weeks. | ||||
| Inferred from live births or fertility histories | 8 | 10 | 12 | |
| Schneider, 2017: Using 19th century data, liveborn infants in “high” stillbirth rate years show a heavier than expected birthweight distribution. | Catalano, Bruckner, Marks, & Eskenazi, 2006: Sex ratio in New York City fell months after the terrorist attacks of September 11, 2001. | |||
| Cai & Feng, 2005: Increased risk of self-reported pregnancy loss during the Great Leap Forward Famine and Cultural Revolution in China. | Torche & Kleinhaus, 2011: Decline in sex ratio among those exposed to Tarapaca earthquake at three months’ gestation. | Auger et al., 2017: Among > 700,000 pregnancies in Quebec, extreme ambient heat exposure during 1st trimester varies positively with risk of live-born atrial septal (heart) defect. | ||
| Almond & Mazumder, 2011: Decline in male births among Arab mothers exposed to Ramadan fast in the 1st month gestation. | ||||
| Bruckner et al., 2015: Males born to low sex ratio cohorts show better health than do males in other cohorts. | ||||
The first dimension of the typology separates papers into those concerned with the endemic, or baseline, level of pregnancy loss and others focused on epidemic loss. The baseline level of pregnancy loss reflects the observed, but not necessarily desired, level. We do not limit the endemic category to any period in gestation. If, alternatively, the article examines exposures, or time-variant characteristics, that increase the incidence of pregnancy loss above expected levels, we categorize it as contributing to our understanding of epidemic pregnancy loss. For the purposes of our typology, “epidemic” does not refer to loss due to infectious pathogens, although exposure to such pathogens could increase selection in utero above baseline levels.
The second dimension of our typology separates papers into two groups defined by whether authors infer selection in utero from (1) observed pregnancy losses or (2) characteristics of births cohorts (e.g., the secondary sex ratio) or fertility histories. In cases where articles fall into multiple categories, we classify them into the cell in which the article makes the primary contribution.
The third dimension of the typology describes the population of gestations “searched” for evidence of selection in utero. Some papers start with all gestations, observe which abort, and then identify, post hoc, the risk factors for abortion. By contrast, other articles examine specific subgroups suggested by a priori considerations. Among the articles that examine subgroups, we delineate “males” as a separate cell given the abundant literature in this area. We assign all other subgroup analyses to an “other at risk group” cell.
5. Endemic selection in utero
Theoretical arguments for selection in utero as an endemic property of human reproduction converge across diverse fields including evolutionary biology, anthropology, and epidemiology. This work traces its origin from Darwin’s groundbreaking theory of natural selection (Darwin, 1859). Hamilton (1966) asserts that natural selection must conserve mutations that maximize the expected frequency of a mother’s genetic material in future generations. Each decision to conceive requires parental investment that could, alternatively, have gone to other gestations or offspring. If a conceptus or fetus somehow signals that it will unlikely thrive if born, natural selection would conserve mechanisms that avoid further maternal investment in the pregnancy. Such termination would not only reduce the time to the “sibling replacement” but also allow maternal allocation of energy and resources to existing children. Hamilton further notes that selection in utero represents a subcategory of maternal strategies of reproductive suppression which include, for instance, inhibition of ovulation during lactation to prevent close spacing of live births.
Vitzthum (2008) builds on Hamilton’s assertion and incorporates a life history perspective. According to life history theory, a mother not only chooses between current and future reproduction but also between reproductive effort and somatic (i.e., her non reproductive biology) maintenance. Somatic maintenance may allow the mother to live longer but diverts energy from reproductive effort. In addition, given that each pregnancy carried beyond the 2nd trimester involves a substantial risk to maternal morbidity and/or death, extended reproductive effort imperils maternal lifespan.
Baird (2009) proposes that early (relative to late) pregnancy loss reflects an adaptive strategy to eliminate less fit gestations well before spontaneous abortion poses a morbidity risk to the mother. The continued health of the mother benefits not only her future reproductive effort but also the survival probability of offspring who rely on maternal care. This “quality control” argument coheres with empirical findings that a disproportionate share of spontaneous abortions and resorbed embryos include chromosomal anomalies or congenital malformations (Roberts and Lowe, 1975).
5.1. Cell 1: Endemic selection in utero inferred from observed losses
Wilcox and colleagues’ (1988) study of pregnancy loss, summarized earlier, ranks among the most highly influential papers to estimate endemic selection in utero. Their key contribution relative to other literature involves early, sensitive detection via hCG before clinical recognition of pregnancy. Their estimate of 31% loss among healthy women is greater than numerous gestational life table estimates conducted in the 1950s and 1960s (summarized expertly by Leridon, 1977). Wilcox and colleagues likely underestimate the true population incidence of pregnancy loss in that they examined healthy, highly educated women who wanted to conceive. The fact that (in the US) ~45% of pregnancies appear unintended (Finer & Zolna, 2016) may limit the external validity of this small cohort study.
Ammon Avalos and colleagues’ recent systematic review (2012) compiled results from four studies of pregnancy loss among women who sought prenatal care before the eighth week of gestation. They report a risk of pregnancy loss after the eighth week which ranges from 11 to 22% depending on the study. The risk of loss converges after the 14th week for all four studies, which indicates that the discrepancy in loss estimates arise mainly from differences in sensitivity of detecting losses in the first trimester. In a separate analysis, Cousens and colleagues (2011) used official fetal death registration data from 193 countries to estimate the worldwide risk of pregnancy loss in the third trimester. They report ~2 percent loss of third trimester pregnancies (i.e., between 2.14 million to 3·82 million losses in 2009). Cousens and colleagues acknowledge that a disproportionate share of these losses occur in countries with unreliably low levels of fetal death registration.
5.2. Cell 2: Endemic selection in utero inferred from live births or fertility histories
Interest in variations in fertility and reproductive timing across societies has led demographers and anthropologists to collect data on mothers’ fertility histories. These fertility history surveys typically ask a mother to recall the number and timing of previous pregnancies. Benefits of these surveys include the ability to estimate pregnancy loss in populations with no formal fetal death registry and/or relatively few interactions with a formal health care system. These conditions describe much of the low-income country context. Such surveys also confer the benefit of obtaining information on sensitive topics including elective pregnancy termination.
Casterline (1989) reports results from the World Fertility Survey, a retrospective survey of mothers in 40 low and middle income countries. He finds that prevalence of spontaneous pregnancy loss ranges from 3.7 to 14.9 percent. These estimates appear substantially lower than prospective clinic-based studies but appear in line with other retrospective estimates based on maternal report. Casterline notes that maternal self-report will underestimate the true proportion of pregnancy losses, given that (i) they do not capture 1st trimester losses that were undetected by mothers; and (ii) mothers may not recall the loss. In addition, mothers may misclassify elective terminations as spontaneous pregnancy loss to avoid cultural stigma associated with elective termination.
We do not include additional literature in this cell given our agreement with Casterline’s point that self-reported fertility histories underestimate selection in utero and cannot distinguish spontaneous pregnancy loss from elective termination. The utility of these fertility surveys—especially in the low and middle income country context, where fertility data remain sparse — involves their ability to provide a baseline estimate of pregnancy loss and to permit comparisons across subgroups (see Cell 6).
5.3. Sex-specific endemic selection in utero
Trivers and Willard’s paper on the sex ratio (1973) remains the most cited theoretical article on ultimate causes of selection against males in utero. They invoke empirical evidence from non-humans to advance a model of a male-skewed mammalian sex ratio at birth. They reason, consistent with earlier work from Fisher (1930), that natural selection favors a birth sex ratio of 1.0 when parents invest equally in sons and daughters. However, given a set of assumptions, parents may bias the sex ratio to maximize the yield of offspring that thrive and produce grand-offspring.
Trivers and Willard assume the following: (i) the condition of the offspring at the end of the mother’s parental investment correlates positively with the mother’s condition during parental investment; (ii) differences in offspring quality at the end of parental investment will persist well into reproductive ages; and (iii.) parental investment will benefit reproductive success of male more than female offspring. They note that, in sexually dimorphic species, males more than females show greater variance in reproductive success. For instance, they cite evidence that male caribou in excellent condition out-compete other males for multiple mates, thereby leaving some males unable to find mates. By contrast, female caribou in excellent condition may only modestly increase reproductive success relative to less-fit females. For these reasons, Trivers and Willard argue that mothers with slight advantages in condition should bias production toward males. Mothers with slight endemic disadvantages in condition, however, should disproportionately terminate male gestations that, if born, have a low likelihood of survival and reproduction. Mechanisms of sex-biased termination would act most efficiently either via mechanisms at conception or via excess male fetal loss.
Theoretical extensions to the Trivers-Willard hypothesis to humans include predictions about epidemic selection against males in utero (discussed in the “epidemic” sections below), endemic characteristics that may bias the sex ratio (see review by James & Grech, 2017), and the health of males once born. Wells (2000) views the persistent male morbidity and mortality disadvantage after birth—especially among low-weight, preterm, or nutritionally deficient males—as consistent with the Trivers-Willard hypothesis. According to Wells, given that maternal parental investment does not end at birth, selection against frail males during “epidemic” environmental stressors in infancy would remain elevated because of its ability to maximize overall maternal reproductive success. We discuss such “epidemic” stressors in cells 7 through 12.
Evolutionary biologists contend that the “… Trivers-Willard hypothesis is also the most misunderstood and incorrectly applied idea in the field of sex allocation.” (p. 206, in West, 2009). Whereas we agree that much controversy surrounds the Trivers-Willard hypothesis, we view the controversy as involving other scholars’ overly simplistic empirical testing and over-interpretation of results. This concern appears quite prevalent in research on human sex ratios. Such work does not diminish the logic of Trivers and Willard’s argument.
5.4. Cell 3: Observed endemic sex-specific selection in utero
For reasons that remain unclear, males appear more sensitive than do females to selection in utero. The literature in this area tends to converge on a male excess in detected losses especially >20 weeks gestation. Owing to the lack of high quality, population-based data on the sex of losses before the 20th week, we know of few direct estimates of losses by sex over the entire gestational age range.
Macdorman and colleagues (MacDorman, Munson, & Kirmeyer, 2007) calculated the sex ratio of fetal deaths >20 weeks’ gestation for the United States from 2002–2004. They report a 10% excess of male fetal deaths. The sex difference in fetal loss is smaller with advancing gestational age, which indicates that their discovered male excess arises due to disproportionately more male losses in gestational weeks 20 through 23. The estimated magnitude of the male excess in fetal deaths >20 weeks coheres with a recent meta-analysis by Mondal and colleagues (Mondal, Galloway, Bailey, & Mathews, 2014) of over 30 million losses worldwide, as well as McMillen’s study (1979) in the mid-20th century in the US. Mondal and colleagues (2014) find that the strength of the male “exposure” for the risk of fetal deaths >20 weeks is equivalent to the population attributable risk of stillbirth due to smoking.
Using a clinical series of 683 cases of spontaneous pregnancy loss in New York City as early as the eighth week of gestation, Byrne and Warburton (1987) determined the anatomic sex of singleton embryos and fetuses. They discovered a male excess which appeared stronger at earlier gestational ages (i.e., weeks 8 to 11, 12–15, and 16–19 relative to weeks 20–23 and 24–28). The male excess appeared confined to anatomically and chromosomally normal losses. Within this group, the male:female ratio of losses was 299:230 (sex ratio=1.30). Byrne and Warburton note their inability to estimate the sex ratio of losses before the eighth week of gestation.
Orzack and colleagues (Orzack et al., 2015) estimate the sex ratio at conception and meta-analyze earlier studies to arrive at sex differences in losses across gestational ages ranging from conception to full term. They genotype over 139,000 embryos three to six days of age from in vitro fertilization clinics and report that sex ratio at conception is either not sex biased or slightly male biased (50.2 percent of embryos are male). They also compile studies of induced abortions in the 1st and 2nd trimester and show a female excess in these induced abortions. The Authors conclude that the conception sex ratio is close to unity, followed by an excess loss of females in the 1st and early 2nd trimester, and a subsequent excess loss of males in the late 2nd and 3rd trimester. The magnitude of sex difference in early losses remains unclear. In addition, using induced abortions to approximate sex difference of spontaneous losses appears questionable. To the extent that fetal sex and family circumstances are not statistically independent events (see, for example, Hamoudi & Nobles 2014), results by Orzack and colleagues have unknown external validity to spontaneous pregnancy losses.
5.5. Cell 4: Sex-specific endemic selection in utero inferred from live births
Researchers have examined the characteristics of frail births at the threshold of viability to make inferences about endemic levels of selection against males in utero. The logic underpinning such inference involves the notion that advances in modern medicine in the 20th century now save a fraction of frail gestations which, absent such advances, would have resulted in a fetal death. Based on this logic, these “periviable” frail live births provide insight into characteristics of late fetal deaths. Tyson and colleagues (2008) collected information on over 4000 live infants born between 22 and 25 weeks of gestation, of which 49% died before two years of age. They find a male overrepresentation in these births (i.e., 54%) and heightened risk of mortality among males relative to females. In a separate analyses, Stevenson and colleagues (2000) examined over 6000 infants born very low birth weight (<1500 g) and report 22% infant mortality for boys relative to 15% for girls.
Larger studies of preterm birth (<37 weeks) which include infants at the threshold of viability show an excess of male preterm births. Zeitlin and colleagues (2002) collected data from four original sources and combined these with extracted data from 20 earlier studies. They find a 12% increased odds of preterm delivery among males. The male excess appears stronger for spontaneous (rather than induced) preterm births before 33 weeks of gestational age, which we argue closely aligns with frail gestations close to the threshold of viability.
5.6. Endemic selection in utero against frail subgroups
Theories of population variation in selection in utero attempt to explain frailty within narrower subgroups. Forbes (1997) offers an evolutionary perspective to the observation that incidence of Down syndrome (trisomy 21) among live births rises with advanced maternal age. He forwarded the relaxed filter hypothesis in which aging nulliparous mothers, nearing the end of their reproductive lifespan, reduce the stringency of their maternal screen against frail or low-quality gestations and thereby allow them to progress to a live birth. By contrast, high parity mothers, who already enjoy a rich reproductive history, would retain a high screen against frail gestations and terminate them spontaneously early in gestation. Forbes reasons that mothers above 35 years in particular may adapt the stringency of the fetal screen based on the relative life history tradeoffs involved in carrying the current pregnancy to term. Intriguingly, two empirical reports appear consistent with Forbes’ hypothesis (Neuhauser and Krackow, 2007, Bruckner et al., 2012).
Quenby and colleagues (2002) incorporate results among women with recurrent miscarriage to suggest a paradigm shift in the clinical perspective of recurrent loss. Recurrent miscarriage involves three consecutive pregnancy losses before 24 gestational weeks; prevalence estimates range from 0.5 to 1% among fertile couples of reproductive age. Whereas prior work attributes recurrent miscarriage to selection against fetuses that most women would carry to term and live birth, Quenby and colleagues propose the opposite. Based on results from artificial reproductive technology as well as immunological studies of the mother’s endometrium, they propose that women with recurrent miscarriage fail to prevent the implantation of low-quality embryos, which in turn leads to more frequent but otherwise normal selection against frail fetuses. According to Quenby and colleagues (and initially proposed by Aplin, Hey and Li (1996)), this delay in “nature’s quality control” may impose a substantial cost on mothers in the form of observed recurrent miscarriage rather than undetectable failure to implant. Quenby and colleagues’ argument coheres with that of Baird (presented before Cell 1, above) and with recent empirical results on “superfertile” women with recurrent miscarriage (Salker et al., 2010).
Ellison (1990) uses an evolutionary perspective on reproductive physiology to forward an ecological theory of human ovarian function. He posits a gradient of ovarian function that responds to environmental and other circumstances including maternal energy balance, aerobic capacity, and age. He theorizes that women too poorly fed or otherwise incapable of provisioning fetal or infant growth will bear very frail offspring unlikely to project maternal genes into succeeding generations. Natural selection would, therefore, conserve any mechanism that blocks ovulation among poorly fed women or those unable to maintain healthy balances. Whereas Ellison’s theory offers clear, testable hypotheses regarding the relation between maternal physiological measurements (e.g., weight) and ovarian function, its application to selection in utero remains less developed.
5.7. Cell 5: Observed endemic selection in utero against frail subgroups
Considerable research identifies characteristics of the mother and of the fetus that confer an elevated risk of pregnancy loss. For purposes of this typology, we selected key papers examining characteristics most relevant to population health. We identify maternal risk factors as falling into the endemic category if they remain stable, or relatively stable, during the course of pregnancy. For instance, we consider maternal race/ethnicity, age, and fetal chromosomal profile as endemic characteristics. We consider other risk factors (e.g., maternal smoking, alcohol consumption, and socioeconomic status) as endemic since the literature on these variables typically does not collect sufficient longitudinal information to allow characterization of their variation before conception. If the research does describe variation in these risk factors before and during pregnancy, we assign the work to the “epidemic” category.
Hardy and colleagues (2016) conducted a cytogenetic analysis on 8319 tissue samples of spontaneous abortions from five research sites over a 40 year period. They used standardized methods across all samples and attempted to minimize contamination of maternal tissue in the fetal tissue samples. They report that 60 percent of spontaneous abortions show a chromosomal abnormality. The Authors infer from this prevalence that a disproportionate share of conceptions with a chromosomal abnormality result in a spontaneous abortion. In addition, they find an increased proportion of chromosomally abnormal spontaneous abortions among mothers greater than 35 years of age.
Nybo Andersen and colleagues’ large, population-based study of pregnancy losses (2000) in Denmark supports Hardy and colleagues’ (2016) maternal age finding. In their prospective study of over 1.2 million pregnancies, they find that the risk of loss from spontaneous abortion, ectopic pregnancy, or stillbirth increases rapidly after age 35 years. At 40 years the risk of pregnancy loss exceeds 40 percent, as compared with 17 percent loss at age 30 years. The Authors ruled out the rival explanation of subfecund women selecting into pregnancies at older ages, and conclude that advancing maternal age confers a strong, independent risk of increased selection in utero.
A large number of studies examine maternal alcohol and tobacco consumption as antecedents of pregnancy loss. Harlap and Shiono (1980) followed over 32,000 women and assessed alcohol and cigarette consumption based on maternal self-report in the first prenatal care visit. They find an increased risk of spontaneous abortion in the 2nd but not the 1st trimester of pregnancy among women who reported moderate to heavy alcohol consumption. Efforts to replicate and extend these findings tend to show a relation between high levels of alcohol consumption and pregnancy loss (Abel, 1997). Regarding maternal smoking, Pineles and colleagues’ (2014) recent meta-analysis of 50 studies on active smoking during pregnancy reports an increased risk of miscarriage (relative risk: 1.23, 95% CI:1.16, 1.30). The Authors note heterogeneity in both the definition of miscarriage (i.e., losses ranging anywhere from 12 to 28 weeks) and the measurement of smoking across the studies. However, given the consistency of the evidence, Pineles and colleagues conclude that over a half-century of research shows an increased risk of pregnancy loss among mothers who actively smoked during pregnancy.
Over the last decade, increasing attention focuses on pregnancy loss after the 20th week of gestation as a significant public health problem. The availability of routinely collected stillbirth data (defined as delivery of a non-live pregnancy >20 weeks gestation) in higher income countries indicates that the risk of stillbirth remained relatively constant over the last 20 years (Flenady et al., 2011). The risk of stillbirth varies substantially by race/ethnicity and socioeconomic status. Using US vital statistics data from 2005, MacDorman and Kirmeyer (2009) find that non-Hispanic black mothers show over a two-fold increased risk of stillbirth relative to non-Hispanic white mothers. A careful multi-site, clinical case-control study confirms this large racial disparity (Stillbirth Collaborative Research Network Writing Group, 2011). Racial/ethnic disparities in stillbirth have been documented in other countries as well. In addition, studies in Scandinavia and elsewhere (Rom et al., 2010, Flenady et al., 2011) show a higher incidence of stillbirth among mothers with lower levels of completed education. It remains unclear the extent to which race/ethnicity and maternal behaviors (e.g., smoking) contribute to the discovered educational disparity in stillbirth.
5.8. Cell 6: Endemic selection in utero against frail subgroups inferred from live births or fertility histories
The work using fertility histories and live birth information generally converges with research in Cell 5 in identifying subgroups at elevated risk of pregnancy loss. Leridon (1977) analyzed pregnancy history data from the 1970s and finds a strong relation between advanced maternal age and pregnancy loss. de la Rochebrochard and Thonneau's (2002) retrospective study of over 3000 pregnancies (reported by mother) agrees with Leridon’s findings and further suggests that advanced paternal age increases the risk of miscarriage.
Several papers have used the observation of paradoxical health advantages of low weight, non-Hispanic black liveborn infants to infer elevated fetal selection of frail non-Hispanic black gestations after 20 weeks of pregnancy. Although overall neonatal mortality among non-Hispanic blacks exceeds that of non-Hispanic whites, the prognosis of non-Hispanic blacks given a very preterm gestational age and/or a very low birth weight tends to be better than that for non-Hispanic whites. Gage and colleagues (2010) suggest that excess fetal loss among non-Hispanic blacks could account for the live birth health advantage at the frail end of the birthweight distribution. Platt and colleagues (2004) reach a similar conclusion using a different “fetuses at risk” method. Taken together, this work indicates that paradoxical health advantages at birth of some allegedly “high-risk” pregnancies may arise due to selection against frail fetuses late in gestation. In addition, Wilcox and colleagues (Wilcox, Weinberg, & Basso, 2011) use the fetuses-at-risk framework to illustrate cases in which selection in utero can bias causal inference. They use directed acyclic graphs to show confounding bias in studies that adjust for gestational age in which exposures lead both to (unobserved) fetal loss and (observed) livebirth outcomes such as preterm delivery.
6. Epidemic antecedents of selection in utero
Examination of epidemic pregnancy loss, presumably induced by circumstances that perturb the endemic level of selection in utero, confers the benefit of establishing clear temporal order between exposure and outcome. In addition, to the extent that the epidemic exposure is exogenous, or statistically independent of endemic characteristics of the pregnancy, such research appears less susceptible to confounding bias. These two design aspects strengthen the extent to which studies of epidemic pregnancy loss may estimate causal effects. Challenges of studies in the “epidemic” category involve the requirements of high-quality data and large sample size, as well as unknown external validity when examining rare exposures.
The argument for selection in utero posits, without controversy, that pregnant women vary in their capacity to invest in children and that children vary in their need for maternal investment to thrive in prevailing environments. The argument also assumes that children with greater needs than their mothers can fill will more frequently die before reproductive age than will other children. These circumstances imply that natural selection, over its 150 billion human experiments, would have conserved any mutations that spontaneously aborted gestations in which the needs of the prospective child likely exceeded the resources of the prospective mother.
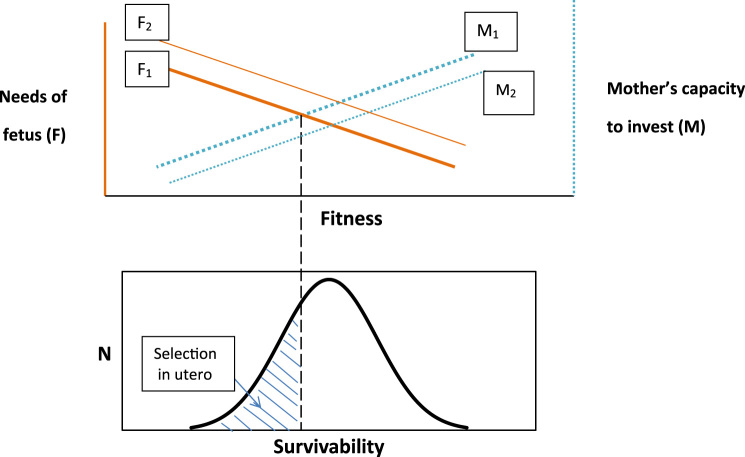
Selection in utero predicts that spontaneous abortion will increase when the distribution of maternal resources among pregnant women shifts downward while the distribution of need for maternal investment among prospective infants remains unchanged (Fig. 1). This increase, referred to in the literature as the Trivers-Willard Effect (Catalano et al., 2017), implies that fewer high need infants will be born when the environment weakens women of reproductive age (Fig. 1: M1 shifts to M2). Selection in utero also predicts that the frequency of spontaneous abortion will increase when the distribution of maternal resources among pregnant women remains unchanged but the distribution of need for maternal investment among prospective infants shifts upward (Fig. 1: F1 shifts to F2). This increase, referred to as the Bruce Effect, implies, that fewer high need infants will be born when the environment increases morbidity and mortality among children (Catalano et al., 2017).
Fig. 1.
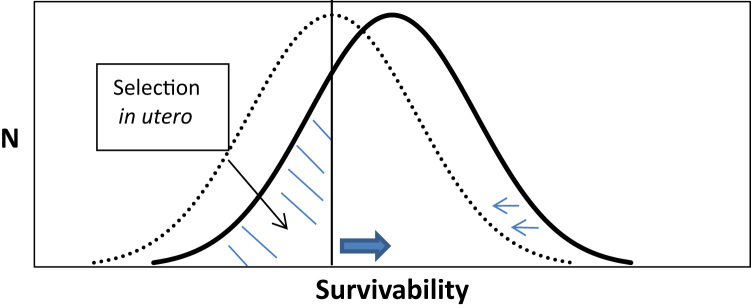
Schematic illustration of fetal needs and maternal capacity to invest. TOP PANEL: X-axis describes Darwinian fitness. Y-axis on the left (orange) indicates level of resource need for fetuses F, which varies across pregnancies. Needs of fetuses F1 decline with increased fitness. Y-axis on the right (blue dash) indicates maternal capacity to invest in children M1; maternal capacity varies positively with maternal fitness. Selection in utero occurs when F1 > M1. Vertical dashed line indicates point at which F1 = M1. BOTTOM PANEL: To the left of this dashed line, the “left tail” of the frequency distribution of fetuses (shaded) undergoes selection in utero. Theory suggests that fewer high need infants will be born when the environment weakens women of reproductive age (i.e., down-shift of M1 to M2 while F1 remains fixed). Selection in utero, moreover, would increase when maternal resources remain fixed but needs of the fetus shift upward (i.e., F1 to F2, with M1 fixed). Perturbations of maternal capacity and fetal need may also occur simultaneously and interact according to parental-offspring conflict theory (Haig, 1993).
Both the Trivers-Willard and Bruce Effects imply “epidemics” of spontaneous abortion in that selection in utero will vary over time with exogenous circumstances that affect either or both infant need for maternal investment and maternal capacity to invest in infants. The frequency of spontaneous abortion could rise when, for example, a famine reduces maternal energy and thereby retards fetal growth. If born, such fetuses would require relatively great transfers of energy from their mothers at a time when mothers have relatively little energy to transfer.
6.1. Cell 7: Observed epidemic selection in utero
Few studies examine pregnant women before the 20th week of gestation and report epidemic antecedents of pregnancy loss. In the infectious diseases field, Haberg and colleagues (2013) tested whether the 2009 H1N1 influenza strain increased risk of fetal death. During this pandemic, pregnant women appeared vulnerable to morbidity and mortality. The Authors linked national registry data on over 113,000 pregnant women in Finland to medical consultation data, which permitted clinical identification of influenza infection. The Authors find that the risk of pregnancy loss after the 12th week increased by almost two-fold if the mother showed a clinical diagnosis of influenza in the second or third trimester of pregnancy.
A larger literature focusing on losses after the 20th week of gestation finds that time-varying ambient environmental factors increase the risk of stillbirth. Strand and colleagues (Strand, Barnett, & Tong, 2011) report an increased risk of stillbirth in Brisbane, Australia when mean temperatures in the last four weeks of pregnancy exceed 21 degrees centrigrade (relative to 15 degrees centigrade). Interestingly, Strand and colleagues find no relation between extreme heat and stillbirth, which they attribute to the habituation of Australian residents to heat waves. Basu, Sarovar, and Malig (2016) report results similar to Strand in that increases in temperature during the end of pregnancy confer an increased risk of stillbirth. Whereas contemporary studies cannot reject the null for cold temperatures, a study of a historical birth cohort in early 20th century Sweden reports an association between cold temperature and stillbirth (Bruckner, Modin, & Vagero, 2013).
Transient rises in ambient air pollution may also confer an increased risk of stillbirth. In a study of New Jersey from 1998 to 2004, Faiz and colleagues (2012) linked over 3,000 stillbirths and over 700,000 livebirths to regional air pollution data and examined the associations among four air pollutants. They find modest increases in risk of pregnancy loss >20 weeks following exposure to higher than expected levels of nitrogen dioxide, sulfur dioxide, and carbon monoxide. No clear pattern emerged across these pollutants regarding specific trimesters of exposure.
A separate line of research indicates that adverse changes in the socioeconomic environment may also precede pregnancy loss. Hogue and colleagues (2013) examine the relation between a mother’s self-reported significant life events, just before or during pregnancy, and the risk of stillbirth (>20 weeks) in over 600 cases and 1300 controls. These significant life events include financial, emotional, traumatic and partner-related events. Hogue and colleagues find in the adjusted analyses a positive dose-response relation between significant life events and the risk of stillbirth. Bruckner and colleagues (Bruckner et al. 2016) build on this result and examine in Denmark whether sudden economic downturns precede an increased risk of spontaneous abortion. They conduct an ecological analysis of over 150,000 clinically detected pregnancies (i.e., >6 weeks of gestation) that ended before live birth. The risk of spontaneous abortion rises one month after unexpected increases in Denmark’s unemployment rate, which supports the notion that ambient stressors adversely affect survival of pregnancies to term.
6.2. Cell 8: Epidemic selection in utero inferred from live births or fertility histories
Schneider (2017) conducted a detailed historical analysis of annual stillbirth rates and birthweight distributions in Boston for the years 1872–1900. He examined the differences in the birthweight distribution of live births in years classified as “high” and “low” rates of stillbirth. He finds a right-shifted (i.e., heavier) birthweight distribution among live infants born in “high” stillbirth rate years. Based on characteristics of the live births, Schneider infers epidemic selection, during high stillbirth years, against frail gestations in utero. The causes of excess stillbirths in Boston, however, remain unexplored.
Despite the limitations of recall, under-reporting, and cultural biases, maternal fertility history surveys have yielded insights on potential causes of selection in utero above endemic levels. Cai and Feng (2005) use the Great Leap Forward famine in 1959-61 and the beginning of Mao Zedong’s cultural revolution in 1967 as natural experiments that plausibly increased the risk of pregnancy loss. They used fertility history information on over 1.5 million pregnancies to mothers surveyed in 1988, which represents the largest fertility survey ever conducted. Cai and Feng find increases in the risk of miscarriage and, to a lesser extent, stillbirth among pregnancies conceived during these severe social disruptions. The famine reportedly induced a greater rise in the risk of miscarriage than did the cultural revolution (57% vs. 27% increase in odds). The elevated risk of miscarriage during the famine, moreover, concentrated among mothers living in rural areas, where food scarcity appeared more severe.
Arbuckle, Lin, and Mery (2001) conducted an exploratory analysis among farmowners and farmworkers on the relation between pesticides and spontaneous pregnancy loss. This sample of ~4,000 pregnancies merged retrospective pesticide exposure and reproductive history data to explore preconception and postconception levels of 17 pesticide variables and risk of pregnancy loss. Whereas most of the results do not reject the null, the Authors report that preconception exposure to glyphosate increased the risk of spontaneous loss. The reader should interpret the results with caution given the lack of the study’s statistical power and based the conclusion from a previous systematic review on this topic which notes flaws in study design (Arbuckle & Sever, 1998). We, as do Arbuckle and colleagues, recommend additional research in this area using larger populations.
6.3. Sex-specific epidemic selection in utero
Trivers and Willard (1973) argued that selection in utero should vary over time with stressors on the population that change the likely return in grandchildren to a mother’s investment in offspring. Differences between birth cohorts subjected to high and low levels of population stressors should reveal a “ranking” of infant genotypes and phenotypes by likely return to maternal investment. Selection should appear greatest against those that require the greatest maternal investment to survive to reproductive age (Fig. 1). The few data available that track grandchildren attributable to specific gestations show that gestations of male twins yield the fewest grandchildren (Lummaa, Haukioja, Lemmetyinen, & Pikkola, 1998). This circumstance presumably arises from the relatively high likelihood that small males, and nearly all male twins, appear small for gestational age and die in infancy. Indeed, male infants more likely die than any other age by sex group before the completion of reproductive life. This relatively high death rate appears for every society and every year for which we have dependable vital statistics (http://www.mortality.org/). The death rate among small male infants, particularly twins, exceeds that for other male infants. This circumstance leads theorists (Wells, 2000) to hypothesize that conception cohorts in gestation during stressful times will exhibit high ratios of males to females among clinically detected fetal deaths and low ratios of male to female live births.
The theory that males will suffer greater selection in utero than females seems inconsistent with recent literature arguing that conception cohorts begin with essentially equal numbers of males and females (Orzack et al., 2015). This literature posits that many more female than male gestations must end early in gestation because clinically detectable fetal loss (i.e., typically that starting in the sixth through eighth week) includes more males than females yet the ratio of male to female live births typically exceeds 1. Early fetal loss presumably selects against fetuses with chromosomal and genetic abnormalities that preclude maturation into infants likely to thrive even in benign post-natal environments and with great maternal investment. Before the eighth week of gestation, female fetuses exhibit cells that mature into the eggs of every child a woman could ever bear. Female fetuses, therefore, are at risk of selection not only from their own chromosomal and genetic defects, but also from those of every offspring they could ever yield. They would suffer greater selection early in gestation than males. Selection later in gestation, however, causes the spontaneous abortion of many fetuses without detectable chromosomal or genetic abnormalities. These gestations would otherwise proceed to live birth had not poorly understood mechanisms sensed that the post-natal environment posed a greater threat to infant survival than the mother could overcome with her resources. These circumstances put small males at greatest risk of clinically detected spontaneous abortion because their relative frailty as infants make them a relatively risky investment for mothers “programmed” by natural selection to further their reproductive fitness (Wells, 2000).
In sum, selection in utero affects both sexes but female fetuses more than male. Female fetuses appear at greater risk of loss because they present more information, in the form of early-stage ova, about their capacity to yield reproductively competent grandchildren. Unlike males who signal only their own chromosomal and genetic integrity, females present that of their children as well. The relatively great frailty of male infants signals in utero as small for gestational age. Unlike chromosomal and genetic abnormalities, present throughout gestation, the signal of male frailty (i.e., small for gestational age) may grow stronger as gestation proceeds. The sex ratio of fetal loss should appear low early in gestation and rise as time passes. This, of course, implies that males predominate among clinically observed, and “treated,” pregnancies. For these reasons, tests of the dose-response of fetal loss to epidemic stressors will focus more on males than females.
6.4. Cell 9: Observed sex-specific epidemic selection in utero
Technical challenges and lack of systematic sex determination among early pregnancy losses limits the number of sex-specific analyses <20th week of pregnancy. A few articles use registry data to examine antecedents of male fetal loss after the 20th week. Catalano and colleagues (2005) test the “economy as a stressor” hypothesis in California in that they posit a sudden rise in male relative to female fetal deaths in months after ambient economic decline. They apply time-series methods to monthly aggregate data and find this male sensitivity one month after rises in the unemployment rate. The magnitude of the result indicates that rises in unemployment account for over three percent of overall male fetal deaths. Using a similar time-series approach, Bruckner, Catalano, and Ahern (2010) test whether the terrorist attacks of September 11, 2001 coincided with an epidemic rise of male fetal death in the US in the month of September. They reason that the terrorist attacks distressed the broader US population including those living in regions not directly targeted by the attacks. The Authors find an acute increase in male relative to female fetal deaths in the US in September 2001.
6.5. Cell 10: Sex-specific epidemic selection in utero inferred from live births
Much literature uses variation over time in the odds of a male live birth to infer epidemic selection in utero against male gestations. Researchers in this area typically examine an acute, large, and unexpected population exposure. This approach confers the benefit of minimizing confounding by inherent maternal/fetal characteristics. In addition, the focus on male sensitivity, and the use of female live births as a referent population which (based on theory) appears less sensitive to epidemic selection in utero, precludes bias due to systematic over- or under-reporting of live births that is shared across both sexes.
Catalano and colleagues (2006) examine the birth sex ratio (i.e., ratio of male to female live births) in New York City in months immediately following the terrorist attacks of September 11, 2001. They find that the sex ratio fell below 1.0 in January 2002, which was the lowest value recorded of all 91 monthly cohorts in the time series. Based on the acute timing of the terrorist attacks and the four month lag in sex ratio response, the Authors infer excess male fetal loss among gestations in weeks 20–24 at the time of the terrorist attacks. Results in California following 9/11 converge with those of New York and show that fewer than expected very low weight males (i.e., <1500 gm) and fewer than expected males with selected birth defects were born in months after the attacks (Catalano et al., 2005, Singh et al., 2017). These results cohere with the argument that the 9/11 events preceded a rise in selection in utero against frail male gestations. This study team has also reported declines in the sex ratio in months immediately following other population stressors, which indirectly supports the notion of epidemic male fetal loss (Catalano, Zilko, Saxton, & Bruckner, 2010).
Earthquakes and the Ramadan fast also precede an increase in selection in utero against males. Torche and Kleinhaus (2011) use a quasi-experimental approach to examine sex ratios and birth outcomes before and after the large Tarapaca earthquake in Chile. They find that, after the earthquake, the gestational age distribution of live female (but not male) births shifted to earlier liveborn deliveries. After adjustment for this gestational age shift, the Authors find a decline in the sex ratio among pregnancies exposed to the earthquake at three months’ gestation. In a separate study, Almond and Mazumder (2011) analyze the odds of a live male birth among Muslim mothers in Michigan who were pregnant during Ramadan. Given that Muslims tend to fast each day during the lunar month of Ramadan, Almond and Mazumder use the timing of Ramadan as a proxy for nutritional disruption among pregnant women with a reported Arab ancestry. In their intent-to-treat analysis, they find a 26 percent decline in male (but not female) births among Arab mothers with peak exposure to the Ramadan fast in the first month of gestation. The timing of this result supports excess male loss early in the pregnancy, rather than pre-conceptional factors that could affect fetal sex.
Research on other population stressors using annual data on live births or maternal fertility histories converges with the literature reviewed above (Valente, 2015, Sanders and Stoecker, 2015). Aggregate annual data, however, cannot distinguish whether sex ratio variation arises from factors that affect the sex ratio at conception or from selection against males in utero. Nevertheless, research using cohort life tables (Catalano & Bruckner, 2006), microdata on historical populations (Bruckner, Helle, Bolund, & Lummaa, 2015), and contemporary surveys (Bruckner & Nobles, 2013) supports the argument that males born to low sex ratio cohorts show, on average, better health than do males born to other cohorts. This line of work indicates that epidemic selection in utero against males disproportionately affects frail gestations.
6.6. Epidemic selection in utero against frail subgroups
Selection in utero implies “decisional biology” that mothers cannot describe using but that assesses the “fit” among environmental circumstances, fetal needs, and maternal resources. As with the decisional biology that men and women can describe using, that applied to gestation likely varies not only among women but also over time, in response to changes in the quality of the ambient environment. Declines in the ambient environment may threaten viability especially among frail fetuses that, absent the environmental stressor, already faced low odds of survival to birth.
6.7. Cell 11: Observed epidemic selection in utero against frail subgroups
We know of no exemplary article on epidemic antecedents of pregnancy loss that focuses on frail subgroups. We suspect that the lack of empirical work in this area arises in part from challenges with data collection. State or national registers do not routinely collect cause-of-fetal death data or other information on pregnancy losses (e.g., congenital defects, growth restriction, or twin status) that may identify frail subpopulations. In addition, hospitals that collect cause-of-death data on pregnancy losses note the inherent difficulty of accurate classification.
6.8. Cell 12: Epidemic selection in utero against frail subgroups inferred from live births
As with Cell 11, we know of scant research which examines frail live births or maternal histories to infer epidemic selection in utero of subgroups. In two papers, Auger and colleagues (Auger et al., 2016, Auger et al., 2017) examine the risk of one frail subgroup of pregnancies—live birth defects—following high ambient temperatures in utero. They focus on congenital heart defects and neural tube defects which, based on other literature, may respond to ambient heat during critical developmental periods in the first trimester. Using registry data on over 700,000 live births in Quebec, the Authors find positive associations between ambient temperatures at 30 °C and selected defects on particular days (for neural tube defects) and weeks (for atrial septal defects) of gestational exposure. The Authors note limitations including the weak associations discovered and the low statistical power to identify associations among defect subgroups. We, like the Authors, interpret these results cautiously given the lack of data on pregnancies that ended in elective or spontaneous termination. The pattern of results, however, indicates that ambient heat in the first trimester may induce teratogenic effects on neural tube and atrial septal defects, rather than epidemic selection in utero on a separate set of male-skewed birth defects as reported by others (Singh et al., 2017, Bruckner et al., 2017; see text for Cell 10).
7. Summary, implications, and future directions
Between 30 to 70 percent of pregnancies do not end in a live birth. In addition, among those carried to clinical detection (i.e., ~5 weeks), 10 to 22 percent end in a spontaneous pregnancy loss. These losses do not occur randomly across the distribution of likely fetal fitness. Consistent with evolutionary and life history theory, selection appears greatest against fetuses likely to yield the fewest grandchildren per unit of maternal investment. Literature from diverse fields identifies several markers of fetal fitness (e.g., chromosomal anomaly, growth restriction, low hCG, male sex) that appear associated with selection in utero. The extent of selection in utero also varies across populations, places and times. For this reason, the health of live birth cohorts depends in part on the strength of selection in utero. Our typology of research summarizes key empirical work in this field and identifies gaps in the literature for scholars wishing to contribute to this area.
Whereas human health and cohort lifespan have improved dramatically over the last 150 years, historical evidence shows relatively scant improvements in fetal health over the same period (Schneider, 2017). This circumstance, combined with the literature summarized in this typology, suggests that selection in utero remains an important process in shaping cohort health. The increasing availability of population-based data on the course of pregnancy—including pregnancy loss—makes clear that live births represent a highly selected sample of conceptions. This selection process, according to the theory of natural selection, appears conserved over generations such that it may maximize maternal reproductive success by focusing reproductive effort on gestations that would likely thrive if born. The implications of this theory for public health and medicine in the 21st century, however, remain understudied.
Key enduring themes of empirical research involve estimating endemic and epidemic selection in utero across the entire population as well as male gestations. Whereas data limitations often lead researchers to focus on stillbirths >20 weeks, a few countries (e.g., Denmark, Sweden, Norway) now make available longitudinal data on pregnancies greater than six weeks. Absent dataset availability of large pregnancy cohorts, and the demise of the planned US National Children’s Study (https://www.nichd.nih.gov/research/NCS/Pages/default.aspx), we anticipate that scholars interested in describing selection in utero, either for the general population or for subgroups, will benefit from broader use of Scandinavian registries.
Despite notable challenges in assessing causes of fetal death, advances in fetal monitoring, as well as availability of sex, biomarker, and anomaly information on pregnancy losses under 20 weeks in various databases (Hardy, Hardy, Jacobs, Lewallen, & Hassold, 2016), may elucidate high-risk pregnancies subject to selection. In addition, basic research which aims to identify biological and/or behavioral signals of selection and fetal hardiness (e.g., hCG) may hold implications for clinical medicine (Devaseelan et al. 2010). Furthermore, increasing interest by funding agencies on understanding causes of periviable birth (i.e., parturitions at 20–26 weeks of gestation) could stimulate data collection efforts at the threshold between selection in utero and fetal viability (https://grants.nih.gov/grants/guide/pa-files/PA-15-200.html).
Analyses of epidemic antecedents of selection in utero confer the study design benefit of establishing temporal order between putative exposures and pregnancy loss. In addition, to the extent that the data permit estimation of responses to a time resolution of less than one year, results may distinguish sudden changes in selection in utero from the important compositional changes in who decides to conceive (Dehejia and Lleras-Muney, 2004). For these reasons, research in cells 9 through 12 with these design features provide evidence for the existence of causal antecedents of selection in utero. Many of these empirical results, moreover, cohere with well-developed evolutionary theory about population responses to ecological perturbations.
One clear gap in the literature lies in theoretical and empirical work on epidemic selection against frail subgroups other than males. Socio-demographic (e.g., age, income, education level), maternal health (e.g., over- or under- weight) or early fetal health (e.g., detected visual anomalies) characteristics could identify subgroups of gestations that theory suggests may respond to time-varying exposures. In addition, certain registries (e.g., http://www.eurocat-network.eu/) collect congenital anomaly and defect data on gestations that end before a live birth. To the extent that these case registries include multiple time points and draw from a sufficiently large catchment population of pregnancies, research on temporal variation in selection in utero on these cases represents a novel area of inquiry. We note, however, the myriad number of subgroups that population health researchers may consider frail will inflate type I error owing to multiple testing. We suggest that scholars interested in this area develop a priori hypotheses which, consistent with theory, identify subgroups on the frail end of the viability distribution.
In particular, we echo the recent sentiment of Wells and colleagues (2017) and Jasienska, Bribiescas, Furberg, Helle, and Nunez-de la Mora (2017). They note that scholars studying, for example, the relation between maternal and infant health and poverty and nutrition, should consider grounding their inquiries in evolutionary theories of human reproduction. The “subgroup” Cell 11 of epidemic selection in utero may also benefit from novel theory development, for two reasons. First, clinical interventions often focus on subgroups or persons with specific conditions. Second, research which attempts to redress perinatal health disparities does not yet converge on a set of predictions regarding epidemic selection in utero.
Although we focused our typology on selection in utero due to factors not requiring maternal awareness, a substantial fraction of conceptions end in elective termination. In countries with legal procedures and reliable data collection, the prevalence of elective termination ranges from 15 to 20 percent (Morbidity and Mortality Weekly Report, 2016, Bruckner et al., 2017). Elective terminations represent another mechanism of selection in utero and, analytically, a “competing risk” to spontaneous pregnancy loss. Interestingly, the prevalence of elective and spontaneous terminations varies positively over time, which indicates shared processes (Catalano, Bruckner, Karasek, Adler, & Mortensen, 2016). In addition, several theories, which we summarize above, may pertain to understanding antecedents and sequelae of elective termination. We await future research in this area before speculating on the relevance of empirical results on spontaneous losses to those on elective terminations.
The large share of pregnancies in developing countries dictates that, from a population health perspective, future research on selection in utero may want to focus on these countries. Some scholars have pursued careful analyses in developing countries despite general lack of fertility and natality data of known provenance and quality. We recommend against over-interpretation of results in these regions especially when using maternal fertility histories to infer characteristics of livebirths and pregnancy losses. Smith-Greenaway and Sennott (2016), for instance, provide cautionary evidence on the unreliable nature of fertility variables derived from maternal recall, such as those from Africa’s Demographic Health Survey. We encourage future work in developing countries which use study designs to minimize recall bias, conduct validation exercises that correct estimates of precision for the extent of bias, or—ideally—create reliable national registers of livebirths and pregnancy losses.
As noted in the Introduction, scholars comparing the health of birth cohorts over the life course often assume that the strength of selection in utero remains constant over time and place. Data limitations likely dictate this simplifying assumption. “Adjusting” the health of birth cohorts for selection in utero would require converting them not only into conception cohorts, but also knowing the fraction, and ideally gestational age, of members lost to spontaneous and intentional abortion. Approximations of such data can come only from registries of gestations entering prenatal care. Such registries, with known provenance and quality, remain rare. As a result, scholars may need to use birth registries to indirectly gauge cohort variation in selection in utero. Our typology provides several examples of such indirect measurement (see Cells 8, 10, and 12).
8. Fetal origins vs. Selection in utero?
As noted at the outset, the “fetal origins” hypothesis has spawned an apparently popular (https://www.nytimes.com/2018/01/31/science/dutch-famine-genes.html) narrative in which the maternal stress response interacts with fetal plasticity to yield “scarred” cohorts that exhibit chronic or episodic poor health over the life course. The literature summarized in our typology, however, challenges this intuitive narrative. Combining the literature we summarize with the scholarly work on fetal origins suggests that pregnancy provides an opportunity for both plasticity and selection to shape cohort health. Fig. 2 illustrates the hypothetical influence of both processes on the frailty distribution of cohorts that survive to birth. In this figure, a right-shift of the frailty distribution reflects scarring, whereas a right-shift of the maternal criterion for pregnancy termination reflects selection. Note that scarring may occur not only among pregnancies at the margin of the maternal criterion for termination. Selection and scarring may occur simultaneously but at different points of the gestational distribution of frailty. Whereas selection in utero could cull those at the frail “left-tail” of a distribution of one variable which signals frailty, scarring may compromise remaining gestations at other points of the distribution which appear hardy enough to survive to birth (Fig. 2). Another possibility involves a non-linear or non-monotonic response to the “dose” of the stressor (Bozzoli, Deaton, & Quintana-Domeque, 2009). These possibilities represent but two of the many research agendas that would provide a more complete understanding of how gestation shapes cohort health. The increasing availability of data during pregnancy—combined with the improved capacity to model cohort frailty (van den Berg & Drepper, 2011)—make possible empirical tests that may quantify the relative effect of selection and scarring during pregnancy on health over the life course.
Fig. 2.
Schematic illustration of selection in utero and fetal origins “damage” in response to a stressful environment. The solid curve shows the frequency distribution of fetal survivability in an average ambient environment. The vertical solid line represents the criterion below which a mother spontaneously terminates the pregnancy. An elevated stress-level environment, in which the maternal capacity to invest declines and the criterion for termination therefore shifts right, would increase selection in utero. Alternatively, in an elevated stress environment; the survivability distribution for fetuses may shift left, resulting in the dashed-line frequency distribution and cohort “damage” among the surviving births. In response to population stressors, both selection and damage in utero may occur but at different quantiles of the survivability distribution. In addition, the maternal criterion for spontaneous termination likely varies across the population, which suggests that selection and damage may be observed in the same conception cohorts.
Natural selection has had at least 150 billion gestations, or “experiments,” from which to conserve mutations that increase the likelihood of a woman yielding reproductively fit offspring. Wells and colleagues (2017) recently argued that population health research would benefit from application of evolutionary principles to understanding health over the life course. Based on our review of the literature concerned with selection in utero, we fully agree. Rigorous application, testing, and refinement of these principles may illuminate insights into maternal-fetal responses to phenomena which interest social scientists.
References
- Abel E.L. Maternal alcohol consumption and spontaneous abortion. Alcohol and Alcoholism. 1997;32(3):211–219. doi: 10.1093/oxfordjournals.alcalc.a008260. [DOI] [PubMed] [Google Scholar]
- Almond D., Mazumder B.A. Health capital and the prenatal environment: The effect of Ramadan observance during pregnancy. American Economic Journal: Applied Economics. 2011;4:56–85. [Google Scholar]
- Ammon Avalos L., Galindo C., Li D.K. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94(6):417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- Aplin J.D., Hey N.A., Li T.C. MUC1 as a cell surface and secretory component of endometrial epithelium: Reduced levels in recurrent miscarriage. American Journal of Reproductive Immunology. 1996;35(3):261–266. doi: 10.1111/j.1600-0897.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Arbuckle T.E., Lin Z., Mery L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environmental Health Perspectives. 2001;109(8):851–857. doi: 10.1289/ehp.01109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle T.E., Sever L.E. Pesticide exposures and fetal death: A review of the epidemiologic literature. Critical Reviews in Toxicology. 1998;28(3):229–270. doi: 10.1080/10408449891344218. [DOI] [PubMed] [Google Scholar]
- Auger N., Fraser W.D., Sauve R., Bilodeau-Bertrand M., Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environmental Health Perspectives. 2017;125(1):8–14. doi: 10.1289/EHP171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger N., Fraser W.D., Arbour L., Bilodeau-Bertrand M., Kosatsky T. Elevated ambient temperatures and risk of neural tube defects. Occupational and Environmental Medicine. 2016;74(5):315–320. doi: 10.1136/oemed-2016-103956. [DOI] [PubMed] [Google Scholar]
- Baird D.D. The gestational timing of pregnancy loss: Adaptive strategy? American Journal of Human Biology. 2009;21(6):725–727. doi: 10.1002/ajhb.20935. [DOI] [PubMed] [Google Scholar]
- Barker D.J. In utero programming of chronic disease. Clinical Science. 1998;95(2):115–128. [PubMed] [Google Scholar]
- Basu R., Sarovar V., Malig B.J. Association Between High Ambient Temperature and Risk of Stillbirth in California. American Journal of Epidemiology. 2016;183(10):894–901. doi: 10.1093/aje/kwv295. [DOI] [PubMed] [Google Scholar]
- Bateson P., Barker D., Clutton-Brock T., Deb D., D’Udine B. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- van den Berg, G.J., & Drepper, B. (2011). Inference for Shared-Frailty Survival Models with Left-Truncated Data. IZA Discussion Paper No. 6031 (available at: 〈http://ftp.iza.org/dp6031.pdf〉).
- Boklage C.E. The survival probability of human conceptions from fertilization to term. International Journal of Fertility. 1990;35:75–94. [PubMed] [Google Scholar]
- Bozzoli C., Deaton A., Quintana-Domeque C. Adult height and childhood disease. Demography. 2009;46(4):647–669. doi: 10.1353/dem.0.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch D.W., Gibson M., Silver R.M. Clinical practice. Recurrent miscarriage. New England Journal of Medicine. 2010;363(18):1740–1747. doi: 10.1056/NEJMcp1005330. [DOI] [PubMed] [Google Scholar]
- Bruckner T.A., Catalano R., Ahern J. Male fetal loss in the U.S. following the terrorist attacks of September 11, 2001. BMC Public Health. 2010;10:273. doi: 10.1186/1471-2458-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner T.A., Helle S., Bolund E., Lummaa V. Culled males, infant mortality and reproductive success in a pre-industrial Finnish population. Proceedings. Biological Sciences. 2015;282(1799):20140835. doi: 10.1098/rspb.2014.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner T.A., Karasek D., Yang W., Shaw G.M., Catalano R.A. Cohort variation in selection during pregnancy and risk of selected birth defects among males. Epidemiology. 2017;28(4):580–586. doi: 10.1097/EDE.0000000000000661. [DOI] [PubMed] [Google Scholar]
- Bruckner T.A., Modin B., Vagero D. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Annals of Epidemiology. 2013;24(2):116–121. doi: 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Bruckner T.A., Mortensen L.H., Catalano R.A. Spontaneous pregnancy loss in denmark following economic downturns. American Journal of Epidemiology. 2016;183(8):701–708. doi: 10.1093/aje/kww003. [DOI] [PubMed] [Google Scholar]
- Bruckner T.A., Mortensen L.H., Catalano R.A. Social and demographic drivers of trend and seasonality in elective abortions in Denmark. BMC Pregnancy Childbirth. 2017;17(1):214. doi: 10.1186/s12884-017-1397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner T.A., Nobles J. Intrauterine stress and male cohort quality: The case of September 11, 2001. Social Science & Medicine. 2013;76(1):107–114. doi: 10.1016/j.socscimed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Bruckner T.A., Saxton K.B., Pearl M., Currier R., Kharrazi M. A test of maternal human chorionic gonadotropin during pregnancy as an adaptive filter of human gestations. Proceedings. Biological Sciences. 2012;279(1747):4604–4610. doi: 10.1098/rspb.2012.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G.J., Fowden A.L. The placenta: A multifaceted, transient organ. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2015;370(1663):20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss L., Tolstrup J., Munk C., Bergholt T., Ottesen B., Gronbaek M. Spontaneous abortion: A prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. ACTA Obstetricia Et Gynecologica Scandinavica. 2006;85(4):467–475. doi: 10.1080/00016340500494887. [DOI] [PubMed] [Google Scholar]
- Byrne J., Warburton D. Male excess among anatomically normal fetuses in spontaneous abortions. American Journal of Medical Genetics. 1987;26(3):605–611. doi: 10.1002/ajmg.1320260315. [DOI] [PubMed] [Google Scholar]
- Cai Y., Feng W. Famine, social disruption, and involuntary fetal loss: Evidence from Chinese survey data. Demography. 2005;42(2):301–322. doi: 10.1353/dem.2005.0010. [DOI] [PubMed] [Google Scholar]
- Casterline J.B. Collecting data on pregnancy loss: A review of evidence from the world fertility survey. Studies in Family Planning. 1989;20(2):81–95. [PubMed] [Google Scholar]
- Catalano R., Bruckner T. Secondary sex ratios and male lifespan: Damaged or culled cohorts. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1639–1643. doi: 10.1073/pnas.0510567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano R., Bruckner T., Anderson E., Gould J.B. Fetal death sex ratios: A test of the economic stress hypothesis. International Journal of Epidemiology. 2005;34(4):944–948. doi: 10.1093/ije/dyi081. [DOI] [PubMed] [Google Scholar]
- Catalano R., Bruckner T., Gould J., Eskenazi B., Anderson E. Sex ratios in California following the terrorist attacks of September 11, 2001. Human Reproduction. 2005;20(5):1221–1227. doi: 10.1093/humrep/deh763. (Epub 2005 Feb 1225) [DOI] [PubMed] [Google Scholar]
- Catalano R., Bruckner T.A., Karasek D., Adler N.E., Mortensen L.H. Shared risk aversion in spontaneous and induced abortion. Human Reproduction. 2016;31(5):1113–1119. doi: 10.1093/humrep/dew031. [DOI] [PubMed] [Google Scholar]
- Catalano R., Bruckner T., Marks A.R., Eskenazi B. Exogenous shocks to the human sex ratio: The case of September 11, 2001 in New York City. Human Reproduction. 2006;21(12):3127–3131. doi: 10.1093/humrep/del283. [DOI] [PubMed] [Google Scholar]
- Catalano R., Gemmill A., Casey J., Karasek D., Stewart H., Saxton K. Separating the Bruce and Trivers-Willard effects in theory and in human data. American Journal of Human Biology. 2017 doi: 10.1002/ajhb.23074. (Epub ahead of print)) [DOI] [PubMed] [Google Scholar]
- Catalano R.A., Saxton K.B., Gemmill A., Hartig T. Twinning in Norway following the Oslo massacre: Evidence of a ‘Bruce Effect’in humans. Twin Research and Human Genetics. 2016;19(5):485–491. doi: 10.1017/thg.2016.58. [DOI] [PubMed] [Google Scholar]
- Catalano R., Yorifuji T., Kawachi I. Natural selection in utero: Evidence from the Great East Japan Earthquake. American Journal of Human Biology. 2013;25(4):555–559. doi: 10.1002/ajhb.22414. [DOI] [PubMed] [Google Scholar]
- Catalano R., Zilko C.E., Saxton K.B., Bruckner T. Selection in utero: A biological response to mass layoffs. American Journal of Human Biology. 2010;22(3):396–400. doi: 10.1002/ajhb.21011. [DOI] [PubMed] [Google Scholar]
- Cousens S., Blencowe H., Stanton C., Chou D., Ahmed S., Steinhardt L. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: A systematic analysis. Lancet. 2011;377(9774):1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- Dancause K.N., Laplante D.P., Fraser S., Brunet A., Ciampi A. Prenatal exposure to a natural disaster increases risk for obesity in 5½-year-old children. Pediatric Research. 2012;71(1):126–131. doi: 10.1038/pr.2011.18. [DOI] [PubMed] [Google Scholar]
- Darwin C. J. Murray; London: 1859. On the origin of species by means of natural selection, or, the preservation of favoured races in the struggle for life. [PMC free article] [PubMed] [Google Scholar]
- Dehejia R., Lleras-Muney A. Booms, busts, and babies' health. Quarterly Journal of Economics. 2004;119(3):1091–1130. [Google Scholar]
- Devaseelan P., Fogarty P., Regan L. John Wiley & Sons, Ltd; 2010. Human chorionic gonadotrophin for threatened miscarriage. (In Cochrane Database of Systematic Reviews) [DOI] [PubMed] [Google Scholar]
- Ellison P.T. Human ovarian function and reproductive ecology: New hypotheses. American Anthropologist. 1990;92:933–952. [Google Scholar]
- Faiz A.S., Rhoads G.G., Demissie K., Kruse L., Lin Y., Rich D.Q. Ambient air pollution and the risk of stillbirth. American Journal of Epidemiology. 2012;176(4):308–316. doi: 10.1093/aje/kws029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer L.B., Zolna M.R. Declines in Unintended Pregnancy in the United States, 2008–2011. New England Journal of Medicine. 2016;374(9):843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. The Clarendon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Flenady V., Koopmans L., Middleton P., Froen J.F., Smith G.C., Gibbons K. Major risk factors for stillbirth in high-income countries: A systematic review and meta-analysis. Lancet. 2011;377(9774):1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- Forbes L.S. The evolutionary biology of spontaneous abortion in humans. Trends in Ecology & Evolution. 1997;12(11):446–450. doi: 10.1016/s0169-5347(97)01179-8. [DOI] [PubMed] [Google Scholar]
- Gage T.B., Fang F., O’Neill E.K., DiRienzo A.G. Racial disparities in infant mortality: What has birth weight got to do with it and how large is it? BMC Pregnancy Childbirth. 2010;10:86. doi: 10.1186/1471-2393-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl L., Krantz D., Simpson J.L., Silver R.K., Zachary J.M., Pergament E., Platt L.D., Mahoney M.J., Wapner R.J. Pregnancy-associated plasma protein A, free beta-hCG, nuchal translucency, and risk of pregnancy loss. Obstetrics & Gynecology. 2004;104(1):30–36. doi: 10.1097/01.AOG.0000129969.78308.4f. [DOI] [PubMed] [Google Scholar]
- Goldhaber M.K., Fireman B.H. The fetal life table revisited: Spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology. 1991;2(1):33–39. [PubMed] [Google Scholar]
- Haberg S.E., Trogstad L., Gunnes N., Wilcox A.J., Gjessing H.K., Samuelsen S.O. Risk of fetal death after pandemic influenza virus infection or vaccination. New England Journal of Medicine. 2013;368(4):333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Genetic conflicts in human pregnancy. The Quarterly Review of Biology. 1993;68(4):495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. The moulding of senescence by natural selection. Journal of Theoretical Biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hamoudi A., Nobles J. Do daughters really cause divorce? Stress, pregnancy, and family composition. Demography. 2014;51(4):1423–1449. doi: 10.1007/s13524-014-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K., Hardy P.J., Jacobs P.A., Lewallen K., Hassold T.J. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. American Journal of Medical Genetics Part A. 2016;170(10):2671–2680. doi: 10.1002/ajmg.a.37795. [DOI] [PubMed] [Google Scholar]
- Harlap S., Shiono P.H. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2(8187):173–176. doi: 10.1016/s0140-6736(80)90061-6. [DOI] [PubMed] [Google Scholar]
- Hogue C.J., Parker C.B., Willinger M., Temple J.R., Bann C.M., Silver R.M. A population-based case-control study of stillbirth: The relationship of significant life events to the racial disparity for African Americans. American Journal of Epidemiology. 2013;177(8):755–767. doi: 10.1093/aje/kws381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W.H., Grech V. A review of the established and suspected causes of variations in human sex ratio at birth. Early Human Development. 2017;109:50–56. doi: 10.1016/j.earlhumdev.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Jasienska G., Bribiescas R.G., Furberg A.S., Helle S., Nunez-de la Mora A. Human reproduction and health: An evolutionary perspective. Lancet. 2017;390(10093):510–520. doi: 10.1016/S0140-6736(17)30573-1. [DOI] [PubMed] [Google Scholar]
- Jauniaux E., Farquharson R.G., Christiansen O.B., Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Human Reproduction. 2006;21(9):2216–2222. doi: 10.1093/humrep/del150. [DOI] [PubMed] [Google Scholar]
- Kamper-Jorgensen F. Vol. 39. 2011. Chief Editor’s introductory comments; p. 7. (Scandinavian Journal of Public Health). [Google Scholar]
- Koletzko B., Brands B., Chourdakis M., Cramer S., Grote V., Hellmuth C. The Power of Programming and the Early Nutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Annals of Nutrition and Metabolism. 2014;64(3–4):187–196. doi: 10.1159/000365017. [DOI] [PubMed] [Google Scholar]
- Leridon H. In: Human fertility: The basic components. Helzner Judith B., editor. The University of Chicago Press; London: 1977. [Google Scholar]
- Lumey L.H., Stein A.D., Susser E. Prenatal famine and adult health. Annual Review of Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummaa V., Haukioja E., Lemmetyinen R., Pikkola M. Natural selection on human twinning. Nature. 1998;394(6693):533–534. doi: 10.1038/28977. [DOI] [PubMed] [Google Scholar]
- Lyall F., Bulmer J.N., Duffie E., Cousins F., Theriault A., Robson S.C. Human trophoblast invasion and spiral artery transformation: The role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. American Journal of Pathology. 2001;158(5):1713–1721. doi: 10.1016/S0002-9440(10)64127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDorman M.F., Kirmeyer S. The Challenge of Fetal Mortality. NCHS Data Brief. 2009;(16) 〈https://www.cdc.gov/nchs/data/databriefs/db16.pdf〉 (available at) [PubMed] [Google Scholar]
- MacDorman M.F., Munson M.L., Kirmeyer S. Fetal and perinatal mortality, United States, 2004. National Vital Statistics Reports. 2007;56(3):1–19. [PubMed] [Google Scholar]
- McMillen M.M. Differential mortality by sex in fetal and neonatal deaths. Science. 1979;204(4388):89–91. doi: 10.1126/science.571144. [DOI] [PubMed] [Google Scholar]
- Menon R., Bonney E.A., Condon J., Mesiano S., Taylor R.N. Novel concepts on pregnancy clocks and alarms: Redundancy and synergy in human parturition. Human Reproduction Update. 2016;22(5):535–560. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D., Galloway T.S., Bailey T.C., Mathews F. Elevated risk of stillbirth in males: Systematic review and meta-analysis of more than 30 million births. BMC Medicine. 2014;12:220. doi: 10.1186/s12916-014-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbidity and Mortality Weekly Report Abortion surveillance — United States, 2013. Surveillance Summaries. 2016;65(12):1–44. doi: 10.15585/mmwr.ss6512a1. (Anon.) [DOI] [PubMed] [Google Scholar]
- Morris J.K., Wald N.J., Watt H.C. Fetal loss in Down syndrome pregnancies. Prenatal Diagnosis. 1999;19(2):142–145. [PubMed] [Google Scholar]
- Neuhauser M., Krackow S. Adaptive-filtering of trisomy 21: Risk of Down syndrome depends on family size and age of previous child. Naturwissenschaften. 2007;94(2):117–121. doi: 10.1007/s00114-006-0165-3. [DOI] [PubMed] [Google Scholar]
- Nybo Andersen A.M., Wohlfahrt J., Christens P., Olsen J., Melbye M. Maternal age and fetal loss: Population based register linkage study. BMJ. 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack S.H., Stubblefield J.W., Akmaev V.R., Colls P., Munne S., Scholl T. The human sex ratio from conception to birth. Proceedings of National Academy of Science United States of America. 2015;112(16):E2102–E2111. doi: 10.1073/pnas.1416546112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone S., Santacroce A., Picardi A., Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World Journal of Clinical Pediatrics. 2016;5(2):172. doi: 10.5409/wjcp.v5.i2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles B.L., Hsu S., Park E., Samet J.M. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. American Journal of Epidemiology. 2014;184(2):87–97. doi: 10.1093/aje/kwv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt R.W., Joseph K.S., Ananth C.V., Grondines J., Abrahamowicz M., Kramer M.S. A proportional hazards model with time-dependent covariates and time-varying effects for analysis of fetal and infant death. American Journal of Epidemiology. 2004;160(3):199–206. doi: 10.1093/aje/kwh201. [DOI] [PubMed] [Google Scholar]
- Quenby S., Vince G., Farquharson R., Aplin J. Recurrent miscarriage: A defect in nature’s quality control? Human Reproduction. 2002;17(8):1959–1963. doi: 10.1093/humrep/17.8.1959. [DOI] [PubMed] [Google Scholar]
- Rai R., Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- Roberts C.J., Lowe C.R. Where have all the conceptions gone? Lancet. 1975;305:498–499. [Google Scholar]
- de la Rochebrochard E., Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Human Reproduction. 2002;17(6):1649–1656. doi: 10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- Rom A.L., Mortensen L.H., Cnattingius S., Arntzen A., Gissler M., Andersen A.M. A comparative study of educational inequality in the risk of stillbirth in Denmark, Finland, Norway and Sweden 1981–2000. Journal of Epidemioligy and Community Health. 2010;66(3):240–246. doi: 10.1136/jech.2009.101188. [DOI] [PubMed] [Google Scholar]
- Salker M., Teklenburg G., Molokhia M., Lavery S., Trew G., Aojanepong T. Natural selection of human embryos: Impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5(4):e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders N.J., Stoecker C. Where have all the young men gone? Using sex ratios to measure fetal death rates. Journal of Health Economics. 2015;41:30–45. doi: 10.1016/j.jhealeco.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Ladner D.G., Cole L.A. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertility and Sterility. 2008;89(6):1781–1786. doi: 10.1016/j.fertnstert.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Schneider E.B. Fetal health stagnation: Have health conditions in utero improved in the United States and Western and Northern Europe over the past 150 years? Social Science & Medicine. 2017;179:18–26. doi: 10.1016/j.socscimed.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Schulz L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R.M., Branch D.W., Goldenberg R., Iams J.D., Klebanoff M.A. Nomenclature for pregnancy outcomes: Time for a change. Obstetrics & Gynecology. 2011;118(6):1402–1408. doi: 10.1097/AOG.0b013e3182392977. [DOI] [PubMed] [Google Scholar]
- Singh P., Yang W., Shaw G.M., Catalano R., Bruckner T.A. Selected birth defects among males following the United States terrorist attacks of 11 September 2001. Birth Defects Research. 2017;109(16):1277–1283. doi: 10.1002/bdr2.1072. [DOI] [PubMed] [Google Scholar]
- Smith-Greenaway E., Sennott C. Death and desirability: Retrospective reporting of unintended pregnancy after a child’s death. Demography. 2016;53(3):805–834. doi: 10.1007/s13524-016-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson D.K., Verter J., Fanaroff A.A., Oh W., Ehrenkranz R.A., Shankaran S. Sex differences in outcomes of very low birthweight infants: The newborn male disadvantage. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2000;83(3):F182–F185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillbirth Collaborative Research Network Writing Group Association between stillbirth and risk factors known at pregnancy confirmation. JAMA. 2011;306(22):2469–2479. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L.B., Barnett A.G., Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. American Journal of Epidemiology. 2011;175(2):99–107. doi: 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- Torche F., Kleinhaus K. Prenatal stress, gestational age and secondary sex ratio: The sex-specific effects of exposure to a natural disaster in early pregnancy. Human Reproduction. 2011;27(2):558–567. doi: 10.1093/humrep/der390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L., Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179(68):90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tyson J.E., Parikh N.A., Langer J., Green C., Higgins R.D. Intensive care for extreme prematurity--moving beyond gestational age. New England Journal of Medicine. 2008;358(16):1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente C. Civil conflict, gender-specific fetal loss, and selection: A new test of the Trivers–Willard hypothesis. Journal of Health Economics. 2015;39:31–50. doi: 10.1016/j.jhealeco.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Vitzthum V. Evolutionary models of women’s reproductive functioning. Annual Reviews of Anthropology. 2008;37:53–73. [Google Scholar]
- Wasser S.K., Barash D.P. Reproductive suppression among female mammals: Implications for biomedicine and sexual selection theory. The Quarterly Review of Biology. 1983;58(4):513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Wells J. Natural selection and sex differences in morbidity and mortality in early life. Journal of Theoretical Biology. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- Wells J.C.K., Nesse R.M., Sear R., Johnstone R.A., Stearns S.C. Evolutionary public health: Introducing the concept. Lancet. 2017;390(10093):500–509. doi: 10.1016/S0140-6736(17)30572-X. [DOI] [PubMed] [Google Scholar]
- West S. Princeton University Press; Princeton, NJ: 2009. Sex allocation. (Monographs in Population Biology, No. 44) [Google Scholar]
- Wijesuriya M., Williams R., Yajnik C. The Kathmandu Declaration: “Life Circle” approach to prevention and care of diabetes mellitus. Diabetes Research and Clinical Practice. 2010;87(1):20–26. doi: 10.1016/j.diabres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Wilcox A.J., Weinberg C.R., Basso O. On the pitfalls of adjusting for gestational age at birth. American Journal of Epidemiology. 2011;174(9):1062–1068. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox A.J., Weinberg C.R., O’Connor J.F., Baird D.D., Schlatterer J.P., Canfield R.E. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Zeitlin J., Saurel-Cubizolles M.J., De Mouzon J., Rivera L., Ancel P.Y., Blondel B. Fetal sex and preterm birth: Are males at greater risk? Human Reproduction. 2002;17(10):2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]