Abstract
Rationale: The efficacy of disease management programs in the treatment of patients with chronic obstructive pulmonary disease (COPD) remains uncertain.
Objectives: To study the effect of disease management (DM) added to recommended care (RC) in ambulatory patients with COPD.
Measurements and Main Results: In this trial, 1,202 patients with COPD (age, ≥40 yr), with moderate to very severe airflow limitation were randomly assigned either to DM plus RC (study intervention) or to RC alone (control intervention). RC included follow-up by pulmonologists, inhaled long-acting bronchodilators and corticosteroids, smoking cessation intervention, nutritional advice and psychosocial support when indicated, and supervised physical activity sessions. DM, delivered by trained nurses during patients’ visits to the designated COPD centers and by remote contacts with the patients between these visits, included patient self-care education, monitoring patients’ symptoms and adherence to treatment, provision of advice in case of acute disease exacerbation, and coordination of care vis-à-vis other healthcare providers. The primary composite endpoint was first hospital admission for respiratory symptoms or death from any cause. During 3,537 patient-years, 284 patients (47.2%) in the control group and 264 (44.0%) in the study intervention group had a primary endpoint event. The median (range) time elapsed until a primary endpoint event was 1.0 (0–4.0) years among patients assigned to the study intervention and 1.1 (0–4.1) years among patients assigned to the control intervention; adjusted hazard ratio, 0.92 (95% confidence interval, 0.77–1.08).
Conclusions: DM added to RC was not superior to RC alone in delaying first hospital admission or death among ambulatory patients with COPD.
Keywords: chronic obstructive pulmonary disease, disease management, pulmonary rehabilitation, hospitalization(s), mortality
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) is a common disorder that is associated with significant morbidity and mortality, poor health-related quality of life, and a heavy societal burden. Patients with COPD have higher rates of health services use, including primary care consultations, visits to emergency departments, and hospital admissions for acute exacerbations.
Care recommended for patients with stable COPD includes inhaled long-acting bronchodilators and corticosteroids, influenza vaccines, smoking cessation for patients who are active smokers, and pulmonary rehabilitation. The efficacy of disease management programs for the treatment of patients with COPD who have access to recommended care is still unknown.
What This Study Adds to the Field
This study provides evidence that a disease management program delivered by trained nurses for a median period of 3 years did not significantly reduce hospital admissions or mortality among ambulatory patients with COPD (GOLD classification 2–4) who had access to recommended care, including pulmonary rehabilitation.
Chronic obstructive pulmonary disease (COPD) is a common disorder that is associated with significant morbidity and mortality, poor health-related quality of life, and a heavy societal burden (1–3). Patients with COPD have higher rates of health services use, including primary care consultations, visits to emergency departments, and hospital admissions for acute exacerbations (4). COPD accounted for 3.5% of the total hospital admissions among Medicare beneficiaries in the United States, of whom 20% were readmitted within 30 days (5). In Norway and Canada, hospital admissions account for about one-half of the total COPD healthcare costs (6, 7). COPD ranks third for direct attributable healthcare costs, after cancer and chronic kidney disease (8).
Various nonpharmacological interventions aimed to improve prognosis, reduce hospital admissions, and improve health-related quality of life among ambulatory patients with COPD were tested. A systematic review of 26 randomized controlled trials showed that integrated disease management programs were associated with better disease-related quality of life and functional exercise capacity, and reduced the number of respiratory-related hospital admissions and in-hospital days for all causes among patients with COPD (9). Nevertheless, another study showed that a comprehensive chronic care program for patients with COPD was associated with excess disease-related mortality and had a neutral effect on the number of hospital admissions (10).
In most clinical trials, the efficacy of disease management interventions for ambulatory patients with COPD was tested against “usual care,” which may vary considerably by the local healthcare setting (9).
Care recommended for patients with stable COPD includes inhaled long-acting bronchodilators and corticosteroids, influenza vaccines, smoking cessation for patients who are active smokers, and pulmonary rehabilitation (11). Pulmonary rehabilitation, although proven efficacious in decreasing the severity of disease-related symptoms and improving health-related quality of life (12), is still not offered to many patients with COPD as a part of usual care.
We aimed to evaluate whether disease management delivered by trained nurses in addition to recommended care is more effective than recommended care alone in the treatment of ambulatory patients with COPD. The intervention was tested in Israel, a country that provides universal health insurance to all its residents, covering primary, secondary, and tertiary healthcare services, medications, and other healthcare technologies, and laboratory and imaging services.
Methods
Study Design
In this multicenter, open-label randomized trial of parallel group design, we compared the efficacy of a comprehensive program that included recommended care plus disease management (study intervention) versus recommended care alone (control intervention) for the treatment of ambulatory patients with COPD ensured with Clalit Health Services, the largest health plan in Israel.
Potential candidates were identified on the basis of electronic patient records in the health plan database, using information on relevant ICD-9 (International Classification of Diseases, 9th revision)–coded chronic diagnoses and purchases of prescribed drugs (i.e., inhaled β2-adrenergic agonists, corticosteroids, or anticholinergic agents; and oral xanthines).
Eligibility screening was conducted in two designated community-based COPD centers, established for the purpose of the study, and included obtaining post-bronchodilator spirometry results and medical history. COPD diagnosis was based on typical signs and symptoms, a history of exposure to cigarette smoking or occupational dust or chemicals, and objective evidence of airflow limitation (11).
Patients were classified as having unstable disease if they had at least one hospital admission or at least two visits to an emergency room for respiratory symptoms within 12 months of recruitment. Otherwise, patients were considered as having stable disease.
Adult patients (age, ≥40 yr) with severe or very severe airflow limitation (Global Initiative for Chronic Obstructive Lung Disease [GOLD] classification 3 or 4 [11]) were eligible. Patients with moderate airflow limitation (GOLD classification 2) were also eligible, if they had unstable disease, a history of current or past exposure to cigarette smoking, and no history of childhood-onset asthma.
Patients with permanent tracheostomy, heart failure with left ventricular ejection fraction less than 40%, severe comorbidity, significant functional or cognitive impairment, communication problems, or substance abuse, or patients who were participating in another trial, were excluded (for more information on the study protocol, see the online supplement).
Interventions
Recommended care
After baseline evaluation by pulmonologists, patients enrolled in both study arms received recommended care for ambulatory patients with COPD (11). This care included follow-up by pulmonologists, treatment with inhaled long-acting bronchodilators and corticosteroids, long-term oxygen treatment where indicated, dietary advice, and psychosocial support where needed. Patients who were cigarette smokers were referred to smoking cessation group sessions and received prescriptions for smoking cessation medications. At enrollment, all patients received self-care education from the nurses at the COPD centers. Unless contraindicated, patients were also referred to supervised physical exercise sessions at the COPD center, twice per week, for a total period of 3 months per year, for the first 2 years. Patients who wished to extend their participation in the physical exercise sessions beyond 2 years were allowed to do so until the end of the study. Except for visits to the pulmonologists and medical therapy, all other activities were considered as components of pulmonary rehabilitation. The frequency of follow-up visits to the pulmonologist at the COPD center for patients assigned to the study intervention was determined according to each patient’s needs, but was not less than once every 6 months. Patients assigned to the control intervention were referred to their consultant pulmonologist in the community. If a patient was not assigned to a consultant pulmonologist, a recommendation for referral was sent to the patient’s primary practitioner. The frequency of visits to the consultant pulmonologist for these patients was determined according to the patients’ needs. In addition, the pulmonologists at the COPD centers evaluated these patients every 6 months for the follow-up study assessments.
Disease management
Patients allocated to the recommended care plus disease management intervention were assigned to trained COPD nurses. These nurses had face-to-face sessions with the patients during their scheduled visits to the COPD center and maintained remote contacts with the patients between these visits. The number of remote sessions was individualized according to the patients’ needs.
During these face-to-face and remote sessions, the nurses monitored disease signs and symptoms; provided advice in case of acute disease exacerbation, following designated protocols and pulmonologists’ guidance; monitored and motivated patients’ adherence to medical therapy, healthy lifestyle (e.g., smoking cessation), and other components of the treatment plan (e.g., participation in physical exercise sessions); and coordinated care vis-à-vis other healthcare professional caregivers. Where possible, nonprofessional caregivers (e.g., family members) were involved in the educational sessions for self-care delivered by the nurses (see also the online supplement). The disease management activities focused mainly on the patient’s respiratory disease and did not routinely address the patient’s comorbidities.
During off-hours, patients assigned to the disease management intervention were instructed to consult with an on-call disease management nurse in case of an acute change in their respiratory symptoms. They were also provided with a prescription for inhaled short-term bronchodilators, oral antibiotics, and steroids (e.g., “crisis medication pack”), and were instructed to take these drugs in such events.
All disease management activities were recorded and supervised by the nurse program director.
Measures taken to avoid contamination of the control intervention
Except for a single self-care education session delivered on enrollment, the contact between the nurses and patients assigned to the control intervention during their follow-up visits to the COPD centers did not include any component of disease management listed above.
Patients assigned to the control intervention were instructed to refer to their family physician for evaluation and treatment in the case of acute exacerbation of respiratory symptoms, and did not receive a “crisis medication pack” prescription.
Follow-up assessment visits, pulmonary rehabilitation sessions, and smoking cessation meetings were conducted on separate days according to the patients’ assigned intervention.
Randomization
After completing eligibility and baseline assessment and providing signed informed consent, patients were randomly assigned either to the study intervention or to the control intervention, using a computerized randomization program with permuted-block design linked to the patients’ electronic medical record. Randomization was done at a 1:1 ratio and stratified by the COPD center.
The study was approved by the Clalit Health Services and the Sheba Medical Center research ethics committees.
Study Assessments and Outcomes
Assessments, performed in the COPD centers at baseline and every 6 months thereafter, included obtaining patient history and patient-completed questionnaires, 6-minute-walk test results, and post-bronchodilator spirometry results.
After randomization, the study personnel at the COPD centers were not blinded to the patients’ assigned intervention during follow-up assessments.
The primary composite outcome was the time elapsed from enrollment until first hospital admission for acute exacerbation of COPD or until death from any cause. Secondary endpoints included the individual components of the primary composite outcome, the total number of hospital admissions and in-hospital days for COPD exacerbations, follow-up assessments of 6-minute-walk test, GOLD classification based on spirometry (11), health-related quality of life assessed with the 12-item Short-Form Health Survey (SF-12 [13]), health status measured with the St. George’s Respiratory Questionnaire (14), depression symptoms assessed with the nine-item Patient Health Questionnaire (PHQ-9 [15]) depression scale, post-bronchodilator FEV1 and FEV1/FVC ratio, and severity of respiratory symptoms assessed with the Medical Research Council (MRC) dyspnea scale (16). Patients were also instructed to keep a log documenting episodes of COPD exacerbations occurring between the 6-month visits to the COPD center, but their adherence to this request was low. Instead, information on acute exacerbations that did not end up in hospital admission during follow-up was derived from data on concomitant purchases of oral antibiotics and corticosteroids, recorded in the Clalit Health Services electronic database.
Information on hospital admissions and deaths during follow-up was obtained from the Clalit Health Services electronic database. Discharge summaries, available for 3,381 (98.4%) of 3,437 hospital admissions, were classified by two independent investigators, blinded to the patients’ assigned intervention, as due either to COPD exacerbation or other causes. Disagreement between the investigators occurred for 368 (10.9%) of the hospital discharge summaries. These 368 summaries were classified by a third blinded investigator, with the final classification following the majority opinion.
Safety Committee
A safety committee monitored the cumulative event rates of hospital admissions and deaths from all causes among the study participants, every 6 months. The committee members were not blinded to the participants’ assigned intervention. The committee discussions were not disclosed to the investigators during the trial. Guided by a predefined level of statistical significance (17), the mandate of the committee was to provide warning in the event of an excess in deaths or hospital admissions from all causes in the study intervention arm. In practice, no such excess was found and the study continued to its planned conclusion.
Sample Size Consideration
The study sample was chosen to detect an intervention effect of 30% reduction in the likelihood of hospital admission for COPD during the first 12 months (18, 19). We assumed that 45% of patients in the control group would be hospitalized for COPD at least once in the first 12 months; that the mortality rate would be 12%, and that one-half of these deaths would not occur among patients admitted to the hospital for COPD (20). Thus, the anticipated rate of the combined outcome in the control group would be 51% (i.e., 45% + 0.5 × 12%). According to this assumption, a sample size of 1,200 (600 in each study arm) would provide 84% statistical power to detect an odds ratio (OR) of 0.7, using a two-sided 0.05 significance level.
Statistical Analysis
The statistical analysis was performed according to a prespecified plan (see statistical analysis plan, in the online supplement). Unadjusted comparisons between the two treatment groups with respect to the primary composite outcome (time to first hospital admission for COPD or death from any cause) and its individual components were made using the log-rank statistic.
The Cox proportional hazards model was used to compare the two treatment groups with respect to the primary composite outcome, adjusted for baseline characteristics (sex, age, COPD center, GOLD classification, and smoking status at baseline). In supportive analyses, adjustment was also made for baseline variables that were significantly imbalanced between the treatment groups, that is, were significantly different at the 10% significance level.
Interactions between treatment group and baseline variables such as center, year of entry, GOLD classification, and smoking were tested, as prespecified in the statistical analysis plan. Also, the assumption of proportional hazards between the treatment groups was tested.
Hospital admission data summarized in 6-month subperiods were compared between treatments, using nonlinear mixed models that included a random subject intercept term. This adjusted for any correlation between the serial observations on each patient. A negative binomial distribution was assumed to account for the overdispersion often occurring in hospital admission frequencies (21). These analyses were conducted using the NLMIXED procedure (SAS, version 9.4; SAS Institute), to compare between treatments the frequency of hospital admission and the days in hospital for COPD and for all causes. The comparisons were adjusted for sex, center, age, GOLD classification, year of entry, and time since entry. The last of these factors adjusted for the different lengths of follow-up due mostly to staggered entry. The treatment group coefficient in this model estimated the adjusted log rate ratio over a 6-month period.
Several other outcomes were dichotomized as attaining (or not) a clinically important change from baseline. These were defined as follows: for the physical and mental component summary scores of the SF-12, an increase of at least 2.5 points (22); for the summary score of St. George’s Respiratory Questionnaire, a decrease of at least four points (23); for the MRC dyspnea scale, a decrease of at least one point (24); for the 6-minute-walk test, an increase of at least 50 m (24, 25); for moderate-to-severe depression symptoms, a PHQ-9 score equal to or exceeding 10 (14); and for FEV1, an increase of at least 100 ml and an increase of at least 5% from baseline (25). These serial binary variables were analyzed using the same mixed-model approach as for hospital admissions, but using a logistic model. The treatment coefficient was the adjusted odds ratio of success.
All analyses were performed according to the intention-to-treat principle, at a critical two-sided significance level of 0.05.
Results
Participants were recruited between October 2009 and June 2012, and monitored until death or end of study (July 2014). The median time of follow-up was 3.0 years (range, 0–4.7), and did not differ significantly between the study groups (see Table 2) (P = 0.50).
Table 2.
Healthcare Services Use and Deaths during Follow-Up
| Total (N = 1,202) | RC plus DM (n = 600) | RC (n = 602) | |
|---|---|---|---|
| Length of follow-up, yr, mean (SD) | 2.94 (0.99) | 2.96 (0.97) | 2.92 (1.02) |
| Primary endpoint events, n (%)* | 548 (45.6) | 264 (44.0) | 284 (47.2) |
| Deaths from all causes, n (%) | 163 (13.6) | 72 (12.0) | 91 (15.1) |
| Hospital admissions for all causes, n | n = 867 | n = 432 | n = 435 |
| Total number of admissions | 3,437 | 1,645 | 1,792 |
| Total number of hospital days | 24,406 | 11,489 | 12,917 |
| Hospital admissions for COPD, n | n = 498 | n = 244 | n = 254 |
| Total number of admissions | 1,617 | 762 | 855 |
| Total number of hospital days | 10,596 | 5,082 | 5,514 |
| Visits to a pulmonologist during follow-up, mean (SD), n/yr | 4.5 (7.0) | 5.2 (4.2) | 3.8 (8.9) |
| Visits to a primary practitioner during follow-up, mean (SD), n/yr | 13.0 (11.0) | 12.6 (9.2) | 13.4 (12.6) |
| Supervised physical exercise sessions attended during follow-up | |||
| None, n (%) | 460 (38.3) | 175 (29.2) | 285 (47.4) |
| Number of sessions attended | n = 742 | n = 425 | n = 317 |
| Median (interquartile range)† | 78 (12–224) | 107 (15–251) | 28 (8–172) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; DM = disease management; RC = recommended care.
Primary endpoint was defined as first hospital admission for COPD or death from any cause.
Among patients who attended at least one session.
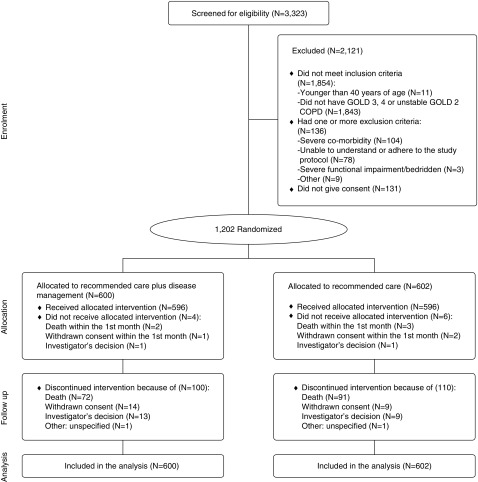
Of 3,323 people screened for eligibility, 1,864 did not meet the inclusion criteria, 136 met one or more exclusion criteria, 131 refused participation, and 1,202 were randomly assigned either to recommended care plus disease management (n = 600) or to recommended care alone (n = 602). Ten participants did not receive the allocated intervention: three because of withdrawn consent and two because of the investigator’s decision, and five participants died within 1 month of randomization. Forty-seven patients discontinued the intervention during follow-up: 23 because of withdrawn consent, 22 because of the investigator’s decision, and two for other causes (Figure 1; see also the online supplement). All randomized patients were included in the analyses.
Figure 1.
Screening, randomization, and completion of follow-up. COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
The mean age (SD) of participants was 67.5 (10.0) years, and 855 (71.1%) were men. Current and past cigarette smoking was reported in 34% and 49% of the participants, respectively. Most patients (77%) had GOLD classification 3. At enrollment, 80% of the participants were receiving inhaled long-acting β2-adrenergic agonists or anticholinergic agents, and 76% were treated with inhaled corticosteroids. Patients assigned to recommended care plus disease management were, on average, 1.6 years younger than patients assigned to recommended care alone. Otherwise, the baseline characteristics of the patients were similar across the two intervention groups (Table 1).
Table 1.
Baseline Characteristics of Study Participants
| RC plus DM (n = 600) | RC (n = 602) | P Value | |
|---|---|---|---|
| Age, yr, mean (SD) | 66.7 (9.9) | 68.3 (10.0) | 0.005 |
| Male, n (%) | 414 (69.0) | 441 (73.3) | 0.103 |
| COPD status at recruitment: unstable, n (%) | 179 (29.8) | 171 (28.4) | 0.586 |
| Comorbidity, n (%) | |||
| Heart failure | 32 (5.3) | 37 (6.2) | 0.535 |
| Diabetes | 174 (29.0) | 197 (32.8) | 0.157 |
| Hypertension | 317 (52.8) | 324 (54.0) | 0.685 |
| Ischemic heart disease | 138 (23.0) | 159 (26.4) | 0.170 |
| Peripheral heart disease | 48 (8.1) | 59 (9.8) | 0.286 |
| Stroke/TIA | 52 (8.7) | 50 (8.3) | 0.84 |
| Renal failure* | 21 (3.5) | 28 (4.7) | 0.313 |
| Cigarette smoking, n (%) | 0.134 | ||
| Never | 96 (16.0) | 108 (17.9) | |
| Past | 283 (47.2) | 305 (50.7) | |
| Current | 221 (36.8) | 189 (31.4) | |
| FEV1, L, mean (SD) | 1.14 (0.36) | 1.13 (0.35) | 0.87 |
| FEV1% predicted, mean (SD) | 43.8 (10.8) | 44.1 (10.0) | 0.601 |
| FVC, L, mean (SD) | 2.11 (0.68) | 2.11 (0.63) | 0.98 |
| FEV1/FVC, mean (SD) | 0.54 (0.09) | 0.54 (0.09) | 0.50 |
| GOLD class, n (%) | 0.36 | ||
| 2 | 81 (13.5) | 80 (13.3) | |
| 3 | 455 (75.8) | 472 (78.4) | |
| 4 | 64 (10.7) | 50 (8.3) | |
| 6-min-walk test, m, mean (SD) | 282.5 (147.7) | 273.8 (151.1) | 0.31 |
| MRC score, mean (SD)† | 2.73 (0.99) | 2.75 (1.00) | 0.65 |
| Medical treatment, n (%) | |||
| Inhaled long-acting bronchodilators‡ | 483 (80.5) | 482 (80.1) | 0.85 |
| Inhaled corticosteroids | 459 (76.5) | 456 (75.7) | 0.45 |
| Ambulatory oxygen therapy, n (%) | 68 (11.3) | 83 (13.8) | 0.20 |
| Health-related quality of life | |||
| SF-12 physical component summary score, mean (SD) | 35.0 (9.7) | 34.5 (9.7) | 0.43 |
| SF-12 mental component summary score, mean (SD) | 47.6 (12.3) | 46.3 (12.8) | 0.083 |
| Total score of the St. George’s Respiratory Questionnaire, mean (SD)† | 51.9 (21.1) | 53.2 (20.7) | 0.25 |
| Depression symptoms (PHQ-9 score ≥ 10), n (%) | 140 (23.3) | 148 (24.6) | 0.60 |
| Past enrollment in pulmonary rehabilitation program, n (%) | 27 (4.5) | 29 (4.8) | 0.59 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; DM = disease management; GOLD = Global Initiative for Chronic Obstructive Lung Disease; MRC = Medical Research Council dyspnea scale (lower scores indicate fewer symptoms); PHQ-9 = nine-item Patient Health Questionnaire depression scale; RC = recommended care; SF-12 = 12-item Short-Form Health Survey (higher scores indicate better quality of life); TIA = transient ischemic attack.
Renal failure defined as plasma creatinine ≥ 2.0 mg/dl.
Lower MRC dyspnea scale scores indicate better functioning/status.
Long-acting β2-adrenergic agonists or anticholinergic agents.
Endpoints
During 3,537 patient-years, there were 3,437 hospital admissions for any cause in 867 participants and 1,617 hospital admissions for COPD in 498 participants; and 163 participants died (Table 2).
Primary Outcome and Its Components
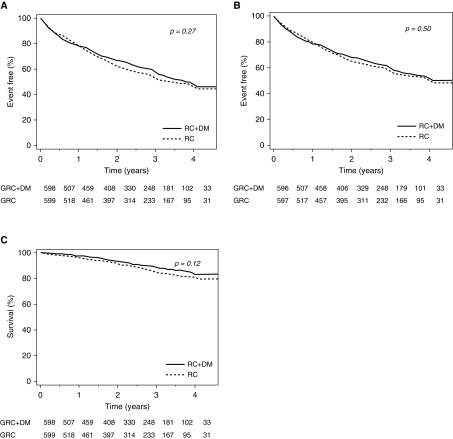
The primary composite outcome, first hospital admission for COPD or death from any cause, occurred in 284 patients (47.2%) assigned to recommended care, and in 264 patients (44.0%) assigned to recommended care plus disease management. The median time to a primary outcome event was 1.0 (range, 0–4.0) years among patients assigned to the study intervention, and 1.1 (range, 0–4.1) years among patients assigned to the control intervention (hazard ratio [HR], 0.91; 95% confidence interval [CI], 0.77–1.08) (Table 3 and Figure 2A). Further adjustment for age, sex, study center, baseline GOLD classification, and smoking status did not show a statistically significant advantage of the study intervention over the control intervention: adjusted HR, 0.92; 95% CI, 0.77–1.08 (Table 3).
Table 3.
Effect of Study Intervention (Recommended Care plus Disease Management) versus Control Intervention (Recommended Care Alone) on Primary Endpoint and Its Components
| Endpoint | Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI)* |
|
|---|---|---|---|
| Model 1 | Model 2 | ||
| Time to first COPD hospital admission or death from all causes (primary composite outcome) | 0.91 (0.77–1.08) | 0.916 (0.77–1.08) | 0.94 (0.80–1.12) |
| Time to death from all causes | 0.78 (0.58–1.07) | 0.82 (0.60–1.12) | 0.82 (0.60–1.13) |
| Time to first COPD hospital admission | 0.94 (0.79–1.12) | 0.93 (0.78–1.11) | 0.96 (0.80–1.15) |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; SF-12 = 12-item Short-Form Health Survey.
Cox proportional hazard models were adjusted for model 1 (sex, study center, baseline age, cigarette smoking status, and GOLD classification) and model 2 (all covariates included in model 1 plus the baseline score on the SF-12 mental component).
Figure 2.
The primary composite endpoint and its components by study group. (A) First hospital admission for chronic obstructive pulmonary disease (COPD) or death from all causes. (B) First hospital admission for COPD. (C) Death from any cause. DM = disease management; GRC = Guideline recommended care; RC = recommended care.
Likewise, the study intervention was not found superior to the control intervention with respect to the individual components of the primary composite outcome, that is, time to first COPD hospital admission (adjusted HR, 0.93; 95% CI, 0.78–1.11) and time to death from all causes (adjusted HR, 0.82; 95% CI, 0.60–1.12) (Table 3 and Figures 2B and 2C).
None of the prespecified interactions tested were found statistically significant, with respect to the intervention effect on the primary outcome or its components.
Secondary Outcomes
There were no significant differences between the two intervention groups with respect to the total number of hospital admissions and in-hospital days, either due to COPD or to any cause (Table 4).
Table 4.
Effect of Study Intervention (Recommended Care plus Disease Management) versus Control Intervention (Recommended Care Alone) on Hospital Admission Endpoints
| Endpoint | Crude Incidence Rate Ratio (95% CI) | Adjusted Rate Ratio* (95% CI) |
|---|---|---|
| Number of hospital admissions for COPD | 0.95 (0.75–1.19) | 0.92 (0.73–1.16) |
| Total number of in-hospital days for COPD | 0.94 (0.68–1.31) | 0.89 (0.61–1.31) |
| Number of hospital admissions for all causes | 0.96 (0.84–1.11) | 0.97 (0.85–1.12) |
| Number of total in-hospital days for all causes | 0.94 (0.74–1.20) | 0.94 (0.75–1.20) |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Rate ratios between the expected mean number of hospital admissions and in-hospital days among patients assigned to disease management and patients assigned to usual care were derived from negative binomial nonlinear mixed models, adjusted for study center, study period, year at recruitment, sex, baseline age, and GOLD classification.
Patients assigned to recommended care plus disease management were more likely to report clinically important improvement in their health status (measured with the St. George’s Respiratory Questionnaire): adjusted OR, 1.32; 95% CI, 1.01–1.73; P = 0.046 (Table 5).
Table 5.
Effect of Study Intervention (Recommended Care plus Disease Management) versus Control Intervention (Recommended Care Alone) on Secondary Outcome and Process Endpoints during Follow-up, Adjusted for Baseline Characteristics
| Endpoint | Adjusted Odds Ratio (95% CI)* |
|---|---|
| Depression symptoms† | 0.99 (0.77–1.27) |
| Health-related quality of life‡ | |
| Physical component summary | 1.14 (0.89–1.45) |
| Mental component summary | 0.94 (0.75–1.18) |
| Health status§ | 1.32 (1.01–1.73) |
| Severity of respiratory symptomsǁ | 1.22 (0.90–1.66) |
| 6-min-walk test¶ | 1.06 (0.79–1.42) |
| FEV1** | 1.05 (0.74–1.48) |
| Quit smoking (n = 410) | 1.72 (1.05–2.82)†† |
Definition of abbreviations: CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Unless otherwise specified, odds ratios are derived from nonlinear mixed models, adjusted for age, sex, study center, year at recruitment, study period, and the baseline value of the endpoint variable.
Measured with the nine-item Patient Health Questionnaire depression scale (PHQ-9). Odds ratios were calculated for PHQ-9 scores of 10 or more (signifying moderate to severe depression) during follow-up.
Measured with the 12-item Short-Form Health Survey (SF-12). Odds ratios were calculated for an increase of at least 2.5 points in physical component summary and mental component summary scores.
Measured with the St. George’s Respiratory Questionnaire. Odds ratios were calculated for a decrease of at least four points in summary score.
ǁMeasured with the Medical Research Council dyspnea scale. Odds ratios were calculated for a decrease of at least one point in summary score.
Odds ratios were calculated for an increase of at least 50 m in 6-minute-walk distance.
An increase of at least 100 ml and an increase of at least 5% from the baseline value.
Odds ratio derived from binary logistic model, adjusted for age, sex, study center, year at recruitment, and GOLD classification.
The two treatment groups did not significantly differ with respect to the likelihood of attaining clinically important changes in health-related quality of life, depression, 6-minute-walk test, FEV1, and severity of respiratory symptoms during follow-up (Table 5). See also Table E1 in the online supplement.
Among patients who were active smokers at enrollment (n = 410), patients assigned to recommended care plus disease management were more likely than patients assigned to recommended care alone to report that they were no longer smoking cigarettes on their last follow-up visit (57 of 221 [25.8%] vs. 32 of 189 [16.9%], respectively; OR, 1.72; 95% CI, 1.05–2.82; P = 0.031) (Table 5).
The median (interquartile range) number of acute exacerbations of COPD that did not end with hospital admission did not differ significantly between the two treatment groups during follow-up; 2.9 (1.7–4.8) per year among patients assigned to disease management plus recommended care, versus 2.7 (1.5–4.4) per year among patients assigned to recommended care alone (P = 0.20).
Process Variables
The mean (SD) number of remote contacts with a nurse for patients assigned to the study intervention was 9.1 (3.8) per year.
The mean (SD) number of annual visits to a pulmonologists during the total follow-up period was 5.2 (4.2) for patients assigned to study intervention, and 3.8 (8.9) for patients assigned to the control intervention (P < 0.001). There was no significant difference in the mean number of visits to a primary practitioner between patients assigned to the study intervention and those assigned to the control intervention (mean [SD] number of visits per year: 12.6 [9.2] and 13.4 [12.6], respectively; P = 0.157) (Table 2).
The proportion of patients who attended the supervised physical activity sessions was larger in the study intervention group compared with the control group (71% vs. 53%, respectively), and the number of sessions attended was higher (P < 0.0001; Table 2).
Sixty-five percent of the patients assigned to the study intervention had at least one consultation with a dietitian, and 58.2% had at least one consultation with a social worker.
Discussion
In this randomized controlled trial, disease management added to recommended care (the study intervention) was not shown to be superior to recommended care alone (the control intervention) in the treatment of ambulatory patients with moderate to very severe COPD, with respect to the primary composite outcome, the time until first hospital admission for COPD or death from any cause, its individual components, or most of the secondary outcomes (e.g., total number of hospital admissions and in-hospital days for respiratory symptoms, and clinically important change in health-related quality of life, depression, exercise capacity, and FEV1). Patients assigned to the study intervention were more likely to report a clinically important improvement in disease status (measured with the St. George’s Respiratory Questionnaire) than were patients assigned to the control intervention.
Disease management is a complex intervention. Typically, these programs include some or all of the following components: delivery of care by multidisciplinary teams, patient empowerment and self-care education, coordination of care, reorganization of care delivery systems, use of information systems, and reliance on evidence-based practices (26). Some studies on disease management programs in the treatment of ambulatory patients with COPD were negative (10, 20). Differences in the components included and in the implementation of the intervention may explain the inconsistent results. In some of the trials included in a systematic review (9), interventions recommended as standard of care for patients with stable COPD (e.g., exercise, nutritional advice, and smoking cessation intervention [11]) were included as part of the disease management intervention and were not offered to patients assigned to the control group. Variations in the study comparator intervention may also explain some of this inconsistency (9).
In our study, the comparator intervention was care recommended for ambulatory patients with COPD, including follow-up by a pulmonologist, inhaled long-acting bronchodilators and corticosteroids, guided physical exercise sessions, smoking cessation intervention, psychosocial support, and dietary advice when needed. This type of care may not reflect what was considered “usual care” in most clinical trials.
Smoking cessation is the only intervention that slows the accelerated rate of pulmonary function decline among patients with COPD (27). Drug therapy combined with intensive behavioral treatment is the most effective intervention for achieving sustainable smoking cessation among these patients (28). Many of the randomized controlled trials testing the efficacy of an integrated disease management program in patients with COPD included a smoking cessation intervention (9). The change in smoking status during follow-up was not reported in some studies (19, 29–32), whereas others showed no effect on the smoking cessation rate (33–36). We found that patients assigned to the study intervention were about 70% more likely to report quitting smoking by the end of follow-up, compared with patients assigned to the control intervention. If proved consistent in further studies, this may be the most significant benefit gained from an integrated disease management intervention in the treatment of patients with COPD who have access to recommended care.
Our study has a few limitations. The evaluation of the intervention effect on smoking cessation was based on patients’ reports and not on objective measurement (e.g., serum or urinary cotinine). Thus, we cannot exclude that reporting on smoking status at the end of the study differed by study intervention.
Despite using a computerized randomization protocol and maintaining strict allocation concealment, patients assigned to the study intervention group were, on average, 1.6 years younger than patients assigned to the control intervention. This age difference was not large, and was accounted for in the multivariable analyses.
The pulmonologists and nurses who were involved in follow-up assessments at the COPD centers were unmasked to the patients’ assigned intervention. Efforts to minimize potential observer bias and contamination of the control intervention were therefore imbedded in the study design. Where relevant (e.g., 6-min-walk test, forms completed by the physician or nurse), the study assessments were conducted according to standard protocols and procedures. As aforementioned, we also took other measures to avoid contamination of the control intervention by assigning separate days for follow-up visits for study assessments, pulmonary rehabilitation, and smoking cessation sessions, according to the patient’s assigned intervention.
Finally, recommended care may not represent usual care given to patients with COPD in various healthcare settings. Thus, we cannot rule out that the disease management intervention tested in this study would be efficacious for ambulatory patients with COPD who do not have access to all components of recommended care, including pulmonary rehabilitation.
This study, based on a large sample, long follow-up period, and near complete ascertainment of the main study endpoint, failed to demonstrate conclusively that disease management added to recommended care is superior to recommended care alone in reducing mortality and hospital admissions for respiratory symptoms, or improving exercise capacity, health-related quality of life, or depression among ambulatory patients with COPD. The effect of the intervention on smoking cessation should be confirmed in further studies.
Acknowledgments
Acknowledgment
The authors thank the study nurses and members of the Computing and Information Technology Division in Clalit Health Services, and the patients who participated in the study.
Chronic Obstructive Pulmonary Disease Community Disease Management (COPD-CDM) Investigators: Anat Amital, Ahmad Atamna†, Michal Benderly, Tali Cukierman-Yaffe, Said Elkrinawi, Laurence S. Freedman, Gershon Fink, Leonardo Fuks, Irena Fomin, Avi Gilad, Meri Gluch, Orit Jacobson, Ofra Kalter-Leibovici, Galit Kaufman, Calanit Key, Havi Murad, Tchiya Molcho Falkenberg Luft, Abdel Rahman Nader, Liraz Olmer, Shmuel Poreh, Mordechai Shani, David Segev, and Manuel Szwarcman.
Study committees:
I. Members of the Study Steering Committee: Michal Benderly, Gershon Fink, Meri Gluch, Orit Jacobson, Ofra Kalter-Leibovici, Galit Kaufman, Calanit Key, Tchiya Molcho Falkenberg Luft, Mordechai Shani, and David Segev.
II. Members of the Study Safety Committee: Baruch Chen, Laurence S. Freedman, and Tamy Shohat.
III. Members of the Data Management Committee: Michal Benderly, Yaacov Hacham, and Vered Yayon.
IV. Members of the Endpoint Adjudication Committee: Tali Cukierman-Yaffe, Said ElKrenawi, Avi Gilad, and Ofra Kalter-Leibovici.
V. Members of the Data Analysis Committee: Michal Benderly, Laurence S. Freedman, Ofra Kalter-Leibovici, Havi Murad, and Liraz Olmer.
Footnotes
A complete list of Chronic Obstructive Pulmonary Disease Community Disease Management (COPD-CDM) Investigators may be found before the beginning of the References.
Supported by the Medical Research Infrastructure Development and Health Services Fund by the Sheba Medical Center (Tel-Hashomer, Israel). The funder approved the study protocol but had no role in the study design, data analysis, or manuscript preparation and submission.
Author Contributions: O.K.-L., G.F., M.B., and L.S.F. conceived and designed the study; G.K., T.M.F.L., and M.B. acquired the data; L.S.F., H.M., L.O., M.B., O.K.-L., T.C.-Y., A.G., and S.E. interpreted and analyzed the data; O.K.-L., G.K., T.M.F.L., M.B., L.S.F., B.C., C.K., O.J., D.S., M.G., and M.S. handled funding and supervision; O.K.-L. drafted the manuscript; L.S.F., H.M., M.B., L.O., and G.F. critically reviewed the manuscript for important intellectual content; all authors approved the version submitted. O.K.-L. and G.F. are accountable for all aspects of the study.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201711-2182OC on March 1, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Chronic Obstructive Pulmonary Disease Community Disease Management (COPD-CDM) Investigators, Anat Amital, Ahmad Atamna, Michal Benderly, Tali Cukierman-Yaffe, Said Elkrinawi, Laurence S. Freedman, Gershon Fink, Leonardo Fuks, Irena Fomin, Avi Gilad, Meri Gluch, Orit Jacobson, Ofra Kalter-Leibovici, Galit Kaufman, Calanit Key, Havi Murad, Tchiya Molcho Falkenberg Luft, Abdel Rahman Nader, Liraz Olmer, Shmuel Poreh, Mordechai Shani, David Segev, Manuel Szwarcman, Michal Benderly, Gershon Fink, Meri Gluch, Orit Jacobson, Ofra Kalter-Leibovici, Galit Kaufman, Calanit Key, Tchiya Molcho Falkenberg Luft, Mordechai Shani, David Segev, Baruch Chen, Laurence S. Freedman, Tamy Shohat, Michal Benderly, Yaacov Hacham, Vered Yayon, Tali Cukierman-Yaffe, Said ElKrenawi, Avi Gilad, Ofra Kalter-Leibovici, Michal Benderly, Laurence S. Freedman, Ofra Kalter-Leibovici, Havi Murad, and Liraz Olmer
References
- 1.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The top 10 causes of death. Geneva, Switzerland: World Health Organization; 2017 [accessed 2017 Mar 1]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 3.Huber MB, Wacker ME, Vogelmeier CF, Leidl R. Comorbid influences on generic health-related quality of life in COPD: a systematic review. PLoS One. 2015;10:e0132670. doi: 10.1371/journal.pone.0132670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson GC, Wedzicha JA. COPD exacerbations. 1. Epidemiology. Thorax. 2006;61:164–168. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen R, Johannessen A, Omenaas ER, Bakke PS, Askildsen JE, Gulsvik A. Excessive costs of COPD in ever-smokers: a longitudinal community study. Respir Med. 2011;105:485–493. doi: 10.1016/j.rmed.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Khakban A, Sin DD, FitzGerald JM, Ng R, Zafarí Z, McManus B, et al. Ten-year trends in direct costs of COPD: a population-based study. Chest. 2015;148:640–646. doi: 10.1378/chest.15-0721. [DOI] [PubMed] [Google Scholar]
- 8.Muka T, Imo D, Jaspers L, Colpani V, Chaker L, van der Lee SJ, et al. The global impact of non-communicable diseases on healthcare spending and national income: a systematic review. Eur J Epidemiol. 2015;30:251–277. doi: 10.1007/s10654-014-9984-2. [DOI] [PubMed] [Google Scholar]
- 9.Kruis AL, Smidt N, Assendelft WJJ, Gussekloo J, Boland MRS, Rutten-van Mölken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD009437. doi: 10.1002/14651858.CD009437.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of COPD—2016. Available from: http://goldcopd.org/
- 12.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B, editors. User’s manual for the SF-12v2 Health Survey. Lincoln: RI: QualityMetric; 2009. [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:1–7. [Google Scholar]
- 16.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocock SJ, Geller NL, Tsiatis AA. The analysis of multiple endpoints in clinical trials. Biometrics. 1987;43:487–498. [PubMed] [Google Scholar]
- 18.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Chronic Obstructive Pulmonary Disease Axis of the Respiratory Network Fonds de la Recherche en Santé du Québec. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 19.Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34:608–614. doi: 10.1111/j.1445-5994.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SJC, Candy B, Bryar RM, Ramsay J, Vrijhoef HJM, Esmond G, et al. Effectiveness of innovations in nurse led chronic disease management for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ. 2005;331:485–491. doi: 10.1136/bmj.38512.664167.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers JK, Pocock SJ, McMurray JJV, Granger CB, Michelson EL, Östergren J, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40. doi: 10.1002/ejhf.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s manual for the SF-36v2 Health Survey. Lincoln: RI: QualityMetric; 2007. Determining important differences in scores; pp. 125–133. [Google Scholar]
- 23.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85:25–31, discussion 33–37. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 24.de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–1098. doi: 10.1378/chest.121.4.1092. [DOI] [PubMed] [Google Scholar]
- 25.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. American Thoracic Society; European Respiratory Society Task Force on Outcomes of COPD. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 26.Scott IA. Chronic disease management: a primer for physicians. Intern Med J. 2008;38:427–437. doi: 10.1111/j.1445-5994.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 28.van Eerd EAM, van der Meer RM, van Schayck OCP, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;8:CD010744. doi: 10.1002/14651858.CD010744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dheda K, Crawford A, Hagan G, Roberts CM. Implementation of British Thoracic Society guidelines for acute exacerbation of chronic obstructive pulmonary disease: impact on quality of life. Postgrad Med J. 2004;80:169–171. doi: 10.1136/pgmj.2003.012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlieb V, Lyngsø AM, Nybo B, Frølich A, Backer V. Pulmonary rehabilitation for moderate COPD (GOLD 2): does it have an effect? COPD. 2011;8:380–386. doi: 10.3109/15412555.2011.610393. [DOI] [PubMed] [Google Scholar]
- 31.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182:890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 32.van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Mölken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. 2010;65:7–13. doi: 10.1136/thx.2009.118620. [DOI] [PubMed] [Google Scholar]
- 33.Bendstrup KE, Ingemann Jensen J, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J. 1997;10:2801–2806. doi: 10.1183/09031936.97.10122801. [DOI] [PubMed] [Google Scholar]
- 34.Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2008;63:194–200. doi: 10.1136/thx.2007.077578. [DOI] [PubMed] [Google Scholar]
- 35.Zwar NA, Hermiz O, Comino E, Middleton S, Vagholkar S, Xuan W, et al. Care of patients with a diagnosis of chronic obstructive pulmonary disease: a cluster randomised controlled trial. Med J Aust. 2012;197:394–398. doi: 10.5694/mja12.10813. [DOI] [PubMed] [Google Scholar]
- 36.Zwar NA, Bunker JM, Reddel HK, Dennis SM, Middleton S, van Schayck OC, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Fam Pract. 2016;33:663–670. doi: 10.1093/fampra/cmw077. [DOI] [PubMed] [Google Scholar]