Abstract
Lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1), a cell surface receptor expressed in endothelial cells, is known to mediate oxidized LDL-induced vascular inflammation and atherogenesis. Although the role of LOX-1 in vascular inflammation has been well established, its involvement in acute lung inflammation and injury remains unclear. In the present study, we examined the effects of a LOX-1-blocking antibody on lung inflammation in a mouse endotoxin lipopolysaccharide (LPS)-induced acute lung injury model. We demonstrated that intraperitoneal challenge with LPS induced a rapid and robust increase in LOX-1 expression in mouse lung. Pre-treatment of mice with anti-LOX-1-blocking antibody significantly inhibited LPS-induced lung inflammation as indicated by decreased neutrophil accumulation in the lung. Furthermore, anti-LOX-1 was capable of inhibiting LPS-induced inflammatory responses, including NF-κ B activation, ICAM-1 expression and apoptotic signaling, in mouse lung. Collectively, these results indicate that LOX-1 may serve as a valuable therapeutic target in the prevention of acute lung inflammation and injury in sepsis.
Keywords: Endotoxin, Inflammation, LOX-1, Lung, Neutrophil
Introduction
Studies have shown that increased blood levels of endotoxin lipopolysaccharide (LPS) due to Gram-negative bacterial infection may lead to acute lung injury (ALI) in sepsis and endotoxemia patients [1]. Despite significant progress in the study of ALI during the last decade, the mortality rate remains high among patients with ALI [2]. Therefore, improved therapeutic strategies are needed in order to reduce the mortality rate associated with the disease. A better understanding of the pathogenesis of ALI would help to identify potential targets for therapeutic intervention.
ALI is usually associated with excessive pulmonary inflammation [3]. Neutrophil accumulation in the lung plays a crucial role in both the onset and progression of ALI [4]. Production and upregulation of proinflammatory mediators such as ICAM-1, P-selectin, TNF-α, and NF-κ B have been reported to contribute to LPS-induced lung inflammation [5, 6]. The extensive neutrophil influx to the lung requires proinflammatory signals capable of supporting both the early recruitment and persistent influx of neutrophils [6–8]. The initial recruitment of neutrophils to the lung may require early signals such as the expression of ICAM-1 and P-selectin in lung endothelial cells [6], and persistent neutrophil influx may require additional mediators such as proinflammatory cytokine CXCR4/SDF-1 [9].
Despite the considerable progress made in elucidating the proinflammatory mediators that contribute to ALI, it is likely that some of the key mediators remain unidentified. In the present study, we examined the role of lectinlike oxidized LDL receptor-1 (LOX-1) in acute lung inflammation in a LPS-induced ALI mouse model. LOX-1, primarily expressed in endothelial cells, is a receptor for oxidized low-density lipoprotein (LDL) (a well-known mediator of atherogenesis) [10]. LOX-1 expression can be regulated by a variety of proinflammatory cytokines, oxidized LDL, oxidative stress and shear stress [11–13]. Increased LOX-1 expression has been observed in atherosclerosis-susceptible regions [14]. Deletion of LOX-1 or inhibition of LOX-1 function by a blocking antibody was able to prevent proinflammatory, pro-oxidant responses in endothelial cells and reduce atherogenesis in mouse atherosclerosis models [15, 16]. In addition, LOX-1 has been reported to recognize apoptotic cells, bacteria and activated platelets which may contribute to the pathogenesis of vascular disorders [17–19].
Blockade of LOX-1 has been shown to prevent animal death in a rat endotoxemia model and inhibited leukocyte-endothelium interaction in retinal blood vessels in low-dose endotoxin-induced uveitis [20]. Furthermore, in an in vitro flow model, LOX-1 has been shown to directly act as a leukocyte adhesion molecule [20]. Nevertheless, the function of LOX-1 in acute lung inflammation and injury remains unknown. In this study, we assessed the effects of a LOX-1-blocking antibody on LPS-induced acute lung inflammation and injury. Specifically, the effects of anti-LOX-1 on LPS-induced proinflammatory responses including ICAM-1 expression, NF-κB activation and apoptotic signals were examined.
Materials and Methods
Animals
C57BL/6 (20–25 g) male mice were purchased from NCI (National Cancer Institute) and housed in cages with access to food and water in a temperature-controlled room with a 12-hour dark/ light cycle. All experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee of University of Texas Health Center at Tyler.
Reagents
LPS from Escherichia coli 0111:B4 was obtained from Sigma (St. Louis, Mo., USA). Goat anti-mouse LOX-1-blocking antibody, control goat IgG, and recombinant mouse LOX-1 protein were purchased from R&D Systems (Minneapolis, Minn., USA). Cleaved caspase-3 (Asp175) polyclonal antibody and Bcl-xl monoclonal antibody were purchased from Cell Signaling Technologies (Beverly, Mass., USA). ICAM-1 (m-19) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif., USA).
Mouse ALI Model
The mouse ALI model was generated based on a previously described procedure [21, 22]. Briefly, mice were first injected with 100 µg/kg anti-LOX-1, IgG control or phosphate-buffered saline (PBS) via the tail vein, and challenged, 2 h later, with 2.5 mg LPS/kg by intraperitoneal injection. At the end of the experiments, the mice were killed by carbon dioxide inhalation.
Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from removed lung tissue with Trizol (Invitrogen, Carlsbad, Calif., USA). 5 µg RNA was used in each reverse transcription-polymerase chain reaction (RT-PCR). Forward primer (5′-ccaagcgaaccttactcagc-3′) and reverse primer (5′-gctccgtcttgaaggtatgc-3′) targeting the LOX-1 sequence were obtained from Integrated DNA Technologies (Coralville, Iowa, USA).
Lung Myeloperoxidase Assay
Sequestration of polymorphonuclear leukocytes (PMNs) in lung tissues was assessed by determining lung myeloperoxidase (MPO) activity in lung homogenate based on our previously developed procedure [23]. Briefly, 6 h after LPS challenge, the lungs were perfused with 3 ml of sterile PBS via the right ventricle, and snap-frozen in liquid nitrogen and stored at −70 ° C. MPO activity was measured using a 3,3′,5,5′-tetramethylbenzidine substrate solution (TMB; Pierce, Rockford, Ill., USA). MPO activity was presented as OD450 nm per milligram lung tissue.
Bronchoalveolar Lavage and Cell Counts
Six hours after LPS challenge, bronchoalveolar lavage (BAL) fluids were collected (3 times with 0.8 ml aliquot of sterile saline). The BAL fluids were centrifuged at 2,000 g for 10 min at 4 ° C. Cells in BAL fluid were counted, and MPO activity was measured using the TMB substrate solution. Mouse albumin levels in BAL fluids were measured using a mouse albumin ELISA kit purchased from Bethyl Laboratories (Montgomery, Tex., USA).
Western Blot Analysis
Lungs were lysed in a buffer containing 10 mm Tris-HCl (pH 7.6), 50 mm NaCl, 1% Triton X-100, and a protease inhibitor cocktail (Sigma). Samples were extracted on ice for 30 min, sonicated twice for 5 s and centrifuged at 14,000 g for 10 min. Protein concentrations of the cell lysates were determined. Aliquots (each containing 100 µg of proteins) of the lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) (12 or 7.5%) and transferred onto a polyvinylidene fluoride membrane. Immunoblotting was performed using primary antibodies against mouse ICAM-1, cleaved caspase-3, Bcl-xl, LOX-1.
Histopathology
Six hours after LPS challenge, mouse lungs were inflated with 0.8 ml 10% formaldehyde in PBS and fixed in 10% formaldehyde for 72 h. Lungs were then embedded in paraffin for sectioning. Lung sections were stained with hematoxylin and eosin.
LOX-1-Mediated Adhesion Assay
96-well plates were first coated with mouse recombinant LOX-1 (50 µg/ml) at 4 ° C. After an overnight incubation, unbound LOX-1 was washed off, and the plates were blocked with 3% BSA overnight at 4 ° C. Neutrophils were isolated from mouse peripheral blood with Polymorphprep (Accurate Chemical and Scientific, Westbury, N.Y., USA) by density gradient centrifugation. Isolated neutrophils were then suspended in PBS at 2 × 106 cells/ml. Immediately before the experiments, BSA solution was removed from the individual wells of the 96-well plate. 100 µl anti-LOX-1 antibody or PBS (as control) was added to individual wells, and incubated for 30 min at 37 °C. Afterwards, neutrophils were added to the wells at 2 × 105 cells/well. The plates were incubated at 5% CO2, 37 °C. After a 30-min incubation, unbound cells were gently washed off with PBS. Bound neutrophils were counted by phase-contrast microscopy. Eight microscopic fields were randomly selected for each assay and quantified as bound cells/microscopic field.
NF-κB Assay
Nuclear proteins were extracted from freshly removed lungs using a Nuclear Extract kit purchased from Active Motif (Carlsbad, Calif., USA). NF-κB DNA-binding activity in the nuclear extract was measured by using NF-κB p65 Transcription Factor Assay kit from Active Motif.
Statistics
Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni test, and expressed as mean ± SEM. A p value <0.05 was considered statistically significant.
Results
Upregulation of LOX-1 Expression in Mouse Lung following LPS Challenge
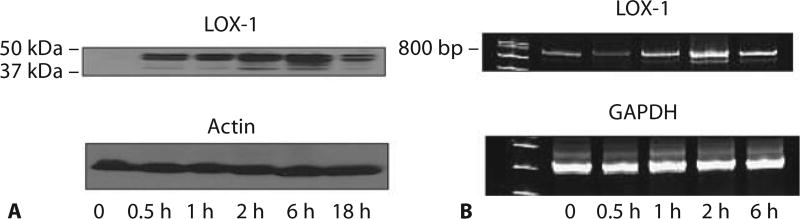
We first examined the time course of LOX-1 expression in mouse lung following LPS challenge. Both LOX-1 protein and mRNA expression were rapidly upregulated following LPS challenge (fig. 1). Increased LOX-1 protein expression was observed as early as 30 min after LPS treatment, peaked at 6 h, decreased to a much lower level at 18 h which, nevertheless, was still considerably higher than that detected prior to LPS challenge. LOX-1 mRNA expression was also increased with time after LPS challenge and peaked at around 2 h after LPS treatment. These results showing rapid induction of LOX-1 upon LPS challenge suggest that LOX-1 is one of the early genes which can quickly respond to inflammatory stimuli.
Fig. 1.
Rapid upregulation of LOX-1 expression in mouse lung following LPS challenge. LPS induced rapid expression of LOX-1 in mouse lung. Western blot analysis showed LOX-1 protein expression (A), and RT-PCR analysis showed LOX-1 mRNA expression (B) in mouse lung tissues at different time points following LPS treatment. Mice were challenged intraperitoneally with 2.5 mg LPS/kg. The results are representative of 3 independent experiments.
Anti-LOX-1 Prevents Neutrophil Accumulation and Increased Albumin Permeability in Mouse Lung after LPS Challenge
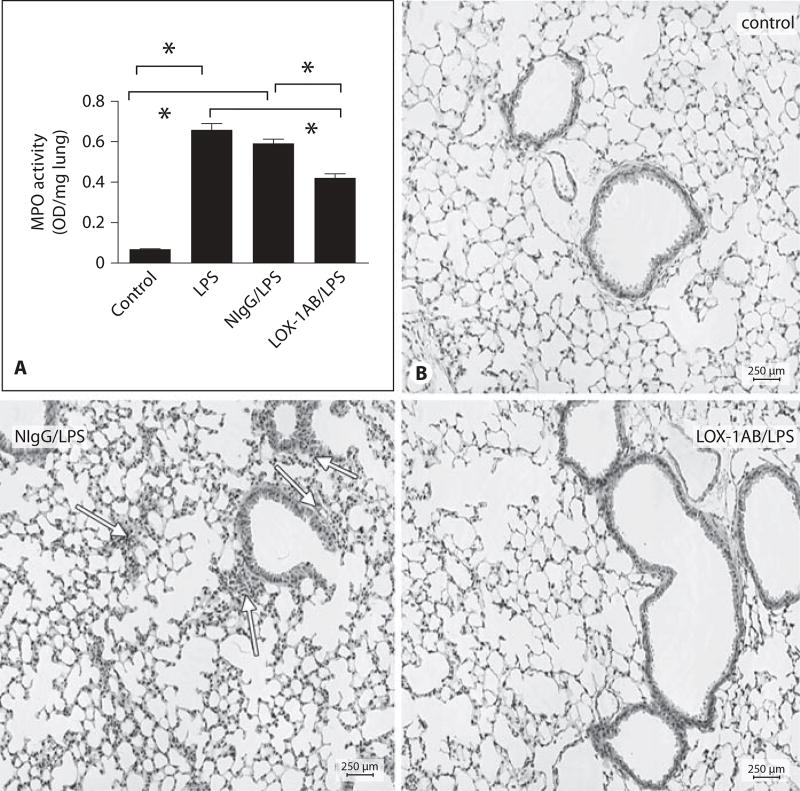
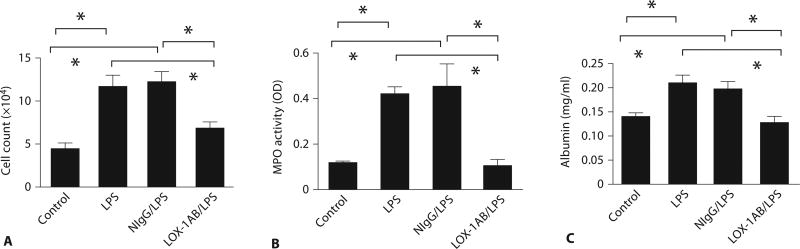
We first examined the effect of a LOX-1-blocking antibody on LPS-induced neutrophil recruitment in the lung. LPS challenge induced marked increase in neutrophil infiltration in the lung as evidenced by elevated MPO activity. Interestingly, pretreatment of the mice with LOX-1 antibody significantly inhibited MPO buildup in the lung (fig. 2A) whereas the control IgG showed no inhibitory effect. Histologic examination also indicated that LPS-induced sequestration of inflammatory cells around the lung vasculature and infiltration of leukocytes into the lung interstitium were significantly inhibited upon LOX-1 antibody treatment (fig. 2B). The increase in lung MPO activity was clearly associated with the increase in both leukocyte count and MPO activity in BAL fluids (fig. 3A, B) following LPS challenge. In contrast, LOX-1 antibody-treated mice exhibited both lower BAL leukocyte count and MPO activity than mice treated with LPS alone or pretreated with control IgG during the same time course. Increased lung vascular permeability in LPS-challenged mouse lung was determined by measuring leakage of mouse albumin into BAL fluids (fig. 3C). Six hours after LPS challenge, albumin levels in BAL fluids from LOX-1 antibody-treated mice were significantly lower than those of mice without LOX-1 antibody treatment or pretreated with control IgG. Collectively, these data suggest that LOX-1 blockade can prevent LPS-induced lung inflammation and injury.
Fig. 2.
Blockade of LOX-1 inhibits neutrophil accumulation in LPS-challenged mouse lung. A Anti-LOX-1 (LOX-1AB/LPS) inhibited LPS-induced lung MPO activity at 6 h after LPS challenge compared with mice treated with LPS alone (LPS) or pretreated with control IgG (NIgG/LPS). Data are presented as means ± SEM. Asterisk indicates a statistical difference between two groups (n = 12 for each group, p < 0.05). B Hematoxylin and eosin staining of lung tissues obtained from control mice without LPS challenge (Control), pretreated with control IgG (NIgG/LPS) or anti-LOX-1 (LOX-1AB/LPS) at 6 h after LPS challenge. Neutrophil accumulation in the lung was observed after LPS challenge. LOX-1-blocking antibody inhibited LPS-induced neutrophil infiltration compared with mice pretreated with control IgG.
Fig. 3.
Blockade of LOX-1 inhibits leukocyte accumulation in BAL and prevents LPS-induced lung permeability. Leukocytes were enumerated (A) and MPO activity (B) was measured in mouse BAL at 6 h after LPS challenge. Anti-LOX-1 (LOX-1AB/LPS) reduced LPS-induced increases in leukocytes and MPO activity in BAL compared with mice treated with LPS alone (LPS) or pretreated with control IgG (NIgG/LPS) (n = 13 for each group, p < 0.05). C Anti-LOX-1 also decreased albumin leakage in mouse BAL at 6 h after LPS challenge. Data are presented as means ± SEM. Asterisk indicates a statistical difference between two groups (n = 13 for each group, p < 0.05).
LOX-1 Is a Neutrophil Adhesion Molecule
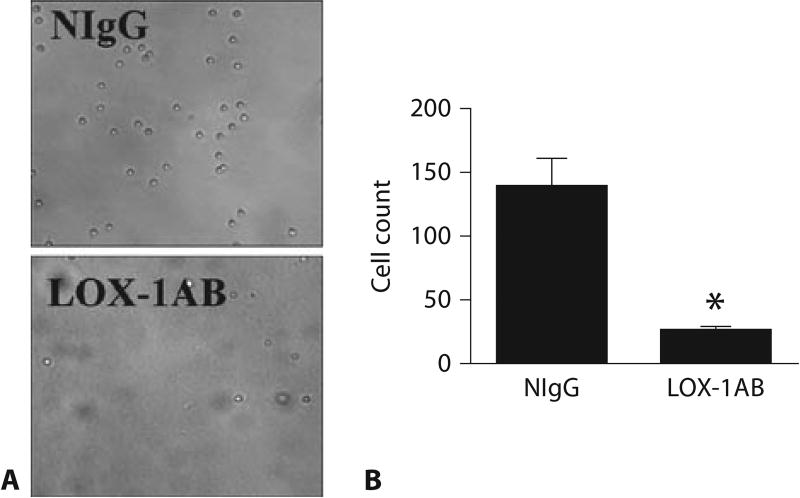
Adhesion of neutrophils to the endothelium of vascular wall is one of the early steps in vascular inflammation and injury. Although previous studies have identified several classes of molecules capable of mediating neutrophil rolling, adhesion and transendothelial migration, unidentified mechanisms may still exist to regulate neutrophil-endothelial cell interactions. Other than serving as a receptor for oxidized-LDL, LOX-1 has been shown to recognize apoptotic cells, bacteria and activated platelets [17–19]. These latter findings prompted us to test whether LOX-1 can act as a neutrophil adhesion molecule. Mouse neutrophils were added to individual wells of a 96-well plate coated with mouse recombinant LOX-1. Adhesion of neutrophils was observed in LOX-1-coated wells (fig. 4) whereas no binding of neutrophils was observed in the wells treated with PBS only (data not shown). Anti-LOX-1 but not control IgG significantly decreased the number of adherent neutrophils, suggesting that neutrophil binding to the recombinant LOX-1 was specifically disrupted by anti-LOX-1.
Fig. 4.
LOX-1-mediated neutrophil adhesion. A Representative images showing adhesion of neutrophils to the surface coated with recombinant mouse LOX-1. Neutrophil adhesion was blocked by LOX-1 antibody (anti-LOX-1) but not by normal IgG control (NIgG). B Quantitative analysis of LOX-1-mediated neutrophil adhesion. Data are presented as means ± SEM. Asterisk indicates a value significantly different from that pretreated with control IgG (n = 3, p < 0.05).
Blockade of LOX-1 Signaling Prevents LPS-Induced Inflammatory Responses
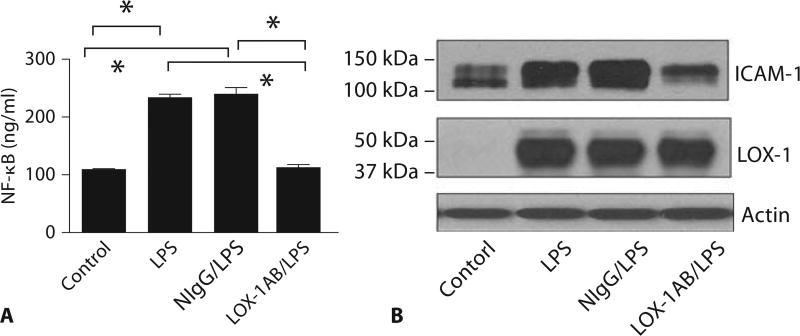
Studies have shown that the expression of ICAM-1 in the lung in response to LPS requires NF-κB activation [22]. To investigate the effects of anti-LOX-1 on LPS-induced inflammatory responses in the lung, we examined NF-κB activation and the expression of ICAM-1 after anti-LOX-1 treatment. NF-κB DNA-binding activity and ICAM-1 expression in the lung were measured. Both LPS-induced NF-κB activity (fig. 5A) and ICAM-1 expression (fig. 5B) in LPS-treated mouse lungs were significantly inhibited by pretreatment with LOX-1 antibody but not with control IgG.
Fig. 5.
Anti-LOX-1 prevents LPS-induced lung NF-κB activity and ICAM-1 expression. At 6 h after LPS challenge, anti-LOX-1 (LOX-1AB/LPS) inhibited LPS-induced NF-κB activity (A) and ICAM-1 expression (B) in mouse lung compared with mice treated with LPS alone (LPS) or pretreated with control IgG (NIgG/LPS). Anti-LOX-1 showed no effects on LOX-1 expression. The results are representative of 3 independent experiments. Asterisk indicates a statistical difference between two groups (n = 12 for each group, p < 0.05).
Anti-LOX-1 Inhibits Apoptotic Signaling in Injured Mouse Lung
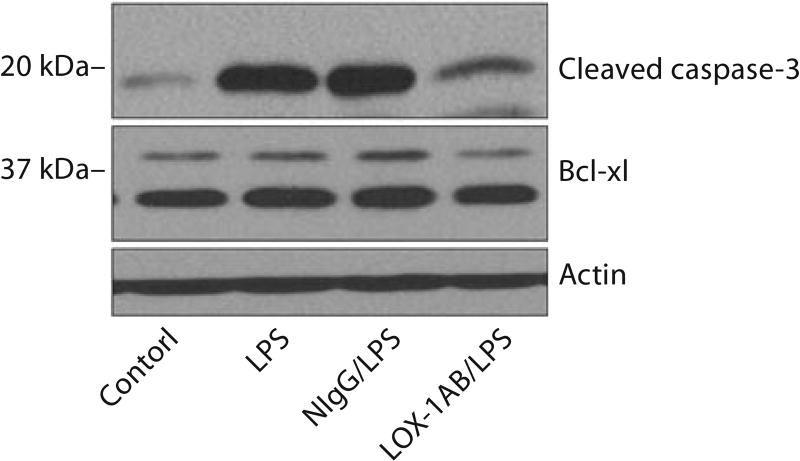
Endothelial apoptosis has been suggested as an important mechanism of LPS-induced vascular injury [24]. To assess the effects of anti-LOX-1 on apoptotic signaling induced by LPS in injured lung, we measured LPS-induced activation of caspases after anti-LOX-1 treatment. Caspase-3 is responsible for the proteolytic cleavage of many key cellular proteins. Activation of caspase-3 requires proteolytic processing of its inactive precursor into an activated form [25]. Activation of caspase-3 (proteolytic cleavage of caspase-3) was analyzed by immunoblotting. Results showed that LPS induced the activation of pro-apoptosis protein caspase-3 (fig. 6) in mouse lung, whereas no effect was observed for anti-apoptosis protein Bcl-xl. Importantly, LOX-1 antibody but not control IgG pretreatment inhibited LPS-induced activation of caspase-3, indicating that anti-LOX-1 was able to inhibit LPS-induced apoptosis in mouse lung.
Fig. 6.
Anti-LOX-1 inhibits LPS-induced apoptotic signaling in mouse lung. Western blot analysis showing expression of cleaved caspase-3 and Bcl-xl in mouse lung at 6 h after LPS challenge. Anti-LOX-1 (LOX-1AB/LPS) inhibited LPS-induced pro-apoptosis protein caspase-3 activation (cleaved caspase-3) compared with mice treated with LPS alone (LPS) or pretreated with control IgG (NIgG/LPS). Anti-LOX-1 showed no effects on the expression of anti-apoptosis protein Bcl-xl. The results are representative of 3 independent experiments.
Discussion
In the present study, we showed that LOX-1 was rapidly upregulated in mouse lung following LPS challenge. Furthermore, inhibition of LOX-1 using a functional blocking antibody prevented acute lung inflammation and injury as evidenced by a decrease in the influx of neutrophils into the lung and inhibition of lung vascular leakage. Our results demonstrated for the first time that LOX-1 blockade was able to inhibit LPS-induced inflammatory lung injury.
In our mouse model, increased LOX-1 expression in mouse lung was observed at both protein and mRNA levels in response to LPS challenge. Rapid upregulation of LOX-1 expression was observed in the lung within 2 h after LPS challenge, and LOX-1 expression peaked at 6 h after LPS treatment. The recruitment of neutrophils to the lung may involve a rapid, early influx of neutrophils, followed by a slower sustained recruitment phase. The temporal pattern of LOX-1 expression indicates that LOX-1 expression may support the early phase of neutrophil recruitment. While our studies focused on the expression of LOX-1 in the lung, upregulation of LOX-1 by LPS may not be a lung-specific phenomenon. Further studies are needed to provide a more detailed analysis of LOX-1 expression in different tissues and organs after LPS challenge. The mechanisms governing LOX-1 expression in the lung remain undetermined. Early proinflammatory mediators including TNF-α and IL-1β have been shown to regulate LOX-1 expression in vascular cells [26, 27]. However, their potential roles in LOX-1 expression in injured lung still await investigation.
Several adhesion molecules are required for the initial interactions of neutrophils with the lung vasculature: selectins for rolling and early attachment, and VCAM-1 or ICAM-1 for firm adhesion [6]. In our studies, we demonstrated that LPS-induced neutrophil accumulation in the lung was partially inhibited by the LOX-1 blocking antibody, which was associated with the inhibition of LPS-induced ICAM-1 expression by LOX-1 blockade. Since LOX-1 can also serve as a neutrophil adhesion molecule, upregulation of both ICAM-1 and LOX-1 expression by LPS challenge may play important roles in early recruitment of neutrophils to the lung.
The functional involvement of LOX-1 in lung vascular inflammation is probably more complex than just the induction of vascular adhesiveness. LOX-1 appears to play diverse roles in proinflammatory signaling and endothelial dysfunction [28–31]. Binding of oxidized LDL to LOX-1 has been reported to inhibit intracellular nitric oxide synthesis and increase superoxide production in endothelial cells [28]. LOX-1 activation is associated with increased expression of proinflammatory mediators TNF-α and IL-1 [31]. LOX-1-dependent activation of PKC has been shown to stimulate the expression of matrix metalloproteinases (MMPs) [30]. MMPs are recognized as critical players in lung inflammation [32, 33]. Furthermore, LOX-1 activation and the resultant oxidant and cytokine production have been linked to pro-apoptotic signaling in endothelial cells [31]. Endothelial apoptosis is a major contributing factor in LPS-induced lung vascular injury [34]. Activation of caspases is one of the intracellular events leading to apoptosis [34]. We showed that LPS-induced caspase-3 activation in mouse lung, which may lead to lung endothelial apoptosis and vascular injury. Importantly, LPS induced caspase-3 activation was strongly inhibited by LOX-1 blockade, implicating an important role of LOX-1 in LPS-induced apoptotic signaling in the lung.
The role of NF-κB in LPS-induced transcriptional regulation of proinflammatory mediators including cytokines, chemokines and adhesion molecules is well established [35, 36]. LPS has been reported to induce NF-κB-dependent ICAM-1 expression in mouse lung [22]. In the present study we showed that LPS-induced NF-κB activation and ICAM-1 expression in the lung were both significantly inhibited by LOX-1 blocking antibody. Interestingly, LOX-1 expression in the injured lung was not affected by anti-LOX-1 antibody treatment, suggesting that the expression of LOX-1 is regulated by a different mechanism. LOX-1 activation has been shown to induce NF-κB activation via oxidant production in endothelial cells [37]. The induction of oxidative stress in injured lung could be a potential mechanism in LOX-1-mediated NF-κB activation. Neutrophil accumulation is a major cause of NF-κB activation in the lung [38]. Our studies demonstrated that LPS-induced neutrophil recruitment to the injured lung was inhibited by anti-LOX-1 antibody, which may at least partially explain the decreased lung NF-κB activation by anti-LOX-1 treatment.
Neutrophil recruitment to the lung vasculature has also been reported to disrupt endothelial junctions and promote vascular leakage, which in turn leads to increased lung vascular permeability as observed in LPS-induced ALI [39, 40]. Indeed, we observed a positive association between lung MPO activity and BAL albumin concentration in LPS-induced lung injury. Importantly, LOX-1 blockade inhibited the increase in both lung MPO activity and BAL albumin concentration, suggesting that one of the mechanisms by which LOX-1 mediates LPS-induced increase in lung vascular permeability is through the induction of neutrophil influx into the lung. This postulation is consistent with our finding that LPS-induced expression of ICAM-1 is significantly inhibited by LOX-1 blockade in LPS-challenged lung. This conclusion is further supported by a previous finding that LOX-1 can function directly as an adhesion molecule to promote leukocyte binding [20]. Nevertheless, other LOX-1-mediated signaling mechanisms may also be involved in LPS-induced increase in lung vascular permeability. A systematic analysis is warranted in order to clarify the mechanisms of LOX-1 signaling in pulmonary edema formation.
In summary, we demonstrated that LOX-1-blocking antibody was able to prevent LPS-induced lung inflammation and injury in a mouse ALI model. Our results also suggest that LOX-1 could be a key mediator of lung inflammation and injury in response to intraperitoneal challenge with LPS. From this standpoint, LOX-1 might serve as a valuable target in the prevention of inflammatory lung injury in sepsis.
References
- 1.Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986;133:913–927. [PubMed] [Google Scholar]
- 2.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Tate RM, Repine JE. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- 5.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 6.Kamochi M, Kamochi F, Kim YB, Sawh S, Sanders JM, Sarembock I, Green S, Young JS, Ley K, Fu SM, Rose CE., Jr P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am J Physiol. 1999;277:L310–L319. doi: 10.1152/ajplung.1999.277.2.L310. [DOI] [PubMed] [Google Scholar]
- 7.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 8.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 9.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, Suratt BT. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–8157. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Masaki T, Sawamura T. Lox-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 11.Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, Masaki T, Kita T. Inducible expression of lox-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci. 2000;902:323–327. doi: 10.1111/j.1749-6632.2000.tb06332.x. [DOI] [PubMed] [Google Scholar]
- 12.Murase T, Kume N, Korenaga R, Ando J, Sawamura T, Masaki T, Kita T. Fluid shear stress transcriptionally induces lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:328–333. doi: 10.1161/01.res.83.3.328. [DOI] [PubMed] [Google Scholar]
- 13.Nagase M, Ando K, Nagase T, Kaname S, Sawamura T, Fujita T. Redox-sensitive regulation of LOX-1 gene expression in vascular endothelium. Biochem Biophys Res Commun. 2001;281:720–725. doi: 10.1006/bbrc.2001.4374. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 15.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic APOE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 17.Kakutani M, Masaki T, Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci USA. 2000;97:360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- 20.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100:1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW, Tiruppathi C, Malik AB. E3 ubiquitin ligase CBLB regulates the acute inflammatory response underlying lung injury. Nat Med. 2007;13:920–926. doi: 10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 22.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 23.Gao XP, Zhu X, Fu J, Liu Q, Frey RS, Malik AB. Blockade of class Ia phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J Biol Chem. 2007;282:6116–6125. doi: 10.1074/jbc.M610248200. [DOI] [PubMed] [Google Scholar]
- 24.Koh H, Tasaka S, Hasegawa N, Yamada W, Shimizu M, Nakamura M, Yonemaru M, Ikeda E, Adachi Y, Fujishima S, Yamaguchi K, Ishizaka A. Protective role of vascular endothelial growth factor in endotoxin-induced acute lung injury in mice. Respir Res. 2007;8:60. doi: 10.1186/1465-9921-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 26.Hofnagel O, Luechtenborg B, Stolle K, Lorkowski S, Eschert H, Plenz G, Robenek H. Proinflammatory cytokines regulate LOX-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1789–1795. doi: 10.1161/01.ATV.0000140061.89096.2b. [DOI] [PubMed] [Google Scholar]
- 27.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V, Sawamura T. The binding of oxidized low density lipoprotein (OX-LDL) to OX-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276:13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 29.Iwai-Kanai E, Hasegawa K, Sawamura T, Fujita M, Yanazume T, Toyokuni S, Adachi S, Kihara Y, Sasayama S. Activation of lectinlike oxidized low-density lipoprotein receptor-1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation. 2001;104:2948–2954. doi: 10.1161/hc4901.100381. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- 31.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized lowdensity lipoprotein receptor-1 activation: Prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- 32.Carney DE, Lutz CJ, Picone AL, Gatto LA, Ramamurthy NS, Golub LM, Simon SR, Searles B, Paskanik A, Snyder K, Finck C, Schiller HJ, Nieman GF. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation. 1999;100:400–406. doi: 10.1161/01.cir.100.4.400. [DOI] [PubMed] [Google Scholar]
- 33.D’Ortho MP, Jarreau PH, Delacourt C, Macquin-Mavier I, Levame M, Pezet S, Harf A, Lafuma C. Matrix metalloproteinase and elastase activities in LPS-induced acute lung injury in guinea pigs. Am J Physiol. 1994;266:L209–L216. doi: 10.1152/ajplung.1994.266.3.L209. [DOI] [PubMed] [Google Scholar]
- 34.Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8:493–500. doi: 10.2174/138945007780362764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 36.Kang JL, Lee HW, Lee HS, Pack IS, Chong Y, Castranova V, Koh Y. Genistein prevents nuclear factor-kappa b activation and acute lung injury induced by lipopolysaccharide. Am J Respir Crit Care Med. 2001;164:2206–2212. doi: 10.1164/ajrccm.164.12.2104017. [DOI] [PubMed] [Google Scholar]
- 37.Matsunaga T, Hokari S, Koyama I, Harada T, Komoda T. NF-kappa b activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem Biophys Res Commun. 2003;303:313–319. doi: 10.1016/s0006-291x(03)00308-5. [DOI] [PubMed] [Google Scholar]
- 38.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 39.Chignard M, Balloy V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1083–L1090. doi: 10.1152/ajplung.2000.279.6.L1083. [DOI] [PubMed] [Google Scholar]
- 40.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]