Abstract
The b-wave is a major component of the electroretinogram that reflects the activity of depolarizing bipolar cells (DBCs). The b-wave is used diagnostically to identify patients with defects in DBC signaling or in transmission from photoreceptors to DBCs. In mouse models, an abnormal b-wave has been used to demonstrate a critical role of a particular protein in the release of glutamate from photoreceptor terminals, in establishing the structure of the photo-receptor-to-DBC synapse, in DBC signal transduction, and also in DBC development, survival, or metabolic support. The purpose of this review is to summarize these models and how they have advanced our understanding of outer retinal function.
Keywords: Electroretinogram, Retina, b-Wave, Depolarizing bipolar cell
Introduction
The electroretinogram (ERG) is a field potential that reflects the electrical response of the retina to a light stimulus. Under most recording conditions, the ERG is comprised of multiple components, each of which reflects the activity of distinct cellular generators. Because ERGs can be recorded using non-invasive procedures, they are used in a wide variety of applications to examine how experimental manipulation, genetic disease, and other factors impact retinal function [1].
The b-wave is a large amplitude component of the ERG, with a positive polarity when recorded at the corneal surface. In response to strong stimulus flashes, the b-wave follows the a-wave, which is initiated by the light-induced closure of ion channels along the photoreceptor outer segment [2]. For many years, the b-wave was thought to reflect the activity of inward rectifying K+ (Kir) channels in Müller cells, evoked as a secondary response to bipolar cell activity [3–5]. More recent evidence indicates that the mammalian b-wave is generated primarily by bipolar cell activity, based on current-source density analysis of b-wave currents [6], the retention of the b-wave in mice lacking Kir4.1 expression [7], and pharmacological blockade of Kir channel activity by barium [7–10]. The b-wave has now been firmly linked to the activity of depolarizing bipolar cells (DBCs) [11–16]. As a consequence, b-wave abnormalities in conjunction with normal photoreceptor function provide insights into the molecular components that are involved in the cellular processes required to support b-wave generation [17]. As diagramed in Fig. 1, these include the pre-synaptic release of glutamate, structural properties of the photoreceptor-to-DBC synapse, and the DBC signal transduction cascade. The purpose of this review is to summarize mouse b-wave mutants that involve these molecules, as well as others that are not included on this diagram but impact b-wave generation due to a role in DBC development, metabolic support, survival, or some other physiological process. In reviewing the types of b-wave abnormalities that have been reported in mouse mutants, we will not encompass mouse models where the b-wave is reduced secondary to photoreceptor degeneration. The review is organized by the extent of b-wave reduction, which corresponds generally to whether a mutant involves a molecule involved in the pre- or post-synaptic aspects of the photoreceptor-to-DBC synapse, and notes how mouse b-wave mutants have played important roles in elucidating this initial stage of information transfer in the visual system and in the identification of human disease genes.
Fig. 1.
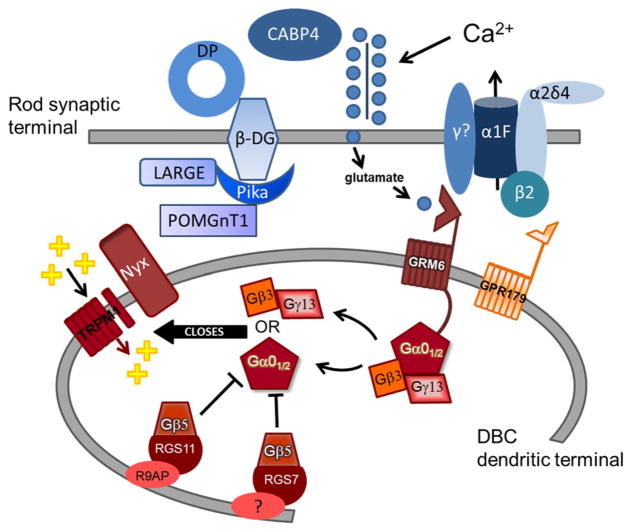
Diagram of molecules required for normal signaling between photoreceptors and DBCs. Pre-synaptic proteins (blue symbols) include the L-type voltage-dependent calcium channel composed of α1F, β2, γ, and α2δ4 subunits and the dystrophin–glycoprotein (DG) complex consisting of β subunit, the glycosyltransferase gene LARGE, pikachurin (Pika), Protein O-mannose beta1,2-N-acetylglucosaminyltransferase 1 (POM-GnT1), and dystrophin (DP). Post-synaptic proteins (red symbols) include metabotropic glutamate receptor 6 (GRM6), the orphan G-protein receptor GPR179, members of the G-protein regulating complex (Gβ5, RGS7, RGS11, and R9AP), G-protein subunits Gβ3, Gγ13, Gα01/2, the transient receptor potential melastatin 1 (Trpm1) cation channel, and nyctalopin (Nyx). Mutants for post-synaptic proteins lack the ERG b-waves, while this response component is reduced but retained in mutants for pre-synaptic proteins
Typical ERG responses from wild-type (WT) mice
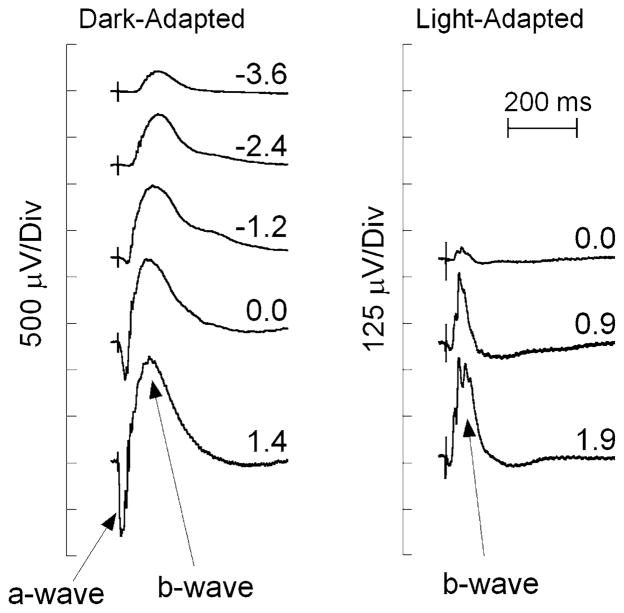
Figure 2 presents a series of ERGs recorded from a C57BL/6 WT mouse to strobe flashes obtained under dark-adapted (left) and light-adapted (right) conditions. At the lower stimulus levels, the dark-adapted ERG is dominated by the positive polarity b-wave, as the negative polarity a-wave component is hidden by the b-wave, which is of larger amplitude with similar kinetics [18–20]. The a-wave only becomes apparent when it precedes the b-wave at higher flash strengths. ERGs evoked by stimuli presented in the dark are dominated by activity of the rod pathway. Cone ERGs may be isolated by superimposing stimulus flashes upon a steady light-adapting field that desensitizes the rod pathway [21]. In the mouse, the overall amplitude of the cone ERG is substantially smaller than the dark-adapted ERG, and the response is dominated by the positive polarity b-wave across these and other stimulus conditions [22, 23]. The cone ERG b-wave nevertheless provides a measure of cone DBC activity [15].
Fig. 2.
Representative ERGs recorded from the corneal surface of a WT mouse in response to strobe flash stimuli presented to the dark-adapted (left) or light-adapted eye (right). The b-wave is seen as a cornea positive potential, which increases in amplitude with increasing flash strength, indicated by the values next to each waveform, in log cd s/m2
Mouse mutants that lack b-waves due to post-synaptic defects
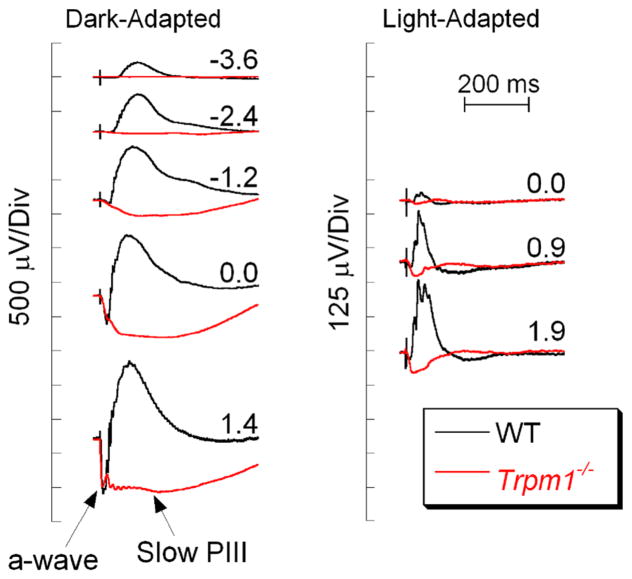
An extreme b-wave abnormality is the absence of this response component, referred to as a no b-wave (nob) ERG. Figure 3 compares WT responses with those of a Trpm1−/− mouse lacking the non-specific cation channel transient receptor potential melastatin 1 (TRPM1). At all flash levels, the dark-adapted Trpm1−/− ERGs lack the b-wave component [24–26]. As a consequence, the ERG is comprised of the initial a-wave followed by a slow negative polarity component. This component is slow PIII and reflects Kir4.1 channel activity in Müller glial cells [7, 27–30]. In comparison with the b-wave, higher light levels are required to evoke these responses (Fig. 3). Nevertheless, in these and other nob mice, the absence of the b-wave allows Müller cell function to be studied in vivo, by crossing an allele of interest to a nob background and examining the response properties of slow PIII [28, 31, 32].
Fig. 3.
Comparison of WT and Trpm1−/− ERGs. WT ERGs are replotted from Fig. 1. The absence of the b-wave reveals slow PIII in the Trpm1−/− responses. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
A number of nob mutants with this phenotype have been described (Table 1), and the nob ERG phenotype has been instrumental in linking-specific proteins to post-synaptic proteins in the DBC signal transduction process. For example, mutations in the glutamate receptor, mGluR6, encoded by Grm6 have a nob ERG phenotype [33], as do other mutants for Grm6 (Grm6nob4 [34]; Grm6nob3 [35]). A nob ERG phenotype is seen in mouse mutants for several other genes involved in DBC signal transduction. These include Trpm1 (Fig. 3) [24–26, 36]; Nyx [37, 38], which encodes nyctalopin, a leucine-rich repeat proteoglycan that appears to have restricted expression in DBCs and may play a key role in TRPM1 trafficking or stabilizing TRPM1 in the DBC dendritic terminal [39]; and Gpr179 [40], encoding an orphan G-protein receptor that may interact with RGS (regulator of G-protein signaling) proteins [41]. A nob ERG phenotype has also been observed in mutants for several G-protein subunits located in the DBCs including Gna01 [42, 43]; Gnb5 [44], and Gnb3 [45], implicating these proteins in DBC signal transduction (Fig. 1) [46, 47]. Because the DBC signal transduction process remains incompletely understood, ERG studies will continue to be important to confirm that deletion of a new candidate member of the DBC cascade leads to a nob ERG phenotype.
Table 1.
Mouse no b-wave mutants for DBC signal transduction/modulation
| Allele | Type of mutation | References |
|---|---|---|
| Gna01−/− | Knockout | [42, 43] |
| Gnb3−/− | Knockout | [45] |
| Gnb5−/− | Knockout | [44] |
| Gpr179nob5 | 6.5-kb insertion | [40] |
| Grm6−/− | Knockout | [33] |
| Grm6nob3 | 65-bp insertion | [35] |
| Grm6nob4 | Point (S207P) | [34] |
| Nyxnob | 85-bp insertion | [37, 38] |
| RGS7−/−/RGS11−/− | Double knockout | [51, 56] |
| Trpm1−/− | Knockout | [24–26] |
| Trpm1tvrm27 | Point (A1068T) | [36] |
A remarkable feature shared by many nob models is that retinal anatomy is normal, including a normal OPL and INL as well as intact ribbon synapses between photoreceptors and DBCs and horizontal cells, although the expression of other proteins in the DBC cascade may be altered [48]. When a transgenic approach was used to deliver a wild-type copy of Nyx to Nyxnob DBCs, the b-wave and inner retinal function were restored [38]. In Gregg et al. [38], the transgene constructed was driven by the GABAc ρ1 promoter, which is active early in development [49]. Further studies are needed to determine whether gene replacement will restore DBC function to an adult retina or in any of the other nob mouse models.
Like all G-protein coupled receptor cascades, the DBC signal transduction cascade needs to be deactivated. The expression of RGS7 and RGS11 in DBCs [50–54] suggested that these proteins may play a role in DBC signaling deactivation. When single mutants for Rgs7 or Rgs11 were examined, however, only modest ERG b-wave delays were observed [53, 55]. More recently, a nob ERG was noted in Rgs7/Rgs11 double mutants [51, 56], indicating that RGS7 and RGS11 are functionally redundant in DBC deactivation.
A nob ERG phenotype has played an important role in the discovery of genes underlying the human condition complete congenital stationary night blindness (cCSNB). For example, the nob ERG phenotype of Grm6−/− mice [33] motivated the evaluation of GRM6 as a cCSNB gene [57, 58]. Similarly, the nob ERG phenotype of animal models for Trpm1 [24–26, 59] and Gpr179 [40] led to the discovery of TRPM1 and GPR179 mutations in patients with cCSNB [40, 60–63]. NYX, the first gene identified for cCSNB, was identified through gene mapping [64, 65]. To date, none of the other proteins listed in Table 1 have been implicated in human retinal disease.
Mouse mutants with reduced b-waves
Gene defects at the photoreceptor terminal
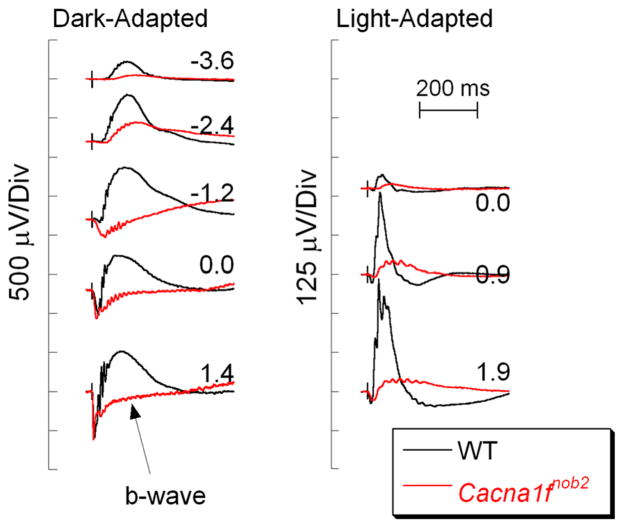
Rod and cone photoreceptors use glutamate as the neurotransmitter [66, 67], which is released by photoreceptor terminals at a specialized ribbon synapse [68]. Glutamate release is mediated by calcium entry through slowly inactivating L-type voltage-dependent calcium channels (VDCCs; Fig. 1) [69]. The VDCC pore is defined by the α1 subunit, the activity of which is regulated by β, α2δ, and γ subunits [70, 71]. Defects in VDCC subunits impair VDCC function and synaptic transmission [72], and b-wave reductions implicate a critical role of specific VDCC subunits in the control of glutamate release from photoreceptor terminals. Reduced b-wave amplitudes have been recorded in mice with defects in genes that encode VDCC subunits or calcium regulatory proteins (Table 2). Mutations in CACNA1F, encoding a photoreceptor-specific VDCC α1F subunit, were identified in patients with incomplete congenital stationary night blindness (iCSNB) in whom ERG b-wave amplitudes are reduced but not absent [73–75]. Subsequently two Cacna1f mouse mutants have been described: Cacna1fnob2 [76] and Cacna1f−/− [77]. Figure 4 compares ERGs of a Cacna1fnob2 mutant with those of a WT mouse. The dark-adapted b-wave is greatly reduced, but not absent, as observed in the nob phenotype. The reduction in the dark-adapted b-waves is also noted in the Cacna1f−/− mouse [77]. The light-adapted ERGs are, however, distinct in these two models. While a measurable cone ERG is observed in Cacna1fnob2 mutants (Fig. 4, right) [76], cone ERGs of Cacna1f−/− mice are more drastically reduced [77]. Both mutants display abnormalities in retinal architecture, including a thin OPL and ectopic neurites that elaborate from DBCs and horizontal cells [76, 77]. A similar phenotype is observed in mouse mutants for the β2 (Cacnb2) [78] or α2δ4 (Cacna2d4) [79, 80] VDCC subunits, implicating these specific subunits in the VDCC used to control glutamate release at the photoreceptor terminal. The γ subunit used in the photoreceptor VDCC has not been identified.
Table 2.
Pre-synaptic mouse mutants with reduced b-waves
Fig. 4.
Comparison of WT (black) and Cacna1fnob2 (red) ERGs. Note that the b-wave is reduced but not absent in the Cacna1fnob2 responses. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
In several instances, the b-wave reduction observed in a mouse mutant led to the identification of a human disease gene. For example, the ERG phenotype of Cacna2d4C57BL/10 mice led to the evaluation of CAC-NA2D4 as a candidate gene for iCSNB [81] and the identification of CACNA2D4 mutations in patients with cone–rod dystrophy [82]. The reduced ERG b-wave phenotype of calcium binding protein 4-deficient (Cabp4−/−) mice [83, 84] led to the identification of CABP4 mutations in patients with iCSNB [85] or a cone–rod synaptic disorder [86, 87]. CACNB2 has not been linked to retinal disease, most likely because it is expressed in skeletal muscle and viable Cacnb2−/−mice were only obtained when the protein was replaced in skeletal muscle using a transgenic approach [78].
A reduced b-wave is also seen in mutants for other proteins expressed at the photoreceptor terminal. These include the pre-synaptic ribbon component Bassoon [88] and CAST, a component of the pre-synaptic active zone [89]. In Bsn−/− mice, the absence of these pre-synaptic ribbon components is accompanied by ectopic neurites from horizontal and bipolar cells toward the outer nuclear layer and floating ribbons [90] BSN or CAST mutations have not been associated with human retinal disease.
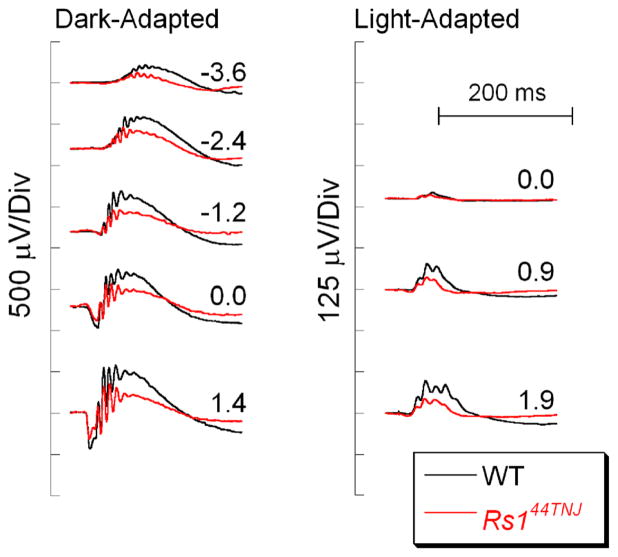
ERG b-waves are also reduced in patients with X-linked juvenile retinoschisis [91, 92], which is caused by mutations in retinoschisin (RS1). Figure 5 plots data obtained from a WT mouse with that of a Rs144TNJ mutant that was identified in a ERG screen of a mutagenesis program [93]. Similar results have been reported for Rs1−/− mice [94, 95], and gene replacement has normalized the b-wave and anatomical defects associated with this model [96, 97]. The function of RS1 is not completely understood, but it may play a role in the retention of VDCC α subunits in the photoreceptor terminal membrane [98].
Fig. 5.
Comparison of WT (black) and Rs144TNJ (red) ERGs. Note that ERG b-waves are reduced in the Rs144TNJ mutant. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
Gene defects in the dystrophin–glycoprotein complex
α and β dystroglycan, along with proteins that glycosylate dystroglycans, and several other members of the dystrophin–glycoprotein complex (DGC) play critical roles in skeletal muscle and the nervous system, and mutations in DGC elements underlie several complex disorders [99]. In the retina, dystroglycans are expressed in the endfeet of Müller glial cells, in photoreceptor ribbons, and on the photoreceptor cell membrane, connecting to DBCs (Fig. 1) [100–103]. Mutations of members of the DGC result in delayed and reduced b-waves (Table 3).
Table 3.
Mutants of the dystroglycan complex with reduced and delayed b-waves
| Allele | Type of mutation | References |
|---|---|---|
| B laminin−/− | Knockout | [118] |
| Largemyd | Spontaneous deletion | [104, 105] |
| Largevls | Premature stop at aa37 | [32, 104] |
| Dmdmdx–Cv3 | Point mutation, intron 53 | [113–115] |
| Nestin-CRE/DG−/− | Müller cell-specific knockout of dystroglycan | [117] |
| GFAP-CRE/DG−/− | Müller cell-specific knockout of dystroglycan | [117] |
| DGβcyt/βcyt | Transgene lacking cytoplasmic region of β-dystroglycan | [117] |
| POMGnT1−/− | Knockout | [138] |
| Pikachurin−/− | Knockout | [139] |
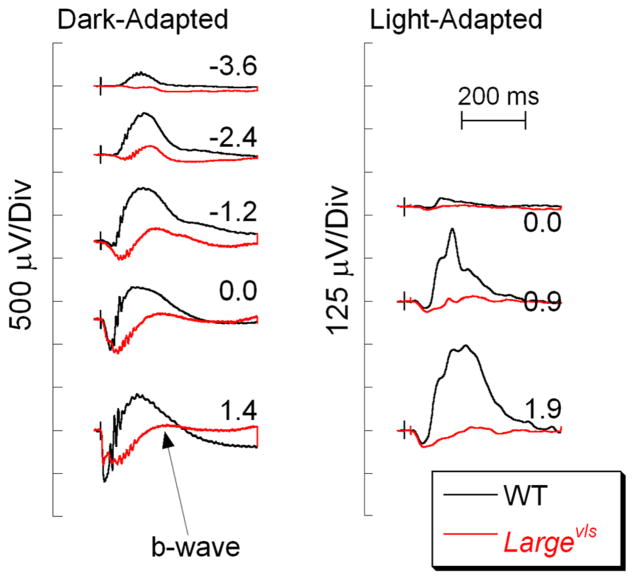
Glycosylation of α-dystroglycan by LARGE, the glycosyltransferase gene, is critical for the formation of a stable OPL [104]. Figure 6 contrasts ERGs of Largevls and WT mice. In comparison with WT, the dark- and light-adapted b-waves of the Largevls mutant are reduced in amplitude and have slow kinetics. A striking feature of the Largevls ERG phenotype is the slow onset of the dark-adapted b-wave. The ERG phenotype shown in Fig. 6 is also shared by Largemyd mice, a second intragenic deletion within LARGE [104, 105] and by mouse mutants for other components of the dystroglycan complex (Table 3). A recent study of the Pikachurin−/− ribbon synapse indicates that DBC invaginations into photoreceptor terminals are abnormal, resulting in a larger gap between the pre-synaptic active zone and the post-synaptic membrane [103]. A larger synaptic gap could delay clearance of glutamate and thus result in a delayed b-wave onset. It remains to be determined whether other mutants for components of the dystroglycan complex have comparable structural abnormalities of the ribbon synapse.
Fig. 6.
Comparison of WT (black) and Largevls (red) ERGs. Note that the b-wave is reduced but not absent in the Largevls responses, and has a slow onset. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
ERG b-wave reductions were noted in some but not all patients with Duchenne muscular dystrophy which had DGC mutations [106–112]. Pillers and colleagues examined the Dmdmdx; DmdCv2–Cv5 series of dystrophin mouse mutants, and noted normal ERG b-waves in some (e.g., Dmdmdx, Dmdmdx–Cv5) and a selective b-wave reduction in Dmdmdx–Cv3 mice, which lack expression of the Dp260 isoform [113–115]. This group subsequently defined a similar relationship between the mutation site and the b-wave phenotype in human patients [116].
Satz et al. [117] described a series of mouse models which lack dystroglycan expression in Müller cells or which express a mutant form of dystroglycan that lacked the C-terminal region. In each of these mutants, the ERG b-wave was reduced in amplitude [117]. Libby et al. [118] described b-wave reductions in mice lacking B laminin.
Defects in development of the retinal vasculature
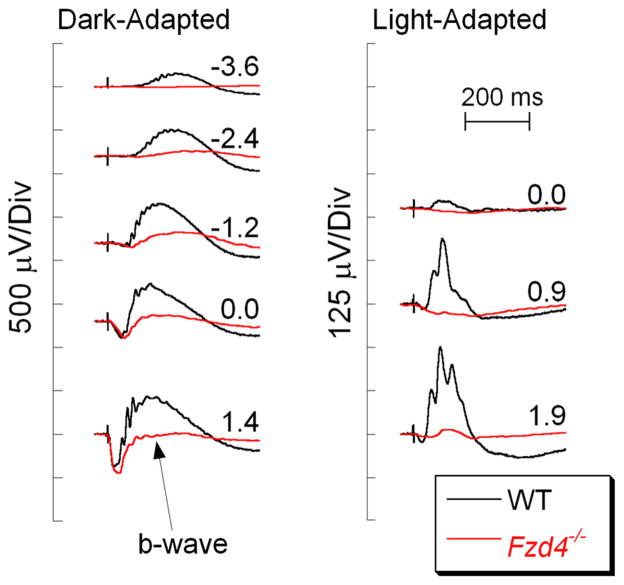
The retinal blood supply consists of a set of major arteries and veins that enter the eye from the optic disk and course across the vitreal face of the retina [119]. These vessels generate a series of smaller vessels that penetrate the retina and connect to a pair of capillary beds which flank the inner nuclear layer, the cell layer in which DBCs reside. The Norrin/Frizzled 4 signaling pathways have been implicated in retinal vascular development and diseases such as diabetic retinopathy and age-related macular degeneration [120]. Retinal vascular abnormalities and reduced b-waves (Fig. 7) are observed in mice lacking normal gene expression of Norrin (Ndp), a cystine knot protein [121–123]; Frizzled 4 (Fzd4), an integral membrane receptor [124, 125], or low-density lipoprotein receptor-related protein 5 (Lrp5), a Fzd4 co-receptor [126, 127]. When a conditional knockout approach was used to eliminate Fzd4 expression, elimination of Fzd4 in epithelial, but not neuronal cells, recapitulated the many aspects of the phenotype observed in the systemic knockout, including the reduced ERG b-wave [125]. Table 4 lists the mutants impacting development of the inner retinal vasculature, in which an ERG b-wave reduction has been documented.
Fig. 7.
Comparison of WT (black) and Fzd4−/− (red) ERGs. Note that the b-wave is reduced but not absent in the Fzd4−/−responses. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
Table 4.
Mutants impacting inner retinal vascularization
Mouse mutant with hypernormal b-waves
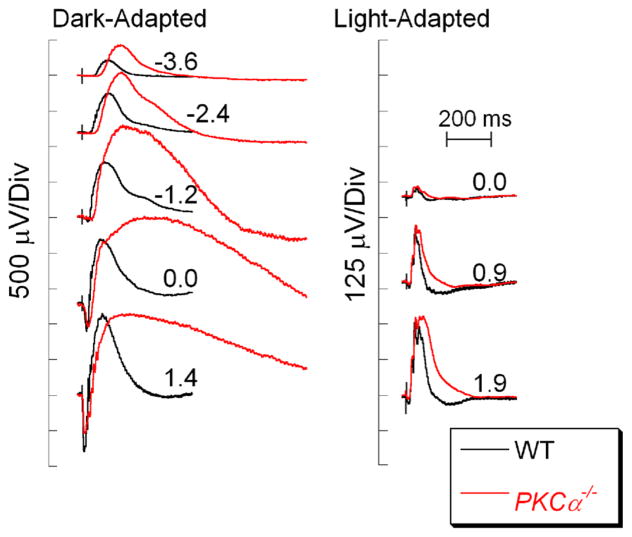
The sections above have been organized around groups of mouse models involving proteins that are involved in a specific cellular process, such as DBC signal transduction, that when mutated or eliminated result in abnormal DBC function. A distinct ERG b-wave phenotype was reported in mice lacking PKCα, a protein that has long been targeted by immunohistochemistry to label rod DBCs [128]. As shown in Fig. 8, PKCα−/− mice have a large amplitude b-wave with an extremely slow recovery phase. The explanation for this unusual pattern is currently not known, but has been reported by two independent research groups [129, 130]. Consistent with the restriction of PKCα expression to rod DBCs [128], cone ERGs of PKCα−/− mice have normal response properties [129].
Fig. 8.
Comparison of WT (black) and PKCα−/− (red) ERGs. Note that the dark-adapted PKCα−/− b-waves are larger in amplitude and have a very prolonged time course and recovery. Flash strength (log cd s/m2) is indicated by the values next to each pair of waveforms
Mouse mutants for other retinal neurons or for bipolar cell development
While this review has mainly focused on alternations to the DBCs, loss of other inner retinal cells also impacts the b-wave. ERG b-wave reductions have been reported in transgenic mice that express an oncogene [131, 132] or a diphtheria toxin gene in horizontal cells [132]. Additionally, dark- and light-adapted b-waves were reduced in mice lacking the transcription factors Bhlhb4, which is required for rod DBC maturation [133], or Math5, which is required for development of retinal ganglion cells and of several classes of bipolar cell [134]. A selective reduction in the cone ERG b-wave, with normal dark-adapted b-waves, was noted in mice lacking Vsx1, a transcription factor required for cone bipolar cell development [135]. Finally, a nob ERG phenotype is observed in mice in which DBCs have been eliminated due to transgenic expression of an oncogene [136]. A similar ERG phenotype is seen in mutants for Cpe, encoding carboxypeptidase E [137]. A remarkable feature of the Cpe mutant phenotype is that it is age-related. The b-wave is retained in young Cpefat/fat mice and is only lost when mice reached 6 months of age [137]. The underlying mechanism for this age-related abolition of the b-wave remains to be determined.
Conclusions
DBCs play an instrumental role in transmitting the visual signal initiated by photoreceptors centrally. The ability to monitor DBC activity via the ERG b-wave has greatly facilitated our understanding of the mechanisms of DBC signal transduction/regulation and the processes that are required for DBC development and for maintaining normal DBC function. As is the case in many other biomedical fields, mouse models have been instrumental to much of this progress. It is likely that analysis of ERG b-waves of mouse mutants will yield further insights into DBC physiology.
Acknowledgments
Work in the author’s laboratories has been supported by grants from the Department of Veterans Affairs, National Institutes of Health, a Foundation Fighting Blindness Center Grant to the Cole Eye Institute, and unrestricted grants from Research to Prevent Blindness to the Departments of Ophthalmology of Emory University and the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Contributor Information
Machelle T. Pardue, Rehabilitation Research and Development Center of Excellence, Atlanta VA Medical Center, 1670 Clairmont Road, Decatur, GA 30033, USA. Department of Ophthalmology, Emory University School of Medicine, 1365B Clifton Road NE, Atlanta, GA 30322, USA
Neal S. Peachey, Research Service (151W), Louis Stokes Cleveland VA Medical Center, 10701 East Boulevard, Cleveland, OH 44106, USA. Cole Eye Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA. Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, 9500 Euclid Avenue, Cleveland, OH 44195, USA
References
- 1.Heckenlively JR, Arden GB. Principles and practice of clinical electrophysiology of vision. 2. MIT Press; Cambridge: 2006. [Google Scholar]
- 2.Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- 3.Newman EA. Regulation of extracellular potassium by glial cells in the retina. Trends Neurosci. 1985;8:156–159. [Google Scholar]
- 4.Newman EA, Odette LL. Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J Neurophysiol. 1984;51:164–182. doi: 10.1152/jn.1984.51.1.164. [DOI] [PubMed] [Google Scholar]
- 5.Frishman LJ. Origins of the electroretinogram. In: Heckenlively JR, Arden GB, editors. Principles and practice of clinical electrophysiology of vision. 2. MIT Press; Cambridge: 2006. pp. 139–183. [Google Scholar]
- 6.Karwoski CJ, Xu X. Current-source density analysis of light-evoked field potentials in rabbit retina. Vis Neurosci. 1999;16:369–377. doi: 10.1017/s0952523899162163. [DOI] [PubMed] [Google Scholar]
- 7.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frishman LJ, Steinberg RH. Light-evoked increases in [K+]o in proximal portion of the dark-adapted cat retina. J Neurophysiol. 1989;61:1233–1243. doi: 10.1152/jn.1989.61.6.1233. [DOI] [PubMed] [Google Scholar]
- 9.Green DG, Kapousta-Bruneau KV. A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Vis Neurosci. 1999;16:727–741. doi: 10.1017/s0952523899164125. [DOI] [PubMed] [Google Scholar]
- 10.Lei B, Perlman I. The contributions of voltage- and time-dependent potassium conductances to the electroretinogram in rabbits. Vis Neurosci. 1999;16:743–754. doi: 10.1017/s0952523899164137. [DOI] [PubMed] [Google Scholar]
- 11.Bush RA, Sieving PA. Inner retinal contributions to the primate photopic flash flicker electroretinogram. J Opt Soc Am A. 1996;13:557–565. doi: 10.1364/josaa.13.000557. [DOI] [PubMed] [Google Scholar]
- 12.Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- 13.Robson JG, Frishman LJ. Photoreceptor and bipolar-cell contributions to the cat electroretinogram: a kinetic model for the early part of the flash response. J Opt Soc Am A. 1996;13:613–622. doi: 10.1364/josaa.13.000613. [DOI] [PubMed] [Google Scholar]
- 14.Hood DC, Birch DG. b Wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am A. 1996;13:623–633. doi: 10.1364/josaa.13.000623. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Ball S, Peachey NS. Pharmacological studies of the mouse cone electroretinogram. Vis Neurosci. 2005;22:631–636. doi: 10.1017/S0952523805225129. [DOI] [PubMed] [Google Scholar]
- 16.Sieving PA, Murayama K, Naarendorp F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing bipolar neurons in shaping the b-wave. Vis Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- 17.McCall MM, Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. J Physiol. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepperberg DR, Birch DG, Hood DC. Photoresponses of human rods in vivo derived from paired-flash electroretinograms. Vis Neurosci. 1997;14:73–82. doi: 10.1017/s0952523800008774. [DOI] [PubMed] [Google Scholar]
- 19.Kang Derwent JJ, Qtaishat NM, Pepperberg DR. Excitation and desensitization of mouse rod photoreceptors in vivo following bright adapting light. J Physiol. 2002;541:201–218. doi: 10.1113/jphysiol.2001.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang Derwent JJ, Saszik SM, Maeda H, Little DM, Pardue MT, Frishman LJ, Pepperberg DR. Test of the paired-flash electroretinographic method in mice lacking b-waves. Vis Neurosci. 2007;24:141–149. doi: 10.1017/S0952523807070162. [DOI] [PubMed] [Google Scholar]
- 21.Peachey NS, Goto Y, Al-Ubaidi MR, Naash MI. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neurosci Lett. 1993;162:9–11. doi: 10.1016/0304-3940(93)90547-x. [DOI] [PubMed] [Google Scholar]
- 22.Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19:442–455. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Quiambao AB, Roveri L, Pardue MT, Marx JL, Röhlich P, Peachey NS, Al-Ubaidi MR. Degeneration of cone photoreceptors induced by expression of the Mas1 oncogene. Exp Neurol. 2000;163:207–219. doi: 10.1006/exnr.2000.7370. [DOI] [PubMed] [Google Scholar]
- 24.Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Heimel JA, Kammermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witkovsky P, Dudek FE, Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975;65:119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Marmorstein AD, Kofuji P, Peachey NS. Contribution of Kir4.1 to the mouse electroretinogram. Mol Vis. 2004;10:650–654. [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg RH, Miller S. Aspects of electrolyte transport in frog pigment epithelium. Exp Eye Res. 1973;16:365–372. doi: 10.1016/0014-4835(73)90130-9. [DOI] [PubMed] [Google Scholar]
- 30.Oakley B, II, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976;39:1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- 31.Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS. Light-evoked responses of the retinal pigment epithelium: changes accompanying photoreceptor loss in the mouse. J Neurophysiol. 2010;104:391–402. doi: 10.1152/jn.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peachey NS, Sturgill-Short GM. Response properties of slow PIII in the Largevls mutant. Doc Ophthalmol. 2012;125:203–209. doi: 10.1007/s10633-012-9347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGIuR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 34.Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Balannik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Glawe B, Cantrell DR, Inayat S, Olvera MA, Vessey KA, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci. 2007;24:111–123. doi: 10.1017/S0952523807070149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. J Physiol. 2008;586:4409–4424. doi: 10.1113/jphysiol.2008.157289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peachey NS, Pearring JN, Bojang P, Jr, Hirschtritt ME, Sturgill-Short G, Ray TA, Furukawa T, Koike C, Goldberg AF, Shen Y, McCall MA, Nawy S, Nishina PM, Gregg RG. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J Neurophysiol. 2012;108:2442–2451. doi: 10.1152/jn.00137.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally-occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998;39:2443–2449. [PubMed] [Google Scholar]
- 38.Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol. 2007;98:3023–3033. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearring JN, Bojang P, Jr, Shen Y, Koike C, Furukawa T, Nawy S, Gregg RG. A role for nyctalopin, a small leucine rich repeat protein, in localizing the TRPM1 channel to retinal depolarizing bipolar cell dendrites. J Neurosci. 2011;31:10060–10066. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev N, Iuvone PM, Bojang P, Jr, Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AFX, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AAB, Kamermans M, Gregg RG. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90:331–339. doi: 10.1016/j.ajhg.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG, Martemyanov KA. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012;197:711–719. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Gα0. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Gα0. J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007;27:14199–14204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A, Vardi N. Gβ3 is required for normal light ON responses and synaptic maintenance. J Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koike C, Numata T, Ueda H, Mori Y, Furukawa T. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium. 2010;48:95–101. doi: 10.1016/j.ceca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Morgans CW, Brown RL, Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T, Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012;107:948–957. doi: 10.1152/jn.00933.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur J Neurosci. 2002;15:1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc Natl Acad Sci USA. 2012;109:7905–7910. doi: 10.1073/pnas.1202332109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen FS, Shim H, Morhardt D, Dallman R, Krahn E, McWhinney L, Rao A, Gold SJ, Chen CK. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest Ophthalmol Vis Sci. 2010;51:686–693. doi: 10.1167/iovs.09-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–7765. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgans CW, Liu W, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, Duvoisin RM. Gβ5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. Eur J Neurosci. 2007;26:2899–2905. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Jeffrey BG, Morgans CW, Burke NS, Haley TL, Duvoisin RM, Brown RL. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010;51:1121–1129. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shim H, Wang CT, Chen YL, Chau VQ, Fu KG, Yang J, McQuiston AR, Fisher RA, Chen CK. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J Biol Chem. 2012;287:14873–14879. doi: 10.1074/jbc.M112.345751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci USA. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Mátyás G, Hoyng CB, Riemslag F, Meire F, Cremers FP, Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]
- 59.Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Saïd S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Léveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS, Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85:720–729. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Sergouniotis PI, Michaelides M, Mackay DS, Wright GA, Devery S, Moore AT, Holder GE, Robson AG, Webster AR. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet. 2009;85:711–719. doi: 10.1016/j.ajhg.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura M, Sanuki R, Yasuma TR, Onishi A, Nishiguchi KM, Koike C, Kadowaki M, Kondo M, Miyake Y, Furukawa T. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis. 2010;16:425–437. [PMC free article] [PubMed] [Google Scholar]
- 64.Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- 65.Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- 66.Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;342:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- 67.Marc RE, Liu W-LS, Kallionatis M, Raiguel SF, van Haesendonck E. Patterns of glutamate immunore-activity in the goldfish retina. J Neurosci. 1990;10:4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Mol Neurobiol. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witkovsky P, Schmitz Y, Akopian A, Krizaj D, Tranchina D. Gain of rod to horizontal cell synaptic transfer: relation to glutamate release and a dihydropyridine-sensitive calcium current. J Neurosci. 1997;17:7297–7306. doi: 10.1523/JNEUROSCI.17-19-07297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 71.Catterall WA. Structure and regulation of voltage-gated Ca2+channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 72.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–1020. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- 74.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 75.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 76.Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 78.Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- 79.Ruether K, Grosse J, Matthiessen E, Hoffmann K, Hartmann C. Abnormalities of the photoreceptor-bipolar cell synapse in a substrain of C57BL/10 mice. Invest Ophthalmol Vis Sci. 2000;41:4039–4047. [PubMed] [Google Scholar]
- 80.Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci. 2006;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- 81.Zeitz C, Labs S, Lorenz B, Forster U, Uksti J, Kroes HY, De Baere E, Leroy BP, Cremers FP, Wittmer M, van Genderen MM, Sahel JA, Audo I, Poloschek CM, Mohand-Saïd S, Fleischhauer JC, Hüffmeier U, Moskova-Doumanova V, Levin AV, Hamel CP, Leifert D, Munier FL, Schorderet DF, Zrenner E, Friedburg C, Wissinger B, Kohl S, Berger W. Genotyping microarray for CSNB-associated genes. Invest Ophthalmol Vis Sci. 2009;50:5919–5926. doi: 10.1167/iovs.09-3548. [DOI] [PubMed] [Google Scholar]
- 82.Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, Berger W. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet. 2006;79:973–977. doi: 10.1086/508944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nature Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maeda T, Lem J, Palczewski K, Haeseleer F. A critical role of CaBP4 in the cone synapse. Invest Ophthalmol Vis Sci. 2005;46:4320–4327. doi: 10.1167/iovs.05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, Mátyás G, Borruat FX, Schorderet DF, Zrenner E, Munier FL, Berger W. Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet. 2006;79:657–667. doi: 10.1086/508067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Littink KW, Koenekoop RK, van den Born LI, Collin RW, Moruz L, Veltman JA, Roosing S, Zonneveld MN, Omar A, Darvish M, Lopez I, Kroes HY, van Genderen MM, Hoyng CB, Rohrschneider K, van Schooneveld MJ, Cremers FP, den Hollander AI. Homozygosity mapping in patients with cone-rod dystrophy: novel mutations and clinical characterizations. Invest Ophthalmol Vis Sci. 2010;51:5943–5951. doi: 10.1167/iovs.10-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Littink KW, van Genderen MM, Collin RW, Roosing S, de Brouwer AP, Riemslag FC, Venselaar H, Thiadens AA, Hoyng CB, Rohrschneider K, den Hollander AI, Cremers FP, van den Born LI. A novel homozygous nonsense mutation in CABP4 causes congenital cone-rod synaptic disorder. Invest Ophthalmol Vis Sci. 2009;50:2344–2350. doi: 10.1167/iovs.08-2553. [DOI] [PubMed] [Google Scholar]
- 88.Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 89.Tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Hagiwara A, Brandstätter JH, Löwel S, Gollisch T, Ohtsuka T, Moser T. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32:12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Hagiwara A, Brandstätter JH, Löwel S, Gollisch T, Ohtsuka T, Moser T. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2010;32:12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirose T, Wolf E, Hara A. Electrophysiological and psychophysical studies in congenital retinoschisis of X-linked recessive inheritance. Doc Ophthalmol Proc Ser. 1977;13:173–184. [Google Scholar]
- 92.Peachey NS, Fishman GA, Derlacki DJ, Brigell MG. Psychophysical and electroretinographic findings in X-linked juvenile retinoschisis. Arch Ophthalmol. 1987;105:513–516. doi: 10.1001/archopht.1987.01060040083038. [DOI] [PubMed] [Google Scholar]
- 93.Jablonski MM, Dalke C, Wang X, Lu L, Manly KF, Pretsch W, Favor J, Pardue MT, Rinchik EM, Williams RW, Goldowitz D, Graw J. An ENU-induced mutation in Rs1h causes disruption of retinal structure and function. Mol Vis. 2005;11:569–581. [PubMed] [Google Scholar]
- 94.Weber BH, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, Jaissle GB, Friedburg C, Tamm E, Molday RS. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci USA. 2002;99:6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeng Y, Takada Y, Kjellstrom S, Hiriyanna K, Tanikawa A, Wawrousek E, Smaoui N, Caruso R, Bush RA, Sieving PA. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–3285. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- 96.Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1−/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci. 2008;49:3677–3686. doi: 10.1167/iovs.07-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi L, Jian K, Ko ML, Trump D, Ko GY. Retinoschisin, a new binding partner for L-type voltage-gated calcium channels in the retina. J Biol Chem. 2009;284:3966–3975. doi: 10.1074/jbc.M806333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 100.Montanaro F, Carbonetto S, Campbell KP, Lindenbaum M. Dystroglycan expression in the wild type and mdx mouse neural retina: synaptic colocalization with dystrophin, dystrophin-related protein but not laminin. J Neurosci Res. 1995;42:528–538. doi: 10.1002/jnr.490420411. [DOI] [PubMed] [Google Scholar]
- 101.Blank M, Koulen P, Blake DJ, Kröger S. Dystrophin and beta-dystroglycan in photoreceptor terminals from normal and mdx3Cv mouse retinae. Eur J Neurosci. 1999;11:2121–2133. doi: 10.1046/j.1460-9568.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 102.Jastrow H, Koulen P, Altrock WD, Kröger S. Identification of a beta-dystroglycan immunoreactive subcompartment in photoreceptor terminals. Invest Ophthalmol Vis Sci. 2006;47:17–24. doi: 10.1167/iovs.05-0597. [DOI] [PubMed] [Google Scholar]
- 103.Omori Y, Araki F, Chaya T, Kajimura N, Irie S, Terada K, Muranishi Y, Tsujii T, Ueno S, Koyasu T, Tamaki Y, Kondo M, Amano S, Furukawa T. Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J Neurosci. 2012;32:6126–6137. doi: 10.1523/JNEUROSCI.0322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, Maddatu TP, Smith RS, Naggert JK, Peachey NS, Nishina PM. Ocular abnormalities in Largemyd and Largevls mice, spontaneous models for muscle, eye and brain diseases. Mol Cell Neurosci. 2005;30:160–172. doi: 10.1016/j.mcn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Holzfeind PJ, Grewal PK, Reitsamer HA, Kechvar J, Lassmann H, Hoeger H, Hewitt JE, Bittner RE. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle–eye–brain disorders. Hum Mol Genet. 2002;11:2673–2687. doi: 10.1093/hmg/11.21.2673. [DOI] [PubMed] [Google Scholar]
- 106.Cibis GW, Fitzgerald KM, Harris DJ, Rothberg PG, Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Invest Ophthalmol Vis Sci. 1993;34:3646–3652. [PubMed] [Google Scholar]
- 107.De Becker I, Riddell DC, Dooley JM, Tremblay F. Correlation between electroretinogram findings and molecular analysis in the Duchenne muscular dystrophy phenotype. Br J Ophthalmol. 1994;78:719–722. doi: 10.1136/bjo.78.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fitzgerald KM, Cibis GW, Giambrone SA, Harris DJ. Retinal signal transmission in Duchenne muscular dystrophy: evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. J Clin Invest. 1994;93:2425–2430. doi: 10.1172/JCI117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pillers DA, Bulman DE, Weleber RG, Sigesmund DA, Musarella MA, Powell BR, Murphey WH, Westall C, Panton C, Becker LE, Worton RG, Ray PN. Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nat Genet. 1993;4:82–86. doi: 10.1038/ng0593-82. [DOI] [PubMed] [Google Scholar]
- 110.Sigesmund DA, Weleber RG, Pillers DA, Westall CA, Panton CM, Powell BR, Héon E, Murphey WH, Musarella MA, Ray PN. Characterization of the ocular phenotype of Duchenne and Becker muscular dystrophy. Ophthalmology. 1994;101:856–865. doi: 10.1016/s0161-6420(13)31249-4. [DOI] [PubMed] [Google Scholar]
- 111.Tremblay F, De Becker I, Riddell DC, Dooley JM. Duchenne muscular dystrophy: negative scotopic bright-flash electroretinogram and normal dark adaptation. Can J Ophthalmol. 1994;29:280–283. [PubMed] [Google Scholar]
- 112.Tremblay F, De Becker I, Dooley JM, Riddell DC. Duchenne muscular dystrophy: negative scotopic bright-flash electroretinogram but not congenital stationary night blindness. Can J Ophthalmol. 1994;29:274–279. [PubMed] [Google Scholar]
- 113.D’Souza VN, Nguyen TM, Morris GE, Karges W, Pillers DA, Ray PN. A novel dystrophin isoform is required for normal retinal electrophysiology. Hum Mol Genet. 1995;4:837–842. doi: 10.1093/hmg/4.5.837. [DOI] [PubMed] [Google Scholar]
- 114.Pillers DA, Weleber RG, Woodward WR, Green DG, Chapman VM, Ray PN. mdxCv3 mouse is a model for electroretinography of Duchenne/Becker muscular dystrophy. Invest Ophthalmol Vis Sci. 1995;36:462–466. [PubMed] [Google Scholar]
- 115.Pillers DA, Weleber RG, Green DG, Rash SM, Dally GY, Howard PL, Powers MR, Hood DC, Chapman VM, Ray PN, Woodward WR. Effects of dystrophin isoforms on signal transduction through neural retina: genotype-phenotype analysis of Duchenne muscular dystrophy mouse mutants. Mol Genet Metab. 1999;66:100–110. doi: 10.1006/mgme.1998.2784. [DOI] [PubMed] [Google Scholar]
- 116.Pillers DA, Fitzgerald KM, Duncan NM, Rash SM, White RA, Dwinnell SJ, Powell BR, Schnur RE, Ray PN, Cibis GW, Weleber RG. Duchenne/Becker muscular dystrophy: correlation of phenotype by electroretinography with sites of dystrophin mutations. Hum Genet. 1999;105:2–9. doi: 10.1007/s004399900111. [DOI] [PubMed] [Google Scholar]
- 117.Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R, Riker MJ, Hernández J, Weiss RM, Anderson MG, Mullins RF, Moore SA, Stone EM, Campbell KP. Visual impairment in the absence of dystroglycan. J Neurosci. 2009;29:13136–13146. doi: 10.1523/JNEUROSCI.0474-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berger W, van de Pol D, Bachner D, Oerlemans F, Winkens H, Hameister H, Wieringa B, Hendriks W, Ropers HH. An animal model for Norrie disease (ND): gene targeting of the mouse ND gene. Hum Mol Genet. 1996;5:51–59. doi: 10.1093/hmg/5.1.51. [DOI] [PubMed] [Google Scholar]
- 122.Ruether K, van de Pol D, Jaissle G, Berger W, Tornow RP, Zrenner E. Retinoschisis like alterations in the mouse eye caused by gene targeting of the Norrie disease gene. Invest Ophthalmol Vis Sci. 1997;38:710–718. [PubMed] [Google Scholar]
- 123.Ohlmann A, Scholz M, Goldwich A, Chauhan BK, Hudl K, Ohlmann AV, Zrenner E, Berger W, Cvekl A, Seeliger MW, Tamm ER. Ectopic norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J Neurosci. 2005;25:1701–1710. doi: 10.1523/JNEUROSCI.4756-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 125.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia CH, Liu H, Cheung D, Wang M, Cheng C, Du X, Chang B, Beutler B, Gong X. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17:1605–1612. doi: 10.1093/hmg/ddn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xia CH, Yablonka-Reuveni Z, Gong X. LRP5 is required for vascular development in deeper layers of the retina. PLoS One. 2010;5:e11676. doi: 10.1371/journal.pone.0011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haverkamp S, Wassle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- 129.Ruether K, Feigenspan A, Pirngruber J, Leitges M, Baehr W, Strauss O. PKCα is essential for the proper activation and termination of rod bipolar cell response. Invest Ophthalmol Vis Sci. 2010;51:6051–6058. doi: 10.1167/iovs.09-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xiong W-H, Tekmen-Clark M, Lolich S, Duvoisin RM, Morgans CW. The effect of PKCα on the electro-retinogram. ARVO. 2013 Abstr #6162. [Google Scholar]
- 131.Peachey NS, Roveri L, Messing A, McCall MA. Functional consequences of oncogene-induced horizontal cell degeneration in the retinas of transgenic mice. Vis Neurosci. 1997;14:627–632. doi: 10.1017/s0952523800012591. [DOI] [PubMed] [Google Scholar]
- 132.Sonntag S, Dedek K, Dorgau B, Schultz K, Schmidt KF, Cimiotti K, Weiler R, Löwel S, Willecke K, Janssen-Bi-enhold U. Ablation of retinal horizontal cells from adult mice leads to rod degeneration and remodeling in the outer retina. J Neurosci. 2012;32:10713–10724. doi: 10.1523/JNEUROSCI.0442-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bramblett DE, Pennesi ME, Wu SM, Tsai M-J. The transcription factor Blhlb4 is required for rod bipolar cell maturation. Neuron. 2004;43:779–793. doi: 10.1016/j.neuron.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 134.Brzezinski JA, Brown NL, Tanikawa A, Bush RA, Sieving PA, Vitaterna MH, Takahashi JS, Glaser T. Loss of circadian photoentrainment and abnormal retinal electro-physiology in Math5 mutant mice. Invest Ophthalmol Vis Sci. 2005;46:2540–2551. doi: 10.1167/iovs.04-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ohtoshi A, Wang SW, Maeda H, Saszik SM, Frishman LJ, Klein WH, Behringer RR. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14:530–536. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 136.Peachey NS, Quiambao AB, Xu X, Pardue MT, Roveri L, McCall MA, Al-Ubaidi MR. Loss of bipolar cells resulting from the expression of bcl-2 directed by the IRBP promoter. Exp Eye Res. 2003;77:477–483. doi: 10.1016/s0014-4835(03)00149-0. [DOI] [PubMed] [Google Scholar]
- 137.Zhu X, Wu K, Rife L, Cawley NX, Brown B, Adams T, Teofilo K, Lillo C, Williams DS, Loh P, Craft CM. Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J Neurochem. 2005;95:1351–1362. doi: 10.1111/j.1471-4159.2005.03460.x. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose beta1,2-N-acetylglucosaminyltransferase (POMGnT1) Mech Dev. 2006;123:228–240. doi: 10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]