Soundscape-level acoustic recordings revealed delay in arrival of songbird community to arctic breeding grounds.
Abstract
Bioacoustic networks could vastly expand the coverage of wildlife monitoring to complement satellite observations of climate and vegetation. This approach would enable global-scale understanding of how climate change influences phenomena such as migratory timing of avian species. The enormous data sets that autonomous recorders typically generate demand automated analyses that remain largely undeveloped. We devised automated signal processing and machine learning approaches to estimate dates on which songbird communities arrived at arctic breeding grounds. Acoustically estimated dates agreed well with those determined via traditional surveys and were strongly related to the landscape’s snow-free dates. We found that environmental conditions heavily influenced daily variation in songbird vocal activity, especially before egg laying. Our novel approaches demonstrate that variation in avian migratory arrival can be detected autonomously. Large-scale deployment of this innovation in wildlife monitoring would enable the coverage necessary to assess and forecast changes in bird migration in the face of climate change.
INTRODUCTION
Shifts in phenology across floral and faunal taxa are among the mostly widely documented biotic responses to global climate change (1). Migratory birds show strong phenological responses to changing climate (2), with many populations arriving to their breeding grounds earlier in association with rising spring temperatures (3). These shifts can influence their reproductive success (4) and may lead to adaptations to climate change (5). These population-specific and often local responses, although important for monitoring biotic climate change impacts, are limited in their ability to provide global-scale assessments of phenological responses of avian communities to climate change. Large-scale spatial and temporal heterogeneity in climate change and taxonomic variability among avian species requires a global approach (6). The absence of this long-term, global-scale information hampers understanding of the relative influences of meteorological conditions, extreme events, and modes of climate variability (for example, El Niño Southern Oscillation), which is necessary to identify the avian species, populations, communities, and ecosystems most vulnerable to projected shifts in climate (7, 8).
To date, wildlife responses to climate change have been measured using in situ censuses and Global Positioning System (GPS) tracking, both of which function poorly in monitoring whole avian communities. In situ censuses provide only point-based information, are conducted at infrequent snapshots in time and space, primarily due to their labor-intensive nature, and are subject to large sampling bias because of limited access to remote areas and observer differences (9). Although GPS tracking provides dynamic data, tagging remains costly, and current tracking units are too large to place on most avian species (10). Automated bioacoustic recorders offer a more cost-effective alternative to sample at larger spatial, temporal, and taxonomic scales (9) but have yet to be widely deployed because bioacoustic data are complex and, despite significant advances in automated analytical methods, comprehensive toolsets remain largely undeveloped. Single recorders provide highly localized information, but recording arrays are being deployed across landscapes, recording sounds at the landscape level, or recording what constitutes a soundscape (11).
Soundscapes are rich in information relating to wildlife abundance, community assemblage, behavior, and communication [for example, (12–15)]. As such, the use of bioacoustics to answer ecological questions has been increasing steadily (9). Many methodological papers have focused on comparing tallies of species presence/absence determined by experts listening to acoustic recordings versus traditional field surveys [for example, (16)]. Other studies use acoustic data to test ecological hypotheses, relying on listener input from trained experts to identify species from recordings [for example, (17)]. Although listening to recorded data has proven a valuable technique (9), recorders typically generate enormous data sets too large to listen to. Considerable effort has gone into automating the extraction of bioacoustic information from large volumes of recorded data for use in ecological studies. For example, researchers developed automated signal processing and machine learning techniques to identify species-specific vocalizations with great success in the study of marine mammals [for example, (18)], elephants [for example, (19)], and nocturnal avian migration [for example, (20)]. Although valuable, these techniques are fine-tuned to individual species of interest (20), which narrows their broad application. Further, these approaches often rely on recordings with limited background noise—a condition atypical of soundscape-level recordings (20, 21). Other studies sidestep the direct identification of vocalizations and examine community-level dynamics through various “acoustic indices” [for example, (22–25)]. This approach has proven powerful because acoustic indices are relatively straightforward to calculate and rapidly synthesize complex soundscapes.
We took a novel approach to analyzing bioacoustic data by leveraging signal processing and machine learning techniques—borrowed from human speech recognition applications—to develop automated monitoring of migratory songbird communities breeding in arctic Alaska. Specifically, we quantified spatiotemporal dynamics in vocal activity and estimated the date on which songbird communities arrived to their breeding grounds in each of five consecutive springs.
RESULTS AND DISCUSSION
Quantifying songbird community vocal activity
We programmed autonomous acoustic recorders to collect 1200 hours of soundscape-level data on a subdaily basis, over 30 consecutive days during the springs of 2010 through 2014, at four sites in the vicinity of Toolik Field Station (TLFS), Alaska (fig. S1) (see the Supplementary Materials). We explored both supervised (includes listener input) and unsupervised (no listener input) classification approaches (Fig. 1) to build seasonal time series of daily avian vocal activity (Fig. 2). The supervised classification yielded a score of the relative proportion of segments containing songbird vocalizations each day—the Vocal Activity Index (VAI)—which agreed well with our listener-generated scores [R2 = 0.65, root mean square error (RMSE) = 0.19] (fig. S2). The unsupervised classification yielded a weighted sum of principal components that were strongly related to results from the supervised approach (R2 = 0.7, RMSE = 0.11) (fig. S2). Time series generated by both approaches showed substantial variation in the songbird community vocal activity levels among days, weeks, years, and recording sites (Fig. 2 and figs. S3 to S5).
Fig. 1. Outline of bioacoustic methodology.
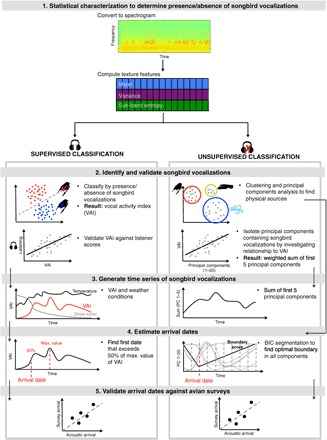
We present two analytical approaches, supervised and unsupervised classifications. Both approaches rely on the same initial statistical characterization of the acoustic data set to identify songbird vocalizations, regardless of species. The supervised approach used a linear classifier to classify every 4-s segment of the data set for the presence/absence of songbird vocalizations, trained on a subset of listener-determined scores (<1% of data set). We used the proportion of segments per day containing songbird vocalizations as a relative score, referred to as the VAI. We estimated the arrival dates as the first date that exceeded 50% of the maximum value of the VAI. The unsupervised approach used a series of signal processing and machine learning techniques to cluster the acoustic data into potential physical sources (for example, vocalizations, wind, and trucks) without training from listener input. Because the number of physical sources is not known a priori, we initially clustered the data into 100 clusters. We then performed principal components analysis on the histograms of cluster assignments to reduce data to 20 dimensions. We estimated the arrival dates as the optimal segmentation boundary in principal components, as measured by the fit of Gaussian distributions on either side of the boundary (see the Supplementary Materials).
Fig. 2. Songbird community vocal activity estimated by supervised and unsupervised approaches.
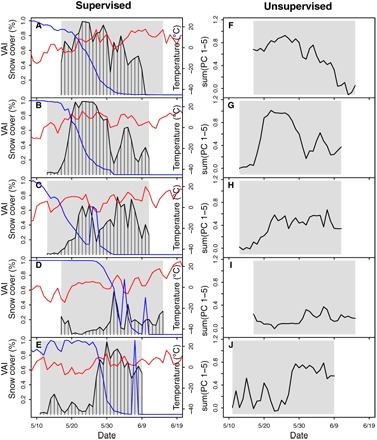
(A to E) Songbird daily VAI, snow cover (blue), and air temperature (red) near TLFS between 2010 and 2014. (F to J) Weighted sums of the first five principal components at the same site and time. Gray boxes identify the available recording period for acoustic data. Daily VAI and weighted sums for the entire data set at all field sites can be found in the Supplementary Materials (figs. S3 to S5).
Influence of environmental conditions and breeding phenology on vocal activity
We found that daily fluctuations in snow cover, air temperature, wind speed, atmospheric pressure, and precipitation had a significant impact on the VAI and explained a large proportion of variance (R2 = 0.52 ± 0.06) (Fig. 3 and fig. S6). Our acoustically derived findings agree with previous in situ work showing that breeding songbirds require snow-free patches of tundra to supply critical food and shelter (26–28), while cold conditions exacerbate the high energetic costs associated with singing (29, 30), suggesting that songbirds are either absent or silent during unfavorable conditions.
Fig. 3. Influence of environmental conditions and breeding phenology on a songbird community vocal activity.
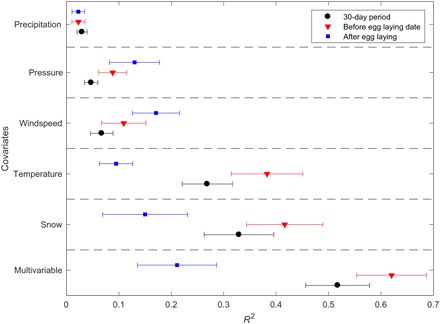
Proportion of variance in the VAI explained by environmental covariates, as determined by linear models. To identify environmental covariates that were significantly predictive (P < 0.1) of the VAI, we used stepwise regression with backward variable selection based on a F test to build linear models for each recording period independently. We also built single-variable linear models with each environmental covariate in isolation. We built the same suite of linear models for the period before and after egg laying dates for the two most abundant songbird species. Points represent mean R2 (across sites and years) ± SE. Black circles indicate linear models built with data over the entire 30-day study period. Red triangles and blue squares indicate linear models built considering the period before and after the mean egg laying dates, respectively.
We found that the VAI was more strongly influenced by environmental conditions before egg laying dates, rather than by conditions after egg laying, for two of the most abundant songbird species in the region—Lapland longspurs (Calcarius lapponicus) and Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) (R2 = 0.62 ± 0.07 versus 0.21 ± 0.08, respectively; P < 0.1). We attribute this pattern to the fact that vocal activity changes with male pairing status and breeding phenology, with singing decreasing markedly after egg laying (31). Our findings demonstrate that the correct interpretation of avian vocal activity to estimate relative songbird abundance requires pairing of acoustic data collection with meteorological data, as well as consideration of the study communities’ breeding phenology. This analytical need could be partially realized by leveraging existing environmental monitoring networks [for example, National Science Foundation’s Arctic Observing Network (AON), National Ecological Observing Network, and Long Term Ecological Research network], which could be expanded to include and power microphone and recording arrays.
Songbird community arrival dates
Across our five study years, acoustically derived estimates of arrival dates were strongly related to those determined via traditional avian surveys of two of the most locally abundant species (supervised: RMSE, 3.02 days; unsupervised: RMSE, 1.88 days) (Fig. 4 and fig. S7). This success derives from the fact that migratory songbirds vocalize intensely soon after they arrive to suitable breeding territories because of the immense pressure to initiate breeding in the Arctic (26). To assess the accuracy of our acoustically derived arrival date estimates, we compared them to an alternative method to estimate arrival timing based on traditional avian surveys. Differences in arrival estimates between these two methods may be due, in part, to the fact that acoustic sensors were able to sample more frequently than human observers. However, the inability of bioacoustic methods to distinguish absence from silence limits their accuracy. Using multiple techniques in tandem could help quantify uncertainty in available methods in the short term and inform interpretation of results. This approach is particularly important for assessing variability and trends in songbird phenology in light of global climate change.
Fig. 4. Acoustically derived estimates of songbird arrival to breeding grounds and relationship to snow-free dates.
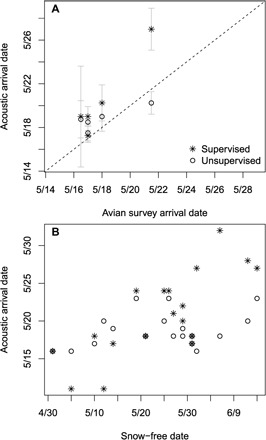
(A) Songbird community mean arrival dates to their breeding grounds near TLFS, Alaska over a 5-year period (2010–2014) using supervised and unsupervised bioacoustic approaches compared to traditional avian surveys. SE bars reflect averages across four recording sites for acoustically derived estimates. (B) Songbird community arrival dates for at each site over a 5-year period (2010–2014) estimated from supervised and unsupervised bioacoustics approaches compared to the date on which the landscape surrounding the recording unit fell below 10% snow coverage.
Further, in each study year, acoustically derived arrival dates differed among our four recording sites, which were spread along a 70-km transect, with the earliest arrivals occurring at the southernmost site in almost all cases. Relative to other study years, in 2013 [a spring characterized by persistent snow cover and cold temperatures (28, 30)], our bioacoustic approaches identified a 1- to 9-day delay in the arrival of songbird communities to their breeding grounds (Fig. 4). In addition, using our supervised approach (R2 = 0.59, P < 0.01) interannual and spatial differences in arrival dates were strongly related to the date on which the landscape surrounding each microphone became snow-free (that is, snow cover of <10%) (Fig. 4). The relationship between snow-free and arrival dates for the unsupervised approach was relatively weak (R2 = 0.13, P = 0.15), suggesting that the unsupervised approach is less sensitive to small variations in snow-free dates than the supervised approach. This disparity is most likely due to methodological differences in estimating arrival dates between the supervised and unsupervised approaches. Arrival date estimates from the supervised approach are based solely on the VAI, whereas the unsupervised approach estimates arrival by incorporating information from other acoustic sources, which may dampen the seasonal transition in songbird vocal activity (for more information, see Materials and Methods). Our results suggest that, while both bioacoustic methods successfully estimated large differences in arrival dates, the supervised approach may be superior to the unsupervised approach in estimating arrival timing in response to small spatiotemporal differences or changes in snow melt timing resulting from climate change. Again, quantifying error associated with arrival estimates by comparing alternate methods could improve our ability to assess trends related to global change.
Millions of songbirds migrate each spring to breed in the Arctic (32). Although the onset of spring migration is cued by photoperiod (33), arrival and settlement are influenced by environmental conditions en route and on breeding grounds (28, 30, 34). Shifts in the arrival of spring due to climate change may be spatially heterogeneous along migratory routes (35). While traditional avian surveys as far back as the 19th century [for example, (36)] have used timing of the onset of singing to estimate dates of migratory species’ arrival to breeding grounds, efforts to extract arrival timing from digital acoustic data sets have been rare [for example, (37)]. We contend that arctic ecosystems in particular merit autonomous methods of data collection because they are changing rapidly and are vast, remote regions that are difficult to survey (38). Climate impacts on arrival timing, which may influence breeding success, could reverberate globally, as Arctic-breeding songbirds perform important ecosystem services worldwide (39).
CONCLUSIONS
The direct application of our automated analytical approaches to monitoring avian phenology may be possible in other ecosystems and for the study of other vocal taxa (for example, insect or amphibian species). Our unsupervised approach is likely to work best in ecosystems with similar high seasonality in vocal activity, such as along migratory stopovers or in other ecosystems with strong seasonality in environmental conditions. Our supervised approach can be easily calibrated for other ecosystems and species via some initial listener input and training. Automated bioacoustic networks present an advantage over traditional surveys because they can be deployed to sample more economically over longer periods and in more remote areas and they preserve a long-term observational data set that can be reanalyzed and thus reduce observer biases (9). Our success demonstrates that automated bioacoustic networks are well poised to integrate with ground-based and remotely sensed observations of environmental conditions and vegetation to enhance understanding of how climate influences phenological responses of wildlife that uses vocal forms of signaling and communication such as birds, amphibians, social mammals, insects, and many other species.
MATERIALS AND METHODS
Study design
This study was focused on four research sites in the foothills of the Brooks Range, Alaska (68°38′N, 149°34′W; elevation, 760 m) in a 35-km radius of TLFS (fig. S1). Data were collected over a 5-year period (2010–2014) at Roche Moutonee Creek (ROMO), Imnavait Creek (IMVT), TLFS, and Sagavanirktok Department of Transportation (SDOT). For a full description of sites, see (40).
Although the acoustic analyses presented in this study did not discriminate between species, we did compare our results to traditional avian surveys of the two most abundant species in our study region. Lapland longspurs (C. lapponicus) and Gambel’s white-crowned sparrows (Z. leucophrys gambelii) are both long-distance migratory passerines, which winter in the contiguous United States (33, 41) and migrate to breed in northern Alaska (32, 40) where they capitalize on the brief but large pulse of food resources and the relatively low predatory risk that Arctic summers offer as compared to more southern ecosystems (32, 41, 42). Typically, arrival occurs in early to mid-May (26, 28). Arrival timing is of critical importance to Arctic-breeding birds who must quickly initiate clutches and complete their breeding cycles before winter’s onset (~90 days) and ensure that their young hatch at the peak of nutritious arthropod biomass (3, 43).
Automated collection of landscape-level acoustic data
Acoustic recordings were taken over five breeding seasons (2010–2014) between early May and mid/late June of each study year, thereby including the arrival, territory establishment, and clutch initiation of our two focal species (28). Thirty-minute recordings were made four times daily (2:00, 6:00, 9:00, and 21:00) to capture diurnal variation in vocal activity. Recordings were made using a digital audio recorder (722, Sound Devices LLC) and two microphones (MKH-30 and MKH-40, Sennheiser Electronic GmbH and Co. KG) at a 48-kHz sample rate. The acoustic data set contained 1200 hours of recordings capturing sounds from a range of typical local sources including rain, wind, truck traffic along the nearby Dalton Highway, mosquitoes, and a variety of bird species.
Traditional avian surveys
The dates of mean arrival of Lapland longspurs and Gambel’s white-crowned sparrows to the study region were determined by the mean date on which individuals were captured in mist nests at the four sites in 2011–2014. No Lapland longspurs were captured in 2010, so the mean arrival date was determined from road surveys in that year. The date of mean arrival of the songbird community to the region was determined by calculating the mean between species and sites. The mean dates of egg laying for all located nests of each species were determined on the basis of observations of egg laying, hatching, and fledging in 2011–2014. The mean date of egg laying of the songbird community was determined by calculating the mean dates between species for each year. For full details, see (28).
Environmental data collection
Air temperature, precipitation, atmospheric pressure, and wind speed data at ROMO and SDOT were collected. Environmental data for TLFS and IMVT were downloaded from the Environmental Data Center (2014) at TLFS and Imnavait AON Tussock Site, respectively. Snow cover was determined as the percentage of ground covered by snow in automated photographs. Snow cover data were only collected at two study sites (ROMO and TLFS) in 2010. For full details, see (28).
Acoustic analysis overview
Our primary objectives were to (i) estimate the arrival date of the songbird community to their arctic breeding grounds in each of our five study years and (ii) determine the influence of both environmental conditions and songbird phenology on estimates of songbird vocalizations through the breeding season. We presented two analytical approaches, supervised (includes listener input) and unsupervised classifications (no listener input), using a data set collected at subdaily intervals over five consecutive breeding seasons (Fig. 1). Both approaches relied on the same initial statistical characterization of the acoustic data set to identify the presence of songbird vocalization, regardless of species.
The supervised classification approach used a linear classifier, trained on a subset of listener-determined presence/absence of songbird vocalizations, to classify every 4-s segment of the data set for the presence/absence of songbird vocalizations. The proportion of segments per day containing songbird vocalizations was then used as a relative measure of daily songbird vocal activity, referred to as the VAI. The performance of the classifier was assessed by the relationship between the VAI and the training data set (fig. S2). Daily time series of VAI was created for each recording site and study year (Fig. 2 and figs. S3 to S5). To understand and interpret daily variation the VAI, we used linear models to quantify relationships between the VAI and local environmental conditions and how these relationships change based on songbird breeding phenology (Fig. 3 and fig. S6). Finally, we used the VAI to estimate the arrival date of songbirds to their breeding grounds in each study year and compared these estimates to avian surveys conducted concurrently with acoustic recordings (Fig. 4).
In contrast, the unsupervised classification approach used a series of signal processing techniques and machine learning algorithms to cluster the acoustic data into potential physical sources (for example, bird vocalizations, wind, and trucks on the nearby Dalton Highway) without any training from listener input. Because the number of physical sources, and thus clusters, was not known a priori, we initially clustered the data into 100 clusters and calculated the proportion of recording segments that fell into each cluster. We then performed principal components analysis on the cluster assignment histograms to reduce the cluster assignment histograms to 20 dimensions. To identify the principal components that contained information about songbird vocalizations, we quantified the relationship between the principal components, added in succession, and the VAI (fig. S2). This approach resulted in a time series of songbird vocalizations, as determined by a weighted sum of the first five principal components (Fig. 2 and figs. S3 to S5). Independent of this procedure, we used the transition in acoustic sources, as measured by the first 20 principal components, over time to estimate arrival date of songbirds in each study year by finding the optimal boundary and compared these estimates to avian surveys (Fig. 4).
Statistical characterization to determine the presence/absence of songbird vocalizations
To identify the presence of songbird vocalizations, regardless of species, we segmented the acoustic data set into 4-s segments (the typical duration of a songbird vocalization phrase) with 2 s between consecutive clips. Each 30-min recording contributed 898 4-s segments, and thus a 30-day period at a single recording site amounted to more than 100,000 segments. Each 4-s segment was described by a set of 54 statistical texture features known to be important for human auditory recognition: mean, variance, and sub-band entropy within auditory-scaled frequency bands (44). The lowest five frequency bands (0 to 630 Hz) were excluded from the analysis because songbird vocalizations are not produced at these frequencies.
Identifying songbird vocalizations
Supervised classification
We used linear discriminant analysis to develop a linear classifier to determine the presence/absence of songbird vocalizations in each segment based on their associated texture features. The linear classifier was trained using a random subset of recording segments manually scored for the presence/absence of songbird vocalizations based on listener input. The training data set consisted of 6000 example segments (<1% of the total data set).
A hyperplane was fit to the training data set to separate the two classes (presence/absence of songbird vocalizations) based on their texture features. A receiver operating characteristic curve was used to investigate the performance of the classifier as the decision threshold was varied and to find the area under the curve, a measure of the classifier’s performance above random classification. The classifier’s decision threshold was adjusted to the equal area rate, where the true-positive rate and true-negative rate are equal. The resulting classifier was used to classify the entire acoustic data set for the presence/absence of songbird vocalizations. Calculating the proportion of 4-s segments per day containing songbird vocalizations gave a score of relative vocal activity, the VAI. The VAI ranges from 0 to 1, where 0 represents no songbird vocalizations in any segments recorded in a day, and 1 means that all segments contained vocalizations.
The performance of the classifier was assessed by comparing the proportion of segments containing songbird vocalizations in a 30-min recording, as determined by the classifier and by listening. The closeness of fit to a linear relationship was quantified with a linear regression. The difference between the values from the classification output and the manual listening was measured as the RMSE. Although each 4-s segment was scored for the presence/absence of songbird vocalization, for the remaining analysis, we reduced the temporal resolution to a daily VAI.
Unsupervised classification
To identify 4-s segments with similar acoustic characteristics without listener input, we used vector quantization to cluster segments based on their associated texture features for each 30-day recording period independently. Vector quantization reduced multidimensional data by grouping neighboring vectors, in this case, texture features, to a predefined number of prototype vector codewords or clusters (45, 46). A codebook of 100 characteristic vectors was trained using 10,000 randomly selected texture feature vectors from each 30-day recording period. The training vectors were grouped using the K-means clustering algorithm, which iteratively updates the location of the codeword vectors until the average Euclidean distance to the associated training vectors falls below a predetermined threshold (46). The entire 30-day recording period was then quantized into the 100 codewords by minimizing the Euclidean distance of each 4-s segment’s texture feature vector to the codewords. This associated each 4-s segment with a codeword. The codeword assignments of the entire data set were summarized by histograms of codeword assignments over 100 4-s segments (approximately 10 min).
The codeword histograms were reduced through principal components analysis via singular value decomposition, which lowered the dimensionality of a data set by finding the optimal subspace, based on minimizing the sum of the square perpendicular distances of the given set of points to the subspace (47). Principal components analysis was performed for each 30-day recording period independently. On average, the first 20 principal components explained 72% of the variability in the codeword data sets; we thus restricted each 30-day period to the first 20 principal components.
To investigate the relationship of the resulting principal components to the presence of songbird vocalizations, we compared their scores to the VAI. To match the temporal resolution of the VAI, principal component scores were averaged to give mean daily scores. A series of linear models were used to quantify the relationship between the VAI and the principal components added in succession for each 30-day recording period independently. For example, the VAI of each 30-day recording period was predicted by linear models based on the following: (i) the first principal component’s scores, (ii) the first two principal components’ scores, etc. The mean and SE of R2 values were found for model input configuration replicates across study years and sites. The appropriate number of principal components to use in a time series of songbird vocalizations was considered the first combination that, on average, explained 70% of the variance in the VAI. The fitted values from the multivariable models were used to generate a time series of the weighted sum of the selected principal components. The ability of the weighted sum of principal components to replicate the VAI was measured by the RMSE.
Arrival date estimation
Supervised classification
Arrival date of the bulk of the songbird community breeding in the vicinity of our four recording sites was calculated as the first recording date on which a given site’s VAI exceeded 50% of its maximum value for that year. The sensitivity of arrival date estimates to thresholds ranging from 30 to 70% was investigated.
Unsupervised classification
The songbird community arrival date was estimated by analyzing scores of the first 20 principal components over time and finding the optimal segmentation boundary at each site for each year. We constrained our analysis to the time period before May 25th because songbirds are known to arrive to our study site in this time window (26, 28). Despite identifying the principal components that were strongly related to the VAI, and thus songbird vocalization, we included the first 20 principal components in this portion of the analysis to develop an arrival date estimation procedure that is independent of any listener input.
The optimal segmentation boundary in the principal components scores was found using a Bayesian Information Criterion (BIC)–based algorithm, a common approach to segmenting audio information (48). Our BIC segmentation tested all possible segmentation boundaries up to May 25th by fitting Gaussian mixture models on either side of the boundary. Boundary placements were scored by the sum of the negative log of the likelihoods of the associated models. We considered the optimal boundary placement to be that which minimized the score criterion. Songbird community arrival date was estimated as the date of the optimal segmentation boundary.
To validate both the supervised and unsupervised classification approaches to estimating the songbird community arrival date, we averaged arrival date estimates among recording sites for each year and compared them to avian survey estimates conducted concurrently (although at a coarser spatial resolution; see “Traditional avian surveys” section). The RMSE was used to quantify the ability of both classification approaches to replicate survey estimates.
The influence of environmental conditions and songbird phenology on VAI
Relationships between the VAI and environmental conditions were investigated at each site and year (20 replicates) with linear models. To identify which covariates were significantly predictive of the VAI, we used stepwise regression with backward variable selection. Multivariable linear models were built for each 30-day recording period independently with the following suite of environmental covariates as predictor variables: snow cover, temperature, wind speed, atmospheric pressure, and precipitation. Predictor variables were iteratively removed by the following procedure: (i) generating a linear model using all available environmental covariates, (ii) performing a F test, and (iii) eliminating variables one at a time that were not statistically significant (P < 0.1). This procedure was repeated until only the environmental covariates that had a statistically significant linear relationship to the VAI remained. Models for 2010 at the SDOT and IMVT sites did not include snow cover as a potential covariate because data were not available (see “Environmental data collection” section).
Linear models were built for each of the 20 (four sites, 5 years) 30-day recording periods using only the environmental covariate(s) determined to be statistically significant, hereafter referred to as significant multivariable models. Linear models were also built for each 30-day period between VAI and each environmental covariate in isolation, hereafter referred to as single-variable models.
Songbirds’ propensity to vocalize is known to change throughout their breeding season—with higher levels of singing when individuals are searching for mates and lower levels after clutches are initiated (31). We explored how relationships between the VAI and environmental conditions changed on the basis of songbird phenology. The 30-day recording periods were segmented on the basis of the mean clutch initiation date for Lapland longspurs and white-crowned sparrows, as determined by avian surveys (see “Traditional avian surveys” section). The same suite of significant multivariable and single-variable linear models, described above, was constructed for the time window before clutch initiation (on average, 22 days) and the time window following clutch initiation (on average, 8 days). This analysis was only performed for the years 2011–2014 because clutch initiation dates were not available for 2010 (see “Traditional avian surveys” section).
The proportion of variance (R2) explained by the predictor variables was found for each model. The mean and SE of R2 values were found for model input configuration replicates. Two-sample t tests of mean R2 values were performed to compare model input configurations.
Supplementary Material
Acknowledgments
We thank TLFS (Institute of Arctic Biology, University of Alaska Fairbanks) for sharing the data on meteorological conditions. We thank both TLFS and CH2M HILL for providing support and logistics. We thank Sanchez Industrial Design for providing the acoustic recording equipment. We thank A. Farnsworth and S. Naeem for providing valuable feedback. Funding: This project was funded by a collaborative NSF grant from the Office of Polar Programs (ARC 0908444 to N.T.B., ARC 0908602 to L.G., and ARC 0909133 to J.C.W.) and the NSF Graduate Research Fellowship Program (DGE 16-44869 to R.Y.O. and 1148897 to H.E.C.). This project received funding from Columbia University’s Data Science Institute through the Research Opportunities and Approaches to Data Science program. This project is further supported by NASA’s Arctic-Boreal Vulnerability Experiment (NNX15AV92A to N.T.B.). Author contributions: R.Y.O. wrote the manuscript with feedback from all coauthors. N.T.B., J.C.W., L.G., R.Y.O., and D.P.W.E. co-conceived, designed, and executed this study. H.E.C., J.S.K., J.H.P., and S.K.S. collected all the data sets. R.Y.O. conducted the acoustic analyses in collaboration with D.P.W.E. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/6/eaaq1084/DC1
Supplementary Text
fig. S1. Map of Alaska (inset) and TLFS with approximate locations of acoustic recording units.
fig. S2. Performance of supervised and unsupervised classification approaches.
fig. S3. Songbird community vocal activity estimated by supervised and unsupervised approaches near IMVT.
fig. S4. Songbird community vocal activity estimated by supervised and unsupervised approaches near ROMO.
fig. S5. Songbird community vocal activity estimated by supervised and unsupervised approaches near SDOT.
fig. S6. Comparison of the VAI to linear model predictions using only environmental covariates found to be statistically significant.
fig. S7. Threshold sensitivity of arrival date estimates from supervised approach.
REFERENCES AND NOTES
- 1.Parmesan C., Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006). [Google Scholar]
- 2.Root T. L., Price J. T., Hall K. R., Schneider S. H., Rosenzweig C., Pounds J. A., Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Rubolini D., Møller A. P., Rainio K., Lehikoinen E., Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Climate Res. 35, 135–146 (2007). [Google Scholar]
- 4.Both C., Bouwhuis S., Lessells C. M., Visser M. E., Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Charmantier A., Gienapp P., Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evol. Appl. 7, 15–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordo O., Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Res. 35, 37–58 (2007). [Google Scholar]
- 7.Morisette J. T., Richardson A. D., Knapp A. K., Fisher J. I., Graham E. A., Abatzoglou J., Wilson B. E., Breshears D. D., Henebry G. M., Hanes J. M., Liang L., Tracking the rhythm of the seasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 7, 253–260 (2009). [Google Scholar]
- 8.Turner W., Sensing biodiversity. Science 346, 301–302 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Shonfield J., Bayne E. M., Autonomous recording units in avian ecological research: Current use and future applications. Avian Conserv. Ecol. 12, 14 (2017). [Google Scholar]
- 10.Kays R., Crofoot M. C., Jetz W., Wikelski M., Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Krause B., Anatomy of the soundscape: Evolving perspectives. J. Audio Eng. Soc. 56, 73–80 (2008). [Google Scholar]
- 12.Poole J. H., Tyack P. L., Stoeger-Horwath A. S., Watwood S., Animal behaviour: Elephants are capable of vocal learning. Nature 434, 455–456 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Farina A., Pieretti N., Piccioli L., The soundscape methodology for long-term bird monitoring: A Mediterranean Europe case-study. Eco. Inform. 6, 354–363 (2011). [Google Scholar]
- 14.Frommolt K.-H., Tauchert K.-H., Applying bioacoustic methods for long-term monitoring of a nocturnal wetland bird. Eco. Inform. 21, 4–12 (2014). [Google Scholar]
- 15.Stoeger A. S., Zeppelzauer M., Baotic A., Age-group estimation in free-ranging African elephants based on acoustic cues of low-frequency rumbles. Bioacoustics 23, 231–246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vold S. T., Handel C. M., Mcnew L. B., Comparison of acoustic recorders and field observers for monitoring tundra bird communities. Wildl. Soc. Bull. 41, 566–576 (2017). [Google Scholar]
- 17.Hart P. J., Hall R., Ray W., Beck A., Zook J., Cicadas impact bird communication in a noisy tropical rainforest. Behav. Ecol. 26, 839–842 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford K. M., Fox C. G., Clark D. S., Long-range acoustic detection and localization of blue whale calls in the northeast Pacific Ocean. J. Acoust. Soc. Am. 104, 3616–3625 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Zeppelzauer M., Hensman S., Stoeger A. S., Towards an automated acoustic detection system for free-ranging elephants. Bioacoustics 24, 13–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salamon J., Bello J. P., Farnsworth A., Robbins M., Keen S., Klinck H., Kelling S., Towards the automatic classification of avian flight calls for bioacoustic monitoring. PLOS ONE 11, e0166866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardeli R., Wolff D., Kurth F., Koch M., Tauchert K.-H., Frommolt K.-H., Detecting bird sounds in a complex acoustic environment and application to bioacoustic monitoring. Pattern Recognit. Lett. 31, 1524–1534 (2010). [Google Scholar]
- 22.Boelman N. T., Asner G. P., Hart P. J., Martin R. E., Multi-trophic invasion resistance in Hawaii: Bioacoustics, field surveys, and airborne remote sensing. Ecol. Appl. 17, 2137–2144 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Sueur J., Pavoine S., Hamerlynck O., Duvail S., Rapid acoustic survey for biodiversity appraisal. PLOS ONE 3, e4065 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pieretti N., Farina A., Morri D., A new methodology to infer the singing activity of an avian community: The Acoustic Complexity Index (ACI). Ecol. Indic. 11, 868–873 (2011). [Google Scholar]
- 25.Gage S. H., Axel A. C., Visualization of temporal change in soundscape power of a Michigan lake habitat over a 4-year period. Eco. Inform. 21, 100–109 (2014). [Google Scholar]
- 26.Wingfield J. C., Owen-Ashley N., Benowitz-Fredericks Z. M., Lynn S. E., Hahn T. P., Wada H., Breuner C., Meddle S. L., Romero L. M., Arctic spring: The arrival biology of migrant birds. Acta Zool. Sinica 50, 948–960 (2004). [Google Scholar]
- 27.Grabowski M. M., Doyle F. I., Reid D. G., Mossop D., Talarico D., Do Arctic-nesting birds respond to earlier snowmelt? A multi-species study in north Yukon, Canada. Polar Biol. 36, 1097–1105 (2013). [Google Scholar]
- 28.Boelman N. T., Krause J. S., Sweet S. K., Chmura H. E., Perez J. H., Gough L., Wingfield J., Extreme spring conditions in the Arctic delay spring phenology of long-distance migratory songbirds. Oecologia 185, 69–80 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Gaunt A. S., Bucher T. L., Gaunt S. L. L., Baptista L. F., Is singing costly? The Auk 113, 718–721 (1996). [Google Scholar]
- 30.Krause J. S., Pérez J. H., Chmura H. E., Meddle S. L., Hunt K. E., Sweet S. K., Gough L., Boelman N., Wingfield J. C., The effects of extreme spring weather on body condition and stress physiology in Lapland longspurs and white-crowned sparrows breeding in the Arctic. Gen. Comp. Endocrinol. 237, 10–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson D. M., Bart J., Reliability of singing bird surveys: Effects of song phenology during the breeding season. Condor 87, 69–73 (1985). [Google Scholar]
- 32.E. C. Pielou, A Naturalist’s Guide to the Arctic (University of Chicago Press, 1994). [Google Scholar]
- 33.Ramenofsky M., Wingfield J. C., Regulation of migration. Bioscience 57, 135–143 (2007). [Google Scholar]
- 34.Tøttrup A. P., Klaassen R. H. G., Kristensen M. W., Strandberg R., Vardanis Y., Lindström Å., Rahbek C., Alerstam T., Thorup K., Drought in Africa caused delayed arrival of European songbirds. Science 338, 1307 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Buitenwerf R., Rose L., Higgins S. I., Three decades of multi-dimensional change in global leaf phenology. Nat. Clim. Chang. 5, 364–368 (2015). [Google Scholar]
- 36.Gordo O., Sanz J. J., Lobo J. M., Geographic variation in onset of singing among populations of two migratory birds. Acta Oecol. 34, 50–64 (2008). [Google Scholar]
- 37.Buxton R. T., Brown E., Sharman L., Gabriele C. M., McKenna M. F., Using bioacoustics to examine shifts in songbird phenology. Ecol. Evol. 6, 4697–4710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon A. W. R., Macias-Fauria M., Long P. R., Benz D., Willis K. J., Sensitivity of global terrestrial ecosystems to climate variability. Nature 531, 229–232 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Sekercioglu C. H., Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Boelman N. T., Gough L., Wingfield J., Goetz S., Asmus A., Chmura H. E., Krause J. S., Perez J. H., Sweet S. K., Griffin K. C., Greater shrub dominance alters breeding habitat and food resources for migratory songbirds in Alaskan arctic tundra. Glob. Chang. Biol. 21, 1508–1520 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Piersma T., Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80, 623–631 (1997). [Google Scholar]
- 42.McKinnon L., Smith P. A., Nol E., Martin J. L., Doyle F. I., Abraham K. F., Gilchrist H. G., Morrison R. I. G., Bêty J., Lower predation risk for migratory birds at high latitudes. Science 327, 326–327 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Pérez J. H., Krause J. S., Chmura H. E., Bowman S., McGuigan M., Asmus A. L., Meddle S. L., Hunt K. E., Gough L., Boelman N. T., Wingfield J. C., Nestling growth rates in relation to food abundance and weather in the Arctic. The Auk 133, 261–272 (2016). [Google Scholar]
- 44.D. P. Ellis, X. Zeng, J. H. McDermott, Classifying soundtracks with audio texture features, in 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22 to 27 May 2011 (IEEE, 2011). [Google Scholar]
- 45.Gray R., Vector quantization. IEEE ASSP Mag. 1, 4–29 (1984). [Google Scholar]
- 46.R. Rabiner, B. H. Juang, Fundamentals of Speech Recognition (Prentice-Hall, 1993). [Google Scholar]
- 47.W. J. Krzanowski, Principles of Multivariate Analysis (Oxford Univ. Press, 1988). [Google Scholar]
- 48.Cettolo M., Vescovi M., Rizzi R., Evaluation of BIC-based algorithms for audio segmentation. Comput. Speech Lang. 19, 147–170 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/6/eaaq1084/DC1
Supplementary Text
fig. S1. Map of Alaska (inset) and TLFS with approximate locations of acoustic recording units.
fig. S2. Performance of supervised and unsupervised classification approaches.
fig. S3. Songbird community vocal activity estimated by supervised and unsupervised approaches near IMVT.
fig. S4. Songbird community vocal activity estimated by supervised and unsupervised approaches near ROMO.
fig. S5. Songbird community vocal activity estimated by supervised and unsupervised approaches near SDOT.
fig. S6. Comparison of the VAI to linear model predictions using only environmental covariates found to be statistically significant.
fig. S7. Threshold sensitivity of arrival date estimates from supervised approach.


