Highlights
-
•
Sternoclavicular septic arthritis is a rare type of septic arthritis.
-
•
Symptoms can be insidious and vague and most commonly include fever and chest pain.
-
•
Common organisms include Staphylococcus aureus and Neisseria gonorrhea, among others.
-
•
Moraxella nonliquefaciens is a Gram-negative organism in the respiratory and urogenital tracts.
-
•
Septic arthritis by this organism was never incidentally in the sternoclavicular joint.
Keywords: Moraxella nonliquefaciens, Incidental, Sternoclavicular septic arthritis, F-18 FDG, PET/CT
Abstract
An 83-year old man presented acutely to the emergency department with generalized weakness and subjective fevers. A month earlier he had undergone resection of a large intramuscular sarcoma from his thigh. The cancer staging work-up was still underway and a decision about adjuvant therapy was still pending. Although initial laboratory assessment showed leukocytosis, this normalized soon after admission without the use of antimicrobials. No fevers were documented. During the admission an 18F-FDG PET/CT was performed in continuation of his sarcoma staging workup. This revealed unexpected abnormal radiotracer uptake in the left sternoclavicular joint with fluid collections extending into the sternocleidomastoid muscle and the mediastinum. Imaging findings were consistent with septic arthritis and abscess formation, despite lack of fever or localizing symptoms. Ultrasound-guided aspiration revealed purulent fluid that grew Moraxella nonliquefaciens. Given the unusual presentation, ongoing clinical uncertainty about the true cause of the septic joint, and concern for an occult sarcoma metastasis, surgical debridement and resection of the joint was carried out. Pathology and microbiology evaluation confirmed septic arthritis with osteomyelitis and abscess extension into the mediastinum. No tumor cells were identified. Postoperative course was complicated by hematoma, but otherwise the patient responded well to antimicrobial therapy.
Case report
An 83-year old man presented to the emergency department with a one-week history of generalized weakness and subjective fevers. One month prior, he underwent resection of an intramuscular intermediate grade pleomorphic sarcoma of the right thigh. A staging computed tomography (CT) of the chest, abdomen, and pelvis at that time was significant for 2 pulmonary nodules which were indeterminate for metastasis and were still pending further workup for completion of staging. His history was otherwise significant for various cardiopulmonary problems including chronic obstructive pulmonary disease, chronic heart failure, severe aortic stenosis, atrial fibrillation on long-term anticoagulation, and sick sinus syndrome with a pacemaker.
On presentation vital signs and physical exam were normal except for bilateral pitting edema. Laboratory values were significant for mild leukocytosis (14,400 cells/mm3), elevated NT-proBNP (5412 pg/mL without prior comparison value), and elevated D-dimer (1.36 μg/mL). Chest radiograph showed mild pulmonary congestion. Chest CT angiogram redemonstrated the pulmonary nodules and was negative for acute findings, within the limitations of streak artifacts in the left shoulder region from the contrast bolus.
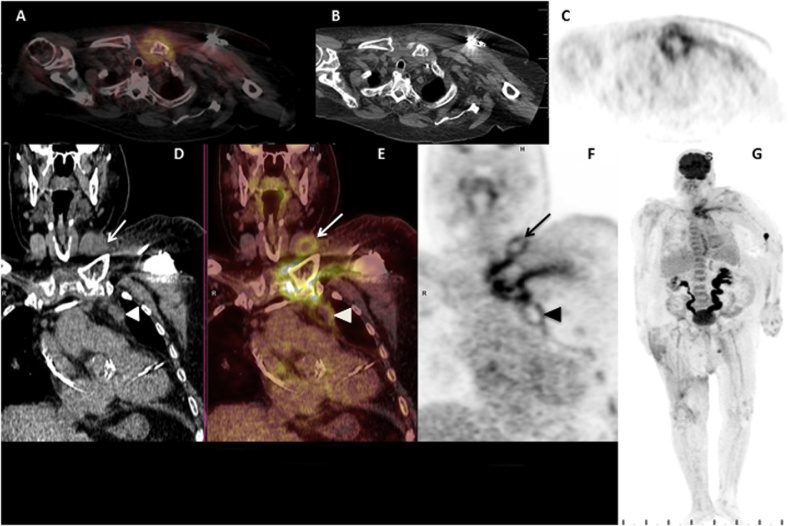
The patient was admitted for the management of decompensated heart failure. The weakness and leukocytosis present on admission normalized the next day without antimicrobials. He remained afebrile with no localizing symptoms. On admission day 3 the patient underwent 18F-FDG PET-CT (Fig. 1) in continuation of his staging work-up for sarcoma. The known pulmonary nodules showed minimal FDG uptake, suggesting either benign etiology or hypometabolic malignancy. The right thigh mass resection bed also showed mild uptake attributable to recent surgery. The most notable and concerning finding, however, was abnormally intense FDG uptake in the left sternoclavicular joint (SCJ) with direct extension into the sternocleidomastoid muscle (SCM) superiorly and mediastinum inferiorly. Associated fluid collections were visible in the SCM and along the mediastinal pleuropericardial reflection, consistent with septic arthritis and abscess (Fig. 1A–F). C-reactive protein (CRP) was found to be elevated (15.1 mg/dL). On exam a palpable nontender mass with mild erythema was evident at the region of the left SCJ. Magnetic resonance imaging (MRI) could not be performed due to presence of the pacemaker. Ultrasound showed a thick-walled collection which was aspirated revealing purulent fluid for which staining showed many polymorphonuclear cells and no organisms, and culture grew Moraxella nonliquefaciens. The patient was started on intravenous (IV) vancomycin and later changed to IV ceftriaxone. Blood cultures from the day of admission and day of the scan were negative.
Fig. 1.
(A) Fluorine-18 Fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) axial fused slice shows an area of markedly increased FDG uptake involving the left sternoclavicular joint. Increased FDG uptake is demonstrated layering between the fascial planes of the left pectoralis muscles, with a focus of increased uptake extending posterior to the left sternoclavicular joint into the left anterior mediastinum. (B) Non-contrast axial slice at the same level as A, demonstrates increased subcutaneous fat stranding in the anterior left chest. The left chest pacemaker is partially visualized. (C) Axial PET slice at the same level as A and B highlights the hypermetabolic uptake with high maximum standardized uptake value (SUVmax 6). (D) Coronal CT slice centered at the left sternoclavicular joint shows subtle lobulated abnormalities in the region of the left sternoclavicular joint (arrow) and the left anterior mediastinum (arrowhead). The left chest pacemaker wires are partially visualized in the right atrium and ventricle. (E) Coronal fused F-18 PET/CT slice shows extensive increased FDG uptake centered at and around the left sternoclavicular joint with linear involvement at the left pectoralis myofascial plane. Also seen are areas of peripheral increased uptake and central photopenia in the inferior left sternocleidomastoid muscle (arrow) and the left anterior mediastinum (arrowhead). (F) Coronal PET slice highlights the fluid collections each with an FDG-avid rim and central photopenia consistent with abscess formation. (G) Maximum intensity projection (MIP) PET image of the whole body shows the findings highlighted above in the left sternoclavicular region. The patient’s urinary system’s dilation is a chronic finding. Mild increase in FDG uptake at the right anterior thigh is postsurgical in nature. Incidental note is made of a tiny focus of increased uptake in the region of the left parotid gland, likely benign.
Given the paucity of infectious or localizing signs/symptoms, the clinical team remained doubtful about infection as the sole etiology of the joint abnormality. Given the unusual presentation, extensive nature of the infection, ongoing uncertainty about the true cause of the joint abnormality, and concern for occult metastatic sarcoma, cardiothoracic surgery performed SCM and mediastinal debridement and SCJ resection. The nearby pacemaker was found not to be involved and was left in place. Antimicrobial beads were placed in the surgical bed. Aerobic, anaerobic, fungal, and acid-fast bacterial cultures of debrided tissues were negative. Surgical pathology examination of the medial clavicle and adjacent tissues revealed acute osteomyelitis with reactive changes without evidence of malignancy. Hospital course was complicated by development of a large neck and chest wall hematoma that required surgical re-exploration and evacuation. The patient was eventually discharged on a 6-week course of IV ceftriaxone (2 g daily). In follow-up 2 months later, CRP had nearly normalized (2.2 mg/dL). The patient was asymptomatic and the wound was healing well.
Discussion
Septic arthritis of the sternoclavicular joint is rare in adults, and represents less than 0.5% of all bone and joint infections, occurring commonly in diabetics, IV drug users, and those with rheumatoid arthritis, renal failure, and the human immunodeficiency virus patients [[1], [2], [3]]. Usually monoarticular and insidious in onset, it may take place due to hematogenous spread or spread from a nearby infection [[1], [4]]. The proximity of the sternoclavicular joint to the mediastinum increases the likelihood of the infection spreading to and from the mediastinum. In fact, mediastinitis is the most feared complication, occurring in about 15% of patients [1]. Sternoclavicular septic arthritis may also be complicated by osteomyelitis of the clavicles or sternum and abscess formation, which can migrate into the surrounding soft tissue.
The diagnosis of sternoclavicular septic arthritis can be delayed, due to insidiousness of symptoms, by days to weeks. Fever is noted in 65% of cases, and patients are likely to complain of chest or shoulder pain [[1], [2], [5]]. Imaging evaluation may start with chest radiography, although CT and MRI are vital for early diagnosis. On CT, findings include widening of the joint space, articular destruction, and the collection of fluid and air in the joint space, chest wall, and mediastinum. MRI demonstrates bone destruction, edema, capsular distention, and inflammatory changes in the surrounding soft tissues [4]. Ultrasound shows joint effusion and pockets of fluid in the soft tissues, and is useful for planning joint aspiration.
Approximately half the patients with SCJ septic arthritis demonstrate leukocytosis. Joint aspiration and culture are useful to define the pathogen, and about two-thirds have positive blood cultures, most commonly Staphylococcus aureus 1,3. Other organisms include Neisseria gonorrhoeae (in patients at risk for sexually-transmitted diseases) and Gram-negative organisms such as Pseudomonas and Escherichia coli (particularly in immunosuppressed individuals) [1].
Patients with septic arthritis are typically managed with long-term IV antimicrobials and repeated joint aspirations or surgical drainage [6]. When there are no complications (e.g. abscess formation or mediastinal spread), SCJ septic arthritis is typically managed with long-term intravenous antimicrobials alone [1]. Surgical intervention is generally reserved for those with extensive osteomyelitis, soft tissue complications such as abscess formation or mediastinitis, and those with immunosuppression or with failure of medical therapy [1].
Moraxella nonliquefaciens is a Gram-negative coccobacillus generally considered a commensal organism residing in the upper respiratory tract and occasionally the urogenital tract [7]. Its pathogenic potential is considered low in the immunocompetent population but has been described as pathogenic in patients with respiratory tract illness, conjunctivitis, keratitis, and endophthalmitis [7]. However, isolated cases of unusual infection by M. nonliquefaciens have been described in peer-reviewed literature, including meningitis, endocarditis, lung abscess, discitis, and septic knee arthritis [[7], [8], [9], [10], [11], [12], [13], [14], [15]]. To our knowledge this case is the first case of M. nonliquefaciens causing SCJ septic arthritis in an asymptomatic immunocompetent patient. While a handful of cases of M. nonliquefaciens septic arthritis exist in peer-reviewed literature, patients were symptomatic and none had septic arthritis of the SCJ.
Conclusion
SCJ septic arthritis is rare and symptoms at presentation are variable in severity. This case demonstrates that unequivocal imaging findings of SCJ septic arthritis should lead to further investigation, even when symptoms are lacking. Atypical or absent symptoms may also point to an unusual pathogen, as seen in this case with the causative organism M. nonliquefaciens.
Declaration of interest
All authors have no relevant conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Ehab Saad Aldin, Email: ehab-saadaldin@uiowa.edu.
Poorani Sekar, Email: poorani-sekar@uiowa.edu.
Zein Saad Eddin, Email: saadeddinzm@upmc.edu.
Jaclyn Keller, Email: jaclyn-keller@uiowa.edu.
Janet Pollard, Email: janet-pollard@uiowa.edu.
References
- 1.Womack J. Septic arthritis of the sternoclavicular joint. J Am Board Fam Med. 2012;25(6):908–912. doi: 10.3122/jabfm.2012.06.110196. [DOI] [PubMed] [Google Scholar]
- 2.Yood R.A., Goldbenberg D.L. Sternoclavicular joint arthritis. Arthritis Rheum. 1980;23:232–239. doi: 10.1002/art.1780230215. [DOI] [PubMed] [Google Scholar]
- 3.Ross J.J., Shamsuddin H. Sternoclavicular septic arthritis: review of 180 cases. Medicine. 2004;83:189–195. doi: 10.1097/01.md.0000126761.83417.29. [DOI] [PubMed] [Google Scholar]
- 4.Restrepo C.S., Martinez S., Lemos D.F., Washington L., McAdams H.P., Vargas D. Imaging appearances of the sternum and sternoclavicular joints. Radiographics. 2009;29(3):839–859. doi: 10.1148/rg.293055136. [DOI] [PubMed] [Google Scholar]
- 5.Guillén Astete C., Aranda García Y., de la Casa Resino C., Carpena Zafrilla M., Braña Cardeñosa A., Roldan Moll F. Sternoclavicular septic arthritis: a series of 5 cases and review of the literature. Reumatol Clin. 2015;11(1):48–51. doi: 10.1016/j.reuma.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Ross J.J., Saltzman C.L., Carling P., Shapiro D.S. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:427–431. doi: 10.1086/345954. [DOI] [PubMed] [Google Scholar]
- 7.Duployze C., Loïez C., Ledoux G., Armand S., Jaillette E., Wallet F. A fatal endocarditis case due to an emerging bacterium: Moraxella nonliquefaciens. IDCases. 2017;8:12–13. doi: 10.1016/j.idcr.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafiq I., Parthasarathy H., Tremlett C., Freeman L.J., Mullin M. Infective endocarditis caused by Moraxella nonliquefaciencs in a percutaneous aortic valve replacement. Cardiovasc Revasc Med. 2011;12(3):184–186. doi: 10.1016/j.carrev.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 9.Sudar J.M., Alleman M.J., Jonkers G.J., de Groot R., Jongejan C. Acute thyroiditis caused by Moraxella nonliquefaciens. Neth J Med. 1994;45(4):170–173. [PubMed] [Google Scholar]
- 10.Lefebvre S., Van Bosterhaut B., Wauters G. Moraxella nonliquefaciens spondylodiscitis. Rev Rhum Ed Fr. 1994;61(7–8):564–565. [PubMed] [Google Scholar]
- 11.Kavkalo D.N., Gorshevikova E.V., Andreeshchev S.A., Stadil’naia T.E. Sepsis caused by Moraxella nonliquefaciens. Klin Khir. 1985;1:68. [PubMed] [Google Scholar]
- 12.Brorson J.E., Falsen E., Nilsson-Ehle H., Rödjer S., Westin J. Septicemia due to Moraxella nonliquefaciens in a patient with multiple myeloma. Scand J Infect Dis. 1983;15(2):221–223. doi: 10.3109/inf.1983.15.issue-2.16. [DOI] [PubMed] [Google Scholar]
- 13.Brauner E., Turcu T., Neguţ M., Dănilă F., Secu A., Vîntu A. Neonatal meningitis due to Moraxella nonliquefaciens. Rev Med Chir Soc Med Nat Iasi. 1982;86(4):637–640. [PubMed] [Google Scholar]
- 14.Bechard D.L., LeFrock J.L., Tillotson J.R. Endocarditis caused by Moraxella nonliquefaciens. South Med J. 1979;72(11):1485–1487. doi: 10.1097/00007611-197911000-00045. [DOI] [PubMed] [Google Scholar]
- 15.Rosett W., Heck D.M., Hodges G.R. Pneumonitis and pulmonary abscess associated with Moraxella nonliquefaciens. Chest. 1976;70(5):664–665. doi: 10.1378/chest.70.5.664. [DOI] [PubMed] [Google Scholar]