Abstract
Sulforaphane (SFN), a compound derived from cruciferous vegetables that has been shown to be safe and nontoxic, with minimal/no side effects, has been extensively studied due to its numerous bioactivities, such as anticancer and antioxidant activities. SFN exerts its anticancer effects by modulating key signaling pathways and genes involved in the induction of apoptosis, cell cycle arrest, and inhibition of angiogenesis. SFN also upregulates a series of cytoprotective genes by activating nuclear factor erythroid-2- (NF-E2-) related factor 2 (Nrf2), a critical transcription factor activated in response to oxidative stress; Nrf2 activation is also involved in the cancer-preventive effects of SFN. Accumulating evidence supports that epigenetic modification is an important factor in carcinogenesis and cancer progression, as epigenetic alterations often contribute to the inhibition of tumor-suppressor genes and the activation of oncogenes, which enables cells to acquire cancer-promoting properties. Studies on the mechanisms underlying the anticancer effects of SFN have shown that SFN can reverse such epigenetic alterations in cancers by targeting DNA methyltransferases (DNMTs), histone deacetyltransferases (HDACs), and noncoding RNAs. Therefore, in this review, we will discuss the anticancer activities of SFN and its mechanisms, with a particular emphasis on epigenetic modifications, including epigenetic reactivation of Nrf2.
1. Introduction
Numerous studies have suggested that high dietary intake of cruciferous vegetables is correlated with a low risk of cancer [1]. The anticancer activity of cruciferous vegetables has been mainly attributed to isothiocyanates, which are a product of myrosinase-mediated glucosinolate degradation. Sulforaphane (SFN) is a naturally occurring isothiocyanate derived from the consumption of cruciferous vegetables, such as broccoli, cabbage, and kale. Because of its efficacy, safety, nontoxicity, lack of side effects, and low cost, bioactive SFN is widely recognized as a promising chemopreventive agent with effects against many kinds of cancers, such as cervical [2], breast [3], and bladder cancer [4]; renal cell carcinoma (RCC) [5]; non-small-cell lung cancer (NSCLC) [6]; and colon and prostate cancers [7]. SFN has also been reported to improve the efficacy of low-dose cisplatin (CDDP), a commonly used chemotherapeutic drug [8].
Studies on the mechanisms underlying the anticancer activities of SFN indicate that its regulatory effects on the tumor cell cycle, apoptosis, and angiogenesis are mediated by modulation of the related signaling pathways and genes. Cell cycle analysis showed that SFN caused G2/M phase arrest leading to inhibition of tumor proliferation/growth, which was associated with downregulation of cyclin B1 [2] and cyclin D1 genes [9], as well as increased protein levels of p21WAF1/CIP1 (an inhibitor of cyclin-dependent kinases) [9]. SFN also increased the expression of the proapoptotic protein Bax and decreased expression of the antiapoptotic protein Bcl-x to induce apoptosis in cancer cells [10]. By suppressing the expression and activity of hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF), SFN inhibited the angiogenesis and metastasis of ovarian and colon cancers [11, 12].
SFN was also reported to be a strong activator of nuclear factor erythroid-2 (NF-E2-) related factor 2 (Nrf2). It is well known that long-term exposure to oxidative stress is an important carcinogenesis-promoting factor that induces DNA damage, mutations, and inflammation [13]. Nrf2 is a critical transcription factor in the antioxidant stress response. Activation of Nrf2 by SFN induced the expression of a battery of cytoprotective genes with anticarcinogenesis activities [14–16]. Those Nrf2-mediated cytoprotective genes include the antioxidants and phase II enzymes, such as NAD(P)H:quinone oxidoreductase-1 (NQO1), heme oxygenase 1 (HO-1), catalase, glutamate-cysteine ligase (GCL), glutathione S transferase (GST), UDP-glucuronosyltransferases (UGT), epoxide hydrolase, and superoxide dismutase (SOD). A number of studies revealed that the effects of SFN on Nrf2 and its downstream cytoprotective genes are through modification of Keap1 cysteine residues [17]; activation of mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and protein kinase C (PKC) pathways; and epigenetic modifications, which resulted in the phosphorylation, nuclear accumulation, and increased transcription and stability of Nrf2 [18–21].
In recent years, the epigenetic mechanisms underlying the anticancer effects of SFN have received increasing attention [22]. Epigenetic modification refers to the heritable changes in gene expression that do not affect the DNA sequence itself. In mammals, epigenetic modifications mainly include DNA methylation, histone modifications (acetylation, phosphorylation, and methylation), and noncoding RNA regulation. Epigenetic changes are reversible and can readily respond to natural bioactive dietary compounds [23], such as SFN. SFN was shown to regulate the gene activation or silencing involved in cancer through epigenetic modifications [22]. Therefore, in this review, we present the anticancer activities of SFN and its epigenetic mechanisms, including epigenetic reactivation of Nrf2. This information will help facilitate the discovery and development of novel anticancer drugs.
2. Epigenetics and Cancer
In the classic view, cancer results from genetic alterations including mutations, insertions, deletions, copy number gains, recombination, genomic instability, and single-nucleotide polymorphisms (SNPs) [24, 25]. The mutations of tumor suppressor genes and/or oncogenes contribute to the loss of normal function or gain of abnormal expression in cancers. For example, mutations of tumor suppressors of P53 and PTEN (phosphatase and tensin homolog deleted on chromosome ten) or BRCA 1/2 (crucial proteins involved in homologous recombination) were associated with colorectal [26–28], breast, and ovarian cancer [29, 30]. In addition, TP53 and CTNNB1 (encoding β-catenin) exhibited point mutations and small deletions in hepatocellular carcinoma [31]. However, emerging evidence indicates that cancer can occur without a change in the nucleotide sequence, through so-called epigenetic alterations. In fact, a combinational crosstalk between genetic and epigenetic alterations has been observed in cancer development, progression, and recurrence [32]. Both gene mutations and epigenetic alterations can be caused by exposure to various environmental factors, such as dietary components, smoke, and chemicals.
Epigenetic dysregulation, such as increased activity of histone deacetyltransferases (HDACs) and DNA methyltransferases (DNMTs) and changes in noncoding RNA expression, may lead to alterations in the transcription and expression of genes involved in the regulation of cell proliferation and differentiation, cell cycle, and apoptosis [32–37]. The present studies indicated that the level of HDAC5 expression was increased in human glioma and hepatocellular carcinoma, which promoted the proliferation of tumor cells via upregulating Six 1 (Sineoculis homeobox homolog 1) and Notch 1, respectively [38, 39]. A combination of HDAC and DNMT inhibitors contributed to cell cycle arrest in the G2/M phase and suppressed the growth of endometrial cancer through downregulation of Bcl-2 [37]. There are also multiple studies on miRNAs involved in regulating cell activities. For example, the upregulation of miR-96 and miR-153 promoted proliferation and colony formation of human prostate cancer cells [35, 36]. Evidence suggests that half of the tumor-suppressor genes are often inactivated via epigenetic, rather than genetic, mechanisms in sporadic cancers [23]. In addition, alterations in epigenetic processes mostly activate oncogenes, which enable cells to acquire cancer-promoting properties, such as uncontrolled proliferation, escape from apoptosis, and invasiveness. Accumulating evidence has suggested that targeting epigenetic modifications is a potent strategy for cancer prevention [23].
3. Epigenetic Mechanisms Underlying the Preventive Effects of SFN on Cancer
3.1. Histone Acetylation and Phosphorylation
Histone acetyltransferase (HAT) acetylates histones by adding acetyl groups to lysine residues in the N-terminal tail; this facilitates gene transcription by relaxing the chromatin structure to allow the transcription machinery to access the DNA. Conversely, HDACs repress transcription by removing acetyl groups. Many malignant neoplasms are characterized by increased expression and activity of HDACs. HDAC overexpression and overactivity are closely associated with transcriptional repression of the tumor-suppressor genes that are responsible for dysregulation of cell cycle, proliferation, differentiation, and apoptosis in malignances [23, 40, 41].
The food-based compound SFN, which is considered to be a HDAC inhibitor, has been shown to exert cancer preventive effects [22, 40, 41]. Treatment of various cancers, such as prostate [42], colon [43], and lung cancer [44], with SFN attenuated cell growth through inhibition of HDACs, accompanied by an increase in global or local histone acetylation. Moreover, SFN-mediated inhibition of HDACs contributed to reactivation of the tumor suppressor gene p21 and the proapoptotic protein Bax. In the LnCaP and PC-3 prostate cancer cell lines, 15 μM SFN treatment caused reexpression of p21WAF1/CIP1 due to reduced expression of class I and II HDACs and subsequent increases in acetylated histone H3 and H4 levels at the p21WAF1/CIP1 promoter, which resulted in cell cycle arrest [42]. Interestingly, SFN also upregulated transcription of the Bax gene to induce apoptosis in prostate cancer cells by accelerating acetylation of histone H4 at the Bax promoter [45]. Similar changes in p21 and Bax reactivation, resulting from inhibition of HDACs and upregulation of acetylated histone H3 and H4, were observed in SFN-treated lung cancer cell lines and tumor tissues [44]. Ultimately, SFN with different concentrations (in vitro 15 μM, in vivo 9 mM/mice/day) suppressed lung cancer growth in vitro and in vivo [44].
Additionally, HDACs can affect DNA damage and repair by altering the acetylation status of c-terminal-binding protein interacting protein (CtIP), a critical DNA repair protein [46]. In human colon cancer cells, coincident with inhibition of HDAC3 activity, SFN induced DNA damage and cell apoptosis via upregulation of CtIP acetylation and its subsequent degradation [43]. However, evidence for a direct interaction between HDACs and CtIP is lacking.
Inhibitory effects of SFN on HDACs were also observed in vivo [44, 47, 48]. In these studies, ingestion of SFN reduced the volume of prostate, breast, and lung tumors, accompanied by enhanced global histone acetylation and reduced HDAC activity [44, 47]. In human subjects, consumption of SFN-rich broccoli sprouts induced acetylation of histone H3 and H4, which was mainly attributed to inhibition of HDAC activity in circulating peripheral blood mononuclear cells (PBMCs) [48, 49].
The discrepancy in the concentration-effect relationship from in vitro to in vivo is a significant problem in the studies of natural phytochemicals, like SFN. To achieve the effective inhibition of HDAC activity, it was reported that the concentration of SFN used in vitro experiments was from 3 to 15 μM, a single oral dose of 10 μmol in mice, and 68 g broccoli sprouts in human [50]. An important factor determining the discrepancies is the conversion of glucosinolate to SFN by myrosinase-mediated hydrolysis. Isothiocyanate SFN was stored in broccoli sprouts as the precursor parent compound of glucosinolate, which was hydrolyzed to isothiocyanate by myrosinases released from the plants when raw vegetables were chopped, cut, or chewed, or by other myrosinase enzymes present in our gut [51, 52]. Therefore, mammalian tissues and cells in vitro cannot convert glucosinolate to SFN due to loss of endogenous myrosinase activity. However, glucosinolate is indeed converted to SFN by the myrosinases existing in gut microbial flora of animals and humans in vivo. Moreover, the bioavailability of SFN was about six times more than glucosinolates, which indicated the minimal conversion [53]. As an example, SFN at a concentration around 10 μM effectively inhibited HDAC activity in mouse colonic mucosa in vivo, and humans would consume about 106 g/day of broccoli sprouts to achieve similar plasma levels [54]. Altogether, the content of myrosinase in plants and variability of gut microbial flora are key factors of determining the discrepancies in the bioavailability of SFN from in vitro to in vivo.
In addition to acetylation modification, histones also undergo phosphorylation. A previous study demonstrated that increased phosphorylation of histone H1 is positively correlated with bladder cancer carcinogenesis and progression [55]. SFN reduced histone H1 phosphorylation by enhancing protein phosphatase 1β and 2A (PP1β and PP2A).
Collectively, these findings suggest that SFN may exert its anticancer effects through inhibition of HDACs and enhancement of phosphatases.
3.2. DNA Methylation
DNA methylation is an important epigenetic modification, mainly occurring within CPG islands in gene promoter regions. The establishment and maintenance of DNA methylation patterns requires the function of several DNA methyltransferases (DNMTs), which catalyze DNA methylation reactions, including DNMT1, which maintains methylation, and DNMT3a and DNMT3b, which catalyze de novo methylation [56]. Aberrant DNA methylation, such as promoter hypermethylation or hypomethylation, can lead to inactivation or activation of specific genes involved in tumorigenesis or progression, respectively. Aberrant DNA methylation is a reversible process and is often caused by the overexpression of DNMTs [57]. Therefore, DNMTs have become attractive targets for cancer chemoprevention.
Growing evidence indicates that SFN is a potential modulator of DNA methylation in cancer development and progression [22, 23, 40, 41]. As previously described, the expression levels of DNMTs, primarily DNMT1, 3a, and 3b, are decreased in SFN-treated breast, prostate, and cervical cancer cells [58–60]. Furthermore, the inhibitory effects of SFN on DNMTs can restore the expression and activation of silenced or repressed genes in cancer cells via promoter demethylation. Silencing of the cell cycle regulatory gene cyclin D2 by promoter hypermethylation was reported to be positively correlated with prostate cancer progression, and restoration of cyclin D2 expression induced cancer cell death [59]. An experiment with LNCap prostate cancer cells showed that SFN treatment reduced the expression of DNMT1 and 3b, resulting in a decrease in the global DNA methylation profile and cyclin D2 promoter methylation [59]. In addition, exposure of breast cancer cells to 10 μM SFN reduced DNMT1 expression, which was accompanied by elevated expression of P21, the tumor suppressor phosphatase and tensin homologue (PTEN), and retinoic acid receptor beta 2 (RARbeta2) due to promoter demethylation [61]. Importantly, combining anticancer drugs, such as clofarabine (ClF) and withaferin A (WA), with SFN enhanced their anticancer effects, as reflected in the stronger growth arrest and apoptosis of cancer cells [61, 62]. Another study, using the same breast cancer cells, aimed at assessing the effects of SFN on human telomerase reverse transcriptase (hTERT), the catalytic regulatory subunit of telomerase. The results showed that SFN, at a dosage of 10 μM, induced inhibition of DNMT1 and DNMT3a causing site-specific CpG demethylation in the first exon of the hTERT gene, thereby facilitating binding of the CTCF transcription repressor and hTERT repression [58]. This downregulation of hTERT expression promoted apoptosis in the breast cancer cells [58].
In the human cervical cancer cell line HeLa, SFN concentration significantly upregulated the expression of the tumor suppressor genes RARβ, CDH1, DAPK1, and GSTP1, as well as the expression of the proapoptosis protein Bax through inhibition of DNMT3b activity in a time-dependent manner, leading to the induction of cell cycle arrest and apoptosis [60].
The above results show that SFN functions as a cancer chemopreventive agent by modulating the expression of tumor-related genes through DNA methylation modification. Further studies in animal models of cancer are required to confirm and enhance the understanding of SFN on DNA methylation.
3.3. Regulation of Noncoding RNAs
A noncoding RNA (ncRNA) is an RNA molecule that functions without being translated into a protein. Abundant and functionally important ncRNAs include transfer RNAs and ribosomal RNAs, as well as small RNAs, such as microRNAs (miRNAs) and long ncRNAs (lncRNAs).
miRNAs are approximately 22 nucleotides in length and bind to complementary sites in the 3′-UTR of target messenger RNAs (mRNAs), leading to posttranscriptional repression or degradation [63]. miRNAs are negative regulators of target genes, and several miRNAs have been shown to be involved in the regulation of tumor cell proliferation, apoptosis, invasion, and metastasis. In addition, miRNA dysregulation has been shown to play an essential role in the development and progression of various cancers [64].
Several miRNAs, such as miR200c, miR-616-5p, and microRNA-21 (miR-21), have been shown to be targets of SFN in some human cancers [6, 65–67]. It is noteworthy that cancer stem cells (CSCs) are considered to be the driving force of carcinogenesis in oral squamous cell carcinoma (OSCC). In one study, miR200c targeting of Bmi1 was shown to be involved in the regulation of cancer stemness in OSCC-CSCs, including their self-renewal and tumor initiation properties [65]. SFN treatment (20 μM) impaired cancer stemness by inducing the tumor-suppressive miRNA miR200c, which subsequently inhibited the migration, invasion, and clonogenicity of OSCC-CSCs in mouse models [65]. In addition to its effect on CSCs, SFN enhanced temozolomide-induced glioblastoma cell apoptosis [67] and reduced the viability and induced apoptosis of colon cancer cells [66] through downregulation of miR-21. In addition, SFN may specifically target miR616-5p to suppress the metastasis of non-small-cell lung cancer (NSCLC) cells [6]. Another study showed that miR-616-5p levels were increased in tissue samples of late-stage NSCLC, as well as three human NSCLC cell lines (H1299, 95C, and 95D) [6]. SFN downregulated miR-616-5p levels, which was accompanied by inactivation of the GSK3β/β-catenin pathway and inhibition of EMT to prevent NSCLC recurrence and metastasis [6].
lncRNAs are transcripts longer than 200 nucleotides that function as crucial regulators of gene transcription through their association with chromatin remodeling complexes [68]. Their aberrant expression endows cells with tumor initiation, high proliferation, and metastasis abilities [68]. Studies on the effects of SFN on lncRNAs are limited. However, a recent study showed that the lncRNA LINC01116 was upregulated in the human prostate cancer cell lines LNCaP and PC-3, and this upregulation was decreased by SFN (15 μM), which was accompanied by inhibition of proliferation [69].
These studies suggest SFN as a promising chemopreventive agent and demonstrate that its anticancer effects partially involve epigenetic mechanisms, which are summarized in Table 1.
Table 1.
The epigenetic regulation of sulforaphane (SFN) in cancer.
| Epigenetic mechanisms | Cancer types | Epigenetic functions | Target genes/proteins | Anticancer effects | References |
|---|---|---|---|---|---|
| Histone acetylation | Prostate cancer cells (LnCaP and PC-3) and PC-3 cell xenografts | Inhibition of class I and II HDACs | Reactivation of p21 and Bax | Cell cycle arrest and apoptosis↑ | [42, 45, 49] |
| Colon cancer cells (HCT116) | Inhibition of HDAC3 | CtIP: a critical DNA repair protein | DNA damage and apoptosis↑ | [43] | |
| Acetylation of CtIP and its degradation | |||||
| Lung cancer cells (A549 and H1299) and A549 cell xenografts | Inhibition of HDAC activity | Reactivation of p21 and Bax | Cell growth↓ | [44] | |
| Apoptosis↑ | |||||
|
| |||||
| Histone phosphorylation | Bladder cancer cells (RT4, J82, and UMUC3) and UMUC3 cell xenografts | Inhibition of histone H1 phosphorylation | Increased PP1β and PP2A phosphatase | Carcinogenesis and progression↓ | [55] |
|
| |||||
| DNA methylation | Prostate cancer cells (LNCap) | Decreased expression of DNMT1 and 3b | Restoration of cyclin D2 | Cancer cell death↑ | [59] |
| Human breast cancer cells (MCF-7 and MDA-MB-231) | Inhibition of DNMT1 expression | Restoration of P21, PTEN, and RARbeta2 | Cell growth arrest and apoptosis↑ | [61] | |
| Human breast cancer cells (MCF-7 and MDA-MB-231) | Decrease in DNMT1 and 3a expression and activity | Downregulation of hTERT expression | Apoptosis↑ | [58] | |
| Cervical cancer cells (HeLa) | Inhibition of DNMT3b activity | Upregulation of RARβ, CDH1, DAPK1 and Bax | Cell cycle arrest and apoptosis↑ | [60] | |
|
| |||||
| Noncoding RNA regulation | Oral squamous carcinoma cells (SAS and GNM); cancer stem cell xenografts (SAS and GNM) | Induction of miR-200c | Suppression of Bmi1 | Cell migration, invasiveness, and growth↓ | [65] |
| 95D and H1299 cells and in vivo xenografts | Downregulation of miR-616-5p | Inactivation of the GSK3β/β-catenin pathway | EMT and metastasis↓ | [6] | |
| Human glioma cell lines (H4, SNB19, LN229, and U251) and colorectal cancer cells | Downregulation of miR 21 | Inhibition of the Wnt/β-catenin pathway | Apoptosis↑ | [66, 67] | |
| Cell viability↓ | |||||
| Prostate cancer cells (LNCaP and PC-3) | Decreased expression of the lncRNA LINC01116 | Cell proliferation↓ | [69] | ||
|
| |||||
| CPG demethylation and histone acetylation at the Nrf2 promoter | Mouse skin epidermal JB6 (JB6 P+) cells and prostate cancer (TRAMP C1) cells | Inhibition of DNMT1, 3a, and 3b and HDAC1–5 and HDAC7 | The reactivation of Nrf2 | Cell transformation and development↓ | [20, 21] |
4. The Keap1/Nrf2 Antioxidant Pathway and Its Epigenetic Modification
4.1. The Keap1/Nrf2 Antioxidant Pathway and Cancer
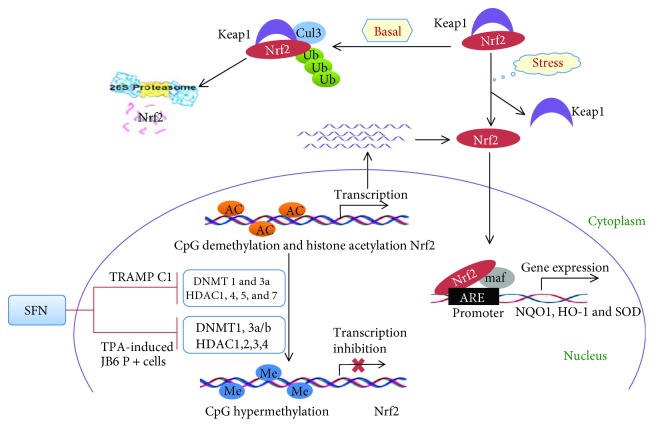
Carcinogenesis is often associated with long-term exposure to oxidative stress resulting from the overproduction of high reactive oxygen species (ROS) and/or the impairment of the antioxidation system [70]. Nuclear factor erythroid-2- (NF-E2-) related factor 2 (Nrf2) is best known as a key transcription factor regulating the expression of antioxidant and detoxification genes, such as heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase-1 (NQO1), and glutathione S-transferases (GST) [70]. A proposed model of Keap1-Nrf2 interaction is as follows: Under basal conditions, Nrf2 binds to its repressor Keap1 in the cytoplasm and subsequently undergoes proteasomal degradation via ubiquitination. Under oxidative stress, Nrf2 dissociates from Keap1 and then translocates to the nucleus. Intranuclear Nrf2 binds with the small protein Maf to antioxidant response element (ARE) sequences on target gene promoters, which drives the transcription of cytoprotective genes and provides protection against oxidative stress [71] (Figure 1).
Figure 1.
The Keap1/Nrf2 pathway and its epigenetic modification by SFN. Under basal conditions, Keap1 binds to Nrf2 in the cytoplasm, which promotes its proteasomal degradation via ubiquitination. Under oxidative stress, Nrf2 dissociates from Keap1 and then translocates into the nucleus and binds with the small protein Maf at ARE sequences in the promoter regions of target genes. This drives the expression of several cytoprotective genes, such as HO-1, NQO1, and SOD. In TRAMP C1 prostate cancer cells, SFN can inhibit the expression and activity of enzymes involved in epigenetic regulation, including DNMT1 and 3a, as well as HDAC1, 4, 5, and 7. Significant inhibition of DNMT1, DNMT3a/b, and HDAC1, 2, 3, and 4 has also been observed in TPA-induced mouse skin JB6 P+ cells treated by SFN, which reduces the CpG methylation and elevates histone acetylation of the Nrf2 promoter. Ultimately, epigenetic regulation by SFN promotes the transcription of Nrf2 and its subsequent nuclear translocation and activation.
Nrf2 has been traditionally regarded as a tumor suppressor. Low expression of cytoprotective genes, due to inactivation of Nrf2, has been shown to be related to tumor formation and progression. For example, Nrf2-deficient mice showed dramatically increased susceptibility to carcinogens and elevated lung metastasis, which was accompanied by increased ROS levels [72, 73]. The incidence, multiplicity, and size of colorectal tumors were increased in Nrf2-knockout mice [74]. Moreover, there are multiple studies describing the beneficial effects of Nrf2 activation in cancer chemoprevention [75]. These results suggest that activation of Nrf2 may be an important strategy in cancer prevention.
However, recent studies demonstrate that Nrf2 protects the survival of normal as well as cancer cells. The constitutive activation of Nrf2 creates an advantageous environment that favors the survival of malignant cells by preventing them from oxidative stress, chemotherapeutic drugs, and radiotherapy. This phenomenon has been called the “dark side of Nrf2.” The elevated Nrf2 expression induced by Keap1 mutation has been found in lung, gallbladder, liver [76], and prostate cancers [77], as well as malignant melanoma [78]. Moreover, the overexpression of Nrf2 contributed to clinical drug resistance and tumor growth, which was associated with poor prognosis of patients with cancer [77–80]. Additionally, inhibition of Nrf2 sensitized DU-145 prostate cancer cells to chemotherapeutic drugs, such as cisplatin and etoposide, and enhanced radiotherapy responsiveness [77]. These findings suggest that Nrf2 has a dual role in cancer development and therapy. Based on a body of studies, it seems that transient activation of Nrf2 in normal cells (where the Nrf2-Keap1 axis is intact) is protective; however, constitutive activation of Nrf2 (mutation of Keap1) promotes the survival and progression of malignant cells.
4.2. Epigenetic Modification of Nrf2 in Cancer Prevention
It has been shown that SFN induces Nrf2 to upregulate expression of its target genes, including antioxidant genes and phase II detoxification enzymes, to prevent carcinogenesis [14]. SFN not only modifies Keap1 cysteine residues, resulting in Nrf2 activation, but also restores Nrf2 expression through epigenetic mechanisms, including inhibition of DNMTs and HDACs [17, 20, 21]. In TRAMP C1 prostate cancer cells, it was reported that Nrf2 and its target gene NQO1 were significantly decreased, resulting in extensive oxidative stress and DNA damage. SFN treatment upregulated the expression of Nrf2 and NQO1 by inhibiting DNMTs (DNMT1 and DNMT3a) and HDACs (HDAC1, HDAC4, HDAC5, and HDAC7) [20], which reduced the methylation level of CpGs and increased histone 3 acetylation at the Nrf2 promoter. It was also observed that reactivation of Nrf2 and its target genes by SFN, via downregulation of CpG methylation at Nrf2, significantly inhibited TPA-induced JB6 P+ cellular transformation, with concomitant attenuation of the expression of DNMTs (DNMT1, DNMT3a, and DNMT3b) and HDACs (HDAC1, HDAC2, HDAC3, and HDAC4) [21] (Figure 1 and Table 1).
In addition, other natural phytochemicals of Nrf2 agonist, such as curcumin, 3,3′-diindolylmethane (DIM), Z-Ligustilide, apigenin, or Tanshinone IIA, have displayed the anticancer effect through epigenetic modification of Nrf2 [81–85]. For instance, curcumin, DIM, or Z-Ligustilide demethylated the CpGs in the Nrf2 promoter and reactivated Nrf2 in the prostate of TRAMP mice and TRAMP C1 cells by the inhibition of DNMTs. Moreover, the hypermethylation of the Nrf2 promoter could be reduced by apigenin or Tanshinone IIA in mouse skin epidermal JB6 P+ cells. Additionally, human prostate cancer cells treated with 5-aza/TSA (DNMT/HDAC inhibitor) restored the expression of Nrf2 [86]. These findings suggest that epigenetic restoration of Nrf2 may be an important strategy in cancer prevention.
5. The Clinical Studies and Future Perspectives
Human clinical studies have supported the chemopreventive effects of SFN on carcinogenesis. Firstly, several clinical trials evaluated the safety and tolerance of SFN at the doses employed. Two clinical phase I studies showed that broccoli sprout extracts containing SFN were well tolerated and caused no significant adverse effects (toxicities) when administered orally by healthy volunteers at a dose of 15 μM for 7 days or women with breast cancer received 200 μmol of on average 50 min prior to the surgery [87, 88]. A recent phase II clinical study on men with recurrent prostate cancer also confirms the safety of SFN [89]. In addition, another study assessed the clinical effectiveness of SFN in the patients with advanced pancreatic ductal adenocarcinoma (PDA). The data indicated that 90 mg/day of active SFN effectively inhibited tumor growth and increased the sensitivity of cancer cells to chemotherapeutics [90]. In human subjects, consumption of SFN-rich broccoli sprouts significantly inhibited HDAC activity in PBMCs [48, 49]. These clinical studies further suggest SFN as a promising anticancer agent and its potential epigenetic mechanisms.
Based on the above-mentioned studies, it is clear that the dietary compound SFN, which has little or no adverse side effects, exerts anticancer activities through multiple mechanisms, including epigenetic regulation. Thus, daily consumption of cruciferous vegetables rich in SFN is not only a healthy diet choice but also an effective chemopreventive strategy. SFN, as an inducer of Nrf2, shows the capacity to reactivate Nrf2 expression and its target cytoprotective genes to prevent carcinogenesis through epigenetic mechanisms, namely, CPG demethylation and histone acetylation of the Nrf2 promoter, via inhibition of DNMTs and HDACs. These studies have prompted us to propose epigenetic restoration of Nrf2 by SFN as an important strategy against oxidative damage-related diseases, including cancer, which may provide new research directions and preventive approaches for oxidative damage-related diseases.
Acknowledgments
This work was supported by projects in part from the National Science Foundation of China (81570344 to Ying Xin), the Norman Bethune Program of Jilin University (2015225 to Ying Xin and 2015203 to Xin Jiang), the Jilin Provincial Science and Technology Foundations (20180414039GH to Ying Xin and 20150204093SF to Xin Jiang), the Education Department of Jilin Province Foundations (2016-448 to Xin Jiang), and the Health and Family Planning Commission of Jilin Province Foundations (2016Q034 to Ying Xin and 2015Q010 to Xin Jiang).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Xuling Su and Xin Jiang contributed equally to this work.
References
- 1.Abdull Razis A. F., Noor N. M. Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pacific Journal of Cancer Prevention. 2013;14(3):1565–1570. doi: 10.7314/APJCP.2013.14.3.1565. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y. M., Tsai C. C., Hsu Y. C. Sulforaphane, a dietary isothiocyanate, induces G2/M arrest in cervical cancer cells through cyclinB1 downregulation and GADD45β/CDC2 association. International Journal of Molecular Sciences. 2016;17(9) doi: 10.3390/ijms17091530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng X., Zhou Y., Tian H., et al. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells. Oncology Reports. 2015;34(3):1565–1572. doi: 10.3892/or.2015.4098. [DOI] [PubMed] [Google Scholar]
- 4.Leone A., Diorio G., Sexton W., et al. Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach. Oncotarget. 2017;8(21):35412–35424. doi: 10.18632/oncotarget.16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juengel E., Maxeiner S., Rutz J., et al. Sulforaphane inhibits proliferation and invasive activity of everolimus-resistant kidney cancer cells in vitro. Oncotarget. 2016;7(51):85208–85219. doi: 10.18632/oncotarget.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D. X., Zou Y. J., Zhuang X. B., et al. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3β/β-catenin signaling pathways. Acta Pharmacologica Sinica. 2017;38(2):241–251. doi: 10.1038/aps.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke J. D., Dashwood R. H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Letters. 2008;269(2):291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Li Y., Dai Y., et al. Sulforaphane improves chemotherapy efficacy by targeting cancer stem cell-like properties via the miR-124/IL-6R/STAT3 axis. Scientific Reports. 2016;6(1, article 36796) doi: 10.1038/srep36796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuryn A., Litwiniec A., Safiejko-Mroczka B., et al. The effect of sulforaphane on the cell cycle, apoptosis and expression of cyclin D1 and p21 in the A549 non-small cell lung cancer cell line. International Journal of Oncology. 2016;48(6):2521–2533. doi: 10.3892/ijo.2016.3444. [DOI] [PubMed] [Google Scholar]
- 10.Kim S. H., Park H. J., Moon D. O. Sulforaphane sensitizes human breast cancer cells to paclitaxel-induced apoptosis by downregulating the NF-κB signaling pathway. Oncology Letters. 2017;13(6):4427–4432. doi: 10.3892/ol.2017.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastorek M., Simko V., Takacova M., et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. International Journal of Oncology. 2015;47(1):51–60. doi: 10.3892/ijo.2015.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D. H., Sung B., Kang Y. J., et al. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells. International Journal of Oncology. 2015;47(6):2226–2232. doi: 10.3892/ijo.2015.3200. [DOI] [PubMed] [Google Scholar]
- 13.Narendhirakannan R. T., Hannah M. A. C. Oxidative stress and skin cancer: an overview. Indian Journal of Clinical Biochemistry. 2013;28(2):110–115. doi: 10.1007/s12291-012-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kensler T. W., Egner P. A., Agyeman A. S., et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Topics in Current Chemistry. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson G. S., Li J., Beaver L. M., et al. A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells. Molecular Nutrition & Food Research. 2017;61(4) doi: 10.1002/mnfr.201600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Yao X. D., Li W., et al. Nrf2 sensitizes prostate cancer cells to radiation via decreasing basal ROS levels. BioFactors. 2015;41(1):52–57. doi: 10.1002/biof.1200. [DOI] [PubMed] [Google Scholar]
- 17.Hu C., Eggler A. L., Mesecar A. D., van Breemen R. B. Modification of keap1 cysteine residues by sulforaphane. Chemical Research in Toxicology. 2011;24(4):515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Medicinal Research Reviews. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin S., Hou D. X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Molecular Nutrition & Food Research. 2016;60(8):1731–1755. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Su Z.-Y., Khor T. O., Shu L., Kong A.-N. T. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochemical Pharmacology. 2013;85(9):1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Z.-Y., Zhang C., Lee J. H., et al. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prevention Research. 2014;7(3):319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- 22.Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(4):405–410. doi: 10.1097/MCO.0b013e328362014e. [DOI] [PubMed] [Google Scholar]
- 23.Meeran S. M., Ahmed A., Tollefsbol T. O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clinical Epigenetics. 2010;1(3-4):101–116. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma M., Seminara D., Arena F. J., John C., Iwamoto K., Hartmuller V. Genetic and epigenetic biomarkers in cancer: improving diagnosis, risk assessment, and disease stratification. Molecular Diagnosis & Therapy. 2006;10(1):1–15. doi: 10.1007/BF03256438. [DOI] [PubMed] [Google Scholar]
- 25.You J. S., Jones P. A. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22(1):9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sopik V., Phelan C., Cybulski C., Narod S. A. BRCA1 and BRCA2 mutations and the risk for colorectal cancer. Clinical Genetics. 2015;87(5):411–418. doi: 10.1111/cge.12497. [DOI] [PubMed] [Google Scholar]
- 27.Day F. L., Jorissen R. N., Lipton L., et al. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clinical Cancer Research. 2013;19(12):3285–3296. doi: 10.1158/1078-0432.CCR-12-3614. [DOI] [PubMed] [Google Scholar]
- 28.Li X. L., Zhou J., Chen Z. R., Chng W. J. p53 mutations in colorectal cancer - molecular pathogenesis and pharmacological reactivation. World Journal of Gastroenterology. 2015;21(1):84–93. doi: 10.3748/wjg.v21.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoniou A., Pharoah P. D. P., Narod S., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. American Journal of Human Genetics. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva F. C., Lisboa B. C. G., Figueiredo M. C. P., et al. Hereditary breast and ovarian cancer: assessment of point mutations and copy number variations in Brazilian patients. BMC Medical Genetics. 2014;15(1):p. 55. doi: 10.1186/1471-2350-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozen C., Yildiz G., Dagcan A. T., et al. Genetics and epigenetics of liver cancer. New Biotechnology. 2013;30(4):381–384. doi: 10.1016/j.nbt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Verma M. Cancer control and prevention: nutrition and epigenetics. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(4):376–384. doi: 10.1097/MCO.0b013e328361dc70. [DOI] [PubMed] [Google Scholar]
- 33.Reichert N., Choukrallah M. A., Matthias P. Multiple roles of class I HDACs in proliferation, differentiation, and development. Cellular and Molecular Life Sciences. 2012;69(13):2173–2187. doi: 10.1007/s00018-012-0921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Shang Y. P., Chen H. Y., Li J. Histone deacetylases function as novel potential therapeutic targets for cancer. Hepatology Research. 2017;47(2):149–159. doi: 10.1111/hepr.12757. [DOI] [PubMed] [Google Scholar]
- 35.Yu J. J., Wu Y. X., Zhao F. J., Xia S. J. miR-96 promotes cell proliferation and clonogenicity by down-regulating of FOXO1 in prostate cancer cells. Medical Oncology. 2014;31(4):p. 910. doi: 10.1007/s12032-014-0910-y. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z., He B., He J., Mao X. Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer. Prostate. 2013;73(6):596–604. doi: 10.1002/pros.22600. [DOI] [PubMed] [Google Scholar]
- 37.Yi T. Z., Li J., Han X., et al. DNMT inhibitors and HDAC inhibitors regulate E-cadherin and Bcl-2 expression in endometrial carcinoma in vitro and in vivo. Chemotherapy. 2012;58(1):19–29. doi: 10.1159/000333077. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q., Zheng J. M., Chen J. K., et al. Histone deacetylase 5 promotes the proliferation of glioma cells by upregulation of Notch 1. Molecular Medicine Reports. 2014;10(4):2045–2050. doi: 10.3892/mmr.2014.2395. [DOI] [PubMed] [Google Scholar]
- 39.Feng G. W., Dong L. D., Shang W. J., et al. HDAC5 promotes cell proliferation in human hepatocellular carcinoma by up-regulating Six 1 expression. European Review for Medical and Pharmacological Sciences. 2014;18(6):811–816. [PubMed] [Google Scholar]
- 40.Tortorella S. M., Royce S. G., Licciardi P. V., Karagiannis T. C. Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition. Antioxidants & Redox Signaling. 2015;22(16):1382–1424. doi: 10.1089/ars.2014.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman-Szymczyk A., Majewski G., Lubecka-Pietruszewska K., Fabianowska-Majewska K. The role of sulforaphane in epigenetic mechanisms, including interdependence between histone modification and DNA methylation. International Journal of Molecular Sciences. 2015;16(12):29732–29743. doi: 10.3390/ijms161226195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke J. D., Hsu A., Yu Z., Dashwood R. H., Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Molecular Nutrition & Food Research. 2011;55(7):999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajendran P., Kidane A. I., Yu T. W., et al. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics. 2013;8(6):612–623. doi: 10.4161/epi.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang L. L., Zhou S. J., Zhang X. M., Chen H. Q., Liu W. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomedicine & Pharmacotherapy. 2016;78:74–80. doi: 10.1016/j.biopha.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Myzak M. C., Hardin K., Wang R., Dashwood R. H., Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27(4):811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert T., Vanoli F., Chiolo I., et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471(7336):74–79. doi: 10.1038/nature09803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atwell L. L., Beaver L. M., Shannon J., Williams D. E., Dashwood R. H., Ho E. Epigenetic regulation by sulforaphane: opportunities for breast and prostate cancer chemoprevention. Current Pharmacology Reports. 2015;1(2):102–111. doi: 10.1007/s40495-014-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke J. D., Riedl K., Bella D., Schwartz S. J., Stevens J. F., Ho E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. Journal of Agricultural and Food Chemistry. 2011;59(20):10955–10963. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myzak M. C., Tong P., Dashwood W. M., Dashwood R. H., Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Experimental Biology and Medicine. 2007;232(2):227–234. [PMC free article] [PubMed] [Google Scholar]
- 50.Dashwood R., Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Seminars in Cancer Biology. 2007;17(5):363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bricker G. V., Riedl K. M., Ralston R. A., Tober K. L., Oberyszyn T. M., Schwartz S. J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Molecular Nutrition & Food Research. 2014;58(10):1991–2000. doi: 10.1002/mnfr.201400104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saha S., Hollands W., Teucher B., et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Molecular Nutrition & Food Research. 2012;56(12):1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro T. A., Fahey J. W., Wade K. L., Stephenson K. K., Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:501–508. [PubMed] [Google Scholar]
- 54.Myzak M. C., Dashwood W. M., Orner G. A., Ho E., Dashwood R. H. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apcmin mice. The FASEB Journal. 2006;20(3):506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbaoui B., Telu K. H., Lucas C. R., et al. The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer. Journal of Proteomics. 2017;156:94–103. doi: 10.1016/j.jprot.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denis H., Ndlovu '. M. N., Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Reports. 2011;12(7):647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson M. A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Meeran S. M., Patel S. N., Tollefsbol T. O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5(7, article e11457) doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu A., Wong C. P., Yu Z., Williams D. E., Dashwood R. H., Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clinical Epigenetics. 2011;3(1):p. 3. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali Khan M., Kedhari Sundaram M., Hamza A., et al. Sulforaphane reverses the expression of various tumor suppressor genes by targeting DNMT3B and HDAC1 in human cervical cancer cells. Evidence-based Complementary and Alternative Medicine. 2015;2015:12. doi: 10.1155/2015/412149.412149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lubecka-Pietruszewska K., Kaufman-Szymczyk A., Stefanska B., Cebula-Obrzut B., Smolewski P., Fabianowska-Majewska K. Sulforaphane alone and in combination with clofarabine epigenetically regulates the expression of DNA methylation-silenced tumour suppressor genes in human breast cancer cells. Journal of Nutrigenetics and Nutrigenomics. 2015;8(2):91–101. doi: 10.1159/000439111. [DOI] [PubMed] [Google Scholar]
- 62.Royston K., Udayakumar N., Lewis K., Tollefsbol T. A novel combination of withaferin A and sulforaphane inhibits epigenetic machinery, cellular viability and induces apoptosis of breast cancer cells. International Journal of Molecular Sciences. 2017;18(5) doi: 10.3390/ijms18051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartel D. P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Leva G., Garofalo M., Croce C. M. MicroRNAs in cancer. Annual Review of Pathology. 2014;9(1):287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C. M., Peng C. Y., Liao Y. W., et al. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. Journal of the Formosan Medical Association. 2017;116(1):41–48. doi: 10.1016/j.jfma.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Martin S. L., Kala R., Tollefsbol T. O. Mechanisms for inhibition of colon cancer cells by sulforaphane through epigenetic modulation of microRNA-21 and human telomerase reverse transcriptase (hTERT) down-regulation. Current Cancer Drug Targets. 2017;18(1) doi: 10.2174/1568009617666170206104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lan F., Pan Q., Yu H., Yue X. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/β-catenin signaling in glioblastoma. Journal of Neurochemistry. 2015;134(5):811–818. doi: 10.1111/jnc.13174. [DOI] [PubMed] [Google Scholar]
- 68.Yang G., Lu X., Yuan L. LncRNA: a link between RNA and cancer. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 69.Beaver L. M., Kuintzle R., Buchanan A., et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. The Journal of Nutritional Biochemistry. 2017;42:72–83. doi: 10.1016/j.jnutbio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kansanen E., Kuosmanen S. M., Leinonen H., Levonen A. L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biology. 2013;1(1):45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki T., Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radical Biology & Medicine. 2015;88(Part B):93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Ramos-Gomez M., Kwak M. K., Dolan P. M., et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Satoh H., Moriguchi T., Taguchi K., et al. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31(10):1833–1843. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- 74.Khor T. O., Huang M. T., Prawan A., et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prevention Research. 2008;1(3):187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayes J. D., McMahon M., Chowdhry S., Dinkova-Kostova A. T. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxidants & Redox Signaling. 2010;13(11):1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 76.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes to Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang P., Singh A., Yegnasubramanian S., et al. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Molecular Cancer Therapeutics. 2010;9(2):336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miura S., Shibazaki M., Kasai S., et al. A somatic mutation of the KEAP1 gene in malignant melanoma is involved in aberrant NRF2 activation and an increase in intrinsic drug resistance. The Journal of Investigative Dermatology. 2014;134(2):553–556. doi: 10.1038/jid.2013.343. [DOI] [PubMed] [Google Scholar]
- 79.Jiang T., Chen N., Zhao F., et al. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Research. 2010;70(13):5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sporn M. B., Liby K. T. NRF2 and cancer: the good, the bad and the importance of context. Nature Reviews Cancer. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khor T. O., Huang Y., Wu T. Y., Shu L., Lee J., Kong A. N. T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochemical Pharmacology. 2011;82(9):1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 82.Wu T.-Y., Khor T. O., Su Z.-Y., et al. Epigenetic modifications of Nrf2 by 3,3′-diindolylmethane in vitro in TRAMP C1 cell line and in vivo TRAMP prostate tumors. The AAPS Journal. 2013;15(3):864–874. doi: 10.1208/s12248-013-9493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su Z.-Y., Khor T. O., Shu L., et al. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chemical Research in Toxicology. 2013;26(3):477–485. doi: 10.1021/tx300524p. [DOI] [PubMed] [Google Scholar]
- 84.Paredes-Gonzalez X., Fuentes F., Su Z.-Y., Kong A.-N. T. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. The AAPS Journal. 2014;16(4):727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Zhang C., Guo Y., et al. Blocking of JB6 cell transformation by tanshinone IIA: epigenetic reactivation of Nrf2 antioxidative stress pathway. The AAPS Journal. 2014;16(6):1214–1225. doi: 10.1208/s12248-014-9666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khor T. O., Fuentes F., Shu L., et al. Epigenetic DNA methylation of antioxidative stress regulator NRF2 in human prostate cancer. Cancer Prevention Research. 2014;7(12):1186–1197. doi: 10.1158/1940-6207.CAPR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapiro T. A., Fahey J. W., Dinkova-Kostova A. T., et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutrition and Cancer. 2006;55(1):53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 88.Cornblatt B. S., Ye L., Dinkova-Kostova A. T., et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28(7):1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 89.Alumkal J. J., Slottke R., Schwartzman J., et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investigational New Drugs. 2015;33(2):480–489. doi: 10.1007/s10637-014-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lozanovski V. J., Houben P., Hinz U., Hackert T., Herr I., Schemmer P. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial) - study protocol for a randomized controlled trial. Trials. 2014;15(1):p. 204. doi: 10.1186/1745-6215-15-204. [DOI] [PMC free article] [PubMed] [Google Scholar]