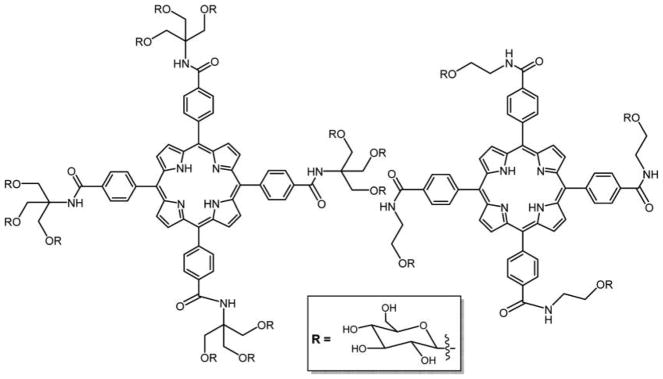
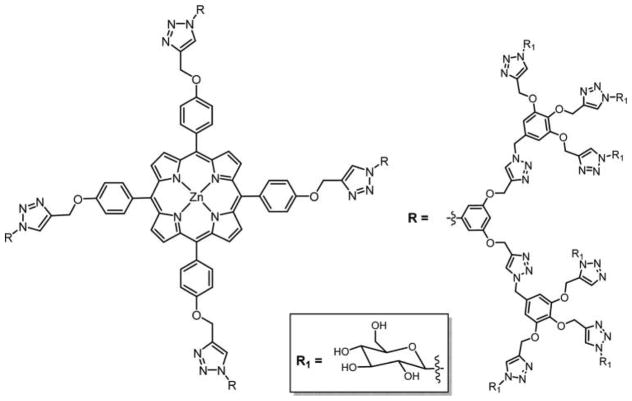
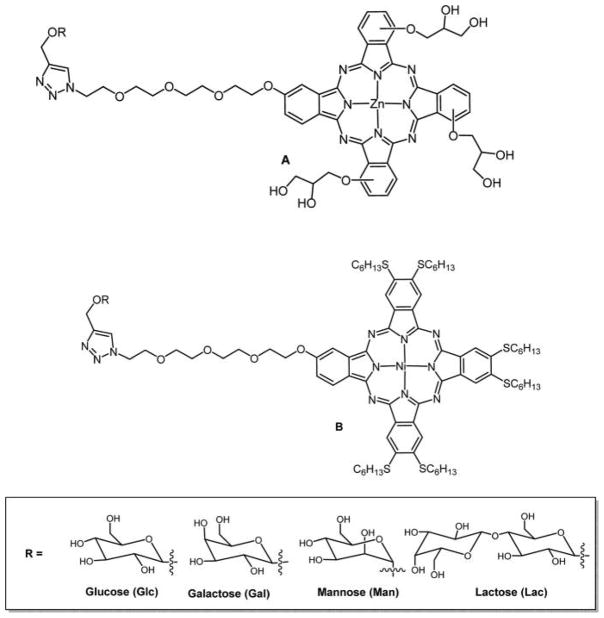
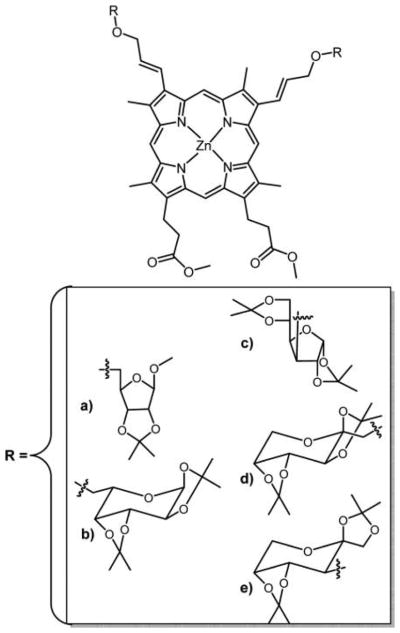
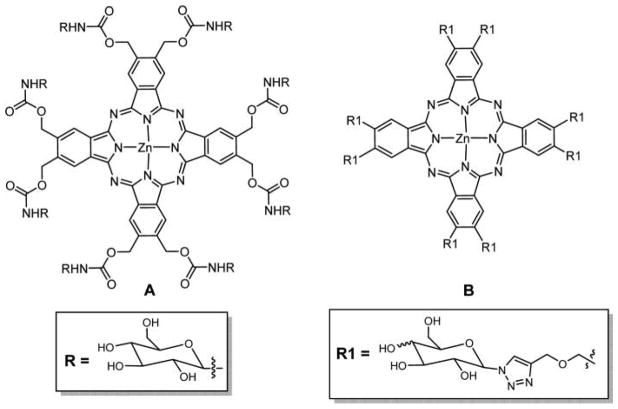
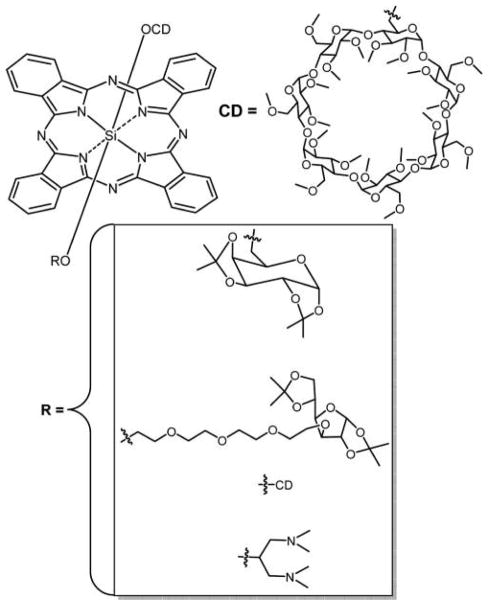
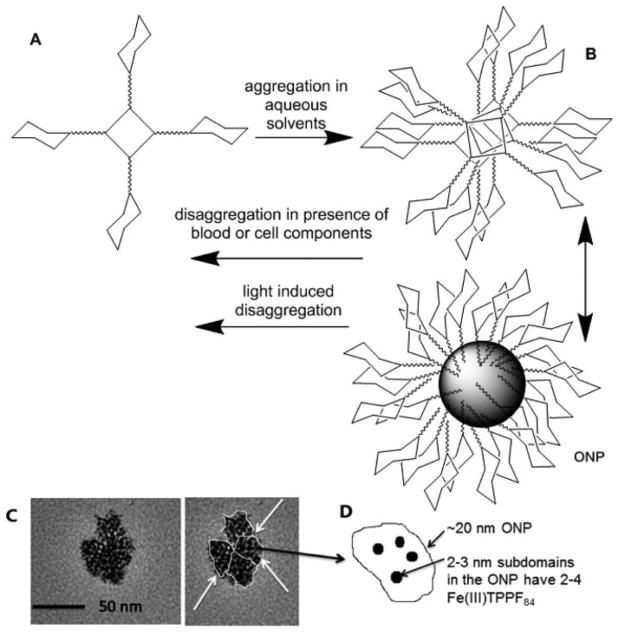
Graphical Abstract
1. INTRODUCTION
As deaths from preventable diseases abate, cancer is becoming one of the leading causes of death in the world. Photodynamic therapy (PDT) is a noninvasive treatment for cancer involving the interactions of light with suitable frequency, a photosensitizer (PS), and molecular oxygen that results in generation of highly reactive oxygen species (ROS) such as singlet oxygen, hydroxyl radicals, the superoxide anion, and hydrogen peroxide.1–3 These ROS react with diffusion limited kinetics with a range of biochemical structures in cells such as lipids, aromatic amino acids, the heterocyclic bases, the backbone of nucleic acids, and flavonoids to induce oxidative damage to the cell, thereby causing cell death via apoptosis or necrosis.4,5 It was proposed that multiple sites of cellular damage may enable more efficient PDT effects.6–9 Selectivity arises from the high reactivity and short lifetime of the ROS, so the cytotoxic response is limited to the irradiated area containing the PS dye.10–12 Because the diffusion distance of singlet oxygen is approximately 2 μm, it reacts at the intracellular level because the diameter of eukaryotic cells is in the range of 10–30 μm. The localization of the PS in the cell influences the mechanism of cell death and is a determinant of biological efficacy.13,14
PDT has been used as a treatment for a variety of cancers including bladder, brain, breast, skin, lung, esophagus, and bronchial cancer.15,16 It also has nononcological applications, such as treatment for age-related macular degeneration, hair growth, acne, and psoriasis.17–23 PDT has several advantages over conventional therapies because this treatment is selective via the selective irradiation of light, PDT is noninvasive for tumors that can be irradiated, it is repeatable, of low cost, and has minimal side effects.24 Because of these properties, applications of PDT can enhance both the quality of life and lengthen survival rate for patients with advanced diseases. Current limitations of PDT are that this treatment may cause skin photosensitivity, and is generally limited to treating tumors that are on or just under the skin because the light that is used to activate the PS cannot pass through more than few millimeters of tissue. Because the irradiation of the whole body with appropriate doses of light is not possible, PDT cannot be used for treating advanced disseminated diseases.25
Porphyrins and phthalocyanines (Pc’s) are the most common and efficient photosensitizers (PSs) used in PDT because of their absorption in the visible range of the electromagnetic spectrum, long-lived triplet excited state, and efficient phototoxicity toward cancer cells.26,27 Porphyrinoid-based PSs have several disadvantages such as poor water solubility, poor light absorption, poor selectivity, and light sensitivity after treatment because they do not efficiently target cancer cells. Current research addresses these pitfalls to make a next-generation PDT agent: (1) synthesis of pure PS in high yields, (2) improve water solubility, (3) fine-tune photophysical properties to efficiently make reactive oxygen species, (4) PSs that selectively target tumors, (5) strong light absorption in the red 650–750 nm for PDT deeper in tissues and for cancer imaging, and (6) minimize dark toxicity and skin sensitivity.
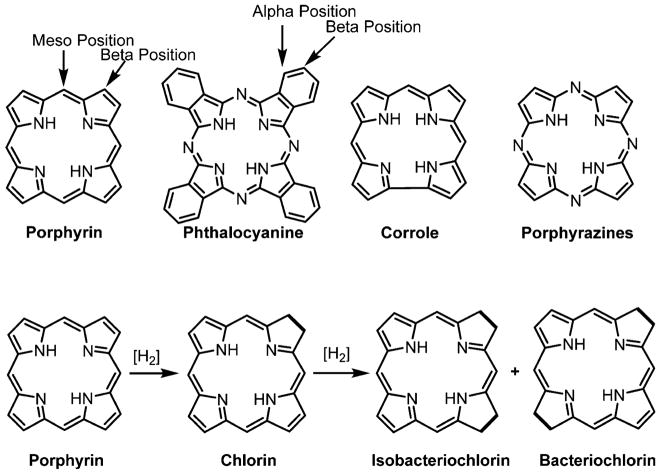
Numerous derivatives can be prepared from porphyrins and phthalocyanines because of the stability of the core macrocycle (Figure 1). In recent years, new methods were developed to synthesize dihydroporphyrins (chlorins) and tetrahydroporphyrins (isobacteriochlorins and bacteriochlorins) (Figure 1).28–30 Chlorins and bacteriochlorins have absorptions bands at longer wavelength than porphyrins (for the lowest energy Q bands, λmax = 650–670 nm for chlorins and λmax = 730–800 nm for bacteriochlorins), yet still have high singlet oxygen quantum yields. Chlorin compounds are in various stages of evaluation for PDT. Pandey and co-workers, Hasan and co-workers, and Senge and co-workers recently reviewed the role of porphyrin derivatives in tumor imaging and PDT,15,31,32 and there are more reviews on PDT.33 Older reviews shed light on the development of the field over the last 20 years.28,34–37 Boron-dipyrromethene, BODIPY (Figure 2), is a related class of fluorescent dyes that are under investigation for PDT38 but is not included in this Review. Recently, the corrole macrocycle, a cognate molecule to the porphyrin, has also been studied as a PS.39,40
Figure 1.
Structures of common porphyrinoid macrocycles (top). Structures of reduced porphyrins, such as chlorin, isobacteriochlorin, and bacteriochlorin (bottom).
Figure 2.
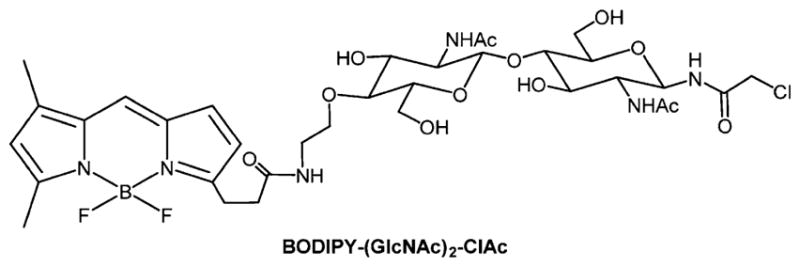
A representative example of a BODIPY conjugated to a simplified chloroacetamidyl chitobiose derivative.41
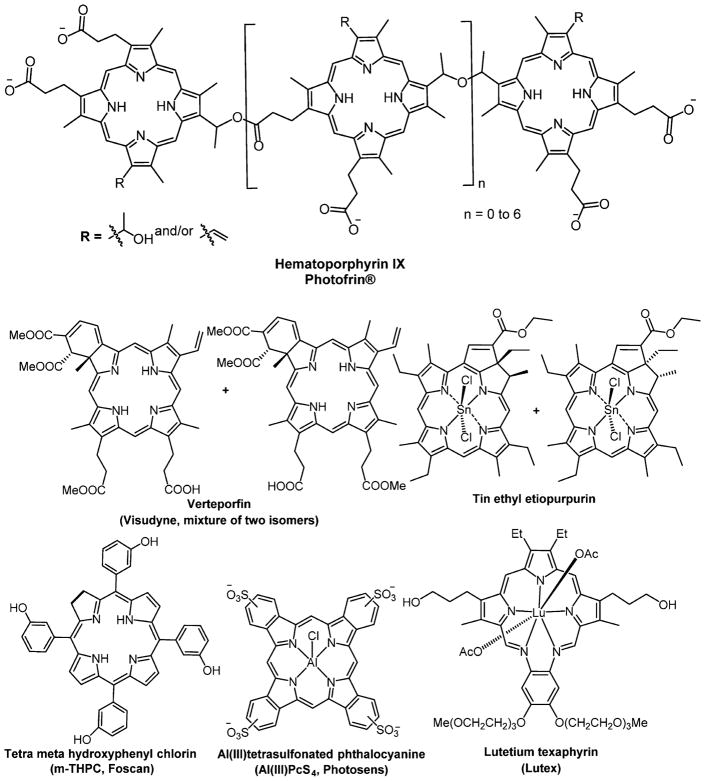
Porfimer sodium (Photofrin), a complex mixture of hematoporphyrin derivatives, was the first drug approved by the United States Food and Drug Administration (FDA) for PDT treatment of various forms of cancers such as lung, bladder, gastric, and cervical cancer (Figure 3). In addition to Photofrin, another PDT compound currently approved for cancer treatment in Europe is Foscan (m-THPC, meta-tetra(hydroxyphenyl)chlorin), which is a mixture of four atropisomers that have somewhat different solubility and aggregation properties in aqueous environments. Foscan was approved in Europe in 2001 for the treatment of head and neck cancers (Table 1).42 Other PSs currently approved for clinical applications or in human trials include (Figure 3): 5-ALA (5-aminolevulinic acid), which is a porphyrin precursor, Metvix (5-aminolevulinic acid methyl ester), which can be used for warts, acne, and fungal infections,8 Lu-Tex (lutetium texaphyrin), and Purlytin (tin ethyl etiopurpurin).43–47 Lutex combines the advantage of water solubility and selective localization, and is capable of being activated by deeply penetrating far-red light.48,49 Light fluences of 50–500 J/cm2 of red light are needed in clinical PDT with Photofrin, whereas chlorins such as m-THPC that have larger extinction coefficients in the red fluences of 10 J/cm2 are typically used.16 White light, white light with red band-pass filters, pulsed and continuous lasers, and photodiodes are all viable light sources. The physics, biophysics, and technology of PDT are well reviewed.24 The light dosimetry depends on the optical cross section of the dye at a given wavelength, the absorption and scattering of light by tissues, the light intensity and duration, and that light can be applied multiple times.
Figure 3.
Structures of photosensitizers in clinical or preclinical trials for PDT.
Table 1.
Photophysical Properties of Some Approved PDT Agents or Those in Clinical Trials
| dye photosensitizer | λmax abs (nm) | ε (M−1 cm−1) | 1O2 quantum yield (ΦΔ)a | year approved by FDA/EMEA/clinical trials |
|---|---|---|---|---|
| hematoporphyrin | 630 | 1170 | 0.25–0.89 | (FDA) 1990s38,50 |
| protoporphyrin IX | 635 | 5000 | 0.22–0.54 | (FDA) 200015,38 |
| m-THPC | 650 | 39 000 | 0.22–0.50 | (EMEA) 200151 |
| verteporfin | 689 | 31 200 | 0.79 | (FDA) 200015 |
| chlorin e6 | 654 | 50 000 | 0.75 | clinical trials (Phase I/II)38 |
| ethyl etiopurpurin Sn(IV) | 666 | 33 900 | 0.70 | clinical trials (Phase I/II)15,52 |
| Al(III) tetrasulfonated phthalocyanine | 675, also two-photon | 100 000 | 0.36 | –53–56 |
ΦΔ is less in aqueous, polar, or protic solvents versus organic solvents or in surfactants.
The benzoporphyrin derivative verteporfin (Visudyne) is approved by the FDA for the treatment of age-related macular degeneration, subfoveal choroidal, neovascularization, and glocoma.57–59 An aluminum phthalocyanine (Al(III)PcS4) drug under clinical trial for age-related macular degeneration is (Photosens),55 and a silicon phthalocyanine (Si(IV)PcS4) is under clinical trial for cutaneous skin cell lesions and sterilization of blood products. The broad success of Photofrin is tempered by several factors including that it is a mixture of porphyrin oligomers, is poorly soluble in water, and has low selectivity toward tumor cells. Additionally, the molecular absorption coefficient of Photofrin is low (ε = 1170 M−1 cm−1) at the clinically used wavelength of 630 nm,19 so this PS cannot be used to treat deep cancers.6 The photophysics of PS are reviewed,24,60 and there are photoactivatable porphyrin designs.61
In terms of molecular design, there are several important considerations that are germane to the photophysics of the porphyrinoids (Scheme 1). When the eight α positions bear hydrocarbon substituents, the phthalocyanine (Pc) macrocycle becomes distorted because of steric crowding. Similarly, when both the β pyrrole and the meso positions of porphyrins have hydrocarbon substituents, the porphyrin becomes distorted. Distortions in the otherwise planar macrocycles realign the molecular orbitals and reduce the HOMO–LUMO energy gap as observed by substantial red shifts and broadening of the bands in the electronic spectra. These distortions also lower the barrier to out-of-plane macrocycle vibrational dynamics, thereby increasing the amount of excited-state energy dissipated by internal conversion and concomitantly reducing fluorescence and inter system crossing to the triplet manifold.62 Thus, the all α-substituted Pc, and the dodeca-substituted porphyrins may be good for photothermal applications but not well suited to PDT and luminescent sensors. Closed-shell metal ions, most notably Zn(II), enhance intersystem crossing to the triplet state by the heavy atom effect, as do Pt(II) and Pd(II). Most open-shell first row transition metals, for example, Ni(II) complexes of porphyrinoids, are minimally or non luminescent because the excited-state energy rapidly goes to low energy d,d states.64–66
Scheme 1. Jablonski Diagram Adapted from Pimenta et al.63a.
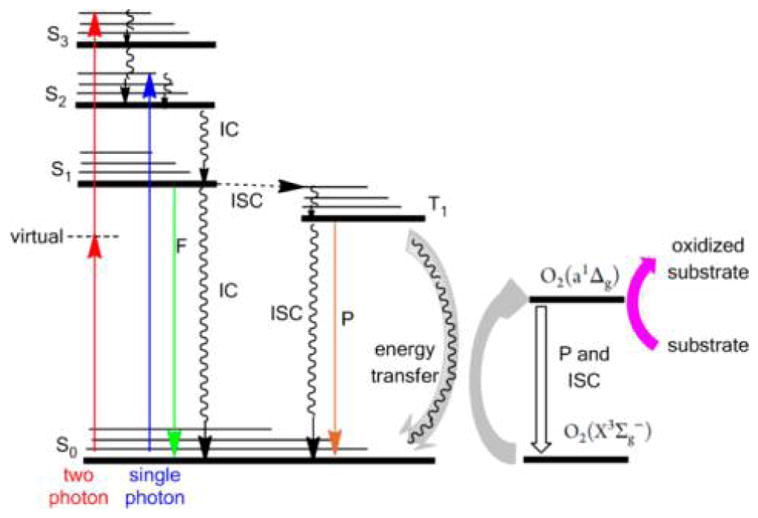
aS is the singlet manifold and T is the triplet manifold, IC is internal conversion by heat loss, F is fluorescence, P is phosphorescence, ISC is intersystem crossing from one manifold to another, and collision of the dye in the triple state with ground-state triplet oxygen photosensitizes the formation of singlet oxygen. Singlet oxygen and hydroxyl radicals react with cell constituents such as double bonds in lipids, flavins, heterocycles, sugar–phosphate backbones of nucleic acids, as well as systems to mitigate oxidative stress like super oxide dismutase.
The major drawback of Photofrin is that it leaves the skin photosensitive for a prolonged period of time. Pharmacokinetics studies in patients have shown that the active oligomeric component in Photofrin has a biological half-life time of about 19 days. This leads to the patient with prolonged skin photosensitivity after treatment, due to accumulation and retention of the drug in skin tissues.67 To overcome the limitations associated with Photofrin, much research has been devoted to develop PSs with improved photophysical properties and fewer side effects. Next-generation PS should meet certain requirements for use in PDT.19,29,68,69 (i) The PS should possess significant absorption in the near-infrared or infrared region between 700 and 1100 nm because biological tissues have low absorption in this region, thus enabling treatment of deeper cancers.70,71 (ii) The PS should minimally aggregate intracellularly, or disaggregate upon entering the cell, thereby maximizing the singlet oxygen quantum yield. (iii) The PS should have high selectivity toward tumor cells and favor intracellular localization68,72,73 such as in mitochondrial membranes and the endoplasmic reticulum to achieve maximum cellular damage within the small diffusion radius of singlet oxygen. (iv) The PS should be chemically stable and be stable to photobleaching.74 (v) It should have a high triplet quantum yield to maximize PDT, or a balance between intersystem crossing to the triplet manifold and fluorescence for dual function imaging and therapy agents.
In 1999, Redmond et al.75 compiled singlet oxygen (1O2) quantum yields (ΦΔ) of several biological molecules and PSs that include: solvent used, intersystem quantum yield (Φisc), fraction of oxygen quenching reactions that leads to O2(SΔ), values of the rate constant (kq), excitation wavelengths (λex) of PS, and methods or techniques used to measure 1O2 quantum yield. Four techniques used to calculate 1O2 quantum yields described by Wilkinson et al.76 follow: (1) time-resolved luminescence upon relaxation of singlet oxygen, (2) steady-state direct detection of the luminescence produced on relaxation of singlet oxygen, (3) photoacoustic calorimetry, and (4) time-resolved thermal lensing calorimetric techniques calculated on the basis of oxygen uptake or loss of absorbance or fluorescence. These organized data serve as a reference for the 1O2 quantum yield calculations of many dye molecules to date.
In a classic paper in 1956, Warburg outlined the metabolic differences displayed by cancer cells, and what we now term the “Warburg effect” in terms of glycolysis, and the strategy of adding saccharides to drugs as a means to target cancer is reviewed.77–82 In the late 1980s, it was recognized that the coupling of sugar molecules to hydrophobic porphyrin dyes can make them amphiphilic, thereby improving their solubility in physiological fluids, and promoting cellular recognition via specific carbohydrate protein interactions on cell surfaces.83–89 The hypothesis was that this strategy would increase PDT efficiency by increasing targeted uptake and subcellular localization into critical areas such as mitochondria and the endoplasmic reticulum. However, the hydrolysis of the sugars diminished the effectiveness of the approach, vide infra. Herein, we will focus on saccharide conjugates of porphyrinoids (porphyrins, Pc’s, corroles, tetrabenzoporphyrins) made to address the tumor targeting and photophysical requirements of PDT. The saccharide(s) can be appended via a direct coupling to the dye or via intervening linkers/spacers. Correlation of chemical structure with targeting, biological activity, and aggregation properties will be a particular focus. Indeed, the cellular uptake of these dyes can be improved through glycoconjugation because various types of sugar transporters, specific for different monosaccharides, are overexpressed in cancer cells.90–92 Also, the presence of chiral functionalities, for example, the sugar moieties, on these macrocycles imparts interesting stereochemical properties in terms of chiral recognition, self-recognition, and aggregation. The biological evaluation of these conjugates reveals that they can be both efficient PSs for PDT93–97 and efficient antibiotic and antiviral agents.98,99
PDT can also be used as an alternative treatment for microbial infections and is shown to be effiective in killing pathogenic microorganisms. The mechanism of destruction of microorganisms is similar to the PDT of cancer in that light activation of certain PSs leads to generating singlet oxygen, which then compromises the bacterial membranes. Several porphyrin and Pc-based PSs are reported to be effective for the photodynamic inactivation (PDI) of bacteria and viruses.99–106 The work reported so far is promising for the deployment of novel glycosylated porphyrinoids to obtain therapies with a large spectrum of antibacterial activity.
The charge and charge distribution on the macrocycle play an important role in cell uptake as well, where it is generally observed that cationic compounds more strongly interact with the negatively charged membrane, but there are some conflicting conclusions in that there are reports that two charges on the same side of the porphyrinoid are better than opposite sides and vice versa.6,107 Our observations using MDA-MB-231, HeLa, and other cell lines are that one cationic N-methylpyridinium is likely optimal with the sugar or other solubilizing groups, and that two cationic groups on the same side are better than on opposite sides of the macrocycle. These observations are consistent with the notion that the amphipathic character of the macrocycle is as important and that the hydrophobic part of the molecule confined to one end or side imparts lipid-like properties. Second, while tetracationic porphyrins certainly bind to cells, they are not actively or passively taken up because of the high energetic costs of traversing the membrane arising from both the +40–60 mV barrier in the center of the membrane arising from the orientation of ions and dipoles of the head groups (electrostatic repulsion of cationic molecules from the membrane core),108 and the hydrophobic membrane core does not accommodate hydrophilic ionic compounds. Cationic compounds that are more hydrophobic, for example, N-alkylpyridinium (where the alkyl group is a long chain) or with lipophilic counterions mitigate the solvation issue, but the large positive potential in the membrane core remains a barrier. An intriguing experiment might be to assess preformed nanoaggregates of cationic and anionic porphyrinoids because lipophilic cations and anions of porphyrins are known to form ionic chain assemblies in lipid bilayers.109
Porphyrinoids offer unique platforms to construct diagnostic and therapeutic agents because of the tunable photophysical properties and the diverse array of methods to append multiple copies of targeting agents, or combinations of targeting and biocompatibility moieties, to result in multifunctional systems. For instance, multifunctional systems that serve as diagnostics and concomitantly as therapeutics, the portmanteau theranostics used hereafter, have many advantages over using one system to diagnose and another to treat disease because the same compound is used, thereby obviating the differences in localization and uptake of a diagnostic agent and a separate therapeutic agent.110–112
2. GLYCOSYLATED PORPHYRINS
2.1. Sugars Appended to meso-Tetraphenylporphyrins
The synthetic strategies to form meso-substituted porphyrin–carbohydrate conjugates include the synthesis of the dye precursors bearing the sugar, for example, aldehydes for meso-substituted porphyrins. Appending the sugar, for example, on meso substituents, to the preformed macrocycle has the distinct advantage of minimizing losses of sometimes precious saccharides, and has been accomplished using substituion, click chemistry, and other approaches.7,113 The sugar units can be covalently linked to the porphyrin macrocycle, or to a tether, via several heteroatom functional groups: N-,114 S-,7,86,115,116 C-,89,117,118 and O-,84,99,119–127 as well as by a 1,2,3-triazole.128,129 In addition to phenyl spacer derivatives on meso-arylporphyrins, glycosides can also serve as spacers88,130–133 (Figure 4).
Figure 4.
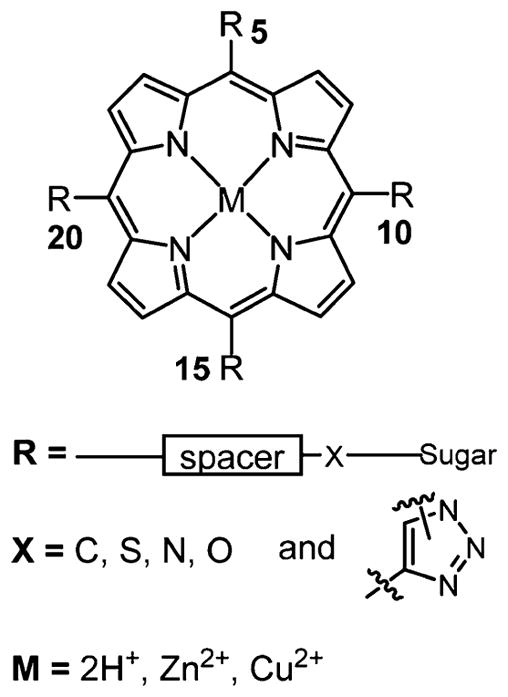
Generalized structure representation of meso-5,10,15,20-substituted porphyrin–carbohydrate conjugates. Spacers vary widely and include phenyl, phenylalkyl, and polyethylene glycol (PEG). Many reports examine the role of the substitution pattern on biochemical properties, for example, the six possible compounds using two different meso substituents.7
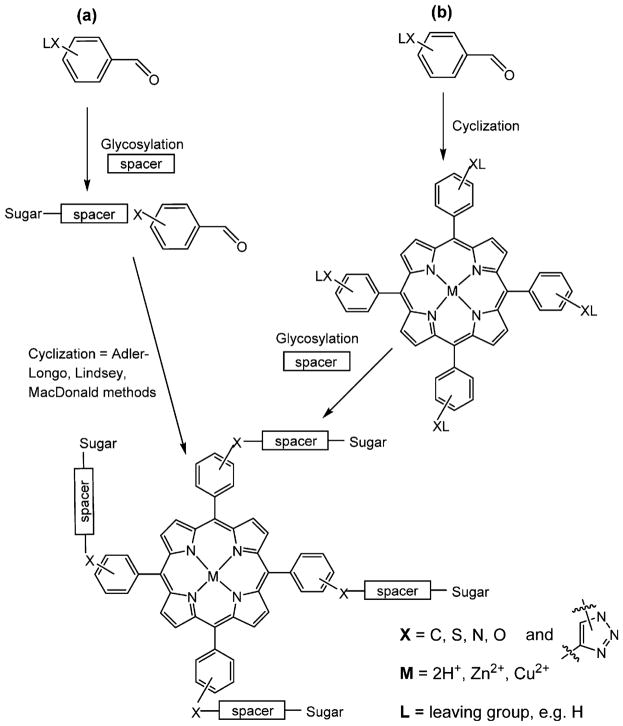
Two general synthetic strategies are currently used to obtain glycosylated porphyrins. (1) The first is cyclization of glycosylated benzaldehydes with pyrroles into the corresponding porphyrins either by the Lindsey or by the Adler method.89,124,134–138 By changing the structure of the benzaldehyde, meso-substituted porphyrins can be prepared with different numbers, types, and positions of sugar units, and may also incorporate other substituents. This method gives low yields in the cyclization step that may be exasperated by the steric hindrance caused by the sugar or other moieties on the 2-and/or 6-position on the aldehyde (Figure 5a). However, this is a versatile synthetic approach that affords a rich array of meso-aryl porphyrin compounds to evaluate photosensitizing efficacy and can be easily adapted to yield specific substitution patterns.119,139,140 (2) Benzaldehydes bearing amine, hydroxy-, carboxy-, halo-, haloalkyl-, or other functional groups enable glycosylation of premade meso-arylporphyrins using appropriate reactions with spacers or carbohydrates to yield the conjugates. Similarly, the six possible compounds with two functionalized aldehydes can be made statistically (Table 2) or by design, to yield small libraries of compounds to assess the role of the number and position of the carbohydrate on uptake and efficacy.7 The second method is free from the problems associated with the porphyrin forming cyclization step, but the efficiency of the coupling chemistry must significantly increase with the number of carbohydrates to be appended to avoid separation of the complex statistical mixtures resulting from low yield reactions (Figure 5b).119,141,142 In general, to develop useful PSs for PDT that can be translated into clinical use, it is important that the carbohydrate units should be introduced onto the porphyrin systems using straightforward and nearly quantitative reactions.
Figure 5.
General scheme of the two major synthetic routes toward multiglycosylated porphyrins: (a) cyclization of glycosylated benzaldehydes by the Lindsey, Adler-Longo, or MacDonald methods;119,139,140,176 (b) glycosylation of porphyrins by reactions with functional groups on the meso-aryl groups.141,142 The spacers may or may not be used.
Table 2.
Six-Member Librariesa
| 5 | 10 | 15 | 20 |
|---|---|---|---|
| A | A | A | A |
| A | A | A | B |
| A | A | B | B |
| A | B | A | B |
| A | B | B | B |
| B | B | B | B |
Six compounds result when two different aldehydes, A and B, are used to synthesize meso porphyrins, and similarly there are six compounds that result from the mixed condensation reactions of phthalocyanines, for example, with two different phthalonitriles A and B. These can be separated by chromatography. Many porphyrin and phthalocyanine cores are constructed using this approach. Note that the meso aryl group on porphyrins is nominally orthogonal to the dye so that substitutions on any of the 2′ or 3′ positions that result in an aryl group that is not symmetric result in a set of four atropisomers; see Foscan, Figure 3. Because of the symmetry of phthalocyanines, isoindoles with one substituent are made as a mixture of four positional isomers; see Photosens, Figure 3.
2.1.1. Direct Linkage of Sugars on meso-Tetraphe-nylporphyrins without Spacer
Glycoporphyrins without spacers are mostly synthesized via substitution of sugars onto preformed porphyrins to achieve better yields, and very few are synthesized with the Lindsey or Alder methods using glycobenzaldehydes and pyrroles. Direct linkage of glucose on porphyrin with O, S, N, C and 1,2,3-triazole without spacer is discussed below.
2.1.1.1. Ether and Ester Linkages
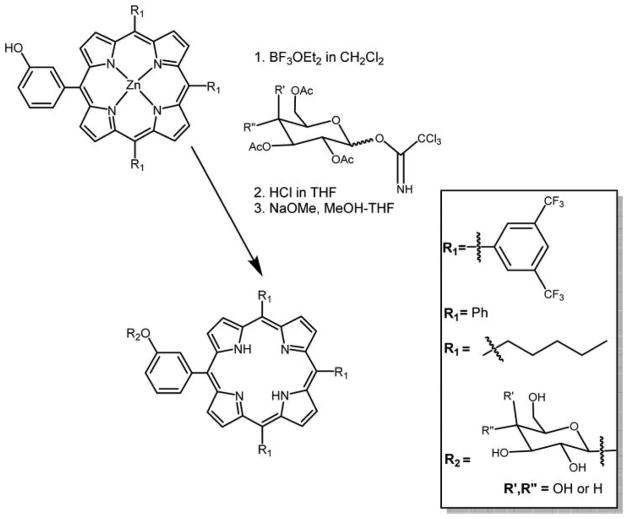
Heretofore, much of the research effort was devoted to appending sugar moieties on the meso-aryl groups of the tetraarylporphyrins via ether or O-glycoside linkages.6,99,119,122–127,138,143 Recently, a simple and highly efficient method for the preparation of glycoporphyrins using trichloroacetimidates as glycosyl donors (Figure 6) was developed by Aicher et al.144 They reported that the presence of Zn(II) in the macrocycle and use of well-matched Lewis acids were necessary for this procedure. This method has advantages as compared to prior methods84,119,120,145–147 because one or more sugar units can be append onto the porphyrin with short reaction times, high yields, and with high purities. This may be a potential method to append other monoglycosylated to polyglycosylated conjugates on porphyrin macrocycle.
Figure 6.
Glycosylated derivatives of hydroxyphenylporphyrin using trichloroacetimidate reagents reported by Aicher et al.144
Several meso-substituted glycoporphyrin conjugates, where glucose is attached to tetraphenylporphyrin via an ester bond without any spacer, were also reported.114,148–150 One typical example is discussed here. The commercially available cationic water-soluble tetra cationic porphyrins such as 5,10,15,20-tetrakis(N-methyl-4-pyridinum)porphyrin and 5,10,15,20-tetrakis[4-(trimethylamonium)-phenyl]porpyrin are active PSs.151,152 Cationic porphyrins and their derivatives can bind with the negatively charged cell membrane and with DNA through intercalation and electrostatic interactions.
Depending on the molecular structure, cationic dyes often times are retained preferentially in cancer cells as compared to normal cells,151,153 thereby enhancing light-induced damage to mitochondria and other cell components and causing cell death.154 Because many cationic porphyrins have significant dark toxicity, there continues to be significant interest in developing porphyrins with low dark toxicity bearing both sugars and cationic groups.6
Molecular design of porphyrins that focuses on increased water solubility and membrane permeability also needs to consider selectivity to make them better PSs.155–157 Studies demonstrated that cationic porphyrins and glycoconjugated porphyrins can both be efficient PSs in PDT and exhibit strong antiviral and antibacterial activity after photoactivation.158 To explore this further, Tome et al.148 reported the synthesis of neutral and cationic tripyridylporphyrin-D-galactose (Figure 7) and their antiviral activity against herpes simplex virus type 1 (HSV-1). The in vitro studies of these compounds showed that the porphyrins were significantly active under noncytotoxic dark concentrations, but photoactivation revealed potential antiherpetic activity.148
Figure 7.
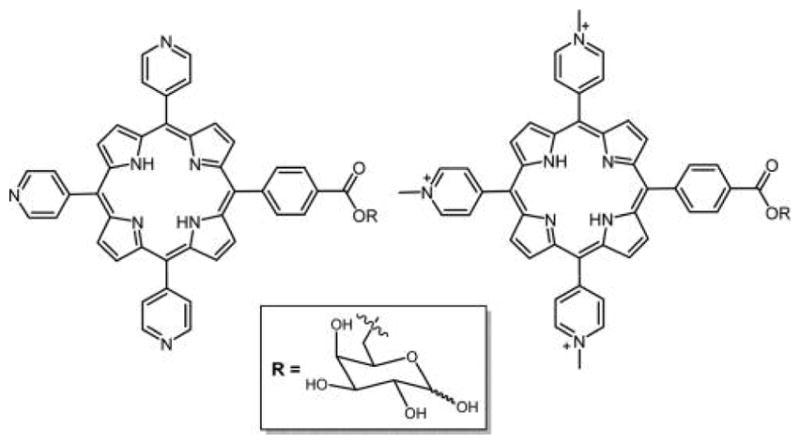
Structure of neutral and cationic tripyridyl porphyrin appended with D-galactose.148
2.1.1.2. Thioether Linkage
Thioglycosides were first reported by Fischer et al. in 1909,159 and many are readily synthesized as glycosylating agents and for polysaccharide formation.160,161 Replacement of the oxygen atom with a sulfur atom at the anomeric position yields reagents that can be used to efficiently make thioglycosylated porphyrins. The S-glycosylated porphyrins are important because these are resistant to endogenous hydrolysis catalyzed by glycosidases, and exhibit greater stability in both acidic and basic media as compared to the corresponding O-glyco analogues,6,137,162 and so are stable under physiological conditions including the reduced pH around cancer cells.
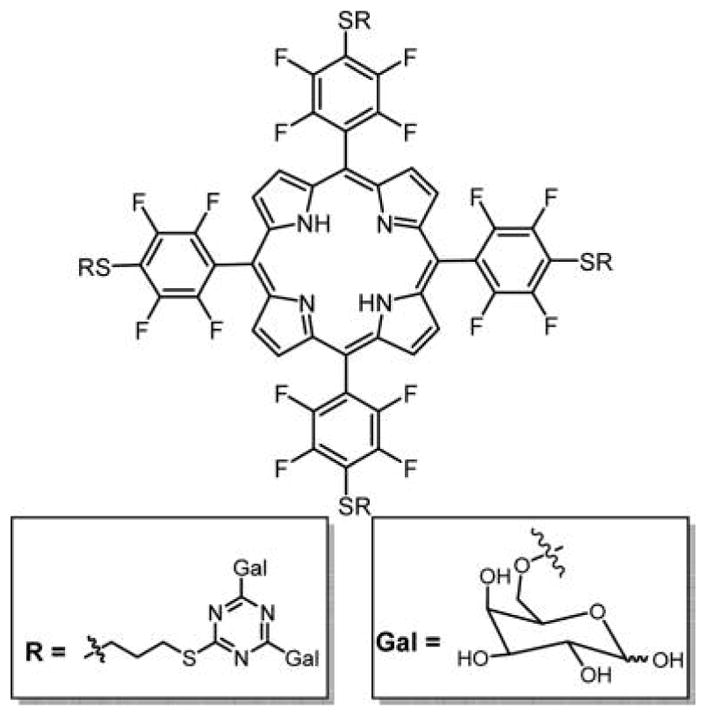
The core 5,10,15,20-tetrakis(2,3,4,5,6-pentafluorophenyl)-porphyrin (TPPF20, Figure 8) chromophore has proven to be a remarkably versatile platform on which a wide variety of biotargeting, biocompatibility, and functional motifs can be rapidly appended in excellent yields; therefore, it has been adopted by many laboratories for evaluation of different molecular design concepts with diverse functionalities.7,163–166 The facile nucleophilic aromatic substitution of the 4-fluorine group by primary S, N, and O groups enables rapid synthesis of dyes appended with diverse substituents including small polylysine peptides, boron clusters, PEGs, and polyamines.7,167–170 This approach can also afford combinatorial libraries of porphyrins wherein the statistical mixture is obtained when the substituents all have the same nucleophile.7,163 Increasing temperatures are needed as the nucleophile becomes harder. This is an improvement over the synthesis of combinatorial libraries from a mixture of aldehydes and pyrroles because of the variable reactivity of the aldehydes, the low yield of the porphyrin, and the purification.151
Figure 8.
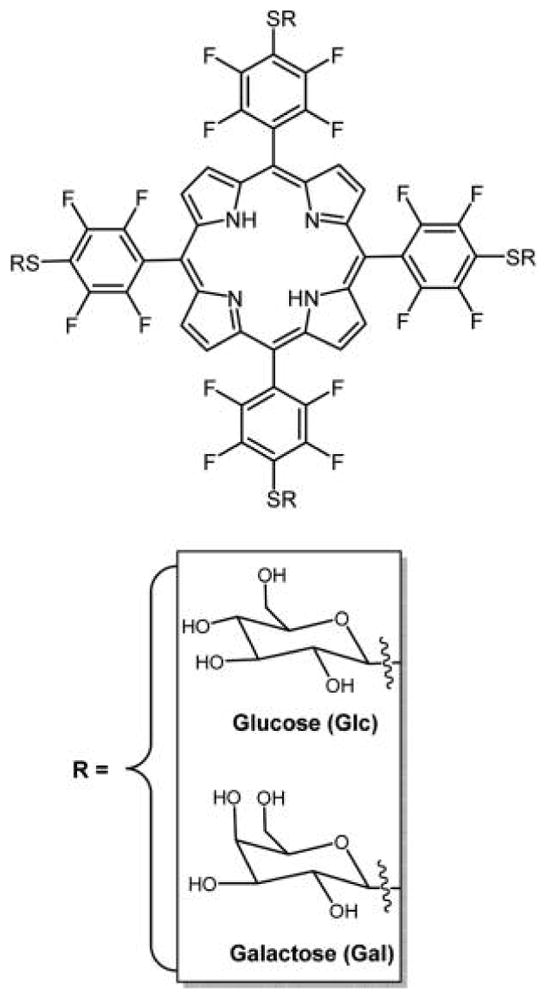
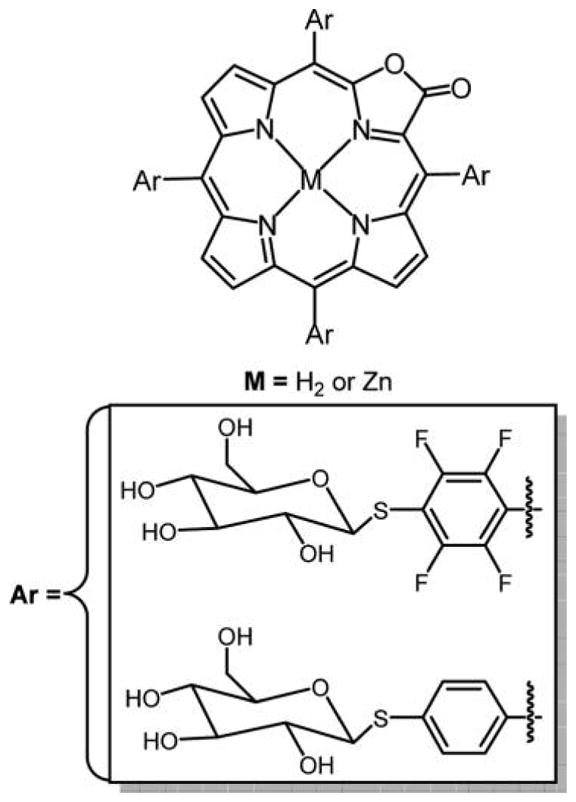
Nearly any primary thiol and unencumbered secondary thiol can substitute for the 4-fluoro group of the commercially available TPPF20 in high yields under mild conditions in this click-type reaction.163 Here, the tetra glycosyl- and tetra galactosyl- conjugates are shown, PGlc4 and PGal4, respectively, reported by Drain and co-workers.86,89
In 2001, our group reported a facile method to append nonhydrolyzable thioglucose and thiogalactose units on TPPF20 to yield PGlc4 and PGal4 (Figure 8) in high yield using this click-type chemistry.89 These compounds were shown to be selectively taken up by several cancer cell lines86 and exert PDT effects by damaging multiple cellular components, especially the endoplasmic reticulum.171 These studies demonstrated the differences in selectivity and uptake resulting from glucose versus galactose targeting moieties, and that uptake of a particular porphyrin–carbohydrate conjugate is proportional to the expression of carbohydrate receptors on the cell. They also demonstrated selectivity for cancerous cell versus the corresponding normal cells. For example, human breast cancer cells (MDA-MB-231) take up PGlc4 conjugate over the corresponding PGal4 conjugate, and PGlc4 predominantly accumulates in the endoplasmic reticulum.
Furthermore, PGlc4 and PGal4 are effective PDT agents as they induce cell death by necrosis and/or apoptosis, depending on the concentration of the conjugate and on the light exposure.86,171 Tanihara and co-workers172 synthesized the mono-, 5,10 and 5,15 di-, and tri- thio-glycosylated porphyrins along with PGlc4, and cell uptake and phototoxicity on HeLa cells were conducted. The cis 5,15-dithio-glycosylated TPPF20 displayed the greatest cellular uptake and phototoxicity among the series. This is in contrast to previous results showing that the 5,10-dithio-glycosylated compounds bearing either phenyl or 4-N-methylpyridinium on the 15,20 positions were taken up and more active than the cis compounds using MDA-MB-231 cells.6,115 The differences may be due to the cell lines, the hydrophobicity, and the substituents.
Recently, Vicente and co-workers reported the synthesis of an asymmetric porphyrin containing three p-carborane and a glucose unit substituted on the para-phenyl position using the same chemistry (Figure 9).173 This compound showed continuous uptake over 24 h by T98G human glioma cells, with low darktoxicity and low phototoxicity, which makes it a good boron neutron capture therapy (BNCT) agent but not a good PS for PDT. In vitro blood brain barrier (BBB) studies on hCMEC/D3 human brain capillary endothelial cell of this compound were also carried out, where a moderate permeability was observed.
Figure 9.
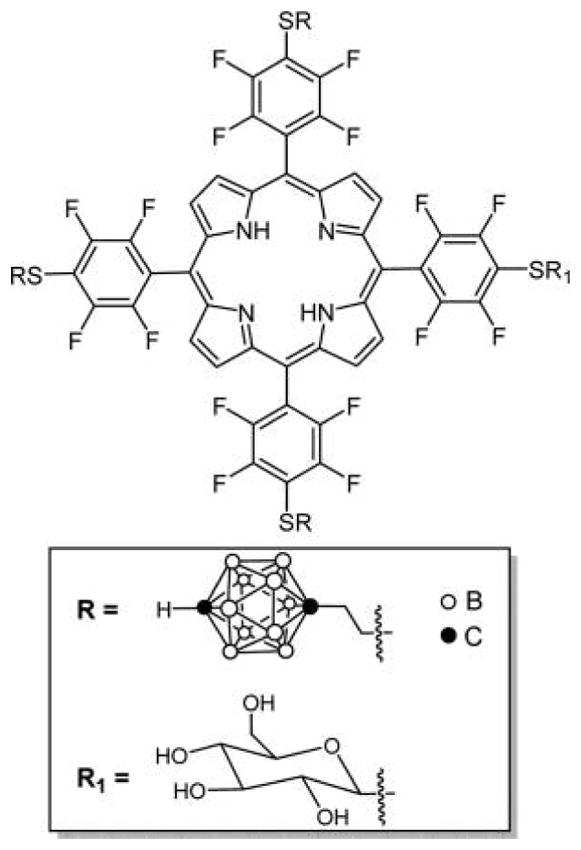
Glucose carboranylporphyrin conjugate reported by Vicente and co-workers made by first appending the thiol carborane and then the thiol glucose.173
Boyle and co-workers reported a mild method for the synthesis of water-soluble porphyrins appended with three thioglycosyl units and pyridyl substituent (Figure 10).115 The dark toxicity observed for these compounds was less as compared to cationic porphyrins with no sugar residues, indicating that the sugars play an important role in moderating dark toxicity. A new series of cationic and neutral water-soluble dimers of glycosylated porphyrins linked at the meso-position via a flexible hydrocarbon spacer were developed by Krausz and co-workers to understand the nature and organization of substituents around the porphyrin macrocycle.136 Here, six glycosylated porphyrin dimers were synthesized differing in the nature, number, and position of the glycosyl units on macrocycle. The preliminary, in vitro biological data suggest that the number of glycosyl units on the porphyrin macrocycle modulates the hydrophilic/lipophilic balance and hence are essential features for an efficient photodynamic activity.99,115,116
Figure 10.
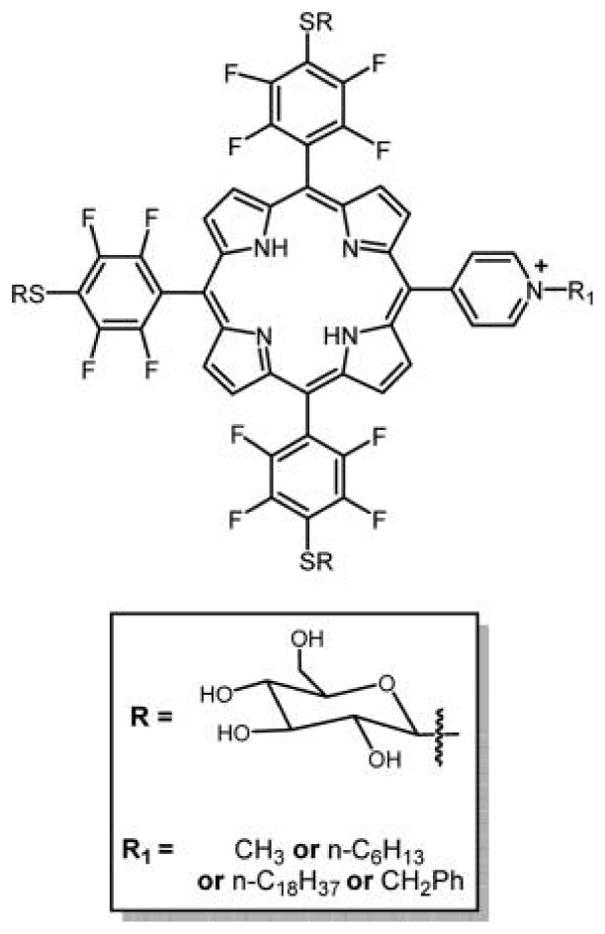
Porphyrin substituted by three glycosyl units and one pyridinium substituent reported by Boyle and co-workers probed lipophilic balance and synergy between glycosylation and cationic moieties.115 The core porphyrin was made from a mixed aldehyde reaction using the Adler and Longo method, and the compounds separated.
2.1.1.3. Amide Linkage
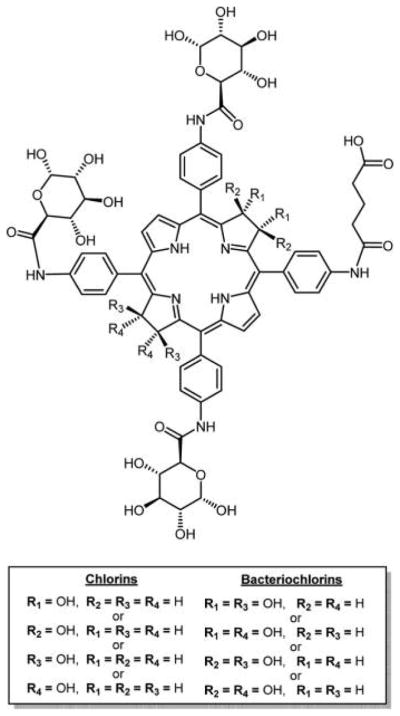
The number of sugars and the position on the macrocycle are determinants of photobiological activity.130 In this study, glycosamide porphyrins and the corresponding chlorins were synthesized by DiStasio et al. (Figure 11).137 The effects of structural modifications, influenced by symmetric or asymmetric position of the glycoconjugation, in these compounds were correlated with the photophysical and photosensitizing properties. As expected, higher photodynamic efficacy was achieved for these compounds as compared to tetraphenylporphyrin (TPP), when incubated with HT29 human adenocarcinoma cells. These cells were found to be more sensitive to asymmetric, monoglycosylated conjugates during survival measurements using photocytotoxicity assays.
Figure 11.
Glycosamide porphyrins and the corresponding chlorins studied reported by DiStasio et al.137 The mono carboxylic acid porphyrins were synthesized via mixed aldehyde condensation using the Adler and Longo method, and the compounds separated. The carboxylic acid chlorin was obtained by diimide reduction of corresponding porphyrin. Amino sugars were conjugated to carboxylic porphyrin and chlorin using a typical amide coupling reaction. trans-Bis-glucose porphyrin was obtained using the [2+2] Mcdonald condensation method starting with O-acetylated glucosamine benzaldehyde.
2.1.1.4. C-Linkage
A novel class of glycoporphyrins use C-glycoside linkages to append the sugar moieties to meso-aryl groups on porphyrin macrocycles, as demonstrated by Franck and co-workers in 2001,89 and later by others.174 These chemically and metabolically robust carbon–carbon bonds retain much of the bond angles and conformations found in the O-glycoside linkages. The approaches to synthesize C-linked (versus O- and S-linked) porphyrin–carbohydrate conjugates remain a challenge and often result in low yields of the target compounds. C-Glycosylated porphyrins conjugated with xylofuranose, glucofuranose, and galactopyranose were also reported by Maillard et al.175 Another example of the synthesis of meso-C-glycoconjugated porphyrins was reported by Casiraghi et al.117 where the porphyrins were prepared by the Lindsey method176 in 6–16% yield, through condensation of suitable dipyrrylglycosides with aryl aldehydes in the presence of Lewis acids such as trifluoroacetic acid or BF3.
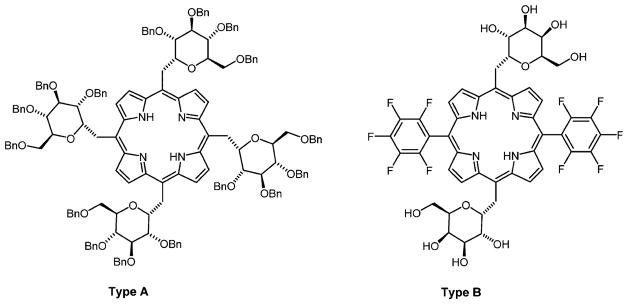
A second class of porphyrin–sugar conjugate with carbon–carbon linkages via methylene bridges was reported by Stepanek et al.118 Porphyrin derivatives containing “C-glycoside” units, either in the 5,10,15,20-meso positions resulting from the direct cyclization of sugar aldehydes with pyrrole (Figure 12, type A), or in 5,15-meso-positions, resulting from the sequential construction of the dyes from the dipyrromethane precursors (Figure 12, type B), were synthesized. The presence of the sugar moieties on these porphyrin macrocycles imparts amphiphilic properties and enables self-organization into chiral suprastructures upon solvent-driven self-aggregation in different aqueous–organic solvent mixtures. The latter property of these compounds was further studied for potential use as building blocks for more elaborate and functional architectures in development of supramolecular chemistry.118,177 Although these types of conjugates have not yet been studied for PDT, they are expected to have important applications as PSs for PDT as well as for other therapeutics and diagnostics.
Figure 12.
meso-C-Glycosylated porphyrins reported by Drasar and co-workers.118,177
2.1.1.5. 1,3-Cyclo Addition Reaction
Sharpless and coworkers178 proposed an effective 1,3-dipolar cycloaddition of azides and alkynes (click reaction) to give a stable 1,2,3-triazole cross-link between two molecules. Because of the ease of the reaction and high yields, this chemistry was employed to conjugate several carbohydrates to porphyrins, chlorins, and Pc’s. Vicente and co-workers reported the expeditious preparation of tetraphenylporphyrin (TPP) (Figure 13) and tetraphenylbenzoporphyrin (TBP) (see Figure 41) appended with lactose or galactose moieties in high yield from readily available sugar azides using Cu(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition click chemistry.129 The four galactose moieties were linked via triazole units to a meso-phenyl group of a TPP and TBP macrocycles. The time-dependent uptake and subcellular distribution of these conjugates were evaluated in human carcinoma HEp2 cells. These assays indicated that the TPP conjugates localized mainly in the ER and endosomes, but the TBP–galactose conjugate was taken up by the HEp2 cells ca. 5-fold more than the TPP conjugates, and localized preferentially within the cell lysosomes. The methodology developed here is highly regioselective and uses milder reaction conditions that are compatible with a large number of functional groups and allows the synthesis of highly water-soluble carbohydrate-substituted TBPs.
Figure 13.
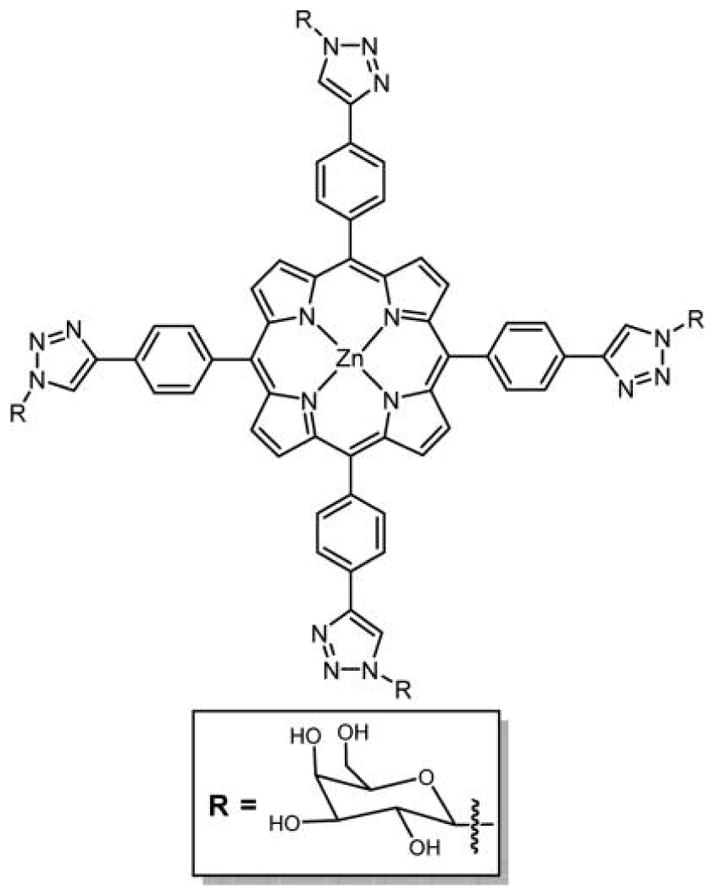
Structure of TPP–galactose conjugate linked via triazole unit.129
Figure 41.
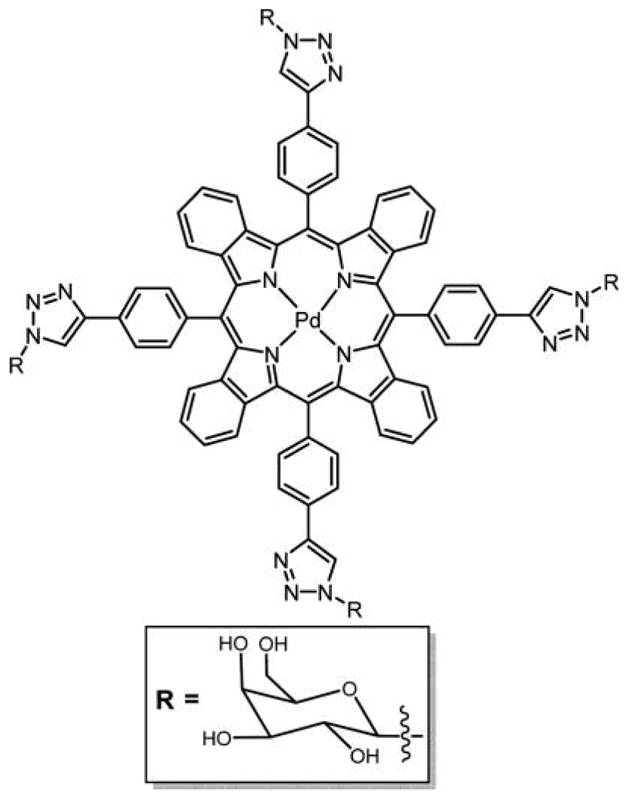
Structure of tetraphenylbenzoporphyrin (TBP)–galactose conjugate linked via triazole unit.129 In these and similar compounds, the rotational barriers between the phenyl and triazole units are likely low enough that interconversion between atropisomers is facile at room temperature, such that they cannot be isolated.
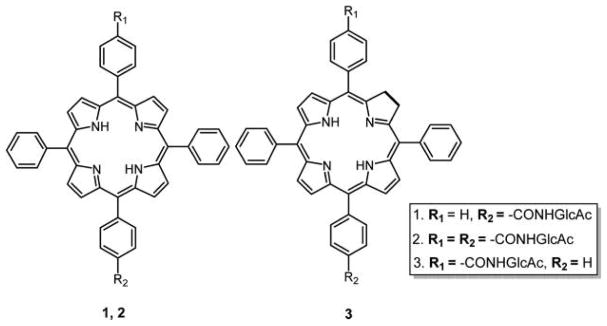
Scanlan and co-workers128 successfully optimized the synthesis of glycoporphyrins without any spacer using 1,3-cyclo addition reaction under microwave (MW) conditions. They could obtain the desired glucose and mannose-substituted tetraphenylporphyrins in quantitative yields within 20 min as opposed to 3 days of conventional heating.
2.1.2. Sugars Linked to meso-Tetraphenylporphyrins with Spacer
The synthesis of glycoporphyrins with different length spacers was achieved, which reduces the steric interactions between the sugars and the macrocycle and improves the carbohydrate recognition when tested in vitro and in vivo. Using PEG spacers would have an added advantage of solubility and stability of the glycoporphyrin drugs. Here, we discuss the glycoporphyrins with spacers that were synthesized by reacting sugars to preformed porphyrins.
2.1.2.1. Ether Linkage
In the 1990s, Krausz and coworkers135,179 reported glycoporphyrin conjugates with a propyl spacer, where the sugar moiety is attached to porphyrin via an ether linkage. Recently, Blais and co-workers88 reported O-glycoporphyrin conjugates with diethylene glycol (DEG) spacers. Human retinoblastoma cells are known to express sugar receptors that have preferential affinity for galactose and mannose residues.180,181 Exploiting this property, Blais and coworkers synthesized a series of glycoconjugate porphyrin-based PSs for a potential PDT treatment of retinoblastoma.88 The glycoconjugated porphyrins include TPP(p-DEG-O-α-GalOH)3, TPP(p-DEG-O-β-GalOH)3, TPP(p-DEG-O-α-ManOH)3 (Figure 14), and their S-analogues in which the sugar motif and porphyrin core were linked by a DEG spacer. The biological and photobiological properties of these DEG-linked O- and S-galacto/mannoconjugated meso-tetraphenyl porphyrins (TPPs) were tested in vitro on a human retinoblastoma cell line (Y79). The photo induced toxicity of these glycosylated derivatives was compared to those of the parent unconjugated DEG porphyrin, TPP(p-DEG–OH)3, and the corresponding monoethylene glycol (MEG)-linked man-nose appended porphyrin, TPP(p-MEG-O-α-ManOH)3. The photobiological activities of these glycosylated porphyrin conjugates depend on the nature and length of the spacer and anomeric configuration of the sugar unit. The increase in the length of the spacer linking the porphyrin with the sugar moiety resulted in higher cellular uptake of these glycosylated porphyrin conjugates relative to that of nonglycoconjugated compounds.88,182
Figure 14.
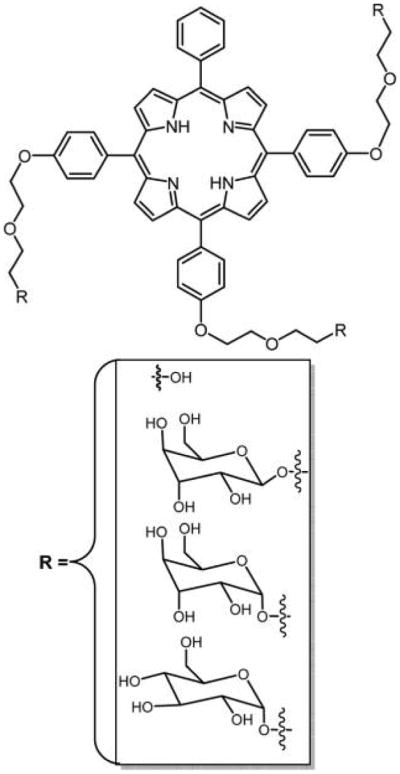
Structures of the parent unconjugated DEG porphyrin and corresponding sugar conjugate; the latter binds to human retinoblastoma cells.88 The porphyrin core was synthesized via mixed aldehyde condensation using the Adler and Longo method. The DEG with or without sugars was substituted onto the porphyrin core via a Williamson type reaction.
2.1.2.2. Thioether Linkage
S-Glycosyl bonds were found to be stable to enzymatic hydrolysis by glycosidase enzymes in vitro and in vivo. Thiosugars linked to symmetrical tetra-substituted porphyrin with DEG spacer was reported by Blais and co-workers.88 Krausz and co-workers116 reported the synthesis of thioglycoporphyrins with propyl linkage/spacer. Three of such derivatives containing glucose, galactose, and mannose were reported (Figure 15). In vitro phototoxicity studies of these conjugates against K562 human chronic myelogenous leukemia cell line were carried out. Cells that had taken up the compounds were irradiated with white light for 0–2 h. An increase in cell death was observed with increased irradiation time, and subsequent incubation of cells in dark resulted in continued death of the cells presumably by apoptosis. Among the series, all of the ortho-isomers were found to be more photoactive.
Figure 15.
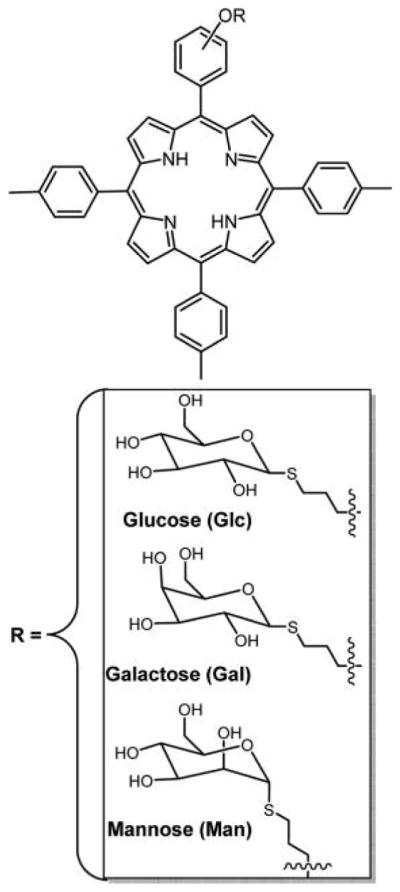
Thioglycosylated meso-porphyrins reported by Krausz and co-workers synthesized via condensation of 1-thioacetylated sugars with monobromotritolyl-porphyrins followed by deprotection of acetate groups using NaOMe.116
2.1.2.3. Amide Linkage
Another approach for the synthesis of derivatives of tetraphenylporphyrin substituted by eight galactose or glucose units with amide linkages containing ethyl spacer was developed by Fujimoto et al.87 These porphyrin glycoconjugates were remarkably water-soluble and fluorescent. Consistent with other reports, the cell uptake studies of these compounds indicate that appending different sugar moieties on porphyrin macrocycle may direct the chromophore toward different cell types and play a significant part in the photosensitizing properties.6
Krausz and co-workers136,183,184 reported the synthesis of tetraphenylporphyrins appended with both amino acids and O-glycosyl, as well as two different glucose porphyrin (para and ortho-substituted) conjugates connected via an amide bond. A propyl group was used as a spacer between the glucose and tetraphenylporphyrin core (Figure 16).183,184 Phototoxicity studies of these compounds were tested on K562 human chronic myelogenous leukemia cells, and the ortho-substituted glycoporphyrin was more PDT active as compared to the para-substituted derivative.
Figure 16.
Glycosylated porphyrin reported by Krausz and co-workers starts by using a mixed aldehyde condensation to yield the monohydroxyphenyltritolylporphyrin.183,184
Asayama and co-workers185 reported Mn(II) porphyrin–lactose conjugates linked by an amine with propyl spacer. Human hepatoma HepG2 cells were used to test this compound for its superoxide dismutase (SOD) activity, dark toxicity, and cellular recognition. Results showed a very good SOD activity, low dark toxicity, and significant cellular recognition.
2.1.2.4. 1,3-Cyclo Addition Reaction
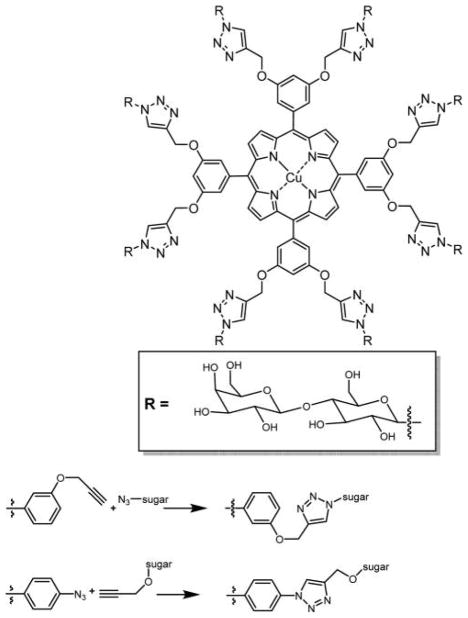
Large numbers of sugar units were appended onto the porphyrin via 1,3-cyclo addition click chemistry with different spacers.121,128,129,186–189 One embodiment of click chemistry takes the advantage of Cu(I)-catalyzed chemoselective coupling between organic azides and terminal alkynes in a simple, convenient, and quantitative method.190,191 Although this is a good method to append multiple sugar units on porphyrin macrocycle, simultaneous insertion of Cu(II) ions into the porphyrin core is disadvantageous for PDT, because Cu(II) porphyrins have poor singlet oxygen quantum yield and therefore serve as poor PSs (Figure 17).142
Figure 17.
Top: Octa-β-lactoglycosylated porphyrinatocopper (PorCu-Lac8) prepared by click chemistry.142 Bottom: Two general routes to azide click chemistry to append sugars onto meso-arylporphyrins.190,191
Garcia et al. reported the synthesis, biological, and photobiological studies of a series of glycosylated porphyrins linked by triazole spacer to target the tumor cells over-expressing lectin type membrane receptors.189 The triazole spacer group is relatively stable toward metabolic degradation and does not pose toxicity problems.192 These porphyrins were prepared by click chemistry under microwave heating and were found to have good singlet oxygen quantum yield. Various factors were investigated such as the nature of the sugar moieties, length of the spacer, and position and orientation of the triazole group to assess the photocytotoxicity of these chromophores. The photocytotoxicity was tested on two different cell lines, HT29 (colorectal adenocarcinoma cell line) and Y79 (human retinoblastoma cell line). Some of these compounds exhibited a good activity in particular against the Y79 cell line and are reported to have less photocytotoxicity as compared to molecules bearing diethylene glycol spacers, for example, those reported by Blais and co-workers (Figure 14).88 meso-Arylporphyrins can be grafted onto cotton fabric via cellulose azidation followed by a click reaction with an acetylenic porphyrin to yield a material with antibacterial activity against representative strains of Escherichia coli and Staphylococcus aureus.128,193
Mukosera et al. recently used Cu(II)-catalyzed 1,3-dipolar cycloaddition reaction for the synthesis of per-O-acetylated glucose, galactose, lactose, and glucosamine conjugates appended directly to 5,15-[p-(ethynyl)diphenyl]porphyrinato zinc(II) and 5,10,15,20-[p-(ethynyl)-diphenyl]porphyrinato zinc(II) compounds.194
Scanlan and co-workers reported the synthesis of a library of glycosylated porphyrin conjugates by using Cu(I)-catalyzed 1,3-dipolar click methodology.121 Here, the ligation reactions are between meso 4-azidophenyl moieties on the porphyrin and the propargylic carbohydrate. In this case, using the Zn(II) metalloporphyrin diminished metalation by the copper catalyst. The reaction conditions were optimized to allow the efficient coupling of porphyrins with biologically active fully protected or deprotected carbohydrates. Synthetic sugars such as an amido derivative of lactose (N-acetylated lactosamine) and the histo blood-group antigen trisaccharide, Lewisx, were ligated to the porphyrin macrocycle for the first time. The PDT activities of these compounds were tested against human esophageal cancer cells.121 A Cu(I)-catalyzed click reaction was used in the synthesis of other triazole-linked mono-, di-, tri-, and tetra-modified glycoporphyrins under microwave-heating conditions,128 wherein a sequential “double-click” reaction sequence yielded bis-modified 5,10-diglycoporphyrins appended with different sugars (Figure 18).
Figure 18.
5,10-Bis-glycoporphyrin synthesized via a microwave heated sequential double click reaction. Core 5,10-bis-azido porphyrin was obtained first by synthesizing 5,10-bis-nitroporphyrin using the Adler and Longo method followed by reduction of nitro groups.128
2.1.3. Glycodendrimeric Porphyrins
Appending sugars on a porphyrin core modifies the amphiphilicity of the macrocycle and makes them specific for binding with lectin-type receptors that are overexpressed in many types of cancer cells.83,195,196 Because the glycoconjugates of the dye are too large for sugar transporters, cell uptake must go by other mechanisms, and these likely depend strongly on the specific molecule under investigation. Passive diffusion of lipophilic species, endocytosis, and other mechanisms may all play a part in uptake to different degrees, again depending on the specific molecular structure. The identification of the transport mechanisms through the biological membranes is challenging, yet is important for optimization of the PSs targeted toward the malignant cells.88,197 For example, the PGlc4 species in Figure 9 has been shown to enter cells both by diffusive and by endocytotic processes.198
The use of glycodendrimers as recognition motifs is a promising avenue toward understanding uptake.199 Carbohydrate–protein interactions play an important role in a large number of biological processes, because not only sugar moieties but also proteins are also active components in cell recognition.131,200–202 Stoddart and co-workers reported the synthesis of two symmetric tetrasubstituted porphyrin glycoconjugated dendrimers with four and 12 β-D-glucopyranosyl residues present on the periphery of the tetrapyrrolic macrocycle (Figure 19).203
Figure 19.
Glycoconjugated dendrimers symmetrically appended to a porphyrin core with 4 and 12 β-D-glucopyranosyl residues reported by Stoddart and co-workers.203
Rosilio and co-workers reported a family of glycoconjugated PS with only one glycodendrimer moiety on the para position of one meso-phenyl group attached to the porphyrin.204,205 Here, the mannose sugar unit is attached to the macrocycle with variable length spacers (Figure 20) to create a lipid-like structure where the dye is the hydrophobic end. The interactions of these glycodendrimeric porphyrins with phospholipids were studied at the air–water interface and in liposome bilayers. These studies found that the conjugate with the longer spacer interacted well with the lipid bilayer with the sugar moieties protruding into the surrounding aqueous phase, which aggregated with Concanavalin A (Con A), a mannose-specific lectin.206 This work highlights the role of complex interactions between the lipid molecules, the sugar moieties, and the hydrophobic porphyrin cores in the passive diffusion of glycoconjugates across cancer cell membranes. The dendrimeric structure was reported to show high binding affinity to plasma proteins and phototoxicity against retinoblastoma cells Y79.207 From another perspective, these and similar glycoporphyrin constructs may constitute efficient targeting carriers for other drugs in addition to PDT. Other porphyrin-based glycoconjugates made by azide–alkyne click chemistry were used in sensors for specific binding of two bacterial lectins that present different carbohydrate preference such as Concanavalin A.208
Figure 20.
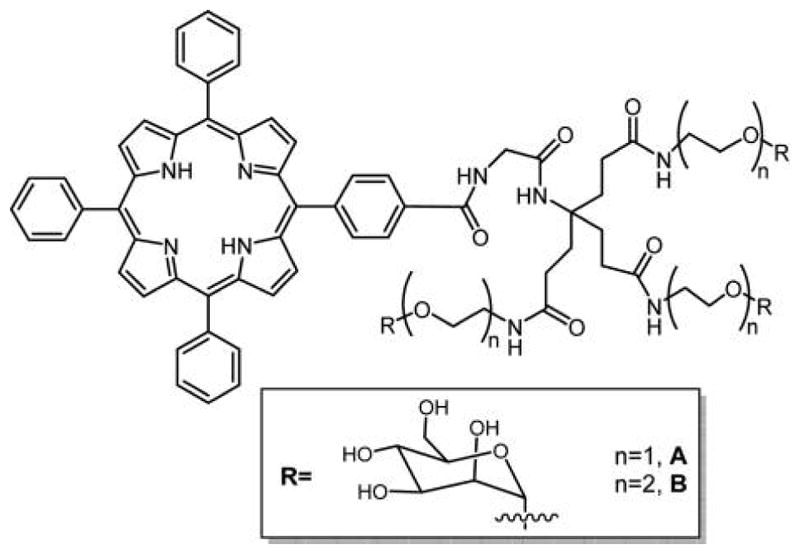
Structures of glycodendrimeric porphyrins reported by Rosilio and co-workers,204 where the porphyrin core was made by mixed aldehyde condensations using the Adler and Longo method.
Kushwaha and Tiwari reported the synthesis of a series of azide-functionalized glycodendrimer porphyrins having 8, 12, 16, and 24 β-glucopyranose units at the peripheral position using azide click chemistry; the structure of 24 β-glucopyranose porphyrin is shown here (Figure 21).209
Figure 21.
Structure of azide-functionalized glycodendrimers of porphyrin having 24 β-glucopyranose units.209
Other examples of glycodendritic conjugates of porphyrins and Pc’s were reported by Silva et al., bearing 8 and 16 D-galactopyranose units, respectively (Figures 22 and 53).132 Both of these conjugates were reported to have high singlet oxygen production, suggesting the potential to be used as PDT agents.
Figure 22.
Structure of porphyrin glycodendritic conjugate with eight galactopyranose units.132
Figure 53.
Structures of glycosylated glycerol-Zn-phthalocyanine (A) with an intervening triethylene glycol spacer and glycosylated thiol-hexane-Ni-phthalocyanine329,332 and (B) with an intervening triethylene glycol spacer prepared via 1,3-dipolar cycloaddition reported by Lafont and coworkers,330 wherein the core Pc was made from a mixed condensation reaction or from a core hydroxyl Pc.
2.1.4. Polysaccharide Porphyrin Conjugates
Although several porphyrin carbohydrate conjugates are reported to have good in vitro PDT efficacy, many of these compounds show lack of selectivity and the moderate solubility in water leads to aggregation (see section 9), thus complicating in vivo testing and analysis. In this section, strategies to overcome aggregation via appending polysaccharides to the porphyrin core are discussed. Cyclodextrins (CDs) are a family of natural compounds widely used in the pharmaceutical and cosmetic industries as insipients, the more hydrophobic interior allows CD to host small molecules, and as covalently attached carriers. Porphyrin–CD conjugates as supramolecular systems were reviewed.210 Supramolecular chemistry is chemistry beyond the covalent bond; therefore, supramolecular systems result from the spontaneous association of molecules driven by intermolecular interactions. The covalent attachment of molecules into macromolecules does not result in supramolecular systems a priori. Self-assembly refers to the formation of discrete systems, for example, porphyrin arrays,211,212 whereas self-organization refers to formation of open or nondiscrete systems,213,214 and the supramolecular porphyrinoid materials are reviewed.215–217 Many of the porphyrin–CD supramolecular systems are designed to examine electron and energy transfer or as potentially catalytic systems, but herein we focus on the conjugates designed for therapeutic applications. For example, meso-tetrakis(4-sulfonatophenyl)porphyrin spontaneously assembles with CDs in aqueous solutions.218
Kral and co-workers219,220 reported several porphyrin–CD conjugates as a potential PDT agent as well as a drug delivery system for cancer therapy (Figure 23). Binding studies of several chemotherapy drugs on porphyrin–CD conjugates showed that doxorubicin had good binding affinity toward porphyrin γ-CD 3 and paclitaxel had good binding affinity toward porphyrin β-CD conjugates 1, 2, and 4. In vitro studies using mouse mammary carcinoma 4T1 cells and human chronic myelogenous leukemia K562 cells, and in vivo studies using BALB/c mice transplanted with 4T1 cells on the supramolecular carrier–chemotherapy drug complexes were tested. These studies showed that this system works as an efficient combination therapy (PDT and chemotherapy) to treat cancer.
Figure 23.
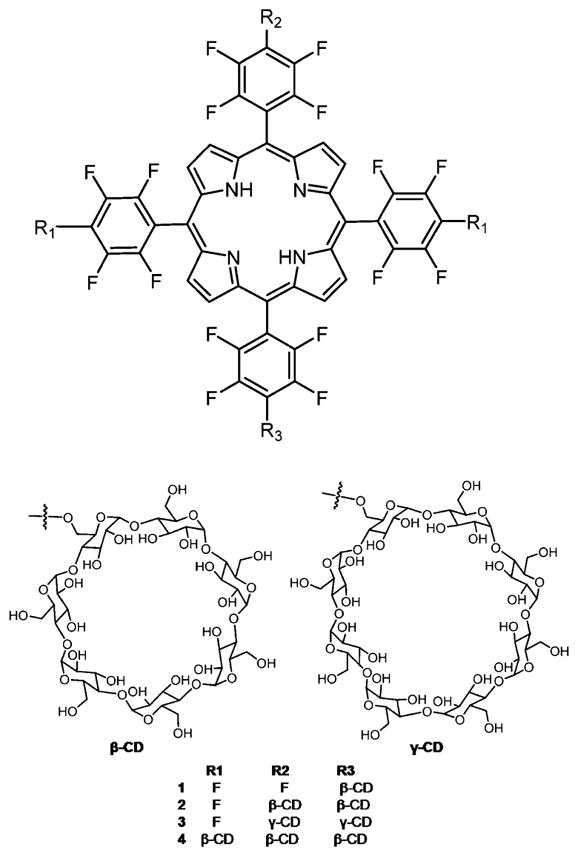
Porphyrin–CD conjugates used as carrier–drug complex for therapy.219,220 Other porphyrin–CD conjugates formed using click chemistry and amide linkers are reported, and there are various strategies that self-assemble CD with porphyrins into supramolecular materials.210
Kun and co-workers221,222 reported acetyl chondroitin sulfate chlorin e6 and acetyl hyaluronic acid porphyrin derivatives as nanodrugs for PDT. The triplet quantum yield of nanogels of pullulan/folate-pheophorbide-a conjugates was suppressed in PBS due to self-quenching of the PS, but when the nanogel was coincubated with esterase in the presence of HeLa cancer cells, the PDT activity was restored.223 In vitro studies of acetyl chondroitin sulfate porphyrin drug were conducted on HeLa cells.222 Continuous uptake of the compound was observed when monitored by confocal microscopy, and a moderate phototoxicity was observed. In vitro cell uptake and phototoxicity studies of acetyl hyaluronic acid porphyrin conjugates (Figure 24)221 were also tested on HeLa cells. A rapid uptake of the compound was observed, suggesting an internalization of compound via endocytosis. Low phototoxicity was also observed with this compound. Low phototoxicity of both conjugates revealed self-quenching due to proximity of the dyes on the hyaluronic acid and chondroitin sulfate backbones.
Figure 24.
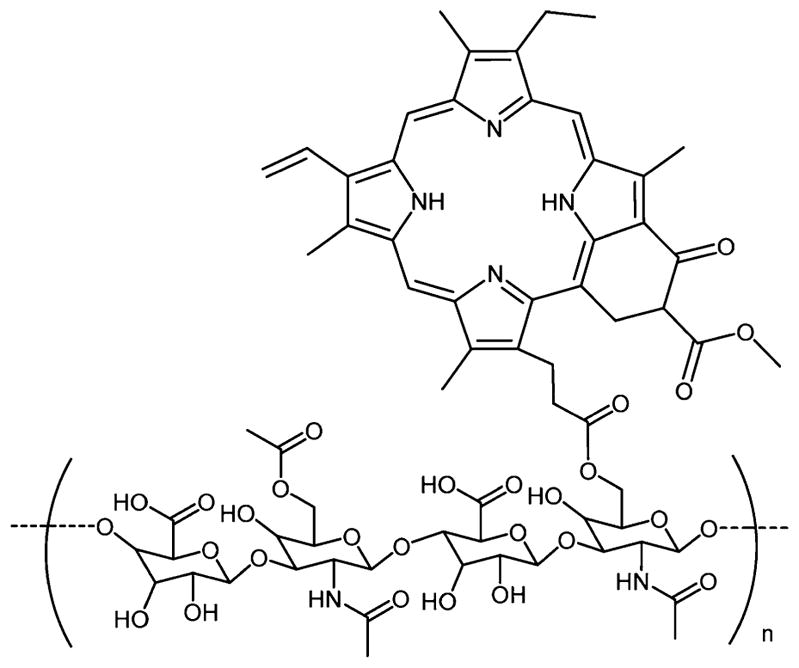
Hyaluronic acid porphyrin conjugate as a PDT agent.221
Mikata et al. reported the synthesis of a series of free base and Zn(II) derivatives of porphyrin bearing 1–4 maltohexoses units at the meso positions. The maltohexoses unit on the porphyrin macrocycle is reported to increase the solubility of the PS. Out of all of the conjugates, only the mono-substituted maltohexaosylated glycoconjugate was reported to be efficient PS using HeLa cells.224 This is another example of how the exocyclic motifs are only part of the story and that amphipathicity, as measured by octanol/water partition coefficients, is a key parameter in designing PDT agents.
2.2. β-Pyrrole-Substituted Porphyrin Sugars
Kupriyanov and co-workers225 first reported the synthesis of porphyrin sugar derivatives substituted at the β-pyrrolic position in 1978 using ester bonds, and the first example of a C-glycosylated porphyrin in which four sugar molecules were attached to the β-pyrrole positions of the porphyrin macrocycle was reported by Maruyama and co-workers in 1992.96 Following these reports, several meso-substituted glycoporphyrins were studied along with a few more β-pyrrolic-substituted sugar porphyrin conjugates.113 Among these, the sugars were substituted on porphyrin via sulfur,116 nitrogen,114 and carbon groups.226 One such example was recently reported by Cavaleiro and co-workers shown in Figure 25.226 Five different allyl sugars were conjugated to zinc(II)protoporphyrin-IX dimethyl ester via cross-metathesis using Grubbs catalyst resulting in two carbohydrate units appended to the core metalloporphyrin.
Figure 25.
Recently reported β-substituted porphyrin sugar by Cavaleiro and co-workers.226
3. GLYCOSYLATED CHLORINS, ISOBACTERIOCHLORINS, AND BACTERIOCHLORINS
Hydroporphyrin-type derivatives, such as chlorins, isobacteriochlorins, and bacteriochlorins, have considerably stronger absorptions in the red and/or near-infrared (NIR) region of the electromagnetic spectrum. The near IR absorption and tunable optical properties make hydroporphyrins promising for the next generation PS for PDT and NIR bioimaging agents. While m-THPC is in clinical use, there remain issues of selectivity; see section 1. Over the past few years, new synthetic methods were developed to obtain novel hydroporphyrin derivatives such as Diels–Alder reaction,227,228 1,3-dipolar cycloadditions,30,229,230 electrocyclizations,231,232 and cyclopropanation233 reactions.
3.1. Sugars Substituted on meso-Phenyl Groups
3.1.1. Thioether Linkage
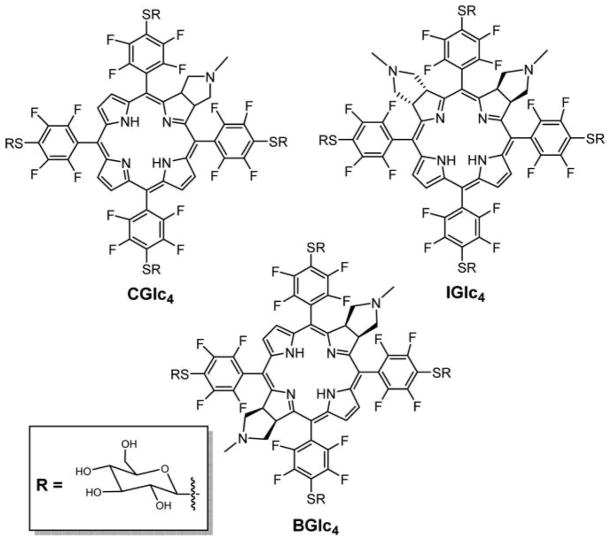
Recently, Drain and co-workers reported the facile synthesis, photophysical properties, and in vitro studies of three nonhydrolyzable, tetrathioglycosylated porphyrinoids: chlorin (CGlc4), isobacteriochlorin (IGlc4), and bacteriochlorin (BGlc4) (Figure 26).234 By tuning the photophysical properties relative to TPPF20 and glycosylated conjugates PGal4 and PGlc4,86,235 this work expands the toolbox of core platforms that can be used for therapeutics, theragnostics, diagnostics, and tracker dyes. The conjugation of biotargeting motifs, for example, appending thio-sugars, on all four platforms is facile; therefore, this allows most laboratories to rapidly synthesize and evaluate new conjugate dyes for a wide array of applications.
Figure 26.
Structures of thioglycosylated chlorin (CGlc4), isobacteriochlorin (IGlc4), and bacteriochlorin (BGlc4) appended with four thioglucose units reported by Singh et al.234
TPPF20 can be used as a starting material because it is readily available and can serve as a platform to append a host of biomolecules by substitution of the para fluoro group with a variety of nucleophiles to form bioconjugates (the reactivity is dictated by the nucleophile: S > N > O, primary > secondary).86,163,168,236 The photophysical properties of this macrocycle can be fine-tuned by adjusting the number of double bonds. The perfluorophenylchlorin (CF20), perfluorophenylisobacteriochlorin (IF20), and perfluorophenylbacteriochlorin (BF20) were prepared by 1,3-dipolar cycloaddition reaction.30 As compared to the parent glycosylated porphyrin conjugate, CGlc4 and IGlc4 have stronger red absorption bands, and the fluorescence quantum yield increases by 6- and 12-fold, respectively, in phosphate buffered saline (PBS). On the other hand, the fluorescence quantum yield of the BGlc4 conjugate is ca. 5% and similar to PGlc4, but has the lowest energy Q-band is considerably red-shifted to near 730 nm with nearly 50-fold greater absorbance. The singlet oxygen quantum yield for PGlc4, CGlc4, IGlc4, and BGlc4 was found to be 0.85, 0.32, 0.59, and 0.28, respectively, in methanol-d1 solvent.234 The uptake of these glycosylated conjugates into cells such as human breast cancer cells MDA-MB-231 and mouse embryonic fibroblast cells K:Molv NIH 3T3 can be observed at nanomolar concentrations and is reported to be selectively taken up by cancer cells over the normal cells (Figures 27 and 28). These platforms will enable the development of new multifunctional imaging, sensing, and therapeutic agents for specific targets.237 Following this work, Pd(II) complexes of thioglycosylated porphyrin and chlorin were prepared by Hirohara and coworkers to study the heavy atom effect on in vitro photocytotoxicity.238 As expected from numerous prior studies,239,240 the presence of the heavy atom enhanced the singlet oxygen quantum yield of the Pd(II) complexes. Somewhat surprisingly, insertion of Pd(II) did not improve the in vitro photocytotoxity as compared to the corresponding free base porphyrin and chlorin thioglucose conjugates, perhaps due to differences in octanol/water partition coefficients or axial binding to an endogenous ligand.
Figure 27.
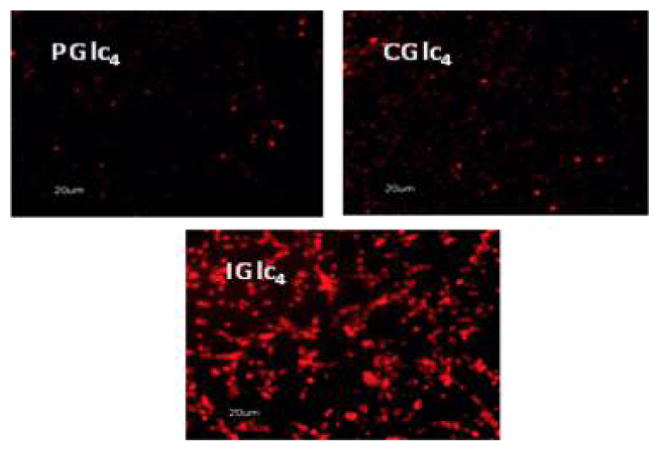
Fluorescence microscopy of K:Molv NIH 3T3 cells treated with 2.5 μM PGlc4, CGlc4, and IGlc4. K:Molv NIH 3T3 cells were incubated for 20 h with porphyrinoids, followed by removal of unbound dye from the cell culture by repeated rinsing with PBS, and the cells were imaged under identical microscope settings and not enhanced; magnification 10×. Reproduced with permission from ref 234. Copyright 2010 American Chemical Society.
Figure 28.
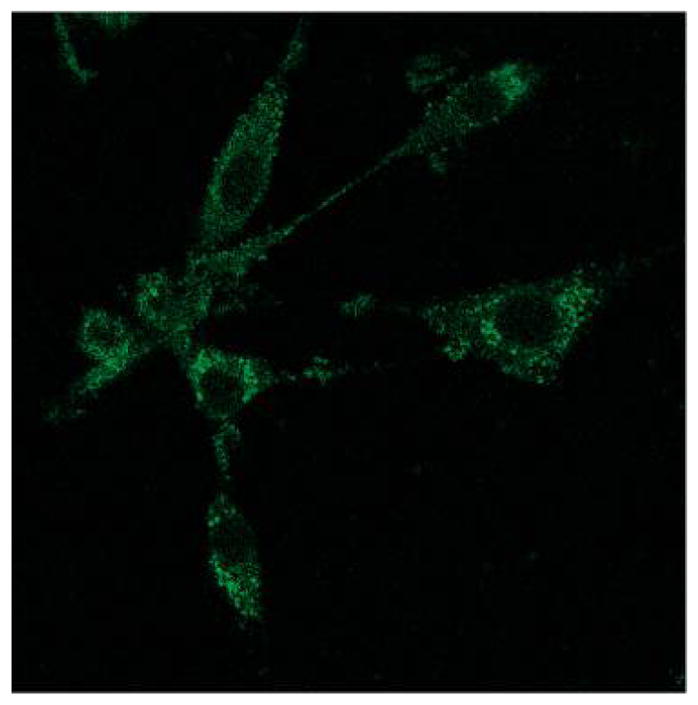
K:Molv NIH 3T3 cells were incubated with 10 μM BGlc4 for 24 h, rinsed three times with PBS buffer, and fixed with 4% paraformaldehyde solution. Confocal microscope excitation at 514 nm, emission monitored with a 710–750 band-pass filter. Under similar conditions using the IGlc4, CGlc4, or PGlc4, no fluorescence images are observed using a 610–650 nm emission band-pass filter. The image is not enhanced; magnification is 60×. Reproduced with permission from ref 234. Copyright 2010 American Chemical Society.
Sakuma et al. have shown that the thioglycosylated chlorins (e.g., CGlc4) have more photodynamic efficacy over the nonglycosylated conjugate (H2TFPC) and aspartyl chlorin (NPe6) using several different human cancer cell lines.241 This reports that in a xenograft tumor model, H2TFPC-SGlc-mediated PDT suppresses tumor growth and shows no adverse effect on the surrounding tissues.242
All of the tetraglycosylated porphyrins reported by Drain and co-workers, and likely most of the others reported, aggregate to some extent in aqueous solutions such as PBS buffers when concentrations are greater than a few μM (Table 3).234 This is evidenced by broadening of the UV–visible spectral bands, fluorescence quenching, and dynamic light scattering (DLS). Aggregation and the consequences of aggregation are discussed in section 9.
Table 3.
Aggregation of Selected Glycosylated Dyes in Figure 26 in PBS Buffer Measured by DLS
| conc.
|
Soret λmax, nm | octanol/water partition coefficient | ||
|---|---|---|---|---|
| 4.53 μM, nm | 1.18 μM, nm | |||
| IGlc4 | 47 ± 8 | 4 ± 2 | 385 | 9.1 ± 1 |
| BGlc4 | 357 | 12.7 ± 1 | ||
| CGlc4 | 48 ± 6 | 10 ± 3 | 409 | 28.5 ± 3 |
| PGlc4 | 49 ± 4 | 20 ± 6 | 410 | 43.9 ± 5 |
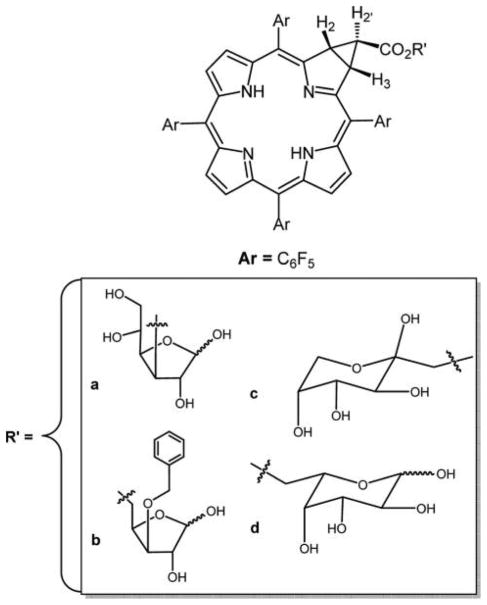
Tang et al.243 reported another class of glycoconjugate of a chlorin in which the β–β′ pyrrole double bond is substituted by an electron-withdrawing lactone unit, which is known to decrease the lipophilicity of the conjugate (Figure 29). This glucose conjugated porpholactone was shown to have high binding affinity with low density lipoprotein (LDL), which enhances the cellular uptake efficacy and localization within the lysosomes. The lactone moiety on the glycoconjugated porpholactone was reported to increase the singlet oxygen quantum yield associated with increasing intracellular ROS levels, and thus this is a new approach for PSs for PDT.243
Figure 29.
Structure of gluco-conjugate of porpholactone.243
3.1.2. Amide Linkage
Recently, McCarthy et al. reported glucose-modified chlorin and isobacteriochlorins-based PS intended for PDT.244 These glucose-substituted conjugates were synthesized in high yields from meso-tetra(p-aminophenyl)porphyrin and resulted in the formation of neutral, hydrophilic chromophores (Figure 30). The presence of sugar groups on these conjugates increases their polarity, and the carboxylic acid-functionalized linker allows facile conjugation of the PS to the biomolecules. The presence of sugar moieties on the macrocycle increases the number of dyes that can be conjugated or cross-linked to dextran-coated nanoparticles and maintains the stability of the suspension. These glycosylated conjugates have potential to be useful in the development of a number of the next generation of targeted nanotherapeutic systems.
Figure 30.
Glycosylated chlorins and bacteriochlorins synthesized from 5,10,15-tris(4-1′,2′,3′,4′-O-acetyl-glucopyranuron-N-phenylamide)-20-[4-(5′methoxy-1′,5′-dioxopentyl) aminophenyl]porphyrin, reported by McCarthy et al.,244 use OsO4 to make the chlorin and bacteriochlorin.
3.2. β-Pyrrole Conjugation
3.2.1. Ester Linkage
Cavaleiro and co-workers reported the synthesis of new glyco–hydroporphyrin conjugates by reaction of sugar-substituted α-diazoacetates with zinc(II) meso-tetrakis(pentafluorophenyl)porphyrin in the presence of a catalytic amount of CuCl.245 The major products of these reactions are chlorins, which, when acidified, afford the corresponding free bases with deprotected sugar units (Figure 31). These chlorin derivatives are reported to be better singlet oxygen producers than methylene blue (MB). This new synthetic methodology can lead to new glycoporphyrin derivatives with the location of the sugars on the pyrroles rather than meso aryl moieties.
Figure 31.
Cavaleiro and co-workers245 made chlorins by reactions of meso-tetrakis(pentafluorophenyl) porphyrinatozinc(II) with α-diazoacetates derived from diacetonides of the glucofuranose (a), monoacetonide of xylofuranose (b), fructopyranose (c), and galactopyranose (d).
3.2.2. Amide Linkage
Zhang et al.246 reported the synthesis of an amide linked glycosylated porphyrin, pyropheophorbide 2-deoxyglucosamide (pyro-2DG, Figure 32), that can serve as both a targeted PDT agent and a NIR fluorescence imaging agent. The intravenous administration of the pyro-2DG theragnostic into a 9L glioma rat model selectively accumulates the compound into the tumor area as indicated by fluorescence imaging studies. Photo illumination of the pyro-2DG accumulated in the tumor area causes selective mitochondrial damage to the tumor region, without affecting nearby areas lacking the PDT agent or in tissues treated with the dye but not irradiated with light. The pyro-2DG theragnostic is tumor selective for tumor-targeted NIR fluorescence imaging and as a good PDT agent.246
Figure 32.
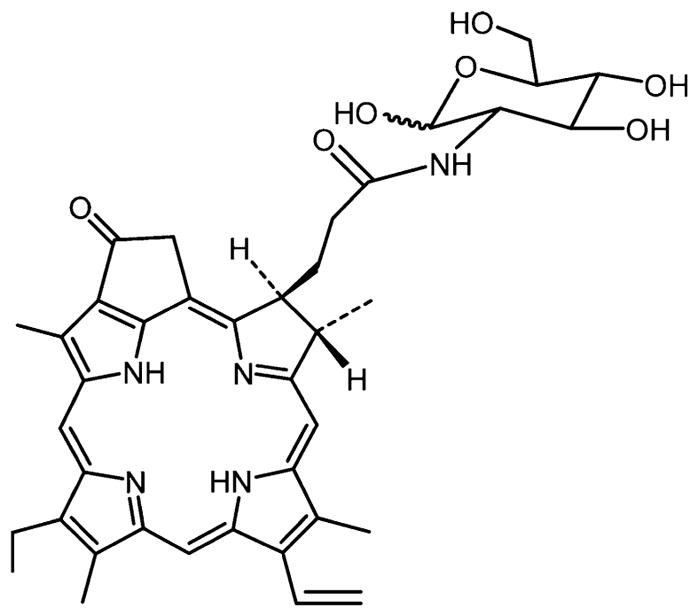
Structure of pyropheophorbide 2-deoxyglucosamide (Pyro-2DG) theragnostic reported by Zhang et al.246
3.2.3. 1,3-Cyclo Addition Reaction
“Click chemistry” exploits one or more high yield reactions to generate a library of organic compounds for creating therapeutics, imaging agents, and other biochemically active molecules. Click chemistry has significantly increased the rate of development of new biologically active compounds.167,247,248 Key features include that the starting compounds are readily accessible, mild reaction conditions, high yields, and minimal side products.7 The core porphyrin platforms for many click reactions (e.g., the tetra-4-X-phenylporphyrins where X = carboxy, amino, fluoro) are easily made or commercially available. Following the combinatorial approach, Mironov and co-workers used the “click chemistry” methodology to synthesize chlorin e6-based PS appended with β-lactose.248,249 1,3-Dipolar cyclo addition reaction of a sugar azide was carried out on a propargyl derivative of chlorin e6249,250 (Figure 33). The lactose conjugates display a high singlet oxygen quantum yield (φ 1O2 ≈ 0.75) and enhanced selectivity toward cancer cells, and so may serve as a PDT photosensitizer.
Figure 33.
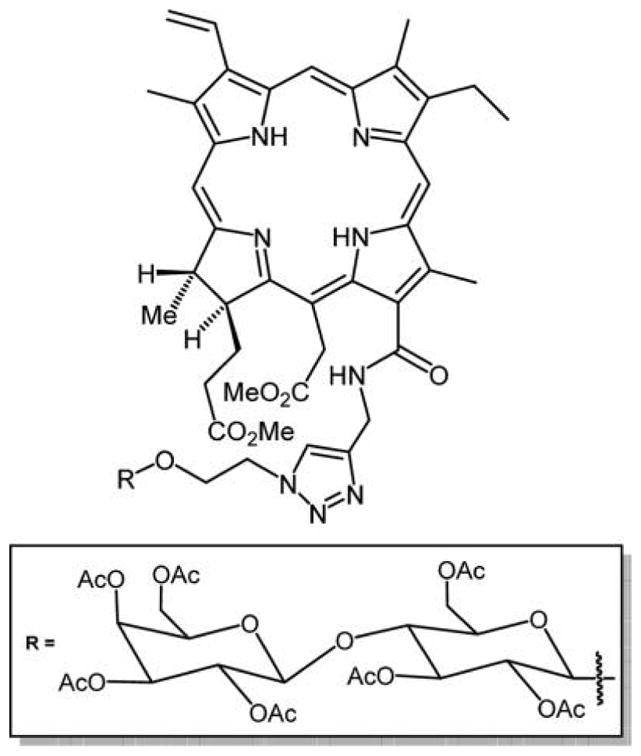
Structure of the chlorin e6–carbohydrate conjugate reported by Grin et al.250
4. GLYCOSYLATED CORROLES
Corroles are a tetrapyrrolic macrocycle with 18-π conjugated electrons. Like porphyrins, corroles are tetradentate ligands that chelate to a large variety of metal ions, and because they are trianionic ligands they stabilize +3 oxidation states better than the porphyrins.251 The corrole macrocycle has been extensively studied as a new generation PS for applications in PDT.39,40 The absorption spectrum of the corroles is similar to that of the porphyrins with a Soret band around 420 nm and several Q absorption bands, which are much stronger than that of porphyrins between 450 and 650 nm. Thus, corroles have larger optical cross sections in the red regoin as compared to porphyrins. Preliminary studies reveal that many corroles are metabolized faster in vivo252,253 and can have high singlet oxygen generation quantum efficacy, and so can act as good cancer therapeutic agents.254 Ventura et al. reported the photophysical properties of a series of meso-substituted corroles in toluene. They were reported to be quite thermally and photochemically stable and have high singlet oxygen quantum yields, 0.51–0.77.252 Agadjanian et al. have shown that pyrrole-substituted sulfonated corroles form stable complexes with protein carriers by a noncovalent assembly and can pass into breast cancer cells and induce toxicity.255
To date, only a few examples of sugar conjugates of corroles are reported (Figure 34) wherein the thioglucose was appended using the substitution chemistry on the perfluorophenyl group,7,163 and an O-glycosyl derivative.256 In vitro uptake and phototoxicity studies of a galactose appended corrole were carried out on human acute T cell leukemia (Jurkat cells). The results showed an increase in cell uptake of the compound but a low PDT efficacy. The low PDT effect was attributed to less efficient singlet oxygen production of the compound. In the future, other glycocorroles with better triplet quantum yields will be needed that can have good cellular uptake, exhibit strong absorption in the red region of the optical spectra, and can be good sensitizers for singlet oxygen generation, making them promising PDT agents.
Figure 34.
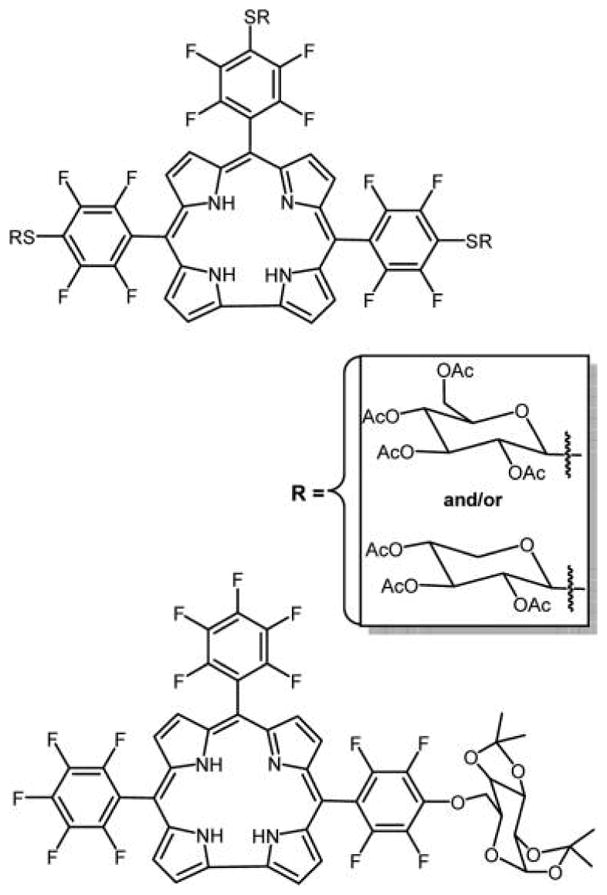
Top: Three thioglucose or three thioxyloses appended to corroles reported by Samaroo et al.,163 made by substitution of the para fluoro group. Note that the para fluoro groups on opposite perfluorophenyls substitute somewhat faster than the central. The bottom pentafluorophenylcorrole-D-galactose conjugate was reported by Röder and co-workers.256
5. PORPHYRIN–CARBOHYDRATE CONJUGATES WITH TWO-PHOTON ABSORPTION PROPERTIES
The currently approved PSs have one-photon absorption peaks in the visible region of the optical spectra. The chlorin macrocycle of Foscan, for example, was developed to improve light absorption in the red region of the UV–visible spectrum near 630 nm as compared to Photofrin. Other chlorins, bacteriochlorins, isobacteriochlorins, phthalocyanines, and porphyrin dimers were prepared because of the stronger absorption cross section in the red to NIR region.234,257,258 However, the significant absorption at wavelengths less than about 650 nm by biological tissues substantially diminishes the effectiveness of PSs for treatment of cancers deeper into tissues.71 To surmount this limitation, several groups embarked on the design of PS with absorption bands in the near-infrared or infrared region, between 700 and 1100 nm, where the absorption and scattering of these wavelengths in tissues are much less than higher energy photons. This range is also known as “the window of biological tissues”.259 Bacteriochlorins and Pc’s are dyes that have significant absorption in this region, but another strategy is to design dyes with good two-photon cross sections. In two-photon absorption (TPA), the dye simultaneously absorbs two lower energy photons to arrive at the same excited state as single photon absorptions. TPA is a nonlinear optical process where the combined energy of two low energy photons is sufficient to produce the excited state, and afterward the normal photophysics occurs. The advantage of TPA is that the molecule can be excited in the NIR region, facilitating deeper light penetration into tissues, but the disadvantages include the high light flux needed to excite the molecule and the small excitation areas of ca. 1 mm2.260–263
Recently, in vivo experiments demonstrated that PDT performed with PS with high TPA enables the treatment of deep and large sized tumors by conventional one-photon excitation.264,265 Covalently linked multiporphyrin arrays, especially those connected with acetylene linkers266,267 or that are fused together,268,269 have attracted considerable attention because of their remarkable photophysical properties such as high polarizability and high nonlinear optical (NLO) properties such as TPA. Several types of multiporphyrin compounds are reported,270–274 some having sugars appended to the chromophores (Figure 35).267
Figure 35.
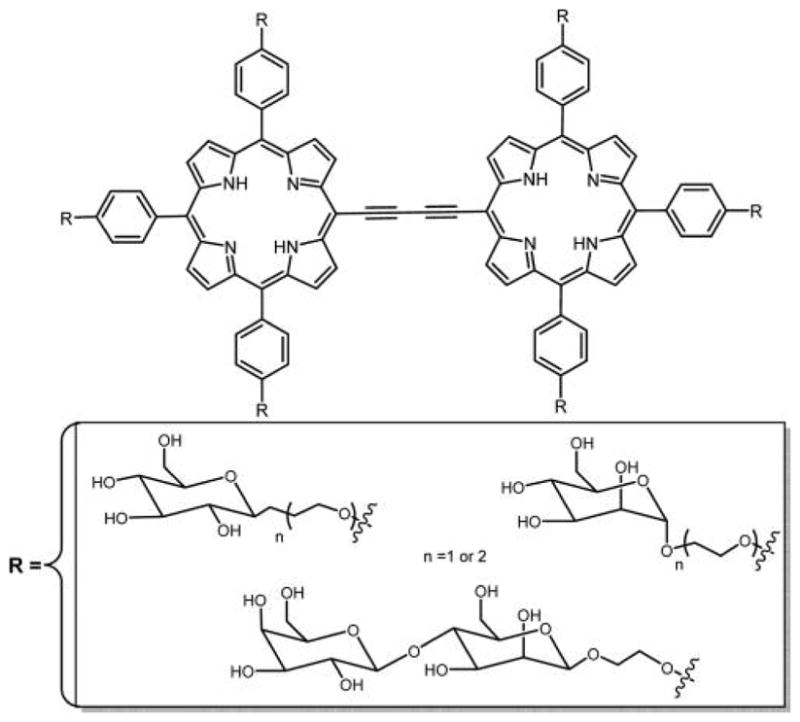
Porphyrin dimers conjugated to carbohydrates were evaluated for potential applications in one-photon and two-photon PDT by Maillard and co-workers.267
Achelle et al. reported the synthesis of series of zinc porphyrin oligomers appended with α-mannose that have good TPA cross sections and high singlet oxygen quantum yields (Figure 36).71 To obtain the conjugated oligomers, porphyrin monomers were linked with bridges that do not twist out of plane with the porphyrin macrocycle. For this reason, sterically hindered neutral π-conjugated cores, ethynyl, butadiyne, diethynylbenzene, and electron-donor triphenyl amine were incorporated between the porphyrin monomers because ethynyl bridges are one of the most effective ways of making strong electronic connections to the meso position of the porphyrin.274–277
Figure 36.
Structure of conjugated zinc porphyrin oligomer reported by Achelle et al. with good two-photon cross sections.71
Singh et al.169 recently reported the synthesis and phototoxicity of NIR dye hexathioglucose triply bridged fused porphyrin (Figure 37). The singlet oxygen quantum yield of this compound in DMSO was found to be 0.78 ± 0.03. In vitro darktoxicity and phototoxicity studies were carried using the MDA-MB-231 breast cancer cell line. No dark toxicity was observed, while the phototoxicity results were in agreement with the singlet oxygen quantum yield. Significant cell death (IC50 = 13 μM) was observed with light exposure for 20 min, and after 24 h 75% of cells were necrotic.
Figure 37.
Triply bridged hexaglycosylated fused porphyrin dye with large two-photon cross section reported by Singh et al.169
Maillard and co-workers reported the synthesis, spectroscopic, and biological properties of carbohydrate-vectorized porphyrin–triphenylamine hybrids, which may have potential to be two-photon PSs (Figure 38).278 The scaffold of these compounds is based on the electronic conjugation of a porphyrin core and a triphenylamine group via an alkyne spacer and shows high singlet oxygen quantum yields as well as good two-photon cross sections. These compounds are inactive against the HT29 cancer cell line as well as retinoblastoma cells Y79 using one-photon phototoxicity assays, which was attributed to aggregation of these compounds in biological media due to their hydrophobicity. Further efforts are being made to overcome this problem by attaching hydrophilic groups on the triphenylamime part of the conjugates.278
Figure 38.
Structure of triphenylamine porphyrin bearing three α-mannose groups attached via DEG tether for two-photon activity reported by Millard and co-workers.278
Recently, Drain and Coworkers279 studied CGlc4, IGlc4, and BGlc4 (Figure 26)234 for two-photon fluorescence imaging on Chinese hamster ovary cells and found that IGlc4 has a good TPA between 760–880 nm, and the cross section is 24.5 GM at 860 nm. Figure 39 shows two-photon microscopy of a 5:1 mixture of IGlc4 and BGlc4 on Chinese hamster ovary cells excited at 860 nm.
Figure 39.
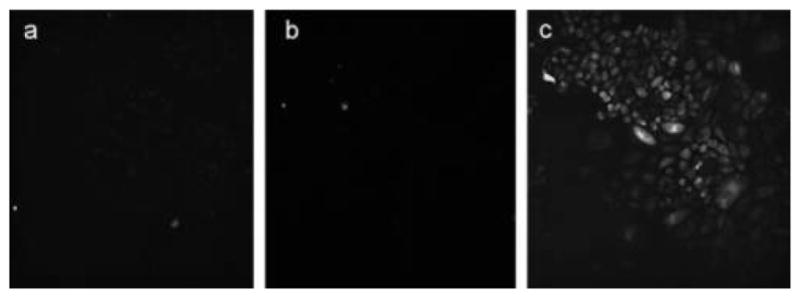
Two-photon microscope images of (a) PGlc4, (b) CGlc4, and (c) IGlc4:BGlc4 (5:1) on Chinese hamster ovary cells excited at 860 nm.279 Reproduced with permission from ref 279. Copyright 2014 John Wiley and Sons, Inc.
6. GLYCOSYLATED TETRABENZOPORPHYRINS
Tetrabenzoporphyrins (TBP) comprise four benzene rings fused to the pyrroles on a porphyrin, with no phenyl rings on meso positions.280,281 These compounds may be promising candidates for PDT because they have strong absorption in the red region of the optical spectra.282 TBPs are chemically stable due to the extended π-conjugated systems. As compared to porphyrins, TBP compounds are poorly soluble due to increased conjugation and π-stacking;281,283,284 however, the solubility of meso-substituted tetraaryltetrabenzoporphyrin (Ar4TBP) in water can be increased by appending sulfonic acid, carboxylic acid, or nido-carborane functional groups.166,285,286 Taking into account the data, TBP bearing glucosyl or polyamine units on meso positions to improve the targeting of cancer cells were synthesized and characterized by Krausz and co-workers (Figure 40).280 The synthesis of these glycosylated tetrabenzoporphyrins was done by condensation of tetrahydroisoindole with aromatic aldehyde followed by oxidation with DDQ.287,288
Figure 40.
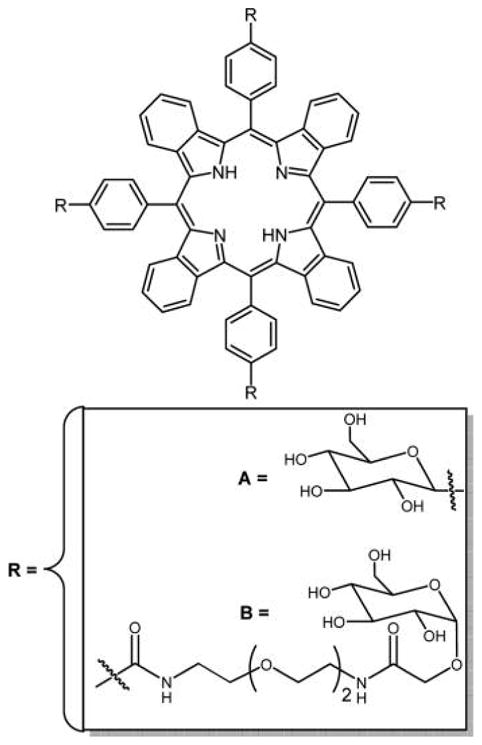
Structures of the glycosylated tetrabenzoporphyrins reported by Krausz and co-workers.280
Two meso-tetraglycosylaryltetrabenzoporphyrins were synthesized by appending glucose units directly on the macrocycle or attached by a tetraethylene glycol spacer (Figure 40). These two compounds were prepared by different methods: compound A was prepared by condensation of glucosylated aldehydic precursors with tetrahydroisoindole, and compound B was prepared by amidation followed by glycosylation of synthetic tetrabenzoporphyrin. The first approach opens interesting prospects for the synthesis of new functionalized asymmetric benzoporphyrins. A strong absorption band was obtained for both of these compounds around 700 nm, and both were capable of producing singlet oxygen, but due to the hydrophilic nature of the core dye they have propensity to aggregate in methanol and water as observed from UV–vis and fluorescence. Compound B with its tetraethylene glycol spacer is more hydrophilic and therefore less efficient as a PDT agent because it lacks the amphiphilic character required for an efficient cell uptake.289 Cell incubation studies using two different human cancer cell lines, MCF-7 and HaCaT, were also done for these compounds, and preliminary results show that these compounds have very low photocytotoxicity and are even weaker than Photofrin II, which was used as a reference.280 TBP–galactose conjugate formed using click chemistry (Figure 41) was reported by Vicente and co-workers,129 and the chemistry is outlined in previous sections.
7. GLYCOSYLATED PHTHALOCYANINES
Pc’s are promising second generation PSs. Since their discovery in 1907 by Brown and Tcheriac,290 numerous work has been done on their synthesis and functionalization to tune their photochemical and photophysical properties.291 Pc’s are well suited as PSs for PDT and for bioimaging agents because of the tunable photophysical properties, and they can be more efficient in generating reactive oxygen species than porphyrins.53,292,293 Pc’s often exhibit low dark toxicity, are chemically stable, and have a strong absorption peak in the red region of the optical spectra (λmax ≈ 670 nm, ε ≈ 105 L mol−1 cm−1) where light penetrates deeper into tissues relative to the weaker absorption of porphyrins (λmax ≈ 610 nm, ε ≈ 5 × 103 L mol−1 cm−1). This absorption can be fine-tuned both by the coordination chemistry and redox properties of the metal present in the central cavity and by the number and type of substituents on the periphery of the macrocycle.25,294 To date, fewer Pc-based PS were tested in vivo for PDT studies than porphyrin-based PS, due in part to the complex purification of the compounds with one moiety per isoindole, or the statistical mixture of four isomers can be used.295 Second, Pc’s have a strong tendency to aggregate in buffers and physiological fluids even with eight substituents on the periphery. Thus, numerous new pathways for the synthesis of Pc’s are under investigation to solve the problems of purification and make them more water-soluble and less prone to aggregation. Significant research aimed at improving the selectivity of the Pc’s toward cancerous cells has resulted in limited success because these compounds show aggregation in physiological fluids and have unfavorable intracellular localization.296
Pc’s that may act as potential PS for PDT include derivatives of zinc(II)phthalocyanine (Zn(II)Pc),297–300 silicon(IV)-phthalocyanine (Si(IV)Pc),301–303 and aluminum(III)-phthalocyanine (Al(III)Pc).304,305 An advantage of the latter two is an increased solubility brought on by a decrease in aggregation where the +4 or +3 oxidation state of the metal ion weakens intermolecular interaction via electrostatic repulsion and the axial ligation of alkoxide counterions prevents H aggregation and mitigates J aggregation. While substitution of these compounds at the peripheral position by bulky or charged groups prevents the stacking of the hydrophobic Pc core and aggregation,306,307 this may also inhibit cell uptake. The conjugation of Pc’s with carbohydrate units increases water solubility, avoids the use of a delivery system to tumor cells, and provides better tumor specificity.85,301,308–311 Herein, Pc carbohydrates are discussed in two sections: one where the carbohydrate units are attached at the axial position of metallophthalocyanines and the other where the carbohydrate units are attached at the α and β positions.
7.1. Axial Phthalocyanine–Carbohydrate Conjugates
To improve the steric shielding of the Pc core, alkoxide axial ligands can be strongly coordinated to tri- or tetravalent chelated oxophilic metal ions, such as Al(III), Ga(III), or Si(IV), which can improve solubility in the aqueous media.301,312,313 Lee et al. reported the preparation and in vitro photodynamic activity of Si(IV)Pc with one or two axial acetal-protected galactose substituents (Figure 42).301 The galactose groups were introduced by substitution reactions with the goal of improving the hydrophilic properties and inhibiting the self-aggregation of these compounds. These axially coordinated sugar Si(IV)Pc’s display a good photodynamic activity against HepG2 human hepatocellular carcinoma, which may be due to high cellular uptake and high singlet oxygen production (1O2,ΦΔ = 0.94).301 Ng and co-worker reported the photodynamic efficacy of two glycosylated Si(IV)Pc in which the D-glucofuranose unit binds axially to the Si center through the tetraethylene glycol spacer (Figure 43).314 The compounds were reported to be highly phototoxic against HT29 human colorectal carcinoma and HepG2 human hepatocarcinoma cells.314
Figure 42.
Galactose-containing Si(IV)Pc reported by Lee et al.301
Figure 43.
Structure of glycosylated Si(IV)Pc from a mixed condensation reaction in which the D-glucofuranose unit binds axially to the Si(IV) center through the tetraethylene glycol spacer.314
7.2. Sugar Substitution on α and β Positions of Phthalocyanine
Several glycophthalocyanine conjugates were reported where the carbohydrate was substituted on the α and β positions of Pc. Substitution of carbohydrates was achieved with S-, O-, N-heteroatoms and 1,2,3-triazole moiety.293 These are discussed in the following sections where sugars are attached to Pc with and without spacers. Synthetic approaches to glycophthalocyanines are reviewed.293
7.2.1. Direct Sugar Substitution
The crucial step in the development of peripheral, directly linked glycosylated Pc’s is the efficient synthesis of their precursors, that is, glycosylated phthalonitriles prepared via SNAr reaction of nitro-phthalonitriles or halo-phthalonitriles with anomerically unprotected glycopyranoses and 1-thio-glycopyranoses.315 Thus, a series of peripherally substituted mono-, di-, tri-, tetra-, and octa-glycosylated Pc’s can be synthesized with carbohydrates such as D-glucopyranose, β-D-galactopyranose, 1-thio-β-D-glucopyranose, D-mannose, β-D-cellulose, β-D-lactobiose, 1-thio-β-D-cello-, and lactobiose.85,293,310,316–321 In vitro studies using human amniotic epithelial WISH cells showed that tetraglycosylated PC’s can be good lead compounds for PDT.320
7.2.1.1. Ether Linkage
Choi et al.319 and Iqbal et al.322 reported several water-soluble asymmetrical tetra O-glycosylated Pc isomers. One such example is shown in Figure 44A322 where glucose was substituted at the peripheral (α and/or β) positions via the anomeric carbon and the classical Pc template reactions with unprotected phthalonitriles were applied.320 Similarly, an asymmetrical Zn(II)Pc bearing three hydrophilic glucosyl groups and a lipophilic octadecyloxy group was synthesized by Zhang et al. (Figure 44B).298
Figure 44.
(A) Asymmetric anomeric glycoconjugated Zn(II)Pc reported by Iqbal et al.322 (B) Asymmetric Zn(II)Pc bearing octadecyloxy and glucosyl groups by Zhang et al.298 The way of representing the peripheral-OR groups indicates that a mixture of positional isomers was used, including α or β.
Kimani et al. reported an isopropylidene protected tetra-β-glycosylated Zn(II)Pc that has 100-fold better cellular uptake than the tetra-β-glycosylated Zn(II)Pc and a 10-fold increase in uptake as compared to second generation PS Al(III)PcS4 (Figure 3) using MCF-7 cancer cells, and this compound has better PDT efficacy than both the corresponding deprotected conjugate (Figure 45) and the Al(III)PcS4.323 The low biological efficacy of tetra-β-glycosylated Zn(II)Pc as compared to protected conjugate was attributed to the aggregation caused by the hydrophobic stacking between the aromatic rings of Pc and H-bonding between the free OH groups on glucose.
Figure 45.
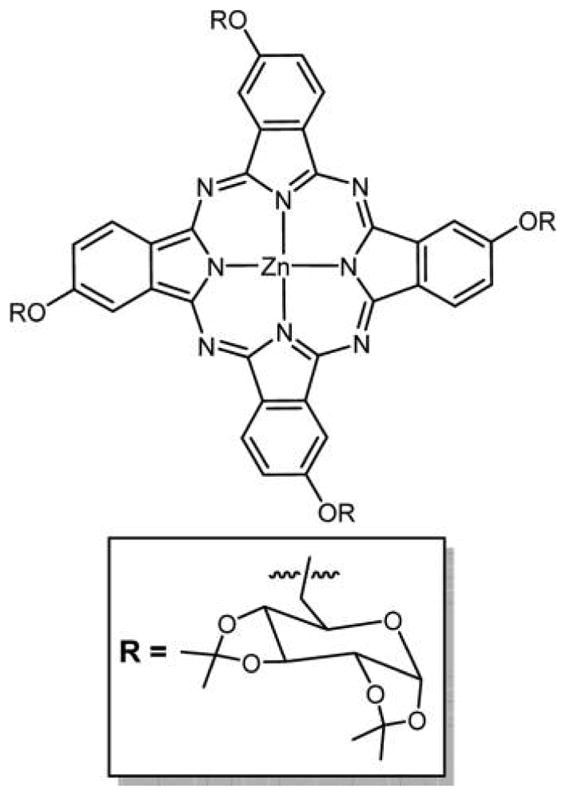
Structure of isopropylidene-protected tetra-β-glycosylated Zn(II)Pc (one isomer shown).323
Tetra-substituted Pc such as Al(III)PcS4 and the tetra glucose conjugates have significant potential to be used as a PS for PDT, but the structural characterization can be difficult because these are obtained as mixtures of positional isomers that are difficult to separate. Mixtures of compounds and/or isomers may have advantages in terms of PDT, because each may partition or localize in different parts of cell or tissue resulting in oxidative damage at multiple sites. Thus, the small physical and chemical differences, for example, the octanol/water partition coefficients and photophysics, of the four isomers with one substituent on each isoindole or the four atropisomers on Foscan, may result in synergistic PDT activities. For Pc’s, derivatives with 0, 8 α, 8 β, and 16 substituents can be pure compounds without isomers. Therefore, to avoid the formation of isomers upon tetramerization of monoglycosylated phthalonitriles, Iqbal et al. reported the preparation of octa-glycosylated Pc’s synthesized from 4,5-diglycosylated phthalonitriles, which is one of the first examples of a Pc symmetrically octasubstituted with D-galactose residues (Figure 46B).317 In vitro studies using human amniotic epithelial WISH cells showed that tetragalactophthalocyanines can be good lead compounds for PDT. Later, they reported several symmetrically pure octaglycophthalocyanines.320 Cavaleiro and co-workers prepared asymmetrically pure tetra-substituted glyco conjugated Pc’s bearing four galactose units (Figure 46A).310 Choi et al.319 recently observed that mono-substituted, isomerically pure O-glucose and O-galactose Pc’s had better in vitro PDT efficacy as compared to isomeric mixtures of tetra O-glucose and O-galactose Pc’s, when tested on HT29 human colon adenocarcinoma and HepG2 human hepatocarcinoma cells.
Figure 46.
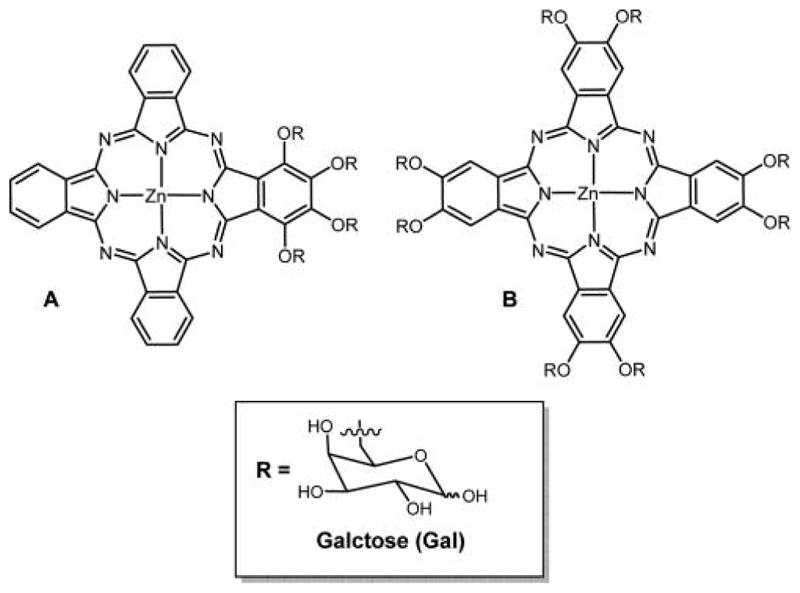
Structure of pure glycophthalocyanine appended with (A) four galactose units from a mixed condensation reaction,310 and (B) eight galactose units made from the phthalonitrile317 in 21% overall yield.
7.2.1.2. Thioether Linkage
Hanack and co-workers320 reported several tetra-substituted S-glycosylphthalocyanine derivatives obtained by cyclization of the S-glycosylated phthalonitrile. All of these compounds were obtained as their isomeric forms, which are difficult to purify.
Recently, Drain and co-workers reported the facile synthesis, photophysical, and cell uptake studies of a Zn(II)Pc appended with eight nonhydrolysable thioglucose units, ZnPcGlc8 (Figure 47).257 This work is the first example of base-promoted substitution of Pc with thioglucose units using the commercially available ZnPcF16 and takes advantage of the leaving group character of fluoride and different reactivities of the F atoms present on the α versus β positions. Zn(II)Pc’s are of great interest to develop Pc-based new generation PS for PDT because of the high triplet quantum yield and long triplet lifetimes.324,325 The longer lifetimes of the PS are advantageous as they promote the diffusional encounter between the excited triplet state of the PS and endogeneous molecular oxygen.
Figure 47.
Symmetrical octa-substituted Zn(II)Pc’s with thioglucose units derived from a core perfluorophthalocyanine platform made in ca. 70% yield in two steps.257 Similar conjugates are also reported using different linkers, for example, Figure 46.320,325,326
ZnPcGlc8 is amphiphilic in nature and was found to be chemically stable. In dry DMSO the compound is highly soluble, but was found to aggregate in water, PBS, or in DMSO–water mixtures as indicated by the significantly quenched fluorescent signal. The low fluorescence quantum yield (Φf = 0.06 in DMSO) for ZnPcGlc8 and correspondingly high singlet oxygen generation quantum yield (1O2, ΦΔ = 0.41 in DMSO) indicate the potential for this compound to be a good PS for PDT. Even though this PS aggregates in aqueous solutions, the small, diffuse spots initially observed by fluorescence microscopy indicate that ZnPcGlc8 is taken up by MDA-MB-231 breast cancer cells mostly as poorly fluorescent nanoaggregates (Figure 48). After 4 days, the slides of the same fixed cells show a significant increase in the fluorescence because of the disaggregation of PS nanoparticles; however, the cell morphology remains the same. These observations clearly indicated uptake of the chromophore into these cells, and the role of nanoaggregated PS is discussed in section 9 in terms of uptake, disaggregation in cancer cells, and the implications for viable strategies for new PDT agents.
Figure 48.
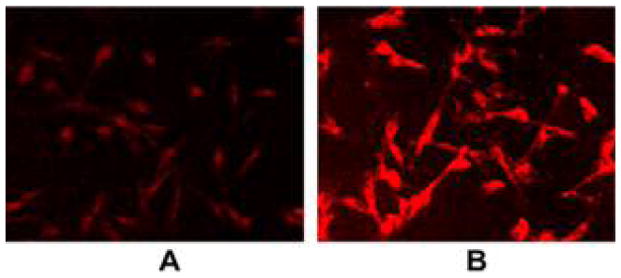
MDA-MB-231 cells were incubated with 50 nM ZnPcGlc8 for 24 h, rinsed three times with PBS buffer to remove unbound dye, and fixed with 4% paraformaldehyde solution. Fluorescence images were captured by exciting at 540–580 nm, magnification 20× under identical conditions. (A) Just after preparation of the fixed cells slide, and (B) 4 days after later. The contrast of each was enhanced by 40% for publication.257 Reproduced with permission from ref 257. Copyright 2011 Elsevier, Inc.
7.2.2. Sugars Linked to Phthalocyanines with Spacers
Glycosyl–phthalocyanine conjugates with short and long spacers between sugar and Pc linked using O-,71,316,327–330 N-331 heteroatoms, and 1,2,3-triazole link330–333 are reported. As noted above, several studies suggest that the presence of appropriate spacers can improve the biological efficacy of the drug, but the reasons for this are not clear.
7.2.2.1. Ether Linkage
Two series of water-soluble Pc–carbohydrate conjugates with tetraethylene glycol spacers, linked via an O-, were reported by Lafont et al. and Ermeydan et al.329,330 Both of these series of compounds have only one sugar moiety appended to the dye and were synthesized by appending sugar moieties on preformed Pc by direct glycosylation of a terminal hydroxyl unit. One glycosylphthalocyanine containing glycerol on three α-positions reported by Lafont et al. is shown in Figure 49A. Asymmetric amphiphilic glycosylphthalocyanines containing thiol-hexane groups on the six β positions of Pc, which is a single isomer as shown in Figure 49B, were reported by Ermeydan et al.330 The biological efficacies of these compounds are discussed in section 7.2.2.3. Other isomeric mixtures of tetra glycosylated pure asymmetric substituted O-glycosyl Pc with different ethylene glycol spacers were reported by Álvarez-Micó et al.316 and Liu et al.328 In vitro PDT efficacy studies on a series of mono-, di-, and tetra-substituted O-glycosyl Pc conjugates were reported by Liu et al. (Figure 50),328 to study the effect of the number and position of the substituents. HT29 human colon adenocarcinoma and HepG2 human hepatocarcinoma cells were used in this study, and the results revealed that isomerically pure disubstituted O-glycosylated Pc’s, in particular the di-α-substituted O-glycosylated Pc’s, had the best PDT efficacy, followed by pure mono-substituted conjugate, as compared to the isomeric mixture of tetra substituted O-glycosylated Pc’s. These results contrast with other studies involving mixtures of isomers, wherein the mixtures of glycosylated Pc perform better than the individual components; this may also reflect differences in octanol/water partition coefficients. A single isomer of an octaglycophthalocyanine with methylene linker was reported by Soares et al. (Figure 51).327 The glycophthalonitrile precursor was prepared by nucleophilic substitution of 4,5-bis(bromomethyl) phthalonitrile followed by the condensation reaction and is reported to have sufficient hydrophilicity and specificity toward tumors, potentially increasing the PDT efficacy.
Figure 49.
Structures of glycosylated glycerol-Zn-phthalocyanine (A) with an intervening tetraethylene glycol spacer, and glycosylated thiol-hexane-Ni-phthalocyanine329 (B) with an intervening tetraethylene glycol spacer; the core Pc was made by a mixed condensation reaction. Ni(II)Pc generally have unfavorable photophysical properties for PDT because of a low-lying d,d state.330
Figure 50.
Mono-, di-, and tetra-O-glycosyl-substituted Pc containing tetraethylene glycol spacer synthesized via condensation of glycosyl phthalonitrile reported by Liu et al.328
Figure 51.
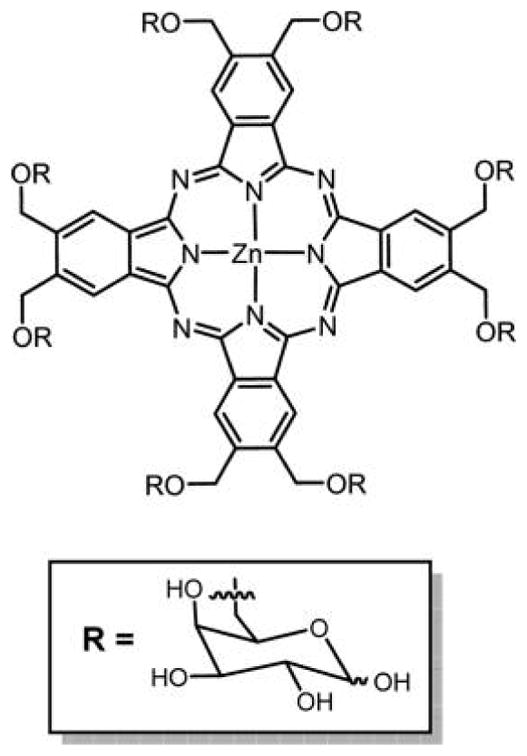
Pc’s bearing eight D-galactose units on the periphery synthesized via condensation of 4,5-(di-D-galactose) phthalonitrile.327
7.2.2.2. Carbamoylation Reaction
Glycoconjugated Pc’s (Figure 52A) were reported by Schotten and co-workers.331 Substitution of the preformed Zn(II)Pc with sugars was achieved by carbamoylation and 1,3-dipolar cycloaddition reactions. The approach of appending targeting moieties to preformed Pc can diminish isomer and purification problems as long as high-yield reactions are used on symmetrically substituted Pc, and, as applied here, can lead to the formation of diverse Pc’s bearing different biomolecules such as amino acids, peptides, proteins, nucleic acids, and steroids. These bioconjugated Pc’s can be useful in generating structure–activity relationships (SAR) for the development of new PSs for PDT, but can provide key starting materials toward the incorporation of Pc cores into functional supramolecular biological matrixes.331
Figure 52.
(A) Structure of octacarbamoyl glycosylated-substituted Zn(II)Pc made from the isocyanate and the hydroxymethylphthalocyanine, and (B) click chemistry to prepare the octaglycosylated Pc starting with the Zn(II)Pc-octa-β-CH2OCH2C≡CH.331
7.2.2.3. 1,3-Cyclo Addition Reaction
After Berthold et al.331 reported the substitution of glycosyl groups on preformed Pc’s using a 1,3-cycloaddition reaction (Figure 52B), Lafont and coworkers329,330,332 reported two different series of monoglycosylated Pc’s linked by 1,2,3-triazole with triethylene glycol spacers and O-glycosylphthalocyanine (Figure 53) to assess the role of the click unit along with two series of Pc–carbohydrate conjugates with tetraethylene glycol spacers, linked via an O-(Figure 49). Two sets of water-soluble monoglycosyl phthalocyanines (Figures 49A and 53A) were investigated for their PDT activity using HT29 human colon adenocarcinoma cells.329 The most active compounds were the O-mannose and O-galactose sugars appended on the terminal hydroxyl unit on preformed Pc with tetraethylene glycol spacer shown in Figure 49A. Thus, the triazole link may play a role in diminishing the effectiveness when compared to the O-linkage sugars, perhaps because of the increased molecular size of the triazole of the conjugates.
The physical and photophysical properties of Pc’s depend upon the central metal ion, the nature of the substituents, and the number and position of these substituents. These distorted Pc’s likely do not have optimal photophysical properties for PDT because of the increased internal conversion. To achieve good solubility and limit the aggregation of Pc’s in most solvents, one strategy is to incorporate branched or bulky substituents or those on the α positions to inhibit π-stacking. Kanat and Dincer prepared the α-substituted tetra terminal alkynyl-substituted Pc’s by the tetramerization of the corresponding precursor having a terminal alkyne function at the C-3 position in the presence of Zn and Co metal salts and/or DBU without protection/deprotection (Figure 54).334 The glycoconjugation of alkyne-functionalized ZnPc prepared was easily achieved via the click reaction between the Pc and azido functional glucopyranosyl in high yield. The glucose units promote water solubility of this ZnPc, and its applications as a PS are currently under investigation.334
Figure 54.
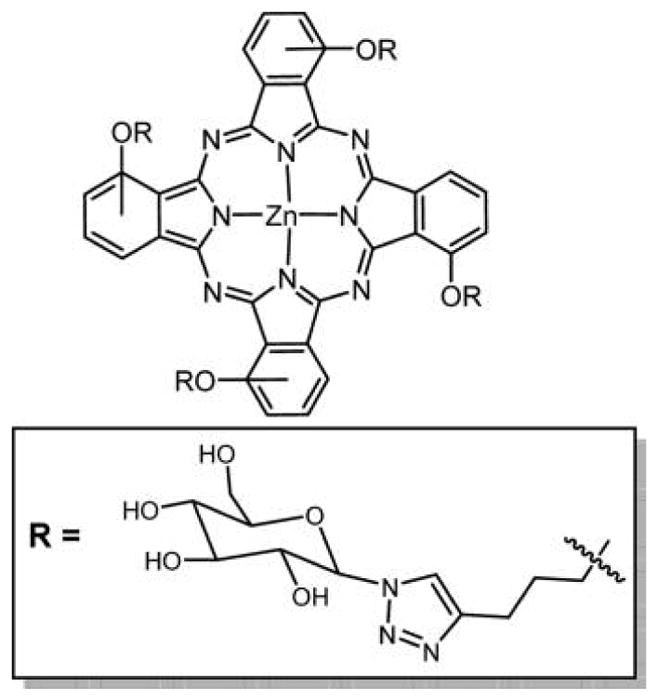
Structure of tetra glycosyl-substituted Zn(II)Pc on the α position.334 The core Pc was obtained by cyclotetramerization of 3-pent-4-ynyloxy phthalonitrile.
7.2.3. Phthalocyanine Dendrimers
Tome and coworkers132,196 reported the synthesis of hexadeca-galacto Pc dendrimer (Figure 55), and recently in vitro studies of this compound were carried on two different human bladder cancer cell lines UM-UC-3 and HT-1376 for their PDT efficacy.196,335 This compound was found to be nontoxic in dark but showed good uptake and has high phototoxicity in both cell lines. Good cell uptake and PDT efficacy of the compound were attributed to the presence of galectin-1 and GLUT1 receptors on the two aforementioned bladder cell lines.
Figure 55.
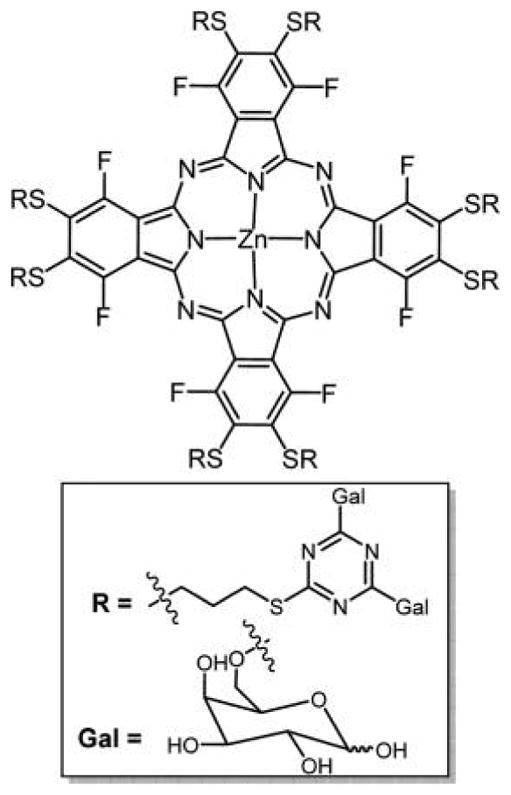
Structure of Zn(II)Pc glycodendritic conjugate with 16 galactopyranose units.132
7.2.4. Phthalocyanine–CD Conjugates
Liu and coworkers reported the synthesis of a glucopyranose conjugated Zn(II)Pc for application as a NIR fluorescent imaging agent.261 The compound was tested as an optical probe on liver tumor bearing nude mice using in vivo fluorescence imaging. The water solubility, stability to photo bleaching, and good emission quantum yield of the compound in the NIR region show that this glucopyranose conjugate of Zn(II)Pc has potential in cancer diagnosis as a NIR optical probe or possibly fluorescence guided surgery.261,321,333,336 Torres and co-workers proposed a new method to obtain water-soluble Pc’s using a covalent linkage to the β-CD, a cyclic glucose heptameric polysaccharide.337 Here, CD provides good amphiphilic character to these compounds because the hydrophilic properties of the CD combined with the hydrophobic cavity in the center of the macrocycle allow these sugar conjugated Pc’s to be soluble in water.338,339 These compounds were prepared via a statistical cross condensation of a 4-(β-CD)phthalonitrile with an excess of phthalonitriles or 4,5-dibutoxypthalonitrile and were characterized by MALDI-TOF-MS (Figure 56).337 The same group also reported a methodology to synthesize asymmetrical sugar appended water-soluble Pc’s by statistical cross condensation of galactosyl-phthalonitrile with phthalonitrile. These CD conjugates have good properties as PSs, and may be useful for constructing supramolecular systems for molecular recognition, with potential applications in optical sensing,340 and in the construction of Pc-based nanostructured materials.210,341,342
Figure 56.
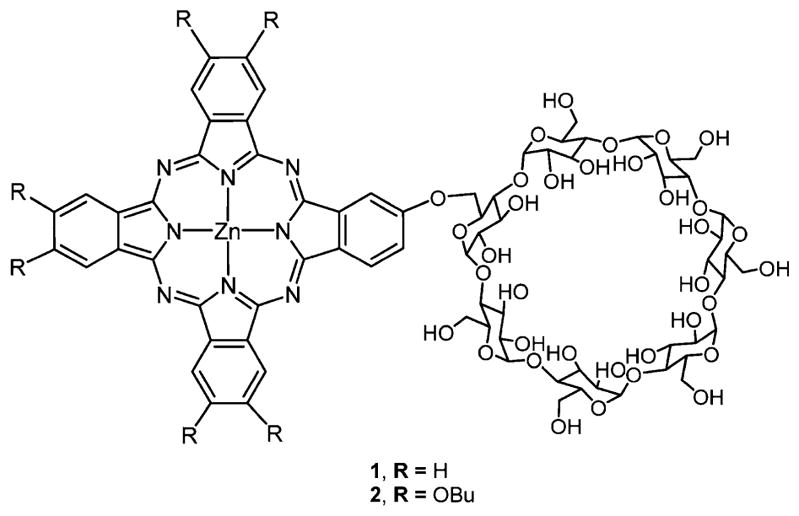
A Zn(II)Pc functionalized with β-CD from a mixed condensation reaction.337
CD can also be attached axially to the metallophthalocyanines. Soft, 5- or 6-coordinate metal ions in Pc can axially bind amines on sugars, albeit with relatively weak binding constants. Conceivably, Pc with both exocyclic and axial sugars can be used to administer the drug, and the axial ligands can exchange with amines on albumin or other biological molecules in vivo, which then can carry the drug to the disease site. Alternatively, Pc coordinating oxophilic metal ions, especially Sn(IV) and Si(IV), can robustly bind oxygen ligands as counterions in a strategy used to self-assemble multichromophore arrays of these dyes.343 Examples of the latter were reported by Ng and co-workers where two CD units attached axially to form a symmetric analogue via an oxomethylene bridge to Si(IV) Pc’s, and unsymmetrical analogues with CD on one side and sugar units on the other side (Figure 57).303 The in vitro photodynamic activities of these symmetrical and unsymmetrical analogues were tested against HT29 human colorectal carcinoma and HepG2 human hepatocarcinoma cells. Because the unsymmetrical analogue is more efficient in generating intracellular ROS, it is thus reported to exhibit greater photocytotoxicity than the symmetrical analogue.303
Figure 57.
Structure of a series of symmetrical and unsymmetrical Si(IV)Pc with a permethylated β-CD unit and a sugar as axial substituents.303
Recently, Tome and co-workers reported three hydrophilic phthalocyanine–CD conjugates, Pc-α-CD, Pc-β-CD, and Pc-γ-CD, via nucleophilic substitution of oxygen atoms of cyclo-maltohexaose (α-CD), cyclo-maltoheptaose (β-CD), and cyclo-maltooctaose (γ-CD) on two fluorine atoms of PcF16.344 The UM-UC-3 bladder cancer cell line was used to study the phototoxicity of these conjugates. Among these conjugates, Pc-α-CD and Pc-γ-CD exhibited higher phototoxicity as compared to Pc-β-CD.
Similar strategies can be used to append other biocompatible motifs such as amino acids, polylysine, peptides, etc., to increase the Pc’s selectivity and targeting for specific cells and tissues.345 For example, palladium-catalyzed Sonogashira cross-coupling reactions afford mono iodotriglycerol-substituted Zn(II)Pc’s.247
8. GLYCOSYLATED PORPHYRAZINES
Porphyrazines (tetra-azaporphyrins, Pz’s) meet all of the requirements to be a good PDT agent, but they are notoriously insoluble in most common solvents. Peripheral-carbohydrate functionalization and core-metal ion complexes can enhance the solubility of the Pz and thus can increase the cellular uptake and phototoxicity. Novel free-base and metallo-Zn(II) or Ni(II) carbohydrate-functionalized Pz (Pz-galactopyranose/ribose derivatives) derivatives were reported by Horne et al.346 and Williams et al.347 for potential use in the administration of PDT (Figure 58). These conjugates proved insoluble in most of the conventional organic solvents except dichloromethane (DCM) and tetrahydrofuran (THF). Pz-galactopyranose/ribose derivatives were solubilized using DCM-based PEG-DSPE5000-PBS encapsulation for biological studies. Only Zn(II) Pz showed sufficient aqueous solubility and low toxicity usig MCF-7 cancer cells, while the free base and Ni(II) derivatives showed persistent aggregation. Further studies are being done on Zn(II)Pz for possible induction of cell death and cell type specificity.346
Figure 58.
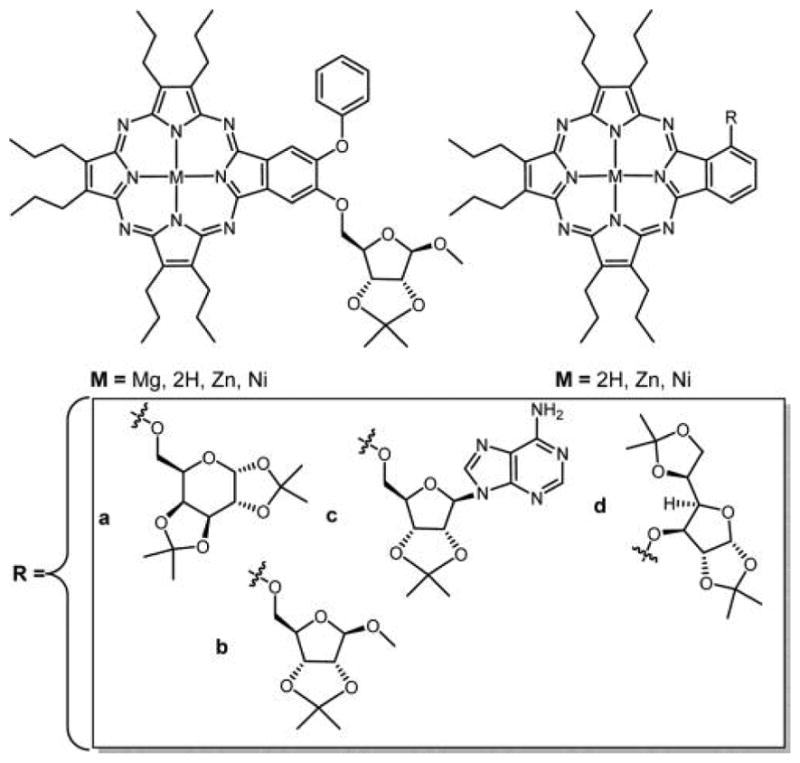
Structure of Pz’s substituted with (a) galactopyranose and (b–d) ribose derivatives.346,347
9. NANOAGGREGATES OF SUGAR-SUBSTITUTED PORPHYRINOIDS AND RELATED MACROCYCLE
The supramolecular chemistry of porphyrinoids is well reviewed,216,343 and the explosive growth of reports on porphyrinoid materials in the last three decades is driven by the facile formation and diverse applications of new photonic and catalytic materials for modern technologies. These technologies include sensors in optical devices, catalysts, drug delivery systems, therapeutics for treatment of variety of diseases, and PSs for photodynamic therapeutic treatment of a variety of diseases such as cancers. To improve the amphiphilicity of the macrocycle, significant effort continues to be invested in appending a variety of biocompatible motifs to the PS, such as sugars, peptides, polylysine, PEGs, and amino acid groups wherein these motifs are also designed to target cancer or bacteria.7,101,348–352 Despite being appended with sugars, the large size and the hydrophobic nature of porphyrinoid cores often cause a rapid aggregation in aqueous media (Figure 59). The size and stability of the aggregate depend on a variety of factors, including number and position of the sugars, the core macrocycle (e.g., π–π interactions between porphyrin, Pc, corrole), other substituents, and whether the compound is the free base or metalated. If the metalloporphyrinoid is used, the charge on the metal ion influences electrostatic interactions, and axial ligands also contribute to the degree of aggregation by inhibiting π–π interactions.213,353–356 The specific intermolecular interactions and size of the nanoaggregates dictate to a large extent cellular uptake and subcellular localization wherein <50 nm diameter nanoaggregates can be endocytosed by cells. The hierarchical structure of the dyes within the nanoaggregates also dictates the photosensitizing properties of the porphyrinoid, wherein the quantum yield of singlet oxygen generation and fluorescence are usually quenched relative to the individual dyes.
Figure 59.
Hydrophobic core of porphyrinoid dyes (A) often drives aggregation in aqueous media into small π-stacked aggregates (B), which can further aggregate into larger organic nanoparticles (ONP). The nanoaggregates can be driven to disassemble by interaction with cellular components such as lipid head groups and protein and/or photothermal processes. While the free bases do not have sufficient contrast in transmission electron microscope studies, the Fe(III) complexes of a fluorous porphyrin do, and reveal the presence of small domains within a larger ONP (C and D).353 Reproduced with permission from ref 353. Copyright 2012 John Wiley and Sons, Inc. If <ca. 50 nm, the aggregate can be taken up by the cell, for example, by endocytosis, where it can disaggregate because of interactions with cellular components, photothermally by internal conversion, and PDT damage to the endosome. Also, the loosely bound subdomains may be induced to break out of the ONP by the same processes and be taken up by the cells. Polysaccharide possessing in the cell will also contribute to the dye distributions, and is a key factor in why nonhydrolyzable bioconjugates can be more effective therapeutics.
Because aggregates of porphyrinoids can be quite stable356,357 and intermolecular interactions affect the photophysical properties of the chromophore, the study of PS aggregates is key to deployment as photonic materials. The degree of aggregation is indicated by the shift, broadening, or the appearance of shoulders on one or both sides of the optical bands, typically focusing on the Soret band. Blue shifts or shoulders indicate face-to-face H- aggregates, and red shifts or shoulders indicate edge-to-edge J-aggregates. Often in porphyrinoid aggregates, one observes both red and blue features, indicating diversity in the intermolecular interactions. In addition to shading effects in aggregates that reduce the relative optical cross sections, aggregates of isoenergetic dyes facilitate excited-state energy transfer, and the resulting branching kinetics significantly diminishes the fluorescence and triplet quantum yields. Thus, aggregates can have limited use as luminescent biochemical tags and PS for PDT. It is difficult to predict the aggregation behavior of these molecules in terms of size and stability because of the aforementioned factors.356 Herein, we focused only on the aggregates of the sugar conjugates of the porphyrinoids and related macrocycles.
Because the porphyrinoid nanoaggregates are self-organized358 by supramolecular interactions,215 they can be induced to disaggregate by a combination of factors. Many of the sugar-coated porphyrins and Pc’s spontaneously aggregate into 10–50 nm aggregates in PBS and other biological buffer systems commonly employed in in vitro and in vivo (Figure 59) studies. These particle sizes can, for example, be endocytosed by cancer cells. Disaggregation can be induced by interactions with proteins in the blood such as albumin, high density lipoproteins (HDL), low density lipoproteins (LDL), and by interactions with a variety of cellular components inside the cell such as proteins, polar head groups of membranes, etc. We have postulated that the sugars on the outside of the aggregates induce the cell to take up the aggregates and then decompartmentalize and disassemble the aggregate to allow the distribution of the dyes in the cell by mechanisms similar for large polysaccharides.171 Because the strength of the supramolecular interactions within an aggregate depends on the specific dye under investigation, the rate of disaggregation is highly dependent on molecular structure and specific interactions with cellular components. Typically, the Pc’s, Pz’s, and benzoporphyrins form more robust aggregates than meso-arylporphyrins and corroles.
Individual porphyrinoids, especially those that have flat molecular architectures, for example, when the sugars are appended to the para positions of meso-arylporphyrins versus the ortho or meta positions, can passively diffuse through the membrane driven by concentration gradients and amphipathic properties. Conversely, passive diffusion is much less likely with bulky molecules and aggregates that have a hydrophobic shell (sugars) around a hydrophilic core (dye). At low concentrations of sugar-appended porphyrins and Pc’s, the dyes can be largely nonaggregated. However, as these dyes collect around the target cells driven by the receptors expressed on the membrane exterior, the local dye concentration substantially increases in the cell micro environment, and this can result in the self-organization of nanometer scale aggregates that are near the cell or attached via the receptor(s) (Figure 60). Thus, there are two possible mechanisms for dye aggregation, and they are not mutually exclusive.
Figure 60.
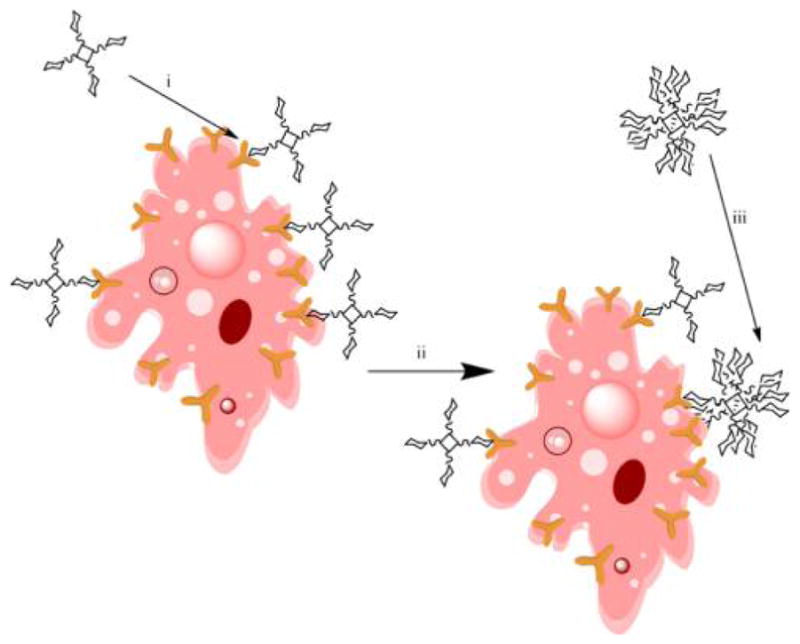
(i) At low concentrations, the porphyrinoid dyes are solvated and not aggregated. (ii) Upon binding to the target cell, the local concentration increases and the dyes then aggregate. (iii) Nanoaggregates in aqueous media have sugar-coated shells around the dye core, which then can bind to cell receptors. Either or both processes can be present depending on the specific dye and cell types and media.
When dyes are aggregated, the excitation energy is largely dissipated by internal conversion (vibrations as heat), which then can reduce the strength of the intermolecular interaction in the nanoaggregates. Photoinduced heating to initiate disaggregation of the dyes may also cause release from endosomes and similar structures, and some photothermal stress to the cell similar to other nanoparticles used in photothermal therapy (PTT).359 Although reduced, the singlet oxygen that is formed in the nanoaggregates can damage the vesicle and cause the dyes to be released. The extent of each of these factors will depend on the specific system under investigation. Both in vitro and in vivo studies have shown that a short light irradiation followed by an induction period and then the therapeutic light treatment significantly increases the PDT efficacy of a variety of dyes.5,171,360 PDT is postulated to have a role in overcoming drug resistance by cancer.361
9.1. Sugar Porphyrin Aggregates
Self-aggregation of porphyrinoids in aqueous media or in mixed solvent systems is common and is mainly driven by π–π interactions between the macrocycles and is a complex process that may involve the formation of dimers, trimers, oligomers, and/or large-scale aggregates. The particle size distribution also varies with both dye and environment.
Sugar appended porphyrinoids, in which the sugar group is attached through C-, S-, and O- atom to the macrocycle, are known to form aggregates in buffer, polar and nonpolar organic solvents, and in mixed solvent systems.118,142,147,157 The size and structural organization of organic nanoparticles of sugar porphyrinoids depend on a variety of factors including concentration, solvent, mixing technique, number of appended sugar groups, and the architecture of the component molecule, for example, relative orientation of the sugars and structure of tethers. Formation of aggregates of sugar porphyrinoid conjugates in aqueous solvents such as PBS or buffers with serum albumin quenches the fluorescence and thereby decreases the singlet oxygen generation efficiency. Singh et al. have investigated the aggregation of tetra-thioglycosylated porphyrin conjugates (PGlc4), the chlorin (CGlc4), and isobacteriochlorin (IGlc4) in PBS.234 The aggregates of these chromophores were characterized by UV–visible spectroscopy, and the sizes were measured by dynamic light scattering. These conjugates aggregate to a different extent to form various sizes range particles. However, all of these are taken up by cancer cells as shown by fluorescence microscopy (see Figures 27 and 28).
Hirohara et al. reported the aggregation behavior in PBS of a series of sugar conjugates on the meta position of tetraarylchlorins (Figure 61) including 5,10,15,20-tetrakis[3-(β-D-glucopyranosyloxy)phenyl]chlorin, 5,10,15,20-tetrakis[3-(β-D-galactopyranosyloxy)phenyl]chlorin, 5,10,15,20-tetrakis-[3-(β-D-xylopyranosyloxy)phenyl]chlorin, and 5,10,15,20-tetrakis[3-(β-D-arabinopyranosyloxy)phenyl]chlorin. Their data suggest that the mode of aggregation, H-aggregate versus J-aggregate, depends on the number of carbon atoms in the sugar units. Hexose conjugates of chlorin such as glucose and galactose predominantly formed H-aggregates, while sugar conjugates of chlorin with five carbon atoms such as xylose and arabinose form J-aggregates.147 The steric bulk and orientation of these sugars are roughly equivalent, so it may be that cooperative H-bonding interactions give rise to the differences in aggregation.
Figure 61.
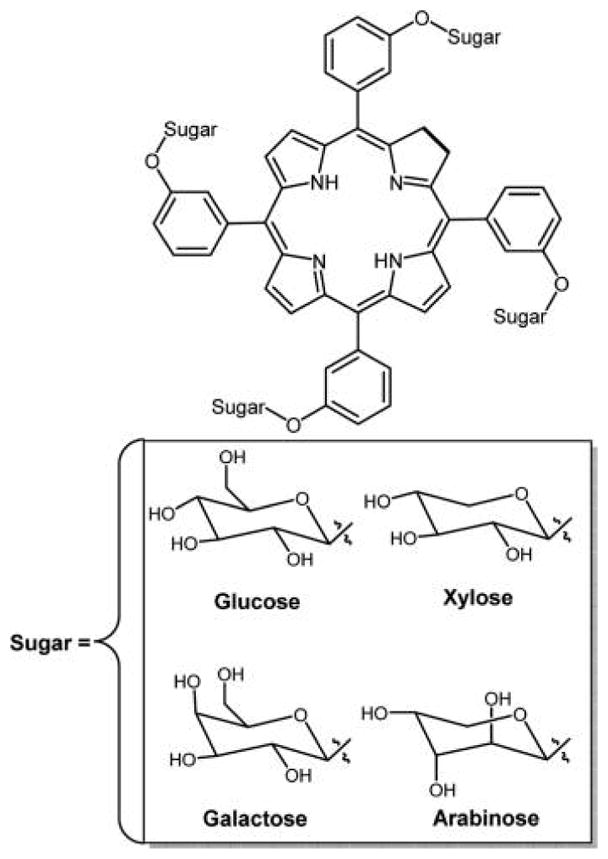
Aggregation properties were correlated with the 5- and 6-carbon sugars, where the former is reported to have a propensity to form H-aggregates and the latter J-aggregates.147
Mikata et al. reported the aggregation behavior of a series of tris(maltohexoses) linked to tetraarylporphyrins with an alkyl chain of varying number of C atoms. Tris(maltohexoses) porphyrins without the alkyl group undergo H-aggregation predominantly where the porphyrin macrocycles are rotated relative to each other (see Figure 59), while the compounds with 2, 4, 6, 10, and 16 C atoms in the alkyl chain predominantly show J-aggregation where the porphyrin macrocycles are further away from each other.362
Song et al. studied the size-controlled aggregates of tetrakis(4-carboxyphenyl)porphyrin–pullulan conjugate and 5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin–pullulan conjugate in PBS buffer. They observed a smaller aggregate (~100 nm) for tetrakis(4-carboxyphenyl)porphyrin-pullulan conjugate as compared to the other conjugate (~320 nm). This was attributed due to the core tetrakis(4-carboxyphenyl)-porphyrin molecule, which is more hydrophilic with three extra carboxylic groups as compared to 5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin.363
Drasar and co-workers reported the aggregation of a series of glycosylated porphyrins with glycosylated groups directly attached to the meso position via a C atom linkage, and 5,15-diglycosylated compounds with 10,20-meso-perfluorophenyl groups in mixed solvent systems such as DMF–water, DMSO–water, and CH3CN–water.118,177
These studies indicate that the self-aggregation of sugar porphyrins also depends on the position of sugar moiety attached. These chromophores show good solubility in that they stay in the monomeric form in polar solvents such as DMSO, DMF, MeCN, CH3OH but form H-type aggregates in mixed solvent system with water, such as CH3CN–H2O and CH3OH–H2O. The degree of aggregation also depends on the % composition of water in the solvent mixture. The critical aggregation solvent composition is ≥~45% water for tetra meso-C-D-glucose porpphyrin, ≥45% for di meso-C-D-glucose porphyrin, ≥50% for tetra meso-C-D-galacto poprhyrin, and ≥60% for di meso-C-D-galacto porphyrin. The formation of aggregates in solvent mixture containing water is due to hydrophobic effects, for example, π–π interactions and dispersion forces, as well as specific carbohydrate–π interactions. Ibrahim et al. reported the tetra para glycosylated derivative of TPP aggregates rapidly in aqueous media as compared to the corresponding tetra meta glycosylated derivative of TPP, and the mono glycosylated compounds insert into liposomes and albumin.364 This makes sense because the ortho and meta positions direct the sugars to one face or the other, and in both cases the compounds exist as statistical mixtures of four atropisomers.
9.2. Sugar Phthalocyanine Nanoaggregates
Drain and co-workers recently reported that octa thioglycosylated Zn(II)phthalocyanine, ZnPcGlc8 does not aggregate in pure DMSO solvent,257 but forms different sized aggregates in other organic solvents such as ethyl acetate, toluene, ethanol, PBS, and also in mixed solvent systems such as DMSO:H2O. About 250 nm diameter aggregates were observed without sonication, and after sonication of this solution for ca. 15 min these large sized aggregates reorganized to form ca. 65 nm sized aggregates in mixed solvent system such as DMSO–water, and in PBS. For Pc’s, the lowest energy Q-band is blue-shifted upon aggregation, indicative of the formation of H-aggregates.4,257 Lyubimtsev et al.365 reported the aggregation of both tetra- and octa-glycosylated Pc bearing the sugars at the α and β positions through O- and S- atoms in aqueous solutions and mixed DMSO–water solvent system. These authors concluded that the degree of aggregation of O-glycosylated ZnPc is greater than that of the S-glycosylated compound because of electronic effects. The degree of aggregation of sugar Pc’s also depends on the nature and number of sugar moieties attached. Choi et al.319 reported that the tetra 1,2:5,6-di-O-isopropylidene-α-D-glucofuranose or 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose-substituted Zn(II)Pc’s made from the glycosubstituted phthalonitriles were less active than the corresponding mono-substituted derivatives against HepG2 human hepatocarcinoma and HT29 human colon adenocarcinoma cells because of decreased cell uptake. Somewhat counterintuitively, the tetra glycosylated derivatives significantly aggregate in aqueous solution as compared to the mono substituted; however, it may be that the mono glyco-Pc forms small aggregates suspended in water that were not detected.
The biomedical application of aggregates of glycosylated porphyrinoids such as porphyrins and Pc’s is complicated by the delivery of the chromophore to target tissues and into target cells. Where aggregates bind to cells and how, or if, they disaggregate is dependent on the specific molecular component. The octanol/water partition coefficient is representative of the lipophilicity of the glycosylated macrocycle but does not reveal the nature of the aggregation in either solvent. Because <50 nm nanoaggregates can be endocytosized into cells, we found that the nanoaggregates of ZnPcGlc8 (Figure 47) can go into MDA-MB-231 human breast cancer cells, but exhibit poor fluorescent signaling evidenced by the mapping of the emission energy between the various chromophoric units in the nanoaggregates. However, ZnPcGlc8 disaggregates with time, and as a result the fluorescence signals significantly increase in confocal microscopy. The uptake of aggregates of sugar conjugated porphyrins and Pc’s into cancer cells, the subsequent disaggregation, and concomitant increase in PDT and fluorescence activities indicate that these compounds may be viable PDT agents.
Corroles and corrolazines are the cognates of the porphyrinoid compounds. Amphiphilic corroles appended with phenyl carboxylic acid moieties at the meso position are reported to aggregate in mixed water ethanol solvent system.366 Miao et al. reported the formation of self-aggregates of corroles dimers on surfaces such as highly ordered pyrolytic graphite (HOPG), mica, and Au.367,368 Hameren et al. reported the self-aggregation of corroles trimers in n-hexane solution.369,370 To date, characterization of the aggregates of sugar-coated corroles or sugar-coated corrolazines is not reported. However, we speculate that sugar conjugates of corroles and corrolazines will exhibit aggregation behavior similar to that of the sugar porphyrin and Pc compounds. Because of the missing meso position, corroles with sugars have polar and nonpolar regions, and so presumably aggregate in water.256
10. CONCLUSIONS
Despite vigorous research efforts, the optimal PSs for PDT and a fluorophore for bioimaging that fulfills the entire set of requirements for in vivo applications have not been created. For theranostics, a balance between fluorescence for optical imaging and singlet oxygen quantum yield for PDT is needed, and the chlorins seem to be the best candidates. However, the optical cross section between 650 and 750 nm of the Pc’s is much larger than for the chlorins, but the fluorescence is significantly less than chlorins. Both chlorins and Pc’s are stable to photo bleaching, but there are few if any quantitative studies on the fate of the sugars during and after PDT, which are likely oxidized and hydrolyzed. In this regard, the C- and S- glycoside bonds likely enhance the activity of the sugar–porphyrinoid conjugate.
One promising aspect of recent research is that there are now a variety of organic synthetic strategies that are easy, straightforward, and of high yield. Efficient syntheses are needed for the construction of porphyrinoid conjugates to any biomolecular species or biocompatible motif to rapidly assess the role of the targeting moiety rather than spending time on the synthesis of the dye. Second, straightforward syntheses facilitate scaling up the synthesis for trials and afford commercial viability. In this regard, the fluorine substitution chemistry can be done on an array of porphyrinoids.7 The presence of fluorine on a PS, for example, derivatives of perfluorophenylporphyrin TPPF20, perfluorophenyl corrole (CorF15), and perfluorophthalocyanine (PcF16) platforms, imparts several properties and opens several new functionalities. Fluorine groups are known to modulate pharmacokinetics, increase photostability to bleaching, enhance singlet oxygen formation, and alter lipophilicity.371,372 The 16 remaining fluoro groups on the porphyrin or chlorin or bacteriochlorin, 12 remaining on the corrole, and eight remaining on the Pc may be suitable for 19F NMR imaging, which can be correlated with fluorescence imaging. Reactions of the above fluorous dyes with K18F and the K+ chelator kryptofix at elevated temperatures may allow F-18 labeling and enable positron emission tomography (PET) without altering the photophysics for fluorescence imaging or PDT.
Over the last three decades, four PS drugs for PDT were approved in the U.S. and Europe. There are more PS under clinical trials for treating different kinds of diseases. One of the most important aspects that needs to be addressed is to increase the efficacy of the PS while decreasing the side effects. So, different targeting and delivery systems have been employed, either on the PS or on vesicles or nanoparticles to effectively deliver it to the targeted site.373 These involve silicon nanoparticle,374,375 hyaluronic acid-PS nanoparticle,376 gold nanoconjugates,377 micelle nanoparticle,378 liposomes,379,380 peptides,381,382 and sugars to improve the water solubility, stability, targeting efficiency, and improve the pharmacokinetics or prolonged release of the PS.383,384 In combination with other therapies such as BNCT, PDT is particularly attractive for the treatment of cancers because it targets different mechanisms of cancer cell destruction and increases the overall therapeutic effect. Recently, age related macular degeneration has been treated using combination of anti-VGFR and PDT.385 Also reported recently is PDT combined with the intravitreal ranibizumab (IVR) technique for the treatment of eye related polypoidal choroidal vasculopathy (macular degeneration) disease resulting in visual and anatomical improvements.386
Because the porphyrinoids cores are poorly soluble or insoluble in physiological conditions of cells/tissues, they aggregate quickly into highly stable precipitates. In addition to targeting, appending biocompatible moieties to the macrocycles increases solubility in aqueous environments, and therefore diminishes aggregation and destabilizes the intermolecular interactions. Clearly, aggregation needs to be considered, but can be exploited because the aggregates are less photo active, which might reduce unwanted side effects such as light sensitivity. The sugar moieties can be attached to the macrocycle either covalently at the peripheral positions or coordinately, axially to the central metal ion, especially to oxophilic metal ion centers. In comparison with O-glycosylated PSs, CONH-glycosyl, thio-glycosyl, and C-glycosyl bonds should resist endogenous hydrolysis catalyzed by glycosidases and lower pH.137 Appending cyclodextrin moieties on these macrocycles may be a potential strategy to make new generation PS for PDT and has proven capacity as a vehicle for the drug delivery. Because of the new chemistries allowing rapid synthesis, many targeting motifs can be assayed.
The fact that Photofrin is a complex mixture may be the single best reason that it remains the gold standard for PDT because different components can partition into different cellular and tumor environments. Given that mixtures of different PS may have synergistic effects for PDT, one can envision designed mixtures using the same platform dye but with different groups that target quite different cellular structures or that are activated in different parts of the cell. This designed mixture would then synergistically and simultaneously disrupt or destroy essential cellular functions and structures to affect tumor necrosis or antiviral or antibacterial activities.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation – United States (NSF), through CHE-0847997 and CHE-1213962 to C.M.D. Hunter College science infrastructure is supported by the NSF, the National Institute on Minority Health and Health Disparities– United States (8G12 MD007599), and the City University of New York. We would like to thank Aaron Dolor for helping with the initial literature searches.
ABBREVIATIONS
- PDT
photodynamic therapy
- PS
photosensitizer
- ROS
reactive oxygen species
- BODIPY
boron-dipyrromethene
- FDA
Food and Drug Administration
- EMEA
European Medicines Evaluation Agency
- 5-ALA
5-aminolevulinic acid
- Metvix
5-aminolevulinic acid methyl ester
- Lu-Tex
lutetium texaphyrin
- Glc
glucose
- Gal
galactose
- Man
mannose
- TPP
tetraphenylporphyrin
- TPPF20
5,10,15,20-tetrakis(2,3,4,5,6-pentafluorophenyl)-porphyrin
- CF20
perfluorophenylchlorin
- IF20
perfluorophenylisobacteriochlorin
- BF20
perfluorophenylbacteriochlorin
- PGlc4
thioglycosylated porphyrin
- CGlc4
thioglycosylated chlorin
- IGlc4
thioglycosylated isobacteriochlorin
- BGlc4
thioglycosylated bacteriochlorin
- PGal4
thiogalactosylated porphyrin
- m-THPC
meta-tetra(hydroxyphenyl)chlorin
- PorCu-Lac8
octa-β-lactoglycosylated porphyrinato copper
- Pc
phthalocyanine
- PcF16
perfluorophthalocyanine
- Zn(II)Pc
zinc(II) phthalocyanine
- Si(IV)Pc
silicon(IV) phthalocyanine
- Al(III)Pc
aluminum(III) phthalocyanine
- ZnPcGlc8
octa thioglycosylated zinc(II) phthalocyanine
- TBP
tetraphenylbenzoporphyrin
- Ar4TBP
tetraaryltetrabenzoporphyrin
- CorF15
perfluorophenyl corrole
- Pz
porphyrazines
- MEG
monoethylene glycol
- DEG
diethylene glycol
- PEG
polyethylene glycol
- CD
cyclodextrin
- MB
Methylene blue
- pyro-2DG
pyropheophorbide-2-deoxyglucosamide
- DMSO
dimethyl sulfoxide
- DCM
dichloromethane
- PBS
phosphate buffered saline
- HOPG
highly ordered pyrolytic graphite
- ONP
organic nanoparticles
- 1O2
singlet oxygen
- ΦΔ
quantum yield
- Φisc
intersystem quantum yield
- HSV-1
Herpes simplex virus type 1
- IC
internal conversion
- ISC
intersystem crossing
- MW
microwave
- SOD
superoxide dismutase
- Con A
Concanavalin A
- NIR
near infrared
- DLS
dynamic light scattering
- LDL
low density lipoproteins
- HDL
high density lipoproteins
- TPA
two-photon absorption
- NLO
non linear optical
- BNCT
boron neutron capture therapy
- BBB
blood brain barrier
- PTT
photothermal therapy
Biographies

Sunaina Singh received her Masters in Chemistry (Honors School) from Punjab University, Chandigarh, India, in 2002. She received her Ph.D. in 2011 from Hunter College of the City University of New York under the supervision of Prof. Charles Michael Drain, laboratory of Supramolecular Photonics. She then pursued her postdoctoral research work from 2011–2013 with Dr. Ronald Koder at The City College of New York, CUNY. In August 2013, she joined as a faculty member at the department of Natural Sciences, LaGuardia Community College, where her research focuses on the synthesis of porphyrin-based photosensitizers for photodynamic therapy and imaging.

Amit Aggarwal received his M.Sc. in Chemistry from D. A. V. College, Jalandhar, India, in 2000. He then joined the Department of Chemistry, Lyallpur Khalsa College as a lecturer and worked there from 2002–2005. In 2006 he joined Hunter College of The City University of New York for his graduate studies and received his Ph.D. in 2011 under the supervision of Prof. Charles Michael Drain. His doctoral research focused on the study of catalytic properties and photophysics of porphyrinoid-based materials. He conducted his Postdoctoral Research in the Department of Biochemistry at Weill Cornell Medical College, New York, from 2011–2013. Currently, he is an Assistant Professor in the department of Natural Sciences at LaGuardia Community College of The City University of New York. His research focuses on the preparation of porphyrinoid-based nanomaterials for their catalytic properties and also to develop new porphyrin-based photosensitizer for both imaging and photodynamic therapeutic treatment of cancers.

N. V. S. Dinesh K. Bhupathiraju was born in Andhrapradesh, India. He obtained his Bachelor’s degree in chemistry (2005) and Master’s degree in physical organic chemistry (2008) from Osmania University, Hyderabad, India. In 2013 he obtained his Ph.D. in organic chemistry from Louisiana State University, LA, under the guidance of Prof. Maria Graça H. Vicente. Presently, he is working as a postdoctoral associate at Hunter College of the City University of New York in Prof. Charles Michael Drain’s laboratory of Supramolecular Photonics and also as a visiting lecturer in the Department of Chemistry and Biochemistry, Hunter College of the City University of New York. His research projects in Prof. Drain’s laboratory involve theranostic nanomedicines for cancer and the development of new dyes for solar energy harvesting materials for coating on windows in urban applications.

Gianluca Arianna began research under the supervision of Dr. Charles Michael Drain in 2008, during his junior year of high school. His research project focused on the dynamic aggregation and cellular uptake of three novel porphyrinoid therapeutics designed for clinical photodynamic therapy and as cancer killing agents. He presented his research in 2009 at NYSCEF, reaching the semifinals, and was awarded the Ezra Levy Award for research in clinical chemistry for his work. From 2009–2013 Mr. Arianna attended CUNY Hunter College, where he worked to complete the ACS certification major and continued his research in the application of porphyrins for cancer treatment, studying their phototoxicity in cancer cell lines. In 2014, after completing his ACS requirements, he received his Bachelor of Arts, graduating magna cum laude. Currently, he is an Adjunct Lecturer in the chemistry department at Hunter College and a volunteer at Bellevue Hospital. He is also conducting research at Weill Cornell Medical College in the Department of Biochemistry and at Memorial Sloan Kettering Cancer Center. His research at Cornell focuses on the elucidation of neuronal protein pathways that regulate synaptic transmission using the C. elegans genetic model, while his research at MSKCC focuses on developing computer models for simulating growth of gold nanocrystals for use in cancer imaging.

Kirran Tiwari was born in Oklahoma City, OK, and was raised in Floral Park, NY. He is currently a junior at the Macaulay Honors College at Hunter College and is pursuing a Biochemistry major. In 2014, he joined Charles Michael Drain’s lab where he is developing a mild extraction method to improve the extraction of pigments from avian eggshells to understand how eggshell pigmentation chemistry is exploited by avian in different ecological contexts. Previously, he studied the antimicrobial properties and the relative toxicity of various medicinal herbs in the West Indies.

Charles Michael Drain is Professor of Chemistry at Hunter College of the City University of New York and adjunct faculty at Rockefeller University. He started his career in chemistry at the University of Missouri in St. Louis. He received his Ph.D. from Tufts University in the laboratory of Barry B. Corden where he worked on porphyrin synthesis. Afterwards, he did postdoctoral work in the laboratory of David Mauzerall at The Rockefeller University where he examined self-organizing systems composed of porphyrins and lipid bilayers and developed one of the first examples of a purely organic, synthetic phototransistor. He also examined the interactions between chiral ion channels helices and chiral centers in lipids with R. Bruce Merrifield. The following two years he was a guest researcher in the laboratory of Jean-Marie Lehn at the University Louis Pasteur in Strasbourg, France, where he developed methodologies to self-assemble porphyrins. Afterward, he spent two years as a research fellow in the Dewey Holten Chris Kirmaier laboratory at Washington University studying the complex dynamics of nickel porphyrins. Since joining Hunter College, 1996, his research continues to focus on the design, synthesis, and characterization of self-assembled and self-organized photonic systems.
Footnotes
DEDICATION
This work is dedicated to the memory of Dr. Clifford E. Soll, all around excellent chemist and colleague.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrev.5b00244.
Appendix with references (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Ogilby PR. Singlet oxygen: there is indeed something new under the sun. Chem Soc Rev. 2010;39:3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- 2.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002;233–234:351–371. [Google Scholar]
- 3.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, et al. Photodynamic therapy of cancer: An update. Ca-Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001;5:105–129. [Google Scholar]
- 5.Henderson BW, Dougherty TJ. How Does Photodynamic Therapy Work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Drain CM. Photodynamic Therapy using Carbohydrate Conjugated Porphyrins. Drug Des Rev–Online. 2004;1:215–234. [Google Scholar]
- 7.Drain CM, Singh S. Combinatorial libraries of porphyrins: Chemistry and applications. In: Kadish K, Smith KM, Guilard R, editors. The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Vol. 3. World Scientific Publisher; Singapore: 2010. pp. 485–537. [Google Scholar]
- 8.Sharma SK, Mroz P, Dai T, Huang YY, Denis TGS, Hamblin MR. Photodynamic Therapy for Cancer and for Infections: What Is the Difference? Isr J Chem. 2012;52:691–705. doi: 10.1002/ijch.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rosa A, Naviglio D, Di Luccia A. Advances in Photodynamic Therapy of Cancer. Curr Cancer Ther Rev. 2011;7:234–247. [Google Scholar]
- 10.Skovsen E, Snyder JW, Lambert JDC, Ogilby PR. Lifetime and Diffusion of Singlet Oxygen in a Cell. J Phys Chem B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 11.Snyder JW, Lambert JDC, Ogilby PR. 5,10,15,20-Tetrakis(N-Methyl-4-Pyridyl)-21 H,23H-Porphine (TMPyP) as a Sensitizer for Singlet Oxygen Imaging in Cells: Characterizing the Irradiation-dependent Behavior of TMPyP in a Single Cell. Photochem Photobiol. 2006;82:177–184. doi: 10.1562/2005-05-30-RA-553. [DOI] [PubMed] [Google Scholar]
- 12.Snyder JW, Skovsen E, Lambert JDC, Ogilby PR. Subcellular, Time-Resolved Studies of Singlet Oxygen in Single Cells. J Am Chem Soc. 2005;127:14558–14559. doi: 10.1021/ja055342p. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz AA, Jans DA, Sobolev AS. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunol Cell Biol. 2000;78:452–464. doi: 10.1046/j.1440-1711.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 14.Balaz M, Collins HA, Dahlstedt E, Anderson HL. Synthesis of hydrophilic conjugated porphyrin dimers for one-photon and two-photon photodynamic therapy at NIR wavelengths. Org Biomol Chem. 2009;7:874–888. doi: 10.1039/b814789b. [DOI] [PubMed] [Google Scholar]
- 15.Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic Therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jichlinski P, Leisinger HJ. Photodynamic therapy in superficial bladder cancer: past, present and future. Urol Res. 2001;29:396–405. doi: 10.1007/s002400100215. [DOI] [PubMed] [Google Scholar]
- 18.Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 19.Detty MR, Gibson SL, Wagner SJ. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J Med Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch C, Goerz G, Ruzicka T. Photodynamic Therapy in Dermatology. Arch Dermatol. 1998;134:207–214. doi: 10.1001/archderm.134.2.207. [DOI] [PubMed] [Google Scholar]
- 21.Szeimies RM, Landthaler M, Karrer S. Non-oncologic indications for ALA-PDT. J Dermatol Treat. 2002;13:s13–s18. doi: 10.1080/095466302317414654. [DOI] [PubMed] [Google Scholar]
- 22.Bonneau S, Vever-Bizet C. Tetrapyrrole photosensitisers, determinants of subcellular localisation and mechanisms of photodynamic processes in therapeutic approaches. Expert Opin Ther Pat. 2008;18:1011–1025. [Google Scholar]
- 23.Phillips D. Light relief: photochemistry and medicine. Photochem Photobiol Sci. 2010;9:1589–1596. doi: 10.1039/c0pp00237b. [DOI] [PubMed] [Google Scholar]
- 24.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 25.Taquet JP, Frochot C, Manneville V, Barberi-Heyob M. Phthalocyanines Covalently Bound to Biomolecules for a Targeted Photodynamic Therapy. Curr Med Chem. 2007;14:1673–1687. doi: 10.2174/092986707780830970. [DOI] [PubMed] [Google Scholar]
- 26.Davia K, King D, Hong Y, Swavey S. A porphyrin-ruthenium photosensitizer as a potential photodynamic therapy agent. Inorg Chem Commun. 2008;11:584–586. doi: 10.1021/ic8015589. [DOI] [PubMed] [Google Scholar]
- 27.Ko YJ, Yun KJ, Kang MS, Park J, Lee KT, Park SB, Shin JH. Synthesis and in vitro photodynamic activities of water-soluble fluorinated tetrapyridylporphyrins as tumor photosensitizers. Bioorg Med Chem Lett. 2007;17:2789–2794. doi: 10.1016/j.bmcl.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 28.Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev. 1995;24:19–33. [Google Scholar]
- 29.Sternberg ED, Dolphin D, Brückner C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151–4202. [Google Scholar]
- 30.Silva AMG, Tomé AC, Neves MGPMS, Silva AMS, Cavaleiro JAS. 1, 3-Dipolar Cycloaddition Reactions of Porphyrins with Azomethine Ylides. J Org Chem. 2005;70:2306–2314. doi: 10.1021/jo048349i. [DOI] [PubMed] [Google Scholar]
- 31.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moylan C, Scanlan EM, Senge MO. Chemical Synthesis and Medicinal Applications of Glycoporphyrins. Curr Med Chem. 2015;22:2238–348. doi: 10.2174/0929867322666150429113104. [DOI] [PubMed] [Google Scholar]
- 33.Garland MJ, Cassidy CM, Woolfson D, Donnelly RF. Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med Chem. 2009;1:667–691. doi: 10.4155/fmc.09.55. [DOI] [PubMed] [Google Scholar]
- 34.Konan YN, Gurny R, Allémann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol, B. 2002;66:89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 35.Calzavara-Pinton PG, Venturini M, Sala R. Photodynamic therapy: update 2006 Photochemistry Photobiology. J Eur Acad Dermatol Venereol. 2007;21:293–302. doi: 10.1111/j.1468-3083.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 36.Boyle RW, Dolphin D. Structure and Biodistrlbution Relationships of Photodynamic Sensitizers. Photochem Photobiol. 1996;64:469–485. doi: 10.1111/j.1751-1097.1996.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 37.Bonnett R, Martínez G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron. 2001;57:9513–9547. [Google Scholar]
- 38.Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. BODIPY dyes in photodynamic therapy. Chem Soc Rev. 2013;42:77–88. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gryko DT, Koszarna B. Refined methods for the synthesis of meso-substituted A3- and trans-A2B-corroles. Org Biomol Chem. 2003;1:350–357. doi: 10.1039/b208950e. [DOI] [PubMed] [Google Scholar]
- 40.Liu HY, Lai TS, Yeung LL, Chang CK. First Synthesis of Perfluorinated Corrole and Its MnO Complex. Org Lett. 2003;5:617–620. doi: 10.1021/ol027111i. [DOI] [PubMed] [Google Scholar]
- 41.Hagihara S, Miyazaki A, Matsuo I, Tatami A, Suzuki T, Ito Y. Fluorescently labeled inhibitor for profiling cytoplasmic peptide:N-glycanase. Glycobiology. 2007;17:1070–1076. doi: 10.1093/glycob/cwm079. [DOI] [PubMed] [Google Scholar]
- 42.Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discovery Today. 1999;4:507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 43.Lovell JF, Liu TWB, Chen J, Zheng G. Activatable Photosensitizers for Imaging and Therapy. Chem Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 44.Josefsen LB, Boyle RW. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met-Based Drugs. 2008;2008(276109):1–24. doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234–1245. doi: 10.1039/b705461k. [DOI] [PubMed] [Google Scholar]
- 46.O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 47.Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012;326:8–16. doi: 10.1016/j.canlet.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finlay JC, Zhu TC, Dimofte A, Stripp D, Malkowicz SB, Busch TM, Hahn SM. Interstitial Fluorescence Spectroscopy in the Human Prostate During Motexafin Lutetium-Mediated Photodynamic Therapy. Photochem Photobiol. 2006;82:1270–1278. doi: 10.1562/2005-10-04-RA-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu TC, Finlay JC, Hahn SM. Determination of the distribution of light, optical properties, drug concentration, and tissue oxygenation in-vivo in human prostate during motexafin lutetium-mediated photodynamic therapy. J Photochem Photobiol, B. 2005;79:231–241. doi: 10.1016/j.jphotobiol.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ormond A, Freeman H. Dye Sensitizers for Photodynamic Therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senge MO, Brandt JC. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)chlorin)—A Second-generation Photosensitizer. Photochem Photobiol. 2011;87:1240–1296. doi: 10.1111/j.1751-1097.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 52.Pogue BW, Redmond RW, Trivedi N, Hasan T. Photophysical Properties of Tin Ethyl Etiopurpurin I (SnET2) and Tin Octaethylbenzochlorin (SnOEBC) in Solution and Bound to Albumin. Photochem Photobiol. 1998;68:809–815. [PubMed] [Google Scholar]
- 53.Sekkat N, Bergh Hvd, Nyokong T, Lange N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules. 2011;17:98–144. doi: 10.3390/molecules17010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Li W, Yu HB, Cheung NH, Chen JY. Sulfonated aluminum phthalocyanines for two-photon photodynamic cancer therapy: the effect of the excitation wavelength. Laser Phys. 2014;24:035602. [Google Scholar]
- 55.Amin RM, Hauser C, Kinzler I, Rueck A, Scalfi-Happ C. Evaluation of photodynamic treatment using aluminum phthalocyanine tetrasulfonate chloride as a photosensitizer: new approach. Photochem Photobiol Sci. 2012;11:1156–1163. doi: 10.1039/c2pp05411f. [DOI] [PubMed] [Google Scholar]
- 56.Idowu M, Chen JY, Nyokong T. Photoinduced energy transfer between water-soluble CdTe quantum dots and aluminium tetrasulfonated phthalocyanine. New J Chem. 2008;32:290–296. [Google Scholar]
- 57.Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA Damage in the Human Trabecular Meshwork: Clinical Correlation in Patients With Primary Open-Angle Glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 58.Harding S. Photodynamic therapy in the treatment of subfoveal choroidal neovascularisation. Eye. 2001;15:407–412. doi: 10.1038/eye.2001.145. [DOI] [PubMed] [Google Scholar]
- 59.Chen E, Brown DM, Wong TP, Benz MS, Kegley E, Cox J, Fish RH, Kim RY. Lucentis using Visudyne study: determining the threshold-dose fluence of verteporfin photodynamic therapy combined with intravitreal ranibizumab for exudative macular degeneration. Clin Ophthalmol. 2010;4:1073–1079. doi: 10.2147/OPTH.S13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnaut LG. Design of porphyrin-based photosensitizers for photodynamic therapy. Adv Inorg Chem. 2011;63:187–233. [Google Scholar]
- 61.Bhaumik J, Weissleder R, McCarthy JR. Synthesis and Photophysical Properties of Sulfonamidophenyl Porphyrins as Models for Activatable Photosensitizers. J Org Chem. 2009;74:5894–5901. doi: 10.1021/jo900832y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuyama T, Satoh K, Kushiya T, Kobayashi N. Design, Synthesis, and Properties of Phthalocyanine Complexes with Main-Group Elements Showing Main Absorption and Fluorescence beyond 1000 nm. J Am Chem Soc. 2014;136:765–776. doi: 10.1021/ja411016f. [DOI] [PubMed] [Google Scholar]
- 63.Pimenta FM, Jensen RL, Holmegaard L, Esipova TV, Westberg M, Breitenbach T, Ogilby PR. Singlet-Oxygen-Mediated Cell Death Using Spatially-Localized Two-Photon Excitation of an Extracellular Sensitizer. J Phys Chem B. 2012;116:10234–10246. doi: 10.1021/jp304954m. [DOI] [PubMed] [Google Scholar]
- 64.Gunaratne TC, Gusev AV, Peng X, Rosa A, Ricciardi G, Baerends EJ, Rizzoli C, Kenney ME, Rodgers MAJ. Photophysics of Octabutoxy Phthalocyaninato-Ni(II) in Toluene: Ultrafast Experiments and DFT/TDDFT Studies. J Phys Chem A. 2005;109:2078–2089. doi: 10.1021/jp0457444. [DOI] [PubMed] [Google Scholar]
- 65.Drain CM, Kirmaier C, Medforth CJ, Nurco DJ, Smith KM, Holten D. Dynamic Photophysical Properties of Conformationally Distorted Nickel Porphyrins. 1. Nickel(II) Dodecaphenylporphyrin. J Phys Chem. 1996;100:11984–11993. [Google Scholar]
- 66.Drain CM, Gentemann S, Roberts JA, Nelson NY, Medforth CJ, Jia S, Simpson MC, Smith KM, Fajer J, Shelnutt JA, et al. Picosecond to Microsecond Photodynamics of a Nonplanar Nickel Porphyrin: Solvent Dielectric and Temperature Effects. J Am Chem Soc. 1998;120:3781–3791. [Google Scholar]
- 67.Vernon DI, Walker I. Tetrapyrroles in Photodynamic Therapy. In: Warren MJ, Smith AG, editors. Tetrapyrroles: Birth, Life and Death. Vol. 1. Landes Bioscience; TX: 2009. pp. 128–148. [Google Scholar]
- 68.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allison RR, Downie GH, Cuenca R, Hu XH, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiagn Photodyn Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 70.Welch AJ, van Gemert MJC. Lasers in Medicine. In: Waynant RW, Ediger MN, editors. Electrooptics Handbook. 2. Vol. 1. McGraw-Hill; New York: 2000. pp. 24.1–24.32. [Google Scholar]
- 71.Achelle S, Couleaud P, Baldeck P, Teulade-Fichou MP, Maillard P. Carbohydrate–Porphyrin Conjugates with Two-Photon Absorption Properties as Potential Photosensitizing Agents for Photodynamic Therapy. Eur J Org Chem. 2011;2011:1271–1279. [Google Scholar]
- 72.Hofman JW, van Zeeland F, Turker S, Talsma H, Lambrechts SAG, Sakharov DV, Hennink WE, van Nostrum CF. Peripheral and Axial Substitution of Phthalocyanines with Solketal Groups: Synthesis and In Vitro Evaluation for Photodynamic Therapy. J Med Chem. 2007;50:1485–1494. doi: 10.1021/jm061136w. [DOI] [PubMed] [Google Scholar]
- 73.Morgan J, Oseroff AR. Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Delivery Rev. 2001;49:71–86. doi: 10.1016/s0169-409x(01)00126-0. [DOI] [PubMed] [Google Scholar]
- 74.Yang E, Diers JR, Huang YY, Hamblin MR, Lindsey JS, Bocian DF, Holten D. Molecular Electronic Tuning of Photosensitizers to Enhance Photodynamic Therapy: Synthetic Dicyanobacteriochlorins as a Case Study. Photochem Photobiol. 2013;89:605–618. doi: 10.1111/php.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. 1999;70:391–475. [PubMed] [Google Scholar]
- 76.Wilkinson F, Helman WP, Ross AB. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J Phys Chem Ref Data. 1993;22:113–262. [Google Scholar]
- 77.Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 78.Airley RE, Mobasheri A. Hypoxic Regulation of Glucose Transport, Anaerobic Metabolism and Angiogenesis in Cancer: Novel Pathways and Targets for Anticancer Therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 79.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 81.Kim J-w, Dang CV. Cancer’s Molecular Sweet Tooth and the Warburg Effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 82.Toschi A, Lee E, Thompson S, Gadir N, Yellen P, Drain CM, Ohh M, Foster DA. Phospholipase D-mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett. 2010;299:72–79. doi: 10.1016/j.canlet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monsigny M, Roche AC, Kieda C, Midoux P, Obrénovitch A. Characterization and biological implications of membrane lectins in tumor, lymphoid and myeloid cells. Biochimie. 1988;70:1633–1649. doi: 10.1016/0300-9084(88)90299-4. [DOI] [PubMed] [Google Scholar]
- 84.Fülling G, Schröder D, Franck B. Water-Soluble Porphyrin Diglycosides with Photosensitizing Properties. Angew Chem, Int Ed Engl. 1989;28:1519–1521. [Google Scholar]
- 85.Maillard P, Gaspard S, Guerquin-Kern JL, Momenteau M. Glycoconjugated tetrapyrrolic macrocycles. J Am Chem Soc. 1989;111:9125–9127. [Google Scholar]
- 86.Chen X, Hui L, Foster DA, Drain CM. Efficient Synthesis and Photodynamic Activity of Porphyrin-Saccharide Conjugates: Targeting and Incapacitating Cancer Cells. Biochemistry. 2004;43:10918–10929. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujimoto K, Miyata T, Aoyama Y. Saccharide-Directed Cell Recognition and Molecular Delivery Using Macrocyclic Saccharide Clusters: Masking of Hydrophobicity to Enhance the Saccharide Specificity. J Am Chem Soc. 2000;122:3558–3559. [Google Scholar]
- 88.Laville I, Pigaglio S, Blais JC, Doz F, Loock B, Maillard P, Grierson DS, Blais J. Photodynamic Efficiency of Diethylene Glycol-Linked Glycoconjugated Porphyrins in Human Retinoblastoma Cells. J Med Chem. 2006;49:2558–2567. doi: 10.1021/jm0580151. [DOI] [PubMed] [Google Scholar]
- 89.Pasetto P, Chen X, Drain CM, Franck RW. Synthesis of hydrolytically stable porphyrin C- and S-glycoconjugates in high yields. Chem Commun. 2001:81–82. [Google Scholar]
- 90.Zamora-León SP, Golde DW, Concha II, Rivas CI, Delgado-López F, Baselga J, Nualart F, Vera JC. Expression of the fructose transporter GLUT5 in human breast cancer. Proc Natl Acad Sci U S A. 1996;93:1847–1852. doi: 10.1073/pnas.93.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumamoto K, Goto Y, Sekikawa K, Takenoshita S, Ishida N, Kawakita M, Kannagi R. Increased Expression of UDP-Galactose Transporter Messenger RNA in Human Colon Cancer Tissues and Its Implication in Synthesis of Thomsen-Friedenreich Antigen and Sialyl Lewis A/X Determinants. Cancer Res. 2001;61:4620–4627. [PubMed] [Google Scholar]
- 92.Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 93.Cavaleiro JAS, Tomé JPC, Faustino MAF. Synthesis of Glycoporphyrins. In: El Ashry E, editor. Heterocycles from Carbohydrate Precursors. Vol. 7. Springer Berlin; Heidelberg: 2007. pp. 179–248. [Google Scholar]
- 94.Mikata Y, Onchi Y, Tabata K, Ogura S-i, Okura I, Ono H, Yano S. Sugar-dependent photocytotoxic property of tetra- and octa-glycoconjugated tetraphenylporphyrins. Tetrahedron Lett. 1998;39:4505–4508. [Google Scholar]
- 95.Li G, Pandey SK, Graham A, Dobhal MP, Mehta R, Chen Y, Gryshuk A, Rittenhouse-Olson K, Oseroff A, Pandey RK. Functionalization of OEP-Based Benzochlorins To Develop Carbohydrate-Conjugated Photosensitizers. Attempt To Target β-Galactoside-Recognized Proteins. J Org Chem. 2004;69:158–172. doi: 10.1021/jo030280b. [DOI] [PubMed] [Google Scholar]
- 96.Ono N, Bougauchi M, Maruyama K. Water-Soluble Porphyrins with Four Sugar Molecules. Tetrahedron Lett. 1992;33:1629–1632. [Google Scholar]
- 97.Aksenova AA, Sebyakin YL, Mironov AF. Conjugates of Porphyrins with Carbohydrates. Russ J Bioorg Chem. 2003;29:201–219. doi: 10.1023/a:1023924213863. [DOI] [PubMed] [Google Scholar]
- 98.Sol V, Branland P, Granet R, Kaldapa C, Verneuil B, Krausz P. Nitroglycosylated meso-arylporphyrins as photoinhibitors of gram positive bacteria. Bioorg Med Chem Lett. 1998;8:3007–3010. doi: 10.1016/S0960-894X(98)00536-8. [DOI] [PubMed] [Google Scholar]
- 99.Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Mendonça AF, Pegado IN, Duarte R, Valdeira ML. Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg Med Chem. 2005;13:3878–3888. doi: 10.1016/j.bmc.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Trannoy LL, Lagerberg JWM, Dubbelman TMAR, Schuitmaker HJ, Brand A. Positively charged porphyrins: a new series of photosensitizers for sterilization of RBCs. Transfusion. 2004;44:1186–1196. doi: 10.1111/j.1537-2995.2004.03275.x. [DOI] [PubMed] [Google Scholar]
- 101.Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Soncin M, Magaraggia M, Ferro S, Jori G. Synthesis and Antibacterial Activity of New Poly-S-lysine–Porphyrin Conjugates. J Med Chem. 2004;47:6649–6652. doi: 10.1021/jm040802v. [DOI] [PubMed] [Google Scholar]
- 102.Mesquita MQ, Menezes JCJMDS, Neves MGPMS, Tomé AC, Cavaleiro JAS, Cunha Â, Almeida A, Hackbarth S, Röder B, Faustino MAF. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorg Med Chem Lett. 2014;24:808–812. doi: 10.1016/j.bmcl.2013.12.097. [DOI] [PubMed] [Google Scholar]
- 103.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of Uptake of a Cationic Water-Soluble Pyridinium Zinc Phthalocyanine across the Outer Membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merchat M, Bertolini G, Giacomini P, Villaneuva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol, B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 105.Minnock A, Vernon DI, Schofield J, Griffiths J, Howard Parish J, Brown SB. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J Photochem Photobiol, B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 106.Hanakova A, Bogdanova K, Tomankova K, Pizova K, Malohlava J, Binder S, Bajgar R, Langova K, Kolar M, Mosinger J, et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol Res. 2014;169:163–170. doi: 10.1016/j.micres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Jensen TJ, Vicente MGH, Luguya R, Norton J, Fronczek FR, Smith KM. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in HEp2 cells. J Photochem Photobiol, B. 2010;100:100–111. doi: 10.1016/j.jphotobiol.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mauzerall D, Drain CM. Photogating of ionic currents across the lipid bilayer: Electrostatics of ions and dipoles inside the membrane. Biophys J. 1992;63:1544–1555. doi: 10.1016/S0006-3495(92)81738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drain CM, Mauzerall D. Photogating of ionic currents across the lipid bilayer: Hydrophobic ion conduction by an ion chain mechanism. Biophys J. 1992;63:1556–1563. doi: 10.1016/S0006-3495(92)81739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yano S, Hirohara S, Obata M, Hagiya Y, Ogura S-i, Ikeda A, Kataoka H, Tanaka M, Joh T. Current states and future views in photodynamic therapy. J Photochem Photobiol, C. 2011;12:46–67. [Google Scholar]
- 111.Josefsen LB, Boyle RW. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics. 2012;2:916–966. doi: 10.7150/thno.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y, Lovell JF. Porphyrins as Theranostic Agents from Prehistoric to Modern Times. Theranostics. 2012;2:905–915. doi: 10.7150/thno.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Titov DV, Gening ML, Tsvetkov YE, Nifantiev NE. Glycoconjugates of porphyrins with carbohydrates: methods of synthesis and biological activity. Russ Chem Rev. 2014;83:523–554. [Google Scholar]
- 114.Fuhrhop JH, Demoulin C, Boettcher C, Koening J, Siggel U. Chiral micellar porphyrin fibers with 2-aminoglycosamide head groups. J Am Chem Soc. 1992;114:4159–4165. [Google Scholar]
- 115.Ahmed S, Davoust E, Savoie H, Boa AN, Boyle RW. Thioglycosylated cationic porphyrins–convenient synthesis and photodynamic activity in vitro. Tetrahedron Lett. 2004;45:6045–6047. [Google Scholar]
- 116.Sylvain I, Zerrouki R, Granet R, Huang YM, Lagorce JF, Guilloton M, Blais JC, Krausz P. Synthesis and biological evaluation of thioglycosylated porphyrins for an application in photodynamic therapy. Bioorg Med Chem. 2002;10:57–69. doi: 10.1016/s0968-0896(01)00255-3. [DOI] [PubMed] [Google Scholar]
- 117.Casiraghi G, Cornia M, Zanardi F, Rassu G, Ragg E, Bortolini R. Synthesis and Characterization of Porphyrin-Sugar Carbon Conjugates. J Org Chem. 1994;59:1801–1808. [Google Scholar]
- 118.Stepanek P, Dukh M, Saman D, Moravcova J, Kniezo L, Monti D, Venanzi M, Mancini G, Drasar P. Synthesis and solvent driven self-aggregation studies of meso-“C-glycoside”-porphyrin derivatives. Org Biomol Chem. 2007;5:960–970. doi: 10.1039/b616096d. [DOI] [PubMed] [Google Scholar]
- 119.Oulmi D, Maillard P, Guerquin-Kern JL, Huel C, Momenteau M. Glycoconjugated Porphyrins. 3. Synthesis of Flat Amphiphilic Mixed meso-(Glycosylated aryl)arylporphyrins and Mixed meso-(Glycosylated aryl)alkylporphyrins Bearing Some Mono- and Disaccharide Groups. J Org Chem. 1995;60:1554–1564. [Google Scholar]
- 120.Hombrecher HK, Schell C, Thiem J. Synthesis and investigation of a galactopyranosyl-cholesteryloxy substituted porphyrin. Bioorg Med Chem Lett. 1996;6:1199–1202. [Google Scholar]
- 121.Daly R, Vaz G, Davies AM, Senge MO, Scanlan EM. Synthesis and Biological Evaluation of a Library of Glycoporphyrin Compounds. Chem - Eur J. 2012;18:14671–14679. doi: 10.1002/chem.201202064. [DOI] [PubMed] [Google Scholar]
- 122.Hirohara S, Obata M, Ogura S-i, Okura I, Higashida S, Ohtsuki C, Ogata S-i, Nishikawa Y, Takenaka M, Ono H, et al. Hydrophobicity parameters (Log P) of glycoconjugated porphyrins for photodynamic therapy evaluated by reversed phase HPLC. J Porphyrins Phthalocyanines. 2004;8:1289–1292. [Google Scholar]
- 123.Hirohara S, Obata M, Saito A, Ogata S, Ohtsuki C, Higashida S, Ogura S, Okura I, Sugai Y, Mikata Y, et al. Cellular uptake and photocytotoxicity of glycoconjugated porphyrins in HeLa cells. Photochem Photobiol. 2004;80:301–308. doi: 10.1562/2004-03-07-RA-103. [DOI] [PubMed] [Google Scholar]
- 124.Amessou M, Carrez D, Patin D, Sarr M, Grierson DS, Croisy A, Tedesco AC, Maillard P, Johannes L. Retrograde Delivery of Photosensitizer (TPPp-O-β-GluOH)3 Selectively Potentiates Its Photodynamic Activity. Bioconjugate Chem. 2008;19:532–538. doi: 10.1021/bc7003999. [DOI] [PubMed] [Google Scholar]
- 125.Desroches MC, Bautista-Sanchez A, Lamotte C, Labeque B, Auchere D, Farinotti R, Maillard P, Grierson DS, Prognon P, Kasselouri A. Pharmacokinetics of a tri-glucoconjugated 5,10,15-(meta)-trihydroxyphenyl-20-phenyl porphyrin photosensitizer for PDT. A single dose study in the rat. J Photochem Photobiol, B. 2006;85:56–64. doi: 10.1016/j.jphotobiol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 126.Kus P, Knerr G, Czuchajowski L. First representatives of porphyrinylnucleosides. Tetrahedron Lett. 1990;31:5133–5134. [Google Scholar]
- 127.Czuchajowski L, Habdas J, Niedbala H, Wandrekar V. Synthesis of porphyrinyl-nucleosides. J Heterocycl Chem. 1992;29:479–486. [Google Scholar]
- 128.Locos OB, Heindl CC, Corral A, Senge MO, Scanlan EM. Efficient Synthesis of Glycoporphyrins by Microwave-Mediated “Click” Reactions. Eur J Org Chem. 2010;2010:1026–1028. [Google Scholar]
- 129.Hao E, Jensen TJ, Vicente MGH. Synthesis of porphyrin-carbohydrate conjugates using “click” chemistry and their preliminary evaluation in human HEp2 cells. J Porphyrins Phthalocyanines. 2009;13:51–59. [Google Scholar]
- 130.Zheng G, Graham A, Shibata M, Missert JR, Oseroff AR, Dougherty TJ, Pandey RK. Synthesis of β-Galactose-Conjugated Chlorins Derived by Enyne Metathesis as Galectin-Specific Photosensitizers for Photodynamic Therapy. J Org Chem. 2001;66:8709–8716. doi: 10.1021/jo0105080. [DOI] [PubMed] [Google Scholar]
- 131.Ballut Sv, Naud-Martin D, Loock B, Maillard P. A Strategy for the Targeting of Photosensitizers. Synthesis, Characterization, and Photobiological Property of Porphyrins Bearing Glycodendrimeric Moieties. J Org Chem. 2011;76:2010–2028. doi: 10.1021/jo102185d. [DOI] [PubMed] [Google Scholar]
- 132.Silva S, Pereira PMR, Silva P, Almeida Paz FA, Faustino MAF, Cavaleiro JAS, Tome JPC. Porphyrin and phthalocyanine glycodendritic conjugates: synthesis, photophysical and photochemical properties. Chem Commun. 2012;48:3608–3610. doi: 10.1039/c2cc17561d. [DOI] [PubMed] [Google Scholar]
- 133.Pandey SK, Zheng X, Morgan J, Missert JR, Liu TH, Shibata M, Bellnier DA, Oseroff AR, Henderson BW, Dougherty TJ, et al. Purpurinimide Carbohydrate Conjugates: Effect of the Position of the Carbohydrate Moiety in Photosensitizing Efficacy. Mol Pharmaceutics. 2007;4:448–464. doi: 10.1021/mp060135x. [DOI] [PubMed] [Google Scholar]
- 134.Gaud O, Granet R, Kaouadji M, Krausz P, Blais JC, Bolbach G. Synthèse et analyse structurale de nouvelles méso-arylporphyrines glycosylées en vue de l application en photothérapie des cancers. Can J Chem. 1996;74:481–499. [Google Scholar]
- 135.Carré V, Gaud O, Sylvain I, Bourdon O, Spiro M, Biais J, Granet R, Krausz P, Guilloton M. Fungicidal properties of meso-arylglycosylporphyrins: influence of sugar substituents on photo-induced damage in the yeast Saccharomyces cerevisioe. J Photochem Photobiol, B. 1999;48:57–62. doi: 10.1016/s1011-1344(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 136.Sol V, Chaleix V, Champavier Y, Granet R, Huang YM, Krausz P. Glycosyl bis-porphyrin conjugates: Synthesis and potential application in PDT. Bioorg Med Chem. 2006;14:7745–7760. doi: 10.1016/j.bmc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 137.Di Stasio B, Frochot C, Dumas D, Even P, Zwier J, Müller A, Didelon J, Guillemin F, Viriot ML, Barberi-Heyob M. The 2-aminoglucosamide motif improves cellular uptake and photodynamic activity of tetraphenylporphyrin. Eur J Med Chem. 2005;40:1111–1122. doi: 10.1016/j.ejmech.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 138.Hirohara S, Obata M, Alitomo H, Sharyo K, Ogata S-i, Ohtsuki C, Yano S, Ando T, Tanihara M. Structure and Photodynamic Effect Relationships of 24 Glycoconjugated Photosensitizers in HeLa Cells. Biol Pharm Bull. 2008;31:2265–2272. doi: 10.1248/bpb.31.2265. [DOI] [PubMed] [Google Scholar]
- 139.Rao PD, Dhanalekshmi S, Littler BJ, Lindsey JS. Rational Syntheses of Porphyrins Bearing up to Four Different Meso Substituents. J Org Chem. 2000;65:7323–7344. doi: 10.1021/jo000882k. [DOI] [PubMed] [Google Scholar]
- 140.Zaidi SHH, Fico RM, Lindsey JS. Investigation of Streamlined Syntheses of Porphyrins Bearing Distinct Meso Substituents. Org Process Res Dev. 2006;10:118–134. [Google Scholar]
- 141.Hirohara S, Obata M, Alitomo H, Sharyo K, Ando T, Yano S, Tanihara M. Synthesis and Photocytotoxicity of S-Glucosylated 5,10,15,20-Tetrakis(tetrafluorophenyl)porphyrin Metal Complexes as Efficient 1O2-Generating Glycoconjugates. Bioconjugate Chem. 2009;20:944–952. doi: 10.1021/bc800522y. [DOI] [PubMed] [Google Scholar]
- 142.Okada M, Kishibe Y, Ide K, Takahashi T, Hasegawa T. Convenient Approach to Access Octa-Glycosylated Porphyrins via Click Chemistry. Int J Carbohydr Chem. 2009;2009(305276):1–9. [Google Scholar]
- 143.Chaleix V, Sol V, Huang YM, Guilloton M, Granet R, Blais; Jean C, Krausz P. RGD-Porphyrin Conjugates: Synthesis and Potential Application in Photodynamic Therapy. Eur J Org Chem. 2003;2003:1486–1493. [Google Scholar]
- 144.Aicher D, Wiehe A, Stark CBW. Synthesis of Glycoporphyrins Using Trichloroacetimidates as Glycosyl Donors. Synlett. 2010;2010:395–398. [Google Scholar]
- 145.Laville I, Pigaglio S, Blais JC, Loock B, Maillard P, Grierson DS, Blais J. A study of the stability of tri-(glucosyloxyphenyl)chlorin, a sensitizer for photodynamic therapy, in human colon tumoural cells: a liquid chromatography and MALDI-TOF mass spectrometry analysis. Bioorg Med Chem. 2004;12:3673–3682. doi: 10.1016/j.bmc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 146.Hirohara S, Obata M, Ogata S-i, Ohtsuki C, Higashida S, Ogura S-i, Okura I, Takenaka M, Ono H, Sugai Y, et al. Cellular uptake and photocytotoxicity of glycoconjugated chlorins in HeLa cells. J Photochem Photobiol, B. 2005;78:7–15. doi: 10.1016/j.jphotobiol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 147.Hirohara S, Obata M, Ogata S-i, Kajiwara K, Ohtsuki C, Tanihara M, Yano S. Sugar-dependent aggregation of glycoconjugated chlorins and its effect on photocytotoxicity in HeLa cells. J Photochem Photobiol, B. 2006;84:56–63. doi: 10.1016/j.jphotobiol.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 148.Tomé JPC, Silva EMP, Pereira AMVM, Alonso CMA, Faustino MAF, Neves MGPMS, Tomé AC, Cavaleiro JAS, Tavares SAP, Duarte RR, et al. Synthesis of neutral and cationic tripyridylporphyrin-d-galactose conjugates and the photoinactivation of HSV-1. Bioorg Med Chem. 2007;15:4705–4713. doi: 10.1016/j.bmc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 149.Hombrecher HK, Ohm S, Koll D. Synthesis of galactopyranosyl substituted porphyrins. Tetrahedron. 1996;52:5441–5448. [Google Scholar]
- 150.Davoust E, Granet R, Krausz P, Carré V, Guilloton M. Synthesis of glycosyl strapped porphyrins. Tetrahedron Lett. 1999;40:2513–2516. [Google Scholar]
- 151.Drain CM, Gong X, Ruta V, Soll CE, Chicoineau PF. Combinatorial Synthesis and Modification of Functional Porphyrin Libraries: Identification of New, Amphipathic Motifs for Biomolecule Binding. J Comb Chem. 1999;1:286–290. doi: 10.1021/cc990011s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drain CM, Ruta V, Gong X. Porphyrin - DNA interactions via hydrogen bonding. Biophys J. 1998;74:A136. [Google Scholar]
- 153.Pandey RK, Smith NW, Shiau F-Y, Dougherty TJ, Smith KM. Syntheses of cationic porphyrins and chlorins. J Chem Soc, Chem Commun. 1991:1637–1638. [Google Scholar]
- 154.Oseroff AR, Ohuoha D, Ara G, McAuliffe D, Foley J, Cincotta L. Intramitochondrial dyes allow selective in vitro photolysis of carcinoma cells. Proc Natl Acad Sci U S A. 1986;83:9729–9733. doi: 10.1073/pnas.83.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woodburn KW, Vardaxis NJ, Hill JS, Kaye AH, Phillips DR. Subcellular Localization of Porphyrins Using Confocal Laser Scanning Microscopy. Photochem Photobiol. 1991;54:725–732. doi: 10.1111/j.1751-1097.1991.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 156.Driaf K, PK, Verneuil B, Spiro M, Blais JC, Bolbach G. Glycosylated cationic porphyrins as potential agents in cancer phototherapy. Tetrahedron Lett. 1993;34:1027–1030. [Google Scholar]
- 157.Kaldapa C, Blais JC, Carré V, Granet R, Sol V, Guilloton M, Spiro M, Krausz P. Synthesis of new glycosylated neutral and cationic porphyrins dimers. Tetrahedron Lett. 2000;41:331–335. [Google Scholar]
- 158.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fischer E, Delbrück K. Synthese neuer Disaccharide vom Typus der Trehalose. Ber Dtsch Chem Ges. 1909;42:2776–2785. [Google Scholar]
- 160.Garegg PJ. Thioglycosides as Glycosyl Donors in Oligosaccharide Synthesis. In: Derek H, editor. Advances in Carbohydrate Chemistry and Biochemistry. Vol. 52. Academic Press; New York: 1997. pp. 179–205. [DOI] [PubMed] [Google Scholar]
- 161.Zhu X, Schmidt RR. New Principles for Glycoside-Bond Formation. Angew Chem, Int Ed. 2009;48:1900–1934. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- 162.Sylvain I, Benhaddou R, Carre V, Cottaz S, Driguez H, Granet R, Guilloton M, Krausz P. Synthesis and Biological Evaluation of Thioglycosylated meso-Arylporphyrins. J Porphyrins Phthalocyanines. 1999;3:1–4. [Google Scholar]
- 163.Samaroo D, Vinodu M, Chen X, Drain CM. meso-Tetra(pentafluorophenyl)porphyrin as an efficient platform for combinatorial synthesis and the selection of new photodynamic therapeutics using a cancer cell line. J Comb Chem. 2007;9:998–1011. doi: 10.1021/cc070067j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samaroo D, Soll CE, Todaro LJ, Drain CM. Efficient microwave-assisted synthesis of amine-substituted tetrakis (pentafluorophenyl) porphyrin. Org Lett. 2006;8:4985–4988. doi: 10.1021/ol060946z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Samaroo D, Drain CM. Solution phase combinatorial libraries for wholecell selection assays. Biochemistry. 2003;42:208. [Google Scholar]
- 166.Gottumukkala V, Ongayi O, Baker DG, Lomax LG, Vicente MGH. Synthesis, cellular uptake and animal toxicity of a tetra(carboranylphenyl)-tetrabenzoporphyrin. Bioorg Med Chem. 2006;14:1871–1879. doi: 10.1016/j.bmc.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 167.Mironov AF, Grin MA. Synthesis of chlorin and bacteriochlorin conjugates for photodynamic and boron neutron capture therapy. J Porphyrins Phthalocyanines. 2008;12:1163–1172. [Google Scholar]
- 168.Bhupathiraju NV, Vicente MG. Synthesis and cellular studies of polyamine conjugates of a mercaptomethyl-carboranylporphyrin. Bioorg Med Chem. 2013;21:485–495. doi: 10.1016/j.bmc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh S, Aggarwal A, Bhupathiraju NV, Newton B, Nafees A, Gao R, Drain CM. Synthesis and cell phototoxicity of a triply bridged fused diporphyrin appended with six thioglucose units. Tetrahedron Lett. 2014;55:6311–6314. doi: 10.1016/j.tetlet.2014.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Králová J, Bříza T, Moserová I, Dolenský B, Vašek P, Poučková P, Kejík Z, Kaplánek R, Martásek P, Dvořák M, et al. Glycol Porphyrin Derivatives as Potent Photodynamic Inducers of Apoptosis in Tumor Cells. J Med Chem. 2008;51:5964–5973. doi: 10.1021/jm8002119. [DOI] [PubMed] [Google Scholar]
- 171.Thompson S, Chen X, Hui L, Toschi A, Foster DA, Drain CM. Low concentrations of a non-hydrolysable tetra-S-glycosylated porphyrin and low light induces apoptosis in human breast cancer cells via stress of the endoplasmic reticulum. Photochem Photobiol Sci. 2008;7:1415–1421. doi: 10.1039/b806536e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hirohara S, Nishida M, Sharyo K, Obata M, Ando T, Tanihara M. Synthesis, photophysical properties and photocytotoxicity of mono-, di-, tri- and tetra-glucosylated fluorophenylporphyrins. Bioorg Med Chem. 2010;18:1526–1535. doi: 10.1016/j.bmc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Bhupathiraju NV, Hu X, Zhou Z, Fronczek FR, Couraud PO, Romero IA, Weksler B, Vicente MG. Synthesis and in vitro evaluation of BBB permeability, tumor cell uptake, and cytotoxicity of a series of carboranylporphyrin conjugates. J Med Chem. 2014;57:6718–6728. doi: 10.1021/jm500786c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vedachalam S, Choi BH, Pasunooti KK, Ching KM, Lee K, Yoon HS, Liu XW. Glycosylated porphyrin derivatives and their photodynamic activity in cancer cells. MedChemComm. 2011;2:371–377. [Google Scholar]
- 175.Maillard P, Huel C, Momenteau M. Synthesis of new meso-tetrakis (glycosylated) porphyrins. Tetrahedron Lett. 1992;33:8081–8084. [Google Scholar]
- 176.Lindsey JS. Synthesis of meso-Substituted Porphyrins. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. Vol. 1. Academic Press; New York: 2000. pp. 67–118. [Google Scholar]
- 177.Monti D, Venanzi M, Gatto E, Mancini G, Sorrenti A, Stepanek P, Drasar P. Study of the supramolecular chiral assembly of meso-“C-glucoside”-porphyrin derivatives in aqueous media. New J Chem. 2008;32:2127–2133. [Google Scholar]
- 178.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem, Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 179.Bourhim A, Gaud O, Granet R, Krausz P, Spiro M. Synthesis of New Glycosylated Porphyrin Derivatives with a Hydrocarbon Spacer Arm. Synlett. 1993;1993:563–564. [Google Scholar]
- 180.Griegel S, Rajewsky MF, Ciesiolka T, Gabius HJ. Endogenous sugar receptor (lectin) profiles of human retinoblastoma and retinoblast cell lines analyzed by cytological markers, affinity chromatography and neoglycoprotein-targeted photolysis. Anticancer Res. 1989;9:723–730. [PubMed] [Google Scholar]
- 181.Chauvin B, Iorga BI, Chaminade P, Paul JL, Maillard P, Prognon P, Kasselouri A. Plasma distribution of tetraphenylporphyrin derivatives relevant for Photodynamic Therapy: Importance and limits of hydrophobicity. Eur J Pharm Biopharm. 2013;83:244–252. doi: 10.1016/j.ejpb.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 182.Maillard P, Loock B, Grierson DS, Laville I, Blais J, Doz F, Desjardins L, Carrez D, Guerquin-Kern JL, Croisy A. In vitro phototoxicity of glycoconjugated porphyrins and chlorins in colorectal adenocarcinoma (HT29) and retinoblastoma (Y79) cell lines. Photodiagn Photodyn Ther. 2007;4:261–268. doi: 10.1016/j.pdpdt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 183.Sol V, Charmot A, Krausz P, Trombotto S, Queneau Y. Synthesis of New Glucosylated Porphyrins Bearing an α-d-Linkage. J Carbohydr Chem. 2006;25:345–360. [Google Scholar]
- 184.Sol V, Blais JC, Carré V, Granet R, Guilloton M, Spiro M, Krausz P. Synthesis, Spectroscopy, and Photocytotoxicity of Glycosylated Amino Acid Porphyrin Derivatives as Promising Molecules for Cancer Phototherapy. J Org Chem. 1999;64:4431–4444. [Google Scholar]
- 185.Asayama S, Mizushima K, Nagaoka S, Kawakami H. Design of metalloporphyrin-carbohydrate conjugates for a new superoxide dismutase mimic with cellular recognition. Bioconjugate Chem. 2004;15:1360–1363. doi: 10.1021/bc049865i. [DOI] [PubMed] [Google Scholar]
- 186.Cecioni S, Matthews SE, Blanchard H, Praly JP, Imberty A, Vidal S. Synthesis of lactosylated glycoclusters and inhibition studies with plant and human lectins. Carbohydr Res. 2012;356:132–141. doi: 10.1016/j.carres.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 187.Cecioni S, Faure S, Darbost U, Bonnamour I, Parrot-Lopez H, Roy O, Taillefumier C, Wimmerová M, Praly JP, Imberty A, et al. Selectivity among Two Lectins: Probing the Effect of Topology, Multivalency and Flexibility of “Clicked” Multivalent Glycoclusters. Chem - Eur J. 2011;17:2146–2159. doi: 10.1002/chem.201002635. [DOI] [PubMed] [Google Scholar]
- 188.Cecioni S, Praly JP, Matthews SE, Wimmerová M, Imberty A, Vidal S. Rational Design and Synthesis of Optimized Glycoclusters for Multivalent Lectin–Carbohydrate Interactions: Influence of the Linker Arm. Chem - Eur J. 2012;18:6250–6263. doi: 10.1002/chem.201200010. [DOI] [PubMed] [Google Scholar]
- 189.Garcia G, Naud-Martin D, Carrez D, Croisy A, Maillard P. Microwave-mediated ‘[]click-chemistry’ synthesis of glycoporphyrin derivatives and in vitro photocytotoxicity for application in photodynamic therapy. Tetrahedron. 2011;67:4924–4932. [Google Scholar]
- 190.Lee LV, Mitchell ML, Huang SJ, Fokin VV, Sharpless KB, Wong CH. A Potent and Highly Selective Inhibitor of Human α-1,3-Fucosyltransferase via Click Chemistry. J Am Chem Soc. 2003;125:9588–9589. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 191.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 192.Whiting M, Muldoon J, Lin YC, Silverman SM, Lindstrom W, Olson AJ, Kolb HC, Finn MG, Sharpless KB, Elder JH, et al. Inhibitors of HIV-1 Protease by Using In Situ Click Chemistry. Angew Chem, Int Ed. 2006;45:1435–1439. doi: 10.1002/anie.200502161. [DOI] [PubMed] [Google Scholar]
- 193.Ringot C, Sol V, Granet R, Krausz P. Porphyrin-grafted cellulose fabric: New photobactericidal material obtained by “Click-Chemistry” reaction. Mater Lett. 2009;63:1889–1891. [Google Scholar]
- 194.Mukosera GT, Adams TP, Rothbarth RF, Langat H, Akanda S, Barkley RG, Dolewski RD, Ruppel JV, Snyder NL. Synthesis of glycosylated zinc (II) 5,15-diphenylporphyrin and zinc (II) 5,10,15,20-tetraphenylporphyrin analogs using Cu-catalyzed azide-alkyne 1,3-dipolar cycloaddition reactions. Tetrahedron Lett. 2015;56:73–77. [Google Scholar]
- 195.Laville I, Figueiredo T, Loock B, Pigaglio S, Maillard P, Grierson DS, Carrez D, Croisy A, Blais J. Synthesis, cellular internalization and photodynamic activity of glucoconjugated derivatives of tri and tetra(meta-hydroxyphenyl)chlorins. Bioorg Med Chem. 2003;11:1643–1652. doi: 10.1016/s0968-0896(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 196.Figueira F, Pereira PMR, Silva S, Cavaleiro JAS, Tomé JPC. Porphyrins and Phthalocyanines Decorated with Dendrimers: Synthesis and Biomedical Applications. Curr Org Synth. 2014;11:110–126. [Google Scholar]
- 197.Phillips D. Toward targeted photodynamic therapy. Pure Appl Chem. 2011;83:733–748. [Google Scholar]
- 198.Thompson S. Thioglycosylated Phorphyrin, Chlorin, Bacteriochlorin and Isobacterichlorin as Photodynamic Therapeutic Agents and Their Possible Use as Bioimaging Agents. City University of New York; New York: 2009. [Google Scholar]
- 199.Maes W, Dehaen W. Synthetic Aspects of Porphyrin Dendrimers. Eur J Org Chem. 2009;2009:4719–4752. [Google Scholar]
- 200.Wells L, Vosseller K, Hart GW. Glycosylation of Nucleocytoplasmic Proteins: Signal Transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 201.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the Immune System. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 202.Ballut S, Makky A, Chauvin B, Michel JP, Kasselouri A, Maillard P, Rosilio V. Tumor targeting in photodynamic therapy. From glycoconjugated photosensitizers to glycodendrimeric one. Concept, design and properties. Org Biomol Chem. 2012;10:4485–4495. doi: 10.1039/c2ob25181g. [DOI] [PubMed] [Google Scholar]
- 203.Ballardini R, Colonna B, Gandolfi MT, Kalovidouris SA, Orzel L, Raymo FM, Stoddart JF. Porphyrin-Containing Glycodendrimers. Eur J Org Chem. 2003;2003:288–294. [Google Scholar]
- 204.Ballut S, Makky A, Loock B, Michel J-P, Maillard P, Rosilio V. New strategy for targeting of photosensitizers. Synthesis of glycodendrimeric phenylporphyrins, incorporation into a liposome membrane and interaction with a specific lectin. Chem Commun. 2009:224–226. doi: 10.1039/b816128c. [DOI] [PubMed] [Google Scholar]
- 205.Makky A, Michel JP, Maillard P, Rosilio V. Biomimetic liposomes and planar supported bilayers for the assessment of glycodendrimeric porphyrins interaction with an immobilized lectin. Biochim Biophys Acta, Biomembr. 2011;1808:656–666. doi: 10.1016/j.bbamem.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 206.Makky A, Michel JP, Kasselouri A, Briand E, Maillard P, Rosilio V. Evaluation of the Specific Interactions between Glycodendrimeric Porphyrins, Free or Incorporated into Liposomes, and Concanavaline A by Fluorescence Spectroscopy, Surface Pressure, and QCM-D Measurements. Langmuir. 2010;26:12761–12768. doi: 10.1021/la101260t. [DOI] [PubMed] [Google Scholar]
- 207.Wang ZJ, Chauvin B, Maillard P, Hammerer F, Carez D, Croisy A, Sandré C, Chollet-Martin S, Prognon P, Paul JL, et al. Glycodendrimeric phenylporphyrins as new candidates for retinoblastoma PDT: Blood carriers and photodynamic activity in cells. J Photochem Photobiol, B. 2012;115:16–24. doi: 10.1016/j.jphotobiol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 208.Vedala H, Chen Y, Cecioni S, Imberty A, Vidal Sb, Star A. Nanoelectronic Detection of Lectin-Carbohydrate Interactions Using Carbon Nanotubes. Nano Lett. 2011;11:170–175. doi: 10.1021/nl103286k. [DOI] [PubMed] [Google Scholar]
- 209.Kushwaha D, Tiwari VK. Click Chemistry Inspired Synthesis of Glycoporphyrin Dendrimers. J Org Chem. 2013;78:8184–8190. doi: 10.1021/jo4012392. [DOI] [PubMed] [Google Scholar]
- 210.Vinodh M, Alipour FH, Mohamod AA, Al-Azemi TF. Molecular Assemblies of Porphyrins and Macrocyclic Receptors: Recent Developments in Their Synthesis and Applications. Molecules. 2012;17:11763–11799. doi: 10.3390/molecules171011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Drain CM, Nifiatis F, Vasenko A, Batteas J. Porphyrin tessellation by design: Metal mediated self-assembly of large arrays and tapes. Angew Chem, Int Ed. 1998;37:2344–2347. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2344::AID-ANIE2344>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 212.Drain CM, Lehn J-M. Self-assembly of square multi-porphyrin arrays by metal ion coordination. J Chem Soc, Chem Commun. 1994:2313–2315. [Google Scholar]
- 213.Aggarwal A, Qureshy M, Johnson J, Batteas JD, Drain CM, Samaroo D. Responsive porphyrinoid nanoparticles: development and applications. J Porphyrins Phthalocyanines. 2011;15:338–349. [Google Scholar]
- 214.Singh S, Aggarwal A, Farley C, Hageman BA, Batteas JD, Drain CM. Hierarchical Organization of a Robust Porphyrin Cage Self-Assembled by Hydrogen Bonds. Chem Commun. 2011;47:7134–7136. doi: 10.1039/c1cc11553g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Drain CM. Self-organization of self-assembled photonic materials into functional devices: Photo-switched conductors. Proc Natl Acad Sci U S A. 2002;99:5178–5182. doi: 10.1073/pnas.062635099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Drain CM, Varotto A, Radivojevic I. Self-Organized Porphyrinic Materials. Chem Rev. 2009;109:1630–1658. doi: 10.1021/cr8002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jurow M, Schuckman AE, Batteas JD, Drain CM. Porphyrins as molecular electronic components of functional devices. Coord Chem Rev. 2010;254:2297–2310. doi: 10.1016/j.ccr.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ribó JM, Farrera JA, Valero ML, Virgili A. Self-assembly of cyclodextrins with meso-tetrakis(4-sulfonatophenyl)-porphyrin in aqueous solution. Tetrahedron. 1995;51:3705–3712. [Google Scholar]
- 219.Kralova J, Synytsya A, Pouckova P, Koc M, Dvorak M, Kral V. Novel Porphyrin Conjugates with a Potent Photodynamic Antitumor Effect: Differential Efficacy of Mono- and Bis-β-cyclodextrin Derivatives In Vifro and In Vivo. Photochem Photobiol. 2006;82:432–438. doi: 10.1562/2005-05-06-RA-516. [DOI] [PubMed] [Google Scholar]
- 220.Kralova J, Kejik Z, Briza T, Pouckova P, Kral A, Martasek P, Kral V. Porphyrin-cyclodextrin conjugates as a nanosystem for versatile drug delivery and multimodal cancer therapy. J Med Chem. 2010;53:128–138. doi: 10.1021/jm9007278. [DOI] [PubMed] [Google Scholar]
- 221.Li F, Bae BC, Na K. Acetylated hyaluronic acid/photosensitizer conjugate for the preparation of nanogels with controllable phototoxicity: synthesis, characterization, autophoto-quenching properties, and in vitro phototoxicity against HeLa cells. Bioconjugate Chem. 2010;21:1312–1320. doi: 10.1021/bc100116v. [DOI] [PubMed] [Google Scholar]
- 222.Li F, Na K. Self-assembled chlorin e6 conjugated chondroitin sulfate nanodrug for photodynamic therapy. Biomacromolecules. 2011;12:1724–1730. doi: 10.1021/bm200115v. [DOI] [PubMed] [Google Scholar]
- 223.Bae B-c, Na K. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials. 2010;31:6325–6335. doi: 10.1016/j.biomaterials.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 224.Mikata Y, Shibata M, Baba Y, Kakuchi T, Nakai M, Yano S. Synthesis and photodynamic properties of maltohexaose-conjugated porphyrins. J Porphyrins Phthalocyanines. 2012;16:1177–1185. [Google Scholar]
- 225.Mironov AF, Isaeva GM, Shvets VI, Evstigneeva RP, Stepanov AN, Perov AA, Eupriyanov SE. Glycosylation of Hydroxyalkylsubstituted Porphyrins. Bioorg Khim. 1978;4:1410–1413. [Google Scholar]
- 226.de C da Silva F, Ferreira VF, de Souza MCBV, Tomé AC, Neves MGPMS, Silva AMS, Cavaleiro JAS. Synthesis of Glycoporphyrins by Cross-Metathesis Reactions. Synlett. 2008;2008:1205–1207. [Google Scholar]
- 227.Zhao S, Neves MGPMS, Tomé AC, Silva AMS, Cavaleiro JAS, Domingues MRM, Ferrer Correia AJ. Reaction of meso-tetraarylporphyrins with pyrazine ortho-quinodimethanes. Tetrahedron Lett. 2005;46:2189–2191. [Google Scholar]
- 228.Silva AMG, Tomé AC, Neves MGPMS, Cavaleiro JAS, Kappe CO. Porphyrins in Diels–Alder reactions. Improvements on the synthesis of barrelene-fused chlorins using microwave irradiation. Tetrahedron Lett. 2005;46:4723–4726. [Google Scholar]
- 229.Li X, Zhuang J, Li Y, Liu H, Wang S, Zhu D. Synthesis of isoxazoline-fused chlorins and bacteriochlorins by 1,3-dipolar cycloaddition reaction of porphyrin with nitrile oxide. Tetrahedron Lett. 2005;46:1555–1559. [Google Scholar]
- 230.Silva AMG, Tomé AC, Neves MGPMS, Silva AMS, Cavaleiro JAS, Perrone D, Dondoni A. Porphyrins in 1, 3-dipolar cycloaddition reactions with sugar nitrones. Synthesis of glycoconjugated isoxazolidine-fused chlorins and bacteriochlorins. Tetrahedron Lett. 2002;43:603–605. [Google Scholar]
- 231.Silva AMG, de Oliveira KT, Faustino MAF, Neves MGPMS, Tomé AC, Silva AMS, Cavaleiro JAS, Brandão P, Felix V. Chemical Transformations of Mono- and Bis(buta-1, 3-dien-1-yl)porphyrins: A New Synthetic Approach to Mono-and Dibenzoporphyrins. Eur J Org Chem. 2008;2008:704–712. [Google Scholar]
- 232.Silva AMG, Faustino MAF, Tome AC, Neves MGPMS, Silva AMS, Cavaleiro JAS. A novel approach to the synthesis of mono- and dipyrroloporphyrins. J Chem Soc, Perkin Trans 1. 2001:2752–2753. [Google Scholar]
- 233.Gomes ATPC, Neves MGPMS, Cavaleiro JAS. Diazo compounds in the functionalization of porphyrin macrocycles. J Porphyrins Phthalocyanines. 2011;15:1–13. [Google Scholar]
- 234.Singh S, Aggarwal A, Thompson S, Tomé JoPC, Zhu X, Samaroo D, Vinodu M, Gao R, Drain CM. Synthesis and Photophysical Properties of Thioglycosylated Chlorins, Isobacteriochlorins, and Bacteriochlorins for Bioimaging and Diagnostics. Bioconjugate Chem. 2010;21:2136–2146. doi: 10.1021/bc100356z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hirohara S, Obata M, Alitomo H, Sharyo K, Ando T, Tanihara M, Yano S. Synthesis, photophysical properties and sugar-dependent in vitro photocytotoxicity of pyrrolidine-fused chlorins bearing S-glycosides. J Photochem Photobiol, B. 2009;97:22–33. doi: 10.1016/j.jphotobiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 236.Costa JIT, Tomé AC, Neves MGPMS, Cavaleiro JAS. 5, 10, 15, 20-tetrakis(pentafluorophenyl)porphyrin: a versatile platform to novel porphyrinic materials. J Porphyrins Phthalocyanines. 2011;15:1116–1133. [Google Scholar]
- 237.Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia ACS, Burda C, Oleinick NL. Fluorescence Resonance Energy Transfer Reveals a Binding Site of a Photosensitizer for Photodynamic Therapy. Cancer Res. 2003;63:5194–5197. [PubMed] [Google Scholar]
- 238.Hirohara S, Kawasaki Y, Funasako R, Yasui N, Totani M, Alitomo H, Yuasa J, Kawai T, Oka C, Kawaichi M, et al. Sugar and Heavy Atom Effects of Glycoconjugated Chlorin Palladium Complex on Photocytotoxicity. Bioconjugate Chem. 2012;23:1881–1890. doi: 10.1021/bc300223j. [DOI] [PubMed] [Google Scholar]
- 239.Gorman A, Killoran J, O’Shea C, Kenna T, Gallagher WM, O’Shea DF. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J Am Chem Soc. 2004;126:10619–10631. doi: 10.1021/ja047649e. [DOI] [PubMed] [Google Scholar]
- 240.Azenha ElG, Serra AC, Pineiro M, Pereira MM, Seixas de Melo J, Arnaut LG, Formosinho SJ, Rocha Gonsalves AMdA. Heavy-atom effects on metalloporphyrins and polyhalogenated porphyrins. Chem Phys. 2002;280:177–190. [Google Scholar]
- 241.Sakuma S, Otake E, Torii K, Nakamura M, Maeda A, Tujii R, Akashi H, Ohi H, Yano S, Morita A. Photodynamic therapy with glycoconjugated chlorin photosensitizer. J Porphyrins Phthalocyanines. 2013;17:331–342. [Google Scholar]
- 242.Tanaka M, kataoka H, Mabuchi M, Sakuma S, Takahashi S, Tujii R, Akashi H, Ohi H, Yano S, Morita A, et al. Anticancer Effects of Novel Photodynamic Therapy with Glycoconjugated Chlorin for Gastric and Colon Cancer. Anticancer Res. 2011;31:763–770. [PubMed] [Google Scholar]
- 243.Tang J, Chen JJ, Jing J, Chen JZ, Lv H, Yu Y, Xu P, Zhang JL. beta-Lactonization of fluorinated porphyrin enhances LDL binding affinity, cellular uptake with selective intracellular localization. Chem Sci. 2014;5:558–566. [Google Scholar]
- 244.McCarthy JR, Bhaumik J, Merbouh N, Weissleder R. High-yielding syntheses of hydrophilic conjugatable chlorins and bacteriochlorins. Org Biomol Chem. 2009;7:3430–3436. doi: 10.1039/b908713c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gomes ATPC, Leao RAC, da Silva FC, Neves MGPMS, Faustino MAF, Tomé AC, Silva AMG, Pinheiro S, de Souza MCBV, Ferreira VF, et al. Synthesis of new glycoporphyrin derivatives through carbohydrate-substituted α-diazoacetates. JPorphyrins Phthalocyanines. 2009;13:247–255. [Google Scholar]
- 246.Zhang M, Zhang Z, Blessington D, Li H, Busch TM, Madrak V, Miles J, Chance B, Glickson JD, Zheng G. Pyropheophorbide 2-Deoxyglucosamide: A New Photosensitizer Targeting Glucose Transporters. Bioconjugate Chem. 2003;14:709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 247.Dumoulin F, Ahsen V. Click chemistry: the emerging role of the azide-alkyne Huisgen dipolar addition in the preparation of substituted tetrapyrrolic derivatives. J Porphyrins Phthalocyanines. 2011;15:481–504. [Google Scholar]
- 248.Grin MA, Lonin IS, Likhosherstov LM, Novikova OS, Plyutinskaya AD, Plotnikova EA, Kachala VV, Yakubovskaya RI, Mironov AF. “Click chemistry” in the synthesis of the first glycoconjugates of bacteriochlorin series. J Porphyrins Phthalocyanines. 2012;16:1094–1109. [Google Scholar]
- 249.Grin MA, Lonin IS, Makarov AI, Lakhina AA, Toukach FV, Kachala VV, Orlova AV, Mironov AF. Synthesis of chlorin-carbohydrate conjugates by ‘click chemistry’. Mendeleev Commun. 2008;18:135–137. [Google Scholar]
- 250.Grin MA, Lonin IS, Lakhina AA, Ol’shanskaya ES, Makarov AI, Sebyakin YL, Guryeva LY, Toukach PV, Kononikhin AS, Kuzmin VA, et al. 1,3-dipolar cycloaddition in the synthesis of glycoconjugates of natural chlorins and bacteriochlorins. J Porphyrins Phthalocyanines. 2009;13:336–345. [Google Scholar]
- 251.Gross Z, Galili N, Saltsman I. The First Direct Synthesis of Corroles from Pyrrole. Angew Chem, Int Ed. 1999;38:1427–1429. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1427::AID-ANIE1427>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 252.Ventura B, Degli Esposti A, Koszarna B, Gryko DT, Flamigni L. Photophysical characterization of free-base corroles, promising chromophores for light energy conversion and singlet oxygen generation. New J Chem. 2005;29:1559–1566. [Google Scholar]
- 253.Lili Y, Han S, Lei S, GuoLiang Z, HaiYang L, Hui W, LiangNian J. Photophysical properties of the Corrole photosensitizers. Sci China: Phys, Mech Astron. 2010;53:1491–1496. [Google Scholar]
- 254.Aviezer D, Cotton S, David M, Segev A, Khaselev N, Galili N, Gross Z, Yayon A. Porphyrin Analogues as Novel Antagonists of Fibroblast Growth Factor and Vascular Endothelial Growth Factor Receptor Binding That Inhibit Endothelial Cell Proliferation, Tumor Progression, and Metastasis. Cancer Res. 2000;60:2973–2980. [PubMed] [Google Scholar]
- 255.Agadjanian H, Weaver JJ, Mahammed A, Rentsendorj A, Bass S, Kim J, Dmochowski IJ, Margalit R, Gray HB, Gross Z, et al. Specific delivery of corroles to cells via noncovalent conjugates with viral proteins. Pharm Res. 2006;23:367–377. doi: 10.1007/s11095-005-9225-1. [DOI] [PubMed] [Google Scholar]
- 256.Cardote TAF, Barata JFB, Faustino MAF, Preuß A, Neves MGPMS, Cavaleiro JAS, Ramos CIV, Santana-Marques MGO, Röder B. Pentafluorophenylcorrole–d-galactose conjugates. Tetrahedron Lett. 2012;53:6388–6393. [Google Scholar]
- 257.Aggarwal A, Singh S, Zhang Y, Anthes M, Samaroo D, Gao R, Drain CM. Synthesis and photophysics of an octathioglycosylated zinc(II) phthalocyanine. Tetrahedron Lett. 2011;52:5456–5459. doi: 10.1016/j.tetlet.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Drain CM, Singh S, Samaroo D, Thompson S, Vinodu M, Tome JPC. New Porphyrin Glyco-conjugates. Proc SPIE. 2009;7380:73902K-1–9. [Google Scholar]
- 259.Boyle RW, Leznoff CC, van Lier JE. Biological activities of phthalocyanines – XVI. Tetrahydroxy- and tetraalkylhydroxy zinc phthalocyanines. Effect of alkyl chain length on in vitro and in vivo photodynamic activities. Br J Cancer. 1993;67:1177–1181. doi: 10.1038/bjc.1993.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lv F, He X, Lu L, Wu L, Liu T. A novel water-soluble near-infrared glucose-conjugated porphyrin: synthesis, properties and its optical imaging effect. J Porphyrins Phthalocyanines. 2011;15:217–222. [Google Scholar]
- 261.Lv F, He X, Lu l, Wu L, Liu T. Synthesis, properties and near-infrared imaging evaluation of glucose conjugated zinc phthalocyanine via Click reaction. J Porphyrins Phthalocyanines. 2012;16:77–84. [Google Scholar]
- 262.Ogawa K, Kobuke Y. Recent Advances in Two-Photon Photodynamic Therapy. Anti-Cancer Agents Med Chem. 2008;8:269–279. doi: 10.2174/187152008783961860. [DOI] [PubMed] [Google Scholar]
- 263.Spangler CW, Starkey JR, Liang B, Fedorka S, Yang H, Jiang H. Development of image-guided targeted two-photon PDT for the treatment of head and neck cancers. Proc SPIE. 2014;8931:89310C-1–8. [Google Scholar]
- 264.Collins HA, Khurana M, Moriyama EH, Mariampillai A, Dahlstedt E, Balaz M, Kuimova MK, Drobizhev M, YangVictor XD, Phillips D, et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat Photonics. 2008;2:420–424. [Google Scholar]
- 265.Hammerer F, Achelle S, Baldeck P, Maillard P, Teulade-Fichou MP. Influence of Carbohydrate Biological Vectors on the Two-Photon Resonance of Porphyrin Oligomers. J Phys Chem A. 2011;115:6503–6508. doi: 10.1021/jp202436x. [DOI] [PubMed] [Google Scholar]
- 266.Hisaki I, Hiroto S, Kim KS, Noh SB, Kim D, Shinokubo H, Osuka A. Synthesis of Doubly β-to-β 1,3-Butadiyne-Bridged Diporphyrins: Enforced Planar Structures and Large Two-Photon Absorption Cross Sections. Angew Chem, Int Ed. 2007;46:5125–5128. doi: 10.1002/anie.200700550. [DOI] [PubMed] [Google Scholar]
- 267.Garcia G, Hammerer F, Poyer F, Achelle S, Teulade-Fichou MP, Maillard P. Carbohydrate-conjugated porphyrin dimers: Synthesis and photobiological evaluation for a potential application in one-photon and two-photon photodynamic therapy. Bioorg Med Chem. 2013;21:153–165. doi: 10.1016/j.bmc.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 268.Yoon MC, Noh SB, Tsuda A, Nakamura Y, Osuka A, Kim D. Photophysics of meso-β Doubly Linked Ni(II) Porphyrin Arrays: Large Two-Photon Absorption Cross-Section and Fast Energy Relaxation Dynamics. J Am Chem Soc. 2007;129:10080–10081. doi: 10.1021/ja0735655. [DOI] [PubMed] [Google Scholar]
- 269.Kim DY, Ahn TK, Kwon JH, Kim D, Ikeue T, Aratani N, Osuka A, Shigeiwa M, Maeda S. Large Two-Photon Absorption (TPA) Cross-Section of Directly Linked Fused Diporphyrins. J Phys Chem A. 2005;109:2996–2999. doi: 10.1021/jp050747h. [DOI] [PubMed] [Google Scholar]
- 270.Vicente MGH, Jaquinod L, Smith KM. Oligomeric porphyrin arrays. Chem Commun. 1999:1771–1782. [Google Scholar]
- 271.Tsuda A, Furuta H, Osuka A. Syntheses, Structural Characterizations, and Optical and Electrochemical Properties of Directly Fused Diporphyrins. J Am Chem Soc. 2001;123:10304–10321. doi: 10.1021/ja0110933. [DOI] [PubMed] [Google Scholar]
- 272.Hiroto S, Furukawa K, Shinokubo H, Osuka A. Synthesis and Biradicaloid Character of Doubly Linked Corrole Dimers. J Am Chem Soc. 2006;128:12380–12381. doi: 10.1021/ja062654z. [DOI] [PubMed] [Google Scholar]
- 273.Schwab PFH, Levin MD, Michl J. Molecular Rods. 1. Simple Axial Rods. Chem Rev. 1999;99:1863–1934. doi: 10.1021/cr970070x. [DOI] [PubMed] [Google Scholar]
- 274.Anderson HL. Building molecular wires from the colours of life: conjugated porphyrin oligomers. Chem Commun. 1999:2323–2330. [Google Scholar]
- 275.Arnold DP, James DA. Dimers and Model Monomers of Nickel(II) Octaethylporphyrin Substituted by Conjugated Groups Comprising Combinations of Triple Bonds with Double Bonds and Arenes. 1. Synthesis and Electronic Spectra. J Org Chem. 1997;62:3460–3469. [Google Scholar]
- 276.Kuimova MK, Collins HA, Balaz M, Dahlstedt E, Levitt JA, Sergent N, Suhling K, Drobizhev M, Makarov NS, Rebane A, et al. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy. Org Biomol Chem. 2009;7:889–896. doi: 10.1039/b814791d. [DOI] [PubMed] [Google Scholar]
- 277.Odom SA, Webster S, Padilha LA, Peceli D, Hu H, Nootz G, Chung SJ, Ohira S, Matichak JD, Przhonska OV, et al. Synthesis and Two-Photon Spectrum of a Bis(Porphyrin)-Substituted Squaraine. J Am Chem Soc. 2009;131:7510–7511. doi: 10.1021/ja901244e. [DOI] [PubMed] [Google Scholar]
- 278.Hammerer F, Garcia G, Chen S, Poyer F, Achelle S, Fiorini-Debuisschert C, Teulade-Fichou MP, Maillard P. Synthesis and Characterization of Glycoconjugated Porphyrin Triphenylamine Hybrids for Targeted Two-Photon Photodynamic Therapy. J Org Chem. 2014;79:1406–1417. doi: 10.1021/jo402658h. [DOI] [PubMed] [Google Scholar]
- 279.Aggarwal A, Thompson S, Singh S, Newton B, Moore A, Gao R, Gu X, Mukherjee S, Drain CM. Photophysics of glycosylated derivatives of a chlorin, isobacteriochlorin and bacteriochlorin for photodynamic theragnostics: discovery of a two-photon-absorbing photosensitizer. Photochem Photobiol. 2014;90:419–430. doi: 10.1111/php.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ménard F, Sol V, Ringot C, Granet R, Alves S, Morvan CL, Queneau Y, Ono N, Krausz P. Synthesis of tetraglucosyl- and tetrapolyamine-tetrabenzoporphyrin conjugates for an application in PDT. Bioorg Med Chem. 2009;17:7647–7657. doi: 10.1016/j.bmc.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 281.Lash TD. Synthesis of Novel Porphyrinoid Chromophores. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook: Applications of Phthalocyanines. Vol. 2. Academic Press; New York: 2000. pp. 125–199. [Google Scholar]
- 282.Valles MA, Biolo R, Bonnett R, Canete M, Gomez AM, Jori G, Juarranz A, McManus KA, Okolo KT. Benzoporphyrins as photosensitizers for the photodynamic therapy of cancer. Proc SPIE. 1996;2625:11–22. [Google Scholar]
- 283.Graça M, Vicente H, Smith KM. Porphyrins with fused exocyclic rings. J Porphyrins Phthalocyanines. 2004;8:26–42. [Google Scholar]
- 284.Lash TD. Modification of the porphyrin chromophore by ring fusion: identifying trends due to annelation of the porphyrin nucleus. J Porphyrins Phthalocyanines. 2001;5:267–288. [Google Scholar]
- 285.Murashima T, Tsujimoto S, Yamada T, Miyazawa T, Uno H, Ono N, Sugimoto N. Synthesis of water-soluble porphyrin and the corresponding highly planar benzoporphyrin without meso-substituents. Tetrahedron Lett. 2005;46:113–116. [Google Scholar]
- 286.Vinogradov SA, Wilson DF. Metallotetrabenzoporphyrins. New phosphorescent probes for oxygen measurements. J Chem Soc, Perkin Trans 2. 1995:103–111. [Google Scholar]
- 287.Finikova OS, Cheprakov AV, Beletskaya IP, Carroll PJ, Vinogradov SA. Novel Versatile Synthesis of Substituted Tetrabenzoporphyrins. J Org Chem. 2004;69:522–535. doi: 10.1021/jo0350054. [DOI] [PubMed] [Google Scholar]
- 288.Filatov MA, Lebedev AY, Vinogradov SA, Cheprakov AV. Synthesis of 5,15-Diaryltetrabenzoporphyrins. J Org Chem. 2008;73:4175–4185. doi: 10.1021/jo800509k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Momenteau M, Oulmi D, Maillard P, Croisy AF. In vitro photobiological activity of a new series of photosensitizers: the glycogonjugated porphyrins. Proc SPIE. 1995;2325:13–23. [Google Scholar]
- 290.Braun A, Tcherniac J. Über die Produkte der Einwirkung von Acetanhydrid auf Phthalamid. Ber Dtsch Chem Ges. 1907;40:2709–2714. [Google Scholar]
- 291.Leznoff CC, Lever ABP. Phthalocyanines, Properties and Applications. Vol. 1 Wiley, VCH; New York: 1989. [Google Scholar]
- 292.Roeder B, Naether D, Lewald T, Braune M, Nowak C, Freyer W. Photophysical properties and photodynamic activity in vivo of some tetrapyrroles. Biophys Chem. 1990;35:303–312. doi: 10.1016/0301-4622(90)80017-2. [DOI] [PubMed] [Google Scholar]
- 293.Lourenc LMO, Neves MGPMS, Cavaleiro JAS, Tome JPC. Synthetic approaches to glycophthalocyanines. Tetrahedron. 2014;70:2681–2698. [Google Scholar]
- 294.Varotto A, Nam CY, Radivojevic I, Tomé JPC, Cavaleiro JAS, Black CT, Drain CM. Phthalocyanine Blends Improve Bulk Heterojunction Solar Cells. J Am Chem Soc. 2010;132:2552–2554. doi: 10.1021/ja907851x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Xu P, Chen J, Chen Z, Zhou S, Hu P, Chen X, Huang M. Receptor-Targeting Phthalocyanine Photosensitizer for Improving Antitumor Photocytotoxicity. PLoS One. 2012;7(5):e37051. doi: 10.1371/journal.pone.0037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Allen CM, Sharman WM, Van Lier JE. Current status of phthalocyanines in the photodynamic therapy of cancer. J Porphyrins Phthalocyanines. 2001;5:161–169. [Google Scholar]
- 297.Lyubimtsev A, Iqbal Z, Crucius G, Syrbu S, Ziegler T, Hanack M. Synthesis of glycosylated metal phthalocyanines and naphthalocyanines. J Porphyrins Phthalocyanines. 2012;16:434–463. [Google Scholar]
- 298.Zhang P, Zhang S, Han G. Synthesis of Novel Asymmetric Zinc (II) Phthalocyanines Bearing Octadecyloxyl and Glucosyl Groups. Molecules. 2009;14:3688–3693. doi: 10.3390/molecules14093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.van Hillegersberg R, Kort WJ, Wilson JHP. Current Status of Photodynamic Therapy in Oncology. Drugs. 1994;48:510–527. doi: 10.2165/00003495-199448040-00003. [DOI] [PubMed] [Google Scholar]
- 300.Novakova V, Kobak RZU, Kucera R, Kopecky K, Miletin M, Krepsova V, Ivincova J, Zimcik P. The effect of the number of carbohydrate moieties on the azaphthalocyanine properties. Dalton Trans. 2012;41:10596–10604. doi: 10.1039/c2dt30971h. [DOI] [PubMed] [Google Scholar]
- 301.Lee PPS, Lo PC, Chan EYM, Fong WP, Ko WH, Ng DKP. Synthesis and in vitro photodynamic activity of novel galactose-containing phthalocyanines. Tetrahedron Lett. 2005;46:1551–1554. [Google Scholar]
- 302.Lo PC, Huang JD, Cheng DYY, Chan EYM, Fong WP, Ko WH, Ng DKP. New Amphiphilic Silicon(IV) Phthalocyanines as Efficient Photosensitizers for Photodynamic Therapy: Synthesis, Photophysical Properties, and in vitro Photodynamic Activities. Chem - Eur J. 2004;10:4831–4838. doi: 10.1002/chem.200400462. [DOI] [PubMed] [Google Scholar]
- 303.Lau JTF, Lo PC, Tsang YM, Fong WP, Ng DKP. Unsymmetrical [small beta]-cyclodextrin-conjugated silicon(iv) phthalocyanines as highly potent photosensitisers for photodynamic therapy. Chem Commun. 2011;47:9657–9659. doi: 10.1039/c1cc13783b. [DOI] [PubMed] [Google Scholar]
- 304.Chan WS, Brasseur N, Madeleine GL, Quellet R, van Lier JE. Efficacy and mechanism of aluminium phthalocyanine and its sulphonated derivatives mediated photodynamic therapy on murine tumours. Eur J Cancer. 1997;33:1855–1859. doi: 10.1016/s0959-8049(97)00220-7. [DOI] [PubMed] [Google Scholar]
- 305.Brasseur N, Ouellet R, Madeleine CL, Lier JEv. Water-soluble aluminium phthalocyanine-polymer conjugates for PDT: photodynamic activities and pharmacokinetics in tumour-bearing mice. Br J Cancer. 1999;80:1533–1541. doi: 10.1038/sj.bjc.6690557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kernag CA, McGrath DV. Non-aggregating octasubstituted dendritic phthalocyanines. Chem Commun. 2003:1048–1049. doi: 10.1039/b301157g. [DOI] [PubMed] [Google Scholar]
- 307.De Filippis MP, Dei D, Fantetti L, Roncucci G. Synthesis of a new water-soluble octa-cationic phthalocyanine derivative for PDT. Tetrahedron Lett. 2000;41:9143–9147. [Google Scholar]
- 308.Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 309.Dwek RA. Glycobiology: Toward Understanding the Function of Sugars. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 310.Ribeiro AO, Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Iamamoto Y, Torres T. 1, 2, 3, 4-Tetrakis-(alpha/beta-d-galactopyranos-6-yl)phthalocyaninato]zinc(II): a water-soluble phthalocyanine. Tetrahedron Lett. 2006;47:9177–9180. [Google Scholar]
- 311.Soares ARM, Neves MGPMS, Tomé AC, Iglesias-de la Cruz MC, Zamarrón A, Carrasco E, González S, Cavaleiro JAS, Torres T, Guldi DM, et al. Glycophthalocyanines as Photosensitizers for Triggering Mitotic Catastrophe and Apoptosis in Cancer Cells. Chem Res Toxicol. 2012;25:940–951. doi: 10.1021/tx300035a. [DOI] [PubMed] [Google Scholar]
- 312.Huang JD, Fong WP, Chan EYM, Choi MTM, Chan WK, Chan MC, Ng DKP. Photodynamic activities of a dicationic silicon(IV) phthalocyanine and its bovine serum albumin conjugates. Tetrahedron Lett. 2003;44:8029–8032. [Google Scholar]
- 313.Lau JTF, Lo PC, Fong WP, Ng DKP. Preparation and Photodynamic Activities of Silicon(IV) Phthalocyanines Substituted with Permethylated β-Cyclodextrins. Chem - Eur J. 2011;17:7569–7577. doi: 10.1002/chem.201100621. [DOI] [PubMed] [Google Scholar]
- 314.Lo PC, Chan CMH, Liu JY, Fong WP, Ng DKP. Highly Photocytotoxic Glucosylated Silicon(IV) Phthalocyanines. Effects of Peripheral Chloro Substitution on the Photophysical and Photodynamic Properties. J Med Chem. 2007;50:2100–2107. doi: 10.1021/jm061415j. [DOI] [PubMed] [Google Scholar]
- 315.Álvarez-Micó X, Calvete MJF, Hanack M, Ziegler T. A new glycosidation method through nitrite displacement on substituted nitrobenzenes. Carbohydr Res. 2007;342:440–447. doi: 10.1016/j.carres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 316.Álvarez-Micó X, Calvete MJF, Hanack M, Ziegler T. Expeditious Synthesis of Glycosylated Phthalocyanines. Synthesis. 2007;2007:2186–2192. [Google Scholar]
- 317.Iqbal Z, Hanack M, Ziegler T. Synthesis of an octasubstituted galactose zinc(II) phthalocyanine. Tetrahedron Lett. 2009;50:873–875. [Google Scholar]
- 318.Zorlu Y, Ermeydan MA, Dumoulin F, Ahsen V, Savoie H, Boyle RW. Glycerol and galactose substituted zinc phthalocyanines. Synthesis and photodynamic activity. Photochem Photobiol Sci. 2009;8:312–318. doi: 10.1039/b817348f. [DOI] [PubMed] [Google Scholar]
- 319.Choi CF, Huang JD, Lo PC, Fong WP, Ng DKP. Glycosylated zinc(ii) phthalocyanines as efficient photosensitisers for photodynamic therapy. Synthesis, photophysical properties and in vitro photodynamic activity. Org Biomol Chem. 2008;6:2173–2181. doi: 10.1039/b802212g. [DOI] [PubMed] [Google Scholar]
- 320.Iqbal Z, Lyubimtsev A, Herrmann T, Hanack M, Ziegler T. Synthesis of Octaglycosylated Zinc(II) Phthalocyanines. Synthesis. 2010;18:3097–3104. [Google Scholar]
- 321.Lv F, Li Y, Cao B, Liu T. Galactose substituted zinc phthalocyanines as near infrared fluorescence probes for liver cancer imaging. J Mater Sci: Mater Med. 2013;24:811–819. doi: 10.1007/s10856-012-4820-2. [DOI] [PubMed] [Google Scholar]
- 322.Iqbal Z, Lyubimtsev A, Hanack M, Ziegler T. Synthesis and characterization of 1,8(11),15(18),22(25)-tetraglycosylated zinc-(II) phthalocyanines. J Porphyrins Phthalocyanines. 2010;14:494–498. [Google Scholar]
- 323.Kimani SG, Shmigol TA, Hammond S, Phillips JB, Bruce JI, MacRobert AJ, Malakhov MV, Golding JP. Fully Protected Glycosylated Zinc (II) Phthalocyanine Shows High Uptake and Photodynamic Cytotoxicity in MCF-7 Cancer Cells. Photochem Photobiol. 2013;89:139–149. doi: 10.1111/j.1751-1097.2012.01204.x. [DOI] [PubMed] [Google Scholar]
- 324.van Lier JE, Spikes JD. The Chemistry, Photophysics and Photosensitizing Properties of Phthalocyanines. In: Dougherty TJ, Block G, Harnett S, editors. Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use (CIBA Foundation Symposium 146) Vol. 17. Wiley; Chichester: 1989. pp. 17–39. [DOI] [PubMed] [Google Scholar]
- 325.Iqbal Z, Masilela N, Nyokong T, Lyubimtsev A, Hanack M, Ziegler T. Spectral, photophysical and photochemical properties of tetra- and octaglycosylated zinc phthalocyanines. Photochem Photobiol Sci. 2012;11:679–686. doi: 10.1039/c2pp05348a. [DOI] [PubMed] [Google Scholar]
- 326.Iqbal Z, Ogunsipe A, Nyokong T, Lyubimtsev A, Hanack M, Ziegler T. Photophysics and photochemistry of octaglucosylated zinc phthalocyanine derivatives. J Porphyrins Phthalocyanines. 2012;16:413–422. [Google Scholar]
- 327.Soares ARM, Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Torres T. Synthesis of water-soluble phthalocyanines bearing four or eight d-galactose units. Carbohydr Res. 2009;344:507–510. doi: 10.1016/j.carres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 328.Liu JY, Lo PC, Fong WP, Ng DKP. Effects of the number and position of the substituents on the in vitro photodynamic activities of glucosylated zinc(ii) phthalocyanines. Org Biomol Chem. 2009;7:1583–1591. doi: 10.1039/b822128f. [DOI] [PubMed] [Google Scholar]
- 329.Lafont D, Zorlu Y, Savoie H, Albrieux F, Ahsen V, Boyle RW, Dumoulin F. Monoglycoconjugated phthalocyanines: Effect of sugar and linkage on photodynamic activity. Photodiagn Photodyn Ther. 2013;10:252–259. doi: 10.1016/j.pdpdt.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 330.Ermeydan MA, Dumoulin F, Basova TV, Bouchu D, Gurek AG, Ahsen V, Lafont D. Amphiphilic carbohydrate-phthalocyanine conjugates obtained by glycosylation or by azide-alkyne click reaction. New J Chem. 2010;34:1153–1162. [Google Scholar]
- 331.Berthold HJ, Franke S, Thiem J, Schotten T. Ex Post Glycoconjugation of Phthalocyanines. J Org Chem. 2010;75:3859–3862. doi: 10.1021/jo100362n. [DOI] [PubMed] [Google Scholar]
- 332.Zorlu Y, Dumoulin F, Bouchu D, Ahsen V, Lafont D. Monoglycoconjugated water-soluble phthalocyanines. Design and synthesis of potential selectively targeting PDT photosensitisers. Tetrahedron Lett. 2010;51:6615–6618. [Google Scholar]
- 333.Lv F, He X, Wu L, Liu T. Lactose substituted zinc phthalocyanine: A near infrared fluorescence imaging probe for liver cancer targeting. Bioorg Med Chem Lett. 2013;23:1878–1882. doi: 10.1016/j.bmcl.2012.12.103. [DOI] [PubMed] [Google Scholar]
- 334.Kanat Z, Dincer H. The synthesis and characterization of nonperipherally tetra terminal alkynyl substituted phthalocyanines and glycoconjugation via the click reaction. Dalton Trans. 2014;43:8654–8663. doi: 10.1039/c4dt00238e. [DOI] [PubMed] [Google Scholar]
- 335.Pereira PM, Silva S, Cavaleiro JA, Ribeiro CA, Tome JP, Fernandes R. Galactodendritic phthalocyanine targets carbohydrate-binding proteins enhancing photodynamic therapy. PLoS One. 2014;9:e95529–e95541. doi: 10.1371/journal.pone.0095529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lu L, Lv F, Cao B, He X, Liu T. Saccharide Substituted Zinc Phthalocyanines: Optical Properties, Interaction with Bovine Serum Albumin and Near Infrared Fluorescence Imaging for Sentinel Lymph Nodes. Molecules. 2014;19:525–537. doi: 10.3390/molecules19010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ribeiro AO, Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Serra OA, Torres T. First phthalocyanine-[beta]-cyclodextrin dyads. Tetrahedron Lett. 2006;47:6129–6132. [Google Scholar]
- 338.Ruebner A, Yang Z, Leung D, Breslow R. A cyclodextrin dimer with a photocleavable linker as a possible carrier for the photosensitizer in photodynamic tumor therapy. Proc Natl Acad Sci U S A. 1999;96:14692–14693. doi: 10.1073/pnas.96.26.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tau P, Ogunsipe AO, Maree S, Maree MD, Nyokong T. Influence of cyclodextrins on the fluorescence, photostability and singlet oxygen quantum yields of zinc phthalocyanine and naphthalocyanine complexes. J Porphyrins Phthalocyanines. 2003;7:439–446. [Google Scholar]
- 340.Claessens CG, González-Rodríguez D, Torres T. Subphthalocyanines: Singular Nonplanar Aromatic CompoundsSynthesis, Reactivity, and Physical Properties. Chem Rev. 2002;102:835–854. doi: 10.1021/cr0101454. [DOI] [PubMed] [Google Scholar]
- 341.de la Escosura A, Martínez-Díaz MV, Thordarson P, Rowan AE, Nolte RJM, Torres T. Donor–Acceptor Phthalocyanine Nanoaggregates. J Am Chem Soc. 2003;125:12300–12308. doi: 10.1021/ja030038m. [DOI] [PubMed] [Google Scholar]
- 342.Guldi DM, Gouloumis A, Vázquez P, Torres T, Georgakilas V, Prato M. Nanoscale Organization of a Phthalocyanine–Fullerene System: Remarkable Stabilization of Charges in Photoactive 1-D Nanotubules. J Am Chem Soc. 2005;127:5811–5813. doi: 10.1021/ja043283u. [DOI] [PubMed] [Google Scholar]
- 343.Beletskaya I, Tyurin VS, Tsivadze AY, Guilard R, Stern C. Supramolecular Chemistry of Metalloporphyrins. Chem Rev. 2009;109:1659–1713. doi: 10.1021/cr800247a. [DOI] [PubMed] [Google Scholar]
- 344.Lourenco LMO, Pereira PMR, Maciel E, Valega M, Domingues FMJ, Domingues MRM, Neves MGPMS, Cavaleiro JAS, Fernandes R, Tome JPC. Amphiphilic phthalocyanine-cyclodextrin conjugates for cancer photodynamic therapy. Chem Commun. 2014;50:8363–8366. doi: 10.1039/c4cc02226b. [DOI] [PubMed] [Google Scholar]
- 345.Ranyuk E, Cauchon N, Klarskov K, Guérin B, van Lier JE. Phthalocyanine–Peptide Conjugates: Receptor-Targeting Bifunctional Agents for Imaging and Photodynamic Therapy. J Med Chem. 2013;56:1520–1534. doi: 10.1021/jm301311c. [DOI] [PubMed] [Google Scholar]
- 346.Horne TK, Cronjé MJ. Novel Porphyrazine Derivatives show Promise for Photodynamic Therapy despite Restrictions in Hydrophilicity. Photochem Photobiol. 2014;90:648–658. doi: 10.1111/php.12231. [DOI] [PubMed] [Google Scholar]
- 347.Williams DBG, Mbatha GB. The synthesis and characterisation of carbohydrate-functionalised porphyrazines. Dyes Pigm. 2011;88:65–74. [Google Scholar]
- 348.Nawalany K, Rusin A, Kepczynski M, Filipczak P, Kumorek M, Kozik B, Weitman H, Ehrenberg B, Krawczyk Z, Nowakowska M. Novel nanostructural photosensitizers for photodynamic therapy: In vitro studies. Int J Pharm. 2012;430:129–140. doi: 10.1016/j.ijpharm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 349.Tuncel S, Dumoulin F, Gailer J, Sooriyaarachchi M, Atilla D, Durmus M, Bouchu D, Savoie H, Boyle RW, Ahsen V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(ii) phthalocyanines: synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011;40:4067–4079. doi: 10.1039/c0dt01260b. [DOI] [PubMed] [Google Scholar]
- 350.Lkhagvadulam B, Kim JH, Yoon I, Shim YK. Synthesis and photodynamic activities of novel water soluble purpurin-18-N-methyl-D-glucamine photosensitizer and its gold nanoparticles conjugate. J Porphyrins Phthalocyanines. 2012;16:331–340. [Google Scholar]
- 351.Kobata K, Ogawa J, Pandey SS, Oshima H, Arai T, Kato T, Nishino N. Synthesis and characterization of dendritic poly(l-lysine) containing porphyrin–fullerene moieties. Synth Met. 2007;157:311–317. [Google Scholar]
- 352.Sibrian-Vazquez M, Jensen TJ, Hammer RP, Vicente MGH. Peptide-Mediated Cell Transport of Water Soluble Porphyrin Conjugates. J Med Chem. 2006;49:1364–1372. doi: 10.1021/jm050893b. [DOI] [PubMed] [Google Scholar]
- 353.Aggarwal A, Singh S, Samson J, Drain CM. Adaptive Organic Nanoparticles of a Teflon-Coated Iron (III) Porphyrin Catalytically Activate Dioxygen for Cyclohexene Oxidation. Macromol Rapid Commun. 2012;33:1220–1226. doi: 10.1002/marc.201200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Aggarwal A, Singh S, Drain CM. Nanoaggregates of Mn(III)tetraperfluorophenylporphyrin: a greener approach for allylic oxidation of olefins. J Porphyrins Phthalocyanines. 2011;15:1258–1264. [Google Scholar]
- 355.Smeureanu G, Aggarwal A, Soll CE, Arijeloye J, Malave E, Drain CM. Enhanced Catalytic Activity and Unexpected Products from the Oxidation of Cyclohexene by Organic Nanoparticles of 5,10,15,20-Tetrakis-(2,3,4,5,6-pentafluorophenyl)porphyrinatoiron (III) in Water by Using O2. Chem - Eur J. 2009;15:12133–12140. doi: 10.1002/chem.200901086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Drain CM, Smeureanu G, Patel S, Gong X, Garno J, Arijeloye J. Porphyrin Nanoparticles as Supramolecular Systems. New J Chem. 2006;30:1834–1843. [Google Scholar]
- 357.Gong X, Milic T, Xu C, Batteas JD, Drain CM. Preparation and characterization of porphyrin nanoparticles. J Am Chem Soc. 2002;124:14290–14291. doi: 10.1021/ja027405z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Drain CM. Self-assembly, specific intermolecular interactions, for example, coordination chemistry and H-bonds, result in discrete supramolecular systems with predictable structures and properties; self-organization, nonspecific intermolecular interactions, for example, dispersion forces, results in open systems that are often difficult to predict or are different depending on the conditions used to make the material, for example, lipid structures; hierarchical, different organization or architectures over different size scales in a given material [Google Scholar]
- 359.Lucky SS, Soo KC, Zhang Y. Nanoparticles in Photodynamic Therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 360.Gallagher-Colombo SM, Finlay JC, Busch TM. Tumor Microenvironment as a Determinant of Photodynamic Therapy Resistance. In: Rapozzi V, Jori G, editors. Resistance to Photodynamic Therapy in Cancer. Vol. 5. Springer International Publishing; New York: 2015. pp. 65–97. [Google Scholar]
- 361.Spring BQ, Rizvi I, Xu N, Hasan T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem Photobiol Sci. 2015;14:1476–1491. doi: 10.1039/c4pp00495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mikata Y, Sawaguchi T, Kakuchi T, Gottschaldt M, Schubert US, Ohi H, Yano S. Control of the Aggregation Properties of Tris(maltohexaose)-Linked Porphyrins with an Alkyl Chain. Eur J Org Chem. 2010;2010:663–671. [Google Scholar]
- 363.Song JY, Kong HJ, Choi MS. Size-controlled assemblies of porphyrin-modified pullulan photosensitizers. J Porphyrins Phthalocyanines. 2012;16:1196–1200. [Google Scholar]
- 364.Ibrahim H, Kasselouri A, You C, Maillard P, Rosilio V, Pansu R, Prognon P. Meso-tetraphenyl porphyrin derivatives: The effect of structural modifications on binding to DMPC liposomes and albumin. J Photochem Photobiol, A. 2011;217:10–21. [Google Scholar]
- 365.Lyubimtsev A, Iqbal Z, Crucius G, Syrbu S, Taraymovich ES, Ziegler T, Hanack M. Aggregation behavior and UV-vis spectra of tetra- and octaglycosylated zinc phthalocyanines. J Porphyrins Phthalocyanines. 2011;15:39–46. [Google Scholar]
- 366.Stefanelli M, Monti D, Venanzi M, Paolesse R. Kinetic and spectroscopic studies on the self-aggregation of a meso-substituted amphiphilic corrole derivative. New J Chem. 2007;31:1722–1725. [Google Scholar]
- 367.Miao X, Gao A, Hiroto S, Shinokubo H, Osuka A, Xin H, Deng W. Adsorption characteristic of self-assembled corrole dimers on HOPG. Surf Interface Anal. 2009;41:225–230. [Google Scholar]
- 368.Miao X, Gao A, Li Z, Hiroto S, Shinokubo H, Osuka A, Deng W. First self-assembly study of large π-conjugated corrole dimers on solid substrates. Appl Surf Sci. 2009;255:5885–5890. [Google Scholar]
- 369.van Hameren R, Elemans JAAW, Wyrostek D, Tasior M, Gryko DT, Rowan AE, Nolte RJM. Self-assembly of corrole trimers in solution and at the solid-liquid interface. J Mater Chem. 2009;19:66–69. [Google Scholar]
- 370.van Hameren R, van Buul AM, Castriciano MA, Villari V, Micali N, Schon P, Speller S, Monsu Scolaro L, Rowan AE, Elemans JAAW, et al. Supramolecular Porphyrin Polymers in Solution and at the Solid–Liquid Interface. Nano Lett. 2008;8:253–259. doi: 10.1021/nl072563f. [DOI] [PubMed] [Google Scholar]
- 371.Goslinski T, Piskorz J. Fluorinated porphyrinoids and their biomedical applications. J Photochem Photobiol, C. 2011;12:304–321. [Google Scholar]
- 372.Pandey SK, Gryshuk AL, Graham A, Ohkubo K, Fukuzumi S, Dobhal MP, Zheng G, Ou Z, Zhan R, Kadish KM, et al. Fluorinated photosensitizers: synthesis, photophysical, electrochemical, intracellular localization, in vitro photosensitizing efficacy and determination of tumor-uptake by 19F in vivo NMR spectroscopy. Tetrahedron. 2003;59:10059–10073. [Google Scholar]
- 373.Monge-Fuentes V, Muehlmann LA, Bentes de Azevedo R. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014;5:24381–24394. doi: 10.3402/nano.v5.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhao B, Yin JJ, Bilski PJ, Chignell CF, Roberts JE, He YY. Enhanced photodynamic efficacy towards melanoma cells by encapsulation of Pc4 in silica nanoparticles. Toxicol Appl Pharmacol. 2009;241:163–172. doi: 10.1016/j.taap.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Guo H, Qian H, Idris NM, Zhang Y. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomedicine. 2010;6:486–495. doi: 10.1016/j.nano.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 376.Yoon HY, Koo H, Choi KY, Lee SJ, Kim K, Kwon IC, Leary JF, Park K, Yuk SH, Park JH, et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials. 2012;33:3980–3989. doi: 10.1016/j.biomaterials.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 377.Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a ‘Trojan horse’. Photochem Photobiol Sci. 2006;5:727–734. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- 378.Master AM, Rodriguez ME, Kenney ME, Oleinick NL, Gupta AS. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J Pharm Sci. 2010;99:2386–2398. doi: 10.1002/jps.22007. [DOI] [PubMed] [Google Scholar]
- 379.Oku N, Ishii T. Antiangiogenic Photodynamic Therapy with Targeted Liposomes. In: Nejat D, editor. Methods in Enzymology. Vol. 465. Academic Press; New York: 2009. pp. 313–330. [DOI] [PubMed] [Google Scholar]
- 380.De Leeuw J, Van Der Beek N, Bjerring P, Martino Neumann HA. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in combination with topical keratolytic agents. J Eur Acad Dermatol Venereol. 2010;24:460–469. doi: 10.1111/j.1468-3083.2009.03447.x. [DOI] [PubMed] [Google Scholar]
- 381.van Hell AJ, Fretz MM, Crommelin DJA, Hennink WE, Mastrobattista E. Peptide nanocarriers for intracellular delivery of photosensitizers. J Controlled Release. 2010;141:347–353. doi: 10.1016/j.jconrel.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 382.van Hell AJ, Costa CICA, Flesch FM, Sutter M, Jiskoot W, Crommelin DJA, Hennink WE, Mastrobattista E. Self-Assembly of Recombinant Amphiphilic Oligopeptides into Vesicles. Biomacromolecules. 2007;8:2753–2761. doi: 10.1021/bm0704267. [DOI] [PubMed] [Google Scholar]
- 383.Jia X, Jia L. Nanoparticles improve biological functions of phthalocyanine photosensitizers used for photodynamic therapy. Curr Drug Metab. 2012;13:1119–1122. doi: 10.2174/138920012802850074. [DOI] [PubMed] [Google Scholar]
- 384.Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV. Nanodrug applications in photodynamic therapy. Photodiagn Photodyn Ther. 2011;8:14–29. doi: 10.1016/j.pdpdt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 385.Kawczyk-Krupka A, Bugaj AM, Potempa M, Wasilewska K, Latos W, Sieroń A. Vascular-targeted photodynamic therapy in the treatment of neovascular age-related macular degeneration: Clinical perspectives. Photodiagn Photodyn Ther. 2015;12:161–175. doi: 10.1016/j.pdpdt.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 386.Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, Iwata E, Maruko R Fujisan Study, G. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The Fujisan Study. Retina. 2015;35:1569–1576. doi: 10.1097/IAE.0000000000000526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.