Abstract
The size, functional group diversity and three-dimensional structure of proteins often allow these biomolecules to bind disease-relevant structures that challenge or evade small-molecule discovery. Additionally, folded proteins are often much more stable in biologically relevant environments, compared to their peptide counterparts. We recently showed that helix-grafted-display—extensive resurfacing and elongation of an existing solvent exposed helix in a Pleckstrin Homology (PH) domain—leads to a new protein that binds a surrogate of HIV-1 gp41, a validated target for inhibition of HIV-1 entry. Expanding on this work, we prepared a number of human-derived helix-grafted-display PH domains with varied helix length, and measured properties relevant to therapeutic and basic research applications. In particular, we show that some of these new reagents express well as recombinant proteins in E. coli, are relativey stable in human serum, bind a mimic of pre-fusogenic HIV-1 gp41 in vitro and in complex biological environments, and significantly lower the incidence of HIV-1 infection of CD4-positive cells.
Graphical Abstract

Essentially every biological process relies heavily on a cascade of protein-protein binding events.(1) The macroscopic architecture and complex molecular diversity of protein ligands or receptors often allows them to engage partners whose large surface area and spatially disperse recognition features frustrate discovery of traditional small-molecule ligands. The ubiquity of such supramolecular targets underscores the need for a complementary discovery approach that produces macromolecular agents capable of specific protein recognition. Indeed, biologics constitute a rapidly expanding sector of our pharmaceutical arsenal. With an eye toward the vast array of protein-protein interfaces (PPIs) comprised of an alpha helix bound into a surface cleft, we recently developed a method for helical ligand discovery.(2)
We began with the premise that isolated helical fragments were themselves poor starting points for either discovery or application of new ligand sequences. Typical helix lengths at PPIs are relatively short, which complicates both production and stability of the excised sequences. Short peptides are difficult to express recombinantly, and their chemical synthesis on a therapeutically viable scale remains a significant challenge. Even when successful it dramatically increases the cost of treatment.(3) Meanwhile, such ligands are typically unfolded, significantly enhancing their susceptibility to non-specific degradation in vivo.(3) These shortcomings have fueled the search for alternative structures that mimic native PPI ligands.(4–10) Native sequences have been fitted with conformational constraints (hydrogen bond surrogates, ‘staples’) or backbone modifications (beta peptides), among other strategies. Though often successful, these approaches require non-trivial chemical synthesis that limits throughput and elevates costs. Alternatively, stably folded peptides or small proteins with intrinsic helical domains have been resurfaced (or grafted) with key contact residues from a particular PPI, although the expression and solubility of these proteins has, in some cases, been poor.(11) Though the final ligands are often still prepared via synthesis, the all-natural sequences can be optimized using directed evolution techniques.
In developing a general scaffold for helix display we sought to identify a protein fold that presents a properly folded alpha helix within the confines of a larger structure, in such a way as to permit direct receptor access to one helical face. Ideally, the basic scaffold would be readily expressible in soluble form, tolerant of mutation and/or extension of the helix (to permit sequence optimization), and protective against rapid helix degradation. Once identified, this basic framework could then serve in plug and play fashion as the starting point for optimizing a broad range of future PPI modulators.
Our initial scaffold search identified Pleckstrin Homology (PH) domains as a plausible starting place. PH domains are found in a broad family of lipid-binding proteins whose fold displays a single alpha helix atop a pair of beta sheets, such that one helix face is solvent exposed.(12–14) We reasoned that such structures might serve as a good foundation for helix-grafted systems—in which solvent exposed helix residues are mutated and/or the native helix is extended—that mimic disease-relevant helical ligands. The reliability of expression, folded state stability, and potential for evolving optimized sequences would make such constructs excellent leads for new protein ligand therapeutics. Our initial proof-of-principle report demonstrated that a helix-grafted PH domain could replicate binding of the native helical ligand to an HIV fusion protein model.(2) Here we explore the scope of viable PH scaffolds, and generate constructs capable of inhibiting HIV infection in a live-virus assay.
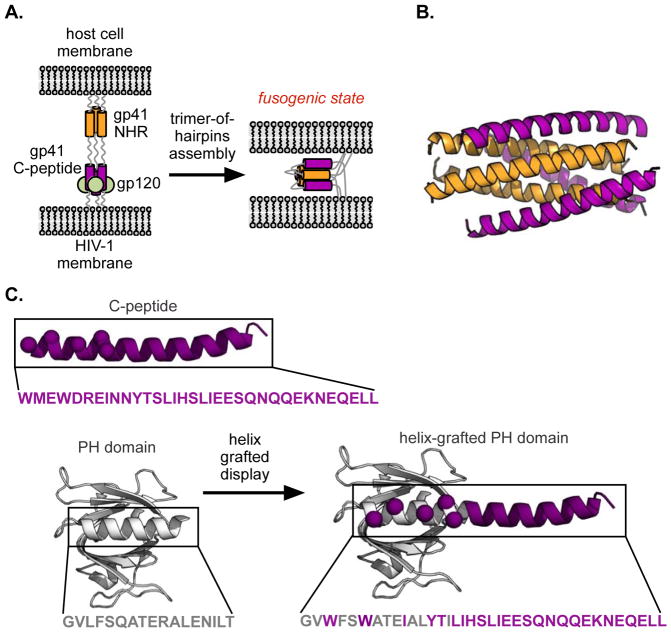
As an enveloped virus, HIV is encased within a host-derived lipid bilayer that must be fused with that of the target cell as a crucial part of the viral life cycle. The envelope glycoprotein responsible for this feat (gp41) operates by inserting an N-terminal fusion peptide anchor into the target cell’s bilayer, establishing an extended bridge between the two membranes (Figure 1A, left).(15) This bridge then contracts by mutual recognition of the trimeric coiled-coil formed by an N-terminal heptad repeat (NHR, Figure 1A and 1B, orange) and C-terminal regions of the fusion protein (C-peptides, Figure 1A and 1B, purple).(16, 17) This ‘trimer-of-hairpins’ helical assembly brings the anchored membranes into closer proximity (Figure 1A, right), ultimately enabling fusion (and thus infection). Disruption of the NHR/C-peptide interaction is a therapeutically validated means to block infection.(18, 19) In particular, various fragments of the C-peptide ligand have proven capable inhibitors, the most well known being enfuvirtide (also referred to as T-20, and marketed as Fuzeon™), a 36-residue C-peptide sequence that is an FDA-approved HIV therapy.(20) Given the demonstrated efficacy and therapeutic relevance of T-20, coupled with its significant drawbacks (extraordinary expense of therapy, half-life of only about 4 hours in vivo)(21), we chose the gp41 C-terminal sequence as our initial target in helix-grafting. In particular, we focused on the 34 residue C-terminal sequence of gp41 (termed C34) whose crystal structure (in complex with a 36 residue NHR peptide) was reported by Kim and coworkers.(17)
Figure 1.
(A) HIV-1 gp41-mediated membrane fusion of HIV-1 with a host cell. Binding of a C-peptide (purple) to an N-terminal coiled coil (NHR, orange) drags viral and cell membranes into close proximity, promoting fusion. (B) Crystal structure of the C-peptide (purple)/NHR (orange) assembly (PDB:1AIK). (C) Helix-grafted display strategy for generating stable C-peptide mimics on a Pleckstrin Homology (PH) domain. The native ligand helix (purple) is overlaid on that of the scaffold protein (grey, PDB:2CAY), and solvent-exposed scaffold residues are mutated to those of the ligand to be displayed (purple spheres).
In our previous report, we described using the PH domain from a yeast-derived protein called GLUE as a scaffold for gp41 C-peptide helix-grafted display (Figure 1C).(2) Surface-exposed GLUE helix residues were mutated to match those of gp41, and the helix was extended to match the native C-peptide length using the pure gp41 sequence. The resulting grafted protein was shown to be stable, well folded, and capable of recognizing a standard gp41 model with fidelity comparable to that of the wild-type peptide as measured by split-GFP reassembly, ELISA, and copurification assays. It also retained much of its efficacy even after 12 hours of exposure to human serum, supporting our initial design hypothesis that positioning the ligand within a larger stably folded structure would protect against rapid degradation.
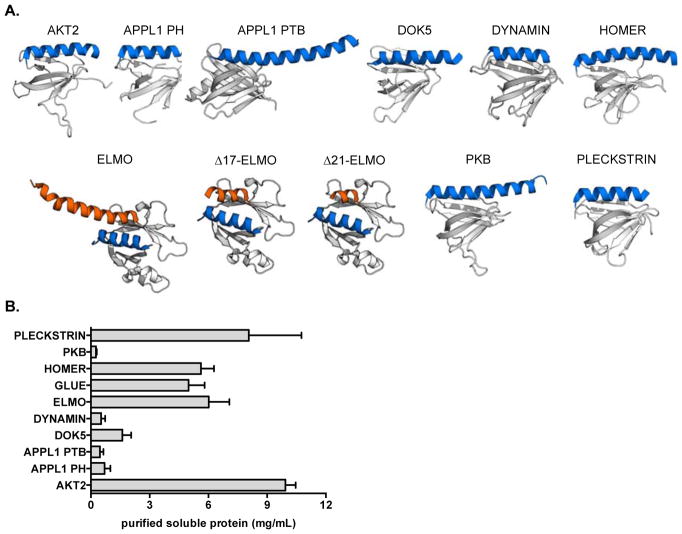
Although our initial effort was quite successful, we were interested both in probing the scope of our chosen scaffold and in testing our gp41 mimics in more demanding environments. We began by selecting a collection of nine PH domains, favoring those of human origin to potentially mitigate downstream immunogenicity. The set of PH domains with reported X-ray structures exhibit considerably diversity in helix length, total protein size, and percentage of total residues involved in the helix. Since we were unsure how each of these variables might impact expression viability and/or helix presentation, we chose an array of candidates covering a range of characteristics (Figure 2A, Supporting Information Table 1). While the canonical PH fold presents a C-terminal helix, the Engulfment and Cell Motility (ELMO)-1 domain has the added benefit of an additional N-terminal helix(22), providing the option to display partially structured ligands that are helical only at one terminus.
Figure 2.
(A) Candidate PH domain scaffolds examined in this work. PDB: 1P6S (AKT2), 2ELA (APPL1 PH), 2ELB (APPL1 PTB), 1J0W (DOK5), 2DYN (DYNAMIN), 1I2H (HOMER), 2VSZ (ELMO, and truncated variants Δ17-ELMO and Δ21-ELMO used in this work), 1UNP (PKB), 2I5F (PLECKSTRIN). Not shown: 2CAY (GLUE). In all images, the native helix is highlighted in blue, while the PH domain scaffold is highlighted in grey. For ELMO and its derivatives, blue is the native N-terminal helix; orange is the native C-terminal helix (or truncated variants). (B) Expression levels of soluble wild type scaffolds following purification by His6/nickel NTA column. Error bars indicate standard deviation of three experiments.
Our initial screen for scaffold suitability was simple expression of the wild-type proteins, since well-expressing systems were expected to better tolerate modification. At this stage, four of our nine next generation candidates were eliminated based on poor expression (Figure 2B, Supporting Information, Figure S1). The remaining ones, including our original GLUE scaffold, were grafted with the gp41 C-peptide sequence. We tested both the N- and C-terminal ELMO sites, and were satisfied to discover that both constructs expressed admirably (Figure 3A, Supporting Information, Figure S1). Although other sequences also worked, ELMO seem to present the most flexibility and thus the most potential to serve as a general scaffold for a series of future helical ligands. We therefore focused on ELMO as our platform of choice moving forward.
Figure 3.
(A) Soluble expression levels (following purification by His6/nickel NTA column purification) of selected scaffolds from Figure 2 with C-terminal C34 helix grafts, along with the N-terminal C26-ELMO graft. Error bars indicate standard deviation of three measurements. The N- and C-terminus of ELMO is labeled for clarity. (B) Models of N-terminal (C26-ELMO) and C-terminal (ELMO-C34) helix grafts on the ELMO scaffold. Helix grafted display strategy on the N- and C-terminal helix of ELMO. Native N- and C-terminal ELMO regions are orange and blue, respectively, while grafted gp41 positions are purple (spheres indicate alpha carbon positions). (C) Sequence of ligand mimic region for N- and C-terminal helix-grafted ELMO (designated L-ELMO and ELMO-L, respectively).
Having centered on ELMO as our most promising display vehicle, we sought to test its ability to effectively present a collection of different sequences, while at the same time probing the sensitivity of our grafted constructs to alterations in ligand sequence. Although the C34 peptide used in our original helix-grafted ligand is taken from the same region of gp41 as enfuvirtide (T-20), a significant portion of each sequence is unique. We thus sought to test both sequences, in addition to the longer one formed by their union (C46, Figure 3B, Figure 3C). To test the intrinsic flexibility of N- versus C-terminal display, we prepared ELMO scaffolds grafted at either terminus (Lig-ELMO or ELMO-Lig) with C34, T-20, and C46. We were especially interested to probe the role of the N-terminal WWI triad on C34, whose sidechains bind into a deep (and highly conserved) hydrophobic groove on the NHR trimer surface. The absence of this interaction in T20 (whose sequence is shifted more toward the C-peptide C-terminus) perhaps reduces barriers to acquired resistance.
The C-terminal grafts were prepared as before, with 5 solvent-exposed positions on the native ELMO helix mutated to correspond to those of the HIV sequence, and the C-terminus of the protein extended the appropriate amount using the pure gp41 sequence. In contrast to the C-terminal ELMO helix, which rests atop the beta sheets, the N-terminal one is more fully exposed, and of considerable length (nearly 20 residues). Fearing that long extensions of this helix might not be well tolerated, we prepared C34-ELMO and T20-ELMO by first excising 17 residues from the native helix, and then simply fusing the appropriate sequence to the N-terminus of the resulting protein (termed Δ17-ELMO). For C46-ELMO 3 additional residues were trimmed (since the fused helix is longer). Finally, as a hedge against even these shorter helices proving unworkable, we prepared C26-ELMO, in which 8 residues were deleted from the C-terminus of C34 before fusing it to the shortened Δ17-ELMO scaffold. To determine the impact of the N-terminal deletions from the core ELMO protein, we prepared both Δ17-ELMO and Δ21-ELMO on their own, and were pleased to discover that both expressed even better than the wild-type platform (Supporting Information, Figure S2), so much so that we used Δ21-ELMO as the starting point for the ELMO-Lig constructs.
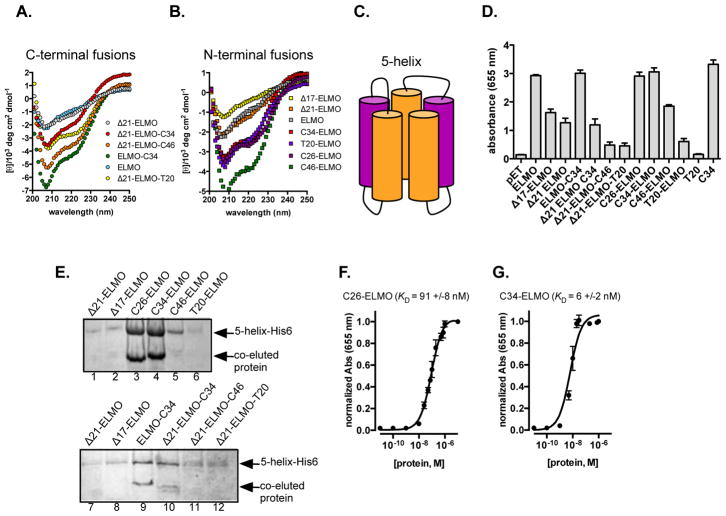
To establish that these various modifications had not compromised the overall protein fold, we examined each grafted protein using circular dichroism (CD) spectroscopy, and compared the results to those of the base proteins (wild type, Δ17-, and Δ21-ELMO). Plots of mean residue ellipticity versus wavelength for the C-terminal fusions are consistent with retention of overall structure, and display expected helicity increases compared to the appropriate control (Figure 4A). Similarly, the N-terminal fusions all exhibit increased helicity with respect to each of the starting proteins (Figure 4B). The helix-grafted proteins also display similar thermal unfolding profiles, consistent with retention of macroscopic structure (Supporting Information, Figure S3). Having determined that the HIV ligand mimic candidates were behaving as expected, we moved on to evaluate their viability as ligands for both model and live-virus receptors.
Figure 4.
(A) Circular dichroism spectra of C-terminal (A) and N-terminal (B) ELMO grafts. (C) A cartoon representation of 5-helix, a single polypeptide that contains the NTR trimer (orange), as well as two C-peptides (purple). D) ELISA data showing binding between 5-helix-His6 and ELMO-derived grafted proteins. (E) Nickel-NTA co-purification of 5-helix-His6 and either N-terminal (top) or C-terminal (bottom) helix-grafted ELMO-derived proteins. (Dissociation constant (KD) for complexes involving 5-helix and (F) C26 ELMO or (G) C34 ELMO.
As in our original report, initial evaluations of ligand/receptor binding employed the 5-helix construct that has been widely used for this purpose.(23, 24) The 5-helix protein links gp41’s central coiled-coil NHR trimer and two CHR ligands into one sequence that assembles to present a single C-peptide binding site. This strategy both simplifies the interaction to be measured (making it a 1:1 rather than 3:1 complex), and eases solubility issues arising from exposing three copies of the significantly hydrophobic ligand binding surface (Supporting Information, Figure S4 and Figure 4C).
We initially performed an ELISA assay to verify binding. Somewhat surprisingly, the native N-helix on ELMO, which we originally feared might compromise expression efficiency, also has some non-specific affinity for 5-helix, at least in this assay. However, this non-specific binding can be abbrogated by truncation of the N-terminal helix. Two shortened variants, Δ17-ELMO (Figure 2A) and Δ21-ELMO (Figure 2A), where 17 or 21 residues were removed from the N-terminal helix, respectively, show substantially decreased affinity for 5-helix in ELISA (Figure 4D). The N-terminal fusions generate stronger signals, particularly those with the WWI residues: C26-ELMO and C34-ELMO are better than C46-ELMO, which in turn is better than T20-ELMO. The two shorter fusions give signals comparable to the native C34 peptide. The C-terminal fusions were weaker, with only Δ21-ELMO-C34 giving a signal even comparable to the analogous control scaffold (Δ21-ELMO).
We next examined the ability of each grafted or control protein to bind 5-helix in the complex environment of E. coli. Cells were transformed with a plasmid encoding both the ligand candidate and His-tagged 5-helix, lysed, and exposed to Ni-NTA agarose resin. Consistent with data points in our ELISA experiment (Figure 4D), SDS-PAGE analysis of the N-terminal fusions (Figure 4E, lanes 1–6) suggested that those bearing the WWI triad (lanes 3–5) were most effective at binding to 5-helix. The two shorter helicies (C26- and C34-) displayed the strongest retention, with a significant reduction for the longer C46-ELMO construct, and very little observable retention for T20-ELMO. The C-terminal fusions (Figure 4E, lanes 7–12) exhibit a similar trend, albeit with an overall reduction in apparent affinity. Comparing those with the native N-helix removed (Δ21-ELMO-Lig), the C34 fusion (lane 10) is again more effective, though the intact ELMO-C34 protein (lane 9) is perhaps even better. Both truncations (Δ17 and Δ21 ELMO do not appreciably bind 5-helix in the co-purifcation assay (Figure 4E)
To better understand the complex involving 5-helix and our best performing binders (C26-ELMO and C34-ELMO), we used ELISA to measure their dissociation constants (KD). Satisfyingly, these proteins tightly bind 5-helix (KD ~ 90 ±8 nM and 6 ±2 nM, respectively, Figure 4F and Figure 4G). In addition to tightly binding 5-helix, C26-ELMO and C34-ELMO are relatively stable in proteolytically active human serum. For both proetins, >50% of the full-length species was detected by Western blot after incubating with proteolytically active human serum for 12 hours (Supporting Information, Figure S5).
Collectively, these initial experiments supported the notion that C-peptide grafted ELMO scaffolds were capable of recognizing the 5-helix model system in even complicated cellular contexts, and further suggested that the most efficient of these systems were the shorter N-terminal fusions bearing the key WWI residues. Encouraged by these data, we set out to test the ability of helix-grafted gp41 mimics to inhibit infection by actual virus, using a previously reported protocol.(25, 26) HIV-1 IIIB was administered to CD4-positive mammalian cells stably integrated with a plasmid that encodes the HIV-1 long-terminal repeat (LTR) upstream of GFP. Thus, if HIV-1 successfully infects these cells, HIV-1 Tat/TAR-dependent transcription ultimately leads to GFP expression. The percentage of cells that express GFP (measured by flow cytometry) is thus equivalent to the percentage infected by the virus. Consistent with the ELISA and co-purification experiments, the N-terminal fusions proved more effective by this measure as well (Figure 5A). Only the three WWI-containing helices (C26-, C34-, and C46-ELMO) exhibited significant inhibition, with the T20-ELMO protein proving largely ineffective. The longer C46 construct again lagged behind the shorter helices, despite containing the WWI triad. The C-terminal fusions (Figure 5B), as before, were overall less efficient, and only Δ21-ELMO-C34 was comparable to any of the N-terminal species. The scaffold controls (Δ17- andΔ21-ELMO) did not materially inhibit infection, despite ELISA data suggesting at least moderate 5-helix affinity. Although both N- and C-terminal T20 fusions were largely ineffective in each of these assays, the isolated T20 peptide itself is significantly more potent in the live virus assay than any of our grafted systems. However, this in vitro assay is blind to the rapid degradation which compromises the in vivo efficacy of C34 peptide and its variants.
Figure 5.
(A) Suppression of HIV-1 entry by ELMO-derived C-terminal fusions Δ21 ELMO (black); T20 peptide (purple); Δ21 ELMO-C34 (red); Δ21 ELMO-C46 (orange); Δ21 ELMO-T20, (green). (B) Suppression by N-terminal fusions Δ17 ELMO (gray); Δ21 ELMO (black); C26 ELMO (red); C34 ELMO (orange); C46 ELMO (green); T20 ELMO (blue); C34 peptide (purple).
Taken together, these results demonstrate the viability of our helix-grafting strategy as a method for presenting gp41 C-peptide helices in a manner that allows them to efficiently recognize their intended receptor. Despite this success, opportunities for improvement remain. One plausible explanation for reduced efficacy of the longer C46 constructs (compared to C26/C34) is steric crowding, especially in the more complex in cellulo experiments with proximal lipid bilayers. It may be that further reduction in scaffold size is required for true generality moving forward. Additional affinity may also be obtained through directed evolution, optimizing the choice of receptor-facing residues. Experiments along both these lines are underway. In the longer term, we are confident that the general strategy of helix-grafted ligand display platforms will prove amenable to the discovery and optimization of new PPI ligands for many different targets.
Supplementary Material
Acknowledgments
We thank the Colorado Center for Drug Discovery for financial support.
Footnotes
Notes
The authors declare no competing financial interest.
Methods
See the Supporting Information for complete methods
Associated Content
Sequence of all proteins described in this work, research materials, experimental methods and supporting data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.De Las Rivas J, Fontanillo C. Protein-protein interactions essentials: key concepts to building and analyzing interactome networks. PLoS Comput Biol. 2010;6:e1000807. doi: 10.1371/journal.pcbi.1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker SN, Tennyson RL, Chapman AM, Kennan AJ, McNaughton BR. GLUE that sticks to HIV: a helix-grafted GLUE protein that selectively binds the HIV gp41 N-terminal helical region. Chembiochem. 2015;16:219–222. doi: 10.1002/cbic.201402531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Davis JM, Tsou LK, Hamilton AD. Synthetic non-peptide mimetics of alpha-helices. Chem Soc Rev. 2007;36:326–334. doi: 10.1039/b608043j. [DOI] [PubMed] [Google Scholar]
- 5.Ross NT, Katt WP, Hamilton AD. Synthetic mimetics of protein secondary structure domains. Philos Trans A Math Phys Eng Sci. 2010;368:989–1008. doi: 10.1098/rsta.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelo NG, Arora PS. Nonpeptidic foldamers from amino acids: synthesis and characterization of 1,3-substituted triazole oligomers. J Am Chem Soc. 2005;127:17134–17135. doi: 10.1021/ja056406z. [DOI] [PubMed] [Google Scholar]
- 7.Chapman RN, Dimartino G, Arora PS. A highly stable short alpha-helix constrained by a main-chain hydrogen-bond surrogate. J Am Chem Soc. 2004;126:12252–12253. doi: 10.1021/ja0466659. [DOI] [PubMed] [Google Scholar]
- 8.Goodman CM, Choi S, Shandler S, DeGrado WF. Foldamers as versatile frameworks for the design and evolution of function. Nature Chemical Biology. 2007;3:252–262. doi: 10.1038/nchembio876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kritzer JA. Stapled peptides: Magic bullets in nature’s arsenal. Nat Chem Biol. 2010;6:566–567. doi: 10.1038/nchembio.407. [DOI] [PubMed] [Google Scholar]
- 10.Gellman SH. Foldamers: A manifesto. Accounts of Chemical Research. 1998;31:173–180. [Google Scholar]
- 11.Kritzer JA, Zutshi R, Cheah M, Ran FA, Webman R, Wongjirad TM, Schepartz A. Miniature protein inhibitors of the p53-hDM2 interaction. Chembiochem. 2006;7:29–31. doi: 10.1002/cbic.200500324. [DOI] [PubMed] [Google Scholar]
- 12.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans. 2004;32:707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 14.Lemmon MA, Ferguson KM. Pleckstrin homology domains. Curr Top Microbiol Immunol. 1998;228:39–74. doi: 10.1007/978-3-642-80481-6_3. [DOI] [PubMed] [Google Scholar]
- 15.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 16.Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 2010;6:e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 18.Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10:1805–1825. doi: 10.2174/1381612043384448. [DOI] [PubMed] [Google Scholar]
- 19.Eckert DM, Kim PS. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc Natl Acad Sci U S A. 2001;98:11187–11192. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalezari JP, Eron JJ, Carlson M, Cohen C, DeJesus E, Arduino RC, Gallant JE, Volberding P, Murphy RL, Valentine F, Nelson EL, Sista PR, Dusek A, Kilby JM. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS. 2003;17:691–698. doi: 10.1097/00002030-200303280-00007. [DOI] [PubMed] [Google Scholar]
- 21.Patel IH, Zhang X, Nieforth K, Salgo M, Buss N. Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide. Clin Pharmacokinet. 2005;44:175–186. doi: 10.2165/00003088-200544020-00003. [DOI] [PubMed] [Google Scholar]
- 22.Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, Cote JF. An alpha-helical extension of the ELMO1 pleckstrin homology domain mediates direct interaction to DOCK180 and is critical in Rac signaling. Mol Biol Cell. 2008;19:4837–4851. doi: 10.1091/mbc.E08-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Root MJ, Kay MS, Kim PS. Protein design of an HIV-1 entry inhibitor. Science. 2001;291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 24.Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH. Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers. Proc Natl Acad Sci U S A. 2009;106:14751–14756. doi: 10.1073/pnas.0902663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gervaix A, West D, Leoni LM, Richman DD, Wong-Staal F, Corbeil J. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4653–4658. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hache G, Shindo K, Albin JS, Harris RS. Evolution of HIV-1 isolates that use a novel Vif-independent mechanism to resist restriction by human APOBEC3G. Curr Biol. 2008;18:819–824. doi: 10.1016/j.cub.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.