Abstract
The interaction of blood proteins with an implant surface is not only a fundamental phenomenon but is also key to several important medical complications. Plasma proteins binding on the surface of intravascular catheters can promote bacterial adhesion leading to the risk of local and systemic complications such as catheter-related blood infections (CRBIs). The incidences of CRBIs in the United States amount to more than 250,000 cases/year with an attributable mortality of up to 35% and an annual healthcare expenditure of $2.3 billion approximately. This demands the development of truly non-thrombogenic and antimicrobial catheters. In the present study, catheters were fabricated by incorporating a nitric oxide (NO) donor molecule, S-nitroso-N-acetyl-penicillamine (SNAP) in a hydrophobic medical grade polymer, Elasteon-E2As. Nitric oxide offers antithrombotic and antibacterial attributes without promoting drug resistance and cytotoxicity. E2As-SNAP catheters were first coated with fibrinogen, a blood plasma protein plays a key role in clot formation and eventual bacterial adhesion to the implant surface. The suitability of the catheters for biomedical applications was tested in vitro for contact angle, NO release kinetics, inhibition of bacteria and absence of cytotoxicity towards mammalian cells. The highly hydrophobic catheters released NO in the physiological range that inhibited > 99 % bacterial viability on fibrinogen-coated catheters in a 24 h study. No toxic response of E2As-SNAP catheters leachate was observed using a standard cytotoxicity assay with mouse fibroblast cells. Overall, the results showed that the E2As-SNAP catheters can inhibit viable bacteria even in the presence of blood proteins without causing a cytotoxic response. The fundamentals of this study are applicable to other blood-contacting medical devices as well.
Keywords: nitric oxide (NO), fibrinogen, catheter-related blood infections (CBRIs), S-nitroso-N-acetyl-penicillamine (SNAP)
1. Introduction
Intravascular catheters are blood contacting devices that are used globally by millions of patients each year. In modern-day medical practices, intravascular catheters enabling direct vascular access are indispensable for patient diagnosis and treatment, particularly in intensive care units (ICUs).1 These catheters include peripheral vascular catheters, such as arterial and venous catheters, central venous catheters (CVCs) like tunneled (long-term) and percutaneous non-tunneled catheters, midline catheters, peripherally inserted central catheters (PICCs) and pulmonary artery catheters.2–5 Although their importance in providing necessary vascular access cannot be denied, they also constitute a breach in the barrier between the outside milieu and the bloodstream, thus increasing the risk of hospital-acquired infections (HAIs). Their use can put the patient at a high risk of local and systemic infectious complications like catheter-related blood infections (CRBIs), septic thrombophlebitis, endocarditis, metastatic infections (e.g., lung abscess, brain abscess, osteomyelitis, and endophthalmitis).6 The CRBIs rank among the most frequent and potentially lethal nosocomial infections.7 More than 11% of all the hospital-acquired infections (HAIs) are caused by central venous catheters (CVCs). The incidences of CRBIs in the United States amount to more than 250,000 cases/year with an attributable mortality of up to 35%.8 With an additional cost of about $35,000 per episode, CRBIs increase annual healthcare expenditure up to $2.3 billion. Catheter-related blood infections can be attributed to contamination of venous catheter prior to insertion, failure to aseptic insertion or maintenance of the catheter exit site or end luminal site during hub manipulation.
Reports have shown that biofilm formation can occur within three days of catheter insertion.9 Bacteria binding on the catheter surface can be exacerbated in the presence of blood proteins as they provide a favorable surface for bacteria to adhere. The bacterial adhesion on any indwelling device surfaces is a multi-step process that occurs in three phases and can be exacerbated by protein binding in the initial step.10 In phase I, blood plasma proteins rapidly coat the device surface immediately after insertion, followed by bacterial adhesion. Initially, the non-specific interaction between the adsorbed protein layer and bacterial cells is mediated by a combination of forces such as gravitational force, Van der Waals force and Coulomb force.11 This is followed by specific binding between plasma proteins on the device surface, and bacterial membrane proteins and polysaccharides during phase II. In phase III, a protective layer of exopolysaccharide is secreted by certain bacteria, ultimately constituting a biofilm that resists the effect of bactericidal agents like antibiotics.12 Approximately 60% of all hospital-associated infections (out of over one million cases per year) occur due to biofilm formation on indwelling medical devices.3 Different approaches such as silver-doped material, anti-quorum sensing drugs, and antibiotics have been followed over the last few decades, yet CBRIs remain a problem.13–15 Therefore, new approaches to reduce the possibilities of these complications, to create truly non-thrombogenic, and antimicrobial catheters are extremely necessary. The ideal catheter should serve two main purposes: (i) minimize blood protein adhesion and (ii) inhibit bacterial adhesion and/or promote bacterial killing. One of the potential approaches could be to fabricate vascular catheters by combining a hemocompatible polymer with a thromboresistant and antimicrobial agent. While the hemocompatible nature of the polymer will prevent the blood protein adhesion and ultimate clot formation, the antimicrobial agent will inhibit bacterial growth and biofilm formation on catheter surface.
In this study, we developed antithrombic and antibacterial material for venous catheter application. Elaste-Eon-E2As was used as the base polymer and a nitric oxide donor, S-nitroso-N-acetyl-penicillamine (SNAP) was incorporated into it. E2As is a biomedical grade polymer which is highly hydrophobic in nature that demonstrates excellent hemocompatibility. In the past, a comparative study by Handa et al. on thrombus formation by different medical grade hydrophobic polymers suggested that E2As has smallest thrombus area in a 4 h rabbit model when compared to other polymers such as compared to Tecoflex SG80A, Tecophilic SP60D60, and plasticized poly(vinyl chloride) and hence making it suitable for making blood-contacting devices.16 This is in line with other studies which demonstrated excellent hemocompatibility of E2As.17–19
Incorporation of a NO releasing donor molecule such as SNAP in a hydrophobic polymer such as E2As can significantly inhibit the attached viable bacteria on the polymer surface.20–26 Nitric oxide (NO) has inherent antithrombotic and antibacterial properties. It is a cellular signaling molecule which is released at a flux of 0.5–4.0 × 10−10 mol min−1cm−2 in the human body by endothelial cells lining the blood vessels in addition to macrophages and sinus cavities.16,24–28 The unpaired NO electron is extremely reactive and has a very short half-life of < 5 sec, allowing it to only have a localized and immediate impact. 29 The use of NO as an antibacterial agent is not expected to result in any resistance due to its short half-life, rapid reduction of microbial load and non-specific action.27,30–32 Furthermore, studies have shown that the SNAP-doped E2As polymer does not result in any cytotoxicity against mammalian cells in vitro.19,25 The absence of antibiotic resistance and cytotoxicity makes NO based antimicrobial therapy, a better alternative to other commonly used strategies such as antibiotics-based approaches.33–37
The antithrombotic and antibacterial applications of NO releasing polymer has been shown in the past. In the present study, the interdependence of bacterial adhesion on fibrinogen and the ability of NO releasing catheter surface to address this challenge is demonstrated. To achieve this, we developed E2As-SNAP based catheters with varying concentration of SNAP, coated them with blood protein fibrinogen and then characterized them in terms of their biomedical applications. The catheters were examined for properties such as contact angle, NO release kinetics, antimicrobial efficacy, and absence of any cytotoxic response.
2. Materials and Methods
2.1 Materials
Elast-eon E2As was obtained from AorTech International, plc (Scoresby, Victoria, Australia). Potassium phosphate monobasic, sodium phosphate dibasic, potassium chloride and sodium chloride, N-acetyl-D-penicillamine (NAP), sodium nitrite, tetrahydrofuran (THF), Ethylenediaminetetraacetic acid (EDTA), hydrochloric acid, sulfuric acid, fibrinogen from bovine plasma, and N, N-dimethylacetamide (DMAc) were obtained from Sigma-Aldrich (St. Louis, MO). Autoclaved phosphate buffered saline (PBS), pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, and 10 mM sodium phosphate was used for all in vitro experiments. The bacterial strains Escherichia coli (ATCC 11303) and Staphylococcus aureus (ATCC 6538) and mouse fibroblast cells (ATCC 1658) were originally obtained from the American Type Culture Collection (ATCC).
2.2 Methods
2.2.1 Fabrication of nitric oxide-releasing catheters
A total of 36 catheters with a length of 1 cm were fabricated by blending the Elaste-Eon-E2As polymer with SNAP. Elaste-Eon-E2As is a copolymer of mixed soft segments including polydimethylsiloxane and poly hexamethylene oxide. All catheters were prepared by dip-coating stainless steel mandrels of 2.37 mm diameter in the E2As polymer solutions. The control polymer solution consisted of the E2As in THF (70 mg/mL). To prepare the control catheters, the stainless-steel mandrels were dipped into the control solution 35 times with a drying time of 2 minutes between each coat. The SNAP-doped E2As catheters had a tri-layer configuration consisting of an E2As base layer, SNAP-doped middle layer, and E2As top layer. Top/base layers were composed of 70 mg/mL E2As in THF. Two solutions were prepared to contain varying weight percentages of SNAP: 5 wt% and 10 wt% to prepare the active layer. The E2As-SNAP catheters were prepared by dip-coating 4 base layers of the E2As solution, 27 layers of the active, E2As-SNAP solution and 4 top layers of the E2As solution. The top layer not only maintains the hydrophobicity of the surface but prevents any possible leaching of the SNAP from E2As polymer. A drying time of 2 minutes was allowed between each dip coating. All the catheters were dried for a minimum of 24 h at room temperature under a fume hood in ambient lighting. After drying, the catheters were removed from the mandrels and stored in the freezer to prevent NO loss through thermal stimulation.
2.2.2 Contact angle analysis via drop shape analyzer
Owing to the smaller surface area and 3D structure of venous catheter, E2As-SNAP films with similar concentrations as that of E2As-SNAP catheters (tri-layer configuration) were created to have a flat 2D surface for contact angle measurement. The polymeric films of E2As with and without 5 wt% and 10 wt% SNAP were cast in a Teflon mold having a diameter of 2.5 cm. The films were top-coated with E2As and dried similarly to that of catheters. For contact angle analysis, films were placed on top of a glass slide and placed under Kruss DA 100 drop shape analyzer and a ~1 μl drop was placed on films surface. The contact angles for each film type was measured from each frame of the recorded files using the sessile drop approximation.
2.2.3 Binding of fibrinogen protein on the catheter surface
The catheters (length = 1 cm, diameter = 3.27 mm) were soaked in a phosphate buffer solution without chloride salts to bind the plasma protein fibrinogen. Meanwhile, a high concentration fibrinogen protein solution of 20 mg/mL was prepared in PBS as the stock. 100 μL of concentrated fibrinogen solution was added under the surface of 900 μL buffer solution to ensure minimal protein was trapped at the air-water interface. The final concentration of the fibrinogen solution in the buffer was 2 mg/mL. The catheters soaked in the protein solution were kept in an incubator at 37°C for 2h. After 2h, the well was infinitely diluted with deionized water to remove any loosely bound protein. The catheters with bound fibrinogen were used to examine the NO release kinetics and bacterial inhibition.
2.2.4 Nitric oxide release analysis with and without blood protein fibrinogen
The NO release profile was tested with and without exposure to blood protein fibrinogen using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA) 280i (Boulder, CO). The E2As-SNAP catheters were placed in a sample cell and immersed in PBS at pH 7.4 containing 100 μM EDTA (to avoid metallic ions assisted NO release). NO released by the catheter sections in the buffer solution was swept into the NOA chemiluminescence detection chamber using N2 sweep gas and bubbler. Amber color sample cells were used to avoid NO release via light stimulation. Prior to analysis, E2As-SNAP catheter sections with and without fibrinogen exposure were soaked in a 2 mg/mL solution of blood protein fibrinogen for 90 minutes. All catheters were incubated in PBS at 37 °C for the NO release measurement by the NOA. The results were reported as NO flux released per surface area of the catheter sections (×10−10 mol min−1 cm−2).
2.2.5 Bacterial adhesion test
In the past, multiple studies have been done to show the antibacterial effect of NO but in the absence of blood protein fibrinogen.23,25,26 One crucial aspect of the present study was to demonstrate the antibacterial effect of NO on fibrinogen-coated catheters which somewhat imitate the catheter-related blood infection in vitro. In real-life situations also, the attached blood plasma protein fibrinogen can facilitate bacterial adhesion at a tissue-implant interface, leading to blood infections.38–40 In the current study, we intended to show the combined effects of E2As and NO to cause a significant reduction in bacteria bound on fibrinogen-coated catheters.
Using a standard bacteria adhesion test, the potential of the E2As-SNAP catheters to inhibit bacterial growth was tested following standard aseptic techniques.23,41 Fibrinogen was allowed to bind on the catheter’s surface by soaking them in a 20 mg/mL blood protein fibrinogen solution. The gram-positive Staphylococcus aureus (S. aureus) and gram-negative Escherichia coli (E. coli) were selected for the study as they are among the most common pathogens responsible for biofilm formation in vascular catheters.42 In fact, S. aureus is one of the most common causes of catheter-related bloodstream infections (CRBIs), specifically biofilm-associated infections on indwelled biomedical devices.43,44 The procedure began with growing 10 mL liquid suspension of bacteria in a 50 mL tube incubated at 37°C for 14 h at a shaking speed of 120 rpm. The optical density of the grown bacteria was measured at 600 nm (OD600) using the UV-vis spectrophotometer (Thermo Scientific Genesys 10S UV-Vis). To remove the traces of LB medium, the bacteria culture was centrifuged for 7 min at 3500 rpm, the supernatant was discarded and 10 mL of sterile phosphate buffer saline (PBS) was added to the bacterial pellet. This step was once again repeated; optical density was measured and readjusted to get the cell count in the range of 10−7–10−9 colony forming units per mL of suspension (CFU/mL). The catheters were UV sterilized for 30 min prior to using them in the experiment. Thereafter, catheters were exposed to the resulting bacterial suspension for 24 h in a 150-rpm shaker incubator at 37°C. After the 24 h period, the catheters were rinsed with PBS to wash off the bacteria that was not tightly adhered to the catheters’ surface. The catheters were placed in 2 mL of sterile PBS solution and homogenized for 45 s. Serial dilutions in the range of 10−1–10−5 were made and bacteria solutions were plated on solid LB agar plates. The LB agar plates with the bacteria were incubated for approximately 20 h at 37°C. After 20 h, the CFUs per unit surface area (CFU/cm2) were counted using the following formula; dilution and working volumes were considered to back-calculate the exact number.
2.2.6 Cytotoxicity Assay
To provide a proof of concept on the noncytotoxic behavior of the E2As-SNAP polymer, a standard cell culture assay was conducted in accordance with the ISO 10993 standard. 3T3 Mouse fibroblast cells (ATCC 1658) were exposed to the leachates obtained from E2As-SNAP catheter in a sterile environment in a stepwise manner following standard protocol.25,41
Cell culture
In a 75 cm2 T-flask containing complete DMEM medium with 1% penicillin-streptomycin antibiotic solution and 10% fetal bovine serum (FBS), mouse fibroblast cells were inoculated from a cryopreserved vial. After inoculation, the cells were transferred in controlled conditions using a cell culture grade incubator (37°C in a humidified atmosphere with 5% CO2) to favor their growth on T-flask surface. The incubation continued, and the medium was changed every second day until cells confluence 80–90% of the T-flask surface.
Leachate collection from E2As-SNAP catheters
The catheter was cut into small pieces and weighed. 10 mg of the catheter piece was transferred in 10 mL of complete DMEM medium and incubated at 37°C to allow leaching of possible extract from the material. After 24 h the catheter pieces were removed, and the leachates were stored in the fridge before used in the experiment.
Leachate exposure to mammalian cells
After reaching a confluence of 80–90%, fibroblast cells were enzymatically detachment from the T-flask using 2.5 mL trypsin based (0.18% trypsin and 5 mM EDTA). Using a dye exclusion method, cells were counted in a hemocytometer by first diluting them with 0.4% trypan blue solution (1:10 ratios of cells: trypan blue) and then transferring 50 μL of the mixture in the hemocytometer and observed under a microscope. After this, the cells from the original source were diluted to get 5000 cells/mL using DMEM and 100 μL of cells were seeded in each of the well of a 96 well plate (n=7 for each sample type). The leachate collected from the E2As-SNAP catheters were transferred to these cells (10 μL/well) in each of the wells except for the negative control. The well plate was then incubated for 24 h in the cell culture grade incubator. After 24 h, 10 μL of a water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt] dye based Cell Culture Kit solution (CCK-8) was added to each well. Live mammalian cells secrete dehydrogenases enzymes which can reduce WST-8 into formazan which is bright orange in color and can be detected by a spectrophotometric plate reader. Thus, the amount of orange color formazan that can be detected at 450 nm is directly proportional to the number of live cells (i.e. lesser the formazan means less the viable cells). The background created by DMEM was normalized by using plane DMEM as a reference (blank). Relative mammalian cell viability with respect to the negative control was measured using the following formula.
2.2.7 Statistical Analysis
Data for all the experiments are expressed as mean ± SEM (standard error of the mean). Comparison of means using a two-tailed student’s t-test assuming unequal variances was utilized to analyze a statistical difference between the samples. Values of p < 0.05 were considered statistically significant and graphically illustrated.
3. Results and Discussion
3.1 Hydrophobicity of E2As-SNAP material
Binding of blood plasma protein such as albumin and fibrinogen to the polymeric surface leads to platelet activation, which ultimately causes thrombus formation.45,46 Fibrinogen is a positive acute phase protein related to the blood infection, inflammatory disease and tissue damage ultimately causing patient mortality via trauma coagulopathy.47,48 Thus a reduction in fibrinogen adhesion is not only expected to reduce thrombus formation but also minimize infection. One way to reduce fibrinogen interaction is by altering the surface properties of a polymer in terms of its hydrophobicity/hydrophilicity. The contact angle measurement provides an easy way to determine the relative hydrophobicity and hydrophilicity of a material surface. It is the measurement of solid-liquid interfacial tension that is performed by establishing the tangent (angle) of a liquid drop with a solid surface at the base. This surface property of a polymer can control the interaction of blood proteins and bacteria with the catheter. In general, a surface with a contact angle higher than 90° is considered hydrophobic.49 A highly hydrophobic surface with low surface energy can exhibit a contact angle as high as ~ 120°.50
In this experiment, the change in hydrophobicity of E2As in the absence and presence of NO donor (SNAP) was tested (n=3) using a Kruss DA100 Drop Shape Analyzer. The control E2As without any SNAP incorporation showed a contact angle of 110.78° ± 0.73°. The 5 wt% SNAP-E2As and 10 wt% E2As-SNAP catheters showed similar contact angles of 113.81° ± 0.23° and 114.53° ± 0.14° respectively. The results confirmed the highly hydrophobic nature of the E2As polymer in combination with SNAP (Figure 1) and are in line with the past report.19 This property is particularly significant in the reduction of clot formation and eventual inhibition of bacterial infection. We have earlier shown that due to its highly hydrophobic nature, E2As forms a smaller thrombus area as compared to another biomedical grade polymer.16 This can be attributed to E2As higher affinity to albumin as compared to fibrinogen.17 Due to the abundance of albumin protein in blood, it competes with fibrinogen protein to bind to the polymer surface.51–53 An inverse correlation between the binding affinity of fibrinogen and albumin to the polymer has been observed in the past.54 Fibrinogen plays a pivotal role in the coagulation cascade ultimately leading to clot formation.17 At the same time, fibrinogen also mediates bacterial adhesion on a biomaterial surface.55,56 E2As higher affinity towards albumin would ultimately result in a decreased fibrinogen binding and hence reduced clotting and bacterial adhesion.
Figure 1.
Thus, the contact angle study provides supporting evidence that E2As catheters due to their inherent hydrophobic surface will ultimately result in reduction in blot clots and bacterial interaction on the polymer surface. Ultimately any bound bacteria will be killed in the presence of NO released by the incorporated SNAP.
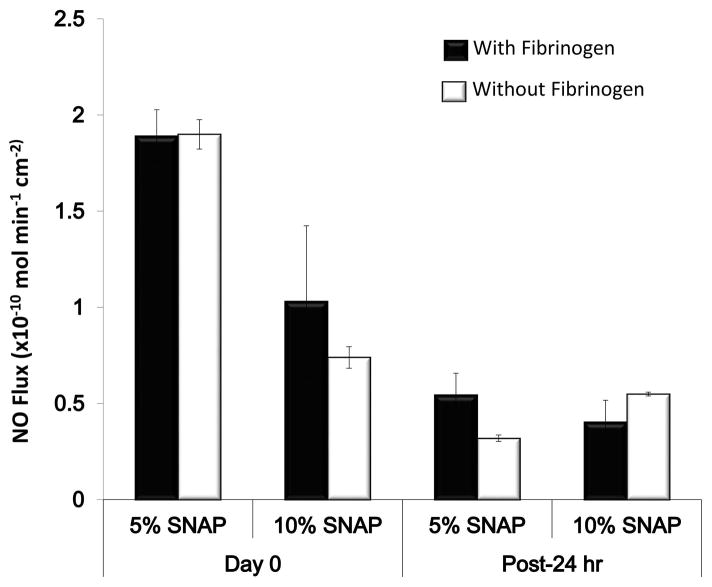
3.2 NO release with and without exposure to plasma fibrinogen
S-nitroso-N-acetyl-penicillamine (SNAP) is an S-nitrosothiol which decomposes to release antimicrobial NO by homolytic cleavage of S-N bond under appropriate conditions.57 The NO release profiles of the 5 wt% and 10 wt% E2As-SNAP catheters were measured both with and without previous exposure to plasma fibrinogen over a 24 h period. The results indicated no significant effect on NO release (Figure 2) due to prior exposure to fibrinogen and were found to be consistent with the previous findings. 58–60 The fibrinogen-coated catheters exhibited NO release in the physiological flux range of 0.5–4.0 × 10−10 mol min−1 cm−2. It is important that the catheters maintain an NO flux in the physiological range even after exposure to plasma fibrinogen for NO to show its antibacterial properties towards bacteria attached to the plasma protein. Differences in NO flux between the 5 wt% and 10 wt% catheters can be attributed to differences in SNAP crystallization due to SNAP solubility within the E2As polymer.1 These crystalline regions are responsible for the extended storage stability and release rates when used at higher weight percentages. While increasing the loading of SNAP is beneficial for the stability and extended release, increasing loading of the donor can begin to have significant effects on the mechanical properties of the material. 19 It is noteworthy, that although the NO flux in the case of 5 wt% E2As-SNAP catheters was higher than 10 wt% E2As-SNAP towards the beginning (represented as 0 h in the graph), while 10 wt% E2As-SNAP showed higher NO flux longer time scales (at 24 h). This can be attributed to the slow and sustained release of NO from the crystallized SNAP structure at higher SNAP loading in a hydrophobic polymer. No significant differences between fibrinogen-coated and non-coated catheters was observed which is in line with the existing literature.16
Figure 2.
The incorporation of SNAP into biomedical grade polymers such as E2As can yield remarkably stable biomaterials, with excellent storage stability and mechanical strength.20 In the past, E2As-SNAP has been shown to release NO for 20 days as measured by chemiluminescence NO analyzer with very low levels of leaching.61 We have recently shown in a stability study that E2As-SNAP can retain 82% of the SNAP during a 2 months period at 37°C.19 The simplicity and flexibility of E2As-SNAP catheters’ fabrication method allow the fine tuning of polymer and NO donor concentration. Hence higher NO flux levels than those shown in the present study can be achieved if needed.
3.3 Inhibition of gram-positive and gram-negative bacterial growth on catheter surface
An increase in bacterial adhesion in the presence of blood protein mainly happens due to the non-specific interactions such as gravitational, Van der Walls, and Coulomb forces between adsorbed protein layer and bacterial cells.10 Additionally, some catheters due to their surface properties are more susceptible to thrombus formation as compared to others which predispose them to increased bacterial adhesion.16,62,63 Catheters with irregular surfaces facilitate the binding of bacteria to the catheter’s surface.62,64 Besides the surface properties of the polymer used in catheter fabrication, the adhesive properties of bacteria are also crucial for the pathogenesis of bacterial related infections. For instance, as compared to other blood pathogens, Staphylococci has greater binding ability to the implant surface that leads to the formation of a slimy extracellular polysaccharide.65 This extracellular matrix protects the bacteria from the action of antibiotics, silver nanoparticles, and other microbicidal agents. However, NO due to its small size can penetrate through the matrix and kill the bacteria embedded in the biofilms. In this study, we could successfully show a significant reduction in the population of S. aureus as discussed below.
As a proof of concept to show the bacterial inhibition properties, fibrinogen-coated E2As-SNAP catheters were exposed to gram-positive S. aureus and gram-negative E. coli bacteria. The amount of bacterial inhibition was directly proportional to the amount of NO flux (see Table 1). When 5 wt% E2As-SNAP and 10 wt% E2As-SNAP catheters were compared with each other, 10 wt% E2As-SNAP killed twice the number of bacteria due to sustaining its NO flux for the full 24 h period. Overall, a bacterial inhibition up to 2 logs was observed for both S. aureus (Figure 3) and E. coli (Figure 4) using the NO releasing catheters. On a percentage scale, the efficiency of S. aureus and E. coli inhibition was up to 99.20 ± 0.11 % and 99.10 ± 0.20 % respectively. Such a high level of reduction in bacterial colony forming units on a polymeric surface is a significant stride in direction of development of new class of biopolymers needed for biomedical device fabrication.
Table 1.
Comparative analysis of NO flux and adhered viable bacteria cells (CFU/cm2)
| Sample | NO flux (10−10 molmin−1cm−2) | S. aureus (CFU/cm2) | E. coli (CFU/cm2) | |
|---|---|---|---|---|
| 0 hour | 24 hour | |||
| E2As-Control | -- | -- | 6.7 ± 2.2 × 107 | 1.6 ± 0.2 × 108 |
| 5% SNAP-E2As-Fibrinogen | 1.9 ± 0.1 | 0.40 ± 0.1 | 1.3 ± 0.3 × 106 | 4.2 ± 0.6 × 106 |
| 10% SNAP-E2As-Fibrinogen | 1.03 ± 0.4 | 0.55 ± 0.1 | 5.5 ± 2.2 × 105 | 1.4 ± 0.3 × 105 |
Figure 3.
Figure 4.
The NO-dependent bacterial killing mechanism includes DNA cleavage, lipid peroxidation, tyrosine nitration and nitrosation of amines, and thiols 66,67. In the recent years, we and other scientific groups have demonstrated that the antimicrobial activity of the various NO delivery systems could be used against a variety of microorganisms to prevent infection in blood contacting devices. 20,68,69 In this regard, antibacterial properties of NO donors have been proven against pathogens such as Staphylococcus aureus, 25,70–72 Staphylococcus epidermis, 70 Pseudomonas aeruginosa, 25,27,71 Escherichia coli, 70–75 Acinetobacter baumanii, 76,77 Listeria monocytogenes, and Enterococcus faecalis.26 Along these lines, the results from the current study provide an important proof of concept for the killing of bacteria attached to surface-bound plasma proteins on E2As-SNAP catheters via the bactericidal action of NO. Furthermore, NO due to its short half-life (< 5 secs), rapid and nonspecific bactericidal action is not expected to trigger any resistance in bacteria, unlike antibiotics.27,30–32 One of the major advantages of this strategy is that it can be combined with other antimicrobial agents such as quaternary ammonium ions, silicone oil, and nanoparticles to bring about a more enhanced antibacterial effect without negatively impacting the NO release. 23,25,78
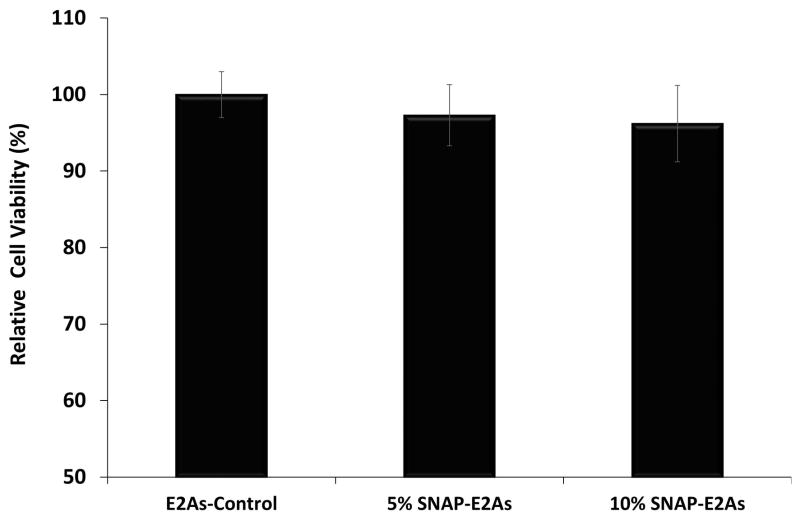
3.4 Cytotoxicity assay
The absence of any cytotoxic response is inevitable for the success of any biomedical device application. In the current study, leachates from control E2As and E2As-SNAP catheters were collected and mouse fibroblast cells (ATCC 1658) were exposed to them for a 24 h period and the viability of the cells was compared. As Figure 5 shows, the cells exposed to leachates collected from E2As-SNAP catheters and control E2As catheters (n=7) demonstrated similar cell viability when compared to cells without any leachate exposure (positive control). The results from our current study are in agreement with another study where SNAP incorporation in another biomedical grade polymer, Carbosil also showed to possess bactericidal properties with minimal platelet activation and no cytotoxicity.41
Figure 5.
This is an important attribute for the safety and efficacy since many therapeutic agents pose remarkable antibacterial properties but at the same time can cause toxicity to the host cells. For instance, antibiotics application are often used in popular lock solutions for venous catheters but at the compromise of antibiotic resistance and cytotoxicity towards mammalian cells.13–15 Nitric oxide due to its endogenous nature, rapid action, non-specific bactericidal mechanism, and short half-life is not expected to allow development of resistant bacterial strains and cytotoxic response. 27,31,32 E2As-SNAP catheters which can inhibit bacteria up to 2 logs in the presence of blood protein without causing cytotoxicity to provide an initial proof of concept for their potential biocompatibility. The results from this study overlap with other studies which show NO releasing surfaces to be highly effective in their antibacterial potential without any cytotoxic response, hemolysis, and platelet activation 16,19,41,79
4. Conclusions
Blood clotting and infection are two common problems associated with vascular catheters. E2As with its inherent hydrophobic nature can bind higher amounts of albumin and hence lower fibrinogen attachment. Fibrinogen is a blood plasma protein that plays a key role in clot formation and eventual bacterial adhesion to the implant surface. Based on this concept, E2As-SNAP catheters were developed, coated with blood protein fibrinogen and their antibacterial potential was tested against gram-positive and gram-negative bacteria. The NO releasing E2As catheters owing to its hydrophobic surface were expected to show reduced bacterial adhesion because of reduction in fibrinogen binding. As expected, the NO releasing E2As-SNAP catheters resulted in > 99 % both grams positive and negative as compared to control E2As catheters (with fibrinogen coating). This result stems from the fact that, the highly hydrophobic nature of E2As helps to reduce the adhesion of fibrinogen, while the free radical NO killed bacteria via lipid peroxidation, DNA cleavage, tyrosine nitration and nitrosation of amines and thiols. The fabricated E2As-SNAP catheters exposed to fibrinogen protein exhibited a NO flux in the physiological range. The cell viability assay on mouse fibroblast cells confirmed that E2As-SNAP catheters can prevent bacterial adhesion without causing cytotoxicity towards mammalian cells thus providing supporting evidence for their biocompatibility. From a translational perspective, E2As-SNAP based biomaterials have excellent storage stability, can undergo popular sterilization methods and have excellent mechanical strength.19 The results from the present study can be used as a proof of concept to fabricate other blood contacting medical devices that utilize similar NO chemistry.
Acknowledgments
This work is supported by National Institutes of Health, USA grant K25HL111213, R01HL134899, and Centers for Disease Control and Prevention, USA contract 200-2016-9193.
References
- 1.Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, Xi C, Bartlett RH, Matzger AJ, Meyerhoff ME. Origin of long-term storage stability and nitric oxide release behavior of carbosil polymer doped with S-nitroso-N-acetyl-D-penicillamine. ACS applied materials & interfaces. 2015;7(40):22218–22227. doi: 10.1021/acsami.5b07501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard S, Maki D, Masur H, McCormick R, Mermel L, Pearson M. Draft guideline for the prevention of intravascular catheter-related infections. Centers for Disease Control; Atlanta, GA: 2001. [PubMed] [Google Scholar]
- 3.Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device—related bloodstream infection. I Pathogenesis and short-term devices. Clinical Infectious Diseases. 2002;34(9):1232–1242. doi: 10.1086/339863. [DOI] [PubMed] [Google Scholar]
- 4.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Elsevier; 2006. pp. 1159–1171. [DOI] [PubMed] [Google Scholar]
- 5.Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. The Lancet infectious diseases. 2007;7(10):645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 6.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML. Guidelines for the prevention of intravascular catheter–related infections. Clinical infectious diseases. 2002;35(11):1281–1307. doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- 7.Mirijello A, Impagnatiello M, Zaccone V, Ventura G, Pompa L, ADDOLORATO G, LANDOLFI R. Catheter-related bloodstream infections by opportunistic pathogens in immunocompromised hosts. European review for medical and pharmacological sciences. 2015;19(13):2440–2445. [PubMed] [Google Scholar]
- 8.Daniels KR, Frei CR. The United States’ progress toward eliminating catheter-related bloodstream infections: Incidence, mortality, and hospital length of stay from 1996 to 2008. American journal of infection control. 2013;41(2):118–121. doi: 10.1016/j.ajic.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Anaissie E, Samonis G, Kontoyiannis D, Costerton J, Sabharwal U, Bodey G, Raad I. Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. European Journal of Clinical Microbiology and Infectious Diseases. 1995;14(2):134–137. doi: 10.1007/BF02111873. [DOI] [PubMed] [Google Scholar]
- 10.Pascual A. Pathogenesis of catheter-related infections: lessons for new designs. Clinical microbiology and infection. 2002;8(5):256–264. doi: 10.1046/j.1469-0691.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 11.Katsikogianni M, Missirlis Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8(3) doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 12.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung KK, Schumacher JF, Sampson EM, Burne RA, Antonelli PJ, Brennan AB. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases. 2007;2(2):89–94. doi: 10.1116/1.2751405. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. Jama. 1999;281(3):261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 15.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML. Guidelines for the prevention of intravascular catheter-related infections. Clinical infectious diseases. 2011;52(9):e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. Journal of Materials Chemistry B. 2014;2(8):1059–1067. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozzens D, Luk A, Ojha U, Ruths M, Faust R. Surface characterization and protein interactions of segmented polyisobutylene-based thermoplastic polyurethanes. Langmuir. 2011;27(23):14160–14168. doi: 10.1021/la202586j. [DOI] [PubMed] [Google Scholar]
- 18.Simmons A, Padsalgikar AD, Ferris LM, Poole-Warren LA. Biostability and biological performance of a PDMS-based polyurethane for controlled drug release. Biomaterials. 2008;29(20):2987–2995. doi: 10.1016/j.biomaterials.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Goudie MJ, Brisbois EJ, Pant J, Thompson A, Potkay JA, Handa H. Characterization of an S-nitroso-N-acetylpenicillamine–based nitric oxide releasing polymer from a translational perspective. International Journal of Polymeric Materials and Polymeric Biomaterials. 2016;65(15):769–778. doi: 10.1080/00914037.2016.1163570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, Handa H. Reduction in thrombosis and bacterial adhesion with 7 day implantation of S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As catheters in sheep. Journal of Materials Chemistry B. 2015;3(8):1639–1645. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisbois EJ, Handa H, Davis R, Jones A, Bartlett RH, Meyerhoff ME. S-Nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon Catheters Reduce Thrombosis and Bacterial Adhesion. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter AW, Schoenfisch MH. Nitric oxide release: Part II. Therapeutic applications Chemical Society Reviews. 2012;41(10):3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pant J, Gao J, Goudie MJ, Hopkins S, Locklin J, Handa H. A Multi-defense Strategy: Enhancing Bactericidal Activity of a Medical Grade Polymer with a Nitric Oxide Donor and Surface-immobilized Quaternary Ammonium Compound. Acta Biomaterialia. 2017 doi: 10.1016/j.actbio.2017.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pant J, Goudie M, Brisbois E, Handa H. Nitric oxide-releasing polyurethanes. Advances in Polyurethane Biomaterials. 2016:417. [Google Scholar]
- 25.Pant J, Goudie MJ, Hopkins SP, Brisbois EJ, Handa H. Tunable nitric oxide release from S-nitroso-N-acetylpenicillamine via catalytic copper nanoparticles for biomedical applications. ACS Applied Materials & Interfaces. doi: 10.1021/acsami.7b01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundaram J, Pant J, Goudie MJ, Mani S, Handa H. Antimicrobial and Physicochemical Characterization of Biodegradable, Nitric Oxide-Releasing Nanocellulose-Chitosan Packaging Membranes. Journal of agricultural and food chemistry. 2016 doi: 10.1021/acs.jafc.6b01936. [DOI] [PubMed] [Google Scholar]
- 27.Hetrick EM, Schoenfisch MH. Antibacterial nitric oxide-releasing xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials. 2007;28(11):1948–1956. doi: 10.1016/j.biomaterials.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunological reviews. 2000;173(1):17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DD, Liu X, Kantrow SP, Lancaster JR. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proceedings of the National Academy of Sciences. 2001;98(1):355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heilman BJ, Halpenny GM, Mascharak PK. Synthesis, characterization, and light-controlled antibiotic application of a composite material derived from polyurethane and silica xerogel with embedded photoactive manganese nitrosyl. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;99(2):328–337. doi: 10.1002/jbm.b.31904. [DOI] [PubMed] [Google Scholar]
- 31.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg’s archives of pharmacology. 1998;358(1):113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan C. Nitric oxide and the immune response. Nature immunology. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y-H, Cheng F-Y, Chiu H-W, Tsai J-C, Fang C-Y, Chen C-W, Wang Y-J. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35(16):4706–4715. doi: 10.1016/j.biomaterials.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Park EJ, Yi J, Kim Y, Choi K, Park K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicology In Vitro. 2010;24(3):872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Baldi C, Minoia C, Di Nucci A, Capodaglio E, Manzo L. Effects of silver in isolated rat hepatocytes. Toxicology Letters. 1988;41(3):261–268. doi: 10.1016/0378-4274(88)90063-x. [DOI] [PubMed] [Google Scholar]
- 36.AshaRani PV, Mun GLK, Hande MP, Valiyaveettil Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2009;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 37.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 38.Que Y-A, Haefliger J-A, Piroth L, François P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. Journal of Experimental Medicine. 2005;201(10):1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho S-H, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. Journal of Allergy and Clinical Immunology. 2001;108(2):269–274. doi: 10.1067/mai.2001.117455. [DOI] [PubMed] [Google Scholar]
- 40.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of biomedical materials research. 1998;43(3):338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Pant J, Goudie MJ, Hopkins SP, Brisbois EJ, Handa H. Tunable Nitric Oxide Release from S-Nitroso-N-acetylpenicillamine via Catalytic Copper Nanoparticles for Biomedical Applications. ACS Applied Materials & Interfaces. 2017;9(18):15254–15264. doi: 10.1021/acsami.7b01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donlan RM. Biofilms and device-associated infections. Emerging infectious diseases. 2001;7(2):277. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allan ND, Giare-Patel K, Olson ME. An in vivo rabbit model for the evaluation of antimicrobial peripherally inserted central catheter to reduce microbial migration and colonization as compared to an uncoated PICC. BioMed Research International. 2012:2012. doi: 10.1155/2012/921617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annual review of medicine. 2013;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 45.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fässler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. Journal of Experimental Medicine. 2007;204(13):3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. Journal of Thrombosis and Haemostasis. 2003;1(7):1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 47.Kushner I. The acute phase response: an overview. Methods in enzymology. 1988;163:373. doi: 10.1016/0076-6879(88)63037-0. [DOI] [PubMed] [Google Scholar]
- 48.Fries D, Martini W. Role of fibrinogen in trauma-induced coagulopathy. British journal of anaesthesia. 2010;105(2):116–121. doi: 10.1093/bja/aeq161. [DOI] [PubMed] [Google Scholar]
- 49.Förch R, Jenkins ATA. Surface design: applications in bioscience and nanotechnology. John Wiley & Sons; 2009. [Google Scholar]
- 50.Zisman WA. Relation of the equilibrium contact angle to liquid and solid constitution. ACS Publications; 1964. [Google Scholar]
- 51.Tang L, Thevenot P, Hu W. Surface chemistry influences implant biocompatibility. Current topics in medicinal chemistry. 2008;8(4):270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. The Journal of Cell Biology. 1986;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warkentin P, Wälivaara B, Lundström I, Tengvall P. Differential surface binding of albumin, immunoglobulin G and fibrinogen. Biomaterials. 1994;15(10):786–795. doi: 10.1016/0142-9612(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 54.Ben-Ami R, Barshtein G, Mardi T, Deutch V, Elkayam O, Yedgar S, Berliner S. A synergistic effect of albumin and fibrinogen on immunoglobulin-induced red blood cell aggregation. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(6):H2663–H2669. doi: 10.1152/ajpheart.00128.2003. [DOI] [PubMed] [Google Scholar]
- 55.Elgalai I, Foster H. Comparison of adhesion of wound isolates of Staphylococcus aureus to immobilized proteins. Journal of applied microbiology. 2003;94(3):413–420. doi: 10.1046/j.1365-2672.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 56.Taylor FB, Jr, Wada H, Kinasewitz G. Description of compensated and uncompensated disseminated intravascular coagulation (DIC) responses (non-overt and overt DIC) in baboon models of intravenous and intraperitoneal Escherichia coli sepsis and in the human model of endotoxemia: toward a better definition of DIC. Critical care medicine. 2000;28(9):S12–S19. doi: 10.1097/00003246-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 57.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. Journal of Pharmacology and Experimental Therapeutics. 1981;218(3):739–749. [PubMed] [Google Scholar]
- 58.Brisbois EJ, Major TC, Goudie MJ, Meyerhoff ME, Bartlett RH, Handa H. Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta biomaterialia. 2016;44:304–312. doi: 10.1016/j.actbio.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-doped poly (vinyl chloride) matrix with poly (lactide-co-glycolide) additive. Journal of Materials Chemistry B. 2013;1(29):3578–3587. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, Annich GM. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31(10):2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials. 2013;34(28):6957–6966. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nachnani GH, Lessin LS, Motomiya T, Jensen WN. Scanning electron microscopy of thrombogenesis on vascular catheter surfaces. New England Journal of Medicine. 1972;286(3):139–140. doi: 10.1056/NEJM197201202860306. [DOI] [PubMed] [Google Scholar]
- 63.Stillman RM, Soliman F, Garcia L, Sawyer PN. Etiology of catheter-associated sepsis: correlation with thrombogenicity. Archives of Surgery. 1977;112(12):1497–1499. doi: 10.1001/archsurg.1977.01370120087011. [DOI] [PubMed] [Google Scholar]
- 64.Locci R, Peters G, Pulverer G. Microbial colonization of prosthetic devices. IV. Scanning electron microscopy of intravenous catheters invaded by yeasts. Zentralblatt fur Bakteriologie, Mikrobiologie und Hygiene. 1. Abt Originale B, Hygiene. 1981;173(6):419–424. [PubMed] [Google Scholar]
- 65.Gray E, Verstegen M, Peters G, Regelmann W. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. The Lancet. 1984;323(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 66.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. Journal of Clinical Investigation. 1997;99(12):2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang FC. Antimicrobial actions of nitric oxide. Nitric Oxide. 2012;27(Supplement):S10. [Google Scholar]
- 68.Jitendra Pant MJG, Brisbois Elizabeth J, Handa Hitesh. Nitric oxide-releasing polyurethanes Advances in Polyurethane. Biomaterials. 2016;1:417–449. [Google Scholar]
- 69.Reynolds MM, Frost MC, Meyerhoff ME. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radical Biology and Medicine. 2004;37(7):926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Charville GW, Hetrick EM, Geer CB, Schoenfisch MH. Reduced bacterial adhesion to fibrinogen-coated substrates via nitric oxide release. Biomaterials. 2008;29(30):4039–4044. doi: 10.1016/j.biomaterials.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engelsman AF, Krom BP, Busscher HJ, van Dam GM, Ploeg RJ, van der Mei HC. Antimicrobial effects of an NO-releasing poly(ethylene vinylacetate) coating on soft-tissue implants in vitro and in a murine model. Acta Biomaterialia. 2009;5(6):1905–1910. doi: 10.1016/j.actbio.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 72.Cai W, Wu J, Xi C, Meyerhoff ME. Diazeniumdiolate-doped poly(lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings. Biomaterials. 2012;33(32):7933–7944. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlsson S, Weitzberg E, Wiklund P, Lundberg JO. Intravesical nitric oxide delivery for prevention of catheter-associated urinary tract infections. Antimicrobial Agents and Chemotherapy. 2005;49(6):2352–2355. doi: 10.1128/AAC.49.6.2352-2355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regev-Shoshani G, Ko M, Crowe A, Av-Gay Y. Comparative Efficacy of Commercially Available and Emerging Antimicrobial Urinary Catheters Against Bacteriuria Caused by E-coli In Vitro. Urology. 2011;78(2):334–339. doi: 10.1016/j.urology.2011.02.063. [DOI] [PubMed] [Google Scholar]
- 75.Regev-Shoshani G, Ko M, Miller C, Av-Gay Y. Slow Release of Nitric Oxide from Charged Catheters and Its Effect on Biofilm Formation by Escherichia coli. Antimicrobial Agents and Chemotherapy. 2010;54(1):273–279. doi: 10.1128/AAC.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010;1(2):62–67. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- 77.Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomaterialia. 2014;10(10):4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goudie MJ, Pant J, Handa H. Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Scientific Reports. 2017;7(1):13623. doi: 10.1038/s41598-017-14012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singha P, Pant J, Goudie MJ, Workman CD, Handa H. Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomaterials Science. 2017 doi: 10.1039/c6bm00948d. [DOI] [PMC free article] [PubMed] [Google Scholar]