Abstract
Background
Resting energy expenditure (REE), adjusted for total lean mass (LM), is lower in African American (AA) than Caucasian American (CA) children. Some adult studies suggest AA-CA differences in lean mass compartments explain this REE difference. Similar data are limited in children.
Objective
To evaluate differences in compartment-specific lean mass between AA and CA children and examine the individual contributions of high-metabolic rate-at-rest trunk lean mass (TrLM) and low-metabolic-rate-at-rest appendicular lean mass (AppLM) for AA-CA differences in REE.
Methods
We studied a convenience sample of 594 AA (n=281) and CA (n=313) children. REE was measured using indirect calorimetry; DXA was used to assess body composition. ANCOVAs were performed to examine AA-CA differences in TrLM, AppLM, and REE. After accounting for age, sex, height, pubertal development, bone mass, and adiposity, REE was evaluated adjusting for total LM (model A) and separately adjusting for TrLM and AppLM (model B).
Results
AA children had greater adjusted AppLM (17.8±0.2 [SE] vs 16.0±0.2kg, p<0.001) and lower TrLM (17.2±0.2 vs 17.7±0.2kg, p=0.022) than CA children. REE adjusted for total LM was 77±16 kcal/day lower in AA than CA (p<0.001). However, after accounting separately for AppLM and TrLM, the discrepancy in REE between the groups declined to 28±19 kcal/day (p=0.14). In the adjusted model, both TrLM (p<0.001) and AppLM (p<0.027) were independently associated with REE.
Conclusion
In children, AA-CA differences in REE appear mostly attributable to differences in body composition. Lower REE in AA children is likely due to lower TrLM and greater AppLM.
Keywords: Metabolic Rate, Energy Metabolism, Child, Race, Lean Mass, Racial differences, Body Composition, African American, Caucasian
Introduction
Obesity is a leading cause of morbidity and mortality that disproportionally impacts non-Hispanic black children and adults in the United States (1, 2). The higher rates of obesity of African Americans (AA) compared to Caucasian Americans (CA) has been hypothesized to be multifactorial, due to a combination of genetic, metabolic, socioeconomic and lifestyle differences. Some studies have suggested that differences in resting energy expenditure (REE) may contribute to the increased prevalence of obesity in AA adults and children (3). The primary determinant of REE is fat free mass (FFM) (4) and thus a standard approach in evaluating REE is through normalizing or adjusting with FFM. FFM is comprised of high metabolic rate lean mass (LM) and low metabolic rate bone. Since AA children have been shown to have greater bone mineral content compared to CA peers (5), it may be more appropriate to normalize REE by lean mass (LM) instead of FFM. When compared to CA, AA children and adults have lower REE, even after adjustment for total LM, fat mass (FM), and bone mass (6–9). Lower REE in AA individuals could contribute to excessive weight gain as well as to resistance to weight loss, given that an individual’s REE is a substantial contributor to daily energy expenditures (10, 11). Improved understanding why AA children have lower REE might then help provide individualized diagnostic information needed for more targeted obesity interventions.
Lean mass can be differentiated between skeletal muscle, a high metabolic rate tissue during motion but a lower metabolic rate tissue during rest, and tissues that are metabolically active even during rest: brain and the remaining organ tissues, which have a metabolic expenditure 15–30 times higher than that of resting skeletal muscle (12). Individual variation in REE has been attributed to differential distribution of body mass, specifically trunk lean mass (TrLM), or the summative mass of high energy expending organs. Organs such as the brain, liver, heart and kidneys are responsible for 70% of REE in adults but only about 50% of total lean mass, whereas skeletal muscle accounts only for 30% of REE but ~50% of total lean mass (4). Some studies find AA adults have lower TrLM, consistent with the notion that lower visceral organ mass or differential lean mass distribution could help explain lower REE in AA compared to matched CA peers (13–15). Additionally, other studies suggest greater appendicular lean mass (AppLM) in AA, which expends little energy at rest, may explain the lower REE of AA vs CA (16–19).
Despite findings that children and adolescents also show the same difference in REE between AA and CA races adjusted for total lean mass as adults (9, 20, 21), there are few pediatric data evaluating differences in TrLM and AppLM or the contribution of compartment-specific effects on REE between these groups. One prior study in children with obesity (22) suggested that the lower REE in AA compared to CA was attenuated when analyses were adjusted for TrLM instead of total lean mass, consistent with the importance of high-energy expenditure organ lean mass for REE in adolescents.
To better understand the contributions of compartment-specific lean mass for racial differences in REE in children, we evaluated the relationship of REE and lean mass compartments in lean, healthy-weight, and overweight AA and CA children. We tested the hypothesis that separately accounting for TrLM (primarily composed of high energy expenditure organs), AppLM (skeletal muscle, which has a relatively low energy expenditure at rest) and head area (reflecting high energy expenditure brain mass) would ameliorate the race-associated difference in REE in children.
Methods
Participants & Variables
Baseline data from a convenience sample of 594 children ages 5–18 years old were collected from current and past research cohorts (registered at clinicaltrials.gov: NCT00001522; NCT00001723; NCT00005669; and NCT00001195) studied at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD) and from participants of the Baton Rouge Children's Study (23) at the Pennington Biomedical Research Center (Baton Rouge, LA). REE from some study participants has been used in a previous analysis examining prediction equations for REE (24). Race was obtained via parental report. At both sites, families were queried to assure that all 4 grandparents of participants were believed to be of the same race. Weight was measured via calibrated electronic scales to the nearest 0.1kg, height was measured via stadiometer in triplicate to the nearest millimeter, and Tanner staging for breast development and pubic hair as well as testicular volume by orchidometry were performed by trained examiners (see Supplemental Text for reference). Whole body composition was obtained by dual energy x-ray absorptiometry (DXA). This provided bone mineral density (BMD), bone mineral content (BMC), bone area, fat mass (FM), and total lean mass (LM) as well as lean mass compartments and head size (calculated as BMD/BMC and used as a proxy for brain mass). Lean mass compartments (including trunk and appendicular) were delineated with linear planes manually placed by experienced radiologists and subsequently calculated by each DXA-specific software. Three DXA machines (Hologic, Waltham, MA) were used for body composition (Hologic QDR 2000 (pencil beam mode) & 4500A (array mode) at the National Institutes of Health, and Hologic QDR 2000 (array mode) at the Pennington Biomedical Research Center). Cross-calibration between instruments was not performed; however, the instruments were periodically calibrated in the same way at each institution by using a commercially available anthropomorphic phantom or other block of tissue-equivalent material. Adjustment for the under-estimation of body fat observed with the QDR 4500A instruments relative to criterion methods (25) was applied as previously reported (26).
Resting energy expenditure was assessed at both research centers as previously described in detail by McDuffie et al (24). In brief, REE was assessed using open-circuit indirect calorimetry (Sensor-Medics 2900Z (Pennington and NIH) or Deltatrac (NIH); SensorMedics Corp, Yorba Linda, CA) after an overnight fast beginning at 10pm. Carts were calibrated independently as previously described (24). Subjects were awake, and resting watching videos; approximately 15–20 minutes of adequate collection (i.e. minutes with non-movement and a respiratory quotient less than 0.95) were used for analysis (see Supplemental Text for complete details). The equation of de Weir (27) was used to calculate REE for each valid test minute and the values were averaged.
Statistical Methods
Descriptive statistics and models were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA). Univariate analyses of covariance (ANCOVAs) were used to examine the differences in TrLM and AppLM for AA and CA, accounting for sex, age, height, pubertal stage, DXA machine, and total FM. Univariate analyses of covariance (ANCOVAs) were also used to examine the differences in REE for AA and CA, accounting for sex, age, height, pubertal stage, DXA machine, total FM, subtotal bone mineral content, head area (as a proxy for brain size), and for either total LM (total bone-free LM, model A) or for both TrLM and AppLM (instead of total LM; model B). Covariates were chosen a priori for their expected impact on body composition and energy expenditure. Calorimetry machine type was evaluated as a covariate and found non-significant and thus was removed from the final models. Additionally, because total fat mass demonstrated a bivariate distribution, the main analyses were also performed after dividing the sample in two distributions of higher (n = 343) and lower total fat mass (n=258). This analysis was done to confirm that, despite the non-normal distribution of the total fat mass covariate, findings were consistent within each subgroup. Significance was interpreted with a p<0.05 and unadjusted means are reported with standard deviation whereas adjusted means are reported with standard error.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. Among the 594 children studied, 281 (47%) were African American and 313 (53%) were Caucasian American with similar distributions at both sites. Overall, participants had mean age of 11.1±2.6y [SD] years and included children of lean and healthy weight (n=258) as well as children with overweight and obesity (n=343). BMI-z scores ranged from −2.73 to +3.06±1.19 [SD]. The AA group had a slightly but significantly greater mean age (11.4 ±2.8 [SD] years) than the CA group (10.7±2.5 years).
Table 1.
Participant Characteristics
| African American (n=281) |
Caucasian American (n=313) |
||
|---|---|---|---|
| Age (y)† | 11.4 ± 2.8* | 10.7 ± 2.5 | |
| Sex (%) | Male | 35.2 | 46.1 |
| Female | 64.8 | 56.9 | |
| Pubertal Stage (%) | Pre-Pubertal | 32.4 | 47.6 |
| Mid-Pubertal | 40.9 | 37.1 | |
| Late-Pubertal | 26.7 | 15.3 | |
| Weight (Kg)† | 70.9 ± 34.5 | 59.2 ± 29.4 | |
| Height (cm)† | 150.2 ± 14.3* | 146.6 ± 14.5 | |
| BMI (kg/m2)† | 30.1 ± 11.3* | 26.1 ± 8.9 | |
| BMI-z Score† | 1.70 ± 1.23 | 1.46 ± 1.15 | |
| Total Percentage Body Fat (%) | 36.6 ± 11.8 | 35.7 ± 11.4 | |
| Total Body Fat Mass (kg) | 29.3 ± 19.1* | 23.9 ± 16.6 | |
| Total Lean Mass (kg) | 41.0 ± 16.4* | 34.7 ± 13.7 | |
| Trunk Lean Mass (kg)† | 18.4 ± 7.5* | 16.6 ± 6.7 | |
| Appendicular Lean Mass (kg)† | 19.1 ± 8.5* | 14.9 ± 6.7 | |
| Subtotal+ Bone Mineral Content (kg)† | 1.35 ± 0.56* | 1.08 ± 0.46 | |
| Head area (cm2)† | 218.1 ± 19.7 | 214.0 ± 19.7 | |
| DXA Machine Used (%) | Hologic 2000 (NIH) | 29.2 | 37.7 |
| Hologic 4500/Discovery A (NIH) | 48.0 | 40.9 | |
| Hologic 2000 (Pennington) | 22.8 | 21.4 | |
| Resting Energy Expenditure (Kcal/d)† | 1574.3 ± 380.0 | 1532.2 ± 367.3 |
BMI, body mass index; AA, African American; CA, Caucasian; DXA, dual-energy x-ray absorptiometry. Unadjusted values shown.
Mean ± SD;
Significant differences between groups (p<0.05);
Total body less head
Fat, Lean, and Bone Compartments
In unadjusted analyses, the AA group had significantly greater total fat mass, total LM, and bone mass than the CA group (Table 1). TrLM and AppLM were also significantly greater in the AA than the CA group (Table 1). After accounting for age, sex, pubertal stage, height, fat mass, and DXA machine, AA children had greater total LM (38.5 ± 0.3 [SE] kg) compared to CA children (36.9 ± 0.3 [SE] kg, p=0.001). AA children had greater adjusted AppLM (17.8±0.2 [SE] vs 16.0±0.2 [SE] kg respectively, p<0.001) but lower TrLM than CA children (17.2±0.2 [SE] vs 17.7±0.2 [SE] kg respectively, p=0.022).
Resting Energy Expenditure
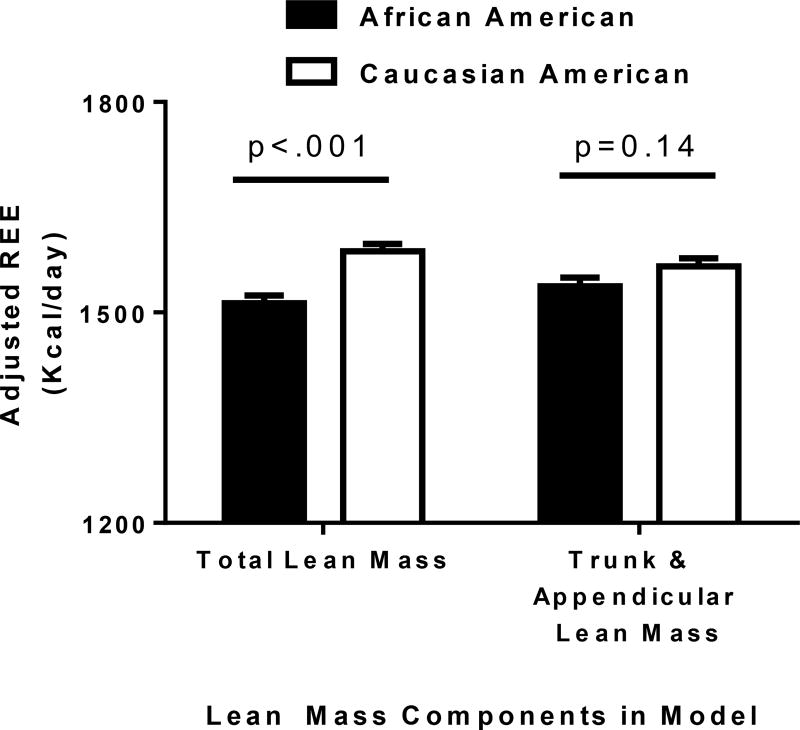
In unadjusted analyses, there were no significant differences in REE between the AA (1574.3 ± 22 [SE] kcal/day) and CA (1532.2 ± 21 [SE] kcal/day) groups even though the AA children had significantly higher LM (Table 1). After accounting for relevant covariates including total LM, REE was 77±16 [SE] kcal/day lower in AA (1511.6 ±11.2 [SE]) than CA (1588.5 ± 10.6 [SE]) (Figure 1A; Table 2; p<0.001). However, after including both AppLM and TrLM in the model instead of total LM, the discrepancy in REE between AA (1537.3 ±12.4 [SE]) and CA (1565.4 ± 11.6 [SE]) groups (−28 ±19 [SE] kcal/day) was no longer significantly different (Figure 1B; Table 2; p=0.14). AppLM was marginally negatively associated with REE in this model (B = −11.0±4.9 [SE], p=0.027) while TrLM was strongly positively associated with REE (B = +32.5±5.3 [SE], p<0.001). See Supplemental Table 1a–1c for additional models evaluating the contribution of all covariates with and without head area. These models demonstrate that the same trends were identified when weight is used in place of lean mass and with and without head area. In evaluating for a LM*Race interaction we identified that Total LM*Race was significant whereas AppLM*Race and TrLM*Race interactions were not significant in the compartment specific models (Supplemental Table 1d, Models 5a and 5b). In these models, Race was no longer a significant main factor predicting REE.
Figure 1.
ANCOVA results comparing resting energy expenditure differences in African American & Caucasian American children adjusted for total lean mass versus adjustment for trunk & appendicular lean mass.
Table 2.
Resting Energy Expenditure (REE) as a Function of Race controlled for standard covariates in addition to Total Lean Mass vs. Appendicular (App) + Trunk Lean Mass (n=594)
| Main Model A: Total Lean Mass Adjusted R2: 0.774 | |||||||
| Parameter | B | Std. Error |
t | Sig. | 95% Confidence Interval |
Partial eta Squared |
|
| Lower | Upper | ||||||
| Intercept | 90.823 | 172.986 | 0.525 | 0.600 | −248.930 | 430.575 | 0.000 |
| Height | 7.274 | 1.321 | 5.509 | 0.000 | 4.681 | 9.868 | 0.050 |
| Age | −37.333 | 6.338 | −5.890 | 0.000 | −49.781 | −24.884 | 0.056 |
| Sex | −52.926 | 16.940 | −3.124 | 0.002 | −86.196 | −19.655 | 0.016 |
| Breast/Genital Maturitya | −33.982 | 17.764 | −1.913 | 0.056 | −68.871 | 0.908 | 0.006 |
| Pubic Hair Maturitya | −32.938 | 10.534 | −3.127 | 0.002 | −53.628 | −12.249 | 0.017 |
| DXA Machine | −52.182 | 11.610 | −4.495 | 0.000 | −74.985 | −29.380 | 0.034 |
| Subtotalb BMCc | 130.874 | 52.003 | 2.517 | 0.012 | 28.737 | 233.011 | 0.011 |
| Total Lean Mass | 10.483 | 1.587 | 6.606 | 0.000 | 7.366 | 13.599 | 0.070 |
| Head Area | 1.388 | 0.479 | 2.898 | 0.004 | 0.447 | 2.329 | 0.014 |
| Total Fat Mass | 7.751 | 0.999 | 7.758 | 0.000 | 5.788 | 9.713 | 0.094 |
| [RACE=[AA] | −76.962 | 16.145 | −4.767 | 0.000 | −108.671 | −45.252 | 0.038 |
| [RACE=CA] | 0d | ||||||
| Main Model B: Lean Mass divided into Trunk and Appendicular compartments Adjusted R2: 0.779 | |||||||
| Parameter | B | Std. Error |
t | Sig. | 95% Confidence Interval |
Partial eta Squared |
|
| Lower | Upper | ||||||
| Intercept | 110.628 | 171.163 | 0.646 | 0.518 | −225.547 | 446.802 | 0.001 |
| Height | 6.920 | 1.310 | 5.284 | 0.000 | 4.348 | 9.493 | 0.046 |
| Age | −39.507 | 6.280 | −6.291 | 0.000 | −51.842 | −27.172 | 0.064 |
| Sex | −57.095 | 16.723 | −3.414 | 0.001 | −89.941 | −24.250 | 0.020 |
| Breast/Genital Maturitya | −38.383 | 17.591 | −2.182 | 0.030 | −72.933 | −3.832 | 0.008 |
| Pubic Hair Maturitya | −33.690 | 10.427 | −3.231 | 0.001 | −54.168 | −13.212 | 0.018 |
| DXA Machine | −61.758 | 11.767 | −5.248 | 0.000 | −84.869 | −38.647 | 0.045 |
| Subtotalb BMC | 160.764 | 51.520 | 3.120 | 0.002 | 59.577 | 261.952 | 0.016 |
| Head Area | 1.481 | 0.475 | 3.118 | 0.002 | 0.548 | 2.414 | 0.016 |
| Total Fat Mass | 8.249 | 0.974 | 8.468 | 0.000 | 6.335 | 10.162 | 0.110 |
| Trunk Lean Mass | 32.453 | 5.321 | 6.100 | 0.000 | 22.003 | 42.903 | 0.060 |
| App Lean Mass | −10.963 | 4.932 | −2.223 | 0.027 | −20.649 | −1.276 | 0.008 |
| [RACE=AA] | −28.175 | 19.103 | −1.475 | 0.141 | −65.694 | 9.345 | 0.004 |
| [RACE=CA] | 0d | ||||||
Pubertal Rating;
Total body less head;
Bone Mineral Content;
This parameter is set to zero because it is redundant; AA = African American; CA = Caucasian American; Model A covariates: height, age, sex, pubertal stage (breast/genital & pubic hair independently), DXA Machine/site; subtotal bone mineral content, head area, Total fat mass, and total lean mass Model B covariates: height, age, sex, pubertal stage (breast/genital & pubic hair independently), DXA Machine/site; subtotal bone mineral content, head area, total fat mass, and trunk lean mass & Appendicular lean mass independently.
Sensitivity analyses were performed given the bivariate distribution of total fat content (lean/healthy weight vs participants who were overweight/obese). We performed our primary analyses in each weight-based group separately to be assured this bivariate distribution did not alter our outcome. We identified the same relationships in each weight-based subgroup as shown for the full cohort.
DISCUSSION
Using a large convenience sample of children, we have demonstrated that differences in lean mass distribution can largely account for the observed lower REE in AA compared to CA children. We found AA children had, on average, 77kcal/day lower REE when data were adjusted for total LM and other relevant covariates. However, when the analysis accounted for TrLM and AppLM separately, this difference decreased to 28kcal/day. Additionally, when REE was adjusted for total LM, there was a significant total LM*Race interaction and Race was no longer a significant main effect. These two analyses provide evidence for the underlying etiology of REE differences between AA and CA children: There are important differences in body composition, namely relatively lower TrLM and higher AppLM in AA children compared to CA children, which in essence can explain why total LM has differential effects on REE observations in AA and CA children (the total LM*Race interaction).
It has been well documented that AA adults appear to have lower REE than CA peers when matched for age, fat mass, and total (or subtotal) fat free mass or total LM, by approximately 100 kcal/day (3, 9, 20, 28–30). Many studies in children and adolescents additionally identify lower REE in AA children when adjusting for total LM (9, 20, 31), but usually have not specifically accounted for compartment specific lean mass (9, 20). Two prior pediatric studies (22, 31) have examined this issue. Among 203 children with obesity ages 5–17y, Tershakovec et al (22) found that accounting for total fat-free mass (as well as for age, sex and fat mass), AA children demonstrated an 111.2kcal/d lower REE than CA children (22); however, after accounting for TrLM, the difference declined to 76.6kcal/day (22). Delany et al. (31) also evaluated compartment specific lean mass contribution to REE differences in a combined AA and CA cohort of 114 children and found that the best model to explain REE variance included trunk lean mass over total fat free mass, and with this variable included, race no longer significantly contributed to REE. In evaluating the contribution of AppLM, Tershakovec et al. also noted that limb fat-free mass was a significant contributor to REE, but they did not evaluate a model including both trunk and appendicular lean mass together (22). Our data demonstrate that AppLM is higher in AA than CA children and TrLM is lower in AA children. When REE is standardized to total LM, these compartment specific differences are not accounted for.
Fat-free mass in the human body is largely comprised of bone, skeletal muscles and organ tissues, each with significantly different metabolic profiles. While bone is relatively low in energy expenditure relative to resting skeletal muscle, organ tissues have approximately 15–33 times more metabolic expenditure than resting skeletal muscle (12). Between AA and CA adults, it is apparent that AA have lower organ mass (i.e. trunk lean mass) and greater appendicular skeletal muscle mass (14, 16). Much less is known about potential body composition differences in AA and CA children. Yanovski et al. found that there were significant differences in body composition between AA girls and CA girls of similar BMI and weight (8). Sun et al. identified a significant difference in relative TrLM between AA and CA children as children were monitored through puberty (29). This study reported 1.0k higher TrLM in CA children compared to AA children (p<0.001) and a −1.6kg lower limb-lean mass in CA compared to AA children (p<0.001) (29). Therefore, it is a logical concern that by grouping skeletal lean mass and TrLM together, total LM might appear similar, or even greater in AA compared to CA individuals, and yet contain less of the most metabolically active organ tissues when assessed at rest (29). Indeed, Sun et al. reported 1.4kg greater total LM in their AA vs. their CA cohort.
In adults, Gallagher et al. evaluated REE with respect to organ volume and found that the difference between AA and CA adults in REE can be explained by lower summative mass of the high-metabolic rate organs (14). In their study sample of 99 individuals, AA adults had an average of 103 kcal/day less REE compared to CA adults matched for total fat, total fat-free mass, age and sex, p<0.001 (14). This difference decreased to 40 kcal/day, p=0.25 when total organ mass was added to the model (14). To determine if this finding is transferrable to children, Sun et al. took a sample of children and monitored body content as well as REE prospectively throughout puberty (29). In contrast to Gallagher et al., Tershakovec et al. and the findings of the current study, Sun et al. did not identify attenuation in REE racial differences by adjusting for compartment specific lean mass (14, 22). Sun et al. reported a 65kcal/day difference between late pubertal AA and CA children, which was essentially unchanged after controlling for the differences in compartment specific lean mass. Of note, Sun et al. did not find a significant racial difference in REE within pre and early-pubertal children (29). Additionally, rather than using TrLM and limb-lean mass as independent adjustments, their study used principal component analysis to create a single variable representing TrLM and limb-lean mass under one variable (29). It is unclear why Sun et al. found neither racial differences in pre- and early pubertal REE nor identified the same attenuation we and others did, but these disparities may perhaps be simply due to their smaller sample size or to the use of the summary variable for lean mass compartments. Additionally, Sun et al. did not account for brain size (29). Brain tissue is highly metabolically active at rest (4) and DEXA head size was a significant contributor to our models (indicating that head area was a significant predictor of REE but it did not explain racial differences in REE.
The strengths of this study include the large sample size, precise methodologies employed to determine REE and tissue compartment, and the wide range of age and body size within the sample. To our knowledge this is the largest data set assembled to evaluate how lean mass compartments affect REE in pediatric subjects. We also included potential confounders in models, most notably pubertal staging, which is variably accounted for directly in past pediatric data. The study is limited by the use of a convenience, rather than a fully-representative sample. However, each of the research protocols that contributed data used very similar techniques for data collection and included only baseline data, before any study intervention was applied. Our sample also included participants evaluated with different DXA machines. Variability between DXA machines is well understood to be a potential source of biases. We have minimized machine variability and site effect to the best of our ability by including DXA machine as a factor in our statistical models (which indeed demonstrated DXA machine to be a significant contributor (Table 2). We also relied on multiple analysts at both sites who observed REE assessments, allowed the subjects to watch videos during data collection, and did not have machine cross calibration; however, a calorimetry-machine variable was not statistically significant within our models. Additionally, watching television seems to affect REE measurement in pediatric samples minimally (see Supplemental Appendix Text for supportive references). Finally, we used a proxy for brain mass, DXA-derived head cross-sectional area, rather than a true volumetric measurement. Although this approach has not been validated in children, it has been used in adult samples; further, head circumference measurement in children and adults correlates with brain mass (see Supplemental Appendix Text for supportive references). Future investigations using, for example, total body MRI could produce much more precise measurements of organ mass, and thus delineate organ-specific contributions to REE between AA and CA children much more precisely.
In summary, this study confirmed that AA children have lower REE than CA children when REE data are adjusted for total LM, which is the standard approach to assessing REE (Main Model A). To evaluate the underlying etiology, we hypothesized and confirmed that AA children demonstrate decreased TrLM and increased AppLM when compared to CA children. When these differences in compartment-specific lean mass were accounted for (either by controlling for TrLM and AppLM individually, Main Model B, or by controlling for the Total LM*Race interaction, Model 5a), the AA-CA difference in REE was no longer statistically significant. Thus, these findings suggest that the etiology of lower REE in AA compared to CA children is due to lower metabolically active-at-rest lean mass (i.e. lower TrLM) and higher metabolically inactive-at-rest lean mass (i.e. higher AppLM).
Supplementary Material
What is already known about this subject
African American (AA) children and adults have lower resting energy expenditure (REE) when compared to Caucasian Americans (CA) when REE is adjusted for total lean mass.
Differences in lean mass sub-compartments have been suggested to be at least partially responsible for the lower REE of AA vs. CA.
What this study adds
This study helps further elucidate why REE appears to be different between AA and CA children.
After separately accounting for trunk lean mass and appendicular lean mass, each of which have different metabolic rates, AA-CA REE differences are substantially diminished.
AA vs. CA differences in REE in children appear to be largely a function of differences in body composition.
Acknowledgments
This study was supported by the Intramural Research Programs of NICHD (Z1A-HD-00641 to JAY) at NIH with supplemental funding from the National Institute on Minority Health and Health Disparities for the research performed in Bethesda, MD and R01HD028020 for the research performed in Baton Rouge. The funding agencies played no role in the decision to submit the manuscript. FS, MMB, and JAY had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JAY is a Commissioned Officer in the United States Public Health Service (PHS). The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS or the Department of Human Health Services.
Footnotes
Conflicts of Interest Statement
The authors report no conflicts of interest for this manuscript. JAY has received grant funds from Zafgen, Inc. and Rhythm Pharmaceuticals, Inc. for pharmacologic treatment studies of obesity that are unrelated to this paper.
An abstract reporting study results was presented at the 10th International Meeting on Pediatric Endocrinology.
Author Contributions
SZY, JPD, and JAY designed the studies. JAY, JPD, MMB, FZ, SEM, and SMB collected data. FZ. MMB, and JAY analyzed the data and wrote the first draft of the manuscript. All authors provided critical review of, and approval for, the manuscript.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glickman D, Parker L, Sim LJ, Del Valle Cook H, Miller EA. Institute of Medicine Committee on Accelerating Progress in Obesity Prevention Food and Nutrition Board. The National Academies Press; Washington, DC: 2012. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. [PubMed] [Google Scholar]
- 3.Kaplan AS, Zemel BS, Stallings VA. Differences in resting energy expenditure in prepubertal black children and white children. J Pediatr. 1996;129(5):643–7. doi: 10.1016/s0022-3476(96)70143-9. [DOI] [PubMed] [Google Scholar]
- 4.Javed F, He Q, Davidson LE, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr. 2010;91(4):907–12. doi: 10.3945/ajcn.2009.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA, DeLany JP, Volaufova J, Harsha DW, Champagne C. Prediction of body fat in 12-y-old African American and white children: evaluation of methods. The American journal of clinical nutrition. 2002;76(5):980–90. doi: 10.1093/ajcn/76.5.980. [DOI] [PubMed] [Google Scholar]
- 6.Luke A, Dugas L, Kramer H. Ethnicity, energy expenditure and obesity: are the observed black/white differences meaningful? Current opinion in endocrinology, diabetes, and obesity. 2007;14(5):370–3. doi: 10.1097/MED.0b013e3282c48a7c. [DOI] [PubMed] [Google Scholar]
- 7.Shook RP, Hand GA, Wang X, et al. Low fitness partially explains resting metabolic rate differences between African American and white women. The American journal of medicine. 2014;127(5):436–42. doi: 10.1016/j.amjmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. The American journal of clinical nutrition. 1996;64(6):833–9. doi: 10.1093/ajcn/64.6.833. [DOI] [PubMed] [Google Scholar]
- 9.Yanovski SZ, Reynolds JC, Boyle AJ, Yanovski JA. Resting metabolic rate in African-American and Caucasian girls. Obesity research. 1997;5(4):321–5. doi: 10.1002/j.1550-8528.1997.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 10.Torun B, Davies PS, Livingstone MB, Paolisso M, Sackett R, Spurr GB. Energy requirements and dietary energy recommendations for children and adolescents 1 to 18 years old. Eur J Clin Nutr. 1996;50(Suppl 1):S37–80. discussion S80-1. [PubMed] [Google Scholar]
- 11.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. The American journal of clinical nutrition. 2010;92(6):1369–77. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter GR, Weinsier RL, Darnell BE, Zuckerman PA, Goran MI. Racial differences in energy expenditure and aerobic fitness in premenopausal women. The American journal of clinical nutrition. 2000;71(2):500–6. doi: 10.1093/ajcn/71.2.500. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher D, Albu J, He Q, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr. 2006;83(5):1062–7. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne NM, Weinsier RL, Hunter GR, et al. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: comparison of responses in white and black women. Am J Clin Nutr. 2003;77(6):1368–73. doi: 10.1093/ajcn/77.6.1368. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. Journal of applied physiology. 1997;83(1):229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz O, Russell M, Daley TL, et al. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. The American journal of clinical nutrition. 1992;55(1):8–13. doi: 10.1093/ajcn/55.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. The American journal of clinical nutrition. 2000;71(6):1392–402. doi: 10.1093/ajcn/71.6.1392. [DOI] [PubMed] [Google Scholar]
- 19.Rahman M, Berenson AB. Racial difference in lean mass distribution among reproductive-aged women. Ethn Dis. 2010;20(4):346–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison JA, Alfaro MP, Khoury P, Thornton BB, Daniels SR. Determinants of resting energy expenditure in young black girls and young white girls. J Pediatr. 1996;129(5):637–42. doi: 10.1016/s0022-3476(96)70142-7. [DOI] [PubMed] [Google Scholar]
- 21.Wong WW, Butte NF, Ellis KJ, et al. Pubertal African-American girls expend less energy at rest and during physical activity than Caucasian girls. The Journal of clinical endocrinology and metabolism. 1999;84(3):906–11. doi: 10.1210/jcem.84.3.5517. [DOI] [PubMed] [Google Scholar]
- 22.Tershakovec AM, Kuppler KM, Zemel B, Stallings VA. Age, sex, ethnicity, body composition, and resting energy expenditure of obese African American and white children and adolescents. Am J Clin Nutr. 2002;75(5):867–71. doi: 10.1093/ajcn/75.5.867. [DOI] [PubMed] [Google Scholar]
- 23.DeLany JP, Bray GA, Harsha DW, Volaufova J. Energy expenditure in preadolescent African American and white boys and girls: the Baton Rouge Children's Study. The American journal of clinical nutrition. 2002;75(4):705–13. doi: 10.1093/ajcn/75.4.705. [DOI] [PubMed] [Google Scholar]
- 24.McDuffie JR, Adler-Wailes DC, Elberg J, et al. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr. 2004;80(2):365–73. doi: 10.1093/ajcn/80.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81(5):1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 26.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, et al. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–9. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turell DJ, Alexander JK. Experimental Evaluation of Weir's Formula for Estimating Metabolic Rate in Man. J Appl Physiol. 1964;19:946–8. doi: 10.1152/jappl.1964.19.5.946. [DOI] [PubMed] [Google Scholar]
- 28.Foster GD, Wadden TA, Vogt RA. Resting energy expenditure in obese African American and Caucasian women. Obesity research. 1997;5(1):1–8. doi: 10.1002/j.1550-8528.1997.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI. A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. The American journal of clinical nutrition. 2001;73(2):308–15. doi: 10.1093/ajcn/73.2.308. [DOI] [PubMed] [Google Scholar]
- 30.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. The American journal of clinical nutrition. 1999;70(1):13–20. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- 31.DeLany JP, Bray GA, Harsha DW, Volaufova J. Energy expenditure in African American and white boys and girls in a 2-y follow-up of the Baton Rouge Children's Study. The American journal of clinical nutrition. 2004;79(2):268–73. doi: 10.1093/ajcn/79.2.268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.