Abstract
Eosinophil secretory (specific) granules have a unique morphology and are both a morphologic hallmark of eosinophils and fundamental to eosinophil-mediated responses. Eosinophil mediators with multiple functional activities are pre-synthesized and stored within these granules, poised for very rapid, stimulus-induced secretion. The structural organization and changes of eosinophil specific granules are revealing in demonstrating the complex and diverse secretory activities of this cell. Here, we review our current knowledge on the architecture, composition and function of eosinophil specific granules as highly elaborated organelles able to produce vesiculotubular carriers and to interplay with the intracellular vesicular trafficking. We reconsider prior identifications of eosinophil cytoplasmic granules, including “primary”, “secondary”, “microgranules”, and “small granules”; and consonant with advances, we provide a contemporary recognition that human eosinophils contain a single population of specific granules and their developmental precursors and derived secretory vesicles.
Keywords: inflammation, immune responses, degranulation, cell secretion, Eosinophil Sombrero Vesicles, vesicular trafficking, transmission electron microscopy
INTRODUCTION
Eosinophils are remarkable secretory cells able to release a large and varied collection of immune mediators, including cationic proteins, cytokines, chemokines and growth factors, which underlie eosinophil functions during inflammatory, allergic and immunoregulatory situations (reviewed in [1, 2]). How these mediators exit the cell is still not well understood. In many eukaryotic cells, synthesis, transportation and externalization of proteins occur through the canonical secretory trafficking pathway associated with endoplasmic reticulum (ER) and Golgi. In mature cells from the immune system, such as eosinophils, the scenario is much more complex (reviewed in [3, 4]). Eosinophil immune mediators constitute a group of unconventionally secreted proteins, that is, proteins that are released via ER/Golgi-independent mechanisms, also known as nonclassical protein export.
Eosinophil cytokines and other immune mediators are predominantly stored as preformed pools within secretory (specific) granules, a major population present in the eosinophil cytoplasm, from where they are mobilized and released in response to cell activation (reviewed in [5–7]). Thus, export routes in eosinophils involve their ability to “degranulate”, an event that has long been described during varied human diseases, immune homeostasis and different experimental conditions. However, neither eosinophil degranulation is an ordinary cell biological phenomenon nor eosinophil specific granules are mere containers for packing of compounds.
Eosinophil secretion of granule-derived products encompasses a multitude of events such as interaction between specific granules and vesicular trafficking for differential secretion of cytokines (reviewed in [7]); increased production of large, morphologically distinct vesiculotubular carriers from specific granules (reviewed in [8]); granule-granule fusions and even interactions of fused granules with vesicular compartments [9]; amplified release of extracellular vesicles [10, 11]; and extrusion of DNA nets associated with release of granule products [12] or with secretion-competent free extracelular granules (FEGs) [13, 14]. The complete identity of the molecular machinery coordinating the complexity of the eosinophil secretory activities remains to be established.
Here, we review current knowledge of the nomenclature, structure and function of eosinophil specific granules as highly active and intricate organelles associated with immune responses in human mature eosinophils.
ON THE NOMENCLATURE AND COMPOSITION OF EOSINOPHIL CYTOPLASMIC GRANULES
A brief history of the eosinophil specific granules and their contents
By examining inflammatory exudates from human and other species during the first half of the 19th century, early investigators noticed the presence of so-called “granule blood cells” or “compound inflammatory globules” (reviewed in [15]). These distinct granular cells were certainly the first observations of eosinophils. However, the term “eosinophil” was introduced only in 1879 by Paul Ehrlich to describe cells with granules having a high affinity for eosin and other acid dyes [15]. Ehrlich provided the first nomenclature for this robust population of cytoplasmic granules, which he called “alpha-granules” and speculated appropriately that their contents were secretory products [15]. In fact, the “alpha-granules” were secretory granules termed later specific granules, which constitute the central morphologic feature of mature eosinophils. The acidophilic nature of these granules is due to the large amount of four cationic (basic) proteins stored within them: major basic protein 1 (MBP-1) (also known as MBP and PRG2), eosinophil cationic protein (ECP) (also known as RNase3), eosinophil-derived neurotoxin (EDN) (also known as RNase2) and eosinophil peroxidase (EPX) (also known as EPO) (reviewed in [2]).
During the 1950’s, an intriguing and unique feature of eosinophil specific granules was revealed in both human and other species by transmission electron microscopy (TEM): these granules were described as “biconvex discs bounded by a membrane and containing usually in their equatorial region, inclusions of a dense, “crystalloid” material embedded in a less dense matrix” [16–18]. Pioneer works elegantly showed details of the crystalline lattice of the specific granules in human and rodent eosinophils [19]. For this reason, these granules are also frequently referred to as crystalloid or crystalline granules and the presence of these morphologically unique organelles in a cell cytoplasm or even free in tissues allows the prompt identification of eosinophils by TEM (Table 1 and Fig. 2A). During the 1970’s, Gleich’s group demonstrated that MBP is the major constituent of the crystalloid cores of eosinophil specific granules in both experimental models [20–22] and humans [23]. More recently, it was demonstrated that MBP-1 is packed within specific granules of human eosinophils as distinctive nanocrystals with amyloid-like structures, which act as inert deposits of this cationic protein, thus enabling its intracellular safe storage [24]. Interestingly, once released in the extracellular medium under cell activation, MBP self-aggregation mediates its toxic effect [24].
Table 1.
Cytoplasmic granules in human eosinophils: past and present
| Past nomenclature | Current nomenclature | Ultrastructural features | Composition |
|---|---|---|---|
| “Secondary granules” | Specific granules |
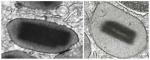 Mature granules with a centrally located crystalloid electron-dense core and an outer electron-lucent matrix, all bounded by a typical membrane. They are large (> 0.5 μm; ~700–1000 nm) and generally ellipsoid and occupy most of the cytoplasm in mature eosinophils [5, 7]. This distinctive granule morphology is found only in eosinophils. |
Cationic proteins, cytokines, chemokines, receptors, enzymes and growth factors [2, 6]. |
| “Primary granules” | Immature specific granules |
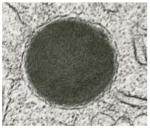 Core-free granules of precursor cells of the eosinophil lineage. They are spherical, large (> 0.5 μm; ~600–1200 nm) and with a moderately dense or very dense amorphous material delimited by a typical membrane [34, 85]. Some immature granules can show intragranular vesicles. They are absent or rare in the cytoplasm of mature eosinophils but can be sometimes seen especially in newly formed eosinophils in response to diseases that recruit these cells. |
Cationic proteins [41], precursors of MBP [40], enzymes [34] and other proteins that are being formed in specific granules. |
| “Microgranules” “Small granules” |
EoSVs (Eosinophil Sombrero Vesicles) |
 EoSVs are not granules but vesiculotubular structures with cross-sectional diameters of ~150–300 nm. They appear as tubular, elongated, curved (C-shaped) and/or circular structures in thin sections [8, 54]. Frequently seen in contact or around specific granules because they are derived from these organelles and transport granule contents for extracellular release. Increased number in activated eosinophils. |
Positive for several granule compounds such as enzymes, IL-4 [54], MBP [51] and CD63 [9]. Also positive for IL-4Rα [55]. |
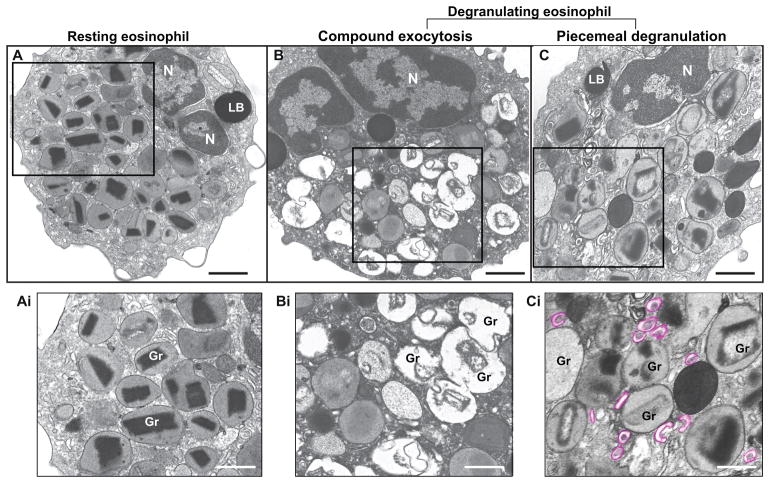
Figure 2. Ultrastructure of eosinophil specific granules in humans.
(A) A representative resting eosinophil show the cytoplasm packed with granules full of contents with typical morphology. Each granule has a central electron-dense core surrounded by an electron-lucent matrix and a delimiting membrane. (B and C) Degranulating eosinophils show morphological pattern of compound exocytosis (B), characterized by large channels formed by granule-granule fusions and wholesale release of granule contents, and piecemeal degranulation (PMD) (C), characterized by progressive emptying of the secretory granules in the absence of granule fusions and specific release of granule contents. Granule enlargement, disarrangement of the cores and matrices and increased formation of EoSVs (highlighted in pink) are observed during PMD. The boxed areas in (A–C) are shown in high magnification in (Ai-Ci). Eosinophils were isolated from the peripheral blood by negative selection, stimulated for one hour with TNF-α (B) or CCL11 (C), immediately fixed and processed for conventional TEM. N, nucleus; Gr, specific granules; LB, lipid body. Scale bars, 900 nm (A, C); 1.0 μm (B); 600 nm (Ai, Bi); 500 nm (Ci).
Notably, works conducted from 1990’s’ [25, 26] have been demonstrating that, in addition to cationic proteins, eosinophil specific granules contain many cytokines, chemokines and growth factors. A detailed list of these mediators and their biological properties in human eosinophils was recently summarized [2]. Moreover, multiple receptors such as chemokine receptor type 3 (CCR3), interleukin 4 alpha chain receptor (IL-4Rα), interferon gamma alpha chain receptor (INF-γ-αR), cysteinyl leukotriene receptor type 1 (cysLT1R), cysteinyl leukotriene receptor type 2 (cysLT2R), purinergic receptor (P2Y12R) (reviewed in [6]), Notch 1 receptor [27] and N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs): the R-SNARes vesicle-associated membrane protein 7 and 8 (VAMP7 and VAMP8) [28] and the Qa-SNARE syntaxin-17 [29] are expressed in specific granules of human eosinophils. SNAREs are critical components of the intracellular trafficking machinery involved in membrane fusion events [30] and are likely associated with the transport of granule-derived specific cargos.
Eosinophil specific granules are also unanticipated sites of certain enzymes such as neuronal nitric oxide synthase (nNOS) [31] and protein disulfide isomerase (PDI) [32], which is a typical ER protein, involved in the oxidative folding of proteins (reviewed in [33]). The functional activity of these enzymes in specific granules from human eosinophils awaits further investigation.
On “primary” and “secondary” granules
The cytoplasm of mature human eosinophils is packed with specific granules, but occasionally some large granules lacking a typical crystalline core can be observed [34]. Based on studies of rat and rabbit eosinophil maturation, it is believed that these core-free granules comprise a separate granule population referred to as “primary” granules [35], analogously to that found in mature neutrophils in which primary (azurophilic) granules persist in mature cells [36, 37]. Thus, two major types of large granules were considered to exist within mature human eosinophils: “primary” (coreless granules) and “secondary” (specific, cored granules) [34, 38, 39].
Several lines of evidence, however, have indicated that “primary” granules do not represent a separate granule population in eosinophils. It is well documented that during granule formation and granule protein genesis, cationic proteins, notably MBP, undergo progressive processing passing from precursor forms, such as proMBP, to finally MBP that forms the signature crystalline core in eosinophil specific granules [40, 41]. Studies of eosinophil differentiation in IL-5-stimulated umbilical cord stem cells cultured for 24 days localized, by immunoEM, proMBP predominantly in large uncondensed granules whereas condensing granules contained both proMBP and mainly MBP [41]. ProMBP was expressed in promyelocytes, myelocytes and metamyelocytes of the eosinophilic lineage that were actively forming specific granules. These findings led the authors to propose that “primary” (coreless) granules are either granules containing unprocessed proMBP or condensing granules containing proMBP and MBP [41]. Other cationic proteins - ECP and EPO – were demonstrated in both coreless and specific granules in human eosinophil progenitors of the bone marrow [42].
Studies in mice have shown that granule formation is closely associated with the maturation/terminal differentiation of eosinophils from marrow progenitors [43, 44]. For example, combined loss of both MBP-1 and EPX gene expression caused the disruption of eosinophilopoiesis with rare eosinophils being found in the peripheral blood [43]. Interestingly, these cells showed specific granules-like structures with limiting membranes but devoid of electron-dense cores [43]. Accordingly, it was demonstrated that a requisite proteolytic processing of granule cationic proteins, including MBP and EPX, during eosinophil cell maturation are required both for specific granule formation and eosinophil survival [44]. Thus, as the eosinophil matures, its granules undergo condensation and crystallization of their centers, which means that coreless granules develop into core-containing granules (Fig. 1). [42]
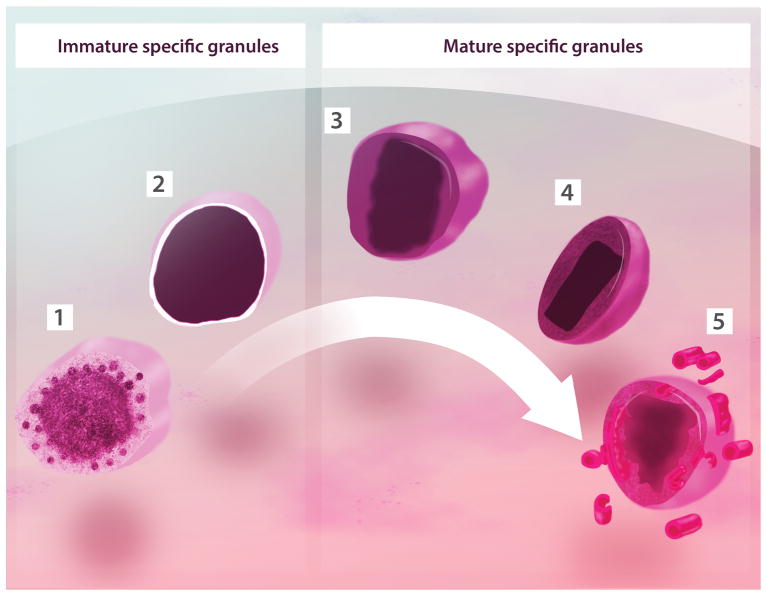
Figure 1. Developmental morphology of specific granules within human eosinophils.
Cored granules of mature human eosinophils are of a single type derived by transition from spherical coreless granules. During maturation in the bone marrow, immature specific granules undergo condensation and crystallization of their cores. (1) Immature granule in process of condensation showing intragranular vesicles surrounding the dense granule content; (2) Round immature granule with homogenously dense content; (3) Spherical core-containing granule seen with an electron-dense central area surrounded by an less dense region; (4) Resting, elliptical granule with a well-defined electron-dense core and an electron-lucent matrix; (5) An activated granule shows disassembling of its core and matrix and formation of vesiculotubular structures (EoSVs). All granules are delimited by a phospholipid bilayer membrane. A mixture of granules (1), (2) and (3) is observed in precursor cells (eosinophilic myelocytes) of the eosinophil lineage. The density of these granules is variable. Initially, small numbers of granule (3) are interspersed among large numbers of coreless granules (1) and (2) [34, 85]. Granules (4) and (5) are typical of mature eosinophils from the peripheral blood and tissues [5, 74].
Our group has been studying the ultrastructure of eosinophils from humans and experimental models during different conditions. In normal donors, the amount of crystalline granules in eosinophils isolated from the peripheral blood corresponds to almost 100% of the granules. The occurrence of true core-free (immature) granules in these cells is rare, considering that some oblique sections of specific granules may not exhibit cores. On the other hand, coreless granules can be more frequently seen in eosinophils from peripheral blood and tissues in situations in which there is an overproduction of these cells such as hypereosinophilic syndrome (HES) and experimental Schistosoma mansoni infection (unpublished observations). Thus, exacerbated recruitment of eosinophils may lead to the release of a cell population from the blood marrow still in process of maturation/terminal differentiation and hence with noticeable numbers of immature granules.
Charcot-Leyden crystal protein (CLC-P), an autocrystallizing protein, is a hallmark of the eosinophil involvement in allergic inflammation and has been identified as a member of the carbohydrate-binding family of galectin 10 (GAL-10) (reviewed in [45]). The function of CLC-P/Gal-10 is not well understood and its intracellular distribution is still intriguing. “Primary” granules, but not specific granules within mature human eosinophils were formerly determined as sites for CLC-P [46] and, for this reason, they have been typically referred to as CLC-P-positive granules. Nevertheless, CLC-P cannot solely be derived from or localized to “primary” granules. First, CLC-P account for ~7–10% of the total eosinophil protein in mature eosinophils (reviewed in [45]), being abundant in the eosinophil cytoplasm [47, 48]. Therefore, the presence of a small population of “primary” granules cannot account for such high concentration and cytoplasmic distribution of CLC-P. Second, quantitative analysis of CLC-P-containing granules in immature at myelocyte stage and mature eosinophils showed that the number of CLC-P-positive granules increases with maturation [48]. This discards the possibility that these granules represent “primary” granules since their number would be higher in the myelocyte stage compared to mature eosinophils [48]. Third, when double labeling of CLC-P and EPO was performed in eosinophils from the peripheral blood, the majority of the granules labeled for CLC-P was also positive for EPO, a typical cationic protein stored in specific granules [48]. Fourth, CLC-P also co-localized with CD63 [48], a member of the transmembrane-4 glycoprotein superfamily (tetraspanins) (reviewed in [49]), that is a marker for eosinophil specific granules [9, 50–53].
Altogether, accumulated data provide evidence that “primary” granules are indeed early “secondary” cored granules. Thus, distinct populations of “primary” and “secondary” granules are misinterpreted - there are no “primary” and no “secondary” granules - there are only immature and mature specific granules (Fig. 1 and Table 1).
On “microgranules” and “small granules”
In the past, several membrane-limited organelles distinct from the crystalloid granules were recognized by TEM in mature human eosinophils. “Microgranules” is a term quoted in the earlier eosinophil literature to describe one population of these organelles frequently seen in the eosinophil cytoplasm as small, round and generally electron-lucent structures [56]. However, this term is not appropriate since “microgranules” are not granules and indeed one of the forms assumed by membrane-bound vesiculotubular structures (Table 1), which have long been recognized in the cytoplasm of mature eosinophils (reviewed in [8]). Only in 2005, the 3D structure and functional activities of these vesiculotubular structures, named Eosinophil Sombrero Vesicles (EoSVs), were unraveled and received attention due to their remarkable ability to interact with and to bud from specific granules [54] as discussed later.
EoSVs are tubular carriers with substantial membrane surfaces and considerable plasticity. These distinct vesicles are easily identifiable because of their typical ‘mexican hat’ (sombrero) appearance in cross sections (~150–300 nm in diameter), with a central area of cytoplasm and a brim of circular membrane-delimited vesicle (Table 1). They also can show a curved shaped morphology [8, 54] (Table 1). The curved morphology of EoSVs provides a higher surface-to-volume ratio system likely suitable for the specific transport of membrane-bound proteins [57].
The morphology of EoSVs is so unique in eosinophils that their presence in the cytoplasm of granulocytes, devoid of specific granules, is useful for lineage assignment of granule-poor activated cells [58]. Moreover, the presence of persistent, free EoSVs is a common finding in biopsies after eosinophil lysis [59], and might be also used as a morphological marker for eosinophil activity.
Another “granule population” described in the early eosinophil literature was termed “small granules” and characterized as round, electron-dense or electron-lucent structures, present in tissue eosinophils found in human diseases such as Hodgkin’s disease [60], Crohn’s disease [61] and the Chédiak-Higashi syndrome [62] and in blood eosinophils isolated from asthmatic patients [63]. By reviewing the morphology of these structures referred to as “small granules” [62, 63], it is evident that they are the same as or very similar to EoSVs [8] (Table 1).
During the 1970’s, “small granules” were described as sites for acid phosphatase and aB [64]. Because specific granules did not exhibit cytochemical activity for arylsulfatase B, this enzyme was considered a marker for “small granules” in human eosinophils [64]. Later, by using immunoelectron EM, it was demonstrated that arylsulfatase B was in fact primarily present within specific granules likely in an inactive form (detected by antibodies) in addition to “small granules” [65]. These authors correctly predicted that “small type granules” might derive from specific granules during secretion when then the inactive form of arylsulfatase would be converted to an enzymatically active form [65]. The morphology of arylsulfatase-positive “small granules” is indeed the same showed by now named EoSVs, which, as noted above, can originate from specific granules [54]. Another piece of evidence that “small granules” are EoSVs came from observations that “small granules” are present in increasing numbers in activated tissue eosinophils [61, 64] and that human eosinophils contain many “small granules” which appeared to fuse with emptying specific granules [63], both features identified later as being of large tubular carriers (EoSVs) being formed from activated specific granules [54] (Table 1).
EOSINOPHIL SPECIFIC GRANULES ARE MEMBRANOUS COMPARTMENTALIZED ORGANELLES
In 1981, Okuda and colleagues emphasized that “elucidation of the eosinophil functions would require an intensive study of their specific granules” [66]. Based on an underappreciated ultrastructural study, these authors conjectured that specific granules of human eosinophils seemed to be much more complex organelles, both morphologically and functionally. By studying human eosinophils activated both in vitro, with immunocomplexes, and in vivo (tissues from patients with nasal allergy), they described a very intriguing finding: a tubular membranous structure in the matrix of the specific granules [66]. These authors reported that emptying, activated specific granules had a characteristic membranous tubular pattern, which, when viewed from different angles in thin sections, appeared as small tubules or round vesicles and suggested that the tubular structure would serve as canal for excreting the enzymatic contents from the specific granules [66]. The presence of similar structures within specific granules was also noted in tissue eosinophils from patients affected with Hodgkin’s disease [60], Crohn’s disease [61] and eosinophilic gastroenteritis [67] as well as in eosinophils isolated from the peripheral blood from patients with asthma [63] and helminthic infections [68], after stimulation with platelet activating factor (PAF) or aerosol, respectively. However, little attention had been given to these earlier findings.
In 2005, by using different TEM approaches, including optimal morphology and antigen preservation with pre-embedding immunonanogold EM [69] and electron tomography, we demonstrated that specific granules from human eosinophils indeed contain internal membranes, observed as tubules and vesicles, mainly in the granule matrix, after stimulation with CCL11, CCL5 or PAF [70]. Quantitative EM analyses showed that, in parallel with a significant increase of emptying granule numbers, there were significant increases in numbers of granules showing internal membrane domains in response to these agonists [70]. The tubular structures that were noted within specific granules in activated eosinophils are likely an integral structural component of these granules whose presence can be mainly detected after disarrangement of the granule contents. Interestingly, subcompartments within specific granules showing clear trilaminar structure, which is typical of phospholipid membranes, were seen delimiting granule products, including MBP-1 [51, 70] and collapse of these internal membranous domains was observed after treatment with brefeldin-A, which affects vesicular trafficking [70].
The presence of membranous domains within specific granules was confirmed by immunonanogold EM, a technique that provides precise epitope preservation [69], which revealed pools of CD63 within granules undergoing depletion of their contents [9, 70] and by electron tomographic analyses, which enabled 3D visualization of granules in high resolution [70]. Application of electron tomography brought a new view of specific granules as organelles with membranous subcompartments organized as an aggregate of flattened tubular networks and tubules with interconnections in some planes as well as connections with the granule limiting membrane [70]. This vesiculotubular network is likely involved in the formation of EoSVs from specific granules [70].
EOSINOPHIL ACTIVATION TRIGGERS STRUCTURAL CHANGES OF SPECIFIC GRANULES
Specific granules of human eosinophils undergo distinct structural changes in response to both in vitro stimulation and numerous diseases. These granule alterations can be identified in detail only at high resolution by TEM and are informative of the complex and diverse secretory activities of these cells (reviewed in [71]).
Morphological patterns of eosinophil degranulation, recently reviewed in ref. [2] include: i) Compound exocytosis (Fig. 2B): fusion of a population of granules with each other to form large open channels for granule cargo release;[72][73] Fusion of single granules with the plasma membrane (classical exocytosis) can also be observed; ii) Piecemeal degranulation (PMD) (Fig. 2C): characterized by a “piece by piece” release of secretory granule contents, which are differentially packed into secretory vesicles, including EoSVs, that bud off from the granules and traffics in the cytoplasm to deliver specific granule proteins to the extracellular space (reviewed in [74]). PMD occurs in the absence of granule fusions and retains the delimiting membranes of specific granules, which appear as emptying containers [74] and; iii) Cytolysis: granule deposition in tissues after cell death, and that may be associated or not with DNA-formed EETs [13, 14, 75]. FEGs remain ligand-responsive and competent to secrete and, for this reason, are considered functionally active [13, 52].
Exocytosis is an uncommon in vivo eosinophil degranulation event, but can be observed during in vitro interaction of eosinophils with different parasitic helminths [72] and environmental fungi [73] or after in vitro stimulation with high concentration of tumor necrosis factor alpha (TNF-α) [9]. Cytolysis and PMD are the most frequent modes of eosinophil degranulation in vivo, being well-documented in numerous human diseases such as nasal polyposis [76–78], allergic rhinitis [77, 79], atopic dermatitis [80], asthma [77], ulcerative colitis [77], Crohn’s disease [77], shigellosis [81], cholera [82], gastric carcinoma [83] and eosinophilic esophagitis [59].
Interestingly, human eosinophils can show different degranulating patterns in vivo, in response to a specific eosinophilic disease or condition. For example, quantitative ultrastructural studies in biopsies showed that, while PMD is the predominant eosinophil degranulation process in allergic rhinitis [77, 79], most infiltrating eosinophils exhibit morphological evidence of cytolysis in eosinophilic esophagitis [59]. In vitro, we observed that structural changes indicative of exocytosis and PMD may coexist in the same cell in response to activation. This is the case of human eosinophils stimulated with TNF-α, which show predominantly compound exocytosis (65%) but also PMD to a lesser degree (28%) [9] while stimulation with eosinophil agonists such as CCL5, CCL11 or PAF triggers PMD and rare fusion of the granules [70].
INTERPLAY BETWEEN SECRETORY GRANULES AND VESICULAR TRAFFICKING
Large vesiculotubular carriers (EoSVs) have been extensively observed within the cytoplasm of human eosinophils, especially around or attached to emptying granules (reviewed in [8, 84] (Fig. 2C). As noted, even when the identity of “small granules” was unknown, the association of these structures with specific granules was reported in activated eosinophils [63]. This intriguing intracellular distribution was better understood with the use of automated electron tomography applied to very thin serial slices (just 4 nm thick) of human eosinophils. Computer reconstructions revealed that EoSVs emerge from granules in process of secretion through PMD, as observed after stimulation with CCL11 [54] (Movie 1, available online). Thus, one remarkable characteristic of eosinophil specific granules is that they are able to generate large carriers for transportation of granule-derived products such as IL-4 [54], MBP-1 [51], CD63 [9] and INF-γ (unpublished observation).
As noted, amplified formation of EoSVs is a feature of activated eosinophils. Quantitative studies clearly demonstrated that the total number of EoSVs significantly increases in response to cell activation with CCL11 [54] or TNF-α [9]. Naturally activated eosinophils, such as those found in patients with HES, also show higher numbers of EoSVs compared to normal donors [51].
It is now clear that EoSVs take part in the eosinophil secretory pathway and that the increased formation of EoSVs in activated eosinophils reflects the immune secretory responses of these cells [8, 9, 54]. In fact, EoSVs are not just implicated with PMD but likely have a more complex role in human eosinophils. A work from our group demonstrated by immunonanogold EM that EoSVs show a differential distribution in the cytoplasm trafficking in concert with the movement of granules in process of secretion by both PMD and compound exocytosis [9]. Thus, when large chambers of fused granules are formed in the cell periphery in response to TNF-α activation, most CD63-positive EoSVs are concentrated in this region of the cytoplasm. In contrast, CD63-positive EoSVs show a more uniform distribution in the cytoplasm of CCL11-stimulated eosinophils, in association with granules undergoing PMD [9]. Our data strongly indicate that EoSVs are acting in the translocation of CD63 pools from and/or to specific granules in response to stimulation, in order to facilitate/regulate secretion [9].
FINAL REMARKS AND FUTURE DIRECTIONS
Eosinophils have the ability to secrete numerous immune mediators, which are pre-synthesized and stored within their specific granules, the single population of granules within mature human eosinophils. However, nowadays, these organelles cannot be considered just storage stations for eosinophil products. The detection of signaling molecules at them, such as specific cytokines receptors [52, 55] and SNAREs [28, 29], in conjunction with the high-resolution, structural findings of internal, organized membranes and ability to release tubular vesicles (EoSVs) [8], makes it clear that specific granules are intricate organelles, able to select their products for release in response to a specific stimulus. However, much remains to be investigated to understand the entire molecular machinery involved. Moreover, there is increasing evidence for a consistent interplay between specific granules and vesicular system in human eosinophils. Better characterization of this interaction, including detection of a probable retrograde traffic of EoSVs from the plasma membrane to specific granules, remains to be investigated. Finally, as recently discussed in ref. [2], several issues in comparing specific granules from mouse and human eosinophils are unsolved. For example, the ability of specific granules from mice in acting as storage sites for cytokines and to release vesicular carriers in response to cell activation is still unknown. Future studies are needed to better understanding of the structural and functional aspects of mouse specific granules.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH grants, USA- R37AI020241, R01AI022571, R01HL095699) and by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil-469995/2014-9, 311083/2014-5) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil-CBB-APQ-03647-16). We gratefully acknowledge the helpful discussions with Dr. Ann M. Dvorak (Department of Pathology, BIDMC, Harvard Medical School) and with the Cell Biology Group (Laboratory of Cellular Biology, ICB, Federal University of Juiz de Fora, MG, Brazil); the skillful assistance of Ellen Morgan, Rita Monahan-Earley, Tracey Sciutto and Kit Pyne (Electron Microscopy Unit, Department of Pathology, BIDMC, Harvard Medical School) during numerous studies conducted on the eosinophil ultrastructure and Kennedy Bonjour for assistance with the illustration (Fig. 1) used in this paper. We apologize to investigators whose relevant work has not been cited because of space constraints.
Abbreviations
- CLC-P
Charcot-Leyden crystal protein
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- EoSV
Eosinophil Sombrero Vesicle
- EPX
eosinophil peroxidase
- ER
endoplasmic reticulum
- FEGs
free extracelular granules
- GAL
galectin
- HES
hypereosinophilic syndrome
- IL-4
interleukin 4
- INF-γ
interferon gamma
- MBP1
major basic protein major 1
- PAF
platelet activating fator
- PMD
piecemeal degranulation
- TEM
transmission electron microscopy
- TNF-α
tumor necrosis factor alpha
Footnotes
Conflict of Interest Disclosure
The authors declare no conflict of interest.
References
- 1.Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570. doi: 10.3389/fimmu.2014.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stow JL, Murray RZ. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013;24:227–39. doi: 10.1016/j.cytogfr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Lacy P. Editorial: secretion of cytokines and chemokines by innate immune cells. Front Immunol. 2015;6:190. doi: 10.3389/fimmu.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo RCN, Dvorak AM, Weller PF. Eosinophil Ultrastructure. In: Lee J, Rosenberg H, editors. Eosinophils in health and disease. Vol. 1. Elsevier; New York: 2012. pp. 20–27. [Google Scholar]
- 6.Muniz VS, Weller PF, Neves JS. Eosinophil crystalloid granules: structure, function, and beyond. J Leukoc Biol. 2012;92:281–8. doi: 10.1189/jlb.0212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer LA, Bonjour K, Melo RCN, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melo RCN, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–36. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmo LAS, Bonjour K, Ueki S, Neves JS, Liu L, Spencer LA, Dvorak AM, Weller PF, Melo RCN. CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J Leukoc Biol. 2016;100:391–401. doi: 10.1189/jlb.3A1015-480R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzeo C, Canas JA, Zafra MP, Rojas Marco A, Fernandez-Nieto M, Sanz V, Mittelbrunn M, Izquierdo M, Baixaulli F, Sastre J, Del Pozo V. Exosome secretion by eosinophils: A possible role in asthma pathogenesis. J Allergy Clin Immunol. 2015;135:1603–13. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Akuthotha P, Carmo LA, Bonjour K, Murphy RO, Silva TP, Gamalier JP, Capron KL, Tigges J, Toxavidis V, Camacho V, Ghiran I, Ueki S, Weller PF, Melo RCN. Extracellular microvesicle production by human eosinophils activated by “inflammatory” stimuli. Front Cell Dev Biol. 2016;4:117. doi: 10.3389/fcell.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 13.Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–83. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Kay AB. The early history of the eosinophil. Clin Exp Allergy. 2015;45:575–82. doi: 10.1111/cea.12480. [DOI] [PubMed] [Google Scholar]
- 16.Kautz J, Demarsh QB. An electron microscope study of sectioned cells of peripheral blood and bone marrow. Blood. 1954;9:24–38. [PubMed] [Google Scholar]
- 17.Pease DC. An electron microscopic study of red bone marrow. Blood. 1956;11:501–26. [PubMed] [Google Scholar]
- 18.Goodman JR, Reilly EB, Moore RE. Electron microscopy of formed elements of normal human blood. Blood. 1957;12:428–42. [PubMed] [Google Scholar]
- 19.Miller F, de Harven E, Palade GE. The structure of eosinophil leukocyte granules in rodents and in man. J Cell Biol. 1966;31:349–62. doi: 10.1083/jcb.31.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleich GJ, Loegering DA, Kueppers F, Bajaj SP, Mann KG. Physiochemical and biological properties of the major basic protein from guinea pig eosinophil granules. J Exp Med. 1974;140:313–32. doi: 10.1084/jem.140.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis DM, Loegering DA, Gleich GJ. Isolation and partial characterization of a major basic protein from rat eosinophil granules. Proc Soc Exp Biol Med. 1976;152:512–5. doi: 10.3181/00379727-152-39429. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DM, Lewis JC, Loegering DA, Gleich GJ. Localization of the guinea pig eosinophil major basic protein to the core of the granule. J Cell Biol. 1978;77:702–13. doi: 10.1083/jcb.77.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleich GJ, Loegering DA, Mann KG, Maldonado JE. Comparative properties of the Charcot-Leyden crystal protein and the major basic protein from human eosinophils. J Clin Invest. 1976;57:633–40. doi: 10.1172/JCI108319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, Schmid I, Radonjic-Hoesli S, Boutet S, Williams GJ, Messerschmidt M, Seibert MM, Cascio D, Zatsepin NA, Burghammer M, Riekel C, Colletier JP, Riek R, Eisenberg DS, Simon HU. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Molecular Cell. 2015;57:1011–21. doi: 10.1016/j.molcel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem. 1993;41:1611–5. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- 26.Moqbel R, Ying S, Barkans J, Newman TM, Kimmitt P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan CJ, Durham SR, Kay AB. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–47. [PubMed] [Google Scholar]
- 27.Radke AL, Reynolds LE, Melo RCN, Dvorak AM, Weller PF, Spencer LA. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood. 2009;113:3092–101. doi: 10.1182/blood-2008-05-155937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan MR, Lacy P, Odemuyiwa SO, Steward M, Davoine F, Kita H, Moqbel R. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy. 2006;61:777–84. doi: 10.1111/j.1398-9995.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 29.Carmo LA, Dias FF, Malta KK, Amaral KB, Shamri R, Weller PF, Melo RCN. Expression and subcellular localization of the Qa-SNARE syntaxin17 in human eosinophils. Exp Cell Res. 2015;337:129–35. doi: 10.1016/j.yexcr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–29. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 31.Saluja R, Saini R, Mitra K, Bajpai VK, Dikshit M. Ultrastructural immunogold localization of nitric oxide synthase isoforms in rat and human eosinophils. Cell Tissue Res. 2010;340:381–8. doi: 10.1007/s00441-010-0947-y. [DOI] [PubMed] [Google Scholar]
- 32.Dias FF, Amaral KB, Carmo LA, Shamri R, Dvorak AM, Weller PF, Melo RCN. Human eosinophil leukocytes express protein disulfide isomerase in secretory granules and vesicles: ultrastructural studies. J Histochem Cytochem. 2014;62:450–459. doi: 10.1369/0022155414531437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurindo FR, Pescatore LA, de Fernandes DC. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–69. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 34.Dvorak AM, Ackerman SJ, Weller PF. Subcellular morphology and biochemistry of eosinophils. In: Harris JR, editor. Blood Cell Biochemistry. Megakaryocytes, Platelets, Macrophages, and Eosinophils. Vol. 2. Plenus Press; New York: 1991. [Google Scholar]
- 35.Bainton DF, Farquhar MG. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970;45:54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bainton DF, Farquhar MG. Origin of granules in polymorphonuclear leukocytes. Two types derived from opposite faces of the Golgi complex in developing granulocytes. J Cell Biol. 1966;28:277–301. doi: 10.1083/jcb.28.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bainton DF, Farquhar MG. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968;39:286–98. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dvorak AM, Ishizaka T. Human eosinophils in vitro. An ultrastructural morphology primer. Histol Histopathol. 1994;9:339–74. [PubMed] [Google Scholar]
- 39.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker RL, Gleich GJ, Pease LR. Acidic precursor revealed in human eosinophil granule major basic protein cDNA. J Exp Med. 1988;168:1493–8. doi: 10.1084/jem.168.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popken-Harris P, Checkel J, Loegering D, Madden B, Springett M, Kephart G, Gleich GJ. Regulation and processing of a precursor form of eosinophil granule major basic protein (ProMBP) in differentiating eosinophils. Blood. 1998;92:623–31. [PubMed] [Google Scholar]
- 42.Egesten A, Calafat J, Weller PF, Knol EF, Janssen H, Walz TM, Olsson I. Localization of granule proteins in human eosinophil bone marrow progenitors. Int Arch Allergy Immunol. 1997;114:130–8. doi: 10.1159/000237657. [DOI] [PubMed] [Google Scholar]
- 43.Doyle AD, Jacobsen EA, Ochkur SI, Willetts L, Shim K, Neely J, Kloeber J, Lesuer WE, Pero RS, Lacy P, Moqbel R, Lee NA, Lee JJ. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. doi: 10.1189/jlb.0213089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, Glimcher LH. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16:829–37. doi: 10.1038/ni.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–15. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dvorak AM, Letourneau L, Login GR, Weller PF, Ackerman SJ. Ultrastructural localization of the Charcot-Leyden crystal protein (lysophospholipase) to a distinct crystalloid-free granule population in mature human eosinophils. Blood. 1988;72:150–8. [PubMed] [Google Scholar]
- 47.Dvorak AM, Furitsu T, Letourneau L, Ishizaka T, Ackerman SJ. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- 48.Calafat J, Janssen H, Knol EF, Weller PF, Egesten A. Ultrastructural localization of Charcot-Leyden crystal protein in human eosinophils and basophils. Eur J Haematol. 1997;58:56–66. doi: 10.1111/j.1600-0609.1997.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 49.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–92. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–47. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 51.Melo RCN, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–81. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neves JS, Perez SA, Spencer LA, Melo RCN, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–83. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JD, Willetts L, Ochkur S, Srivastava N, Hamburg R, Shayeganpour A, Seabra MC, Lee JJ, Moqbel R, Lacy P. An essential role for Rab27a GTPase in eosinophil exocytosis. J Leukoc Biol. 2013;94:1265–74. doi: 10.1189/jlb.0812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melo RCN, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–57. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer LA, Melo RCN, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103:3333–8. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaefer HE, Hubner G, Fischer R. Specific microgranules in eosinophils. A comparative electronmicroscopic study on various mammals for the characterization of a special form of granulation in eosinophil granulocytes. Acta Haematol. 1973;50:92–104. doi: 10.1159/000208335. [DOI] [PubMed] [Google Scholar]
- 57.Melo RCN, Dvorak AM, Weller PF. Electron tomography and immunonanogold electron microscopy for investigating intracellular trafficking and secretion in human eosinophils. J Cell Mol Med. 2008;12:1416–9. doi: 10.1111/j.1582-4934.2008.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melo RCN, Weller PF, Dvorak AM. Activated human eosinophils. Int Arch Allergy Immunol. 2005;138:347–9. doi: 10.1159/000089189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saffari H, Hoffman LH, Peterson KA, Fang JC, Leiferman KM, Pease LF, 3rd, Gleich GJ. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;133:1728–34e1. doi: 10.1016/j.jaci.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 60.Parmley RT, Spicer SS. Altered tissue eosinophils in Hodgkin’s Disease. Exp Mol Pathol. 1975;23:70–82. doi: 10.1016/0014-4800(75)90007-6. [DOI] [PubMed] [Google Scholar]
- 61.Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn’s disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980;11:606–19. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- 62.Hamanaka SC, Gilbert CS, White DA, Parmley RT. Ultrastructural morphology, cytochemistry, and morphometry of eosinophil granules in Chediak-Higashi syndrome. Am J Pathol. 1993;143:618–27. [PMC free article] [PubMed] [Google Scholar]
- 63.Kroegel C, Dewar A, Yukawa T, Venge P, Barnes PJ, Chung KF. Ultrastructural characterization of platelet-activating factor-stimulated human eosinophils from patients with asthma. Clin Sci (Lond) 1993;84:391–9. doi: 10.1042/cs0840391. [DOI] [PubMed] [Google Scholar]
- 64.Parmley RT, Spicer SS. Cytochemical and ultrastructural identification of a small type granule in human late eosinophils. Lab Invest. 1974;30:557–67. [PubMed] [Google Scholar]
- 65.Egesten A, Weller PF, Olsson I. Arylsulfatase B is present in crystalloid-containing granules of human eosinophil granulocytes. Int Arch Allergy Immunol. 1994;104:207–10. doi: 10.1159/000236732. [DOI] [PubMed] [Google Scholar]
- 66.Okuda M, Takenaka T, Kawabori S, Ogami Y. Ultrastructural study of the specific granule of the human eosinophil. J Submicrosc Cytol. 1981;13:465–71. [PubMed] [Google Scholar]
- 67.Torpier G, Colombel JF, Mathieu-Chandelier C, Capron M, Dessaint JP, Cortot A, Paris JC, Capron A. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin Exp Immunol. 1988;74:404–8. [PMC free article] [PubMed] [Google Scholar]
- 68.El-Hashimi W. Charcot-Leyden crystals. Formation from primate and lack of formation from nonprimate eosinophils. Am J Pathol. 1971;65:311–24. [PMC free article] [PubMed] [Google Scholar]
- 69.Melo RCN, Morgan E, Monahan-Earley R, Dvorak AM, Weller PF. Pre-embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nature protocols. 2014;9:2382–94. doi: 10.1038/nprot.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melo RCN, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–79. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melo RCN, Dvorak AM, Weller PF. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc Microanal. 2010;16:653–60. doi: 10.1017/S1431927610093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLaren DJ, Mackenzie CD, Ramalho-Pinto FJ. Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasitic helminths (Schistosoma mansoni, Trichinella spiralis and Nippostrongylus brasiliensis) Clin Exp Immunol. 1977;30:105–18. [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–47. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 74.Melo RCN, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25:1341–54. doi: 10.14670/hh-25.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radonjic-Hoesli S, Wang X, de Graauw E, Stoeckle C, Styp-Rekowska B, Hlushchuk R, Simon D, Spaeth PJ, Yousefi S, Simon HU. Adhesion-induced eosinophil cytolysis requires the receptor-interacting protein kinase 3 (RIPK3)-mixed lineage kinase-like (MLKL) signaling pathway, which is counterregulated by autophagy. J Allergy Clin Immunol. 2017;140:1632–1642. doi: 10.1016/j.jaci.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 76.Erjefalt JS, Greiff L, Andersson M, Matsson E, Petersen H, Linden M, Ansari T, Jeffery PK, Persson CG. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med. 1999;160:304–12. doi: 10.1164/ajrccm.160.1.9809048. [DOI] [PubMed] [Google Scholar]
- 77.Erjefalt JS, Greiff L, Andersson M, Adelroth E, Jeffery PK, Persson CG. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax. 2001;56:341–4. doi: 10.1136/thorax.56.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Armengot M, Garin L, Carda C. Eosinophil degranulation patterns in nasal polyposis: an ultrastructural study. Am J Rhinol Allergy. 2009;23:466–70. doi: 10.2500/ajra.2009.23.3357. [DOI] [PubMed] [Google Scholar]
- 79.Ahlstrom-Emanuelsson CA, Greiff L, Andersson M, Persson CG, Erjefalt JS. Eosinophil degranulation status in allergic rhinitis: observations before and during seasonal allergen exposure. Eur Respir J. 2004;24:750–7. doi: 10.1183/09031936.04.00133603. [DOI] [PubMed] [Google Scholar]
- 80.Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, Leiferman KM. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J Allergy Clin Immunol. 1997;99:683–92. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- 81.Raqib R, Moly PK, Sarker P, Qadri F, Alam NH, Mathan M, Andersson J. Persistence of mucosal mast cells and eosinophils in Shigella-infected children. Infect Immun. 2003;71:2684–92. doi: 10.1128/IAI.71.5.2684-2692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qadri F, Bhuiyan TR, Dutta KK, Raqib R, Alam MS, Alam NH, Svennerholm AM, Mathan MM. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut. 2004;53:62–9. doi: 10.1136/gut.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caruso RA, Ieni A, Fedele F, Zuccala V, Riccardo M, Parisi E, Parisi A. Degranulation patterns of eosinophils in advanced gastric carcinoma: an electron microscopic study. Ultrastruct Pathol. 2005;29:29–36. doi: 10.1080/019131290882303. [DOI] [PubMed] [Google Scholar]
- 84.Melo RCN, Weller PF. Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy. Exp Cell Res. 2016;347:385–90. doi: 10.1016/j.yexcr.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dvorak AM, Saito H, Estrella P, Kissell S, Arai N, Ishizaka T. Ultrastructure of eosinophils and basophils stimulated to develop in human cord blood mononuclear cell cultures containing recombinant human interleukin-5 or interleukin-3. Lab Invest. 1989;61:116–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.