Abstract
Introduction
It is unclear, how pelvic floor supporting structures might be affected by the absence of the vagina. It was the aim of this prospective study to analyze the magnetic resonance imaging (MRI) morphology of pelvic support prior and after a Vecchietti procedure in women suffering Mullerian agenesis (Mayer-Rokitansky-Küster-Hauser syndrome).
Material and methods
N=26 women with a diagnosis of Mayer-Rokitansky-Küster-Hauser syndrome associated vaginal agenesis were recruited prospectively prior to the laparoscopic creation of a neovagina according to the Vecchietti procedure. Primary outcome measure was the MRI morphology of supporting structures. Secondary outcome measures were anatomical and functional vaginal length. Follow up was six months after surgery.
Results
N=26 women were subject to analysis. Mean age was 19.8 ± 4.4 years (mean ± sd), mean body mass index was 23.7 ± 4.3 kg/m2 (mean ± sd). All were Caucasian. Supporting structures consistent with cardinal and uterosacral ligaments were visible on MRI in all cases (100%). There were no levator ani defects. The vaginal apex could be visualized postoperatively in n=12 (46.2%) reaching up to Level I. The vagina was visible in both Level II and III with normal relationships to the pelvic walls in all cases. On gynecological examination, vaginal length was 8.8 ± 2.1cm (mean ± sd) anatomically and 10.2 ± 2.2cm (mean ± sd) functionally.
Conclusions
The pre-operative presence of pelvic support structures into which the vagina is lengthened by the surgery likely explains the uncommon occurrence of vaginal prolapse in women having had the Vecchietti procedure.
Keywords: Perineum, prolapse, surgical techniques, urogynecology, endoscopic surgery, Mayer-Rokitansky-Küster-Hauser, Mullerian agenesis
Introduction
The Mayer-Rokitansky-Küster-Hauser syndrome (MRKHS) is a rare disease but still the second most common cause of primary amenorrhea and affects at least one in 4,500 females (1, 2). It is characterized by congenital absence of the uterus and the upper two thirds of the vagina in women with a normal female karyotype. Due to functional ovaries, women affected have physiological hormone levels and normal secondary sexual characteristics (3, 4). The MRKHS may occur in isolation (type I), or can be associated with renal or skeletal malformations, and, to a lesser extent, auditory and cardiac defects (type II) (5). At present, the etiology and pathogenesis of MRKHS remain to be clarified.
The creation of a functional neovagina that enables the woman to have sexual intercourse is currently considered the primary therapeutic goal in women with congenital vaginal agenesis (6, 7). As one of the centers specializing in the diagnosis and treatment of MRKHS and other malformations of the female genital tract, we developed and optimized a laparoscopically assisted technique using vaginoabdominal blunt perforation and intraabdominal traction to create a neovagina in a standardized, controlled manner (8). In a proof-of-principle study in 101 women we demonstrated that our procedure produced better functional results and caused fewer complications than the standard laparoscopic Vecchietti procedure with vesicorectal tunneling (8). A longterm study including 240 women has shown, that our technique creates a neovagina of adequate size and secretory capacity for normal coitus, requiring no prolonged dilation postoperatively, even in the absence of sexual intercourse. The procedure is fast, effective and minimally traumatic, has a very low long-term complication rate and provides very satisfactory long-term functional results (9).
Although prolapse can occur after most of the treatments (10–18) (McIndoe, Sigmoid, self-dilation, Shears) it is very uncommon. The reasons why the vagina rarely prolapses are unknown. No prolapse has been described so far after the Vecchietti procedure and its modifications. The anatomical supports of the normal vagina are well known (19) but whether or not these structures exist in women with MRKHS and how the elongated vagina relates to them is unknown.
The purpose of the present study is to analyze magnetic resonance imaging (MRI) morphology of supporting structures prior and after the Vecchietti procedure in women suffering Mullerian agenesis (MRKHS) and to correlate findings with clinical measures such as postoperative anatomical and functional vaginal length in this unique cohort of women.
Material and methods
The methods of recruitment and MRI have been described earlier in a secondary analysis regarding the magnetic resonance (MR) visibility of the rectovaginal septum (20).
All MRKHS women scheduled for the Vecchietti procedure were prospectively enrolled (n=26). Inclusion criteria were diagnosis of MRKHS and opting for a laparoscopic creation of a neovagina according to the Vecchietti procedure at our institution. Exclusion criteria were gynecologic surgery within the last six months or contraindications for MRI, however, none of the recruited women met the exclusion criteria.
Prior to surgery all participants were examined clinically including a measurement of the vaginal length by palpating during gynecologic examination, demographic data were obtained. Written consent was acquired for all women.
As described previously, scans were acquired prior to and six months after surgery with women resting (not straining). For image analysis unenhanced multiplanar, 2D, T2-weighted turbo spin-echo MRI sequences were obtained using a 1.5-T scanner (Achieva, Philips Medical Systems®, Best, The Netherlands) using a 4 channel SENSE body coil in supine position (20). Axial and coronal images (echo time (TE) 90 ms, repetition time (TR) at least 4,000 ms, 2 averages, slice thickness 4 mm, gap 0.4 mm, field of view 28 cm, matrix 424 × 340 mm2) as well as sagittal images (echo time 90 ms, repetition time at least 4,000 ms, 4 averages, slice thickness 4 mm, gap 0.4 mm, field of view 25 cm, matrix 256 × 256 mm2) were obtained. Postoperatively a vaginal obturator was used to maintain the vaginal length and diameter until epithelialization was complete for at least six months. At the time of the postoperative MRI scan women decided to use the obturator by their own desire. Some of the women felt more comfortable to have the obturator inside their vagina during the scan, others were concerned about removing the probe.
Scans were reviewed by the first (MH) and second (JOLD) author, three interrater differences were solved by discussion and experience of the second author (JOLD). The MRI analysis was performed without any bias since the procedure itself had been performed by different authors (SYB and KKR). There was no option of blinding the scans prior to analysis since the postoperative situation could be identified clearly on the scans. The levator ani defect status was judged according to a previously described scoring system (21), visibility and morphology of supporting structures such as the pelvic sidewall and the endopelvine fascia, cardinal and uterosacral ligaments prior to the Vecchietti procedure and postoperatively, their close relationship to the created neovagina was based on prior work with the pelvic support structures. All three levels of support were analyzed separately (19).
After a follow up of six months, women were scheduled again for the postoperative MRI scan using the same protocol as described above. In addition anatomical and functional vaginal length was evaluated using a finger and a ruler with and without gentle inward pressure. Women were asked about the sexual history after surgery using the Female Sexual Function Index-questionnaire as a brief, self-report measure of female sexual function with a 6-domain structure. Numbers lower than 26.55 indicate a risk for sexual dysfuction (22, 23).
Statistical analyses
Descriptive statistics included means and standard deviation as appropriate. A 4x4 cross tab had been used in addition to chi-square test using IBM SPSS Statistics Version 22 (IBM Corp., Armonk, NY, USA).
Ethical approval
The study was approved by the local ethical committee (274/2009BO1, Oct. 27th, 2009)
Results
Regarding demographics, mean age was 19.8 ± 4.5 years (±sd), mean body mass index (BMI) was 23.7 ± 4.3 kg/m2 (± sd). All 26 women were Caucasian. Prior to surgery, vaginal length could be determined as 1.0 ± 0.9 cm (mean ± sd), whereas after the Vecchietti procedure the vaginal length was 8.8 ± 2.1cm (mean ± sd) anatomically and 10.2 ± 2.2cm (mean ± sd) functionally. Mean follow up for all 26 women was 6.6 ± 1.2 months (mean ± sd) according to the study design. All 26 participants had MR scans preoperatively and postoperatively, 18 without a vaginal obturator (69.2%), 8 with an obturator (30.8%).
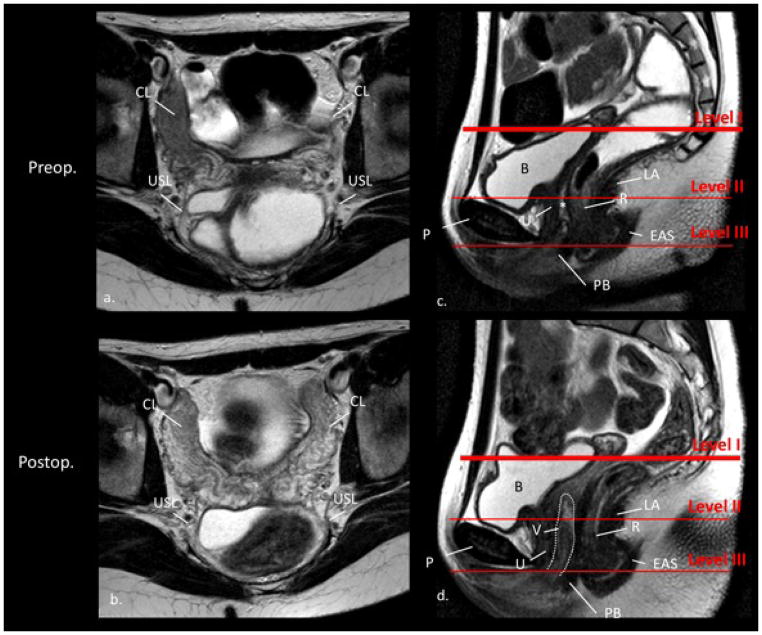
Normal MR anatomy of the M. levator ani was visible in all 26 cases without any defects (Defect status 0 for all 26 cases). Apical supporting structures (Figure 1) (cardinal and uterosacral ligaments) could be identified in all 26 cases (100%).
Figure 1.
Overview. Axial magnetic resonance images preoperatively and postoperatively (same subject); with and without vaginal obturator (different women). U, urethra; PB, perineal body; EAS, external anal sphincter; R, rectum; V, vagina; LA, M. levator ani; CL, cardinal ligament; USL, uterosacral ligament; VO, vaginal obturator; P, pubis; B, bladder). See the vaginal apex reaching all the way up to Level I (right scan on the bottom, marked with an asterix). Sagittal scan for orientation.
The following results could be found regarding MR morphology according to the different levels of support:
Level I
In Level I, the neurovascular structures that comprise the cardinal and uterosacral ligaments were visible pre- and post-operatively in all cases. The vaginal apex could be visualized in Level I overall in n = 12 (46.2%) with close relationship to the supporting structures (see Figure 1, right scan on the bottom). The vagina reached this level more often with an obturator in place (n=6 out of 8, 75%) than without (n=6 out of 18, 33.3%, p=.049). See Figure 2 for details.
Figure 2.
Level I. Axial (a. and b.) and sagittal (c. and d.) MR images preoperatively and postoperatively (same subject); without vaginal obturator. The red lines in the sagittal scan (c. and d.) define the levels of support. The axial scan plane was acquired at level I. U, urethra; B, bladder; PB, perineal body; EAS, external anal sphincter; R, rectum; V, vagina; LA, M. levator ani; CL, cardinal ligament; USL, uterosacral ligament, including the deep uterosacral ligaments, also referred to as the mesorectal fascia; P, pubis; *, rectovaginal septum. Notice the vagina (dotted white line) in scan d. reaching Level II but not Level I in this subject.
Level II
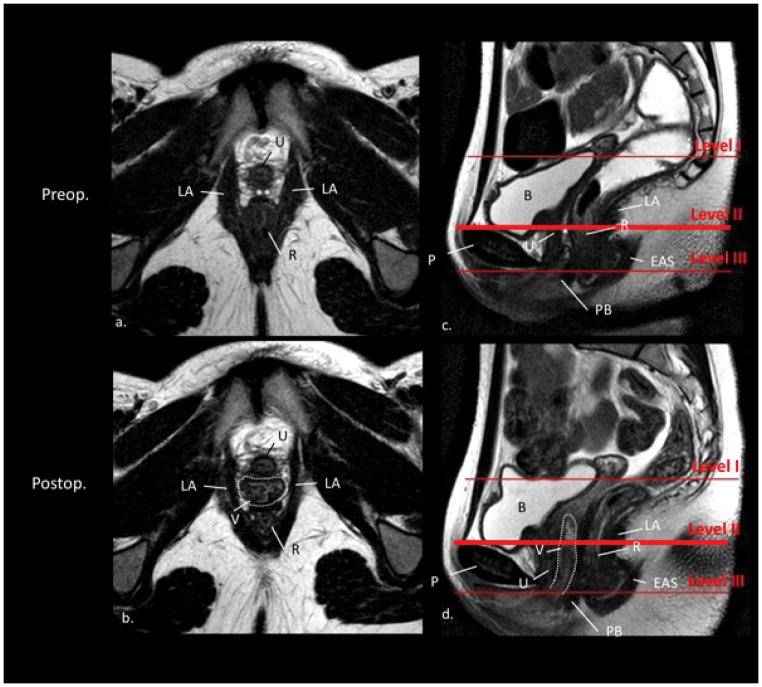
The vagina could be seen postoperatively in lateral relationship to the levator ani muscle with similar relationships as are seen in normal women in all 26 women (Figure 3).
Figure 3.
Level II. Axial (a. and b.) and sagittal (c. and d.) MR images preoperatively and postoperatively (same subject); without vaginal obturator. The red lines in the sagittal scan (c. and d.) define the levels of support. The axial scan plane was acquired at level II. U, urethra; B, bladder; PB, perineal body; EAS, external anal sphincter; R, rectum; V, vagina; LA, M. levator ani; P, pubis; *, rectovaginal septum. In the axial scan a. the area where the vagina is missing is labeled with two asterices. Notice the vagina (dotted white line) in scan b. and d. reaching Level II but not Level I in this subject. Notice in scan b. the typical vaginal morphology with its connection to the pelvic sidewall as known from healthy controls.
Level III
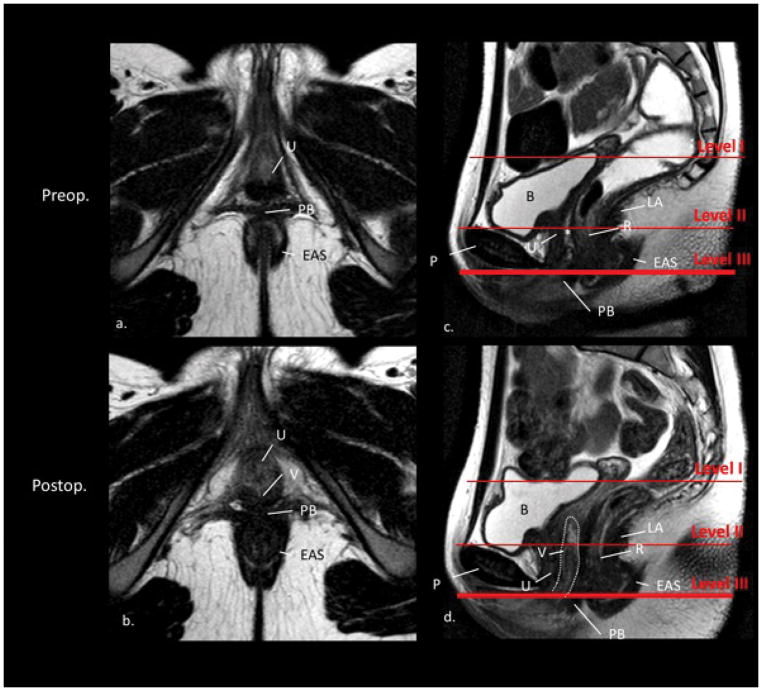
No differences could be found pre- and postoperatively in Level III (Figure 4). There was a close relationship between the anal canal, the perineum, the vagina (or the vaginal indentation preoperatively) as well as the urethra.
Figure 4.
Level III. axial (a. and b.) and sagittal (c. and d.) MR images preoperatively and postoperatively (same subject); without vaginal obturator. The red lines in the sagittal scan (c. and d.) define the levels of support. The axial scan plane was acquired at level III. U, urethra; B, bladder; PB, perineal body; EAS, external anal sphincter; R, rectum; V, vagina; LA, M. levator ani; P, pubis; *, rectovaginal septum. In the axial scan a. the area where the vagina is missing is labeled with two asterices. Notice the vagina (dotted white line) in scan b. and d. reaching Level II but not Level I in this subject. Notice in scan b. the typical vaginal morphology with its connection to the pelvic sidewall as known from healthy controls.
There was no correlation between visibility in Levels of support and functional or anatomical vaginal length.
Postoperatively the anatomical vaginal length was 8.8 ± 2.1 cm. Functional vaginal length was 10.2 ± 2.2 cm (mean ± standard deviation). At follow up, none of the women presented with anatomical signs of prolapse according to the Baden-Walker-System (n= 26: Stage 0) (24). There were no postoperative complications. Six months postoperatively, 17 out of 26 women stated satisfying sexual intercourse by evaluating their detailed sexual history, while the others were not sexually active yet. However, only six of those fully completed the Female Sexual Function Index questionnaire with a mean Female Sexual Function Index total score of 29,3 (range 23,5–32) within the normal range.
Discussion
As our principle findings, MRKHS women do have intact supporting structures of the pelvic floor. Both levator ani muscle and cardinal and uterosacral ligaments with their deep part, that are also referred to as the mesorectal fascia are clearly visible in those women with vaginal agenesis prior to any surgical procedure. In addition, the Vecchietti procedure creates a neovagina with adequate anatomical and functional lengths that allow women to have sexual intercourse. Postoperatively, MRI visualizes the close relationship between those supporting structures and the neovagina that potentially allows tissue fibers to interact.
This is the first study describing the MR anatomy of basic fundamentals of pelvic floor support in a unique cohort of MRKHS women. In order to better understand the results, it might be worth to discuss the embryological aspects of both urogenital sinus and levator ani muscle:
Urogenital sinus
MRKHS is suggested to result from a non-fusion of the Mullerian ducts (MD) with the Wolffian ducts. This explains the fact that in the majority of cases the fallopian tube together with a small rudimentary uterine horn extends only as far as the connection with the round ligament (25).
The initial segment of the MD is an independent formation. After connection with the Wolffian ducts the MD splits off from it during the caudal development in direction of the urogenital sinus (25). Around postovulatory day 57 the MDs reach the dorsal wall of the urogenital sinus and fuse to form the uterovaginal (more correctly cervicovaginal) canal (25). It is generally known and presented in several common, contemporary textbooks of embryology, that the caudal part of the vagina, the urethra, vaginal vestibule and the local glands develop from the urogenital sinus and not from the MD. This is the reason why MRKHS women are usually only diagnosed in adolescence as they cannot be distinguished from healthy females in terms of external genitalia. Accordingly, we do not see differences in level 3 in our MRKHS women compared to healthy women (26). The vaginal rudiment can be a shallow indentation with a relatively wide urethra, which is the commonest case, but conditions range from hypoplasia to rudimentary vaginas separated from the introitus by a hymen (25).
Levator ani
During early fetal development, the levator ani muscle can already be subdivided into three portions: the pubococcygeus, the iliococcygeus and the puborectalis. Differences between the male and female levator ani muscles are already marked before birth (27). The cloacal sphincter and the levator ani are derived from the third and fourth sacral myotomes (28). The levator ani primordium at the sixth week is recognizable in form of some promyoblasts and myoblasts scattered into mesenchymal tissue around the rectum. The medial part of the levator ani at the end of the second month adheres to the longitudinal muscular fibres. Some myoblasts more medially interpose between the urogenital sinus and the primitive rectum (29). There is no evidence that the development of the levator ani is dependent on the MD. As the levator ani morphology in our MRKHS women is still like in other women, therefore it is not the development of the Müllerian tract that is responsible for the difference between males and females.
This is to our knowledge the first analysis of the MR-relationship of pelvic floor supporting structure levels and a neovagina created on a Vecchietti based laparoscopic procedure. All 26 women were available at follow up, even though treatment of a rare disease with only few centers in the country is associated with large traveling efforts. Nevertheless, we do have to admit that a follow up period of six months might be adequate to evaluate the procedures primary goal, to create a neovagina in order to allow sexual intercourse, but might be too short to look for the long-term prevalence of vaginal prolapse, which might be a minor problem in this group of women. In addition, in a group of nulliparous women at the age of 20.4 ± 4.4 years (mean ± sd, time of the postoperative MRI scan) the prevalence of prolapse is very low anyway. Pregnancy and delivery induced alterations to the pelvic floor are not present in these women. Nygaard et al. described a weighted prevalence of prolapse in a nulliparous cohort of 0.6% (95% confidence interval 0.0–1.5) and in a “young” group of women between 20–39 years of 1.6% (95% confidence interval 0.6–2.6) (30). In addition, the fact that women were allowed to perform the scan with or without the obturator by their own desire did not alter the results since the supporting structures were visible in any case.
However, this is the first MRI analysis of women prior and after the surgical procedure, which makes this database unique.
Our study establishes that the levator ani muscle is normal in appearance. So the question arises, as to why prolapse in Vecchietti women has never been described, while it has been described after several other techniques. However, it has to be clearly stated, that the follow-up-period of six months is insufficient to answer this question.
The support structures are present in these women preoperatively and the Vecchietti procedure simply extends the vagina into these areas. Levator structure is normal and not affected by the anomaly so there would be excellent pelvic floor closure. Scar tissue at the apex as a result of the peritoneal tunneling during the Vecchietti procedure might enforce apical support of both cardinal and uterosacral ligaments. Missing scar tissue might explain why prolapse has been described quite often after self-dilatation (10).
The prevalence of prolapse after different treatment option are established in the literature. Swenson et al. described a sacrospinous ligament suspension after recurrent sigmoid neovagina prolapse (18). Kuhn et al. showed 11 out of 43 women with asymptomatic grade I cystocele, rectocele or apical descent after neovagina according to Shears eight years ago (15). In our institution, we performed laparoscopic sacrocolpopexy in two cases of prolapse after self-dilatation (25 years ago) and sigmoid vaginoplasty (24 years ago) (13).
Vaginal agenesis does not mean any absence of pelvic organ support structures. The Vecchietti procedure pulls the vagina close to those already existing structures. The fact that neovaginas rarely prolapse is an observation that challenges many principles of our understanding of pelvic organ support and has general importance for our understanding of prolapse in general. The fact that the levator ani muscles are normal and can be expected to maintain pelvic floor closure would result in reduced loads on vaginal attachments. In addition, if vaginal support is predicated on the vagina’s attachment to surrounding structures, then the absence of the vagina might also signal the absence of support structures. We demonstrate that the cardinal/uterosacral complex is visibly present in these women consistent with its structure. These are not ligaments, but neurovascular mesenteries that also supply the bladder. The Vecchietti procedure moves the vagina into these pre-formed areas potentially allowing fibres to interact. As a future perspective, a longterm analysis is planned.
Key message.
Support structures in each level are visible in women with Mayer-Rokitansky-Küster-Hauser syndrome prior and after Vecchietti procedure and are in close relationship to the neovagina.
Acknowledgments
The authors acknowledge the contribution in recruitment.
Funding
There was no funding or compensation.
Abbreviations
- MD
Mullerian ducts
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MRKHS
Mayer-Rokitansky-Küster-Hauser syndrome
Footnotes
Conflicts of Interest statement
Dr. DeLancey’s effort is supported by NIH Office of Research on Women’s Health Special Center of Research P50 HD 44406. The other authors report no conflicts of interest.
References
- 1.Aittomaki K, Eroila H, Kajanoja P. A population-based study of the incidence of Mullerian aplasia in Finland. Fertil Steril. 2001;76(3):624–5. doi: 10.1016/s0015-0282(01)01963-x. [DOI] [PubMed] [Google Scholar]
- 2.Herlin M, Bjorn AM, Rasmussen M, Trolle B, Petersen MB. Prevalence and patient characteristics of Mayer-Rokitansky-Kuster-Hauser syndrome: a nationwide registry-based study. Hum Reprod. 2016;31(10):2384–90. doi: 10.1093/humrep/dew220. [DOI] [PubMed] [Google Scholar]
- 3.Folch M, Pigem I, Konje JC. Mullerian agenesis: etiology, diagnosis, and management. Obstet Gynecol Surv. 2000;55(10):644–9. doi: 10.1097/00006254-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Rall K, Barresi G, Walter M, Poths S, Haebig K, Schaeferhoff K, et al. A combination of transcriptome and methylation analyses reveals embryologically-relevant candidate genes in MRKH patients. Orphanet J Rare Dis. 2011;6:32. doi: 10.1186/1750-1172-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledig S, Schippert C, Strick R, Beckmann MW, Oppelt PG, Wieacker P. Recurrent aberrations identified by array-CGH in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil Steril. 2011;95(5):1589–94. doi: 10.1016/j.fertnstert.2010.07.1062. [DOI] [PubMed] [Google Scholar]
- 6.Committee on Adolescent Health Care. Committee opinion: no. 562: müllerian agenesis: diagnosis, management, and treatment. Obstet Gynecol. 2013;121:1134–7. doi: 10.1097/01.AOG.0000429659.93470.ed. [DOI] [PubMed] [Google Scholar]
- 7.Routh JC, Laufer MR, Cannon GM, Jr, Diamond DA, Gargollo PC. Management strategies for Mayer-Rokitansky-Kuster-Hauser related vaginal agenesis: a cost-effectiveness analysis. J Urol. 2010;184(5):2116–21. doi: 10.1016/j.juro.2010.06.133. [DOI] [PubMed] [Google Scholar]
- 8.Brucker SY, Gegusch M, Zubke W, Rall K, Gauwerky JF, Wallwiener D. Neovagina creation in vaginal agenesis: development of a new laparoscopic Vecchietti-based procedure and optimized instruments in a prospective comparative interventional study in 101 patients. Fertil Steril. 2008;90(5):1940–52. doi: 10.1016/j.fertnstert.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 9.Rall K, Schickner MC, Barresi G, Schonfisch B, Wallwiener M, Wallwiener CW, et al. Laparoscopically assisted neovaginoplasty in vaginal agenesis: a long-term outcome study in 240 patients. J Pediatr Adolesc Gynecol. 2014;27(6):379–85. doi: 10.1016/j.jpag.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Calcagno M, Pastore M, Bellati F, Plotti F, Maffucci D, Boni T, et al. Early prolapse of a neovagina created with self-dilatation and treated with sacrospinous ligament suspension in a patient with Mayer-Rokitansky-Kuster-Hauser syndrome: a case report. Fertil Steril. 2010;93(1):267, e1–4. doi: 10.1016/j.fertnstert.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Coulon C, Orazi G, Nayama M, Cosson M. Prolapse of neovagina created with labia minora: a case report. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(5):409–11. doi: 10.1007/s00192-004-1262-5. [DOI] [PubMed] [Google Scholar]
- 12.Fedele L, Frontino G, Motta F, Peruzzi E. Davydov’s procedure for the treatment of neovaginal prolapse in Rokitansky syndrome. J Minim Invasive Gynecol. 2011;18(4):503–6. doi: 10.1016/j.jmig.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Henninger V, Reisenauer C, Brucker SY, Rall K. Laparoscopic nerve-preserving colposacropexy for surgical management of neovaginal prolapse. J Pediatr Adolesc Gynecol. 2015;28(5):e153–5. doi: 10.1016/j.jpag.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kondo W, Ribeiro R, Tsumanuma FK, Zomer MT. Laparoscopic promontofixation for the treatment of recurrent sigmoid neovaginal prolapse: case report and systematic review of the literature. J Minim Invasive Gynecol. 2012;19(2):176–82. doi: 10.1016/j.jmig.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn A, Neukomm C, Dreher EF, Imobersteg J, Mueller MD. Prolapse and sexual function 8 years after neovagina according to Shears: a study of 43 cases with Mayer-von Rokitansky-Kuster-Hauser syndrome. Int Urogynecol J. 2013;24(6):1047–52. doi: 10.1007/s00192-012-1980-z. [DOI] [PubMed] [Google Scholar]
- 16.Muir TW, Walters MD. Surgical management of vaginal vault prolapse in a woman with a neovagina and pelvic kidneys. Obstet Gynecol. 2004;104(5 Pt 2):1199–201. doi: 10.1097/01.AOG.0000133534.85084.ea. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer J, Fabricant C, Carr BR. Vaginal vault prolapse after nonsurgical and surgical treatment of MAAdullerian agenesis. Obstet Gynecol. 2002;99(5 Pt 2):947–9. doi: 10.1016/s0029-7844(02)01969-5. [DOI] [PubMed] [Google Scholar]
- 18.Swenson CW, DeLancey JO, Schimpf MO. Left-sided sacrospinous ligament suspension for treating recurrent sigmoid neovagina prolapse. Int Urogynecol J. 2014;25(11):1593–5. doi: 10.1007/s00192-014-2415-9. [DOI] [PubMed] [Google Scholar]
- 19.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717–24. doi: 10.1016/0002-9378(92)91562-o. discussion 24–8. [DOI] [PubMed] [Google Scholar]
- 20.Huebner M, Rall K, Brucker SY, Reisenauer C, Siegmann-Luz KC, DeLancey JO. The rectovaginal septum: visible on magnetic resonance images of women with Mayer-Rokitansky-Kuster-Hauser syndrome (Mullerian agenesis) Int Urogynecol J. 2014;25(3):323–7. doi: 10.1007/s00192-013-2214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan DM, Umek W, Stein T, Hsu Y, Guire K, DeLancey JO. Interrater reliability of assessing levator ani muscle defects with magnetic resonance images. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):773–8. doi: 10.1007/s00192-006-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 23.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 24.Baden WF, Walker TA, Lindsey JH. The vaginal profile. Tex Med. 1968;64(5):56–8. [PubMed] [Google Scholar]
- 25.Ludwig KS. The Mayer-Rokitansky-Kuster syndrome. An analysis of its morphology and embryology. Part II: Embryology. Arch Gynecol Obstet. 1998;262(1–2):27–42. doi: 10.1007/s004040050225. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Betschart C, Ashton-Miller JA, DeLancey JO. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int Urogynecol J. 2016;27(7):1087–95. doi: 10.1007/s00192-016-2949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritsch H, Frohlich B. Development of the levator ani muscle in human fetuses. Early Hum Dev. 1994;37(1):15–25. doi: 10.1016/0378-3782(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 28.Power RM. Embryological development of the levator ani muscle. Am J Obstet Gynecol. 1948;55(3):367–81. doi: 10.1016/s0002-9378(15)32955-0. [DOI] [PubMed] [Google Scholar]
- 29.Levi AC, Borghi F, Garavoglia M. Development of the anal canal muscles. Dis Colon Rectum. 1991;34(3):262–6. doi: 10.1007/BF02090167. [DOI] [PubMed] [Google Scholar]
- 30.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]