ABSTRACT
The interactions between Klebsiella pneumoniae and the host environment at the site of infection are largely unknown. Pulmonary surfactant serves as an initial point of contact for inhaled bacteria entering the lung and is thought to contain molecular cues that aid colonization and pathogenesis. To gain insight into this ecological transition, we characterized the transcriptional response of K. pneumoniae MGH 78578 to purified pulmonary surfactant. This work revealed changes within the K. pneumoniae transcriptome that likely contribute to host colonization, adaptation, and virulence in vivo. Notable transcripts expressed under these conditions include genes involved in capsule synthesis, lipopolysaccharide modification, antibiotic resistance, biofilm formation, and metabolism. In addition, we tested the contributions of other surfactant-induced transcripts to K. pneumoniae survival using engineered isogenic KPPR1 deletion strains in a murine model of acute pneumonia. In these infection studies, we identified the MdtJI polyamine efflux pump and the ProU glycine betaine ABC transporter to be significant mediators of K. pneumoniae survival within the lung and confirmed previous evidence for the importance of de novo leucine synthesis to bacterial survival during infection. Finally, we determined that pulmonary surfactant promoted type 3 fimbria-mediated biofilm formation in K. pneumoniae and identified two surfactant constituents, phosphatidylcholine and cholesterol, that drive this response. This study provides novel insight into the interactions occurring between K. pneumoniae and the host at an important infection site and demonstrates the utility of purified lung surfactant preparations for dissecting host-lung pathogen interactions in vitro.
KEYWORDS: pneumonia, pulmonary surfactant, polyamines, putrescine, spermidine, colonization, type 3 fimbriae, metabolism
INTRODUCTION
Klebsiella pneumoniae is a Gram-negative opportunistic pathogen that causes an estimated 8 to 10% of nosocomial infections in the United States and Europe (1–3). K. pneumoniae is often found in the environment (4–6) and is also a frequent colonizer of the human gastrointestinal tract (7, 8). Infections by this bacterium occur in a range of tissues within immunocompromised individuals, with the tissues of the urinary and respiratory tracts being the most prevalent sites of infection (1, 2, 9). Pulmonary infections caused by K. pneumoniae are particularly concerning and are associated with high levels of morbidity and mortality. Unfortunately, treatment options for combating these infections are becoming increasingly limited due to the widespread development of drug resistance (10–12). The recent emergence of colistin resistance in K. pneumoniae, coupled with the increasing prevalence of extended-spectrum-beta-lactamase (ESBL)- and carbapenemase-producing strains, suggests that new therapeutics are urgently needed (13–15). Despite the clinical significance of K. pneumoniae, little is known about its interaction with the host lung environment during infection. K. pneumoniae transcriptional changes occurring following inhalation and deposition into the lung are likely associated with adaptation and niche colonization. Therefore, characterizing this ecological transition is critical to our understanding of the infection process.
One of the first aspects of the host lung environment encountered by inhaled bacteria is pulmonary surfactant. This phospholipid-rich mixture coats the alveolar surfaces at the air-liquid interface and serves to reduce surface tension within the lung to prevent collapse following expiration (16, 17). Aside from this mechanophysical role, lung surfactant also modulates the activity of inflammatory cells and directly participates in the innate immune response via two surfactant-associated collectins (SP-A and SP-D) (18–20). Lung surfactant contains roughly 100 unique components, including a minor proteinaceous fraction consisting of four surfactant-associated proteins (SP-A, SP-B, SP-C, SP-D), as well as a much larger lipid fraction comprising nearly 90% of the dry weight of this substance. Within the lipid fraction, dipalmitoylphosphatidylcholine and mixed-tail phosphatidylcholines are the major constituents, making up nearly 80% of the total lipid content, followed by phosphatidylglycerol, phosphatidylinositol, phosphatidylethanolamine, and sphingomyelin. Also present within the lipid fraction are fatty acids, free triglycerides, and neutral lipids, such as cholesterol (17, 21, 22).
Pathogenic bacteria entering the host lung must generate an appropriate transcriptional response to successfully transition to this environment and avoid clearance by the innate immune system. Recognition of components within lung surfactant has been associated with the survival and virulence of several other opportunistic pathogens, perhaps, unsurprisingly, given the locale of this substance at the respiratory surfaces of the alveoli and terminal bronchioles. Previous transcriptional profiling studies by our group with purified lung surfactant led to the determination that both the detection of sphingosine and the metabolism of the choline moiety of phosphatidylcholine by Pseudomonas aeruginosa are independently required for full virulence in a mouse model of acute pneumonia (23–25). Similarly, work by Ishii et al. concluded that fatty acids within lung surfactant invoked a membrane stress response in Staphylococcus aureus and identified a novel virulence determinant implicated in this process (26).
On the basis of these studies, purified lung surfactant represents a critical, yet experimentally tractable, aspect of the host lung environment that offers an attractive in vitro model to examine host-pathogen interactions occurring during the onset of infection. Here, we characterized the transcriptional response of K. pneumoniae MGH 78578, a multidrug-resistant clinical isolate (27), to purified bovine lung surfactant (Survanta). This transcriptome-based strategy allowed us to determine that numerous characterized virulence- and fitness-related genes of K. pneumoniae are expressed in response to lung surfactant, including those involved in capsule synthesis, biofilm formation, antibiotic resistance, lipopolysaccharide (LPS) modification, and metabolism (1, 2, 9). We also tested the contributions of some of the identified genes to survival in a mouse model of acute pneumonia. We identified the MdtJI polyamine efflux pump and the ProU glycine betaine ABC transporter to be significant mediators of K. pneumoniae survival within the lung and confirmed the importance of endogenous leucine synthesis for K. pneumoniae survival during infection. An additional goal of this study was to identify the constituents within lung surfactant that induced expression of K. pneumoniae virulence-associated transcripts. Here, we show that at least two components of lung surfactant, phosphatidylcholine and cholesterol, promote type 3 fimbria expression.
RESULTS
Lung surfactant alters expression of K. pneumoniae metabolic pathways and virulence factors.
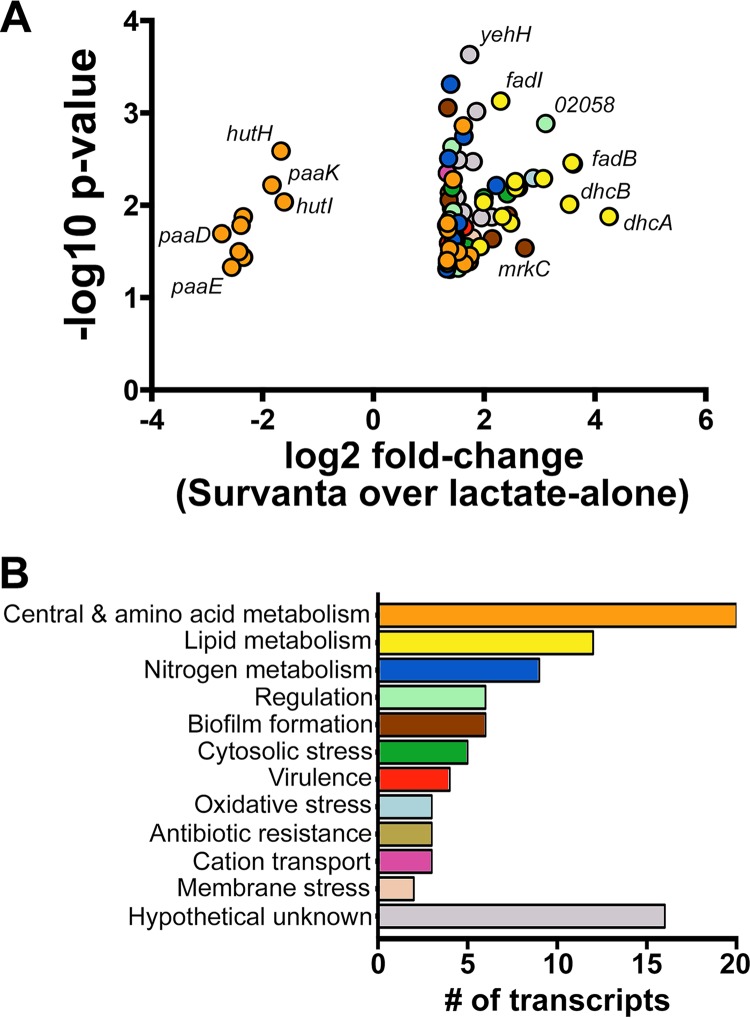
Our goal was to characterize the transcriptional changes occurring within K. pneumoniae as a result of exposure to purified lung surfactant. To accomplish this, we performed microarray analysis using a custom Affymetrix GeneChip designed for the K. pneumoniae MGH 78578 (ATCC 700721) genome and RNA collected from cells that were cultured in MOPS (morpholinepropanesulfonic acid) minimal medium containing lactate as a carbon source with or without purified bovine lung surfactant (Survanta). Under these conditions, 89 transcripts exhibited more than a 2.5-fold change in expression (P < 0.05) between the presence and absence of lung surfactant. Eighty of these genes increased in expression in response to surfactant, while nine genes were repressed. A summary of these changes is shown in Fig. 1A and B, with the transcripts being categorized into groups reflecting their known or bioinformatically predicted function (28–30). The 25 most highly expressed transcripts are also shown in Table 1, while a full list of the transcriptional changes occurring within K. pneumoniae in response to Survanta can be found in Table S1 in the supplemental material.
FIG 1.
K. pneumoniae MGH 78578 transcriptome changes in response to lung surfactant. (A) Volcano plot of transcripts detected through the microarray as exhibiting more than a 2.5-fold change in expression (P < 0.05) following exposure to Survanta. (B) Survanta-regulated transcripts were categorized into groups reflecting their known or bioinformatically predicted functions. The color coding of the categories is the same for both panels.
TABLE 1.
Summary of the 25 most highly induced transcripts expressed by K. pneumoniae MGH 78578 in response to lung surfactant
| Fold increase in expression | Gene | Alternate name | Function |
|---|---|---|---|
| 19.16 | Kpn_02053 | dhcA | Acetyl-CoAa transferase alpha subunit |
| 12.25 | Kpn_04340 | fadB | 3-Hydroxyacyl-CoA dehydrogenase |
| 12.06 | Kpn_00235 | fadE | Acyl-CoA dehydrogenase |
| 11.69 | Kpn_02054 | dhcB | Acetyl-CoA transferase beta subunit |
| 8.68 | Kpn_02058 | LysR-family transcriptional regulator | |
| 8.41 | Kpn_02055 | atoB | Beta-ketothiolase |
| 7.41 | Kpn_01989 | nemA | N-Ethylmaleimide reductase |
| 6.67 | Kpn_03278 | mrkC | Type 3 fimbrial assembly chaperone |
| 6.16 | Kpn_02505 | Kp52D | Glycosyltransferase: capsule synthesis |
| 5.96 | Kpn_04339 | fadA | Acetyl-CoA acetyltransferase |
| 5.94 | Kpn_02057 | bdhA | Short-chain dehydrogenase |
| 5.58 | Kpn_01635 | yneI | Putative aldehyde dehydrogenase |
| 5.40 | Kpn_01159 | Cyclic di-GMP phosphodiesterase | |
| 5.36 | Kpn_00406 | queC | 7-Cyano-7-deazaguanine synthase |
| 5.04 | Kpn_02056 | bdhB | 3-Hydroxybutyryl-CoA dehydrogenase |
| 4.93 | Kpn_02724 | fadI | Acetyl-CoA acetyltransferase |
| 4.68 | Kpn_01565 | mdtJ | Polyamine efflux pump subunit |
| 4.44 | Kpn_03277 | mrkB | Type 3 fimbrial usher protein |
| 4.41 | Kpn_01316 | Hypothetical protein | |
| 4.00 | Kpn_03008 | proV | Glycine betaine ABC transporter subunit |
| 3.99 | Kpn_02723 | fadJ | Enoyl-CoA hydratase |
| 3.88 | Kpn_pKpn5p08207 | Hypothetical protein | |
| 3.82 | Kpn_03510 | fadH | 2,4-Dieonyl-CoA reductase |
| 3.65 | Kpn_01727 | Hypothetical protein | |
| 3.49 | Kpn_01676 | Hypothetical protein |
CoA, coenzyme A.
Fifteen percent of the genes expressed by K. pneumoniae in response to Survanta are predicted to function in phospholipid and fatty acid metabolism. FadR regulon members are well represented among this group, with six β-oxidation-related genes (fadBA, fadHIJ, fadE) (31) exhibiting between 3.8- and 12.3-fold increases in transcript abundance in response to surfactant. In addition, the six genes within the Kpn_02053-Kpn_02057 operon displayed between 5- and 19.2-fold increases in abundance under these conditions. Encoded within this operon are a predicted citrate permease-like transporter (which we designate Kpn_02056.5, as it was not annotated in the original genome numbering) and orthologs of genes found within the dehydroxycarnitine and 3-hydroxybutryate metabolism gene clusters of P. aeruginosa PAO1, reflecting their probable function in the uptake and metabolism of short-chain fatty acids (32, 33).
Other changes within the K. pneumoniae transcriptome reflect global alterations in nitrogen metabolism. The genes for glutamate synthase (gltD), the glutamine ABC transporter permease (glnP), and the nitrogen regulatory protein (glnK) exhibited between 2.6- and 3.1-fold increases in transcript abundance in response to lung surfactant, indicative of fluctuations in nitrogen pool homeostasis. Other transcriptional changes reflected the accumulation, metabolism, and excretion of polyamines during growth in lung surfactant. Notably, increases in the abundance of several putrescine-inducible transcripts (34, 35), including the mdtJI polyamine efflux pump and yneI succinate semialdehyde dehydrogenase (5.6- to 2.7-fold increases), were observed.
Exposure to lung surfactant also altered the expression of metabolic transcripts in K. pneumoniae in unexpected ways. Interestingly, Survanta stimulated the transcription of genes involved in the synthesis of branched-chain amino acids (BCAA), including the gene for valine-pyruvate transaminase (avtA; 2.6-fold increase) and the leuABCD leucine synthesis operon (2.9- to 2.3-fold increase). In addition, repression of the phenylacetic acid (paaCDFEFIK) and histidine (hutUIH) catabolism gene clusters was observed (6.7- to 3-fold decrease).
Numerous oxidative stress-related transcripts were also induced by K. pneumoniae in lung surfactant, potentially in response to the elevated amounts of reactive oxygen species generated through the β-oxidation of fatty acids. The levels of transcription of nemAR, encoding the oxidative stress-responsive regulator NemR and the reactive-electrophile neutralizing N-ethylmaleimide reductase NemA (36, 37) increased 2.8- and 7.4-fold, respectively, under these conditions. Other oxidative stress response genes were also expressed, including ybbL (2.5-fold increase) and a hydrogen peroxide-inducible gene of unknown function, ybjM (2.9-fold increase) (38, 39).
Other aspects of the K. pneumoniae transcriptional response to lung surfactant are reflective of metabolic/cytosolic stress. Notably, transcription of the multiple drug resistance and acid response regulator (40–42) gene evgA increased 3.5-fold in response to surfactant exposure. Transcription of the genes for the glycine betaine ABC transporter, proVWX (43), also increased 4-fold, suggesting that the lipid-rich environment of lung surfactant invokes osmotic stress in K. pneumoniae. Two tRNA nucleotide modification enzymes were also induced under these conditions, with queC and gidA exhibiting 5.4- and 2.8-fold increases in transcript abundance, respectively (44, 45). Finally, transcription of genes associated with antibiotic resistance were also upregulated, including the genes for the 23S rRNA methylation enzyme (yfgB) and aminoglycoside 3′-phosphotransferase (strB) (2.6- to 2.7-fold increase) (46, 47).
Lung surfactant also induced transcriptional changes within K. pneumoniae associated with colonization, virulence, and immune evasion. Exposure to Survanta induced the expression of type 3 fimbriae encoded by the mrkABCDF gene cluster (6.7- to 2.6-fold increase) (48). Similarly, a 5.4-fold increase in transcript abundance was observed for a cyclic-di-GMP phosphodiesterase (KPN_01159) that has been implicated in promoting mrK operon expression in vitro (49). Increased transcription of genes encoded within the capsular polysaccharide synthesis (cps) region was also observed following exposure to surfactant, including ugd, Kp52D, and Kpn_02483 (2.6- to 6.2-fold increase). Two other capsule synthesis genes, Kpn_02503 and Kpn_02506 (50), also exhibited statistically significant increases in transcript abundance but failed to surpass our 2.5-fold change cutoff for inclusion in this study (2.0- and 2.1-fold increases, respectively). Lung surfactant also invoked transcriptional changes within K. pneumoniae indicative of LPS modification. A 3.1-fold increase in transcript abundance was observed for arnA, whose product participates in conferring resistance to cationic peptides and polymyxin B through the addition of 4-amino-4-deoxy-l-arabinose (Ara4N) to lipid A (51–53).
Validation of microarray data.
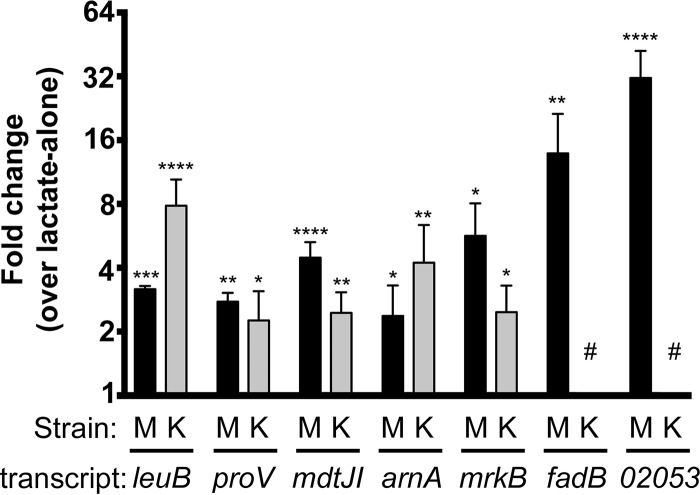
Quantitative reverse transcription-PCR (qRT-PCR) was used to confirm the Survanta-induced transcriptional changes within K. pneumoniae that we identified through the microarray and to determine the conservation of these responses in KPPR1. To accomplish this, reverse transcription-PCR (RT-PCR) was performed on K. pneumoniae MGH 78578 and KPPR1 RNA collected from three additional Survanta induction experiments, as described in the Materials and Methods section. The relative abundances of seven transcripts, representing nearly 10% of the genes identified through the microarray to be induced under these conditions, were examined. The genes for analysis were chosen to represent a range of cellular functions, including fatty acid and phospholipid metabolism (fadB and Kpn_02053), biofilm formation (mrkA), branched-chain amino acid synthesis (leuA), nutrient uptake (proV), polyamine efflux (mdtJ), and LPS modification (arnA). As shown in Fig. 2, all transcripts examined exhibited greater than a 2-fold increase in expression in response to Survanta, in close agreement with the microarray data. For the five primers that produced correct amplicons, all showed induction, with leuB and arnA showing higher relative induction in KPPR1.
FIG 2.
qRT-PCR validation of induced transcripts in K. pneumoniae following exposure to lung surfactant. The relative abundance of seven Survanta-induced transcripts detected through the microarray were reexamined using quantitative RT-PCR with RNA collected from three independent Survanta induction experiments as described in the Materials and Methods section. Genes for analysis were chosen to represent a range of cellular functions, including lipid metabolism (fadB and Kpn_02053 [dhcA]), biofilm formation (mrkA), branched-chain amino acid synthesis (leuA), nutrient uptake (proV), polyamine efflux (mdtJ), and LPS modification (arnA). Raw transcript expression values were normalized to those for Kpn_04184, which exhibited no change in expression between conditions in our microarrays. We examined expression in both MGH 78578 (M) and KPPR1 (K), though our primers designed for fadB and Kpn_02053 in MGH 78578 were not usable in KPPR1 due to multiple products (noted with the # symbol). Statistical analysis was conducted via two-way analysis of variance with a Sidak posttest analyzing in-strain changes comparing expression under conditions with Survanta additions to expression under the lactate-alone condition. The data shown summarize those from three independent Survanta induction experiments, and statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Surfactant-induced transcripts contribute to K. pneumoniae fitness during lung infection.
The contributions of other surfactant-induced transcripts to K. pneumoniae lung pathogenesis were explored using engineered K. pneumoniae gene deletion strains in a mouse oropharyngeal aspiration model of acute pneumonia. Due to the historic usage of KPPR1 as the model for Klebsiella lung infection, gene deletions were engineered into KPPR1 (ATCC 43816) (1, 54–58). KPPR1 and MGH 78578 share a high level of gene conservation, with 88% of their open reading frames being considered orthologous (59). More importantly, this genetic similarly is reflected within the surfactant microarray data, where 81% of transcripts expressed by MGH 78578 under these conditions are also encoded within the genome of KPPR1.
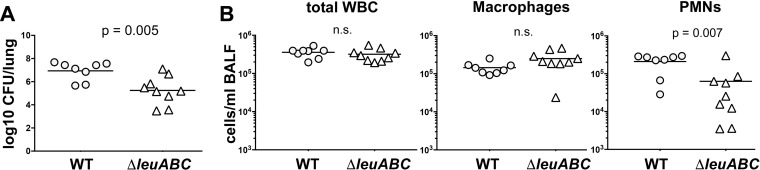
Exposure to lung surfactant induced the expression of the leucine synthesis gene cluster (leuABCD) in both strains of K. pneumoniae (Fig. 2). The importance of branched-chain amino acid synthesis to K. pneumoniae during pulmonary infection was recently demonstrated through an in vivo genetic screen that recognized that the ilvADE isoleucine and valine synthesis gene clusters are required for pathogenesis and that also noted that leuABCD disruption mutants display competitive fitness defects in vivo (60). Therefore, we generated a leuABCD deletion strain to determine if the defect was absolute or manifests only in competition with the wild type (WT). Deletion of leuABCD resulted in a nearly 50-fold decrease in the number of bacterial CFU in the lung compared to the number for the WT strain (a 48.98-fold decrease in the number of CFU; P = 0.0048) (Fig. 3A). Interestingly, although the total number of immune cells in bronchoalveolar lavage fluid (BALF) collected from mice infected with the ΔleuABCD strain was similar to those in BALF collected from mice infected with the KPPR1 WT, the composition of the infiltrating leukocytes differed. BALF collected from mice infected with the deletion strain demonstrated a reduction in the neutrophilic response to the mutant strain, likely as a consequence of the reduced number of bacterial CFU (Fig. 3B).
FIG 3.
Leucine biosynthesis by K. pneumoniae is required for virulence during acute pneumonia. (A) Adult male C57BL/6J mice were infected via oropharyngeal aspiration with either K. pneumoniae KPPR1 WT or KPPR1 ΔleuABCD. The numbers of CFU per lung were measured at 24 h postinstillation. (B) White blood cells (WBC) within the bronchoalveolar lavage fluid collected from each infected mouse were enumerated. The total white blood cell, macrophage, and PMN counts are shown. The data shown summarize those from three independent experiments, with statistical significance being determined through an unpaired t test. The arithmetic mean is depicted for each strain, with the counts from individual mice being represented by individual points.
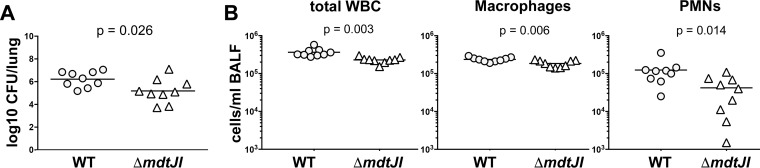
The mdtJI operon encodes a small multidrug resistance (SMR-family) efflux pump that was first implicated in resistance to deoxycholate and SDS in Escherichia coli (61). More recent reports have indicated that MdtJI primarily functions in the excretion of the polyamines spermidine and putrescine (35, 62). Polyamines have been recognized as important mediators of virulence in numerous bacterial genera, including Shigella, Salmonella, and Staphylococcus (63), leading to our interest in exploring the potential contribution of MdtJI to K. pneumoniae fitness during infection of the lung. As shown in Fig. 4A, deletion of mdtJI resulted in a more than 10-fold decrease in the bacterial lung burden relative to that of the WT strain at 24 h postinoculation (a 10.86-fold decrease in the number of CFU). Interestingly, BALF collected from mice infected with KPPR1 ΔmdtJI contained significantly fewer infiltrating leukocytes, neutrophils, and macrophages than BALF collected from mice infected with the WT strain (Fig. 4B).
FIG 4.
The MdtJI polyamine efflux pump contributes to K. pneumoniae fitness during acute pneumonia. (A) Adult male C57BL/6J mice were infected via oropharyngeal aspiration with either K. pneumoniae KPPR1 WT or KPPR1 ΔmdJI. The numbers of CFU per lung were measured at 24 h postinstillation. (B) The white blood cells within the bronchoalveolar lavage fluid collected from each infected mouse were enumerated. The total white blood cell, macrophage, and PMN counts are shown. The data shown summarize those from three independent experiments, with statistical significance being determined through an unpaired t test. The arithmetic mean is depicted for each strain, with the counts from individual mice being represented by individual points.
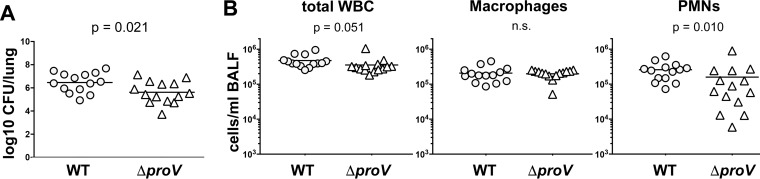
The ProU (proVWX) ABC transporter was the most highly induced metabolite acquisition system expressed by K. pneumoniae following exposure to Survanta. The role of this transporter has been extensively studied in Escherichia coli and Salmonella eneterica serovar Typhimurium and participates in the uptake of glycine betaine from the environment during periods of osmotic stress (43, 64). Phosphatidylcholine is the most abundant phospholipid in lung surfactant and has previously been shown to serve as an important source of the osmoprotectant glycine betaine, which is required for Pseudomonas aeruginosa fitness within the lung (24). We were therefore curious to determine if ProU-mediated glycine betaine uptake also contributed to K. pneumoniae fitness during lung infection. As shown in Fig. 5A, deletion of proV resulted in a significant decrease in the bacterial lung burden compared to that of the WT KPPR1 strain at 24 h postinoculation (a 6.99-fold decrease in the number of CFU). Examination of immune cells in BALF collected from these mice indicated that deletion of proV altered polymorphonuclear leukocyte (PMN) recruitment (Fig. 5B). It is important to note for the changes in the number of CFU reported for these three strains that although we suggest a survival difference, we did not enumerate the CFU in the BALF or other body compartments, and it therefore remains a formal possibility that localization is affected instead of or in addition to survival.
FIG 5.
The ProU glycine betaine ABC transporter (proVWX) contributes to K. pneumoniae fitness during acute pneumonia. (A) Adult male C57BL/6J mice were infected via oropharyngeal aspiration with either K. pneumoniae KPPR1 WT or KPPR1 ΔproV. The numbers of CFU per lung were measured at 24 h following instillation. (B) The white blood cells within the bronchoalveolar lavage fluid collected from each infected mouse were enumerated. The total white blood cell, macrophage, and PMN counts are shown. The data shown summarize those from four independent experiments, with statistical significance being determined through an unpaired t test. In each panel, the arithmetic mean is depicted for each strain, with the counts from individual mice being represented by individual points.
KPPR1 and isogeneic deletion strains exhibit wild-type growth kinetics in TSB.
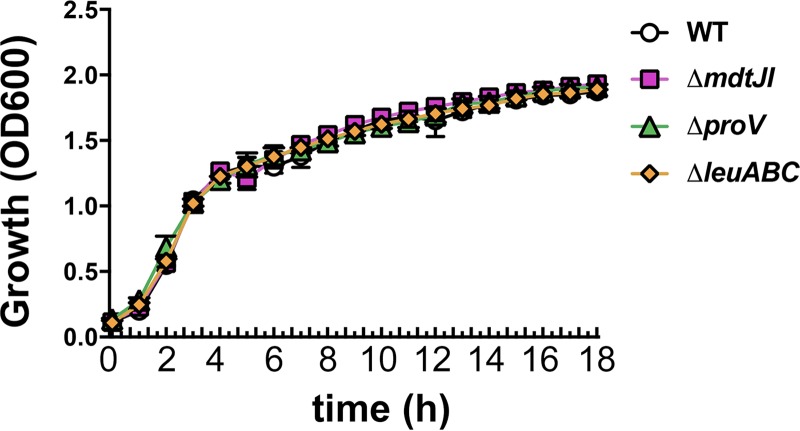
Our K. pneumoniae gene deletion strains were assessed for general growth defects in nutrient-rich media to ensure that the decreased CFU counts that we observed in vivo were not a consequence of generalized growth defects. In order to address this question, the growth of the KPPR1 WT and engineered gene deletion strains were measured in tryptic soy broth (TSB), the medium used to culture the bacteria prior to inoculation into mice. As shown in Fig. 6, the growth kinetics of all deletion strains closely mirrored those of the WT strain. We were also interested in exploring the impact that deletion of these genes had on the ability of K. pneumoniae to grow in the presence of Survanta. However, both K. pneumoniae MGH 78578 and KPPR1 failed to effectively utilize Survanta as a nutrient source in a range of media, including lysogeny broth (LB), TSB, R2A, MOPS, and M63 (data not shown). Specifically, addition of Survanta to either these rich or minimal medium formulations did not result in an increase or a decrease of the CFU counts compared to those in the media without Survanta.
FIG 6.
KPPR1 isogenic deletion strains exhibit wild-type growth kinetics in TSB. The growth of the KPPR1 WT and gene deletion strains in TSB was measured via measurement of the OD600 over an 18-h period. The growth curves shown are representative of those from three independent experiments, with error bars indicating standard deviation.
Some Survanta-induced transcripts are expressed in response to specific lung surfactant components.
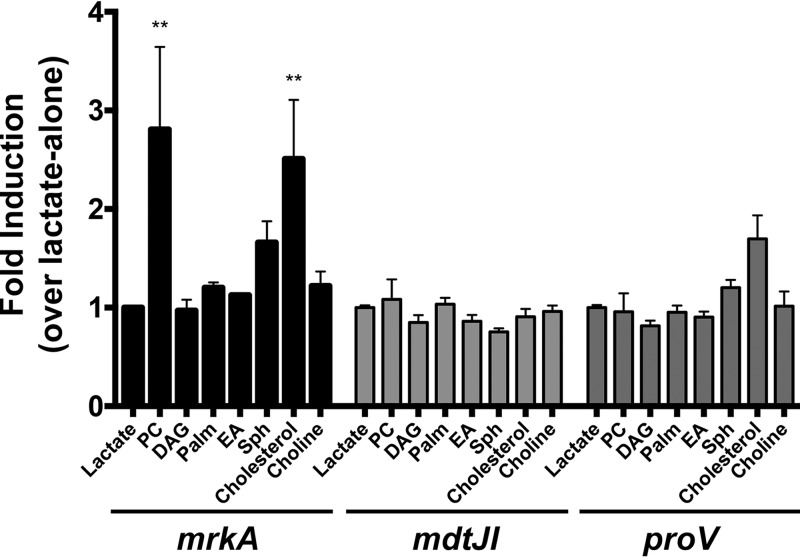
An additional goal of this study was to identify the molecules within lung surfactant that promote K. pneumoniae virulence gene expression. The ability of individual constituents of lung surfactant to stimulate mrkA, proV, and mdtJ transcription was examined through quantitative RT-PCR. For these experiments, RNA was collected from K. pneumoniae MGH 78578 cells grown in MOPS minimal medium and subsequently exposed to the individual components found within lung surfactant or lactate as a control. The compounds tested included phosphatidylcholine, diacylglycerol, palmitate, sphingosine, and cholesterol, in addition to choline and ethanolamine, which have previously been shown to induce fimbria expression in enterohemorrhagic E. coli (65). Exposure to cholesterol and phosphatidylcholine stimulated the transcription of mrkA, but none of the individual compounds tested significantly induced the transcription of mdtJ or proV (Fig. 7).
FIG 7.
Constituents of lung surfactant stimulate K. pneumoniae gene expression. Gene induction assays were performed for 4 h with K. pneumoniae MGH 78578 in MOPS minimal medium containing lactate and individual compounds found within PS. RNA collected from these inductions was then used for quantitative RT-PCR, with the raw transcript values being normalized to those for Kpn_04184. The data shown encompass those from three separate experiments. Statistical analysis was performed via two-way analysis of variance and Dunnett's multiple-comparison test, using the uninduced (lactate-alone) condition as the comparator. For this analysis, the Kpn_04184-adjusted transcript values under the uninduced (lactate-alone) condition were first set equal to 1 for comparison. Statistical significance is depicted as follows: **, P < 0.01. PC, phosphatidylcholine; DAG, diacylglycerol; Palm, palmitate; EA, ethanolamine; Sph, sphingosine.
Surfactant-induced biofilm formation is mediated by type 3 fimbriae.
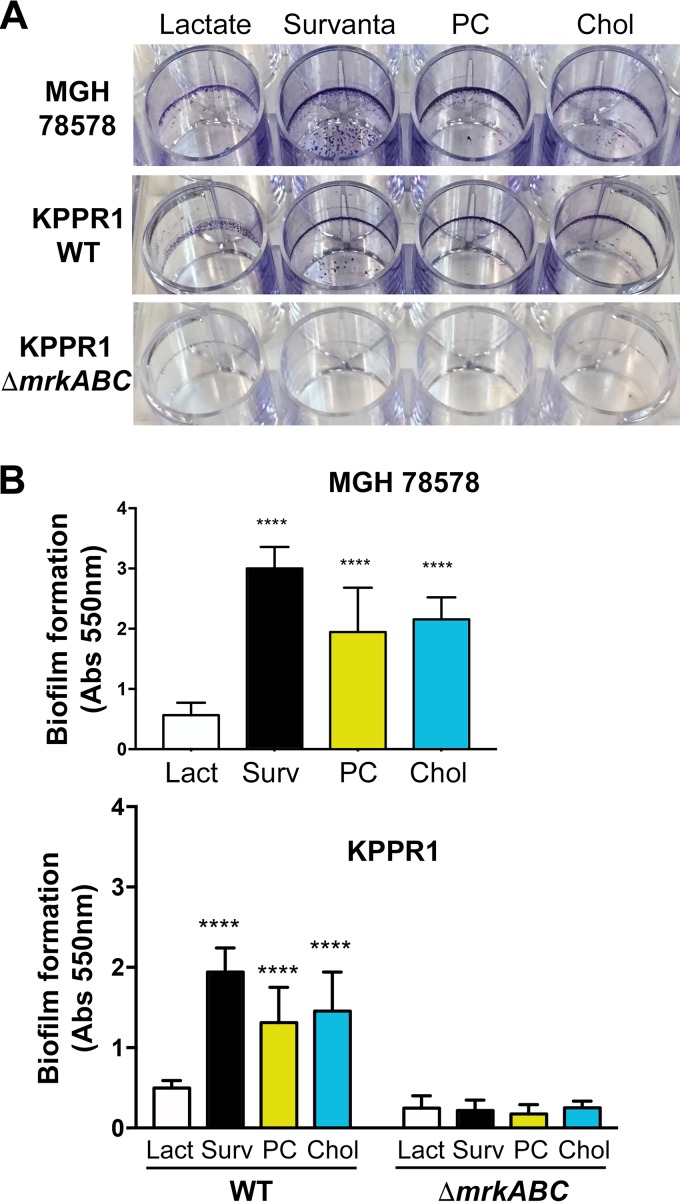
Both our microarrays and subsequent qRT-PCR revealed that the type 3 fimbria gene cluster (mrkABCDF) is expressed by K. pneumoniae MGH 78578 and KPPR1 following exposure to lung surfactant (Fig. 1 and 2 and Table 1). In addition, our gene induction experiments indicated that two constituents of lung surfactant, phosphatidylcholine and cholesterol, induced transcription from the mrkA promoter (Fig. 7). We were therefore curious to determine if these observations were reflected through increased biofilm production. To address this question, we cultured K. pneumoniae MGH 78578, the KPPR1 WT, and KPPR1 ΔmrkABC in minimal medium in the presence and absence of Survanta, phosphatidylcholine, or cholesterol and then on the following day quantified the resultant biofilm material that adhered to the plastic culture wells through a crystal violet staining assay.
As shown in Fig. 8A, exposure to Survanta, phosphatidylcholine, and cholesterol resulted in significant increases in biofilm production in K. pneumoniae MGH 78578 and KPPR1. The biofilms generated by KPPR1 under these conditions were notably less robust than those produced by MGH 78578. These observations can be explained in part by the hypermucoid phenotype of KPPR1 (cpsK2 serotype [56]) relative to the phenotype of MGH 78578 (cpsK52 serotype [66]), since capsule production is known to negatively impact biofilm formation in K. pneumoniae (67, 68). Furthermore, deletion of the mrkABC fimbria genes in KPPR1 disrupted biofilm formation at the air-liquid interface in the presence of lung surfactant and resulted in a substantial reduction in adhered biofilm material compared to that for the WT strain under every condition tested (Fig. 8A). These data indicate that lung surfactant-induced biofilm formation is primarily mediated by type 3 fimbriae.
FIG 8.
Type 3 fimbriae mediate biofilm formation in response to lung surfactant. K. pneumoniae MGH 78578, KPPR1, and KPPR1 ΔmrkABC were cultured in MOPS minimal medium containing 20 mM lactate in the presence and absence of Survanta, phosphatidylcholine (PC), and cholesterol (Chol). After 18 h, the extracellular material remaining adhered to the culture dish was stained with 0.1% crystal violet. (A) Representative crystal violet-stained biofilms generated by each strain under the culturing conditions described above. (B) Biofilm-adhered crystal violet was solubilized with 30% glacial acetic acid and quantified through measuring the absorbance at 550 nm. The data shown are the summary of those from four individual experiments that were performed in technical triplicate, with error bars representing standard deviations. Lact, lactate; Surv, Survanta. Statistical analysis was performed using the MOPS-lactate condition of each strain as the comparator via one-way analysis of variance and Dunnett's multiple-comparison test for K. pneumoniae MGH 78578 and two-way analysis of variance with Sidak's multiple-comparison test for the KPPR1 WT and KPPR1 ΔmrkABC. Statistical significance is specified as follows: ****, P < 0.0001.
DISCUSSION
Our understanding of the genetic factors influencing Klebsiella pneumoniae pathogenesis has significantly improved in recent years (1, 9). In vivo genetic screens and deep sequencing have been particularly effective in identifying genes associated with K. pneumoniae fitness during infection in a range of tissue types (42, 69–72). Bachman and colleagues recently applied this methodology to uncover numerous K. pneumoniae genes that contribute to pathogenesis within the lung (60). Despite these advances, there is still much that we do not understand regarding the role that K. pneumoniae's response to the host environment plays in shaping colonization and pathogenesis.
Lung surfactant serves as an initial point of contact for inhaled bacteria entering the lung, particularly those in small aerosol droplets, and likely contains molecular cues that influence colonization and pathogenesis. Our group has demonstrated the utility of the lung surfactant preparation Survanta for dissecting host-lung pathogen interactions in P. aeruginosa. We previously showed that lung surfactant leads to induction of transcripts involved in the detection of sphingosine and the metabolism of choline and that both of these pathways are required for P. aeruginosa survival in a mouse model of acute pneumonia (23, 24). We also showed that the utilization of phosphatidylcholine metabolites in lung surfactant by P. aeruginosa promoted virulence factor expression (25) and directly contributed to the loss of surfactant function during murine infection (73).
In this study, we expanded on our previous efforts with P. aeruginosa and characterized the transcriptional changes within Klebsiella pneumoniae MGH 78578 resulting from exposure to Survanta. We observed numerous alterations within the K. pneumoniae transcriptome that likely promote colonization, adaptation to the host, and virulence in vivo. Notable transcripts expressed by K. pneumoniae under these conditions included genes involved in capsule synthesis, LPS modification, antibiotic resistance, and biofilm formation (Fig. 1 and 2). Furthermore, a sizeable fraction of the transcripts identified through this work indicates that the lipid-rich environment of lung surfactant invokes significant membrane, cytosolic, and oxidative stress in K. pneumoniae (Fig. 1B and Table 1). These results parallel our earlier findings in P. aeruginosa (23, 25) and support similar observations in Staphylococcus aureus (26) suggesting that lung surfactant likely promotes the expression of virulence and stress-related genes in a range of lung pathogens.
Lung surfactant-induced transcripts contribute to K. pneumoniae survival during acute murine pneumonia.
We also demonstrated that lung surfactant-induced transcripts contribute to K. pneumoniae survival and the resulting inflammation during acute pneumonia. For these experiments, we focused on metabolism-related genes induced by surfactant.
Our interest in BCAA synthesis and the role that these genes play in bacterial fitness during infection stems from our observation that lung surfactant specifically stimulates transcription of the leucine synthetic operon (leuABCD) in K. pneumoniae. In contrast, the expression of genes for other amino acid anabolic pathways was not altered by lung surfactant under these conditions. The mechanism driving this induction is unclear, but lung surfactant metabolism by K. pneumoniae could invoke a specific, previously unknown need for increased leucine synthesis. The ability to synthesize BCAAs during infection is known be critical for the survival and virulence of several bacterial lung pathogens, given its scarcity in the lung environment (60, 74–76). The necessity of BCAA synthesis for K. pneumoniae during pulmonary infection was recently highlighted through an in vivo transposon mutant screen that recognized that ilvADE and leuABCD gene disruption mutants displayed competitive fitness defects in the murine lung (60). Results from our mouse infections with an engineered leucine auxotroph of K. pneumoniae support these earlier findings and confirm that BCAA biosynthesis is required for both fitness and survival in the absence of competition during lung infection (Fig. 3), suggesting that, like in other bacterial lung pathogens, the loss of leucine synthesis is deleterious to survival in the lungs.
Polyamines have been recognized to be significant mediators of bacterial virulence and often have pleiotropic effects on pathogenesis (63, 77). Within enteric species, the accumulation of putrescine and spermidine has been shown to promote biofilm formation in Yersinia pestis, type 3 secretion system expression in Salmonella Typhimurium, and increased resistance to reactive oxygen species in Shigella flexneri during macrophage infection (78–80). Surprisingly, however, the influence of polyamines on K. pneumoniae survival had not previously been explored. Here, we have shown that deletion of the genes encoding the spermidine and putrescine efflux pump mdtJI (35, 62) resulted in a significant defect in K. pneumoniae survival relative to that of the parental WT strain in our murine model of pneumonia (Fig. 4). We propose two potential explanations for these observations. First, putrescine and spermidine are present on the outer membranes of enteric species and have been shown to alter membrane permeability through modifying the charge and shape of porins in E. coli (81, 82). Likewise, the presence of these polyamines on the outer membrane surface of P. aeruginosa has been shown to protect against oxidative stress and antibiotic-mediated killing (83). Therefore, MdtJI-mediated polyamine efflux could similarly facilitate resistance against oxidative killing in K. pneumoniae. Second, the polyamines secreted by bacteria and fungi have been shown to interfere with the innate immune response by disrupting polymorphonuclear leukocyte (PMN) function (84–88).
Our lung surfactant lipid induction experiments failed to reveal any individual components within surfactant that stimulated transcription of mdtJI (Fig. 7). The expression of this pump is primarily regulated by the intracellular concentration of putrescine (35). However, transcription of mdtJI has also been shown to be stimulated by deoxycholate and bile salts in S. flexneri (35), suggesting that this efflux pump could also be induced by membrane stress or other environmental cues. We predict that the expression of mdtJI in K. pneumoniae under these conditions could be a consequence of either membrane stress or the metabolism of multiple components within lung surfactant.
The ProU (proVWX) ABC transporter has been well characterized in E. coli and S. Typhimurium and functions in the uptake of the osmoprotectant glycine betaine under periods of osmotic stress (43, 64). Phosphatidylcholine is the most abundant phospholipid within lung surfactant and serves as a vital precursor of glycine betaine for P. aeruginosa, the accumulation of which is required for bacterial survival in the lung (24). The survival defect that we observed in the K. pneumoniae ΔproV strain in our acute murine pneumonia model is in close agreement with these earlier findings and suggests that the ability to obtain glycine betaine from phosphatidylcholine is likely important for other Gram-negative respiratory pathogens as well (Fig. 5). Host-derived glycine betaine has additionally been shown to promote K. pneumoniae success at other sites of infection. An in vivo screen previously revealed that proV gene disruption mutants displayed a competitive fitness defect in the colon and liver (42), indicating that glycine betaine likely serves as a preferred osmoprotectant for K. pneumoniae during infection.
It is important to note that not all surfactant-induced transcripts expressed by K. pneumoniae contribute to bacterial fitness during lung infection. The products of the six-gene Kpn_02053-Kpn_02057 operon are predicted to function in the uptake and metabolism of short-chain fatty acids (28, 29), and these genes represent the most highly induced transcripts expressed by K. pneumoniae in response to lung surfactant (Fig. 1 and Table 1). Despite the dramatic increase in transcription of this operon in response to lung surfactant, the deletion strain exhibited no defect in bacterial lung burden compared to the WT strain at 24 h postinoculation. Similar results were also observed in a ΔfadBA strain, indicating that the metabolism of fatty acids within lung surfactant does not directly contribute to K. pneumoniae fitness during acute pneumonia (data not shown). The lack of a phenotype for these highly expressed transcripts is not unexpected, as there is no evidence of a direct relationship between gene expression and fitness phenotype in bacterial lung infections to date (89).
Lung surfactant promotes type 3 fimbria expression and biofilm formation in K. pneumoniae.
Exposure to lung surfactant induced type 3 fimbria-mediated biofilm formation in K. pneumoniae MGH 78578 and KPPR1 (Fig. 8). Type 3 fimbriae (Mrk fimbriae) have been extensively studied in K. pneumoniae and facilitate cell adhesion to a range of biotic and abiotic substrates, including type IV and type V collagen, silicone, and hard plastics (90–93). Although type 3 fimbriae are not directly involved in K. pneumoniae virulence, their requirement for colonization and persistence in catheter-associated urinary tract infections (CAUTI) has been demonstrated by multiple groups (94, 95). The transcriptional regulation of type 3 fimbria expression in K. pneumoniae is complex and governed by multiple integrated regulatory networks, including being dependent on the coordinated activities of MrkH and MrkI in response to the intracellular accumulation of the secondary messenger cyclic-di-GMP (96, 97). Surprisingly, the environmental signals and regulatory networks acting upstream of MrkHI that drive type 3 fimbria expression are largely unknown, particularly in the context of infection. Recent reports have identified iron- and oxidative stress-responsive transcription regulators that modulate mrk fimbria expression (98–100), and Chen et al. also identified bile salts to be stimulators of type 3 fimbria-mediated biofilm formation (101). Here, we expand on these previous findings and report that at least two components of lung surfactant, phosphatidylcholine and cholesterol, promote type 3 frimbria transcription and biofilm formation in K. pneumoniae (Fig. 7 and 8).
Conclusions.
In summary, we characterized the transcriptional response of K. pneumoniae MGH 78578 to the lung surfactant preparation Survanta. This work revealed numerous transcripts expressed by K. pneumoniae in response to lung surfactant that reflect metabolic adaptation, stress resistance, virulence, and host colonization. We also demonstrated that some surfactant-induced transcripts contribute to bacterial survival in vivo in a mouse model of acute pneumonia. Through this effort we confirmed the necessity of BCAA synthesis to K. pneumoniae success during infection and provided novel evidence suggesting that glycine betaine uptake and polyamine efflux also contribute to Klebsiella survival during respiratory tract infection. Finally, we identified multiple components within lung surfactant that stimulate type 3 fimbria-mediated biofilm formation. This study provides novel insight into the interactions occurring between K. pneumoniae and the host at an important infection site. This work, together with our previous studies in P. aeruginosa, highlights the utility of using lung surfactant to uncover important aspects of host-lung pathogen interactions in vitro.
MATERIALS AND METHODS
Bacterial strains and compounds.
K. pneumoniae KPPR1 (ATCC 43816) and K. pneumoniae MGH 78578 (ATCC 700721) were maintained on lysogeny broth (LB), Lennox formulation, supplemented with 200 μg/ml of hygromycin B when appropriate. All cloning steps were performed with E. coli DH5α λpir, while E. coli S17-1 λpir was used for conjugation with K. pneumoniae. Both E. coli strains were maintained in LB, supplemented with 150 μg/ml of hygromycin B when appropriate. The strains and plasmids used in this study are described in Table 2. The purified bovine pulmonary surfactant preparation Survanta (Beractant; AbbVie, Lake Bluff, IL) was utilized for our surfactant-response microarrays and biofilm experiments. Survanta is an organic extraction of lung surfactant from cows and as such is missing the polar surfactant proteins involved in pulmonary defense (SP-A and SP-D) as well as most antimicrobial peptides (including defensins) and antimicrobial proteins present in the lung lining fluid (e.g., lysozyme); thus, it is composed of the lipids naturally present in lung surfactant along with the hydrophobic proteins SP-B and SP-C. Because it is an organic extraction product, physiological concentrations of salts and dissolved polar compounds are added back by dilution of this product into minimal medium. The lung surfactant constituents used in our gene induction assays were purchased from Avanti Polar Lipids (Alabaster, AL) and Sigma-Aldrich (St. Louis, MO).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| K. pneumoniae strains | ||
| GGW112 | MGH 78578 | ATCC 700721 |
| GGW231 | KPPR1 | ATCC 43816 |
| GGW178 | ΔproV in GGW231 | This study |
| GGW180 | ΔleuABCD in GGW231 | This study |
| GGW192 | ΔmrkABC in GGW231 | This study |
| GGW194 | ΔmdtJI in GGW231 | This study |
| E. coli strains | ||
| DH5α λpir | sup E44 ΔlacU169 ϕ80Δ(lacZ)M15 recA1 endA1 hsdR17 thi-1 gyrA96 relA λpir | Bio-Rad |
| NEB5α | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 ϕ80Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | NEB |
| S17-1 λpir | thi pro hsdR negative hsdM positive ΔrecA RP4-2::TcMu-Km::Tn7 λpir | 112 |
| GGW166 | pGW74 in S17-1 λpir | This study |
| GGW168 | pGW76 in S17-1 λpir | This study |
| GGW172 | pGW78 in S17-1 λpir | This study |
| GGW186 | pGW79 in S17-1 λpir | This study |
| Plasmids | ||
| pGW65 | Suicide vector, R6Kγ ori Hmr sacB | This study |
| pGW74 | proV-SOE in pGW65 | This study |
| pGW76 | leuABCD-SOE in pGW65 | This study |
| pGW78 | mdtJI-SOE in pGW65 | This study |
| pGW79 | mrkABC-SOE in pGW65 | This study |
SOE, splice overlap extension products used for gene deletion.
Construction of K. pneumoniae gene deletion strains.
Gene deletion strains in K. pneumoniae KPPR1 were generated through allelic exchange facilitated by the suicide vector pGW65. To create pGW65, pMQ310 and pMQ30 (102, 103) were first digested with NcoI and KpnI (New England BioLabs, Ipswich, MA). The 3.9-kbp fragment of pMQ310 carrying the hygromycin B resistance cassette and R6Kγ origin and the 4.6-kbp fragment of pMQ30 carrying the sacB counterselectable marker were gel extracted using Thermo Fisher's GeneJET kit (Waltham, MA) and subsequently ligated together before transformation into chemically competent DH5α λpir cells. Gene deletion constructs were engineered into this vector using the molecular cloning methodology previously described with pMQ30 (103, 104). Briefly, ∼1-kbp fragments immediately upstream and downstream of the gene (or genes) targeted for deletion were amplified using the primers listed in Table S2 in the supplemental material. For each deletion construct, tailed primers were used to facilitate the fusion of each fragment via overlap extension PCR as well as ligation into pGW65 through incorporated flanking restriction sites. The ligation reaction mixtures were then chemically transformed into DH5α λpir cells, and transformants were selected for on LB supplemented with 150 μg/ml of hygromycin B. Plasmid DNA was harvested from these colonies by use of a miniprep kit (Qiagen) and verified by restriction digestion.
Deletion constructs were subsequently transformed into chemically competent E. coli S17-1 λpir cells and mobilized into K. pneumoniae KPPR1 via conjugation (105). Following overnight incubation at 37°C, merodiploids were selected by plating on MOPS (morpholinepropanesulfonic acid) minimal agar medium supplemented with 200 μg/ml of hygromycin B and 25 mM sodium pyruvate. To select for the ΔleuABCD strain, 0.5% Casamino Acids was added to this medium. KPPR1 merodiploids that arose the next day were then restreaked onto this medium to ensure that E. coli S17-1 λpir cells were not carried over. A second round of recombination was then permitted by first growing hygromycin B-resistant colonies overnight in LB containing 200 μg/ml of the antibiotic, diluting the overnight culture 1:500, and growing the culture to mid-log phase in LB in the absence of hygromycin B. Dilutions of this culture were then plated on low-salt LB agar containing 6% sucrose and incubated overnight at 25°C, as suggested previously (105). Sucrose-resistant colonies arising 24 h later were screened for deletion of the gene(s) of interest via PCR with the primers listed in Table S2.
Growth conditions and RNA purification for microarrays/qRT-PCR.
K. pneumoniae MGH 78578 was grown overnight at 37°C in modified MOPS minimal medium (106, 107) supplemented with 25 mM lactate and 5 mM d-glucose. On the following day, cells were collected by centrifugation, washed with 1 ml of MOPS medium, and resuspended in MOPS medium containing 4 mM lactate to achieve an optical density at 600 nm (OD600) of 0.6. These cultures were then mixed 1:1 with MOPS medium containing 4 mM lactate or the same medium supplemented with Survanta (AbbVie, Lake Bluff, IL) at a dilution of 1:50 to reflect the physiological concentration of pulmonary surfactant in the airway surface liquid (15 mg/ml). Cultures were incubated at 37°C with shaking at 170 rpm for 4 h, at which point the cells were harvested via centrifugation, immediately lysed in ∼85°C RNAzol reverse transcriptase (Sigma-Aldrich, St. Louis, MO), and frozen at −80°C. RNA extractions were first performed using Zymo Research's RNA miniprep kit (Irvine, CA) following the manufacturer's provided protocol. The resulting RNA was then incubated for 1 h with DNase I (NEB) before being repurified using RNeasy columns (Qiagen) to remove small RNAs in preparation for their use in the microarrays, as we have done previously (23, 108). The quality of each RNA sample was then assessed via an Agilent BioAnalyzer and quantified through a Qubit fluorometer.
Survanta microarray methodology.
Microarray analyses were performed by the UVM Advanced Genome Technology Core using a custom Affymetrix chip containing probes specific to the genomes of Klebsiella pneumoniae MGH 78578, Stenotrophomonas maltophilia K279A, Burkholderia thailandensis E264, and Pseudomonas aeruginosa PA14 (109). Analyses with the arrays were performed in biological duplicate, with RNA being collected from two independent Survanta induction experiments that were performed on separate days. K. pneumoniae cDNA hybridization was performed simultaneously with a 1:1 mixture of Survanta-induced Stenotrophomonas maltophilia K279A cDNA (cultured under the same conditions) per the manufacturer's recommendation. Each condition was analyzed in duplicate, with the intensity of the probes for each gene being averaged into the intensity of one probe using the Affymetrix Expression Console and Transcriptome Analysis Console software packages (version 3.0). Surfactant-altered transcripts were identified as those exhibiting at least a 2.5-fold change in signal between the two conditions, as determined using robust multiarray average (RMA) analysis, and a P value of <0.05.
Quantitative RT-PCR.
Total RNA was prepared from three additional Survanta inductions with K. pneumoniae MGH 78578 as described above. Twenty nanograms of RNA from each sample was then utilized as the template for cDNA synthesis using SuperScript IV reverse transcriptase and random hexamers (Thermo Fisher) per the manufacturer's instruction. Quantitative PCR was performed using the resulting cDNA in technical duplicate with the primers listed in Table S1 and NEB's Q5 2× master mix supplemented with SYBR green I nucleic acid gel stain (Thermo Fisher) at a concentration of 0.2×, as we have done previously (108). A standard curve dilution series was generated for each primer set to determine transcript abundance (110). The values for each reaction were normalized to those for Kpn_04184, which exhibited no change in expression between conditions in the Survanta microarrays. The fold change in expression for each transcript was determined by dividing the normalized surfactant-exposed values by their corresponding control condition values. The absence of reverse transcriptase during cDNA synthesis resulted in no product from any primer set when the isolated RNA was used.
Mouse infections.
Mouse infections were performed as previously described (57, 73). Briefly, K. pneumoniae KPPR1 WT and isogenic deletion strains were grown in TSB overnight, and the growth was normalized by the OD600, harvested via centrifugation, washed in 2 ml of phosphate-buffered saline (PBS), and finally, resuspended in PBS to achieve 2 × 103 CFU per 50 μl. For each strain, the actual input inoculum was determined by serial dilution plating on LB agar. Eight- to 10-week-old adult male C57BL/6J mice (The Jackson Laboratory, Detroit, MI) were briefly anesthetized with isoflurane and inoculated with 2 × 103 CFU of either the KPPR1 WT or isogenic deletion strains through oropharyngeal aspiration. Twenty-four hours later, the mice were euthanized with sodium pentobarbital, delivered through intraperitoneal injection. Bronchoalveolar lavage fluid was then collected, and the lungs were then quickly removed, placed into 1 ml of cold PBS, and immediately homogenized.
Serial dilutions of the resulting lung homogenates were plated on LB agar to determine the bacterial burden by counting the number of CFU. The white blood cell content within the bronchoalveolar lavage fluid was enumerated manually. Infections were performed at least three times with 3 to 4 mice per strain per experiment. In each case, paired infections were performed with one gene deletion strain and the parental WT strain for comparison of the number of lung CFU. The protocol for animal infection was approved by the University of Vermont Institutional Animal Care and Use Committee, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines (Animal Welfare Assurance A3301-01).
Growth assays.
Growth assays were conducted with K. pneumoniae KPPR1 WT and isogenic deletion strains as we have done previously with P. aeruginosa (104). Briefly, the KPPR1 WT and our isogenic deletion strains were grown overnight at 37°C on a roller drum in MOPS minimal medium supplemented with 20 mM lactate and 5 mM d-glucose. In the case of KPPR1 ΔleuABC, 0.5% Casamino Acids was added to this medium to permit growth. On the following day, cells were collected via centrifugation, washed with 1 ml MOPS medium, and resuspended in TSB at a final optical density of 0.05 OD600 unit. Growth assays were performed three times, each with technical triplicates, in a 48-well tissue culture plate, and growth was determined by measurement of the OD600 using a Synergy 2 plate reader (Biotek). Growth assays in Survanta were conducted as described above, except that growth was quantified by serial dilution plating, as Survanta is a colloidal suspension and prevents growth assessment by measurement of the OD600.
Gene induction assays with components of lung surfactant.
To identify the transcript-inducing molecules within lung surfactant, quantitative RT-PCR was performed on K. pneumoniae MGH 78578 RNA collected from cells exposed to 1 mM phosphatidylcholine, sphingosine, cholesterol, diacylglycerol, palmitate, choline, or ethanolamine or no compound as a control. For these experiments, K. pneumoniae was first grown overnight in MOPS minimal medium as described above. On the following day, cells were collected by centrifugation, washed in 1 ml MOPS, and resuspended in MOPS–20 mM lactate to achieve a final OD600 of 0.3. One-milliliter aliquots of this culture were then added to a plastic culture dish with wells containing these compounds, deposited via the evaporation of ethanol, and incubated for 4 h at 37°C and 170 rpm. Following the induction period, RNA was purified from these cells, cDNA was synthesized, and quantitative PCR was performed as described above.
Biofilm assay.
K. pneumoniae MGH 78578, the KPPR1 WT, and KPPR1 ΔmrkABC were grown overnight at 37°C on a roller drum in MOPS minimal medium supplemented with 20 mM sodium pyruvate and 5 mM glucose. On the following day, cells were collected by centrifugation, washed in 1 ml of MOPS medium, and adjusted to an OD600 of 0.1. Each strain was then added 1:1 to MOPS medium containing 20 mM sodium lactate, in addition to the same medium supplemented with Survanta, to achieve a final surfactant dilution of 1:50. The OD600-adjusted cultures were also diluted 1:1 in the same medium (MOPS, 20 mM sodium lactate) and added to the wells of a 48-well dish containing phosphatidylcholine or cholesterol that had been deposited the night prior through ethanol evaporation. These cultures were incubated for 18 h at 37°C and agitated at 170 rpm to loosely reflect the continuous aeration and mixing of surfactant that occurs within the lung. Following the incubation, the cell suspension was removed from the wells and the remaining biofilm material was stained using 0.1% crystal violet, followed by a water rinse and solubilization of the remaining crystal violet in 30% acetic acid (111). Biofilm was quantified by measuring the A550 using a Biotek Synergy 2 plate reader. This experiment was performed four times with technical triplicates of each experiment.
Statistical analysis and data visualization.
All statistical analyses and figure generation were performed using GraphPad Prism software (version 7.0), unless otherwise noted. Microarray analysis and statistical assessment were performed through RMA using Affymetrix's Expression Console and Transcriptome Analysis Console software packages (version 3.0) as described above. Gene functional classification was done by manually combining related Gene Ontology, Clusters of Orthologous Groups, and KEGG predictions into more general functions.
Accession number(s).
The array data have been submitted to the GEO database under accession number GSE110628.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by AI103003 to M.J.W., AI117069 to M.J.W. and B.T.S., pilot funding through the Vermont Center for Immunology and Infectious Diseases to M.J.W. (GM118228), and internal funding from the Department of Medicine, Larner College of Medicine, to B.T.S. G.G.W. was supported by fellowship T32 HL076122, awarded through the Vermont Lung Center.
The funders had no role in experimental design or the collection and interpretation of data.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00135-18.
REFERENCES
- 1.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown C, Seidler RJ. 1973. Potential pathogens in the environment: Klebsiella pneumoniae, a taxonomic and ecological enigma. Appl Microbiol 25:900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsen JM, Spindler JA, Blosser RO. 1974. Characterization of Klebsiella isolates from natural receiving waters and comparison with human isolates. Appl Microbiol 28:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edberg SC, Piscitelli V, Cartter M. 1986. Phenotypic characteristics of coliform and noncoliform bacteria from a public water supply compared with regional and national clinical species. Appl Environ Microbiol 52:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung CP, Lin YT, Lin JC, Chen TL, Yeh KM, Chang FY, Chuang HC, Wu HS, Tseng CP, Siu LK. 2012. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis 18:1322–1325. doi: 10.3201/eid1808.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selden R, Lee S, Wang WL, Bennett JV, Eickhoff TC. 1971. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med 74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 9.Broberg CA, Palacios M, Miller VL. 2014. Klebsiella: a long way to go towards understanding this enigmatic jet-setter. F1000Prime Rep 6:64. doi: 10.12703/P6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehn BM. 2013. “Nightmare” bacteria on the rise in US hospitals, long-term care facilities. JAMA 309:1573–1574. doi: 10.1001/jama.2013.2922. [DOI] [PubMed] [Google Scholar]
- 12.Iredell J, Brown J, Tagg K. 2016. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ 352:h6420. doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez GV, Master RN, Clark RB, Fyyaz M, Duvvuri P, Ekta G, Bordon J. 2013. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998-2010. Emerg Infect Dis 19:133–136. doi: 10.3201/eid1901.120310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapp RP, Urban C. 2012. Klebsiella pneumoniae carbapenemases in Enterobacteriaceae: history, evolution, and microbiology concerns. Pharmacotherapy 32:399–407. doi: 10.1002/j.1875-9114.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 15.Antoniadou A, Kontopidou F, Poulakou G, Koratzanis E, Galani I, Papadomichelakis E, Kopterides P, Souli M, Armaganidis A, Giamarellou H. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother 59:786–790. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuizen R, Nag K, Orgeig S, Possmayer F. 1998. The role of lipids in pulmonary surfactant. Biochim Biophys Acta 1408:90–108. doi: 10.1016/S0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 17.Goerke J. 1998. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta 1408:79–89. doi: 10.1016/S0925-4439(98)00060-X. [DOI] [PubMed] [Google Scholar]
- 18.Chiba H, Piboonpocanun S, Mitsuzawa H, Kuronuma K, Murphy RC, Voelker DR. 2006. Pulmonary surfactant proteins and lipids as modulators of inflammation and innate immunity. Respirology 11(Suppl):S2–S6. doi: 10.1111/j.1440-1843.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 19.McCormack FX, Whitsett JA. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 109:707–712. doi: 10.1172/JCI0215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Mallampalli RK. 2015. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc 12:765–774. doi: 10.1513/AnnalsATS.201411-507FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernhard W, Hoffmann S, Dombrowsky H, Rau GA, Kamlage A, Kappler M, Haitsma JJ, Freihorst J, von der Hardt H, Poets CF. 2001. Phosphatidylcholine molecular species in lung surfactant: composition in relation to respiratory rate and lung development. Am J Respir Cell Mol Biol 25:725–731. doi: 10.1165/ajrcmb.25.6.4616. [DOI] [PubMed] [Google Scholar]
- 22.Wright JR, Clements JA. 1987. Metabolism and turnover of lung surfactant. Am Rev Respir Dis 136:426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 23.LaBauve AE, Wargo MJ. 2014. Detection of host-derived sphingosine by Pseudomonas aeruginosa is important for survival in the murine lung. PLoS Pathog 10:e1003889. doi: 10.1371/journal.ppat.1003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wargo MJ. 2013. Choline catabolism to glycine betaine contributes to Pseudomonas aeruginosa survival during murine lung infection. PLoS One 8:e56850. doi: 10.1371/journal.pone.0056850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. 2009. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to choline catabolites. Infect Immun 77:1103–1111. doi: 10.1128/IAI.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii K, Adachi T, Yasukawa J, Suzuki Y, Hamamoto H, Sekimizu K. 2014. Induction of virulence gene expression in Staphylococcus aureus by pulmonary surfactant. Infect Immun 82:1500–1510. doi: 10.1128/IAI.01635-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 28.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL. 2017. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. 2015. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price MN, Arkin AP. 2017. PaperBLAST: text mining papers for information about homologs. mSystems 2(4):e00039-. doi: 10.1128/mSystems.00039-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry MF, Cronan JE Jr. 1991. Escherichia coli transcription factor that both activates fatty acid synthesis and represses fatty acid degradation. J Mol Biol 222:843–849. doi: 10.1016/0022-2836(91)90574-P. [DOI] [PubMed] [Google Scholar]
- 32.Wargo MJ, Hogan DA. 2009. Identification of genes required for Pseudomonas aeruginosa carnitine catabolism. Microbiology 155:2411–2419. doi: 10.1099/mic.0.028787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundgren BR, Harris JR, Sarwar Z, Scheel RA, Nomura CT. 2015. The metabolism of (R)-3-hydroxybutyrate is regulated by the enhancer-binding protein PA2005 and the alternative sigma factor RpoN in Pseudomonas aeruginosa PAO1. Microbiology 161:2232–2242. doi: 10.1099/mic.0.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara S, Kato K, Asada K, Kumagai H, Suzuki H. 2010. A putrescine-inducible pathway comprising PuuE-YneI in which gamma-aminobutyrate is degraded into succinate in Escherichia coli K-12. J Bacteriol 192:4582–4591. doi: 10.1128/JB.00308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leuzzi A, Di Martino ML, Campilongo R, Falconi M, Barbagallo M, Marcocci L, Pietrangeli P, Casalino M, Grossi M, Micheli G, Colonna B, Prosseda G. 2015. Multifactor regulation of the MdtJI polyamine transporter in Shigella. PLoS One 10:e0136744. doi: 10.1371/journal.pone.0136744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umezawa Y, Shimada T, Kori A, Yamada K, Ishihama A. 2008. The uncharacterized transcription factor YdhM is the regulator of the nemA gene, encoding N-ethylmaleimide reductase. J Bacteriol 190:5890–5897. doi: 10.1128/JB.00459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray MJ, Wholey WY, Parker BW, Kim M, Jakob U. 2013. NemR is a bleach-sensing transcription factor. J Biol Chem 288:13789–13798. doi: 10.1074/jbc.M113.454421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karash S, Liyanage R, Qassab A, Lay JO Jr, Kwon YM. 2017. A comprehensive assessment of the genetic determinants in Salmonella Typhimurium for resistance to hydrogen peroxide using proteogenomics. Sci Rep 7:17073. doi: 10.1038/s41598-017-17149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda N, Church GM. 2002. Escherichia coli gene expression responsive to levels of the response regulator EvgA. J Bacteriol 184:6225–6234. doi: 10.1128/JB.184.22.6225-6234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z, Masuda N, Foster JW. 2004. Characterization of EvgAS-YdeO-GadE branched regulatory circuit governing glutamate-dependent acid resistance in Escherichia coli. J Bacteriol 186:7378–7389. doi: 10.1128/JB.186.21.7378-7389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC. 2009. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun 77:2657–2671. doi: 10.1128/IAI.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins CF, Sutherland L, Cairney J, Booth IR. 1987. The osmotically regulated proU locus of Salmonella typhimurium encodes a periplasmic betaine-binding protein. J Gen Microbiol 133:305–310. [DOI] [PubMed] [Google Scholar]
- 44.Shippy DC, Eakley NM, Lauhon CT, Bochsler PN, Fadl AA. 2013. Virulence characteristics of Salmonella following deletion of genes encoding the tRNA modification enzymes GidA and MnmE. Microb Pathog 57:1–9. doi: 10.1016/j.micpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Heroven AK, Nuss AM, Dersch P. 2017. RNA-based mechanisms of virulence control in Enterobacteriaceae. RNA Biol 14:471–487. doi: 10.1080/15476286.2016.1201617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob Agents Chemother 53:1998–2004. doi: 10.1128/AAC.01355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toh SM, Xiong L, Bae T, Mankin AS. 2008. The methyltransferase YfgB/RlmN is responsible for modification of adenosine 2503 in 23S rRNA. RNA 14:98–106. doi: 10.1261/rna.814408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerlach GF, Clegg S, Allen BL. 1989. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol 171:1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy CN. 2014. The role of cyclic di-GMP in regulating type 3 fimbriae: a colonization factor of Klebsiella pneumonia. PhD dissertation. The University of Iowa, Ames, IA. [Google Scholar]
- 50.Pan YJ, Lin TL, Chen CT, Chen YY, Hsieh PF, Hsu CR, Wu MC, Wang JT. 2015. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci Rep 5:15573. doi: 10.1038/srep15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem 76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breazeale SD, Ribeiro AA, Raetz CR. 2002. Oxidative decarboxylation of UDP-glucuronic acid in extracts of polymyxin-resistant Escherichia coli. Origin of lipid A species modified with 4-amino-4-deoxy-l-arabinose. J Biol Chem 277:2886–2896. [DOI] [PubMed] [Google Scholar]
- 53.Shafer WM, Casey SG, Spitznagel JK. 1984. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun 43:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, Karagouni E, Tzelepi E, Daikos GL. 2013. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 57:5144–5146. doi: 10.1128/AAC.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong H, Carter RA, Leiner IM, Tang YW, Chen L, Kreiswirth BN, Pamer EG. 2015. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect Immun 83:3418–3427. doi: 10.1128/IAI.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broberg CA, Wu W, Cavalcoli JD, Miller VL, Bachman MA. 2014. Complete genome sequence of Klebsiella pneumoniae strain ATCC 43816 KPPR1, a rifampin-resistant mutant commonly used in animal, genetic, and molecular biology studies. Genome Announc 2(5):e00924-. doi: 10.1128/genomeA.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, Parsons PE, Dixon AE, Wargo MJ, Littenberg B, Wouters EF, Suratt BT. 2016. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 1:e82101. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ubags ND, Burg E, Antkowiak M, Wallace AM, Dilli E, Bement J, Wargo MJ, Poynter ME, Wouters EF, Suratt BT. 2016. A comparative study of lung host defense in murine obesity models. Insights into neutrophil function. Am J Respir Cell Mol Biol 55:188–200. doi: 10.1165/rcmb.2016-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry CS, Rotman E, Lathem WW, Tyo KE, Hauser AR, Mandel MJ. 2017. Generation and validation of the iKp1289 metabolic model for Klebsiella pneumoniae KPPR1. J Infect Dis 215:S37–S43. doi: 10.1093/infdis/jiw465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bachman MA, Breen P, Deornellas V, Mu Q, Zhao L, Wu W, Cavalcoli JD, Mobley HL. 2015. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 6:e00775-15. doi: 10.1128/mBio.00775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishino K, Yamaguchi A. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol 183:5803–5812. doi: 10.1128/JB.183.20.5803-5812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higashi K, Ishigure H, Demizu R, Uemura T, Nishino K, Yamaguchi A, Kashiwagi K, Igarashi K. 2008. Identification of a spermidine excretion protein complex (MdtJI) in Escherichia coli. J Bacteriol 190:872–878. doi: 10.1128/JB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Martino ML, Campilongo R, Casalino M, Micheli G, Colonna B, Prosseda G. 2013. Polyamines: emerging players in bacteria-host interactions. Int J Med Microbiol 303:484–491. doi: 10.1016/j.ijmm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Stirling DA, Hulton CS, Waddell L, Park SF, Stewart GS, Booth IR, Higgins CF. 1989. Molecular characterization of the proU loci of Salmonella typhimurium and Escherichia coli encoding osmoregulated glycine betaine transport systems. Mol Microbiol 3:1025–1038. doi: 10.1111/j.1365-2958.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 65.Gonyar LA, Kendall MM. 2014. Ethanolamine and choline promote expression of putative and characterized fimbriae in enterohemorrhagic Escherichia coli O157:H7. Infect Immun 82:193–201. doi: 10.1128/IAI.00980-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shu HY, Fung CP, Liu YM, Wu KM, Chen YT, Li LH, Liu TT, Kirby R, Tsai SF. 2009. Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology 155:4170–4183. doi: 10.1099/mic.0.029017-0. [DOI] [PubMed] [Google Scholar]
- 67.Schembri MA, Blom J, Krogfelt KA, Klemm P. 2005. Capsule and fimbria interaction in Klebsiella pneumoniae. Infect Immun 73:4626–4633. doi: 10.1128/IAI.73.8.4626-4633.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang TW, Lam I, Chang HY, Tsai SF, Palsson BO, Charusanti P. 2014. Capsule deletion via a lambda-Red knockout system perturbs biofilm formation and fimbriae expression in Klebsiella pneumoniae MGH 78578. BMC Res Notes 7:13. doi: 10.1186/1756-0500-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 70.Lau HY, Clegg S, Moore TA. 2007. Identification of Klebsiella pneumoniae genes uniquely expressed in a strain virulent using a murine model of bacterial pneumonia. Microb Pathog 42:148–155. doi: 10.1016/j.micpath.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maroncle N, Balestrino D, Rich C, Forestier C. 2002. Identification of Klebsiella pneumoniae genes involved in intestinal colonization and adhesion using signature-tagged mutagenesis. Infect Immun 70:4729–4734. doi: 10.1128/IAI.70.8.4729-4734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Struve C, Forestier C, Krogfelt KA. 2003. Application of a novel multi-screening signature-tagged mutagenesis assay for identification of Klebsiella pneumoniae genes essential in colonization and infection. Microbiology 149:167–176. doi: 10.1099/mic.0.25833-0. [DOI] [PubMed] [Google Scholar]
- 73.Wargo MJ, Gross MJ, Rajamani S, Allard JL, Lundblad LK, Allen GB, Vasil ML, Leclair LW, Hogan DA. 2011. Hemolytic phospholipase C inhibition protects lung function during Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 184:345–354. doi: 10.1164/rccm.201103-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR Jr, Bloom BR. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun 68:2888–2898. doi: 10.1128/IAI.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim GL, Lee S, Luong TT, Nguyen CT, Park SS, Pyo S, Rhee DK. 2017. Effect of decreased BCAA synthesis through disruption of ilvC gene on the virulence of Streptococcus pneumoniae. Arch Pharm Res 40:921–932. doi: 10.1007/s12272-017-0931-0. [DOI] [PubMed] [Google Scholar]
- 76.Subashchandrabose S, LeVeque RM, Wagner TK, Kirkwood RN, Kiupel M, Mulks MH. 2009. Branched-chain amino acids are required for the survival and virulence of Actinobacillus pleuropneumoniae in swine. Infect Immun 77:4925–4933. doi: 10.1128/IAI.00671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah P, Swiatlo E. 2008. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol 68:4–16. doi: 10.1111/j.1365-2958.2008.06126.x. [DOI] [PubMed] [Google Scholar]
- 78.Wortham BW, Oliveira MA, Fetherston JD, Perry RD. 2010. Polyamines are required for the expression of key Hms proteins important for Yersinia pestis biofilm formation. Environ Microbiol 12:2034–2047. doi: 10.1111/j.1462-2920.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jelsbak L, Thomsen LE, Wallrodt I, Jensen PR, Olsen JE. 2012. Polyamines are required for virulence in Salmonella enterica serovar Typhimurium. PLoS One 7:e36149. doi: 10.1371/journal.pone.0036149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbagallo M, Di Martino ML, Marcocci L, Pietrangeli P, De Carolis E, Casalino M, Colonna B, Prosseda G. 2011. A new piece of the Shigella pathogenicity puzzle: spermidine accumulation by silencing of the speG gene [corrected]. PLoS One 6:e27226. doi: 10.1371/journal.pone.0027226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koski P, Vaara M. 1991. Polyamines as constituents of the outer membranes of Escherichia coli and Salmonella typhimurium. J Bacteriol 173:3695–3699. doi: 10.1128/jb.173.12.3695-3699.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iyer R, Delcour AH. 1997. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J Biol Chem 272:18595–18601. doi: 10.1074/jbc.272.30.18595. [DOI] [PubMed] [Google Scholar]
- 83.Johnson L, Mulcahy H, Kanevets U, Shi Y, Lewenza S. 2012. Surface-localized spermidine protects the Pseudomonas aeruginosa outer membrane from antibiotic treatment and oxidative stress. J Bacteriol 194:813–826. doi: 10.1128/JB.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lamster IB, Mandella RD, Zove SM, Harper DS. 1987. The polyamines putrescine, spermidine and spermine in human gingival crevicular fluid. Arch Oral Biol 32:329–333. doi: 10.1016/0003-9969(87)90087-2. [DOI] [PubMed] [Google Scholar]
- 85.Walters JD, Miller TJ, Cario AC, Beck FM, Marucha PT. 1995. Polyamines found in gingival fluid inhibit chemotaxis by human polymorphonuclear leukocytes in vitro. J Periodontol 66:274–278. doi: 10.1902/jop.1995.66.4.274. [DOI] [PubMed] [Google Scholar]
- 86.Walters JD, Chapman KJ. 1995. Polyamines found in gingival fluid enhance the secretory and oxidative function of human polymorphonuclear leukocytes in vitro. J Periodont Res 30:167–171. doi: 10.1111/j.1600-0765.1995.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 87.Mariggio MA, Vinella A, Pasquetto N, Curci E, Cassano A, Fumarulo R. 2004. In vitro effects of polyamines on polymorphonuclear cell apoptosis and implications in the pathogenesis of periodontal disease. Immunopharmacol Immunotoxicol 26:93–101. doi: 10.1081/IPH-120029947. [DOI] [PubMed] [Google Scholar]
- 88.Lasbury ME, Merali S, Durant PJ, Tschang D, Ray CA, Lee CH. 2007. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J Biol Chem 282:11009–11020. doi: 10.1074/jbc.M611686200. [DOI] [PubMed] [Google Scholar]
- 89.Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. 2014. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet 10:e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jagnow J, Clegg S. 2003. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149:2397–2405. doi: 10.1099/mic.0.26434-0. [DOI] [PubMed] [Google Scholar]
- 91.Tarkkanen AM, Allen BL, Westerlund B, Holthofer H, Kuusela P, Risteli L, Clegg S, Korhonen TK. 1990. Type V collagen as the target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol 4:1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 92.Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A. 2003. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res Microbiol 154:9–16. doi: 10.1016/S0923-2508(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 93.Sebghati TA, Korhonen TK, Hornick DB, Clegg S. 1998. Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect Immun 66:2887–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. 2013. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun 81:3009–3017. doi: 10.1128/IAI.00348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stahlhut SG, Struve C, Krogfelt KA, Reisner A. 2012. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol Med Microbiol 65:350–359. doi: 10.1111/j.1574-695X.2012.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson JG, Murphy CN, Sippy J, Johnson TJ, Clegg S. 2011. Type 3 fimbriae and biofilm formation are regulated by the transcriptional regulators MrkHI in Klebsiella pneumoniae. J Bacteriol 193:3453–3460. doi: 10.1128/JB.00286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilksch JJ, Yang J, Clements A, Gabbe JL, Short KR, Cao H, Cavaliere R, James CE, Whitchurch CB, Schembri MA, Chuah ML, Liang ZX, Wijburg OL, Jenney AW, Lithgow T, Strugnell RA. 2011. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog 7:e1002204. doi: 10.1371/journal.ppat.1002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin TH, Tseng CY, Lai YC, Wu CC, Huang CF, Lin CT. 2017. IscR regulation of type 3 fimbriae expression in Klebsiella pneumoniae CG43. Front Microbiol 8:1984. doi: 10.3389/fmicb.2017.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu CC, Lin CT, Cheng WY, Huang CJ, Wang ZC, Peng HL. 2012. Fur-dependent MrkHI regulation of type 3 fimbriae in Klebsiella pneumoniae CG43. Microbiology 158:1045–1056. doi: 10.1099/mic.0.053801-0. [DOI] [PubMed] [Google Scholar]
- 100.Hennequin C, Forestier C. 2009. oxyR, a LysR-type regulator involved in Klebsiella pneumoniae mucosal and abiotic colonization. Infect Immun 77:5449–5457. doi: 10.1128/IAI.00837-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen KM, Chiang MK, Wang M, Ho HC, Lu MC, Lai YC. 2014. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb Pathog 77:89–99. doi: 10.1016/j.micpath.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Kalivoda EJ, Horzempa J, Stella NA, Sadaf A, Kowalski RP, Nau GJ, Shanks RM. 2011. New vector tools with a hygromycin resistance marker for use with opportunistic pathogens. Mol Biotechnol 48:7–14. doi: 10.1007/s12033-010-9342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wargo MJ, Szwergold BS, Hogan DA. 2008. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J Bacteriol 190:2690–2699. doi: 10.1128/JB.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Aartsen JJ, Rajakumar K. 2011. An optimized method for suicide vector-based allelic exchange in Klebsiella pneumoniae. J Microbiol Methods 86:313–319. doi: 10.1016/j.mimet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J Bacteriol 119:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.LaBauve AE, Wargo MJ. 2012. Growth and laboratory maintenance of Pseudomonas aeruginosa. Curr Protoc Microbiol Chapter 6:Unit 6E.1. doi: 10.1002/9780471729259.mc06e01s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Willsey GG, Wargo MJ. 2016. Sarcosine catabolism in Pseudomonas aeruginosa is transcriptionally regulated by SouR. J Bacteriol 198:301–310. doi: 10.1128/JB.00739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.English EL, Schutz KC, Willsey GG, Wargo MJ. 2018. Transcriptional responses of Pseudomonas aeruginosa to potable water and freshwater. Appl Environ Microbiol 84:e02350-17. doi: 10.1128/AEM.02350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hampel KJ, LaBauve AE, Meadows JA, Fitzsimmons LF, Nock AM, Wargo MJ. 2014. Characterization of the GbdR regulon in Pseudomonas aeruginosa. J Bacteriol 196:7–15. doi: 10.1128/JB.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O'Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp 30:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for invivo genetic-engineering-transposon mutagenesis in gram-negative bacteria. BioTechnology 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.