ABSTRACT
Long-lasting and sterile homologous protection against malaria can be achieved by the exposure of malaria-naive volunteers under chemoprophylaxis to Plasmodium falciparum-infected mosquitoes (chemoprophylaxis and sporozoite [CPS] immunization). While CPS-induced antibodies neutralize sporozoite infectivity in vitro and in vivo, antibody-mediated effector mechanisms are still poorly understood. Here, we investigated whether complement contributes to CPS-induced preerythrocytic immunity. Sera collected before and after CPS immunization in the presence of active or inactive complement were assessed for the recognition of homologous NF54 and heterologous NF135.C10 sporozoites, complement fixation, sporozoite lysis, and possible subsequent effects on in vitro sporozoite infectivity in human hepatocytes. CPS immunization induced sporozoite-specific IgM (P < 0.0001) and IgG (P = 0.001) antibodies with complement-fixing capacities (P < 0.0001). Sporozoite lysis (P = 0.017), traversal (P < 0.0001), and hepatocyte invasion inhibition (P < 0.0001) by CPS-induced antibodies were strongly enhanced in the presence of active complement. Complement-mediated invasion inhibition in the presence of CPS-induced antibodies negatively correlated with cumulative parasitemia during CPS immunizations (P = 0.013). While IgG antibodies similarly recognized homologous and heterologous sporozoites, IgM binding to heterologous sporozoites was reduced (P = 0.023). Although CPS-induced antibodies did not differ in their abilities to fix complement, lyse sporozoites, or inhibit the traversal of homologous and heterologous sporozoites, heterologous sporozoite invasion was more strongly inhibited in the presence of active complement (P = 0.008). These findings demonstrate that CPS-induced antibodies have complement-fixing activity, thereby significantly further enhancing the functional inhibition of homologous and heterologous sporozoite infectivity in vitro. The combined data highlight the importance of complement as an additional immune effector mechanism in preerythrocytic immunity after whole-parasite immunization against Plasmodium falciparum malaria.
KEYWORDS: chemoprophylaxis, sporozoites, IgG, Plasmodium falciparum, antibodies, complement, controlled human malaria infection, immunization, liver stage, sporozoites
INTRODUCTION
Malaria is one of the world's chief causes of morbidity and mortality by infectious diseases and has a significant impact on public and economic health worldwide. Nearly half of the world's population is at risk of malaria, and in 2015, there were roughly 200 million clinical cases and nearly half a million deaths attributed to malaria (1). Malaria is caused by the protozoan parasite Plasmodium and is characterized by a complex multistage life cycle in the human host. Sporozoites deposited into the skin by a female Plasmodium falciparum-infected Anopheles mosquito first travel to the liver by gliding motility (2) and cross cell barriers by breaching host cell membranes (3). After an invasion-and-maturation step in the liver, merozoites progress to invade erythrocytes, leading to the clinical symptoms of malaria (4). Although a significant decrease in malaria mortality rates has been observed in the last 15 years (1), malaria control efforts are threatened by the emergence of drug-resistant parasites (5) and insecticide-resistant mosquitoes (6), stressing the need for a highly effective vaccine.
While achieving sterile immunity by subunit vaccination has proven to be difficult (7–9), long-lasting and sterile protection against a homologous P. falciparum malaria infection can be accomplished experimentally by whole-parasite immunization with live attenuated sporozoites. For instance, this can be achieved in healthy human volunteers by the intravenous injection of 150,000 cryopreserved sporozoites (PfSPZ-CVac) (10) or by exposure to bites of 30 to 45 P. falciparum-infected mosquitoes under chloroquine chemoprophylaxis (chemoprophylaxis and sporozoites [CPS]) (11, 12). This immunity is long-lasting, involving effector memory T-cell responses as well as memory B-cell and antibody responses recognizing preerythrocytic-stage antigens (12–17). CPS-induced antibodies show neutralizing activity against sporozoite and liver-stage parasites and are capable of reducing liver-stage infection in hepatocytes in vitro and in vivo in a human liver-chimeric mouse model (18). CPS-induced antibodies show a much stronger effect on reducing liver-stage infection in vivo than in vitro, suggesting that additional effector mechanisms besides the direct neutralization of sporozoites by antibodies may be involved.
One possible mechanism is the activation of complement, representing a system of heat-sensitive, soluble, and cell surface-associated proteins that are involved in pathogen opsonization, the recruitment of phagocytes, and pathogen lysis via downstream C3 complement protein deposition (19). The complement pathway plays a key role in the antibody-mediated inhibition of P. falciparum merozoite invasion, thereby reducing P. falciparum blood-stage replication and preventing clinical disease (20). Additionally, P. falciparum sporozoites are susceptible to complement activation by human antibodies that are naturally acquired after exposure to multiple infections in areas where malaria is endemic. Naturally acquired antibodies are able to promote complement deposition and activation, resulting in enhanced antibody-mediated traversal inhibition in vitro (21). Here, we studied whether antibody-dependent complement activation contributes to preerythrocytic-antibody-mediated protective immunity against P. falciparum malaria sporozoites induced by CPS immunization. To this end, CPS-induced antibodies were assessed for their functional capacity to fix complement proteins on sporozoites, induce sporozoite lysis, and further impact in vitro hepatocyte traversal and invasion by sporozoites in the presence of active complement, tested for both the homologous NF54 strain the genetically and geographically distinct NF135.C10 parasite clone.
RESULTS
CPS immunization induces sporozoite-specific IgG and IgM antibodies.
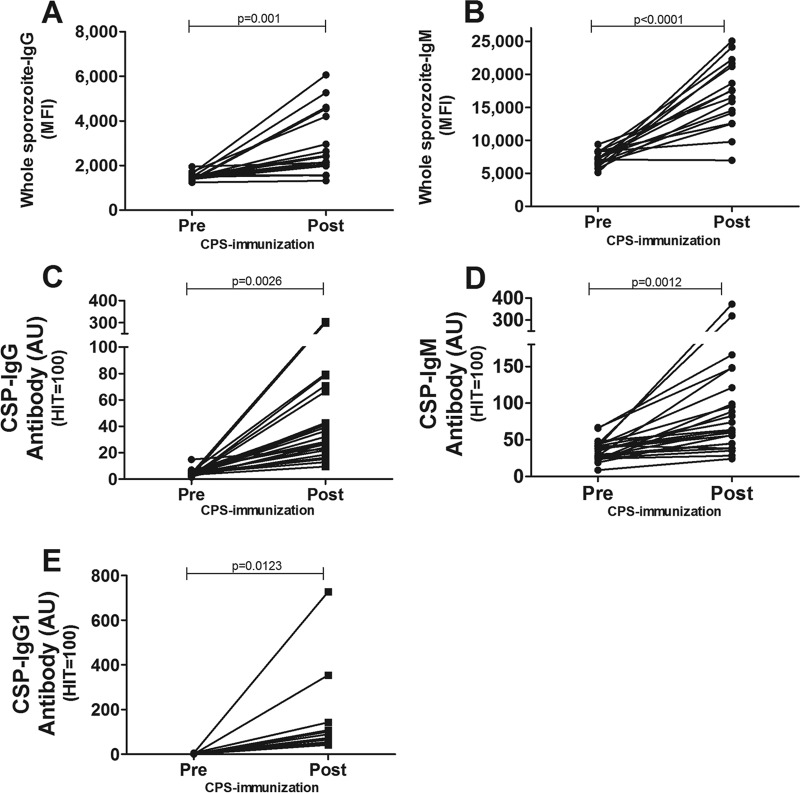
Sporozoite-specific IgG antibodies were specifically induced in 15 out of 16 volunteers after completed CPS immunization using NF54-infected mosquitoes, with a median fold increase of 1.6 and an interquartile range (IQR) of 1.2 to 3.1 (P < 0.0001 [Fig. 1A] and P = 0.004 [see Fig. S1A and S1C in the supplemental material]) compared to the baseline. CPS immunization also strongly induced sporozoite-specific IgM antibodies in 15 out of 16 volunteers, with a median fold increase of 2.3 (IQR, 1.8 to 2.9; P < 0.0001 [Fig. 1B] and P < 0.0001 [Fig. S1B and S1D]). There was no correlation between IgG and IgM antibodies (P = 0.192) (Fig. S2A). Specific IgG and IgM antibodies against the dominant circumsporozoite protein (CSP) were induced (P = 0.0026 and P = 0.0012) (Fig. S1C and S1D) and were positively correlated (P = 0.0194) (Fig. S2B). While whole-sporozoite-specific IgG antibodies did not correlate with anti-CSP-specific IgG (anti-CSP-IgG) antibody levels (P = 0.269) (Fig. S2C), sporozoite-specific IgM antibodies correlated with anti-CSP-IgM antibody levels (P = 0.003) (Fig. S2D).
FIG 1.
Recognition of homologous P. falciparum NF54 sporozoites by CPS-induced antibodies. Homologous NF54 sporozoites were preincubated with 10% inactive complement and 10% heat-inactivated pre- or postimmunization serum from CPS-immunized volunteers (n = 16). (A and B) The amounts of CPS-induced IgG (A) and IgM (B) antibodies recognizing sporozoites were determined by flow cytometry and are shown as geometric mean fluorescence intensities (MFI). (C and D) Levels of IgG (C) and IgM (D) antibodies to CSP before (Pre) and after (Post) completed CPS immunization in CPS-immunized volunteers (n = 24) were determined by ELISAs and are shown as arbitrary units (AU), as defined by serial dilutions of a reference standard serum pool with high antibody concentrations. (E) CSP-specific IgG1 antibodies in pre- and postimmunization samples from CPS-immunized volunteers (n = 15) were determined by CSP-specific IgG1 subclass ELISAs and are shown as arbitrary units. Differences between pre- and postimmunization samples were determined by paired Student's t test, and a P value of <0.05 was considered statistically significant.
IgG1 and IgM isotype antibodies to CSP are most prevalent in CPS-immunized volunteers.
Antibody isotype is a major factor in the subsequent activation of the classical complement pathway. Levels of both anti-CSP-specific IgM (P = 0.0012) (Fig. 1D) and IgG1 (P = 0.0123) (Fig. 1E) antibodies were significantly increased, while IgG2, IgG3, and IgG4 antibodies against CSP remained undetectable after CPS immunization (data not shown). Anti-CSP-IgG1 levels strongly correlated with total CSP-specific IgG, confirming that CSP-IgG1 antibodies are primarily induced in CPS-immunized volunteers (P = 0.0001) (Fig. 1E). The combined data demonstrate that CPS immunization predominantly induces IgG1 and IgM antibodies, both of which are known to be potent activators of the complement pathway (22).
CPS-induced antibodies fix complement and lyse homologous P. falciparum NF54 sporozoites.
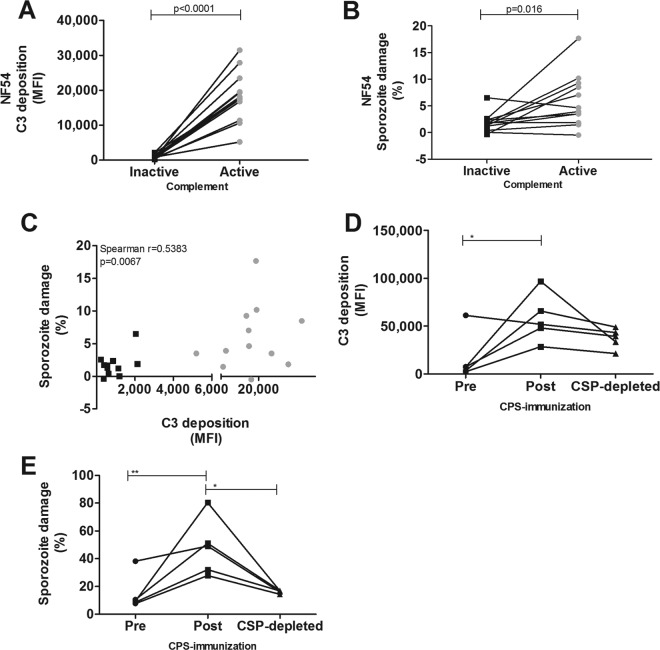
C3 complement protein deposition on sporozoites and sporozoite membrane permeability were strongly enhanced in the presence of postimmunization antibodies and active complement (P < 0.0001 and P = 0.016) (Fig. 2A and B). As expected, there was a strong correlation between C3 deposition and sporozoite membrane permeability, suggesting that antibody-dependent C3 deposition on sporozoites results in functional sporozoite lysis (P = 0.0067) (Fig. 2C).
FIG 2.
Complement activation and lysis of homologous P. falciparum NF54 sporozoites by CPS-induced antibodies. Homologous NF54 sporozoites were preincubated with 10% inactive or active complement and 10% heat-inactivated pre- or postimmunization serum from CPS-immunized volunteers. (A) C3 complement protein deposition on NF54 sporozoites in 10% postimmunization serum (n = 12 volunteers) in the presence of inactive or active of complement was assessed and is shown as MFI. C3 deposition by postimmunization serum was corrected for baseline responses by subtracting C3 deposition by that for preimmunization serum. (B) Sporozoite damage by CPS-induced antibodies (n = 12 volunteers) in the presence of 10% inactive or active complement, shown as percent sporozoite damage and corrected by subtracting the percent sporozoite damage in the presence of preimmunization antibodies. (C) Scatter plots showing C3 complement protein deposition. The percent damaged sporozoites per donor was corrected for preimmunization values and analyzed by Spearman correlation analysis (n = 12 CPS-immunized volunteers). Samples in the presence or absence of active complement are shown with gray circles and black squares, respectively. (D and E) NF54 sporozoites were preincubated with 10 mg/ml of purified preimmunization IgGs, postimmunization IgGs, and postimmunization IgGs depleted from CSP-specific antibodies (n = 5 volunteers) in the presence of 10% active complement. C3 complement protein deposition (D) and sporozoite damage by purified IgGs (E) are shown as C3 deposition (MFI) and the percentage of sporozoite damage, respectively. Comparisons between multiple groups were performed by one-way ANOVA with a Bonferroni multiple-comparison post hoc test. Data are shown as the means of results from duplicate measurements and presented as black squares or gray circles for samples tested in the presence of inactive or active complement, respectively. Asterisks represent P values of <0.05 (*) and <0.01 (**).
Activation of the complement pathway can also occur via antibody-independent pathways. Indeed, C3 deposition and sporozoite lysis also occurred in the presence of preimmunization serum, with median fold increases of 12.2 (IQR, 10.2 to 13.7) and 1.30 (IQR, 1.1 to 1.6), respectively (P < 0.0001 and P = 0.0009) (see Fig. S3A and S3B in the supplemental material). However, this effect was much weaker than that in the presence of CPS-induced antibodies (P < 0.0001 and P = 0.002) (Fig. S3C and S3D), with median fold increases of 16.2 (IQR, 14.5 to 20.0) and 1.8 (IQR, 1.4 to 2.2). Thus, complement activation against sporozoites via the antibody-dependent classical pathway is more potent than those via antibody-independent pathways.
We next investigated to which degree complement activation is mediated by anti-CSP-specific IgG antibodies. C3 deposition on sporozoites and sporozoite lysis were enhanced in the presence of postimmunization rather than preimmunization IgG (P < 0.05 and P < 0.01) (Fig. 2D and E). The level of sporozoite lysis in the presence of anti-CSP-depleted postimmunization IgG was also significantly lower than that in the presence of postimmunization IgG (P < 0.05) (Fig. 2E). The combined data suggest that anti-CSP-IgG antibodies contribute to the functional CPS-induced complement-mediated antibody response but also show that antibodies to other sporozoite surface-expressed proteins may be involved as well.
Complement-dependent inhibition of homologous P. falciparum NF54 sporozoite infectivity.
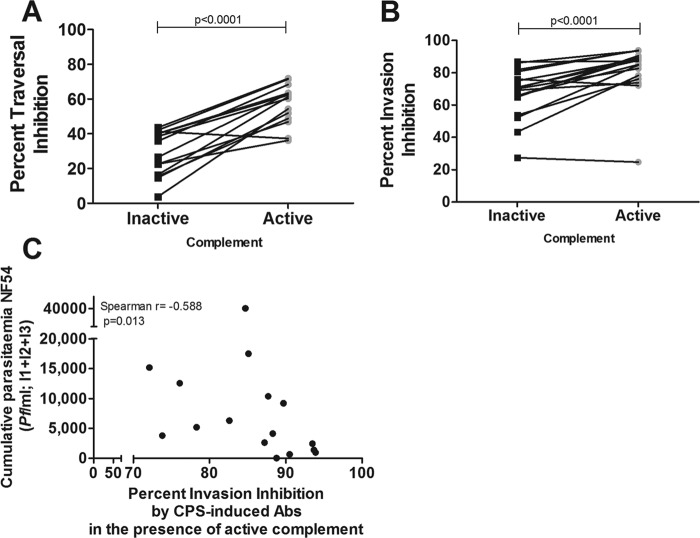
Antibody-mediated inhibition of sporozoite traversal was enhanced in the presence of active complement, with median traversal inhibition percentages of 31.2% (IQR, 16.2 to 40.4%) and 60.8% (IQR, 48.3 to 64.6%) for inactive and active complement, respectively (P < 0.0001) (Fig. 3A). Similarly, sporozoite invasion was reduced more efficiently in the presence of active complement, with median invasion inhibition percentages of 87.2% (IQR, 77.2 to 90.1%) and 70.1% (IQR, 59.4 to 78.4%) for active and inactive complement, respectively (P < 0.0001) (Fig. 3B). Cumulative parasitemia during CPS immunizations negatively correlated with NF54 invasion inhibition in vitro by CPS-induced antibodies in the presence of active complement (P = 0.013) (Fig. 3C) but not inactive complement (P = 0.2518) (see Fig. S4A in the supplemental material).
FIG 3.
In vitro traversal and invasion inhibition of homologous P. falciparum NF54 sporozoites by CPS-induced antibodies in the presence or absence of complement. (A and B) The percent inhibition of traversal (14 volunteers) (A) and invasion (17 volunteers) (B) was calculated for 10% postimmunization serum compared to preimmunization serum for each volunteer in the presence of 10% inactive or active complement. (C) Spearman correlation analysis between cumulative parasitemia (P. falciparum parasites per milliliter) during three CPS immunizations and invasion inhibition by CPS-induced antibodies (Abs) in the presence of active complement (uncorrected for HIS) (n = 17 volunteers). Data are shown as the means of results from duplicate measurements and presented as black squares or gray circles for samples tested in the presence of inactive or active complement, respectively.
Complement-dependent inhibition of heterologous P. falciparum NF135.C10 sporozoite infectivity.
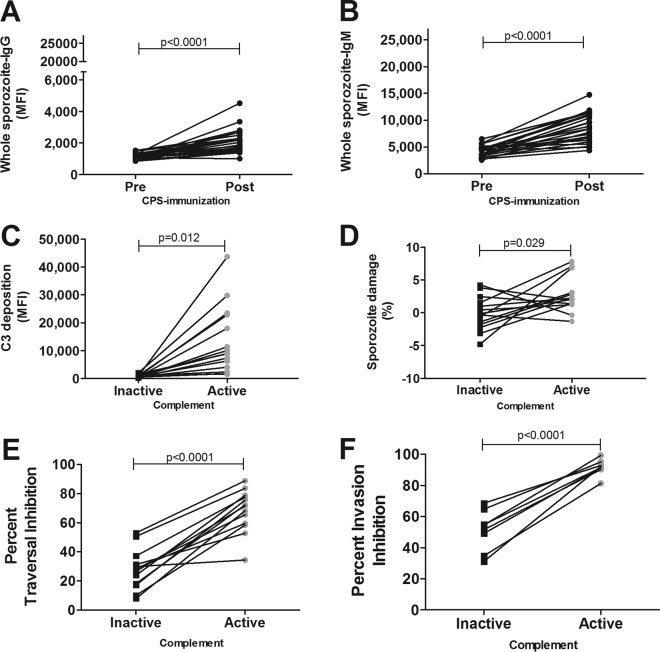
It was shown previously that CPS-induced antibodies can inhibit in vitro hepatocyte invasion by sporozoites of the homologous NF54 strain but also the genetically and geographically distinct NF135.C10 clone (23, 24). Therefore, we next examined whether the effects of complement on NF54 sporozoites also extend to heterologous NF135.C10 sporozoites. Both CPS-induced IgG and IgM antibodies recognized NF135.C10 sporozoites (P < 0.0001) (Fig. 4A and B). Complement fixation and lysis of heterologous sporozoites were also enhanced by postimmunization antibodies in the presence of active complement (P = 0.012 and P = 0.029) (Fig. 4C and D). Similar to NF54 sporozoites, heterologous traversal and invasion were inhibited more efficiently by postimmunization antibodies in the presence of active complement (P < 0.0001) (Fig. 4E and F). Median traversal inhibition percentages were 71.6% (IQR, 59.0 to 78.2%) and 28.2% (IQR, 17.7 to 34.3%), and median invasion inhibition percentages were 91.8% (IQR, 90.5 to 94.5%) and 53.0% (IQR, 38.4 to 62.2%) for active and inactive complement, respectively. There was no correlation between complement-dependent NF135.C10 invasion inhibition by CPS-induced antibodies in vitro and that during the prepatent period following NF135.C10 challenge infection, although only a limited number of volunteers could be tested (P = 0.083; n = 4 volunteers) (see Fig. S4B in the supplemental material). These combined data suggest that broadly neutralizing and complement-fixing antibodies are also potent against heterologous parasites.
FIG 4.
Complement activation and inhibition of heterologous P. falciparum NF135.C10 sporozoites. Heterologous P. falciparum NF135.C10 sporozoites were preincubated with 10% heat-inactivated pre- or postimmunization serum (n = 24 volunteers) and 10% inactive complement. (A and B) The amounts of CPS-induced IgG (A) and IgM (B) antibodies recognizing sporozoites were determined by flow cytometry and are shown as MFI. (C) C3 complement deposition on NF135.C10 sporozoites in the presence of 10% inactive (black) or active (gray) complement by 10% pre- or postimmunization CPS serum (n = 15 volunteers), shown as the MFI. C3 complement protein deposition in the presence of postimmunization serum was corrected for C3 deposition in the presence of preimmunization serum. (D) Sporozoite damage by pre- or postimmunization antibodies (n = 14 volunteers) in the presence of 10% active or inactive complement, shown as the percent sporozoite damage and corrected for the percent sporozoite damage in the presence of preimmunization antibodies. (E and F) The percentages of inhibition of heterologous sporozoite traversal (13 volunteers) (E) and invasion (n = 8 volunteers) (F) were calculated for 10% postimmunization serum compared to preimmunization serum for each volunteer in the presence of 10% inactive or active complement. Data are shown as the means of results from duplicate measurements and presented as black squares or gray circles for samples tested in the presence of inactive or active complement, respectively. Differences between pre- and postimmunization samples or inactive and active complement were determined by paired Student's t test, and a P value of <0.05 was considered statistically significant.
Comparison of complement-mediated effects on P. falciparum NF54 and NF135.C10 sporozoite infectivity.
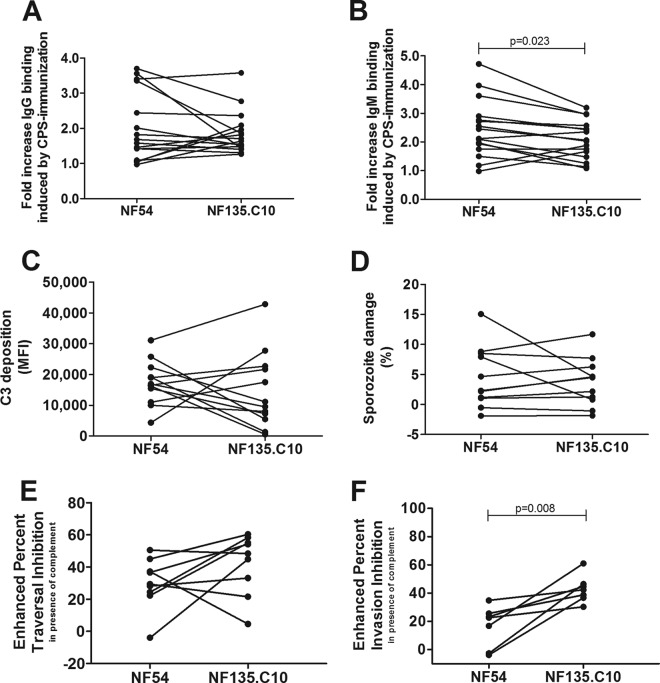
There were no significant differences in the binding of specific IgG antibodies to NF54 and NF135.C10 sporozoites (P = 0.494) (Fig. 5A). In contrast, NF135.C10 sporozoites were less opsonized by specific IgM antibodies than were NF54 sporozoites (P = 0.023) (Fig. 5B). Nevertheless, this did not translate into differences in C3 deposition and lysis (P = 0.53 and P = 0.69) (Fig. 5C and D). Traversal inhibition was not significantly different between the two strains (P = 0.18) (Fig. 5E), with median percent enhanced traversal inhibition values of 29.4% (IQR, 23.4 to 41.1%) and 48.4% (IQR, 27.3 to 57.7%) for NF54 and NF135.C10 sporozoites, respectively. NF54 sporozoite invasion was neutralized more strongly by CPS-induced antibodies in the absence of active complement, with median percent invasion inhibition values of 70.1% (IQR, 59.4 to 78.4%) and 53.0% (IQR, 38.4 to 62.2%) for NF54 and NF135.C10 sporozoites, respectively (P = 0.019). Percent invasion inhibition values for NF54 and NF135.C10 sporozoites in the presence of active complement were not significantly different (medians, 87.2% [IQR, 77.2 to 90.2%] and 91.8% [IQR, 90.5 to 94.5%] for NF54 and NF135.C10, respectively). However, the invasion of heterologous sporozoites in the presence of active complement was inhibited more strongly than was the invasion of NF54 sporozoites (P = 0.008) (Fig. 5F), with median enhanced invasion inhibition values of 22.5% (IQR, −2.7 to 25.5%) and 42.3% (IQR, 36.4 to 46.4%) for NF54 and NF135.C10 sporozoites, respectively.
FIG 5.
Comparison of complement activation and inhibition between NF54 and NF135.C10 sporozoites. (A and B) Recognition of homologous NF54 and heterologous NF135.C10 sporozoites by postimmunization IgG (A) and IgM (B) antibodies, shown as fold increases over baseline (preimmunization) antibody values (n = 16 CPS-immunized volunteers). (C) Enhanced C3 complement protein deposition on homologous and heterologous sporozoites, shown as the MFI and corrected for complement fixation by preimmunization antibodies and inactive complement (n = 12 volunteers). (D) Enhanced sporozoite damage of homologous and heterologous sporozoites, shown as percent sporozoite damage and corrected for damage in the presence of preimmunization antibodies and inactive complement (n = 11 volunteers). (E and F) The percentages of enhanced inhibition of both homologous and heterologous sporozoite traversal (n = 9 volunteers) (E) and invasion (n = 7 volunteers) (F) in the presence of active complement were calculated for 10% postimmunization serum compared to preimmunization serum for each volunteer in the presence of 10% inactive or active complement. To calculate the percent enhanced inhibition by active complement and postimmunization antibodies, inhibition in the presence of complement was corrected for inhibition in the presence of inactive complement. Data are shown as the means of results from duplicate measurements. Differences between parasite strains were determined by paired Student's t test, and a P value of <0.05 was considered statistically significant.
DISCUSSION
The present study shows that CPS immunization with P. falciparum NF54 sporozoites induces complement-fixing antibodies that are capable of activating the classical complement pathway, resulting in membrane-compromised sporozoites and a further reduction of homologous and heterologous sporozoite infectivity in vitro. This is supported by previous findings from in vitro assays with Plasmodium gallinaceum revealing the importance of the classical complement pathway in inducing sporozoite death (25).
Sporozoite-specific IgM responses contribute to CPS-induced immunity, with the potential benefits of higher avidity for P. falciparum target antigens and efficient complement fixation (26). Some volunteers tend to have a stronger induction of sporozoite-specific IgM than IgG antibodies, while sporozoite-specific IgG antibodies are induced more strongly in other volunteers. As shown in the present study, CSP- and sporozoite-specific IgM antibodies are still present 18 weeks after the last CPS immunization, corroborating previous observations (27). This suggests that an initial IgM response is developed, whereby not all epitope-specific responses might be followed by class switching to IgG over the next 2 to 3 weeks (28). The functional relevance and importance of IgM antibodies were shown previously in the Plasmodium chabaudi model, where Plasmodium-specific IgM-producing memory B cells were readily induced in response to repeated parasite exposure (29). Interestingly, the magnitude and breadth of P. falciparum-specific IgM antibody responses are also higher in African adults with naturally acquired antimalarial immunity who are resistant to malaria than in adults who are susceptible to malaria (30). Additionally, it was shown very recently that immunization of Tanzanian individuals with radiation-attenuated cryopreserved P. falciparum sporozoites (PfSPZ) induces functional antisporozoite IgM antibodies with human hepatocyte invasion-inhibitory and complement-fixing activities (31).
Humoral reactivity to CSP is induced following CPS immunization (16, 17), but the induction of sporozoite-specific IgG does not correlate with anti-CSP-IgG levels. In fact, even the virtually complete depletion of anti-CSP-specific IgG (median percent CSP depletion, 91.7% [IQR, 79.8 to 96.4%]) (see Table S2 in the supplemental material) still leaves complement-fixing activity against sporozoites intact. This may suggest that only a fraction of anti-CSP-specific antibodies may still be sufficient for complement activation. It is more likely, however, that CPS-induced IgG antibodies with different antigenic specificities than CSP (17, 27) might show functional activity.
Showing some variation between volunteers, it is clear that CPS-induced antibodies can efficiently enhance C3 fixation, with subsequent enhanced homologous and heterologous sporozoite lysis. Complement fixation efficacy depends on antibody-intrinsic features, such as the epitope specificity of antibodies, antibody affinity as well as antibody isotype, or Fc receptor glycosylation. High-affinity antibodies can activate complement more efficiently, since antibodies may switch classes in a particular order depending on the degree of affinity maturation (32–35). As for antibody isotype, it is very likely that some individuals with low IgG concentrations may have high IgM levels, while other individuals might rely more on IgGs for complement-fixing activity. While CPS immunization predominantly induces anti-CSP-specific IgG1 and IgM antibodies, RTS,S (CSP) subunit vaccination primarily induces IgG1 antibodies against CSP repeats (36). On the other hand, CSP-specific naturally acquired antibodies are mainly IgG1, IgG3, and IgM (21), showing that the induction of anti-CSP antibody isotypes differs between these immunizations. A possible explanation for the absence of IgG2, IgG3, and IgG4 responses following CPS immunization might be that IgG subclass responses following whole-sporozoite immunization may be polarized toward IgG1, the most abundant immunoglobulin, and induced upon exposure to soluble protein antigens and membrane proteins (22). IgG2 and IgG4 antibody responses are produced only following exposure to bacterial polysaccharide antigens, in response to helminth infections, or following repeated or long-term exposure to noninfectious antigens (allergens) (37).
Alternatively, the observed variation in classical complement activation might be due to variation in the density of fragment crystallizable (Fc) regions, since a minimum threshold concentration of Fc regions is required for stronger classical pathway activation (34). Moreover, Fc receptor glycosylation can affect antigen-binding characteristics and, thus, antibody activity (22, 38).
As a next step, we studied the functional consequences of enhanced complement fixation on sporozoite infectivity in vitro. NF54 human hepatocyte invasion is inhibited by CPS-induced antibodies more strongly than is NF135.C10 invasion in the absence of active complement, as recently reported by us (26). Despite lower levels of IgM binding to NF135.C10 sporozoites, which is most likely due to genetic diversity in the CSP protein sequence (24), there were no differences in complement protein deposition and sporozoite lysis. This can be explained by the fact that IgM antibodies generally have a lower threshold for complement fixation than IgG antibodies and are therefore still very efficient in complement activation, despite the fact that they bind less efficiently to heterologous NF135.C10 sporozoites. Interestingly, the level of NF135.C10 invasion inhibition is twice as high as the level of NF54 inhibition in the presence of active complement. These data suggest that opsonization for complement might also occur independently of strain-specific epitopes and that complement has more added value when antibodies alone are less efficient in binding and neutralization, e.g., due to parasite genetic diversity. Antibody-mediated complement activation may also have additional indirect effects on sporozoite clearance, including modulation of the inflammatory response, induction of antibody-dependent cellular cytotoxicity (ADCC), or phagocytosis (39).
Unexpectedly, some C3 deposition and sporozoite death also take place before immunization, suggesting that some C3 complement proteins may bind directly to sporozoites via the alternative pathway. Some sporozoite proteins may contain carbohydrates that might activate the antibody-independent mannose-binding lectin (MBL) pathway (40–45). While MBL-deficient mice show no altered resistance to liver-stage infection, MBL binding may take place and modulate host defense (46). More likely, malaria-naive volunteers may have cross-reactive antibodies that recognize sporozoites or mosquito salivary gland material and thus are able to interact with complement proteins. Although possibly contributing, our data clearly show that complement activation is activated and functional primarily in the presence of malaria-specific antibodies. Additionally, it was recently shown that C3 deposition on CSP by naturally acquired antibodies strongly correlates with C1q fixation to CSP (21). Here, we show that NF54 invasion inhibition by CPS-induced antibodies and active complement is negatively associated with cumulative parasitemia during CPS immunizations. Previous studies in human and animal models of malaria support a role for complement activation during malaria infection with increased membrane attack complex (47) or reduced serum complement protein (48–53) levels. It was observed previously in a humanized liver-chimeric mouse model that CPS-induced antibodies had a much stronger inhibitory effect on P. falciparum liver-stage infection in vivo than in vitro (18). A possible explanation for this observation might be that CPS-induced IgGs could potentially interact with human complement, which was shown previously to be produced by human hepatocytes in human liver-chimeric mice (54), resulting in a more pronounced effect on liver-stage inhibition in vivo. Additionally, resistance to natural infection with Plasmodium malaria parasites has been associated with IgG1 and IgG3 antibody isotypes, both of which are very potent in fixing complement and activating the complement system (20, 55–57). This is further supported by the fact that children living in an area where P. falciparum is holoendemic in Papua New Guinea who have high levels of complement-fixing antibodies to CSP have a reduced risk of clinical malaria compared to children with undetectable functional antibodies (21). Taken together, data from these studies suggest that the complement system plays a role in antimalarial immunity and protection from malaria infection in vivo.
In summary, these findings demonstrate for the first time that CPS-induced antibodies can interact with the complement system, further reducing homologous and heterologous sporozoite infectivity in vitro. Together, these data highlight the importance of the complement pathway and provide new knowledge on antibody-mediated immune mechanisms involved in preerythrocytic immunity to homologous and heterologous sporozoites after whole-parasite immunization against P. falciparum malaria.
MATERIALS AND METHODS
Study design of experimental controlled human malaria infection.
Citrated plasma and serum samples were used from a double-blind, randomized, placebo-controlled CPS immunization trial conducted at the Radboud University Medical Center in 2015 (Nijmegen, The Netherlands) (ClinicalTrials.gov registration number NCT02098590) (24). Due to limited plasma sample availability, citrated plasma samples from a second open-labeled, randomized, CPS immunization study conducted in 2014 to 2015 (ClinicalTrials.gov registration number NCT02080026) were used. All study subjects provided written informed consent, and both studies were approved by the Central Committee for Research Involving Human Subjects of The Netherlands (CCMO) (approval numbers NL48732.091.14 and NL48301.091.14).
In both CPS trials (ClinicalTrials.gov registration numbers NCT02098590 and NCT02080026), volunteers were subjected to NF54 CPS immunization. While receiving chloroquine in a prophylactic dose, subjects were immunized three times (ClinicalTrials.gov registration number NCT02098590) or four times (ClinicalTrials.gov registration number NCT02080026) at monthly intervals by exposure to bites from 15 P. falciparum NF54-infected Anopheles stephensi mosquitoes. Fourteen weeks after the discontinuation of chloroquine prophylaxis, subjects underwent a primary challenge infection by exposure to bites of 5 mosquitoes infected with the homologous P. falciparum NF54 strain (58) or the genetically distinct NF135.C10 (23) and NF166.C8 (59) clones. Of note, study subjects from the second, open-label, randomized CPS immunization study were exposed only to NF54-infected mosquito bites (ClinicalTrials.gov registration number NCT02080026). All subjects were monitored closely from days 6 to 10 after each CPS immunization and from days 6 to 21 after mosquito bite challenge infection for symptoms and signs of malaria. When the treatment threshold (100 parasites per ml of blood) by quantitative PCR (qPCR) between days 7 and 9 was reached (60, 61), blood-stage parasitemia was treated with a curative regimen of atovaquone and proguanil once daily for 3 days. Cumulative blood-stage parasitemia during all three CPS immunizations (ClinicalTrials.gov registration number NCT02098590) was calculated by summing up the number of parasites per milliliter of blood, as determined by qPCR, from days 6 to 10 after each CPS immunization. NF54 CPS immunization induced sterile protection against NF54, NF135.C10, and NF166.C8 mosquito bite challenge infections in 5/5, 2/10, and 1/9 subjects, respectively. Six of ten NF135.C10-challenged subjects showed a prolonged prepatent period (ClinicalTrials.gov registration number NCT02098590). With respect to the second CPS immunization study (ClinicalTrials.gov registration number NCT02080026), 5/9 study subjects were completely protected from homologous NF54 mosquito bite challenge.
Parasite strains.
P. falciparum NF54 and NF135.C10 sporozoites were used for in vitro sporozoite assays. Plasmodium falciparum NF54 was isolated from an individual near Schiphol Airport (The Netherlands) and most likely originated from West Africa (58, 62). The genetically and geographically distinct P. falciparum NF135.C10 clone originated from a clinical isolate in Cambodia (23).
Parasite culture and generation of P. falciparum-infected mosquitoes.
P. falciparum NF54 and NF135.C10 asexual and sexual blood-stage parasites were cultured in a semiautomated culture system, as described previously (63–65). Anopheles stephensi mosquitoes were reared in the insectary of the Radboud University Medical Center, and female mosquitoes were infected by standard membrane feeding on NF54 or NF135.C10 gametocyte cultures (66). For in vitro sporozoite assays, salivary glands from infected mosquitoes were hand dissected 14 to 28 days after mosquito infection, collected in Leibovitz culture medium without serum, and homogenized in a homemade glass grinder. The number of P. falciparum NF54 or NF135.C10 sporozoites was determined with a Bürker-Türk counting chamber, using phase-contrast microscopy (18).
Human hepatoma HC-04 cell line.
The HC-04 human hepatocyte cell line (Homo sapiens HC-04; MRA-965), deposited by Jetsumon Sattabongkot (67), was acquired through the Malaria Research and Reference Reagent Resource Center (MR4) as part of the Biodefense and Emerging Infections Research Resources Repository (BEI Resources). Cells were maintained in Dulbecco's modified Eagle medium (DMEM)–Ham's F-12 nutrient mixture medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), 1% glutamine (Gibco), and 1% penicillin-streptomycin (Gibco) at 37°C in an atmosphere of 5% CO2.
Citrated plasma, serum samples, and complement source.
Citrated plasma samples and serum samples from 24 CPS-immunized volunteers were collected 11 to 14 days before the first CPS immunization (preimmunization) and 1 day before challenge infection (18 weeks after the last immunization) (postimmunization) (ClinicalTrials.gov registration number NCT02098590) by using citrated Vacutainer cell preparation tubes (CPT Vacutainers; Becton Dickinson) or serum Vacutainer tubes (Becton Dickinson). Samples were used for the determination of immunoglobulin subclasses, antibody opsonization assays (citrated plasma samples), or in vitro sporozoite assays (serum). Samples were stored in aliquots at −20°C. Prior to use in in vitro sporozoite assays, serum aliquots were heat inactivated for 30 min at 56°C, centrifuged at 13,000 rpm for 5 min at room temperature, and kept at 4°C.
Due to limited plasma availability, additional citrated plasma samples were collected 1 week before the first CPS immunization (preimmunization) and 1 day before challenge infection (15 weeks after the last immunization) (postimmunization) from 5 sterilely protected CPS-immunized volunteers (ClinicalTrials.gov registration number NCT02080026) and used for IgG purifications and depletion of CSP-specific antibodies. Purified IgGs were assessed for their ability to activate complement and induce sporozoite lysis in the presence or absence of CSP-specific antibodies.
An external source of human complement was used for all in vitro sporozoite assays to determine the complement-fixing activity of CPS-induced antibodies (either heat-inactivated CPS serum or purified IgGs) independently of possible differences in complement activity present in each CPS-immunized volunteer. This specific batch was consistently used for all experiments and samples and either added fresh (normal human serum [NHS]) (active complement) or heat inactivated (heat-inactivated serum [HIS]) (inactive complement). Heat inactivation was validated previously. This complement source consisted of pooled sera from 5 malaria-naive Australian donors and was validated for the absence of CSP-specific antibodies by a standardized enzyme-linked immunosorbent assay (ELISA), as described previously (21).
Purification of IgG from citrated plasma samples.
Purification of IgG from citrated pre- and postimmunization plasma samples from CPS-immunized volunteers (n = 5) was performed by using a 5-ml HiTrap protein G HP affinity column (Amersham Biosciences) according to the manufacturer's instructions. A fraction of purified postimmunization IgGs was depleted from CSP-specific antibodies by running IgGs three times over a CSP affinity column that was constructed by coupling CSP (Gennova Biotechniques Pvt. Ltd., India) to a 1-ml HiTrap NHS-activated HP affinity column (catalog number 17-0717-01; GE Healthcare). IgGs were purified and depleted from CSP-specific antibodies by using an Äkta prime machine and Unicorn software (version 1.0; GE Healthcare). Purified IgGs were taken up in phosphate-buffered saline (PBS). IgG concentrations and CSP depletion efficacy (see Tables S1 and S2 in the supplemental material) were determined by standardized total IgG and CSP-IgG ELISAs prior to use in in vitro complement deposition and sporozoite damage assays.
Total IgG, CSP-IgG, CSP-IgM, and CSP-specific Ig subclass ELISAs.
Total IgG concentrations and CSP-specific IgG antibodies in purified preimmunization IgGs, postimmunization IgGs, and CSP-depleted postimmunization IgGs and CSP-specific IgM antibody levels in pre- and postimmunization plasma samples were determined by total IgG, CSP-IgG, and CSP-IgM ELISAs. CPS-induced immunoglobulin isotypes to CSP were determined by CSP-specific Ig subclass ELISAs. All these ELISAs are described in detail in supplemental material.
IgG and IgM antibody opsonization of whole sporozoites.
The recognition of whole sporozoites by immunization-induced IgG and IgM antibodies was determined by an in vitro flow cytometry-based antibody opsonization assay, as described in detail in the supplemental material. Briefly, 5 × 104 P. falciparum NF54 or NF135.C10 sporozoites/well in a V-bottom 96-well plate were incubated with 10% heat-inactivated pre- or postimmunization serum and 10% heat-inactivated normal human serum (inactive complement) for 30 min at 37°C. Following incubation, samples were washed with PBS, centrifuged at 3,220 × g for 5 min at room temperature, and stained with fluorescently labeled antibodies targeting sporozoite CSP, IgG, and IgM for 30 min in the dark at 4°C. Unstained sporozoites and single compensation controls were included. Following incubation, samples were fixed with 1% paraformaldehyde (PFA) for 20 min in the dark at 4°C and taken up in PBS. Samples were kept at 4°C in the dark until flow cytometric analysis was performed. Flow cytometric analysis was performed with an LSRII flow cytometer (BD BioSciences), and data analysis was performed with FlowJo software (version 10.0.8; TreeStar).
In vitro complement deposition and sporozoite damage assay.
C3 complement protein deposition on whole sporozoites and sporozoite damage due to complement activation in the presence or absence of immunization-induced antibodies were assessed with an in vitro flow cytometry-based assay, as described in detail in the supplemental material. Briefly, 5 × 104 P. falciparum NF54 or NF135.C10 sporozoites/well in a V-bottom 96-well plate were incubated with 10% heat-inactivated pre- or postimmunization serum and 10% fresh normal human serum (active complement) or 10% inactive complement for 30 min at 37°C. In the case of purified IgGs, sporozoites were incubated with 10 mg/ml preimmunization IgGs, postimmunization IgGs, or postimmunization IgGs depleted from CSP in the presence of 10% active complement. Following incubation, PBS–20 mM EDTA was added to all samples, and plates were incubated at 4°C for 5 min to inactivate complement. Subsequently, sporozoites were stained with fluorescently labeled antibodies targeting sporozoite CSP, C3 complement protein deposition, and a fixable viability dye for 30 min in the dark at 4°C. Unstained sporozoites and single compensation controls were taken along. After incubation, samples were processed and analyzed as described above. The geometric mean fluorescence intensity (MFI) and the percentage of membrane-compromised sporozoites in postimmunization samples were corrected for those for preimmunization responses by subtracting the MFI and percentage of membrane-compromised sporozoites for preimmunization responses from that for postimmunization responses.
In vitro sporozoite hepatocyte traversal inhibition assay.
The antibody-dependent complement-mediated effect of CPS-induced antibodies on further augmenting the inhibition of in vitro human hepatocyte traversal by P. falciparum sporozoites in the presence of active complement was assessed as described previously, with small adaptations (18). Briefly, freshly dissected NF54 and NF135.C10 sporozoites were preincubated with 10% heat-inactivated pre- or postimmunization CPS serum for 30 min at 4°C. Subsequently, 5 × 104 sporozoites/well were added in duplicate to flat-bottom 96-well plates containing monolayers of 5 × 104 HC-04 cells in the presence of 10% active or inactive complement and 0.5 mg/ml fixable tetramethylrhodamine dextran (Thermo Fisher Scientific). HC-04 cells alone in the presence of dextran served as a background control. After 2 h of incubation at 37°C in 5% CO2, cells were washed gently and processed for flow cytometric analysis. Flow cytometric analysis was performed with a cyan ADP flow cytometer (Beckman Coulter), and data were analyzed with FlowJo software (version 9.6.7; TreeStar). The percentage of dextran-positive cells was first corrected for background reactivity by subtracting the background, and the percent traversal inhibition was calculated as follows: 1 − (average percent dextran-positive cells in postimmunization cultures/average percent dextran-positive cells in preimmunization cultures) × 100%.
In vitro sporozoite infectivity assay with a human hepatoma cell line.
The neutralization of P. falciparum sporozoite hepatocyte invasion in vitro by CPS-induced antibodies was assessed by a flow cytometry-based in vitro invasion assay, as described in detail in the supplemental material. Briefly, P. falciparum NF54 or NF135.C10 sporozoites were preincubated with 10% heat-inactivated pre- or postimmunization CPS serum for 30 min at 4°C. Subsequently, 5 × 104 sporozoites/well were added to flat-bottom 96-well plates containing monolayers of 5 × 104 HC-04 cells in the presence of 10% active or inactive complement. After 3 h of incubation at 37°C in 5% CO2, intracellular and invaded parasites were stained with a fluorescently labeled antibody targeting CSP, and cells were processed for flow cytometric analysis. Flow cytometric analysis was performed with a Gallios flow cytometer (Beckman Coulter), and data were analyzed with FlowJo software (version 10.0.8; TreeStar). The percentage of CSP-positive sporozoites was first corrected for background reactivity by subtracting the background (uninfected HC-04 cells in the presence of 3SP2-Alexa Fluor 488 antibody). The percent invasion inhibition was calculated as follows: 1 − (average percent CSP-positive cells in postimmunization cultures/average percent CSP-positive cells in preimmunization cultures) × 100%.
Statistical analysis.
Statistical analysis was performed by using GraphPad Prism software (version 5; GraphPad Software Inc., CA, USA). For analysis of in vitro sporozoite data, antibody binding, C3 complement deposition on sporozoites, and sporozoite lysis, differences between pre- and postimmunization samples, HIS and NHS, or parasite strains were tested by using two-tailed paired Student's t test. Comparisons of two nonmatching groups (controls versus CPS-immunized volunteers) or comparisons between multiple groups (pre- and postimmunization IgGs and CSP-depleted postimmunization IgGs) were tested with unpaired Student's t test or one-way analysis of variance (ANOVA) with a Bonferroni multiple-comparison post hoc test, respectively. Correlation analyses were conducted with Spearman correlation analysis. A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the volunteers who participated in the trial, and we thank Jolanda Klaassen, Laura Pelser-Posthumus, Jacqueline Kuhnen, and Astrid Pouwelsen for technical assistance with the generation of infected mosquitoes and salivary gland dissections. Additionally, we thank Will Roeffen and Karina Teelen for the generation, testing, and validation of the CSP affinity column.
The clinical trials from which samples were used in the present study were funded by the Bill and Melinda Gates Foundation (grants OPP1080385 and OPP1091355). The funder had no role in study design, data collection, analysis or interpretation of the data, the preparation of the manuscript, or the decision to submit the work for publication. M.C.B. is supported by a Nijmegen Institute for Infection, Inflammation, and Immunity (N4i) Ph.D. scholarship.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00920-17.
REFERENCES
- 1.WHO. 2015. World malaria report 2015. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Stewart MJ, Vanderberg JP. 1988. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool 35:389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 3.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. 2001. Migration of Plasmodium sporozoites through cells before infection. Science 291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 4.Menard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. 2013. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol 11:701–712. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 5.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemingway J, Ranson H. 2000. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 7.Casares S, Brumeanu TD, Richie TL. 2010. The RTS,S malaria vaccine. Vaccine 28:4880–4894. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Walsh DS, Pichyangkul S, Gettayacamin M, Tongtawe P, Siegrist CA, Hansukjariya P, Kester KE, Holland CA, Voss G, Cohen J, Stewart AV, Miller RS, Ballou WR, Heppner DG Jr. 2004. Safety and immunogenicity of rts,s+trap malaria vaccine, formulated in the as02a adjuvant system, in infant rhesus monkeys. Am J Trop Med Hyg 70:499–509. [PubMed] [Google Scholar]
- 9.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, Walsh DS, Yoon IK, Prosperi C, Juompan LY, Lanar DE, Krzych U, Hall BT, Ware LA, Stewart VA, Williams J, Dowler M, Nielsen RK, Hillier CJ, Giersing BK, Dubovsky F, Malkin E, Tucker K, Dubois MC, Cohen JD, Ballou WR, Heppner DG Jr. 2010. Recombinant liver stage antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, Held J, Calle CL, Mengue JB, Gebru T, Ibanez J, Sulyok M, James ER, Billingsley PF, Natasha KC, Manoj A, Murshedkar T, Gunasekera A, Eappen AG, Li T, Stafford RE, Li M, Felgner PL, Seder RA, Richie TL, Sim BK, Hoffman SL, Kremsner PG. 2017. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542:445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 12.Bijker EM, Teirlinck AC, Schats R, van Gemert GJ, van de Vegte-Bolmer M, van Lieshout L, IntHout J, Hermsen CC, Scholzen A, Visser LG, Sauerwein RW. 2014. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis 210:1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, Remarque EJ, Roeffen W, Jansens A, Zimmerman D, Vos M, van Schaijk BC, Wiersma J, van der Ven AJ, de Mast Q, van Lieshout L, Verweij JJ, Hermsen CC, Scholzen A, Sauerwein RW. 2013. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A 110:7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 15.Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, van der Ven AJ, Hermsen CC, Luty AJ, Sauerwein RW. 2011. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 7:e1002389. doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahrendorf W, Scholzen A, Bijker EM, Teirlinck AC, Bastiaens GJ, Schats R, Hermsen CC, Visser LG, Langhorne J, Sauerwein RW. 2014. Memory B-cell and antibody responses induced by Plasmodium falciparum sporozoite immunization. J Infect Dis 210:1981–1990. doi: 10.1093/infdis/jiu354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felgner PL, Roestenberg M, Liang L, Hung C, Jain A, Pablo J, Nakajima-Sasaki R, Molina D, Teelen K, Hermsen CC, Sauerwein R. 2013. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci Rep 3:3549. doi: 10.1038/srep03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behet MC, Foquet L, van Gemert GJ, Bijker EM, Meuleman P, Leroux-Roels G, Hermsen CC, Scholzen A, Sauerwein RW. 2014. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar J 13:136. doi: 10.1186/1475-2875-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. 2015. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol 6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, McCarthy JS, Muller I, Marsh K, Anders RF, Beeson JG. 2015. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtovic L, Behet MC, Feng G, Reiling L, Chelimo K, Dent AE, Mueller I, Kazura JW, Sauerwein RW, Fowkes FJI, Beeson JG. 2018. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med 16:61. doi: 10.1186/s12916-018-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidarsson G, Dekkers G, Rispens T. 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teirlinck AC, Roestenberg M, van de Vegte-Bolmer M, Scholzen A, Heinrichs MJ, Siebelink-Stoter R, Graumans W, van Gemert GJ, Teelen K, Vos MW, Nganou-Makamdop K, Borrmann S, Rozier YP, Erkens MA, Luty AJ, Hermsen CC, Sim BK, van Lieshout L, Hoffman SL, Visser LG, Sauerwein RW. 2013. NF135.C10: a new Plasmodium falciparum clone for controlled human malaria infections. J Infect Dis 207:656–660. doi: 10.1093/infdis/jis725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walk J, Reuling IJ, Behet MC, Meerstein-Kessel L, Graumans W, van Gemert GJ, Siebelink-Stoter R, van de Vegte-Bolmer M, Janssen T, Teelen K, de Wilt JHW, de Mast Q, van der Ven AJ, Diez Benavente E, Campino S, Clark TG, Huynen MA, Hermsen CC, Bijker EM, Scholzen A, Sauerwein RW. 2017. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med 15:168. doi: 10.1186/s12916-017-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touray MG, Seeley DC Jr, Miller LH. 1994. Plasmodium gallinaceum: differential lysis of two developmental stages of malaria sporozoites by the alternative pathway of complement. Exp Parasitol 78:294–301. doi: 10.1006/expr.1994.1031. [DOI] [PubMed] [Google Scholar]
- 26.Stone SL, Lund FE. 2016. IgM memory cells: first responders in malaria. Immunity 45:235–237. doi: 10.1016/j.immuni.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Peng K, Goh YS, Siau A, Franetich JF, Chia WN, Ong AS, Malleret B, Wu YY, Snounou G, Hermsen CC, Adams JH, Mazier D, Preiser PR, Sauerwein RW, Gruner AC, Renia L. 2016. Breadth of humoral response and antigenic targets of sporozoite-inhibitory antibodies associated with sterile protection induced by controlled human malaria infection. Cell Microbiol 18:1739–1750. doi: 10.1111/cmi.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarlinton D, Good-Jacobson K. 2013. Diversity among memory B cells: origin, consequences, and utility. Science 341:1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, Pepper M. 2016. Somatically hypermutated plasmodium-specific IgM(+) memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity 45:402–414. doi: 10.1016/j.immuni.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arama C, Skinner J, Doumtabe D, Portugal S, Tran TM, Jain A, Traore B, Doumbo OK, Davies DH, Troye-Blomberg M, Dolo A, Felgner PL, Crompton PD. 2015. Genetic resistance to malaria is associated with greater enhancement of immunoglobulin (Ig)M than IgG responses to a broad array of Plasmodium falciparum antigens. Open Forum Infect Dis 2:ofv118. doi: 10.1093/ofid/ofv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zenklusen I, Jongo S, Abdulla S, Ramadhani K, Sim BKL, Cardamone H, Flannery EL, Nguyen T, Fishbaugher M, Steel RWJ, Betz W, Carmago N, Mikolajczak S, Kappe SHI, Hoffman SL, Sack BK, Daubenberger C. 8 February 2018. Immunization of malaria pre-exposed volunteers with PfSPZ vaccine elicits long-lived IgM invasion-inhibitory and complement-fixing antibodies. J Infect Dis doi: 10.1093/infdis/jiy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson KJ, Kidd MJ, Wang Y, Collins AM. 2013. The shape of the lymphocyte receptor repertoire: lessons from the B cell receptor. Front Immunol 4:263. doi: 10.3389/fimmu.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson KJ, Wang Y, Collins AM. 2014. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol 92:729–733. doi: 10.1038/icb.2014.44. [DOI] [PubMed] [Google Scholar]
- 34.Burton DR. 1985. Immunoglobulin G: functional sites. Mol Immunol 22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 35.Harris CL, Heurich M, Rodriguez de Cordoba S, Morgan BP. 2012. The complotype: dictating risk for inflammation and infection. Trends Immunol 33:513–521. doi: 10.1016/j.it.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, Krzych U, Delchambre M, Voss G, Dowler MG, Palensky J, Wittes J, Cohen J, Ballou WR, RTS,S Malaria Vaccine Evaluation Group. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis 183:640–647. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 37.Lucas AH. 1990. IgG subclass-restricted immune responses to allergens. Springer Semin Immunopathol 12:385–400. doi: 10.1007/BF00225325. [DOI] [PubMed] [Google Scholar]
- 38.Wright A, Morrison SL. 1997. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol 15:26–32. doi: 10.1016/S0167-7799(96)10062-7. [DOI] [PubMed] [Google Scholar]
- 39.Peipp M, Beyer T, Dechant M, Valerius T. 2008. Chapter 8. Molecular engineering III: Fc engineering. In Dubel S. (ed), Handbook of therapeutic antibodies. Wiley & Sons Ltd, Chichester, United Kingdom. [Google Scholar]
- 40.Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. 2001. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem 276:6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- 41.Han KK, Martinage A. 1992. Possible relationship between coding recognition amino acid sequence motif or residue(s) and post-translational chemical modification of proteins. Int J Biochem 24:1349–1363. doi: 10.1016/0020-711X(92)90060-E. [DOI] [PubMed] [Google Scholar]
- 42.Marshall RD. 1972. Glycoproteins. Annu Rev Biochem 41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- 43.Gowda DC, Davidson EA. 1999. Protein glycosylation in the malaria parasite. Parasitol Today 15:147–152. doi: 10.1016/S0169-4758(99)01412-X. [DOI] [PubMed] [Google Scholar]
- 44.Dunkelberger JR, Song WC. 2010. Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 45.Swearingen KE, Lindner SE, Shi L, Shears MJ, Harupa A, Hopp CS, Vaughan AM, Springer TA, Moritz RL, Kappe SH, Sinnis P. 2016. Interrogating the Plasmodium sporozoite surface: identification of surface-exposed proteins and demonstration of glycosylation on CSP and TRAP by mass spectrometry-based proteomics. PLoS Pathog 12:e1005606. doi: 10.1371/journal.ppat.1005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SJ, Gonzalez-Aseguinolaza G, Nussenzweig MC. 2002. Disseminated candidiasis and hepatic malarial infection in mannose-binding-lectin-A-deficient mice. Mol Cell Biol 22:8199–8203. doi: 10.1128/MCB.22.23.8199-8203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roestenberg M, McCall M, Mollnes TE, van Deuren M, Sprong T, Klasen I, Hermsen CC, Sauerwein RW, van der Ven A. 2007. Complement activation in experimental human malaria infection. Trans R Soc Trop Med Hyg 101:643–649. doi: 10.1016/j.trstmh.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Greenwood BM, Brueton MJ. 1974. Complement activation in children with acute malaria. Clin Exp Immunol 18:267–272. [PMC free article] [PubMed] [Google Scholar]
- 49.Neva FA, Howard WA, Glew RH, Krotoski WA, Gam AA, Collins WE, Atkinson JP, Frank MM. 1974. Relationship of serum complement levels to events of the malarial paroxysm. J Clin Invest 54:451–460. doi: 10.1172/JCI107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glew RH, Atkinson JP, Frank MM, Collins WE, Neva FA. 1975. Serum complement and immunity in experimental simian malaria. I. Cyclical alterations in C4 related to schizont rupture. J Infect Dis 131:17–25. doi: 10.1093/infdis/131.1.17. [DOI] [PubMed] [Google Scholar]
- 51.Srichaikul T, Puwasatien P, Karnjanajetanee J, Bokisch VA, Pawasatien P. 1975. Complement changes and disseminated intravascular coagulation in Plasmodium falciparum malaria. Lancet i:770–772. [DOI] [PubMed] [Google Scholar]
- 52.Phanuphak P, Hanvanich M, Sakulramrung R, Moollaor P, Sitprija V, Phanthumkosol D. 1985. Complement changes in falciparum malaria infection. Clin Exp Immunol 59:571–576. [PMC free article] [PubMed] [Google Scholar]
- 53.Berg A, Otterdal K, Patel S, Gonca M, David C, Dalen I, Nymo S, Nilsson M, Nordling S, Magnusson PU, Ueland T, Prato M, Giribaldi G, Mollnes TE, Aukrust P, Langeland N, Nilsson PH. 2015. Complement activation correlates with disease severity and contributes to cytokine responses in Plasmodium falciparum malaria. J Infect Dis 212:1835–1840. doi: 10.1093/infdis/jiv283. [DOI] [PubMed] [Google Scholar]
- 54.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. 2005. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 55.Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Perignon JL, Druilhe P. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, Kumar S, Chitnis CE, Narum DL, Michon P, Siba PM, Cowman AF, Mueller I, Beeson JG. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51:e50–e60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 58.Delemarre BJ, van der Kaay HJ. 1979. Tropical malaria contracted the natural way in the Netherlands. Ned Tijdschr Geneeskd 123:1981–1982. (In Dutch.) [PubMed] [Google Scholar]
- 59.McCall MBB, Wammes LJ, Langenberg MCC, van Gemert GJ, Walk J, Hermsen CC, Graumans W, Koelewijn R, Franetich JF, Chishimba S, Gerdsen M, Lorthiois A, van de Vegte M, Mazier D, Bijker EM, van Hellemond JJ, van Genderen PJJ, Sauerwein RW. 2017. Infectivity of Plasmodium falciparum sporozoites determines emerging parasitemia in infected volunteers. Sci Transl Med 9:eaag2490. doi: 10.1126/scitranslmed.aag2490. [DOI] [PubMed] [Google Scholar]
- 60.Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW. 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol 118:247–251. doi: 10.1016/S0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 61.Walk J, Schats R, Langenberg MC, Reuling IJ, Teelen K, Roestenberg M, Hermsen CC, Visser LG, Sauerwein RW. 2016. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J 15:398. doi: 10.1186/s12936-016-1434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delemarre-van de Waal HA, de Waal FC. 1981. A 2d patient with tropical malaria contracted in a natural way in the Netherlands. Ned Tijdschr Geneeskd 125:375–377. (In Dutch.) [PubMed] [Google Scholar]
- 63.Ifediba T, Vanderberg JP. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 64.Ponnudurai T, Lensen AH, Leeuwenberg AD, Meuwissen JH. 1982. Cultivation of fertile Plasmodium falciparum gametocytes in semi-automated systems. 1. Static cultures. Trans R Soc Trop Med Hyg 76:812–818. doi: 10.1016/0035-9203(82)90116-X. [DOI] [PubMed] [Google Scholar]
- 65.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. 1986. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology 93(Part 2):263–274. doi: 10.1017/S003118200005143X. [DOI] [PubMed] [Google Scholar]
- 66.Ponnudurai T, Lensen AH, Van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98(Part 2):165–173. doi: 10.1017/S0031182000062065. [DOI] [PubMed] [Google Scholar]
- 67.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, Udomsangpetch R, Cui L, Brewer TG. 2006. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg 74:708–715. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.