Abstract
Tetrapyrroles such as chlorophylls and bacteriochlorophylls play a fundamental role in the energy absorption and transduction activities of photosynthetic organisms. Because of these molecules, however, photosynthetic organisms are also prone to photooxidative damage. They had to evolve highly efficient strategies to control tetrapyrrole biosynthesis and to prevent the accumulation of free intermediates that potentially are extremely destructive when illuminated. In higher plants, the metabolic flow of tetrapyrrole biosynthesis is regulated at the step of δ-aminolevulinic acid synthesis. This regulation previously has been attributed to feedback control of Glu tRNA reductase, the first enzyme committed to tetrapyrrole biosynthesis, by heme. With the recent discovery of chlorophyll intermediates acting as signals that control both nuclear gene activities and tetrapyrrole biosynthesis, it seems likely that heme is not the only regulator of this pathway. A genetic approach was used to identify additional factors involved in the control of tetrapyrrole biosynthesis. In Arabidopsis thaliana, we have found a negative regulator of tetrapyrrole biosynthesis, FLU, which operates independently of heme and seems to selectively affect only the Mg2+ branch of tetrapyrrole biosynthesis. The identity of this protein was established by map-based cloning and sequencing the FLU gene. FLU is a nuclear-encoded plastid protein that, after import and processing, becomes tightly associated with plastid membranes. It is unrelated to any of the enzymes known to be involved in tetrapyrrole biosynthesis. Its predicted features suggest that FLU mediates its regulatory effect through interaction with enzymes involved in chlorophyll synthesis.
In higher plants, at least three distinct classes of tetrapyrroles can be distinguished, Mg2+ porphyrins [chlorophylls (Chl)], Fe2+/3+ porphyrins (hemes), and phycobilins (phytochromobilin; ref. 1). All of them strongly absorb light and may act as photosensitizers (2, 3). Chls, hemes, and phycobilins are bound to proteins and in this state may use various quenching mechanisms to dissipate absorbed light energy. Their biosynthetic precursors, however, occur mostly in a free form and are potentially much more destructive when illuminated (3–5). Angiosperms, the most highly evolved group of plants, use a very efficient strategy to prevent the accumulation of such intermediates by regulating the metabolic flow at the step of δ-aminolevulinic acid (ALA) synthesis (1, 6). In the dark, the Chl synthesis pathway leads only to the formation of protochlorophyllide (Pchlide), the immediate precursor of chlorophyllide (Chlide). Once a critical level of Pchlide has been reached, ALA synthesis slows down. Only after illumination, when Pchlide has been photoreduced to Chlide by the NADPH–Pchlide-oxidoreductase (POR), does Chl biosynthesis resume (7). This regulation of Chl biosynthesis has been attributed to feedback control of ALA synthesis. In analogy to its regulatory role in animals and yeast (8, 9), heme has been proposed to act also in plants as an effector of feedback inhibition of tetrapyrrole biosynthesis (1). Several lines of evidence support this assumed function of heme. The activity of Glu tRNA reductase, the first enzyme committed to ALA synthesis and the most likely target of feedback control, has been shown to be inhibited in vitro by heme (10, 11). Inactivation of a heme oxygenase gene perturbs the breakdown of heme, attenuates the rate of ALA synthesis, and suppresses Pchlide accumulation in etiolated seedlings (12–14). Conversely, removal of free Fe2+/3+ by the addition of an iron chelator leads to a decline of the heme level and causes an increase in the level of Pchlide (15).
Even though these data emphasize the potential importance of free heme as an effector of feedback control, it seems unlikely that heme alone is sufficient to coordinate and adjust all activities of the various branches of tetrapyrrole biosynthesis in higher plants. The synthesis of hemes, phycobilins, and Chls share a common pathway until the level of protoporphyrin IX. At the point of metal ion insertion the pathway diverges, one route being directed to the synthesis of hemes and phycobilins and the other giving rise to the formation of Chl (1). Because Chl and heme are being synthesized by plants in very different amounts depending on the developmental stages and/or light conditions, the relative activities of the first enzymes of the Mg2+ and Fe2+ branches of tetrapyrrole biosynthesis, Mg2+ chelatase and ferrochelatase, must undergo drastic changes under these varying conditions. Although in etiolated seedlings roughly similar amounts of Pchlide and heme accumulate, in the light the major resource allocation of tetrapyrrole biosynthesis is directed toward the massive accumulation of Chl (16, 17). Chl in its free form would cause extensive photooxidative damage if exposed to light. Thus, formation of Chl has to be closely coordinated and linked to the synthesis of Chl-binding proteins. Because nuclear genes encode most of these proteins, Chl accumulation requires an intimate interaction between the plastid compartment and the nucleus. Intermediates of tetrapyrrole synthesis such as Mg2+ protoporphyrin IX, protoporphyrinogen IX, and linear tetrapyrroles as well as subunit H of Mg2+ chelatase have been implicated in signaling and retrograde control of nuclear gene activities (18–21). These components can be expected to form part of a more complex regulatory network involved in coordinating the activities of the various branches of tetrapyrrole biosynthesis within the plastid and tuning them to the expression of nuclear genes. Here we describe the discovery of a negative regulator of tetrapyrrole biosynthesis that seems to affect selectively only the Mg2+ branch.
Methods
Isolation of the flu Mutant.
Chemical mutagenesis of seeds from Arabidopsis thaliana ecotype Landsberg erecta (Ler) with ethyl methanesulfonate (EMS) was performed as described (22). Seeds from each M1 plant were collected separately. For the subsequent screening, a total of 1,500 seed families were analyzed. From each of the seed families, ≈100 etiolated seedlings were illuminated with blue light and examined under the Leica MZ12 fluorescence microscope with a Leica FM blue 10446146 filter. Mutants that emitted a bright red fluorescence in their cotyledons were identified. Homozygous mutants could be rescued by growing them under continuous light.
Cloning of the FLU Gene.
The information about PCR-based cleaved amplified polymorphic sequence (CAPS) and simple sequence length polymorphism (SSLP) genetic markers was obtained from The Arabidopsis Information Resource (TAIR) database (http://www.arabidopsis.org/). Nine new genetic markers were generated by using A. thaliana ecotype Columbia (Col) DNA sequence information available from the Kazusa DNA Research Institute (http://www.kazusa.or.jp/). To find DNA polymorphisms between the Ler and Col ecotypes, specific primers were designed for PCR amplification from Ler and Col genomic DNA, and the resulting DNA fragments were digested with various restriction enzymes. The bacterial artificial chromosome (BAC) contig, including marker nga162, is available from TAIR database (23). The BAC clones were obtained from the Arabidopsis Biological Resource Center (ABRC) and checked by PCR with specific primers. BAC DNA was partially digested with HindIII and subcloned in the cosmid vector pBIC20 (24). The cosmid contig was assembled by using three sets of data: the restriction pattern of individual cosmid clones fully digested with HindIII, computer restriction analysis of the region, and PCR analysis of cosmid clones. Cosmid DNA of seventeen selected clones was transferred to Agrobacterium tumefaciens C58 by using electroporation (25). Homozygous flu plants grown under continuous light were transformed by using Agrobacterium as described (26). Primary transformants were selected on kanamycin-containing medium under continuous light and tested for the insertion of the complete T-DNA by histochemical β-glucuronidase (GUS)-staining of leaves (24). The ORFs within the complementing DNA fragment were predicted by the Kazusa DNA Research Institute (annotation for clone MAG2). The probes for gene expression studies were obtained by reverse transcription–PCR from total mRNA of light-grown Ler seedlings with specific primers designed by using the predicted exon-intron structures of the genes. Single base pair exchanges in the four allelic flu mutants were found by direct sequencing of the PCR products amplified from genomic DNA. Products of four independent PCR reactions were sequenced in each case. For the final complementation assay, a 4.6-kb ScaI-ScaI genomic DNA fragment from the complementing cosmid 28 was subcloned in the plant transformation vector pRD400 [kindly provided by S. Melzer, Institute for Plant Sciences, Swiss Federal Institute of Technology (ETH), Zürich].
Localization of the FLU Protein.
The FLU cDNA was amplified from total Ler cDNA with primers designed according to the predicted translation start and stop sites, subcloned in the pET21d vector (Novagen), and sequenced. FLU was translated in vitro in the presence of [35S]methionine by using a coupled transcription/translation system (Promega) containing reticulocyte lysate. Isolation of pea chloroplasts and the import of in vitro synthesized precursor proteins were performed as described (27). Separation of chloroplasts into soluble and membrane fractions was performed as described (28).
Other Methods.
For homology searches and protein structure predictions, proteomics tools collected in the ExPASy Molecular Biology Server (http://www.expasy.ch/) were used. The rate of ALA synthesis was determined according to ref. 29, Pchlide was measured spectroscopically (22), and the level of free heme was measured enzymatically (30).
Results and Discussion
Isolation of the flu Mutant.
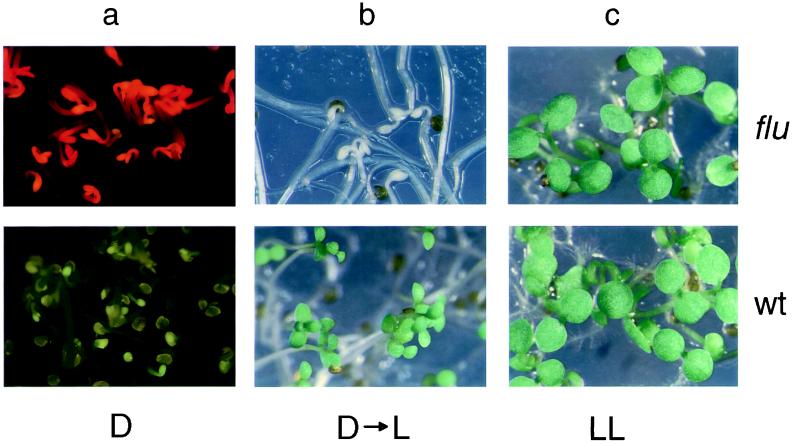
We have used a genetic approach to dissect the control mechanism of tetrapyrrole biosynthesis in A. thaliana. First, mutants were identified that are no longer able to restrict the accumulation of Pchlide in the dark. Such mutants can be distinguished from wild-type (wt) seedlings by the strong Pchlide fluorescence that etiolated mutant seedlings emit after they have been exposed to blue light (Fig. 1a). Because of this trait, these mutants have been named flu (fluorescent). They resemble dark-grown seedlings that were fed exogenous ALA (6) and etiolated tigrina mutants of barley (31). Of a total of 1,500 seed families of ethyl methanesulfonate-mutagenized Arabidopsis plants, four independent flu mutants were identified. They were rescued by identifying heterozygous plants derived from the same seed family. These M2 plants segregated flu mutants in a 1:3 ratio. When etiolated flu seedlings were transferred from the dark to the light, they rapidly bleached and died (Fig. 1b). The homozygous mutants could be rescued, however, by germinating the seedlings under constant light (Fig. 1c). Crosses between the four flu mutants revealed that they were allelic and hence represented a single gene.
Figure 1.
flu and wt seedlings of A. thaliana grown in the dark (a), under nonpermissive dark to light (b), or permissive continuous light (c) conditions. (a) Etiolated mutant and wt seedlings were exposed to blue light (400–450 nm), and the emitted fluorescence was recorded. The bright red fluorescence emitted by the mutant is caused by the excitation of free Pchlide. (b) Etiolated mutant and wt seedlings were transferred from the dark to continuous white light for 12 h.
Identification of FLU by Map-Based Cloning.
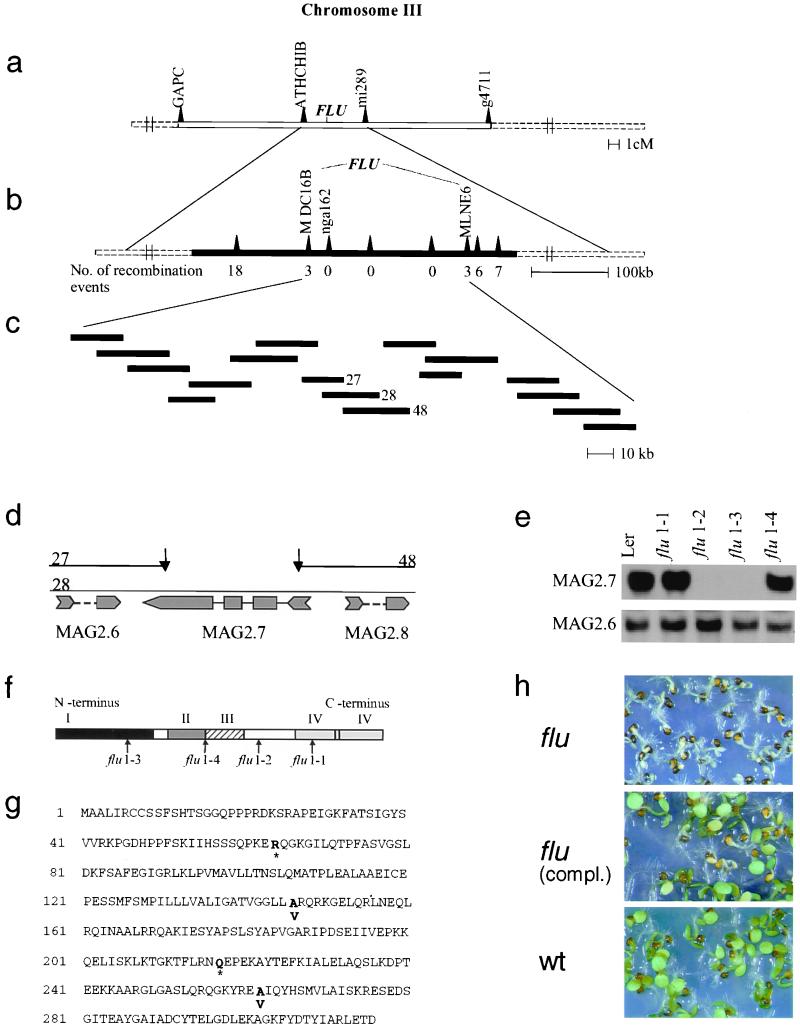
As a first step toward its functional characterization, we have used a map-based cloning strategy to isolate FLU. We genetically mapped FLU in F2 plants from a cross between the flu/flu mutant Ler and wt FLU/FLU plants of ecotype Col. Tests on 80 plants for genetic linkage between the flu phenotype and cleaved amplified polymorphic sequence (CAPS) and simple sequence length polymorphism (SSLP) markers placed FLU on chromosome 3 (Fig. 2a). For the subsequent fine mapping, the size of the mapping population was increased to 960 F2 plants. FLU was located on a genomic fragment of ≈210 kb (Fig. 2b). A contig consisting of 17 overlapping cosmid clones that encompassed this chromosomal region was generated (Fig. 2c). Each of the 17 genomic fragments was stably integrated into the genome of homozygous flu/flu plants by using A. tumefaciens for transformation. Seeds from each of the primary transformants were collected and germinated under light/dark cycles. The flu phenotype could easily be scored by the photobleaching of the homozygous mutant plants. One of the cloned genomic fragments (clone 28) complemented the flu mutation (data not shown). The neighboring cosmid clones 27 and 48 of the contig that overlapped to a large extent with the insert of the cosmid clone 28 did not complement the flu mutation. The nonoverlapping part of the complementing genomic fragment has a size of 1.4 kb. It contains a single ORF (MAG 2.7; Fig. 2d). Specific primers for this and the two adjacent genes (MAG 2.6 and MAG 2.8) were used to generate gene-specific probes for the detection of the corresponding transcripts in the four allelic flu mutants by Northern blot analysis. Two of the three genes, MAG 2.6 and 2.7, were expressed. Although the transcript levels of the MAG 2.6 gene were similar in all four mutants and the wt, transcripts of the MAG 2.7 gene were detectable only in the flu 1–1 and flu 1–4 mutants at levels similar to those in wt plants, but not in the flu 1–2 and 1–3 mutants (Fig. 2e). The DNA regions of all four allelic flu mutants covering the ORF of this gene and an additional 200 bp upstream were sequenced. In all four mutants, single point mutations were detected in this region. In two of the mutants, flu 1–2 and 1–3, these changes led to the formation of stop codons within the ORF that might explain why the corresponding transcripts were not detectable. mRNAs with premature stop codons have been shown to be recognized by a surveillance complex and to be selectively degraded (32). In flu 1–1 and 1–4, single base exchanges resulted in an amino acid exchange from alanine to valine (Fig. 2 f and g) without affecting the apparent abundance of the mRNA. A 4.6-kb genomic fragment of wt DNA, containing the ORF of MAG 2.7, a 1.7-kb promoter region, and a 1.5-kb downstream sequence, was used for a final complementation test to confirm the identification of the FLU gene. Primary transformants were selected on kanamycin. Seeds of these plants were collected and germinated under light/dark cycles. Seedlings of nontransformed flu plants were photobleached under these conditions, whereas seedlings of flu complemented with MAG 2.7 segregated the wt phenotype in a 3:1 ratio (Fig. 2h).
Figure 2.
Identification of the FLU gene. Genetic (a) and physical (b) map of the DNA region on chromosome III of A. thaliana that contains the FLU gene. (c) The region between the markers MDC16B and MLNE6 was encompassed by 17 partially overlapping cosmid clones that were used for the complementation test. Four bacterial artificial chromosome (BAC) clones fully covered this region containing the FLU gene. (d) Details of the physical map of the nonoverlapping part of the cosmid clones 27, 28, and 48. Three ORFs, MAG 2.6, MAG 2.7, and MAG 2.8, have been deduced by the Kazusa DNA Research Institute. (e) Northern blot analysis of RNA extracted from wt (Ler) and the four allelic flu mutants 1–1, -2, -3, -4 grown under continuous white light. Gene-specific probes of MAG 2.6, -2.7, and -2.8 were used for hybridization. Transcripts of MAG 2.8 were not detectable (data not shown). (f) A schematic presentation of the structure of the FLU protein with four domains (I-IV): I, a putative chloroplast signal peptide; II, a hydrophobic region; III, a coiled coil motif; and IV, a TPR region with two TPR motives. Arrows indicate the locations of mutations in the four allelic flu mutants. The extent of the putative chloroplast signal peptide was deduced from the difference in apparent molecular mass of the FLU precursor protein and its mature form that was present inside the plastid (see also Fig. 3). (g) The derived amino acid sequence of FLU deduced from the FLU cDNA sequence. Bold letters indicate positions of mutational changes of the ORF that lead to the premature termination of the polypeptide chain (*) or to amino acid exchanges. Single base pair exchanges in the four allelic flu mutants were found by direct sequencing of PCR products amplified from genomic DNA. (h) The identification of the FLU gene was confirmed by the complementation of the flu mutant with a genomic fragment of wt DNA containing MAG 2.7.
FLU Encodes a Protein with Two Putative Protein-Binding Domains That Is Imported into Plastids and Tightly Associated with Membranes.
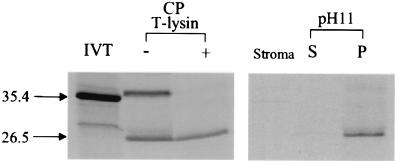
A FLU cDNA was synthesized from cDNA derived from total RNA of Arabidopsis seedlings by using gene-specific primers. The ORF of the FLU cDNA predicts a protein of 316 amino acids that is unrelated to any of the enzymes known to be involved in tetrapyrrole biosynthesis and that has not been described previously. Nevertheless, the predicted FLU protein reveals features that may be important for its presumptive function during feedback control. It contains an N-terminal extension that resembles import signal sequences of nuclear-encoded plastid proteins (33). Furthermore, the central part of FLU ranging from amino acid position 125 to 146 consists of a hydrophobic domain that may be important for anchoring the protein within a membrane, whereas the C-terminal part is hydrophilic (Fig. 2 f and g). We tested these predictions deduced from the sequence of the FLU gene. FLU was synthesized in a coupled transcription/translation system in the presence of [35S]methionine and then imported into chloroplasts isolated from 12-day-old pea seedlings. At the end of the incubation, the plastid proteins were dissolved and separated electrophoretically. Two major radioactively labeled proteins could be detected by autoradiography, one with an apparent molecular mass (MM) of 35.4 kDa similar to the size of the in vitro-synthesized product of the FLU gene and the second with an apparent MM of 26.5 kDa (Fig. 3). After treatment of chloroplasts with thermolysin, most of the 35.4-kDa polypeptide had been digested, whereas the 26.5-kDa protein was protected against proteolytic attack. This latter protein seems to represent the imported and processed form of the FLU protein (Fig. 3). After import, chloroplasts were lysed, and the membranes were separated from the soluble stroma fraction by centrifugation at 100,000 × g for 20 min. FLU was recovered only in the membrane but not in the stroma fraction (Fig. 3), whereas the imported small subunit of ribulose 1,5-bis-P-carboxylase that served as a positive control was mostly recovered from the stroma fraction, as expected (data not shown). When the membranes were extracted with an alkaline buffer (200 mM Na2CO3, pH 11), the FLU protein was not released but remained tightly associated with plastid membranes (Fig. 3).
Figure 3.
The import of FLU into isolated chloroplasts of pea. FLU was synthesized by coupled in vitro transcription/translation of the FLU cDNA (IVT) and incubated with isolated intact chloroplasts (CP) of light-grown pea seedlings. Two major radioactively labeled protein bands of apparent molecular masses of 35.4 and 26.5 kDa were separated electrophoretically (− T-lysin). After thermolysin treatment of chloroplasts, only the 26.5-kDa protein was protected against proteolytic digestion and thus seemed to represent the imported mature form of the FLU protein (+ T-lysin). After the lysis of these chloroplasts, FLU was not detectable in the stroma fraction but was bound to membranes. After extracting the membranes with an alkaline buffer (pH 11) and centrifugation at 100,000 × g for 20 min, FLU was not released to the supernatant (S) but remained tightly associated with membranes (P).
Database searches indicate that the hydrophilic half of FLU contains two different regions implicated in protein–protein interactions. Two tetratricopeptide repeats (TPRs) were predicted for the C-terminal region. Based on structural studies, the minimum number of TPR motives required for protein–protein interactions has been proposed to be 3 (34). Because TPR motives are highly degenerate and are difficult to recognize, a third cryptic motif may exist in FLU that is able to complement the predicted pair of TPR motives to form a functional unit. This interpretation fits with the observed amino acid substitution in the flu 1–1 mutant. The alanine residue at position 20 in the first predicted TPR motif is one of the few conserved amino acids of TPR motives (35). Its replacement by a valine leads to the inactivation of FLU. The second region possibly engaged in protein–protein interaction is a short coiled coil motif adjacent to the hydrophobic membrane anchor of FLU. Notably, the amino acid substitution in flu 1–4 is found in this region.
FLU-Dependent Regulation of Chl Synthesis: A Model.
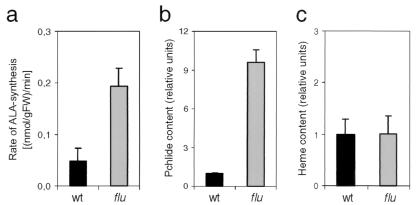
If FLU forms part of the feedback loop that down-regulates ALA synthesis in dark-grown seedlings, its inactivation should result in an enhanced rate of ALA formation. As shown in Fig. 4a, the rate of ALA synthesis in the flu mutant does indeed exceed that of the wt by a factor of 3 to 4. Two different mechanisms have been considered previously to explain the regulation of ALA formation: light-induced changes in the synthesis of enzymes required for its formation (36, 37) and the removal of an inhibitor affecting the activity of one of these enzymes (1, 38). We have tested the former possibility by measuring the concentrations of mRNAs for the two enzymes committed exclusively to ALA synthesis: Glu tRNA reductase and Glu 1-semialdehyde aminotransferase (GSA). Drastic light/dark fluctuations of these two mRNAs have been described earlier for Arabidopsis (39). These changes in mRNA levels during a light/dark shift could form the basis for the restriction of ALA synthesis in the dark. In such a case, the enhanced rate of ALA synthesis in flu mutants transferred to the dark could be caused by a constitutive up-regulation of the two mRNAs. However, in the mutant the two transcripts showed the same fluctuations during a light to dark transition as the wt plants (data not shown). Also in the tigrina-d12 mutant of barley that resembles closely the flu mutant of Arabidopsis, accumulation of Pchlide in etiolated mutant seedlings could not be explained by a change in the concentrations of enzymes involved in ALA synthesis and their mRNAs (40). Therefore, we favor the second model that predicts direct metabolic feedback inhibition of tetrapyrrole biosynthesis. In one of the first models of metabolic feedback control, Pchlide was proposed to act as a regulatory factor and to inhibit one of the steps leading to ALA synthesis (1, 41). The validity of this model was tested after the putative target enzymes of feedback control, Glu tRNA reductase and GSA, had been identified and purified. As it turned out, the activities of both enzymes were not affected by Pchlide; instead, Glu tRNA reductase was shown to be inhibited by heme at a site distinct from the catalytic center of the enzyme (10, 11). Based on these in vitro studies, the proposed regulatory role of Pchlide was abandoned in subsequent models, and control of tetrapyrrole biosynthesis in higher plants was exclusively attributed to heme (42). Only recently has this proposed supreme role of heme been questioned by the results of studies with plants with a reduced activity of Mg2+ chelatase, the first enzyme committed to the synthesis of Mg2+ porphyrins (43). This block in the pathway did not result in the overaccumulation of the enzyme's substrate but to its down-regulation (43). At the same time, the rate of ALA synthesis was also reduced in these plants. Because the concentration of heme was significantly lower than in control plants, this inhibition of tetrapyrrole biosynthesis had to result from a control mechanism that operates separately from the heme-dependent regulation. A similar dual-negative control of tetrapyrrole biosynthesis had been proposed earlier, based on studies of double-mutant lines of trigrina-d12 and two xantha mutants of barley that were blocked in the conversion of protoporphyrin IX to Mg2+ protoprophyrin IX (44).
Figure 4.
A comparison of the rates of ALA synthesis (a), Pchlide (b), and heme (c) contents of wt (black bars) and flu (gray bars). The rates of ALA synthesis were measured in seedlings grown for 6 days in continuous light and returned to the dark for 30 min. Pchlide was measured spectroscopically, and the level of free heme was measured enzymatically in etiolated seedlings. Each of the experiments was repeated 3 times.
If FLU forms part of the heme-dependent feedback loop, its inactivation in the flu mutant should not only enhance the level of Pchlide (Fig. 4b) but also the level of free heme. However, in etiolated flu mutants only the level of Pchlide but not that of free heme was higher than in wt seedlings (Fig. 4c). Pchlide would be an attractive candidate for a tetrapyrrole intermediate that operates within the second metabolic feedback control circuit. Pchlide has been proposed to inhibit Mg2+ chelatase once the pigment has reached a critical level in the dark (1). One could envisage this negative signal being passed on to one of the two enzymes committed to ALA synthesis, Glu tRNA reductase or GSA, either by Mg2+ chelatase (43) or by protoporphyrin IX, which has been proposed to act as a strong feedback inhibitor of Chl synthesis (44). The proposed inhibition of Mg2+ chelatase by Pchlide could not be confirmed, however, by in vitro studies (45). The discovery of FLU as a negative regulator of Pchlide accumulation may explain this apparent discrepancy (Fig. 5). Pchlide has been shown to form part of a photoactive ternary complex in plastids together with Pchlide-oxidoreductase (POR) and NADPH and to be localized in the hydrophobic environment of prolamellar bodies, plastid envelopes, and thylakoid membranes. Direct interaction with Glu tRNA reductase, GSA, or Mg2+ chelatase may not be feasible. Although Glu tRNA reductase and GSA seem to be localized exclusively within the hydrophilic stroma (46), subunit H of Mg2+ chelatase is retained at the membrane surface at higher concentrations of Mg2+, although at lower concentrations it is released to the stroma (47). Changes in Mg2+ concentration that affect this reversible attachment of subunit H to the membrane surface are within the physiological concentration range in the stroma with 1–3 mM in the dark and an increase to 3–6 mM in the light (48). The FLU protein could be necessary to bridge the gap between the membrane and the stroma and to facilitate the interaction between the putative effector of feedback inhibition and hydrophilic target enzymes. Within the membrane, Pchlide may associate with the hydrophobic membrane anchor of FLU, whereas the hydrophilic part of FLU with its two putative protein-interacting domains may interact with the Glu tRNA reductase, GSA, and/or subunit H of Mg2+ chelatase (Fig. 5). Although purely speculative at this time, this model is amenable to experimental tests.
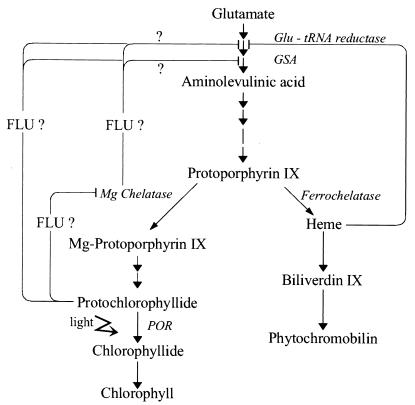
Figure 5.
A simplified scheme of the tetrapyrrole biosynthesis pathway of higher plants. It predicts two metabolic feedback loops, one that is controlled by free heme and a second that depends on FLU. Enzymes that may be subject to control have been indicated. As shown by question marks, there are several possibilities of how FLU could regulate ALA synthesis.
Acknowledgments
We thank E. Grill and G. Benning (Technical University, Munich) for their advice and support concerning experimental procedures, W. Gruissem and G. Armstrong for critical comments, D. Rubli for photography, and R. Langjahr for editorial work. This work was supported by grants from the Swiss Federal Institute of Technology (ETH) and the Swiss National Science Foundation. A major part of this work has been performed by R.M. in partial fulfillment of the requirements for her Ph.D. degree.
Abbreviations
- Chl
chlorophyll
- Pchlide
protochlorophyllide
- ALA
δ-aminolevulinic acid
- GSA
Glu 1-semialdehyde aminotransferase
- wt
wild type
- TPR
tetratricopeptide repeat
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beale S I, Weinstein J D. In: Biosynthesis of Heme and Chorophyll. Dailey H A, editor. New York: McGraw–Hill; 1990. pp. 287–391. [Google Scholar]
- 2.Spikes J D, Bommer J C. In: Chlorophylls. Scheer H, editor. Boca Raton, FL: CRC; 1991. pp. 1181–1204. [Google Scholar]
- 3.Rebeiz C A, Montazer-Zouhoor A, Mayasich J M, Tripathay B C, Wu S-M, Rebeiz C. CRC Crit Rev Plant Sci. 1988;6:385–436. [Google Scholar]
- 4.Matringe M, Camadro J M, Labbe P, Scalla R. Biochem J. 1989;260:231–235. doi: 10.1042/bj2600231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mock H-P, Keetman U, Kruse E, Rank B, Grimm B. Plant Physiol. 1998;116:107–116. [Google Scholar]
- 6.Granick S. Plant Physiol. 1959. , Suppl. 34, 18. [Google Scholar]
- 7.Reinbothe S, Reinbothe C, Lebedev N, Apel K. Plant Cell. 1996;8:763–769. doi: 10.1105/tpc.8.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrew T, Riley P G, Dailey H A. In: Biosynthesis of Heme and Chlorophyll. Dailey H A, editor. New York: McGraw–Hill; 1990. pp. 163–200. [Google Scholar]
- 9.Labbe-Bois R, Labbe P. In: Biosynthesis of Heme and Chlorophyll. Dailey H A, editor. New York: McGraw–Hill; 1990. pp. 235–285. [Google Scholar]
- 10.Pontoppidan B, Kannangara C G. Eur J Biochem. 1995;225:529–537. doi: 10.1111/j.1432-1033.1994.00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Vothknecht U C, Kannangara C G, v. Wettstein D. Phytochemistry. 1998;47:513–519. doi: 10.1016/s0031-9422(97)00538-4. [DOI] [PubMed] [Google Scholar]
- 12.Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman H M. Plant Cell. 1999;11:335–347. doi: 10.1105/tpc.11.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis S J, Kurepa J, Vierstra R D. Proc Natl Acad Sci USA. 1999;96:6541–6546. doi: 10.1073/pnas.96.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terry M J, Kendrick R E. Plant Physiol. 1999;119:143–152. doi: 10.1104/pp.119.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggan J, Gassman M L. Plant Physiol. 1974;53:206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelfranco P A, Jones O T G. Plant Physiol. 1975;55:485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stillman L C, Gassman M. Plant Physiol. 1978;62:182–184. doi: 10.1104/pp.62.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kropat J, Oster U, Rüdiger W, Beck C F. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery B L, Yeh K-C, Crepeau M, Lagarias J C. Plant Physiol. 1999;121:629–639. doi: 10.1104/pp.121.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moeller S G, Kunkel T, Chua N-H. Genes Dev. 2001;15:90–103. doi: 10.1101/gad.850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochizuki N, Brusslan J A, Larkin B, Nagatani A, Chory J. Proc Natl Acad Sci USA. 2001;98:2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Runge S, v. Cleve B, Lebedev N, Armstrong G, Apel K. Planta. 1995;197:490–500. doi: 10.1007/BF00196671. [DOI] [PubMed] [Google Scholar]
- 23.Mozo T, Fischer S, Shizuya H, Altmann T. Mol Gen Genet. 1998;259:562–570. doi: 10.1007/s004380050769. [DOI] [PubMed] [Google Scholar]
- 24.Meyer K, Leube M P, Grill E. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 25.Ausubel F M, Brent R, Konston R E, Moore D D, Seiodman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 26.Bechtold N, Ellis J, Pelletier G. C R Acad Sci Paris, Life Sciences. 1993;316:1194–1199. [Google Scholar]
- 27.Chen D, Schnell D J. J Biol Chem. 1997;272:6614–6620. doi: 10.1074/jbc.272.10.6614. [DOI] [PubMed] [Google Scholar]
- 28.Schnell D J, Blobel G. J Cell Biol. 1993;120:103–115. doi: 10.1083/jcb.120.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauzerall D, Granick S. J Biol Chem. 1956;219:435–446. [PubMed] [Google Scholar]
- 30.Thomas J, Weinstein J D. Plant Physiol Biochem. 1992;30:285–292. [Google Scholar]
- 31.Nielsen O F. Hereditas. 1974;76:269–304. doi: 10.1111/j.1601-5223.1974.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Czaplinski K, Rao Y, Peltz S W. EMBO J. 2001;20:880–890. doi: 10.1093/emboj/20.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Das A K, Cohen P W, Barford D. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano T, Kinoshita N, Morikawa K, Janagida M. Cell. 1990;60:319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- 36.Nadler K, Granick S. Plant Physiol. 1970;46:240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papenbrock J, Mock H-P, Kruse E, Grimm B. Planta. 1999;208:264–273. [Google Scholar]
- 38.Fluhr R, Harel E, Klein S, Meller E. Plant Physiol. 1975;56:497–501. doi: 10.1104/pp.56.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilag L L, Kumar A M, Söll D. Plant Cell. 1994;6:265–275. doi: 10.1105/tpc.6.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansson M, Gough S P, Kannangara C G, v. Wettstein D. Plant Physiol Biochem. 1997;35:827–836. [Google Scholar]
- 41.Bogorad L. In: Chemistry and Biochemistry of Plant Pigments. 2nd Ed. Goodwin T W, editor. New York: Academic; 1976. pp. 64–148. [Google Scholar]
- 42.Beale S I. Photosynth Res. 1999;60:43–73. [Google Scholar]
- 43.Papenbrock J, Mock H-P, Tanaka R, Kruse E, Grimm B. Plant Physiol. 2000;122:1161–1169. doi: 10.1104/pp.122.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn A, Avivi-Bleiser N, v. Wettstein D. In: Genetics and Biogenesis of Chloroplasts and Mitochondria. Bŭcher T, Neŭpert W, Sebald W, Werner S, editors. Amsterdam: Elsevier/North-Holland Biomedical; 1976. pp. 119–131. [Google Scholar]
- 45.Walker C J, Weinstein J D. Plant Physiol. 1991;95:1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.v. Wettstein D, Gough S, Kannangara C G. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson L C D, Marrison J L, Leech R M, Jensen P E, Bassham D C, Gibson M, Hunter C N. Plant Physiol. 1996;111:61–71. doi: 10.1104/pp.111.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malkin R, Niyogi K. In: Biochemistry and Molecular Biology of Plants. Buchanan B, Gruissem W, Jones R, editors. Rockville, MD: Amer. Soc. Plant Biol.; 2000. pp. 568–628. [Google Scholar]