Abstract
Background/aim
Ulcerative colitis (UC) patients are at increased risk for colorectal carcinoma (CRC). It is suggested that cyclooxygenase-2 (COX-2) plays a role in sporadic CRC. The p53 gene is a tumor-suppressor gene and the most frequent site of genetic alteration found in human cancer. The aim of this study was to analyze the immunoexpression of proinflammatory enzyme COX-2 and p53 in UC, UC-associated dysplasia, and CRC, in comparison with each other and with different clinical and histopathological parameters, to clarify if they have a possible role in the pathogenesis of CRC in UC patients.
Materials and methods
In this cross-sectional study, 98 patients were divided into three groups: 39 patients with UC without dysplasia, 32 patients with UC with dysplasia, and 27 patients with colorectal cancer on top of UC, in addition to 10 healthy controls. All patients underwent colonoscopy, and multiple biopsies were taken for histopathological and COX-2 and p53 immunohistochemical studies.
Results
There was significant difference in the expression of COX-2 and p53 in UC-related dysplasia either without or with CRC, compared with their expression in the UC group without dysplasia.
Conclusion
Adding immunohistochemical analysis of COX-2 enzyme and p53 gene to routine histological assessment may improve the accuracy of early detection of dysplasia and colorectal cancer. COX-2 and p53 can be promising chemotherapeutic/chemopreventive targets in UC patients.
Keywords: colitis-associated dysplasia, colorectal carcinoma, cyclooxygenase-2, p53, ulcerative, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disorder of the large intestine that is characterized by mucosal inflammation of the rectum, which extends proximally through the colon in a continuous manner but to a variable extent [1]. Although the exact cause of UC remains undetermined, the condition appears to be related to a combination of genetic and environmental factors. Diagnosis of UC is based on a combination of laboratory, endoscopic, histological, and radiographic investigations [2]. Chronic UC is associated with six- to sevenfold increased risk of colorectal cancer [3]. The risk of developing cancer, or its precursor lesion dysplasia, increases exponentially with the duration of the disease [4].
Cyclooxygenase (COX) is an enzyme important for the synthesis of prostaglandins. Cyclooxygenase-2 (COX-2) is highly inducible in response to cellular activation by hormones, proinflammatory cytokines, growth factors, and tumor promoters [5]. COX-2 activates procarcinogens, promotes angiogenesis, and indirectly increases free radical production [6].
The tumor-suppressor gene p53 is a nuclear protein involved in the control of the cell cycle, apoptosis, and the maintenance of genomic stability [7]. P53 plays an active role in both DNA repair and induction of apoptosis. It is mutated in a variety of cancers including colorectal carcinoma (CRC) [8]. Abnormal p53 expression, detected by immunohistochemistry, is often used as a marker of p53 mutation and thus found in dysplastic or cancerous tissue. Repeated and continuous inflammation and oxidative stress induce activation and overload of the p53 checkpoint system, possibly resulting in p53 mutation. In patients with UC, increased proliferation of epithelial cells and overexpression of p53 predispose the bowel mucosa to dysplasia and the development of carcinoma [9].
1.1. Aim of the work
The aim of this work was to analyze the immunoexpression of proinflammatory enzyme COX-2 and p53 in UC, UC-associated dysplasia, and CRC, in comparison with each other and with different clinical and histopathological parameters, to clarify if they have a possible role in the pathogenesis of CRC in UC patients.
2. Materials and methods
In this cross-sectional study, the participants comprised 98 adult patients, who were prospectively recruited from the Departments of Tropical Medicine and Infectious Diseases and Clinical Oncology, Tanta University, Tanta, Egypt. All participants provided informed consent, and the study was approved by Tanta University Ethical Committee. Besides, 10 participants had functional symptomatic irritable bowel and were diagnosed as having healthy endoscopic and histopathological colonic findings served as control group.
All patients were subjected to full history taking and thorough clinical examination. Laboratory investigations, including complete blood pictures, liver function tests, Erythrocyte Sedimentation Rate (ESR), blood urea and serum creatinine, and stool analysis, were performed to exclude bacterial causes of colitis. Radiological investigations included plain X-ray of the abdomen and pelviabdominal ultrasound. Colonoscopy was done for all patients, and multiple biopsies were taken for histopathological and immunohistochemical procedures. The endoscopic assessment of UC severity was performed using the Ulcerative Colitis Colonoscopic Index of Severity (UCCIS). The vascular pattern, ulcerations, bleeding friability, and granularity were used to obtain the UCCIS [10] (Table 1).
Table 1.
Ulcerative Colitis Colonoscopic Index of Severity.
| Score | Definition |
|---|---|
| Vascular pattern | |
| 0 | Normal, clear vascular pattern |
| 1 | Partially visible vascular pattern |
| 2 | Complete loss of vascular pattern |
| Granularity | |
| 0 | Normal, smooth, and glistening |
| 1 | Fine |
| 2 | Coarse |
| Ulceration | |
| 0 | Normal, no erosion or ulcer |
| 1 | Erosions or pinpoint ulcerations |
| 2 | Numerous shallow ulcers with mucopus |
| 3 | Deep, excavated ulcerations |
| 4 | Diffusely ulcerated with >30% involvement |
| Bleeding/friability | |
| 0 | Normal, no bleeding, no friability |
| 1 | Friable, bleeding to light touch |
| 2 | Spontaneous bleeding |
| Grading of SAES and GAES | |
| 0 | Normal/quiescent: visible vascular pattern with no bleeding, erosions, ulcers or friability (includes altered vascular pattern in quiescent disease) |
| 1 | Mild: erythema, decreased or loss of vascular pattern, fine granularity, but no friability or spontaneous bleeding |
| 2 | Moderate: friability with bleeding to light touch, coarse granularity, erosions or pinpoint ulcerations |
| 3 | Severe: spontaneous bleeding or gross ulcers |
The GAES also includes a 10-cm visual analog scale of severity.
GAES = global assessment of endoscopic severity; SAES = segmental assessment of endoscopic activity.
Biopsies taken during colonoscopy were fixed in 10% formaldehyde for a period of 24–48 hours, embedded in paraffin, and then sectioned together with control blocks at 3–4 μm for histopathological and immunohistochemical studies.
Endoscopy and microscopic examination of collected biopsies allowed the grouping and classification of studied cases into four groups:
Group I: 39 UC patients without dysplasia
Group II: 32 UC patients with dysplasia
Group III: 27 patients with CRC on top of UC
Group IV: 10 individuals as the control group. They underwent colonoscopy in the Endoscopy Unit at the Tropical Medicine and Infectious Diseases department, which revealed healthy endoscopic and histopathological findings
Immunohistochemistry staining was performed using the Lab Vision's Ultra Vision Detection Kit (TP-015-HD, Fremont, CA 94539, USA), according to the manufacturer's protocol. Sections were incubated for 10 minutes using Ultra V block to prevent nonspecific background staining, followed by rinsing the sections with Phosphate Buffered Saline (PBS). Afterward, overnight incubation was done in a humidity chamber with rabbit polyclonal antibody against COX-2 (Lab Vision Catalog # RB-9072-R7, Fremont, CA 94539, USA) and mouse monoclonal antibody against p53 (Lab Vision p53 Ab-8, clone DO-7, Fremont, CA 94539, USA at 1:100 dilution), followed by washing in PBS. Finally, sections were counterstained using Mayer's hematoxylin, dehydrated in alcohol, and mounted in Distyrene, Plasticizer, and Xylene (DPX). Negative controls had primary antibody replaced by buffer.
2.1. Scoring methods
2.1.1. COX-2 scoring
COX-2 was expressed as granular cytoplasmic staining. COX-2 reactivity was evaluated according to staining intensity, which was graded as 1 (weak), 2 (moderate), or 3 (strong), and the positively stained area, which was graded as 0 (<10%), 1 (10–40%), 2 (40–70%), and 3 (≥70%). Both parameters were evaluated in the cytoplasm of epithelial and inflammatory cells. Total scores for grade and stained area of ≥ 3 were defined as positive and those of < 3 as negative [11].
2.1.2. P53 scoring
Positive tumor cells were quantified and expressed as the percentage of the total number of tumor cells. The presence of p53 mutation (p53 positivity) was defined as ≥ 50% of tumor cells with unequivocal moderate/strong nuclear staining, as recommended for improved specificity. The absence of p53 mutation (p53 negativity) was defined as either absent/weak staining or < 50% of tumor cells with moderate/strong staining [12].
2.2. Statistical analysis
The collected data were organized, tabulated, and statistically analyzed using SPSS software statistical computer package, Version 19. Analysis of variance (ANOVA) was used to test the difference between more than two groups; also Dunnett test was used to compare the other groups with the control group. Spearman correlation test was used to test the correlation between different variables. Significance was adopted at p < 0.05 for interpretation of results of tests of significance.
3. Results
This study included 108 participants distributed into four groups. Group I included 39 UC patients without dysplasia (21 male patients and 18 female patients, with a mean age of 38.2 ± 11 years). Group II included 32 UC patients with dysplasia (15 male patients and 17 female patients, with a mean age of 45.6 ± 9 years). Group III included 27 patients with UC with colorectal cancer (18 male patients and 9 female patients, with a mean age of 49.5 ± 16 years). Group IV (control) included 10 age-matched healthy individuals (6 male individuals and 4 female individuals, with a mean age of 40.3 ± 15.2 years). The demographic and clinical data are shown in Table 2.
Table 2.
Demographic data of all studied groups.
| Patient data | Ulcerative colitis without dysplasia (n = 39) | Ulcerative colitis with dysplasia (n = 32) | Colorectal carcinoma on top of ulcerative colitis (n = 27) | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex: male | 21 | 54 | 15 | 47 | 18 | 67 | 0.307 |
| Clinical presentation | 5 | 13 | 4 | 13 | 7 | 26 | 0.284 |
| Fever | 6 | 15 | 5 | 16 | 4 | 15 | 0.975 |
| Abdominal pain | 16 | 41 | 18 | 56 | 16 | 59 | 0.267 |
| Tenesmus and\or diarrhea | 7 | 18 | 9 | 28 | 6 | 22 | 0.593 |
| Extraintestinal manifestation | |||||||
| Age | 38.2 ± 11 | 45.6 ± 9 | 49.5 ± 16 | 0.001 | |||
| Hemoglobin | 11 ± 2.45 | 9 ± 3.87 | 8 ± 4.77 | 0.003 | |||
| ESR | 26 ± 9.98 | 43 ± 6.77 | 64 ± 8.99 | <0.001 | |||
ESR = Erythrocyte Sedimentation Rate.
Colonoscopic examination showed a significant difference between the three studied patient groups with respect to an endoscopic scoring system (UCCIS) for UC (p = 0.001).
There was a significant difference between the four studied groups as regards COX-2 (p < 0.001), as demonstrated in Table 3. On comparing the control group with the other three groups, it had no significant difference with Group I, whereas it was significantly different from the other two groups, which had UC associated with dysplasia (Group II) and UC with CRC (Group III) (0.661, < 0.001, and < 0.001, respectively) as shown in Table 3. Figures 1 and 2 demonstrate COX-2 expression in the different studied groups.
Table 3.
Comparison of COX-2 expression of patients in the studied groups.
| COX-2 | Control (n = 10) |
Ulcerative colitis without dysplasia (n = 39) | Ulcerative colitis with dysplasia (n = 32) | Colorectal carcinoma on top of ulcerative colitis (n = 27) |
|---|---|---|---|---|
| Negative | 7 | 20 | 7 | 6 |
| Positive | 3 | 19 | 25 | 21 |
| X2 | 20.737 | |||
| p | <0.0001* | |||
| p1 | 0.155 | |||
| p2 | 0.002* | |||
| p3 | 0.009* |
p: comparing the difference in frequencies between the four groups.
p1: control and ulcerative colitis without dysplasia.
p2: control and ulcerative colitis with dysplasia.
p3: control and colorectal carcinoma on top of ulcerative colitis.
* Significant.
COX-2 = cyclooxygenase-2.
Figure 1.
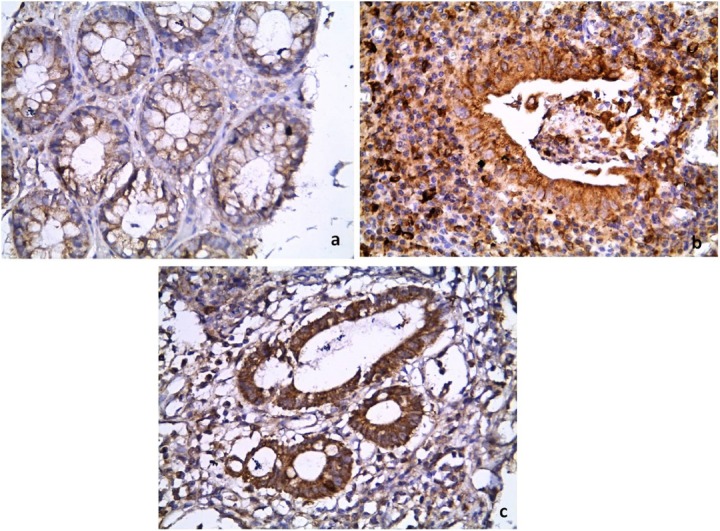
Comparison of the immunohistochemical pattern of COX-2 between: (a) the control, that shows weak cytoplasmic epithelial staining; and (b, c) the UC group, that shows strong cytoplasmic staining in epithelial cells and inflammatory cells. (Immunoperoxidase: 400×).
COX-2 = cyclooxygenase-2; UC = ulcerative colitis.
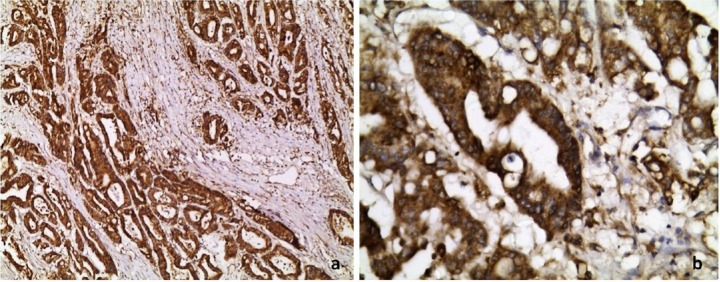
Figure 2.
Profile of COX-2 in colorectal cancer on top of UC showing diffuse strong cytoplasmic staining in neoplastic epithelial cells. (a) 100× and (b) 400× immunoperoxidase.
COX-2 = cyclooxygenase-2; UC = ulcerative colitis.
As regards p53 expression, there was a significant difference (p < 0.001) between the four studied groups (Table 4). The control group differed significantly from the UC-associated dysplasia and CRC groups, but showed no statistically significant difference from the UC group without dysplasia. Expression of p53 in the different studied groups is demonstrated in Figures 3 and 4.
Table 4.
Comparison of p53 expression of patients in the studied groups.
| p53 | Control (n = 10) |
Ulcerative colitis without dysplasia (n = 39) | Ulcerative colitis with dysplasia (n = 32) | Colorectal carcinoma on top of ulcerative colitis (n = 27) |
|---|---|---|---|---|
| Negative | 7 | 21 | 8 | 7 |
| Positive | 3 | 18 | 24 | 20 |
| X2 | 10.721 | |||
| p | 0.017* | |||
| p1 | 0.482 | |||
| p2 | 0.02* | |||
| p3 | <0.023* |
p: comparing the difference in frequencies between the four groups.
p1: control and ulcerative colitis without dysplasia.
p2: control and ulcerative colitis with dysplasia.
p3: control and colorectal carcinoma on top of ulcerative colitis.
* Significant.
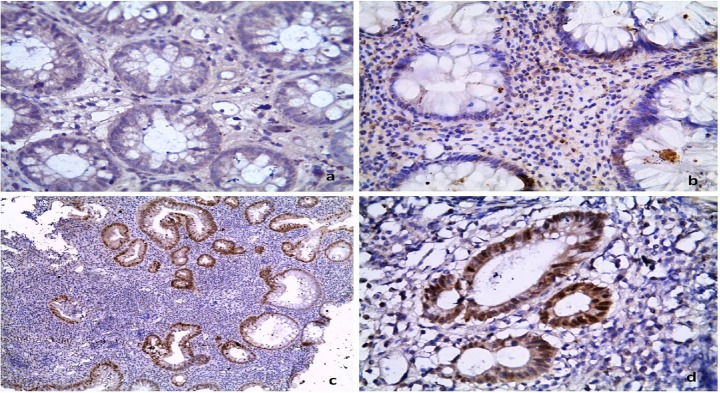
Figure 3.
P53 immunohistochemical expression is: (a) absent in control, (b) infrequent in UC without dysplasia, and (c, d) very frequent in UC with dysplasia. [Immunoperoxidase: 400× in (a, b, d) & 100× in (c)].
UC = ulcerative colitis.
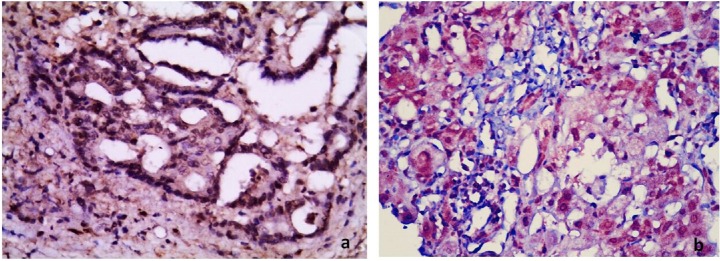
Figure 4.
Photomicrographs showing (a) negative and (b) positive nuclear staining for p53 protein in two cases of colonic adenocarcinoma on top of UC. (Immunoperoxidase: 400×).
UC = ulcerative colitis.
There was a significant negative relationship between p53 and index of severity by colonoscopy, and a significant positive relationship between p53 and duration of illness in years and COX-2 expression (r = 0.2, 0.08, and 0.021; and p = 0.04, 0.01, and 0.001, respectively).
4. Discussion
Patients with Inflammatory Bowel Disease (IBD), both Ulcerative Colitis (UC) and Crohn's Disease (CD), are at increased risk of developing CRC. In fact, IBD ranks among the top three high-risk conditions for CRC, together with hereditary polyposis and nonpolyposis CRC [13]. UC is a chronic disease characterized by diffuse mucosal inflammation limited to the colon. It involves the rectum, and may extend proximally in a symmetrical, circumferential, and interrupted pattern to involve parts or all of the large intestine [14]. The overall incidence of CRC among UC patients is 3.7%, and this incidence increased to reach up to 4.5% in UC patients with pancolitis [15]. UC develops in relatively young adults, and long-term, continuous drug therapy is required to stabilize the disease. However, a substantial number of patients are unable to strictly comply with drug therapy during follow up, increasing the risk of relapse [16]. All patients with UC should have a surveillance colonoscopy every 1–3 years, starting 7–10 years after disease onset [17]. Random biopsies (quadrant biopsies every 10 cm) and targeted biopsies of any visible lesion should be performed if white light endoscopy is used [18]. The aim of our study was to analyze the immunoexpression of proinflammatory enzyme COX-2 and p53 in UC, UC-associated dysplasia, and CRC, in comparison with each other and with different clinical and histopathological parameters, to clarify if they have a possible role in pathogenesis of CRC in UC patients. Colonoscopic examination showed a significant difference between the three studied groups, according to an endoscopic scoring system for UC [10] (p = 0.001). As regards the colonoscopic severity index, there were significant differences between patients with UC with dysplasia and those without dysplasia, indicating that there is a direct correlation between the index of colonoscopic severity and the degree of UC-associated dysplasia. These findings were in concordance with the results of Kiran et al. [19], who concluded that most UC-related CRCs can be diagnosed or suspected on the basis of endoscopic findings and biopsy of areas of colitis. UC-associated colorectal carcinogenesis is believed to follow a multistep process from inflammation and epithelial regeneration to hyperplastic epithelium to flat dysplasia and finally to invasive adenocarcinoma [20]. In this study, patients with CRC on top of dysplasia (Group III) show a significant difference from the other groups with respect to older age but no significant difference in UC-related dysplasia, and this was supported by a Japanese study conducted by Watanabe et al. [21] and another by Sanchez et al. [22], who concluded that demographic data are nonsignificant variables in UC-related dysplasia. As regards the occurrence of carcinoma on top of dysplasia in this study at a mean age (49.5 ± 16), Eaden et al. [23] published a meta-analysis, reporting that the cumulative risk of CRC in patients with UC was 2% at 10 years, 8% at 20 years, and 18% at 30 years. This meta-analysis and other studies showed that the risk of developing CRC in UC patients is increased by specific factors, such as disease duration, extensive mucosal involvement, and concomitant primary sclerosing cholangitis [24]. In our study, patients with CRC on top of dysplasia (Group III) showed a statistically significantly low pretherapy hemoglobin level (8 ± 4.77 mg/dL) compared with the other groups; this fact was explained as early iron-deficiency anemia and has long been recognized as a feature of colorectal cancer [25]. It is present in 11–57% of cancers [26] and is particularly suggestive of cecal tumors [27]. Patients with anemia as their presenting feature of cancer have worse staging [28] and mortality [29]. In our work, the expression of COX-2, especially in lamina propria, was significantly different in the UC with dysplasia group (Group II) and UC with colorectal cancer group (Group III) from the UC without dysplasia and control group. COX-2 immunostaining, according to our work, was intense in cases with dysplasia with or without cancer, and the intensity is directly correlated with the degree of dysplasia. This was supported by the study of Svec et al. [30], which concluded that COX-2 was significantly increased during neoplastic transformation of UC patients' colonic mucosa. In concordance with these were the results of Shaker et al. [31], who concluded that COX-2 may play a role in the pathophysiological process of inflammatory bowel disease and in the development of carcinoma. Thus, it can be used as a marker for premalignant and malignant lesions. They added that COX-2 inhibitors can be used in the targeted therapy of these lesions [31]. Fratila and Ilias [32] found that COX-2 immunostaining was positive in UC-related dysplasia cases and negative in nondysplastic cases, which is supported by our results. Interestingly, by contrast, in the study of Agoff et al. [33], COX-2 overexpression occurred early in UC-associated neoplasia; however, the cancer risk increase could not be explained solely by inflammatory activity. In their study, the overall neoplastic change explained the majority of the variation in COX-2 expression, whereas inflammatory activity explained only 11%. As regards p53, our study showed that there was a significant difference in the expression of p53 between the UC-related dysplasia group (Group II) and CRC on top of UC group (Group III) and the UC group without dysplasia group (Group I) and control group. These results were supported by Kulaylat and Dayton [34], who studied the difference between sporadic CRC and UC-related CRC, with respect to histological abnormalities and genetic alterations, and they concluded that there is an early expression of p53 in UC-related CRC and late expression in sporadic CRC. Another study by Vaji et al. [35] in 2011, concluded that genetic polymorphism of p53 is closely related to UC and subsequently to dysplasia. In concordance with our results are the results of the study by Risques et al. [36] in 2011, who concluded that the expression of p53 was low in nondysplastic biopsies, but progressively increased in low- and high-grade dysplasia. Surprisingly, a high p53 expression has been found in chronic UC patients with severe disease but without cancer [37]. The inflammatory cells in UC generate oxygen radicals and nitrogen species, which lead to oxidative stress and genetic alterations involved in cell repair leading to dysplasia and hence to CRC [4].
Our study showed a significant negative relationship between p53 and the index of severity by colonoscopy, and a significant positive relationship between p53 and duration of illness in years and COX-2 expression (r = 0.2, 0.08, and 0.021; and p = 0.04, 0.01, and 0.001, respectively). COX-2 expression has previously been shown to be repressed by wild-type p53 [38], although p53 is unlikely the only regulator of COX-2. Thus, tumors with wild-type p53 may have a lower frequency of COX-2 overexpression. Indeed, our data support this hypothesis. The frequency of COX-2– tumors was higher among p53– tumors than among p53+ tumors, and COX-2 and p53 statuses were positively correlated with each other. For therapeutic approaches targeting the COX-2 pathway, it may be important to consider the effects of wild-type or mutant p53, potentially modulating signaling through the COX-2 pathway. Further study is necessary to investigate the mechanisms of COX-2 regulation by p53.
The apparent synergistic effect of COX-2 and p53 might also reflect the limitations of immunohistochemical assays in which false positives and false negatives can occur, and the combined analysis for COX-2 and p53 might select tumors that are caused by COX-2 activation, which can, in turn, be caused by mutations and functional loss of p53. It has been shown that the correlation between TP53 gene mutations and p53 positivity by immunohistochemistry is less than perfect [39]. Thus, the combination of COX-2 and p53 immunohistochemistry might be useful to decrease false positives and false negatives, or to select a more homogenous group of colorectal cancer cases. COX-2 expression has been shown to be regulated by wild-type p53, suggesting that TP53 mutation may cause deregulation of COX-2 expression [38]. Previous studies demonstrated that MSI-H tumors, whether in sporadic or in familial setting, are inversely associated with COX-2 overexpression [40]. In addition, Ogino et al. [41] in 2006 suggested a synergistic effect of CpG island methylator phenotype (CIMP) and microsatellite instability— high (MSI-H) on lowering the frequency of COX-2 and p53 overexpression in colorectal cancer. Elucidating the molecular mechanisms of COX-2 overexpression and its action in COX-2-overexpressed tumors, and the alternative mechanisms that may bypass COX-2 overfunction in COX-2– tumors is important for the purpose of developing molecularly targeted treatments against colorectal cancer [42].
Our data may have significant clinical implications because of the emerging importance of both COX-2 and p53 as promising chemotherapeutic/chemopreventive targets. The present study carries two messages that we believe to be important. First, COX-2 is an attractive chemopreventive target [43]. The COX inhibitor aspirin has been known to decrease the risk for colorectal cancer [44], and the more recent COX-2-specific inhibitor celecoxib has been shown to inhibit the growth of colorectal cancer cells in vitro [45], especially for young patients having UC with dysplasia, to avoid developing cancer in their future lifetime. The second message is for the patients with colorectal cancer harboring COX-2 overexpression who should receive molecular target therapy in their adjuvant setting. However, our study had several limitations: it was conducted in a single center and had a small sample size. Further multicenter prospective studies with larger numbers of patients are needed to confirm our findings and to further delineate the clinical significance of COX-2 expression in UC.
5. Conclusion
Adding immunohistochemical analysis of COX-2 enzyme and p53 gene to routine histological assessment may improve the accuracy of early detection of dysplasia and colorectal cancer. COX-2 and p53 can be promising chemotherapeutic/chemopreventive targets in UC patients.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- [1].Kappelman MD, Rifas-Shiman SL, Kleinman K, Carol P, Daniel AO, Robert SS, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [2].Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- [3].Mattar M, Lough D, Pishvaian M, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- [4].Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–24. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- [5].Yu Y, Fan J, Hui Y, Rouzer CA, Marnett LJ, Klein-Szanto AJ, et al. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J Biol Chem. 2007;282:1498–506. doi: 10.1074/jbc.M609930200. [DOI] [PubMed] [Google Scholar]
- [6].Matkowskyj KA, Chen ZE, Rao MS, Yang GY. Dysplastic lesions in inflammatory bowel disease: molecular pathogenesis to morphology. Arch Pathol Lab Med. 2013;137:338–50. doi: 10.5858/arpa.2012-0086-RA. [DOI] [PubMed] [Google Scholar]
- [7].Vogelstein B, Kinzler KW. Cancer genes and the pathways they controi. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- [8].Ullman TA, Itzkowitz SH. Intestina inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- [9].Reuter S, Gupta S, Chaturvedi M, Aggarwal B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thia KT, Loftus Jr EV, Pardi DS, Kane SV, Faubion WA, Tremaine WJ, et al. Measurement of disease activity in ulcerative colitis: interobserver agreement and predictors of severity. Inflamm Bowel Dis. 2011;17:1257–64. doi: 10.1002/ibd.21480. [DOI] [PubMed] [Google Scholar]
- [11].Paiotti AP, Artigiani Neto R, Forones NM, Oshima CT, Miszputen SJ, Franco M. Immunoexpression of cyclooxygenase-1 and -2 in ulcer-ative colitis. Braz J Med Biol Res. 2007;40:911–s8. doi: 10.1590/s0100-879x2006005000128. [DOI] [PubMed] [Google Scholar]
- [12].Schernhammer ES, Ogino S, Fuchs CS. Folate and vitamin B6 intake and risk of colon cancer in relation to p53 expression. Gastroenterology. 2008;135:770–80. doi: 10.1053/j.gastro.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–89. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- [15].Karvellas CJ, Fedorak RN, Hanson J, Wong CK. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can J Gastroenterol. 2007;21:443–6. doi: 10.1155/2007/136406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- [17].Vienne A, Simon T, CosnesJ, Baudry C, Bouhnik Y, Soulé JC, et al. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther. 2011;34:188–95. doi: 10.1111/j.1365-2036.2011.04711.x. [DOI] [PubMed] [Google Scholar]
- [18].Van Assche G, Dignass A, BokemeyerB, Danese S, Gionchetti P, Moser G, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. doi: 10.1016/j.crohns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- [19].Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, Remzi FH. Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn's and ulcerative colitis based on three decades of experience. Ann Surg. 2010;252:330–5. doi: 10.1097/SLA.0b013e3181e61e69. [DOI] [PubMed] [Google Scholar]
- [20].Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflamm. 2012;2012:1–16. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Watanabe T, Konishi T, Kishimoto J, Kotake K, Muto T, Sugihara K, et al. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis. 2011;17:802–8. doi: 10.1002/ibd.21365. [DOI] [PubMed] [Google Scholar]
- [22].Sanchez JA, Dejulius KL, Bronner M, Church JM, Kalady MF. Relative role of methylator and tumor suppressor pathways in ulcerative colitis-associated colon cancer. Inflamm Bowel Dis. 2011;17:1966–70. doi: 10.1002/ibd.21526. [DOI] [PubMed] [Google Scholar]
- [23].Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110(2):331–8. doi: 10.1053/gast.1996.v110.pm8566577. [DOI] [PubMed] [Google Scholar]
- [25].Goodman D, Irvin TT. Delay in the diagnosis and prognosis of carcinoma of the right colon. Br J Surg. 1993;80:1327–9. doi: 10.1002/bjs.1800801037. [DOI] [PubMed] [Google Scholar]
- [26].Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust N Z J Surg. 2000;70:635–8. doi: 10.1046/j.1440-1622.2000.01916.x. [DOI] [PubMed] [Google Scholar]
- [27].Dunne JR, Gannon CJ Osborn TM, Taylor MD, Malone DL, Napolitano LM. Preoperative anemia in colon cancer: assessment of risk factors. Am Surg. 2002;68:582–7. [PubMed] [Google Scholar]
- [28].Gonzalez-Hermoso F, Perez-Palma J, Marchena-Gomez J, Lorenzo-Rocha N, Medina-Arana V. Ca early diagnosis of symptomatic colorectal cancer improve the prognosis? World J Surg. 2004;28:716–20. doi: 10.1007/s00268-004-7232-8. [DOI] [PubMed] [Google Scholar]
- [29].Stapley S, Peters TJ, Sharp D, Hamilton W. The mortality of colorectal cancer in relation to the initial symptom and to the duration of symptoms: a cohort study in primary care. Br J Cancer. 2006;95:1321–5. doi: 10.1038/sj.bjc.6603439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Svec J, Musílková J, Bryndová J, Jirásek T, Mandys V, Kment M, et al. Enhanced expression of proproliferative and antiapoptotic genes in ulcerative colitis-associated neoplasia. Inflamm Bowel Dis. 2010;16:1127–37. doi: 10.1002/ibd.21178. [DOI] [PubMed] [Google Scholar]
- [31].Shaker SS, Hasan AA, El Bostany MZ. The role of cyclooxygenase-2 in ulcerative colitis and colorectal adenocarcinoma. Egypt J Pathol. 2012;32:256–60. [Google Scholar]
- [32].Fratila OC, Ilias TI. COX-2 and Ki-67 immunohistochemical markers in the assessment of long-standing ulcerative colitis associated dysplasia. Rom J Morphol Embryol. 2013;54:143–9. [PubMed] [Google Scholar]
- [33].Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737–45. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706–12. doi: 10.1002/jso.21505. [DOI] [PubMed] [Google Scholar]
- [35].Vaji S, Saleh Z, Aminian K. Association of p53 codon 72 genetic polymorphism with the risk of ulcerative colitis in northern Iran. Int J Colorectal Dis. 2011;26:235–8. doi: 10.1007/s00384-010-1021-7. [DOI] [PubMed] [Google Scholar]
- [36].Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71:1669–79. doi: 10.1158/0008-5472.CAN-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–7. [PubMed] [Google Scholar]
- [38].Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999;274:10911–5. doi: 10.1074/jbc.274.16.10911. [DOI] [PubMed] [Google Scholar]
- [39].Curtin K, Slattery ML, Holubkov R, Edwards S, Holden JA, Samowitz WS. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2004;12:380–6. doi: 10.1097/00129039-200412000-00017. [DOI] [PubMed] [Google Scholar]
- [40].Sinicrope FA, Lemoine M, Xi L, Lynch PM, Cleary KR, Shen Y, et al. Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology. 1999;117:350–8. doi: 10.1053/gast.1999.0029900350. [DOI] [PubMed] [Google Scholar]
- [41].Ogino S, Brahmandam M, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–64. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- [43].Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–66. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- [44].Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, et al. Aspirin and the risk of colorectal cancer in women [see comments] N Engl J Med. 1995;333:609–14. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- [45].Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738–44. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]