Abstract
Background
Interleukin‐15 (IL‐15) is a myokine associated with muscle strength, possibly by attenuating protein breakdown. A variant in the alpha‐receptor (IL‐15Rα 1775 A>C, rs2228059) partially modulates the muscle strength and size response to resistance training. We examined if this polymorphism associated with habitual physical activity among European‐American adults.
Methods
Men (n = 240, 23.7 ± 0.3 year, body mass index [BMI] 25.3 ± 0.3 kg/m2) and women (n = 292, 23.2 ± 0.3 year, 24.0 ± 0.3 kg/m2) were genotyped. Physical activity phenotypes were derived from the Paffenbarger Physical Activity Questionnaire. Analysis of covariance (ancova) tested log‐transformed differences between the IL‐15Rα genotype and physical activity phenotypes by gender with age and BMI as covariates.
Results
Men with the IL‐15Rα 1775AA genotype spent more time in light intensity physical activity (39.4 ± 2.4 hr/week) than men with the CC genotype (28.6 ± 2.3 hr/week, (p = .009).
Conclusion
Further research is needed to confirm our finding and determine the possible mechanisms by which the IL‐15Rα variant modulates light intensity physical activity.
Keywords: cytokine, exercise, polymorphisms
1. INTRODUCTION
A physically active lifestyle is vital to physical and mental well‐being (Haskell et al., 2007). Consequently, the American College of Sports Medicine recommends that most adults engage in moderate‐intensity aerobic exercise for ≥30 min/day on ≥5 day/week for a total of ≥150 min/week; vigorous‐intensity aerobic exercise for ≥20 min/day on ≥3 day/week (≥75 min/week); or a combination of these moderate‐ and vigorous‐intensity exercise recommendations to achieve a total energy expenditure of ≥500–1,000 MET‐min/week (American College of Sports, 2017; Garber et al., 2011). Despite awareness of the myriad of health benefits that result from habitual physical activity, only one in five adults in the United States meet these physical activity recommendations (Troiano et al., 2008). In addition, 25% of Americans do not participate in any leisure time physical activity (Centers for Disease Control and Prevention, 2013). Given these low rates of adherence to a physically active lifestyle, it is imperative to gain insight into factors that may modulate habitual physical activity so that effective interventions can be developed.
Physical activity has a significant genetic component as evident in twin studies with heritability estimates between 50%–90% (Joosen, Gielen, Vlietinck, & Westerterp, 2005; de Moor et al., 2007; Stubbe et al., 2006). “Activity genomics” is a growing field indicating that the genetic basis of physical activity is accounted for by the sum of small gene effects rather than a small number of genes with a large effect (de Moor et al., 2007, 2009). Furthermore, the genetic influence on habitual physical activity appears to be more important in young adulthood than adolescence (Stubbe, Boomsma, & de Geus, 2005; Vink et al., 2011).
A gene that is a logical candidate to explore regarding its effects on habitual physical activity is the interleukin‐15 alpha specific receptor (IL‐15Rα) for the various reasons that follow. Signaling by IL‐15 occurs not only through binding with its trimeric receptor but also through a complex formed between IL‐15 and IL‐15Rα such that the biological activity of IL‐15 in vivo is likely regulated through its interactions with IL‐15Rα (Pistilli et al., 2011). IL‐15 is a myokine that inhibits cell death (i.e., anti‐apoptotic) and promotes the production of many immune cells including natural killer (NK) cells, cluster of differentiation 8 (CD8+) T cells, neutrophils, eosinophils, mast cells, monocytes, and B lymphocytes (Budagian, Bulanova, Paus, & Bulfone‐Paus, 2006).
In addition to its anti‐apoptotic action on immunological cells, IL‐15 plays a role in protein, lipid, and glucose metabolism. IL‐15 reduces fat mass in rodents (Barra et al., 2010; Carbo et al., 2001), and its effect on lipids in white adipose tissue may result via its mediation of calcineurin (Almendro et al., 2009), which decreases white adipose mass by affecting preadipocyte differentiation (Quinn, Strait‐Bodey, Anderson, Argiles, & Havel, 2005) and reducing lipogenesis (Carbo et al., 2001). Cell culture studies indicate IL‐15 also stimulates lipolysis (Ajuwon & Spurlock, 2004) and adiponectin secretion (Quinn et al., 2005). Lastly, IL‐15 regulates glucose metabolism in vitro (Busquets, Figueras, Almendro, Lopez‐Soriano, & Argiles, 2006), and recent work by He et al. (2010) and Wu, He, et al. (2010) has revealed differences in spontaneous cage activity when comparing IL‐15Rα control mice and IL‐15Rα knockout mice, whereby the knockout mice displayed hyperactivity in comparison to the control mice. Collectively, the regulatory actions of IL‐15Rα on inflammatory and metabolic processes on muscle and adipose tissue and IL‐15Rα spontaneous cage activity of mice suggest they could be linked in some fashion.
Furthermore, IL‐15Rα 1775A>C (rs2228059; OMIM *601070; GenBank NC_000010.11) has been reported to be associated with several health and fitness‐related phenotypes including adiposity, muscular size and strength, and lipid‐lipoproteins (Arnett et al., 2009; He et al., 2010; Pistilli et al., 2008; di Renzo et al., 2009; Riechman, Balasekaran, Roth, & Ferrell, 2004; Wu, He, et al. (2010)). Our research group from the Functional Single Nucleotide Polymorphisms Associated with Muscle Size and Strength (FAMuSS NIH R01 NS40606‐02) study found that IL‐15Rα 1775 A>C modulated baseline whole muscle volume and quality and serum cholesterol among European‐American men and women from FAMuSS (Pistilli et al., 2008).
The strongest candidate genes are those whose association with the phenotype in question is supported by multiple lines of evidence (Dipetrillo, Wang, Stylianou, & Paigen, 2005; Lightfoot, 2011). In the case of habitual physical activity, such criteria include genetic variants reported to be associated with chronic diseases and health conditions related to physical inactivity (Arnett et al., 2009; Gokkusu et al., 2010; di Renzo et al., 2009), those with functional relevance to exercise (Riechman et al., 2004; Scanzello et al., 2009), and those previously reported to be associated with exercise performance and health and fitness‐related phenotypes (Bray et al., 2009; Pistilli et al., 2008). IL15Rα 1775A>C meets these criteria. Therefore, the purpose of our study was to test the hypothesis that IL‐15‐Rα 1775 A>C would influence habitual physical activity in a large subsample of 532 young European‐American adults from FAMuSS (Pescatello, Devaney, Hubal, Thompson, & Hoffman, 2013; Thompson et al., 2004).
2. METHODS
2.1. Ethical compliance
The FaMuSS study was conducted by the Exercise and Genetics Collaborative Research Group consisting of researchers from the University of Connecticut, Dublin City University, University of Massachusetts, Central Michigan University, University of Central Florida, Florida Atlantic University, West Virginia University, Yale University, Hartford Hospital, and the Children's National Medical Center. The institutional review boards from all 10 institutions approved the study protocol. The primary aim of FAMuSS was to identify genetic factors that dictated the response of health‐related fitness phenotypes to resistance training (RT). However, we took the opportunity to assess habitual physical activity with the Paffenbarger Physical Activity Questionnaire (PPAQ) (Paffenbarger, Wing, & Hyde, 1978) prior to RT. The experimental design of FAMuSS has been described in detail previously so only the methods related to this substudy are described (Clarkson et al., 2005; Kostek et al., 2007; Pescatello et al., 2006, 2013; Thompson et al., 2004).
2.2. Subjects
All subjects provided written informed consent. Potential study volunteers were excluded if they: (1) had performed RT in the past year; (2) had a chronic condition that would preclude their ability to perform RT; (3) had metal implants in the arms, eyes, head, brain, neck, and/or heart that would be contraindicated to magnetic resonance imaging (MRI); (4) were prescribed and/or taking medications (i.e., corticosteroids, anabolic steroids, antihypertensive or antilipidemic medications, diuretics, Depo‐Provera contraceptive injection, Clenbuterol, Rhinocort nasal inhaler, lithium nonsteroidal anti‐inflammatory medications) known to effect skeletal muscle function; (5) consumed an average of ≥ two alcoholic drinks per day; (6) consumed dietary supplements to enhance muscle strength and size or weight; or (7) gained or lost >2.2 kg within 3 months prior to enrolling in the study. In all, 532 European American adults (≥18 years) were genotyped for IL‐15 A>C and comprised the sample of this substudy.
2.3. Physical activity
Upon entry into FAMuSS, subjects were measured for body weight (kg) and height (cm) to determine body mass index (BMI, kg/m2). Subjects then completed the PPAQ (Ainsworth, Leon, Richardson, Jacobs, & Paffenbarger, 1993; Paffenbarger et al., 1978). The PPAQ is an accurate and reliable measure of leisure time physical activity (Ainsworth et al., 1993; Paffenbarger, Blair, Lee, & Hyde, 1993; Paffenbarger et al., 1978; Simpson et al., 2015). The physical activity phenotypes examined in this substudy were derived from the following question: “On a usual weekday and weekend day over the past year, how much time do you spend in the following activities‐vigorous, moderate, and light intensity physical activity, sitting and sleeping?”. The self‐reported time in hours (hr) spent in vigorous, moderate, and light intensity physical activity and time spent sitting were totaled and used for comparison among the IL‐15 A>C genotypes (Paffenbarger et al., 1993).
2.4. Genotyping
Fasting venous blood samples were taken from each subject in standard EDTA vacutainer tubes (Becton Dickson and Company, Franklin Lakes, NJ, USA). Samples remained anonymous and were sent to Children's National Medical Research Center in Washington, D.C for processing using the Gentra Puregene Blood DNA Purification kit (Qiagen, Valencia, CA, USA). Genotyping of the IL‐15 A>C polymorphism was performed with TaqMan allele discrimination assays employing a 5′ nuclease activity of Taq polymerase detecting a fluorescent reporter signal generated during polymerase chain reactions (PCR) according to the manufacturer's protocol for a 10 μl reaction. The PCR profile was 10 min at 95°C (denaturation), 44 cycles of 15 s at 92°C, and 1 min at an annealing temperature of 60°C. All Taqman allelic discrimination reactions were analyzed using an ABI 7900 real‐time PCR system (Applied Biosystems, Foster City, CA, USA). For quality control, negative controls (water blanks) and duplicate samples covering 5% of the total number of samples analyzed (100% agreement) were included in the genotyping analysis.
2.5. Statistical analysis
Chi‐square analysis determined IL‐15Rα 1775A>C was in Hardy‐Weinberg equilibrium (HWE) (p < .05). Analysis of covariance (ancova) was used to test for differences among IL‐15Rα genotypes and physical activity phenotypes by sex with age and BMI as covariates. In this model, log‐transformation was performed for the physical activity phenotypes to satisfy the underlying assumption of normality. For ancova models reaching statistical significance, post‐hoc pair‐wise comparisons were performed between genotypes with Bonferroni adjustments to control for multiple comparisons. Because IL‐15Rα genotype physical activity phenotype associations revealed differential patterns in men and women, our results are displayed for the total sample and by men and women, separately. The proportion of all variance in physical activity phenotypes attributable to IL‐15Rα genotype was determined using the likelihood test ratio. Alpha levels were set a priori at p < .05, and all statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, Armonk, NY, USA) 24.0.
3. RESULTS
3.1. Subject characteristics
FAMuSS subjects (n = 532) genotyped for the IL‐15Rα (rs2228059) polymorphism consisted of 240 men and 292 women of European‐American decent (Table 1). Age did not differ between men and women (p ≥ .05); however, men had a higher BMI than women (p = .04).
Table 1.
Subject characteristics (X ± SEM) for IL‐15Rα A>C (rs2228059) among the FAMuSS subsample
| Variable | Total sample (n = 532) | Men (n = 240) | Women (n = 292) |
|---|---|---|---|
| Age (year) | 23.4 ± 0.2 | 23.7 ± 0.3 | 23.2 ± 0.3 |
| BMI (kg/m2) | 24.6 ± 0.2 | 25.3 ± 0.3a | 24.0 ± 0.3 |
FAMuSS, functional single nucleotide polymorphisms associate with muscle size and strength study.
Statistically significant difference between men and women (p = .04).
3.2. IL‐15Rα 1775 A>C genotype physical activity phenotype associations by sex
A significant difference was observed amongst IL‐15Rα 1775 A>C genotypes and light intensity physical activity levels (F = 4.65, p = .01). Men with IL‐15Rα 1775AA genotype spent more time in light intensity physical activity (39.4 ± 2.4 hr/week) than men with the CC genotype (28.6 ± 2.3 hr/week), (p = .009) explaining 5.8% of the variability in time spent in light intensity physical activity (Figure 1). However, time spent in sitting and moderate intensity physical activity did not differ by IL‐15Rα 1775 A>C genotype among men. Furthermore, time spent in sitting and light, moderate, or vigorous intensity physical activity did not differ by IL15Rα 1775 A>C genotype among women (p > .05) (Figure 1).
Figure 1.
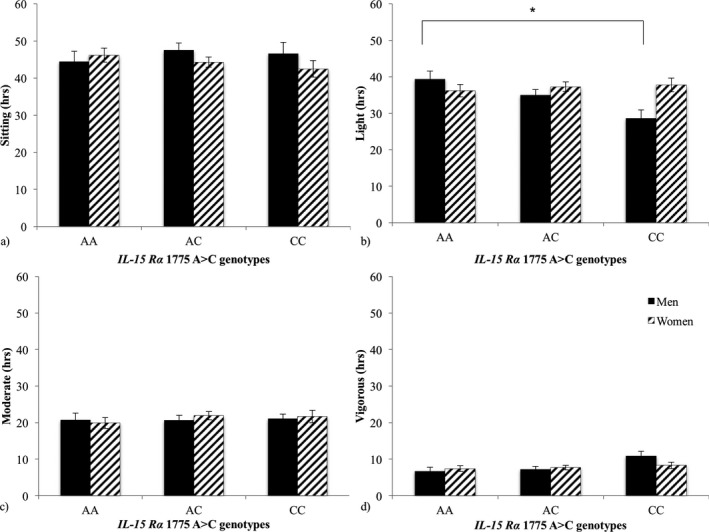
Time spent (X ± SEM) sitting (a) and in light (b), moderate (c), and vigorous (d) physical activity (hr) by IL‐15Rα 1775 A>C (rs2228059) genotype and sex
4. DISCUSSION
The aim of this substudy was to determine whether IL‐15Rα 1775 A>C associated with habitual physical activity in a large subsample of healthy European‐American adults from FAMuSS (Pescatello et al., 2013; Thompson et al., 2004). The most noteworthy finding was the IL‐15Rα 1775 A>C made a small but meaningful contribution to the interindividual differences we observed in habitual physical activity that varied by sex and the intensity of physical activity performed. Men homozygous for the A allele reported spending more time in light intensity physical activity than men homozygous for the C allele, explaining 5.8% of the variability in habitual physical activity; while these associations were not apparent in women. This finding is consistent with much of the work published by FAMuSS study investigators in that a persistent effect modifier of FAMuSS findings has been sex differences in the various phenotypes examined (Pescatello et al., 2013). Furthermore, they are consistent with the “Activity Genomic” literature that physical activity is a polygenetic trait such that there are many genes accounting for small effects (de Moor et al., 2007, 2009).
Previous work by He et al. (2010) using IL15‐Rα knockout rodents revealed an association with locomotor activity, core temperature, and food intake. Similarly, Pistilli et al. (2011) used two different measures to quantify cage activity, cage wheel running and number of photobeam breaks during a 24 hr period. They observed a 6.3 fold increase in wheel revolutions during the 14 hr period with a 74% higher ambulatory count during the 24 hr collection period for IL15‐Rα knockout mice compared to controls. These authors concluded that complete knockout of ‐IL15Rα resulted in a mouse with an increased capacity for activity.
Providing further evidence of IL15‐Rα role in potentially mediating physical activity behavior, Pan et al. (2014) demonstrated that IL‐15 crosses the blood brain barrier, while work by Hsuchou, Pan, Wu, and Kastin (2009) and Hanisch et al. (1997) reported IL‐15 mRNA and its receptor subunits are also constitutively present in various brain regions. These findings indicate an active communication between blood‐borne IL‐15 and the central nervous system (He et al., 2010; Wu, Wang, Yeh, Lu, & Wu, 2010). Considering that biological activity of IL‐15 in vivo is likely regulated through its interactions with IL‐15Rα, the findings of Pistilli et al. (2011) in animals and now ours in humans suggest that differential receptor activity due to IL‐15Rα genetic variation impacts physical activity levels in animals and humans.
There is no obvious explanation for the sex differences observed in the present substudy. However, previous candidate gene association studies involving health and fitness‐related phenotypes by our group have also observed sex‐specific differences (Arnett et al., 2009; Pistilli et al., 2008; di Renzo et al., 2009; Riechman et al., 2004). Although speculative, the sex differences that have been observed in several studies perhaps are partially due to sex‐specific hormonal differences. Work by Bowen, Turner, and Lightfoot (2011) using a rodent model suggests that physical activity in mice is affected by endogenous steroids and may be an important biological factor regulating physical activity. Therefore, due to the vastly different hormonal environments between men and women, it is unlikely that genetic variation influencing our physical activity phenotypes would be identical between sexes.
The public health significance of our findings is of importance considering genetic factors influencing physical activity levels are now viewed as “the core” of the transdisciplinary model of exercise behavior (Bryan et al., 2011). In this model, Bryan et al. (2011) stated that approaches in treating physical inactivity should emphasize elucidating relationships among genetic, physiological, and psychological variables that will aid in our understanding of individual differences in the initiation and maintenance of physical activity behavior (Bryan et al., 2011). Work in “Activity Genomics” may therefore provide an exciting new avenue that may help us better understand individual differences in the initiation and maintenance of physical activity behavior due to genetic predispositions eventually leading to the development of effective interventions to increase physical activity and decrease morbidity and mortality due to sedentary lifestyles (Bryan et al., 2011). Indeed, men with IL‐15Rα 1775AA genotype spent more time in light intensity physical activity (39.4 ± 2.4 hr/week) than men with the CC genotype (28.6 ± 2.3 hr/week), which equates to a potential body weight differential of ~21.9 kg annually.
Similarly, Bann et al. (2015) evaluated the association between light intensity physical activity and BMI and found that a 1 hr/day increase in light physical activity was associated with a 0.46 kg/m2 reduction in BMI. Excessive body weight is a risk factor for numerous chronic diseases and health conditions including cardiovascular disease, diabetes mellitus, and several forms of cancer, among others (Benjamin et al., 2017). From a public health perspective, the genotype differences in light intensity physical activity we found of 10.8 hr/week, have the potential to minimize weight gain as well as lower the adverse health consequences of overweight and obesity.
This FaMUss substudy is not without limitation. Because the primary purpose of FaMuSS was to examine the influence of genetic variation on human muscle size and strength in response to RT, this study was not designed to examine habitual physical activity as a primary outcome measure. We assessed physical activity with a valid and reliable questionnaire (Paffenbarger et al., 1978) on populations with similar physical characteristics as ours to minimize the known limits of self‐report methods. However, the self‐reported recall of habitual physical activity from the previous year may have been limited by inaccuracies in subject reporting and/or social desirability bias (Chastin, Culhane, & Dall, 2014; Paulhus, 1991). We also did not measure biomarkers along the interleukin‐15 and its alpha specific receptor pathway, so further research is warranted to elucidate mechanisms for the sex and intensity dependent associations we observed. Nonetheless, the strengths of our substudy include the large sample of 532 healthy European American adults that was sufficiently powered to detect our observed effect size of f = 0.19 with 98% power at an alpha set to p < .05 (Faul, Erdfelder, Lang, & Buchner, 2007). Our substudy also controlled for the potential confounding variables of age and BMI, which could have influenced the associations found between IL‐15Rα and light physical activity.
5. CONCLUSION
In conclusion, the present results add a variant within IL‐15Rα to a growing list of polymorphisms that have been identified as making contributions to the inter‐individual variation in habitual physical activity levels of humans. Further exploration into the IL‐15Rα and other gene variants associated with physical activity should continue so that we better understand the role of genetic variation and its influence on physical activity.
CONFLICT OF INTEREST
The authors declare no conflict of interest with this manuscript.
ACKNOWLEDGMENTS
This project was funded by NIH R01 NS40606‐02; 2001‐2006 and the University of Connecticut Institute for Health, Intervention and Policy (InCHIP) and Institute for Systems Genomics (ISG). NIH/NIDDK T32DK097718 supported GIA during manuscript preparation.
Bruneau M Jr, Walsh S, Selinsky E, et al. A genetic variant in IL‐15Rα correlates with physical activity among European–American adults. Mol Genet Genomic Med. 2018;6:401–408. https://doi.org/10.1002/mgg3.368
REFERENCES
- Ainsworth, B. E. , Leon, A. S. , Richardson, M. T. , Jacobs, D. R. , & Paffenbarger, R. S. Jr. (1993). Accuracy of the college alumnus physical activity questionnaire. Journal of Clinical Epidemiology, 46, 1403–1411. https://doi.org/10.1016/0895-4356(93)90140-V [DOI] [PubMed] [Google Scholar]
- Ajuwon, K. M. , & Spurlock, M. E. (2004). Direct regulation of lipolysis by interleukin‐15 in primary pig adipocytes. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 287, R608–R611. https://doi.org/10.1152/ajpregu.00192.2004 [DOI] [PubMed] [Google Scholar]
- Almendro, V. , Fuster, G. , Ametller, E. , Costelli, P. , Pilla, F. , Busquets, S. , … Lopez‐Soriano, F. J. (2009). Interleukin‐15 increases calcineurin expression in 3T3‐L1 cells: Possible involvement on in vivo adipocyte differentiation. International Journal of Molecular Medicine, 24, 453–458. [DOI] [PubMed] [Google Scholar]
- American College of Sports . (2017). ACSM's guidelines for exercise testing and prescription Philadelphia Lippincott. Philadelphia, PA: Williams & Wilkins. [Google Scholar]
- Arnett, D. K. , Devereux, R. B. , Rao, D. C. , Li, N. , Tang, W. , Kraemer, R. , … Broeckel, U. (2009). Novel genetic variants contributing to left ventricular hypertrophy: The HyperGEN study. Journal of Hypertension, 27, 1585–1593. https://doi.org/10.1097/HJH.0b013e32832be612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bann, D. , Hire, D. , Manini, T. , Cooper, R. , Botoseneanu, A. , McDermott, M. M. , … Gill, T. (2015). Light intenstiy physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: Cross‐sectional findings from the lifestyle interventions and independence for elders (life) study. PLoS ONE, 10(2), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra, N. G. , Reid, S. , Mackenzie, R. , Werstuck, G. , Trigatti, B. L. , Richards, C. , … Ashkar, A. A. (2010). Interleukin‐15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring), 18, 1601–1607. https://doi.org/10.1038/oby.2009.445 [DOI] [PubMed] [Google Scholar]
- Benjamin, E. J. , Blaha, M. J. , Chiuve, S. E. , Cushman, M. , Das, S. R. , de Ferranti, S. D. , & Muntner, P. (2017). Heart disease and stroke statistics ‐ 2017 update. Circulation, 136(10), e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, R. S. , Turner, M. J. , & Lightfoot, J. T. (2011). Sex hormone effects on physical activity levels: Why doesn't Jane run as much as Dick? Sports Medicine (Auckland, N. Z.), 41, 73–86. https://doi.org/10.2165/11536860-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, M. S. , Hagberg, J. M. , Perusse, L. , Rankinen, T. , Roth, S. M. , Wolfarth, B. , & Bouchard, C. (2009). The human gene map for performance and health‐related fitness phenotypes: The 2006–2007 update. Medicine and Science in Sports and Exercise, 41, 35–73. https://doi.org/10.1249/MSS.0b013e3181844179 [DOI] [PubMed] [Google Scholar]
- Bryan, A. D. , Nilsson, R. , Tompkins, S. A. , Magnan, R. E. , Marcus, B. H. , & Hutchison, K. E. (2011). The big picture of individual differences in physical activity behavior change: A transdisciplinary approach. Psychology of Sport and Exercise, 12, 20–26. https://doi.org/10.1016/j.psychsport.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budagian, V. , Bulanova, E. , Paus, R. , & Bulfone‐Paus, S. (2006). IL‐15/IL‐15 receptor biology: A guided tour through an expanding universe. Cytokine & Growth Factor Reviews, 17, 259–280. https://doi.org/10.1016/j.cytogfr.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Busquets, S. , Figueras, M. , Almendro, V. , Lopez‐Soriano, F. J. , & Argiles, J. M. (2006). Interleukin‐15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochimica et Biophysica Acta, 1760, 1613–1617. https://doi.org/10.1016/j.bbagen.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Carbo, N. , Lopez‐Soriano, J. , Costelli, P. , Alvarez, B. , Busquets, S. , Baccino, F. M. , … Argiles, J. M. (2001). Interleukin‐15 mediates reciprocal regulation of adipose and muscle mass: A potential role in body weight control. Biochimica et Biophysica Acta, 1526, 17–24. https://doi.org/10.1016/S0304-4165(00)00188-4 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2013). One in five adults meet overall physical activity guidelines [Online]. Retrieved from https://www.cdc.gov/media/releases/2013/p0502-physical-activity.html.
- Chastin, S. F. , Culhane, B. , & Dall, P. M. (2014). Comparison of self‐reported measure of sitting time (IPAQ) with objective measurement (activPAL). Physiological Measurement, 35, 2319–2328. https://doi.org/10.1088/0967-3334/35/11/2319 [DOI] [PubMed] [Google Scholar]
- Clarkson, P. M. , Devaney, J. M. , Gordish‐Dressman, H. , Thompson, P. D. , Hubal, M. J. , Urso, M. , … Hoffman, E. P. (2005). ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. Journal of Applied Physiology, 1985(99), 154–163. https://doi.org/10.1152/japplphysiol.01139.2004 [DOI] [PubMed] [Google Scholar]
- Dipetrillo, K. , Wang, X. , Stylianou, I. M. , & Paigen, B. (2005). Bioinformatics toolbox for narrowing rodent quantitative trait loci. Trends in Genetics, 21, 683–692. https://doi.org/10.1016/j.tig.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A. G. , & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Garber, C. E. , Blissmer, B. , Deschenes, M. R. , Franklin, B. A. , Lamonte, M. J. , Lee, I. M. , … Swain, D. P. (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine and Science in Sports and Exercise, 43, 1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Gokkusu, C. , Aydin, M. , Ozkok, E. , Tulubas, F. , Elitok, A. , Pamukcu, B. , & Umman, B. (2010). Influences of genetic variants in interleukin‐15 gene and serum interleukin‐15 levels on coronary heart disease. Cytokine, 49, 58–63. https://doi.org/10.1016/j.cyto.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Hanisch, U. K. , Lyons, S. A. , Prinz, M. , Nolte, C. , Weber, J. R. , Kettenmann, H. , & Kirchhoff, F. (1997). Mouse brain microglia express interleukin‐15 and its multimeric receptor complex functionally coupled to Janus kinase activity. Journal of Biological Chemistry, 272, 28853–28860. https://doi.org/10.1074/jbc.272.46.28853 [DOI] [PubMed] [Google Scholar]
- Haskell, W. L. , Lee, I. M. , Pate, R. R. , Powell, K. E. , Blair, S. N. , Franklin, B. A. , … Bauman, A. (2007). Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise, 39, 1423–1434. https://doi.org/10.1249/mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- He, Y. , Wu, X. , Khan, R. S. , Kastin, A. J. , Cornelissen‐Guillaume, G. G. , Hsuchou, H. , … Pan, W. (2010). IL‐15 receptor deletion results in circadian changes of locomotor and metabolic activity. Journal of Molecular Neuroscience, 41, 315–321. https://doi.org/10.1007/s12031-009-9319-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou, H. , Pan, W. , Wu, X. , & Kastin, A. J. (2009). Cessation of blood‐to‐brain influx of interleukin‐15 during development of EAE. Journal of Cerebral Blood Flow and Metabolism, 29, 1568–1578. https://doi.org/10.1038/jcbfm.2009.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen, A. M. , Gielen, M. , Vlietinck, R. , & Westerterp, K. R. (2005). Genetic analysis of physical activity in twins. American Journal of Clinical Nutrition, 82, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Kostek, M. A. , Pescatello, L. S. , Seip, R. L. , Angelopoulos, T. J. , Clarkson, P. M. , Gordon, P. M. , … Price, T. B. (2007). Subcutaneous fat alterations resulting from an upper‐body resistance training program. Medicine and Science in Sports and Exercise, 39, 1177–1185. https://doi.org/10.1249/mss.0b0138058a5cb [DOI] [PubMed] [Google Scholar]
- Lightfoot, J. T. (2011). Current understanding of the genetic basis for physical activity. Journal of Nutrition, 141, 526–530. https://doi.org/10.3945/jn.110.127290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor, M. H. , Liu, Y. J. , Boomsma, D. I. , Li, J. , Hamilton, J. J. , Hottenga, J. J. , … Deng, H. W. (2009). Genome‐wide association study of exercise behavior in Dutch and American adults. Medicine and Science in Sports and Exercise, 41, 1887–1895. https://doi.org/10.1249/MSS.0b013e3181a2f646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor, M. H. , Posthuma, D. , Hottenga, J. J. , Willemsen, G. , Boomsma, D. I. , & de Geus, E. J. (2007). Genome‐wide linkage scan for exercise participation in Dutch sibling pairs. European Journal of Human Genetics, 15, 1252–1259. https://doi.org/10.1038/sj.ejhg.5201907 [DOI] [PubMed] [Google Scholar]
- Paffenbarger, R. S. Jr. , Blair, S. N. , Lee, I. M. , & Hyde, R. T. (1993). Measurement of physical activity to assess health effects in free‐living populations. Medicine and Science in Sports and Exercise, 25, 60–70. https://doi.org/10.1249/00005768-199301000-00010 [DOI] [PubMed] [Google Scholar]
- Paffenbarger, R. S. Jr. , Wing, A. L. , & Hyde, R. T. (1978). Physical activity as an index of heart attack risk in college alumni. American Journal of Epidemiology, 108, 161–175. https://doi.org/10.1093/oxfordjournals.aje.a112608 [DOI] [PubMed] [Google Scholar]
- Pan, M. , Medina, A. , Romero, M. , Ojeda, S. , Martin, P. , de Lezo, J. S. , & de Lezo, J. S. (2014). Assessment of side branch predilation before a provisional T‐stent strategy for bifurcation lesions. A randomized trial. American Heart Journal, 168, 374–380. https://doi.org/10.1016/j.ahj.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Paulhus, D. (1991). Measurement and control of response bias In Robinson J., Shaver P. & Wrightsman L. (Eds.), Measures of personality and psychological attitudes (pp. 17–59). San Diego, CA: Academic Press. [Google Scholar]
- Pescatello, L. S. , Devaney, J. M. , Hubal, M. J. , Thompson, P. D. , & Hoffman, E. P. (2013). Highlights from the functional single nucleotide polymorphisms associated with human muscle size and strength or FAMuSS study. BioMed Research International, 2013, 643575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello, L. S. , Kostek, M. A. , Gordish‐Dressman, H. , Thompson, P. D. , Seip, R. L. , Price, T. B. , … Hoffman, E. P. (2006). ACE ID genotype and the muscle strength and size response to unilateral resistance training. Medicine and Science in Sports and Exercise, 38, 1074–1081. https://doi.org/10.1249/01.mss.0000222835.28273.80 [DOI] [PubMed] [Google Scholar]
- Pistilli, E. E. , Bogdanovich, S. , Garton, F. , Yang, N. , Gulbin, J. P. , Conner, J. D. , … Khurana, T. S. (2011). Loss of IL‐15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. Journal of Clinical Investigation, 121, 3120–3132. https://doi.org/10.1172/JCI44945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli, E. E. , Devaney, J. M. , Gordish‐Dressman, H. , Bradbury, M. K. , Seip, R. L. , Thompson, P. D. , … Hoffman, E. P. (2008). Interleukin‐15 and interleukin‐15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine, 43, 45–53. https://doi.org/10.1016/j.cyto.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, L. S. , Strait‐Bodey, L. , Anderson, B. G. , Argiles, J. M. , & Havel, P. J. (2005). Interleukin‐15 stimulates adiponectin secretion by 3T3‐L1 adipocytes: Evidence for a skeletal muscle‐to‐fat signaling pathway. Cell Biology International, 29, 449–457. https://doi.org/10.1016/j.cellbi.2005.02.005 [DOI] [PubMed] [Google Scholar]
- di Renzo, L. , Gloria‐Bottini, F. , Saccucci, P. , Bigioni, M. , Abenavoli, L. , Gasbarrini, G. , & de Lorenzo, A. (2009). Role of interleukin‐15 receptor alpha polymorphisms in normal weight obese syndrome. International Journal of Immunopathology and Pharmacology, 22, 105–113. https://doi.org/10.1177/039463200902200112 [DOI] [PubMed] [Google Scholar]
- Riechman, S. E. , Balasekaran, G. , Roth, S. M. , & Ferrell, R. E. (2004). Association of interleukin‐15 protein and interleukin‐15 receptor genetic variation with resistance exercise training responses. Journal of Applied Physiology, 1985(97), 2214–2219. https://doi.org/10.1152/japplphysiol.00491.2004 [DOI] [PubMed] [Google Scholar]
- Scanzello, C. R. , Umoh, E. , Pessler, F. , Diaz‐Torne, C. , Miles, T. , Dicarlo, E. , … Crow, M. K. (2009). Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin‐15 differentiates early from end‐stage disease. Osteoarthritis Cartilage, 17, 1040–1048. https://doi.org/10.1016/j.joca.2009.02.011 [DOI] [PubMed] [Google Scholar]
- Simpson, K. , Parker, B. , Capizzi, J. , Thompson, P. , Clarkson, P. , Freedson, P. , & Pescatello, L. S. (2015). Validity and reliability question 8 of the Paffenbarger Physical Activity Questionnaire among healthy adults. Journal of Physical Activity and Health, 12, 116–123. https://doi.org/10.1123/jpah.2013-0013 [DOI] [PubMed] [Google Scholar]
- Stubbe, J. H. , Boomsma, D. I. , & de Geus, E. J. (2005). Sports participation during adolescence: A shift from environmental to genetic factors. Medicine and Science in Sports and Exercise, 37, 563–570. https://doi.org/10.1249/01.MSS.0000158181.75442.8B [DOI] [PubMed] [Google Scholar]
- Stubbe, J. H. , Boomsma, D. I. , Vink, J. M. , Cornes, B. K. , Martin, N. G. , Skytthe, A. , … de Geus, E. J. (2006). Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS ONE, 1, e22 https://doi.org/10.1371/journal.pone.0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. D. , Moyna, N. , Seip, R. , Price, T. , Clarkson, P. , Angelopoulos, T. , … Hoffman, E. P. (2004). Functional polymorphisms associated with human muscle size and strength. Medicine and Science in Sports and Exercise, 36, 1132–1139. https://doi.org/10.1249/01.MSS.0000132274.26612.23 [DOI] [PubMed] [Google Scholar]
- Troiano, R. P. , Berrigan, D. , Dodd, K. W. , Masse, L. C. , Tilert, T. , & McDowell, M. (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40, 181–188. https://doi.org/10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- Vink, J. M. , Boomsma, D. I. , Medland, S. E. , de Moor, M. H. , Stubbe, J. H. , Cornes, B. K. , … de Geus, E. J. (2011). Variance components models for physical activity with age as modifier: A comparative twin study in seven countries. Twin Research and Human Genetics, 14, 25–34. https://doi.org/10.1375/twin.14.1.25 [DOI] [PubMed] [Google Scholar]
- Wu, X. , He, Y. , Hsuchou, H. , Kastin, A. J. , Rood, J. C. , & Pan, W. (2010). Essential role of interleukin‐15 receptor in normal anxiety behavior. Brain, Behavior, and Immunity, 24, 1340–1346. https://doi.org/10.1016/j.bbi.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. J. , Wang, L. C. , Yeh, C. H. , Lu, C. A. , & Wu, S. J. (2010). Isolation and characterization of the Arabidopsis heat‐intolerant 2 (hit2) mutant reveal the essential role of the nuclear export receptor EXPORTIN1A (XPO1A) in plant heat tolerance. New Phytologist, 186, 833–842. https://doi.org/10.1111/j.1469-8137.2010.03225.x [DOI] [PubMed] [Google Scholar]


