Abstract
Background:
Functional limitations are a major cause for needing care and institutionalization among older adults. Exposure to air pollution has been suggested to be associated with increased functional limitations in older people.
Objective:
Our objective was to assess the association between air pollution and physical functioning in Dutch older adults.
Methods:
We analyzed data on performance-based (walking speed, ability to rise from a chair, putting on and taking off a cardigan, balance test) and self-reported physical functioning for 1,762 participants of the Longitudinal Aging Study Amsterdam, who participated in measurement cycles performed in 2005/2006, 2008/2009, and 2011/2012. Annual average outdoor air pollution concentrations [nitrogen dioxide (), nitrogen oxides (), particulate matter with diameters (), (), and (), and absorbance] at the home address at the start of the first measurement cycle were estimated using land-use regression models. Analyses were performed using mixed models with random participant intercepts adjusting for potential confounders.
Results:
Exposure to most air pollutants was associated with reduced performance-based physical functioning; for example, an interquartile range increase in exposure was associated with a 0.22 (95% confidence interval: 0.03, 0.42) lower performance test score in fully adjusted models, equivalent to the difference in performance score between participants who differed by 9 mo in age. Exposure to air pollution was generally not statistically significantly associated with self-reported functional limitations, and not associated with a faster decline in performance-based physical functioning over the study period.
Conclusion:
This study suggests that exposure to air pollution may adversely affect physical performance of older adults in the Netherlands. https://doi.org/10.1289/EHP2239
Introduction
A substantial increase in the number of older persons has been observed in many geographic regions over the past years and this increase is expected to accelerate in the near future (United Nations 2015). In Europe, the percentage of persons age 65 and older has been estimated to increase from 17% to 30% between 2013 and 2060 (European Commission 2015). These demographic changes will shape future health care use and health care expenditures (European Commission 2015). Functional limitations and disabilities in the activities of daily living are among the major causes of loss of independence and the need for long-term care (Kemper 1992). Being no longer able to live independently is a major concern for older adults and their families. Consequently, there is a growing interest in the determinants of physical functioning and well-being of older people.
Long-term exposure to air pollution is associated with a number of adverse health effects, including increased risks for cardiovascular and respiratory disease and (subclinical) pathophysiological processes that contribute to these conditions, such as hypertension and systemic inflammation (Brook et al. 2010; WHO Regional Office for Europe 2005; Health Effects Institute Panel on the Health Effects of Traffic-Related Air Pollution 2010; Pope and Dockery 2006; Wellenius et al. 2013).
Given that physical limitations are common functional consequences of these subclinical processes and chronic diseases, it is possible that long-term exposure to air pollution also influences physical functioning. Evidence for an association between ambient air pollution exposure and physical functioning is growing. A cross-sectional study among more than 45,000 adults ( of age on average) from China, Ghana, India, Mexico, Russia, and South Africa reported increasing levels of disability, in particular in the domains of cognition and mobility, with increasing concentrations of particulate matter smaller than (Lin et al. 2017). A multilevel prospective cohort study with 13,802 participants (65–105 y of age) from China suggests that exposure to high levels of air pollution, defined as a high air pollution index for the city or prefecture of residence, is associated with fewer years without functional limitations (Wen and Gu 2012). Another prospective cohort study with 6,157 participants () from Chicago, Illinois, USA, concluded that long-term exposure to nitrogen oxides () may be associated with a faster aging-related decline in physical functioning (Weuve et al. 2016).
This study aims to contribute to the currently limited body of evidence regarding the association between air pollution exposure and physical functioning by assessing the association of long-term exposure to ambient nitrogen oxides, soot, and particulate matter mass with performance-based (walking speed, ability to rise from a chair, putting on and taking off a cardigan, balance test) and self-reported physical functioning of older adults in the Dutch Longitudinal Aging Study Amsterdam (LASA). The Netherlands is a densely populated country with a tight network of roads and highways for motorized traffic, which are major sources of these pollutants.
Materials and Methods
Study Design and Sample
The LASA is an ongoing multidisciplinary prospective cohort study on predictors and consequences of changes in physical, cognitive, emotional, and social functioning in older people in the Netherlands (Huisman et al. 2011). In 1992 an age-stratified random sample of adults 55–85 y of age was drawn from population registries of 11 municipalities in three geographical areas (west, northeast, and south) of the Netherlands. These regions were selected to achieve an optimal representation of the older Dutch population. Starting at baseline, every three years data were collected through face-to-face interviews by specially trained interviewers. After the inclusion of a new cohort in 2002/2003, these participants were added to the regular data collection cycles in 2005. For the present study, we used data from the three data collection cycles performed in both the original and the new cohort, that is, data of collection cycles performed in 2005/2006, 2008/2009, and 2011/2012. The study sample for the present analysis consisted of a total of 1,762 participants with estimated air pollution exposure, data on performance-based and/or self-reported physical functioning for at least one of the three cycles of data collection (2005/2006, 2008/2009, and 2011/2012), and complete data on the potential confounders considered in this analysis (see Figure S1). The 2005/2006 cycle is considered as the baseline for this study. The local medical ethics committee approved the study and written informed consent was obtained from all participants.
Long-Term Air Pollution Exposure Assessment
Annual average air pollution concentrations at the participants’ home addresses at the start of the 2005/2006 data collection were estimated by land-use regression models as described elsewhere (Beelen et al. 2013; Eeftens et al. 2012a). In brief, for the land-use regression models, an air pollution monitoring campaign was performed between October 2008 and February 2010 in the study area. Three measurements of over a period of 2 wk were done within 1 y at 80 sites; in the cold, warm, and (in one) intermediate temperature season. Simultaneous measurements of soot ( absorbance, determined as the reflectance of filters), particulate matter with diameters (), (), and (, calculated by subtracting from ) were done at 40 of these sites (Cyrys et al. 2012; Eeftens et al. 2012b). Results from the three measurements were averaged to estimate the annual average concentrations, adjusting for temporal variation of the measurements. Predictor variables of nearby traffic, population, and household density, and land use derived from geographic information systems were evaluated to explain spatial variation in annual average concentrations as described elsewhere (Beelen et al. 2013; Eeftens et al. 2012a). The models explained substantial fractions of the spatial variation in annual average , , absorbance, , and concentrations (leave-one-out-cross validation ), but limited fractions (0.38) for (see Table S1). The regression models were then used to estimate pollution concentrations at the participants’ home addresses at the beginning of the 2005/2006 measurement cycle, for which the same geographic information systems predictor variables were obtained, and exposures were not updated when participants moved to another address. Residents of nursing homes and other long-term health facilities were included in the present analysis and the addresses of these facilities were used as the home addresses for participants who lived in such a facility at the beginning of the 2005/2006 measurement cycle. The percentage of participants who lived in nursing homes and other long-term health facilities was small (4.0%) in 2005/2006 and only another 4.5% of the population was admitted to such facilities between 2006 and 2009 (Alders et al. 2016).
Assessment of Physical Functioning
Self-reported physical functioning.
Functional limitations were assessed during the main interview by asking the respondent questions about the degree of difficulty they had with seven activities of daily living during a normal week: walking up and down a 15-step staircase without resting, walking 5 min outdoors without resting, getting up and sitting down in a chair, (un)dressing oneself, using one’s own or public transportation, showering or bathing oneself, and cutting one’s own toenails. Response categories ranged from “No, I cannot” (1 point) to “Yes, without difficulty” (5 points). The total score was calculated as the sum of the scores for the different activities and ranged from 7 to 35 points. The questions were derived from a widely used questionnaire (Katz et al. 1970; McWhinnie 1981; Van Sonsbeek 1988).
Performance-based physical functioning.
Performance-based physical functioning was assessed by timed measurements of walking speed, rising up from and sitting down in a chair, putting on and taking off a cardigan, and maintaining balance in a tandem stand. For the walking test, respondents were asked to walk back and forth as quickly as possible. For the chair-stand test, respondents were asked to stand up and sit down in a chair five times as quickly as possible, without using their hands. For the cardigan test, respondents were asked to put on and take off a cardigan. For the ability to maintain balance in tandem stand, the respondent was asked to put the heel of one foot in front of the big toe of the other foot and to stand still as long as possible. After 10 s the test was stopped. The time for each test was categorized based on the quartiles of performance for cohort 1 at baseline. The first three tests resulted in scores ranging from 1 (slowest quartile) to 4 (fastest quartile), with score 0 for those not able to do the test. The balance test resulted in a score of 0 (not able), 2 (0–3 s) or 4 (10 s). The overall performance was calculated as the sum of the scores and ranged from 0–16, with higher scores representing a higher level of physical performance (Wicherts et al. 2007).
Both self-reported and performance-based physical functioning have been shown to be highly predictive of subsequent morbidity, hospitalization, and institutionalization (Ferrucci et al. 1997; Guralnik et al. 1994a, 1994b, 1995; Penninx et al. 2000).
Potential Confounders
Information on sex and participants’ educational level were collected at baseline. Information on participants’ age, smoking status, alcohol consumption, physical activity, depression, presence of chronic diseases, and changes in residential address was collected for each cycle. Education was classified as high (higher vocational education, college education, or university education), medium (general intermediate education, intermediate vocational education, or secondary education), and low (elementary education not completed, elementary education, or lower vocational education). Three categories of smoking were created: current, former, and never smoking. Alcohol consumption was divided into four categories: not drinking, light drinking, moderate drinking, and (very) excessive drinking, according to the Garretsen alcohol consumption index (Garretsen 1983). Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression scale (CES-D), which has been validated in the Dutch older population (Beekman et al. 1997; Radloff 1977). The total score of the 20 items of the CES-D, which ranges from 0–60, with higher scores indicating more depressive symptoms, was dichotomized using 16 as the cutoff score because scores of 16 or higher represent a clinically relevant depressive syndrome (Beekman et al. 1997). Physical activity was defined as the time (minutes per day) spent on a set of six physical activities (walking outside, bicycling, performing light and heavy household activities, and a maximum of two sports), calculated from self-reports considering the last 2 wk (Stel et al. 2004). Three categories were created using the first and third quartile as cutoffs. The status score of the four-digit postal code area was used to define area-level socioeconomic status. Status scores are based on the average income, the percentage of residents with a low income, the percentage of residents with a low level of education, and the percentage of unemployed persons (Knol 2012). Higher scores indicate a higher socioeconomic status. We used routine data from a regional site of the National Air quality Monitoring Network (http://www.rivm.nl/milieukwaliteit/lucht/) in the study area to estimate for each participant average exposure to and during the week preceding the performance test. We calculated the number of self-reported physician-diagnosed chronic diseases out of a set of seven conditions that were explicitly asked (cardiac diseases, cerebrovascular disorders, chronic obstructive pulmonary disease, diabetes, cancer, arthritis, and peripheral atherosclerosis) and that have been found to be associated with the decline in physical functioning in the LASA cohort (Kriegsman et al. 2004) and categorized these into 0, 1, and chronic diseases. Self-reported medical histories from the first LASA data collection cycle () were generally consistent with information provided by general practitioners for lung disease, cardiac disease, cerebrovascular disease, diabetes, and malignant neoplasms (), but less consistent for osteoarthritis/rheumatoid arthritis and peripheral atherosclerosis ( and 0.38, respectively) (Kriegsman et al. 1996). Because participants were not able to distinguish properly between osteoarthritis and rheumatoid arthritis, these two were combined.
Statistical Analyses
We assessed the overall relationship between air pollution and physical functioning in the 2005/2006, 2008/2009, and 2011/2012 measurement cycles (performance-based and self-reported) by means of mixed linear models with exposure and exposure–time since baseline interactions (which can be directly interpreted as the association of air pollution exposure with the rate of change in physical functioning) and random participant intercepts, taking into account the correlation between repeated measurements within participants. We performed all analyses with adjustment for age at baseline, time since baseline, and sex (Model 1), with additional adjustment for individual confounders including education level, smoking, alcohol consumption, depression, and physical activity (Model 2), and with additional adjustment for area-level socioeconomic status (Model 3). Covariates were selected from the 2005/2006 measurement cycle, which coincided best with the exposure period, and were not updated. We included interactions with time since baseline to the model for education, alcohol consumption, and depression (in addition to their main effects); other interactions were not included because they did not change air pollution–physical performance association estimates (change in estimate was ). Associations are presented as mean differences in performance score and 6-y change in physical performance (both derived from mixed linear models with exposure and exposure–time since baseline interaction) with 95% confidence intervals (CI). In all analyses, the six pollutants were studied separately, as continuous variables and as quartiles of exposure to assess the linearity of the exposure–health relationships. Associations with air pollution from models with air pollution as continuous variables are presented for an interquartile range increase in exposure to facilitate comparison of estimated effect sizes between pollutants.
Because stronger associations in females than in males have been reported for the study of air pollution and functional limitations in China by Wen and Gu (2012), we assessed sex-specific associations with air pollution in addition to overall associations by adding exposure–sex interaction terms to the mixed models.
As part of a sensitivity analysis, we explored the effect of additional adjustment for short-term air pollution exposure (Stieb et al. 2002) defined as air pollution levels during the week preceding the performance test (not for the self-reported physical functioning, because this does refer to a normal week and not to a specific day). Moreover, we compared the results of our analysis with the results of the same analysis restricted to participants who completed all three cycles of data collection. The impact of moving to another address during the follow-up on the observed associations was explored by repeating the mixed model analysis for the subgroup of participants who did not change address between 3 y prior to the baseline (2005/2006) cycle and the last completed cycle. Attrition (due to mortality) is a concern in longitudinal studies of older persons. Attrition in the present study was primarily due to mortality. Both in 2009 and in 2012, 11% of the participants had died during the previous 3 y and 5% had dropped out for other reasons. Given that air pollution is also a risk factor of mortality, attrition bias of the associations between air pollution and physical functioning due to selective attrition is a concern (Weuve et al. 2012). We explored the potential of bias due to selective attrition for our study by applying the method proposed by Weuve et al. (2012). In brief, we fitted pooled logistic regression models of continuation from study cycle to study cycle and subsequently weighted observations by the inverse of the probability of continuation in mixed model analyses using both nontruncated weights and extreme weights truncated to the first and 99th percentile of the weight distribution. Separate models for continuation were fitted for each pollutant and included all covariates of Model 3 described above, the exposure of interest, and physical performance at the previous visit. The likelihood of continuation increased with increasing prior physical functioning, decreased with age, and was significantly lower in smokers and participants with a low level of education, but was not associated with air pollution exposure. The c-statistics for all models were . Finally, we performed two-pollutant models for pollutants that were significantly () associated with physical functioning in one-pollutant models and assessed potential residual confounding due to unmeasured confounders using the method described by Vanderweele and Arah for a binary confounder with a constant effect on the outcome across treatment and covariate levels and a constant prevalence difference across covariate levels under the stable unit treatment value and consistency assumptions (Vanderweele and Arah 2011).
All analyses were done with SAS (version 9.4; SAS Institute Inc.).
Results
Characteristics of the Study Participants
Distributions of characteristics of the study participants and performance scores (performance-based and self-reported), overall and by quartile of annual average exposure at the home addresses at the beginning of the 2005/2006 cycle, are shown in Table 1. The study sample consisted of more females than males; the average age was 75.5 y for the 2005/2006 cycle. Less than one-fifth of the study participants were highly educated and about two-thirds reported one or more chronic diseases. A total of 1,289 participants did not change address between 3 y prior to the 2005/2006 cycle and the last completed cycle. Participants in the highest quartile of exposure were older, more often highly educated, more often depressed, and less often free from chronic diseases than participants in the lower-exposure quartiles. Furthermore, they lived in postal code areas with lower socioeconomic status scores and had lower performance-based physical functioning scores. Similar associations with participant characteristics were observed for the other air pollutants (data not shown). Younger participants, men, highly educated participants, smokers, drinkers, physically active participants, and participants without depressive symptoms or chronic diseases had higher performance scores (see Table S2).
Table 1.
Characteristics of the study participants by measurement cycle, overall and by quartile of annual average exposure ( participants).
| Covariate | Overall | Quartile of annual average exposure (concentration range ) | p-Valuea | ||||
|---|---|---|---|---|---|---|---|
| First (11.3–18.3) | Second (18.3–22.1) | Third (22.1–27.3) | Fourth (27.1–59.6) | ||||
| Number of participants | 1,762b | 440 | 441 | 440 | 441 | ||
| Age at baselinec (y) () | 0.0059 | ||||||
| Female sex [n (%)] | 970 (55.1) | 54.1 | 52.2 | 58.2 | 55.8 | 0.3211 | |
| Educational leveld [n (%)] | |||||||
| Low | 854 (48.5) | 58.4 | 47.8 | 46.8 | 40.8 | ||
| Medium | 592 (33.6) | 25.9 | 33.6 | 36.8 | 38.1 | ||
| High | 316 (17.9) | 15.7 | 18.6 | 16.4 | 21.1 | ||
| Smoking [n (%)] | 0.1919 | ||||||
| Never smoker | 548 (31.1) | 33.4 | 32.9 | 31.1 | 27.0 | ||
| Ex-smoker | 911 (51.7) | 51.1 | 51.7 | 51.8 | 52.2 | ||
| Current smoker | 303 (17.2) | 15.5 | 15.4 | 17.0 | 20.9 | ||
| Alcohol consumptione [n (%)] | 0.0854 | ||||||
| Nondrinker | 285 (16.2) | 18.2 | 14.5 | 18.6 | 13.4 | ||
| Light drinker | 909 (51.6) | 50.2 | 53.7 | 51.8 | 50.6 | ||
| Moderate drinker | 467 (26.5) | 27.5 | 27.0 | 22.7 | 28.8 | ||
| Excessive drinker | 101 (5.7) | 4.1 | 4.8 | 6.8 | 7.3 | ||
| Physical activity past 2 wk [n (%)] | 0.3181 | ||||||
| 442 (25.1) | 24.5 | 24.0 | 22.7 | 29.0 | |||
| 879 (49.9) | 48.6 | 49.7 | 52.7 | 48.5 | |||
| 441 (25.0) | 26.8 | 26.3 | 24.5 | 22.4 | |||
| Depression [n (%)] | 258 (14.6) | 12.3 | 12.2 | 15.2 | 18.8 | 0.0163 | |
| Chronic diseases [n (%)] | |||||||
| Lung disease | 227 (12.9) | 11.1 | 12.2 | 15.5 | 12.7 | 0.2656 | |
| Cardiac disease | 438 (24.9) | 24.1 | 24.5 | 23.6 | 27.2 | 0.6086 | |
| Peripheral arteriosclerosis | 133 (7.5) | 6.1 | 7.0 | 7.7 | 9.3 | 0.3367 | |
| Diabetes | 205 (11.6) | 9.1 | 14.1 | 10.5 | 12.9 | 0.0858 | |
| Stroke | 106 (6.0) | 4.5 | 5.9 | 6.6 | 7.0 | 0.4314 | |
| Arthritis | 829 (47.0) | 44.5 | 43.1 | 50.2 | 50.3 | 0.0553 | |
| Cancer | 247 (14.0) | 14.3 | 12.2 | 14.1 | 15.4 | 0.5926 | |
| No. of above chronic diseases [n (%)] | 0.0696 | ||||||
| 0 | 488 (27.7) | 30.1 | 29.5 | 28.2 | 22.2 | ||
| 1 | 644 (36.5) | 37.0 | 35.6 | 34.3 | 39.2 | ||
| 630 (35.8) | 32.0 | 34.9 | 37.5 | 38.5 | |||
| Changed addressf [n (%)] | 473 (26.8) | 26.5 | 29.3 | 25.0 | 26.8 | 0.5485 | |
| Status score of 4-digit postal code areag () | 0.0059 | ||||||
| Physical performance | |||||||
| Performance-based () | |||||||
| Self-reported () | 0.0298 | ||||||
F-test for age, status score, and physical performance, and chi-square test otherwise.
Number of participants with estimated air pollution exposure, data on all potential confounders listed in Table 1, and performance-based and/or self-reported physical functioning for at least one measurement cycle.
At the 2005/2006 measurement cycle.
Low: elementary not completed, elementary education, or lower vocational education; medium: general intermediate education, general intermediate education, or general secondary education; high: higher vocational education, college education, or university education.
Light: less than 1 d/mo, 1–3 d/mo and consumption each time, 1–2 d/wk and consumptions each time, days/week and less than 2 consumptions each time; moderate: 1–3 d/mo and consumptions each time, 1–2 d/wk and 4–5 consumptions each time, 3–4 d/wk and 2–4 consumptions each time, or 5–7 d/wk and 2–3 consumptions each time; excessive: 1–4 d/wk and consumptions each or 5–7 d/wk and consumptions each.
Change of address between 3 y prior to the 2005/2006 cycle and the last completed cycle.
Measure of area-level socioeconomic status based on the average income, percentage of low income residents, percentage of residents with low level of education, and percentage of unemployed persons. Higher scores indicate a higher socioeconomic status.
Air Pollution Exposure
The distribution of the estimated annual average air pollution levels at the participants’ home addresses at the beginning of the 2005/2006 cycle is shown in Table 2. Variation in air pollution levels was largest for (maximum/minimum ratio 5.3) and smallest for (maximum/minimum ratio 1.3). Correlations were high between , , , absorbance, and (Pearson correlation 0.82–0.92; see Table S3), and moderate between and the other pollutants (Pearson correlation 0.36–0.69). The distributions of the average and concentrations during the week preceding the performance test are presented in Table S4. Annual average air pollution concentrations and average air pollution concentrations during the week preceding the performance test were not correlated (Pearson correlation , data not shown).
Table 2.
Distribution of estimated annual average air pollution levels at the participants’ home addresses at the beginning of the 2005/2006 cycle for all study participants ().
| Pollutant | Min | Median | Max | IQR | |
|---|---|---|---|---|---|
| () | 11.3 | 22.1 | 59.6 | 8.9 | |
| () | 18.5 | 31.8 | 93.3 | 13.5 | |
| abs () | 0.86 | 1.19 | 2.60 | 0.31 | |
| () | 15.0 | 16.1 | 19.8 | 1.4 | |
| () | 23.7 | 24.5 | 32.2 | 1.5 | |
| () | 7.6 | 8.0 | 13.3 | 0.8 |
Note: IQR: interquartile range; max, maximum; min, minimum.
Association between Air Pollution and Physical Functioning
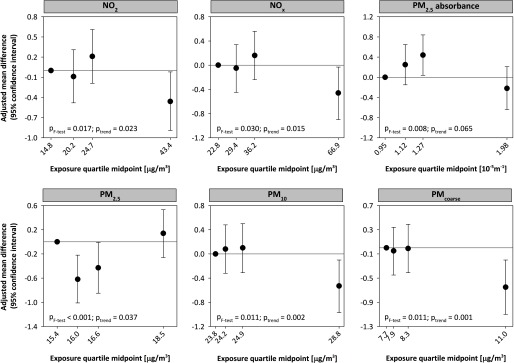
Analyses of associations of performance-based (Figure 1) and self-reported physical functioning (see Figure S2) with air pollution exposure quartiles indicate that the associations with performance-based physical functioning are nonlinear and largely driven by the participants with the highest exposures.
Figure 1.
Adjusted associations between performance-based physical functioning and quartiles of residential air pollution exposure from linear mixed model analyses with p-values of F-tests for equality of means and trend tests using quartile midpoints ( participants, observations). Models were adjusted for age, sex, education level, smoking, alcohol consumption, depression, physical activity, area-level socioeconomic status defined as the status score of the four-digit postal code area, and cross-products of time since baseline with education, alcohol consumption, and depression. Associations are presented as mean difference in physical performance score in the different quartiles as compared with the 1st quartile with 95% confidence intervals and were derived from models with exposure and exposure–time since baseline interaction.
Estimates of the associations of performance-based and self-reported physical functioning with air pollution exposure (continuous) are presented in Table 3. After adjustment for potential individual confounders, we observed a decrease in performance-based physical functioning with increasing levels of all pollutants except , and no association with self-reported physical functioning. The associations with performance-based physical functioning were somewhat attenuated after adjustment for socioeconomic status scores of the four-digit postal code area. The decreases in performance score per interquartile-range increase in exposure varied from 0.13 points (95% CI: , 0.05) for absorbance to 0.22 points (95% CI: , and 95% CI: , ) for and , respectively, in Model 3. Interactions between exposure and time since baseline were not statistically significant (minimum , data not shown), and findings of these analyses suggest that over the 6-y observation period there was no faster decline in performance-based and self-reported physical functioning with increasing air pollution exposure (Table 4). There was also no statistically significant modification of the association between performance-based physical functioning and air pollution by sex (see Figure S3; minimum ).
Table 3.
Associations between physical performance (performance-based and self-reported) and residential air pollution exposure from linear mixed model analyses for an interquartile range increase in exposure.
| Pollutant | Increment | Model 1a | Model 2a | Model 3a | |||
|---|---|---|---|---|---|---|---|
| Mean difference | (95% CI) | Mean difference | (95% CI) | Mean difference | (95% CI) | ||
| Performance-basedb | |||||||
| (, ) | (, ) | (, ) | |||||
| (, ) | (, ) | (, ) | |||||
| abs | 0.31 | (, ) | (, ) | (, 0.05) | |||
| 0.09 | (, 0.33) | 0.12 | (, 0.35) | 0.16 | (, 0.39) | ||
| (, ) | (, ) | (, ) | |||||
| (, ) | (, ) | (, ) | |||||
| Self-reportedc | |||||||
| 0.03 | (, 0.30) | 0.10 | (, 0.35) | 0.21 | (, 0.49) | ||
| 0.04 | (, 0.27) | 0.13 | (, 0.35) | 0.23 | (, 0.47) | ||
| abs | 0.31 | (, 0.22) | 0.03 | (, 0.27) | 0.10 | (, 0.35) | |
| (, 0.18) | (, 0.20) | (, 0.22) | |||||
| 0.03 | (, 0.23) | 0.08 | (, 0.27) | 0.16 | (, 0.37) | ||
| 0.00 | (, 0.18) | 0.09 | (, 0.26) | 0.19 | (, 0.37) | ||
Note: Associations are presented as mean differences in physical performance score with 95% confidence intervals (CI) for an interquartile range increase in air pollution exposure and were derived from models with exposure and exposure–time since baseline interaction.
Model 1: Adjusted for age and sex; Model 2. as in Model 1, also adjusted for education level, smoking, alcohol consumption, depression, physical activity, and cross-products of time since baseline with education, alcohol consumption and depression; Model 3: as in Model 2, also adjusted for area-level socioeconomic status defined as the status score of the four-digit postal code area.
participants, observations.
participants, observations.
Table 4.
Associations between 6-y change in physical performance (performance-based and self-reported) and residential air pollution exposure from linear mixed model analyses for an interquartile range increase in exposure.
| Pollutant | Increment | Model 1a | Model 2a | Model 3a | |||
|---|---|---|---|---|---|---|---|
| Mean difference | (95% CI) | Mean difference | (95% CI) | Mean difference | (95% CI) | ||
| Performance-basedb | |||||||
| 0.06 | (, 0.26) | 0.00 | (, 0.20) | 0.00 | (, 0.20) | ||
| 0.11 | (, 0.28) | 0.07 | (, 0.24) | 0.07 | (, 0.23) | ||
| abs | 0.31 | 0.08 | (, 0.26) | 0.02 | (, 0.20) | 0.02 | (, 0.20) |
| (, 0.13) | (, 0.06) | (, 0.07) | |||||
| 0.12 | (, 0.27) | 0.08 | (, 0.22) | 0.08 | (, 0.22) | ||
| 0.10 | (, 0.23) | 0.07 | (, 0.20) | 0.07 | (, 0.20) | ||
| Self-reportedc | |||||||
| 0.05 | (, 0.27) | (, 0.21) | (, 0.21) | ||||
| 0.05 | (, 0.23) | 0.01 | (, 0.20) | 0.01 | (, 0.19) | ||
| abs | 0.31 | 0.05 | (, 0.26) | (, 0.19) | (, 0.19) | ||
| (, 0.22) | (, 0.16) | (, 0.16) | |||||
| 0.07 | (, 0.24) | 0.02 | (, 0.18) | 0.02 | (, 0.18) | ||
| 0.07 | (, 0.22) | 0.03 | (, 0.18) | 0.03 | (, 0.18) | ||
Note: Associations are presented as mean differences in 6-y change in physical performance score with 95% confidence intervals (CI) for an interquartile range increase in air pollution exposure and were derived from models with exposure and exposure–time since baseline interaction. Negative values represent a faster decline in physical performance.
Model 1: Adjusted for age and sex; Model 2: as in Model 1, also adjusted for education level, smoking, alcohol consumption, depression, physical activity, and cross-products of time since baseline with education, alcohol consumption and depression; Model 3: as in Model 2, also adjusted for area-level socioeconomic status defined as the status score of the four-digit postal code area.
participants, observations.
participants, observation.
Sensitivity Analyses
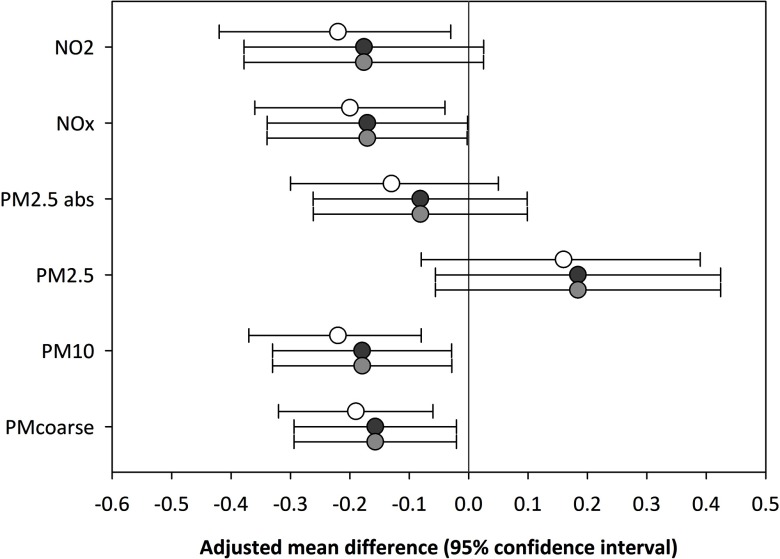
Additional adjustment of the associations between performance-based physical functioning and air pollution exposure for air pollution concentrations during the week preceding the performance test did not change effect estimates (see Table S5). When we restricted our analyses to participants with complete data for all three cycles of data collection, effect estimates were slightly strengthened for performance-based physical functioning; whereas associations with self-reported physical functioning were attenuated (see Table S6). When restricting the analysis to participants who had not changed their address since 3 y prior to the 2005/2006 cycle, effect estimates for performance-based physical functioning increased by about one-third for , , and , and by more than 70% for and absorbance (see Table S7). Adjustment for attrition somewhat reduced associations of performance-based physical functioning with , , absorbance, , and (Figure 2) and did not affect associations with the decline in performance-based physical functioning (i.e., exposure–time since baseline interactions, maximum difference in effect size was 0.003 points, data not shown). Results did not differ between analyses with nontruncated and with truncated weights (Figure 2). Two-pollutant models for performance-based physical functioning with combinations of the pollutants that were significantly associated with performance-based physical functioning resulted in multicollinearity problems (variance inflation factors were 3.2–7.8) and are therefore not presented. We assessed whether an unmeasured confounder could plausibly explain the reported associations between air pollution and performance-based physical functioning by conducting a post hoc sensitivity analysis using the method described by Vanderweele and Arah (2011) where we quantified the characteristics of an unmeasured binary confounder U that would be required to induce the observed difference in performance-based physical functioning of units (fourth vs. first quartile of exposure) and found that assuming U had an effect on the outcome equivalent to that of being 2 or 5 y older, the difference in the prevalence of U across exposure groups would have to be 77 and 31 percentage points, respectively, corresponding to minimum odds ratios of 59.2 and 3.6 (see Table S8).
Figure 2.
Adjusted associations between performance-based physical functioning and residential air pollution exposure without (white circles) and with weighting for attrition (light gray circles represent analyses with uncensored weights; dark gray circles represent analyses with extreme weights truncated to the first and 99th percentiles of the weight distribution). Models were adjusted for age, sex, education level, smoking, alcohol consumption, depression, physical activity, area-level socioeconomic status defined as the status score of the four-digit postal code area, and cross-products of time since baseline with education, alcohol consumption, and depression. Associations are presented as mean differences in physical performance score with 95% confidence intervals (CI) for an interquartile range increase in air pollution exposure and were derived from models with exposure and exposure–time since baseline interaction.
Discussion
This study found that long-term exposure to air pollution was associated with decreased physical functioning in a cohort of older adults from the Dutch general population. The associations with air pollution exposure were more consistent for performance-based physical functioning than for self-reported physical functioning. Air pollution exposure was not associated with a faster decline in physical performance over the 6-y observation period.
Our study adds to the currently limited evidence for an adverse effect of ambient air pollution on physical functioning in older adults. It supports findings from the Chinese Longitudinal Health Longevity Survey (CLHLS) suggesting a decrease in 3-y self-reported health expectancies, defined as life expectancy free of specific health conditions including (instrumental) activities of daily living, with increasing air pollution levels (Wen and Gu 2012), and from the Study on Global Ageing and Adult Health (SAGE) suggesting an increase in self-reported disability in particular in the domains of cognition and mobility among adults from China, Ghana, India, Mexico, Russia, and South Africa (Lin et al. 2017). Moreover, the present study provides additional evidence for associations not being limited to areas with very high levels of air pollution (the range for in the Chinese study estimated from the air pollution index was ; the maximum estimated level in the present study was ). Due to the differences in outcome and exposure assessment, findings of the Chinese study cannot be directly compared with our findings. In contrast to the CLHLS and SAGE studies, associations of air pollution exposure with physical functioning were limited to performance-based physical functioning and were not found with self-reported physical functioning. Self-reports and performance tests measure different dimensions of physical functioning. Self-reported physical functioning measures an individual’s perceived functional capacity in their own social and physical context. Performance-based physical functioning, in particular performance in gait speed and chair-stand tests, has been found to be a good predictor of subsequent disability and mortality (Guralnik et al. 1994a, 1994b). In contrast, self-reports of physical functioning are much more influenced by environment and psychological characteristics (Kempen et al. 1996; Lan et al. 2002) with differing thresholds for admitting difficulty (Melzer et al. 2004). Self-reported physical functioning may therefore be less accurate than performance-based physical functioning, which may explain the consistent associations of air pollution exposure with performance-based physical functioning and the general lack of associations with self-reported physical functioning in the present study. Furthermore, a stronger association of physical functioning with air pollution in females than in males, as reported in the CLHLS and SAGE studies, could not be confirmed in the present study.
Weuve et al. (2016) published a study on the association between air pollution and physical performance of older adults in the Chicago Health and Aging Project. In that cohort, there was no indication of a lower baseline physical performance among those with higher exposures, but higher exposure was found to be associated with a faster decline in physical performance over on average a period of 5–7 y (Weuve et al. 2016). We found no statistically significantly faster decline with increasing exposure in our study. The reason for this is not clear. Performance-based tests (a short battery of tests: balance, lower-extremity strength, and gait speed), mean age at baseline, duration of follow-up, and air pollution levels in the study by Weuve et al. (2016) were very similar to those of the present study; therefore, most likely these do not explain differences in study findings. This does not rule out that, apart from chance, the differences between the findings of the two studies could be related to subtle differences in physical fitness, social support, health behaviors, etc. for which we were unable to account.
Physical limitations are common functional consequences of chronic diseases including cardiovascular and respiratory disease and (subclinical) pathophysiological processes that contribute to these conditions, such as hypertension and systemic inflammation, which have been found to be associated with long-term air pollution exposure (Brook et al. 2010; WHO Regional Office for Europe 2005; Health Effects Institute Panel on the Health Effects of Traffic-Related Air Pollution 2010; Pope and Dockery 2006; Wellenius et al. 2013). Therefore, it has been hypothesized that long-term air pollution exposure may also adversely affect physical functioning. Although there is evidence from our cohort that chronic disease precedes physical disability (van Gool et al. 2005), the observed association between air pollution and performance-based physical functioning remained unchanged after additional adjustment for the chronic diseases studied. However, misclassification of the chronic disease status is possible and may at least partly post hoc explain these findings.
We performed analyses with and without adjustment for attrition and found that associations with performance-based physical functioning were only slightly attenuated after adjustment. Selection of participants into the study at cohort formation may be another source of bias in the observed associations, when selection is related to the outcome and the exposure under study (Weisskopf et al. 2015). It is possible that physical functioning has influenced participation in the study and an association between air pollution exposure and participation cannot be ruled out because of the association between air pollution and participants’ education, which is a determinant of participation. The use of a purely spatial air pollution exposure model might be a limitation of this study. Air pollution levels were modeled by land-use regression for the year 2009, which is a few years after our first cycle of data collection in 2005/2006. However, previous studies from Europe support our assumption of stability of spatial contrasts in air pollution levels, in particular and black carbon levels, over periods of 7 y and more (Cesaroni et al. 2012; Eeftens et al. 2011; Gulliver et al. 2011). Further support for the validity of the estimated air pollution levels for participants who did not change their address comes from the National Air Quality Monitoring Network, where annual average and levels were relatively stable during the relevant period, that is, 2005–2009 (Mooibroek et al. 2013). Some measurement error might still be a concern because we do not, for example, have data on individual time–activity patterns and air pollution concentrations at locations other than at home, where people spend some part of their time.
We adjusted for a large number of potential individual confounders at baseline, for area-level socioeconomic status, and acute air pollution exposure. For some of the individual confounders like physical activity and depression, however, we cannot rule out that their status at baseline is a consequence of prior disability rather than precedes disability status and that this may have caused some bias. We therefore performed sensitivity analyses of residual confounding due to an unmeasured binary confounder assuming an effect of the unmeasured confounder on performance-based physical functioning equivalent to that of 2 or 5 y of age ( and points), which is large, but possible. The difference in prevalence of the confounder between the first and fourth quartile of exposure would need to be at least 77 and 31 percentage points, respectively, to completely eliminate the observed associations after adjustment for other covariates. Such differences in prevalence are considered unlikely because they largely exceed the observed differences across exposure quartiles for a range of potential confounders (Table 1).
We derived associations with performance levels from models with exposure–time since baseline interaction terms. The exposure coefficient from the model with exposure–time since baseline interaction terms is a direct estimate of the association between exposure and physical performance at baseline. However, if the coefficient for the exposure-time cross-product term is small, then the exposure coefficient can also be interpreted as describing the association of exposure with the disability over the entire course of follow-up analyzed. This interpretation is justified in the present study because coefficients for the exposure–time since baseline interaction terms were small. The estimated exposure coefficients for performance-based physical functioning were very similar (maximum 0.03 points smaller for Model 3) in models with and without exposure–time since baseline interaction terms (see Table S9).
Air pollution is a complex mixture. An important issue regarding the health effects of air pollution concerns the issue of pollutant-specific effects, that is, which pollutants are responsible for the observed associations. Because a general air pollution index (instead of pollutant-specific concentrations) was used in the Chinese study (Wen and Gu 2012) and the U.S. study (Weuve et al. 2016) was limited to , this could not be assessed in the previous studies. In the present study, we linked concentrations of different air pollutants including nitrogen oxides, absorbance, and different fractions of particulate matter (, , and ). has been suggested as a marker for the mixture of traffic-related air pollutants in studies such as ours (Brown et al. 2007). A recent review, however, concluded that individually or in combination with other pollutants may cause adverse health effects independent of effects of particulate matter mass metrics (WHO Regional Office for Europe 2013). absorbance characterizes local soot emissions, especially from diesel vehicles. mass concentrations, in contrast to and absorbance, are more attributable to long-range transport. Two traffic variables were included in the land-use regression models, probably reflecting resuspended road dust from tire and brake wear (Eeftens et al. 2012a). Performance-based physical functioning was statistically significantly associated with all pollutants, except in the present study and absorbance in fully adjusted Model 3. Moreover, associations with were positive, whereas associations with the other pollutants were negative. The reason for this lack of association and inconsistency is not clear. is positively correlated with the other pollutants; correlations with , however, are lower than correlations between the other pollutants (Pearson correlation 0.36–0.69 vs. 0.82–0.93) despite no major differences between the model and the models for the other pollutants in terms of predictors (it contains traffic variables in addition to background air pollution concentrations) and model performance (see Table S1). The lack of association with in the presence of associations with , absorbance, and larger particles underline the importance of traffic for the observed associations with physical functioning. However, a limitation of the present study is the smaller contrast in levels as compared with the other pollutants except . The smaller contrast may partly explain the lack of association with , but in the presence of an association with , for which variation is even smaller, this likely does not fully explain the lack of association with . Another limitation of the present study is the very high correlation between , , , absorbance, and (Pearson correlation 0.82–0.93), which makes it impossible to disentangle the effects of these specific pollutants. Moreover, we acknowledge the possibility that the observed associations could (partly) reflect effects of traffic-related air pollutants other than the ones included in the present study.
To assess the relevance of the observed associations, we calculated the average functional decline per year for our study sample assuming a linear decline of physical functioning with age. An increase in age by 20 y within our study sample is associated with a decrease in the physical performance score by about 6 points. The observed effect of a 0.22 decrease in the physical performance score per interquartile range increase in thus corresponds to an age-related decline in performance of about 0.73 y (9 mo).
Conclusion
We conclude from our results that higher air pollution exposure was associated with decreased physical functioning, but not with a faster decline in physical functioning in older adults in the Netherlands. The associations with air pollution exposure were more consistent for performance-based physical functioning than for self-reported physical functioning.
Supplemental Material
Acknowledgments
The Longitudinal Aging Study Amsterdam is largely supported by a grant from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care.
References
- Alders P, Comijs HC, Deeg DJH. 2016. Changes in admission to long-term care institutions in the Netherlands: comparing two cohorts over the period 1996–1999 and 2006–2009. Eur J Ageing 14(2):123–131, PMID: 28579933, 10.1007/s10433-016-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. 1997. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med 27(1):231–235, PMID: 9122304, 10.1017/S0033291796003510. [DOI] [PubMed] [Google Scholar]
- Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, et al. 2013. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe – The ESCAPE project. Atmos Environ 72:10–23, 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brown JS, Graham JA, Chen LC, Postlethwait EM, Ghio AJ, Foster WM, et al. 2007. Panel discussion review: session four—assessing biological plausibility of epidemiological findings in air pollution research. J Expo Sci Environ Epidemiol 17 (suppl 2):S97–S105, PMID: 18079771, 10.1038/sj.jes.7500632. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, et al. 2012. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 11:48, PMID: 22808928, 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrys J, Eeftens M, Heinrich J, Ampe C, Armengaud A, Beelen R, et al. 2012. Variation of NO2 and NOx concentrations between and within 36 European study areas: results from the ESCAPE study. Atmos Environ 62:374–390, 10.1016/j.atmosenv.2012.07.080. [DOI] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. 2012a. Development of land use regression models for PM2.5, PM2.5 Absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–11205, PMID: 22963366, 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. 2011. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med 68(10):765–770, PMID: 21285243, 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- Eeftens M, Tsai MY, Ampe C, Anwander B, Beelen R, Bellander T, et al. 2012b. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2 – results of the ESCAPE project. Atmos Environ 62:303–317, 10.1016/j.atmosenv.2012.08.038. [DOI] [Google Scholar]
- European Commission. 2015. The 2015 Ageing Report. Economic and Budgetary Projections for the 28 EU Member States (2013–2060). Luxembourg:European Commission. [Google Scholar]
- Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. 1997. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 277(9):728–734, PMID: 9042845, 10.1001/jama.1997.03540330050034. [DOI] [PubMed] [Google Scholar]
- Garretsen HFL. 1983. Probleemdrinken: Prevalentiebepaling, beïnvloedende factoren en preventiemogelijkheden: Theoretische overwegingen en onderzoek in Rotterdam [in Dutch]. Lisse, Netherlands:Swets & Zeitlinger. [Google Scholar]
- Gulliver J, Morris C, Lee K, Vienneau D, Briggs D, Hansell A. 2011. Land use regression modeling to estimate historic (1962–1991) concentrations of black smoke and sulfur dioxide for Great Britain. Environ Sci Technol 45(8):3526–3532, PMID: 21446726, 10.1021/es103821y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. 1995. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332(9):556–561, PMID: 7838189, 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. 1994a. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 6(6):410–419, PMID: 7748914. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. 1994b. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94, PMID: 8126356, 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute Panel on the Health Effects of Traffic-Related Air Pollution. 2010. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. Boston, MA:Health Effects Institute. [Google Scholar]
- Huisman M, Poppelaars J, van der Horst M, Beekman AT, Brug J, van Tilburg TG, et al. 2011. Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol 40(4):868–876, PMID: 21216744, 10.1093/ije/dyq219. [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC. 1970. Progress in development of the index of ADL. Gerontologist 10(1):20–30, PMID: 5420677, 10.1093/geront/10.1_Part_1.20. [DOI] [PubMed] [Google Scholar]
- Kempen GI, Steverink N, Ormel J, Deeg DJ. 1996. The assessment of ADL among frail elderly in an interview survey: self-report versus performance-based tests and determinants of discrepancies. J Gerontol B Psychol Sci Soc Sci 51(5):P254–P260, PMID: 8809001, 10.1093/geronb/51B.5.P254. [DOI] [PubMed] [Google Scholar]
- Kemper P. 1992. The use of formal and informal home care by the disabled elderly. Health Serv Res 27(4):421–451, PMID: 1399651. [PMC free article] [PubMed] [Google Scholar]
- Knol F. 2012. Statusontwikkeling van wijken in Nederland 1998–2010 [in Dutch]. The Hague, Netherlands:Sociaal en Cultureel Planbureau. [Google Scholar]
- Kriegsman DM, Deeg DJ, Stalman WA. 2004. Comorbidity of somatic chronic diseases and decline in physical functioning: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol 57(1):55–65, PMID: 15019011, 10.1016/S0895-4356(03)00258-0. [DOI] [PubMed] [Google Scholar]
- Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. 1996. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol 49(12):1407–1417, PMID: 8970491, 10.1016/S0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- Lan TY, Melzer D, Tom BD, Guralnik JM. 2002. Performance tests and disability: developing an objective index of mobility-related limitation in older populations. J Gerontol A Biol Sci Med Sci 57(5):M294–M301, PMID: 11983723, 10.1093/gerona/57.5.M294. [DOI] [PubMed] [Google Scholar]
- Lin H, Guo Y, Zheng Y, Zhao X, Cao Z, Rigdon SE, et al. 2017. Exposure to ambient PM2.5 associated with overall and domain-specific disability among adults in six low- and middle-income countries. Environ Int 104:69–75, PMID: 28453972, 10.1016/j.envint.2017.04.004. [DOI] [PubMed] [Google Scholar]
- McWhinnie JR. 1981. Disability assessment in population surveys: results of the O.E.C.D. Common Development Effort. Rev Epidemiol Sante Publique 29(4):413–419, PMID: 6461907. [PubMed] [Google Scholar]
- Melzer D, Lan TY, Tom BD, Deeg DJ, Guralnik JM. 2004. Variation in thresholds for reporting mobility disability between national population subgroups and studies. J Gerontol A Biol Sci Med Sci 59(12):1295–1303, PMID: 15699529, 10.1093/gerona/59.12.1295. [DOI] [PubMed] [Google Scholar]
- Mooibroek D, Berkhout JPJ, Hoogerbrugge R. 2013. “Air quality in the Netherlands 2012” [in Dutch]. Report 680704023/2013. Bilthoven, Netherlands:National Institute of Public Health and the Environment (RIVM). [Google Scholar]
- Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. 2000. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontology A Biol Sci Med Sci 55(11):M691–M697, PMID: 11078100, 10.1093/gerona/55.11.M691. [DOI] [PubMed] [Google Scholar]
- Pope CA III, Dockery DW. 2006. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56(6):709–742, PMID: 16805397, 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psych Meas 1(3):385, 10.1177/014662167700100306. [DOI] [Google Scholar]
- Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. 2004. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol 57(3):252–258, PMID: 15066685, 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Smith-Doiron M, Brook JR, Burnett RT, Dann T, Mamedov A, et al. 2002. Air pollution and disability days in Toronto: results from the National Population Health Survey. Environ Res 89(3):210–219, PMID: 12176005, 10.1006/enrs.2002.4373. [DOI] [PubMed] [Google Scholar]
- United Nations. 2015. World Population Ageing 2015. New York:United Nations, Department of Economic and Social Affairs, Population Division. [Google Scholar]
- van Gool CH, Kempen GIJM, Penninx BWJH, Deeg DJH, Beekman ATF, van Eijk JTM. 2005. Impact of depression on disablement in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Soc Sci Med 60(1):25–36, PMID: 15482864, 10.1016/j.socscimed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Van Sonsbeek J. 1988. Methodological and substantial aspects of the OECD indicator of chronic functional limitations. (Maandbericht Gezondheid). The Hague: Statistics Netherlands (CBS).
- Vanderweele TJ, Arah OA. 2011. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology 22(1):42–52, 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Sparrow D, Hu H, Power MC. 2015. Biased exposure-health effect estimates from selection in cohort studies: are environmental studies at particular risk? Environ Health Perspect 123(11):1113–1122, PMID: 25956004, 10.1289/ehp.1408888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Boyle LD, Wilker EH, Sorond FA, Coull BA, Koutrakis P, et al. 2013. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke 44(6):1532–1536, PMID: 23709640, 10.1161/STROKEAHA.111.000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Gu D. 2012. Air pollution shortens life expectancy and health expectancy for older adults: the case of China. J Gerontol A Biol Sci Med Sci 67(11):1219–1229, PMID: 22518820, 10.1093/gerona/gls094. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kaufman JD, Szpiro AA, Curl C, Puett RC, Beck T, et al. 2016. Exposure to traffic-related air pollution in relation to progression in physical disability among older adults. Environ Health Perspect 124(7):1000–1008, PMID: 27022889, 10.1289/ehp.1510089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. 2012. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology 23(1):119–128, PMID: 21989136, 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Regional Office for Europe. 2005. Health Effects of Transport-Related Air Pollution. Copenhagen:WHO Regional Office for Europe; http://www.euro.who.int/__data/assets/pdf_file/0006/74715/E86650.pdf [Accessed 18 September 2016]. [Google Scholar]
- WHO Regional Office for Europe. 2013. Review of Evidence on Health Aspects of Air Pollution – REVIHAAP Project. Copenhagen, Denmark:WHO Regional Office for Europe; http://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report-final-version.pdf [Accessed 18 September 2016]. [PubMed] [Google Scholar]
- Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. 2007. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 92(6):2058–2065, PMID: 17341569, 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.