Abstract
Background:
Long-term exposure to particulate matter (PM) air pollution may increase blood pressure and the risk of hypertension. However, epidemiological evidence is scarce and inconsistent.
Objectives:
We investigated the associations between long-term exposure to PM with an aerodynamic diameter (), blood pressure, and incident hypertension in a large Taiwanese cohort.
Methods:
We studied 361,560 adults old from a large cohort who participated in a standard medical examination program during 2001 to 2014. Among this group, 125,913 nonhypertensive participants were followed up. A satellite-based spatiotemporal model was used to estimate the 2-y average concentrations at each participant’s address. Multivariable linear regression was used in the cross-sectional data analysis with the 361,560 participants to investigate the associations between and systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP), and Cox proportional hazard regression was used in the cohort data analysis with the 125,913 participants to investigate the associations between and incident hypertension.
Results:
Each increment in the 2-y average concentration was associated with increases of [95% confidence interval (CI): 0.40, 0.50], (95% CI: 0.04, 0.11), and (95% CI: 0.33, 0.42) in SBP, DBP, and PP, respectively, after adjusting for a wide range of covariates and possible confounders. Each increment in the 2-y average concentration was associated with an increase of 3% in the risk of developing hypertension [ (95% CI: 1.01, 1.05)]. Stratified and sensitivity analyses yielded similar results.
Conclusions:
Long-term exposure to air pollution is associated with higher blood pressure and an increased risk of hypertension. These findings reinforce the importance of air pollution mitigation strategies to reduce the risk of cardiovascular disease. https://doi.org/10.1289/EHP2466
Introduction
Cardiovascular disease (CVD) is a leading cause of death worldwide, contributing to 17.7 million deaths in 2015 (WHO 2017). Many epidemiological studies have shown that long-term exposure to particulate matter (PM) air pollution is associated with increased cardiovascular morbidity and mortality (Brook et al. 2010; Hoek et al. 2013; Pope and Dockery 2006). The American Heart Association states a causal relationship between CVD and PM, particularly PM with an aerodynamic diameter (), which can penetrate deep into the lungs (Brook et al. 2010). However, the underlying mechanisms of the relationship between and CVD remain uncertain. One potential mechanism by which PM may contribute to CVD development is through its effects on blood pressure. PM exposure can lead to imbalances in the autonomic nervous system and to local and systemic inflammation and oxidative stress, which result in elevated blood pressure (Brook et al. 2010). High blood pressure is a well-established determinant of atherosclerosis, which is the underlying pathology of most CVDs (Bondjers et al. 1991).
An increasing number of studies have shown that short-term exposure to PM is associated with increases in blood pressure (Chuang et al. 2010; Cosselman et al. 2012; Delfino et al. 2010; Dvonch et al. 2009; Hoffmann et al. 2012; Wu et al. 2013). However, evidence on the long-term effects of PM on blood pressure is scarce and inconsistent. Some studies have reported that long-term exposure to PM and black carbon is associated with increased blood pressure (Chan et al. 2015; Chen et al. 2015; Chuang et al. 2011; Dong et al. 2013; Fuks et al. 2011; Schwartz et al. 2012), but two large studies in Europe (Fuks et al. 2014) and the United States (Shanley et al. 2016) did not support a significant association. Studies on the associations between PM and incident hypertension are even scarcer, and the results are similarly inconsistent (Chen et al. 2014; Coogan et al. 2012, 2016; Zhang et al. 2016).
Large-scale prospective cohort studies may provide more stable results and allow more precise estimates. Furthermore, although most previous studies were conducted in North America and Europe, little evidence is available from other regions, such as East Asia, where air pollution is often more serious. To our knowledge, only three studies have investigated the association between PM air pollution and blood pressure in this region, and all of these studies are of cross-sectional design (Chen et al. 2015; Chuang et al. 2011; Dong et al. 2013). However, hypertension causes a heavy disease burden in Asian countries, with a population-attributable fraction of 60% for CVD (Martiniuk et al. 2007). We therefore used a satellite-based spatiotemporal model to estimate PM exposure and investigated the effects of long-term exposure to fine particulate matter () on blood pressure and incident hypertension in a large Taiwanese cohort.
Methods
Study Participants
We studied participants from a large cohort in Taiwan, which has been documented elsewhere (MJ Health Research Foundation 2016; Wen et al. 2011; Zhang et al. 2017). In brief, residents from all over Taiwan participated in a standard medical examination program provided by a private firm (MJ Health Management Institution, Taipei, Taiwan) from 1996 to 2014. The participants received a series of medical examinations including general physical examinations, anthropometric measurements, and biochemical tests of blood and urine. A standard self-administered questionnaire was also used to collect demographic and socioeconomic information, lifestyle indicators, and medical history. All participants gave written informed consent before their participation. Ethical approval was obtained from the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee.
In this study, we included participants old who had joined the program between 2001 and 2014, when satellite data used for exposure assessment were available. During this period, blood pressure was available for 424,061 participants. A total of 62,501 participants were excluded because of missing information on key potential confounders (57,412 cases) or on exposure due to missing address information (5,069 cases) or because they had a systolic blood pressure (SBP) or a diastolic blood pressure (DBP) (20 cases), which were likely due to measurement error. Finally, 361,560 participants were included in the data analysis to investigate the effects of on blood pressure. A total of 148,513 participants (148,513 of 361,560; 41.1%) had at least two visits (medical examinations) during the study period. Of these, 125,913 participants (84.8%) without hypertension (defined as having or , or having self-reported physician-diagnosed hypertension) at their first visit were followed up and included in the analysis of the effects of on the development of hypertension.
Air Pollution Exposure Assessment
A satellite-based spatiotemporal model was used for exposure assessment; the details of this model have been described in our previous study (Zhang et al. 2017). Briefly, we developed the model to retrieve ground-level concentrations at a high resolution () based on aerosol optical depth (AOD) data, which were derived from the spectral data from the two Moderate Resolution Imaging Spectroradiometer (MODIS) instruments aboard the U.S. National Aeronautics and Space Administration’s (NASA’s) Terra and Aqua satellites. The model was validated using ground-measured data from monitoring stations in Taiwan. The correlation coefficients between the satellite-retrieved and ground-measured yearly average concentrations ranged from 0.79 to 0.83, and the mean percentage errors were approximately 20% (Zhang et al. 2017).
Each participant’s address (either residence or company) was geocoded into latitude and longitude data, and then address-specific yearly average concentrations were calculated. Afterwards, the 2-y average concentration was calculated (based on the concentrations from the year of the medical examination and the year before the medical examination) as an indicator of long-term exposure to ambient air pollution.
Health Examination
The participants visited the medical center in the morning. After a 10-min rest, SBP and DBP were measured with a computerized auto-mercury sphygmomanometer (CH-5000; Citizen) with the participant seated. If the SBP was or the DBP was , a second measurement was obtained after 10 min, and the records from the second measurement were used for data analysis. The pulse pressure (PP) was computed as the difference between the SBP and the DBP. The weight and barefoot height were measured, and the body mass index (BMI) was calculated as the weight (kg) divided by the square of the height (m). An overnight fasting venous blood sample was taken to measure blood lipids (total cholesterol, triglycerides, and high-density lipoprotein cholesterol) and plasma glucose using an auto-analyzer (model 7150; Hitachi). Blood samples were analyzed at the central laboratory of MJ Health Screening Center by trained technicians. A standard self-administered questionnaire survey was also conducted to collect information on demographic and socioeconomic factors, lifestyle, and disease history. All of the examination procedures were approved according to International Organization for Standardization (ISO) 9001, and the relevant quality-control measures can be found in the technical report of the MJ Health Research Foundation (MJ Health Research Foundation 2016).
For the 125,913 initially nonhypertensive participants, the presence or absence of incident hypertension was identified in their subsequent visits and was defined as an SBP or a DBP or as a self-report of newly physician-diagnosed hypertension after the first visit. The number of visits ranged from 2 to 18 with a mean of 3.4 [standard deviation (SD): 2.0]. The censoring date was the first occurrence of hypertension or the last visit if hypertension did not occur.
Statistical Analysis
Statistical analyses were performed using R (version 3.2.3; R Core Team). A two-tailed p-value was considered statistically significant.
Air pollution and blood pressure.
We used the multivariable linear regression model to conduct a cross-sectional data analysis of the associations between exposure and blood pressure using the baseline (first-visit) data of the 361,560 participants. The following covariates were introduced into the models: age (y), sex, education level [less than high school (), high school (10–12 y), college or university (13–16 y), or postgraduate ()], smoking status (never, former, or current), alcohol drinking (less than once per week, one to three times per week, or more than three times per week), leisure-time physical activity (, , or ), occupational exposure to dust or organic solvents in the workplace (yes or no), season (calendar season), BMI, hypertension (defined above), diabetes (fasting blood glucose or self-reported physician-diagnosed diabetes), hyperlipidemia (total cholesterol , triglycerides , or high-density lipoprotein cholesterol ), any self-reported CVD or stroke (yes or no), and any self-reported form of cancer (yes or no). The participants were categorized into four groups based on the quartiles. When was treated as a continuous variable, effect estimates were calculated as changes in blood pressure for each increment in the 2-y average concentration.
We examined whether the association between blood pressure and exposure was modified by age ( and ), sex (men and women), education level (high school or lower, and above high school), smoking (never and ever smoker), hypertension (hypertensive and nonhypertensive), diabetes (diabetic and nondiabetic), and obesity ( and ) (Wen et al. 2009). Each potential modifier was examined in separate models by adding a multiplicative interaction term for , and p-values for the product terms were calculated. Subgroup analyses stratified by these factors were also performed.
Air pollution and incident hypertension.
Among the 125,913 nonhypertensive participants with follow-up, Cox proportional hazard regression was used to calculate the hazard ratio (HR) for incident hypertension associated with exposure after adjusting for age, sex, educational level, smoking, alcohol drinking, physical activity, occupational exposure, season, BMI, diabetes, hyperlipidemia, self-reported CVD or stroke, and cancer. Effect modification by age, sex, educational level, smoking, diabetes, and obesity was also assessed.
Sensitivity Analysis
We also performed a series of sensitivity analyses. For analysis on and blood pressure, a) we excluded participants who used their company address rather than their home address, and b) we restricted the study participants to those who were free of obesity, hypertension, diabetes, hyperlipidemia, cardiovascular disease, stroke, and cancer to minimize the influence of comorbidities. For analysis of and incident hypertension, we excluded participants with of follow-up to eliminate the potential influence of short follow-up because hypertension development is generally a chronic process.
Results
The general characteristics of the study participants are summarized in Table 1. The mean age was [standard deviation (SD): 13.0], and men were slightly in the minority (48.5%). Most study participants were well-educated, had never been smokers, and did not use alcohol. Generally, the participants selected for incident hypertension analysis were healthier than the overall cohort, with lower SBP, DBP, and PP values and lower prevalence of diabetes, hyperlipidemia, self-reported CVD or stroke, and self-reported cancer. The mean follow-up duration was 4.5 y (SD: 3.1), and 15,587 incident hypertension cases were identified during the study period.
Table 1.
Baseline characteristics of study participants.
| Characteristics | Overall cohort | Nonhypertensive participants with follow-up |
|---|---|---|
| Participants (n) | 361,560 | 125,913 |
| Age (y) | 39.9 (13.0) | 37.6 (10.9) |
| Male | 175,222 (48.5%) | 60,758 (48.3%) |
| Educational level | ||
| Lower than high school | 58,629 (16.2%) | 13,245 (10.5%) |
| High school | 73,261 (20.3%) | 25,279 (20.1%) |
| College or university | 186,963 (51.7%) | 71,389 (56.7%) |
| Postgraduate | 42,707 (11.8%) | 16,000 (12.7%) |
| Cigarette smoking | ||
| Never | 267,562 (74.0%) | 95,022 (75.5%) |
| Former | 20,859 (5.8%) | 6,354 (5.0%) |
| Current | 73,139 (20.2%) | 24,537 (19.5%) |
| Alcohol drinking | ||
| 310,460 (85.9%) | 109,420 (86.9%) | |
| 1–3 times/wk | 339,26 (9.4%) | 11,570 (9.2%) |
| 17,174 (4.7%) | 4,923 (3.9%) | |
| Physical activity | ||
| 163,031 (45.1%) | 58,944 (46.8%) | |
| 1–2 h/wk | 114,015 (31.5%) | 38,815 (30.8%) |
| 84,514 (23.4%) | 28,154 (22.4%) | |
| Occupational exposure | ||
| Dust | 13,493 (3.7%) | 4,644 (3.7%) |
| Organic solvent | 17,733 (4.9%) | 6,508 (5.2%) |
| Body mass index () | 23.0 (3.7) | 22.6 (3.4) |
| Systolic blood pressure () | 117.9 (17.6) | 113.1 (12.7) |
| Diastolic blood pressure () | 70.9 (11.4) | 67.8 (8.9) |
| Pulse pressure () | 47.0 (11.7) | 45.3 (9.6) |
| Hypertension | 56,716 (15.7%) | NA |
| Diabetes | 16,652 (4.6%) | 2,790 (2.2%) |
| Hyperlipidemia | 83,194 (23.0%) | 25,707 (20.4%) |
| Self-reported cancer | 4,226 (1.2%) | 1,174 (0.9%) |
| Self-reported cardiovascular disease or stroke | 10,891 (3.0%) | 2,214 (1.8%) |
Note: Data are the mean (standard deviation) for continuous variables and number (percentage) for categorical variables. NA, not available.
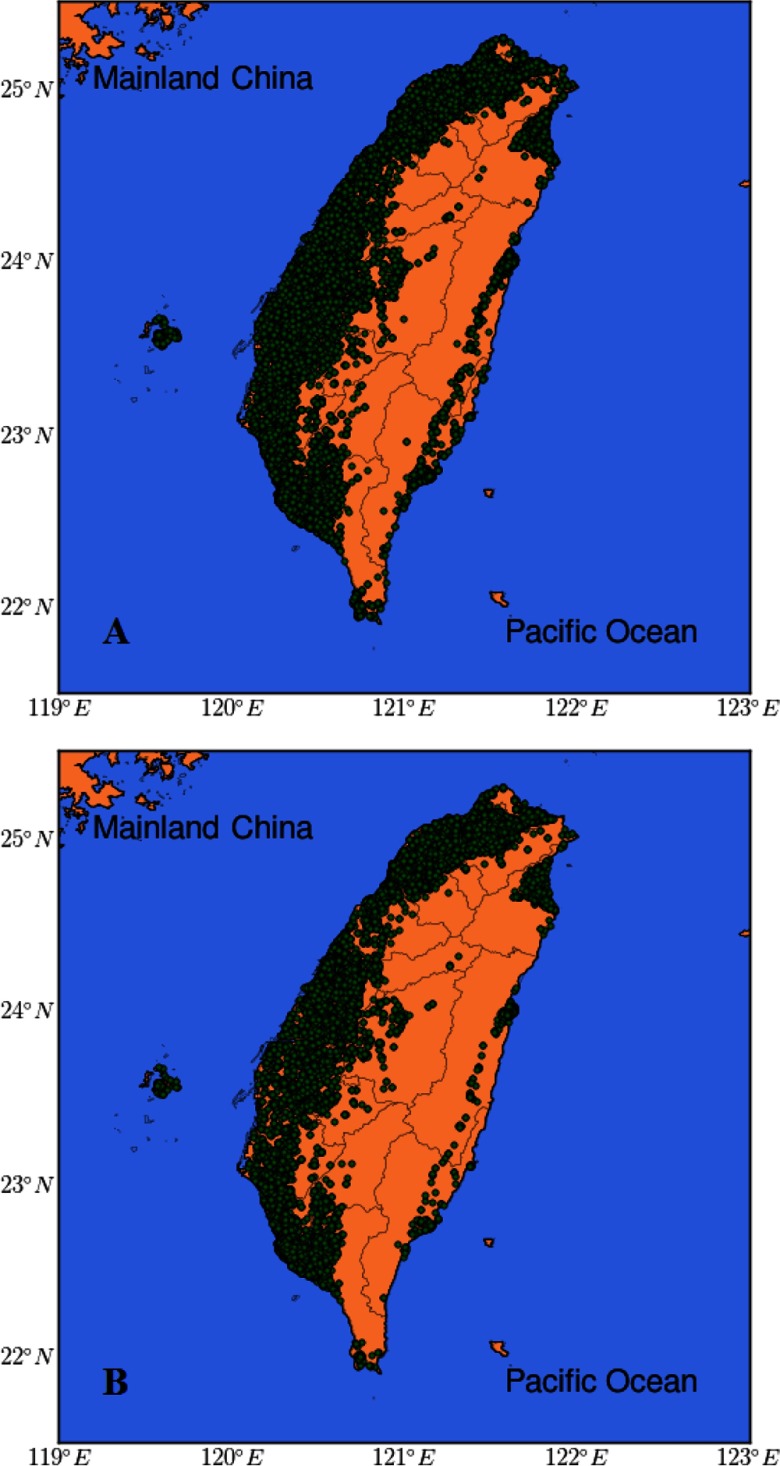
Figure 1 presents the locations of the study participants. The distribution of the 2-y average concentrations of all participants is presented by year in Table 2. The spatial distribution of over Taiwan was generally stable during the study period. No apparent upward or downward trends in were observed over the study period. The mean concentration of the overall cohort was 26.5 (SD: 7.6) , and the median was [interquartile range (IQR): 6.9]. The participants who were followed up for incident hypertension analysis had levels similar to those of the overall cohort.
Figure 1.
Location map of the study participants. (A) 361,560 participants in blood pressure– analysis. (B) 125,913 participants in incident hypertension– analysis. Circles represent the locations of the study participants.
Table 2.
Distribution of 2-y average concentrations () at baseline.
| Year | Overall cohort | Nonhypertensive participants with follow-up | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | 25th percentile | 50th percentile | 75th percentile | n | Mean (SD) | 25th percentile | 50th percentile | 75th percentile | ||||||||||||
| 2001 | 56,688 | 25.0 (7.4) | 19.9 | 22.5 | 28.7 | 27,605 | 24.8 (7.4) | 19.9 | 22.3 | 25.6 | |||||||||||
| 2002 | 42,830 | 26.6 (8.3) | 21.0 | 23.5 | 35.0 | 18,107 | 26.5 (8.3) | 20.8 | 23.4 | 34.4 | |||||||||||
| 2003 | 29,159 | 28.8 (8.4) | 23.0 | 25.8 | 36.0 | 11,178 | 28.8 (8.4) | 23.1 | 25.8 | 36.0 | |||||||||||
| 2004 | 28,942 | 29.9 (8.8) | 23.7 | 26.5 | 38.6 | 11,544 | 29.9 (8.8) | 23.6 | 26.5 | 38.9 | |||||||||||
| 2005 | 28,282 | 27.4 (7.8) | 22.2 | 24.5 | 29.8 | 10,653 | 27.4 (7.6) | 22.3 | 24.8 | 30.9 | |||||||||||
| 2006 | 26,989 | 26.9 (6.9) | 22.5 | 24.6 | 27.7 | 9,512 | 26.9 (6.8) | 22.5 | 24.8 | 27.5 | |||||||||||
| 2007 | 26,882 | 26.8 (6.6) | 22.6 | 24.9 | 27.6 | 8,863 | 26.8 (6.5) | 22.7 | 25.0 | 27.4 | |||||||||||
| 2008 | 23,516 | 26.8 (6.7) | 22.6 | 24.7 | 27.4 | 7,567 | 26.8 (6.6) | 22.6 | 24.8 | 27.3 | |||||||||||
| 2009 | 18,930 | 27.2 (7.2) | 22.5 | 24.9 | 30.0 | 6,125 | 27.7 (7.3) | 22.7 | 25.3 | 31.4 | |||||||||||
| 2010 | 20,471 | 25.9 (7.2) | 21.5 | 23.3 | 26.7 | 6,077 | 26.3 (7.4) | 21.6 | 23.6 | 29.6 | |||||||||||
| 2011 | 18,484 | 25.5 (7.1) | 20.8 | 23.1 | 26.2 | 4,944 | 25.7 (7.1) | 21.0 | 23.4 | 26.2 | |||||||||||
| 2012 | 20,696 | 24.4 (6.2) | 20.3 | 22.8 | 25.3 | 3,123 | 24.3 (5.7) | 20.9 | 22.9 | 25.2 | |||||||||||
| 2013 | 15,366 | 23.7 (5.9) | 19.8 | 22.2 | 25.5 | 615 | 23.8 (5.0) | 20.7 | 22.9 | 25.3 | |||||||||||
| 2014 | 4,325 | 24.4 (4.5) | 21.2 | 24.1 | 26.1 | — | — | — | — | — | |||||||||||
| All | 361,560 | 26.5 (7.6) | 21.6 | 23.9 | 28.5 | 125,913 | 26.7 (7.7) | 21.6 | 24.0 | 28.9 | |||||||||||
Note: —, the end year of the study period is 2014; therefore participants enrolled in 2014 had no follow-up and were not included in the incident analysis. SD, standard deviation.
Based on the crude model, participants with values in the third and fourth quartiles had higher SBP than participants in the lowest quartile of , and those in the second, third, and fourth quartiles had higher PP than participants in the lowest quartile of (Table 3). After fully adjusting for potential confounders (Adjusted Model 3), participants with values in the second, third, and fourth quartiles had increased levels of SBP and PP compared with those who had 2-y average values in the lowest quartile (Table 3). Participants with in the fourth quartile had higher DBP, whereas those with in the second and third quartiles had lower DBP, relative to those in the lowest quartile of exposure. These results remained robust after adjusting for confounders (Table 3). When was treated as a continuous variable, based on the fully adjusted model (Adjusted Model 3), each increment was associated with increases of [95% confidence interval (CI): 0.40, 0.50] in SBP, (95% CI: 0.04, 0.11) in DBP, and (95% CI: 0.33, 0.42) in PP (Table 3).
Table 3.
Associations between long-term exposure to and blood pressure in Taiwanese adults at baseline.
| () | Crude model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | ||||||||||||
| Systolic blood pressure | |||||||||||||||||||
| 1st Quartile | Reference | — | Reference | — | Reference | — | Reference | — | |||||||||||
| 2nd Quartile | (, 0.02) | 0.09 | 0.46 (0.32, 0.60) | 0.46 (0.32, 0.61) | 0.49 (0.38, 0.60) | ||||||||||||||
| 3rd Quartile | 0.28 (0.11, 0.44) | 0.001 | 0.89 (0.75,1.04) | 0.90 (0.76, 1.05) | 0.90 (0.79, 1.02) | ||||||||||||||
| 4th Quartile | 1.44 (1.28, 1.60) | 1.01 (0.86, 1.15) | 0.91 (0.77, 1.05) | 0.85 (0.74, 0.97) | |||||||||||||||
| Trend test | — | — | — | — | |||||||||||||||
| increment | 0.75 (0.68, 0.83) | 0.52 (0.45, 0.58) | 0.47 (0.40, 0.53) | 0.45 (0.40, 0.50) | |||||||||||||||
| Diastolic blood pressure | |||||||||||||||||||
| 1st Quartile | Reference | — | Reference | — | Reference | — | Reference | — | |||||||||||
| 2nd Quartile | (, ) | (, ) | (, ) | (, ) | |||||||||||||||
| 3rd Quartile | (, ) | (, ) | (, ) | (, ) | |||||||||||||||
| 4th Quartile | 0.45 (0.35, 0.56) | 0.25 (0.15, 0.34) | 0.20 (0.11, 0.30) | 0.17 (0.09, 0.24) | |||||||||||||||
| Trend test | — | — | — | — | |||||||||||||||
| increment | 0.21 (0.16, 0.26) | 0.11 (0.06, 0.15) | 0.08 (0.04, 0.13) | 0.07 (0.04, 0.11) | |||||||||||||||
| Pulse pressure | |||||||||||||||||||
| 1st Quartile | Reference | — | Reference | — | Reference | — | Reference | — | |||||||||||
| 2nd Quartile | 0.67 (0.57, 0.78) | 1.04 (0.94, 1.14) | 1.03 (0.93, 1.14) | 1.04 (0.95, 1.14) | |||||||||||||||
| 3rd Quartile | 0.98 (0.87, 1.09) | 1.33 (1.23, 1.43) | 1.33 (1.22, 1.43) | 1.33 (1.23, 1.42) | |||||||||||||||
| 4th Quartile | 0.99 (0.88, 1.10) | 0.76 (0.66, 0.86) | 0.71 (0.61, 0.81) | 0.69 (0.59, 0.78) | |||||||||||||||
| Trend test | — | — | — | — | |||||||||||||||
| increment | 0.54 (0.49, 0.59) | 0.41(0.36, 0.46) | 0.38 (0.34, 0.43) | 0.38 (0.33, 0.42) | |||||||||||||||
Note: Crude Model: no adjustment; Adjusted Model 1: adjusted by age, sex and education level; Adjusted Model 2: also adjusted by smoking, alcohol use, physical activity, occupational exposure to dust/organic solvents and season; Adjusted Model 3: also adjusted by body mass index, hypertension, diabetes, hyperlipidemia, cardiovascular disease/stroke, and cancer. CI, confidence interval; , particulate matter with aerodynamic diameter .
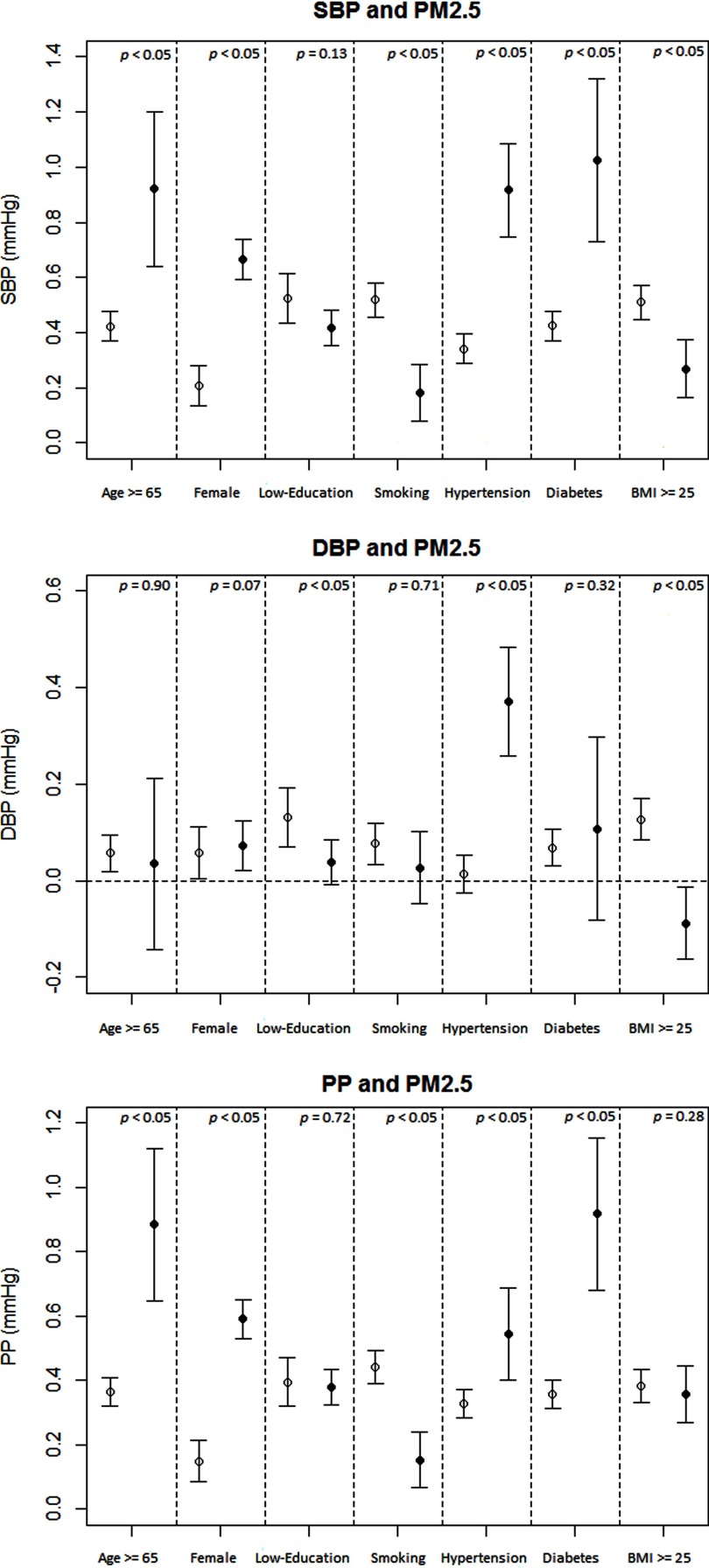
The results of the stratified analysis of blood pressure and are shown in Figure 2. was associated with significantly higher SBP and PP in all subgroups, whereas stratum-specific estimates varied for associations with DBP. Interactions between and hypertension were significant for all three outcomes (p for interaction ), with stronger positive associations in the hypertensive participants. Effect modification by other factors was not consistent across the three blood pressure parameters.
Figure 2.
Stratified analysis on associations between long-term exposure ( increments) and systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) at baseline. Effect estimates (coefficients) are derived from multivariable linear regression analysis, and bars cover 95% confidence intervals. Results were adjusted for age (not in age-stratified analysis), sex (not in sex-stratified analysis), education level (not in education level–stratified analysis), smoking status (not in smoking-stratified analysis), alcohol drinking, leisure-time physical activity, occupational exposure to dust or organic solvents in the workplace, season, body mass index (BMI; not in BMI-stratified analysis), hypertension (not in hypertension-stratified analysis), diabetes (not in diabetes-stratified analysis), hyperlipidemia, and self-reported cardiovascular disease, stroke, or cancer. The lines with hollow circles from left to right represent the participants who were , were males, had a high education level, were nonsmokers, had no hypertension, had no diabetes, and had BMI , respectively. The lines with solid circles from left to right represent the participants who were , were females, had a low education level, were smokers, had hypertension, had diabetes, and had , respectively. p-Values for interaction terms between (continuous variable) and each potential modifier (dichotomous variable) are presented in the figure.
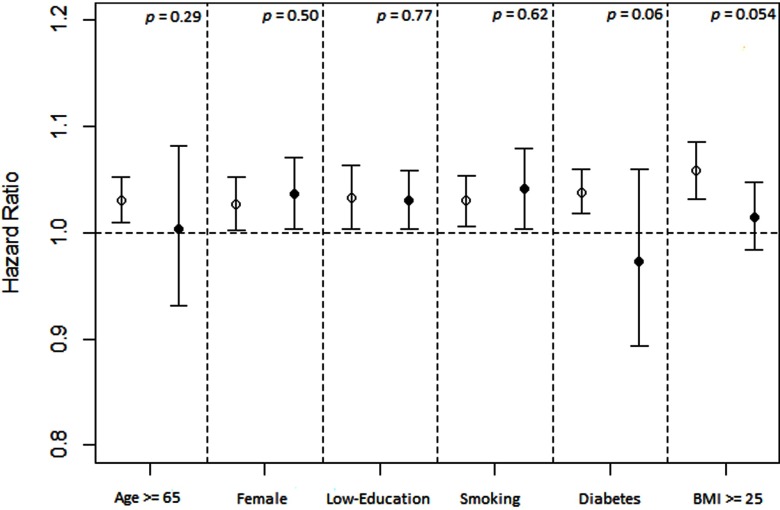
The results for and incident hypertension are shown in Table 4. Participants with higher levels were associated with an increased risk of developing hypertension. Similar results were observed after adjusting for confounders. Hazard ratios (HRs) from the fully adjusted model (Adjusted Model 3) were 1.10 (95% CI: 1.05, 1.15), 1.16 (95% CI: 1.11, 1.22), and 1.11 (95% CI: 1.06, 1.16) for the participants with in the second, third, and fourth quartiles, respectively. When was modeled as a continuous variable, every increment was associated with a 3% increase in risk [ (95% CI: 1.01, 1.05)]. Results were similar between strata of age, sex, educational level, smoking, diabetes, and obesity (all p for interaction ) (Figure 3).
Table 4.
Association between long-term exposure to and incident hypertension in Taiwanese adults.
| () | Crude Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||||||||||||
| 1st Quartile | Reference | — | Reference | — | Reference | — | Reference | — | |||||||||||
| 2nd Quartile | 1.06 (1.01, 1.10) | 0.02 | 1.10 (1.05, 1.15) | 1.10 (1.05, 1.14) | 1.10 (1.05, 1.15) | ||||||||||||||
| 3rd Quartile | 1.11 (1.06, 1.16) | 1.18 (1.13, 1.24) | 1.18 (1.13, 1.24) | 1.16 (1.11, 1.22) | |||||||||||||||
| 4th Quartile | 1.16 (1.11, 1.21) | 1.14 (1.09, 1.19) | 1.14 (1.09, 1.19) | 1.11 (1.06, 1.16) | |||||||||||||||
| Trend test | — | — | — | — | |||||||||||||||
| increment | 1.06 (1.04, 1.08) | 1.05 (1.03, 1.07) | 1.05 (1.03, 1.07) | 1.03 (1.01, 1.05) | 0.001 | ||||||||||||||
Note: Crude Model: no adjustment; Adjusted Model 1: adjusted by age, sex and education level; Adjusted Model 2: also adjusted by smoking, alcohol use, physical activity, occupational exposure to dust/organic solvents and season; Adjusted Model 3: also adjusted by body mass index, diabetes, hyperlipidemia, cardiovascular disease/stroke, and cancer. CI, confidence interval; HR, hazard ratio; , particulate matter with aerodynamic diameter .
Figure 3.
Stratified analysis on associations between long-term exposure ( increments) and incident hypertension. Effect estimates (hazard ratios) are derived from Cox proportional hazard regression analysis, and bars cover 95% confidence intervals. Results were adjusted for age (not in age-stratified analysis), sex (not in sex-stratified analysis), education level (not in education level–stratified analysis), smoking status (not in smoking-stratified analysis), alcohol drinking, leisure-time physical activity, occupational exposure to dust or organic solvents in the workplace, season, body mass index (BMI; not in BMI-stratified analysis), diabetes (not in diabetes-stratified analysis), hyperlipidemia, and self-reported cardiovascular disease, stroke, or cancer. The lines with hollow circles from left to right represent the participants who were , were males, had a high education level, were nonsmokers, had no diabetes, and had BMI , respectively. The lines with solid circles from left to right represent the participants who were , were females, had a low education level, were smokers, had diabetes, and had , respectively. p-Values for interaction terms between (continuous variable) and each potential modifier (dichotomous variable) are calculated and presented in the figure.
The results of the sensitivity analyses are presented in Tables S1 and S2. Exclusion of the participants who used their company address or those with comorbidities did not change the results noticeably (compare Table S1 with Table 3). For incident hypertension, results similar to those of the overall cohort were observed among participants with of follow-up (compare Table S2 with Table 4).
Discussion
To the best of our knowledge, this is the largest study to investigate the health effects of long-term exposure to air pollution on both blood pressure and hypertension development in a same population. We found that the 2-y average was associated with increased levels of SBP, DBP, and PP. In line with these positive associations with blood pressure, exposure was associated with an increased risk of incident hypertension. The results remained robust after adjusting for a wide range of confounders and modifiers and in a series of sensitivity analyses. Our results show that every increment in was associated with a 3% higher risk of developing hypertension. The World Health Organization (WHO) estimated that 1 billion adults were hypertensive worldwide in 2008 (WHO 2013). Based on the formula for calculating population attributable fraction,
where is the level of in micrograms per cubic meter, is the percentage of the population exposed to that level of air pollution, and RR is relative risk (WHO 2014), and on the fact that exposure to air pollution is ubiquitous, we estimated that a universal increment of in may contribute to 29 million hypertensive patients if the effect magnitude of 1.03 is valid globally.
Our findings of a positive association between PM air pollution and blood pressure are in line with some previous studies. Long-term exposure to PM or black carbon, a surrogate for particulate traffic emissions, has been reported to be associated with increases in both SBP and DBP in Taiwan (Chuang et al. 2011), Germany (Fuks et al. 2011), China (Dong et al. 2013), and the United States (Schwartz et al. 2012). However, the results of some other studies have been inconsistent. Chan et al. (2015) reported positive associations of exposure with SBP and PP, but not with DBP. The associations for DBP in the present study were also less consistent than those for SBP and PP. Negative associations were observed for the second and third quartiles, and the reasons for this phenomenon are unclear. In contrast, a recent study in Taiwan reported that long-term exposure to PM was associated with increased DBP but not SBP (Chen et al. 2015). The European Study of Cohorts for Air Pollution Effects (ESCAPE) project in Europe (Fuks et al. 2014) did not find a significant association of PM exposure with either SBP or DBP. In the NHANES III study in the United States, neither SBP, DBP, nor PP was associated with long-term exposure to PM with an aerodynamic diameter () (Shanley et al. 2016). Two studies in children also showed inconsistent findings (Bilenko et al. 2015; Liu et al. 2014). Among 2,368 German children, Liu et al. (2014) reported no consistent associations for exposure to , , or absorbance with SBP or DBP. In a Dutch study, long-term exposure to nitrogen dioxide () and were positively associated with DBP, but not with SBP, and the observed associations were only statistically significant among children who had lived at the same address since birth (Bilenko et al. 2015).
This inconsistency may be due to a number of factors, including the heterogeneity of the study populations and study regions as well as the research methods. Studies on the relationship between PM and blood pressure, including the present one, are generally of cross-sectional design, which may result in greater uncertainties. The accuracy of the exposure estimates may also play an important role in the inconsistency among the studies. Some of the previous studies (Chuang et al. 2011; Dong et al. 2013; Shanley et al. 2016) estimated PM exposure based on the proximity of residences to fixed monitoring stations, with the same exposure level assigned to an entire community (district, county, or city). This community-level exposure assessment may have introduced exposure misclassification. For those studies using individual-level exposure, different modeling methods were applied for PM estimates. Dispersion models (Fuks et al. 2011) and land-use regression models (Bilenko et al. 2015; Chen et al. 2015; Fuks et al. 2014; Liu et al. 2014; Schwartz et al. 2012) were the most commonly adopted methods in these studies. One study in the United States also used satellite-based technology; in this study, a positive association was found between 1-y average and SBP and PP (Chan et al. 2015). Furthermore, the sizes of the effects of on the three blood pressure parameters are very small, and blood pressure may fluctuate because it is easily influenced by a number of factors. The results for the three blood pressure parameters are not as stable as those for incident hypertension in the present study as well.
To date, only four studies have prospectively investigated the associations between long-term exposure and the incidence of hypertension. The Nurses’ Health Study found that every increment in was associated with a 4% increase in the risk of incident hypertension [ (95% CI: 1.00, 1.07)] (Zhang et al. 2016), which is similar to our findings. A Canadian study with 35,303 adults also reported that long-term exposure to was positively associated with hypertension incidence, but the effect size was slightly larger [HR per increment, 1.13 (95% CI: 1.05, 1.22)] (Chen et al. 2014). However, the Black Women’s Health Study of participants living in Los Angeles found no significant association (Coogan et al. 2012). The extension of this analysis to the whole study cohort still failed to reveal any significant associations (Coogan et al. 2016). All of these studies were conducted in North America, and the average air pollution levels were 10.7 (Chen et al. 2014), 13.9 (Coogan et al. 2016), 15.6 (Zhang et al. 2016), and (Coogan et al. 2012), respectively, which were lower than the level in our study (). As for study population, two studies were conducted mainly in Caucasian populations (Chen et al. 2014; Zhang et al. 2016), and the Black Women’s Health Study was in African-American women (Coogan et al. 2012; Coogan et al. 2016). Thus, it may be difficult to compare them directly with our study. A Danish cohort study investigated the traffic-related pollutant nitrogen oxides () rather than , but the authors reported no association with incident hypertension (Sørensen et al. 2012).
The biological mechanisms through which PM can raise blood pressure are not fully understood. One plausible pathway is that PM exposure may lead to raised blood pressure by instigating autonomic imbalance (Brook et al. 2009). Inhaled PM can stimulate receptors and nerve endings in the airways and thereby alter the reflexes of the autonomic nervous system, leading to a blunting of cardiovascular parasympathetic tone and a relative favoring of sympathetic activity, consequently raising the blood pressure (Widdicombe and Lee 2001). Another hypothesized pathway is via impaired endothelial function elicited by the chronic systemic inflammation and oxidative stress induced by PM (Brook et al. 2009). Our previous study (Zhang et al. 2017) and others (Green et al. 2016; Viehmann et al. 2015) have reported positive associations between long-term exposure and markers of systemic inflammation, which partially supports this hypothesis.
In stratified analyses, we found stronger associations between and blood pressure in the elderly, females, never smokers, and participants with hypertension, diabetes, or normal weight (), although the observed modifying effects were not consistent across the three blood pressure measures, particularly for DBP. There is limited information on these potential modifiers at the present time. A German study reported that exposure had smaller effects on blood pressure in smokers (Fuks et al. 2011), which is in line with our results. A study in the United States also found stronger associations between exposure and carotid intima-media thickness, a measure of atherosclerosis, in nonsmokers (Künzli et al. 2005). It was hypothesized that smoking and air pollutants may share the same pathways in mediating cardiovascular effects through oxidative stress and inflammation. Smoking may play a dominant role in smokers. Therefore, the additional exposure to air pollutants might not further enhance the effects along the same pathway (Künzli et al. 2005). Reports on effect modification by obesity have also been inconsistent. Two previous studies (Fuks et al. 2011; Schwartz et al. 2012) did not find a significant effect modification, which is contrast to our findings. On the other hand, in our analysis on and incident hypertension, we did not find any significant effect modification. Because blood pressure is easily fluctuant and the present analysis on and blood pressure is cross-sectional in nature, interpretation regarding modifiers should be made cautiously. Further studies on this issue are warranted.
Our study has some important strengths. We included 361,560 adults in the analysis of the association between PM and blood pressure. Previous studies had sample sizes ranging from 853 to 113,926. For the analysis on the associations between PM and hypertension development, we included 125,913 nonhypertensive adults. The sample sizes of the previous four studies were 3,236 (Coogan et al. 2012), 35,303 (Chen et al. 2014), 33,771 (Coogan et al. 2016), and 74,800 (Zhang et al. 2016). The large sample size in the present study enabled us to detect even small effects of air pollution. We collected a wealth of information on a wide range of potential confounders to better characterize the relationship between exposure and hypertension. The longitudinal nature of the cohort enabled us to examine the effects of exposure on hypertension development prospectively. Another strength is the use of a spatiotemporal model with high resolution () to estimate individual exposure based on the participants’ addresses. This technology enabled us to overcome the problems with spatial coverage that typically occur when only using data from fixed monitoring stations.
This study also has some limitations. First, we only measured participants’ blood pressure once during their visit if their first blood pressure reading was not hypertensive; this is not strictly in accordance with the clinical guidelines, which require multiple measurements. However, a second measurement was obtained if a participant had an SBP or a DBP in the first measurement. The second measurement was intended to minimize “white coat” effects. Another limitation is that information on noise exposure was not available. We cannot exclude the possible confounding effect of noise in this study. However, the wide geographic coverage and the large sample size in our study may have minimized the confounding effects (Tétreault et al. 2013). Finally, the exposure levels were calculated at fixed addresses, and participants’ activity patterns were not taken into account. More advanced technologies are needed for more accurate exposure assessment in future studies.
Conclusion
In conclusion, we found that long-term exposure to air pollution is associated with higher levels of SBP, DBP, and PP after adjusting for a wide range of covariates and possible confounders. In line with the positive associations with blood pressure, long-term exposure to air pollution is also associated with an increased risk of developing hypertension. Our study provides strong evidence of a role for exposure in the development of hypertension, a well-established determinant of CVD morbidity and mortality. Our findings support the urgent need to develop global strategies on air pollution mitigation for the prevention of CVD.
Supplemental Material
Acknowledgments
We would like to thank MJ Health Research Foundation for authorizing the use of MJ health data (authorization code: MJHR2015002A). Any interpretation or conclusion related to this manuscript does not represent the views of MJ Health Research Foundation. This work was partially supported by the Environmental Health Research Fund of the Chinese University of Hong Kong (7104946). Z. Zhang and C. Guo are supported by the PhD Studentship of the Chinese University of Hong Kong. We are grateful to the anonymous reviewers and the editors for their valuable comments.
References
- Bilenko N, van Rossem L, Brunekreef B, Beelen R, Eeftens M, Hoek G. 2015. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. Eur J Prev Cardiol 22(1):4–12, PMID: 24047569, 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- Bondjers G, Glukhova M, Hansson GK, Postnov YV, Reidy MA, Schwartz SM. 1991. Hypertension and atherosclerosis. Cause and effect, or two effects with one unknown cause? Circulation 84(6suppl):VI2–V16, PMID: 1959216. [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54(3):659–667, PMID: 19620518, 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, et al. 2015. Long-term air pollution exposure and blood pressure in the Sister Study. Environ Health Perspect 123(10):951–958, PMID: 25748169, 10.1289/ehp.1408125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. 2014. Spatial association between ambient fine particulate matter and incident hypertension. Circulation 129(5):562–569, PMID: 24190962, 10.1161/CIRCULATIONAHA.113.003532. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wu CF, Lee JH, Hoffmann B, Peters A, Brunekreef B, et al. 2015. Associations between long-term air pollutant exposures and blood pressure in elderly residents of Taipei city: a cross-sectional study. Environ Health Perspect 123(8):779–784, PMID: 25793646, 10.1289/ehp.1408771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Cheng TJ. 2010. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med 52(3):258–262, PMID: 20190657, 10.1097/JOM.0b013e3181ceff7a. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68(1):64–68, PMID: 20833756, 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, et al. 2012. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation 125(6):767–772, PMID: 22219348, 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, White LF, Yu J, Burnett RT, Seto E, Brook RD, et al. 2016. PM2.5 and diabetes and hypertension incidence in the Black Women’s Health Study. Epidemiology 27(2):202–210, PMID: 26595125, 10.1097/EDE.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Krishnan RM, Oron AP, Jansen K, Peretz A, Sullivan JH, et al. 2012. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension 59(5):943–948, PMID: 22431582, 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, et al. 2010. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology 21(3):396–404, PMID: 20335815, 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Qian ZM, Xaverius PK, Trevathan E, Maalouf S, Parker J, et al. 2013. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 61(3):578–584, PMID: 23357184, 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, et al. 2009. Acute effects of ambient particulate matter on blood pressure: Differential effects across urban communities. Hypertension 53(5):853–859, PMID: 19273743, 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, et al. 2011. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect 119(12):1706–1711, PMID: 21827977, 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks KB, Weinmayr G, Foraster M, Dratva J, Hampel R, Houthuijs D, et al. 2014. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Environ Health Perspect 122(9):896–905, PMID: 24835507, 10.1289/ehp.1307725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, et al. 2016. Long- and short-term exposure to air pollution and inflammatory/hemostatic markers in midlife women. Epidemiology 27(2):211–220, PMID: 26600256, 10.1097/EDE.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. 2013. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ Health 12(1):1, PMID: 23714370, 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, Zanobetti A, de Souza C, Foley C, et al. 2012. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect 120(2):241–246, PMID: 22020729, 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. 2005. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 113(2): 201–206, PMID: 15687058, 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Fuertes E, Tiesler CM, Birk M, Babisch W, Bauer C, et al. 2014. The associations between traffic-related air pollution and noise with blood pressure in children: Results from the GINIplus and LISAplus studies. Int J Hyg Environ Health 217(4–5):499–505, PMID: 24183515, 10.1016/j.ijheh.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Martiniuk AL, Lee CM, Lawes CM, Ueshima H, Suh I, Lam TH, et al. 2007. Hypertension: its prevalence and population-attributable fraction for mortality from cardiovascular disease in the Asia-Pacific region. J Hypertens 25(1):73–79, PMID: 17143176, 10.1097/HJH.0b013e328010775f. [DOI] [PubMed] [Google Scholar]
- MJ Health Research Foundation. 2016. “MJ Health Resource Center, Technical Report.” MJHRF-TR-01. http://www.mjhrf.org/File/En/Report/MJHRF-TR-01MJ%20Health%20Database.pdf. [accessed 20 June 2017].
- Pope CA 3rd, Dockery DW. 2006. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc 56(6):709–742, PMID: 16805397, 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Alexeeff SE, Mordukhovich I, Gryparis A, Vokonas P, Suh H, et al. 2012. Association between long-term exposure to traffic particles and blood pressure in the Veterans Administration Normative Aging Study. Occup Environ Med 69(6):422–427, PMID: 22383587, 10.1136/oemed-2011-100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J. 2016. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 27(2):291–298, PMID: 26605815, 10.1097/EDE.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M, Hoffmann B, Hvidberg M, Ketzel M, Jensen SS, Andersen ZJ, et al. 2012. Long-term exposure to traffic-related air pollution associated with blood pressure and self-reported hypertension in a Danish cohort. Environ Health Perspect 120(3):418–424, PMID: 22214647, 10.1289/ehp.1103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault L, Perron S, Smargiassi A. 2013. Cardiovascular health, traffic-related air pollution and noise: Are associations mutually confounded? A systematic review. Int J Public Health 58(5):649–666, PMID: 23887610, 10.1007/s00038-013-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, et al. 2015. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 72(9):656–663, PMID: 26163546, 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- Wen CP, Cheng TYD, Tsai SP, Chan HT, Hsu HL, Hsu CC, et al. 2009. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 12(4):497–506, PMID: 18547457, 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M, et al. 2011. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378(9798):1244–1253, PMID: 21846575, 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. World Health Statistics 2017: monitoring health for the SDGs. http://www.who.int/gho/publications/world_health_statistics/2017/en/ [accessed 15 June 2017].
- WHO. 2013. A global brief on hypertension: silent killer, global public health crisis. http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ [accessed 20 June 2017].
- WHO. 2014. Burden of disease from ambient air pollution for 2012. http://www.who.int/phe/health_topics/outdoorair/databases/AAP_BoD_methods_March2014.pdf?ua=1 (accessed 3 December 2017)
- Widdicombe J, Lee LY. 2001. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect 109(suppl4):579–584, PMID: 11544167, 10.2307/3454673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Huang J, Wang H, Shima M, Wang X, et al. 2013. Blood pressure changes and chemical constituents of particulate air pollution: results from the healthy volunteer natural relocation (HVNR) study. Environ Health Perspect 121(1):66–72, PMID: 23086577, 10.1289/ehp.1104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chang LY, Lau KHA, Chan TC, Chuang YC, Chan J, et al. 2017. Satellite-based estimates of long-term exposure to fine particulate matter are associated with C-reactive protein in 30 034 Taiwanese adults. Int J Epidemiol, PMID: 28541501, 10.1093/ije/dyx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Laden F, Forman JP, Hart JE. 2016. Long-term exposure to particulate matter and self-reported hypertension: A prospective analysis in the Nurses’ Health Study. Environ Health Perspect 124(9):1414–1420, PMID: 27177127, 10.1289/EHP163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.