Abstract
Purpose
Cholangiocarcinoma (CCA) is a malignancy of the bile ducts. The purpose of this discovery study was to identify effective serum markers for surveillance of cholangiocarcinoma.
Experimental design
Using a glycomic method, patients with CCA were determined to have increased levels of alpha-1,3 and alpha-1,6 linked fucosylated glycan. Proteomic analysis of the serum fucosylated proteome identified proteins such as alpha-2-macroglobulin, kininogen, hemopexin, fetuin-A, alpha-1 anti-trypsin, and ceruloplasmin as being hyperfucosylated in HCC. The levels of these glycoproteins in 109 patients with CCA, primary sclerosing cholangitis (PSC), and control patients were compared to the performance of CA-19-9, the current “gold standard” assay for cholangiocarcinoma.
Results
Fucosylated Fetuin-A (fc-Fetuin-A) had the best ability to differentiate CCA from PSC, with an AUROC of 0.812 or 0.8665 at differentiating CCA from those with PSC or other liver disease. CA-19-9 had poor ability to differentiate PSC from cholangiocarcinoma (AUROC of 0.625).
Conclusion and clinical relevance
Using glycomic and proteomic methods we identified a set of proteins that contain altered glycan in the sera of those with CCA. One of these proteins, fucosylated Fetuin-A may have value in the surveillance of people at risk for the development of cholangiocarcinoma.
Keywords: Cholangiocarcinoma, Fetuin-A, Glycosylation
1 Introduction
Cholangiocarcinoma (CCA) is an epithelial cancer arising in the biliary mucosa lining the ducts that carry bile from the liver to the small intestine [1]. Unlike most human cancers, a tissue diagnosis of CCA is difficult because of tumor location, size, and cellular characteristics. In addition, tumor masses are often not identifiable by imaging modalities as a result of the location of the tumor in the hilum of the liver or within the bile ducts. Further, many cholangiocarcinomas consist of small nests of epithelial cancer cells surrounded by a dense stromal reaction of cancer associated fibroblasts, immune cells, and extracellular matrix. It can therefore be difficult to make a confirmed histologic diagnosis of CCA from biopsies or brush cytology obtained from suspicious bile duct strictures. Because of the problems with obtaining a definitive tissue diagnosis, treatment, and management decisions for patients with biliary strictures that may be malignant are difficult.
Unlike hepatocellular carcinoma (HCC), for which measurement of the serum alpha-fetoprotein is used in surveillance programs, no such serum marker for CCA exists. Several groups have reported that serum CA 19-9 determinations are useful for the diagnosis of cholangiocarcinomas arising both in the background of the known predisposing disorder, primary sclerosing cholangitis (PSC), and in patients without PSC [2–4].
We have observed changes in N-linked glycosylation with the development of cirrhosis and HCC [5–9]. Specifically, the amount of fucosylated N-linked glycan derived from total protein preparations isolated from the serum of individuals chronically infected with HCV and/or HBV and from those with a diagnosis of HCC was consistently greater than in healthy subjects or those with HCV/HBV and “inactive” liver disease [9]. Here we perform a similar study in a cohort of patients with a predisposing disorder, primary sclerosing cholangitis (PSC) [2–4] and CCA in an attempt to find novel biomarkers of CCA.
2 Materials and methods
2.1 Sample population
Three sets of serum samples were used in this study. The first set from the University of Osaka, which was collected under an IRB approved protocol, consisted of 8 patients with CCA. This sample set was used for N-linked glycan analysis and was compared with 20 control patients obtained commercially from Biological Specialty Corporation (Colmar, PA). Eight samples with PSC and 2 patients with CCA were obtained from St. Louis University School of Medicine. These samples were used, in combination with the CCA samples from the University of Osaka to validate the identified glycan and proteomic changes. It is noted, that as CCA remains a rare cancer, these were considered convenience samples and used initially for discovery.
Serum samples to be used for validation of markers were obtained from patients seen at the Mayo Hepatobiliary Neoplasia Clinic under an IRB approved protocol. All participants provided informed consent for this study. Samples from 39 patients with cholangiocarcinoma, 39 samples with primary sclerosing cholangitis (PSC), and 39 patients with other liver disease but not CCA or PSC who were seen at Mayo Clinic, Rochester, MN between January 2000 and May 2010 were included in the study. Peripheral blood was collected from each participant at the time of the office visit prior to treatment. Sera were stored at −80°C. The following data elements were abstracted from the medical record: demographics (age, gender, ethnicity, race, weight, height), medical history, etiology of liver disease, laboratory data including CA-19-9 and AFP, and imaging results (US, CT, or MRI). Histopathology results and radiologic findings from medical records of all patients were reviewed to ascertain the diagnosis of CCA and identify tumor location. The diagnosis of CCA of all patients was confirmed by histopathology. The anatomic location of cholangiocarcinomas was categorized as “intrahepatic” if the mass lesion arose within the hepatic parenchyma and did not extend to or involve the secondary branches of the biliary trees as demonstrated either by computerized tomography imaging, magnetic resonance imaging or endoscopic retrograde cholangiopancreatography findings. Etiology of liver disease was based on the laboratory, imaging, and histopathology results and the judgment of the treating physician. For patients with viral hepatitis, anti-HCV antibody, serum HCV RNA, HBV surface antigen, HBV e-antigen, and HBV DNA levels were recorded.
2.2 N-linked glycan analysis
N-linked glycan analysis was performed on total serum using sequential exoglycosidase digestion of fluorescently labeled glycans resolved by ultra high performance liquid chromatography (UPLC) that we, and others, have previously reported [8,10–15].
2.3 LC MS/MS analysis
Serum from 100 μl of pooled CCA patients (set 1) was enriched for fucosylated proteins following extraction of fucosylated glycoproteins using the Aleuria Aurantia Lectin (AAL) as we have done previously [12,16]. Fucosylated proteins were subsequently dialyzed and digested with Trypsin before mass spectrometry. Mass spectrometry was completed on an LTQ ion trap mass spectrometer (ThermoFisher, San Jose, CA) using the microspray source from ThermoFisher, spray voltage was 2 KV. Separation was achieved using an Ultimate 3000 microflow HPLC (Dionex, Sunnyvale, CA). Five microliters of sample was injected onto a C18 trap column (Dionex), and then transferred to a 75 × 150 mm C18 column (Dionex). The gradient was a 5–35% buffer A to B over 2 h, where buffer A is 5% acetonitrile + 0.5% acetic acid and buffer B is 90% acetronitrile + 0.5% acetic acid at a flow rate of 250 nL/min. The LTQ was programmed to perform MS/MS fragmentation on the top 5 ions. Data exclusion was enabled with a peak list of 100, for 360 s, width of 0.75 Da, when ion was seen twice within 30 s.
2.4 Data analysis and interpretation
Mass spectrometry data (spectra) was searched using BioWorks 3.2 (ThermoFisher, San Jose, CA) using the CDS non-redundant database (Celera, Alameda, CA) along with Swissprot additions (expasy.org). Search criteria used were XC > 2.0 for z = 1, XC > 2.5 for z = 2, and XC > 3 for z = 3, percent ions > 50%, peptide probability < 1e−3 using a tryptic digest restriction. Peptides that scored above the threshold value were manually verified.
2.5 Lectin fluorophore-linked immunosorbent assay (Lectin FLISA)
The plate based lectin-ELISA was performed as described in detail in our previous work [13, 17]. Briefly, this assay requires the removal of fucose residues on the capture antibody via mild periodate oxidation and processing of serum to limit interference of heterophilic antibodies [13,17]. In addition, it is noted that the lectin-FLISA detects the amount of fucosylation present on an equal amount of captured molecules from each patient sample and is performed in a manner that is independent of the total amount of protein in any given patient. The relative level of lectin reactive protein as compared to the healthy control sample was used to assign a “fold increase” value for each sample. The sensitivity of the system was determined by a dilution experiment shown in Supporting Information Figs. 1 and 2. In all cases antibodies were purchased from commercial sources, alpha 2 macrogobulin (Invitrogen, Carlsbad, CA), Ceruloplasmin (Abcam, Cambridge, MA), Kininogen (Abcam), Fetuin-A (Abcam), Alpha-1-antitrypsin (Sigma Chemicals, St. Louis, MO).
2.6 GP73 ELISA
GP73 quantitative ELISA was performed using the methods previously reported [18].
2.7 Statistical analysis
Statistical analysis was performed using the Graph Pad Prism 5.0 software package (Graph Pad, Inc, San Diego CA.,) and the open source R-Package. Descriptive statistics for patients were compared using scatter plots that included the outliers. All values were reported as mean values ± standard deviation unless otherwise stated. As the data did not follow typical Gaussian distributions, a non-parametric test (two-tailed, 95% confidence, Mann–Whitney Test) was used to determine statistical significance between groups.
3 Results
3.1 Glycan analysis of total serum reveals increased levels of alpha 1,6 linked core and alpha 1,3 linked outer arm fucosylation with CCA
Comparative N-linked glycan analysis was performed on individual serum samples from 8 patients with CCA and 20 control patients. N-linked glycan attached to serum glycoproteins was removed using PNGase F, the glycans labeled with a fluorescent dye and analyzed by normal phase UPLC [8, 9, 16, 19–24]. In this method, each peak on the UPLC represents either individual glycans or mixtures of glycans [25]. Peaks that vary between patient groups can be collected and subsequently analyzed by sequential exoglycosidase digestion to identify peaks that are altered in the specific patient groups. We refer to this approach as “total” serum glycan analysis, because all (total) N-glycans derived from the serum are analyzed, without any depletions or enrichments. Figure 1 shows an example of a desialylated normal phase UPLC profile from a representative healthy control subject (Fig. 1A) and a patient with CCA (Fig. 1B). The asterisks indicate peaks that are altered in the CCA patient group. Sequential exoglycosidase digestion was used to identify the peaks that were altered in the CCA group. Panels 1C-1E show the desialylated profile of a representative CCA patient after sialidase treatment (Fig. 1C), almond meal fucosidase (Fig. 1D), which removes alpha-1,2,3,4 linked fucose and bovine kidney fucosidase (Fig. 1E), which removes alpha-1,6 linked fucose. As Fig. 1C shows, the peak proposed to be an alpha-1,3 linked out-arm fucosylated glycan (A3F1G3) shifts after treatment with almond meal fucosidase, indicating that this peak contains an outer arm fucosylated structure. Similarly, the peak proposed to contain an alpha-1,6 linked core fucosylated structure (F(6)A2G2) does not move following treatment with the almond meal fucosidase (compare Fig. 1C and D). In contrast, the additional treatment with bovine kidney fucosidase causes a shift in the peak now confirmed to be a core fucosylated bi-antennary glycan (F(6)A2G2). The level of the core fucosylated bi-antennary glycan in the control and CCA patients is shown in the scatter plot in Fig. 1F while the level of the outer arm fucosylated tri-antennary glycan (A3F1G3) is shown in Fig. 1G. The level of core fucosylated bi-antennary glycan was 5.8% (±0.92) in the control group and 8.5% (1.97) in the CCA group. This difference was statistically different (P = 0.001). The level of the outer-arm fucosylated tri-antennary glycan was 2.3% (±1.1) in the control group and 6.3% (±1.6) in the CCA group. This difference was also statistically different (p = 0.001).
Figure 1.
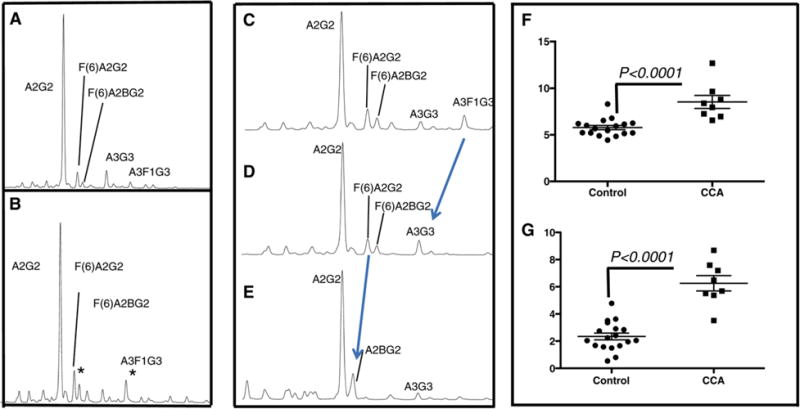
Glycan analysis of total serum reveals increased levels of alpha 1,6 linked core and 1,3 linked outer arm fucosylation with CCA. Representative normal-phase UPLC profile of de-sialylated N-linked glycans from a control sample (A) or CCA sample (B). The asterisks indicate peaks that are altered in the CCA patient group. C) Sequential exoglycosidase digestion was used to identify the peaks that were altered. Panels 1C-1E show the de-sialylated profile of a representative CCA patient after sialidase treatment (Fig. 1C), almond meal fucosidase (Fig. 1D), and bovine kidney fucosidase (Fig. 1E). Scatter plot indicating the level of the core fucosylated bi-antennary glycan in the control and CCA patients (1F) and the level of the outer arm fucosylated tri-antennary glycan (A3F1G3)(1G).
3.2 Identification of fucosylated glycoproteins in CCA patients
In an effort to identify fucosylated proteins in the CCA patients, core and outer arm fucosylated proteins were extracted from pooled CCA serum using the lectin AAL as we have done previously [8]. Proteins were subsequently digested with trypsin and identified by LC MS/MS. Efforts were not made to quantify using LC MS/MS but rather to just identify proteins that would be further analyzed using traditional lectin-FLISA (lectin-Fluorophore-Linked Immunosorbent Assay) based approaches. Supporting Information Table 1 shows the list of protein found to be associated with the CCA fucosylated proteome. As Supporting Information Table 1 shows, the fucosyated serum proteome from CCA patients contained numerous proteins, such as alpha-2-macroglobulin, kininogen, hemopexin, fetuin-A, and ceruloplasmin. Several of these proteins were further examined by Lectin-FLISA techniques to confirm their identification as part of the CCA fucome. A diagram of a typical lectin-FLISA is shown in Fig. 2A. In this case, an antibody to human alpha-1-anti-trypsin, which has been modified through mild periodate oxidation is conjugated to bottom of a 96 well plate. The sample is added and fucosylated glycoforms identified using a biotin-conjugated fucose binding lectin. Bound lectin is then detected using IRDye™ 800 conjugated streptavidin and the signal intensity measured using the Odyssey ® Infrared Imaging System. In all cases, signal intensity is compared to signals detected with commercially purchased human serum (Sigma Chemicals). It is noted that the lectin-FLISA detects the amount of fucosylation present on an equal amount of captured molecules from each patient sample and is performed in a manner that is independent of the total amount of protein in any given patient. Thus, even if protein levels are different in different individuals, this assay will only measure the relative proportion of altered glycoprotein.
Figure 2.
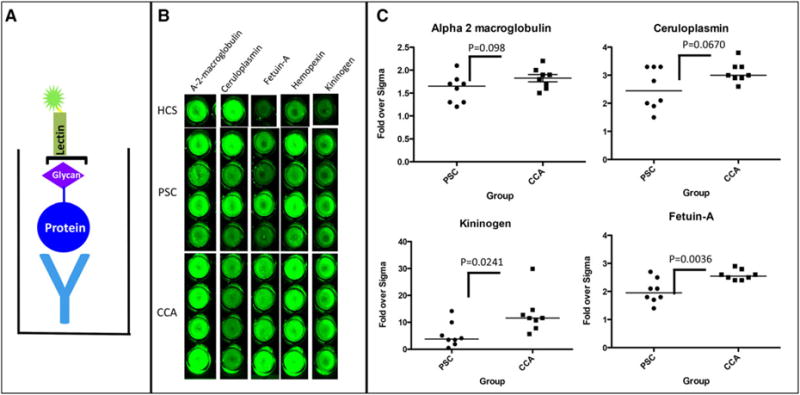
(A). General Lectin-FLISA methodology. (B) Lectin-FLISA for the detection of fucosylated alpha-2 macroglobulin, ceruloplasmin, kininogen and Fetuin-A from a commercially purchased human control serum, 4 patients with PSC, or 4 patients with CCA. (C) The level of fucosylated alpha-2 macroglobulin, ceruloplasmin, kininogen, and Fetuin-A in 8 patients PSC patients or 8 CCA patients. The mean of value of each marker is indicated by the horizontal line.
Figure 2B shows a typical lectin-FLISA confirming the presence of fucosylated alpha-2 macroglobulin, fucosylated ceruloplasmin, fucosylated alpha-2-HS-glycoprotein (referred to as Fetuin-A), fucosylated hemopexin, fucosylated kininogen in the sera of four representative patients with primary sclerosing cholangitis (PSC) and four patients with CCA. As this figure shows, compared to the commercially acquired human control serum (HCS), increased amounts of lectin binding can be observed in the samples following incubation of captured protein with lectin. This is true for the four proteins shown here. As this figure shows, varying levels of lectin reactivity of the fucosylated proteins were observed in PSC and CCA patients. That is, as Fig. 2C shows, high levels of lectin reactive alpha-2-macroglobulin could be observed in the control, PSC and CCA patients, suggesting that this may not be a marker that requires further investigation. Indeed, as this figure shows, the levels of alpha-2-macroglobulin or ceruloplasmin were statistically no different in the CCA samples, as compared to the PSC samples (p>0.05). In contrast, two other proteins found via glycoproteomics, fucosylated Fetuin–A and kininogen, were elevated in patients with CCA, as compared to PSC (p<0.05).
3.3 Correlation of fucosylated Fetuin-A and fucosylated kininogen with cholangiocarcinoma
The results shown in Figs. 1 and 2 were obtained using convenience sample sets and were suggestive that these protein glycoforms could be potential biomarkers of CCA. Thus, the two proteins from Fig. 2 (fucosylated Fetuin A and fucosylated kininogen), along with two other proteins, Golgi-protein 73 and fucosylated alpha-1-anti-trypsin, were subsequently examined in a larger, well defined, independent cohort of CCA patients and compared to the current standard of care, CA-19-9. GP73 was included, as previous reports have shown that this marker is increased in patients with HCC and potentially with CCA [26]. This study was comprised of 117 patients (see Table 1); 39 patients were controls with benign or malignant liver, but not bile duct related, disease, 39 patients had PSC without CCA and 39 patients had cholangiocarcinoma. GP73 was detected by sandwich ELISA [18]. For the analysis of the fucosylated glycoforms of kininogen, A1AT and Fetuin-A, a lectin-FLISA approach was used as shown in Fig. 2 [13,16,27,28].
Table 1.
Samples used for marker validation
| Disease diagnosis: | Controlsa) | PSCb) | Cholangiocarcinomac) | p value |
|---|---|---|---|---|
| Number | 39 | 39 | 39 | – |
| Etiology (n) (HBV/HCV/negative/unknown)d) | 1/10/22/7 | 0/0/29/10 | 1/0/21/17 | – |
| Cirrhosis (n)e) | 9 | 17 | 0 | – |
| Hepatic Mass(n)f) | 11 | 0 | 0 | – |
| Secondary liver cancer(n)g) | 7 | 0 | 0 | – |
| Other liver diseaseh) | 12 | 0 | 0 | |
| Agei) | NA | 47.82 (± 13.87) | 62.0(17.0) | p < 0.001 |
| ALTj) | NA | 115.0 (± 154.4) | 191 (± 723) | p = 0.733 |
| ASTk) | NA | 120.6(± 218.8) | 225 (±1021) | p = 0.528 |
| Alk Phosl) | NA | 477.9 (± 441.1) | 266(± 257) | p = 0.011 |
| Albuminm) | NA | 3.69(± 0.5741) | 3.80 (± 0.65) | p = 0.421 |
Control patients are those with benign liver disease, metastases from other primary sites, or chronic liver disease that is not defined as PSC or cholangiocarcinoma.
Primary sclerosing cholangitis (PSC) was diagnosed as described in methods.
Cholangiocarcinoma (CCA) was diagnosed as described in methods.
Number of patients with positive serology for Hepatitis B virus (HBV) or Hepatitis C virus (HCV), negative serologic markers, or unknown hepatitis status.
The number of patients with liver cirrhosis as defined in material and methods.
Number of patients with non-malignant hepatic masses.
Patients with liver metastases from other primary sites, (n = 7),
Patients with other liver disease not mentioned. These include chronic Hepatitis C without cirrhosis (n = 7), nonalchoholic steatohepatitis (n = 1), non-functioning islet cell tumor with probable liver metastases (n = 1), benign fibrotic gallbladder disease (n = 1), advanced alcoholic liver disease with fluid retention (n = 1), and autoimmune hepatitis with biliary involvement/immune-associated cholangitis (n = 1)
Mean age ± standard deviation.
Mean level of alanine aminotransaminase (ALT) ± standard deviation (IU/mL).
Mean level of aspartate aminotransferase (AST) ± standard deviation (IU/mL).
Mean level of alkaline phosphatase (Alk Phos) ± standard deviation (IU/mL).
Mean level of serum albumin ± standard deviation (IU/mL).
As Fig. 3A shows, compared to commercially purchased serum (from HBV and HCV negative donors), the level of fucosylated -Fetuin-A was 2.9 fold elevated (±1.56) in serum from control patients, 3.5 fold elevated (±1.65) in patients with PSC and 5.8 fold elevated (±2.16) in patients with CCA. Statistical significance was observed between the PSC group and the CCA group (p<0.001) but not between the PSC group and the control group (p = 0.0839).
Figure 3.
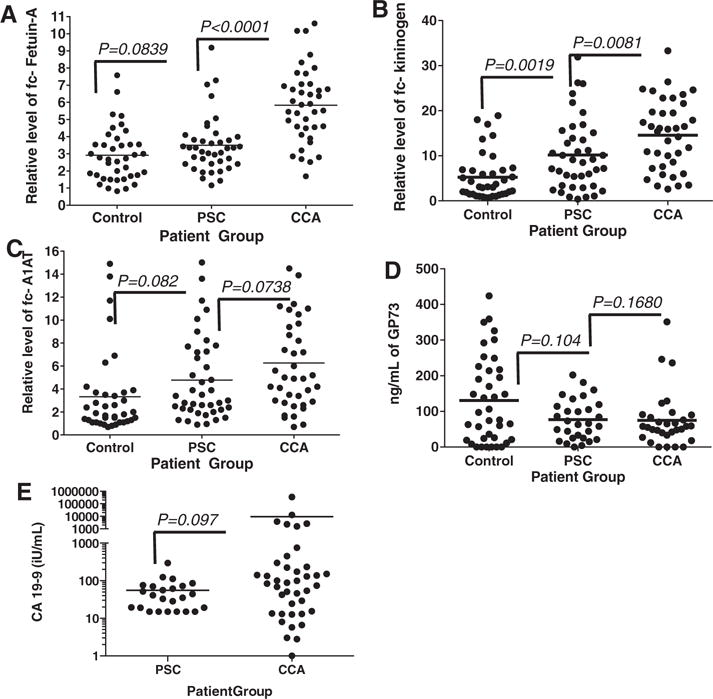
Scatter plots of the relative levels of (A) fucosylated Fetuin-A, (B), fucosylated kininogen, (C) fucosylated A1AT, (D) total GP73 or (E) CA-19-9 in control patients, patients with PSC or patients with cholangiocarcinoma. Fucosylated Fetuin-A, fucosylated kininogen, and fucosylated A1AT, values are presented as fold over commercially purchased sera. GP73 is given as ng/mL, CA-19-9 are given as international units (iU) per mL. In all panels, the line indicates the mean.
Similar to the results obtained with fucosylated-fetuin A, the level of fucosylated -kininogen was 5.2 fold elevated (±5.14) in serum from control patients, 10.2 fold elevated (±7.6) in patients with PSC and 14.58 fold elevated (±7.9) in patients with CCA (Fig. 3B). Statistical significance was observed between the control group and the PSC group (p = 0.0019) and between the CCA group and the control group (p<0.001) and between the PSC and CCA group (p = 0.0081).
Similar changes were observed in several other fucosylated proteins such as fc-A1AT (Fig. 3C) and fucosylated hemopexin (data not shown) but to a lesser extent. For example, the level of fc-A1AT levels was 3.33 fold elevated (±3.58) in serum from control patients, 4.78 fold elevated in patients group and the CCA group (p = 0.0738). In the case of fucosylated hemopexin, it was 2.99 fold elevated (±1.41) in serum from control patients, 4.63 fold elevated in patients with PSC (±1.85) and 5.23 fold (±1.87) in patients with CCA (data not shown). In these three groups, the mean level of fc-A1AT was not statistically significant (p>0.05).
Figure 3D shows the level of GP73 as detected by ELISA in the three patient groups. The control group had a mean of 130.3 ng/mL of GP73 (±121.4), which was similar to that observed in patients with PSC (76.592 ng/mL ±56.296) and CCA(74.842 ng/mL ±77.01). In these three groups, the mean level of GP73 was not statistically significant (p>0.05).
For comparison, the levels of the currently used marker for cholangiocarcinoma, CA-19-9, was also measured in several of these samples (Fig. 3E). CA-19-9 values were available for all CCA patients and for 24 out of 39 PSC patients. Using just these samples, CA-19-9 had a median level of 55.6 U/mL (±60.21) in patients with PSC and 9289 U/mL (±54 459) in patients with cholangiocarcinoma. The difference in the amount of CA-19-9 in the two patient groups was not statistically significant (p = 0.097).
3.4 The performance of the fucosylated proteins in the detection of CCA
Receiver operator characteristic (ROC) curves were plotted to determine overall performance and to identify the sensitivity and specificity for each marker in differentiating PSC from CCA. As CCA often occurs in the absence of PSC, we also examined the ability of these markers to differentiate CCA from other forms of liver disease (Control group; see Table 1). As shown in Fig. 4A, in differentiating PSC from CCA, the AUROC curve for GP73 was 0.536 with a sensitivity of 13% at a fixed specificity of 90%. Comparable results were obtained comparing control group to the CCA group where the AUROC was 0.610 (Fig. 4B). The currently used marker, CA-19-9, had an AUROC of 0.625 at differentiating PSC from CCA (Fig. 4A). CA-19-9 data were not available for the control patient group.
Figure 4.
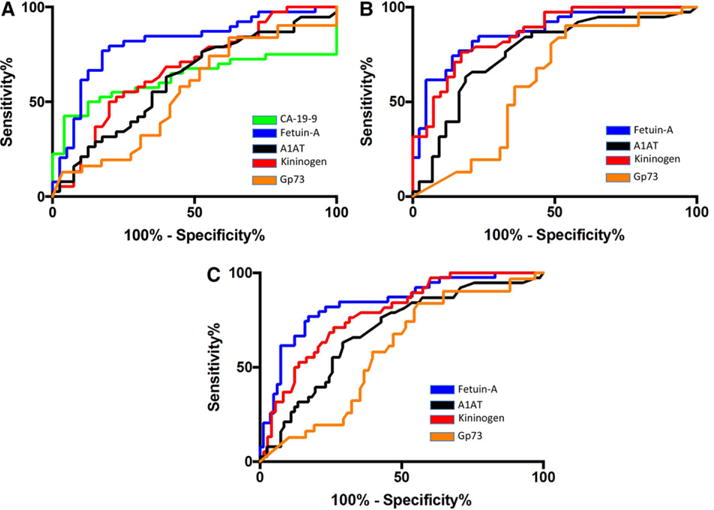
Receiver operator characteristic (ROC) curves for fucosylated Fetuin-A, total GP73, fucosylated A1AT, fucosylated kininogen and CA19-9. (A) ROC analysis of PSC controls versus cholangiocarcinoma. (B) ROC analysis of Non-PSC controls versus cholangiocarcinoma. (C) ROC analysis of CCA versus all other samples. CA19-9 was not available for samples used in panels B and C.
Similar performance was obtained with fc-kininogen (AUROC of 0.673), which, at a cut-off of 22 fold, had a sensitivity of 21% at a fixed specificity of 90% in differentiating PSC from CCA. However, the discriminatory ability of fc-kininogen was greater for distinguishing the control group from CCA (AUROC of 0.854) with a sensitivity of 55% at a fixed specificity of 90%.
The marker fc-Fetuin-A had the best individual performance characteristics for differentiating PSC from CCA, with an AUROC of 0.812 with 62% sensitivity at a fixed specificity of 90% (at a cut-off of 5.2 fold increase). Similar to fc-kininogen, when the CCA group was compared to the control group the AUROC increased to 0.867, with a sensitivity of 62% at a fixed specificity of 90%.
It is noted that the level of some of the clinical markers varied in the larger patient population. That is, there were statistical differences in the age and ALK level in the PSC and CCA patients (see Table 1). To determine if this difference impacted the performance of fc-Fetuin-A, we examined this marker in a subset of these patients, with a greater balance of age and ALK. In this smaller sub-group consisting of 39 patients with PSC and 20 patients with CCA, fc-fetuin-A had an AUROC of 0.815 with 62% sensitivity at a fixed specificity of 90% (at a cut-off of 5.2 fold increase), which is not statistically different than that obtained with the full set (p>0.05).
4 Discussion
We have previously identified several glycoproteins with altered fucosylation in HCC [8, 9]. Others have reported that some of these glycoproteins, such as GP73, may be increased in the serum of patients with cholangiocarcinoma [26]. Surprisingly, GP73 was not elevated in patients with CCA or PSC as compared to control patients, suggesting that this protein may not be a useful marker for the detection of CCA. In contrast, two of the fucosylated glycoforms, fc-kininogen and fc-Fetuin-A were increased in patients with CCA, as compared to both the PSC and/or the control patients. It is noted that the level of fc-kininogen was increased in patients with PSC, suggesting that the increased levels of inflammation and or cellular damage may play a role in the increased levels of these fucosylated glycoforms observed in the serum.
We have previously reported that Fetuin-A levels were increased in patients with HCC as compared to patients with liver cirrhosis and hepatitis infections [16]. However, there was a very obvious quantitative difference in the level of fc-Fetuin-A in patients with CCA as compared with HCC. That is, the level of fc-Fetuin-A observed in the CCA patient set (mean of 5.81 ±2.164) is much greater than that observed either in our previous analysis of an HCC sample set (mean of 2.5 ± 0.73) or in the control sample set (mean of 2.9 fold elevated ±1.56) in this study, which consisted of many patients with hepatic masses (see Table 1) [16]. Thus, fc-Fetuin-A, in contrast to the other fucosylated acute phase proteins, maybe a specific marker for CCA.
In summary, we have developed a lectin-FLISA based method for the analysis of fucosylated glycoforms of several secreted liver glycoproteins. These markers, when used alone or in combination, had an overall performance that was better than CA-19-9 in the detection of CCA. It is postulated that these markers could be used to supplement CA-19-9 as a general screen in those patients at high risk for CCA development either alone or in combination with imaging modalities. It is also important to note that a serum biomarker such as this will not necessarily trigger the diagnosis of cancer but rather a further imaging modality to positively identify the cancer.
These data need to be confirmed in larger cohorts of patients to determine if these markers are true indicators of cholangiocarcinoma, the assay will also need to be optimized further before use in a clinical setting and the accuracy compared with other markers in patients of diverse gender, ethnicity, and etiologies of liver disease, to determine their role in CCA detection.
Supplementary Material
Clinical Relevance.
Cholangiocarcinoma is a cancer of bile duct cells for which no effective biomarker exists. Tumor masses are often not identifiable by imaging modalities as a result of the location of the tumor in the hilum of the liver or within the bile ducts. Because of the problems with obtaining a definitive tissue diagnosis, treatment and management decisions for patients with biliary strictures that may be malignant are difficult. The work presented here highlights the potential role of fucosylated fetuin-A as a biomarker of cholangiocarcinoma and may help in the early detection of this deadly cancer.
Acknowledgments
This work was supported by grants R01 CA120206 (to ASM) and U01 CA168856 (to ASM) from the National Cancer Institute (NCI), the Hepatitis B Foundation, and an appropriation from The Commonwealth of Pennsylvania and also by grants CA100882 and CA128633 from the National Cancer Institute, The Cholangiocarcinoma Foundation (to LRR) and the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567). Dr. Adrian M. Di Bisceglie, Department of Internal Medicine and Saint Louis University Liver Center, Saint Louis University School of Medicine, St Louis, MO is thanked for providing samples.
We have received a patent that contains many of the proteins identified here as biomarkers of liver disease.
Footnotes
References
- 1.Sandhu DS, Roberts LR. Diagnosis and management of cholangiocarcinoma. Curr Gastroenterol Reports. 2008;10:43–52. doi: 10.1007/s11894-008-0008-9. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JC, Gores GJ, LaRusso NF, Wiesner RH, et al. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874–879. doi: 10.1016/s0025-6196(12)60696-x. [DOI] [PubMed] [Google Scholar]
- 3.Ramage JK, Donaghy A, Farrant JM, Iorns R, et al. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865–869. doi: 10.1016/0016-5085(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 4.Patel AH, Harnois DM, Klee GG, LaRusso NF, et al. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 5.Naitoh A, Aoyagi Y, Asakura H. Highly enhanced fucosylation of serum glycoproteins in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:436–445. doi: 10.1046/j.1440-1746.1999.01882.x. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi Y, Isokawa O, Suda T, Watanabe M, et al. The fucosylation index of alpha-fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma. Cancer. 1998;83:2076–2082. [PubMed] [Google Scholar]
- 7.Noda K, Miyoshi E, Gu J, Gao CX, et al. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 2003;63:6282–6289. [PubMed] [Google Scholar]
- 8.Block TM, Comunale MA, Lowman M, Steel LF, et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci USA. 2005;102:779–784. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comunale MA, Lowman M, Long RE, Krakover J, et al. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;6:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 10.Guile GR, Rudd PM, Wing DR, Prime SB, et al. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- 11.Saldova R, Reuben JM, Abd Hamid UM, Rudd PM, et al. Levels of specific serum N-glycans identify breast cancer patients with higher circulating tumor cell counts. Ann Oncol. 2011;22:1113–1119. doi: 10.1093/annonc/mdq570. [DOI] [PubMed] [Google Scholar]
- 12.Comunale MA, Lowman M, Long RE, Krakover J, et al. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;5:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 13.Comunale MA, Rodemich-Betesh L, Hafner J, Wang M, et al. Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. PLoS One. 2010;5:e12419. doi: 10.1371/journal.pone.0012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold JN, Saldova R, Galligan MC, Murphy TB, et al. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–1764. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 15.Saldova R, Wormald MR, Dwek RA, Rudd PM. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 2008;25:219–232. doi: 10.1155/2008/601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comunale MA, Wang M, Hafner J, Krakover J, et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J Proteome Res. 2009;8:595–602. doi: 10.1021/pr800752c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Long RE, Comunale MA, Junaidi O, et al. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomark Prevention. 2009;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morota K, Nakagawa M, Sekiya R, Hemken PM, et al. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49:711–718. doi: 10.1515/CCLM.2011.097. [DOI] [PubMed] [Google Scholar]
- 19.Guile GR, Rudd PM, Wing DR, Prime SB, et al. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- 20.Rudd PM, Dwek RA. Rapid, sensitive sequencing of oligosaccharides from glycoproteins. Curr Opin Biotechnol. 1997;8:488–497. doi: 10.1016/s0958-1669(97)80073-0. [DOI] [PubMed] [Google Scholar]
- 21.Rudd PM, Guile GR, Kuster B, Harvey DJ, et al. Oligosaccharide sequencing technology. Nature. 1997;388:205–207. doi: 10.1038/40677. [DOI] [PubMed] [Google Scholar]
- 22.Rudd PM, Colominas CC, Royle L, Murphy N, et al. A high-performance liquid chromatography based strategy for rapid, sensitive sequencing of N-linked oligosaccharide modifications to proteins in sodium dodecyl sulphate polyacrylamide electrophoresis gel bands. Proteomics. 2001;1:285–289. doi: 10.1002/1615-9861(200102)1:2<285::AID-PROT285>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 24.Mehta AS, Long RE, Comunale MA, Wang M, et al. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol. 2008;82:1259–1270. doi: 10.1128/JVI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold JN, Saldova R, Galligan MC, Murphy TB, et al. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–1764. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 26.Riener MO, Stenner F, Liewen H, Soll C, et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology (Baltimore, Md) 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 27.Comunale MA, Wang M, Rodemich-Betesh L, Hafner J, et al. Novel changes in glycosylation of serum Apo-J in patients with hepatocellular carcinoma. Cancer Epidemiol Biomark Prevent. 2011;20:1222–1229. doi: 10.1158/1055-9965.EPI-10-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Long RE, Comunale MA, Junaidi O, et al. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


