Abstract
Background
Data on the true burden of hyperkalemia in patients with heart failure (HF) in a real‐world setting are limited.
Methods and Results
Incidence rates of hyperkalemia (first blood test with a potassium level >5.0 mmol/L) in primary or hospital care were assessed in a population‐based cohort of patients with incident HF diagnoses in northern Denmark from 2000 to 2012. Risk factors and clinical outcomes were compared in patients with HF with versus without hyperkalemia. Of 31 649 patients with HF, 39% experienced hyperkalemia (mean follow‐up, 2.2 years). Risks of experiencing a second, third, or fourth event were 43%, 54%, and 60%, respectively. Among patients with HF with stage 3A, 3B, 4, or 5 kidney dysfunction, 26%, 35%, 44%, and 48% experienced hyperkalemia within the first year. Important hyperkalemia risk factors included chronic kidney disease (prevalence ratio, 1.46; 95% confidence interval [CI], 1.43−1.49), diabetes mellitus (prevalence ratio, 1.38; 95% CI, 1.32−1.45), and spironolactone use (prevalence ratio, 1.48; 95% CI, 1.42−1.54). In patients with HF who developed hyperkalemia, 53% had any acute‐care hospitalization 6 months before the hyperkalemia event, increasing to 74% 6 months after hyperkalemia (before‐after risk ratio, 1.41; 95% CI, 1.38−1.44). Compared with matched patients with HF without hyperkalemia, adjusted 6‐month hazard ratios in patients with hyperkalemia were 2.75‐fold (95% CI, 2.65–2.85) higher for acute‐care hospitalization and 3.39‐fold (95% CI, 3.19–3.61) higher for death.
Conclusions
Almost 4 in 10 patients with HF develop hyperkalemia, and many patients have recurrent hyperkalemia episodes. Hyperkalemia risk is strongly associated with degree of reduced kidney function and use of spironolactone. Hyperkalemia is associated with severe clinical outcomes and death in HF.
Keywords: chronic kidney disease, cohort study, heart failure, potassium, prognosis
Subject Categories: Heart Failure, Complications, Mortality/Survival, Epidemiology, Nephrology and Kidney
Clinical Perspective
What Is New?
To understand the potential impact of new drug therapies for hyperkalemia, it is important to assess the true burden of hyperkalemia among people with heart failure (HF).
In a population‐based cohort study including 31 649 patients with first hospital‐diagnosed HF, almost 4 in 10 patients developed hyperkalemia (potassium >5.0 mmol/L) over 2.2 years.
The risk of developing hyperkalemia was strongly associated with degree of reduced kidney function and spironolactone use, and recurrent hyperkalemia was frequent.
Compared with matched patients with HF without hyperkalemia, events of hyperkalemia were associated with severe clinical outcomes, including arrhythmias, intensive care admission, and death.
What Are the Clinical Implications?
Among patients with HF in everyday clinical care, attention to the substantial risk of hyperkalemia is prudent.
Efforts to prevent and normalize hyperkalemia should be directed to patients with HF, chronic kidney disease, and diabetes mellitus, and to those who use spironolactone.
Our data support the need for regular potassium measurement to identify patients with HF at risk of serious clinical outcomes and death.
Heart failure (HF) affects >37 million adults worldwide and is a leading cause of hospitalizations and death.1, 2 Despite improvements in HF therapy and prognosis,3 hospitalizations with HF remain frequent, and comorbidities, including diabetes mellitus and renal disease, have become more common.2, 3, 4, 5
Hyperkalemia, usually defined as blood potassium level >5.0 mmol/L, is a concern in patients with HF in everyday clinical practice and can be a life‐threatening condition.6, 7 Hyperkalemia with high potassium levels (eg, >6.0 mmol/L) may lead to cardiac arrhythmias and death,8 but even potassium levels of >5.0 mmol/L have been associated with increased mortality in patients with acute‐care hospital admission6 and in patients with HF.9, 10
Knowledge is scarce on the occurrence of hyperkalemia and associated outcomes in patients with HF in the real‐world setting.11, 12 Patients with kidney disease have an increased risk of hyperkalemia,7 and a dynamic association between heart and kidney dysfunction has been described in the cardiorenal syndrome,13 with evidence of chronic kidney disease in more than half of patients with HF.14 Moreover, several drugs commonly used for treating HF may lead to hyperkalemia15 by interfering with renal potassium excretion, including angiotensin‐converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), and potassium‐sparing diuretics, such as spironolactone and eplerenone.11, 16
To understand the potential impact of new drug therapies for hyperkalemia,8, 17 it is important to assess the true burden of hyperkalemia among people with HF and to assess associated patient characteristics, current treatment practices, and clinical outcomes in real‐world settings. We, therefore, undertook a large population‐based cohort study in Denmark to examine occurrence, risk factors, and outcomes of hyperkalemia in patients with HF.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results. According to Danish legislation, researchers may apply for the data at the Danish Health Data Authority.
Setting
This cohort study was conducted in northern Denmark, by using routine care laboratory test results from both primary and hospital care for the entire region's population (1 851 496 residents [33% of Denmark's population] by the end of 2012).18 The laboratory data were linked using the Civil Personal Registration number with data from the Danish National Patient Registry, which contains dates of admission and discharge (including emergency department and outpatient clinic visits) and procedures.19 Furthermore, data on drugs were retrieved from the Aarhus University Prescription Database, which holds records of type of drug, date of dispensing, and quantity on all prescribed drugs dispensed from Danish pharmacies in northern Denmark.20
Patients With HF and Covariates
From the source population of all individuals living in northern Denmark, we identified a cohort of individuals with a first‐time inpatient hospital admission with a primary or secondary discharge diagnosis of HF between 2000 and 2012. The HF diagnosis has a positive predictive value of ≈80% in the Danish National Patient Registry.21 On the basis of the lowest estimated glomerular filtration rate (eGFR) measured up to and including the date of first HF admission (or presence of dialysis), patients were classified into kidney disease stage 1 and 2, eGFR ≥60 mL/min per 1.73 m2; stage 3A, eGFR of 45 to 59 mL/min per 1.73 m2; stage 3B, eGFR of 30 to 44 mL/min per 1.73 m2; stage 4, eGFR of 15 to 29 mL/min per 1.73 m2; stage 5, eGFR of <15 mL/min per 1.73 m2; and dialysis.22 Baseline characteristics at time of the first HF admission were based on dispensed drugs from pharmacies within the past 12 months and on diagnoses and procedures recorded in the National Patient Registry any time up to and including the date of HF discharge. Patients were followed up for hyperkalemia events from the time of the first HF hospital admission until migration, death, or end of follow‐up.
All codes and variable algorithms used in the study are provided in Table S1.
Statistical Analysis
Incidence of hyperkalemia
An incident episode of hyperkalemia was defined as an elevated blood potassium level >5.0 mmol/L not preceded by a prior episode of elevated potassium within the previous month. Incidence of hyperkalemia events with cut points potassium >5.5 mmol/L and potassium >6.0 mmol/L was examined separately. Incidence rates of a first hyperkalemia event per 1000 person‐years were calculated. Cumulative incidence function curves were constructed, and risks (cumulative incidence proportions) of developing hyperkalemia within 1 and 3 years were estimated. The risk of developing a second, third, and fourth hyperkalemia event was also described.
Risk factors at the time of hyperkalemia
To examine risk factors before the hyperkalemia/index date, one comparison patient without hyperkalemia was matched to each corresponding patient with hyperkalemia on the index date, according to sex, age, calendar time, and time since first HF admission. Prevalence ratios (PRs) for the association of specified risk factors were calculated.
Outcomes associated with hyperkalemia
In a before‐after analysis among patients with HF who all had experienced hyperkalemia, the cumulative incidence proportion ratios (risk ratios [RRs]) of experiencing each individual clinical outcome during the 6 months after, versus 6 months before, the hyperkalemia event date were calculated, accounting for competing risk of death within 6 months after the hyperkalemia date.
Second, a matched cohort design with Cox regression was used to estimate the hazard ratio (HR) of clinical outcomes 6 months after the hyperkalemia/index date in patients with hyperkalemia versus age, sex, and HF duration matched comparisons without hyperkalemia. Factors potentially associated with both hyperkalemia risk and outcomes were adjusted for, including intensity of HF treatment regimen and number of acute HF hospitalizations 6 months before the hyperkalemia/index date as marker of HF severity, eGFR category, Charlson Comorbidity Index score, presence of specific hyperkalemia risk factors (diabetes mellitus/chronic kidney disease/hypertension), and use of ACEis/ARBs, spironolactone, or potassium supplements.
Additional and Sensitivity Analyses
Although potassium tests were generally frequent in the HF cohort (mean, 23 tests; median, 15 tests; interquartile range, 5–31 tests per patient during the observation period), bias by indication for blood testing may be a concern (ie, sicker patients possibly receiving more frequent blood testing with higher likelihood of hyperkalemia being detected). Therefore, the analyses were repeated using a randomly selected set of 5 potassium tests per patient among patients with HF with at least 5 tests the year after HF. Second, to study hyperkalemia impact in a subcohort more likely to have chronic HF, and thus be more closely followed up, patients who were alive at 6 months after HF discharge had an echocardiography performed and were treated with both ARBs or ACEis and β blocker up to this date were separately examined. Third, as an alternative method to account for remaining unmeasured confounding when comparing outcomes in patients with and without hyperkalemia with HF, the ratio of the 2 matched HRs observed after versus before the hyperkalemia/index date (ie, the prior event rate ratio adjusted rate ratio) was estimated.23 This method is based on the assumption that differences in outcomes between hyperkalemia‐exposed and matched hyperkalemia‐unexposed patients present already before experiencing hyperkalemia reflect the combined effect of remaining unmeasured confounders, independent of any effect of hyperkalemia. Fourth, to exclude reverse causality (ie, acute‐care hospitalization outcomes, such as intensive care unit admission, leading to secondary hyperkalemia, versus being a consequence of hyperkalemia), outcomes were also examined separately for hyperkalemia detected on the first admission date of any hospitalization. Fifth, analyses were stratified by initial HF diagnosis being primary (first‐listed) versus secondary diagnosis. Sixth, we evaluated outcomes separately for elevated potassium detected on dates on which patients were hospitalized versus out of hospital, and we repeated the outcome analyses separately in patients with <10 potassium tests versus patients with ≥10 tests before the hyperkalemia event. Finally, the influence of potential unmeasured confounding was quantified by means of a rule‐out approach.24 We estimated how strongly an unmeasured binary confounder would need to be associated with hyperkalemia and outcomes to fully explain our findings, and we illustrated this association graphically.
The study was approved by the Danish Data Protection Agency. The study was purely registry based and did not involve any contact with patients or interventions; therefore, according to Danish legislation, no informed consent was required.
Results
Baseline Characteristics
Overall, the analysis comprised 31 649 individuals with a first incident hospital record of HF in northern Denmark from 2000 to 2012. Median age was high, at 78 (interquartile range, 69–85) years, and 47% were women (Table 1). Diagnosed cardiomyopathy at time of first HF diagnosis was relatively rare (4%), whereas 14% had known valvular heart disease, 21% had prior MI, 40% had any ischemic heart disease, 35% had atrial fibrillation, and 62% had hypertension. In total, two thirds (68%) of the patients had a history of hospital‐diagnosed comorbidities included in the Charlson Comorbidity Index on admission; 19% had diabetes mellitus, 41% had chronic kidney disease, and 18% had chronic pulmonary disease. At baseline before first admission, 24% of the patients were treated with ACEis, 11% were treated with ARBs, 31% were treated with βblockers, 11% were treated with spironolactone, and 30% were treated with potassium supplements. Of the patients, 57% had been examined with echocardiography. Three months after initial HF diagnoses, proportions with echocardiography and receiving drug treatment had increased considerably in our cohort, as expected (for subpopulation with chronic HF, see later).
Table 1.
Baseline Characteristics Among Patients With First Hospital‐Diagnosed HF Stratified by eGFR Category and Subsequent Incidence of Hyperkalemia
| Characteristics | eGFR Category, mL/min per 1.73 m2 a | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| eGFR ≥60 | eGFR 45–59 | eGFR 30–44 | eGFR 15–29 | eGFR <15 | Dialysis | No eGFR Recorded | ||
| No. of patients with HF (row %) | 7679 (24.3) | 6931 (21.9) | 5800 (18.3) | 3707 (11.7) | 1166 (3.7) | 298 (0.9) | 6068 (19.2) | 31 649 (100) |
| Female sex | 2602 (33.9) | 3241 (46.8) | 3198 (55.1) | 2016 (54.4) | 612 (52.5) | 93 (31.2) | 3047 (50.2) | 14 809 (46.8) |
| Age, median (quartiles), y | 70 (60.2–79.0) | 78 (69.7–84.3) | 82 (74.6–87.0) | 83 (76.1–87.7) | 81 (73.5–86.4) | 70 (60.3–78.6) | 79 (71.5–85.4) | 78 (69.1–84.9) |
| Echocardiography performed before/at first HF admission | 5021 (65.4) | 4222 (60.9) | 3381 (58.3) | 2262 (61.0) | 735 (63.0) | 223 (74.8) | 2281 (37.6) | 18 125 (57.3) |
| HF as primary diagnosis | 2977 (38.8) | 2586 (37.3) | 2109 (36.4) | 1277 (34.4) | 412 (35.3) | 124 (41.6) | 2423 (39.9) | 11 908 (37.6) |
| Conditions underlying HFb | ||||||||
| Cardiomyopathy | 494 (6.4) | 312 (4.5) | 195 (3.4) | 124 (3.3) | 24 (2.1) | 12 (4.0) | 180 (3.0) | 1341 (4.2) |
| Valvular heart disease | 867 (11.3) | 947 (13.7) | 931 (16.1) | 629 (17.0) | 189 (16.2) | 51 (17.1) | 648 (10.7) | 4262 (13.5) |
| Myocardial infarction | 1506 (19.6) | 1513 (21.8) | 1406 (24.2) | 1034 (27.9) | 324 (27.8) | 99 (33.2) | 905 (14.9) | 6787 (21.4) |
| Ischemic heart disease | 2780 (36.2) | 2819 (40.7) | 2540 (43.8) | 1810 (48.8) | 530 (45.5) | 162 (54.4) | 2093 (34.5) | 12 734 (40.2) |
| Atrial fibrillation | 2393 (31.2) | 2585 (37.3) | 2243 (38.7) | 1426 (38.5) | 402 (34.5) | 81 (27.2) | 1964 (32.4) | 11 094 (35.1) |
| Other comorbidities | ||||||||
| Diabetes mellitus | 1222 (15.9) | 1162 (16.8) | 1225 (21.1) | 1007 (27.2) | 360 (30.9) | 107 (35.9) | 993 (16.4) | 6076 (19.2) |
| Chronic kidney diseasec | 59 (0.8) | 3524 (50.8) | 4579 (78.9) | 3224 (87.0) | 1068 (91.6) | 298 (100) | 243 (4.0) | 12 995 (41.1) |
| Hypertension | 3942 (51.3) | 4399 (63.5) | 4195 (72.3) | 2968 (80.1) | 955 (81.9) | 270 (90.6) | 2852 (47.0) | 19 581 (61.9) |
| Peripheral vascular disease | 566 (7.4) | 721 (10.4) | 768 (13.2) | 656 (17.7) | 254 (21.8) | 99 (33.2) | 609 (10.0) | 3673 (11.6) |
| Cerebrovascular disease | 948 (12.3) | 1155 (16.7) | 1229 (21.2) | 865 (23.3) | 294 (25.2) | 58 (19.5) | 940 (15.5) | 5489 (17.3) |
| Chronic pulmonary disease | 1315 (17.1) | 1301 (18.8) | 1156 (19.9) | 761 (20.5) | 205 (17.6) | 56 (18.8) | 1044 (17.2) | 5838 (18.4) |
| Peptic ulcer disease | 514 (6.7) | 582 (8.4) | 641 (11.1) | 514 (13.9) | 178 (15.3) | 54 (18.1) | 546 (9.0) | 3029 (9.6) |
| Any malignant disease | 963 (12.5) | 1052 (15.2) | 1035 (17.8) | 751 (20.3) | 246 (21.1) | 66 (22.1) | 665 (11.0) | 4778 (15.1) |
| Alcoholism‐related disorders | 775 (10.1) | 438 (6.3) | 372 (6.4) | 311 (8.4) | 121 (10.4) | 43 (14.4) | 360 (5.9) | 2420 (7.6) |
| Medical obesity | 577 (7.5) | 433 (6.2) | 370 (6.4) | 311 (8.4) | 124 (10.6) | 22 (7.4) | 322 (5.3) | 2159 (6.8) |
| Drug treatment before first HF diagnosis | ||||||||
| ACEis overall | 1588 (20.7) | 1625 (23.4) | 1516 (26.1) | 1109 (29.9) | 353 (30.3) | 93 (31.2) | 1146 (18.9) | 7430 (23.5) |
| Ramipril | 650 (8.5) | 652 (9.4) | 570 (9.8) | 440 (11.9) | 122 (10.5) | 39 (13.1) | 241 (4.0) | 2714 (8.6) |
| Enalapril | 437 (5.7) | 450 (6.5) | 440 (7.6) | 324 (8.7) | 109 (9.3) | 33 (11.1) | 284 (4.7) | 2077 (6.6) |
| Other ACEis | 422 (5.5) | 426 (6.1) | 422 (7.3) | 289 (7.8) | 92 (7.9) | 20 (6.7) | 600 (9.9) | 2271 (7.2) |
| ACEi/diuretic combination | 156 (2.0) | 175 (2.5) | 160 (2.8) | 118 (3.2) | 51 (4.4) | 4 (1.3) | 67 (1.1) | 731 (2.3) |
| ARBs overall | 647 (8.4) | 724 (10.4) | 756 (13.0) | 595 (16.1) | 215 (18.4) | 70 (23.5) | 396 (6.5) | 3403 (10.8) |
| Losartan | 271 (3.5) | 302 (4.4) | 308 (5.3) | 241 (6.5) | 95 (8.1) | 27 (9.1) | 193 (3.2) | 1437 (4.5) |
| Candesartan | 114 (1.5) | 123 (1.8) | 120 (2.1) | 93 (2.5) | 40 (3.4) | 32 (10.7) | 57 (0.9) | 579 (1.8) |
| Other ARBs | 93 (1.2) | 115 (1.7) | 119 (2.1) | 83 (2.2) | 30 (2.6) | 11 (3.7) | 61 (1.0) | 512 (1.6) |
| ARB/diuretic combination | 235 (3.1) | 232 (3.3) | 252 (4.3) | 223 (6.0) | 63 (5.4) | 6 (2.0) | 118 (1.9) | 1129 (3.6) |
| β Blockers | 2046 (26.6) | 2110 (30.4) | 1945 (33.5) | 1500 (40.5) | 491 (42.1) | 175 (58.7) | 1475 (24.3) | 9742 (30.8) |
| Spironolactone | 522 (6.8) | 595 (8.6) | 759 (13.1) | 618 (16.7) | 195 (16.7) | 7 (2.3) | 755 (12.4) | 3451 (10.9) |
| Potassium supplements | 1447 (18.8) | 1852 (26.7) | 2011 (34.7) | 1557 (42.0) | 457 (39.2) | 62 (20.8) | 2151 (35.4) | 9537 (30.1) |
| Loop diuretics | 1890 (24.6) | 2332 (33.6) | 2525 (43.5) | 2187 (59.0) | 734 (63.0) | 182 (61.1) | 2634 (43.4) | 12 484 (39.4) |
| NSAIDs | 1685 (21.9) | 1466 (21.2) | 1221 (21.1) | 904 (24.4) | 282 (24.2) | 28 (9.4) | 1490 (24.6) | 7076 (22.4) |
| Trimethoprim | 91 (1.2) | 140 (2.0) | 176 (3.0) | 168 (4.5) | 68 (5.8) | 3 (1.0) | 152 (2.5) | 798 (2.5) |
| Macrolides | 911 (11.9) | 808 (11.7) | 627 (10.8) | 463 (12.5) | 136 (11.7) | 41 (13.8) | 788 (13.0) | 3774 (11.9) |
| Hyperkalemia event >5.0 mmol/L | ||||||||
| Total with a first hyperkalemia event | 2706 (35.2) | 2884 (41.6) | 2823 (48.7) | 1985 (53.5) | 641 (55.0) | 167 (56.0) | 1134 (18.7) | 12 340 (39.0) |
| Time to event in patients with event, median, y | 0.53 | 0.43 | 0.19 | 0.08 | 0.07 | 0.17 | 3.91 | 0.34 |
| Potassium tests before first event, median | 9 | 9 | 7 | 5 | 6 | 8 | 7 | 8 |
| 1‐y Cumulative incidence, % (95% CI) | 21.3 (20.1–22.4) | 26.1 (24.7–27.5) | 35.0 (33.1–36.9) | 43.6 (40.8–46.5) | 48.1 (42.6–53.6) | 47.7 (36.8–58.5) | 2.8 (2.4–3.2) | 25.2 (24.5–25.8) |
| Incidence rate per 1000 person‐years | 125.7 | 189.3 | 325.5 | 569.8 | 786.2 | 827.4 | 58.5 | 178.0 |
| Hyperkalemia event >5.5 mmol/L | ||||||||
| Total with a first hyperkalemia event | 1135 (14.8) | 1365 (19.7) | 1472 (25.4) | 1177 (31.8) | 433 (37.1) | 129 (43.3) | 513 (8.5) | 6224 (19.7) |
| Time to event in patients with event, median, y | 1.13 | 0.87 | 0.47 | 0.16 | 0.08 | 0.25 | 4.29 | 0.61 |
| Potassium tests before first event, median | 16 | 13 | 11 | 8 | 8 | 11 | 12 | 11 |
| 1‐y Cumulative incidence, % (95% CI) | 7.1 (6.5–7.7) | 10.3 (9.5–11.1) | 15.7 (14.6–16.8) | 23.3 (21.5–25.1) | 30.4 (26.6–34.2) | 32.6 (24.7–40.4) | 1.0 (0.8–1.3) | 11.2 (10.8–11.6) |
| Incidence rate per 1000 person‐years | 42.7 | 68.5 | 123.9 | 234.4 | 367.9 | 465.9 | 24.4 | 72.5 |
| Hyperkalemia event >6.0 mmol/L | ||||||||
| Total with a first hyperkalemia event | 491 (6.4) | 593 (8.6) | 688 (11.9) | 603 (16.3) | 233 (20.0) | 81 (27.2) | 245 (4.0) | 2934 (9.3) |
| Time to event in patients with event, median, y | 1.50 | 1.30 | 0.70 | 0.22 | 0.09 | 0.38 | 4.48 | 0.84 |
| Potassium tests before first event, median | 20 | 16 | 13 | 9 | 8 | 14 | 17 | 14 |
| 1‐y Cumulative incidence, % (95% CI) | 2.7 (2.3–3.1) | 3.8 (3.4–4.3) | 6.7 (6.0–7.4) | 11.7 (10.5–12.9) | 15.7 (13.2–18.2) | 18.5 (13.1–23.9) | 0.4 (0.2–0.6) | 4.9 (4.7–5.2) |
| Incidence rate per 1000 person‐years | 17.5 | 27.3 | 51.4 | 102.1 | 161.2 | 213.8 | 11.4 | 31.7 |
Data are given as number (percentage) unless otherwise indicated. Percentages are based on the first row. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor II blocker; CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure.
Patients categorized according to lowest measured eGFR before or on admission date with HF; presence of dialysis overrules any eGFR measurement result.
As present at the time of HF diagnosis.
Manifest chronic kidney disease was defined on the date of the second of 2 measurements >90 days apart of a creatinine value corresponding to an eGFR <60 mL/min per 1.73 m2 or on the first date of a hospital diagnosis.
Incidence of Hyperkalemia
During 69 318 person‐years of follow‐up (mean, 2.2 years), 39% (n=12 340) of the patients experienced a first hyperkalemia event with potassium of >5.0 mmol/L, resulting in an incidence rate of 178 per 1000 person‐years (Table 1), increasing with age and comorbidity (Table S2). The cumulative risk of hyperkalemia was 25% during the first year and 32% within 3 years after HF diagnosis. Among patients with stage 3A, 3B, 4, or 5 kidney disease, or dialysis, 26%, 35%, 44%, 48%, and 48% experienced hyperkalemia within the first year. Median time to first hyperkalemia event in those who experienced an event was 0.34 years; 7787 events (63%) were hospital diagnosed.
The panels of Figure 1 each display cumulative incidence curves of first event of potassium >5.0 mmol/L, potassium >5.5 mmol/L, or potassium >6.0 mmol/L by decreasing kidney function. Almost half (43%) of all patients with a first hyperkalemia event had a second event, whereas risks of experiencing a third event or fourth event were 54% and 60%, respectively, with successively shorter time between the episodes (Figure 2).
Figure 1.
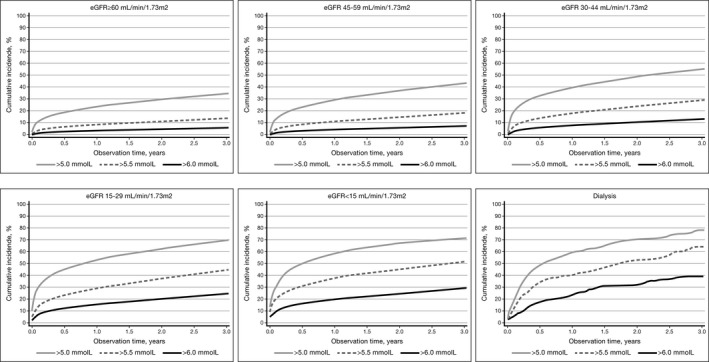
Cumulative incidence curves for first occurrence of potassium >5.0 mmol/L, potassium >5.5 mmol/L, or potassium >6.0 mmol/L in patients with first hospital diagnosed heart failure, according to estimated glomerular filtration rate (eGFR) category.
Figure 2.
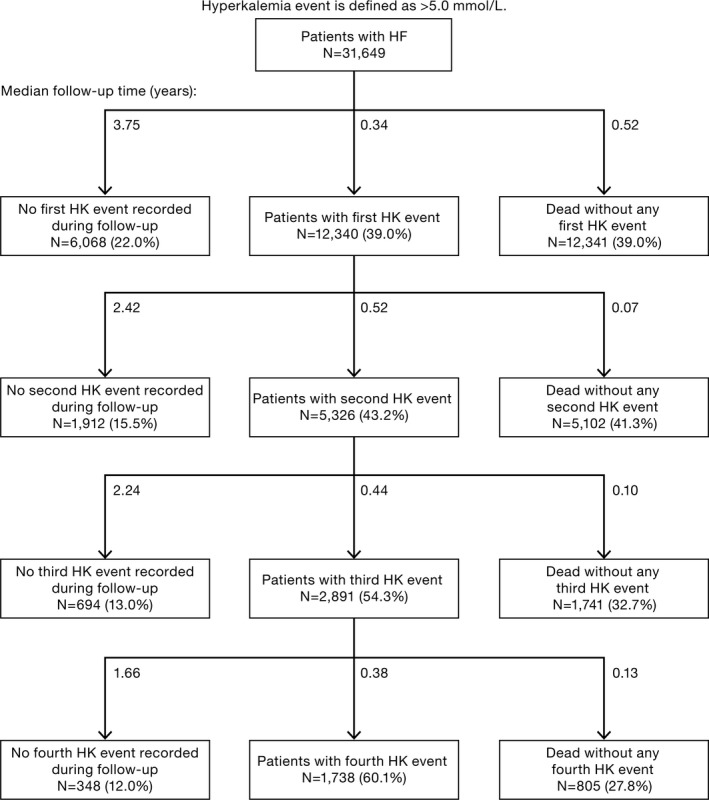
Proportions and median follow‐up time for patients with heart failure (HF) experiencing recurrent hyperkalemia (HK) events.
Risk Factors of Hyperkalemia
Patients who developed hyperkalemia had similar underlying HF causes as those who did not develop hyperkalemia, but most comorbidities were more common in those with hyperkalemia (Table 2). Use of spironolactone was also increased, whereas use of other hyperkalemia‐associated medications at the time of hyperkalemia was not higher. There was an association between hyperkalemia and decreasing eGFR values (eGFR 30–44 mL/min per 1.73 m2: PR, 1.38; 95% confidence interval [CI], 1.33–1.45; eGFR 15–29 mL/min per 1.73 m2: PR, 2.05; 95% CI, 1.94–2.17; eGFR <15 mL/min per 1.73 m2: PR, 2.83; 95% CI, 2.49–3.21), and with presence of chronic kidney disease overall (PR, 1.46; 95% CI, 1.43−1.49), diabetes mellitus (PR, 1.38; 95% CI, 1.32−1.45), peripheral vascular disease (PR, 1.34; 95% CI, 1.30−1.43), and use of spironolactone (PR, 1.48; 95% CI, 1.42−1.54). The relative importance of risk factors increased, by higher potassium level (Table S3).
Table 2.
Prevalence of Risk Factors at Time of Hyperkalemia/Index Date Among Patients With HF With Hyperkalemia and Matched Comparisons Without Hyperkalemia
| Variable | Patients With HF With First Hyperkalemia >5.0 mmol/L | Matched HF Comparisons Without Hyperkalemiaa | Matched PR (95% CI) |
|---|---|---|---|
| No. of patients | 12 340 (100) | 12 151 (100) | … |
| Female sex | 5670 (45.9) | 5581 (45.9) | … |
| Age, median (quartiles), y | 78.6 (70.2–85.1) | 78.7 (70.6–85.0) | … |
| Potassium tests 6 mo before hyperkalemia/index date, median (quartiles) | 5.0 (2.0–11.0) | 2.0 (0.0–6.0) | … |
| Acute HF hospitalizations 6 mo before hyperkalemia/index date, median (quartiles) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | … |
| Conditions underlying HFb | |||
| Cardiomyopathy | 925 (7.5) | 806 (6.6) | 1.13 (1.03–1.24) |
| Valvular heart disease | 2182 (17.7) | 1754 (14.4) | 1.22 (1.16–1.30) |
| Myocardial infarction | 3551 (28.8) | 3236 (26.6) | 1.08 (1.0–1.13) |
| Any ischemic heart disease | 5796 (47.0) | 5268 (43.4) | 1.08 (1.05–1.11) |
| Atrial fibrillation | 4843 (39.2) | 4679 (38.5) | 1.02 (0.99–1.05) |
| Lowest eGFR measured before hyperkalemia/index date | |||
| No values <60 mL/min per 1.73 m2 | 1636 (13.3) | 2530 (20.8) | 0.64 (0.60–0.67) |
| eGFR 45–59 mL/min per 1.73 m2 | 2502 (20.3) | 3062 (25.2) | 0.80 (0.77–0.84) |
| eGFR 30–44 mL/min per 1.73 m2 | 3702 (30.0) | 2633 (21.7) | 1.38 (1.33–1.45) |
| eGFR 15–29 mL/min per 1.73 m2 | 3119 (25.3) | 1498 (12.3) | 2.05 (1.94–2.17) |
| eGFR <15 mL/min per 1.73 m2 | 896 (7.3) | 312 (2.6) | 2.83 (2.49–3.21) |
| Dialysis | 235 (1.9) | 73 (0.6) | 3.17 (2.44–4.12) |
| No eGFR measurement recorded | 250 (2.0) | 2043 (16.8) | 0.12 (0.11–0.14) |
| Selected predefined risk factors for hyperkalemia | |||
| Diabetes mellitus | 3425 (27.8) | 2440 (20.1) | 1.38 (1.32–1.45) |
| Chronic kidney diseasec | 8139 (66.0) | 5489 (45.2) | 1.46 (1.43–1.49) |
| Hypertension | 10 180 (82.5) | 9454 (77.8) | 1.06 (1.05–1.07) |
| Other comorbidities | |||
| Peripheral vascular disease | 2050 (16.6) | 1503 (12.4) | 1.34 (1.3–1.43) |
| Cerebrovascular disease | 2483 (20.1) | 2333 (19.2) | 1.05 (1.0–1.10) |
| Chronic pulmonary disease | 3327 (27.0) | 2797 (23.0) | 1.17 (1.1–1.22) |
| Peptic ulcer disease | 1483 (12.0) | 1214 (10.0) | 1.20 (1.1–1.29) |
| Any malignant disease | 2143 (17.4) | 1881 (15.5) | 1.12 (1.06–1.19) |
| Alcoholism‐related disorders | 1193 (9.7) | 937 (7.7) | 1.25 (1.16–1.36) |
| Medical obesity | 1110 (9.0) | 881 (7.3) | 1.24 (1.14–1.35) |
| Use of hyperkalemia‐associated medications | |||
| ACEis overall | 5580 (45.2) | 5113 (42.1) | 1.07 (1.04–1.11) |
| Ramipril | 2700 (21.9) | 2337 (19.2) | 1.14 (1.08–1.20) |
| Enalapril | 1284 (10.4) | 1059 (8.7) | 1.19 (1.10–1.29) |
| Other ACEis | 1624 (13.2) | 1717 (14.1) | 0.93 (0.87–0.99) |
| ACEi/diuretic combination | 306 (2.5) | 299 (2.5) | 1.01 (0.86–1.18) |
| ARBs overall | 1779 (14.4) | 1663 (13.7) | 1.05 (0.99–1.12) |
| Losartan | 775 (6.3) | 794 (6.5) | 0.96 (0.87–1.06) |
| Candesartan | 414 (3.4) | 297 (2.4) | 1.37 (1.19–1.59) |
| Other ARBs | 308 (2.5) | 252 (2.1) | 1.20 (1.02–1.42) |
| ARB/diuretic combination | 425 (3.4) | 468 (3.9) | 0.89 (0.79–1.02) |
| Spironolactone | 4125 (33.4) | 2753 (22.7) | 1.48 (1.42–1.54) |
| Potassium supplements | 6229 (50.5) | 6100 (50.2) | 1.01 (0.98–1.03) |
| Loop diuretics | 8130 (65.9) | 7683 (63.2) | 1.04 (1.02–1.06) |
| NSAIDs | 2727 (22.1) | 2705 (22.3) | 0.99 (0.95–1.04) |
| Trimethoprim | 428 (3.5) | 371 (3.1) | 1.14 (0.99–1.30) |
| Macrolides | 1598 (12.9) | 1413 (11.6) | 1.11 (1.04–1.19) |
Data are given as number (percentage) unless otherwise indicated. Percentages are based on the first row. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor II blocker; CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; NSAIDS, nonsteroidal anti‐inflammatory drugs; PR, prevalence ratio.
HF comparison patients without hyperkalemia matched on age, sex, and duration of HF on the hyperkalemia/index date.
As present at the time of HF diagnosis.
Manifest chronic kidney disease was defined on the date of the second of 2 measurements >90 days apart of a creatinine value corresponding to an eGFR <60 mL/min per 1.73 m2 or on the first date of a hospital diagnosis of chronic kidney disease (see Table S1 for codes).
Clinical Outcomes of Hyperkalemia
In patients with hyperkalemia, the incidence proportion with any acute‐care hospitalization was 53% within 6 months before the hyperkalemia event, increasing to 74% 6 months after the hyperkalemia event, corresponding to a competing risk of death adjusted before‐after RR of 1.41 (95% CI, 1.38−1.44) (Figure 3). The risk for any cardiac hospital diagnosis increased from 44% to 61% (before‐after RR, 1.46; 95% CI, 1.43−1.50), for HF hospitalization from 33% to 43% (RR, 1.42; 95% CI, 1.37–1.46), for ventricular arrhythmias from 2.4% to 3.6% (RR, 1.90; 95% CI, 1.65−2.18), for intensive care unit admissions from 3.3% to 14.9% (RR, 5.29; 95% CI, 4.77−5.86), for ventilator therapy from 1.3% to 8.0% (RR, 7.17; 95% CI, 6.10–8.43), and for cardiac arrest from 0.2% to 1.3% (RR, 9.89; 95% CI, 6.17–15.84). Six‐month mortality after hyperkalemia was 36%. Among patients who survived after hyperkalemia, discontinuation of hyperkalemia‐associated drugs was rarely seen (eg, adjusted RRs were 1.02, 1.11, and 1.10 for ARBs, ACEis, and spironolactone, yet 0.90 for potassium supplements) (Figure 3). Reductions in average defined daily doses were rarely observed in those who continued drugs (eg, for ACEis, 2.0 and 1.9 defined daily doses before and after; for ARBs, 1.4 defined daily doses both before and after; data not shown).
Figure 3.
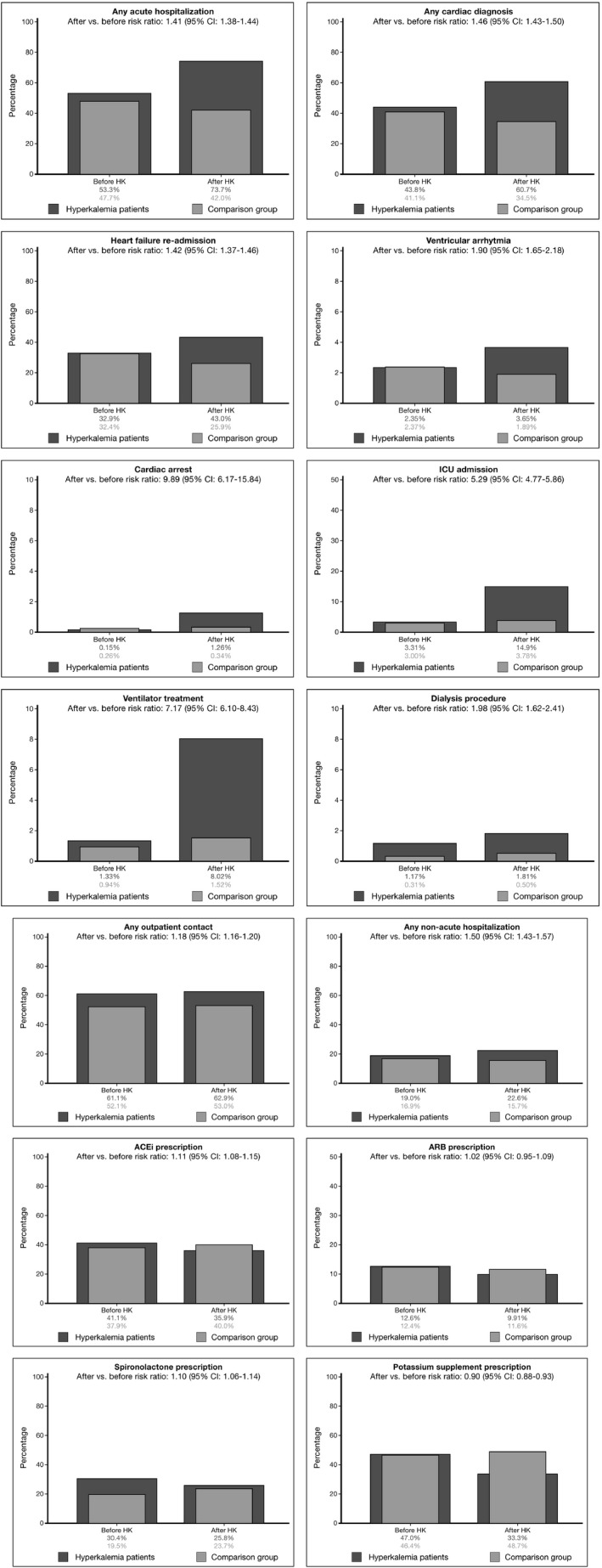
Clinical outcomes before and after hyperkalemia (HK). Dark gray bars show outcomes 6 months before and after the HK date in patients with heart failure with HK. Corresponding after vs before risk ratios are shown, adjusted for competing risk of death after HK. Light gray bars show outcomes in age‐, sex‐, and heart failure duration–matched patients with heart failure without HK as a point of comparison. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor II blocker; CI, confidence interval; ICU, intensive care unit.
When compared with matched patients without hyperkalemia, and adjusting for the prior event increased HRs, 6‐month HRs for clinical outcomes after the index date were higher in patients with hyperkalemia: 2.3‐fold higher for any acute‐care hospitalization, 2.3‐fold higher for ventricular arrhythmias, 4.4‐fold higher for intensive care unit admission, and 7.3‐fold higher for cardiac arrest. The 6‐month adjusted HR of death was 3.39 (95% CI, 3.19–3.61) (Table 3). Outcome RRs were generally higher for more pronounced potassium elevations (Table S4).
Table 3.
HRs for Clinical Outcomes 6 Months After Hyperkalemia Versus Fully Matched Comparisons Without Hyperkalemia
| Outcome | Patients With HF With First Hyperkalemia >5.0 mmol/L | Matched HF Comparisons Without Hyperkalemiaa | Fully Adjusted HR (95% CI)b | Prior Event Rate Ratio Adjusted HR (95% CI)c |
|---|---|---|---|---|
| n (Rate per 1000 Person‐Years) | n (Rate per 1000 Person‐Years) | |||
| Any hospital outpatient contact | 7760 (3204.49) | 6444 (1717.40) | 1.64 (1.58–1.70) | 1.39 (1.34–1.46) |
| Any acute‐care hospitalization | 9100 (4499.29) | 5101 (1261.17) | 2.75 (2.65–2.85) | 2.26 (2.17–2.35) |
| Any non–acute‐care hospitalization | 2786 (778.41) | 1913 (381.40) | 1.93 (1.81–2.06) | 1.65 (1.53–1.78) |
| Any cardiac diagnosis | 7492 (3017.17) | 4198 (980.49) | 2.47 (2.37–2.58) | 2.19 (2.09–2.29) |
| Ventricular arrhythmia | 450 (104.85) | 230 (41.67) | 2.27 (1.91–2.69) | 2.33 (1.88–2.88) |
| Cardiac arrest | 156 (35.42) | 41 (7.35) | 5.34 (3.70–7.73) | 7.30 (3.55–16.01) |
| Dialysis procedure | 223 (51.06) | 61 (10.95) | 1.75 (1.30–2.35) | 1.18 (0.85–1.60) |
| Ventilator treatment | 990 (233.90) | 185 (33.38) | 6.80 (5.77–8.02) | 4.46 (3.29–5.86) |
| ICU admission | 1837 (460.02) | 459 (83.93) | 5.12 (4.60–5.71) | 4.42 (3.80–5.29) |
| HF readmission | 5309 (1718.65) | 3147 (692.21) | 2.15 (2.05–2.26) | 1.96 (1.88–2.05) |
| ACEi prescription | 4430 (1572.70) | 4863 (1311.79) | 1.05 (1.01–1.10) | 0.99 (0.95–1.02) |
| ARB prescription | 1223 (305.03) | 1406 (276.36) | 1.00 (0.92–1.09) | 1.03 (0.97–1.09) |
| Spironolactone prescription | 3182 (943.22) | 2876 (625.89) | 1.07 (1.02–1.13) | 0.84 (0.81–0.88) |
| Potassium supplement prescription | 4109 (1326.70) | 5919 (1713.53) | 0.81 (0.78–0.84) | 0.75 (0.72–0.78) |
| Death | 4457 (1006.59) | 1542 (276.03) | 3.39 (3.19–3.61) | – |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor II blocker; CI, confidence interval; HF, heart failure; HR, hazard ratio; ICU, intensive care unit.
HF comparisons without hyperkalemia individually matched to patients with HF with hyperkalemia on age, sex, and HF duration (see Statistical Analysis section).
Adjusted for age, sex, and HF duration by matched design and, by Cox regression analyses, for HF treatment regimen, number of acute HF hospitalizations 6 months before the hyperkalemia/index date, estimated glomerular filtration rate category, Charlson Comorbidity Index score, presence of diabetes mellitus/chronic kidney disease/hypertension, use of ACEis/ARBs, spironolactone, or potassium supplements.
The prior event rate ratio adjusted HR is the ratio of the 2 age, sex, and HF duration matched rate ratios observed 6 months after vs 6 months before the hyperkalemia/index date. (see Additional and Sensitivity Analyses section).
When restricted to the subcohort of patients with chronic HF, incidences of hyperkalemia increased less steeply during early follow‐up, but total hyperkalemia incidence rates were close to those in all patients with HF, with similar risk factors, whereas outcome RRs after hyperkalemia were less high (Tables S5 through S7). When repeating analyses using 5 random potassium tests per person (Tables S8 through S10), incidences of hyperkalemia were modestly reduced versus the main analysis (1‐year cumulative incidence decreased from 25.2% to 20.9%), risk factor estimates were similar, and outcome associations with hyperkalemia were weaker (eg, adjusted HR for acute‐care hospitalization, 1.99 [main analysis, 2.75]; adjusted HR for intensive care unit, 3.18 [main analysis, 5.12)]. When repeating analyses for those with initial HF as primary diagnosis, all results remained robust and were similar to the main analysis (Tables S11 through S13). The associations between hyperkalemia and clinical outcomes were generally stronger when potassium was measured on in‐hospital days than out‐of‐hospital days (Tables S14 and S15) and in patients with ≥10 potassium tests than in patients with <10 potassium tests (Tables S16 and S17). Finally, when quantifying the influence of potential unmeasured confounding (Figure S1), we found that to explain an adjusted HR of ≈2.0 for acute‐care hospitalizations associated with hyperkalemia, a confounder that was 4 times more frequent among patients with hyperkalemia, compared with patients without hyperkalemia, would need to increase the hazard of acute‐care hospitalization by a factor of ≥10 to explain our findings fully (if no increased hazard actually existed).
Discussion
This population‐based cohort study provides a longitudinal overview of hyperkalemia occurrence, risk factors, and prognosis in patients with incident HF in Denmark over 13 years. Approximately 1 in 4 patients with incident hospital‐diagnosed HF developed hyperkalemia within the first year, whereas risk of hyperkalemia was even higher in patients with both HF and kidney disease. Recurrent hyperkalemia episodes were common. Individuals treated with spironolactone and those with diabetes mellitus and renal failure were at particularly increased risk. Hyperkalemia was strongly associated with adverse clinical outcomes in patients with HF, including ≈1.4‐fold higher risk of acute‐care and cardiac hospitalizations compared with the time period before hyperkalemia in the same patients, and ≈2.2‐fold higher rates of these outcomes and 3.2‐fold higher mortality compared with matched patients with HF without hyperkalemia.
Our findings are consistent with previous studies, but they suggest that the real‐world burden of hyperkalemia is even greater in patients with cardiorenal syndrome than anticipated.25 In the PARADIGM‐HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) study, ≈15% in both treatment groups developed hyperkalemia within a median follow‐up of 27 months,26 whereas the risks of hyperkalemia in patients with HF were generally lower in a UK observational study11 and in several other randomized controlled trials.27 The risk of hyperkalemia was substantially higher in our study, which likely mirrors a different universal healthcare system with inclusion of all hospital contacts with HF, including also elderly, comorbid, and frail patients. Our findings indicate that the true real‐world burden of hyperkalemia is even greater than anticipated and highlight that patients with the cardiorenal syndrome are at particular high risk of hyperkalemia. In our current analysis, risk factors for hyperkalemia included chronic kidney disease, diabetes mellitus, and use of spironolactone, as corroborated by others’ findings.11, 12, 28, 29, 30
Our study adds to the literature on the prognostic impact of hyperkalemia among patients with HF. In a Danish cohort study of 2596 patients with myocardial infarction receiving loop diuretics (as a proxy for HF), all levels of elevated potassium, including slightly increased levels, predicted death from any cause (4.6–5.0 mmol/L: adjusted HR, 1.6; 5.1–5.5 mmol/L: adjusted HR, 2; >5.5 mmol/L, adjusted HR, 5.6).9 Similarly, among 7788 patients with chronic HF in the Digitalis Investigation Group trial, mild hyperkalemia was associated with ≈15% increased mortality.10 However, mild hyperkalemia was not associated with cardiovascular or HF mortality or all‐cause or cardiovascular hospitalization. Among 3900 patients admitted with acute HF in the Patients Hospitalized with acute heart failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) and Coordinating Study Evaluating Outcomes of Advising and Counseling Failure (COACH) trials, admission potassium levels of >5.0 mEq/L versus normal potassium level were associated with a 2.36‐fold increased risk of death after 6 months.30 However, the association weakened considerably and became nonsignificant after multivariate adjustment for kidney dysfunction and other predictors. Comparison with our study is difficult because we examined hyperkalemia as a risk factor for being acutely hospitalized with different conditions, and for mortality as a separate outcome, rather than studying hyperkalemia as a prognostic factor among patients already acutely hospitalized with HF. Our findings extend current knowledge, suggesting that elevated potassium levels >5.0 mmol/L may be associated with higher risk of cardiovascular hospitalizations and arrhythmias in patients with HF.
Although most current guidelines for HF recommend clinicians to consider stopping or reducing dose of ACEi/ARB or potassium‐sparing diuretics if potassium increases >5.5 mmol/L,15 the proportion of users and the dosing of these drugs remained broadly unchanged after the initial hyperkalemia event. Potential explanations may include that some patients had potassium levels <5.5 mmol/L or that some patients had transient elevated potassium levels not requiring cessation of treatment.
Any conclusions on causal mechanisms in our study should be made with caution. Hyperkalemia measured in association with acute‐care hospitalization may be a consequence of the condition leading to hospital contact (eg, infection, dehydration, or deteriorating kidney function), rather than causing the hospitalization. The exact order of events in the pathophysiological pathway leading to a acute‐care hospitalization is difficult to disentangle. It is impossible to determine in our registry‐based study, in which patients with hyperkalemia did not have any bearing on the acute‐care hospitalization and its course. However, the findings that outcome RRs were higher for more severe versus mild hyperkalemia events and that specific diagnoses, such as cardiac arrhythmia or cardiac arrest, were associated with high levels of potassium argue for a causal association.
Interestingly, the results of our analysis were consistent in a subset of patients with chronic HF receiving ACEi/ARB blockade and β blockers in whom ≈40% had chronic kidney disease (eGFR <45 mL/min per 1.73 m2). This indicates that careful surveillance of potassium levels is important in patients with chronic HF, and future studies should address the role for novel prevention and treatment strategies.
Our real‐world observational study of the burden of hyperkalemia in patients with HF has both strengths and weaknesses. The Danish public healthcare system permitted a population‐based design with inclusion of all patients with incident HF and subsequent incident hyperkalemia in a well‐defined geographical region. This largely eliminated some of the selection problems encountered in clinic‐based or health insurance–based observational studies.
The validity of our findings depends on the completeness and quality of registry data. We relied on potassium and other tests ordered in everyday clinical practice (ie, by medical indication for measurement). Sicker patients may have had a higher likelihood of any hyperkalemia being detected because of higher frequency of testing, leading to potential overestimation of hyperkalemia incidence and possibly also its clinical consequences. However, our patients all had in‐hospital–diagnosed HF and are all likely to receive regular blood tests in a universally covering healthcare system, as demonstrated by the high average number of potassium tests observed. Moreover, we were able to control for several HF severity markers and other prognostic predictors. Our registry data did not allow for further adjustment for potential differences in left ventricular ejection fraction or New York Heart Association class.
Hyperkalemia incidence and adverse outcomes decreased modestly but remained high when only examining several random potassium tests per person. Moreover, associations between hyperkalemia and clinical outcomes were generally stronger, not weaker, when focusing on people with many potassium tests, arguing against potassium elevations being casual findings. The less severe outcome observed with hyperkalemia measured on out‐of‐hospital versus in‐hospital days is more difficult to interpret. Although this might suggest that “hyperkalemia in primary care” is a less severe condition, our analysis immanently selects for patients who are not sick enough to be hospitalized on the hyperkalemia index day (unfortunately, we had only data on hyperkalemia test dates, not on the caregiver requesting the test). Finally, methods for blood potassium analysis have not been uniform in all laboratories over the entire study period, including both serum‐ and plasma‐based tests over the years, which is an inevitable limitation. False‐positive hyperkalemia was presumably rare because all laboratories took various precautions to avoid falsely elevated potassium values, which included nonreporting of potassium values in the presence of hemolysis.
The validity of medical diagnoses and disease algorithms that we used in our study has been documented as high.21 Data on diagnoses set by general practitioners were not available, only general practitioner prescribed drugs, and our data thus may have been incomplete for less severe lifestyle‐treated conditions followed up in primary care. Because all reimbursed prescriptions have to be redeemed at pharmacies in Denmark, obtaining drugs from other sources is unlikely. Nonetheless, prescription redemption is only a marker of consumption of any drug and noncompliance is possible.
Conclusion
In this study, a substantial proportion of patients with HF developed hyperkalemia, and recurrences were common. In particular, those with kidney disease and those treated with spironolactone were at high risk. Hyperkalemia was associated with severe clinical outcomes and death in patients with HF.
Sources of Funding
This work was supported by a research grant from AstraZeneca to Aarhus University and by the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation.
Disclosures
Hasvold and Garcia‐Sanchez are employees of AstraZeneca. Thomsen, Nicolaisen, Pedersen, Adelborg, Egfjord, Egstrup, and Sørensen have reported no personal conflicts of interest relevant to this article. The Department of Clinical Epidemiology is, however, involved in studies with funding from various companies as research grants to (and administered by) Aarhus University, including the present study.
Supporting information
Table S1. Codes Used to Identify Study Variables
Table S2. Baseline Characteristics of Patients With Incident Heart Failure in Northern Denmark, 2000–2012. Incidences of Any (>5.0 mmol/L), Moderate (>5.5 mmol/L), and Severe (>6.0 mmol/L) Hyperkalemia (HK) Events are Shown According to Each Characteristic
Table S3. Prevalence and Prevalence Ratios (PR) for Risk Factors Prior to the Hyperkalemia/Index Date Among Heart Failure Patients With Hyperkalemia Versus Matched Comparison Patients Divided in Moderate (>5.5 mmol/L) and Severe (>6.0 mmol/L) Hyperkalemia
Table S4. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK Separate for Moderate and (>5.5 mmol/L) and Severe (>6.0 mmol/L) HK
Table S5. Chronic Heart Failure: Baseline Characteristics Among Patients With First Hospital Diagnosed Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S6. Chronic Heart Failure: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S7. Chronic Heart Failure. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK
Table S8. Restricted Analysis to Patients With 5 K+ Tests: Baseline Characteristics Among Patients With First Hospital Diagnosed Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S9. Restricted Analysis to Patients With 5 K+ Tests: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S10. Restricted Analysis to Patients With 5 K+ Tests: Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK
Table S11. Primary Heart Failure Diagnosis: Baseline Characteristics Among Patients With a First Primary Hospital Diagnosis of Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S12. Primary Heart Failure Diagnosis: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S13. Primary Heart Failure Diagnosis: Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK
Table S14. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK, Restricted to Potassium Measured In‐Hospital
Table S15. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Potassium Measured in the Primary Health Care Sector
Table S16. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Patients With Less Than 10 Potassium Tests
Table S17. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Patients With 10 or More Potassium Tests
Figure S1. Required Strength of An Unmeasured Confounder to Explain Our Associations Assuming That 50% of the Heart Failure Population Had Hyperkalemia and that the Prevalence of the Unmeasured Confounder was 25%. *HR indicates hazard ratio for the association between hyperkalemia and the different outcomes. For example, to explain an adjusted HR of ≈2.0 (brown line) for acute hospitalization associated with hyperkalemia, a confounder that is four times more frequent among hyperkalemia than non‐hyperkalemia patients would need to increase the hazard of acute hospitalization by a factor of 10 or more to explain our findings fully, if no increased hazard actually existed.
(J Am Heart Assoc. 2018;7:e008912 DOI: 10.1161/JAHA.118.008912.)29789332
References
- 1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp‐Pedersen C, Andersson C. Age‐specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Sørensen HT. Thirty‐year trends in heart failure hospitalization and mortality rates and the prognostic impact of co‐morbidity: a Danish nationwide cohort study. Eur J Heart Fail. 2016;18:490–499. [DOI] [PubMed] [Google Scholar]
- 4. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway R, Creagh D, Byrne DG, O'Riordan D, Silke B. Serum potassium levels as an outcome determinant in acute medical admissions. Clin Med (Lond). 2015;15:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128:1281–1287. [DOI] [PubMed] [Google Scholar]
- 8. Ingelfinger JR. A new era for the treatment of hyperkalemia? N Engl J Med. 2015;372:275–277. [DOI] [PubMed] [Google Scholar]
- 9. Krogager ML, Eggers‐Kaas L, Aasbjerg K, Mortensen RN, Køber L, Gislason G, Torp‐Pedersen C, Søgaard P. acute‐care mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2015;1:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed MI, Ekundayo OJ, Mujib M, Campbell RC, Sanders PW, Pitt B, Perry GJ, Bakris G, Aban I, Love TE, Aronow WS, Ahmed A. Mild hyperkalemia and outcomes in chronic heart failure: a propensity matched study. Int J Cardiol. 2010;144:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michel A, Martín‐Pérez M, Ruigómez A, García Rodríguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case‐control study in UK general practice. Eur J Heart Fail. 2015;17:205–213. [DOI] [PubMed] [Google Scholar]
- 12. Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long‐term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real‐life data: a population‐ and insurance‐based cohort. Pharmacoepidemiol Drug Saf. 2015;24:406–413. [DOI] [PubMed] [Google Scholar]
- 13. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; Acute Dialysis Quality Initiative (ADQI) consensus group . Cardio‐renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 16. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin‐angiotensin‐aldosterone system. N Engl J Med. 2004;351:585–592. [DOI] [PubMed] [Google Scholar]
- 17. Packham DK, Rasmussen HS, Lavin PT, El‐Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. [DOI] [PubMed] [Google Scholar]
- 18. Grann AF, Erichsen R, Nielsen AG, Frøslev T, Thomsen RW. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pottegård A, Schmidt SA, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46:798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denburg MR, Haynes K, Shults J, Lewis JD, Leonard MB. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20:1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: population based study. BMJ. 2016;355:i5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. [DOI] [PubMed] [Google Scholar]
- 25. Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 27. Desai AS. Hyperkalemia in patients with heart failure: incidence, prevalence, and management. Curr Heart Fail Rep. 2009;6:272–280. [DOI] [PubMed] [Google Scholar]
- 28. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD; Randomized Aldactone Evaluation Study (RALES) Investigators . Incidence, predictors, and outcomes related to hypo‐ and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7:573–579. [DOI] [PubMed] [Google Scholar]
- 29. Khan SS, Campia U, Chioncel O, Zannad F, Rossignol P, Maggioni AP, Swedberg K, Konstam MA, Senni M, Nodari S, Vaduganathan M, Subacius H, Butler J, Gheorghiade M; EVEREST Trial Investigators . Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115:790–796. [DOI] [PubMed] [Google Scholar]
- 30. Tromp J, Ter Maaten JM, Damman K, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017;119:290–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Codes Used to Identify Study Variables
Table S2. Baseline Characteristics of Patients With Incident Heart Failure in Northern Denmark, 2000–2012. Incidences of Any (>5.0 mmol/L), Moderate (>5.5 mmol/L), and Severe (>6.0 mmol/L) Hyperkalemia (HK) Events are Shown According to Each Characteristic
Table S3. Prevalence and Prevalence Ratios (PR) for Risk Factors Prior to the Hyperkalemia/Index Date Among Heart Failure Patients With Hyperkalemia Versus Matched Comparison Patients Divided in Moderate (>5.5 mmol/L) and Severe (>6.0 mmol/L) Hyperkalemia
Table S4. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK Separate for Moderate and (>5.5 mmol/L) and Severe (>6.0 mmol/L) HK
Table S5. Chronic Heart Failure: Baseline Characteristics Among Patients With First Hospital Diagnosed Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S6. Chronic Heart Failure: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S7. Chronic Heart Failure. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK
Table S8. Restricted Analysis to Patients With 5 K+ Tests: Baseline Characteristics Among Patients With First Hospital Diagnosed Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S9. Restricted Analysis to Patients With 5 K+ Tests: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S10. Restricted Analysis to Patients With 5 K+ Tests: Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK
Table S11. Primary Heart Failure Diagnosis: Baseline Characteristics Among Patients With a First Primary Hospital Diagnosis of Heart Failure, Stratified By eGFR Category and Subsequent Incidence of Hyperkalemia
Table S12. Primary Heart Failure Diagnosis: Prevalence of Risk Factors At Time of Hyperkalemia/Index Date Among Heart Failure Patients and Matched Comparisons Without Hyperkalemia
Table S13. Primary Heart Failure Diagnosis: Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK
Table S14. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) vs Fully Matched Comparisons Without HK, Restricted to Potassium Measured In‐Hospital
Table S15. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Potassium Measured in the Primary Health Care Sector
Table S16. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Patients With Less Than 10 Potassium Tests
Table S17. Hazard Ratios for Clinical Outcomes 6 Months After Hyperkalemia (HK) Versus Fully Matched Comparisons Without HK, Restricted to Patients With 10 or More Potassium Tests
Figure S1. Required Strength of An Unmeasured Confounder to Explain Our Associations Assuming That 50% of the Heart Failure Population Had Hyperkalemia and that the Prevalence of the Unmeasured Confounder was 25%. *HR indicates hazard ratio for the association between hyperkalemia and the different outcomes. For example, to explain an adjusted HR of ≈2.0 (brown line) for acute hospitalization associated with hyperkalemia, a confounder that is four times more frequent among hyperkalemia than non‐hyperkalemia patients would need to increase the hazard of acute hospitalization by a factor of 10 or more to explain our findings fully, if no increased hazard actually existed.


