Abstract
Background
Both the adrenergic and renin‐angiotensin systems contribute to orthostatic circulatory homeostasis, which is impaired in postural orthostatic tachycardia syndrome (POTS). Activating autoantibodies to the α1‐adrenergic and β1/2‐adrenergic receptors have previously been found in sera from patients with POTS. We hypothesized that patients with POTS might also harbor activating autoantibodies to the angiotensin II type 1 receptor (AT1R) independently of antiadrenergic autoimmunity. This study examines a possible pathophysiological role for AT1R autoantibodies in POTS.
Methods and Results
Serum immunoglobulin G from 17 patients with POTS, 6 patients with recurrent vasovagal syncope, and 10 normal controls was analyzed for the ability to activate AT1R and alter AT1R ligand responsiveness in transfected cells in vitro. Of 17 subjects with POTS, 12 demonstrated significant AT1R antibody activity in immunoglobulin G purified from their serum. No significant AT1R antibody activity was found in the subjects with vasovagal syncope or healthy subjects. AT1R activation by POTS immunoglobulin G was specifically blocked by the AT1R blocker losartan. Moreover, POTS immunoglobulin G significantly shifted the angiotensin II dosage response curve to the right, consistent with an inhibitory effect. All subjects with POTS were positive for one or both autoantibodies to the AT1R and α1‐adrenergic receptor.
Conclusions
Most patients with POTS harbor AT1R antibody activity. This supports the concept that AT1R autoantibodies and antiadrenergic autoantibodies, acting separately or together, may exert a significant impact on the cardiovascular pathophysiological characteristics in POTS.
Keywords: angiotensin II type 1 receptor, autoimmunity, autonomic nervous system, postural orthostatic tachycardia syndrome, vasoconstriction
Subject Categories: Arrhythmias, ACE/Angiotension Receptors/Renin Angiotensin System, Translational Studies
Clinical Perspective
What Is New?
Activating autoantibodies to the posture‐related angiotensin II type 1 receptor were present in a cohort with postural orthostatic tachycardia syndrome.
Angiotensin II type 1 receptor–activating autoantibodies had a negative allosteric effect on angiotensin II action.
What Are the Clinical Implications?
These data support previous reports for an impaired responsiveness to angiotensin II in postural orthostatic tachycardia syndrome and a rationale for the beneficial impact of salt loading on blood pressure and pulse responsiveness in postural orthostatic tachycardia syndrome.
Pharmacological or physiological suppression of relevant autoantibodies may have a therapeutic benefit in postural orthostatic tachycardia syndrome.
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a multisymptomatic disorder characterized by an abnormally increased heart rate during standing.1, 2 It is frequently accompanied by other vascular and nonvascular symptoms. The syndrome affects predominantly young women (70–80%), but its cause has not been established.3, 4, 5 In 2014, our laboratory demonstrated that a marked relationship of activating autoantibodies (AAbs) to the α1‐adrenergic (α1AR) and β1/2‐adrenergic (β1/2AR) receptors existed in 14 of 14 such patients.6 A second confirmatory report demonstrated a similar AAb pattern in most of 17 additional patients with POTS; data were collected independently in Sweden and assayed in blinded manner in the same laboratory as before but using modified assay techniques for the α1AR‐AAb.7 We previously demonstrated that α1AR‐AAbs possess the orthosteric ability to directly activate the target receptor; however, they also produce an allosteric inhibition of α1AR, resulting in a net inhibitory effect on receptor responsiveness to its orthosteric ligand phenylephrine in rat resistance cremaster arteries.6 In contrast, there was an AAb‐mediated allosteric facilitation of β1/2AR responsiveness to the βAR agonist isoproterenol in transfected cells. We believe the opposing effects of these AAbs on their specific receptors may account in part for many of the cardiovascular manifestations in POTS. In the second report, we observed 4 of 17 subjects with POTS had β1/2AR‐AAbs, but their α1AR‐AAbs were not measureable.7
The renin‐angiotensin‐aldosterone system is involved in the homeostatic control of blood pressure (BP) in the upright position,8 and the angiotensin II (Ang II) type 1 receptor (AT1R) belongs to the same family of G‐protein–coupled receptors as the adrenergic receptors. We therefore hypothesized that the presence of AAbs to the AT1R could potentially contribute to the cardiovascular instability observed in POTS. For the purpose of this study, we have included patients with POTS and 2 different control groups, one being autonomic controls with classic recurrent vasovagal syncope (VVS) who previously had failed to demonstrate any known relationship to α1AR‐AAbs or β1/2AR‐AAbs, and the other being healthy subjects without a history of syncope and with normal postural hemodynamics.
Methods
The Health Insurance Portability and Accountability Act–sensitive data, including sera and proprietary protected method, cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure. All other data are available in the article.
Study Patients
Sera from 6 subjects with POTS in our original article6 from Vanderbilt University (Nashville, TN) were screened for the presence of AT1R‐AAbs. These studies had been approved by the Vanderbilt University Institutional Review Board. These positive data encouraged us to study in more detail 17 patients with POTS, 6 patients with VVS, and 10 normal controls who were obtained through the SYSTEMA (Syncope Study of Unselected Population in Malmö) in Sweden. These subjects have been studied for their α1AR‐AAb and β1/2AR‐AAb activity, as previously reported.7 Briefly, patients with POTS reported typical symptom onset at least 1 year before the index examination. The average period from onset to examination was 6±6 years; none of patients with POTS had the symptoms from birth (ie, in each case, it was an acquired syndrome). The average age at the onset was 18±6 years; and at the index examination, 24±6 years. Both the Institutional Review Board at the Oklahoma University Health Sciences Center (Oklahoma City, OK) and the Regional Ethical Review Board in Lund, Sweden, approved the study protocol. Each subject gave his or her signed informed consent.
Sample Preparation
Blood samples for the present study were obtained after overnight fasting and were from the same group, as previously described.7 Serum was separated by centrifugation and stored at −80°C before shipping of duplicate aliquots in dry ice to the laboratory in Oklahoma City. The frozen integrity of each aliquot was confirmed on arrival, and samples were immediately placed in a −80°C freezer before thawing of one aliquot for the first assay. The identity of each aliquot was blinded to the laboratory personnel until after the assays were completed. Serum immunoglobulin G (IgG) was purified using the NAb Protein A/G Spin Kit (Pierce Biotechnology).
Cell‐Based AT1R Assay
AT1R autoantibody activity was measured in AT1R‐transfected Chinese hamster ovary cells using the PathHunter eXpress β‐arrestin G‐protein–coupled receptor assay kit (DiscoveRx, Fremont, CA), as previously described.9 Briefly, 10 000 AT1R Chinese hamster ovary cells were dispensed into each well of a 96‐well culture plate and incubated for 48 hours. Assay buffer containing serum IgG (0.125–1 mg/mL) in the presence and absence of the selective AT1R blocker losartan (10 μmol/L) was then added and incubated for 90 minutes. Negative (buffer) and positive Ang II controls were included in each assay. All samples were tested in triplicate. After sample treatment, PathHunter detection reagents were added and the luminescence signal was quantified to determine β‐arrestin recruitment. The β‐arrestin values were expressed as percentage of buffer baseline to normalize the data.
Dosage response curves of Ang II (10−9–10−6 mol/L) in the absence or presence of serum IgG (0.5 mg/mL) from 4 AT1R‐AAb–positive subjects with POTS were constructed to examine the allosteric effect of IgG on the Ang II–induced response.
Complementary Analyses
In a subset of patients with POTS (13/17) and vasovagal controls, we measured plasma sodium, potassium, and creatinine, according to routine laboratory methods at the Swedish center, and renin concentration using an immunoradiometric assay (Renin III Generation; Cisbio Bioassays International, Codolet, France). These assays were performed in the Lund University (Malmö, Sweden) laboratory.
Statistical Analyses
Data were expressed as mean±SD. Group comparisons were performed using Student t test for comparison of 2 groups or 1‐way ANOVA, followed by post hoc Tukey's test, for multiple group comparisons. The positivity of bioactive autoantibodies was defined as values above the mean+2 SDs from the normal subject control group. The number of samples for the dosage responses was limited partly because of limited available sera and the consistent data, despite the number limitation. The data in all cases were symmetrical and without outliers. Statistical significance was set at P<0.05.
Results
Clinical Characteristics
The demographic and clinical characteristics for each group have been previously published.7 The mean age for the Swedish patients with POTS was not significantly different from those with VVS and normal control subjects. One Hashimoto thyroiditis case was reported in each group (ie, POTS positive and negative; P=0.5).
Clinical Laboratory Values
There was no significant difference between patients with POTS and vasovagal controls in regard to sodium (140.2±1.6 versus 139.4±2.1 mmol/L; P=0.31), potassium (3.8±0.2 versus 3.7±0.2 mmol/L; P=0.58), creatinine (76.4±10.9 versus 69.9±18.2 μmol/L; P=0.24), and renin (24.4±17.0 versus 15.7±8.6 mU/L; P=0.24) plasma levels. There was no significant difference in renin plasma levels between AT1R‐AAb–positive (n=9) and AT1R‐AAb–negative (n=4) patients (18.3±7.7 versus 34.5±27.2 mU/L; P=0.42).
Autoantibody Activity
In the pilot study, remaining sera from 6 subjects with POTS previously submitted from Vanderbilt University6 were tested for α1AR and AT1R autoantibody‐mediated contractile activity using an isolated rat cremaster arteriole assay. After the α1AR blocker prazosin was added, residual contractility in 4 subjects was suppressed by addition of the AT1R blocker losartan. These data were compatible with the copresence of circulating α1AR‐AAb and AT1R‐AAb in these patients (data not shown).
Serum IgG from the subjects with POTS and control subjects, submitted from Lund University, was examined for AT1R‐AAb activity in AT1R‐transfected Chinese hamster ovary cells. IgG from 3 of the patients with POTS was tested at 4 different concentrations to determine an optimal concentration for study. There was a significant dose effect of IgG on AT1R activation, and the maximal effect appeared to occur at ≈0.5 mg/mL of IgG (Figure 1).
Figure 1.
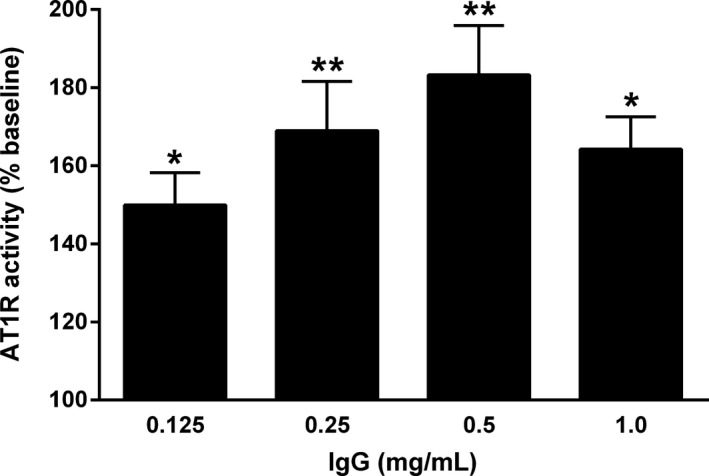
Dose effect of serum immunoglobulin G (IgG) from 3 subjects with postural orthostatic tachycardia syndrome (POTS) on angiotensin II type 1 receptor (AT1R) activation in AT1R‐transfected Chinese hamster ovary cells. Values are expressed as percentage of buffer baseline. There was a significant dosage‐dependent increase in AT1R activation with a maximal effect at 0.5 mg/mL. n=3. *P<0.05, **P<0.01 vs baseline.
IgG from all 17 patients with POTS was then tested at a concentration of 0.5 mg/mL and demonstrated a variable, but significant, capacity to activate AT1R. Twelve of these patients (70%) showed elevated AT1R‐AAb activity (Table and Figure 2A). No AT1R‐AAb activity was found in patients with VVS or healthy subjects. The mean AT1R‐AAb activity value was significantly higher in the POTS group (113.9±9.8%) than for either subjects with VVS (100.3±3.9%, P=0.001) or healthy subjects (103.6±3.2%, P=0.0007) (Figure 2B). There was no significant difference between the latter 2 groups.
Table 1.
Sex/Age, Plasma Renin Concentrations, and Autoantibody Test Positivity (Direct Activating and/or Ligand‐Modulating Activity) Among Patients With POTS
| Patient No. | Sex/Age, y | Renin, mU/L | α1AR Aba | AT1R Ab | β1AR Aba | β2AR Aba |
|---|---|---|---|---|---|---|
| 1 | F/23 | 22 | x | x | x | |
| 2 | F/29 | 30 | x | x | ||
| 3 | F/19 | 12 | x | x | x | x |
| 4 | M/34 | 14 | x | x | x | x |
| 5 | F/21 | 24 | x | x | x | |
| 6 | F/18 | 24 | x | x | x | |
| 7 | F/17 | 6 | x | x | x | |
| 8 | F/36 | 12 | x | x | x | |
| 9 | F/32 | 22 | x | x | x | |
| 10 | M/16 | ··· | x | x | x | |
| 11 | F/25 | ··· | x | x | x | x |
| 12 | F/33 | ··· | x | x | x | x |
| 13 | F/22 | 9 | x | x | ||
| 14 | F/21 | 65 | x | x | ||
| 15 | F/20 | 10 | x | |||
| 16 | F/35 | 54 | x | x | x | |
| 17 | F/20 | ··· | x | x | x | |
| Total | ··· | ··· | 13/17 | 12/17 | 13/17 | 12/17 |
α1AR indicates α1‐adrenergic receptor; β1AR, β1‐adrenergic receptor; β2AR, β2‐adrenergic receptor; Ab, antibody; AT1R, angiotensin II type 1 receptor; F, female; M, male; and POTS, postural orthostatic tachycardia syndrome.
Reproduced in part from Fedorowski et al7 with permission. Copyright ©2017, Oxford University Press.
Figure 2.
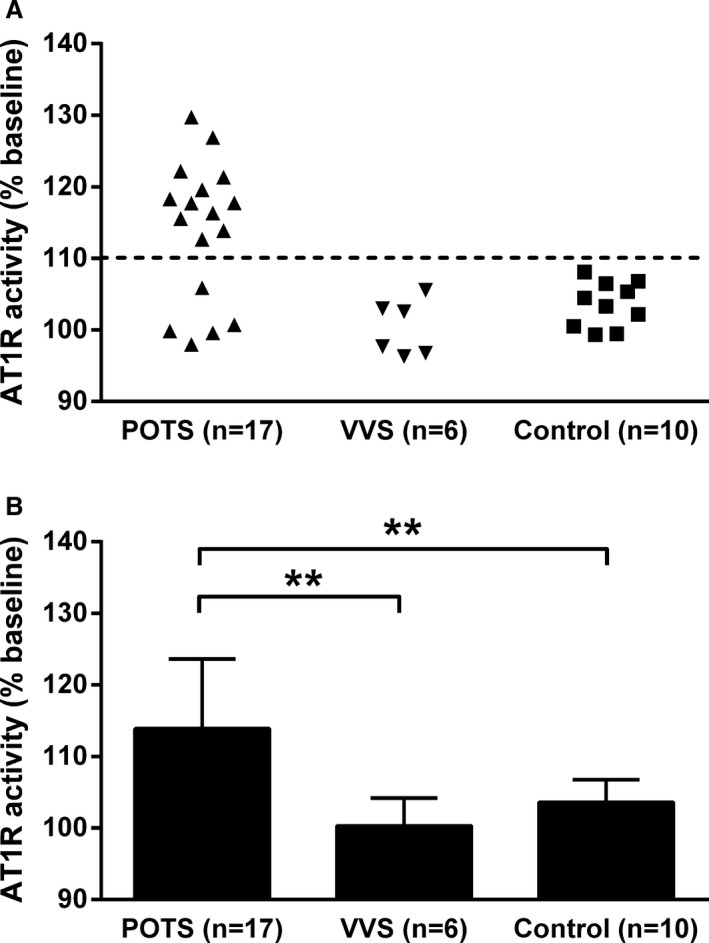
Effects of serum immunoglobulin G (IgG) from subjects with postural orthostatic tachycardia syndrome (POTS), subjects with vasovagal syncope (VVS), and healthy control subjects on activation of angiotensin II type 1 receptor (AT1R) in AT1R‐transfected Chinese hamster ovary cells. A, Most subjects with POTS demonstrated elevated AT1R antibodies, whereas none of these antibodies were found in the VVS and normal control groups. B, The mean activity values of AT1R antibodies were significantly higher in the POTS group than for both subjects with VVS and normal controls. No significant difference between the VVS and normal controls was observed. IgG was tested at a concentration of 0.5 mg/mL. The dashed line is the threshold derived from the mean activity values+2 SDs of the healthy controls. Values are expressed as percentage of buffer baseline. **P<0.01.
In the previous study, 4 of the 17 subjects with POTS did not demonstrate α1AR‐AAb activity.7 Each of these 4 subjects demonstrated significant AT1R‐AAb–mediated orthosteric activation of AT1R (Table).
The effect of the selective AT1R blocker losartan on AT1R‐AAb–induced AT1R activation in vitro was tested in 13 patients with POTS (Figure 3A). Losartan markedly suppressed AT1R‐AAb activity in IgG from the POTS group (124.0±8.2% to 111.4±3.3%, P<0.001) (Figure 3B). No significant effect of losartan was observed in IgG from the normal subject control group.
Figure 3.
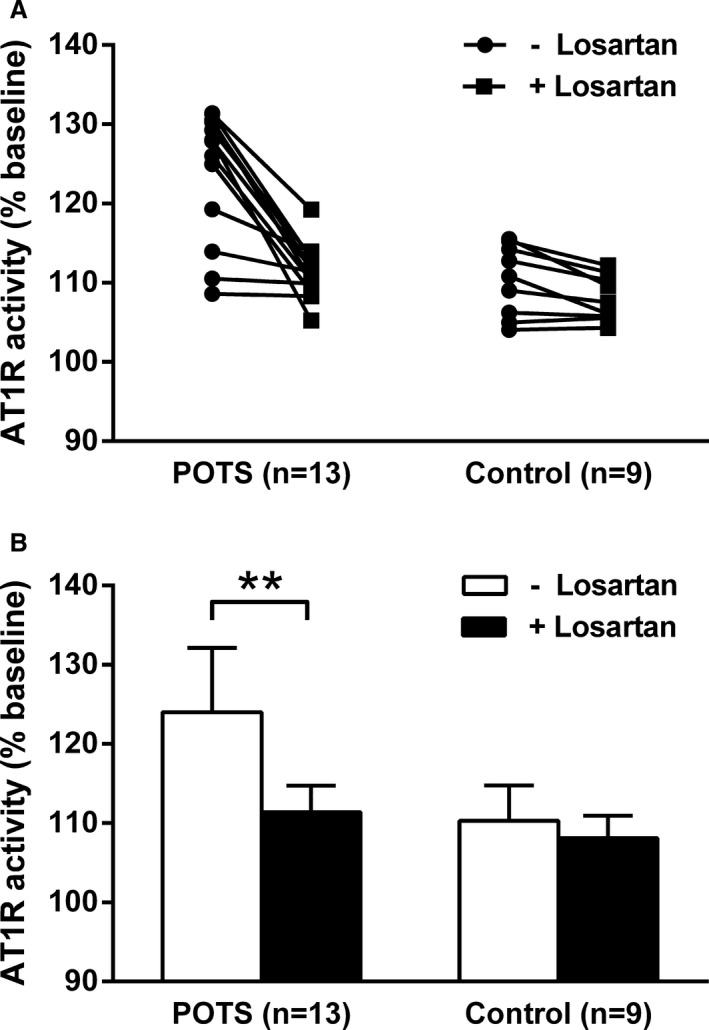
Effects of angiotensin II type 1 receptor (AT1R) blockade on immunoglobulin G–induced AT1R activation in AT1R‐transfected Chinese hamster ovary cells. A and B, The addition of the selective AT1R blocker losartan (10 μmol/L) suppressed the AT1R activity values observed in the subjects with postural orthostatic tachycardia syndrome (POTS) to control levels. Losartan produced no significant change in the AT1R activity in the control subjects. Values are expressed as percentage of buffer baseline. **P<0.01.
AT1R‐AAb Activity and Hemodynamics
There was a nonsignificant trend toward a lower supine BP in AT1R‐AAb–positive patients with POTS compared with those with AT1R‐AAb–negative POTS (118/69 mm Hg versus 130/76 mm Hg; P=0.11). There was no difference in supine heart rates (73 versus 73 beats per minute; P=0.94). During head‐up tilt testing, minimal BP was significantly lower in AT1R‐AAb–positive patients with POTS compared with AT1R‐AAb–negative patients with POTS (107/73 versus 120/83 mm Hg; P=0.043 for diastolic BP). There was no significant difference in maximal heart rate (110 versus 115 beats per minute; P=0.44).
Effect of AT1R‐AAb on Ang II Dose Response
Dosage response curves for Ang II (10−9–10−6 mol/L) with and without POTS IgG (0.5 mg/mL) were constructed and compared. IgG samples from 4 patients with POTS who were positive for AT1R‐AAb were used for curve comparisons. In the presence of POTS IgG, the Ang II response curve was significantly shifted to the right, demonstrating a decreased AT1R response (Figure 4). Control IgG showed minimal modulating effects.
Figure 4.
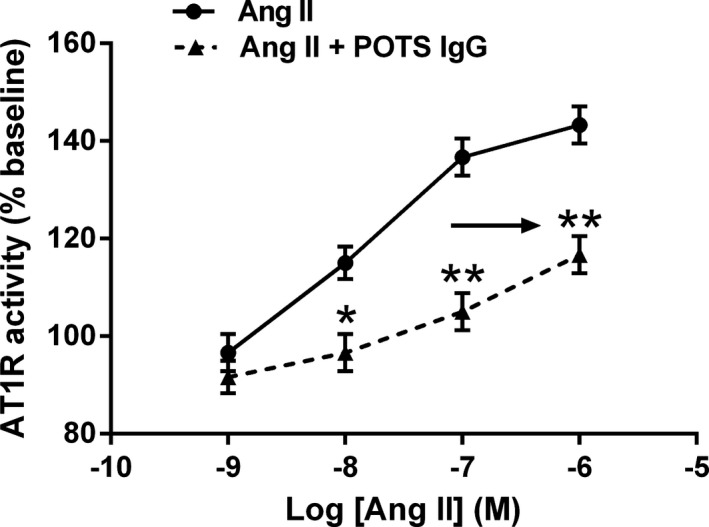
Effects of serum immunoglobulin G (IgG) from patients with postural orthostatic tachycardia syndrome (POTS) on angiotensin II (Ang II) responses in Ang II type 1 receptor (AT1R)–transfected Chinese hamster ovary cells. IgG from 4 patients with POTS with positive AT1R antibodies shifted the Ang II dosage response curve to the right, indicating an inhibitory allosteric effect. Control IgG failed to alter these curves. n=4. *P<0.05, **P<0.01 vs Ang II alone.
Discussion
We have demonstrated that most of 17 patients with POTS have circulating autoantibodies to the AT1R. These autoantibodies may exert a direct agonist effect in the absence of their normal ligands. They also demonstrated the ability to alter the orthosteric ligand response. Autoantibodies to the AT1R were not detected in the current subjects with recurrent VVS and normal controls.
We previously reported serum IgG from patients with POTS contains circulating autoantibodies, some with a direct stimulatory effect on adrenergic receptors. Most also exerted a positive allosteric effect on β1AR and a negative allosteric effect on α1AR activity. We hypothesized these and possibly other autoantibodies directed toward other receptors within the G‐protein–coupled receptor family may exert variable biological effects and explain, in part, the different constellations of symptoms presenting in patients with POTS. Although there was a significant positive correlation between β1AR‐AAb activity and the cardiac response in POTS, the expected correlation of the α1AR‐AAb activity and heart rates was present but at a lower level of significance than for the β1AR‐AAb activity.7 The absence of any apparent α1AR‐AAb activity, by either direct assay or allosteric modulation of ligand activity, in 4 of the 17 subjects with POTS clearly contributed to this apparent decrease in significance. Our present study, using IgG from these 4 α1AR‐AAb–negative subjects with POTS, demonstrated significant direct in vitro activation of AT1R. We also demonstrated significant AT1R‐AAb activity, along with α1AR‐AAb activity, in another 8 of the original 17 subjects. IgG from 4 of the patients with POTS who were positive for AT1R‐AAb showed a significant shift of their dosage response curve to the right compared with that from Ang II alone and confirmed our original report of an inhibitory allosteric impact of AT1R‐AAb on the natural receptor ligand Ang II.10 The AT1R‐AAbs, therefore, appear to act as a partial agonist in vitro.
Because the renin‐angiotensin‐aldosterone system is involved in the subacute and prolonged responses to upright posture, we believe that AT1R‐AAbs may confound normal orthostatic circulatory homeostasis. AT1R‐AAb produced in a rabbit model attenuated the Ang II–induced vasoconstriction10 and may lead to an impaired vascular response, similar to that induced by α1AR‐AAb.6, 7 In the present study, the minimal BP during passive orthostatic challenge was significantly lower in AT1R‐AAb–positive patients with POTS compared with AT1R‐AAb–negative patients with POTS. These data would support a negative impact on upright BP of this partial agonist. There was no difference in maximal heart rates between the groups, suggesting the compensatory βAR adrenergic response in the 2 groups was comparable. Renin concentration assays were performed but failed to provide any significant correlations. Unfortunately, sodium intake and/or excretion was not measured in this study and can vary widely in subjects with POTS. These differences may have variably affected the renin values and their interpretation. Serum aldosterone values were not obtained.
There are clinical circumstances to demonstrate the relative importance of the renin‐angiotensin‐aldosterone system in maintenance of normal postural homeostasis.11, 12 This relationship has been examined previously before our discovery of AT1R‐AAbs. Two groups have documented elevated Ang II levels in POTS13, 14; of these groups, one also demonstrated relatively low serum aldosterone levels.13 This group posited possible receptor problems to explain the inadequate transduction from Ang II to aldosterone. An interfering inhibitory AAb could be the missing link. Another report on AT1R‐AAb in subjects with primary aldosteronism demonstrated different effects of these AAbs on the vascular and adrenal AT1R, depending on sodium intake.9 The Vanderbilt group has also reported an impaired vascular pressor response to Ang II in patients with POTS.15
Sodium loading is frequently used to ameliorate some of the cardiovascular symptoms associated with POTS. Such salt loading would normally suppress endogenous Ang II concentrations and concurrently increase the sensitivity of vascular AT1R to their circulating autoantibodies. This would allow circulating AT1R‐AAbs to maintain (or elevate) vascular tone and moderate the impairment of cardiovascular responsiveness to upright posture mediated by α1AR‐AAbs. The variability in presentation of these 2 separate autoantibodies in complementary receptor‐driven systems that modulate cardiovascular homeostasis could partially account for the different cardiovascular responses in POTS.
The primary objective of this study was to determine if cardiovascular autoantibodies other than the α1AR‐AAb or β1/2AR‐AAb were present, with the capacity to modify their functionality for postural homeostasis. We have provided direct evidence for the copresence of AT1R‐AAbs in POTS. It would be of interest to determine in an animal model if these autoantibodies can individually or in combination reproduce the characteristic cardiovascular effects observed in POTS. We have previously documented an impact of M2/3 muscarinic receptor autoantibodies on the cardiovascular system in diverse conditions, such as idiopathic postural hypotension16, 17; for β1AR and M2 receptor autoantibodies in atrial fibrillation associated with Graves disease18; and now autoantibodies directed toward at least 3 cardiovascular receptor targets in POTS, including the α1AR, β1/2AR, and AT1R. Recent presentations by Dubey et al have provided preliminary evidence for autoantibodies to the M1/2 muscarinic receptors in POTS sera.19 These autoantibodies likely will affect cardiovascular function at their putative sites of action. Other groups have recently provided data confirming this hypothesis.20, 21, 22
POTS has challenged afflicted patients, their families, and caregivers. The variable presentation and associated autonomic disorders have led to various symptom‐based therapies, some of which have partially ameliorated the patients’ condition. However, a pathophysiologically based effective treatment against POTS is still not available. Thus, it is important to define the cause of POTS and develop a rationale for specific interventions. A high percentage of subjects with POTS examined by our group presented with an apparent autoimmune diathesis, which supports the concept that autoantibodies play an important role in POTS pathophysiological characteristics.
Although the identified AT1R‐AAbs have demonstrated in vitro activity, it will be important to establish their relevance in vivo in animal models and eventually in human subjects who harbor these suspect antibodies. Ultimately, successful removal or inactivation of the antibodies and concurrent improvement of their signs and symptoms will be needed to conclusively document their role in POTS. The marked presence of either or both AT1R‐AAbs and α1AR‐AAbs in concordance with β1/2AR‐AAbs provides a rational framework to support an autoimmune pathophysiological condition in POTS. This concept, if validated, will provide the basis for development of novel therapeutic approaches against POTS based on immunotherapy.
These data support an autoimmune relationship to the cardiovascular components of POTS. This relationship may also relate to other manifestations of this otherwise perplexing disease. Because POTS has several presentations that are similar to those observed in chronic fatigue syndrome, in several other autonomic dyscrasias, and even with some people with otherwise unexplained protean diseases, it is important that the clinician who cares for these patients be aware of possible relationships that may exist and provide important clues in our future understanding of their pathophysiological characteristics.
These data are of interest because they demonstrate that activating autoantibodies may present with differing and opposing effects on a given receptor complex. They, therefore, provide an enigma requiring the observer to determine which or whether both effects are operative. The absence of applicable animal models is also a roadblock to such studies. It appears likely that final resolution of the autoantibodies’ relative importance to the pathophysiological characteristics will depend on our ability to remove the antibodies from limited animal models and, eventually, patients with POTS and to determine whether they will permit a return to normal homeostasis.
Sources of Funding
Work at the Oklahoma site was supported in part by funding from the National Institutes of Health (R01HL128393), anonymous individual grants directed through Dysautonomia International, the American Heart Association, and an individual grant from Will and Helen Webster. Support for the Lund University site was provided by the Swedish Heart and Lung Foundation, Medical Faculty of Lund University, Region Skåne, Crafoord Foundation, and Eva and Carl‐Eric Larsson Foundation.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008351 DOI: 10.1161/JAHA.117.008351.)29618472
This work was presented in part at the Annual Meeting of the American Autonomic Society, November 2 to 5, 2016, in San Diego, CA.
References
- 1. Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117:2814–2817. [DOI] [PubMed] [Google Scholar]
- 2. Sheldon RS, Grubb BP II, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Low PA, Opfer‐Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 4. Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127:2336–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Yu X, Liles C, Khan M, Vanderlinde‐Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, Hill MA, Raj SR, Okamoto LE, Cunningham MW, Aston CE, Kem DC. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755 DOI: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, Murphy TA, Quadri SMS, Scofield RH, Sutton R, Melander O, Kem DC. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen EL, Conn JW, Rovner DR. Postural augmentation of plasma renin activity and aldosterone excretion in normal people. J Clin Invest. 1967;46:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kem DC, Li H, Velarde‐Miranda C, Liles C, Vanderlinde‐Wood M, Galloway A, Khan M, Zillner C, Benbrook A, Rao V, Gomez‐Sanchez CE, Cunningham MW, Yu X. Autoimmune mechanisms activating the angiotensin AT1 receptor in “primary” aldosteronism. J Clin Endocrinol Metab. 2014;99:1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Kem DC, Zhang L, Huang B, Liles C, Benbrook A, Gali H, Veitla V, Scherlag BJ, Cunningham MW, Yu X. Novel retro‐inverso peptide inhibitor reverses angiotensin receptor autoantibody‐induced hypertension in the rabbit. Hypertension. 2015;65:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol. 2014;5:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sassard J, Vincent M, Annat G, Bizollon CA. A kinetic study of plasma renin and aldosterone during changes of posture in man. J Clin Endocrinol Metab. 1976;42:20–27. [DOI] [PubMed] [Google Scholar]
- 13. Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011;8:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond). 2006;110:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustafa HI, Raj SR, Diedrich A, Black BK, Paranjape SY, Dupont WD, Williams GH, Biaggioni I, Robertson D. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ Arrhythm Electrophysiol. 2012;5:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu X, Stavrakis S, Hill MA, Huang S, Reim S, Li H, Khan M, Hamlett S, Cunningham MW, Kem DC. Autoantibody activation of beta‐adrenergic and muscarinic receptors contributes to an “autoimmune” orthostatic hypotension. J Am Soc Hypertens. 2012;6:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Kem DC, Reim S, Khan M, Vanderlinde‐Wood M, Zillner C, Collier D, Liles C, Hill MA, Cunningham MW, Aston CE, Yu X. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stavrakis S, Yu X, Patterson E, Huang S, Hamlett SR, Chalmers L, Pappy R, Cunningham MW, Morshed SA, Davies TF, Lazzara R, Kem DC. Activating autoantibodies to the beta‐1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker. J Clin Neuromuscul Dis. 2016;17:S‐9 (abstr). [Google Scholar]
- 20. Thieben MJ, Sandroni P, Sletten DM, Benrud‐Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK, Low PA. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. [DOI] [PubMed] [Google Scholar]
- 21. Wang XL, Chai Q, Charlesworth MC, Figueroa JJ, Low P, Shen WK, Lee HC. Autoimmunoreactive IgGs from patients with postural orthostatic tachycardia syndrome. Proteomics Clin Appl. 2012;6:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang XL, Ling TY, Charlesworth MC, Figueroa JJ, Low P, Shen WK, Lee HC. Autoimmunoreactive IgGs against cardiac lipid raft‐associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res. 2013;162:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


