Abstract
Capillary endothelial cells can be switched between growth and apoptosis by modulating their shape with the use of micropatterned adhesive islands. The present study was carried out to examine whether cytoskeletal filaments contribute to this response. Disruption of microfilaments or microtubules with the use of cytochalasin D or nocodazole, respectively, led to levels of apoptosis in capillary cells equivalent to that previously demonstrated by inducing cell rounding with the use of micropatterned culture surfaces containing small (<20 μm in diameter) circular adhesive islands coated with fibronectin. Simultaneous disruption of microfilaments and microtubules led to more pronounced cell rounding and to enhanced levels of apoptosis approaching that observed during anoikis in fully detached (suspended) cells, indicating that these two cytoskeletal filament systems can cooperate to promote cell survival. Western blot analysis revealed that the protein kinase Akt, which is known to be critical for control of cell survival became dephosphorylated during cell rounding induced by disruption of the cytoskeleton, and that this was accompanied by a decrease in bcl-2 expression as well as a subsequent increase in caspase activation. This ability of the cytoskeleton to control capillary endothelial cell survival may be important for understanding the relationship among extracellular matrix turnover, cell shape changes, and apoptosis during angiogenesis inhibition.
INTRODUCTION
Endothelial cells deprived of attachment to their extracellular matrix (ECM) substrate undergo apoptosis in vitro (Meredith et al., 1993; Brooks et al., 1994; Frisch and Francis, 1994). This process, known as anoikis, can be attributed to decreased ECM binding with associated inhibition of integrin signaling (Schwartz et al., 1991). However, cell death also can be triggered by promoting cell retraction and rounding within endothelial cells that remain adherent to ECM (Re et al., 1994; Chen et al., 1997). For example, adherent capillary cells can be induced to switch from growth to apoptosis with the use of micropatterned substrates containing micrometer-sized adhesive islands. These islands restrict the degree to which the cells can extend independently of the local density of ECM ligand or the concentration of soluble growth factors; the more retracted and round the cell, the greater the apoptotic rate (Chen et al., 1997). Capillary regression induced by angiogenesis inhibitors in vivo is also accompanied by the presence of rounded cells that die even though they remain in contact with large ECM fragments (Ingber et al., 1986).
Although many of the molecular pathways involved in adhesion-dependent survival have been well characterized, little is known about the molecular machinery that transduces a structural signal, such as that associated with cell rounding or retraction, into an apoptotic response. Cell shape is governed by the cytoskeleton that acts as a mechanical supporting framework (Ingber, 1993; Wang et al., 1993) as well as an orienting foundation for much of the cell's signal transduction machinery (Miyamoto et al., 1995; Plopper et al., 1995). Disruption of the actin cytoskeleton has been shown to induce cell rounding and inhibit cell cycle progression in capillary endothelial cells (Ingber et al., 1995; Huang et al., 1998). Mechanical coupling between microtubules and actin microfilaments also has been shown to play an important role in cell shape stability as well as control of proliferation in these cells (Wang et al., 1993; Ingber et al., 1995). Yet, it is not clear whether cytoskeletal filaments contribute to shape-dependent apoptotic control and, if they do, how they couple to relevant biochemical transduction pathways.
One possible signal-transducing molecule that may be involved in this form of apoptotic control is the serine threonine kinase, Akt/protein kinase B, which is known to promote adhesion-dependent survival in endothelial cells (Khwaja et al., 1997; Fujio and Walsh, 1999). Akt is activated by phosphatidylinositol-3 kinase (PI3K), which physically associates with the cytoskeleton within focal adhesions (Chen and Guan, 1994; Plopper et al., 1995; Downward, 1998; Gillham et al., 1999). To become activated, Akt is bound by PI3K-generated 3-phosphoinositides and translocated to the plasma membrane, where it is phosphorylated by phosphoinositide-dependent kinases PDK-1 and PDK-2 (Klippel et al., 1997; Downward, 1998). Phosphorylated Akt can down-regulate apoptotic factors, such as caspase-9 (Cardone et al., 1998) and BAD (Datta et al., 1997; Tang et al., 1999) and up-regulate survival factors, such as nitric oxide (Dimmeler et al., 1999; Fulton et al., 1999) and nuclear factor-κB (Ozes et al., 1999; Romashkova and Makarov, 1999), thereby promoting cell survival. In addition, Akt has been implicated in several cytoskeleton-mediated processes, such as regulation of actin reorganization during vascular endothelial growth factor-induced migration of endothelial cells (Morales-Ruiz et al., 2000) and signaling by the cytoskeleton-plasma membrane linker protein ezrin, which also interacts with PI3K (Gautreau et al., 1999). PTEN, a protein with homology to the cytoskeletal protein tensin, also negatively regulates Akt activity and promotes apoptosis (Stambolic et al., 1998).
Akt confers survival signals, at least in part, through phosphorylation of proapoptotic members of the bcl-2 protein family (Datta et al., 1997; Adams et al., 1998; Tang et al., 1999) or through altering bcl-2 expression (Pugazhenthi et al., 2000). Bcl-2 family members can protect against apoptosis conferred by integrins (Frisch and Francis, 1994; Zhang et al., 1995; Stromblad et al., 1996; Gilmore et al., 2000) and constituitive up-regulation of bcl-2 expression prevents endothelial cell apoptosis in a model of capillary network formation (Pollman et al., 1999). Moreover, inactivation of bcl-2 by its phosphorylation appears to mediate apoptosis induced by microtubule disruption in carcinoma cells (Haldar et al., 1997). Thus, in the present study, we set out to explore whether actin microfilaments, microtubules, Akt, and bcl-2 contribute to shape-dependent control of apoptosis in capillary endothelial cells and, if they do, whether they are part of a common pathway.
MATERIALS AND METHODS
Experimental System
Bovine capillary endothelial cells were cultured on bacteriological plastic dishes or glass coverslips that were precoated with a saturating density (1 μg/cm2) of fibronectin (Collaborative Research, Bedford, MA) in carbonate buffer, as described (Ingber, 1990). Gold-coated glass slides containing 10-μm-diameter circular islands prepared by microcontact printing (Chen et al., 1997,1998) were coated with a similar saturating density of fibronectin in phosphate-buffered saline (PBS). Nonspecific attachment sites were blocked with 1% bovine serum albumin in DMEM for 1 h at 37°C before use. The cells were dissociated into single cells by brief exposure to trypsin-EDTA, washed in 1% bovine serum albumin/DMEM, and resuspended in chemically defined medium (DMEM, 10 μg/ml high-density lipoprotein, 5 μg/ml transferrin, and 5 ng/ml basic fibroblast growth factor). Cell suspensions were incubated with or without cytochalasin D (Cyto D, 1 μg/ml; Sigma, St. Louis, MO), nocodazole (Noc, 10 μg/ml; Sigma), butanedione monoxime (BDM, 10 mM; Sigma), rho kinase inhibitor (Y27632; 20 μM; kindly provided by Welfide, Osaka, Japan), or wortmannin (100 nM; Sigma) for 15 min before plating as well as during subsequent incubations. Wortmannin was also added to the cells every 4 h to ensure continued activity during the entire time course of the experiment. Approximately 1–10 × 105 cells were either plated onto dishes coated with fibronectin or maintained in suspension in 2% methylcellulose (supplemented with chemically defined medium) at 37°C for the indicated times. For caspase activity assays, cells were allowed to spread overnight before treatment with drugs. For caspase inhibition, cell monolayers were pretreated with the caspase inhibitor z-VAD.fmk (100 μM; Calbiochem, San Diego, CA) for 1 h at 37°C before the start of the experiment, as well as after replating. Importantly, all of the cytoskeletal modifiers induce optimal effects on the cytoskeleton within 10–15 min after addition in our cells and thus, the cytoskeletal modifications preceded the time zero point measured in all experiments on Akt, Bcl-2, caspase activation, and apoptosis. The specificity of these cytoskeletal-disrupting agents has been demonstrated in past studies with the same cell type (Wang et al., 1993; Ingber et al., 1995).
Detection of Apoptosis
At the indicated times, adherent cells on coverslips and suspended cells that were collected by centrifugation were washed in PBS and fixed with 4% paraformaldehyde/PBS for 30 min at room temperature. Fixed suspended cells were immobilized by drying onto prewarmed (55°C) gelatin-coated slides. To detect apoptosis, cells were permeablized with 0.1% sodium citrate/0.1% Triton X-100 in PBS, and stained with terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) enzyme reagent (In Situ Cell Death Detection kit; Roche Molecular Biochemicals, Indianapolis, IN) and 4,6-diamidino-2-phenylindole; fluorescently labeled nuclei were counted on a Zeiss fluorescence microscope.
Measurement of Caspase Activity
Cells were washed in PBS, snap-frozen as cell pellets in liquid nitrogen, and stored at −80°C. For caspase activity analysis, the cells were thawed and lysed in ice-cold lysis buffer (ApoAlert Fluorometric Caspase-3 Activity Assay; CLONTECH, Palo Alto, CA), centrifuged, and the supernatants were transferred to a 96-well plate. Lysates were incubated for 1 h at 37°C with the caspase-3–specific fluorescent substrate DEVD-AFC (50 μM; CLONTECH) or the caspase-8-specific substrate IETD-AFC, and fluorescence was measured with the use of a fluorometric plate reader (Bio-Rad, Hercules, CA) at 380-nm excitation and 460-nm emission.
Quantitation of Akt Phosphorylation and bcl-2 Expression
Cells were washed in PBS and lysed in sample buffer containing 50 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 6% β-mercaptoethanol, and 0.1% bromophenol blue, and boiled for 10 min. Equal amounts of protein were loaded onto a 10% SDS-polyacrylamide gel and electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). The membranes were blocked in Tris-buffered saline, 0.2% Tween 20, and 5% nonfat dry milk, and probed with anti-Akt or phosphorylated Akt (Ser473) polyclonal antibodies (1:1000 dilution; New England Biolabs, Beverly, MA) followed by a horseradish peroxidase-linked anti-rabbit secondary antibody (1:4000 dilution; Vector Laboratories, Burlingame, CA); or with monoclonal antibodies directed against bcl-2 (1:250 dilution; Sigma-Genosys, The Woodlands, TX) or tubulin (clone DM1a, 1:200 dilution; Sigma), followed by a horseradish peroxidase-linked anti-mouse secondary antibody (1:4000 dilution; Vector Laboratories). Proteins were detected with the use of an enhanced chemiluminescence reagent (PerkinElmer Life Science Products, Boston, MA) by autoradiography and developed with the use of Biomax film (Eastman Kodak, Rochester, NY). Ratios of phosphorylated Akt to total Akt, and of the expression of bcl-2 relative to tubulin, were quantitated with the use of digital imaging software (Alpha Innotech, San Leandro, CA).
RESULTS
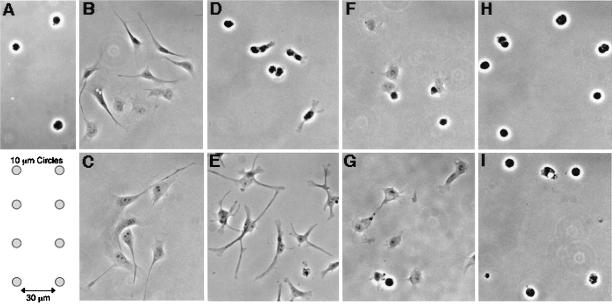
We have previously demonstrated that restricting cell spreading in capillary endothelial cells induces an apoptotic response independent of anoikis (Chen et al., 1997). For example, ∼20% of adherent capillary endothelial cells died by apoptosis when cultured on 10-μm-wide circular adhesive islands coated with fibronectin, which physically restrict cell extension (Figures 1A and 2); this is distinct from the majority (>95%) of cells that survive on fibronectin in the same medium when spreading is permitted and from the 60% of cells that undergo anoikis when completely detached from a substrate and cultured in suspension (Figure 2). To determine whether the cytoskeleton plays a role in this shape-dependent apoptotic response, we measured apoptosis within cells that were cultured on unpatterned fibronectin substrates in the absence or presence of the cytoskeleton-disrupting drugs Cyto D (1 μg/ml) and Noc (10 μg/ml), both alone and in combination. Capillary endothelial cells normally spread within 1–3 h after plating (Figure 1B) and extend even further >24 h of culture (Figure 1C) on standard fibronectin substrates. Although disrupting the actin microfilament system with Cyto D did not interfere with cell-ECM adhesion, it completely prevented spreading for at least 3 h (Figure 1D). After 24 h of continuous exposure to Cyto D, the cell bodies still remained retracted, although extension of some thin spindle-like projections could be observed (Figure 1E). Some of the cells cultured for 3 h in the presence of Noc, which disrupts microtubules, remained rounded, whereas other cells took on a partially spread, polygonal shape (Figure 1F); after 24 h, Noc-treated cells still failed to extend fully and appeared largely polygonal in form (Figure 1G). In contrast, simultaneous administration of Cyto D and Noc caused cells to remain completely rounded for the entire 24 h time course examined (Figure 1, H and I).
Figure 1.
Different degrees of cell rounding induced by growing cells on circular micropatterns or by cytoskeletal disruption. Cells were plated on fibronectin-coated adhesive islands or on fibronectin-coated coverslips in the presence of drugs, as described in MATERIALS AND METHODS. Cells maintained a round shape when grown on 10-μm-diameter circular adhesive islands for 24 h (A; diagram showing micropattern layout below). Control cells spread on fibronectin within 3 h (B) and extended further by 24 h (C). Disruption of microfilaments with the use of 1 μg/ml Cyto D (D and E), or of microtubules with the use of 10 μg/ml Noc (F and G) led to mostly rounded cells at 3 h of treatment (D and F), but only partially rounded cells after 24 h (E and G). In contrast, disruption of microfilaments and microtubules with the use of a combination of Cyto D and Noc led to sustained cell rounding up to 24 h (H and I).
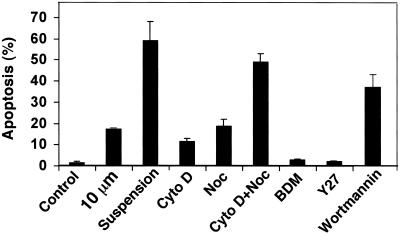
Figure 2.
Induction of apoptosis with the use of cytoskeleton-disrupting drugs. Apoptosis was detected after 24 h in cells that were plated on fibronectin-coated 10-μm circular patterns (10 μm) or on standard fibronectin-coated dishes in the absence (control) or presence of 1 μg/ml Cyto D, 10 μg/ml Noc, 10 mM BDM, 20 μM Y27632 (Y27), 100 nM wortmannin, or grown in suspension (suspension). Results are expressed as the percentage of nuclei that stained positive with the TUNEL enzyme reagent (± SEM).
Importantly, treatment of cells with Cyto D or Noc for 24 h mimicked the effect of cell shape restriction on apoptosis in that between 10 and 20% of cells exhibited apoptosis when quantitated with the use of TUNEL staining (Figure 2). Interestingly, combination of Cyto D and Noc led to an additive effect on apoptosis, with >50% of cells undergoing programmed cell death. The level of apoptosis induced by disruption of both microfilaments and microtubules approached that displayed by cells grown in suspension (∼60%) at 24 h (Figure 2). In contrast, treatment of endothelial cells with inhibitors of cytoskeletal tension generation (BDM and the rho kinase inhibitor Y27632) that do not disrupt cytoskeletal integrity or alter cell shape did not significantly alter cell apoptotic rates (Figure 2).
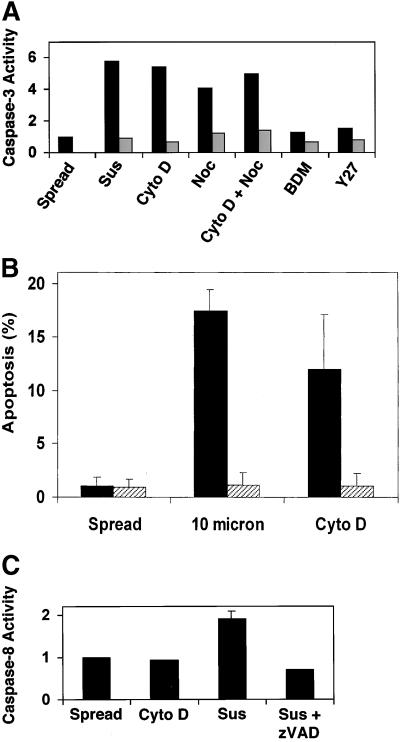
Apoptosis induced by cytoskeletal disruption and measured by TUNEL staining also was accompanied by up-regulation of caspase-3 activity, the major effector caspase responsible for cellular proteolysis associated with the apoptotic cascade (Cryns and Yuan, 1998). After 24 h of treatment with Cyto D, Noc, or Cyto D + Noc, caspase-3 activity was elevated ∼4–5-fold relative to that exhibited by control spread cells (Figure 3A). This was similar to the up-regulation of caspase-3 activity in suspended cells, even though a greater percentage of suspended cells eventually died (Figures 2 and 3A). Furthermore, shape-dependent apoptosis appeared to be caspase dependent, because the general caspase inhibitor z-VAD.fmk blocked apoptosis of cells treated with Cyto D or grown on 10-μm circles (Figure 3B). Because recently it was shown that activity of caspase-8, an initiator caspase important in fas-mediated cell death, is up-regulated in endothelial cells in suspension (Rytomaa et al., 1999), we also tested whether caspase-8 activity was increased in capillary endothelial cells after cytoskeletal disruption. In fact, caspase-8 activity did increase in our suspended endothelial cells and this increase could be inhibited with the use of z-VAD.fmk; however, we failed to detect any increase in caspase-8 activity in adherent cells treated with Cyto D (Figure 3C).
Figure 3.
Involvement of caspase activity in endothelial cell apoptosis induced by shape restriction. (A) Representative experiment showing caspase-3 activity in suspended cells (sus) or in cells on fibronectin-coated dishes without (spread) or with exposure to Cyto D (1 μg/ml), Noc (10 μg/ml), BDM (10 mM), or Y27632 (Y27; 20 μg/ml) for 24 h; similar results were obtained in two or more experiments. Black bars, untreated cells; striped bars, cells pretreated with the caspase inhibitor z-VAD.fmk (100 μM) for 1 h and then continually exposed to the inhibitor during the 24-h experiment. (B) Prevention of apoptosis in shape-restricted cells treated with a caspase inhibitor. Capillary endothelial cells were grown on 10-μm circles (10 micron) or treated with Cyto D (1 μg/ml) for 24 h in the presence (striped bars) or absence (black bars) of the caspase inhibitor z-VAD.fmk (100 μM). Apoptosis was measured as the percentage of nuclei stained positively by the TUNEL enzyme reagent. (C) Caspase-8 activity in adherent cells treated in the absence (control) or presence of Cyto D (1 μg/ml), or in cells grown in suspension without (sus) or with the caspase inhibitor, z-VAD.fmk (100 μM), for 20 h. Caspase activities are expressed as the ratio of fluorescence units between treated and control cells.
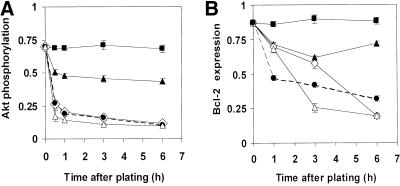
Because PI3K and phosphorylation of its downstream target Akt have been reported to confer adhesion-dependent survival signals (Khwaja et al., 1997; Fujio and Walsh, 1999), we then set out to determine whether survival signals mediated by the cytoskeleton and cell shape modulation also involve this pathway. Treatment of cells with a pharmacological inhibitor of PI3K, wortmannin (100 nM), induced apoptosis within ∼40% of adherent cells (Figure 2) and this was associated with down-regulation of Akt phosphorylation as previously demonstrated by others (King et al., 1997; Downward, 1998). Cells treated with Noc or grown in suspension also almost completely dephosphorylated their Akt, compared with spread cells, as early as 30 min after plating, and Akt phosphorylation levels continued to decrease up to 6 h after plating (Figure 4A). Treatment with Cyto D produced a smaller, but still significant decrease in Akt phosphorylation by 3 h, with phosphorylation levels being ∼50% of those exhibited by spread cells at 6 h (Figure 4A). This partial microfilament-dependent decrease in Akt phosphorylation corresponded to ∼10–15% of cells undergoing apoptosis by 24 h (Figure 2). Disruption of microtubules with Noc led to both a more marked decrease in Akt phosphorylation at 6 h (Figure 4A), and a slightly higher level of apoptosis at 24 h (Figure 2). However, although suspension produced similar suppression of Akt phosphorylation as Noc (Figure 4A), it led to ∼3 times higher levels of anoikis at 24 h (Figure 2).
Figure 4.
Effects of cytoskeletal disruption on Akt phosphorylation (A) and bcl-2 expression (B). Akt phosphorylation and bcl-2 protein levels were detected by Western blots in cells treated with Cyto D (1 μg/ml), Noc (10 μg/ml), or a combination of the two, or grown in suspension for the times indicated. Akt phosphorylation is expressed as the ratio of phosphorylated Akt to total Akt protein. Bcl-2 expression levels are presented as the ratio of bcl-2 to a control protein (α-tubulin), which did not significantly change in expression levels during the course of the experiment. ▪, control cells spread on fibronectin-coated dishes; ▴, Cyto D-treated cells; open diamonds, Noc-treated cells; ●/----, cells treated with a combination of Cyto D and Noc; ▵, suspended cells.
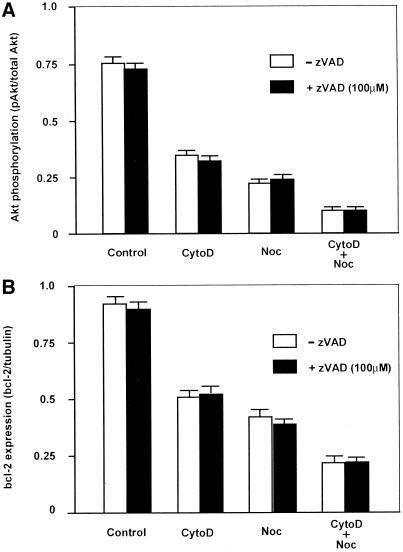
An increased ratio of bcl-2/bax is involved in survival conferred by binding of integrin αvβ3 in endothelial cells (Stromblad et al., 1996), and Akt was recently shown to regulate bcl-2 expression in neuronal PC12 cells (Pugazhenthi et al., 2000). We therefore set out to determine whether expression of bcl-2 was affected by disruption of the cytoskeleton and whether it correlated with Akt dephosphorylation in our system. Treatment of cells with Cyto D led to a small but significant decrease in bcl-2 protein levels that was sustained >6 h (Figure 4B). Treatment with Noc led to a much more marked decrease in bcl-2 expression at 6 h. Although addition of Cyto D to Noc appeared to produce a slight additive effect on bcl-2 suppression at early times (1–3 h), there was no significant difference between the combination and Noc alone at 6 h (Figure 4B). Again, Noc and suspension both produced similar effects on bcl-2 expression at 6 h (Figure 4B), even although they exhibited significantly different effects on apoptosis measured at 24 h (Figure 2). Interestingly, the inhibitory effect of Cyto D was only 20–50% of the effect of nocodazole in the cases of both Akt phosphorylation and bcl-2 expression. Furthermore, the timecourse of bcl-2 down-regulation in Noc-treated cells closely followed that of Akt dephosphorylation. The largest jump in Akt dephosphorylation was seen between 0 and 30 min, whereas bcl-2 expression decreased steadily over a 6-h period, suggesting that bcl-2 is downstream of Akt in this pathway. In contrast, caspase 3 activation was not detectable until >8 h after cytoskeletal disruption by cytochalasin D or nocodazole. Furthermore, inhibition of caspase activation with the use of the general inhibitor, zVAD, did not interfere with the effects of cytoskeletal modifiers on Akt phosphorylation or bcl-2 (Figure 5). Thus, Akt and bcl-2 appear to be upstream of caspase activation in the apoptotic cascade in bovine capillary endothelial cells, as previously observed in other cell types (Kluck et al., 1997; Cardone et a., 1998).
Figure 5.
Effects of the general caspase inhibitor, zVAD, on Akt phosphorylation (A) and bcl-2 expression (B) induced by cytoskeletal disruption. Measurements were carried out at 3 h with the use of methods and drug concentrations described in Figure 4. White bars, absence of zVAD; black bars, presence of 100 μM zVAD.
DISCUSSION
Structural cues associated with endothelial cell rounding appear to be able to induce endothelial cells to undergo apoptosis; however, the underlying mechanism is unknown. In this study, we showed that disruption of cytoskeletal microfilaments could induce both cell rounding and a concomitant increase in apoptosis analogous to that observed in cells whose spreading was restricted in the past with the use of a micropatterning technique (Chen et al., 1997). Disruption of microtubules with the use of Noc also induces apoptosis, but with relatively minor inhibition of spreading. Combination of both drugs resulted in additive effects on apoptosis, such that the cells exhibited an anoikis-like response even although they remained adherent to the ECM-coated dish. Thus, loss of both microfilament and microtubule networks rendered the cells incapable of generating the structural cues necessary for survival, whereas loss of one structural element alone merely reduced the probability for survival. These results suggest that intact cytoskeletal filaments normally cooperate to promote cell survival and that structural alterations in the cytoskeleton induced by modulating cell-ECM binding and cell shape may actively control the apoptotic signaling pathway.
These results differ from a past study (Pollman et al., 1999) in which microtubule disruption with the use of colchicine led to endothelial cell apoptosis in Matrigel where cells partially retracted and formed capillary tubes, whereas the cells did not die when treated with colchicine in a two-dimensional monolayer (Pollman et al., 1999). It is possible that microtubule disruption has different effects when cells form lateral junctional complexes or when these filaments are disrupted within preexisting monolayers that have formed other types of stabilizing structures (i.e., rather than during the spreading of single cells as in the present study). A different ECM molecule (collagen as opposed to fibronectin) was used in that study; this also may have contributed to these different effects on cell survival. In any case, our results with Noc and Cyto D clearly indicate that disruption of structural elements and cell rounding are enough to induce apoptosis within individual capillary endothelial cells cultured under defined ECM conditions. Thus, a change in cell shape that alters cytoskeletal structure can itself be a signal for apoptosis. These findings are consistent with the observation that HIV-1 vpr protein appears to prevent anoikis by promoting actin microfilament assembly (Matarrese et al., 2000) and that microtubule disassembly similarly induces apoptosis in fibroblasts (Kook et al., 2000).
Analysis of the biochemical basis of this cytoskeletal control revealed that disruption of the cytoskeleton in capillary endothelial cells mimicked the known apoptosis-inducing effects of the PI3K inhibitor wortmannin, by producing dephosphorylation of Akt, down-regulation of bcl-2 expression, and subsequent caspase activation. These findings are important because overexpression of bcl-2 has been shown to prevent endothelial cell apoptosis as well as capillary involution (Pollman et al., 1999). Akt became partially dephosphorylated upon microfilament disruption with Cyto D, and almost completely dephosphorylated upon microtubule disruption with Noc, as early as 30 min after treatment. Similarly, bcl-2 expression was partially down-regulated by Cyto D, and completely down-regulated by Noc.
Taken together, these results indicate that Akt may be an early player in the transduction of structural cues into survival signals. Akt is known to be activated by integrin signaling through focal adhesion kinase (FAK) and PI3K (Khwaja et al., 1997; King et al., 1997; Fujio and Walsh, 1999). We observed that Akt became dephosphorylated by disruption of the cytoskeleton and by inhibition of PI3K with the use of wortmannin, suggesting that PI3K may be involved in the shape- and cytoskeleton-dependent apoptotic cascade. Akt dephosphorylation induced by cytoskeletal disruption also could be related to inhibition of FAK signaling, which has been demonstrated under these conditions (Burridge and Chrzanowska-Wodnicka, 1996). Treatment of fibroblasts with Noc similarly leads to deactivation of FAK and increased apoptosis (Kook et al., 2000), whereas PTEN, which inhibits FAK, negatively regulates Akt (Tamura et al., 1999). Thus, one mechanism by which changes in cell shape may lead to endothelial cell apoptosis could be through disruption of cytoskeletal signaling within the focal adhesion complex that contains FAK as well as PI3K (Miyamoto et al., 1995; Plopper et al., 1995). Furthermore, it was shown recently that Akt is directly phosphorylated by the integrin-linked kinase, which binds to the cytoplasmic domains of integrins and prevents anoikis (Delcommenne et al., 1998); integrin-linked kinase may also become dislodged upon cytoskeletal disruption.
Recently, cell spreading and activated Rho-GTP were shown to lead to increased bcl-2 expression and prevent epithelial cell apoptosis (Fiorentini et al., 1998). We observed that bcl-2 expression was higher in spread cells than in round. Thus, it is possible that Akt provides a link between integrins, the cytoskeleton, and bcl-2 expression in providing structural context-dependent antiapoptotic signals. This mechanism may involve regulation of the bcl-2/Bax or bcl-2/BAD ratio and subsequent regulation of cytochrome c release from mitochondria (Datta et al., 1997; Kluck et al., 1997; Adams et al., 1998) or direct regulation of cytochrome c release by Akt through an as yet unknown mechanism (Kennedy et al., 1999). Interestingly, Akt also can regulate cell survival at the postmitochondrial level (Zhou et al., 2000). Our data show that at least in the case of cytoskeletal perturbation, Akt appears to act upstream of bcl-2 in the induction of apoptosis, because Akt dephosphorylation occurred earlier than bcl-2 down-regulation in our system (Figure 4). The ability of Akt to up-regulate nitric oxide production and nuclear-κB activity (Dimmeler et al., 1999; Fulton et al., 1999; Ozes et al., 1999; Romashkova and Makarov, 1999) also could contribute to its survival-promoting activity in our system.
Interestingly, the activity of caspase-3, an effector caspase, was up-regulated during shape-dependent apoptosis, whereas the activity of caspase-8, an initiator caspase involved in regulating apoptosis induced by the fas death receptor (reviewed by Cryns and Yuan, 1998) during anoikis, was not activated. Although matrix detachment results in endothelial cell susceptibility to fas-mediated cell death via caspase-8 signaling (Rytomaa et al., 1999), adherent cells are resistant (Aoudjita and Vuoria, 2001). Our finding that cells treated with cyto D that did not fully detach from the ECM substrate also did not activate caspase-8 is consistent with this work. Added involvement of the caspase-8 pathway also could account for the greater levels of apoptosis seen in suspension than in rounded cells, despite apparently similar increases in caspase-3 activity in round adherent cells versus cells in suspension. Most importantly, these results show that apoptosis induced by cell rounding or cytoskeletal disruption within cells anchored to ECM differs mechanistically from apoptosis (anoikis) that results from substrate detachment.
The past finding that cells switch between proliferation and death when their shape is varied over a wide range (Chen et al., 1997) suggests that there could be a link between growth and apoptotic signaling pathways. For example, cell cycle arrest induced by cell rounding and microfilament disruption correlates with up-regulation of the cell cycle inhibitor p27Kip1 (Huang et al., 1998). Ectopic expression of PTEN, which modulates Akt activity (Stambolic et al., 1998), also produces growth arrest through elevation of p27 Kip1 (Sun et al., 1999), and Akt has been implicated in regulation of cell proliferation in other systems (Zimmermann and Moelling, 1999). Thus, one possibility is that disruption of the actin cytoskeleton could lead to both cell cycle arrest and apoptosis through Akt-dependent up-regulation of the cell cycle inhibitor p27 Kip1. However, although Noc produced profound down-regulation of Akt in the present study, it does not induce cell cycle arrest in our capillary endothelial cells (Ingber et al., 1995). Thus, Akt dephosphorylation apparently does not cause cell cycle arrest in these cells. Furthermore, although mechanical force interactions between microtubules and microfilaments and specifically, tension generated within the actin cytoskeleton, appear to play a key role in the shape-dependent cell cycle checkpoint in capillary endothelial cells (Huang et al., 1998), pharmacological inhibitors of cytoskeletal tension generation did not increase apoptosis in this study. Apoptosis was only induced when the structural integrity of the cytoskeleton was compromised and/or cell shape was altered.
The cytoskeleton has previously been shown to be involved in the execution phase of apoptosis, by regulating membrane bleb formation (Huot et al., 1998; Mills et al., 1998b;) and release of apoptosis-inducing phosphatases from disassembled microtubules (Mills et al., 1998b). Actin and the actin-associated protein gelsolin have also been shown to be cleaved during apoptosis, leading to the release of DNase 1 and subsequent DNA cleavage (Kayalar et al., 1996; Kothakota et al., 1997). However, the results of the present study go further and suggest that adhesion-dependent changes in cytoskeletal structure may play an active role in the initiation of the apoptosis signal transduction cascade in endothelial cells. Cytoskeletal rearrangement also has been suggested to be a critical step in the pathway to apoptosis induced by stress or heat shock (DeMeester et al., 1998), and cytoskeletal disruption leads to apoptosis in a variety of other cell types (Sauman and Berry, 1993; Zhang et al., 1997; Korichneva and Hammerling, 1999; Suria et al., 1999). Thus, the cytoskeleton apparently plays a supporting role in both the induction and execution of the apoptotic program.
In summary, these data provide a conceptual link between cell-ECM adhesion, cytoskeletal structure, cell shape, and Akt-mediated control of cell survival during angiogenesis. The results reveal that the cytoskeletal components that stabilize cell structure are critical for the cell's ability to survive, and suggest that shape-dependent apoptosis is due at least in part to disorganized cytoskeletal architecture. Elucidation of how cell geometry conveys survival signals will contribute to our understanding of the mechanism underlying regression of tissues, such as capillary blood vessels, which undergo apoptosis without completely detaching from their insoluble ECM attachment scaffolds in vivo (Ingber et al., 1986).
ACKNOWLEDGMENTS
We thank Dr. George Whitesides, his lab group, and the Harvard Materials Research Science and Engineering Center for assistance in the micropatterning studies. This work was supported by National Institutes of Health grants HL-57669 and CA-45548.
REFERENCES
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Aoudjita F, Vuoria K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells. A role for c-Flip and implications for anoikis. J Cell Biol. 2001;152:633–644. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeester SL, Cobb JP, Hotchkiss RS, Osborne DF, Karl IE, Tinsley KW, Buchman TG. Stress-induced fractal rearrangement of the endothelial cell cytoskeleton causes apoptosis. Surgery. 1998;124:362–371. [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Matarrese P, Straface E, Falzano L, Fabbri A, Donelli G, Cossarizza A, Boquet P, Malorni W. Toxin-induced activation of Rho GTP-binding protein increases bcl-2 expression and influences mitochondrial homeostasis. Exp Cell Res. 1998;242:341–350. doi: 10.1006/excr.1998.4057. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton J, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham H, Golding MCHM, Pepperkok R, Gullick WJ. Intracellular movement of green fluorescent protein-tagged phosphatidylinositol 3-kinase in response to growth factor receptor signaling. J Cell Biol. 1999;146:869–880. doi: 10.1083/jcb.146.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation, and subcellular localization. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- Huang S, Chen CS, Whitesides GM, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Jamieson JD. Basement membrane as a spatial organizer of polarized epithelia. Am J Pathol. 1986;122:129–139. [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Kayalar C, Ord T, Testa MP, Zhong L-T, Bredesen DE. Cleavage of actin by interleukin 1beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci USA. 1996;93:2234–2238. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Kandel E, Cross T, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase B/Akt cellular survival pathway. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kook S, Shim SR, Kim JI, Ahnn JH, Jung YK, Paik SG, Song WK. Degradation of focal adhesion proteins during nocodazole-induced apoptosis in rat-1 cells. Cell Biochem Funct. 2000;18:1–7. doi: 10.1002/(SICI)1099-0844(200001/03)18:1<1::AID-CBF840>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Korichneva I, Hammerling U. F-actin as a functional target for retro-retinoids: a potential role in anhydroretinol-triggered cell death. J Cell Sci. 1999;112:2521–2528. doi: 10.1242/jcs.112.15.2521. [DOI] [PubMed] [Google Scholar]
- Kothakota S, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Matarrese P, Conti L, Varano B, Gauzzi MC, Belardelli F, Gessani S, Malorni W. The HIV-1 vpr protein induces anoikis-resistance by modulating cell adhesion process, and microfilament assembly. Cell Death Differ. 2000;7:25–36. doi: 10.1038/sj.cdd.4400616. [DOI] [PubMed] [Google Scholar]
- Meredith JEJ, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Lee VM-Y, Pittman RN. Activation of a PP2A-like phosphatase and dephosphorylation of tau protein characterize onset of the execution phase of apoptosis. J Cell Sci. 1998a;111:625–636. doi: 10.1242/jcs.111.5.625. [DOI] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998b;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization, and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999;178:359–370. doi: 10.1002/(SICI)1097-4652(199903)178:3<359::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE-B. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signaling in detachment-induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- Sauman I, Berry SJ. Cytochalasin D treatment triggers premature apoptosis of insect ovarian follicle and nurse cells. Int J Dev Biol. 1993;37:441–450. [PubMed] [Google Scholar]
- Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha5beta1, independent of cell shape. Proc Natl Acad Sci USA. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. P.T.E.N. modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B. signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suria H, Chau LA, Negrou E, Kelvin DJ, Madrenas J. Cytoskeletal disruption induces T cell apoptosis by a caspase-3 mediated mechanism. Life Sci. 1999;65:2697–707. doi: 10.1016/s0024-3205(99)00538-x. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- Tang K, Nie D, Cai Y, Honn KV. The beta4 integrin subunit rescues A431 cells from apoptosis through a PI3K/Akt kinase signaling pathway. Biochem Biophys Res Commun. 1999;264:127–132. doi: 10.1006/bbrc.1999.1496. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Zhang X, Minale L, Zampella A, Smith CD. Microfilament depletion and circumvention of multiple drug resistance by sphinxolides. Cancer Res. 1997;57:3751–3758. [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Li X-M, Meinkoth J, Pittman RN. Akt regulates cell survival, and apoptosis at a postmitochondrial Level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and Regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]