Abstract
Abstract concepts play a central role in human behaviour and constitute a critical component of the human conceptual system. Here, we investigate the neural basis of four types of abstract concepts, examining their similarities and differences through neuroimaging meta-analyses. We examine numerical and emotional concepts, and two higher-order abstract processes, morality judgements and theory of mind. Three main findings emerge. First, representation of abstract concepts is more widespread than is often assumed. Second, representations of different types of abstract concepts differ in important respects. Each of the domains examined here was associated with some unique areas. Third, some areas were commonly activated across domains and included inferior parietal, posterior cingulate and medial prefrontal cortex. We interpret these regions in terms of their role in episodic recall, event representation and social–emotional processing. We suggest that different types of abstract concepts can be represented and grounded through differing contributions from event-based, interoceptive, introspective and sensory-motor representations. The results underscore the richness and diversity of abstract concepts, argue against single-mechanism accounts for representation of all types of abstract concepts and suggest mechanisms for their direct and indirect grounding.
This article is part of the theme issue ‘Varieties of abstract concepts: development, use and representation in the brain’.
Keywords: semantics, abstract concepts, embodiment, events, emotions, numbers
1. Introduction
In investigations of the semantic system, two broad categories of concepts have been used. Concrete concepts, such as tree, hammer or lion, have attracted a lion's share of research on the neural basis of the semantic system. These concepts have an identifiable, bounded referent that can be experienced with the five senses. They also tend to fall into taxonomic, hierarchical categories, such as animals, tools and living things, owing to clusters of co-occurring features (‘has legs' and ‘has eyes’), and are intuitively easier to reason about. A large body of evidence now indicates that such concepts are based, at least partially, in action and perception systems of the brain, a view referred to as embodiment, or more generally, grounded cognition [1–5]. Abstract concepts, such as democracy, philosophy or evaluation, on the other hand, are messier and are more difficult to characterize and categorize. They evoke complex and rich experiences involving episodes and situations, emotions, relations, introspection and interoception,1 but lack a clear sensory-motor experience. However, such concepts are very frequently encountered in language and represent one of the most sophisticated and important abilities of the human mind. Based on some behavioural findings, such as the fact that people list fewer features for abstract than concrete words, and require higher response times for abstract words, it is suggested that abstract concepts are more impoverished and ‘less semantic’. But it is not clear that there is anything impoverished about many abstract concepts such as justice or opinion. Semantic richness may be largely a matter of the methods used to measure it, which may be more or less suitable for certain types of concepts. The apparent semantic poverty of abstract concepts may be an artefact of methods suited to evaluating concrete concepts [6,7].
While abstract concepts have been studied for at least three decades, neuropsychological investigations of patients with conditions such as aphasia and semantic dementia typically define ‘semantics’ entirely in terms of concrete concepts, often only concrete objects. This is driven, in part, by the popularity of tasks such as picture naming and picture association in standardized test batteries, which are suited to concrete concepts. This tendency also trickles down to neuroimaging studies. Even today, it is not uncommon to see studies that use a few concrete object categories such as animals and tools as stimuli, and assume that the results speak to the entire semantic system. The bias against abstract concepts starts with the definition itself, in that they are defined negatively by what they lack rather than by what they contain. They are essentially ‘everything that is not concrete’. This is a bit like dividing buildings into ‘dwellings’ and ‘non-dwellings’. This can be useful especially if we are mainly interested in dwellings, but it shortchanges the diversity of non-dwellings that can vary from hospitals to museums to shopping centres. The most important feature of a movie theatre is not that people do not live there.
Over the last decade, there has been a growing realization of the importance and diversity of abstract concepts. Intense debate has been generated relating to the representation of abstract concepts, driven mainly by the observation that they do not seem suitable for grounding in action and perception systems. This has resulted in a number of valuable theoretical proposals which range in their degree of embodiment and a number of distinct mechanisms that are required to account for concrete and abstract concepts [8]. In general, these theories treat abstract concepts as a single entity to be explained by a particular mechanism. To be sure, concrete and abstract concepts do not form a clean dichotomy, but vary on a continuum that is commonly implemented using the continuous measure of concreteness rating (and the closely related measure of imageability). Researchers have typically used the high end of the continuum for the concrete category, and mid- to low range concepts are classified under ‘abstract’.
Neuroimaging and neuropsychological research has consistently suggested that abstract and concrete concepts differ. The particular brain regions involved and the reasons for these differences remain controversial, but the existence of differences is consistent. Neuropsychological double dissociations have pointed to reliance of concrete concepts on featural or taxonomic, and that of abstract concepts on associative or thematic information [7,9–13] (see [8] for a discussion). With regard to neuroanatomy, a meta-analysis of 19 studies [14] found that two regions were consistently activated for abstract over concrete words or sentences: the left anterior temporal lobe (ATL) and the inferior frontal gyrus (IFG). This contrasts with the findings for concrete concepts, which revealed a more extensive network that includes bilateral angular gyrus (AG), posterior lateral, medial and ventral temporal lobe, and posterior cingulate (pCi) and precuneus [14,15].
These findings are consistent with both of the classical theories of abstract/concrete concept representation. According to the Context Availability Theory [16–18], abstract words are more reliant on the context in which they occur. Abstract word processing requires greater activation of, and integration with, the context. This is consistent with the left IFG activation, which is associated with strategic semantic access and integration. The Dual Coding Theory [19,20] posits that only concrete concepts have a direct connection to image-based or visual representations, while abstract concepts are represented only through verbal associations. Activation of a number of areas associated with higher-order visual processing, such as the parahippocampal gyrus, fusiform gyrus and middle occipital gyrus (MOG), as well as greater bilateral activation, supports this view. Left lateralized activation for abstract concepts, including the traditional language processing area of IFG, also supports the notion of importance of verbal associations for abstract concepts.
According to the meta-analyses, abstract concepts would appear to have a significantly smaller footprint than concrete concepts, involving only the left IFG and lateral ATL, whereas concrete concepts reveal a much more extensive territory. This apparent small footprint of abstract concepts may be misleading for multiple reasons. First, note that these activations only reveal areas activated to a greater extent for abstract concepts relative to concrete ones. They do not include areas that may be equally activated by both types of concepts, or those that are activated by abstract concepts, but even more so by concrete concepts. Secondly, meta-analyses are designed to detect consistent activation across studies that identify neural correlates of a particular psychological process. Activations found in a smaller proportion of included studies are assumed to be unreliable or false positives, likely resulting from statistical accidents or a flaw in the experiment such as a confound. However, this is not necessarily the case. Variable results between different experiments can exist if categories with different underlying representations are lumped together. For example, concepts that refer to mental processes (thought and consideration), those that refer to social structures and constructs (democracy and conspiracy) and those that have a strong emotional component (praise and anger) could potentially have different neural representations. Legitimate variability resulting from these differences is eliminated in a meta-analysis.
Apart from the heterogeneity, there is an additional methodological issue that adds variability to concepts that are classified as ‘abstract’ in different studies, and hence in the results of these studies. As Pollock [21] has recently pointed out, concepts with intermediate concreteness ratings tend to have high standard deviations. They are not truly rated as ‘intermediate’ by many raters, but as clearly concrete by some and clearly abstract by others. Abstract stimuli used in many studies contain words with intermediate and high standard deviation ratings, and hence, their abstractness is in question. Concrete concepts selected in many studies tend to have low standard deviations and tightly cluster near the high end of the concreteness scale. Using parametric variation in concreteness (e.g. [22]) can mitigate this, but the high variability related to intermediate ratings is still problematic. This can potentially explain some of the discrepancy in neuroimaging and behavioural results, such as the advantage of concrete over abstract words, or the importance of taxonomic versus thematic information for concrete and abstract words, respectively.
Here, we meta-analyse neuroimaging studies from four categories of abstract concepts, with two aims. The first is to examine the similarities and differences between the neural correlates of types of abstract concepts, to test whether different subcategories differ substantially, or whether a single representation format for not-concrete words is likely. A related second aim is to examine the possibility that at least some types of abstract concepts can be grounded in brain circuits subserving other functions, such as emotional or spatial processing, and event representations. We review emotional and numerical concepts expressed symbolically and two ‘higher-order’ processes that reflect abstract thought: morality judgements and theory of mind (ToM). Apart from the salience and controversial nature of these domains, they are chosen because of the substantial body of neuroimaging work on these domains that can be used to draw inferences across a variety of stimuli and tasks.
2. Numerical concepts
Numerical magnitude can be conveyed through symbolic (e.g. Arabic numerals or written number words) or non-symbolic stimuli (e.g. dot patterns), and has been pointed out as a key example of abstract semantics. The question of how abstract numerical magnitude information can be represented, or grounded in sensory-motor systems, therefore forms one of the key challenges for any theory of cognition. Previous evidence has suggested that semantic information of the domain of numerical cognition can be grounded through its systematic association with left-to-right coordinates of space [23,24]. Numerous studies have indeed demonstrated that numerical cognition shares computational processes with spatial processing. Dehaene et al. [23] demonstrated that subjects were faster in making parity judgements to small numbers when responding with their left hand, while judgements to large numbers were faster in the case of a right-hand response. Such a demonstration of a common representational format underlying numerical magnitude information and the spatial characteristics of a response was termed the SNARC effect (Spatial–Numerical Association of Response Codes) and has been taken as evidence for a spatial nature of numerical magnitude representations analogue to the location on a ‘mental number line’. In line with the observation that the SNARC effect is typically not observed earlier than in 9-year-old children and becomes stronger from childhood to elderly age [25,26], it has been suggested that it reflects the long-term practice of using spatial information in processing numerical magnitude [27,28] and establishment of a reading–writing system [29]. The exact nature of the spatial information recruited during numerical magnitude processing seems to be modulated by cross-cultural differences in the direction of the writing system [30–32]. Furthermore, it has been shown that the SNARC effect is modulated by finger counting habits and hand preference [33,34]. This finding might reflect the fact that the direction of the spatial–numerical association given by a writing system seems to be an important determinant in shaping finger counting habits [35]. Moreover, an influence of finger counting habits and hand preference might signify the coexistence of multiple number–space mappings that are based on either extra-personal physical space or finger counting habits. Findings from studies that tried to disentangle these influences are mixed, with some studies reporting a stronger influence of body-based than space-based representations [36,37], while other studies found the reverse pattern [38,39]. Altogether, findings have shown that numerical magnitude information shares computational processes with spatial representations that are based on either personal or extra-personal number–space mappings.
In line with the idea that numerical cognition overlaps with computational processes that underlie spatial processing and/or finger counting actions, spatial processing areas in the posterior parietal cortex play a role in the processing of numerical magnitude information across a wide range of tasks like mental calculation, approximation and number comparison [40,41] and across cultures [42]. Similarly, patient studies show that lesions to the parietal cortex are associated with deficits in numerical processing ranging from magnitude comprehension to number subtraction or bisection [43], and repetitive transcranial magnetic stimulation (rTMS) over posterior parietal regions has been shown to reduce the SNARC effect in a parity judgement task [44]. To investigate if the abstract domain of numbers is grounded in brain areas involved in spatial processing, we conducted an activation likelihood estimation (ALE) meta-analysis on peak coordinates reported in neuroimaging studies on number processing.
(a). Methods
First, we describe general methods that were used for meta-analyses in all domains. Peer-reviewed articles were obtained from literature search or existing meta-analyses, to which additional inclusion criteria were applied. Specific contrasts were included if they contained a whole-brain neuroimaging analysis and contrasts with linguistic baseline conditions (with the exception of numbers, where non-linguistic baselines such as scrambled letters are commonly used). This excluded contrasts that used ‘rest’, fixation or a similar low-level condition. Comparisons with nonverbal materials such as pictures were also excluded, as they do not control for orthographic and phonological processes. Coordinates for all the included contrasts across the different domains were extracted and, where necessary, converted from Montreal Neurological Institute (MNI) to Talairach space using the icbm2tal transformation tool provided in GingerALE 2.3.6 [45,46]. In the ALE analysis using GingerALE, we used a corrected cluster-level p < 0.05, 1000 permutations, uncorrected p < 0.005. A region of interest (ROI) analysis was also included to examine the left ATL and left AG, two areas that often emerge during semantic processing, which were also corrected using the same parameters.
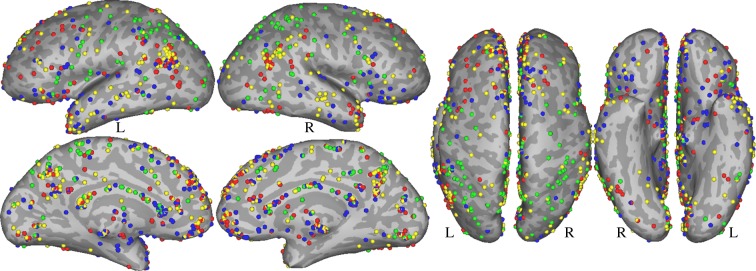
Studies included for the numerical concepts domain were selected on the basis of meta-analyses by Arsalidou & Taylor [47] and Sokolowski et al. [48]. From the 146 studies included across these two meta-analyses, we only included studies that investigated numerical magnitude processing by means of symbolic stimuli (i.e. Arabic numerals like ‘1, 5’ or written number words like ‘three, nine’, in either the visual or auditory modality) and that did not involve any calculation of some sort (e.g. multiplication or addition). Contrasts that involved judging numerosity or counting (e.g. five circles versus seven circles) were removed. Furthermore, we excluded positron emission tomography (PET) studies, studies that used children as subjects, studies that did not include a control for general executive processing (e.g. number comparison versus rest), studies that used tasks that heavily relied on other cognitive processes (e.g. delayed-number matching) and studies that only used contrasts that focus on higher-order numerical processing (e.g. positive versus negative integers, congruent versus incongruent, comparing different number ratios). After screening all studies, 23 separate contrasts remained, with a total of 126 foci from 375 participants (figure 1; electronic supplementary material, table S1).
Figure 1.
Peaks from all contrasts used in the meta-analyses. Green—numbers, blue—emotion, red—morality, yellow—theory of mind.
(b). Results
Three significant clusters of activation, with a total of 12 peaks, were found for numerical cognition (table 1 and figure 2). These fell in the right anterior intraparietal sulcus (IPS), extending into the posterior IPS and the superior parietal lobule (SPL), and the left anterior IPS, extending into the inferior parietal lobule (IPL) and the left posterior IPS.
Table 1.
Clusters for the numerical cognition meta-analysis. Coordinates are reported in Talairach space and refer to the location of the maximum observed ALE value. clust., cluster number; hem., hemisphere; BA, approximate Brodmann area; ALE, activation likelihood estimate; vol., volume.
| clust. | hem. | brain area | BA | x | y | z | ALE | vol. (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | R | inferior parietal lobule | 40 | 36 | −44 | 40 | 0.024 | 5792 |
| middle temporal gyrus | 39 | 30 | −60 | 30 | 0.014 | |||
| superior parietal lobule | 7 | 24 | −64 | 42 | 0.014 | |||
| 28 | −52 | 44 | 0.013 | |||||
| 36 | −64 | 48 | 0.009 | |||||
| 2 | L | inferior parietal lobule | 40 | −42 | −44 | 38 | 0.020 | 3824 |
| −48 | −38 | 48 | 0.013 | |||||
| −34 | −40 | 44 | 0.013 | |||||
| 3 | L | superior parietal lobule | 7 | −24 | −58 | 42 | 0.015 | 2632 |
| −24 | −54 | 44 | 0.015 | |||||
| precuneus | 19 | −30 | −66 | 36 | 0.013 | |||
| 31 | −26 | −76 | 24 | 0.008 |
Figure 2.
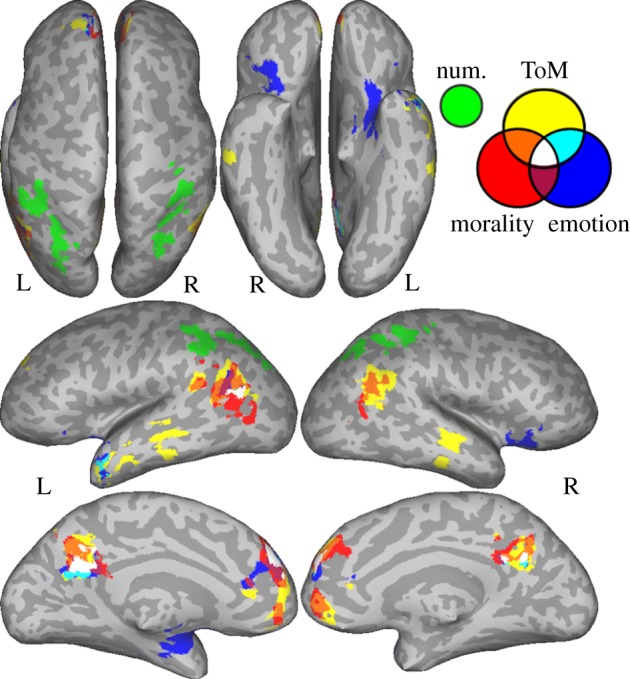
Results of the meta-analysis showing significant ALE values within each domain, and overlap.
(c). Discussion
The IPS has been consistently linked to spatial processing and attention as well as visually guided grasping [49–54]. It is part of the classic where/how dorsal visual pathway [53,55], although the binary distinction between dorsal/ventral pathways has been challenged [56], with evidence that the dorsal pathway may also contain object representations that subserve actions. Regarding number processing, Eger et al. [57] showed that, in a task that did not require the explicit activation of magnitude information, processing of numeric symbols across the visual and auditory modality elicited activation in a bilateral region of the horizontal section of IPS (hIPS). In addition, imaging studies have provided evidence that the hIPS plays a role in the coding of non-symbolic numerical magnitude [58] and shows a distance-dependent recovery of activation for newly presented numbers irrespective of the symbolic or non-symbolic nature of the stimulus material [59]. The finding of an involvement of the hIPS in the processing of numerical magnitude information has been replicated across a wide range of tasks such as number comparison, mental calculation and approximation [40,41,60] (although we eliminated calculation tasks here) and has been replicated across cultures [42]. Patient studies have demonstrated that parietal lesions are associated with deficits in numerical processing ranging from magnitude comprehension to number subtraction or bisection [43]. These results have led to the proposal that the hIPS contains a representation of numerical magnitude that is independent of the modality of presentation (i.e. visual or auditory, [57,61]). The finding of an important role of the hIPS in the processing of numbers is in line with the idea that numerical magnitude is grounded in brain regions that code for extra-personal spatial representations.
Alternatively, or in addition to spatial representations, these results may reflect the activation of body-based representations acquired through finger counting habits. It is difficult to distinguish between these two proposals on the basis of these neuroimaging findings, given that the hIPS has traditionally also been strongly linked with the control of hand and finger movements [62–65]. A number of studies have provided evidence for a role of finger and hand movement representations in the grounding of numerical knowledge by showing that numerical magnitude processing not only involves parietal regions but also extends to the primary motor cortex [41,60,66,67] and increases corticospinal excitability of hand muscles [68]. Several peaks were observed in the current analysis in the motor cortex, but did not survive the meta-analysis owing to their distributed nature.
IPS involvement, especially in the right hemisphere, could reflect the activation of a generalized magnitude system, as proposed by A Theory of Magnitude (ATOM) [69,70]. ATOM suggests a single system responsible for many types of magnitude estimations, including those for time, space and number, with the right parietal lobe as the key component. Neuroanatomy suggests that such common representations are grounded in action–perception systems. To argue that these magnitude representations are abstract, positive evidence would need to be provided to the effect that they do not contain any spatial or action components.
The lack of frontal activation in the current meta-analysis might reflect the fact that we only selected studies that used ‘simple’ number tasks like numerical comparison, whereas studies of numerical cognition typically include a wide array of tasks that involve additional arithmetic processing [47]. Similarly, while AG was seen in the three other domains meta-analysed below, its absence from the current domain is noteworthy, given its strong association with numerical cognition [71]. This can also be explained by the fact that AG is most commonly seen in tasks that involve calculation. During calculation, it is involved with recall of memorized mathematical facts (such as 3 × 4 = 12) [72] and transfer of facts between calculations [73]. Involvement of AG is likely minimized here owing to the inclusion criteria that eliminated calculation tasks.
In addition to confirming the role of IPS in number processing, these results demonstrate that it also holds for purely symbolic stimuli, and without the use of higher-order tasks. The bilateral extent of IPS, SPL and IPL activation for number processing suggests grounding of numerical concepts in the processing of physical space and the spatial/non-spatial characteristics of hand movements. However, it should be noted that the precise nature of conceptual grounding of numerical magnitude information seems to vary extensively between individuals and cultures [30,31,35], reflecting interindividual and task-specific differences in the disposition to map numbers to different body parts, to personal versus extra-personal space or even to non-spatial force parameters [74]. These interindividual differences in number–space mappings can likely be explained by factors such as handedness, counting habits and writing system direction [33–35]. For example, some cultures use counting systems that use specific body parts in sequence, like counting on fingers but also using elbows, knees and eyes [75, p. 242]. These systems can top out at 12, or go as high as 74, and can even cycle through these two or three times to obtain even higher values. Such systems can be neurobiologically grounded in cortical representations of body parts that go well beyond fingers.2
3. Emotion concepts
From the perspective of semantic information, emotion refers to the emotional or affective experience elicited by the use of a word. Based on the circumplex model [76,77], two dimensions are often used to describe emotion semantics: arousal describes the amount of emotional ‘energy’ or the level of engagement of the organism, and valence describes the hedonic tone of the emotional state, ranging from positive to negative. Although more highly valenced stimuli are also frequently more highly arousing [78], this pattern is stronger for negative than for positive words [79]. Other dimensions have also been proposed to describe emotional stimuli. For example, a word can be classified according to whether it is ‘biological’, that is, if its emotional content is relevant to evolutionary success [80]. However, arousal and valence are the most commonly studied in psycholinguistic evaluations of emotion.
Emotional arousal and valence are often cited as playing a critical role in the difference between abstract semantics and concrete semantics [7,81,82]. Abstract words, in general, tend to be more emotionally valenced than concrete words [83] and, within the domain of abstract words, highly arousing words elicit different behaviours from emotionally neutral words; the distribution of lexical decision reaction times is narrower and earlier for emotional words [84]. There is even evidence for a specific role of emotion in processing abstract concepts that is absent in concrete concepts: behavioural performance [85,86] and deterioration of semantic memory in Alzheimer's dementia patients [87] show effects of emotional content within abstract words but not within concrete words. This suggests that the emotional information associated with abstract words is uniquely critical to how their meanings are stored in the brain. We performed a meta-analysis of neuroimaging studies that examined affect through verbal materials.
(a). Methods
Studies or contrasts were selected that investigated emotion by means of verbal stimuli only, excluding studies that used materials such as pictures, videos or music, either as the condition of interest or as the control condition. The dimension of valence has been experimentally isolated from emotional arousal by a few neuroimaging studies, but it is often confounded with emotional arousal. In the current analysis, the majority of studies that purport to study valence contrasted either positively or negatively valenced words with emotionally neutral words. The neutral word baseline is also low-arousal relative to either positive or negative words; hence, it is difficult to distinguish arousal from valence across these studies. Thus, the peaks included in the current analysis are restricted to experiments that contrast emotional arousal, even if some may also be contrasting valence. We included foci from experiments that contrasted emotionally valenced words with neutral words, or an explicitly valenced semantic task with a non-emotional semantic task. Thirty-three separate experiments were included, with a total of 205 foci (figure 1) from 560 participants (electronic supplementary material, table S2).
(b). Results
Four significant clusters of activation, with a total of 10 peaks, were found in the ALE meta-analysis for the emotion domain (table 2 and figure 2). Activations fell in the bilateral dorsomedial prefrontal cortex (dmPFC) and anterior insula, the left anterior cingulate, medial superior frontal gyrus (SFG), amygdala, hippocampus and precuneus as well as the right orbitofrontal cortex (OFC) extending into the pars orbitalis.
Table 2.
Clusters for the emotional cognition meta-analysis. *Area that was significant only in the ROI analysis.
| clust. | hem. | brain area | BA | x | y | z | ALE | vol. (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | L | dorsomedial prefrontal cortex | 9 | −6 | 48 | 34 | 0.020 | 4608 |
| −4 | 48 | 26 | 0.017 | |||||
| 8 | 50 | 16 | 0.014 | |||||
| anterior cingulate | 32 | −4 | 38 | 18 | 0.014 | |||
| middle frontal gyrus | 9 | −14 | 38 | 28 | 0.012 | |||
| 2 | L | amygdala | −26 | −4 | −12 | 0.020 | 2752 | |
| hippocampus | −28 | −14 | −14 | 0.015 | ||||
| 3 | L | precuneus | −4 | −56 | 24 | 0.027 | 2448 | |
| 4 | R | IFG/orbitofrontal gyrus | 47 | 32 | 20 | −12 | 0.020 | 1808 |
| 38 | 26 | −2 | 0.011 | |||||
| 5 | L | anterior temporal lobe* | −47 | −6 | −23 | |||
| −53 | −7 | −5 | ||||||
| 6 | L | angular gyrus/TPJ* | −49 | −61 | 18 |
(c). Discussion
Of the areas that emerged in the ALE meta-analysis, the amygdala is the most specific to emotion processing relative to other forms of abstract semantics. Neuroimaging studies consistently report the amygdala as being sensitive to emotional arousal across many types of stimuli (for review, see [88]). It emerges in both contrasts comparing negative words with neutral words [89–91] and those comparing positive words with neutral words [92,93]. Traditionally, amygdala is considered to be part of the subcortical pathway that is dedicated to processing emotions, especially fear. This view has been challenged [94], and instead, it is suggested that it is part of a broader network that evaluates the emotional significance of (especially visual) stimuli and also the possible responses to it [95,96]. While amygdala has an important role in emotion processing under this model, it suggests that cortical areas such as the OFC and anterior cingulate cortex (ACC) have a greater role in evaluating emotional significance than is traditionally assumed [96].
The OFC is also unique to emotional content in the current meta-analysis. The OFC is often associated with decision-making and predicting rewards (for review, see [97]), and in the left hemisphere, this may include emotional decision-making, specifically ‘counterfactual emotions', e.g. regret following emotional decisions [98]. A recent meta-analysis of emotion semantics found left OFC activation for verbal emotion stimuli compared with nonverbal emotion [99]. Although the literature included in the current analysis does reveal some peaks in the left OFC [93,100], only the right OFC emerges as significant for emotion semantics. This area emerges in the few studies that specifically test emotional valence and/or attempt to decorrelate or control for the effects of emotional arousal [101–103]. The right OFC plays a role in social cognition; patients with social anxiety disorder have impaired functional connectivity between the right OFC and amygdala, suggesting that both areas are important for successful emotional and social processing [104]. Even in healthy adults, grey matter volume in the bilateral OFC correlates negatively with symptoms of emotional dysregulation, supporting the theory that both hemispheres of the OFC are important to emotional processing [105]. OFC specifically has a role in computation of valence. Rapid electrophysiological responses (100–150 ms) to discrimination of valence of a visual stimulus are observed in OFC [106]. An fMRI study [107] used representational similarity analysis to show that both positive and negative valence stimuli, across sensory modality, showed distinct patterns in bilateral OFC. Furthermore, subjective valence ratings could be classified based on activation similarity patterns in OFC and generalized across subjects.
The region of ACC activated by emotion concepts, pregenual ACC, is considered to be part of the emotion processing network for both linguistic and non-linguistic stimuli [108–110], and is specifically associated with valence [111]. It is connected to amygdala [112] and to autonomic nuclei associated with visceromotor control in monkeys [113]. More recently, the role of ACC in emotion processing is thought to involve modulating the activity of amygdala [109,114].
In contrast to the emotion-specific activation patterns in the amygdala, OFC and anterior insula, a wide region of medial frontal areas, as well as the pCi and precuneus, were commonly activated across abstract semantic domains. Apart from general semantics, these regions are also associated with emotion processing from multivariate classification studies [115]. For example, using movies and mental imagery, Saarimaki et al. [116] found distinct signatures of six basic emotions in dmPFC, ventromedial prefrontal cortex (vmPFC), ACC and precuneus/pCi, in addition to amygdala and OFC, in a multivoxel analysis. Classification generalized between movies and imagery methods. We suggest that the medial frontal regions and precuneus/pCi are unlikely to be specialized for emotion processing, although they may be upregulated by emotional content. Domains with extensive activation in these regions (emotion, ToM and moral judgements) take place during social interactions or elicit memories of interactions in the form of episodes or events. The fact that emotional movies and mental imagery activate these regions, and the activity generalizes across the modalities, supports this view. A meta-analysis comparing social cognition, emotional processing and introspection found overlapping areas between emotional and social cognition that included the orbitofrontal and medial frontal cortex [117]. The ROI analysis also revealed some activation for the left AG during emotion processing, which overlaps with the larger temporo-parietal junction (TPJ) clusters for morality and ToM, and is discussed later.
The left ATL also emerged in the ROI analysis. Among its many associated functions, such as general semantics, sentence processing, short-term memory, social cognition and processing unique entities, the ATL has previously been implicated in emotional arousal [118,119] and valence [90,120] as well as social processing such as detection of familiar faces [121]. Research on non-human primate lesions suggests that the ATL's role in social processing may, at least in part, rely on its role in emotional processing [122,123]. There is also evidence that the ATL is involved in emotional processing even when controlling for the presence of social cognition [124,125]. The connectivity of the temporal pole is similar to that of amygdala, and damage to the pole causes Klüver–Bucy syndrome. Some symptoms of this syndrome include social withdrawal, blunted affect, tameness or diminished fear, which are produced by lesions to either temporal pole, OFC or amygdala [122,126].
In summary, emotion semantics consistently activated regions of the network implicated in the experience and processing of emotions. ‘Core’ regions of this network, amygdala and OFC, were activated uniquely for emotion words (with respect to the domains evaluated here). Other regions, such as the ACC, ATL, AG, precuneus/pCi and medial PFC (mPFC), overlapped with other domains and may be involved in episodic memory, social cognition and general semantics (see General discussion, §7). These results are consistent with Barrett's theory of constructed emotion [127,128]. Briefly, this view suggests that the brain runs an internal model based on past experiences to perform allostasis, which refers to the regulation of the body's internal environment by anticipating physiological needs. Interoception is the representation and utilization of these internal sensations. Past experiences are implemented as concepts, which are embodied representations that predict what is about to happen in the sensory environment. When the internal model creates an emotion concept, a situated conceptualization (or categorization) results in an instance of emotion. The activation of visceromotor regions involved in interoception (amygdala, anterior insula, OFC, ACC and mPFC) and those associated with representation of events and situations (AG, precuneus/pCi and ATL) is broadly consistent with this view (see also [129], where evidence for situated conceptualizations for emotions was found in a neuroimaging study using a task context where situations were described explicitly).
4. Morality
Morality has been defined as a set of guiding principles that includes standards of right and wrong conduct, or rules that guide us in our everyday choices and actions [130]. Moral judgements involve decisions about what we would do when faced with a moral dilemma, or evaluations of the decisions and actions of others in situations that involve moral principles like harm, justice and fairness. Deciding what to do when faced with a moral dilemma or deciding whether an action is right or wrong might involve slightly different processes [131], with the former relying on additional self-referential processes [132]. Theoretical accounts have argued for a role of abstract-inferential, self-referential and emotional processes in moral judgements. For example, studies suggest that moral cognition is influenced by the perceived intentionality of agents' actions [133–135]. To judge whether an agent's action is intentional requires the ability to infer other's desires, thoughts and behavioural dispositions [136,137] often captured by the term ToM. Support for the idea that abstract-inferential processes play a role in moral cognition comes from neuroimaging studies, showing that moral evaluations and decisions share common neural resources with brain areas engaged in ToM, including medial frontal and temporo-parietal brain regions [138]. Evidence for a role of emotional processes in making moral judgements comes from studies showing that experiencing empathy promotes moral behaviour towards other people [139], and morally inappropriate behaviour can be attributed to a deficiency in empathic skills [140,141]. In line with this proposal, neuroimaging and lesion studies have provided evidence that moral decision-making relies on the vmPFC [142,143]. It has been argued that the vmPFC plays a role in emotion regulation and that its involvement in studies on morality therefore reflects a role of emotional processes in making moral judgements [144]. To investigate neural regions underpinning moral cognition mediated verbally, and to examine their relationship to other abstract domains, we conducted an ALE meta-analysis using neuroimaging studies on moral decision-making.
(a). Methods
Studies included for the morality domain were selected on the basis of a meta-analysis by Garrigan et al. [132]. From the 28 studies included in this meta-analysis, we selected studies that investigated morality by means of written sentences, statements and scenarios (excluding studies that used animated stimuli, pictures or vignettes). After screening, 20 separate experiments remained for inclusion, with a total of 182 foci from 395 participants (figure 1; electronic supplementary material, table S3).
(b). Results
Five significant clusters of activation, with a total of 15 peaks, were found across the morality studies. Activations fell in the bilateral dmPFC, vmPFC, AG, supramarginal gyrus (SMG) (or TPJ3; [145]), left MOG and precuneus/pCi (table 3 and figure 2).
Table 3.
Clusters for the morality meta-analysis.
| clust. | hem. | brain area | BA | x | y | z | ALE | vol. (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | L | dorsomedial prefrontal cortex | 9 | −2 | 50 | 26 | 0.024 | 6536 |
| R | 2 | 46 | 30 | 0.024 | ||||
| −4 | 42 | 28 | 0.022 | |||||
| 2 | L | temporo-parietal junction | 22 | −46 | −58 | 16 | 0.034 | 5800 |
| −58 | −50 | 16 | 0.016 | |||||
| middle occipital gyrus | 19 | −48 | −68 | 6 | 0.016 | |||
| inferior parietal lobule | 39 | −46 | −66 | 28 | 0.013 | |||
| −34 | −58 | 22 | 0.010 | |||||
| 3 | L | precuneus | 7 | −4 | −54 | 28 | 0.024 | 4552 |
| −2 | −60 | 34 | 0.021 | |||||
| 0 | −46 | 26 | 0.016 | |||||
| 4 | R | ventromedial prefrontal cortex | 10 | 4 | 52 | 2 | 0.020 | 2432 |
| L | −10 | 48 | 0 | 0.013 | ||||
| 5 | R | temporo-parietal junction | 39 | 48 | −58 | 20 | 0.022 | 2168 |
| superior temporal sulcus | 44 | −60 | 8 | 0.017 |
(c). Discussion
The TPJ is activated by abstract-inferential processes that have been proposed to underlie ToM abilities like inferring other's desires, thoughts and beliefs [138,146–149]. Most theoretical accounts have argued for a role of both the left and right TPJ in ToM. However, contributions to ToM abilities seem to differ between hemispheres [150]. A study by Saxe & Wexler [150] showed that the right TPJ showed a response that was restricted to the attribution of mental states, while the left TPJ showed a response to the social background information of a protagonist that was not significantly different from the response during mental state attribution. Based on these findings, the authors suggested that the left TPJ is involved more broadly in the coding of socially relevant information of a protagonist, whereas the right TPJ is selectively involved in the attribution of mental states. Deciding what to do when faced with a moral dilemma or deciding whether someone's actions can be considered right or wrong requires a general coding of socially relevant information of a protagonist and more specifically the attribution of intentions to others' behaviour. Furthermore, the overlap in activation between morality and ToM tasks suggests that these abilities rely on similar abstract-inferential computational processes, which may involve general semantic knowledge and, more specifically, events and episodic information. TPJ/AG is also commonly found in studies of semantics involving individual concrete or abstract words, and is more strongly activated for concrete words [15], and likely has a more general role. The General discussion further addresses this point.
One region that was uniquely seen for the morality domain was the ventral-most aspect of TPJ bilaterally, extending into the occipito-temporal cortex. This region is similar to the ventral subdivision of AG identified by Seghier et al. [151]. It responds more strongly to picture semantics than to written words, and likely has a role in visual semantics, which is also the function associated with the lateral occipito-temporal cortex. Thus, its role is unlikely to be specific to morality and possibly reflects vivid visual imagery evoked by scenarios used in morality tasks.
Medial frontal areas, dmPFC and vmPFC, are commonly seen in social cognition, abstract-inferential processing and emotion regulation [109,152–156]. It has been suggested that the vmPFC tracks the value of rewards and valence of emotional stimuli [155,157,158], regulates emotions [144,155] and plays a role in mesolimbic reward systems [159,160]. Furthermore, it has been shown that lesions of the vmPFC are associated with deficits in empathic processing and ToM abilities [161]. Studies on moral cognition typically involve situations and moral dilemmas that involve principles like harm, justice and fairness. We suggest that the common activation of the vmPFC across studies on moral decision-making reflects a role of emotional processes in making moral judgements. The dmPFC plays a role in the processing of complex social judgements on visual face stimuli [162], abstract-inferential processes involved in ToM [154,163] and the generation and regulation of emotion [156]. In line with the idea that the dmPFC plays a role in emotion regulation, the dmPFC shows strong functional connectivity with subcortical regions involved in emotion processing [156], and the region homologous to the dmPFC in the macaque monkey has strong neuroanatomical projections to core limbic structures [164,165]. Advanced cognitive abilities such as social cognition also involve dmPFC [152,153]. Recent studies have provided evidence that the dmPFC is hierarchically higher and especially important for highly demanding and uniquely human social cognitive tasks [166,167]. Consistent activation of the dmPFC in studies on moral cognition, and its overlap with emotion, therefore likely reflects the demanding abstract-inferential processes involved in moral judgements, as well as a role of emotional processes in moral decision-making.
Like TPJ, the precuneus/pCi is frequently activated in studies of general semantics, emotions and higher-order processes such as morality. Some authors have interpreted it as being responsible for visual awareness, self-awareness and self-referential processing, or consciousness [168–170]. We suggest that its role is likely more general, and this is further addressed in the General discussion.
In summary, the present meta-analysis shows that moral decision-making based only on verbal stimuli involves a network of areas including medial frontal and temporo-parietal brain regions, and overlaps greatly with areas associated with general semantics, social and episodic memories, and emotion regulation. This suggests a process that is based in retrieval of socially relevant entities and events, as well as emotions.
5. Theory of mind
ToM, often referred to as ‘mentalizing’, is traditionally defined as the process of inferring the beliefs, thoughts or feelings of another person, especially when those beliefs differ from one's own beliefs [136]. In recent years, the term has been used to describe other situations involving the ‘self’ and ‘other’, including abstracted social behaviour (e.g. shapes performing social-like behaviours), trait judgements and strategic social games, among other tasks [171]. It is further complicated by the fact that ToM relies on thinking about another person, making it difficult to experimentally isolate from social cognition. Here, we focus on the original definition of ToM, inferring the beliefs of others, and specifically focus on the use of verbal stimuli, e.g. inferring one's beliefs from a written story about his/her circumstances and behaviours. This is often studied by contrasting responses to ‘false belief’ stories, in which a protagonist acts according to information that the reader knows to be false, against ‘photo’ descriptions, in which an outdated photograph is described with some information that the reader knows is no longer true [172]. Both conditions involve verbal descriptions of some false information, but only the false belief stories involve imagining the beliefs of another person.
As in other domains of abstract semantics, ToM tasks often rely on social cognition and/or emotion perception. There is debate as to how these domains are related. It is generally agreed that emotion perception is an affective process while ToM is a cognitive one; yet, ToM is often required to infer the emotional state of another person, and both processes could reasonably fall under the umbrella of social cognition [173]. Mitchell & Phillips describe several cognitive models comparing ToM and emotion processing. They may be separate mechanisms that differ in how much arousal is required (ToM is ‘cold’, emotions are ‘hot’; [174]); they may be connected processes which are accessed serially, such that emotional processing precedes ToM [175], or they may have a superset–subset relationship, in which ToM is a complex process that includes emotion [176,177]. In all of these models, it is acknowledged that ToM is a more complex, high-level process than emotion processing.
(a). Methods
Only studies that investigated ToM by means of verbal stimuli were included for the ALE meta-analysis. We included foci from experiments that contrasted sentences or vignettes describing the mental states of others (typically beliefs) with a sentence or story about the protagonist's physical circumstances or a non-ToM scene; or that contrasted tasks which require inference about another's beliefs with tasks that require non-ToM comprehension. Twenty-eight separate contrasts were included (electronic supplementary material, table S3), with a total of 250 foci from 554 participants (figure 1).
(b). Results
Seven significant clusters of activation, with a total of 15 peaks, were found across the ToM studies. Activations fell in the bilateral medial frontal cortex (dmPFC and vmPFC), TPJ, middle superior temporal sulcus (STS), precuneus/pCi, left SFG, ACC, ATL and the right middle frontal gyrus (MFG) (table 4 and figure 2).
Table 4.
Clusters for the ToM meta-analysis.
| clust. | hem. | brain area | BA | x | y | z | ALE | vol. (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | ventromedial prefrontal cortex | 10 | 0 | 48 | 2 | 0.030 | 9304 | |
| R | dorsomedial prefrontal cortex | 9 | 2 | 50 | 34 | 0.030 | ||
| L | −4 | 50 | 16 | 0.023 | ||||
| R | middle frontal gyrus | 10 | 2 | 44 | −8 | 0.017 | ||
| L | superior frontal gyrus | 9 | −20 | 50 | 36 | 0.016 | ||
| anterior cingulate | 32 | −2 | 36 | 10 | 0.013 | |||
| 2 | L | temporo-parietal junction | 39 | −50 | −58 | 20 | 0.056 | 6112 |
| 3 | L | precuneus | 7 | −2 | −56 | 32 | 0.036 | 5944 |
| 0 | −58 | 24 | 0.025 | |||||
| 4 | R | temporo-parietal junction | 39 | 48 | −54 | 24 | 0.048 | 5264 |
| 5 | R | superior temporal sulcus | 22 | 50 | −18 | −8 | 0.026 | 2928 |
| 6 | L | −54 | −24 | −6 | 0.023 | 1760 | ||
| 7 | L | anterior temporal lobe | 21 | −50 | 0 | −22 | 0.017 | 1504 |
| −56 | −6 | −12 | 0.015 | |||||
| −42 | 2 | −30 | 0.011 |
(c). Discussion
The areas seen in the domain of ToM have a great deal of overlap with the areas in the moral reasoning meta-analysis, with the addition of the ATL mid-STS. In a meta-analysis, Schurz et al. [178] showed that the TPJ and medial frontal cortex are the most task-indifferent regions that are involved in ToM, and they are also the most consistent areas to emerge during verbal narrative tasks. The STS has also been implicated in the ‘ToM network’, although this area is somewhat less consistent and may rely specifically on social animations [147] or inferring desires, rather than beliefs, of another person [179]. It also emerged in a semantic meta-analysis [15], where most studies did not involve inference about others' beliefs or desires. The description of these areas as a ‘network’ may not be entirely accurate, however; these areas play important roles in the cognitive processes involved in ToM, but their specific functions are likely distinct between regions.
The TPJ, overlapping with both morality and emotion domains, is consistently activated while imagining another's thoughts [146], emotional state [180] or beliefs [147]. The TPJ is also activated across a wide variety of ToM-related modalities and tasks, e.g. during entirely visual tasks that require taking the perspective of another [181] and the interpretation of jokes that require inference about others' beliefs [148,182]. The TPJ is also involved in making moral judgements of others based on their intentions [138,183], even in children [184], consistent with the TPJ's involvement in the morality analysis above. Like in studies of morality, there is some disagreement over whether there are separate lateralized roles for the right and left TPJ in ToM processing. For example, the left TPJ is activated by false belief stories and also by ‘false sign’ stories that involve a protagonist being misled, but do not explicitly require consideration of the protagonist's beliefs [185,186]. The right TPJ, on the other hand, may be involved in detecting an incongruity between the protagonist's behaviour and his/her environment [185,186], or it may be a more domain-general monitor of working memory and attentional demands [187,188]. In either case, it is clear that both left and right TPJ play a role in both ToM and morality domains [189–191]. Its likely functions are more general, which are considered in the General discussion.
The medial frontal cortex is a very consistent area of activation during ToM studies [192,193] using both verbal and nonverbal tasks [194], but also like the TPJ, its role in cognition may not be specific to ToM, because it has also been implicated in other forms of social and emotional processing [122,192]. As discussed above, there are also differences between the specific roles of dmPFC and vmPFC [109,195,196] as well as medial and lateral subregions [179]. In the current analysis, the verbal ToM studies included peaks in all relevant subregions of the medial frontal cortex.
The ACC, in addition to ToM tasks, also emerges during tasks that require introspecting about one's own beliefs or judgements [176,197]. It is also involved in similar higher-order social and especially affective tasks, such as understanding jokes [148] and processing abstract or affective words [108,180], and was seen in our emotion meta-analysis, suggested to play a role in modulating activity in amygdala.
Finally, the ATL emerged during ToM processing, in conjunction with emotional processing in the left hemisphere. This is unsurprising, given that the ATL is involved in detecting the emotional states of others [198,199] or making moral judgements about others [200], which require ToM. This likely reflects the ATL's role in emotional and social processing, as discussed earlier.
Thus, the neural areas involved in ToM overlap substantially with those of other domains, in particular morality and emotional processing. Morality and ToM tasks often entail similar cognitive abilities, namely considering the mental and emotional states of others.
6. Domain-general abstract processing
Finally, an ALE meta-analysis was performed collapsing across peaks from all four domains. Six clusters of activation, with a total of 16 peaks, were found across all domains (104 experiments, 1884 participants and 763 foci). Activations fell in the bilateral TPJ, dmPFC, vmPFC, left MFG, SFG, precuneus/pCi, ATL and the right ACC (table 5 and figure 3, pink/purple).
Table 5.
Areas activated in the meta-analysis across all domains.
| clust. | hem. | brain area | BA | x | y | z | ALE | vol. (mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | R | dorsomedial prefrontal cortex | 9 | 0 | 48 | 32 | 0.054 | 19 376 |
| 0 | 50 | 26 | 0.051 | |||||
| ventromedial prefrontal cortex | 0 | 48 | 2 | 0.045 | ||||
| L | middle frontal gyrus | −8 | 40 | −6 | 0.025 | |||
| R | anterior cingulate | 4 | 34 | 14 | 0.020 | |||
| L | superior frontal gyrus | −14 | 32 | 44 | 0.020 | |||
| 2 | L | temporo-parietal junction | −50 | −58 | 18 | 0.088 | 8680 | |
| 3 | L | precuneus | −4 | −56 | 24 | 0.065 | 8536 | |
| 4 | R | temporo-parietal junction | 39 | 48 | −56 | 24 | 0.071 | 5368 |
| 5 | L | anterior temporal lobe | −50 | 4 | −20 | 0.031 | 2736 | |
| −42 | 10 | −28 | 0.023 | |||||
| −44 | 2 | −28 | 0.022 | |||||
| 6 | L | inferior parietal lobule | 40 | −42 | −44 | 38 | 0.022 | 2192 |
| −48 | −40 | 48 | ||||||
| −50 | −40 | 44 | ||||||
| −52 | −44 | 32 |
Figure 3.
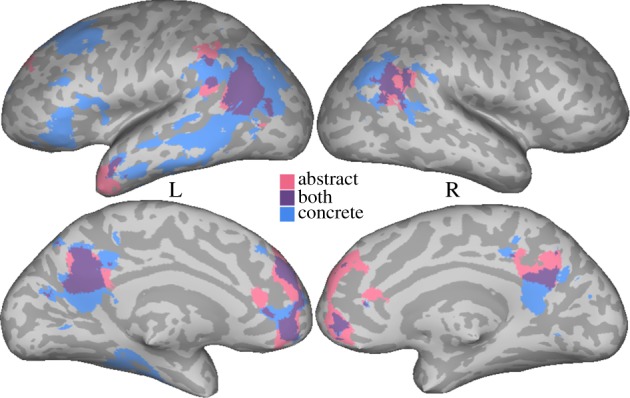
Results of the meta-analysis collapsing across all four abstract domains are shown in pink/purple. Activation from a meta-analysis of peaks from Binder et al. [15], after removing abstract peaks, is depicted in blue/purple, with purple showing the overlap between the two.
These regions overlap with the semantic system identified in Binder et al. [15], which reflected predominantly concrete semantics.4 To perform a direct comparison with the results of Binder et al., and to better identify concrete semantics, we used peaks from that study after eliminating contrasts and tasks that targeted abstract or metaphoric content (e.g. affective judgement). This resulted in 981 peaks (of the original 1145). An ALE analysis using these peaks (using the same threshold used for other domains here) is depicted in figure 3 (blue/purple).
Considering the abstract map, the contrast between figures 1 and 3 (and the circumscribed left ATL/IFG activation in [14]) is striking: the former shows activations that are widely distributed and involves most regions other than primary sensory and ventral temporal regions, while figure 3 shows a relatively circumscribed set of regions. In one sense, the meta-analysis in figure 3 does not contain false negatives by definition, in that it is meant to detect consistent activation controlling for the total number of peaks. But in another sense, such an analysis can create false negatives if heterogeneous tasks and domains are included. A significant proportion of the cortex involved in abstract concept processing does not show up on the map, not necessarily because the peaks from individual studies are false positives or are unreliable, but because they reflect different underlying processes. Even within a single domain, such as emotions, the distribution of peaks is much more extensive than what is obtained in the emotion map in figure 2. In this sense, the conclusion that abstract processing relies on only a small set of regions is misleading. This highlights the importance of the specific stimuli chosen for an experiment, the task and the control conditions, which can lead to variability within and across domains. Any negative conclusions based on a meta-analysis (the lack of involvement of a region in a domain) should be treated with caution if there is a chance that the stimuli, tasks or controls reflect heterogeneous processes.
Regions overlapping between domain-general abstract processing and concrete concepts were bilateral TPJ, precuneus/pCi, dmPFC and vmPFC, with a left > right pattern, and are discussed below.
7. General discussion and conclusion
We examined the representation of concepts related to numerical, emotional, moral and ToM processing. Basic number concepts showed a signature in bilateral IPS that extended into SPL. This supports the idea of spatial and body/finger-based representation of numbers. Verbally mediated emotion concepts activated areas associated with emotion processing and regulation, including the left amygdala and the right OFC, supporting grounding of emotion concepts in emotional experience. Higher-order, inferential processes of morality and ToM show partial overlap with areas associated with emotion processing in medial PFC and ATL, and also show activation in general concrete semantic areas, namely AG, precuneus/pCi and ATL. Apart from partial grounding in emotions, this suggests access to episodic/semantic content, as opposed to a process that is fundamentally different from other types of semantics.
The meta-analysis across the four abstract conceptual domains revealed a map (figure 3) that shows significant overlap with a map based on concrete concepts and includes bilateral AG/TPJ, dmPFC, vmPFC, precuneus/pCi and the left ATL. (Numbers do not significantly contribute to these areas, but the other three domains do.) Using a large set of concepts that varied in their sensory-motor attributes, a recent study [201] identified several ‘hub’ areas that were commonly activated by all attributes, which included all of the areas seen here, with the notable exception of ATL. ATL has also been suggested to be a semantic hub region [202,203].
We suggest that the reason for the similarity of activation patterns between two seemingly very different types of semantics (concrete concepts versus emotions and abstract-inferential processing) can be explained by the role of social and episodic knowledge and the semantic content of the episodes. For precuneus/pCi, two functional subdivisions have been suggested, where the anterior section is involved in mental imagery, and the posterior region in episodic memory retrieval [170]. In a meta-analysis comparing episodic encoding and retrieval, precuneus/pCi was upregulated for retrieval compared with encoding [204]. A related function of this area is in egocentric versus allocentric spatial processing [205,206]. The overlap map revealed that the retrosplenial cortex was activated for concrete but not for abstract concepts, while a more dorsal section in the right precuneus showed the opposite pattern. While retrosplenial cortex plays multiple roles associated with episodic memory, it is especially important for spatial memory [207,208]. Activation of retrosplenial (along with parahippocampal) cortex for concrete but not for abstract concepts suggests a more direct reliance on spatial content for concrete concepts, while abstract concepts evoke episodic memories that are less spatial in nature.
AG has been proposed to be involved in a wide variety of functions (see [71] for a review). It features prominently in concrete concept processing and has been proposed to integrate sensory-motor features [5,15,201,209,210]. While bilateral AG is seen in concrete > abstract contrasts, it also responds to abstract concepts when compared with a non-semantic control condition [108,211]. Beyond integrating features of static concrete concepts, it is likely to have a special role in integrating information across time, in processing events and relations. The left AG has been associated with understanding stories [119,212], verb argument structures [213] and thematic relations [214,215]. Boylan et al. [216] tested the response of AG to several types of two-word combinations, and found that it tracked the presence of an event-denoting verb, supporting the view that AG responds to event structure. Along with precuneus/pCi, AG is also a central node in the default-mode network [217,218] in the resting state, which is rich in episodic, prospective, introspective and semantic content involving objects, relations, plans, emotions, people and events [5,163,219,220]. Recently, Thakral et al. [221] found that applying rTMS to the left AG reduced the amount of episodic details produced during simulation and memory tasks based on event words (but not during a free association task), providing evidence for its role in episodic simulation and episodic memory. The set of common regions identified here was also found during sustained episodic simulations [222]. Spunt et al. [22] found activation of the same network seen here, consisting of AG, ATL, precuneus/pCi and mPFC, when actions (riding a bike) were processed at high levels of abstraction (Why ride a bike? To get exercise) versus low levels of abstraction (How to ride a bike? Grip handlebars). The ‘why’ question brings episodic or situational context into play. Based on a review of such findings, Rugg & King [223] recently proposed that AG represents retrieved episodic information, supported by a more general role in representing multi-modal and multi-domain information. Along the same lines, Ramanan et al. [224] proposed that AG is the critical contributor to episodic reconstruction across past, future and atemporal contexts, and actively represents and integrates disparate sensory-perceptual details into a rich multi-modal ‘contextual layer’. In summary, processes that are common across these abstract domains appear to be related to event and relation processing, episodic recall, imagery and emotion processing, and are also triggered by concrete concepts.
(a). Representing abstract concepts: a proposal and implications for theories
We suggest that event- or situation-based representations provide one mechanism for understanding and grounding of many types of abstract concepts. Exemplars and prototypes for concrete object categories (apple) are objects. Objects with a certain range of feature values (e.g. colours, patterns, shapes, sizes and tastes), or those with a family resemblance to other apples, constitute ‘good’ or acceptable instances of apples. For some abstract concepts, exemplars and prototypes are events, and family resemblance is computed over event structures. Concepts inhabit a space whose dimensions include event-based or situational information, interoception, introspection, and sensory-motor features that contribute to varying degrees. For example, we hypothesize that event-based representations are critical for conceptual processing related to morality and ToM, as well as for concepts related to social constructs (justice and democracy), which is supplemented by interoceptive affect. For emotion concepts, interoception plays a central role, supplemented by event representations. Introspection is most salient for concepts related to cognition (thought and consideration). Number concepts rely on body-part-based and spatial representations, with little contribution from events. There is no categorical distinction between concrete and abstract concepts, but the reliance on event-based, interoceptive, introspective and sensory-motor dimensions differs. Concrete object concepts, in general, rely more strongly on sensory-motor properties, but other dimensions can play a greater role for specific concepts (e.g. interoceptive affect can play a greater role for concrete object concepts with salient emotional features, such as knife; event information contributes to picnic), or due to task demands. An additional dimension that can contribute to some abstract concepts is metaphoric representation, which is not examined here. Abstract concepts can be understood by analogies to concrete domains [225–227], exemplified by metaphors such as grasping an idea or moving up in the company. Here, correspondence is established between understanding and physical grasp, and between power/authority and higher spatial location, respectively.
Space does not permit discussion of the behavioural findings here, but we note that consistent with the current proposal, a number of investigators have pointed out the importance of contextual, and especially relational or social, information for at least some types of abstract concepts, and provided behavioural evidence in healthy subjects [18,85,228–230]. Additionally, in the treatment of persons with aphasia, training on abstract concepts (justice) leads to generalization to related but untrained concrete concepts (jury), but not vice versa [231,232]. This is also consistent with event-based representations of these abstract concepts that activate concrete entities contained therein. The current study builds on this body of work and provides new evidence from neuroimaging that is not limited by behavioural tasks such as property generation, or explicit consideration of a situation.
While we have provided preliminary neuroimaging evidence in support of some of these hypotheses, a very limited set of abstract concepts was examined here, and it is clear that much work remains to be done to devise ways of partitioning abstract concepts, and to delineate the dimensions and their relative contributions to their representation. Nonetheless, these findings provide constraints on theories of concept representation. Both classical theories, Dual Coding and Context Availability, assume two differing mechanisms that apply to the entire categories of abstract and concrete concepts. This trend of a hybrid approach is also seen in recent theories. A single mechanism dedicated to abstract concept processing carries with it an implicit assumption that not being concrete is a good unifying principle. For example, some models combine embodied and distributional information that is used predominantly for concrete and abstract concepts, respectively [233–235]. Similar to the Dual Coding theory, Dove [236,237] proposed a view where abstract concepts rely on amodal symbols, which acquire meaning only from their relationship with other symbols. Accounts that propose a single and amodal mechanism for abstract concept representation are challenged by the current results, for the two reasons outlined above: (i) there are important differences in how different types of abstract concepts are represented; and (ii) the areas that are commonly activated across abstract domains overlap with those for concrete concepts, and those implicated in episodic memory, event processing, imagery and emotions. The current results fit most naturally within the framework of situated cognition (e.g. [238–241]) and can be interpreted as being most compatible with situated theories, such as Language and Situated Simulation [241] and the Words as Social Tools (WAT) [230,242] proposals. WAT emphasizes the importance of social information that is accessed through words. Activation of areas involved in event representation, episodic memory, emotions and concrete concepts is consistent with retrieval of social interaction memories that have all of these components. While there was no clear support for the other aspect of the theory which postulates linguistic, mouth-related and phonological basis for all types of abstract concepts, negative results are not necessarily conclusive, as emphasized earlier. The Affective Embodiment view [83,243] proposes that affective experience and emotional development are crucial for abstract concepts, and sensory-motor experience is important for concrete concepts. Emotions provide a bootstrapping mechanism for acquisition of abstract words. Although the ‘core’ regions associated with emotion processing, amygdala and OFC, were not activated by all abstract domains, the common activation of mPFC and ACC, involved in emotion processing and regulation, can be interpreted as being broadly consistent with this view, at least for the types of abstract concepts examined here.
Finally, it is worth keeping in mind that many, if not most, brain areas are highly multi-functional. Areas such as mPFC, OFC, insula and precuneus/pCi are activated in a staggering variety of studies and have numerous functional interpretations. The interpretations that we argue for are supported by a large body of literature, and not just by one or two isolated studies. Nonetheless, caution should be used deriving functional interpretations from these activations, with consideration given to alternative views and possibilities, especially with regard to complex processes such as introspection and interoception, as our understanding of the functions and connectivity of these regions increases.
(b). Conclusions
We have provided evidence that both differences and similarities exist in the representation of the four types of abstract concepts considered here. We show that the representation of abstract concepts is much more widespread in the brain than is often assumed. Emotional and number concepts can be grounded directly in emotional, spatial or action-related brain circuits. Higher-order abstract processes of morality and ToM can also be processed and grounded through events, episodic memories and simulations, which in turn rely on emotions, imagery and lower-order concepts. The differences suggest that the way in which various types of abstract concepts are represented can differ substantially. While ‘abstract’ is a useful label to situate oneself in the general area, faster progress can potentially be made if theory development focuses on varieties of abstract concepts and their characteristics, and explores possibly different formats and mechanisms. Promising initial steps in this direction have been taken by a number of investigators (e.g. [82,244–246]). Theory-driven, top-down approaches (e.g. starting by examining categories devised by linguists, available in a thesaurus) and data-driven bottom-up approaches are both promising ways of teasing apart potential categories of abstract concepts from complex high-dimensional neuroimaging or behavioural data. Based on the similarities in activation between the domains, we also propose that events or situations may constitute an important dimension in representing many types of abstract concepts. Categories of abstract concepts may differentially weight event-based, interoceptive, introspective and sensory-motor information.
Supplementary Material
Endnotes
Here, interoception refers to the sensory perception of internal body states. Introspection is used to refer to reflection on one's mental processes, such as reasoning, thoughts, beliefs and will.
We thank an anonymous reviewer for pointing out these counting systems.
Here, we use the term ‘TPJ’ for consistency with the terminology typically adopted in morality and ToM studies. However, it is worth pointing out that this term is used to refer to a variable set of anatomical regions that includes AG, lateral occipital cortex, STS, SMG and pMTG. Often activations that are entirely contained in one of these regions are described as being in the TPJ, which seems unnecessarily vague and creates potential for incorrect reverse inference.
To rule out activations due to executive processing demands, Binder et al. [15] eliminated contrasts that had greater difficulty for the condition of interest relative to that of the control condition. Because abstract conditions frequently have greater difficulty/reaction time, many such contrasts were excluded, resulting in an increase in bias towards concrete semantics, which stemmed from the fact that there were many more studies of concrete concepts to begin with.
Data accessibility
This article has no additional data.
Authors' contributions
R.H.D. conceived of the topics discussed in the paper. M.R. ran the meta-analyses, reviewed literature and collected peaks on emotions and ToM domains. W.v.D. reviewed literature and collected peaks on numbers and morality domains. All authors wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was supported by the NIH/NIDCD grant R01 DC010783 to R.H.D.
References
- 1.Barsalou LW. 2008. Grounded cognition. Annu. Rev. Psychol. 59, 617–645. ( 10.1146/annurev.psych.59.103006.093639) [DOI] [PubMed] [Google Scholar]
- 2.Kiefer M, Pulvermuller F. 2012. Conceptual representations in mind and brain: theoretical developments, current evidence and future directions. Cortex 48, 805–825. ( 10.1016/j.cortex.2011.04.006) [DOI] [PubMed] [Google Scholar]
- 3.Gallese V, Lakoff G. 2005. The brain's concepts: the role of the sensory-motor system in conceptual knowledge. Cogn. Neuropsychol. 22, 455–479. ( 10.1080/02643290442000310) [DOI] [PubMed] [Google Scholar]
- 4.Meteyard L, Cuadrado SR, Bahrami B, Vigliocco G. 2012. Coming of age: a review of embodiment and the neuroscience of semantics. Cortex 48, 788–804. ( 10.1016/j.cortex.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 5.Binder JR, Desai RH. 2011. The neurobiology of semantic memory. Trends Cogn. Sci. 15, 527–536. ( 10.1016/j.tics.2011.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recchia G, Jones MN. 2012. The semantic richness of abstract concepts. Front. Hum. Neurosci. 6, 315 ( 10.3389/fnhum.2012.00315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reilly M, Desai RH. 2017. Effects of semantic neighborhood density in abstract and concrete words. Cognition 169, 46–53. ( 10.1016/j.cognition.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghi AM, Binkofski F, Castelfranchi C, Cimatti F, Scorolli C, Tummolini L. 2017. The challenge of abstract concepts. Psychol. Bull. 143, 263–292. ( 10.1037/bul0000089) [DOI] [PubMed] [Google Scholar]
- 9.Crutch SJ, Warrington EK. 2005. Abstract and concrete concepts have structurally different representational frameworks. Brain 128, 615–627. ( 10.1093/brain/awh349) [DOI] [PubMed] [Google Scholar]
- 10.Crutch SJ, Warrington EK. 2010. The differential dependence of abstract and concrete words upon associative and similarity-based information: complementary semantic interference and facilitation effects. Cogn. Neuropsychol. 27, 46–71. ( 10.1080/02643294.2010.491359) [DOI] [PubMed] [Google Scholar]
- 11.Crutch SJ, Connell S, Warrington EK. 2009. The different representational frameworks underpinning abstract and concrete knowledge: evidence from odd-one-out judgements. Q. J. Exp. Psychol. 62, 1388–1390. ( 10.1080/17470210802483834) [DOI] [PubMed] [Google Scholar]
- 12.Crutch SJ, Jackson EC. 2011. Contrasting graded effects of semantic similarity and association across the concreteness spectrum. Q. J. Exp. Psychol. 64, 1388–1408. ( 10.1080/17470218.2010.543285) [DOI] [PubMed] [Google Scholar]
- 13.Crutch SJ, Ridha BH, Warrington EK. 2006. The different frameworks underlying abstract and concrete knowledge: evidence from a bilingual patient with a semantic refractory access dysphasia. Neurocase 12, 151–163. ( 10.1080/13554790600598832) [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Conder JA, Blitzer DN, Shinkareva SV. 2010. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum. Brain Mapp. 31, 1459–1468. ( 10.1002/hbm.20950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder JR, Desai RH, Graves WW, Conant LL. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796. ( 10.1093/cercor/bhp055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwanenflugel P. 1991. Why are abstract concepts hard to understand? In The psychology of word meanings (ed. Schwanenflugel P.), pp. 223–250. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 17.Schwanenflugel PJ, Stowe RW. 1989. Context availability and the processing of abstract and concrete words in sentences. Read. Res. Q. 24, 114–126. ( 10.2307/748013) [DOI] [Google Scholar]
- 18.Schwanenflugel PJ, Akin C, Luh WM. 1992. Context availability and the recall of abstract and concrete words. Mem. Cognit. 20, 96–104. ( 10.3758/BF03208259) [DOI] [PubMed] [Google Scholar]
- 19.Paivio A. 1986. Mental representations: a dual-coding approach. New York, NY: Oxford University Press. [Google Scholar]
- 20.Paivio A. 2010. Dual coding theory and the mental lexicon. Mental Lexicon 5, 205–230. ( 10.1075/ml.5.2.04pai) [DOI] [Google Scholar]
- 21.Pollock L. 2017. Statistical and methodological problems with concreteness and other semantic variables: a list memory experiment case study. Behav. Res. Methods 55, 64 ( 10.3758/s13428-017-0938-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spunt RP, Kemmerer D, Adolphs R. 2016. The neural basis of conceptualizing the same action at different levels of abstraction. Soc. Cogn. Affect. Neurosci. 11, 1141–1151. ( 10.1093/scan/nsv084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dehaene S, Bossini S, Giraux P. 1993. The mental representation of parity and number magnitude. J. Exp. Psychol. Gen. 122, 371–396. ( 10.1037/0096-3445.122.3.371) [DOI] [Google Scholar]
- 24.Fias WB. 1996. The importance of magnitude information in numerical processing: evidence from the SNARC effect. Math. Cogn. 2, 95–110. ( 10.1080/135467996387552) [DOI] [Google Scholar]
- 25.Berch DB, Foley EJ, Hill RJ, Ryan PM. 1999. Extracting parity and magnitude from Arabic numerals: developmental changes in number processing and mental representation. J. Exp. Child Psychol. 74, 286–308. ( 10.1006/jecp.1999.2518) [DOI] [PubMed] [Google Scholar]
- 26.Wood G, Willmes K, Nuerk HC, Fischer MH. 2008. On the cognitive link between space and number: a meta-analysis of the SNARC effect. Psychol. Sci. Q. 50, 489–525. ( 10.1027/1618-3169.52.3.187) [DOI] [Google Scholar]
- 27.Knoch D, Brugger P, Regard M. 2005. Suppressing versus releasing a habit: frequency-dependent effects of prefrontal transcranial magnetic stimulation. Cereb. Cortex 15, 885–887. ( 10.1093/cercor/bhh196) [DOI] [PubMed] [Google Scholar]
- 28.Brigman S, Cherry KE. 2002. Age and skilled performance: contributions of working memory and processing speed. Brain Cogn. 50, 242–256. ( 10.1016/S0278-2626(02)00510-9) [DOI] [PubMed] [Google Scholar]
- 29.Fagard J, Dahmen R. 2003. The effects of reading-writing direction on the asymmetry of space perception and directional tendencies: a comparison between French and Tunisian children. Laterality 8, 39–52. ( 10.1080/713754473) [DOI] [PubMed] [Google Scholar]
- 30.Zebian S. 2005. Linkages between number concepts, spatial thinking, and directionality of writing: the SNARC effect and the reverse SNARC effect in English and Arabic monoliterates, bilaterates, and illiterate Arabic speakers. J. Cogn. Cult. 1, 165–190. ( 10.1163/1568537054068660) [DOI] [Google Scholar]
- 31.Shaki S, Fischer MH, Petrusic WM. 2009. Reading habits for both words and numbers contribute to the SNARC effect. Psychon. Bull. Rev. 16, 328–331. ( 10.3758/PBR.16.2.328) [DOI] [PubMed] [Google Scholar]
- 32.Gobel SM, Shaki S, Fischer MH. 2011. The cultural number line: a review of cultural and linguistic influences on the development of number processing. J. Cross Cult. Psychol. 42, 543–565. ( 10.1177/0022022111406251) [DOI] [Google Scholar]
- 33.Fischer MH. 2008. Finger counting habits modulate spatial-numerical associations. Cortex 44, 386–392. ( 10.1016/j.cortex.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Lalain M. 2008. On the relationship between handedness and hand-digit mapping in finger counting. Cortex 44, 393–399. ( 10.1016/j.cortex.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 35.Lindemann O, Alipour A, Fischer MH. 2011. Finger counting habits in Middle-Eastern and Western individuals: an online survey. J. Cross Cult. Psychol. 42, 566–578. ( 10.1177/0022022111406254) [DOI] [Google Scholar]
- 36.Luca SD, Grana A, Semenza C, Seron X, Pesenti M. 2006. Finger-digit compatibility in Arabic numeral processing. Q. J. Exp. Psychol. 59, 1648–1663. ( 10.1080/17470210500256839) [DOI] [PubMed] [Google Scholar]
- 37.Fischer MH, Brugger P. 2011. When digits help digits: spatial–numerical associations point to finger counting as prime example of embodied cognition. Front. Psychol. 2, 260 ( 10.3389/fpsyg.2011.00260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brozzoli C, Ishihara M, Gobel SM, Salemme R, Rossetti Y, Farne A. 2008. Touch perception reveals the dominance of spatial over digital representation of numbers. Proc. Natl Acad. Sci. USA 105, 5644–5648. ( 10.1073/pnas.0708414105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaisier MA, Smeets JB. 2011. Number magnitude to finger mapping is disembodied and topological. Exp. Brain Res. 209, 395–400. ( 10.1007/s00221-011-2562-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chochon F, Cohen L, van de Moortele P, Dehaene S. 1999. Differential contributions of the left and right inferior parietal lobules to number processing. J. Cogn. Neurosci. 11, 617–630. ( 10.1162/089892999563689) [DOI] [PubMed] [Google Scholar]
- 41.Pinel P, Le Clec HG, van de Moortele PF, Naccache L, Le Bihan D, Dehaene S. 1999. Event-related fMRI analysis of the cerebral circuit for number comparison. Neuroreport 10, 1473–1479. ( 10.1097/00001756-199905140-00015) [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Zhang W, Chen K, Feng S, Ji Y, Shen J, Reiman EM, Liu Y. 2006. Arithmetic processing in the brain shaped by cultures. Proc. Natl Acad. Sci. USA 103, 10 775–10 780. ( 10.1073/pnas.0604416103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehaene S, Dehaene-Lambertz G, Cohen L. 1998. Abstract representation of numbers in the animal and human brain. Trends Neurosci. 21, 355–361. ( 10.1016/S0166-2236(98)01263-6) [DOI] [PubMed] [Google Scholar]
- 44.Rusconi E, Turatto M, Umilta C. 2007. Two orienting mechanisms in posterior parietal lobule: an rTMS study of the Simon and SNARC effects. Cogn. Neuropsychol. 24, 373–392. ( 10.1080/02643290701309425) [DOI] [PubMed] [Google Scholar]
- 45.Laird AR, et al. 2010. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage 51, 677–683. ( 10.1016/j.neuroimage.2010.02.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. 2007. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205. ( 10.1002/hbm.20345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arsalidou M, Taylor MJ. 2011. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54, 2382–2393. ( 10.1016/j.neuroimage.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 48.Sokolowski HM, Fias W, Bosah Ononye C, Ansari D. 2017. Are numbers grounded in a general magnitude processing system? A functional neuroimaging meta-analysis. Neuropsychologia 105, 50–69. ( 10.1016/j.neuropsychologia.2017.01.019) [DOI] [PubMed] [Google Scholar]
- 49.Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. ( 10.1038/nrn755) [DOI] [PubMed] [Google Scholar]
- 50.Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. 2001. Functional specificity of superior parietal mediation of spatial shifting. Neuroimage 14, 661–673. ( 10.1006/nimg.2001.0860) [DOI] [PubMed] [Google Scholar]
- 51.Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz R, Freund H. 1998. Human anterior intraparietal area subserves prehension. Neurology 50, 1253–1259. ( 10.1212/WNL.50.5.1253) [DOI] [PubMed] [Google Scholar]
- 52.Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. 2003. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp. Brain Res. 153, 180–189. ( 10.1007/s00221-003-1591-5) [DOI] [PubMed] [Google Scholar]
- 53.Gallivan JP, Culham JC. 2015. Neural coding within human brain areas involved in actions. Curr. Opin. Neurobiol. 33, 141–149. ( 10.1016/j.conb.2015.03.012) [DOI] [PubMed] [Google Scholar]
- 54.Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. 2002. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron 33, 475–487. ( 10.1016/S0896-6273(02)00575-5) [DOI] [PubMed] [Google Scholar]
- 55.Goodale MA, Milner BA. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25. ( 10.1016/0166-2236(92)90344-8) [DOI] [PubMed] [Google Scholar]
- 56.Freud E, Plaut DC, Behrmann M. 2016. ‘What’ is happening in the dorsal visual pathway. Trends Cogn. Sci. 20, 773–784. ( 10.1016/j.tics.2016.08.003) [DOI] [PubMed] [Google Scholar]
- 57.Eger E, Sterzer P, Russ MO, Giraud AL, Kleinschmidt A. 2003. A supramodal number representation in human intraparietal cortex. Neuron 37, 719–725. ( 10.1016/S0896-6273(03)00036-9) [DOI] [PubMed] [Google Scholar]
- 58.Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. 2004. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron 44, 547–555. ( 10.1016/j.neuron.2004.10.014) [DOI] [PubMed] [Google Scholar]
- 59.Piazza M, Pinel P, Le Bihan D, Dehaene S. 2007. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53, 293–305. ( 10.1016/j.neuron.2006.11.022) [DOI] [PubMed] [Google Scholar]
- 60.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. 1999. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284, 970–974. ( 10.1126/science.284.5416.970) [DOI] [PubMed] [Google Scholar]
- 61.Fulbright RK, Manson SC, Skudlarski P, Lacadie CM, Gore JC. 2003. Quantity determination and the distance effect with letters, numbers, and shapes: a functional MR imaging study of number processing. Am. J. Neuroradiol. 24, 193–200. [PMC free article] [PubMed] [Google Scholar]
- 62.Butterworth B. 1999. A head for figures. Science 284, 928–929. ( 10.1126/science.284.5416.928) [DOI] [PubMed] [Google Scholar]
- 63.Simon TJ. 1999. The foundations of numerical thinking in a brain without numbers. Trends Cogn. Sci. 3, 363–365. ( 10.1016/S1364-6613(99)01383-2) [DOI] [PubMed] [Google Scholar]
- 64.Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binder JR, Cox RW. 2000. Specialized neural systems underlying representations of sequential movements. J. Cogn. Neurosci. 12, 56–77. ( 10.1162/08989290051137602) [DOI] [PubMed] [Google Scholar]
- 65.Haslinger B, Erhard P, Weilke F, Ceballos-Baumann AO, Bartenstein P, von Einsiedel HG, Schwaiger M, Conrad B, Boecker H. 2002. The role of lateral premotor–cerebellar–parietal circuits in motor sequence control: a parametric fMRI study. Brain Res. Cogn. 13, 159–168. ( 10.1016/S0926-6410(01)00104-5) [DOI] [PubMed] [Google Scholar]
- 66.Pesenti M, Thioux M, Seron X, De Volder A. 2000. Neuroanatomical substrates of Arabic number processing, numerical comparison, and simple addition: a PET study. J. Cogn. Neurosci. 12, 461–479. ( 10.1162/089892900562273) [DOI] [PubMed] [Google Scholar]
- 67.Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B. 1996. Cerebral activations during number multiplication and comparison: a PET study. Neuropsychologia 34, 1097–1106. ( 10.1016/0028-3932(96)00027-9) [DOI] [PubMed] [Google Scholar]
- 68.Andres M, Seron X., Olivier E. 2007. Contribution of hand motor circuits to counting. J. Cogn. Neurosci. 19, 563–576. ( 10.1162/jocn.2007.19.4.563) [DOI] [PubMed] [Google Scholar]
- 69.Walsh V. 2003. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 7, 483–488. ( 10.1016/j.tics.2003.09.002) [DOI] [PubMed] [Google Scholar]
- 70.Bueti D, Walsh V. 2009. The parietal cortex and the representation of time, space, number and other magnitudes. Phil. Trans. R. Soc. B 364, 1831–1840. ( 10.1098/rstb.2009.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seghier ML. 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. ( 10.1177/1073858412440596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. 2009. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia 47, 604–608. ( 10.1016/j.neuropsychologia.2008.10.013) [DOI] [PubMed] [Google Scholar]
- 73.Ischebeck A, Zamarian L, Schocke M, Delazer M. 2009. Flexible transfer of knowledge in mental arithmetic—an fMRI study. Neuroimage 44, 1103–1112. ( 10.1016/j.neuroimage.2008.10.025) [DOI] [PubMed] [Google Scholar]
- 74.Krause F, Lindemann O, Toni I, Bekkering H. 2014. Different brains process numbers differently: structural bases of individual differences in spatial and nonspatial number representations. J. Cogn. Neurosci. 26, 768–776. ( 10.1162/jocn_a_00518) [DOI] [PubMed] [Google Scholar]
- 75.Henrich J. 2016. The secret to our success. Princeton, NJ: Princeton University Press. [Google Scholar]
- 76.Russell JA. 1980. A circumplex model of affect. J. Pers. Soc. Psychol. 39, 1161–1178. ( 10.1037/h0077714) [DOI] [Google Scholar]
- 77.Posner J, Russell JA, Peterson BS. 2005. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 17, 715–734. ( 10.1017/S0954579405050340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bradley MM, Lang PJ. 1999. Affective norms for English words (ANEW): instruction. Res. Psychophysiol.Tech. Rep. C-1. Gainesville, FL: Center for Research in Psychophysiology, University of Florida.
- 79.Ito TA, Cacioppo JT, Lang PJ. 1998. Eliciting affect using the international affective picture system: trajectories through evaluative space. Pers. Soc. Psychol. Bull. 24, 855–879. ( 10.1177/0146167298248006) [DOI] [Google Scholar]
- 80.Sakaki M, Niki K, Mather M. 2012. Beyond arousal and valence: the importance of the biological versus social relevance of emotional stimuli. Cogn. Affect. Behav. Neurosci. 12, 115–139. ( 10.3758/s13415-011-0062-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghio M, Vaghi MMS, Tettamanti M. 2013. Fine-grained semantic categorization across the abstract and concrete domains. PLoS ONE 8, e67090 ( 10.1371/journal.pone.0067090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Troche J, Crutch S, Reilly J. 2014. Clustering, hierarchical organization, and the topography of abstract and concrete nouns. Front. Psychol. 5, 64 ( 10.3389/fpsyg.2014.00360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kousta ST, Vigliocco G, Vinson DP, Andrews M, Del Campo E. 2011. The representation of abstract words: why emotion matters. J. Exp. Psychol. Gen. 140, 14–34. ( 10.1037/a0021446) [DOI] [PubMed] [Google Scholar]
- 84.Yap MJ, Seow CS. 2014. The influence of emotion on lexical processing: insights from RT distributional analysis. Psychon. Bull. Rev. 21, 526–533. ( 10.3758/s13423-013-0525-x) [DOI] [PubMed] [Google Scholar]
- 85.Moffat M, Siakaluk PD, Sidhu DM, Pexman PM. 2015. Situated conceptualization and semantic processing: effects of emotional experience and context availability in semantic categorization and naming tasks. Psychon. Bull. Rev. 22, 408–419. ( 10.3758/s13423-014-0696-0) [DOI] [PubMed] [Google Scholar]
- 86.Vinson D, Ponari M, Vigliocco G. 2014. How does emotional content affect lexical processing? Cogn. Emot. 28, 737–746. ( 10.1080/02699931.2013.851068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giffard B, Laisney M, Desgranges B, Eustache F. 2015. An exploration of the semantic network in Alzheimer's disease: influence of emotion and concreteness of concepts. Cortex 69, 201–211. ( 10.1016/j.cortex.2015.05.020) [DOI] [PubMed] [Google Scholar]
- 88.Sergerie K, Chochol C, Armony JL. 2008. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 32, 811–830. ( 10.1016/j.neubiorev.2007.12.002) [DOI] [PubMed] [Google Scholar]
- 89.Isenberg N, Silbersweig DA, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC, Stern E. 1999. Linguistic threat activates the human amygdala. Proc. Natl Acad. Sci. USA 96, 10 456–10 459. ( 10.1073/pnas.96.18.10456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. 2001. Neural activity associated with episodic memory for emotional context. Neuropsychologia 39, 910–920. ( 10.1016/S0028-3932(01)00025-2) [DOI] [PubMed] [Google Scholar]
- 91.Stillman PE, Van Bavel JJ, Cunningham WA. 2015. Valence asymmetries in the human amygdala: task relevance modulates amygdala responses to positive more than negative affective cues. J. Cogn. Neurosci. 27, 842–851. ( 10.1162/jocn_a_00756) [DOI] [PubMed] [Google Scholar]
- 92.Hamann SB, Ely TD, Hoffman JM, Kilts CD. 2002. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol. Sci. 13, 135–141. ( 10.1111/1467-9280.00425) [DOI] [PubMed] [Google Scholar]
- 93.Lai VT, Willems RM, Hagoort P. 2015. Feel between the lines: implied emotion in sentence comprehension. J. Cogn. Neurosci. 27, 1528–1541. ( 10.1162/jocn_a_00798) [DOI] [PubMed] [Google Scholar]
- 94.Sander D, Grafman J, Zalla T. 2003. The human amygdala: an evolved system for relevance detection. Rev. Neurosci. 14, 303–316. ( 10.1515/REVNEURO.2003.14.4.303) [DOI] [PubMed] [Google Scholar]
- 95.Pessoa L. 2010. Emotion and cognition and the amygdala: from ‘what is it?’ to ‘what's to be done?’ Neuropsychologia 48, 3416–3429. ( 10.1016/j.neuropsychologia.2010.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pessoa L, Adolphs R. 2010. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 11, 773–783. ( 10.1038/nrn2920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallis JD. 2007. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 30, 31–56. ( 10.1146/annurev.neuro.30.051606.094334) [DOI] [PubMed] [Google Scholar]
- 98.Levens SM, Larsen JT, Bruss J, Tranel D, Bechara A, Mellers BA. 2014. What might have been? The role of the ventromedial prefrontal cortex and lateral orbitofrontal cortex in counterfactual emotions and choice. Neuropsychologia 54, 77–86. ( 10.1016/j.neuropsychologia.2013.10.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brooks JA, Shablack H, Gendron M, Satpute AB, Parrish MH, Lindquist KA. 2017. The role of language in the experience and perception of emotion: a neuroimaging meta-analysis. Soc. Cogn. Affect. Neurosci. 12, 169–183. ( 10.1093/scan/nsw121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuchinke L, Jacobs AM, Grubich C, Võ MLH, Conrad M, Herrmann M. 2005. Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage 28, 1022–1032. ( 10.1016/j.neuroimage.2005.06.050) [DOI] [PubMed] [Google Scholar]
- 101.Colibazzi T, et al. 2010. Supplemental material for neural systems subserving valence and arousal during the experience of induced emotions. Emotion 10, 377–389. ( 10.1037/a0018484) [DOI] [PubMed] [Google Scholar]
- 102.Kensinger EA, Corkin S. 2004. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc. Natl Acad. Sci. USA 101, 3310–3315. ( 10.1073/pnas.0306408101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. 2007. Neural correlates of processing valence and arousal in affective words. Cereb. Cortex 17, 742–748. ( 10.1093/cercor/bhk024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sladky R, Hoflich A, Kublbock M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C. 2015. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for FMRI. Cereb. Cortex 25, 895–903. ( 10.1093/cercor/bht279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petrovic P, et al. 2016. Significant grey matter changes in a region of the orbitofrontal cortex in healthy participants predicts emotional dysregulation. Soc. Cogn. Affect. Neurosci. 11, 1041–1049. ( 10.1093/scan/nsv072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, Hori T, Howard MA III, Adolphs R. 2001. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat. Neurosci. 4, 15–16. ( 10.1038/82850) [DOI] [PubMed] [Google Scholar]
- 107.Chikazoe J, Lee DH, Kriegeskorte N, Anderson AK. 2014. Population coding of affect across stimuli, modalities and individuals. Nat. Neurosci. 17, 1114–1122. ( 10.1038/nn.3749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vigliocco G, Kousta ST, Della Rosa PA, Vinson DP, Tettamanti M, Devlin JT, Cappa SF. 2013. The neural representation of abstract words: the role of emotion. Cereb. Cortex 24, 1767–1777. ( 10.1093/cercor/bht025) [DOI] [PubMed] [Google Scholar]
- 109.Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93. ( 10.1016/j.tics.2010.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. ( 10.1016/S1364-6613(00)01483-2) [DOI] [PubMed] [Google Scholar]
- 111.Phan KL, Wager T, Taylor SF, Liberzon I. 2002. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348. ( 10.1006/nimg.2002.1087) [DOI] [PubMed] [Google Scholar]
- 112.Beckmann M, Johansen-Berg H, Rushworth MF. 2009. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190. ( 10.1523/JNEUROSCI.3328-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiba T, Kayahara T, Nakano K. 2001. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 888, 83–101. ( 10.1016/S0006-8993(00)03013-4) [DOI] [PubMed] [Google Scholar]
- 114.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. 2006. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882. ( 10.1016/j.neuron.2006.07.029) [DOI] [PubMed] [Google Scholar]
- 115.Kragel PA, LaBar KS. 2016. Decoding the nature of emotion in the brain. Trends Cogn. Sci. 20, 444–455. ( 10.1016/j.tics.2016.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saarimaki H, Gotsopoulos A, Jaaskelainen IP, Lampinen J, Vuilleumier P, Hari R, Sams M, Nummenmaa L. 2016. Discrete neural signatures of basic emotions. Cereb. Cortex 26, 2563–2573. ( 10.1093/cercor/bhv086) [DOI] [PubMed] [Google Scholar]
- 117.Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, Manes F, Ibanez A. 2017. Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex 88, 124–142. ( 10.1016/j.cortex.2016.12.019) [DOI] [PubMed] [Google Scholar]
- 118.Dolan RJ, Lane R, Chua P, Fletcher P. 2000. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage 11, 203–209. ( 10.1006/nimg.2000.0538) [DOI] [PubMed] [Google Scholar]
- 119.Ferstl EC, von Cramon DY. 2007. Time, space and emotion: fMRI reveals content-specific activation during text comprehension. Neurosci. Lett. 427, 159–164. ( 10.1016/j.neulet.2007.09.046) [DOI] [PubMed] [Google Scholar]
- 120.Skipper LM, Olson IR. 2014. Semantic memory: distinct neural representations for abstractness and valence. Brain Lang. 130, 1–10. ( 10.1016/j.bandl.2014.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ross LA, Olson IR. 2010. Social cognition and the anterior temporal lobes. Neuroimage 49, 3452–3462. ( 10.1016/j.neuroimage.2009.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olson IR, Plotzker A, Ezzyat Y. 2007. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731. ( 10.1093/brain/awm052) [DOI] [PubMed] [Google Scholar]
- 123.Olson IR, McCoy D, Klobusicky E, Ross LA. 2013. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc. Cogn. Affect. Neurosci. 8, 123–133. ( 10.1093/scan/nss119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crosson B, Cato MA, Sadek JR, Bauer G, Fischler IS, Maron L, Gopinath K. 2002. Semantic monitoring of words with emotional connotation during fMRI: contribution of anterior left frontal cortex. J. Int. Neuropsychol. Soc. 8, 607–622. ( 10.1017/S1355617702801394) [DOI] [PubMed] [Google Scholar]
- 125.Mellem MS, Jasmin KM, Peng C, Martin A. 2016. Sentence processing in anterior superior temporal cortex shows a social-emotional bias. Neuropsychologia 89, 217–224. ( 10.1016/j.neuropsychologia.2016.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kling AS, Tachiki K, Lloyd R. 1993. Neurochemical correlates of the Klüver-Bucy syndrome by in vivo microdialysis in monkey. Behav. Brain Res. 56, 161–170. ( 10.1016/0166-4328(93)90034-N) [DOI] [PubMed] [Google Scholar]
- 127.Barrett LF. 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 12, 1–23. ( 10.1093/scan/nsx060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barrett LF. 2017. How emotions are made: the secret life of the brain. New York, NY: Houghton-Mifflin-Harcourt. [Google Scholar]
- 129.Wilson-Mendenhall CD, Barrett LF, Simmons WK, Barsalou LW. 2011. Grounding emotion in situated conceptualization. Neuropsychologia 49, 1105–1127. ( 10.1016/j.neuropsychologia.2010.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen TR, Montoya RM, Insko CA. 2006. Group morality and intergroup relations: cross-cultural and experimental evidence. Pers. Soc. Psychol. Bull. 32, 1559–1572. ( 10.1177/0146167206291673) [DOI] [PubMed] [Google Scholar]
- 131.Tassy S, Oullier O, Mancini J, Wicker B. 2013. Discrepancies between judgment and choice of action in moral dilemmas. Front. Psychol. 4, 250 ( 10.3389/fpsyg.2013.00250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garrigan B, Adlam AL, Langdon PE. 2016. The neural correlates of moral decision-making: a systematic review and meta-analysis of moral evaluations and response decision judgements. Brain Cogn. 108, 88–97. ( 10.1016/j.bandc.2016.07.007) [DOI] [PubMed] [Google Scholar]
- 133.Knobe J. 2005. Theory of mind and moral cognition: exploring the connections. Trends Cogn. Sci. 9, 357–359. ( 10.1016/j.tics.2005.06.011) [DOI] [PubMed] [Google Scholar]
- 134.Cushman F. 2008. Crime and punishment: distinguishing the roles of causal and intentional analyses in moral judgment. Cognition 108, 353–380. ( 10.1016/j.cognition.2008.03.006) [DOI] [PubMed] [Google Scholar]
- 135.Killen M, Lynn Mulvey K, Richardson C, Jampol N, Woodward A. 2011. The accidental transgressor: morally-relevant theory of mind. Cognition 119, 197–215. ( 10.1016/j.cognition.2011.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Premack D, Woodruff G. 1978. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 4, 515–526. [Google Scholar]
- 137.Frith U, Frith CD. 2003. Development and neurophysiology of mentalizing. Phil. Trans. R. Soc. Lond. B 358, 459–473. ( 10.1098/rstb.2002.1218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. 2012. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 217, 783–796. ( 10.1007/s00429-012-0380-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eisenberger N. 2000. Emotion, regulation, and moral development. Ann. Rev. Psychol. 51, 665–697. ( 10.1146/annurev.psych.51.1.665) [DOI] [PubMed] [Google Scholar]
- 140.Hare RD. 2003. The Hare psychopathy checklist–revised, 2nd edn Toronto, Canada: Multi-Health Systems. [Google Scholar]
- 141.Soderstrom H. 2003. Psychopathy as a disorder of empathy. Eur. Child Adolesc. Psychiatry 12, 249–252. ( 10.1007/s00787-003-0338-y) [DOI] [PubMed] [Google Scholar]
- 142.Blair J, Marsh AA, Finger E, Blair KS, Luo J. 2006. Neuro-cognitive systems involved in morality. Philos. Explor. 9, 13–27. ( 10.1080/13869790500492359) [DOI] [Google Scholar]
- 143.Fumagalli M, Priori A. 2012. Functional and clinical neuroanatomy of morality. Brain 135, 2006–2021. ( 10.1093/brain/awr334) [DOI] [PubMed] [Google Scholar]
- 144.Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. 2001. An fMRI investigation of emotional engagement in moral judgment. Science 293, 2105–2108. ( 10.1126/science.1062872) [DOI] [PubMed] [Google Scholar]
- 145.Schurz M, Tholen MG, Perner J, Mars RB, Sallet J. 2017. Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: a review using probabilistic atlases from different imaging modalities. Hum. Brain Mapp. 38, 4788–4805. ( 10.1002/hbm.23675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saxe R, Powell LJ. 2006. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17, 692–699. ( 10.1111/j.1467-9280.2006.01768.x) [DOI] [PubMed] [Google Scholar]
- 147.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. 2007. Two takes on the social brain: a comparison of theory of mind tasks. J. Cogn. Neurosci. 19, 1803–1814. ( 10.1162/jocn.2007.19.11.1803) [DOI] [PubMed] [Google Scholar]
- 148.Chan YC, Lavallee JP. 2015. Temporo-parietal and fronto-parietal lobe contributions to theory of mind and executive control: an fMRI study of verbal jokes. Front. Psychol. 6, 1285 ( 10.3389/fpsyg.2015.01285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mason RA, Just MA. 2011. Differentiable cortical networks for inferences concerning people's intentions versus physical causality. Hum. Brain Mapp. 32, 313–329. ( 10.1002/hbm.21021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saxe R, Wexler A. 2005. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43, 1391–1399. ( 10.1016/j.neuropsychologia.2005.02.013) [DOI] [PubMed] [Google Scholar]
- 151.Seghier ML, Fagan E, Price CJ. 2010. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci. 30, 16 809–16 817. ( 10.1523/JNEUROSCI.3377-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mitchell JP. 2009. Social psychology as a natural kind. Trends Cogn. Sci. 13, 246–251. ( 10.1016/j.tics.2009.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amodio DM, Frith CD. 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. ( 10.1038/nrn1884) [DOI] [PubMed] [Google Scholar]
- 154.Mar RA. 2011. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 62, 103–134. ( 10.1146/annurev-psych-120709-145406) [DOI] [PubMed] [Google Scholar]
- 155.Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. 2013. Ventromedial prefrontal cortex encodes emotional value. J. Neurosci. 33, 11 032–11 039. ( 10.1523/JNEUROSCI.4317-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. 2008. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 42, 998–1031. ( 10.1016/j.neuroimage.2008.03.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. 2009. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron 64, 431–439. ( 10.1016/j.neuron.2009.09.040) [DOI] [PubMed] [Google Scholar]
- 158.Kable JW, Glimcher PW. 2007. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 10, 1625–1633. ( 10.1038/nn2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rolls ET. 2000. The orbitofrontal cortex and reward. Cereb. Cortex 10, 284–294. ( 10.1093/cercor/10.3.284) [DOI] [PubMed] [Google Scholar]
- 160.Schultz W, Tremblay L, Hollerman JR. 2000. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex 10, 272–284. ( 10.1093/cercor/10.3.272) [DOI] [PubMed] [Google Scholar]
- 161.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. 2009. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132, 617–627. ( 10.1093/brain/awn279) [DOI] [PubMed] [Google Scholar]
- 162.Bzdok D, Langner R, Hoffstaedter F, Turetsky BI, Zilles K, Eickhoff SB. 2012. The modular neuroarchitecture of social judgments on faces. Cereb. Cortex 22, 951–961. ( 10.1093/cercor/bhr166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spreng RN, Mar RA, Kim ASN. 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510. ( 10.1162/jocn.2008.21029) [DOI] [PubMed] [Google Scholar]
- 164.An X, Bandler R, Ongur D, Price JL. 1998. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 401, 455–479. ( 10.1002/(SICI)1096-9861(19981130)401:4%3C455::AID-CNE3%3E3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- 165.Ongur D, An X, Price JL. 1998. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J. Comp. Neurol. 401, 480–505. ( 10.1002/(SICI)1096-9861(19981130)401:4%3C480::AID-CNE4%3E3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 166.Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. 2013. Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosci. 7, 232 ( 10.3389/fnhum.2013.00232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. 2016. Functional segregation of the human dorsomedial prefrontal cortex. Cereb. Cortex 26, 304–321. ( 10.1093/cercor/bhu250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kjaer TW, Lou HC. 2000. Interaction between precuneus and dorsolateral prefrontal cortex may play a unitary role in consciousness—a principal component analysis of rCBF. Conscious Cogn. 9, S59. [Google Scholar]
- 169.Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC. 2001. Precuneus–prefrontal activity during awareness of visual verbal stimuli. Conscious Cogn. 10, 356–365. ( 10.1006/ccog.2001.0509) [DOI] [PubMed] [Google Scholar]
- 170.Cavanna AE, Trimble MR. 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. ( 10.1093/brain/awl004) [DOI] [PubMed] [Google Scholar]
- 171.Schaafsma SM, Pfaff DW, Spunt RP, Adolphs R. 2015. Deconstructing and reconstructing theory of mind. Trends Cogn. Sci. 19, 65–72. ( 10.1016/j.tics.2014.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saxe R, Kanwisher N. 2003. People thinking about thinking people. The role of the temporo-parietal junction in ‘theory of mind’. Neuroimage 19, 1835–1842. ( 10.1016/S1053-8119(03)00230-1) [DOI] [PubMed] [Google Scholar]
- 173.Mitchell RLC, Phillips LH. 2015. The overlapping relationship between emotion perception and theory of mind. Neuropsychologia 70, 1–10. ( 10.1016/j.neuropsychologia.2015.02.018) [DOI] [PubMed] [Google Scholar]
- 174.Brothers L, Ring B. 1992. A neuroethological framework for the representation of minds. J. Cogn. Neurosci. 4, 107–118. ( 10.1162/jocn.1992.4.2.107) [DOI] [PubMed] [Google Scholar]
- 175.Frith CD, Frith U. 2006. The neural basis of mentalizing. Neuron 50, 531–534. ( 10.1016/j.neuron.2006.05.001) [DOI] [PubMed] [Google Scholar]
- 176.Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. 2005. The neural correlates of direct and reflected self-knowledge. Neuroimage 28, 797–814. ( 10.1016/j.neuroimage.2005.06.069) [DOI] [PubMed] [Google Scholar]
- 177.Singer T. 2006. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev. 30, 855–863. ( 10.1016/j.neubiorev.2006.06.011) [DOI] [PubMed] [Google Scholar]
- 178.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. 2014. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 42, 9–34. ( 10.1016/j.neubiorev.2014.01.009) [DOI] [PubMed] [Google Scholar]
- 179.Abraham A, Rakoczy H, Werning M, von Cramon DY, Schubotz RI. 2010. Matching mind to world and vice versa: functional dissociations between belief and desire mental state processing. Soc. Neurosci. 5, 1–18. ( 10.1080/17470910903166853) [DOI] [PubMed] [Google Scholar]
- 180.Bodden ME, Kubler D, Knake S, Menzler K, Heverhagen JT, Sommer J, Kalbe E, Krach S, Dodel R. 2013. Comparing the neural correlates of affective and cognitive theory of mind using fMRI: involvement of the basal ganglia in affective theory of mind. Adv. Cogn. Psychol. 9, 32–43. ( 10.5709/acp-0129-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aichhorn M, Perner J, Kronbichler M, Staffen W, Ladurner G. 2006. Do visual perspective tasks need theory of mind? Neuroimage 30, 1059–1068. ( 10.1016/j.neuroimage.2005.10.026) [DOI] [PubMed] [Google Scholar]
- 182.Feng S, Ye X, Mao L, Yue X. 2014. The activation of theory of mind network differentiates between point-to-self and point-to-other verbal jokes: an fMRI study. Neurosci. Lett. 564, 32–36. ( 10.1016/j.neulet.2014.01.059) [DOI] [PubMed] [Google Scholar]
- 183.Kliemann D, Young L, Scholz J, Saxe R. 2008. The influence of prior record on moral judgment. Neuropsychologia 46, 2949–2957. ( 10.1016/j.neuropsychologia.2008.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Young L, Cushman F, Hauser M, Saxe R. 2007. The neural basis of the interaction between theory of mind and moral judgment. Proc. Natl Acad. Sci. USA 104, 8235–8240. ( 10.1073/pnas.0701408104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G. 2009. Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J. Cogn. Neurosci. 21, 1179–1192. ( 10.1162/jocn.2009.21082) [DOI] [PubMed] [Google Scholar]
- 186.Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G. 2006. Thinking of mental and other representations: the roles of left and right temporo-parietal junction. Soc. Neurosci. 1, 245–258. ( 10.1080/17470910600989896) [DOI] [PubMed] [Google Scholar]
- 187.Callejas A, Shulman GL, Corbetta M. 2011. False belief vs. false photographs: a test of theory of mind or working memory? Front. Psychol. 2, 316 ( 10.3389/fpsyg.2011.00316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mitchell JP. 2008. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb. Cortex 18, 262–271. ( 10.1093/cercor/bhm051) [DOI] [PubMed] [Google Scholar]
- 189.Kobayashi C, Glover GH, Temple E. 2007. Children's and adults' neural bases of verbal and nonverbal ‘theoy of mind’. Neuropsychologia 45, 1522–1532. ( 10.1016/j.neuropsychologia.2006.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saxe R, Moran JM, Scholz J, Gabrieli J. 2006. Overlapping and non-overlapping brain regions for theory of mind and self-reflection in individual subjects. Soc. Cogn. Affect. Neurosci. 1, 229–234. ( 10.1093/scan/nsl034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Young L, Dodell-Feder D, Saxe R. 2010. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia 48, 2658–2664. ( 10.1016/j.neuropsychologia.2010.05.012) [DOI] [PubMed] [Google Scholar]
- 192.Calarge C, Andreasen NC, O'Leary D. 2003. Visualizing how one brain understands another: a PET study of theory of mind. Am. J. Psychiatry 160, 1954–1964. ( 10.1176/appi.ajp.160.11.1954) [DOI] [PubMed] [Google Scholar]
- 193.Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. 1995. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition 57, 109–128. ( 10.1016/0010-0277(95)00692-R) [DOI] [PubMed] [Google Scholar]
- 194.Gallagher A, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. 2000. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38, 11–21. ( 10.1016/S0028-3932(99)00053-6) [DOI] [PubMed] [Google Scholar]
- 195.Mitchell JP, Macrae CN, Banaji MR. 2006. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50, 655–663. ( 10.1016/j.neuron.2006.03.040) [DOI] [PubMed] [Google Scholar]
- 196.Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, Vogeley K. 2006. Being with virtual others: neural correlates of social interaction. Neuropsychologia 44, 718–730. ( 10.1016/j.neuropsychologia.2005.07.017) [DOI] [PubMed] [Google Scholar]
- 197.Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. 1999. In search of the self: a positron emission tomography study. Psychol. Sci. 10, 26–34. ( 10.1111/1467-9280.00102) [DOI] [Google Scholar]
- 198.Farrow TFD, Zheng Y, Wilkinson ID, Spence SA, Deakin JFW, Tarrier N, Griffiths PD, Woodruff PWR. 2001. Investigating the functional anatomy of empathy and forgiveness. Neuroreport 12, 2433–2438. ( 10.1097/00001756-200108080-00029) [DOI] [PubMed] [Google Scholar]
- 199.Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. 2006. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29, 90–98. ( 10.1016/j.neuroimage.2005.07.022) [DOI] [PubMed] [Google Scholar]
- 200.Moll J, de Oliviera-Souza R, Eslinger PJ, Bramati IE, Mourão-Miranda J, Andreiuolo PA, Pessoa L. 2002. The neural correlates of moral sensitivity: a functional magnetic resononance imaging investigation of basic and moral emotions. J. Neurosci. 22, 2730–2736. ( 10.1523/JNEUROSCI.22-07-02730.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fernandino L, Binder JR, Desai RH, Pendl SL, Humphries CJ, Gross WL, Conant LL, Seidenberg MS. 2016. Concept representation reflects multimodal abstraction: a framework for embodied semantics. Cereb. Cortex 26, 2018–2034. ( 10.1093/cercor/bhv020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. 2010. Coherent concepts are computed in the anterior temporal lobes. Proc. Natl Acad. Sci. USA 107, 2717–2722. ( 10.1073/pnas.0907307107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ralph MA, Jefferies E, Patterson K, Rogers TT. 2017. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 18, 42–55. ( 10.1038/nrn.2016.150) [DOI] [PubMed] [Google Scholar]
- 204.Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779. ( 10.1016/j.neuropsychologia.2009.02.028) [DOI] [PubMed] [Google Scholar]
- 205.Burgess N. 2008. Spatial cognition and the brain. Ann. NY Acad. Sci. 1124, 77–97. ( 10.1196/annals.1440.002) [DOI] [PubMed] [Google Scholar]
- 206.Freton M, Lemogne C, Bergouignan L, Delaveau P, Lehericy S, Fossati P. 2014. The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Struct. Funct. 219, 959–968. ( 10.1007/s00429-013-0546-2) [DOI] [PubMed] [Google Scholar]
- 207.Vann SD, Aggleton JP, Maguire EA. 2009. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802. ( 10.1038/nrn2733) [DOI] [PubMed] [Google Scholar]
- 208.Epstein RA, Parker WE, Feiler AM. 2007. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J. Neurosci. 27, 6141–6149. ( 10.1523/JNEUROSCI.0799-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fernandino L, Humphries CJ, Conant LL, Seidenberg MS, Binder JR. 2016. Heteromodal cortical areas encode sensory-motor features of word meaning. J. Neurosci. 36, 9763–9769. ( 10.1523/JNEUROSCI.4095-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bonner MF, Peelle JE, Cook PA, Grossman M. 2013. Heteromodal conceptual processing in the angular gyrus. Neuroimage 71, 175–186. ( 10.1016/j.neuroimage.2013.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Binder JR, Westbury C, McKiernan KA, Possing ET, Medler DA. 2005. Distinct brain systems for processing concrete and abstract concepts. J. Cogn. Neurosci. 17, 905–917. ( 10.1162/0898929054021102) [DOI] [PubMed] [Google Scholar]
- 212.Ferstl EC, Rinck M, von Cramon DY. 2005. Emotional and temporal aspects of situation model processing during text comprehension: an event-related fMRI study. J. Cogn. Neurosci. 17, 724–739. ( 10.1162/0898929053747658) [DOI] [PubMed] [Google Scholar]
- 213.Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM. 2007. Neural correlates of verb argument structure processing. J. Cogn. Neurosci. 19, 1753–1767. ( 10.1162/jocn.2007.19.11.1753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kalenine S, Peyrin C, Pichat C, Segebarth C, Bonthoux F, Baciu M. 2009. The sensory-motor specificity of taxonomic and thematic conceptual relations: a behavioral and fMRI study. Neuroimage 44, 1152–1162. ( 10.1016/j.neuroimage.2008.09.043) [DOI] [PubMed] [Google Scholar]
- 215.Schwartz MF, Kimberg DY, Walker GM, Brecher A, Faseyitan OK, Dell GS, Mirman D, Coslett HB. 2011. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proc. Natl Acad. Sci. USA 108, 8520–8524. ( 10.1073/pnas.1014935108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boylan C, Trueswell JC, Thompson-Schill SL. 2015. Compositionality and the angular gyrus: a multi-voxel similarity analysis of the semantic composition of nouns and verbs. Neuropsychologia 78, 130–141. ( 10.1016/j.neuropsychologia.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. ( 10.1038/nrn2201) [DOI] [PubMed] [Google Scholar]
- 218.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 29, 1860–1873. ( 10.1523/JNEUROSCI.5062-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim H. 2016. Default network activation during episodic and semantic memory retrieval: a selective meta-analytic comparison. Neuropsychologia 80, 35–46. ( 10.1016/j.neuropsychologia.2015.11.006) [DOI] [PubMed] [Google Scholar]
- 220.Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. 2015. Default mode network connectivity during task execution. Neuroimage 122, 96–104. ( 10.1016/j.neuroimage.2015.07.053) [DOI] [PubMed] [Google Scholar]
- 221.Thakral PP, Madore KP, Schacter DL. 2017. A role for the left angular gyrus in episodic simulation and memory. J. Neurosci. 37, 8142–8149. ( 10.1523/JNEUROSCI.1319-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Thakral PP, Benoit RG, Schacter DL. 2017. Imagining the future: the core episodic simulation network dissociates as a function of timecourse and the amount of simulated information. Cortex 90, 12–30. ( 10.1016/j.cortex.2017.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rugg MD, King DR. In press Ventral lateral parietal cortex and episodic memory retrieval. Cortex. ( 10.1016/j.cortex.2017.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ramanan S, Piguet O, Irish M. In press Rethinking the role of the angular gyrus in remembering the past and imagining the future: the contextual integration model. Neuroscientist 1073858417735514 ( 10.1177/1073858417735514) [DOI] [PubMed] [Google Scholar]
- 225.Gibbs J, Raymond W. 1996. Why many concepts are metaphorical. Cognition 61, 309–319. ( 10.1016/S0010-0277(96)00723-8) [DOI] [PubMed] [Google Scholar]
- 226.Lakoff G. 1993. The contemporary theory of metaphor. In Metaphor and thought, 2nd edn (ed. Ortony A.), pp. 202–251. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 227.Lakoff G. 2014. Mapping the brain's metaphor circuitry: metaphorical thought in everyday reason. Front. Hum. Neurosci. 8, 1–14. ( 10.3389/fnhum.2014.00958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barsalou L, Wiemer-Hastings K. 2005. Situating abstract concepts. In Grounding cognition: the role of perception and action in memory, language, and thought (eds Pecher D, Zwaan RA), pp. 129–163. New York, NY: Cambridge University Press. [Google Scholar]
- 229.Wiemer-Hastings K, Xu X. 2005. Content differences for abstract and concrete concepts. Cogn. Sci. 29, 719–736. ( 10.1207/s15516709cog0000_33) [DOI] [PubMed] [Google Scholar]
- 230.Borghi AM, Binkofski F. 2014. Words as social tools: an embodied view of abstract concepts. London, UK: Springer. [Google Scholar]
- 231.Kiran S, Sandberg C, Abbott K. 2009. Treatment for lexical retrieval using abstract and concrete words in persons with aphasia: effect of complexity. Aphasiology 23, 835–853. ( 10.1080/02687030802588866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sandberg C, Kiran S. 2014. How justice can affect jury: training abstract words promotes generalisation to concrete words in patients with aphasia. Neuropsychol. Rehabil. 24, 738–769. ( 10.1080/09602011.2014.899504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Andrews M, Vigliocco G, Vinson D. 2009. Integrating experiential and distributional data to learn semantic representations. Psychol. Rev. 116, 463–498. ( 10.1037/a0016261) [DOI] [PubMed] [Google Scholar]
- 234.Andrews M, Frank S, Vigliocco G. 2014. Reconciling embodied and distributional accounts of meaning in language. Top. Cog. Sci. 6, 359–370. ( 10.1111/tops.12096) [DOI] [PubMed] [Google Scholar]
- 235.Louwerse MM. 2011. Symbol interdependency in symbolic and embodied cognition. Top. Cogn. Sci. 3, 273–302. ( 10.1111/j.1756-8765.2010.01106.x) [DOI] [PubMed] [Google Scholar]
- 236.Dove G. 2009. Beyond perceptual symbols: a call for representational pluralism. Cognition 110, 412–431. ( 10.1016/j.cognition.2008.11.016) [DOI] [PubMed] [Google Scholar]
- 237.Dove G. 2014. Thinking in words: language as an embodied medium of thought. Top. Cogn. Sci. 6, 371–389. ( 10.1111/tops.12102) [DOI] [PubMed] [Google Scholar]
- 238.Clancey WJ. 1993. Situated action—a neuropsychological interpretation response. Cogn. Sci. 17, 87–116. ( 10.1207/s15516709cog1701_7) [DOI] [Google Scholar]
- 239.Clark A. 1997. Being there: putting brain, body, and world together again. Cambridge, MA: MIT Press. [Google Scholar]
- 240.Barsalou L. 2009. Simulation, situated conceptualization, and prediction. Phil. Trans. R. Soc. B 364, 1281–1289. ( 10.1098/rstb.2008.0319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Barsalou LW, Santos A, Simmons WK, Wilson C. 2008. Language and simulation in conceptual processing. In Symbols and embodiment: debates on meaning and cognition (eds De Vega M, Glenberg A, Graesser A), pp. 245–283. Cambridge, MA: Oxford University Press. [Google Scholar]
- 242.Borghi AM, Cimatti F. 2009. Words as tools and the problem of abstract word meanings. In Proc. 31st Ann. Conf. Cogn. Sci. Soc., Amsterdam, The Netherlands, 29 July–1 Aug 2009 (eds N Taatgen, HV Rijn), pp. 2304–2309 Amsterdam, The Netherlands: Cognitive Science Society. [Google Scholar]
- 243.Vigliocco G, Kousta S, Vinson D, Andrews M, Del Campo E. 2013. The representation of abstract words: what matters? Reply to Paivio's (2013) comment on Kousta et al. (2011). J. Exp. Psychol. Gen. 142, 288–291. ( 10.1037/a0028749) [DOI] [PubMed] [Google Scholar]
- 244.Ghio M, Vaghi MM, Perani D, Tettamanti M. 2016. Decoding the neural representation of fine-grained conceptual categories. Neuroimage 132, 93–103. ( 10.1016/j.neuroimage.2016.02.009) [DOI] [PubMed] [Google Scholar]
- 245.Crutch SJ, Troche J, Reilly J, Ridgway GR. 2013. Abstract conceptual feature ratings: the role of emotion, magnitude, and other cognitive domains in the organization of abstract conceptual knowledge. Front. Hum. Neurosci. 7, 186 ( 10.3389/fnhum.2013.00186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wilson-Mendenhall CD, Simmons WK, Martin A, Barsalou LW. 2013. Contextual processing of abstract concepts reveals neural representations of nonlinguistic semantic content. J. Cogn. Neurosci. 25, 920–935. ( 10.1162/jocn_a_00361) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.