Abstract
Animal body armour is often considered an adaptation that protects prey against predatory attacks, yet comparative studies that link the diversification of these allegedly protective coverings to differential predation risk or pressure are scarce. Here, we examine the evolution of body armour, including spines and osteoderms, in Cordylinae, a radiation of southern African lizards. Using phylogenetic comparative methods, we attempt to identify the ecological and environmental correlates of body armour that may hint at the selective pressures responsible for defensive trait diversification. Our results show that species inhabiting arid environments are more likely to possess elaborated body armour, specifically osteoderms. We did not find any effect of estimated predation pressure or risk on the degree of body armour. These findings suggest that body armour might not necessarily evolve in response to direct interactions with predators, but rather as a result of increased habitat-mediated predation risk. Furthermore, we discuss the possibility that osteoderms might have been shaped by factors unrelated to predation.
Keywords: adaptive radiation, body armour, correlated evolution, osteoderm, predation, predator–prey interactions
1. Introduction
The role of predation as an agent of natural selection has been of interest to researchers ever since Darwin's time, with numerous examples showcasing its prevalence in nature [1–4]. In particular, the evolution of defensive morphologies, such as spines [5–8], carapaces [9], thickened keratinous scales [10] and osteoderms (i.e. bony elements embedded in the dermis) [10,11], has received considerable attention in the past because of their presumptive role in protection against predators.
To date, various studies have suggested that predator guilds may play a key role in the diversification process of these defensive traits [12–15]. A straightforward explanation for any disparity in defensive traits is that different predator communities select for different protective measures, i.e. predator-induced defensive trait diversification. Firstly, predator communities may vary in the number of predators belonging to each guild (figure 1b). For instance, in dragonflies, shifts from fish-dominated lakes to lakes in which large dragonfly predators dominate, have resulted in a decline in larval abdominal spine length [14,16]. The differential selection can be attributed to the fact that long abdominal spines are effective against predatory fish [17] but facilitate grasping by arthropod predators [18]. Secondly, the diversification of defensive traits might not be determined by the dominant predator guild present in the novel environment, but instead by the risk each guild poses to the prey (figure 1c).
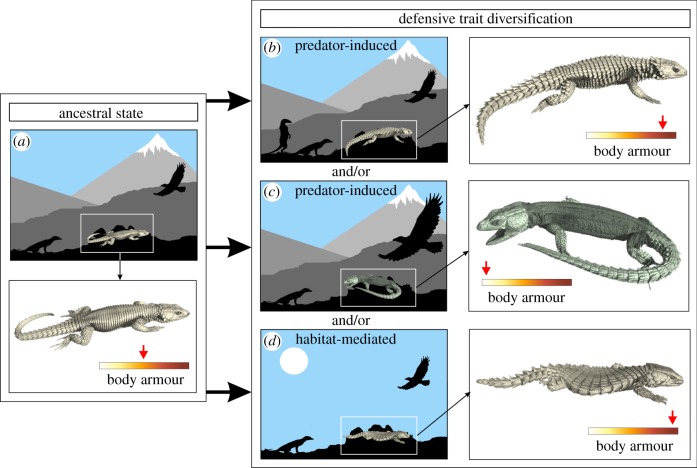
Figure 1.
Schematic representation illustrating the evolutionary trajectory of a defensive trait of an ancestral species (a) following introduction into a novel environment. Under the predator-induced defensive trait diversification scenario, changes in the number of predators belonging to a specific guild (b) or changes in predation risk posed by a specific predator guild (c) in the novel environment lead to the divergence of defensive traits. Under the habitat-mediated defensive trait diversification scenario, the exploitation of novel environments might shift the vulnerability to a specific predator guild (d), and likewise lead to the divergence of defensive traits. It is important to note that all three scenarios can act in synergy with one another. Three-dimensional rendered micro-CT images show possible outcomes of diversification. The colour gradient represents the degree of body armour, ranging from lightly armoured (white) to heavily armoured (dark red).
The risk posed by the different predator guilds might be independent of habitat use (above) or associated with habitat divergence, in which case habitat-mediated defensive trait diversification might take place (figure 1d). For example, Leinonen et al. [19] show that, in threespine sticklebacks, shifts from pelagic habitats (without shelter) to benthic habitats (with shelter) underlie independent transitions from heavy armour to light armour. Given that the probability of survival during encounters with fish predators appears to be associated with shelter availability within each habitat [19], the use of benthic habitats might have decreased the importance of fish predators, but increased the importance of arthropod predators, resulting in reduced body armour. In this context, it is important to note that the above-mentioned mechanisms might act in synergy. For instance, shifts in ancestral threespine sticklebacks from marine environments dominated by fish predators to novel freshwater habitats dominated by arthropod predators, appear to be associated with transitions from a high lateral-plate number and long pelvic spines to a low lateral-plate number and loss of pelvic spines [20,21].
While the results of studies on sticklebacks and dragonflies seem to confirm the notion that defensive traits are induced by predators or shaped by habitat-mediated predation risk, few studies have investigated the effect of these selective pressures on defensive trait diversification at a broader phylogenetic scale. The lizard subfamily Cordylinae, a radiation of predominantly rock-dwelling sit-and-wait foragers endemic to southern Africa [15,22] provides a unique opportunity to test the aforementioned hypotheses. Cordyline lizards demonstrate extensive variation in body armour, particularly in their expression of osteoderms, bony plates embedded in the dermal layer of the skin, and spines, bony protrusions with an overlying keratin sheath [15] (figure 2). They inhabit a large diversity of habitats, ranging from semi-desert to subtropical forest and from lowlands to montane regions at high altitude [22]. Within these diverse habitats, cordyline lizards are exposed to three distinct types of predators that can be grouped into two predation guilds: aerial predators (i.e. predatory birds) and terrestrial predators (i.e. snakes and mammals). Aerial predators are exclusively visually orientated, non-gape limited and equipped with sharp beaks and talons against which armour offers little protection. An increase in running speed, and consequently loss of body armour would be the best strategy to avoid aerial predators [3,15]. In contrast, terrestrial predators make use of olfactory cues in addition to visual cues, but their killing ability is limited by gape size (e.g. snakes) [23] or bite force (e.g. mammals) [24]. Well-developed osteoderms and sharp spines could provide a physical barrier against terrestrial predators [11,25].
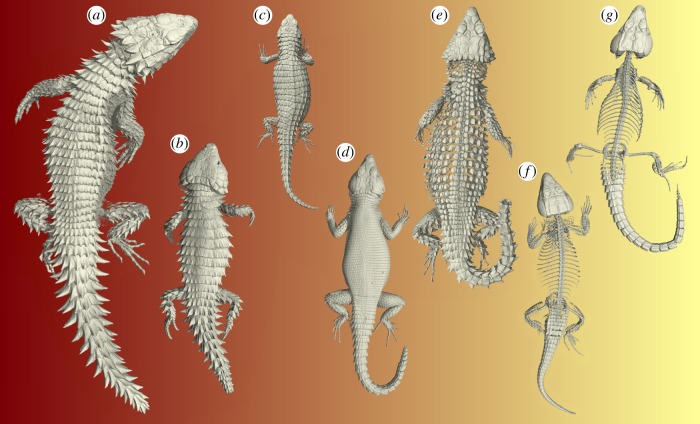
Figure 2.
Three-dimensional rendered micro-CT images of cordyline lizards illustrating the dichotomy in defensive traits. Smaug giganteus (a), as well as members of the genera Ouroborus (b), Cordylus (c) and Karusasaurus (d) are characterized by imbricating rectangular osteoderms covering the body. In other members of the genus Smaug (e), the body is covered with well-developed disc-like osteoderms. In Ninurta (f) and Pseudocordylus (g), the trunk region is completely devoid of osteoderms or osteoderms are extremely reduced.
Here, we examine the evolutionary drivers of defensive trait diversification using cordyline lizards as model organisms. A conceptual framework is shown in figure 1, depicting various potential evolutionary pathways from an ancestral state (figure 1a). Following the predator-induced defensive trait diversification scenario, we expect strong correlated evolution between degree of body armour and the predator guild exerting the highest pressure (figure 1b) or posing the highest risk (figure 1c). Following the habitat-mediated defensive trait diversification scenario, we expect that a strong relationship exists between the degree of body armour and habitat use (figure 1d).
2. Material and methods
(a). Defensive trait morphology
Micro-computed tomography (micro-CT) was used to quantify two important elements of defensive trait morphology: the length of spines and the expression of dorsal osteoderms located above the trunk vertebrae. Preserved specimens from 27 species (range: 2–6 specimens per species, mean: 4.48 ± 0.89 (s.d.)) were obtained from the Ellerman Collection of Stellenbosch University. Whenever possible, an equal number of male and female specimens was selected to reduce the effects of sexual dimorphism in body armour, particularly osteoderm expression [26]. The preserved specimens represented all the genera of Cordylinae, with the exception of the serpentiform genus Chamaesaura, and accounted for approximately 56% of the total cordyline lizard diversity. Specimens included representatives of the genus Smaug (giganteus, breyeri, vandami, depressus), Pseudocordylus (melanotus, subviridis, transvaalensis, spinosus, microlepidotus, langi), Hemicordylus (capensis, nebulosus), Namazonurus (peersi, lawrenci), Cordylus (vittifer, jonesii, niger, oelofseni, cordylus, macropholis, mclachlani, imkeae, minor, aridus), as well as Ninurta coeruleopunctatus, Ouroborus cataphractus, and Karusasaurus polyzonus. All lizards were micro-CT scanned using a GE Phoenix v|tome|x L240 dual tube CT instrument (Phoenix X-ray, General Electric Sensing & Technologies, Wunstorf, Germany) located at Stellenbosch University [27]. Firstly, the entire body of each specimen was micro-CT scanned at 120 kV, 180 µA, and with a spatial resolution of 100 µm. For each micro-CT scan, we determined the length of the spines of the ear (SEAR), neck (SNECK), trunk (STRUNK), front leg (SFLEG), hind leg (SHLEG) and tail (STAIL), by calculating the distance between the posterior tip of the keel and the anterior end of the scale. For specimens in which the keel was less pronounced, the width of the longest anterior–posterior axis of each scale served as spine length. From each of the six body regions, measurements of the five longest spines were taken for each individual and averaged (electronic supplementary material, figure S1). Secondly, the trunk region of each specimen was re-scanned at 120 kV, 180 µA, and with a spatial resolution of 30–35 µm. For each specimen, three region-of-interests (ROIs) measuring 1 cm3 were digitally extracted from the mid-dorsal region of the trunk which contained the dorsal osteoderms. A surface determination was carried out to find the material (i.e. bone) edge, after which the skeletal elements were digitally removed and the osteoderms retained. For each ROI, we determined (i) the total osteoderm volume (OVOL), (ii) the surface area of the osteoderms (OSURF) and (iii) the osteoderm thickness (OTHICK). The latter was estimated by measuring the thickness of the osteoderms using the transverse slice images. For each ROI, 10 thickness measurements were taken along a 1 cm transect through the middle region of the osteoderms (electronic supplementary material, figure S1). All three osteoderm measurements were averaged to obtain a species mean for subsequent analyses. Morphological measurements were obtained using the VGStudio Max 3.1 software (Volume Graphics GmbH, Heidelberg, Germany). In addition, we measured the distance between the posterior end of the cranium and the posterior end of the pelvis, which served as proxy for body size (BS).
(b). Predation pressure/risk
A list of all South African species of snakes, small to medium-sized carnivoran mammals and actively hunting birds (i.e. birds-of-prey, corvids) was compiled using reference literature ([28–30]; see table S1 in the electronic supplementary material). Following Stankowich et al. [31], we then estimated the risk that each predator may impose on each cordyline lizard by scoring ecological variables and integrating geographical data. To do so, an extensive literature study was conducted to obtain information on the diet, activity time, habitat use and hunting method of each of the predators. Based on this information, a score was assigned to each of the four life-history traits (see table S2 in the electronic supplementary material). Next, we calculated the risk (diet × activity time × hunting method × habitat use) that each predator poses for cordyline lizards. To further estimate the predation risk experienced by lizards, we incorporated distribution data as lizards can only be preyed upon by a predator with an overlapping distribution. In brief, species distribution data were obtained for all prey and predator species from BirdLife International [32], the IUCN Red List [33] and the Red List of the Reptiles of South Africa [28]. The ‘pairwiseRangemaps’ function of the R package fuzzySim [34] was used to calculate the pairwise similarities between the range maps. Lizards will experience a higher predation risk from predators that pose a greater threat and have higher spatial similarity. Therefore, we multiplied the risk scores by the percentage of spatial similarity with each lizard species. This resulted in a single score that represents the risk that a potential predator species poses for a given cordyline lizard. Since we hypothesized that the predation risk posed by different predator guilds has shaped antipredator morphology, the scores were summed for snakes + mammals (= terrestrial predation) and birds (= aerial predation). Using these values, we calculated the relative terrestrial predation risk (RTPRISK) which was used as a variable in subsequent statistical analyses. Lastly, we calculated the number of terrestrial and aerial predators that overlap in spatial distribution with each cordyline lizard to obtain an estimate of relative terrestrial predation pressure (RTPPRES). A list of all potential predators and associated scores can be found on Dryad Digital Repository [35].
(c). Environmental data
Geographical coordinates of all cordyline lizards used in the study were obtained from Bates et al. [31]. We compiled 3 972 unique records for 27 species (ranging from three to 793 records per species). Various environmental variables were extracted based upon the geographical coordinates of the species using the R package RASTER version 2.5-8 [36]. (i) Elevation and 12 bioclimatic variables were obtained from the WorldClim database [37], (ii) the global aridity index was obtained from the CGIAR-CSI database (Consortium for Spatial Information) [38], (iii) global cloud cover was obtained from Wilson & Jetz [39], (iv) global surface vegetation cover was obtained from the MODIS vegetation continuous field MOD44B [40], and (v) solar radiation (i.e. direct normal irradiance, DNI and global horizontal irradiance, GHI) was obtained from the SolarGIS database [41]. Bioclimatic variables, elevation, aridity and cloud cover were obtained at a spatial resolution of approximately 1 km2, whereas global surface vegetation cover and solar radiation were obtained at a spatial resolution of 250 m2. The environmental variables were extracted for each distribution record and averaged across all localities to obtain a species mean value.
(d). Phylogenetic comparative analyses
To test for associations between degree of body armour and the ecological/environmental variables while accounting for shared ancestry, all analyses were conducted within a phylogenetic context. A phylogenetic tree with relative divergence times was constructed following the methodology of Broeckhoven et al. [15], which is presented in the electronic supplementary material. Firstly, to account for phylogenetic autocorrelation among sets of variables, i.e. closely related species tend to have more similar trait values than expected at random, phylogenetic principal component analyses (pPCA) [42] were conducted on the morphological and environmental datasets, respectively. Phylogenetic PCA summarizes data into principal components after controlling for the phylogenetic relationships among taxa [42]. A first pPCA was conducted on the three osteoderm measurements to obtain an index of osteoderm expression, whereas a second pPCA was conducted on the six spine measurements to obtain an index of spinosity. Although both osteoderms and spines constitute body armour in cordyline lizards, they were analysed separately because osteoderm expression appears to be shaped by factors unrelated to predation as well (see Broeckhoven et al. [11,25]). A third and last pPCA was conducted on the environmental variables to obtain an index of habitat use. The pPCAs on the correlation matrices were conducted using the function ‘phyl.pca’ implemented in the R package phytools [43].
To address whether, and which, environmental and ecological variables explained the variation in defensive morphology, we chose a twofold approach. Firstly, univariate phylogenetic regression analyses were run using the function ‘phylolm’ of the R package phylolm [44]. Osteoderm expression and spinosity (pPCA scores) were the dependent variables, whereas RTPPRES, RTPRISK and habitat use (pPCA scores), were the explanatory variables. The interaction effects between habitat use and RTPRISK were also included in the model. The diversification of defensive traits in cordyline lizards is best explained by an ‘Early Burst (EB)’ mode of evolution [15], hence all phylogenetic regression analyses were performed assuming an EB process for the residual error. Secondly, a multivariate approach was used to investigate the combination of variables that best explained the degree of body armour in cordyline lizards. Model selection was based on the Akaike information criterion corrected for small sample size (AICc) [45] and the model with the lowest AICc value was considered the ‘best’ model given the current set of explanatory variables. Results from any competing models with ΔAICc < 2 [45] were also taken into account. In addition, stepwise model selection for phylogenetic linear models was conducted using forward selection implemented in the function ‘phylostep’ of the R package phylolm. [44].
3. Results
(a). Morphological variation in defensive traits
Substantial disparity in body armour is present among cordyline lizards (figures 2 and 3). The majority of members of the genus Pseudocordylus, Hemicordylus and Ninurta have reduced spines, whereas others, such as Ouroborus, Cordylus and Namazonurus, are characterized by relatively long spine lengths (figures 2 and 3). With regards to the osteoderm expression in the trunk region, three distinct phenotypes can be discriminated: (i) complete absence of osteoderms, as is the case in Pseudocordylus (figure 2), (ii) isolated disc-like elements, as is the case in Ninurta, Hemicordylus and Smaug (figures 2 and 3), and (iii) imbricating rectangular osteoderms in the trunk region, as is the case in Ouroborus, Cordylus, Namazonurus and Karusasaurus (figures 2 and 3). A summary of the spine and osteoderm measurements of the cordyline species used in this study is provided in the electronic supplementary material (table S3). All measurements (except SNECK, OVOL and OTHICK) showed a significant positive relationship with body size (electronic supplementary material, table S4). Hence, prior to statistical analyses, a phylogenetic size correction was performed on these measurements [42] using the function ‘phyl.resid’ implemented in the R package phytools [43]. A first pPCA conducted on OVOL, OTHICK and the phylogenetic residuals for OSURF, revealed one axis, coined osteoderm expression, which explained 86.2% of the total variation (electronic supplementary material, table S5). A second pPCA conducted on SNECK, as well as the phylogenetic residuals for SEAR, STRUNK, SFLEG, SHLEG and STAIL, revealed one axis, coined spinosity, which explained 79.2% of the total variation (electronic supplementary material, table S6). Both axes describe a gradient from lightly armoured phenotypes (low scores) to heavily armoured phenotypes (high scores).
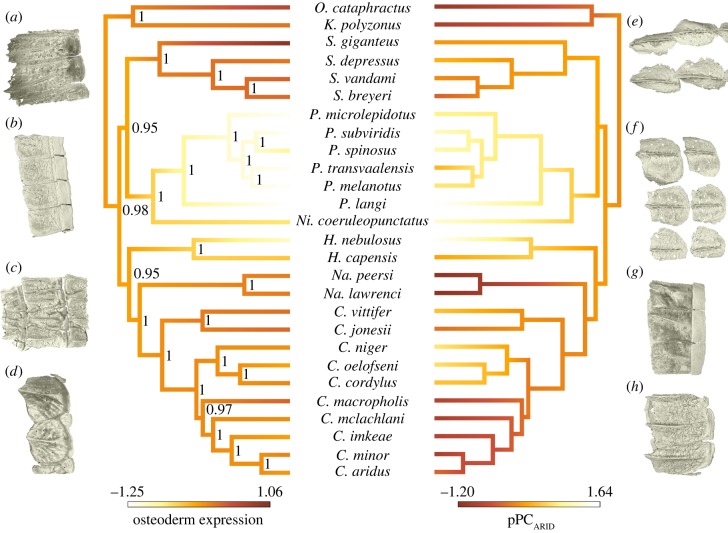
Figure 3.
Reconstruction of the evolution of osteoderm expression and habitat use. Branches are colour-coded with positive values characterizing species with high osteoderm expression in the left phylogram and negative values describing arid environments in the right phylogram. Reconstruction of the ancestral states of given traits were done under ML using the function ‘contMap’ in the R package phytools [43]. Representative three-dimensional rendered micro-CT images of osteoderms illustrate imbricating rectangular osteoderms in O. cataphractus (a), K. polyzonus (b), S. giganteus (c), Na. peersi (g) and C. macropholis (h), and disc-like elements in S. breyeri (d), Ni. coeruleopunctatus (e) and H. capensis (f). Bayesian posterior probabilities are indicated at each node.
(b). Ecological and environmental correlates of body armour
To test for the influence of ecological and environmental variables on variation in body armour, we extracted the scores for osteoderm expression and spinosity from the pPCA and correlated these with the relative terrestrial predation pressure/risk and habitat use. For the latter, we firstly conducted a pPCA on the 18 environmental variables. This analysis revealed four axes that explained 92% of the variation (electronic supplementary material, table S7). The first axis correlated positively with annual precipitation, surface vegetation cover, cloud cover and the aridity score, but negatively with solar radiation (electronic supplementary material, table S7). Hence, this axis described a gradient from arid to humid environments and was coined pPCARID. The second axis was positively correlated with several temperature measures and described a thermal gradient from warm to cold environments (electronic supplementary material, table S7). This axis was consequently coined pPCTEMP. The third and fourth axes were not strongly related to environmental variables meaningful for our study and hence, were not included in the statistical analyses.
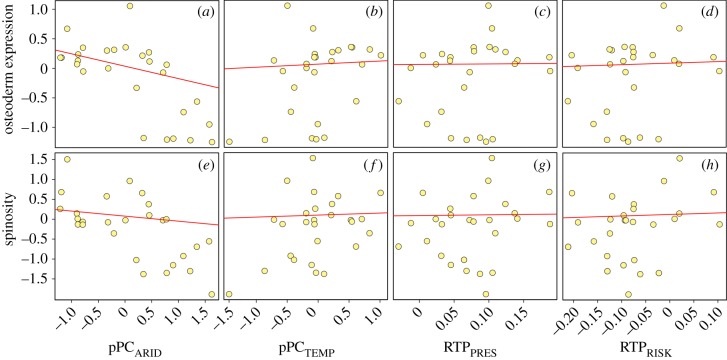
Both univariate and multivariate phylogenetic linear regression analyses demonstrated that aridity (pPCARID) had a significant effect on osteoderm expression (table 1, figure 4). Species inhabiting arid environments, characterized by low rainfall and cloud cover, but high summer temperatures and solar radiation, had a higher osteoderm expression than those inhabiting non-arid environments (figures 3 and 4). Four competing models within 2 ΔAICc units were present: the null (intercept-only) model and three models that added pPCTEMP, RTPPRES and RTPRISK to the model, respectively (table 2). A stepwise model selection procedure for phylogenetic linear models selected a model with only pPCARID as the explanatory variable. In contrast to osteoderm expression, there were no clear relationships between spinosity and any of the environmental/ecological factors (table 1, figure 4), with the null model receiving the best support (table 2). Likewise, the model selection procedure for phylogenetic linear models demonstrated that the intercept-only model had the best support. We further explored the relationship between ecological variables and body armour by separating terrestrial predation risk/pressure into relative snake and mammal predation risk/pressure. Univariate phylogenetic linear regressions, however, did not reveal any novel significant relationships (electronic supplementary material, tables S8 and S9).
Table 1.
Results of univariate phylogenetic linear regression analyses examining the effect of indices of predation risk/pressure and habitat use on osteoderm thickness and spinosity. Statistically significant results are given in italics. a, model parameters of the Early Burst (EB) evolutionary model; σ², maximum-likelihood estimate of the variance rate.
| estimate | StdErr | t-value | p-value | a | σ² | |
|---|---|---|---|---|---|---|
| osteoderm expression | ||||||
| pPCARID | −0.21 | 0.10 | −2.15 | 0.04 | −24.83 | 8.36 |
| pPCTEMP | 0.05 | 0.12 | 0.45 | 0.65 | −31.46 | 14.60 |
| RTPPRES | 0.08 | 0.71 | 0.11 | 0.91 | −31.71 | 14.94 |
| RTPRISK | 0.26 | 0.53 | 0.49 | 0.63 | −31.47 | 14.59 |
| spinosity | ||||||
| pPCARID | −0.13 | 0.14 | −0.88 | 0.39 | −27.35 | 21.26 |
| pPCTEMP | 0.05 | 0.16 | 0.32 | 0.76 | −30.61 | 26.62 |
| RTPPRES | 0.18 | 0.98 | 0.18 | 0.86 | −31.22 | 27.70 |
| RTPRISK | 0.38 | 0.74 | 0.51 | 0.61 | −30.99 | 27.07 |
Figure 4.
Scatterplots depicting the relationships between the two constituents of body armour, i.e. osteoderm expression and spinosity, as well as indices of habitat use (pPCARID and pPCTEMP), relative terrestrial predation pressure (RTPPRES) and relative terrestrial predation risk (RTPRISK). Aridity had a statistically significant effect on osteoderm expression (a), with species inhabiting arid environments being more likely to possess well-developed osteoderms.
Table 2.
The top supported models (ΔAICc < 2) from phylogenetic multivariate regression analyses examining the associations between body armour (i.e. osteoderm expression and spinosity), relative terrestrial predation pressure/risk and habitat use in cordyline lizards. Models are ranked from best to worst, with the best-fitting model given in italics. k, number of parameters; Δ, difference in mean AICc value among models; ω, Akaike weights; a, model parameters of EB evolutionary model; σ2, maximum-likelihood estimate of the variance rate.
| model | k | AICc | ΔAICc | ω | a | σ² |
|---|---|---|---|---|---|---|
| osteoderm | ||||||
| pPCARID | 4 | 25.03 | 0 | 0.22 | −24.83 | 8.35 |
| pPCARID + RTPPRES | 4 | 24.35 | 1.19 | 0.12 | −24.93 | 8.15 |
| pPCARID + RTPRISK | 4 | 24.78 | 1.61 | 0.10 | −24.27 | 7.95 |
| pPCARID + pPCTEMP | 4 | 24.76 | 1.60 | 0.10 | −23.79 | 7.71 |
| null | 3 | 25.03 | 1.86 | 0.08 | −31.83 | 15.05 |
| spines | ||||||
| null | 3 | 43.23 | 0 | 0.26 | −31.30 | 27.87 |
4. Discussion
Predation is believed to be one of the primary selective forces affecting the evolution of defensive strategies and morphologies [6–8], yet evidence is accumulating that defensive traits might not evolve in response to predators directly [46]. We proposed three non-mutually exclusive scenarios that could explain how predators can shape the evolution of defensive traits directly (i.e. predator-driven defensive trait diversification), or indirectly through habitat use (i.e. habitat-mediated defensive trait diversification). The results of our study fail to support a general hypothesis of predator-driven evolution of body armour. Instead, we show that osteoderm expression coevolved with climatic conditions, thereby corroborating the idea that defensive morphologies might not be driven directly by predators. Either the exploitation of novel habitats might secondarily favour defensive trait diversification if the novel habitat favours a different defence mode or shift in defensive morphology [6,47], or body armour is the evolutionary result of another selective pressure unrelated to predation [11,25].
Habitat divergence might be associated with increased vulnerability to predators or shifts in the vulnerability from one predator guild to another [15,19]. For instance, Stankowich & Campbell [10] proposed that vulnerability to predators in general might change as a function of habitat openness. Medium-sized mammals utilizing open habitats are more likely to possess some form of body armour presumably due to the increased exposure to predators [10]. A similar trend is observed in cordyline lizards, showing that species inhabiting open, arid environments, characterized by high summer temperatures, low precipitation and low surface vegetation cover ([48], also see electronic supplementary material, table S7) are more likely to possess elaborated body armour. The observed trend can be attributed to the fact that lizards inhabiting arid environments might spend considerable time hiding in rock crevices (e.g. reduced need for basking [49] or reduced chance of overheating [50]). Within these retreat sites, species might be more susceptible to extraction by terrestrial predators such as snakes and selection should favour adaptations (e.g. body armour) that minimize the risk of extraction by this type of predator. In addition, the availability of suitable retreat sites might be low in arid environments, thereby further increasing terrestrial predation risk. Alternatively, environmental conditions in open, arid environments might cause species to engage in risky behaviours. Ouroborus cataphractus, for example, visits harvester termite colonies far away from the safety of the refuge, rendering individuals prone to terrestrial predation, particularly mongooses [49]. Outrunning a predator might not be an option and selection should favour alternative strategies, such as the evolution of elaborated body armour [11].
Non-arid environments, on the contrary, are characterized by high cloud cover (and mist) and low solar radiation (electronic supplementary material, table S7). These conditions, often experienced by species inhabiting montane habitats, might necessitate individuals to increase basking time to attain optimal body temperatures [51]. Outside of their retreat sites, species might be more susceptible to aerial predators and selection should favour a reduction in body armour to facilitate rapid escape. However, the relationship between body armour and habitat use held only for the expression of dorsal osteoderms, which presumably play an insignificant role in reducing extraction risk. We did not find any association between spinosity and aridity, despite strong coevolution between spinosity and osteoderm expression (pGLS; p < 0.001). This lack of association might be attributed to the fact that, regardless of the risk that the dominant predator guild poses, species are still preyed upon by members of the other predator guild. For instance, lightly armoured taxa, such as Pseudocordylus and Hemicordylus, might be well-adapted to rapidly escape from aerial predators, they remain vulnerable to terrestrial predation within their retreat sites. Cordyline lizards frequently use their tails to prevent predators from accessing the lizard's body when inside of a retreat site [52]. Enlarged spines, especially those on the tail and limbs, might be an effective strategy to prohibit predators from accessing the body, and remain useful in the lightly armoured phenotype. Hence, the absence of an association between spinosity and aridity might solely be attributed to the fact that lightly armoured species have reduced osteoderm expression but retained some degree of spinosity.
Although the above-mentioned results suggest that shifts in vulnerability to predator guilds following the exploitation of novel environments might have been a major selective pressure in the diversification of defensive traits, we cannot preclude that different or additional selective pressures have played an important role. In addition to providing protection against predatory bites [11,25], osteoderms appear to have unique thermal properties [25] and it has been suggested that they play an important role in reducing evaporative water loss (EWL) through the skin [53]. The latter would make the possession of osteoderms highly advantageous to sit-and-wait foraging lizards inhabiting arid environments characterized by long periods of drought. Given that squamates inhabiting (semi-)arid environments might experience higher EWL than those inhabiting more humid environments [54], adaptations that minimize cutaneous water loss are expected to be particularly important to survival [54,55]. The EWL hypothesis is further supported by a recent study by Broeckhoven et al. [56] who found that O. cataphractus individuals inhabiting more arid environments possess relatively thicker osteoderms than those found elsewhere. In support of this argument, populations of the lightly armoured species Hemicordylus capensis that occur in arid environments [26] are always found in close association with water bodies, such as waterfalls [57]. Based on the aforementioned evidence, we propose that osteoderms might have played a key role in the historical colonization of arid environments by cordyline lizards. This idea of a physiological role of osteoderms is further supported by the fact that species inhabiting non-arid environments appear to have completely lost osteoderm expression in the trunk region, despite similar predation pressure/risk as that experienced by species occupying arid environments.
In summary, while several studies have demonstrated the antipredatory function of spines and osteoderms, our results fail to support the hypothesis that the evolutionary trajectory of these defensive morphologies is driven directly by predators. Instead, we suggest that behavioural changes associated with habitat use might shift the balance in predation risk and consequently, determine the type of defence favoured. In addition, climatic conditions might further shape morphology, through their effects on physiology. In light of these results, it must be noted that our approach only allowed for an estimation of the potential predation risk/pressure experienced by cordyline lizards. The presence of few, but significant, predators might constitute a notable strong risk for particular species in particular habitats [11,51]. Exposed (arid) environments are a more dangerous ground than closed (humid) environments and the evolution of robust defensive morphologies is therefore to be expected [10].
Supplementary Material
Acknowledgements
We are grateful to A. du Plessis and S. G. le Roux for their assistance with the micro-CT scanning. We also give special thanks to E. Hartnick for help with the extraction of micro-CT data and to G. Diedericks for the construction of the phylogenetic tree. Rick Blob and two anonymous reviewers are thanked for their comments on an earlier version of the manuscript.
Ethics
No ethical clearance was required because museum specimens were used for the micro-CT scanning.
Data accessibility
The datasets supporting this article are deposited on the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.bv9247d [35].
Authors' contributions
C.B. designed the project; C.B. and Y.E.A. collected the data; C.B., Y.E.A. and R.V.D. analysed the data; C.B. wrote the manuscript with contributions from Y.E.A., R.V.D., C.H. and T.S.
Competing interests
The authors declare no competing interests.
Funding
Funding for this study was provided by the National Research Foundation (C.H.) and the Australian Research Council (C.H.). C.B. is a Postdoctoral Fellow of the Research Foundation – Flanders.
References
- 1.Rundle HD, Vamosi SM, Schluter D. 2003. Experimental test of predation's effect on divergent selection during character displacement in sticklebacks. Proc. Natl Acad. Sci. USA 100, 14 943–14 948. ( 10.1073/pnas.2036360100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. 2004. Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58, 2305–2318. ( 10.1111/j.0014-3820.2004.tb01605.x) [DOI] [PubMed] [Google Scholar]
- 3.Losos JB, Schoener TW, Spiller DA. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432, 505–508. ( 10.1038/nature03039) [DOI] [PubMed] [Google Scholar]
- 4.Vamosi SM. 2005. On the role of enemies in divergence and diversification of prey: a review and synthesis. Can. J. Zool. 83, 894–910. ( 10.1139/z05-063) [DOI] [Google Scholar]
- 5.Losos JB, Mouton PleFN, Bickel R, Cornelius I, Ruddock L. 2002. The effect of body armature on escape behaviour in cordylid lizards. Anim. Behav. 64, 313–321. ( 10.1006/anbe.2002.3051) [DOI] [Google Scholar]
- 6.Vamosi SM, Schluter D. 2004. Character shifts in the defensive armor of sympatric sticklebacks. Evolution 58, 376–385. ( 10.1111/j.0014-3820.2004.tb01653.x) [DOI] [PubMed] [Google Scholar]
- 7.Young KV, Brodie ED. 2004. How the horned lizard got its horns. Science 304, 65 ( 10.1126/science.1094790) [DOI] [PubMed] [Google Scholar]
- 8.Marchinko KB. 2009. Predation's role in repeated phenotypic and genetic divergence of armor in threespine stickleback. Evolution 63, 127–138. ( 10.1111/j.1558-5646.2008.00529.x) [DOI] [PubMed] [Google Scholar]
- 9.Caro T, Shaffer HB. 2010. Chelonian antipredator strategies: preliminary and comparative data from Tanzanian Pelusios. Chelonian Conserv. Biol. 9, 302–305. ( 10.2744/CCB-0812.1) [DOI] [Google Scholar]
- 10.Stankowich T, Campbell LA. 2016. Living in the danger zone: exposure to predators and the evolution of spines and body armor in mammals. Evolution 70, 1501–1511. ( 10.1111/evo.12961) [DOI] [PubMed] [Google Scholar]
- 11.Broeckhoven C, Diedericks G, Mouton PleFN. 2015. What doesn't kill you might make you stronger: functional basis for variation in body armour. J. Anim. Ecol. 84, 1213–1221. ( 10.1111/1365-2656.12414) [DOI] [PubMed] [Google Scholar]
- 12.Johansson F, Samuelsson L. 1994. Fish-induced variation in abdominal spine length of Leucorrhinia dubia (Odonata) larvae? Oecologia 100, 74–79. ( 10.1007/BF00317132) [DOI] [PubMed] [Google Scholar]
- 13.Hoverman JT, Relyea RA. 2009. Survival trade-offs associated with inducible defences in snails: the roles of multiple predators and developmental plasticity. Funct. Ecol. 23, 1179–1188. ( 10.1111/j.1365-2435.2009.01586.x) [DOI] [Google Scholar]
- 14.Mikolajewski DJ, De Block M, Rolff J, Johansson F, Beckerman AP, Stoks R. 2010. Predator-driven trait diversification in a dragonfly genus: covariation in behavioral and morphological antipredator defense. Evolution 64, 3327–3335. ( 10.1111/j.1558-5646.2010.01078.x) [DOI] [PubMed] [Google Scholar]
- 15.Broeckhoven C, Diedericks G, Hui C, Makhubo BG, Mouton PleFN. 2016. Enemy at the gates: rapid defensive trait diversification in an adaptive radiation of lizards. Evolution 70, 2647–2656. ( 10.1111/evo.13062) [DOI] [PubMed] [Google Scholar]
- 16.Petrin Z, Schilling EG, Loftin CS, Johansson F. 2010. Predators shape distribution and promote diversification of morphological defenses in Leucorrhinia, Odonata. Evol. Ecol. 24, 1003–1016. ( 10.1007/s10682-010-9361-x) [DOI] [Google Scholar]
- 17.Mikolajewski DJ, Rolff J. 2004. Benefits of morphological defence demonstrated by direct manipulation in larval dragonflies. Evol. Ecol. Res. 6, 619–626. [Google Scholar]
- 18.Mikolajewski DJ, Johansson F, Wohlfahrt B, Stoks R. 2006. Invertebrate predation selects for the loss of a morphological antipredator trait. Evolution 60, 1306–1310. ( 10.1111/j.0014-3820.2006.tb01208.x) [DOI] [PubMed] [Google Scholar]
- 19.Leinonen T, Herczeg G, Cano JM, Merilä J. 2011. Predation-imposed selection on threespine stickleback (Gasterosteus aculeatus) morphology: a test of the refuge use hypothesis. Evolution 65, 2916–2926. ( 10.1111/j.1558-5646.2011.01349.x) [DOI] [PubMed] [Google Scholar]
- 20.Bell MA. 2001. Lateral plate evolution in the threespine stickleback: getting nowhere fast. Genetica 112, 445–461. ( 10.1023/A:1013326024547) [DOI] [PubMed] [Google Scholar]
- 21.Bell MA, Aguirre WE, Buck NJ. 2004. Twelve years of contemporary armor evolution in a threespine stickleback population. Evolution 58, 814–824. ( 10.1111/j.0014-3820.2004.tb00414.x) [DOI] [PubMed] [Google Scholar]
- 22.Mouton PleFN, van Wyk JH. 1997. Adaptive radiation in cordyliform lizards: an overview. Afr. J. Herpetol. 46, 78–88. ( 10.1080/21564574.1997.9649981) [DOI] [Google Scholar]
- 23.Cundall D, Greene HW. 2000. Feeding in snakes. In Feeding: form, function, and evolution in tetrapod vertebrates (ed. Schwenk K.), pp. 293–333. San Diego, CA: Academic Press. [Google Scholar]
- 24.Dumont ER, Herrel A. 2003. The effects of gape angle and bite point on bite force in bats. J. Exp. Biol. 206, 2117–2123. ( 10.1242/jeb.00375) [DOI] [PubMed] [Google Scholar]
- 25.Broeckhoven C, du Plessis A, Hui C. 2017. Functional trade-off between strength and thermal capacity of dermal armor: insights from girdled lizards. J. Mech. Behav. Biomed. Mater. 74, 189–194. ( 10.1016/j.jmbbm.2017.06.007) [DOI] [PubMed] [Google Scholar]
- 26.Broeckhoven C, de Kock C, Mouton PleFN. 2017. Sexual dimorphism in osteoderm expression and the role of male intrasexual aggression. Biol. J. Lin. Soc. 122, 329–339. ( 10.1093/biolinnean/blx066) [DOI] [Google Scholar]
- 27.Du Plessis A, le Roux SG, Guelpa A. 2016. The CT scanner facility at Stellenbosch University: an open access X-ray computed tomography laboratory. Nucl. Instrum. Methods Phys. Res. B 384, 42–49. ( 10.1016/j.nimb.2016.08.005) [DOI] [Google Scholar]
- 28.Bates MF, Branch WR, Bauer AM, Burger M, Marais J, Alexander GJ, De Villiers MS. 2014. Atlas and red list of the reptiles of South Africa, Lesotho and Swaziland. Pretoria, South Africa: South African National Biodiversity Institute. [Google Scholar]
- 29.Skinner J, Chimimba C. 2005. The mammals of the Southern African sub-region. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Hockey PAR, Dean WRJ, Ryan PG. 2005. Roberts birds of southern Africa. Cape Town, South Africa: The Trustees of the John Voelcker Bird Book Fund. [Google Scholar]
- 31.Stankowich T, Haverkamp PJ, Caro T. 2014. Ecological drivers of antipredator defenses in carnivores. Evolution 68, 1415–1425. ( 10.1111/evo.12356) [DOI] [PubMed] [Google Scholar]
- 32. BirdLife International and Handbook of the Birds of the World, Bird species distribution maps of the world. Version 6.0. 2016 See http://datazone.birdlife.org/species/requestdis. (accessed September 2016)
- 33.IUCN Red List, Red List Spatial Data. 2016. See http://www.iucnredlist.org/technical-documents/spatial-data (accessed September 2016)
- 34.Barbosa AM. 2015. fuzzySim: applying fuzzy logic to binary similarity indices in ecology. Methods Ecol. Evol. 6, 853–858. ( 10.1111/2041-210X.12372) [DOI] [Google Scholar]
- 35.Broeckhoven C, El Adak Y, Hui C, Van Damme R, Stankowich T. 2018. Data from: On dangerous ground: the evolution of body armour in cordyline lizards Dryad Digital Repository ( 10.5061/dryad.bv9247d) [DOI] [PMC free article] [PubMed]
- 36.Hijmans RJ, et al. 2016. Package ‘raster’. R package. See https://cran.R-project.org/web/packages/raster/index.html. (accessed January 2017)
- 37.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 38.Trabucco A, Zomer RJ. 2009. Global aridity index (global-aridity) and global potential evapo-transpiration (global-PET) geospatial database. CGIAR Consortium for Spatial Information. See http://www.cgiar-csi.org. (accessed January 2017)
- 39.Wilson AM, Jetz W. 2016. Remotely sensed high-resolution global cloud dynamics for predicting ecosystem and biodiversity distributions. PLoS Biol. 14, e1002415 ( 10.1371/journal.pbio.1002415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen MC, DeFries SR, Townshend JRG, Carroll M, Dimiceli C, Sohlberg RA. 2003. Global percent tree cover at a spatial resolution of 500 meters: first results of the MODIS vegetation continuous fields algorithm. Earth Interact. 7, 1–15. ( 10.1175/1087-3562(2003)007%3C0001:GPTCAA%3E2.0.CO;2) [DOI] [Google Scholar]
- 41.SolarGIS. 2017. Solar resource maps for South Africa. See http://solargis.com/products/maps-and-gis-data/free/download/south-africa (accessed January 2017)
- 42.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268. ( 10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 43.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 44.Ho LST, Ane C, Lachlan R, Tarpinian K, Feldman R, Yu Q, Ho MLST. 2016. Package ‘phylolm’. See http://cran.r-project.org/web/packages/phylolm/index.html. (accessed February 2018)
- 45.Burnham KP, Anderson DR.. 2002. Model selection and multimodel inference. New York, NY: Springer. [Google Scholar]
- 46.Brock KM, Bednekoff PA, Pafilis P, Foufopoulos J. 2015. Evolution of antipredator behavior in an island lizard species, Podarcis erhardii (Reptilia: Lacertidae): The sum of all fears? Evolution 69, 216–231. ( 10.1111/evo.12555) [DOI] [PubMed] [Google Scholar]
- 47.Abrams PA. 2000. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Evol. Syst. 31, 79–105. ( 10.1146/annurev.ecolsys.31.1.79) [DOI] [Google Scholar]
- 48.Dilts TE, Weisberg PJ, Dencker CM, Chambers JC. 2015. Functionally relevant climate variables for arid lands: a climatic water deficit approach for modelling desert shrub distributions. J. Biogeogr. 42, 1986–1997. ( 10.1111/jbi.12561) [DOI] [Google Scholar]
- 49.Broeckhoven C, Mouton PleFN. 2015. Some like it hot: camera traps unravel the effects of weather conditions and predator presence on the activity levels of two lizards. PLoS ONE 10, e0137428 ( 10.1371/journal.pone.0137428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duran F, Kubisch EL, Boretto JM. 2018. Thermal physiology of three sympatric and syntopic Liolaemidae lizards in cold and arid environments of Patagonia (Argentina). J. Comp. Physiol. B 188, 141–152. ( 10.1007/s00360-017-1116-3) [DOI] [PubMed] [Google Scholar]
- 51.Caldwell AJ, While GM, Wapstra E. 2017. Plasticity of thermoregulatory behaviour in response to the thermal environment by widespread and alpine reptile species. Animal Behav. 132, 217–227. ( 10.1016/j.anbehav.2017.07.025) [DOI] [Google Scholar]
- 52.Cooper W Jr, van Wyk JH, Mouton PleFN, Al-Johany AM, Lemos-Espinal JA, Paulissen MA, Flowers M. 2000. Lizard antipredatory behaviors preventing extraction from crevices. Herpetologica 56, 394–401. [Google Scholar]
- 53.Ruibal R, Shoemaker V. 1984. Osteoderms in anurans. J. Herpetol. 18, 313–328. ( 10.2307/1564085) [DOI] [Google Scholar]
- 54.Cox CL, Cox RM. 2015. Evolutionary shifts in habitat aridity predict evaporative water loss across squamate reptiles. Evolution 69, 2507–2516. ( 10.1111/evo.12742) [DOI] [PubMed] [Google Scholar]
- 55.Bentley PJ, Schmidt-Nielsen K. 1966. Cutaneous water loss in reptiles. Science 151, 1547–1549. ( 10.1126/science.151.3717.1547) [DOI] [PubMed] [Google Scholar]
- 56.Broeckhoven C, Mouton PleFN, Hui C. 2018. Proximate causes of variation in dermal armour: insights from armadillo lizards. Oikos. ( 10.1111/oik.05401) [DOI] [Google Scholar]
- 57.Janse van Rensburg DA, Mouton PleFN. 2009. Foraging behaviour and use of space in the Graceful Crag Lizard, Pseudocordylus capensis: life on large rock surfaces. Afr. J. Herpetol. 58, 106–115. ( 10.1080/21564574.2009.9650030) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Broeckhoven C, El Adak Y, Hui C, Van Damme R, Stankowich T. 2018. Data from: On dangerous ground: the evolution of body armour in cordyline lizards Dryad Digital Repository ( 10.5061/dryad.bv9247d) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are deposited on the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.bv9247d [35].