Abstract
The endogenous opioid system is well known to relieve pain and underpin the rewarding properties of most drugs of abuse. Among opioid receptors, the μ receptor mediates most of the analgesic and rewarding properties of opioids. Based on striking similarities between social distress, physical pain and opiate withdrawal, μ receptors have been proposed to play a critical role in modulating social behaviour in humans and animals. This review summarizes experimental data demonstrating such role and proposes a novel model, the μ opioid receptor balance model, to account for the contribution of μ receptors to the subtle regulation of social behaviour. Interestingly, μ receptor null mice show behavioural deficits similar to those observed in patients with autism spectrum disorder (ASD), including severe impairment in social interactions. Therefore, after a brief summary of recent evidence for blunted (social) reward processes in subjects with ASD, we review here arguments for altered μ receptor function in this pathology.
This article is part of a themed section on Emerging Areas of Opioid Pharmacology. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.14/issuetoc
Abbreviations
- ACC
anterior cingulate cortex
- ASD
autism spectrum disorder
- CPu
caudate putamen
- NAc
nucleus accumbens
- PAG
periaqueductal gray
- PFC
prefrontal cortex
- SAS
social avoidance systems
- USV
ultrasound vocalizations
- VTA
ventral tegmental area
Social reward and social pain: overlapping neurobiological substrates
Depending on internal state and environment, interactions with conspecifics in humans and animals may be experienced as aversive or pleasurable. Indeed, individuals may either compete for resources, territory, mates, social status or parental care (Roughgarden, 2012) when these are scarce or instead cooperate, play, mate, care for offspring, to ensure their survival and genetic legacy. In the latter case, social interactions can elicit pleasure and euphoria (Fehr and Camerer, 2007; Neuhaus et al., 2010; Trezza et al., 2010) and accordingly activate brain regions belonging to the reward processing circuitry. Social interaction or craving for social connection activates the nucleus accumbens (NAc) and medial prefrontal cortex (PFC) in humans (Fareri et al., 2015; Kawamichi et al., 2016; Inagaki et al., 2016a). Conversely, activating reward regions such as ventral tegmental area (VTA) or dopaminergic raphe neurons in rodents promotes social interaction (Gunaydin et al., 2014; Matthews et al., 2016). Pleasure associated with social experience facilitates further social contact and may lead ultimately to the structuration of a social organization (Cacioppo et al., 2011).
As a corollary of social organization, however, rejection by peers or loss of a close relative can be felt in social species as extremely painful, as much as an amputation (Parkes, 1975), and prolonged isolation elicits deleterious psychological and physical damages (Eisenberger et al., 2016; Filipovic et al., 2016). Remarkably, social rejection activates similar brain regions, such as the anterior cingulate cortex (ACC), anterior insula, periaqueductal gray (PAG) and amygdala, as physical pain (Kross et al., 2011; Eisenberger, 2012; Hsu et al., 2013; Papini et al., 2015), suggesting that indeed rejected individuals experience ‘social pain’, which can be relived even more easily and more intensely than physical pain (Chen et al., 2008). Importantly, social context, whether positive (such as images of loved ones in humans) or negative (rejection), can influence physical pain perception (Defeudis et al., 1976; Puglisi‐Allegra and Oliverio, 1983; Coudereau et al., 1997; Younger et al., 2010). Together, these data suggest that affiliative and social behaviours have hijacked primary reward and pain systems to promote social interactions and avoid adverse social contexts (Nelson and Panksepp, 1998).
At the neuronal level, many different neurotransmitters modulate social behaviour, either facilitating or inhibiting social interactions. Remarkably, most of the former are well‐known key players of the reward system, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484, cannabinoids, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2174 or opioids (Dolen et al., 2013; Coria‐Avila et al., 2014; Baribeau and Anagnostou, 2015; Loureiro et al., 2016; Vanderschuren et al., 2016), and have been initially identified as neurochemical mediators of the motivational/rewarding properties of drugs of abuse and/or as pain killers. In contrast, neurobiological substrates of social avoidance [social avoidance systems (SAS)] have been characterized as main factors of the pain, aggression or stress systems and include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5, glucocorticoids and neuropeptides such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=912, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2168, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2098 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=864 (Katsouni et al., 2009; Takahashi et al., 2012; Barik et al., 2013; Katsouni et al., 2013; Gobbroge et al., 2017). Pro‐social and anti‐social neuromodulators thus compete to drive adaptive social behaviour in individuals. In the following section, we will focus on the role of opioids and, more specifically, of the opioid http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319&familyId=50&familyType=GPCR, in this subtle balance.
The μ receptor is a critical neurobiological substrate of social behaviour
The opioid receptors belong to the large family of GPCRs and include three members: μ, δ and κ opioid receptors, whose preferential endogenous ligands are the opioid peptides, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1613, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1643 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1620, respectively. The opioid system is a well‐known key modulator of pain, and exogenous ligands of opioid receptors, the opiates, have been used for thousands of years as pain killers. Medicinal use of opiates, however, has also led to the discovery of their addictive properties, shedding light on a second major physiological role of opioids: modulating reward processes. The identification of opioid peptides, their receptors and respective genes has since allowed a better understanding of their mechanism of action, regarding the control of pain and reward (Le Merrer et al., 2009) as well as many other roles in stress response, respiration, food intake, gastrointestinal transit, endocrine and immune processes (Yamanaka and Sadikot, 2013; Sobczak et al., 2014; Plein and Rittner, 2017).
In 1978, Panksepp and colleagues formulated their ‘Brain Opioid Theory of Social Attachment’ (BOTSA), in which social and affiliative behaviours are proposed to tightly depend on the level of endogenous opioid peptides, based notably on striking similarities between social attachment and drug addiction (Panksepp et al., 1978). BOTSA hypothesizes that social deprivation induces social distress and contact seeking due to insufficient opioid tone (opioid withdrawal). Social contact would relieve this negative affect by triggering opioid (endorphin) release. Since then, experimental evidence has accumulated showing that μ receptors are primarily involved in pro‐social effects of opioids, which are not only exclusively observed under social distress conditions (social deprivation or defeat) but also in more neutral or even positive social situations (social comfort). We review these data in the next subsections.
Role of μ receptors under social distress conditions
With regard to the role of μ receptors under social distress conditions (summary and references in Table 1 and Figure 1), the first evidence came from the observation that μ receptor agonists in infant rodents or monkeys can reduce the severe distress and ultrasound vocalizations (USV) triggered by separation from the mother. Similarly, in young adults, μ receptor agonists restore social interaction deficits and decrease defensive/submissive behaviour and USVs in isolated and defeated animals. Although social play is a highly rewarding and conserved behaviour in young social mammals (Vanderschuren et al., 2016), the common use is to isolate animals prior to its study, to increase social seeking (Table 1). In such mild negative social conditions, μ receptor agonists, delivered systemically, increase social play and motivation to play and reverse social dominance in juvenile rodents or monkeys. Furthermore, direct injection of μ receptor agonists in the NAc is sufficient to enhance social play, in accordance with the hedonic properties of μ receptor activation in this region. Besides, exposure to negative social contexts modifies brain μ receptor expression (Figure 1), notably in the NAc and VTA (Vanderschuren et al., 1995c; Nikulina et al., 2005; Chaijale et al., 2013; Rodriguez‐Arias et al., 2016). In contrast with agonists, opioid antagonists exacerbate social distress and USV and increase the incidence of defensive postures. Together, therefore, pharmacological data in animals suggest that μ receptor activation can relieve distress in animals experiencing aversive social situations, whereas μ receptor blockade exacerbates such distress. In humans, imaging studies have revealed the activation of μ receptors under conditions of social rejection (notably in the NAc, amygdala and PAG) that correlated with lower levels of sadness and feeling of rejection, suggesting that μ receptors in these regions play a protective or adaptive role in social pain (Hsu et al., 2013; Hsu et al., 2015) (Figure 1). Whereas six other rare polymorphisms exist, the A118G polymorphism of the OPRM1 gene (coding for the μ opioid receptor), removing a potential extracellular N‐glycosylation site, is commonly found in European and Asian populations (15–60%) and considered as a gain‐of‐function in vitro and in vivo (Kroslak et al., 2007; Sia et al., 2008). A118G polymorphism carriers exhibit increased sensitivity to dramatic social rejection, leading to higher depression scores in adults (Way et al., 2009; Hsu et al., 2013; Slavich et al., 2014; Carver et al., 2016), more negative personality and impaired emotional, behavioural and social skills in children (Bertoletti et al., 2012; Carver et al., 2016). These last data suggest that μ receptor hyperactivity can be detrimental to coping with highly negative social experiences.
Table 1.
Effects of pharmacological manipulation of μ receptors on social behaviour
| Species | Age | Housing | Drug | Dose | Route | Treatment schedule | Effects on social behaviour | References |
|---|---|---|---|---|---|---|---|---|
| Social distress | ||||||||
| Agonists | ||||||||
| Mouse | Young adults | Isolated 3 weeks | Morphine | +/++ | Systemic | Acute | Decreased timid/defensive behaviour in females | Benton et al., 1985 |
| Rat | Pups | Isolated from mother | DAMGO | + | Intracisternal | Acute | Reduced USVs at all doses | Carden et al., 1991 |
| Rat | Juveniles | Isolated from weaning | Morphine | + | Systemic | Acute | Increased pinning in social play | Panksepp, 1979 |
| Rat | Juveniles | Isolated from weaning | Morphine | + | Systemic | Acute | Increased social dominance slightly | Panksepp et al., 1985 |
| Rat | Juveniles | Isolated from weaning | Morphine | + | Systemic | Acute | Increased wanting to play | Normansell and Panksepp, 1990 |
| Rat | Juveniles | Isolated from weaning | Morphine | + | Systemic | Chronic | Restored social exploration and contact | Van den Berg et al., 1999 |
| Rat | Juveniles | Isolated 3.5 h | Fentanyl | + | Systemic | Acute | Increased social play | Vanderschuren et al., 1995b |
| Rat | Juveniles | Isolated 3.5 h | Morphine | + | Systemic | Acute | increased social play | Vanderschuren et al., 1995a |
| Rat | Juveniles | Isolated 3.5 h | Morphine | + | Systemic | Acute | Increased social play in an unfamiliar environment | Trezza and Vanderschuren, 2008 |
| Rat | Juveniles | Isolated 2 h | Morphine, DAMGO | + | NAc | Acute | Increased social play | Trezza et al., 2011 |
| Rat | Juveniles | Isolated 2 h | Beta‐endorphin, Met‐enkephalin | + | NAc | Acute | Increased social play | |
| Rat | Young adults | Isolated and defeated | DAMGO | + | VTA | Two times | Decreased defeat‐induced inactivity | Nikulina et al., 2005 |
| Rat | Young adults | Isolated from weaning, defeated | Morphine | +/++ | PAG ventrolateral | Acute | Decreased defeat‐induced USVs | Vivian and Miczek, 1998 |
| Macaque | Infants | Isolated from mother | Morphine | + | Systemic | Three times | Reduced USVs and isolation‐induced inactivity | Kalin et al., 1988 |
| Macaque | Infants | Chronic separation with mother | Morphine | + | Systemic | Acute | Increased mother‐infants clinging | Kalin et al., 1995 |
| Marmoset | Juveniles | Transparent physical separation | Morphine | +/− | Systemic | Acute | Increased social play | Guard et al., 2002 |
| Titi monkey | Young adults | 30 min isolation | Morphine | + | Systemic | Acute |
Increased affiliative behaviour in males and decreased female breaks Trend for reduced male approaches and reduced number of males initiating contact with pair‐mate |
Ragen et al., 2015b |
| Antagonists | ||||||||
| Mouse | Young adults | Isolated from weaning | Naloxone | ++ | Systemic | Acute | Increased fearful/defensive postures when exposed to resident mouse | Brain et al., 1985 |
| Rat | Juveniles | Isolated 3.5 h | β‐funaltrexamine | + | Systemic | Acute | Decreased social play | Vanderschuren et al., 1995b |
| Rat | Juveniles | Isolated from weaning | Naloxone | + | Systemic | Acute | Decreased pinning in social play | Panksepp, 1979 |
| Rat | Juveniles | Isolated from weaning | Naloxone | + | Systemic | Acute | Decreased wanting to play | Normansell and Panksepp, 1990 |
| Rat | Juveniles | Isolated from weaning | Naloxone | + | Systemic | Acute | Decreased social dominance | Panksepp et al., 1985 |
| Rat | Juveniles | Isolated 8 days | CTAP | + | NAc | Acute | Decreased social play | Trezza et al., 2011 |
| Rat | Young adults | Isolated and defeated | Naloxone | + | Systemic | Acute | Increased defeat‐induced chewing/teeth chattering and shakes | Chaijale et al., 2013 |
| Macaque | Infants | Isolated from mother | Naloxone | + | Systemic | Three times | Increased USVs | Kalin et al., 1988 |
| Macaque | Infants | Chronic separation with mother | Naltrexone | + | Systemic | Acute | Reduced mother‐infants clinging | Kalin et al., 1995 |
| Titi monkey | Young adults | 30 min isolation | Naloxone | + | Systemic | Acute | Increased female breaks | Ragen et al., 2015b |
| Social comfort | ||||||||
| Agonists | ||||||||
| Rat | Young adults | Groups | Morphine | + | Systemic | Acute | Enhanced social recognition memory | Bianchi et al., 2013 |
| Rat | Young adults | Groups | Heroin | +/++ | Systemic | Acute/pretreatment | Enhanced social recognition memory | Levy et al., 2009 |
| Rat | Young males | Isolated with females |
Endomorphin‐1 Endomorphin‐1 |
+ |
Medial POA Medial amygdala |
Acute Acute |
Increased mount and pursuit duration Increased mount number and ejaculation latency |
Parra‐Gamez et al., 2013 |
| Human | Young adults | Buprenorphine | + | Systemic | Acute |
Increased ratings of images with social context Reduced attention to fear expressions and perceived social rejection |
Bershad et al., 2016 | |
| Human | Young adults | Buprenorphine | + | Systemic | Acute | Improved short term memory for happy expressions | Syal et al., 2015 | |
| Human | Young men | Remifentanil | + | Systemic | Acute | Increased rating of pleasantness for neutral pictures | Gospic et al., 2008 | |
| Human | Young men | Morphine | – | Systemic | 3 days | Increased ‘keep’ presses for the most attractive female faces | Chelnokova et al., 2014 | |
| Antagonists | ||||||||
| Mouse | Pups | Groups | Naltrexone | + | Systemic | First 4 days postnatal |
Abolished preference for own cage and own mother in pups Abolished congener preference and social place preference in adults |
Cinque et al., 2012 |
| Mouse KI Oprm1A112G | Adults | Groups | Naloxone | + | Systemic | Acute | Abolished preference for congener in the three‐chamber test | Briand et al., 2015 |
| Rat | Juveniles | Groups | CTAP | + | Lateral ventricule | Acute | Reduced social investigation time | Smith et al., 2015 |
| Prairie vole | Adult females | Isolated with males | Naltrexone | ++ | Systemic | Acute | Reduced mating bouts and suppressed partner preference | Burkett et al., 2011 |
| Naltrexone | ++ | Systemic | Three times | Reversed preference for stranger versus partner | ||||
| CTAP | ++ | NAc | Acute | No effect on partner preference | ||||
| CTAP | ++ | CPu | Acute | Suppressed partner preference | ||||
| Prairie vole | Adult females | Isolated with males | CTAP | ++ | CPu | Acute | Suppressed partner preference and mating bouts | Resendez et al., 2013 |
| CTAP | ++ | NAc core | Acute | No effect on partner preference and decreased mating bouts | ||||
| CTAP | ++ | NAc dorsomedial shell | Acute | Suppressed partner preference and no effect on mating bouts | ||||
| CTAP | ++ | NAc ventral shell | Acute | No effect on partner preference and mating bouts | ||||
| Macaque | Juveniles | Stable social group | Naloxone | + | Systemic | Acute | Increased proximity with mothers and demands for comfort | Schino and Troisi, 1992 |
| Titi monkey | Young adults | Groups | Naloxone | + | Systemic | Acute | Reduced grooming and male approach | Ragen et al., 2013 |
| Titi monkey | Young adults | Groups | Naloxone | + | Systemic | Acute | Increased female breaks | Ragen et al., 2015b |
| Human | Young adults | Naltrexone | + | Systemic | Acute | Increased negative emotions muscle responses to happy faces | Meier et al., 2016 | |
| Human | Young adults | Naltrexone | + | Systemic | Four times | Reduced feeling of social connections | Inagaki et al., 2016b | |
| Human | Young adults | Naltrexone | + | Systemic | Four times | Decreased female face attractiveness | Chelnokova et al., 2014 | |
| Excessive opioid stimulation | ||||||||
| Mouse | Young adults | Groups | Morphine | +++ | Systemic | 6 days, escalating | Reduced social interaction after 4 weeks of abstinence | Goeldner et al., 2011 |
| Mouse | Young adults | Groups | Heroin | +++ | Systemic | 6 days, escalating | Reduced social interaction after 4 and 7 weeks of abstinence | Lutz et al., 2014 |
| Mouse | Young adults | Isolated from weaning | Morphine | +++ | Systemic | 6 days, escalating | Reduced social interaction after 7 days of abstinence | Zanos et al., 2014 |
| Mouse | Young adults | Groups | Morphine | +++ | Systemic | 6 days, escalating | Reduced social interaction and social preference in three‐chamber test after 4 weeks of abstinence | Becker et al., in press |
| Rat | Young adults | Groups | Morphine | ++/+++ | Systemic | Acute | Decreased social recognition memory | Bianchi et al., 2013 |
| Rat | Young adults | Groups | Morphine | + | Systemic | Chronic | Reduced social exploration | Van den Berg et al., 1999 |
| Rat | Nullipares or lactating females | Isolated from weaning | Morphine | ++ | Dorsal POA | Acute | Decreased care for pups | Rubin and Bridges, 1984 |
| Rat | Juveniles | Isolated 3.5 h | Morphine | ++ | Systemic | Acute | Decreased social play | Vanderschuren et al., 1995a |
| Rat | Neonates | Females with their litters | Morphine | ++ | Systemic | 23 days, escalating | Lag in development of social behaviours | Najam and Panksepp, 1989 |
| Rat | Young adults | Females with their litters | Morphine | +++ | Systemic | Chronic | Increased latencies for full maternal behaviour | Miranda‐Paiva et al., 2001 |
| Rat | Young adults | Females with their litters | Morphine | ++ | Systemic | Chronic | Increased latencies for full maternal behaviour | Miranda‐Paiva et al., 2003 |
| Rat | Young adults | Females with their litters | Morphine | +++ | Systemic | Chronic | Disrupted maternal behaviour | Yim et al., 2006 |
| Prairie vole | Young adults | Isolated 1 week | Morphine | +++ | Systemic | Acute | Reduced huddling duration | Shapiro et al., 1989 |
| Titi monkey | Young adults | Groups, with pair mates | Morphine | + | Systemic | Chronic, 7 days | Reduced grooming, tendency towards reduced contact | Ragen et al., 2013 |
CTAP, D‐Phe‐Cys‐Tyr‐D‐Trp‐Arg‐Thr‐Pen‐Thr‐NH2; DAMGO, [D‐Ala2, N‐MePhe4, Gly‐ol]‐enkephalin, KI, knock‐in; POA, preoptic area of hypothalamus.
Figure 1.
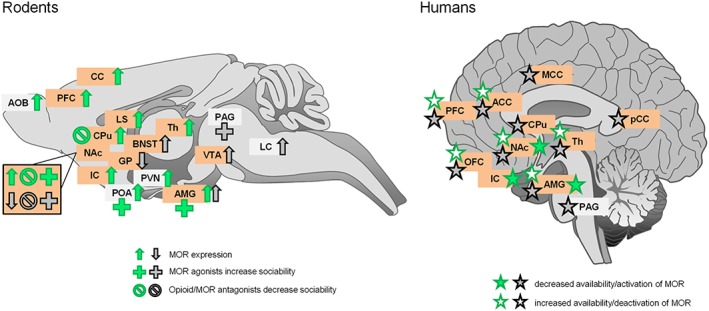
Effects of regional pharmacological manipulation of μ receptors (MOR) on social behaviour and regional μ receptor expression in rodents (left panel) and humans (right panel) under social comfort (green) or social distress (black) conditions. Lateralization of brain responses (right or left hemisphere) was not taken into account for simplification purpose. Pharmacological manipulations of μ receptors and modifications in μ receptor expression (in rodents) or availability (humans) affect similar brain regions, independently from the social context. These structures mostly belong to the reward circuitry (highlighted in orange). ACC, anterior cingulate cortex; AMG, amygdala; AOB, accessory olfactory bulb; BNST, bed nucleus of stria terminalis; CC, cingulate cortex; IC, insular cortex; GP, globus pallidus; LC, locus coeruleus; LS, lateral septum; MCC, middle cingulate cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; POA, preoptic area of hypothalamus; PVN, paraventricular nucleus of hypothalamus; Th, thalamus.
Role of μ receptors under neutral/positive social conditions
The role of μ receptor activation has also been extensively studied under conditions of social comfort (Table 1). In such context, low to moderate μ receptor activation facilitates social behaviour. In rats, μ receptor agonists increase socio‐sexual and sexual behaviours, bias spatial exploration in an unfamiliar environment towards social play and enhance long‐term social memory. In contrast, blockade of μ receptor activity inhibits various social behaviours when administered under neutral social context in animals, including social interaction, social play, affiliative and sexual or socio‐sexual behaviours. The μ receptors in the NAc play a critical role in socio‐sexual behaviour, as their expression in this structure is enriched in monogamous species (Inoue et al., 2013; Ragen et al., 2015a) and intra‐NAc administration of antagonists abolishes partner preference formation (Table 1 and Figure 1). Remarkably, administering an opioid antagonist during the first four post‐natal days in mice leads to social impairment and anhedonia in adult age. In humans, psychopharmacological studies have revealed that a low dose of μ receptor agonist increases female face attractiveness and avoidance of the least attractive female faces in men. Partial agonists improve memory for happy faces, increase pleasantness ratings of neutral or social images and decrease perception of social rejection (Table 1). Moreover, positive social stimuli such as social acceptance or pleasant social touch modify μ receptor availability in common structures (Hsu et al., 2013; Nummenmaa et al., 2016) (Figure 1). In contrast with agonists, opioid antagonists decrease facial emotional responses to happy faces, decrease female face attractiveness and reduce feelings of social connection in young adults. In humans, adult carriers of the A118G OPRM1 polymorphism show less avoidance of affectionate relationships and increased capacity to experience social reward (Troisi et al., 2011), whereas child carriers display improved relationships, better resilience to aversive parental care and enhanced responses to facial expression of social and emotional stimuli (Copeland et al., 2011; Troisi et al., 2011; Bertoletti et al., 2012). Consistent with this, A118G OPRM1 knock‐in mice exhibit increased social interaction, dominance and protection from social defeat (Mague et al., 2009; Briand et al., 2015). Similarly, the C77G polymorphism in macaques, equivalent to the human A118G polymorphism, results in higher affiliative behaviour in mothers and infants (Barr et al., 2008; Higham et al., 2011). All these data thus converge to draw a pro‐social picture for μ receptors under neutral/positive social conditions.
The particular case of excessive and/or prolonged opioid stimulation
Surprisingly, however, several studies have reported that μ receptor agonists administered either acutely at a moderate to high dose or chronically at a low dose inhibit various social behaviours such as socio‐sexual behaviours, maternal behaviour and duration of direct social exploration independently from the social context (Table 1). Interestingly, in the clinics, former opiate addicts under opioid maintenance (chronic low dose) display social interaction/cognition deficits, report feeling of being unrelated and behave in an autistic way (McDonald et al., 2013; Johnson et al., 2014). In other studies, high doses of a μ receptor agonist, given acutely or chronically, reduce socio‐sexual behaviour, social play and impair long‐term social memory in animals. Furthermore, exposure to high doses of opiates affects social behaviour in the long term, as mice exposed to escalating doses of morphine or heroin display severe social interaction deficit up to 7 weeks after cessation of treatment (Table 1). Of note, brain transcription of opioid peptides is reduced after 4 weeks in abstinent animals (Becker et al., in press) suggesting that excessive opioid stimulation results in long lasting adaptive peptide down‐regulation. Accordingly, opiate‐abstinent patients without substitutive therapy show low levels of circulating β‐endorphin (Shi et al., 2009) and display reduced prefrontal response to social stimuli, pointing to social behaviour deficits (Huhn et al., 2016). Together, these data indicate that, when excessive and/or prolonged, μ receptor activation can exert a deleterious influence on social behaviour.
The μ receptor balance model
In an attempt to solve the paradox of divergent effects of μ receptor stimulation on social behaviours depending on species (rodents vs. primates/humans), a ‘State‐dependent μ‐Opioid Modulation of Social Motivation’ (SOMSOM) model was recently proposed. This model postulates that the effects of μ receptor agonists and antagonists depend on the social motivational state of the animals when assessing their behaviour, namely, whether they are trying to reduce distress or seeking a pleasurable experience (Loseth et al., 2014). In this model, the initial social context is the crucial determinant of future effects of opioid manipulation on sociability. However, although this model successfully predicts the consequences of μ receptor stimulation and inhibition under social distress or comfort conditions, it fails to account for negative consequences of intense and/or long‐lasting μ receptor stimulation. Extending the SOMSOM model, we propose a μ receptor balance model (Figure 2), in which the key determinant to predict the consequences of opioid/μ receptor manipulation on social behaviour is the initial μ receptor activity. In this model, both excessive and deficient μ receptor activity negatively influence social behaviour through a classical inverted‐U relationship (Johnson et al., 2014). In a narrow window of optimal functioning, μ receptor activity could be balanced with SAS to allow adaptive social behaviour. In contrast, blunted μ receptor activity, due to social distress or pharmacological antagonism, leads to reduced social reward/motivation and leaves the field clear for SAS to elicit social withdrawal. Similarly, excessive μ receptor stimulation due to intense/prolonged exposure to opioids saturates the reward system and produces social indifference. In this framework, blocking μ receptor activity in the case of excessive tone, or stimulating μ receptor when the tone is too low, can restore normal, adaptive, social behaviour. The μ receptor balance model thus reconciles experimental discrepancies regarding the social consequences of μ receptor stimulation depending on species and social context.
Figure 2.
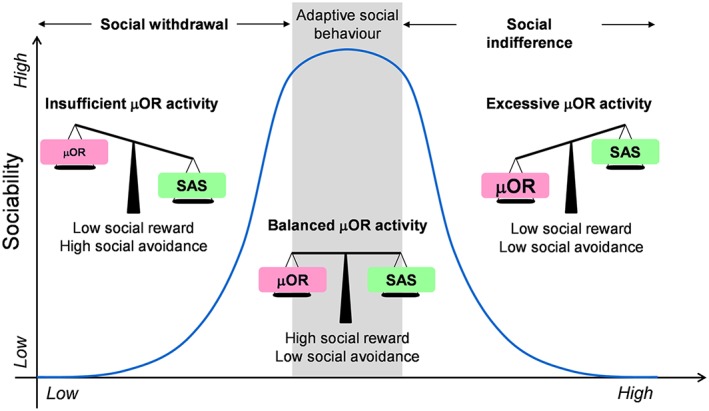
The μ receptor balance model. Activity of μ receptors (μOR) competes with SAS to drive social behaviours. In a narrow window of optimal functioning, μ receptor activity is balanced with SAS to allow adaptive social behaviour. These conditions are ideal to detect social reward (good signal to noise ratio). On the left part of the curve, low μ receptor activity, due to social distress, pharmacological antagonism or genetic anomaly, leads to reduced social reward and/or motivation and leaves the field clear for SAS to elicit social withdrawal (insufficient signal). On the right part of the curve, excessive μ receptor activity, due to intense and/or prolonged exposure to opioid ligands or increased μ receptor expression, saturates the reward system and produces social indifference (excessive noise). Importantly, in this model, blocking μ receptor activity in the case of excessive tone, or stimulating μ receptor when the tone is too low, can restore normal, adaptive, social behaviour.
Manipulation of μ receptors in different social contexts might face some technical issues of other non‐specific functions of the μ receptor (locomotion and sedation) affecting social behaviour. However, our conclusions are drawn on studies involving different doses of μ receptor ligands and studying different kind of social behaviours and also non‐social behaviours, excluding issues regarding other μ receptor functions and their effects on social behaviours.
Genetic manipulations of the μ opioid receptor and autistic‐like syndrome in mice
Genetic knockout of μ receptors (Oprm1 −/−) produces severe alterations of social behaviour in mice. Indeed, mouse pups lacking μ receptors vocalize less when separated from their mother (Moles et al., 2004; Cinque et al., 2012), juvenile Oprm1 −/− mice exhibit reduced interest in social partners (Cinque et al., 2012), young adult Oprm1 −/− males respond less to female vocalizations (Wohr et al., 2011; Gigliucci et al., 2014) and Oprm1 −/− male and female animals display reduced social interaction and preference (Becker et al., 2014). However, when exposed to chronic social defeat, μ receptor knockout animals show less aversion to the social context (Komatsu et al., 2011). These data fit with the μ receptor balance model (insufficient μ receptor tone) and further demonstrate that μ receptors are essential for establishing appropriate social behaviour.
Interestingly, Oprm1 −/− mice were recently proposed as a monogenic mouse model of autism spectrum disorder (ASD) (Oddi et al., 2013; Becker et al., 2014). ASD are complex neurodevelopmental diseases whose diagnosis is based on the detection of two types of behavioural symptoms (core symptoms): impaired social reciprocity and communication together with restricted, repetitive patterns of behaviour, interests or activities (Diagnostic and Statistical Manual of Mental Disorders: DSM‐5). Co‐morbid symptoms, variable in their occurrence and intensity, are frequently associated with ASD and encompass anxiety disorders, cognitive and motor deficits, aggressive behaviour, epileptic episodes, sleep disturbances, increased sensitivity to pain and altered gastrointestinal transit (Johnson and Myers, 2007; Robinson, 2012; Veenstra‐VanderWeele and Blakely, 2012; White et al., 2012; Mazurek et al., 2013; Whyatt and Craig, 2013; Chen et al., 2016). Intriguingly, in addition to deficits in social behaviour, Oprm1 −/− mice show stereotyped and perseverative behaviours, necessary to fulfil ASD criteria, as well as many of the co‐morbid symptoms of ASD, namely, increased aggressiveness, exacerbated anxiety and motor clumsiness (Becker et al., 2014), increased susceptibility to seizures (Jang et al., 2001; Grecksch et al., 2004; Becker et al., 2014), impaired spatial learning (Jamot et al., 2003), lowered nociceptive thresholds (Gaveriaux‐Ruff and Kieffer, 2002) and reduced gastrointestinal motility (Roy et al., 1998). The μ receptor null mice thus reproduce the broadest autistic syndrome ever reported in preclinical research, proving unique face validity. Moreover, Oprm1 −/− mice display anatomical, neurochemical and genetic landmarks of the disease, such as modified neuronal activation in anxiety‐ and reward‐associated brain structures, reduced striatal connectivity, altered synaptic morphology and monoamine levels in the striatum and modified expression of several candidate genes of autism, providing construct validity to the model (Becker et al., 2014; Mechling et al., 2016). Finally, as regards predictive validity, risperidone and oxytocin, compounds that demonstrated efficiency in relieving autistic features in patients, also alleviated such symptoms in μ receptor null mice (Becker et al., 2014; Gigliucci et al., 2014). Together, these results establish the Oprm1 −/− mouse line as a comprehensive model of ASD and demonstrate that genetic invalidation of μ receptors is sufficient to trigger an autistic syndrome in these rodents.
Preclinical studies exploring MECP2‐related genetic diseases further support the hypothesis of a tight link between ASD and μ receptors. The MECP2 gene codes for methyl‐CpG binding protein 2, which binds DNA at methylated sites to either repress or activate transcription (Chahrour et al., 2008). Genetic invalidation of Mecp2 in mice is a model for Rett syndrome, which includes autistic features; conversely, Mecp2 duplication mimics the MECP2 duplication syndrome, which also encompasses autistic symptoms (Lombardi et al., 2015). Remarkably, μ receptor expression is decreased in the striatum of Mecp2 null mice (Kao et al., 2015), consistent with a critical role of μ receptors in the striatum in supporting social behaviour. In mice bearing multiple copies of Mecp2, however, μ receptor expression is instead excessive, and breeding these animals with heterozygous Oprm1 +/− mice corrects both high μ receptor expression and deficient social interaction (Samaco et al., 2012). These data fit the μ receptor balance model, with both deficient and excessive signalling having deleterious consequences (Figure 2). Most importantly, they further establish a connection between μ receptor and ASD, suggesting that defective μ receptor function could account for decreased social reward/motivation in these disorders.
Evidence for blunted reward processes in autism spectrum disorder
In search of the neurobiological underpinnings of disrupted social interactions in ASD, autism research had initially focused on cognitive impairments and involved theory‐of‐mind deficits or altered ability to infer other's mental states. Recently, however, motivational aspects of social skills have received more attention, and a social motivation theory has emerged (Dawson and Bernier, 2007; Chevallier et al., 2012). In this framework, disrupted social interest in ASD patients would result from early deficits in social motivation, including interest in attending social stimuli, as well as enjoying and prolonging reciprocal social interactions. Such interactions being intrinsically rewarding (Fehr and Camerer, 2007; Neuhaus et al., 2010; Trezza et al., 2010), the social motivation theory thus suggests that reward and/or social reward processes are altered in patients with ASD.
Consistent with this hypothesis, numerous psychological studies have shown diminished sensitivity to the positive reward value of social stimuli in subjects with ASD (Scott‐Van Zeeland et al., 2010; Chevallier et al., 2012; Lin et al., 2012; Sasson et al., 2012; Dubey et al., 2015; Kim et al., 2015). Accordingly, electrophysiological (Stavropoulos and Carver, 2014; Gonzalez‐Gadea et al., 2016) and pupillary (Sepeta et al., 2012) responses as well as neural activation in brain areas involved in reward anticipation and/or processing (notably PFC and NAc) (Scott‐Van Zeeland et al., 2010; Kohls et al., 2012; Assaf et al., 2013; Kohls et al., 2014; Richey et al., 2014; Choi et al., 2015; Leung et al., 2015) are diminished in ASD patients presented with social stimuli (Figure 3). Of note, subjects with ASD also display hypo‐activated NAc and caudate putamen (CPu) while anticipating negative social reinforcement (Damiano et al., 2015) and diminished activity in the social brain circuitry (including PFC and amygdala) in response to social touch (Kaiser et al., 2016). As regards the amygdala and insular cortex, also involved in reward processing, studies have reported either hypo‐activation (Kohls et al., 2012; Leung et al., 2015; Kaiser et al., 2016) or hyper‐activation (Dichter et al., 2012b) in response to social stimuli in ASD subjects, possibly due to different experimental settings. Together, these data strongly support the hypothesis of deficient social reward in patients with ASD, as a likely consequence of abnormal activation of the brain reward circuit in social contexts.
Figure 3.
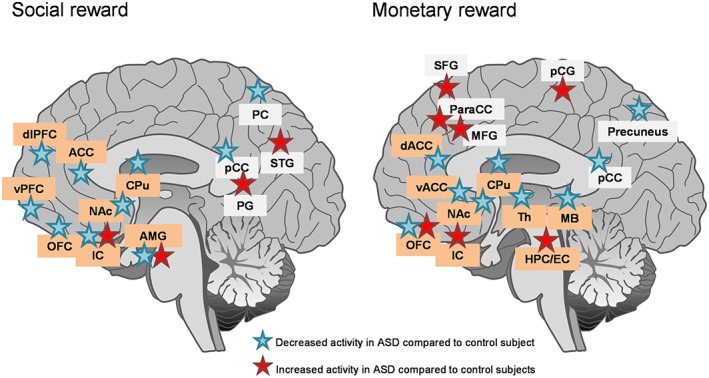
Brain areas differentially activated in patients with ASD versus controls during social (left panel) or monetary (right panel) reward anticipation and/or processing. Lateralization of brain responses (right or left hemisphere) was not taken into account to simplify the proposal. Comparing brain activation patterns for social and monetary reward reveals a common hypoactivation of a frontostriatal circuit including key regions for reward processing that are ACC, NAc and CPu in patients with ASD. The nature of stimuli used for experiments (images of faces, verbal praise and social reinforcement), tasks performed (stimulus presentation vs game or learning task for example) and timing (anticipation vs reward processing) varied across studies, possibly accounting for discrepancies in the level of activation of some structures (IC, AMG and OFC). Brain regions belonging to the reward circuitry are highlighted in orange. ACC, anterior cingulate cortex; AMG, amygdala; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; HPC/EC, hippocampus/enthorinal cortex; IC, insular cortex; MB, midbrain; MFG, medial frontal gyrus; OFC, orbitofrontal cortex; paraCC, paracingulate cortex; PC, parietal cortex; pCC, posterior cingulate cortex; PCG, precentral gyrus; PG, parahippocampal gyrus; SFG, superior frontal gyrus; STG, superior temporal gyrus; vPFC, ventral prefrontal cortex.
Clinical data relative to other (non‐social) rewarding stimuli appear less consistent. Concerning primary rewards, individuals with ASD were reported to display intact hedonic responses to sweet taste (Damiano et al., 2014) and stronger activation in brain reward regions (NAc, orbitofrontal cortex, amygdala and insula) in response to food cues (Cascio et al., 2012a) pointing to increased food reward in these patients. In line with this, children with ASD were shown as less able to delay gratification when tested for a food reward (Faja and Dawson, 2015). In contrast, ASD subjects exhibited diminished brain activation for pleasant (and neutral) tactile texture stimuli (Cascio et al., 2012b) and young children with ASD were impaired in learning an abstract rule from a discrete physical reward (small toy) (Jones et al., 2013). Consistent with this, when presented with an object incentive, adults with ASD showed decreased activation in the dorsal ACC but increased activation in the paracingulate gyrus and other frontal regions (Dichter et al., 2012a). These data suggest that reward processing for these stimuli is altered in ASD patients. Regarding secondary rewards, some studies have reported unchanged anticipation and processing of monetary reward (Dvash et al., 2014) and no modification of neural responses during monetary choices (Gonzalez‐Gadea et al., 2016) in patients with ASD. In other reports, these patients were shown to be less sensitive to monetary incentive (Scott‐Van Zeeland et al., 2010; Lin et al., 2012; Kohls et al., 2014), notably when making effort‐based decisions (Damiano et al., 2012), and more sensitive to monetary loss (South et al., 2014). Accordingly, imaging studies have disclosed hypo‐activation in the mesocorticolimbic circuitry (NAc, CPu and midbrain) during monetary anticipation or outcome in ASD patients (Kohls et al., 2012; Dichter et al., 2012a; Dichter et al., 2012b; Richey et al., 2014) (Figure 3). Moreover, children with ASD were found to display reduced activation of the right CPu when anticipating monetary loss (Damiano et al., 2015). Finally, when reward stimulus was a positive feedback (‘Correct!’ printed on screen) versus negative feedback (‘incorrect!’), young adults with ASD displayed impaired ability to develop an effective reward‐based working memory (Solomon et al., 2015). These data converge towards altered processing of secondary reward in ASD patients. However, ASD subjects can attribute higher positive ratings to non‐social stimuli whenever these stimuli match their restricted interests (trains and electronics) (Sasson et al., 2012; Watson et al., 2015). Non‐social reward processes thus appear unevenly affected by ASD, with food reward and restricted interests showing increased motivational value, whereas other stimuli show blunted motivational properties.
Methodological issues should be mentioned, though, that may limit previous conclusions. Indeed, a major difficulty in clinical ASD research lies in recruiting patients. Notably, sample size in a majority of studies is small and the age of participants is highly heterogeneous (young children, adolescents and adults) as well as patient's use of psychotropic medications or involvement in behavioural therapy programs, which has significant consequences on reward processing (Pankert et al., 2014). Most importantly, clinical studies suffer from a major IQ bias: They focus on a population of subjects with IQ in a normal range, namely, higher functioning ASD patients. This bias is essentially due to technical issues, as most psychological tasks and imaging settings in previous studies are too demanding and restrictive for lower functioning subjects. The possibility that the latter patients present more severe reward deficits has thus never been explored. Of importance though, conditioned learning mechanisms are set very early in the course of human infant development (Rovee‐Collier, 1999) and depend tightly, as regards appetitive aspects, on the integrity of the reward system (Dayan and Balleine, 2002; O'Doherty et al., 2016). Major deficits in reward processing may thus result in cognitive impairment, such as observed in low functioning ASD patients, highlighting the interest of evaluating reward in this population.
In conclusion, clinical data have demonstrated deficient reward processing in patients with ASD, for social and secondary (monetary) stimuli. The neurobiological substrates of this deficit remain to be identified, and one possible candidate is a dysfunction of the opioid system.
The opioid hypothesis in autism
As a straightforward consequence of BOTSA (Panksepp et al., 1978), Panksepp proposed for the first time in 1979 that autism would be an emotional disorder caused by excessive brain opioid activity and, therefore, that opioid antagonists should be beneficial to treat this pathology (Panksepp, 1979). The last hypothesis was later tested by administering http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1639 to ASD patients. Several clinical trials showed beneficial effects of naltrexone in reducing hyperactivity and irritability, but failed to detect an improvement in core autistic symptoms (reviewed in (Roy et al., 2015)), except maybe in a subgroup of patients with high blood levels of β‐endorphins (Leboyer et al., 1992; Bouvard et al., 1995). Consistent with the excessive opioid hypothesis, several studies have reported increased (but also decreased) levels of opioid peptides in plasma, urinary and cerebrospinal fluid samples from patients with ASD (Table 2). Reichelt and colleagues proposed a dietary origin for urine peptides (Reichelt et al., 1991) and therefore recommended a gluten‐ and casein‐free diet to relieve ASD (Knivsberg et al., 1999). Technical issues were raised, however, regarding the concentrations of urinary opioid peptides that challenged Reichelt's hypothesis and led to a still active scientific controversy. In further questioning of the excessive opioid theory, reviews and meta‐analyses have examined clinical evidence for the efficacy of dietary intervention in autism and found a lack of consistent effects (Dosman et al., 2013; Mari‐Bauset et al., 2014; Lange et al., 2015). In sum, technical concerns and disappointing clinical results have argued against the excessive opioid theory of autism and, more generally, dissuaded the scientific community from considering dysfunction in the opioid system as a plausible neurobiological substrate for autism.
Table 2.
Levels of blood, CSF or urinary opioid peptides in subjects with ASD or Rett Syndrome
| Opioid peptide | Fluid | Detection method | Number of subjects | Results | References |
|---|---|---|---|---|---|
| β‐Endorphin | Plasma | RIA | 13 | Similar in ASD and controls | Ernst et al., 1993 |
| β‐Endorphin | Plasma | RIA | 10 | Decreased in ASD compared to controls | Weizman et al., 1984 |
| β‐Endorphin | Plasma | RIA | 22 | Decreased in ASD compared to controls | Weizman et al., 1988 |
| β‐Endorphin | Plasma | RIA | 67 | Increased in ASD compared to controls | Leboyer et al., 1994 |
| 22 | Increased in Rett Syndrome compared to controls | ||||
| β‐Endorphin | Plasma | RIA | 62 | Increased in mothers of children with ASD compared to controls | Leboyer et al., 1999 |
| β‐Endorphin | Plasma | RIA | 10 | Increased in ASD compared to controls | Bouvard et al., 1995 |
| β‐Endorphin | Plasma | RIA | 33 | Increased in ASD compared to controls | Willemsen‐Swinkels et al., 1996 |
| β‐Endorphin | Plasma | RIA | 48 | Increased in ASD compared to controls | Tordjman et al., 1997 |
| β‐Endorphin | Plasma | RIA | 12 | Increased in ASD compared to controls | Brambilla et al., 1997 |
| β‐Endorphin | Plasma | RIA | 11 | Increased in ASD compared to controls | Cazzullo et al., 1999 |
| β‐Endorphin | Plasma | RIA | 73 | Increased in ASD compared to controls | Tordjman et al., 2009 |
| β‐Endorphin | CSF | RIA | 19 | Similar in ASD and controls | Nagamitsu, 1993 |
| β‐Endorphin | 3 | Increased in Rett Syndrome compared to controls | |||
| β‐Endorphin | CSF | RIA | 22 | Similar in ASD and controls | Nagamitsu et al., 1997 |
| β‐Endorphin | CSF | RIA | 9 | Decreased in Rett Syndrome compared to controls | Genazzani et al., 1989 |
| β‐Endorphin | CSF | RIA | 31 | Decreased in ASD compared to controls | Gillberg et al., 1990 |
| β‐Endorphin | 8 | Decreased in Rett Syndrome compared to controls | |||
| β‐Endorphin | CSF | RIA | 9 | Increased in ASD compared to controls | Ross et al., 1987 |
| Met‐Enkephalin | CSF | RIA | 24 | Increased in ASD compared to controls | Gillberg et al., 1985 |
| Opioid Peptides | Urine | HPLC | 54 | Not detected | Dettmer et al., 2007 |
| Opioid Peptides | Urine | Mass Spec | 10 | Not detected | Shattock et al., 2004 |
| Opioid Peptides | Urine | HPLC | 97 | Not detected | Pusponegoro et al., 2015 |
| Opioid Peptides | Urine | Mass Spec | 65 | Similar in ASD and controls | Cass et al., 2008 |
| β‐Casomorphin | Urine | ELISA | 10 | Increased in ASD compared to controls | Sokolov et al., 2014 |
| β‐Casomorphin | Urine | HPLC | 53 | Increased in Rett Syndrome compared to controls | Solaas et al., 2002 |
| 35 | Increased in ASD compared to controls | ||||
| Exorphins | Urine | HPLC | 135 | Increased in ASD compared to controls | Reichelt et al., 2012 |
Mass Spec, mass spectrometry.
The hypothesis of excessive opioid activity in autism fits into the excessive opioid tone window of our μ receptor balance model (Figure 2). However, this model also predicts that ASD could result from insufficient opioid tone. Interestingly, there is clinical evidence supporting both propositions. Dosage studies have examined levels of opioid peptides in various biological samples from patients with autism. As regards plasma samples, most studies have detected an increase in circulating β‐endorphin in subjects with ASD (or their mothers) or Rett syndrome (Table 2). Whether such increase would reflect an excessive opioid tone in these patients or, instead, reflects a compensation for defective opioid signalling, as suggested by abnormal β‐endorphin immunoreactivity (Leboyer et al., 1994) or decreased μ receptor expression in a mouse model of Rett syndrome (Kao et al., 2015), has not been explored yet. Of note however, low doses of naltrexone, known to stimulate μ receptor expression (Brown and Panksepp, 2009), significantly relieved autistic symptoms in several ASD patients (Leboyer et al., 1992; Bouvard et al., 1995). Regarding cerebrospinal fluid and urine samples, levels of β‐endorphin or, more generally, opioid peptides appear inconsistent, possibly due to methodological issues. Urinary exogenous (presumably dietary) opioid peptides have been detected in several studies (Table 2). Together, these data argue for a link between opioid tone and ASD, in either direction (excessive or deficient tone). Genetic studies further support the existence of such connection. We browsed the SFARIgene2.0 database (https://gene.sfari.org/autdb/Welcome.do) in search for mutations affecting opioid genes in ASD patients. We failed to find mutations in genes coding for the precursors of endorphins (POMC) or dynorphins (PDYN) and for δ or κ receptors (OPRD1, OPRK1). We found one patient with a genetic deletion including PENK, coding for proenkephalin (Kaminsky et al., 2011). Strikingly, however, we found 10 patients with mutations affecting the OPRM1 gene (Figure 4). For comparison, mutations in CNR1, coding for the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56, another candidate gene for ASD involved in reward processing (Chakrabarti et al., 2006), are not reported in the SFARIgene2.0 database. In addition, OPRM1 mutations are all associated with intellectual deficiency, suggesting that compromised μ receptor function in humans can lead to IQ impairment. Genetic data thus demonstrate that genetic μ receptor ablation is sufficient to produce ASD in humans, as it is in mice (Becker et al., 2014). Direct genetic mutation, though, is not the only way to impair the expression and function of a GPCR, as illustrated by the effects of Mecp2 genetic manipulation on μ receptor expression in mice. Whether mutations in other candidate genes for autism identified from sequencing studies could affect μ receptor signalling thus needs to be carefully explored.
Figure 4.
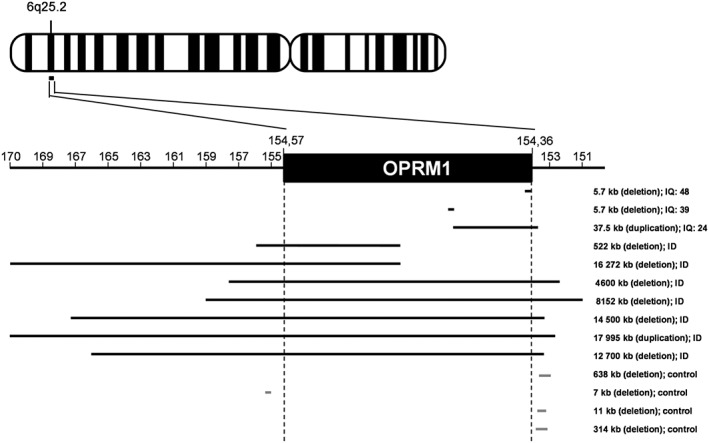
Copy number variation in the OPRM1 gene of patients with ASD. OPRM1 is located on chromosome 6 at the q25.2 position. The map was build based on the integrated catalogue of copy number variants (CNVs) associated with ASD from the Simons Foundation Autism Research Initiative resource website (https://gene.sfari.org/autdb/CNVHome.do; September 2016). We identified 10 patients from this database displaying CNVs that affect the OPRM1 gene (eight deletions and two duplications) (Kaminsky et al., 2011; Sanders et al., 2011; Halgren et al., 2012; Battaglia et al., 2013). No CNVs were detected in controls, some of which showed mutations immediately beyond OPRM1 boundaries. Remarkably, OPRM1 CNVs were all detected in low‐functioning ASD subjects (with intellectual disability). ID, intellectual deficiency; IQ, intellectual quotient.
Conclusions
Recent advances in brain imaging and sustained efforts towards identifying the genetic and neurobiological substrates of social impairment in ASD, including characterization of mouse models, have revived the exciting field of opioids and social behaviour. These studies have provided the demonstration that, among factors of the opioid system, μ receptors are the key substrate for the control of social behaviour. In an attempt to better describe the role of μ receptors in such control, we propose here a new model, the μ receptor balance model, in which both deficient and excessive μ receptor signalling results in social behaviour impairments. How this model could apply to other opioid‐dependent functions would need further investigation. Importantly, altered μ receptor function is clearly sufficient to hamper social abilities, making it a plausible contributor to ASD. Dedicated studies are now required to explore the necessity of μ receptor dysfunction for social impairment in such pathologies. Remarkably, pharmacological treatments can relieve autistic‐like symptoms in μ receptor null mice (Becker et al., 2014; Gigliucci et al., 2014), suggesting that whether or not μ receptor dysfunction is a core mechanism in ASD, promising therapeutic strategies exist that can overcome such dysfunction.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de la Recherche Agronomique (INRA) and Université François Rabelais de Tours. We thank Région Centre‐Val de Loire (ARD2020 Biomédicaments – GPCRAb) for support in this project. L.P. Pellissier acknowledges postdoctoral support from the Marie‐Curie/AgreenSkills Programme.
Pellissier L. P., Gandía J., Laboute T., Becker J. A. J., and Le Merrer J. (2018) μ opioid receptor, social behaviour and autism spectrum disorder: reward matters, British Journal of Pharmacology, 175: 2750–2769, https://doi.org/10.1111/bph.13808.
References
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Hyatt CJ, Wong CG, Johnson MR, Schultz RT, Hendler T et al. (2013). Mentalizing and motivation neural function during social interactions in autism spectrum disorders. Neuroimage Clin 3: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E (2015). Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci 9: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G et al. (2013). Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 339: 332–335. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D et al. (2008). Variation at the mu‐opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci U S A 105: 5277–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia A, Doccini V, Bernardini L, Novelli A, Loddo S, Capalbo A et al. (2013). Confirmation of chromosomal microarray as a first‐tier clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features. Eur J Paediatr Neurol 17: 589–599. [DOI] [PubMed] [Google Scholar]
- Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL (2014). Autistic‐like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology 39: 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Kieffer BL, Le Merrer J (in press). Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addict Biol. https://doi.org/10.1111/adb.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Smoothy R, Brain PF (1985). Comparisons of the influence of morphine sulphate, morphine‐3‐glucuronide and tifluadom on social encounters in mice. Physiol Behav 35: 689–693. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Seiden JA, de Wit H (2016). Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology 63: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti E, Zanoni A, Giorda R, Battaglia M (2012). Influence of the OPRM1 gene polymorphism upon children's degree of withdrawal and brain activation in response to facial expressions. Dev Cogn Neurosci 2: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Menicacci C, Ghelardini C (2013). Dual effect of morphine in long‐term social memory in rat. Br J Pharmacol 168: 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard MP, Leboyer M, Launay JM, Recasens C, Plumet MH, Waller‐Perotte D et al. (1995). Low‐dose naltrexone effects on plasma chemistries and clinical symptoms in autism: a double‐blind, placebo‐controlled study. Psychiatry Res 58: 191–201. [DOI] [PubMed] [Google Scholar]
- Brain PF, Brain S, Benton D (1985). Ethological analyses of the effects of naloxone and the opiate antagonist ICI 154, 129 on social interactions in male house mice. Behav Process 10: 341–354. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Guareschi‐Cazzullo A, Tacchini C, Musetti C, Panerai AE, Sacerdote P (1997). Beta‐endorphin and cholecystokinin 8 concentrations in peripheral blood mononuclear cells of autistic children. Neuropsychobiology 35: 1–4. [DOI] [PubMed] [Google Scholar]
- Briand LA, Hilario M, Dow HC, Brodkin ES, Blendy JA, Berton O (2015). Mouse model of OPRM1 (A118G) polymorphism increases sociability and dominance and confers resilience to social defeat. J Neurosci 35: 3582–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Panksepp J (2009). Low‐dose naltrexone for disease prevention and quality of life. Med Hypotheses 72: 333–337. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ (2011). Activation of mu‐opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology 36: 2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG (2011). Social isolation. Ann N Y Acad Sci 1231: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA (1991). Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res 62: 17–22. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Kim Y (2016). Mu opioid receptor polymorphism, early social adversity, and social traits. Soc Neurosci 11: 515–524. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss‐Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM et al. (2012a). Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moana‐Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT et al. (2012b). Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res 5: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass H, Gringras P, March J, McKendrick I, O'Hare AE, Owen L et al. (2008). Absence of urinary opioid peptides in children with autism. Arch Dis Child 93: 745–750. [DOI] [PubMed] [Google Scholar]
- Cazzullo AG, Musetti MC, Musetti L, Bajo S, Sacerdote P, Panerai A (1999). Beta‐endorphin levels in peripheral blood mononuclear cells and long‐term naltrexone treatment in autistic children. Eur Neuropsychopharmacol 9: 361–366. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J et al. (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale NN, Curtis AL, Wood SK, Zhang XY, Bhatnagar S, Reyes BA et al. (2013). Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology 38: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron‐Cohen S (2006). Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci 23: 1944–1948. [DOI] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Eikemo M, Riegels J, Loseth G, Maurud H et al. (2014). Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry 19: 746–747. [DOI] [PubMed] [Google Scholar]
- Chen C, Hung AY, Fan YT, Tan S, Hong H, Cheng Y (2016). Linkage between pain sensitivity and empathic response in adolescents with autism spectrum conditions and conduct disorder symptoms. Autism Res 10: 267–275. [DOI] [PubMed] [Google Scholar]
- Chen Z, Williams KD, Fitness J, Newton NC (2008). When hurt will not heal: exploring the capacity to relive social and physical pain. Psychol Sci 19: 789–795. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Grezes J, Molesworth C, Berthoz S, Happe F (2012). Brief report: selective social anhedonia in high functioning autism. J Autism Dev Disord 42: 1504–1509. [DOI] [PubMed] [Google Scholar]
- Choi US, Kim SY, Sim HJ, Lee SY, Park SY, Jeong JS et al. (2015). Abnormal brain activity in social reward learning in children with autism spectrum disorder: an fMRI study. Yonsei Med J 56: 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque C, Pondiki S, Oddi D, Di Certo MG, Marinelli S, Troisi A et al. (2012). Modeling socially anhedonic syndromes: genetic and pharmacological manipulation of opioid neurotransmission in mice. Transl Psychiatry 2: e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Sun H, Costello EJ, Angold A, Heilig MA, Barr CS (2011). Child mu‐opioid receptor gene variant influences parent‐child relations. Neuropsychopharmacology 36: 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria‐Avila GA, Manzo J, Garcia LI, Carrillo P, Miquel M, Pfaus JG (2014). Neurobiology of social attachments. Neurosci Biobehav Rev 43: 173–182. [DOI] [PubMed] [Google Scholar]
- Coudereau JP, Monier C, Bourre JM, Frances H (1997). Effect of isolation on pain threshold and on different effects of morphine. Prog Neuropsychopharmacol Biol Psychiatry 21: 997–1018. [DOI] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Burrus C, Garbutt JC, Kampov‐Polevoy AB, Dichter GS (2014). Intact hedonic responses to sweet tastes in autism spectrum disorder. Res Autism Spectr Disord 8: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS (2012). Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort‐based decisions. J Neurodev Disord 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Cockrell DC, Dunlap K, Hanna EK, Miller S, Bizzell J et al. (2015). Neural mechanisms of negative reinforcement in children and adolescents with autism spectrum disorders. J Neurodev Disord 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Bernier R (2007). Development of social brain circuitry in autism In: Coch D, Dawson G, Fischer K. (eds). Human Behavior and the Developing Brain: Atypical Development, 2nd edn. Guilford Press: New York, pp. 28–55. [Google Scholar]
- Dayan P, Balleine BW (2002). Reward, motivation, and reinforcement learning. Neuron 36: 285–298. [DOI] [PubMed] [Google Scholar]
- Defeudis FV, Defeudis PA, Somoza E (1976). Altered analgesic responses to morphine in differentially housed mice. Psychopharmacology (Berl) 49: 117–118. [DOI] [PubMed] [Google Scholar]
- Dettmer K, Hanna D, Whetstone P, Hansen R, Hammock BD (2007). Autism and urinary exogenous neuropeptides: development of an on‐line SPE‐HPLC‐tandem mass spectrometry method to test the opioid excess theory. Anal Bioanal Chem 388: 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW (2012a). Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci 7: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW (2012b). Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord 42: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosman C, Adams D, Wudel B, Vogels L, Turner J, Vohra S (2013). Complementary, holistic, and integrative medicine: autism spectrum disorder and gluten‐ and casein‐free diet. Pediatr Rev 34: e36–e41. [DOI] [PubMed] [Google Scholar]
- Dubey I, Ropar D, Hamilton AF (2015). Measuring the value of social engagement in adults with and without autism. Mol Autism 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash J, Ben‐Zeev A, Noga A, Shamay‐Tsoory S (2014). The road not taken: social vs. private comparisons in Aspergers syndrome and high functioning autism. Psychiatry Res 216: 385–390. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI (2012). The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 13: 421–434. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR (2016). In sickness and in health: the co‐regulation of inflammation and social behavior. Neuropsychopharmacology 42: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Devi L, Silva RR, Gonzalez NM, Small AM, Malone RP et al. (1993). Plasma beta‐endorphin levels, naltrexone, and haloperidol in autistic children. Psychopharmacol Bull 29: 221–227. [PubMed] [Google Scholar]
- Faja S, Dawson G (2015). Reduced delay of gratification and effortful control among young children with autism spectrum disorders. Autism 19: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Chang LJ, Delgado MR (2015). Computational substrates of social value in interpersonal collaboration. J Neurosci 35: 8170–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF (2007). Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci 11: 419–427. [DOI] [PubMed] [Google Scholar]
- Filipovic D, Todorovic N, Bernardi RE, Gass P (2016). Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive‐ and anxiety‐like symptoms. Brain Struct Funct 222: 1–20. [DOI] [PubMed] [Google Scholar]
- Gaveriaux‐Ruff C, Kieffer BL (2002). Opioid receptor genes inactivated in mice: the highlights. Neuropeptides 36: 62–71. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Zappella M, Nalin A, Hayek Y, Facchinetti F (1989). Reduced cerebrospinal fluid B‐endorphin levels in Rett syndrome. Childs Nerv Syst 5: 111–113. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, Leonzino M, Busnelli M, Luchetti A, Palladino VS, D'Amato FR et al. (2014). Region specific up‐regulation of oxytocin receptors in the opioid oprm1 (−/−) mouse model of autism. Front Pediatr 2: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, Terenius L, Hagberg B, Witt‐Engerstrom I, Eriksson I (1990). CSF beta‐endorphins in childhood neuropsychiatric disorders. Brain Dev 12: 88–92. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Terenius L, Lonnerholm G (1985). Endorphin activity in childhood psychosis. Spinal fluid levels in 24 cases. Arch Gen Psychiatry 42: 780–783. [DOI] [PubMed] [Google Scholar]
- Gobbroge KL, Jia X, Liu Y, Wang Z (2017). Neurochemical mediation of affiliation and aggression associated with pair‐bonding. Biol Psychiatry 81: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM et al. (2011). Impaired emotional‐like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Gadea ML, Sigman M, Rattazzi A, Lavin C, Rivera‐Rei A, Marino J et al. (2016). Neural markers of social and monetary rewards in children with attention‐deficit/hyperactivity disorder and autism spectrum disorder. Sci Rep 6: 30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P (2008). Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology (Berl) 197: 295–307. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Becker A, Schroeder H, Kraus J, Loh H, Hollt V (2004). Accelerated kindling development in mu‐opioid receptor deficient mice. Naunyn Schmiedebergs Arch Pharmacol 369: 287–293. [DOI] [PubMed] [Google Scholar]
- Guard HJ, Newman JD, Roberts RL (2002). Morphine administration selectively facilitates social play in common marmosets. Dev Psychobiol 41: 37–49. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157: 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren C, Kjaergaard S, Bak M, Hansen C, El‐Schich Z, Anderson CM et al. (2012). Corpus callosum abnormalities, intellectual disability, speech impairment, and autism in patients with haploinsufficiency of ARID1B. Clin Genet 82: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D (2011). Mu‐opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free‐ranging rhesus macaques Macaca mulatta . Behav Neurosci 125: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ et al. (2015). It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry 20: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H et al. (2013). Response of the mu‐opioid system to social rejection and acceptance. Mol Psychiatry 18: 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Meyer RE, Harris JD, Ayaz H, Deneke E, Stankoski DM et al. (2016). Evidence of anhedonia and differential reward processing in prefrontal cortex among post‐withdrawal patients with prescription opiate dependence. Brain Res Bull 123: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Moieni M, Dutcher JM, Jevtic I, Irwin MR et al. (2016a). Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc Cogn Affect Neurosci 11: 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI (2016b). Opioids and social bonding: naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci 11: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Burkett JP, Young LJ (2013). Neuroanatomical distribution of mu‐opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non‐monogamous meadow voles (Microtus pennsylvanicus). Neuroscience 244: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC (2003). Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav 2: 80–92. [DOI] [PubMed] [Google Scholar]
- Jang CG, Lee SY, Loh HH, Ho IK (2001). Lack of mu‐opioid receptor leads to an increase in the NMDA receptor subunit mRNA expression and NMDA‐induced convulsion. Brain Res Mol Brain Res 94: 105–111. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ulberg S, Shivale S, Donaldson J, Milczarski B, Faraone SV (2014). Fibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Discov Med 18: 209–220. [PubMed] [Google Scholar]
- Johnson CP, Myers SM (2007). Identification and evaluation of children with autism spectrum disorders. Pediatrics 120: 1183–1215. [DOI] [PubMed] [Google Scholar]
- Jones EJ, Webb SJ, Estes A, Dawson G (2013). Rule learning in autism: the role of reward type and social context. Dev Neuropsychol 38: 58–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Yang DY, Voos AC, Bennett RH, Gordon I, Pretzsch C et al. (2016). Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb Cortex 26: 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM (1988). Opiate modulation of separation‐induced distress in non‐human primates. Brain Res 440: 285–292. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE (1995). Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology 20: 735–742. [DOI] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D et al. (2011). An evidence‐based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med 13: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao FC, Su SH, Carlson GC, Liao W (2015). MeCP2‐mediated alterations of striatal features accompany psychomotor deficits in a mouse model of Rett syndrome. Brain Struct Funct 220: 419–434. [DOI] [PubMed] [Google Scholar]
- Katsouni E, Sakkas P, Zarros A, Skandali N, Liapi C (2009). The involvement of substance P in the induction of aggressive behavior. Peptides 30: 1586–1591. [DOI] [PubMed] [Google Scholar]
- Katsouni E, Zarros A, Skandali N, Tsakiris S, Lappas D (2013). The role of cholecystokinin in the induction of aggressive behavior: a focus on the available experimental data (review). Acta Physiol Hung 100: 361–377. [DOI] [PubMed] [Google Scholar]
- Kawamichi H, Sugawara SK, Hamano YH, Makita K, Kochiyama T, Sadato N (2016). Increased frequency of social interaction is associated with enjoyment enhancement and reward system activation. Sci Rep 6: 24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Rosenthal MZ, Gwaltney M, Jarrold W, Hatt N, McIntyre N et al. (2015). A virtual joy‐stick study of emotional responses and social motivation in children with autism spectrum disorder. J Autism Dev Disord 45: 3891–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knivsberg AM, Reichelt KL, Nodland M (1999). Dietary intervention for a seven year old girl with autistic behaviour. Nutr Neurosci 2: 435–439. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte‐Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp‐Becker I et al. (2012). Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 8: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Thonessen H, Bartley GK, Grossheinrich N, Fink GR, Herpertz‐Dahlmann B et al. (2014). Differentiating neural reward responsiveness in autism versus ADHD. Dev Cogn Neurosci 10: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Ohara A, Sasaki K, Abe H, Hattori H, Hall FS et al. (2011). Decreased response to social defeat stress in mu‐opioid‐receptor knockout mice. Pharmacol Biochem Behav 99: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ (2007). The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem 103: 77–87. [DOI] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD (2011). Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A 108: 6270–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Hauser J, Reissmann A (2015). Gluten‐free and casein‐free diets in the therapy of autism. Curr Opin Clin Nutr Metab Care 18: 572–575. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL (2009). Reward processing by the opioid system in the brain. Physiol Rev 89: 1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M, Bouvard MP, Launay JM, Tabuteau F, Waller D, Dugas M et al. (1992). Brief report: a double‐blind study of naltrexone in infantile autism. J Autism Dev Disord 22: 309–319. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bouvard MP, Recasens C, Philippe A, Guilloud‐Bataille M, Bondoux D et al. (1994). Difference between plasma N‐ and C‐terminally directed beta‐endorphin immunoreactivity in infantile autism. Am J Psychiatry 151: 1797–1801. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud‐Bataille M, Bondoux D, Tabuteau F et al. (1999). Whole blood serotonin and plasma beta‐endorphin in autistic probands and their first‐degree relatives. Biol Psychiatry 45: 158–163. [DOI] [PubMed] [Google Scholar]
- Leung RC, Pang EW, Cassel D, Brian JA, Smith ML, Taylor MJ (2015). Early neural activation during facial affect processing in adolescents with autism spectrum disorder. Neuroimage Clin 7: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Choleris E, Leri F (2009). Enhancing effect of heroin on social recognition learning in male Sprague–Dawley rats: modulation by heroin pre‐exposure. Psychopharmacology (Berl) 204: 413–421. [DOI] [PubMed] [Google Scholar]
- Lin A, Rangel A, Adolphs R (2012). Impaired learning of social compared to monetary rewards in autism. Front Neurosci 6: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi LM, Baker SA, Zoghbi HY (2015). MECP2 disorders: from the clinic to mice and back. J Clin Invest 125: 2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loseth GE, Ellingsen DM, Leknes S (2014). State‐dependent mu‐opioid modulation of social motivation. Front Behav Neurosci 8: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Kramar C, Renard J, Rosen LG, Laviolette SR (2016). Cannabinoid transmission in the hippocampus activates nucleus accumbens neurons and modulates reward and aversion‐related emotional salience. Biol Psychiatry 80: 216–225. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Ayranci G, Chu‐Sin‐Chung P, Matifas A, Koebel P, Filliol D et al. (2014). Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive‐like behaviors during heroin abstinence. Neuropsychopharmacology 39: 2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu‐Chen LY, Lerman C, Blendy JA (2009). Mouse model of OPRM1 (A118G) polymorphism has sex‐specific effects on drug‐mediated behavior. Proc Natl Acad Sci U S A 106: 10847–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari‐Bauset S, Zazpe I, Mari‐Sanchis A, Llopis‐Gonzalez A, Morales‐Suarez‐Varela M (2014). Evidence of the gluten‐free and casein‐free diet in autism spectrum disorders: a systematic review. J Child Neurol 29: 1718–1727. [DOI] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS et al. (2016). Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164: 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A et al. (2013). Anxiety, sensory over‐responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol 41: 165–176. [DOI] [PubMed] [Google Scholar]
- McDonald S, Darke S, Kaye S, Torok M (2013). Deficits in social perception in opioid maintenance patients, abstinent opioid users and non‐opioid users. Addiction 108: 566–574. [DOI] [PubMed] [Google Scholar]
- Mechling AE, Arefin T, Lee HL, Bienert T, Reisert M, Ben Hamida S et al. (2016). Deletion of the mu opioid receptor gene in mice reshapes the reward‐aversion connectome. Proc Natl Acad Sci U S A 113: 11603–11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier IM, Bos PA, Hamilton K, Stein DJ, van Honk J, Malcolm‐Smith S (2016). Naltrexone increases negatively‐valenced facial responses to happy faces in female participants. Psychoneuroendocrinology 74: 65–68. [DOI] [PubMed] [Google Scholar]
- Miranda‐Paiva CM, Nasello AG, Yin AJ, Felicio LF (2001). Morphine pretreatment increases opioid inhibitory effects on maternal behavior. Brain Res Bull 55: 501–505. [DOI] [PubMed] [Google Scholar]
- Miranda‐Paiva CM, Ribeiro‐Barbosa ER, Canteras NS, Felicio LF (2003). A role for the periaqueductal grey in opioidergic inhibition of maternal behaviour. Eur J Neurosci 18: 667–674. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR (2004). Deficit in attachment behavior in mice lacking the mu‐opioid receptor gene. Science 304: 1983–1986. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S (1993). CSF beta‐endorphin levels in pediatric neurologic disorders. Kurume Med J 40: 233–241. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S, Matsuishi T, Kisa T, Komori H, Miyazaki M, Hashimoto T et al. (1997). CSF beta‐endorphin levels in patients with infantile autism. J Autism Dev Disord 27: 155–163. [DOI] [PubMed] [Google Scholar]
- Najam N, Panksepp J (1989). Effect of chronic neonatal morphine and naloxone on sensorimotor and social development of young rats. Pharmacol Biochem Behav 33: 539–544. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J (1998). Brain substrates of infant‐mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev 22: 437–452. [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Beauchaine TP, Bernier R (2010). Neurobiological correlates of social functioning in autism. Clin Psychol Rev 30: 733–748. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP Jr (2005). Prolonged effects of repeated social defeat stress on mRNA expression and function of mu‐opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology 30: 1096–1103. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J (1990). Effects of morphine and naloxone on play‐rewarded spatial discrimination in juvenile rats. Dev Psychobiol 23: 75–83. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Tuominen L, Dunbar R, Hirvonen J, Manninen S, Arponen E et al. (2016). Social touch modulates endogenous mu‐opioid system activity in humans. Neuroimage 138: 242–247. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Cockburn J, Pauli WM (2016). Learning, reward, and decision making. Annu Rev Psychol 68: 73–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi D, Crusio WE, D'Amato FR, Pietropaolo S (2013). Monogenic mouse models of social dysfunction: implications for autism. Behav Brain Res 251: 75–84. [DOI] [PubMed] [Google Scholar]
- Pankert A, Pankert K, Herpertz‐Dahlmann B, Konrad K, Kohls G (2014). Responsivity to familiar versus unfamiliar social reward in children with autism. J Neural Transm 121: 1199–1210. [DOI] [PubMed] [Google Scholar]
- Panksepp J (1979). A neurochemical theory of autism. Trends Neurosci 2: 174–177. [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP (1978). The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry 13: 607–618. [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P (1985). Opiates and play dominance in juvenile rats. Behav Neurosci 99: 441–453. [DOI] [PubMed] [Google Scholar]
- Papini MR, Fuchs PN, Torres C (2015). Behavioral neuroscience of psychological pain. Neurosci Biobehav Rev 48: 53–69. [DOI] [PubMed] [Google Scholar]
- Parkes CM (1975). Psycho‐social transitions: comparison between reactions to loss of a limb and loss of a spouse. The British journal of psychiatry : the journal of mental science 127: 204–210. [DOI] [PubMed] [Google Scholar]
- Parra‐Gamez L, Garcia‐Hidalgo A, Paredes RG (2013). Infusion of endomorphin‐1 (EM‐1) in the MPOA and the Me modulate sexual and socio‐sexual behavior in the male rat. Brain Res 1517: 36–43. [DOI] [PubMed] [Google Scholar]
- Plein LM, Rittner HL (2017). Opioids and the immune system – friend or foe. Br J Pharmacol . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi‐Allegra S, Oliverio A (1983). Social isolation: effects on pain threshold and stress‐induced analgesia. Pharmacol Biochem Behav 19: 679–681. [DOI] [PubMed] [Google Scholar]
- Pusponegoro HD, Ismael S, Sastroasmoro S, Firmansyah A, Vandenplas Y (2015). Maladaptive behavior and gastrointestinal disorders in children with autism spectrum disorder. Pediatr Gastroenterol Hepatol Nutr 18: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Freeman SM, Laredo SA, Mendoza SP, Bales KL (2015a). mu and kappa opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): implications for social behavior and endocrine functioning. Neuroscience 290: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL (2015b). The effects of morphine, naloxone, and kappa opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus). Neuroscience 287: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL (2013). Presence of a pair‐mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus). Psychoneuroendocrinology 38: 2448–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt KL, Knivsberg A‐M, Lind G, Nødland M (1991). Probable etiology and possible treatment of childhood autism. Brain Dysfunction 4: 308–319. [Google Scholar]
- Reichelt KL, Tveiten D, Knivsberg AM, Bronstad G (2012). Peptides' role in autism with emphasis on exorphins. Microb Ecol Health Dis 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA et al. (2013). mu‐Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci 33: 9140–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Rittenberg A, Hughes L, Damiano CR, Sabatino A, Miller S et al. (2014). Common and distinct neural features of social and non‐social reward processing in autism and social anxiety disorder. Soc Cogn Affect Neurosci 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ (2012). Childhood epilepsy and autism spectrum disorders: psychiatric problems, phenotypic expression, and anticonvulsants. Neuropsychol Rev 22: 271–279. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Arias M, Navarrete F, Blanco‐Gandia MC, Arenas MC, Bartoll‐Andres A, Aguilar MA et al. (2016). Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict Biol 21: 87–97. [DOI] [PubMed] [Google Scholar]
- Ross DL, Klykylo WM, Hitzemann R (1987). Reduction of elevated CSF beta‐endorphin by fenfluramine in infantile autism. Pediatr Neurol 3: 83–86. [DOI] [PubMed] [Google Scholar]
- Roughgarden J (2012). The social selection alternative to sexual selection. Philos Trans R Soc Lond B Biol Sci 367: 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee‐Collier C (1999). The development of infant memory. Current Directions in Psychological Science 8: 80–85. [Google Scholar]
- Roy A, Roy M, Deb S, Unwin G (2015). Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: a systematic review. J Intellect Disabil Res 59: 293–306. [DOI] [PubMed] [Google Scholar]
- Roy S, Liu HC, Loh HH (1998). mu‐opioid receptor‐knockout mice: the role of mu‐opioid receptor in gastrointestinal transit. Brain research . Mol Brain Res 56: 281–283. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS (1984). Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Res 307: 91–97. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Mandel‐Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY (2012). Crh and Oprm1 mediate anxiety‐related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat Genet 44: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan‐Sencicek AG, Hus V, Luo R, Murtha MT, Moreno‐De‐Luca D et al. (2011). Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Dichter GS, Bodfish JW (2012). Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS One 7: e42457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Troisi A (1992). Opiate receptor blockade in juvenile macaques: effect on affiliative interactions with their mothers and group companions. Brain Res 576: 125–130. [DOI] [PubMed] [Google Scholar]
- Scott‐Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY (2010). Reward processing in autism. Autism Res 3: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeta L, Tsuchiya N, Davies MS, Sigman M, Bookheimer SY, Dapretto M (2012). Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. J Neurodev Disord 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA (1989). Affiliative behavior in voles: effects of morphine, naloxone, and cross‐fostering. Physiol Behav 46: 719–723. [DOI] [PubMed] [Google Scholar]
- Shattock P, Hooper M, Waring R (2004). Opioid peptides and dipeptidyl peptidase in autism. Dev Med Child Neurol 46: 357 author reply 357–358. [DOI] [PubMed] [Google Scholar]
- Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY et al. (2009). Time‐dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse 35: 267–272. [DOI] [PubMed] [Google Scholar]
- Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R et al. (2008). A118G single nucleotide polymorphism of human mu‐opioid receptor gene influences pain perception and patient‐controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 109: 520–526. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Tartter MA, Brennan PA, Hammen C (2014). Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology 49: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Wilkins KB, Mogavero JN, Veenema AH (2015). Social novelty investigation in the juvenile rat: modulation by the mu‐opioid system. J Neuroendocrinol 27: 752–764. [DOI] [PubMed] [Google Scholar]
- Sobczak M, Salaga M, Storr MA, Fichna J (2014). Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol 49: 24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov O, Kost N, Andreeva O, Korneeva E, Meshavkin V, Tarakanova Y et al. (2014). Autistic children display elevated urine levels of bovine casomorphin‐7 immunoreactivity. Peptides 56: 68–71. [DOI] [PubMed] [Google Scholar]
- Solaas KM, Skjeldal O, Gardner ML, Kase FB, Reichelt KL (2002). Urinary peptides in Rett syndrome. Autism 6: 315–328. [DOI] [PubMed] [Google Scholar]
- Solomon M, Frank MJ, Ragland JD, Smith AC, Niendam TA, Lesh TA et al. (2015). Feedback‐driven trial‐by‐trial learning in autism spectrum disorders. Am J Psychiatry 172: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Chamberlain PD, Wigham S, Newton T, Le Couteur A, McConachie H et al. (2014). Enhanced decision making and risk avoidance in high‐functioning autism spectrum disorder. Neuropsychology 28: 222–228. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos KK, Carver LJ (2014). Reward anticipation and processing of social versus nonsocial stimuli in children with and without autism spectrum disorders. J Child Psychol Psychiatry 55: 1398–1408. [DOI] [PubMed] [Google Scholar]
- Syal S, Ipser J, Terburg D, Solms M, Panksepp J, Malcolm‐Smith S et al. (2015). Improved memory for reward cues following acute buprenorphine administration in humans. Psychoneuroendocrinology 53: 10–15. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, Miczek KA (2012). Behavioral and pharmacogenetics of aggressive behavior. Curr Top Behav Neurosci 12: 73–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly‐Tabard S, Perez‐Diaz F, Graignic R et al. (2009). Pain reactivity and plasma beta‐endorphin in children and adolescents with autistic disorder. PLoS One 4: e5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM et al. (1997). Plasma beta‐endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry 38: 705–715. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ (2010). The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ (2011). Nucleus accumbens mu‐opioid receptors mediate social reward. J Neurosci 31: 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ (2008). Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 197: 217–227. [DOI] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D'Amato FR et al. (2011). Social hedonic capacity is associated with the A118G polymorphism of the mu‐opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci 6: 88–97. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Van Ree JM, Spruijt BM, Kitchen I (1999). Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Eur J Neurosci 11: 3023–3032. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Achterberg EJ, Trezza V (2016). The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev 70: 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM (1995a). Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology (Berl) 117: 225–231. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM (1995b). Mu‐ and kappa‐opioid receptor‐mediated opioid effects on social play in juvenile rats. Eur J Pharmacol 276: 257–266. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM (1995c). Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res 680: 148–156. [DOI] [PubMed] [Google Scholar]
- Veenstra‐VanderWeele J, Blakely RD (2012). Networking in autism: leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology 37: 196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA (1998). Effects of mu and delta opioid agonists and antagonists on affective vocal and reflexive pain responses during social stress in rats. Psychopharmacoloy 139: 364–375. [DOI] [PubMed] [Google Scholar]
- Watson KK, Miller S, Hannah E, Kovac M, Damiano CR, Sabatino‐DiCrisco A et al. (2015). Increased reward value of non‐social stimuli in children and adolescents with autism. Front Psychol 6: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI (2009). Variation in the mu‐opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci U S A 106: 15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman R, Gil‐Ad I, Dick J, Tyano S, Szekely GA, Laron Z (1988). Low plasma immunoreactive beta‐endorphin levels in autism. J Am Acad Child Adolesc Psychiatry 27: 430–433. [DOI] [PubMed] [Google Scholar]
- Weizman R, Weizman A, Tyano S, Szekely G, Weissman BA, Sarne Y (1984). Humoral‐endorphin blood levels in autistic, schizophrenic and healthy subjects. Psychopharmacology (Berl) 82: 368–370. [DOI] [PubMed] [Google Scholar]
- White SW, Kreiser NL, Pugliese C, Scarpa A (2012). Social anxiety mediates the effect of autism spectrum disorder characteristics on hostility in young adults. Autism 16: 453–464. [DOI] [PubMed] [Google Scholar]
- Whyatt C, Craig C (2013). Sensory‐motor problems in autism. Frontiers in integrative neuroscience 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen‐Swinkels SH, Buitelaar JK, Weijnen FG, Thijssen JH, Van Engeland H (1996). Plasma beta‐endorphin concentrations in people with learning disability and self‐injurious and/or autistic behaviour. The British journal of psychiatry : the journal of mental science 168: 105–109. [DOI] [PubMed] [Google Scholar]
- Wohr M, Moles A, Schwarting RK, D'Amato FR (2011). Lack of social exploratory activation in male mu‐opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Soc Neurosci 6: 76–87. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Sadikot RT (2013). Opioid effect on lungs. Respirology 18: 255–262. [DOI] [PubMed] [Google Scholar]
- Yim AJ, Miranda‐Paiva CM, Florio JC, Oliveira CA, Nasello AG, Felicio LF (2006). A comparative study of morphine treatment regimen prior to mating and during late pregnancy. Brain Res Bull 68: 384–391. [DOI] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S (2010). Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One 5: e13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky‐Sommerer R et al. (2014). The oxytocin analogue carbetocin prevents emotional impairment and stress‐induced reinstatement of opioid‐seeking in morphine‐abstinent mice. Neuropsychopharmacology 39: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]


