Parkinson’s disease (PD) ranks as the second-most prevalent neurodegenerative disorder that afflicts the aging population (1). Hallmark neuropathological features of PD consist of dopaminergic cell loss in the substantia nigra, striatal dopamine deficiency, and formation of intracellular inclusions, called Lewy bodies, marked by α-synuclein aggregates (2). Although the primary brain deficiency of PD involves nigrostriatal dopaminergic depletion, various cell types undergo degeneration in the brain, as well as in the periphery during disease onset and progression (3). The clinical symptoms of PD have been routinely defined as cardinal motor abnormalities, which include bradykinesia, tremors, muscle rigidity, and freezing of gait (1). However, PD is also associated with many nonmotor symptoms, such as cognitive deficits, that contribute to overall morbidity of the disease (1, 4). In addition to α-synuclein accumulation, several mechanisms postulated to mediate dopaminergic depletion comprise mitochondrial dysfunction, aberrant oxidative stress, calcium imbalance, impaired axonal transport, and neuroinflammation (1). The advent of disease biomarkers may facilitate early diagnosis and treatment initiation for PD. Unfortunately, despite these scientific advances in our knowledge of the disease pathology, there is no cure for PD, only relief from its symptoms. While dopaminergic cells are selectively destroyed in PD patients, what triggers this brain cell death remains poorly understood, thereby relegating treatment intervention to the late, rather than the early stage of the disease. Palliative treatments of PD target pharmacological replacement of striatal dopamine (i.e., Levodopa), coupled with nondopaminergic drugs to attenuate both motor and nonmotor symptoms, and deep brain stimulation (1, 4). Cell-based regenerative medicine, in particular stem cell therapies designed to either exogenously replace dopamine or stimulate endogenous host brain repair machinery (5, 6), represents efforts toward disease-modifying outcomes, such as reduction in α-synuclein aggregates.
A recent PNAS paper by Ren et al. (7) reveals that an enzyme called soluble epoxide hydrolase (sEH) may be key to the demise of the brain dopaminergic cells. The authors report that in multiple animal models of PD, and further confirmed in a group of PD patients with Lewy bodies, this enzyme was highly elevated in specific regions of the brain associated with dopaminergic cell death. Indeed, increased sEH expression positively correlated with phosphorylation of α-synuclein in the striatum. In tandem, expression of sEH mRNA was up-regulated in human induced pluripotent stem cell (iPSC)-derived neurons compared with iPSC-derived neurons from healthy controls. Early pioneering work on the sEH enzyme revealed that many regulatory molecules are controlled by degradation and biosynthesis, with the epoxy fatty acid chemical mediator sEH regulating inflammation (8, 9), which is further reinforced in Ren et al.’s PNAS paper (7), highlighting the important role of sEH-mediated inflammation in the PD pathogenesis of α-synuclein and Lewy bodies. Interestingly, parallel lines of investigation demonstrate α-synuclein in the periphery (10, 11). Whether sEH similarly initiates from the periphery and propagates to the brain in transporting α-synuclein will be of high clinical relevance, as it will allow early peripheral diagnosis of PD, which does not manifest its first motor symptoms until 80% of striatal dopamine is lost (12). It may be possible to detect elevated sEH levels peripherally as a prelude to brain dopamine degeneration, thereby aiding in early intervention of the disease (Fig. 1). Of note, sEH activity can be measured in the intestines in other disease indications (13, 14), suggesting its feasibility as a biomarker for PD.
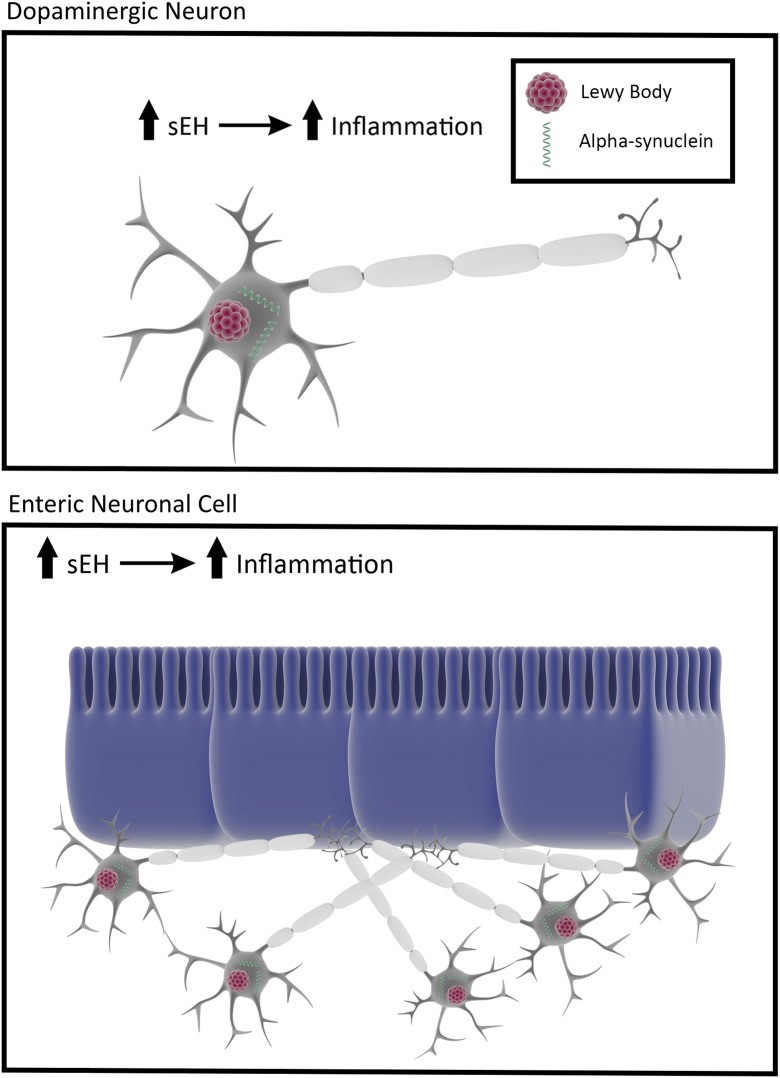
Fig. 1.
Soluble epoxide hydrolase and Parkinson’s disease. sEH elevated levels in dopamine neurons accompany accumulation of α-synuclein and Lewy bodies, which may occur peripherally in the gut before detection of disease symptoms allowing early detection and early treatment of PD.
The PNAS findings of Ren et al. (7) advance our understanding of how PD may evolve, but also point to its novel treatment. Equally compelling evidence shows that using a drug that inhibits sEH can effectively lessen PD-associated toxicity in cell and animal models of the disease (7). Clinical trials of sEH inhibitors in heart and lung diseases have been examined over the last decade (15, 16), and may facilitate the entry of these drugs for PD. Safety and efficacy clinical profiles in these disorders may optimize the treatment regimen for PD and a variety of neurological disorders, as recently seen in animal models of stroke and traumatic brain injury (17, 18), characterized by aberrant inflammation. The assessment of amelioration of behavioral manifestations, coinciding with reduction of sEH levels following inhibitor treatment, will likely provide additional critical guidance in designing the clinical trials that will not only rescue against neurodegeneration, but will also alleviate the debilitating symptoms of PD.
The observation that stem cells derived from PD animals and patients display increased sEH levels and closely accompany dopamine degeneration further supports the intimate role of sEH in PD pathology (7). The use of PD patient-derived stem cells as a model to recapitulate the disease has paved the way for research and development of novel treatments (19, 20). In Ren et al.’s paper (7), the utilization of small and large animal models, including primates—which closely resemble human PD—further probes the pathological contribution of sEH across species. Many positive laboratory findings have failed in clinical trials in part because of poor animal modeling, a translational gap that the present study (7) appears to bridge by employing rigorous animal models and incorporating the human component in the experiments, likely strengthening its laboratory-to-clinic translation toward clinical applications. In parallel to demonstrating that PD iPSCs reveal association of sEH with α-synuclein formation, the results may point to stem cell therapy as an alternative approach in replacing dysfunctional cells, albeit transplanting stem cells with reduced levels of sEH. Indeed, cell therapy has been tested in PD in the past (5, 6), and such cell-based regenerative medicine may be optimized by exploiting sEH as a biological selection criterion in screening transplantable stem cells.
In summary, the recognition of sEH-mediated inflammation in the accumulation of α-synuclein and Lewy bodies in the brain and potentially in the periphery, and in patient-derived stem cells, offers key pathological insights that may improve the diagnosis and treatment of PD. It is conceivable that the development of sEH-based biomarkers, inhibitors, and stem cells may lead to new clinical products for inflammation-plagued disorders.
Footnotes
The author declares no conflict of interest.
See companion article on page E5815.
References
- 1.Poewe W, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.Peng C, et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018 doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolzenberg E, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9:456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantcheva P, Reyes S, Hoover J, Kaelber S, Borlongan CV. Treating non-motor symptoms of Parkinson’s disease with transplantation of stem cells. Expert Rev Neurother. 2015;15:1231–1240. doi: 10.1586/14737175.2015.1091727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli E, Borlongan CV. Cell therapy in Parkinson’s disease: Host brain repair machinery gets a boost from stem cell grafts. Stem Cells. 2017;35:1443–1445. doi: 10.1002/stem.2636. [DOI] [PubMed] [Google Scholar]
- 6.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet. 1999;353:SI29–SI30. doi: 10.1016/s0140-6736(99)90229-5. [DOI] [PubMed] [Google Scholar]
- 7.Ren Q, et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc Natl Acad Sci USA. 2018;115:E5815–E5823. doi: 10.1073/pnas.1802179115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota K, Hammock BD. Cytosolic and microsomal epoxide hydrolases: Differential properties in mammalian liver. Science. 1980;207:1479–1481. doi: 10.1126/science.7361100. [DOI] [PubMed] [Google Scholar]
- 9.Morisseau C, et al. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uemura N, et al. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener. 2018;13:21. doi: 10.1186/s13024-018-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manfredsson FP, et al. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol Dis. 2018;112:106–118. doi: 10.1016/j.nbd.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden CD. Parkinson’s disease. Lancet. 1990;335:948–952. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- 13.Goswami SK, et al. Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. J Pharmacol Exp Ther. 2016;357:529–536. doi: 10.1124/jpet.116.232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, et al. Postprandial effect to decrease soluble epoxide hydrolase activity: Roles of insulin and gut microbiota. J Nutr Biochem. 2017;49:8–14. doi: 10.1016/j.jnutbio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono E, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190:886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, et al. Mechanisms of vascular dysfunction in COPD and effects of a novel soluble epoxide hydrolase inhibitor in smokers. Chest. 2017;151:555–563. doi: 10.1016/j.chest.2016.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu R, et al. Soluble epoxide hydrolase inhibition decreases reperfusion injury after focal cerebral ischemia. Sci Rep. 2018;8:5279. doi: 10.1038/s41598-018-23504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss KI, Gruzdev A, Zeldin DC. Altered behavioral phenotypes in soluble epoxide hydrolase knockout mice: Effects of traumatic brain injury. Prostaglandins Other Lipid Mediat. 2013;104-105:18–24. doi: 10.1016/j.prostaglandins.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devine MJ, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heman-Ackah SM, et al. Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum Mol Genet. 2017;26:4441–4450. doi: 10.1093/hmg/ddx331. [DOI] [PMC free article] [PubMed] [Google Scholar]