Abstract
Stats (signal transducers and activators of transcription) are latent cytoplasmic transcription factors that on a specific stimulus migrate to the nucleus and exert their transcriptional activity. Here we report a novel signaling pathway whereby RhoA can efficiently modulate Stat3 transcriptional activity by inducing its simultaneous tyrosine and serine phosphorylation. Tyrosine phosphorylation is exerted via a member of the Src family of kinases (SrcFK) and JAK2, whereas the JNK pathway mediates serine phosphorylation. Furthermore, cooperation of both tyrosine as well as serine phosphorylation is necessary for full activation of Stat3. Induction of Stat3 activity depends on the effector domain of RhoA and correlates with induction of both Src Kinase-related and JNK activities. Activation of Stat3 has biological implications. Coexpression of an oncogenic version of RhoA along with the wild-type, nontransforming Stat3 gene, significantly enhances its oncogenic activity on human HEK cells, suggesting that Stat3 is an essential component of RhoA-mediated transformation. In keeping with this, dominant negative Stat3 mutants or inhibition of its tyrosine or serine phosphorylation completely abrogate RhoA oncogenic potential. Taken together, these results indicate that Stat3 is an important player in RhoA-mediated oncogenic transformation, which requires simultaneous phosphorylation at both tyrosine and serine residues by specific signaling events triggered by RhoA effectors.
INTRODUCTION
Rho GTPases are members of the Ras superfamily involved in critical cellular functions such as cell growth, development, apoptosis, and cell cytoarchitecture as well as in dysregulatory scenarios such as tumorigenesis and metastasis (Narumiya, 1996; Van Aelst and D'Souza-Schorey, 1997; Hernández-Alcoceba et al., 2000). Although much is known about the pathways that lead to Rho-mediated transformation, the precise mechanisms by which these proteins impinge in regulation of cell proliferation and the subversion that takes place during cell transformation need further in depth study.
It is well established that members of this family of small GTPases induce the activation of intracellular signaling cascades that result in the activation of specific transcription factors. Thus, constitutively active forms of Cdc42Hs and Rac1 can effectively induce the activity of c-Jun N-terminal kinase/stress-activated protein kinases (JNK/SAPK; Coso et al., 1995; Minden et al., 1995). As well, constitutively activated mutants of Rac1 and Cdc42Hs induce p38/Mpk2 activity (Coso et al., 1995). Interestingly, in human embryonic kidney (HEK)-293T cells, RhoA, RhoB, RhoC, and Cdc42Hs, but not Rac1, can activate this kinase (Teramoto et al., 1996). In addition, in human epithelial A459, RhoA is involved in JNK activation in response to specific stimuli (Roberts et al., 1998), suggesting that RhoA might regulate this MAPK in diverse human cell lines.
We have previously described the activation of the nuclear factor κB (NF-κB) by prototypes of the three families of Rho GTPases: RhoA, Rac1, and Cdc42Hs (Perona et al., 1997; Montaner et al., 1998, 1999). This activation was also observed by different members of the family of exchange factors for Rho proteins (Dbl family), mainly Vav, Ost, and Dbl. Furthermore, the JNK/SAPK cascade is involved in this pathway for Rac1 and Cdc42Hs, but not for RhoA. Recently, Zohar et al. (1998) and Sahai et al. (1998) have shown that effector domains within Rho GTPases that are responsible for nuclear signaling and cellular transformation are independent of those involved in cytoskeletal rearrangement functions. Yet, there still is some controversy in this topic because SRF activation by Rho GTPases appears to be linked to actin dynamics (Hill et al., 1995; Sotiropoulos et al., 1999). Furthermore, little is known about the physiological roles of these domains and the effectors they interact with.
Stats are a family of transcription factors implicated in ligand-dependent growth stimulation or differentiation as well as in antiproliferative effects (Darnell, 1997; Schindler, 1998). Although Stats transcription factors were originally identified as components of a DNA-bound complex induced by IFN-α stimulus (Shuai et al., 1992), nowadays it is known that >40 different extracellular polypeptides that activate either receptor tyrosine kinases (RTKs) or cytokine receptors coupled to JAKs trigger Stats activation. Seven mammalian Stat genes have been identified so far, arranged in three chromosomal clusters, and alternative splicing leads to the synthesis of additional Stats. On cytokine stimulation, JAKs are recruited to the oligomerized receptor, phosphorylating it at specific residues that constitute docking sites for Stat monomers (Heim et al., 1995; Stahl et al., 1995). Once recruited to the receptor via their src homology domain-2 (SH-2), Stats are phosphorylated by JAKs on a single tyrosine in the C terminus at position 705. This phosphorylation event enables Stats to homo- or heterodimerize, via their SH2 domains to subsequently migrate to the nucleus where they interact with both DNA motifs located at the promoter region of specific genes as well as other transcription factors and accessory proteins, thereby stimulating transcription (Paulson et al., 1999).
A second phosphorylation event in a single serine (residue 727) has been described recently that modulates transcriptional activity of Stat3 (Sadowski et al., 1993; Ram et al., 1996; Wen et al., 1997; Kuroki et al., 1999). Members of the MAPK and JNK family of serine kinases mediate this serine phosphorylation. Serine phosphorylation appears to be in some cases necessary for maximal transcriptional activity of Stat3 (Ng and Cantrell, 1997; Sengupta et al., 1998), whereas inhibitory in others (Chung et al., 1997; Jain et al., 1998; Lim and Cao, 1999; Woetmann et al., 1999). Furthermore, serine phosphorylation does not seem to be necessary for DNA binding (Wen and Darnell, 1997), and some works have described no DNA binding activity upon serine phosphorylation (Ceresa and Pessin, 1996).
Here, we investigate the functional relationship between Rho proteins and Stat3 transcription factor and its biological implications. With the use of human HEK-293T cells we demonstrate that RhoA and to a lesser extent Cdc42Hs, but not Rac1, can efficiently activate Stat3 transcriptional activity by both tyrosine and serine phosphorylation. As well, RhoA can induce Stat3 activity in other cell systems such as CHO-4 and BRL-4 cells. We provide evidence of a role for a member of the Src family kinase and JAK2 in Stat3 tyrosine phosphorylation and of JNK1 cascade in Stat3 serine phosphorylation upon Rho activation. Finally, we demonstrate that Stat3 cooperates with RhoA for the oncogenic transformation of human cells and might be necessary for RhoA-mediated transformation.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfections, and Chemical Inhibitors
Human embryonic kidney 293T fibroblast cells (HEK293T), Chinese Hamster Ovary 4 cells (CHO4), and Buffalo Rat Liver cells (BRL), were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FCS) and 1 mM glutamine. For transient expression assays, 4–8 × 105 HEK293T or CHO4 cells were transfected in 60-mm dishes by the calcium phosphate method as described previously (Montaner et al., 1998). The amount of plasmidic DNA was kept constant at 1–1.5 μg per 60-mm plate with the corresponding empty vector. BRL4 cells were transfected by Lipofectamine Plus (Life Technologies BRL, Rockville, MD) after the manufacturer's manual. p38 inhibitor SB203580 (Calbiochem) and MEK1 inhibitor PD98059 (Calbiochem, La Jolla, CA) were used at a final concentration of 20 and 50 μM, respectively. cSrc-specific inhibitor PP1 (Alexis, San Diego, CA) was resuspended in DMSO and used at a final concentration of 10 μM. JAK2 specific inhibitor AG-490 (Calbiochem) was resuspended in DMSO and added at the indicated concentrations.
Plasmids
pCDNAIIIB plasmid (Invitrogen, San Diego, CA) and derived expression vectors encoding for constitutively activated RhoA (QL), Rac1 (QL), and Cdc42Hs (QL) proteins and their wild-type versions have been described (Montaner et al., 1998). RhoA QL effector loop mutants were kindly provided by Dr. Gutkind. The 1 × SIE-CAT (TTCCCGTCAA) contains the sequence of the human c-fos promoter spanning from nucleotides −350/−336 inserted into a pBLCAT5 derived plasmid. The pfosCAT and the mutated version in the SIE element were kindly provided by Dr. Ugo Moens. HIV-LUC, 4×SRECAT, Gal4-LUC, Gal4-c-jun reporter plasmids as well as MEKK1-KR expression vector have been reported previously (Montaner et al., 1998). PLNCX-vSrc plasmid and pXEM-Lckwt and Lck Y505F were obtained from Dr. Crespo. Expression vector for dominant negative JAK2 (pRk-JAK2-KE) was a kind gift from Dr. I. M. Kerr. The JNK-1 binding domain (JBD) of JIP-1 spanning residues 127–281, termed JIP-1 Dom. Neg (DN) was subcloned into pXCXCMV expression plasmid. Dominant negative mutants of Stat3 were generated and generously provided by Dr. Masahiko Hibi.
Gene Expression Analysis
Cells, 8 × 105, were transfected with the indicated plasmids. Twenty-four hours after transfection, protein extracts were prepared by lysis with the commercially available reporter lysis buffer (Promega, Madison, WI). The total amount of protein was determined with a commercial kit based on the Bradford method (Bio-Rad, Richmond, CA). Two to 4 μg of protein was assayed for chloranphenicol acetyl transferase (CAT) activity with the use of a xylene-based method. Total volume of each cellular extract was adjusted to 85 μl with 0.25 M Tris-HCl, pH 7.5, to which 40 μl of a mixture containing 32 μl 0.25 M Tris-HCl, 5 μl butyryl CoA (5 mg/ml), 3 μl 14C-chloranphenicol (0.1 mCi/ml) was added. The reaction mixture was incubated for 1–2 h at 37°C, and the reaction was stopped by adding 300 μl of a mixture of 2:1, 2,6,10,14-tetramethylpentadecane (Pristane; Sigma Chemical Co., St. Louis, MO):xylene isomers (Sigma). The mixture was shaken vigorously for 30 s and spun at 14,000 rpm for 5 min. Two hundred microliters of the upper phase was taken and added to 4 ml of Optiphase Highsafe 2 liquid scintillation cocktail (Wallack). Total counts (cpm) were detected with the use of a 1214 RackBeta Liquid scintillation counter (Wallack) and normalized by microgram of protein. Luciferase activity derived from the transfection of the HIVLUC or Gal4-LUC/Gal4-c-jun reporters was detected with the use of 100–400 ng of protein with a commercial kit (Promega). Transfection efficiencies were corrected by cotransfection of the pCMV-CAT plasmid or pCMV-Luc, for luciferase and CAT experiments, respectively.
Western Blot Assays, Immunoprecipitations, and Kinase Activity Assay
For protein expression assays, cells were transfected with the corresponding plasmids and incubated in DMEM 0.5% FCS for the next 24 h. The lysis was performed in a buffer containing 50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 0.5% Triton X-100, 0.5% sodium deoxycholate, 15 mM β-glycerophosphate, 10 mM Na4P2O7, 200 μM orthovanadate, 50 mM NaF, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride. Ten micrograms of total protein was analyzed by SDS-electrophoresis on 10% polyacrylamide gels (SDS-PAGE). After transfer of proteins to Immobilon-P PVDF membrane (Millipore, Bedford, MA), the blots were incubated with the corresponding antibodies. Immunocomplexes were visualized by enhanced chemiluminescence detection (Amersham, Arlington Heights, IL) with the use of a biotinylated anti-rabbit antibody and streptavidin-peroxidase. Immunoprecipitation assays were performed as follows: 200–500 μg of total extracts were incubated with 2 μg of indicated antibody for 1 h at 4°C. Twenty microliters of Protein G-agarose (resuspended in lysis buffer) was added and incubated overnight at 4°C. Conjugates were pelleted by centrifugation at 2500 rpm at 4°C and washed four times with lysis buffer. Supernatant was discarded, and 40 μl of Laemmli buffer was added, boiled at 95°C for 5 min, and subjected to electrophoresis. Antisera against Stat3, phospho-Stat3 (Tyr 705), and phospho-Stat3 (Ser 727) were purchased from New England Biolabs (Beverly, MA). Mouse monoclonal anti phospho-p44/42 MAP kinase (Thr202/Tyr204) and phospho p38 were purchased from New England Biolabs. JAK2- and c-Src–specific polyclonal antibodies were obtained from Sigma. C-Lyn– and c-Lck–specific polyclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For kinase activity assay, rabbit antiserum against p38 was used as previously reported (Sánchez-Perez and Perona, 1999). The rabbit polyclonal antibody specific to Src pY418, which is able to recognize all members of the Src family of kinases, was purchased from Biosource (Camarillo, CA).
Electrophoretic Mobility Shift Assays
For EMSA assays, cells were transfected with the corresponding plasmids and incubated in DMEM 0.5% FBS for 24 h. Nuclear extracts were obtained as described previously (10). Nuclear protein was measured with a commercial kit based on the Bradford method (Bio-Rad). Two micrograms of nuclear protein was then incubated with 0.1 ng of SIE probe (20,000 cpm) or with unlabeled probe and subjected to electrophoresis on a nondenaturing 4% acrylamide: bisacrylamide gel (29:1; Bio-Rad).
Anchorage-independent Growth in Soft Agar
Cells, 8 × 105 (293T), in 60-mm dishes were transiently transfected as indicated above with the indicated expression vectors. Twenty-four hours posttransfection, cells were trypsinized and resuspended in fresh medium. Anchorage-independent growth assay was performed as previously described with the use of 40 × 105 cells (Perona et al., 1993). After 3 weeks of incubation the medium was absorbed, and 500 μl of 0.005% Crystal Violet was added and incubated for 1 h at 37°C. Plates were then washed once with 1× PBS and visualized under a microscope.
RESULTS
Rho Proteins Activate Transcription through SIE Sites in Human Embryonic Kidney Cells
We asked whether Rho proteins are capable of stimulating Stat3-dependent transcription. Because the c-fos promoter contains a SIE element responsive to Stat3 and Stat1 adjacent to the SRE, we used a reporter plasmid containing a single SIE element obtained from the human c-fos promoter spanning from nucleotides −350/−336 inserted into a pBLCAT5-derived plasmid, consequently named SIECAT. A sequence analysis was carried out to verify that indeed no other known element responsive to Rho GTPases was present in this reporter.
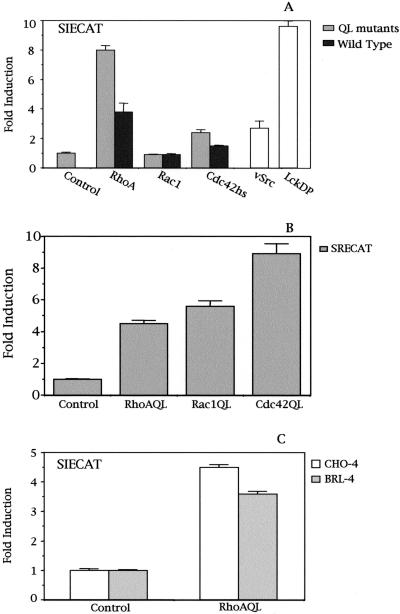
Fibroblast-like HEK293T cells were cotransfected with the SIE-CAT reporter together with expression vectors for constitutively active mutants or the wild-type versions of RhoA, Rac1, and Cdc42Hs (QL; Figure 1A). An eightfold induction of the SIE reporter was obtained in RhoA QL transfectants over control cells. Cdc42Hs QL induced the activation of SIECAT fourfold less than RhoA QL, but no detectable activity was obtained for Rac1 QL. SIE-dependent activation was observed to be dose dependent for both RhoA and Cdc42Hs, but no activation was achieved with Rac1 even at high doses of plasmid (our unpublished results). Furthermore, although to a lower magnitude, similar results where observed for the wild-type versions. RhoA and to a lesser extent Cdc42Hs induced transcriptional activity of the SIE reporter, but not Rac1. As a positive control of reporter activity, vectors encoding either the oncogene v-src or a dominant positive mutant Lck with a Y505F mutation (LckDP) were used.
Figure 1.
Rho proteins activate Stat3-dependent transcription. (A) Constitutively active mutants as well as their wild-type versions for RhoA, and to a lesser extent Cdc42 Hs (QL) but not Rac1, induce transcriptional activation of the SIECAT reporter. RhoA QL, Rac1 QL, Cdc42Hs QL (0.1 μg), v-Src (0.3 μg), or LckDP (1.0 μg) were cotransfected independently along with 0.5 μg of SIECAT reporter. (B) Oncogenic mutants of RhoA, Rac1, and Cdc42 induce SRE-driven transcription in HEK293T to a similar extent. Same amounts as in A of RhoA, Rac1, and Cdc42 QL mutants were transfected, along with 0.5 μg of the SRECAT reporter. (C) RhoA QL efficiently induces SIE-dependent transcription in both BRL-4 and CHO4 cells. SIECAT reporter (0.5 μg) was cotransfected with RhoA QL (1.0 μg each). Data shown in this figure represent the mean of a single experiment performed in triplicate ± SD and are representative of at least five5 independent experiments with similar results.
To verify whether the differences observed in SIE-dependent transcription triggered by RhoA, Rac1, and Cdc42 were not due to differential expression of the vectors, we cotransfected each expression vector along with a SRECAT reporter plasmid responsive to SRF. Both RhoA and Rac1 induced transactivation of the SRE element in HEK-293T cells to the same extent, whereas Cdc42 was more efficient in doing so (Figure 1B). Finally, a similar experiment was carried out with respect to RhoA, with the use of two additional cell lines, BRL-4 and CHO-4, with results similar to the ones obtained with 293T cells (Figure 1C). Thus, Stat3-dependent transcriptional activity by RhoA was not restricted to the HEK-293T cells.
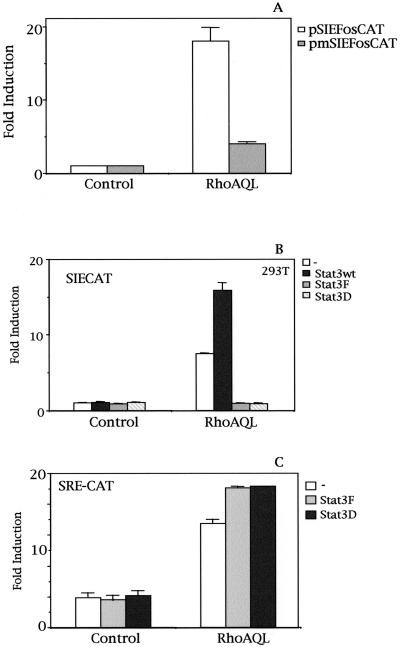
To further confirm the relationship between RhoA and Stat3, we then used a reporter plasmid constructed by cloning the human c-fos promoter fragment spanning from nucleotides −362 to +13 into pBLCAT3 (pfosCAT). This reporter was then mutated by PCR in its SIE element to obtain a reporter containing a c-fos proximal promoter region nonresponsive to Stat. Consequently, we cotransfected both these reporter plasmids, pfosCAT and pmSIEfosCAT, along with the expression vector for RhoA QL (Figure 2A). Transcriptional activation of this c-fos promoter construct was dependent on the SIE site, because mutation of this element reduced its stimulation by 80%. It should be noted that the 20% activity left in c-fos transcription corresponds to a fourfold induction over basal levels, which may correspond to SRE-dependent transcription.
Figure 2.
RhoA-induced SIE transcription is driven by Stat3. (A) RhoA QL (0.1 μg DNA) was cotransfected with 0.5 μg of either the human c-fos promoter fragment spanning from nucleotides −362 to +13 subcloned into pBLCAT3 (pfosCAT) or with a mutant in the SIE element nonresponsive to Stat3 into 293T cells. CAT activity was determined 24 h posttransfection. Data represent the mean of a single experiment carried out in triplicate. Similar results were obtained in three additional independent experiments. (B) Stat3-mediated SIE transcription induced by RhoA QL. RhoA QL, 0.1 μg, was transiently cotransfected with either 1.0 μg of Stat3wt, Stat3F (DN), or Stat3D (DN) expression vectors along with SIECAT reporter. Both Stat3F and Stat3D dominant negative mutants completely inhibit RhoA QL-induced SIE transcription. (C) Dominant negative Stat3 do not inhibit SRF activation by RhoA. The same amounts of RhoAQL and Stat3F/D were transfected as in B along with 0.5 μg of SRECAT reporter. Data shown are representative of four independent experiments in triplicate. All data shown in this figure represent the mean of a single experiment performed in triplicate ± SD.
The above results were generated with the use of the endogenous Stat3 proteins present in the HEK-293T cells. We next coexpressed the wild-type version of Stat3 and RhoA QL along with the SIECAT reporter (Figure 2B). Although expression of Stat3 itself was not capable of inducing any transcriptional activity of the SIE element, coexpression of Stat3 with RhoA-QL enhanced SIE-mediated transcription. Furthermore, coexpression of RhoA with two dominant negative mutants of Stat3, with either a mutated tyrosine 705 to phenylalanine (Stat3F), which lacks tyrosine phosphorylation, or with impaired DNA-binding activity (Stat3D), respectively, completely inhibited RhoA-mediated SIECAT activity. As a control of specificity of inhibition by both Stat3 dominant negative mutants, we tested their ability to inhibit other Rho-dependent transcriptional signals. As shown in Figure 2C, neither mutant are capable of inhibiting SRF activation by RhoA, indicating that their inhibitory action over SIE-dependent transcription is specific.
RhoA Induces Tyrosine Phosphorylation and DNA Binding of Stat3 in HEK-293T Cells
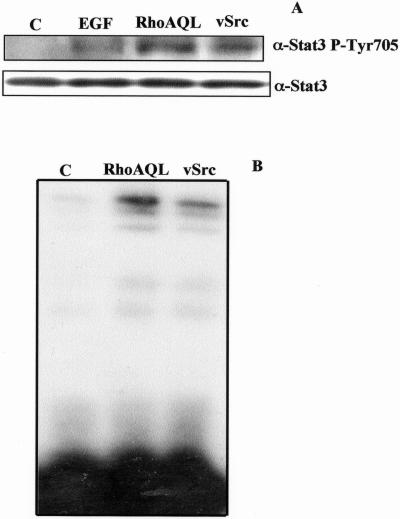
Because RhoA can efficiently induce transcriptional activity of the SIECAT reporter in HEK-293T cells, we investigated the mechanism of Stat3 activation by RhoA. First, we examined whether overexpression of RhoA QL could induce tyrosine phosphorylation of Stat3. To this end, we transiently transfected HEK-293T cells with RhoA QL, and 24 h posttransfection we carried out Western immunoblot analysis against tyrosine phosphorylation of endogenous Stat3 (Figure 3A). Analysis of whole cell extracts with the use of a phosphotyrosine-specific Stat3 (Tyr 705) antibody revealed tyrosine phosphorylation of Stat3 in RhoA-transfected cells to a similar extent as that observed with transfection of v-Src or cells treated with EGF. In keeping with the above results, nuclear extracts of RhoA QL transfected HEK-293T cells were subjected to electrophoretic mobility shift assays (EMSA) with the use of the SIE element of the c-fos promoter as a Stat3 DNA binding specific probe. Both RhoA QL and v-Src induced Stat3 DNA binding to the SIE probe, hence causing a shift of mobility (Figure 3B), an indication of Stat3 activation. Hence RhoA QL induces both tyrosine phosphorylation of Stat3 and its consequent DNA binding.
Figure 3.
RhoA induces tyrosine phosphorylation and DNA-binding of Stat3. (A) Tyrosine phosphorylation of Stat3 induced by RhoA, v-Src, and EGF. RhoA (0.1 μg) and v-Src (0.3 μg) were transiently transfected into 293T cells, and total cell extracts were obtained 24 h posttransfection. As well, cells transfected with the empty vector were treated with 100 ng/ml EGF (Sigma) for 5 min before lysis. Whole cell extracts (10 μg) were then subjected to Western blot, with the use of a phosphotyrosine Stat3 (P-Tyr 705) antiserum. To verify equal loading, the membrane was stripped and incubated with Stat3 antiserum. (B) RhoA QL induces Stat3 DNA-binding. 293T cells were transfected with RhoAQL and vSrc, and 24 h posttransfection (0.5% serum), nuclear extracts were obtained and subjected to EMSA, with the use of the SIE probe. The experiment shown is representative of four independent experiments with similar results.
JAK2 and a Src Family Kinase Member Mediate Transcriptional Activity of the SIE Element Induced by RhoA
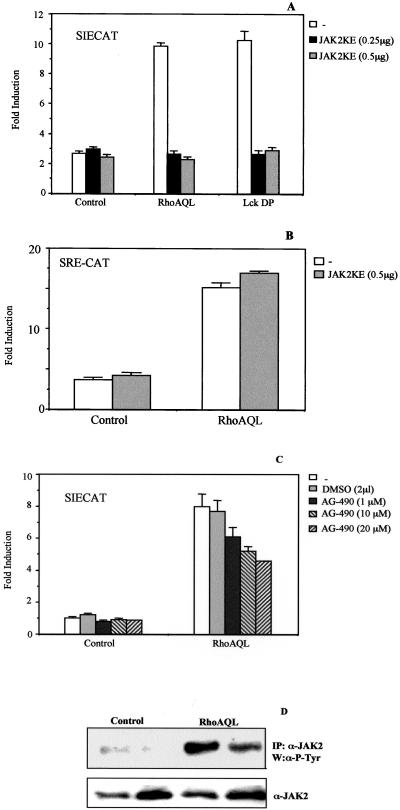
Given that RhoA induces tyrosine phosphorylation, DNA binding, and transcriptional activity of Stat3, we then assessed whether JAK2 could be involved in RhoA-induced Stat3 activation. To verify this, we used both a tyrphostin-derived chemical inhibitor specific to JAK2 (AG-490), and a dominant negative mutant of this kinase with a lysine (K) to glutamate (E) mutation at residue 899 (JAK2-KE). Cotransfection of JAK2-KE mutant together with RhoA QL in 293T cells completely inhibits SIECAT reporter activity induced by both RhoA in a dose-dependent manner (Figure 4A). As a positive control of inhibition, JAK2 KE was cotransfected with LckDP at the same doses used with RhoA QL (Figure 4A). Inhibition of JAK2KE is specific to Stat3-dependent transcription, because it does not inhibit SRF activation by RhoA (Figure 4B). In addition, treatment with different concentrations of AG-490 of HEK-293T cells transfected with RhoA along with the SIECAT reporter led to >60% inhibition of reporter activity (Figure 4C). Thus, both results strongly suggest that JAK2 is a key component in RhoA-induced Stat3 transcriptional activation.
Figure 4.
JAK2 lies downstream of RhoA to induce Stat3 transactivation. (A) Ectopic expression of a dominant negative mutant of JAK2 (K-E) causes total inhibition of RhoA-induced Stat3-dependent transcription. Indicated amounts of JAK2 KE were cotransfected with RhoA QL (0.1 μg) together with the SIECAT reporter. As a control for JAK2 K-E dominant negative effect, LckDP (1.0 μg) was cotransfected with the same amounts of JAK2 K-E as with RhoA QL. (B) Dominant negative JAK2 (KE) does not inhibit RhoAQL-dependent activation of SRF. 0.1 μg of RhoA QL (0.1 μg) and JAK2KE (0.5 μg) were transfected as shown, along with 0.5 μg of SRECAT reporter plasmid. (C) Tyrphostin-based JAK2 specific inhibitor, AG-490, partially inhibits SIECAT reporter activity induced by RhoAQL. 293T RhoA QL transfectants were treated with increasing amounts of DMSO solvent or AG-490 (1, 10, and 20 μM) for 16 h. Data shown for parts A and B represent the mean of a single experiment carried out in triplicate ± SD. Both results shown are representative of three independent experiments. (D) RhoA QL induces tyrosine phosphorylation of JAK2. Immunoprecipitation with an anti-JAK2 antibody of whole cell extracts from quiescent 293T cells transfected with an expression vector for JAK2 or cotransfected with RhoA QL and JAK2 was carried out as described in EXPERIMENTAL PROCEDURES. After SDS-PAGE and transfer to PDVF membrane, tyrosine phosphorylation was detected with an anti-P-Tyr antibody. Similar loading and immunoprecipitation was determined with the use of an anti-JAK2 antibody.
We next investigated whether tyrosine phosphorylation of JAK2 is indeed triggered by RhoA QL transfection. As shown in Figure 4D, immunoprecipitation of JAK2 with a specific anti-JAK2 antibody and subsequent Western blot analysis using a general antiphosphotyrosine antibody show a prominent tyrosine-phosphorylated band in RhoA QL-transfectants extracts versus control cells, which corresponds to JAK2.
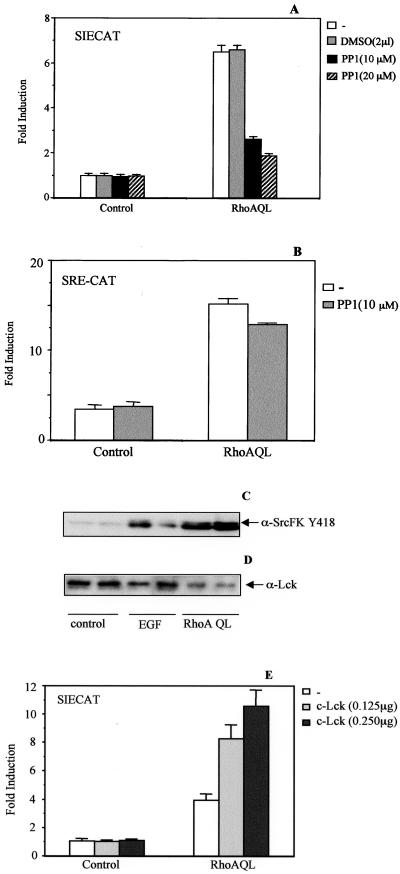
Because both RhoA, v-Src, and LckDP induce Stat3 activation in this cellular system, we studied whether there is any functional interaction between both these proteins and RhoA, as described for other members of the Ras superfamily of GTPases. PP1, a specific inhibitor for c-Src family kinases, was used to verify whether RhoA-mediated Stat3 activation is dependent on a member of this family of kinases. Treatment of RhoA transfectants with PP1 inhibits SIECAT transactivation by 60–70%, consistent with placing an Src family member downstream of RhoA, necessary for Stat3 activity (Figure 5A). No effect is observed with a NF-κB responsive reporter (our unpublished results) and an SRF-dependent element (SRE-CAT) under identical inhibitory conditions, indicating that this effect is specific for Stat3 activation by RhoA (Figure 5B).
Figure 5.
A Src Family Kinase member synergistically cooperates with and acts downstream of RhoA QL in Stat3 activation. (A) c-Src inhibitor PP1 causes a 70% inhibition of RhoA QL-induced Stat3-dependent transcription. 293T cells were transfected with either pcDNA3B or RhoA QL (0.1 μg) together with the SIECAT reporter (0.5 μg). Twenty hours after transfection, DMSO (2 μl) or increasing concentrations of PP1 were added (1, 10, and 20 μM) and incubated for 4 h. Cells were then lysed, and CAT activity was measured. (B) PP1 does not inhibit SRF activation by oncogenic RhoA. RhoAQL was cotransfected with SRECAT reporter, and cells were treated with PP1 as in A. Data in A and B represent the mean of a single experiment ± SD carried out in triplicate and is representative ofthree independent experiments. (C) RhoA QL promotes tyrosine phosphorylation of residue 418 of a member of the Src-FK. Ten micrograms of total extracts from quiescent 293T RhoA QL transfectants and from control cells treated and untreated with EGF was subjected to SDS-PAGE. Immunoblot was carried out with a specific antibody against Tyr-418 of the family of Src kinases (α-SrcFKY418). The experiment shown is from a single experiment carried out in triplicate ± SD and is representative of three independent results. (D) Same blot as in B was blotted against anti-Lck specific antibody. (E) Wild-type Lck (c-Lck) synergizes with RhoA QL to promote Stat3 activation. Indicated amounts of c-Lck were cotransfected with oncogenic RhoA (0.1 μg) along with the SIE reporter. CAT activity was determined 24 h posttransfection. The result shown is representative of three independent experiments.
In the same context, we then investigated whether c-Src could lie downstream of RhoA in Stat3 activation. To this end, we tested the ability of RhoA QL to promote tyrosine phosphorylation of residue 418 of human c-Src, which is conserved in all members of this family and is essential for their activation. As shown in Figure 5C a prominent band appears in extracts obtained from RhoA QL-transfected cells, as well as EGF-treated 293T cells. However, this band migrates in denaturing polyacrylamide gels to an apparent molecular weight that is lower of that of c-Src, indicating that most probably it does not correspond to c-Src. In fact, subsequent immunobloting of the same membrane with a c-Src–specific antibody revealed that this band does not represent c-Src (our unpublished results). Thus, we searched for other members of the Src family as potential mediators of Stat3 activation by RhoA. A negative result was obtained when Lyn was tested (our unpublished results). However, when an Lck-specific antibody was used, two bands were detected that matched the phospho-specific bands detected with the anti–P-Tyr antibody specific against Src family kinases (Figure 5D).
To further investigate the possible involvement of Lck in Stat3 activation, we performed experiments of transient expression of RhoA with or without Lck. As shown in Figure 5E, expression of wild-type Lck itself fails to increase SIECAT activity. However, when Lck was cotransfected with oncogenic RhoA, a significant increase in Stat3 activity was observed with respect to RhoA alone (Figure 5E). Furthermore, coexpression of a dominant negative mutant RhoA (N19) fails to inhibit LckDP-stimulated SIE transcription, whereas completely inhibiting SRF stimulation by 20% fetal calf serum or lysophosphatidic acid (our unpublished results). These results demonstrate a functional cooperation of RhoA and wild-type Lck for Stat3 activation. Furthermore, they also suggest that Lck might be placed downstream of RhoA in this signaling cascade.
Serine Phosphorylation of Stat3 by Rho AQL Is Essential for Its Full Activity and Is Exerted by a MEKK1/JNK1 Pathway
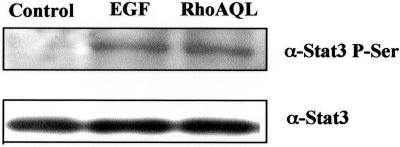
Recent studies have suggested that phosphorylation of serine 727 of Stat3 upon diverse stimuli might be an essential event for the modulation of its activity. Thus, we tested whether oncogenic RhoA could be inducing serine phosphorylation of Stat3 in 293T cells with the use of a phospho-specific antibody that only recognizes its serine phosphorylated form. As shown in Figure 6, both EGF and RhoA QL lead to phosphorylation of Stat3 on serine 727 to a similar extent.
Figure 6.
RhoA induces serine 727 phosphorylation of Stat3 in 293T cells. RhoA QL and pcDNA3B were transfected, and 24-h posttransfection whole cell extracts were obtained for Western blotting. Control pcDNA3B-transfected cells were untreated or treated with 100 ng/ml EGF (5 min). Ten micrograms of total protein was subjected to SDS-PAGE, transferred to PDVF membrane, and immunoblotted with antiphosphoserine-Stat3 antiserum. Equal loading was verified with the use of a specific Stat3 antibody (B). Similar results were obtained in four independent experiments.
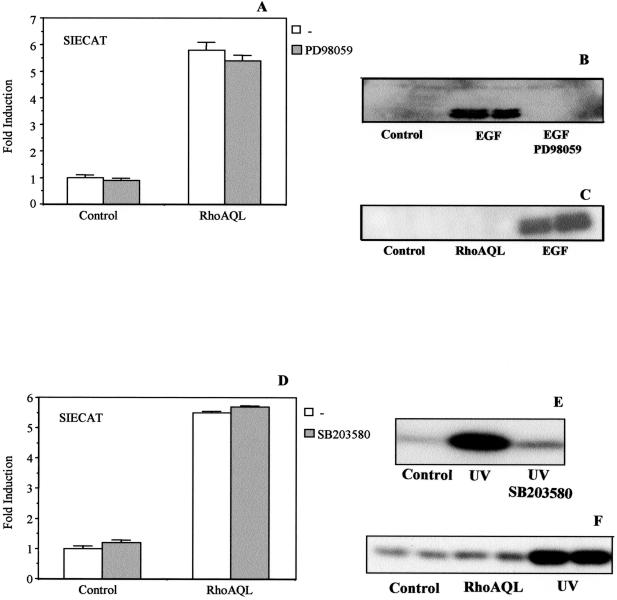
Because previous studies have related Stat3 modulation with the ERK1, p38, and JNK/SAPK kinases, we investigated the possible role of these MAP Kinases signaling cascades in the transcriptional activation of the SIE element by RhoA. To this end, we cotransfected the expression vector of RhoA QL, along with SIECAT plasmid and treated the cells with the specific MEK1 inhibitor, PD98059 (Figure 7A). No effect on SIE transcriptional activation was obtained in PD98059-treated cells, whereas full inhibition of ERK1/2 (p42/44) serine/threonine phosphorylation by EGF was achieved under similar conditions (Figure 7B). This is not surprising because RhoA QL fails to activate MAPK in this cellular system, as in others (Figure 7C). Equal loading was detected with the use of an anti-MAPK antibody (our unpublished results).
Figure 7.
Neither MEK1 nor p38 signaling pathways are involved in RhoA-mediated Stat3 activation. (A) MEK1 specific inhibitor PD98059 does not cause any effect in RhoA QL-induced SIECAT reporter activity. Control vector or its derivative RhoA QL were cotransfected into 293T cells along with the SIECAT reporter. Eight hours before lysis, cells were treated with PD988059 at a final concentration of 50 μM. CAT activity was then assessed as described in EXPERIMENTAL PROCEDURES. The data shown here represent the mean of a single experiment carried out in triplicate ± SD. The same experiment was carried out four times with similar results. (B) PD98059 inhibits EGF-induced MAPK activation in HEK293T cells. Parallel to the experiment above, quiescent cells (24 h in 0.5% FBS) were stimulated with EGF (100 ng/ml) or EGF and PD98059 (50 μM) for 5 min. Whole extracts were obtained, and 10 μg of protein was used for SDS-PAGE. Membranes were incubated with antiphospho-MAPK mAb. Same results were obtained in four independent results. (C) RhoA QL does not activate ERK1/2 in 293T cells. Same extracts as in A, were subjected to SDS-PAGE and transferred to PDVF membranes. Immunobloting was carried out with antiphospho-MAPK antibody. The result shown is representative of three independent results. (D) p38-specific inhibitor SB203580 (Calbiochem) does not alter RhoA-mediated Stat3-dependent transcription. RhoA QL was cotransfected with the SIECAT reporter. Twenty-four hours after transfection cells were treated with SB203580 at a final concentration of 20 μM for 6 h. CAT activity was measured. Data shown are the mean of a single experiment done in duplicate ± SD. Similar results were obtained in four independent experiments. (E) p38 inhibitor, SB203580, efficiently inhibits p38 kinase activity induced by UV light. (F) RhoA QL does not affect p38 activity. Ten micrograms of total extracts from 293T cells unstimulated or stimulated with UV or transfected with oncogenic RhoA QL (0.1 μg) was subjected to SDS-PAGE and transferred to PDVF membranes. Immunoblots were incubated with phospho-specific p38 antibody. Result shown is representative of two independent experiments.
A similar experiment was carried out with the use of the p38 specific inhibitor, SB203580 (Figure 7D). No effect in SIE activity after p38 inhibition was observed in RhoA transfectants. Similarly, coexpression of RhoA QL and a dominant negative mutant MKK6 had no effect over SIE transcriptional levels induced by RhoA, further indicating that this MAPK pathway is not necessary for Stat3 activation by RhoA (our unpublished results). As a control of inhibitor action, we carried out a kinase assay with the use of purified GST-ATF2 as a substrate to p38. As shown in Figure 7E, full inhibition of UV-induced p38 activation was achieved upon conditions similar to those used in RhoA-induced activation of Stat3. Despite the very slight increase in Ser/Thr phosphorylation of p38 in RhoA QL transfectants, this phosphorylation is not relevant to Stat3 activation (Figure 7F). Equal loading was verified with an antibody specific to p38 (our unpublished results). Thus, RhoA involves neither MAPK/ERK nor p38 in Stat3 activation.
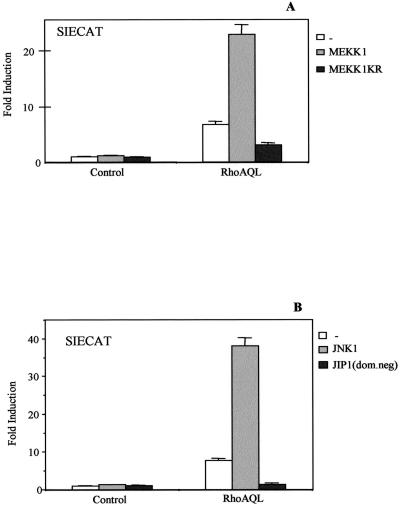
Finally, we sought the role of the JNK/SAPK pathway in Stat3 activation by RhoA. The expression vector for RhoA was cotransfected with the cDNAs for MEKK1 or a kinase dead mutant with dominant negative properties termed MEKK1-K/R, along with the SIECAT reporter in HEK-293T cells. Wild-type MEKK1 enhanced SIE transcriptional activity induced by RhoA by fourfold, whereas its kinase dead mutant caused a 70% decrease in RhoA SIE-induced transcriptional activity (Figure 8A).
Figure 8.
MEKK1/JNK1 signaling lies downstream of RhoA QL and is involved in Stat3 signaling. (A) Ectopic expression of wild-type MEKK1 or its dominant negative mutant, MEKK1KR, synergizes and inhibits respectively Stat3-dependent transcription induced by RhoA QL. 293T cells were cotransfected with either 0.5 μg of MEKK1 or MEKK1 KR together with RhoA QL (0.1 μg) along with the SIECAT reporter. Twenty-four hours after transfection, cells were lysed, and CAT activity was measured. (B) JNK1 pathway is essential for Stat3 activation by oncogenic RhoA. Wild-type JNK1 (1.0 μg) or dominant negative JIP-1 were each cotransfected with RhoA QL (0.1 μg) and control vector along with the SIECAT reporter. CAT activity was assayed 24 h posttransfection. All data shown are the mean of a single experiment carried out in triplicate ± SD. Similar results for all data shown were obtained in three independent experiments.
Activation of the JNK/SAPK cascade by Rho GTPases is cell specific. In this sense, Rac1 and Cdc42 but not RhoA activate JNK in COS-7 cells and NIH3T3, whereas in HEK-293T cells, RhoA and to a lesser extent Cdc42 but not Rac1 activate this cascade (Coso et al., 1995; Teramoto et al., 1996). As previously mentioned, RhoA can also mediate JNK activity in human epithelial A549 cells (Roberts et al., 1998). Accordingly, cotransfection of Gal4-luc/Gal4-jun (a reporter system indicative of JNK activity) with RhoA QL indeed shows JNK activity (our unpublished results). To demonstrate a functional relationship between JNK1 and Stat3 downstream of RhoA, we coexpressed wild-type JNK1 and constitutively active RhoA QL and measured SIECAT transcriptional activity. JNK1 caused more than a fivefold synergism in a dose-dependent manner with RhoA QL to promote SIE-dependent transcription, hence confirming the role of JNK1 in RhoA-mediated Stat3 activation (Figure 8B). In addition, expression of a dominant negative mutant form of the scaffold protein JIP-1 (Dickens et al., 1997) completely inhibited RhoA-induced SIE-dependent transcriptional activity, further indicating that the JNK cascade is essential for full Stat3 activity in the context of oncogenic RhoA (Figure 8B).
The Effector Region of RhoA Is Necessary for Full Activation of Stat3
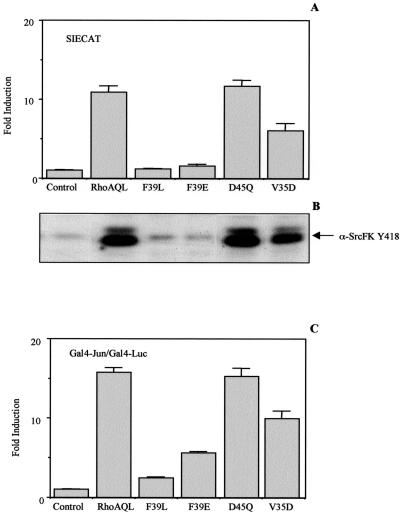
Mutations within the effector loop region have constituted a valuable tool to interconnect the different pathways triggered by small GTPases with specific downstream effects (Sahai et al., 1998; Zohar et al., 1998). A set of different RhoA mutants in the effector region (see MATERIALS AND METHODS) was tested for their ability to activate the SIECAT reporter. Two mutants tested, RhoAQL (F39E) and (F39L), show complete impaired ability to promote SIECAT transcription, and a third mutant (V35D) displayed 50% activity of SIE transcription, whereas a mutant with a D45Q mutation retained full capacity to induce Stat3-dependent transcription when compared with RhoA QL (Figure 9A). Accordingly, both F39L and F39E mutants have lost the capacity to promote tyrosine phosphorylation on residue 418 of Src Family Kinase member, whereas D45Q mutant induced similar levels of phosphorylation of Src Family Kinase member as those induced by the unmutated RhoAQL. V35D mutant showed a partial activation of both phosphorylation and Stat3 transcriptional activity (Figure 9B). Finally, a complete correlation between Stat3 activation and JNK activation was also found. Although a drastic reduction of both JNK and Stat3 activity was achieved with F39L and F39E, the V35D mutation only partially inhibited JNK activation, which would also account for the partially impaired ability of this mutant to promote SIE-dependent transcription (Figure 9C). In keeping with the above results, D45Q mutant showed JNK activation to the same extent as that of RhoA QL. Thus, RhoA activation of Stat3 is dependent on its effector binding region, which is necessary for its ability to promote both tyrosine and serine phosphorylation of Stat3.
Figure 9.
The effector binding region of RhoA is necessary for Stat3 activation. (A) Mutations of residue 39 or 35 within the effector region of RhoA completely or partially impair Stat3 activation, respectively. The indicated mutants were cotransfected along with the SIECAT reporter, and CAT activity was assayed 24 h posttransfection. (B) RhoA, F39L, and F39E mutants fail to induce tyrosine phosphorylation of a SrcFK member activated by oncogenic RhoA, whereas V35D is capable of doing so partially. Western blot analysis of 10 μg with anti-SrcFK Y418, of whole cell extracts from quiescent 293T cells transfected with the indicated expression vectors, was carried out. Equal loading was verified after stripping of the membrane with an anti-Lck antibody (our unpublished results). (C) V35D mutant has a partially impaired ability to promote JNK1 activation. Extracts from quiescent 293T cells transfected with the indicated RhoA mutant's expression vectors along with the Gal4-luc/Gal4-Jun reporters were assayed for luciferase activity. All data shown in parts (A) and (C) are the mean of a single experiment carried out in triplicate ± SD and are representative of nine independent experiments.
RhoA and Stat3 Cooperate for Oncogenic Transformation of the Human HEK-293T Cells
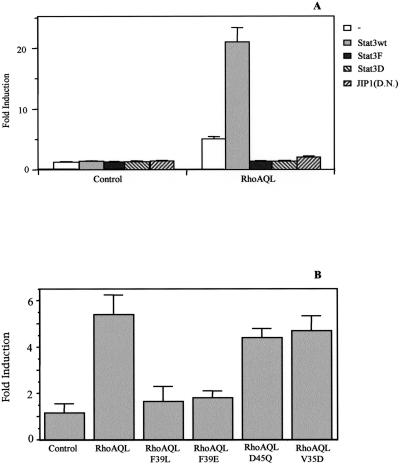
To investigate whether activation of Stat3 had any effect on the biological function of RhoA, a system for testing Stat3 influence in the oncogenic transformation mediated by RhoA was set up as a biological read out. Transient transfection experiments of the human HEK-293T cells were performed as described above with the use of either RhoA alone or in combination with wild-type Stat3, dominant negative Stat3 mutants, Stat3F, and Stat3D as well as dominant negative mutant JIP1 (Figure 10A). Transfected cells were then plated on soft-agar plates, and growth of anchorage-independent clones was estimated after 3 weeks. Expression of RhoA QL is sufficient to induce a 2.7-fold increase in the number of colonies. Also, an increase in the mean size of the colonies was observed with respect to control cells, as determined by their relative spherical volume. Expression of Stat3wt, Stat3F, Stat3D, or JIP1DN alone had no influence over background levels. However, coexpression of Stat3wt with RhoA QL resulted in a further increase of approximately fourfold in the number of colonies over RhoAQL alone. As well, an increased range in colony volume in RhoA transfectants combined with Stat3 is observed with respect to control colonies (our unpublished results). Furthermore, combinations of both Stat3 dominant negative mutants (F and D) or JIP1DN with RhoA QL, reverse the oncogenic potential of the latter back to background levels (Figure 10A). This suggests that both tyrosine and serine phosphorylation of Stat3 are essential to induce full effects in the context of oncogenic signaling.
Figure 10.
Stat3 is necessary for contact-independent growth of RhoA-transfected 293T cells in soft-agar. (A) Stat3wt, Stat3F (DN), Stat3D (DN), or JIP-1 (DN; 1.0 μg each) modulate anchorage-independent growth of RhoA QL-transfected 293T cells. Cells, 8 × 105, were transfected with the indicated expression vectors and maintained in optimal serum conditions for 24 h posttransfection. After trypsin-EDTA treatment, 4 × 104 cells were seeded in a soft agar base and incubated for 21 d. Although RhoA QL and Stat3 synergize to promote anchorage-independent growth, both Stat3 mutants (F and D) completely abrogate RhoA QL oncogenic potential. The same inhibitory effect to the one observed with dominant negative mutants Stat3 was obtained with JIP-1 (DN). No effect was observed with Stat3wt, Stat3F, Stat3D, or JIP-1 alone. (B) RhoA mutants QL-F39E and QL-F39L, which fail to activate Stat3, do not induce anchorage-independent growth in soft agar. Data shown represent a single experiment carried out in triplicate. The result shown is representative of seven independent experiments for A and two independent experiments for B, with similar results.
In addition, the ability of RhoA mutants, F39E, F39L, D45Q, and V35D, to promote anchorage-independent growth was tested (Figure 10B). Both mutants that fail to activate Stat3 (F39L and F39E) do not promote significant growth in soft-agar. However, RhoAQL-D45Q and RhoAQL-V35D promoted anchorage-independent growth but to a lesser extent than oncogenic RhoAQL. Thus, these results further suggest that Stat3 might be an important player in RhoA-mediated oncogenic transformation.
Taken together, these results demonstrate that simultaneous tyrosine and serine phosphorylation of Stat3 plays an important role in the oncogenic activity of RhoA.
DISCUSSION
It is well established that Stat3 activation is based on the phosphorylation of a single tyrosine residue at position 705 (for reviews see Darnell, 1997; and Hoey and Grusby, 1999). This phosphorylation event enables cytoplasmic Stat3 to homo- or heterodimerize via its SH2 domains, to subsequently translocate to the nucleus, and to interact with specific DNA elements, thereby promoting gene transcription. At least two tyrosine kinases have been identified as being directly responsible for Stat3 phosphorylation at tyrosine residues. Cytokine receptor oligomerization brings JAKs in juxtaposition, permitting their cross-phosphorylation and subsequent activation. These tyrosine-phosphorylated residues then constitute docking sites for the SH2 domains for the different Stats, which once recruited become phosphorylated on a single tyrosine residue by JAKs. Receptor tyrosine kinases dimerize upon ligand binding and transphosphorylate each other at tyrosine residues that work as attachment sites for different proteins that contain SH2 domains. For instance, upon EGF stimulation, c-Src is rapidly recruited to the dimerized ErbB receptor via its SH2 domains, where it can interact with Stat/ErbB receptor complexes, hence phosphorylating the transcription factors in a JAK-independent manner (Monilola et al., 1999). In keeping with this, a recent report indicates that upon EGF binding, EGFR activates c-Src through RalA to induce Stat3 activation, providing evidence that this growth factor induces Stat3 activation through different mechanisms depending on which receptor it may bind to (Goi et al., 2000). Here we demonstrate that RhoA and to a lesser extend Cdc42 but not Rac1, activate Stat3 transcriptional function by triggering a novel pathway that involves a member of the Src family of kinases (SrcFK) and JAK2. The role of JAK2 in small GTPases of the Rho family might be a common event, because Rac1 also uses this tyrosine kinase to induce tyrosine phosphorylation of Stat3 in other cellular systems (Simon et al., 2000). In the past years, several works have studied the functional relationship between Src and RhoA in critical cellular functions such as cell growth, cytoskeletal organization, and transformation. Evidence for placing RhoA both upstream and downstream of c-Src activation has been reported (Nagao et al., 1999; Nozu et al., 1999; Tominaga et al., 2000). We have observed a dependence of RhoA on SrcFK activity to promote Stat3 activation, because a chemical inhibitor of this family of kinases greatly reduces transcriptional activation of Stat3 induced by RhoA. It is clear although that neither c-Src nor c-Lyn mediate this process, because oncogenic RhoA fails to activate either of them. By contrary, an antibody raised against c-Lck recognizes the phosphorylated band that appears in extracts of RhoA-transfectants. On the other hand, when c-Lck is coexpressed with RhoA QL, Stat3 activity is enhanced more than twofold with respect to RhoA alone. Whether other members of the SrcFK can also contribute to this effect remains to be elucidated. Experiments in our group are currently in progress to address this issue.
Stat3 and Stat1 are susceptible to serine phosphorylation on a single serine residue (Ser-727) by members of the MAPK, p38, and MEKK1 signaling cascades (Wen et al., 1995; Ceresa and Pessin, 1996; Ceresa et al., 1997; Chung et al., 1997; Wen and Darnell, 1997). Yet, depending on the particular stimulus, serine phosphorylation is capable of either potentiating Stat3 transcriptional activity or to negatively regulate it, without affecting its DNA binding affinity. Here we unambiguously demonstrate that serine phosphorylation of Stat3 by RhoA is executed by the JNK pathway, whereas the ERKs or p38 kinases are not involved. Furthermore, we demonstrate that not only RhoA induces activation of Stat3 by JNK phosphorylation of Ser-727, but also that this event cooperates with tyrosine phosphorylation for full transcriptional activation. Moreover, suppression of either event alone is sufficient to impair activation of Stat3 and RhoA-induced transformation. However, the precise mechanism by which RhoA activates these two converging signaling cascades remains to be fully elucidated. Recently, Simon et al. (2000) have described that Rac1 interacts directly with Stat3 through its effector domain, and is capable of inducing both its tyrosine and serine phosphorylation. Although we observe that Stat3 activation by RhoA is dependent on the effector loop region (Figure 9), we have not tested whether both proteins interact directly or via other proteins.
Much is known about the physiological roles of Stats signaling mediated by cytokines (Darnell, 1997; Hoey and Grusby, 1999). Stats are implicated in ligand-dependent growth stimulation or differentiation as well as in antiproliferative effects. In recent years, Stats have been implicated in growth disregulation and tumorigenesis (Garcia and Jove, 1998; Bromberg et al., 1999; Ram et al., 2000). Cell lines stably transformed with different oncogenes related to tyrosine kinase receptors show constitutive activation of Stat3 proteins (Garcia and Jove,1998). Specifically, Stat3 mediates and is necessary for the transforming phenotype of Src oncoprotein and members of the Src-family of kinases (Cao et al., 1996; Liu et al., 1998; Lund et al., 1999). Constitutively active Stat3 is also observed in a number of different human tumors and cell lines derived from human tumors. Moreover, a constitutively active mutant Stat3 is capable of transforming cultured cell lines and to induce tumors in nude mice, hence constituting an oncoprotein in itself independent of tyrosine kinase signaling (Bromberg et al., 1999).
Rho GTPases have been shown to be oncogenic (Ballestero et al., 1991; Perona et al., 1993; del Peso et al., 1997; Lacal., 1997; Lin et al., 1999; Turkson et al., 1999), but the knowledge of their precise mechanism for oncogenic transformation still contains many gaps. Indeed, transcriptional regulation induced by Rho proteins and their oncogenicity could be intimately related. In this report, we show strong evidence for such a direct link, because RhoA-induced transformation depends on Stat3 transcriptional activity, providing a new clue for the ability of Rho proteins to induce transformation. We demonstrate that both tyrosine and serine phosphorylation of Stat3 induced by RhoA are required to achieve full Stat3 activation. Furthermore, cooperation of RhoA and Stat3 synergies for transformation of human HEK-293T cells. A significant increase in both the number and size of anchorage-independent colonies, a landmark for tumorigenicity, indicates that Stat3 is an important player in transformation mediated by RhoA. Inhibition of tyrosine phosphorylation by a mutated Stat3 (F) proves that phosphorylation at this residue is essential for RhoA-induced transformation. Interestingly, because dominant negative JIP1 completely inhibits Stat3 activation by RhoAQL and it abolishes the ability of RhoA transfectants to grow under anchorage-independent conditions, it can be concluded that serine phosphorylation of Stat3 might also be necessary for RhoA-mediated transformation.
These results may also be relevant to the carcinogenesis process in humans. It has been recently demonstrated that primary human HEK cells need to be genetically altered by al least three concurrent events in order to become fully tumorigenic (Hahn et al., 1999). Our results are consistent with that of Hahn et al., because HEK-293T carry both E1A and Large T antigen, in that these alterations confer very low growth properties in soft agar and fail to induce tumorigenicity in syngeneic mice even upon injection of 2 × 106 cells (our unpublished results). However, additional mutations in signaling pathways involving Rho GTPases, instead of Ras, seem to be sufficient for anchorage-independent growth.
Our results directly implicate Stat3 in RhoA transformation. However, further research will be needed to clarify its relationship with other transcription factors, such as NF-κB, previously reported to be implicated in Rho-mediated transformation (Lin et al., 1999). Furthermore, the possible role of Stat3 in transformation should be determined in human tumors known to contain high levels of RhoA or Rac1 (Fritz et al., 1999). Also the involvement of other genes responsive to Stat3 regulation, such as Bcl-XL, cyclin D, p21WAF1/CIP1, or p19INK4d, should be investigated (Karni et al., 1999; Kiuchi et al., 1999; Narimatsu et al., 1997; O'Farrel, et al., 2000). Finally, the relationship between these transcriptional pathways with both the regulation of the cytoskeleton and cell adhesion by oncogenic RhoA should be determined. Although these distinct effects and functions have been commonly treated as independent events, an increasing amount of evidence points out to an intimate cooperation of these to promote oncogenic transformation (Aznar and Lacal, 2001a, 2001b).
There is strong evidence suggesting that transformation by Ras oncogenes require Rho GTPases (Qiu et al., 1995a, 1995b, 1997; Osada et al., 1997; Nur-E-Kamal et al., 1999; Philips et al., 2000). Because Ras oncogenes are directly involved in at least 25–30% of all human cancers, it places the Rho/Stat3 signaling pathway as a possibly important player in human carcinogenesis. As well, it might play a relevant role in tumors where RhoA (and possibly other Rho GTPases) is overexpressed independently of Ras. Furthermore, the link between Rho- and Src-dependent signaling suggests that Rho may participate in pathways relevant to human cancer other than ras oncogenes. At last, these results could be relevant for novel therapeutic strategies that target Stat3 where Ras and Rho proteins are an issue.
ACKNOWLEDGMENTS
We thank E. López-Collazo and L. Lucas for their help in some of the experiments. We thank Dr. Hibi and Dr. Hirano for kindly providing both dominant negative mutants of Stat3, Dr. Kerr for providing us with dominant negative JAK2, and Dr. Gutkind for the mutated RhoA genes at the effector region. This work was supported by Grant 2FD97–0647 from CICYT, Grants 99/0817 and 00/0862 from FIS, and Grant 08.1/0045.1/98 from Consejería de Educación of Comunidad de Madrid.
REFERENCES
- Aznar S, Lacal. JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001a;165:1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- Aznar, S., and Lacal., J.C. (2001b). Searching new targets for anticancer drug design. The families of Ras and Rho GTPases and their effectors. Prog. Nucleic Acid Res. Mol. Biol. (In press). [DOI] [PubMed]
- Ballestero RP, Esteve P, Perona R, Jiménez B, Lacal. JC. The Superfamily of ras related genes. 1991. Biological function of Aplysia californica rho gene. p. 237–242. NATO Advanced Science Institute Series. A220: 237–242. [Google Scholar]
- Bromberg J, Wresczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Horvath CM, Pessin JE. Signal transducer and activator of transcription-3 serine phosphorylation by insulin is mediated by a Ras/Raf/MEK-dependent pathway. Endocrinology. 1997;138:4131–4137. doi: 10.1210/endo.138.10.5266. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Pessin JE. Insulin stimulates the serine phosphorylation of the signal transducer and activator of transcription (STAT3) isoform. J Biol Chem. 1996;271:12121–12124. doi: 10.1074/jbc.271.21.12121. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- del Peso L, Hernandez-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, Lacal JC. Rho proteins induce metastatic properties in vivo. Oncogene. 1997;15:3047–3053. doi: 10.1038/sj.onc.1201499. [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. RhoGTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Garcia R, Jove R. Activation of STAT transcription factors in oncogenic tyrosine kinase signaling. J Biomed Sci. 1998;5:79–85. doi: 10.1007/BF02258360. [DOI] [PubMed] [Google Scholar]
- Goi T, Shiptsin M, Lu Z, Foster DA, Klinz SG, Feig LA. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity, and substrate specificity. EMBO J. 2000;19:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumor cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Heim MH, Kerr IM, Stark GR, Darnell JE., Jr Contribution of Stat SH2 groups to specific inteferon signaling by the Jak-STAT pathway. Science. 1995;267:1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- Hernández-Alcoceba R, del Peso L, Lacal. JC. The Ras family of GTPases in cancer cell invasion. Cell Mol Life Sci. 2000;57:65–76. doi: 10.1007/s000180050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1 and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hoey T, Grusby MJ. STATs as mediators of cytokine-induced responses. Adv Immunol. 1999;71:145–160. doi: 10.1016/s0065-2776(08)60401-0. [DOI] [PubMed] [Google Scholar]
- Jain N, Zhang T, Fong SL, Lim CP, Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK) Oncogene. 1998;17:3157–3167. doi: 10.1038/sj.onc.1202238. [DOI] [PubMed] [Google Scholar]
- Karni R, Jove R, Levitzki A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene. 1999;18:4654–4662. doi: 10.1038/sj.onc.1202835. [DOI] [PubMed] [Google Scholar]
- Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, O'Flaherty JT. Extracellular signal-regulated protein kinase (ERK)-dependent and ERK-independent pathways target STAT3 on serine-727 in human neutrophils stimulated by chemotactic factors and cytokines. Biochem J. 1999;341:691–696. [PMC free article] [PubMed] [Google Scholar]
- Lacal. JC. Regulation of proliferation and apoptosis by Ras and Rho GTPases through specific phospholipid-dependent signaling. FEBS Lett. 1997;410:73–77. doi: 10.1016/s0014-5793(97)00444-4. [DOI] [PubMed] [Google Scholar]
- Lim CP, Cao X. Serine phosphorylation and negative regulation of Stat3 by JNK. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31055. [DOI] [PubMed] [Google Scholar]
- Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem. 1999;274:23633–23641. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Akajima K, Hirano T, Yang-Yen HF. Activation of Stat3 by v-Src is through a Ras-independent pathway. J Biomed Sci. 1998;5:446–450. doi: 10.1007/BF02255934. [DOI] [PubMed] [Google Scholar]
- Lund TC, Coeman C, Horvath E, Sefton BM, Jove R, Medveczky MM, Medveczky PG. The Src-family kinase Lck can induce STAT3 phosphorylation and DNA binding activity. Cell Signal. 1999;11:789–796. doi: 10.1016/s0898-6568(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Monilola AO, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of Stat transcription factors is mediated by Src tyrosine kinase. J Biol Chem. 1999;274:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal. JC. Multiple signaling pathways lead to the activaiton of the nuclear factor kB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal. JC. Activation of serum response factor by RhoA is mediated by the nuclear factor-kB and C/EBP transcription factors. J Biol Chem. 1999;274:8506–8515. doi: 10.1074/jbc.274.13.8506. [DOI] [PubMed] [Google Scholar]
- Nagao M, Kaziro Y, Itoh H. The Src family tyrosine kinase is involved in Rho-dependent activation of c-Jun N-terminal kinase by Galpha 12. Oncogene. 1999;18:4425–4434. doi: 10.1038/sj.onc.1202832. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Nakajima K, Ichiba M, Hirano T. Association of Stat3-dependent transcriptional activation of p19INK4D with IL-6-induced growth arrest. Biochem Biophys Res Commun. 1997;238:764–768. doi: 10.1006/bbrc.1997.7387. [DOI] [PubMed] [Google Scholar]
- Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem (Tokyo) 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- Ng J, Cantrell D. STAT3 is a serine kinase target in T lymphocytes. Interleukin 2 and T cell antigen receptor signals converge upon serine 727. J Biol Chem. 1997;272:24542–24549. doi: 10.1074/jbc.272.39.24542. [DOI] [PubMed] [Google Scholar]
- Nozu F, Tsunda Y, Ibitayo AI, Bitar KN, Owyang C. Involvement of RhoA and its interaction with protein kinase C and Src in CCK-stimulated pancreatic acini. Am J Physiol. 1999;276:G915–G923. doi: 10.1152/ajpgi.1999.276.4.G915. [DOI] [PubMed] [Google Scholar]
- Nur-E-Kamal MS, Kamal JM, Qureshi MM, Maruta H. The CDC42-specific inhibitor derived from ACK-1 blocks v-Ha-Ras-induced transformation. Oncogene. 1999;18:7787–7793. doi: 10.1038/sj.onc.1203215. [DOI] [PubMed] [Google Scholar]
- O'Farrel AM, Parry D, Zindy F, Roussel MF, Lees E, Moore KW, Mui AL. Stat3-dependent induction of p19INK4D by IL-10 contributes to inhibition of macrophage proliferation. J Immunol. 2000;164:4607–4615. doi: 10.4049/jimmunol.164.9.4607. [DOI] [PubMed] [Google Scholar]
- Osada S, Izawa M, Koyama T, Hirai S, Ohno S. A domain containing the Cdc42/Rac interactive binding (CRIB) region of p65PAK inhibits transcriptional activation and cell transformation mediated by the Ras-Rac pathway. FEBS Lett. 1997;404:227–233. doi: 10.1016/s0014-5793(97)00139-7. [DOI] [PubMed] [Google Scholar]
- Paulson M, Pisharody S, Pan L, Guadagno S, Mui AL, Levy DE. Stat protein transactivation domains recruit p300/CBP through widely divergent sequences. J Biol Chem. 1999;274:25343–25349. doi: 10.1074/jbc.274.36.25343. [DOI] [PubMed] [Google Scholar]
- Perona R, Esteve P, Jimenez B, Ballestero RP, Ramon y Cajal S, Lacal JC. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993;8:1285–1292. [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal. JC. Activation of the nuclear factor kB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Philips A, Roux P, Coulon V, Bellanger JM, Vie A, Vignas ML, Blanchard JM. Differential effect of Rac and Cdc42 on p38 kinase activity and cell cycle progression of nonadherent primary mouse fibroblasts. J Biol Chem. 2000;275:5911–5917. doi: 10.1074/jbc.275.8.5911. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential Role for Rac in Ras transformation. Nature. 1995a;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995b;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram PA, Park S, Choi HK, Waxman DJ. Growth hormone activation of Stat1, Stat3 and Stat5 in rat liver. Differential kinetics of horomone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem. 1996;271:5929–5940. doi: 10.1074/jbc.271.10.5929. [DOI] [PubMed] [Google Scholar]
- Ram PT, Curt MH, Iyengar R. Stat3-mediated transformation of NIH-3T3 cells by the constitutively active Q205L G alpha o protein. Science. 2000;287:142–144. doi: 10.1126/science.287.5450.142. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Cowsert LM. Interleukin-1 beta and reactive oxygen species mediate activation of c-Jun NH2-terminal kinases, in human epithelial cells, by two independent pathways. Biochem Biophys Res Commun. 1998;251:166–172. doi: 10.1006/bbrc.1998.9434. [DOI] [PubMed] [Google Scholar]
- Sadowski HB, Shuai K, Darnell JE, Jr, Gilman MZ. A common nuclear signal transduction pathway activated by growth and cytokine receptors. Science. 1993;261:1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Perez I, Perona R. Lack of c-Jun activity increases survival to cisplatin. FEBS Lett. 1999;453:151–158. doi: 10.1016/s0014-5793(99)00690-0. [DOI] [PubMed] [Google Scholar]
- Schindler C. STATs as activators of apoptosis. Trends Cell Biol. 1998;8:97–98. doi: 10.1016/s0962-8924(98)01233-1. [DOI] [PubMed] [Google Scholar]
- Sengupta TK, Talbot E, Scherle PA, Ivanshkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen activated protein kinase. Proc Natl Acad Sci USA. 1998;95:11107–11112. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Schindler C, Prezioso, Darnell JE., Jr Activation of transcription by IFN gamma: tyrosine phosphorylation of a 91 kDa DNA binding protein. Science. 1992;259:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan K. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290:144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Stahl N, Farruggella T, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1352. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for the mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Pierre ES, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge RhoGTPase, and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Adnane J, Yi Z, Djeu JY, Sekharam M, David AF, Lawrence BH, Jie WM, Said S, Jove R. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol. 1999;19:7519–7528. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. RhoGTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Wen Z, Darnell JE., Jr Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 1997;24:2062–2067. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Woetmann A, Nielsen M, Chrostensen ST, Brockdorff J, Kaltoft K, Engel AM, Skov S, Brender C, Geisler C, Svejgaard A, Rygaard J, Leick V, Odum N. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar M, Teramoto H, Katz B-Z, Yamada KM, Gutkind JS. Effector domain mutants of Rho dissociate cytoskeletal changes from nuclear signaling and cellular transformation. Oncogene. 1998;17:991–998. doi: 10.1038/sj.onc.1202022. [DOI] [PubMed] [Google Scholar]