Abstract
(1) Background: d-alanine-d-alanine ligase (DdlA), an effective target for drug development to combat against Mycobacterium tuberculosis (Mtb), which threatens human health globally, supplies a substrate of d-alanyl-d-alanine for peptidoglycan crosslinking by catalyzing the dimerization of two d-alanines. To obtain a better understanding of DdlA profiles and develop a colorimetric assay for high-throughput inhibitor screening, we focused on explicating and characterizing Tb-DdlA. (2) Methods and Results: Rv2981c (ddlA) was expressed in Escherichia coli, and the purified Tb-DdlA was identified using (anti)-polyhistidine antibody followed by DdlA activity confirmation by measuring the released orthophosphate via colorimetric assay and the yielded d-alanyl-d-alanine through high performance thin layer chromatography (HP-TLC). The kinetic assays on Tb-DdlA indicated that Tb-DdlA exhibited a higher affinity to ATP (KmATP: 50.327 ± 4.652 μmol/L) than alanine (KmAla: 1.011 ± 0.094 mmol/L). A colorimetric assay for Tb-DdlA activity was developed for high-throughput screening of DdlA inhibitors in this study. In addition, we presented an analysis on Tb-DdlA interaction partners by pull-down assay and MS/MS. Eight putative interaction partners of Tb-DdlA were identified. (3) Conclusions: Our dataset provided a valuable resource for exploring Tb-DdlA biology, and developed an easy colorimetric assay for screening of Tb-DdlA inhibitors.
Keywords: Mycobacterium, d-alanine-d-alanine ligase, peptidoglycan
1. Introduction
According to the 2017 statistic report by the WHO [1], Tuberculosis (TB) killed approximately 1.4 million people worldwide in 2016 and an estimated 10.4 million new TB cases occurred annually. The current anti-TB treatment regimens, in particular, remain very unsatisfactory for infection with Mycobacterium tuberculosis (Mtb) strains of MDR (multidrug-resistance) or XDR (extensively drug-resistance) [1]. Mtb, a causative pathogen of TB [1,2,3], has been found to be the dominant strain with MDR or XDR at present. So far, intensive worldwide efforts have been made to discover novel anti-TB drug targets or innovative anti-TB drugs to combat against these evolved Mtb strains.
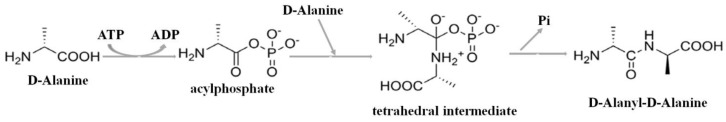
The DdlA (d-alanyl-d-alanine ligase A) in Mtb, catalyzing the dimerization of two d-alanine molecules, has been of particular interest as a potential and attractive anti-TB drug target [4,5,6]. The ATP-dependent dimerization reactions catalyzed by DdlA proceed through the phosphorylation of the d-alanine at the carboxyl group by the γ-phosphate of ATP to produce an acylphosphate intermediate firstly. Then, a tetrahedral intermediate is formed by the nucleophilic attacking of the amino nitrogen of the second d-alanine on the acylphosphate intermediate. Finally, a phosphate group is released from the tetrahedral intermediate to generate the final product of d-alanyl-d-alanine (Figure 1) [7,8,9].
Figure 1.
A series of chemical reactions catalyzed by DdlA.
d-alanyl-d-alanine is involved in the pentapeptide biosynthesis of peptidoglycan (PG) as a dipeptide donor. PG is a universal and pivotal component of the mycobacterial cell wall. It is fundamental for bacteria to maintain the defined cell morphology, withstand the internal osmotic pressure, and affect cell division [10,11,12,13]. The biosynthesis of PG is an ideal target for anti-TB drug design because the whole pathways are not present in mammalian cells [9]. DdlA is one of the potent candidates as an anti-TB drug target among a great deal of factors that affect PG biosynthesis because (1) it utilizes d-alanine as its substrate, which is specific for bacterial PG biosynthesis; (2) it is essential in mycobacteria, and its essentiality was confirmed by means of transposon mutagenesis in 2003; and (3) the product of DdlA catalyzed reaction is d-ananly-d-alanine which promotes the cross-linking of PG by synthesizing the integrated pentapeptide. DdlA is responsible for supplying a substrate of d-alanyl-d-alanine for PG crosslinking.
d-cycloserine (DCS), a cyclic analogue of d-alanine and an effective second-line antibiotic against Mtb, inhibits the biosynthesis of the bacterial cell wall by targeting alanine racemase (Alr) and d-alanine:d-alanine ligase (Ddl). Nowadays, DCS is restricted to TB treatment due to its neurological side effects. DCS can be hydrolyzed to generate d-serine, which is a potent agonist at the glycine site of the NMDAR (N-methyl-d-aspartate-type glutamate receptor) which causes central nervous system side effects, such as headaches, drowsiness, depression, dizziness, vertigo, confusion, paresthesias, dysarthria, hyperirritability, psychosis, convulsions, and tremors. Overdose of DCS may result in paresis, seizures, and coma [6,14,15].
Nevertherless, Tb-DdlA is of particular importance as an effective and attractive anti-TB drug target. To obtain a better understanding of the DdlA profiles and develop a colorimetric assay for high-throughput inhibitor screening, we focused on explicating and characterizing Tb-DdlA which serves as a non-toxic and effective anti-TB compound screening.
2. Results
2.1. The Detection of Soluble Tb-DdlA Protein Expressed in Escherichia coli
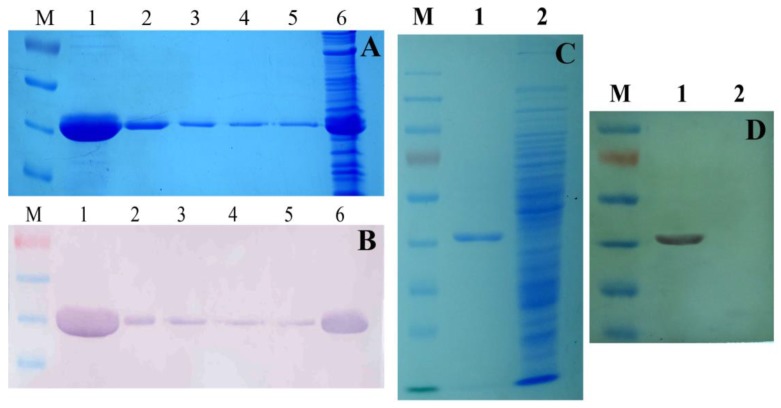
The Tb-ddlA gene was cloned into the HindIII and NdeI sites of pColdII generating a Tb-DdlA fusion protein with an N-terminal histidine tag (His-tag), which serves as a selective and efficient tool for protein purification and detection. The expected molecular weight of the Tb-DdlA fusion protein was about 39.68 kDa of Tb-DdlA protein, plus 1 kDa of the His-tag. The expressed protein was purified by Ni2+-NTA affinity chromatography from supernatant of cell lysates, and was examined using polyacrylamide gel electrophoresis (PAGE) and Western blot. The PAGE and Western blot results displayed that only one band was visible on the gel and nitrocellulose membrane for the purified Tb-DdlA fusion protein, which had a molecular weight around 40 kDa, which was consistent with the expected size of Tb-DdlA fusion protein (about 40 kDa) (Figure 2A,B). Figure 2C,D shows that the monoclonal anti-polyhistidine antibody, which was used to blot the expressed Tb-DdlA, was specific for the expressed Tb-DdlA fusion protein with His-tag compared to its negative control. Therefore, the PAGE and Western blot analysis demonstrated that a soluble Tb-DdlA protein was successfully and highly-efficiently expressed as a fusion protein with histidine tag in E. coli BL21(DE3).
Figure 2.
Analysis of the purified Tb-DdlA fusion protein by PAGE and Western blot. M, PageRuler prestained protein ladder (Fermentas), the sizes of the bands from top to bottom were 180 kDa, 130 kDa, 100 kDa, 70 kDa (red), 55 kDa, 40 kDa, 35 kDa, 25 kDa, and 15 kDa, respectively. (A) PAGE analysis of Tb-DdlA; 1–5, the elute fraction of Tb-DdlA fusion protein (with histidine tag) with an expected molecular weight of around 40 kDa purified by Ni2+-NTA affinity chromatography; 6, cell lysate from E. coli BL21(DE3) harboring pCold-ddlA; (B) Western Blot analysis of Tb-DdlA. The monoclonal anti-polyhistidine antibody at 1 to 5000 dilution was used to blot the expressed Tb-DdlA. The bands on the gel were visualized by BCIP/NBT solution; 1–5, the elute fraction of the Tb-DdlA fusion protein (with the histidine tag) with an expected molecular weight of around 40 kDa purified by Ni2+-NTA affinity chromatography; 6, cell lysate from E. coli BL21(DE3) harboring pCold-ddlA; (C) PAGE analysis of purified Tb-DdlA; 1, purified Tb-DdlA; 2, cell lysate from E. coli BL21(DE3) harboring pColdII; (D) Western Blot analysis of Tb-DdlA. The monoclonal anti-polyhistidine antibody at a 1 to 5000 dilution was used to blot the expressed Tb-DdlA. The bands on the gel were visualized by BCIP/NBT solution; 1, purified Tb-DdlA; 2, cell lysate from E. coli BL21(DE3) harboring pColdII.
2.2. The Profiles of Tb-DdlA d-Alanyl-d-alanine Ligase Activity
2.2.1. Confirmation of DdlA Activity
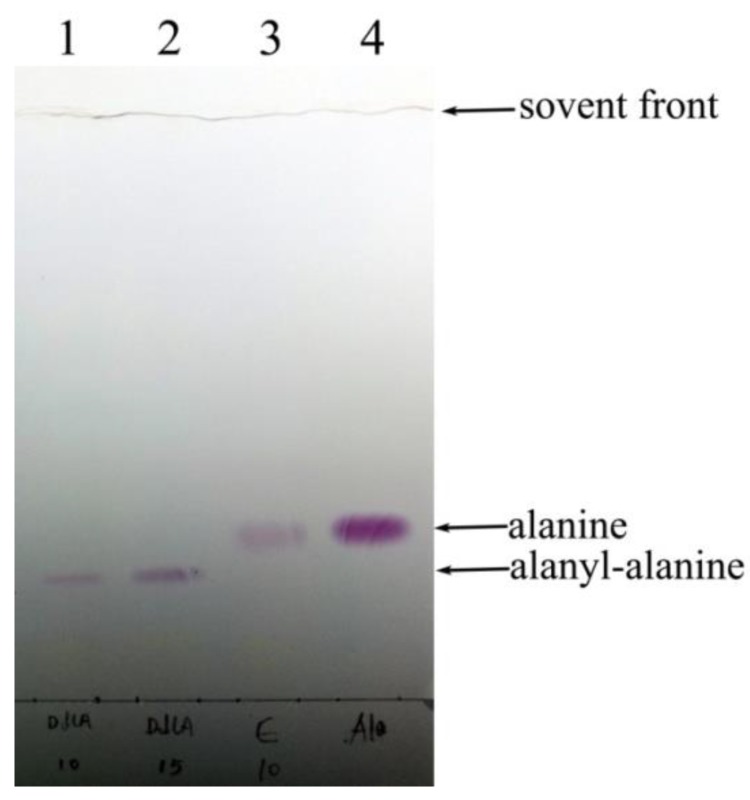
The DdlA that catalyzes the dimerization of two d-alanine molecules typically couples ATP hydrolysis to provide a thermodynamic driving force and exhibits a cleavage of ATP to ADP and orthophosphate. Thus, in this study, the colorimetric assay demonstrated d-alanyl-d-alanine ligase activity of DdlA as evidence from orthophosphate released, which was measured by adding malachite green reagent. Table 1 showed a high d-alanyl-d-alanine ligase activity of DdlA. Thus, the coupled colorimetric assay demonstrated that Tb-DdlA encoded by Rv2981c possessed ligase activity toward d-alanine by liberating a molecule of orthophosphate. In addition, the d-alanyl-d-alanine ligase activity was confirmed by detecting the yielded d-Alanyl-d-alanine using HP-TLC. The HP-TLC was performed in the solvent system of phenol:water (4:1), and the color spots were visualized by spraying 2% ninhydrin solution. The HP-TLC image displays the band of d-alanyl-d-alanine formed by dimerization of two molecules of d-alanine (Figure 3). The d-alanine was completely converted into d-alanyl-d-alanine, so no remaining d-alanine was detected on the plate. The reaction mixture containing all reactants, except Tb-DdlA, was used as a negative control in this study. Therefore, the coupled colorimetric assay and HP-TLC image confirmed that Tb-DdlA encoded by Rv2981c possessed ligase activity toward d-alanine to generate a dipeptide of d-alanyl-d-alanine with liberation of a molecule of orthophosphate.
Table 1.
The assay of d-alanyl-d-alanine ligase activity on Tb-DdlA. The decreasing d-alanine in the reaction mixture was measured by colorimetric reaction coupled with malachite green reagents. The analysis was conducted in quadruplicate.
| Reactions | Components | Mean Values of A620nm ± SD | Net Mean ± SD | Z-Factor | |
|---|---|---|---|---|---|
| Reaction 1 | 100 μmol/L ATP; 2 mmol/L d-Ala | 20 μg/mL DdlA | 0.692 ± 0.099 | 0.452 ± 0.099 | 0.187 |
| 100 μmol/L ATP; 2 mmol/L d-Ala | No DdlA | 0.240 ± 0.023 | |||
| Reaction 2 | 75 μmol/L ATP; 2 mmol/L d-Ala | 10 μg/mL DdlA | 0.459 ± 0.013 | 0.291 ± 0.013 | 0.735 |
| 75 μmol/L ATP; 2 mmol/L d-Ala | No DdlA | 0.168 ± 0.013 | |||
The above values in net mean ± SD had been deducted from values in DdlA group minus ones in its control group.
Figure 3.
The identification of d-alanyl-d-alanine ligase activity of Tb-DdlA by HP-TLC. Phenol:water of 4:1 was used as the developing reagent. The color spots were visualized by spraying ninhydrin; 4, the standard d-alanine; 3, control, no DdlA in the reaction mixture; 2, reactant, d-alanine was incubated with DdlA protein purified by Ni2+-NTA affinity choromatography; 15 μL was loaded; 1, reactant, d-alanine was incubated with DdlA protein purified by Ni2+-NTA affinity choromatography; 10 μL was loaded.
In addition, the Z-factor, a measure of the statistical effect size which has been proposed for use in high-throughput screening, was estimated according to the following equation: Z-factor = 1 − 3(σp + σn)/│μp − μn│ (parameters: μ, means; σ, standard deviation; p, positive of sample; n, negative control of sample). The Table 1 showed that the Z-factor of Reaction 2 was 0.735, which ranged between 0.5 and 1, so it was more reliable for high-throughput screening compared to Reaction 1, whose Z-factor was 0.187.
2.2.2. Kinetic Analysis of Tb-DdlA
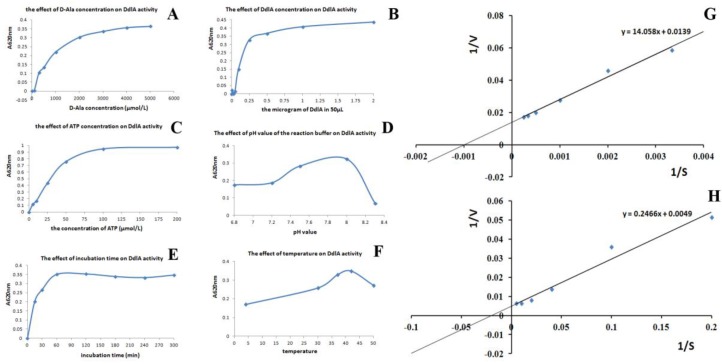
The kinetic parameters of Tb-DdlA were measured using a malachite green reagent-coupled assay. The catalytic activity of Tb-DdlA was measured by varying d-alanine concentration, DdlA concentration, ATP concentration, and pH value in the reaction mixture, respectively, and incubation time and temperature, as well. Tb-DdlA was observed to display a higher d-alanyl-d-alanine ligase activity at more than 1 mmol/L of alanine concentration, more than 0.6 μg/mL of DdlA concentration, 50 μmol/L of ATP concentration, pH 8.0, and cultivating at 37 °C for more than 30 min (Figure 4). Therefore, an optimal enzymatic activity was determined according to the kinetic parameters and Z-factor as follows: 2 mmol/L alanine, 10 μg/mL DdlA, 75 μmol/L of ATP, and cultivating at 37 °C in the pH 8.0 reaction mixture for 60 min. In addition, the Km, Vmax, and Kcat values of the Tb-DdlA for the substrate of D-alanine and ATP were determined by a double-reciprocal plot under the optimal catalytic conditions. The KmATP was 50.327 ± 4.652 μmol/L and the VmATP was 204.082 ± 1.021 mmol/min/mg at pH 8.0 and 37 °C (Figure 4H); KmAla was 1.011 ± 0.094 mmol/L and VmAla was 71.942 ± 0.884 mmol/min/mg under the above optimal conditions of enzyme-catalyzed chemical reaction (Figure 4H). Kcat was 0.044 ± 0.002 min−1. Therefore, the double-reciprocal plot indicated that the enzyme exhibited a higher affinity to ATP than d-alanine.
Figure 4.
Determination of optimal reaction conditions for Mtb DdlA activity assay. (A–F) represented the effect of alanine concentration, DdlA concentration, ATP concentration, pH, incubation time, and temperature on the enzyme activity respectively; (G) The effect of alanine concentration on Tb-DdlA activity analyzed by the double reciprocal plot; (H) The effect of ATP concentration on Tb-DdlA activity analyzed by the double reciprocal plot.
2.2.3. Inhibitory Analysis of Tb-DdlA Using DCS
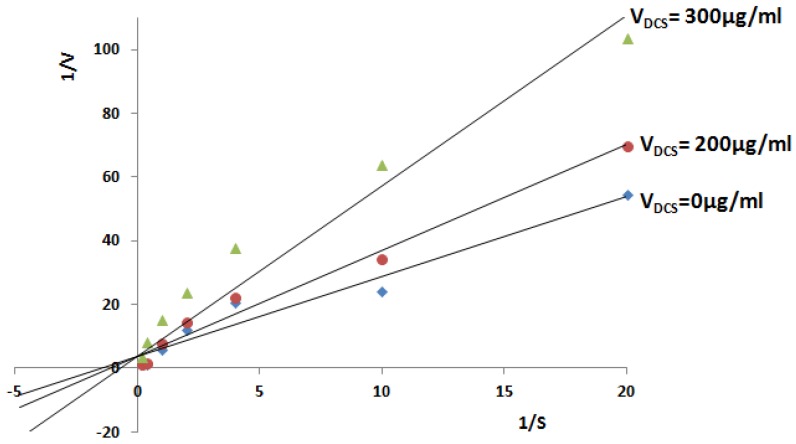
To demonstrate whether the colorimetric assay to measure the released orthophosphate using malachite green reagent can be utilized for screening of Tb-DdlA’s inhibitory compounds, the DCS, a known competitive inhibitor typically with close structural similarities to d-alanine, was used as a positive control in this study. Double-reciprocal plots for the mode of Tb-DdlA inhibition by DCS with respect to the substrate of d-alanine were demonstrated herein. The concentrations of inhibitors including 0, 25, 50, 100, 200, 300 μg/mL were used in this study. However, only a high concentration of DCS (≥200 μg/mL (1.959 mmol/L)) exhibited an obviously inhibitory effect on Tb-DdlA (Figure 5), and the inhibitory effect enhanced as the concentration of DCS increased.
Figure 5.
Steady state inhibition by DCS on d-alanyl-d-alanine ligase activity of DdlA. The double-reciprocal plots were obtained without or with different concentrations of DCS. The Lineweaver-Burk plot analysis of the data also confirmed the competitive inhibition.
2.3. The Identification of Tb-DdlA Potential Interaction Partners
Identifying interaction partners is key to understanding the function of a protein. In this study, the pull-down assay was used to obtain proteins in Msm that complexed with Tb-DdlA using Tb-DdlA-coated magnetic beads. To establish the pull-down assay, we expressed Tb-DdlA as a recombinant His6-fusion protein in E. coli and purified it to homogeneity. The loaded Tb-DdlA fusion protein was covalently coupled to Ni2+-NTA-activated sepharose beads, which were used to pull down putative interacting proteins from cell lysates of Msm. After washing, the putative interacting proteins were eluted and analyzed by PAGE and silver staining. In total, four extra bands were obtained by pull-down techniques (Figure 6).
Figure 6.
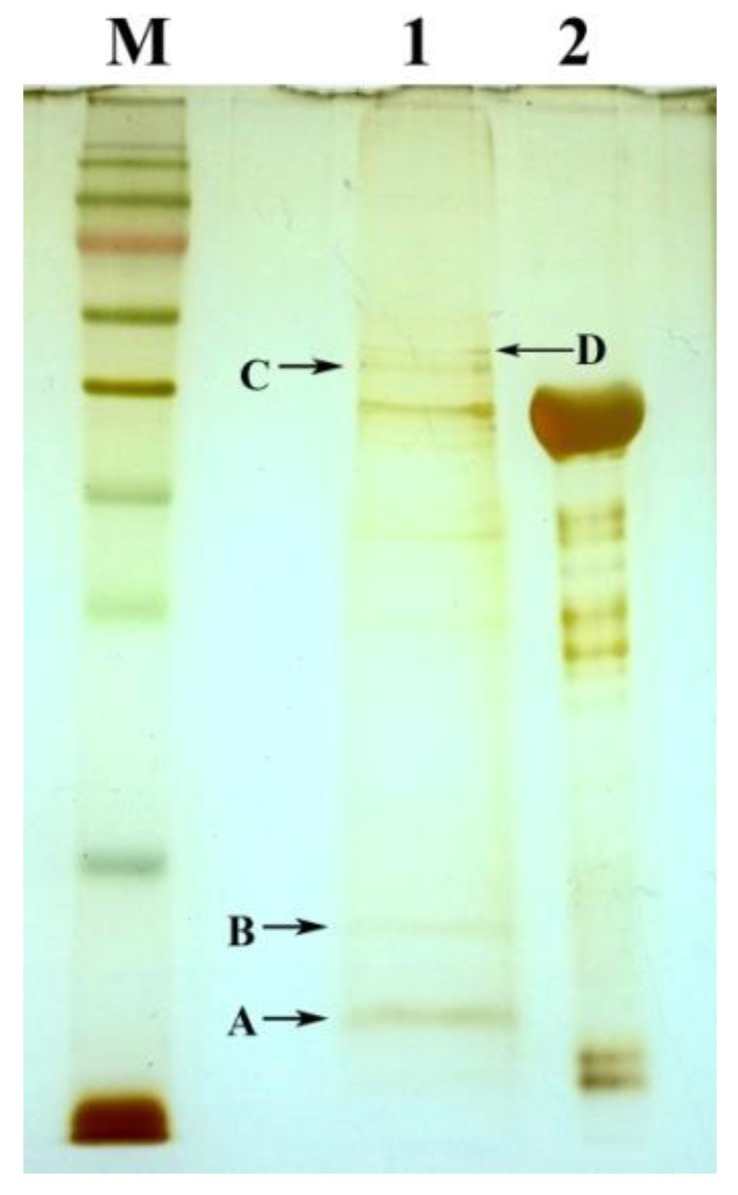
The PAGE analysis on Tb-DdlA interaction partners obtained by pull-down assay. The gel was stained by silver staining. M, PageRuler prestained protein ladder (Fermentas), the band sizes from top to bottom are 180 kDa, 130 kDa, 100 kDa, 70 kDa (red), 55 kDa, 40 kDa, 35 kDa, 25 kDa, 15 kDa, and 10 kDa, respectively; 1, Tb-DdlA interaction protein complexes obtained by pull-down assay; 2, Tb-DdlA purified by Ni2+-NTA affinity chromatography. A–D, the bands selected for MS identification.
The proteins from four bands on PAGE were deciphered as putative interaction partners of Tb-DdlA by mass spectrometry. The MS/MS analysis resulted in identification of eight different potential interacting partners of Tb-DdlA in total (Table 2). The list contained two well-known interactors which were involved in protein expression, including LuxR family transcriptional regulator (cupin) and elongation factor Tu (Tuf). The remaining five putative interacting partners were FAD-dependent oxidoreductase, ornithine-oxo-acid transaminase, diaminopimelate decarboxylase, cyclopropane-fatty-acyl-phospholipid synthase, and carbon-monoxide dehydrogenase large subunit, respectively. The last one was the hypothetical protein LI98_12890.
Table 2.
The list of the putative interaction partners of Tb-DdlA in Msm detected by MS/MS. A, B, C and D were the corresponding bands from the silver-stained polyacrylamide gel.
| ID | Protein Name | Accession No. (NCBI) | Locus Name/Gene Definition | Protein MW (D) | Protein PI | Pep. Count | Protein Score | Protein Score C. I. % |
|---|---|---|---|---|---|---|---|---|
| A | LuxR family transcriptional regulator | AIU17347.1 | MSMEG_5707, cupin | 12,011.3 | 6.36 | 5 | 285 | 100 |
| B | hypothetical protein LI98_12890 | AIU21016.1 | MSMEG_2589 | 15,412.9 | 6.28 | 7 | 314 | 100 |
| C | elongation factor Tu | YP_885786.1 | MSMEG_1401, tuf | 43,708.6 | 5.18 | 18 | 696 | 100 |
| FAD-dependent oxidoreductase | AIU20161.1 | MSMEG_1682, FMO | 46,185.2 | 5.86 | 13 | 74 | 99.697 | |
| ornithine-oxo-acid transaminase | WP_011727654.1 | MSMEG_1413, rocD | 44,661.1 | 5.62 | 14 | 72 | 99.581 | |
| D | diaminopimelate decarboxylase | YP_889210.1 | MSMEG_4958, lysA | 50,317 | 5.25 | 13 | 224 | 100 |
| cyclopropane-fatty-acyl-phospholipid synthase | YP_890503.1 | MSMEG_6284 | 48,608.7 | 5.99 | 13 | 198 | 100 | |
| carbon-monoxide dehydrogenase large subunit | WP_011727162.1 | MSMEG_0746 | 85,807 | 5.21 | 19 | 60 | 93.366 |
3. Discussion
The DdlA, an essential gene product determined by Himar1-based transposon mutagenesis in H37Rv strain [16], can dimerize two d-alanine molecules to generate the dipeptide of d-alanyl-d-alanine, which can be utilized as a substrate for PG biosynthesis and maturation. Tb-DdlA is of particular importance as an effective and attractive anti-TB drug target. In this study, we successfully expressed and purified soluble Tb-DdlA and analyzed its enzymatic characteristics. In addition, according to the kinetic parameters of the enzyme, we developed a colorimetric assay to measure the released orthophosphate for high-throughput screening of Tb-DdlA inhibitory compounds. During enzyme activity test, some disadvantages were found: (1) the color compound formed by malachite green reagent and orthophosphate had low solubility, so that an insoluble precipate was generated in the reaction mixture when it was catalyzed by Tb-DdlA with high activity. According to this property, we optimized the enzymatic parameters and Z-factor as follows: 2 mmol/L alanine, 10 μg/mL DdlA, 75 μmol/L of ATP in the pH 8.0 reaction mixture at 37 °C for 60 min. (2) malachite green reagent easily turned green in color even without adding anything, so we needed to perform color developing carefully in case the malachite green reagent was contaminated. Most importantly, the colorimetric assay to measure the released orthophosphate allowed a fast and accurate determination of Tb-DdlA’s ligase activity. Actually, in order to overcome the disadvantage of enzyme activty determination by adding malachite green reagent, we introduced a colorimetric assay of Tb-DdlA activity to monitor the decreased d-alanine using d-amino acid oxidase mixture (phenol, 4-aminoantipyrine, d-amino acid oxidase, peroxidase). However, the enzyme assay to detect the decreased d-alanine performed far lower sensitivity than to detect the released orthophosphate by adding malachite green reagent.
Usually, for inhibitory compound screening, the persistent enzyme activity was expected. However, during the enzyme activity assay, we found that Tb-DdlA only could maintain its high activity in 3 days after purification. On the second day, Tb-DdlA remained high activity, the absorbance reached to 0.758 ± 0.038 at 620 nm; then the enzyme’s activity started to decline and the absorbance of the assay was read as 0.452 ± 0.099 at 620 nm on the third day; until the fourth day, Tb-DdlA lost almost all of its activity (A620nm was about 0.124 ± 0.002). For inhibitory compound screening of Tb-DdlA, it was required to prepare the fresh enzyme or improve the storage conditions for Tb-DdlA. DCS definitely exhibited an inhibitory effect on mycobacteria. Thus, we had perform the DCS inhibitory effect on Msm cells using various final concentrations of DCS containing 0, 5, 10, 25, 50, 100, and 200 μg/mL. The results showed that 50 μg/mL (0.490 mmol/L) of DCS inhibited the cell growth of Msm efficiently in vivo. In this study, the double reciprocal plot indicated that high concentrations of DCS (≥200 μg/mL) displayed inhibitory effects on Tb-DdlA. DCS, a structural analogues of d-alanine, competitively inhibit the bacterial growth by blocking the activity of DdlA and alanine racemase (Alr), theoretically [17,18], and its inhibitory effect depends on the concentration of DCS and d-alanine. In this study, the optimal concentration of d-alanine was determined as 2 mmol/L, so it required high concentrations of DCS (200 μg/mL (1.959 mmol/L); 300 μg/mL (2.939 mmol/L)) to inhibit Tb-DdlA activity, while less than 100 μg/mL (0.979 mmol/L) did not display any inhibitory effect on Tb-DdlA. The double-reciprocal plot also showed that Tb-DdlA exhibited a higher affinity to ATP (KmATP: 50.327 ± 0.465 μmol/L) than alanine (KmAla: 1.011 ± 0.094 mmol/L).
The study of protein–protein interactions (PPIs) exhibits crucial roles in the field of medicine, pharmaceutical industry, and biology. Identifying protein interaction sites and uncovering the interaction mechanism is closely relevant in the drug development industry. In addition, unveiling of protein interaction partners allows biologists to construct protein interaction networks, which in turn facilitate the understanding of many biological and clinical observations [19,20,21,22]. The data in this study indicated that Tb-DdlA could not maintain its activity long in vitro under the conditions tested, which might be attributable to its synergistic effect with potential interacting proteins that stabilized its activity in vivo. Therefore, we performed a pull-down assay to identify the interaction partners of Tb-DdlA in Msm. Thus, a Tb-DdlA fusion protein with His-tag was expressed for detecting potential interaction partners of Tb-DdlA in Msm using Ni2+-NTA magnetic agarose beads. In this study, Msm has been used as a surrogate model instead of pathogenic and slower-growing mycobacterial species such as Mtb [23]. Msm is a non-pathogenic and fast growing species with highly genetic and antigenic homology with Mtb [24]. Msm had similar unique cell wall to Mtb. Additionally, the homology between Tb-DdlA and its counterpart of Sm-DdlA in Msm had been analyzed. The alignment analysis showed that Tb-DdlA was highly homologous to Sm-DdlA (the identities accounted for 79%, and the positives accounted for 87%). Therefore, it was applicable for pull-down assay to be performed using cell lysates of Msm as a prey.
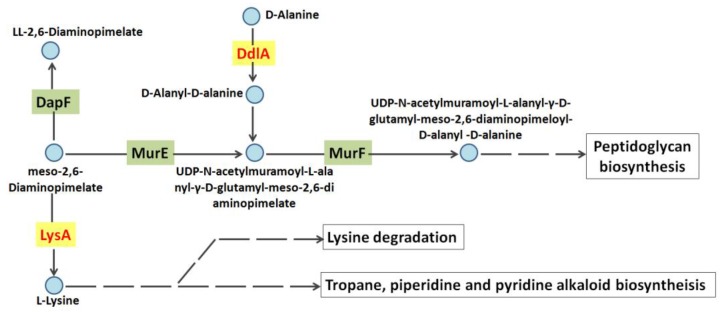
Totally, eight different potential interaction partners of Tb-DdlA had been identified in this study. Among these eight proteins, diaminopimelate decarboxylase (LysA) was of great interests. This enzyme was emphasized because it was particularly important with regard to PG biosynthesis. LysA participates in lysine biosynthesis, and catalyzes the conversion of lysine from meso-2,6-diaminoheptanedioate (DAP) with a liberation of CO2 molecule. DAP metabolic pathway has been attracting considerable attention due to its unique features in prokaryotes and its essentiality to cross-linking of PG which provides strength and rigidity to the cell wall in bacteria. It has been documented that mycobacterial cell walls contained an unusual high content of DAP [13,25,26,27,28]. The dipeptide d-alanyl-d-alanine, which catalyzed by DdlA was known to act as a substrate for the synthesis of petapeptides of PG as well. Figure 7 showed that DdlA and LysA had a close relationship in the pathway of PG synthesis. Therefore, we proposed a hypothesis that DdlA and LysA might act synergistically in PG biosynthesis in prokaryotes under the same regulation networks. The d-amino acids, such as DAP and d-alanine, play a crucial role in defining the cellular growth, cell wall integrity and protein synthesis of bacteria. In view of their importance, the designing of potential inhibitory compounds against any enzyme of the regulation networks might display a novel class of antitubercular agents which inhibit bacterial cell wall synthesis and protein biosynthesis, and are not toxic to mammalian species. Interestingly, LuxR family transcriptional regulator and elongation factor Tu were detected as interaction partners of Tb-DdlA, meanwhile, we performed the pull-down assay for the carboxypeptidase coded by Rv3627c, which hydrolyze the terminal d-alanine from the petapeptides of PG to promote PG crosslink in mycobacteria. The MS identification of Rv3627c interaction partners demonstrated that the above two proteins identified by Tb-DdlA were detected as interaction partners of Rv3627c, as well (data not published). We hypothesized that the LuxR family transcriptional regulator and elongation factor Tu were involved in the transcription and translation of Tb-DdlA and Rv3627c in mycobacteria, thus, it provided a promising view on anti-mycobacterial drug design that a compound which specifically targets LuxR family transcriptional regulator or elongation factor Tu of prokaryotes may be vital to bacterial survival.
Figure 7.
A view of metabolic pathway of DdlA and LysA leading to peptidoglycan biosynthesis. The pathway was summarized from the KEGG website (http://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=mtu00300&keyword=lysA and http://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=mtu01502&keyword=ddlA). Single lines represent the proceeding individual biosynthesis pathway catalyzed by the highlighted gene products. Dashed lines represent a series of successive biosynthesis pathways. Round nodes displayed metabolite intermediates. The genes associated with individual reaction were highlighted in grey color.
In summary, we developed an easy and fast colorimetric assay for Tb-DdlA, which could be utilized for high-throughput screening of Tb-DdlA inhibitory compounds under the reaction conditions determined herein. In addition, we presented an analysis on Tb-DdlA interaction partners by the combination of pull-down assay with MS/MS. Eight putative interaction partners of Tb-DdlA had been identified in this study. Thus, our dataset provided a valuable resource for exploring Tb-DdlA biology.
4. Methods
4.1. Strains, Plasmids, and Growth Conditions
The characters of plasmids and bacterial strains used in this context were summarized in Table 3. Escherichia coli NovaBlue and BL21(DE3) cells were grown in Luria-Bertani (LB) broth or LB agar at 37 °C routinely. Tb-DdlA expression was performed at 16 °C. For the final concentration of antibiotic, 100 μg/mL ampicillin (Amp) was used in this study.
Table 3.
Bacterial strains and plasmids used in this study.
| Strains/Plasmids | Description | Source |
|---|---|---|
| Strains | ||
| E. coli NovaBlue | Used for cloning and propagation of plasmids | Novagen |
| E. coli BL21(DE3) | Used for expressing Tb-DdlA protein | Invitrogen |
| Plasmids | ||
| pMD18-T | Carries ampR gene; used for cloning PCR product with A at 3′ ends | Takara |
| pCold II | Carries ampR gene; contains cold start promoter; used for expressing M. tuberculosis DdlA | Takara |
| pMD18-Tb-ddlA | Carries ampR gene; M. tuberculosis ddlA was cloned to the EcoRV site of pMD18-T | This study |
| pCold II-Tb-ddlA | Carries ampR gene; used for expressing M. tuberculosis DdlA | This study |
4.2. Construction of Tb-ddlA Expression Plasmids
The Mtb ddlA gene (Rv2981c) was amplified from Mtb H37Rv genomic DNA (supplied by Colorado State University through the NIH contract “Tuberculosis research materials and vaccine testing.”) using the forward primer of 5′ AG CATATG AGT GCT AAC GAC CGG CGT G 3′ (underlined sequence was NdeI site) and the reverse primer of AT AAGCTT CTA GTG CAG GCC CAC GCC GCG 3′ (underlined sequence was HindIII site). The purified PCR product was cloned into pMD18-T to generate pMD18-Tb-ddlA recombinant plasmid. Then the sequence-confirmed ddlA was cloned into pCold II, which carries a hexahistidine tag and a cold start promoter. The new derived recombinant plasmid was named as pCold II-Tb-ddlA, which was transformed into Escherichia coli BL21(DE3) for expression of the fusion protein.
4.3. Expression, Purification, and Identification of Tb-DdlA
For protein expression, E. coli BL21(DE3) carrying pCold II-Tb ddlA was grown in 200 mL LB broth containing Amp at 37 °C until the optical density reached 0.5 at 600 nm, followed by induction with 0.25 mmol/L isopropyl-d-thiogalactopyranoside (IPTG) at 16 °C for another 20 h. The cells were harvested and lysed by sonication (30s pulse with 40 s cooling interval) in chilled lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 500 mmol/L NaCl, 20% glycerol, 1 mmol/L phenylmethylsulphonyl fluoride [PMSF]). Lysates were clarified by centrifugation at 27,000× g for 40 min, twice. The cytoplasmic fraction was applied to a pre-equilibrated Ni-NTA column (Qiagen) of 1.0 mL column volume. The column was washed with 20 mL wash buffer (lysis prep buffer with 20 mmol/L imidazole), and eluted with 10 mL elute buffer (lysis prep buffer with 200 mmol/L imidazole), 1 mL was collected for each tube, and the final protein concentration of the elute was determined by BCA kit.
The eluted fractions were analyzed by running a 12% SDS-polyacrylamide gel in a vertical electrophoresis apparatus and transferred to a nitrocellulose membrane in blotting buffer (20 mmol/L Tris-base, 150 mmol/L glycine and 20% methanol) to make the proteins accessible to antibody detection. The probing of the membrane with antibody of (anti)-polyhistidine monoclonal HIS-1 (Sigma, St. Louis, MO, USA) was conducted manually followed by incubation with the secondary antibody of antimouse-IgG conjugated alkaline phosphatase (Sigma, St. Louis, MO, USA), and the colorimetric detection of protein bands were developed by BCIP/NBT solution.
4.4. Functional Assay of Tb-DdlA
The enzyme’s activities were assayed in a 96-well microtiter plate at a total volume of 50 μL at 37 °C for 60 min. The reaction mixture was composed of 100 mmol/L HEPES (pH 8.0, 100 mmol/L KCl, 100 mmol/L MgCl2, 100 μmol/L ATP), and 2 mmol/L d-Ala. The purified Tb-DdlA (20 μg/mL or 10 μg/mL) was added to start the reaction after all assay components except the Tb-DdlA were pre-incubated at 37 °C for 10 min. The enzyme activities were monitored by measuring the release of orthophosphate. The concentration of released orthophosphate was measured by adding malachite green reagent (0.03% (w/v) malachite green, 0.2% (w/v) ammonium molybdate, and 0.05% (v/v) Triton X-100 in 0.7 N HCl) at 37 °C for 5 min. The plates were read at 620 nm by a microplate reader (Thermo Scientific Multiskan Ascent, Thermo Fisher Scientific Oy FI., Vantaa, Finland).
4.5. Kinetic Analysis of Tb-DdlA
The steady-state kinetic parameters were routinely evaluated by measuring the release of orthophosphate in microtiter plate. The optimized assay was performed in a final volume of 50 μL, and the absorbance was measured at 620 nm. The initial velocity was obtained by performing the enzymatic reaction at different incubation times and different concentrations of the purified enzyme. Then, kinetic parameters of Tb-DdlA catalyzed reaction were determined in the range of initial velocity. The optimum pH was determined using HEPES-NaOH at the pH of 7.2, 7.5, 8.0, and 8.3. The thermal stabilities of Tb-DdlA were determined by measuring enzyme activities at 4 °C, 30 °C, 37 °C, 42 °C, and 55 °C in the HEPES-NaOH buffer (pH 8.0). The Michaelis constant (Km) and maximal reaction velocity (Vm) were determined by linear regression analysis based on the Lineweaver-Burk equation. The KmD-Ala and VmD-Ala against d-alanine was determined by carrying out the enzymatic reaction at various d-alanine concentrations including 0, 0.05, 0.1, 0.25, 0.5, 1, 2.5, and 5 mmol/L, and KmATP and VmATP against ATP was determined by performing the enzymatic reaction at various ATP concentrations containing 0, 5, 10, 25, 50, 100, 200, 400, 500, and 1000 μmol/L. In this study, each of enzymatic reactions was conducted in triplicate. To eliminate the interference of buffer components, the background absorbance of the reaction mixture without Tb-DdlA was measured and subtracted from the absorbance values with Tb-DdlA.
4.6. Colorimetric Inhibition Assay of Tb-DdlA by DCS
The antibiotic DCS, a known substrate competitive inhibitor of Tb-DdlA, was used to measure the inhibitory effects on Tb-DdlA protein. A double reciprocal plot was performed in the presence of inhibitor (0, 25, 50, 100, 200, and 300 μg/mL) under the following conditions using 100 mM HEPES (pH 8.0, 100 mmol/L KCl, 100 mmol/L MgCl2, 100 μmol/L ATP and a series of gradient concentrations of d-Ala including 0, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5 mmol/L), and 0.25 μg (5 μL) purified Tb-DdlA in a total volume of 50 μL at 37 °C for 60 min. DdlA activity was monitored by measuring the release of orthophosphate at 620 nm.
4.7. Pull down Assay of Tb-DdlA
Three microgram of purified His6-tagged Tb-DdlA (bait) was bound with Ni-NTA magnetic agarose beads suspension by rotating slowly at 4 °C for 1 h, then the supernatant was removed by using the magnetic MagRack6™ (Qiagen, Hong Kong) to leave only the magnetic beads with bound proteins in a microcentrifuge tube. The bound Tb-DdlA were incubated with 200 μL soluble proteins (prey) from the whole-cell lysates of Msm with gentle rotation at 4 °C for 1 h. The resins were washed to remove non-specific bound proteins. The bound proteins were eluted and electrophoresed by 12% SDS-PAGE. Gel bands were excised for a further MS/MS analysis after visualization by silver staining kit (Sigma, St. Louis, MO, USA). The detailed process of pull-down assay was performed as the manufacturer’s instructions.
4.8. MS/MS Analysis
Silver-stained gel slices were excised into small pieces followed by decolorization in 100 mmol/L NH4HCO3/30%ACN with K3Fe(CN)6 and Na2S2O3. Then, the digestion of decolorized gel was performed in 100 mmol/L NH4HCO3 solution containing 12.5 ng/μL of sequencing-grade trypsin for 20 h at 37 °C according to a modified in-gel trypsin digestion procedure. Mass spectrometry was carried out by using a 5800 MALDI-TOF/TOF MS (AB SCIEX, Foster City, CA, USA) and conducted in positive ionization mode and automatic data acquisition mode. The optimal source/gas parameters were set as follows: the instrument contained a Nd:YAG laser; the mass analyzer scan was 800–4000 Da m/z; the ions were accelerated with a voltage of 2 kV; the list of peptides of interest meant for the fragmentation analyses was manually defined in the equipment’s current settings with 2 kV collision energy and CID off; data were acquired using Mascot 2.2 software (Matrix Science, http://www.matrixscience.com). The obtained MS/MS spectra were searched against the NCBI database.
Acknowledgments
This work was supported by his work was supported by National Natural Science Foundation of China (81272429).
Author Contributions
W.Z. conceived the study, performed the experiments including pull down assay of Tb-DdlA and kinetic analysis of Tb-DdlA, and contributed in writing of the manuscript. S.Y. performed the experiments including the construction of expression vector and the assay of enzyme activities. Y.X. processed and analyzed the data, and provided material support. Y.W. performed the experiments including protein purification. F.R. provided material support. S.L. contributed in editing the manuscript. W.D. provided financial support. Y.M. provided working space and fundamental material support. All authors shared in the responsibility for the final decision to submit it for publication.
Conflicts of Interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The research process was handled objectively and no conflict of interest exists.
Footnotes
Sample Availability: Samples of the pCold II-Tb-ddlAplasmid are available from the authors.
References
- 1.WHO gtbr2017 WHO TB report.pdf. [(accessed on 1 December 2017)]; Available online: http://www.who.int/tb/publications/global_report/en/
- 2.Zhang W., Jones V.C., Scherman M.S., Mahapatra S., Crick D., Bhamidi S., Xin Y., McNeil M.R., Ma Y. Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase. Int. J. Biochem. Cell Biol. 2008;40:2560–2571. doi: 10.1016/j.biocel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ba Diallo A., Ossoga G.W., Daneau G., Lo S., Ngandolo R., Djaibe C.D., Djouater B., Mboup S., de Jong B.C., Diallo A.G., et al. Emergence and clonal transmission of multi-drug-resistant tuberculosis among patients in Chad. BMC Infect. Dis. 2017;17:579. doi: 10.1186/s12879-017-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prosser G.A., de Carvalho L.P. Metabolomics Reveal d-alanine: d-alanine Ligase As the Target of d-Cycloserine in Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2013;4:1233–1237. doi: 10.1021/ml400349n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCoy A.J., Maurelli A.T. Characterization of Chlamydia MurC-Ddl, a fusion protein exhibiting D-alanyl-D-alanine ligase activity involved in peptidoglycan synthesis and D-cycloserine sensitivity. Mol. Microbiol. 2005;57:41–52. doi: 10.1111/j.1365-2958.2005.04661.x. [DOI] [PubMed] [Google Scholar]
- 6.Halouska S., Fenton R.J., Zinniel D.K., Marshall D.D., Barletta R.G., Powers R. Metabolomics analysis identifies d-alanine-d-alanine ligase as the primary lethal target of d-Cycloserine in mycobacteria. J. Proteom. Res. 2014;13:1065–1076. doi: 10.1021/pr4010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura Y., Ebihara A., Agari Y., Shinkai A., Hirotsu K., Kuramitsu S. Structure of D-alanine-D-alanine ligase from Thermus thermophilus HB8: Cumulative conformational change and enzyme-ligand interactions. Acta Crystallogr. Sec. D Biol. Crystallogr. 2009;65:1098–1106. doi: 10.1107/S0907444909029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruning J.B., Murillo A.C., Chacon O., Barletta R.G., Sacchettini J.C. Structure of the Mycobacterium tuberculosis d-alanine:d-alanine ligase, a target of the antituberculosis drug D-cycloserine. Antimicrob. Agents Chemother. 2011;55:291–301. doi: 10.1128/AAC.00558-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.H., Na Y., Song H.E., Kim D., Park B.H., Rho S.H., Im Y.J., Kim M.K., Kang G.B., Lee D.S., et al. Crystal structure of the apo form of d-alanine: d-alanine ligase (Ddl) from Thermus caldophilus: A basis for the substrate-induced conformational changes. Proteins. 2006;64:1078–1082. doi: 10.1002/prot.20927. [DOI] [PubMed] [Google Scholar]
- 10.Scheffers D.J., Pinho M.G. Bacterial cell wall synthesis: New insights from localization studies. Microbiol. Molecular Biol. Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauvage E., Kerff F., Terrak M., Ayala J.A., Charlier P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 12.Macheboeuf P., Contreras-Martel C., Job V., Dideberg O., Dessen A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol. Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 13.Pavelka M.S., Jr., Jacobs J.W., Jr. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J.M., Uplekar S., Gordon S.V., Cole S.T. A point mutation in cycA partially contributes to the D-cycloserine resistance trait of Mycobacterium bovis BCG vaccine strains. PLoS ONE. 2012;7:e43467. doi: 10.1371/journal.pone.0043467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schade S., Paulus W. D-Cycloserine in Neuropsychiatric Diseases: A Systematic Review. Int. J. Neuropsychopharmacol. 2016;19:pyv102. doi: 10.1093/ijnp/pyv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CM S., DH B., EJ R. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng Z., Barletta R.G. Roles of Mycobacterium smegmatis D-Alanine:D-Alanine Ligase and D-Alanine Racemase in the Mechanisms of Action of and Resistance to the Peptidoglycan Inhibitor D-Cycloserine. Antimicrob. Agents Chemother. 2003;47:283–291. doi: 10.1128/AAC.47.1.283-291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halouska S., Chacon O., Fenton R.J., Zinniel D.K., Barletta R.G., Powers R. Use of NMR metabolomics to analyze the targets of D-cycloserine in mycobacteria: Role of D-alanine racemase. J. Proteom. Res. 2007;6:4608–4614. doi: 10.1021/pr0704332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R., Samal S.K., Routray S., Dash R., Dixit A. Identification of oral cancer related candidate genes by integrating protein-protein interactions, gene ontology, pathway analysis and immunohistochemistry. Sci. Rep. 2017;7:2472. doi: 10.1038/s41598-017-02522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampe J.N. Advances in the Understanding of Protein-Protein Interactions in Drug Metabolizing Enzymes through the Use of Biophysical Techniques. Front. Pharmacol. 2017;8:521. doi: 10.3389/fphar.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modell A.E., Blosser S.L., Arora P.S. Systematic Targeting of Protein-Protein Interactions. Trends Pharmacol. Sci. 2016;37:702–713. doi: 10.1016/j.tips.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedan Y., Marcu O., Lyskov S., Schueler-Furman O. Peptiderive server: Derive peptide inhibitors from protein-protein interactions. Nucleic Acids Res. 2016;44:W536–W541. doi: 10.1093/nar/gkw385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal P., Miryala S., Varshney U. Use of Mycobacterium smegmatis deficient in ADP-ribosyltransferase as surrogate for Mycobacterium tuberculosis in drug testing and mutation analysis. PLoS ONE. 2015;10:e0122076. doi: 10.1371/journal.pone.0122076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente W., Pienaar E., Fast A., Fluitt A., Whitney S., Fenton R., Barletta R., Chacon O., Viljoen H. A Kinetic Study of In Vitro Lysis of Mycobacterium smegmatis. Chem. Eng. Sci. 2009;64:1944–1952. doi: 10.1016/j.ces.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehrmann A., Phillipp B., Sahm H., Eggeling L. Different modes of diaminopimelate synthesis and their role in cell wall integrity: A study with Corynebacterium glutamicum. J. Bacteriol. 1998;180:3159–3165. doi: 10.1128/jb.180.12.3159-3165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCoy A.J., Adams N.E., Hudson A.O., Gilvarg C., Leustek T., Maurelli A.T. l,l-diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate/lysine. Proc. Natl. Acad. Sci. USA. 2006;103:17909–17914. doi: 10.1073/pnas.0608643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gokulan K., Rupp B., Pavelka M.S., Jr., Jacobs W.R., Jr., Sacchettini J.C. Crystal structure of Mycobacterium tuberculosis diaminopimelate decarboxylase, an essential enzyme in bacterial lysine biosynthesis. J. Biol. Chem. 2003;278:18588–18596. doi: 10.1074/jbc.M301549200. [DOI] [PubMed] [Google Scholar]
- 28.Consaul S.A., Wright L.F., Mahapatra S., Crick D.C., Pavelka M.S., Jr. An unusual mutation results in the replacement of diaminopimelate with lanthionine in the peptidoglycan of a mutant strain of Mycobacterium smegmatis. J. Bacteriol. 2005;187:1612–1620. doi: 10.1128/JB.187.5.1612-1620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]